- 1Department of Psychiatry, The Brain Hospital of Guangxi Zhuang Autonomous Region, LiuZhou, China
- 2Department of Psychiatry, Chongqing Jiangbei Second Hospital, Chongqing, China
- 3Department of Psychiatry, The Affiliated Brain Hospital of Guangzhou Medical University, Guangzhou, China
- 4Key Laboratory of Neurogenetics and Channelopathies of Guangdong Province and the Ministry of Education of China, Guangzhou Medical University, Guangzhou, China
- 5Department of Psychiatry, Chongqing mental health center, Chongqing, China
- 6Discipline of Psychiatry, University of Tasmania, Hobart, TAS, Australia
- 7Section of Psychiatry, University of Notre Dame Australia, Fremantle, WA, Australia
- 8Division of Psychiatry, School of Medicine, University of Western Australia, Perth, WA, Australia
- 9Unit of Psychiatry, Department of Public Health and Medicinal Administration, & Institute of Translational Medicine, Faculty of Health Sciences, University of Macau, Macao, Macao SAR, China
- 10Centre for Cognitive and Brain Sciences, University of Macau, Macao, Macao SAR, China
Background: The efficacy and safety of deep transcranial magnetic stimulation (dTMS) as an intervention for schizophrenia remain unclear. This systematic review examined the efficacy and safety of dTMS for schizophrenia.
Methods: A systematic search of Chinese (WanFang and Chinese Journal Net) and English databases (PubMed, EMBASE, PsycINFO, and Cochrane Library) were conducted.
Results: Three randomized clinical trials (RCTs) comprising 80 patients were included in the analyses. Active dTMS was comparable to the sham treatment in improving total psychopathology, positive symptoms, negative symptoms, and auditory hallucinations measured by the Positive and Negative Syndrome Scale (PANSS), the Scale for the Assessment of Positive Symptoms (SAPS), the Scale for the Assessment of Negative Symptoms (SANS), and the Auditory Hallucinations Rating Scale (AHRS), respectively. Only one RCT reported the effects on neurocognitive function measured by the Cambridge Neuropsychological Test Automated Battery (CANTAB), suggesting that dTMS may only improve one Stockings of Cambridge measure (i.e., subsequent times for five move problems). All three studies reported overall discontinuation rates, which ranged from 16.7% to 44.4%. Adverse events were reported in only one RCT, the most common being tingling/twitching (30.0%, 3/10), head/facial discomfort (30.0%, 3/10), and back pain (20.0%, 2/10).
Conclusion: This systematic review suggests that dTMS does not reduce psychotic symptoms in schizophrenia, but it shows potential for improving executive functions. Future RCTs with larger sample sizes focusing on the effects of dTMS on psychotic symptoms and neurocognitive function in schizophrenia are warranted to further explore these findings.
Introduction
Schizophrenia is a psychiatric disorder characterized by psychotic symptoms and impaired cognition, emotional life and insight (1, 2). As a chronic illness, schizophrenia imposes a substantial burden on sufferers, their families, and society due to its high rate of disability (3). However, patients and their families often face barriers in seeking treatment, such as limited insight, real or perceived stigma, and a pessimistic prognosis (4, 5). Effective treatment can enhance engagement between patients and their families, improve quality of life, and contribute to the reintegration of patients into society, thereby reducing the incidence of destructive behaviors such as violence and aggression (6).
Psychopharmacological treatment has been the primary approach in treating schizophrenia since the 1950s. However, a significant proportion of patients has poor treatment response or intolerable drug side effect (7), and up to 70% of patients are resistant to first-line antipsychotic treatments (8). Consequently, in addition to electroconvulsive therapy (ECT) (9, 10), there has been growing interest in exploring adjunctive, non-invasive brain stimulation (NIBS) techniques such as transcranial magnetic stimulation (TMS) (11), magnetic seizure therapy (MST) (12), transcranial direct current stimulation (tDCS) (13), and transcranial alternating current stimulation (tACS) (14). There is preliminary evidence that NIBS may improve psychiatric symptoms (15) and neurocognitive function (16) in patients with schizophrenia. However, these techniques also have limitations. For instance, ECT can negatively affect memory and neurocognitive function (17), and the effectiveness and safety of MST for schizophrenia remain uncertain (18).
A promising new development is deep transcranial magnetic stimulation (dTMS), which addresses the limitations of traditional TMS. Traditional TMS is limited in its ability to accurately stimulate localized targets, as in 27% to 32% of patients the intended stimulation site is not reached (19). When compared to traditional TMS using a 8 coil, dTMS with a specialized (H) coil is more precise and allows for deeper stimulation through brief magnetic pulses that induce targeted neuronal depolarization in the cerebral cortex (20). As a novel technique, dTMS enables non-invasive stimulation of deep layers of the prefrontal cortex. Several studies have found a method of modulating insula function through targeted neurostimulation, which can be achieved using dTMS (21, 22). This technique has demonstrated efficacy in various neurological and psychiatric disorders, including depression (23), obsessive-compulsive disorder (24), and substance use disorders (25). dTMS does not require hospitalization or anesthesia and has minimal side effects (26). It has several further advantages, including deeper stimulation, broader range, reduced damage to the surface cortex, effective reduction of static electric field interference, and slower decay rate (27).
However, the therapeutic effect of dTMS in schizophrenia is still inconsistent. An open-label study found that dTMS significantly improved negative symptoms in schizophrenia (28), but recent randomized controlled trials (RCTs) (29–31) concluded that dTMS did not improve psychotic symptoms in schizophrenia. For example, Rosenberg et al. (29) found that low-frequency dTMS did not have a statistically significant effect on auditory hallucinations or other psychopathology in schizophrenia.
A systematic review (32) examined the efficacy and safety of dTMS in schizophrenia based on one RCT (30) and a single-arm perspective study (33), with obvious heterogeneity in methodology between studies; furthermore, this systematic review did not incorporate a recent double-blind RCT (31) on the efficacy and safety of dTMS in schizophrenia. To understand the current literature on the role of dTMS in schizophrenia and provide a more comprehensive and robust basis for clinical application, the present systematic review included three RCTs that evaluated the therapeutic efficacy and safety of dTMS in schizophrenia.
Methods
Search strategy and selection criteria
Three researchers, YM, XJL and CJD, independently conducted a literature search in four international (PubMed, EMBASE, PsycINFO, and Cochrane Library) and two Chinese (WanFang and Chinese Journal Net) databases from their inception to July 6, 2023. The search terms were as follows: (“deep transcranial magnetic stimulation” OR “deep repetitive transcranial magnetic stimulation” OR deep rTMS OR deep TMS OR dTMS OR H-coil) AND (schizophrenia [MeSH] OR schizophrenic disorder OR disorder, schizophrenic OR schizophrenic disorders OR schizophrenia OR dementia praecox) AND (random* OR sham OR placebo OR control). Furthermore, the reference lists of eligible articles (29–31) and a systematic review (32) were screened for additional studies.
In accordance with the PRISMA guidelines, the inclusion criteria of this systematic review were based on the PICOS acronym (34). Participants: adults diagnosed with schizophrenia and schizoaffective disorder according to international criteria. Intervention versus Comparison: active dTMS plus antipsychotics or no antipsychotics versus sham dTMS plus antipsychotics or no antipsychotics. Outcomes: the primary outcome was the change in total psychopathology at post-dTMS, measured using standardized instruments such as the Positive and Negative Syndrome Scale (PANSS) (35) or the Brief Psychiatric Rating Scale (BPRS) (36). Secondary outcomes included positive, negative, and general psychopathology scores of the PANSS, BPRS, the Scale for the Assessment of Positive Symptoms (SAPS) or the Scale for the Assessment of Negative Symptoms (SANS), auditory hallucinations measured by the Auditory Hallucination Rating Scale (AHRS) (37), neurocognitive function, discontinuation due to any reason, and adverse events. Study: published RCTs of dTMS for schizophrenia. Studies on active dTMS versus other forms of NIBS, review articles, and case reports/series were excluded.
Data extraction
Data extraction from each included RCT was carried out independently by the same three researchers. A standardized form was used for data extraction that included authorship, publication year, study design, dTMS protocol, and primary and secondary outcomes. When additional information was required, the study author(s) were contacted to provide missing data.
Study quality assessment
The quality of the RCTs was evaluated independently by the same three researchers using the Jadad scale (38) and the Cochrane risk of bias (39). RCTs with a Jadad score of ≥3 were classified as of “high quality” (40).
Results
Literature search
As shown in Figure 1, 87 studies were identified. After screening the titles, abstracts, and full texts independently by the same three researchers, three RCTs met the inclusion criteria (29–31). Due to the insufficient data, a meta-analysis could not be conducted.
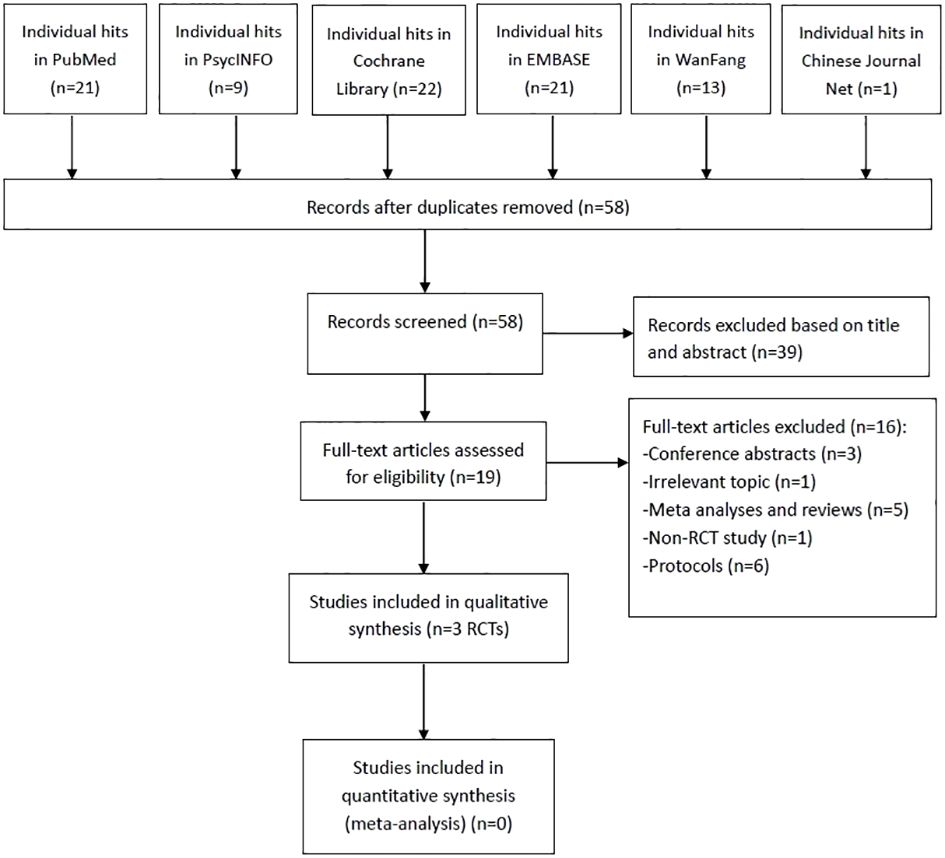
Figure 1 PRISMA flow diagram. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses; RCTs, randomized controlled trials.
Study characteristics
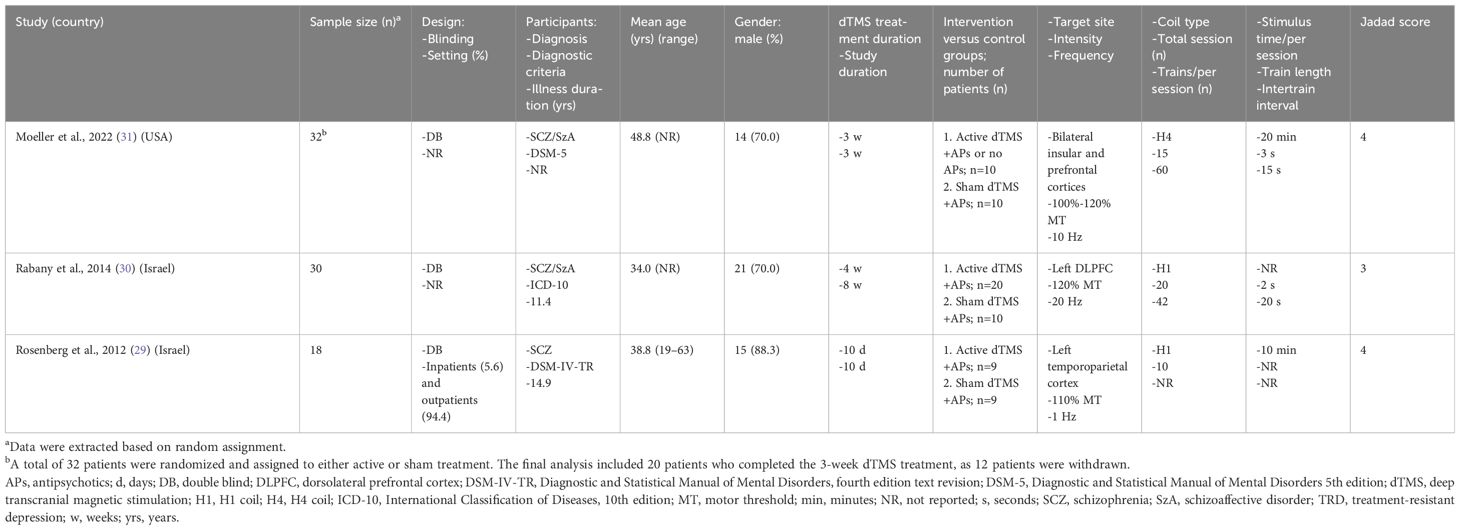
Table 1 shows the participants’ characteristics and dTMS parameters across the three RCTs (n = 80). These studies, published between 2012 and 2022, compared active dTMS (n = 39) with sham tACS (n = 29) in patients with schizophrenia or schizoaffective disorder. Two RCTs (2/3, 66.7%) were conducted in Israel, and one (1/3, 33.3%) in the USA (Table 1). The weighted mean age of the participants was 39.6 years, with 73.5% (range: 70.0%–88.3%) being male. Regarding treatment target, Moeller et al. (31) targeted bilateral insular and prefrontal cortices, while Rabany et al.’ (30) focused on the left dorsolateral prefrontal cortex, and Rosenberg et al.’ (29) aimed at the left temporoparietal cortex. The stimulation intensity and frequency in the protocols were different between studies, with the stimulation intensity ranging from 100% to 120%, and the frequency ranging from 1, 10 and 20 Hz (Table 1).
Quality assessment
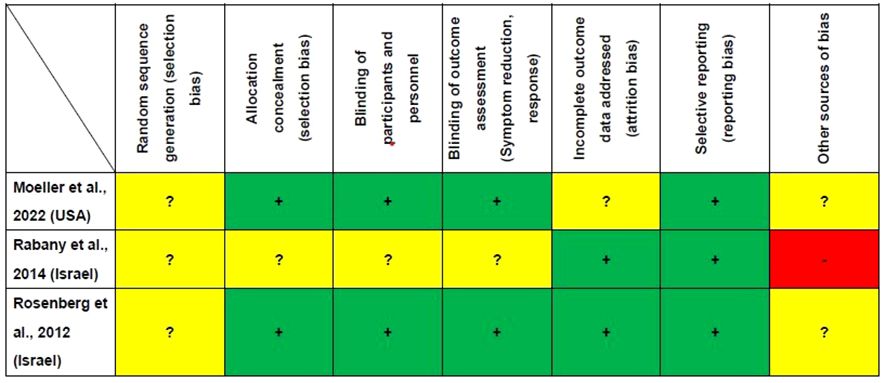
Figure 2 presents the study quality assessment of the three RCTs. Two RCTs (2/3, 66.7%) were deemed ‘low risk’ for allocation concealment, blinding of participants and personnel and blinding of outcome assessment (29, 31). Two RCTs (2/3, 66.7%) were classified as ‘low risk’ for incomplete outcome data (29, 30). All the three RCTs were rated as ‘low risk’ for selective reporting (29–31). The mean Jadad score was 3.7 (range: 3–4), indicating that all the studies were of high quality (Jadad score ≥ 3) (Table 1).
Psychopathology
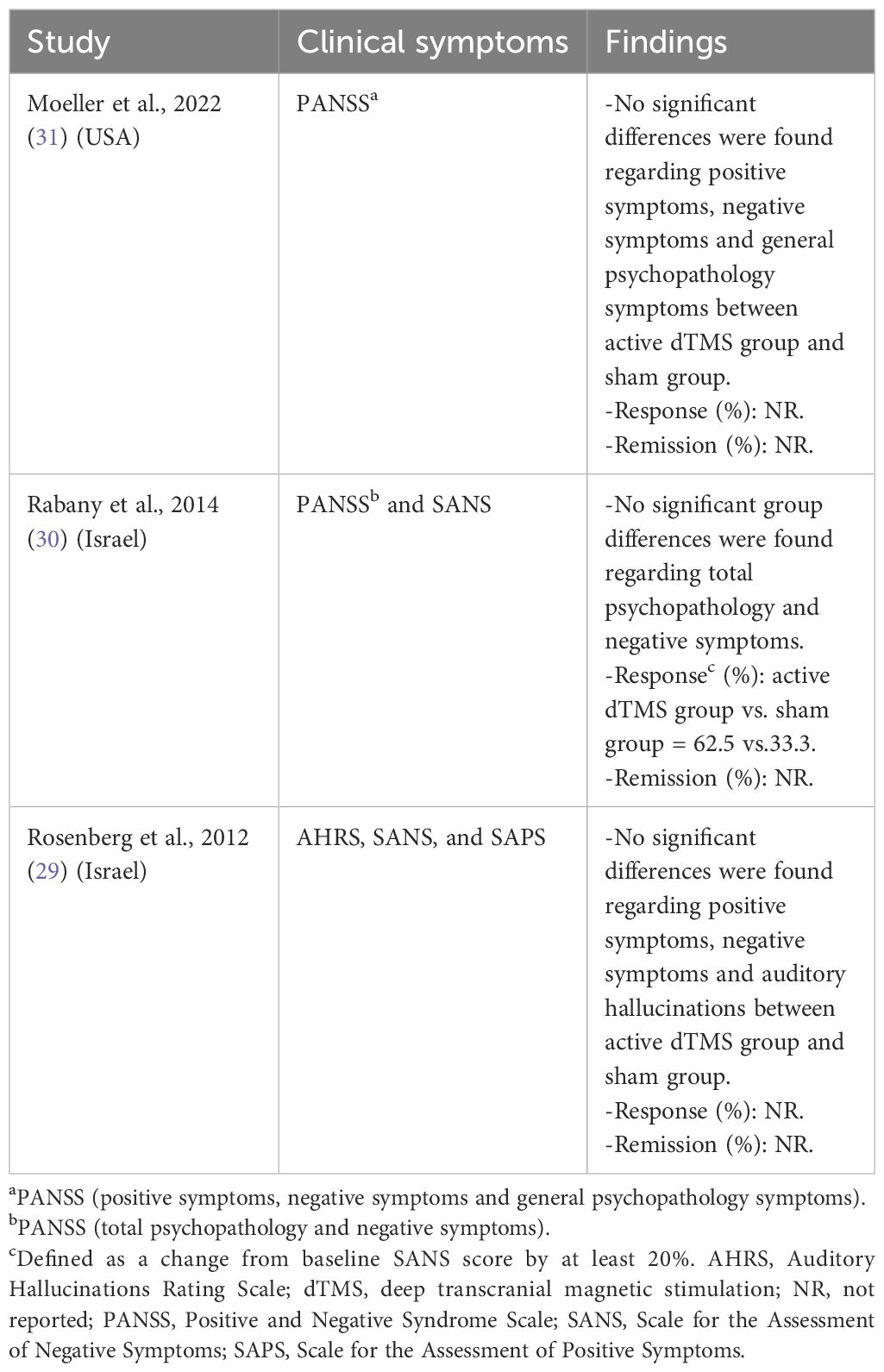
Table 2 displays the findings consistently demonstrating that active dTMS yielded similar improvements in total psychopathology, positive symptoms, negative symptoms, and auditory hallucinations compared to the sham treatment. Rabany et al.’s study (30) reported a significant reduction in negative symptoms in the dTMS group, although no significant differences were observed between the two groups.
Neurocognitive function
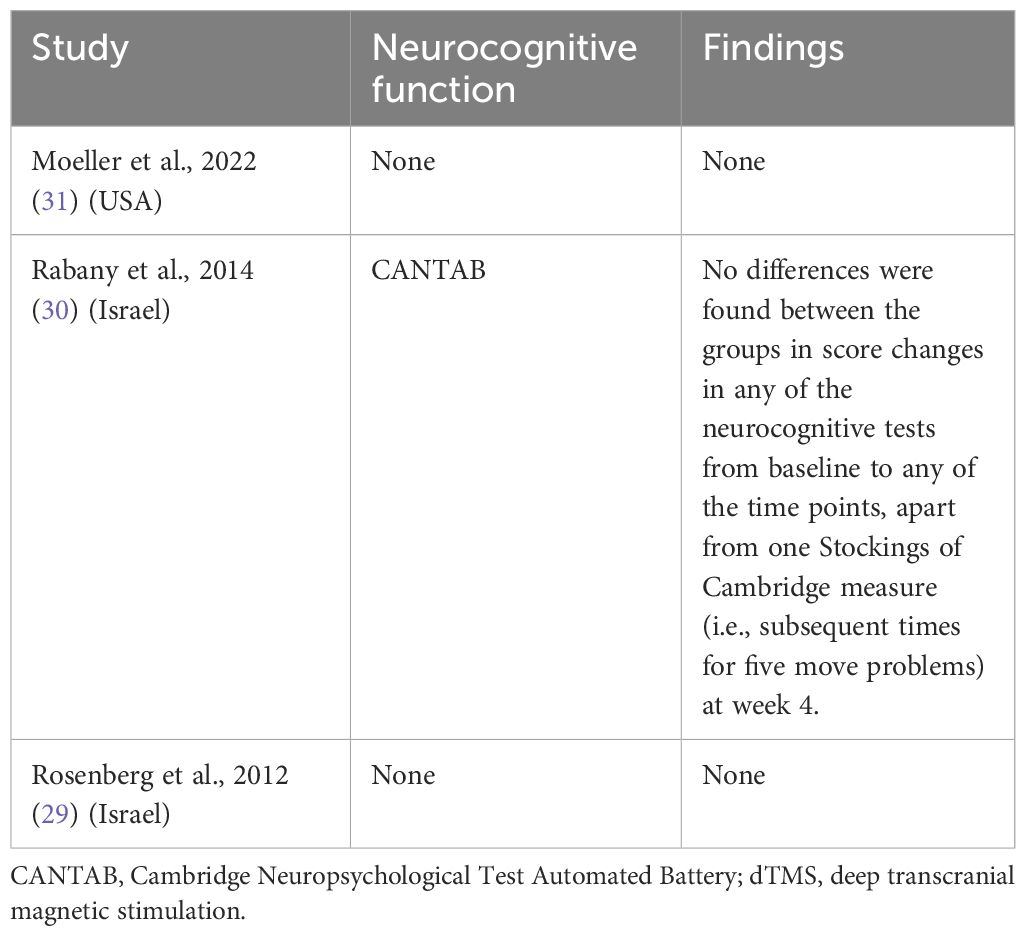
Only one study examined the impact of dTMS on neurocognitive function (30) (Table 3): active dTMS improved only one Stockings of Cambridge measure (subsequent times for five move problems) at week 4 (P < 0.05) using the Cambridge Neuropsychological Test Automated Battery (CANTAB). However, no significant effects were observed on psychomotor speed, visuospatial memory, and sustained attention measured by the CANTAB.
Discontinuation rate and adverse events
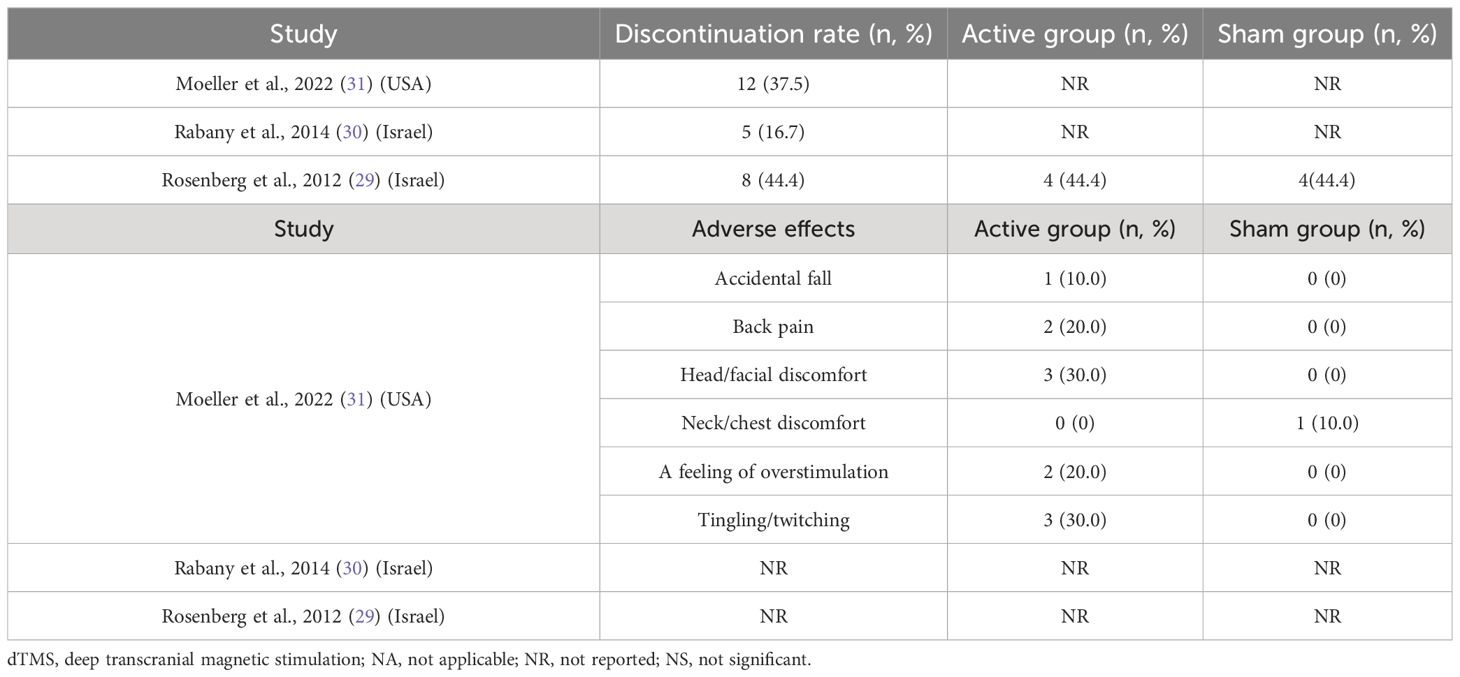
Table 4 presents the discontinuation rates reported in the three RCTs, ranging from 16.7% to 44.4%. Adverse events were assessed in only one RCT (31). Adverse events associated with dTMS included tingling/twitching (3/10, 30.0%), head/facial discomfort (3/10, 30.0%), back pain (2/10, 20.0%), and accidental falls (1/10, 10.0%).
Discussion
This systematic review included three RCTs involving 80 patients with schizophrenia. The main findings indicate that dTMS was not effective in reducing total psychopathology, positive psychopathology, negative psychopathology, or auditory hallucinations in schizophrenia, measured by the PANSS, SAPS, SANS, and AHRS, respectively. One RCT reported a relatively high rate of discontinuation of dTMS for various reasons (29). The most commonly reported adverse effects, assessed in only one RCT (31), included tingling/twitching, head/facial discomfort, and back pain. While dTMS does not appear to be a promising adjunctive therapy for schizophrenia, it shows potential for improving executive functions, suggesting the need for further studies with larger sample sizes to confirm this finding (30).
This systematic review indicates a lack of evidence to support the antipsychotic effects of dTMS in schizophrenia, particularly for refractory auditory hallucinations that may involve multiple brain regions. Previous meta-analyses have shown a negative correlation between the severity of auditory hallucinations and the volume of grey matter in the left insula and right superior temporal gyrus (41, 42). Additionally, a meta-analysis of diffusion-tensor-imaging studies comparing the brain network structure between schizophrenia patients with auditory hallucinations and healthy controls has identified defects in the left arcuate tract in the former group (43). Stimulation of the left temporoparietal cortex alone may not be sufficient, as inadequate activity in the prefrontal cortex and anterior cingulate cortex has also been linked to negative symptoms in schizophrenia (44, 45). High-frequency stimulation may improve activity in these regions, alleviating negative symptoms. However, a meta-analysis revealed that there is insufficient evidence to support or refute the use of TMS for treating psychotic symptoms in schizophrenia (11). This aligns with the findings of the present systematic review, which suggests that inadequate or inappropriate treatment parameters may contribute to the lack of efficacy. A network meta-analysis found that stimulation of the left dorsolateral prefrontal cortex resulted in significant improvements in psychotic symptoms (15). Only one RCT included in this review targeted the left dorsolateral prefrontal cortex for stimulation and reported a significant reduction in negative symptoms in the dTMS group (30). Considering the potential impact of dTMS on depression following schizophrenia, further investigation is warranted, given that a systematic review reported the efficacy of high-frequency dTMS in reducing depressive symptoms in major depression (23).
Neurocognitive function was assessed in only one RCT included in this systematic review (30), which did not find significant differences between the groups in the changes of neurocognitive test scores measured by the CANTAB, except for one executive function test, the Stocks of Cambridge. While dTMS can target deep regions, such as the anterior cingulate cortex (ACC) (46), which are associated with neurocognitive function (47), the results of dTMS on neurocognitive function in various populations have been inconsistent. Some studies suggest that dTMS may improve neurocognitive function in patients with depression (48, 49), while others have found no significant changes (50, 51). Similar results have been observed in studies involving healthy volunteers (52–55). The variability in results may be attributed to the lack of sensitivity of the assessment tools. Further research is needed to investigate the effects of dTMS on neurocognitive function using standardized and highly sensitive tools such as the MATRICS Consensus Cognitive Battery (56) or the Repeatable Battery for the Assessment of Neuropsychological Status (57).
The discontinuation rates for any reason and the occurrence of adverse events ranged from 16.7% to 44.4% in this systematic review, with only one study providing detailed numbers for discontinuation in each group (29). Although the discontinuation rate was high, there was no significant difference between the dTMS and sham groups. Adverse effects reported in one study (31) included tingling/twitching, head/facial discomfort, and back pain. These adverse effects were generally mild and transient.
This systematic review has the following limitations. First, it is worth noting that the sample sizes (n = 80) of the included studies were relatively small, which limits the generalizability of the findings and their ability to detect significant differences. Second, the three studies were too heterogeneous in their methods (e.g., the stimulation frequency of dTMS ranging from 1, 10 and 20 Hz, respectively) to allow for reliable and pertinent results. Third, the stimulation parameters of RCTs included in this systematic review were variable, therefore, the optimal stimulation parameters, such as stimulation time per session and train length, for the dTMS protocol need further exploration. Fourth, only one of the three included RCTs reported the neurocognitive effects of dTMS on schizophrenia patients. Further research is warranted to evaluate the neurocognitive effects of dTMS in patients with schizophrenia.
In conclusion, this systematic review found that dTMS does not appear to be effective in reducing psychopathology, including positive and negative symptoms, and auditory hallucinations in schizophrenia. This may call for further research on this technique and the development of protocols. Further research with larger, well-designed trials is needed to evaluate the efficacy and safety of dTMS in schizophrenia, particularly targeting specific brain regions and neurocognitive domains. Additionally, standardized and sensitive assessment tools should be used to evaluate the neurocognitive effects of dTMS in this population.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
YM: Writing – original draft, Data curation. Z-MS: Writing – original draft, Investigation. X-HY: Writing – review & editing, Writing – original draft, Methodology, Conceptualization. X-JL: Writing – review & editing, Resources, Methodology. C-JD: Writing – review & editing, Investigation, Data curation. X-BH: Writing – review & editing, Supervision, Project administration. X-LT: Writing – review & editing, Resources, Investigation. SP: Writing – review & editing, Supervision, Resources. GU: Writing – review & editing, Validation, Supervision. Y-TX: Writing – review & editing, Supervision. WZ: Writing – review & editing, Validation, Supervision.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by the National Natural Science Foundation of China (82101609), the China International Medical Exchange Foundation (Z-2018-35-2002), the Science and Technology Program of Guangzhou (2023A03J0839, 2023A03J0436), the Science and Technology Planning Project of Liwan District of Guangzhou (202201012), the National Clinical Key specialty construction project [(2023) 33], The Natural Science Foundation Program of Guangdong (2023A1515011383), the Guangzhou Municipal Key Discipline in Medicine (2021-2023), the Guangzhou High-level Clinical Key Specialty, Department of Emergency Medicine of National clinical key specialty and the Guangzhou Research-oriented Hospital. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sabe M, Pillinger T, Kaiser S, Chen C, Taipale H, Tanskanen A, et al. Half a century of research on antipsychotics and schizophrenia: a scientometric study of hotspots, nodes, bursts, and trends. Neurosci Biobehav Rev. (2022) 136:104608. doi: 10.1016/j.neubiorev.2022.104608
2. Uzman Özbek S, Alptekin K. Thought disorder as a neglected dimension in schizophrenia. Alpha Psychiatry. (2022) 23:5–11. doi: 10.1530/alphapsychiatry.2021.21371
3. Collaborators GMD. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. (2022) 9:137–50. doi: 10.1016/s2215–0366(21)00395–3
4. Kim J, Ozzoude M, Nakajima S, Shah P, Caravaggio F, Iwata Y, et al. Insight and medication adherence in schizophrenia: an analysis of the CATIE trial. Neuropharmacology. (2020) 168:107634. doi: 10.1016/j.neuropharm.2019.05.011
5. Zäske H, Linden M, Degner D, Jockers-Scherübl M, Klingberg S, Klosterkötter J, et al. Stigma experiences and perceived stigma in patients with first-episode schizophrenia in the course of 1 year after their first in-patient treatment. Eur Arch Psychiatry Clin Neurosci. (2019) 269:459–68. doi: 10.1007/s00406–018-0892–4
6. Wu Y, Kang R, Yan Y, Gao K, Li Z, Jiang J, et al. Epidemiology of schizophrenia and risk factors of schizophrenia-associated aggression from 2011 to 2015. J Int Med Res. (2018) 46:4039–49. doi: 10.1177/0300060518786634
7. Ellenbroek BA. Psychopharmacological treatment of schizophrenia: what do we have, and what could we get? Neuropharmacology. (2012) 62:1371–80. doi: 10.1016/j.neuropharm.2011.03.013
8. Howes OD, McCutcheon R, Agid O, de Bartolomeis A, van Beveren NJ, Birnbaum ML, et al. Treatment-resistant schizophrenia: treatment response and resistance in psychosis (TRRIP) working group consensus guidelines on diagnosis and terminology. Am J Psychiatry. (2017) 174:216–29. doi: 10.1176/appi.ajp.2016.16050503
9. Grover S, Sahoo S, Rabha A, Koirala R. ECT in schizophrenia: a review of the evidence. Acta neuropsychiatrica. (2019) 31:115–27. doi: 10.1017/neu.2018.32
10. Cavaleri D, Bartoli F. Biomolecular research on electroconvulsive therapy for mental disorders: state of the art and future directions. Alpha Psychiatry. (2022) 23:57–8. doi: 10.5152/alphapsychiatry.2022.0003
11. Dougall N, Maayan N, Soares-Weiser K, McDermott LM, McIntosh A. Transcranial magnetic stimulation (TMS) for schizophrenia. Cochrane Database systematic Rev. (2015) 2015:Cd006081. doi: 10.1002/14651858.CD006081.pub2
12. Zhang XY, Chen HD, Liang WN, Yang XH, Cai DB, Huang X, et al. Adjunctive magnetic seizure therapy for schizophrenia: a systematic review. Front Psychiatry. (2021) 12:813590. doi: 10.3389/fpsyt.2021.813590
13. Jiang WL, Cai DB, Sun CH, Yin F, Goerigk S, Brunoni AR, et al. Adjunctive tDCS for treatment-refractory auditory hallucinations in schizophrenia: A meta-analysis of randomized, double-blinded, sham-controlled studies. Asian J Psychiatry. (2022) 73:103100. doi: 10.1016/j.ajp.2022.103100
14. Pathak H, Sreeraj VS. Transcranial alternating current stimulation (tACS) and its role in schizophrenia: a scoping review. Clin Psychopharmacol Neurosci. (2023) 21:634–49. doi: 10.9758/cpn.22.1042
15. Tseng PT, Zeng BS, Hung CM, Liang CS, Stubbs B, Carvalho AF, et al. Assessment of noninvasive brain stimulation interventions for negative symptoms of schizophrenia: a systematic review and network meta-analysis. JAMA Psychiatry. (2022) 79:770–9. doi: 10.1001/jamapsychiatry.2022.1513
16. Begemann MJ, Brand BA. Efficacy of non-invasive brain stimulation on cognitive functioning in brain disorders: a meta-analysis. psychol Med. (2020) 50:2465–86. doi: 10.1017/s0033291720003670
17. Zheng W, Tong G, Ungvari GS, Ng CH, Chiu HFK, Xiang YQ, et al. Memory impairment following electroconvulsive therapy in chinese patients with schizophrenia: meta-analysis of randomized controlled trials. Perspect Psychiatr Care. (2018) 54:107–14. doi: 10.1111/ppc.12206
18. Wu H, Jiang J, Cao X, Wang J, Li C. Magnetic seizure therapy for people with schizophrenia. Cochrane Database systematic Rev. (2023) 6:Cd012697. doi: 10.1002/14651858.CD012697
19. Johnson KA, Baig M, Ramsey D, Lisanby SH, Avery D, McDonald WM, et al. Prefrontal rTMS for treating depression: location and intensity results from the OPT-TMS multi-site clinical trial. Brain stimulation. (2013) 6:108–17. doi: 10.1016/j.brs.2012.02.003
20. Cheng CM, Li CT, Tsai SJ. Current updates on newer forms of transcranial magnetic stimulation in major depression. Adv Exp Med Biol. (2021) 1305:333–49. doi: 10.1007/978–981-33–6044-0_18
21. Ibrahim C, Rubin-Kahana DS, Pushparaj A, Musiol M, Blumberger DM, Daskalakis ZJ, et al. The insula: A brain stimulation target for the treatment of addiction. Front Pharmacol. (2019) 10:720. doi: 10.3389/fphar.2019.00720
22. Zhang JJQ, Fong KNK, Ouyang R-G, Siu AMH, Kranz GS. Effects of repetitive transcranial magnetic stimulation (rTMS) on craving and substance consumption in patients with substance dependence: a systematic review and meta-analysis. Addiction. (2019) 114:2137–49. doi: 10.1111/add.14753
23. Kedzior KK, Gellersen HM, Brachetti AK, Berlim MT. Deep transcranial magnetic stimulation (DTMS) in the treatment of major depression: an exploratory systematic review and meta-analysis. J Affect Disord. (2015) 187:73–83. doi: 10.1016/j.jad.2015.08.033
24. Ikawa H, Osawa R, Sato A, Mizuno H, Noda Y. A case series of deep transcranial magnetic stimulation treatment for patients with obsessive-compulsive disorder in the tokyo metropolitan area. J Clin Med. (2022) 11:6133. doi: 10.3390/jcm11206133
25. Kedzior KK, Gerkensmeier I, Schuchinsky M. Can deep transcranial magnetic stimulation (DTMS) be used to treat substance use disorders (SUD)? a systematic review. BMC Psychiatry. (2018) 18:137. doi: 10.1186/s12888–018-1704–0
26. Tendler A, Barnea Ygael N, Roth Y, Zangen A. Deep transcranial magnetic stimulation (dTMS) - beyond depression. Expert Rev Med devices. (2016) 13:987–1000. doi: 10.1080/17434440.2016.1233812
27. Roth Y, Levkovitz Y, Pell GS, Ankry M, Zangen A. Safety and characterization of a novel multi-channel TMS stimulator. Brain stimulation. (2014) 7:194–205. doi: 10.1016/j.brs.2013.09.004
28. Linsambarth S, Jeria A, Avirame K, Todder D, Riquelme R, Stehberg J. Deep transcranial magnetic stimulation for the treatment of negative symptoms in schizophrenia: beyond an antidepressant effect. J ECT. (2019) 35:e46–54. doi: 10.1097/yct.0000000000000592
29. Rosenberg O, Gersner R, Klein LD, Kotler M, Zangen A, Dannon P. Deep transcranial magnetic stimulation add-on for the treatment of auditory hallucinations: a double-blind study. Ann Gen Psychiatry. (2012) 11:13. doi: 10.1186/1744–859x-11–13
30. Rabany L, Deutsch L, Levkovitz Y. Double-blind, randomized sham controlled study of deep-TMS add-on treatment for negative symptoms and cognitive deficits in schizophrenia. J Psychopharmacol (Oxford England). (2014) 28:686–90. doi: 10.1177/0269881114533600
31. Moeller SJ, Gil R, Weinstein JJ. Deep rTMS of the insula and prefrontal cortex in smokers with schizophrenia: Proof-of-concept study. Schizophr (Heidelberg Germany). (2022) 8:6. doi: 10.1038/s41537–022-00224–0
32. Kedzior KK, Gierke L, Gellersen HM, Berlim MT. Cognitive functioning and deep transcranial magnetic stimulation (DTMS) in major psychiatric disorders: a systematic review. J Psychiatr Res. (2016) 75:107–15. doi: 10.1016/j.jpsychires.2015.12.019
33. Levkovitz Y, Rabany L, Harel EV, Zangen A. Deep transcranial magnetic stimulation add-on for treatment of negative symptoms and cognitive deficits of schizophrenia: a feasibility study. Int J Neuropsychopharmacol. (2011) 14:991–6. doi: 10.1017/S1461145711000642
34. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PloS Med. (2009) 3:e123–30. doi: 10.1371/journal.pmed.1000097
35. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. (1987) 13:261–76. doi: 10.1093/schbul/13.2.261
36. Faustman WO, Overall JE. The brief psychiatric rating scale. psychol Rep. (1962) 10:799–812. doi: 10.2466/PR0.10.3.799–812
37. Hoffman RE, Hawkins KA, Gueorguieva R, Boutros NN, Rachid F, Carroll K, et al. Transcranial magnetic stimulation of left temporoparietal cortex and medication-resistant auditory hallucinations. Arch Gen Psychiatry. (2003) 60:49–56. doi: 10.1001/archpsyc.60.1.49
38. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled Clin trials. (1996) 17:1–12. doi: 10.1016/0197–2456(95)00134–4
39. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane cllaboration's tool for assessing risk of bias in randomised trials. BMJ (Clinical Res ed). (2011) 343:d5928. doi: 10.1136/bmj.d5928
40. Linde K, Clausius N, Ramirez G, Melchart D, Eitel F, Hedges LV, et al. Are the clinical effects of homeopathy placebo effects? a meta-analysis of placebo-controlled trials. Lancet (London England). (1997) 350:834–43. doi: 10.1016/s0140–6736(97)02293–9
41. Palaniyappan L, Balain V, Radua J, Liddle PF. Structural correlates of auditory hallucinations in schizophrenia: a meta-analysis. Schizophr Res. (2012) 137:169–73. doi: 10.1016/j.schres.2012.01.038
42. Romeo Z, Spironelli C. Hearing voices in the head: two meta-analyses on structural correlates of auditory hallucinations in schizophrenia. NeuroImage Clin. (2022) 36:103241. doi: 10.1016/j.nicl.2022.103241
43. Geoffroy PA, Houenou J, Duhamel A, Amad A, De Weijer AD, Curčić-Blake B, et al. The arcuate fasciculus in auditory-verbal hallucinations: a meta-analysis of diffusion-tensor-imaging studies. Schizophr Res. (2014) 159:234–7. doi: 10.1016/j.schres.2014.07.014
44. Andreasen NC, O'Leary DS, Flaum M, Nopoulos P, Watkins GL, Boles Ponto LL, et al. Hypofrontality in schizophrenia: distributed dysfunctional circuits in neuroleptic-naïve patients. Lancet (London England). (1997) 349:1730–4. doi: 10.1016/s0140–6736(96)08258-x
45. Reid MA, Stoeckel LE, White DM, Avsar KB, Bolding MS, Akella NS, et al. Assessments of function and biochemistry of the anterior cingulate cortex in schizophrenia. Biol Psychiatry. (2010) 68:625–33. doi: 10.1016/j.biopsych.2010.04.013
46. Hayward G, Mehta MA, Harmer C, Spinks TJ, Grasby PM, Goodwin GM. Exploring the physiological effects of double-cone coil TMS over the medial frontal cortex on the anterior cingulate cortex: an H2(15)O PET study. Eur J Neurosci. (2007) 25:2224–33. doi: 10.1111/j.1460–9568.2007.05430.x
47. Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci. (2001) 2:417–24. doi: 10.1038/35077500
48. Levkovitz Y, Harel EV, Roth Y, Braw Y, Most D, Katz LN, et al. Deep transcranial magnetic stimulation over the prefrontal cortex: evaluation of antidepressant and cognitive effects in depressive patients. Brain stimulation. (2009) 2:188–200. doi: 10.1016/j.brs.2009.08.002
49. Naim-Feil J, Bradshaw JL, Sheppard DM, Rosenberg O, Levkovitz Y, Dannon P. Neuromodulation of attentional control in major depression: a pilot deep TMS study. Neural plasticity. (2016) 2016:5760141. doi: 10.1155/2016/5760141
50. Harel EV, Zangen A, Roth Y, Reti I, Braw Y, Levkovitz Y. H-coil repetitive transcranial magnetic stimulation for the treatment of bipolar depression: an add-on, safety and feasibility study. World J Biol Psychiatry. (2011) 12:119–26. doi: 10.3109/15622975.2010.510893
51. Kaster TS, Daskalakis ZJ, Noda Y, Knyahnytska Y, Downar J. Efficacy, tolerability, and cognitive effects of deep transcranial magnetic stimulation for late-life depression: a prospective randomized controlled trial. Neuropsychopharmacology. (2018) 43:2231–8. doi: 10.1038/s41386–018-0121-x
52. Gruberger M, Levkovitz Y, Hendler T, Harel EV, Harari H, Ben Simon E, et al. I think therefore I am: rest-related prefrontal cortex neural activity is involved in generating the sense of self. Consciousness Cogn. (2015) 33:414–21. doi: 10.1016/j.concog.2015.02.008
53. Krause L, Enticott PG, Zangen A, Fitzgerald PB. The role of medial prefrontal cortex in theory of mind: a deep rTMS study. Behav Brain Res. (2012) 228:87–90. doi: 10.1016/j.bbr.2011.11.037
54. Levkovitz Y, Roth Y, Harel EV, Braw Y, Sheer A, Zangen A. A randomized controlled feasibility and safety study of deep transcranial magnetic stimulation. Clin Neurophysiol. (2007) 118:2730–44. doi: 10.1016/j.clinph.2007.09.061
55. Spagnolo PA, Wang H, Srivanitchapoom P, Schwandt M, Heilig M, Hallett M. Lack of target engagement following low-frequency deep transcranial magnetic stimulation of the anterior insula. Neuromodulation: J Int Neuromodulation Soc. (2019) 22:877–83. doi: 10.1111/ner.12875
56. Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK. Identification of separable cognitive factors in schizophrenia. Schizophr Res. (2004) 72:29–39. doi: 10.1016/j.schres.2004.09.007
Keywords: deep transcranial magnetic stimulation, schizophrenia, psychopathology, executive function, systematic review
Citation: Mo Y, Shi Z-M, Yang X-H, Lan X-J, Deng C-J, Huang X-B, Tan X-L, Pridmore S, Ungvari GS, Xiang Y-T and Zheng W (2024) Deep transcranial magnetic stimulation for schizophrenia: a systematic review. Front. Psychiatry 15:1390913. doi: 10.3389/fpsyt.2024.1390913
Received: 24 February 2024; Accepted: 13 May 2024;
Published: 31 May 2024.
Edited by:
Osama Abulseoud, Mayo Clinic Arizona, United StatesReviewed by:
Nicola Acevedo, Swinburne University of Technology, AustraliaDominique Januel, Unité de recherche clinique (URC), France
Copyright © 2024 Mo, Shi, Yang, Lan, Deng, Huang, Tan, Pridmore, Ungvari, Xiang and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu-Tao Xiang, xyutly@gmail.com; Wei Zheng, zhengwei0702@163.com
†These authors have contributed equally to this work
 Yu Mo1†
Yu Mo1† Xin-Hu Yang
Xin-Hu Yang Xian-Jun Lan
Xian-Jun Lan Can-Jin Deng
Can-Jin Deng Xing-Bing Huang
Xing-Bing Huang Saxby Pridmore
Saxby Pridmore Gabor S. Ungvari
Gabor S. Ungvari Yu-Tao Xiang
Yu-Tao Xiang Wei Zheng
Wei Zheng