- 1Laboratory of Aquatic Environmental Science, Faculty of Agriculture and Marine Science, Kochi University, Nankoku, Japan
- 2Usa Marine Biological Institute, Kochi University, Tosa, Japan
- 3Kochi Prefectural Fisheries Experiment Station, Susaki, Japan
- 4Faculty of Agriculture and Marine Science, Kochi University, Nankoku, Japan
Uranouchi Inlet, situated on the Pacific coast of southwestern Japan, has been a highly enclosed inlet known for yellowtail farming since 1959. Since the 1980s, harmful algal blooms (HABs) have repeatedly occurred, resulting in mass mortality of fish and shellfish. In the sediment at the inlet, the resulting cysts of the HAB species may be preserved, which reflects the history of HAB events. However, the vertical distributions of HAB species in sediment have not been elucidated. In this study, core sediment samples were analyzed by metabarcoding. The dating of each sample was cited from previous study dating the same samples. The findings revealed the presence of eleven HAB species, with notable shifts from approximately 1977–1988. The timing of the shifts corresponded to that of the development of aquaculture and the resulting eutrophication. Vertical core metabarcoding provides footprints of how HAB species composition may be influenced by anthropogenic environmental changes.
1 Introduction
Uranouchi Inlet, located on the Pacific coast of southwestern Japan, is a highly enclosed inlet with a narrow bay mouth (Yamaguchi et al., 2018). These characteristics enabled the start of yellowtail farming in 1959. Since then, fish farming has continued in the inlet, with nearly 3,500 tons of farmed fish (mainly yellowtail or red sea bream) produced in 2018 (Ministry of Agriculture, Forestry and Fisheries, 2018; Takahashi et al., 2021a). Harmful algal blooms (HABs) have repeatedly occurred in the inlet since the 1980s, resulting in mass mortality of fish and shellfish (Takahashi et al., 2021a).
Some HAB-causative species are known to form cysts, resulting in the accumulation of these cysts in sediments. These cysts germinate when the surrounding environment is suitable for growth (Borsato et al., 2023; Brosnahan et al., 2020; Ellegaard and Ribeiro, 2018; Zingone and Oksfeldt Enevoldsen, 2000). It has been reported that some cysts in bottom sediments collected by a core sampler and found to be almost a century old could still germinate (Feifel et al., 2012, 2015; Härnström et al., 2011; Lundholm et al., 2011; Miyazono et al., 2012), which enables us to speculate on the history of HABs caused by cyst-forming species by identifying the species of cysts in bottom sediments. However, species identification of HAB cysts is difficult because of the lack of understanding of their morphological characteristics in some cases (Hallegraeff et al., 2003; Thoha et al., 2019; Yamaguchi et al., 1995). Recently, DNA-based species identification by metabarcoding via high-throughput sequencers has been performed to detect HAB-causative species in surface sediment samples because of the fast, work-saving, and comprehensive identification of multiple species (Dzhembekova et al., 2018; Liu et al., 2023; Wang et al., 2022a, 2022b). Studies that attempt to identify HAB species by collecting surface sediment samples at shallow depths (from 0 to 15 cm of collected surface sediments) have been performed to determine the HAB species that have been present recently in the nearby collection area. However, several studies have focused on the detection of HAB-causative species collected from sediment core samples that are more than 15 cm depths (Armbrecht et al., 2024; Boere et al., 2011; Coolen et al., 2013; Siano et al., 2021). Among them, few studies have focused on how marine eukaryotic HAB communities have been influenced by anthropogenic activities, such as heavy metal pollution and agricultural pollution (Siano et al., 2021), climate change (Boere et al., 2009) and nutrient runoff from rivers (Coolen et al., 2013). Under these circumstances, there are no studies on the long-term history of the transition of HAB-causative species due to eutrophication in coastal areas caused by aquaculture. In this study, we aimed to clarify the vertical distribution of cyst-forming HAB species in sediment core samples from Uranouchi Inlet by metabarcoding, where fish farming has been continuously performed since the 1960s and HAB events have repeatedly occurred, and discuss the possibility that environmental changes caused by fish farming have contributed to changes in the community compositions of HAB species.
2 Method
The sediment core sample (0–57 cm depth) was collected as described by Takahashi et al. (2021a) at Menokuso Station in Uranouchi Inlet, Kochi, Japan (33.25.346N, 133.23.522E), on August 22, 2016 (Supplementary Figure 1). The sediment core was sliced into 3-cm layers each with a thread saw, and the nineteen layered samples were named URA01 (0–3 cm) to URA19 (54–57 cm), which were obtained as described previously by Takahashi et al. (2021a). To avoid contamination between each sample, only the center of each sediment sample was collected and peripheral sediment was removed by washing with sterile seawater. Prior to DNA extraction, the samples were stored in the dark at -80°C to prevent degradation of the genomic DNA of cysts of HAB species in the sediment. Radiometric dating of the nineteen samples (Figure 1) was conducted with Pb-210 and Cs-137 by Takahashi et al. (2021a). The result of estimated year of each sample by Takahashi et al. (2021a) was shown in Figure 1.
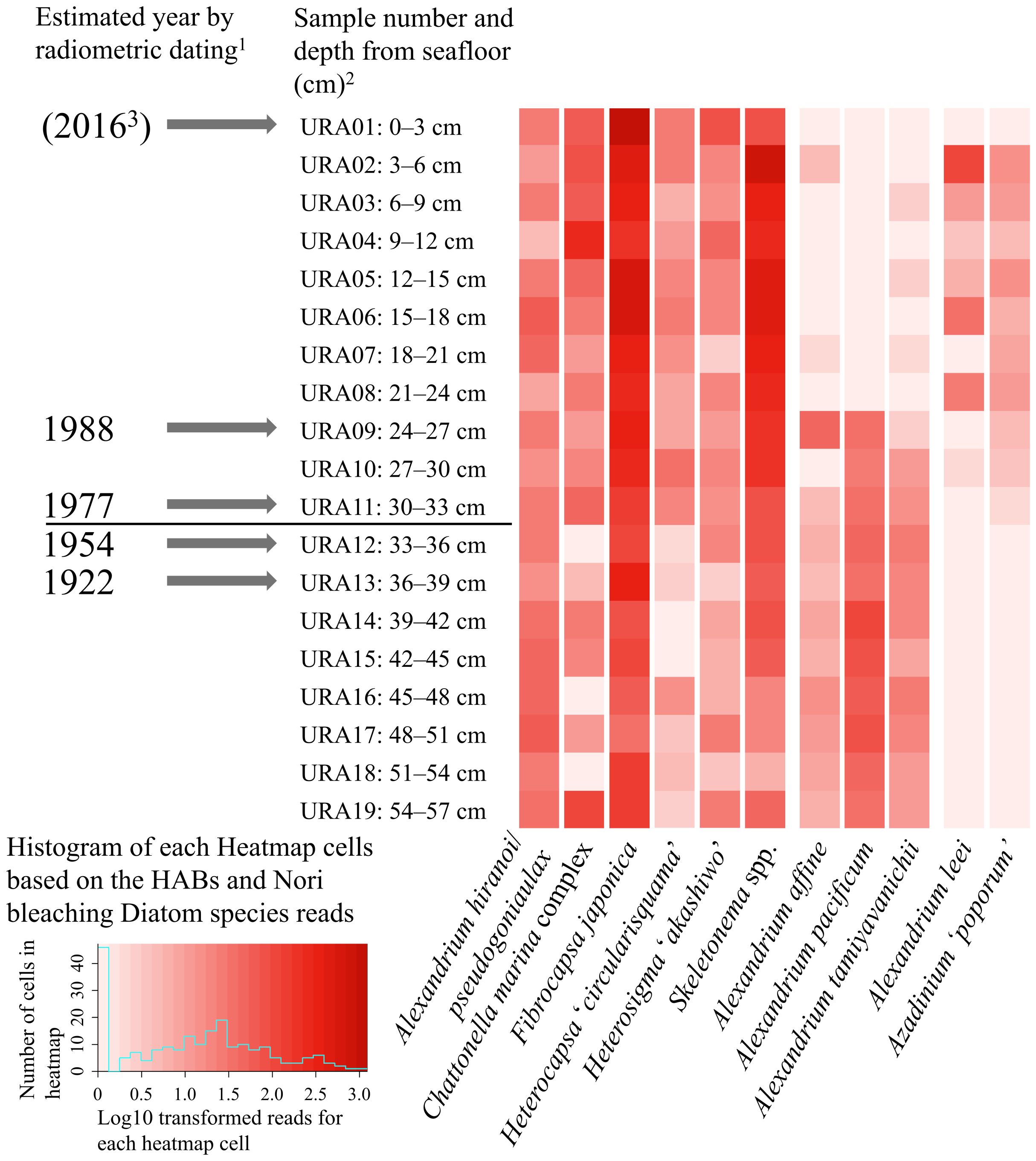
Figure 1. Heatmaps and histogram based on the log10 transformed read numbers of HAB species and nori bleaching diatoms contained in the sediment samples from Uranouchi Inlet, Kochi, Japan. The solid line in the age column on the left side of the figure indicates the start of the aquaculture in Uranouchi Inlet. 1: Radiometric dating using Pb-210 and Cs-137 of sediment samples was determined by Takahashi et al. (2021a). 2: URA01—03, URA04—13 and URA14—19 were surface mixed layers, layers determined by radiometric dating, and layers out of the dating range, respectively. 3: The core sample was collected from Uranouchi inlet in 2016.
The processes required for MiSeq sequencing, such as DNA extraction and MiSeq library preparation, were essentially performed following the methods described by Funaki et al. (2022). DNA was extracted from 250 mg of raw sediment taken from each layer of the core sample in duplicate via a DNeasy® PowerSoil® Kit according to the manufacturer’s protocol (QIAGEN, Hilden, Germany). The eukaryotic universal V8–V9 primer set was used to amplify the V8–V9 region of the 18S rDNA (approximately 350 bp). The primers used were forward primer V8F+adapter 5’- TCGTCGGCAGCGTCAGATGTGTATAAGAGACAG+ATAACAGGTCTGTGATGCCCT-3’ and reverse primer 1510R+adapter 5’-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAG+CCTTCYGCAGGTTCACCTAC-3’ (Funaki et al., 2022). PCR was conducted using the primers and the extracted DNA from core samples as templates, and the amplified products (approximately 400 bp) were purified via the method described in Funaki et al. (2022). Equimolar quantities of the purified amplicons were pooled and subjected to 2 × 250-bp sequencing (MiSeq Reagent Nano Kit v2, Illumina) on an inhouse MiSeq platform (Illumina). The raw data (fastq file) of both the forward and reverse sequences obtained from MiSeq were deposited in the DDBJ Sequence Read Archive under BioProject number PRJDB17880 (DRR Run number: DRR543997–DRR544015).
After MiSeq paired-end sequencing (2 × 250 bp), raw sequences were trimmed in Mothur ver. 1.40.3 (Schloss et al., 2009) on Galaxy (Afgan et al., 2018) and Mothur ver. 1.36.1 (Schloss et al., 2009) in the laboratory based on the bioinformatics method described by Funaki et al. (2022). After trimming the sequences, only the sequences expected for the 18S rDNA V8–V9 region were retrieved, and singleton sequences were also removed based on the method of Funaki et al. (2022).
For HAB species identification of unique sequences, a BLAST (basic local alignment search tool) 2.7.1+ search (Camacho et al., 2009) was conducted via the Protist Ribosomal Reference database ver. 5.0.1 (PR2, Guillou et al., 2013). After a BLAST search against the reference database described above, only sequences showing more than 97% similarity to those of HAB species and more than 281-bp query coverage were selected for further analysis. To understand the full picture of eukaryotes obtained by MiSeq, the total number of reads in all samples of each taxon group from Supergroup to Division was calculated, as well as the relative number of reads in each of the 19 samples for each taxon group. Since this study focused on HAB species in sediment samples, unique sequences associated with HAB genera and species listed in the IOCUNESCO Taxonomic Reference List of Harmful Micro Algae (Lundholm et al., 2009) were retained. In addition, the genus Skeletonema, which is known to cause nori bleaching in Japan (Hori et al., 2019; Imai et al., 2006; 2021, Minamiura and Yamaguchi, 2019; Sakamoto et al., 2021) and caused fish kill events by Skeletonema costatum in British Columbia, Canada (Kent et al., 1995) was also treated as a HAB genus.
To prevent misidentification of HAB species with unique sequences at the genus or species level, maximum likelihood (ML) molecular phylogenetic trees were constructed using unique sequences and reference sequences belonging to each genus obtained from GenBank and PR2. Multiple alignments, best model selection and ML molecular phylogenetic trees were constructed via the methods described by Funaki et al. (2022). If the unique sequence appeared in the same clade as the reference HAB species, it was assigned to the same HAB species. However, if the reference sequences of multiple HAB species were mixed in the same clade, misidentification was avoided by listing multiple species together.
To investigate the vertical distribution of HAB species detected in the sediment samples, a heatmap with hierarchical clustering was generated on the basis of the read numbers of each HAB species in each sediment sample. The detailed heatmap analysis method followed the same approach as that in Funaki et al. (2022) and was performed in R 4.3.1 (R Core Team, 2023) and RStudio 2023.06.0 + 421 (RStudio Team, 2023).
To discuss the occurrence of HAB species detected by metabarcoding, the occurrence species of major HAB species and their cells measured by light microscopy when fishery damage occurred in Uranouchi Inlet from 1984 to 2017 was provided by the Kochi Prefectural Fisheries Experiment Station. Simultaneously, water quality survey results (NH4-N, NO2-N, NO3-N, PO4-P, DIN-N, DON-N, DOP-P, T-N and T-P) from 1980 to 2020 surveyed every 2 to 5 years in Mitsumatsu station near Menokuso Station in Uranouchi Inlet, where the core sample used in this study was collected, was provided by Kochi Prefectural Fisheries Experiment Station and Fisheries Research Institute, Japan Fisheries Research and Education Agency.
3 Results
3.1 Overview of metabarcoding results
A total of 561,941 raw sequences were obtained from MiSeq paired-end sequencing, and all the raw sequences were generated as contig sequences (Supplementary Table 1). The sequences filtered and trimmed via Mothur were 179,827 unique sequences, and 465,118 reads were obtained. Among the sequences, 164,789 unique sequences and a total of 433,983 reads of the 18S rDNA V8–V9 region were obtained. After removal of chimeras and ‘pre.cluster’, 35,897 unique sequences and a total of 332,895 reads were passed through filtering. After singleton sequences were removed, 10,224 unique sequences and a total of 307,222 reads were obtained (Supplementary Table 1).
Eight supergroups of Eukaryota were identified from metabarcoding sequences using BLAST search with PR2 database (Supplementary Table 2 and Supplementary Figure 2). The total read number identified by the BLAST search in the 19 sediment samples was 234,173 reads, with the highest number of 139,129 reads for Alveolata in the TSAR (Supplementary Table 2). Total reads of the supergroups and divisions derived from the 19 sediment samples were varied among 5,124 reads at URA18 to 17,703 reads at URA02 (Supplementary Table 3). Supergroup and division showing the most abundant reads was TSAR and Alveolata, respectively, in URA01–URA14 and URA19 samples (ranged from 33.79% at URA19 to 79.83% at URA09, Supplementary Table 4 and Supplementary Figure 2). In contrast, Obazoa and Opisthokonta were the most dominant supergroup and division in URA15–URA18 (ranged from 33.86% at URA15 to 42.59% at URA17), respectively.
3.2 Molecular phylogenetic position of each identified HAB species
Ten HAB species belonging to the genera Alexandrium, Azadinium, Chattonella, Fibrocapsa, Heterocapsa, and Heterosigma were identified in this study (Table 1). In the case of the genus Skeletonema, the total numbers of unique sequences and reads of the genus were also shown in Table 1 because not only Skeletonema costatum but also many species of the genus are considered to cause nori bleaching in Japan. The HAB species Aureococcus anophagefferens, Azadinium dexteroporum and A. spinosum, Dictyocha fibula, Karenia mikimotoi, Pseudo-nitzschia delicatissima, P. multiseries, P. pseudodelicatissima, and P. pungens were also detected. However, the total read numbers of those HAB species were less than fifty which was difficult to examine for vertical distribution in the sediment samples, so those species were excluded from the subsequent analysis.
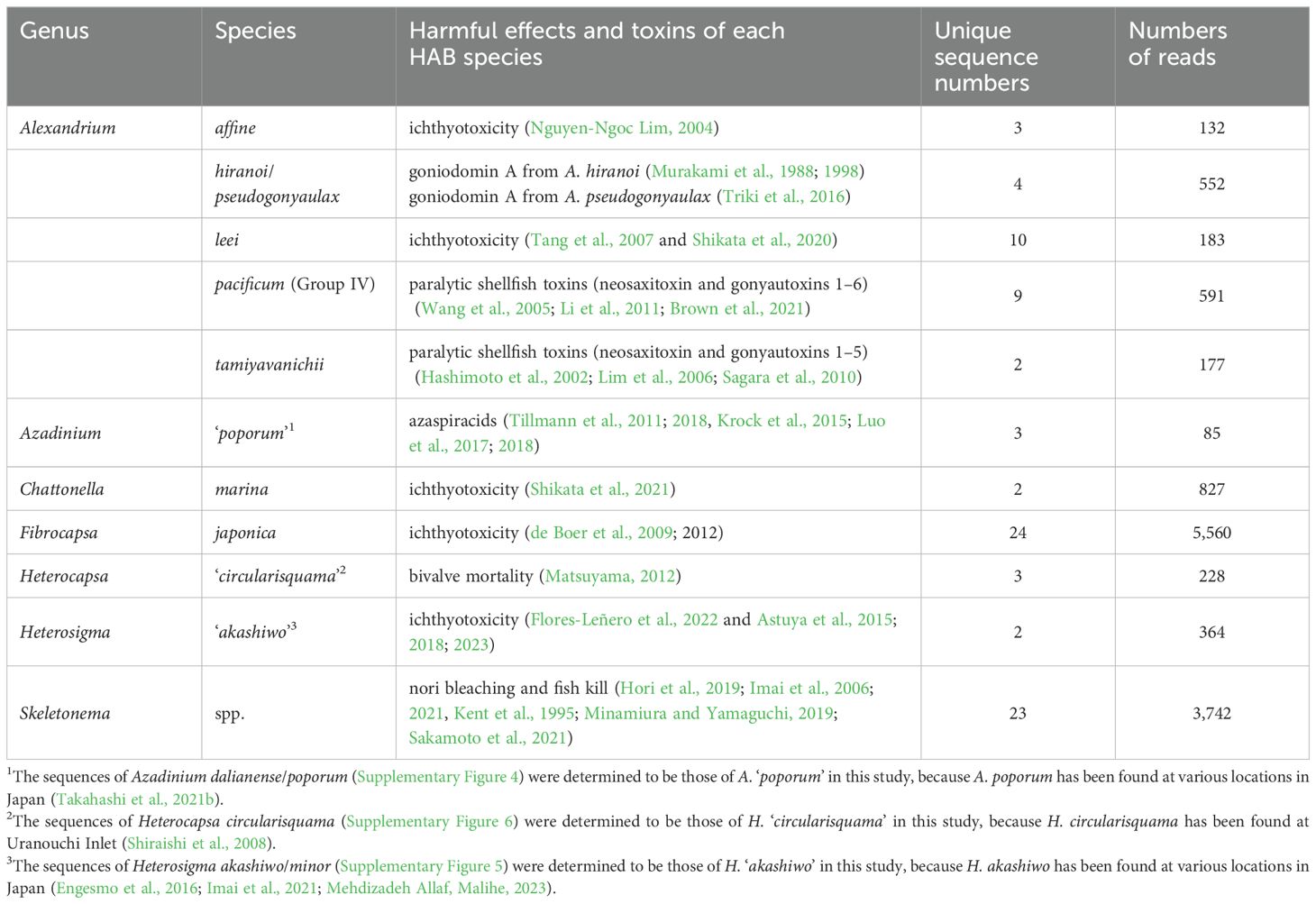
Table 1. List of HAB species and nori bleaching/fish kill species detected by metabarcoding in this study, their harmful effects, and their numbers of unique sequences and reads obtained in this study.
In the molecular phylogenetic tree of the genus Alexandrium, unique sequences of the genus Alexandrium obtained this study were separated into five major clades (Supplementary Figure 3). Four clades contained sequences of A. affine, A. leei, A. pacificum (group IV) and A. tamiyavanichii, whereas the remaining clade contained two species (A. hiranoi and A. pseudogonyaulax) in the molecular phylogenetic tree. A. pacificum (Group IV) and A. tamiyavanichii have been reported as paralytic shellfish toxin (PST) producers (A. pacificum: Brown et al., 2021; Li et al., 2011; Wang et al., 2005, A. tamiyavanichii: Hashimoto et al., 2002; Lim et al., 2006; Sagara et al., 2010, Table 1). A. affine and A. leei have been reported to be associated with fish mortality (A. affine: Nguyen-Ngoc, 2004, A. leei: Shikata et al., 2020; Tang et al., 2007, Table 1). A. hiranoi and A. pseudogonyaulax produce goniodomin A, which is an antifungal polyether macrolide that inhibits actin reorganization and angiogenesis (Murakami et al., 1988, 1998; Triki et al., 2016, Table 1).
Regarding the toxic species of the genus Azadinium, three unique sequences belonging to the clade A. poporum/A. dalianense were obtained (Supplementary Figure 4). Since A. dalianense has not been found and A. poporum has recently been found in Japanese coastal waters via many water samples collected from various locations in Japan (Takahashi et al., 2021b), these unique sequences may be those of A. ‘poporum’, known as an azaspiracid producer (Krock et al., 2015; Luo et al., 2017, 2018; Tillmann et al., 2011, 2018, Table 1). The azaspiracid is known to cause symptoms similar to diarrheal shellfish poisoning, including nausea, vomiting, abdominal pain, and severe diarrhea symptoms in humans who ingest mussels or scallops contaminated with azaspiracids (Twiner et al., 2008).
The unique sequences belonging to the Chattonella marina complex (C. marina var. antiqua, C. marina var. marina, C. marina var. ovata and C. minima) were detected (Supplementary Figure 5). Several strains of the C. marina complex produce reactive oxygen species (ROS), which may affect the gills of fish during red tide outbreaks and cause fish mortality (Shikata et al., 2021, Table 1). Regarding phylogeny, the C. marina complex detected in the sediment sample may also have the potential for ROS production because sequences of the productive ROS strains and those of the C. marina complex detected in this study appeared in the same clade on the tree (Supplementary Figure 5). The unique sequences assigned to Fibrocapsa japonica were detected (Supplementary Figure 5). Fish mortality caused by F. japonica has been reported (Iwasaki, 1971; Landsberg, 2002, Table 1), and some studies have reported that this species produces ROS and hemolysins, which may cause cell clogging in the gills (de Boer et al., 2009, 2012). The unique sequence of Heterosigma ‘akashiwo’, which has been reported as a HAB that causes fish mortality, was detected (Table 1 and Supplementary Figure 5). The genus Heterosigma contains a new species, H. minor, described in 2016 (Engesmo et al., 2016). These two species of Heterosigma are indistinguishable in the 18S rDNA V8–V9 region analyzed in this study. However, H. minor has only been described from a strain isolated from Virginia, USA (strain ARC HA0504-1), and no Japanese strain has been isolated to date (Engesmo et al., 2016; Imai et al., 2021; Mehdizadeh Allaf, 2023). Therefore, we determined the unique sequences in the phylogenetic tree as H. ‘akashiwo’ (Supplementary Figure 5).
Three unique sequences were found in the genus Heterocapsa, but the position of those sequences in the phylogenetic tree of the genus Heterocapsa was difficult to identify at the species level, since those sequences belonged to one clade along with several other species of the genus Heterocapsa (Supplementary Figure 6). This is because the 18S rDNA V8–V9 region, which is the target region for metabarcoding in this study, cannot identify each species of the genus Heterocapsa. However, considering that there are reports of bivalve mortality caused by H. circularisquama in Uranouchi Inlet (Horiguchi, 1995; Imai et al., 2006; Matsuyama, 2012; Sakamoto et al., 2021; Shiraishi et al., 2008), these three sequences were considered as H. circularisquama and named as H. ‘circularisquama’ (Table 1).
The blooms of Skeletonema have been reported to be responsible for the color bleaching of nori (Pyropia spp.) in Japan (Imai et al., 2006, Table 1) and gill lesions and mortality of Atlantic salmon Salmo salar in British Columbia (Kent et al., 1995), unique sequences assigned to this genus were treated as HAB species in this study (Supplementary Figure 7).
3.3 Monitoring data of HAB species and water quality survey
The HAB monitoring data provided by the Kochi Prefectural Fisheries Experiment Station for fishery damage in Uranouchi Inlet between 1984 and 2017 showed that the largest fishery damage on record occurred in 2001 in this inlet, with the damage amounting to 60 million yen (Table 2).
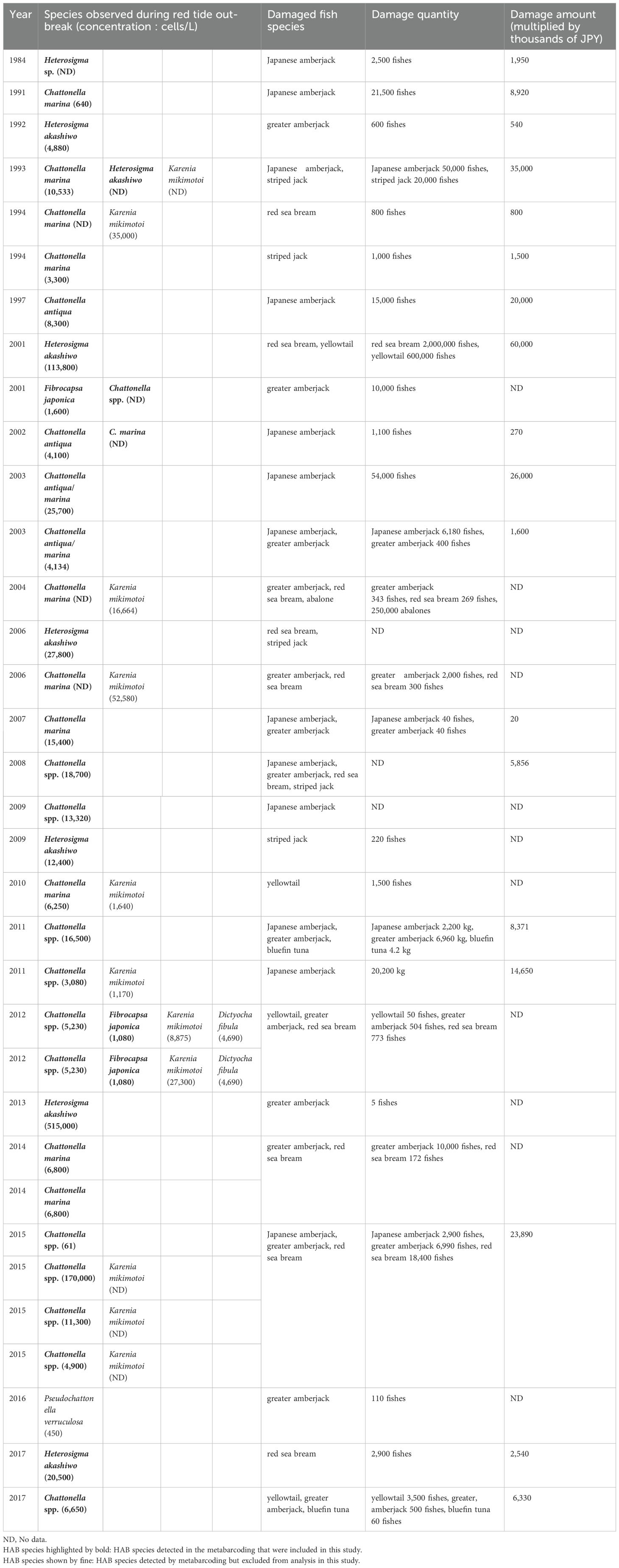
Table 2. Summary of the dominant HAB species of the red tides and the economic damage to fish aquaculture in Uranouchi Inlet, compiled by the Kochi Prefectural Fisheries Experiment Station.
Water quality survey results from 1980 to 2020 showed that DIN-N, DON-N, DOP-P, T-N and T-P stayed high in 2005, while inorganic nitrogen (NH4-N, NO2-N, NO3-N and DIN-N) and inorganic phosphorus (PO4-P) were intermittently high from 1980 to 2020 (Table 3).
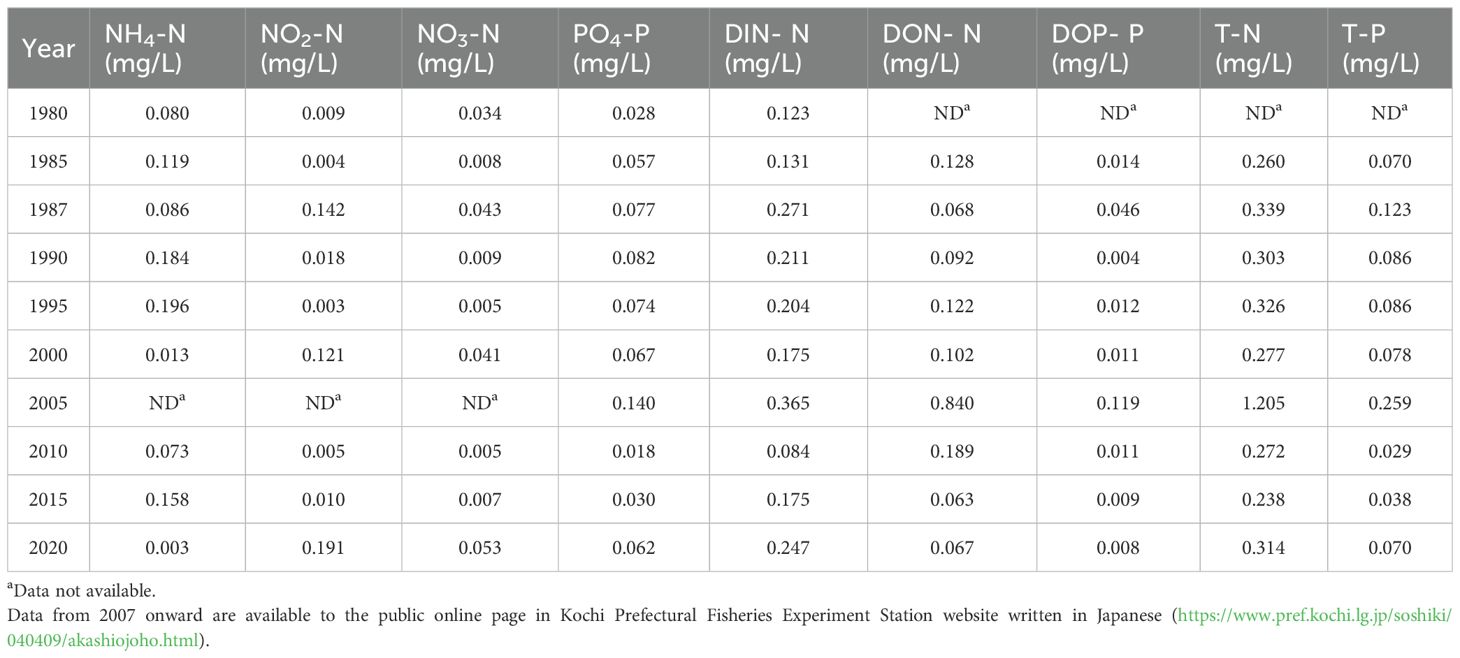
Table 3. Summary of water quality survey results in Mitsumatsu station near Menokuso Station in Uranouchi Inlet, where the core sample used in this study was collected, conducted by Kochi Prefectural Fisheries Experiment Station and Fisheries Research Institute, Japan Fisheries Research and Education Agency.
3.4 Vertical distribution of HAB species
The vertical distribution of HAB species analyzed via a heatmap revealed that the eleven HAB species could be divided into three groups (Figure 1). The first group was composed of six species found in samples from almost all the sediment layers: A. hiranoi/pseudogonyaulax, C. marina complex, F. japonica, H. ‘circularisquama’, H. ‘akashiwo’ and Skeletonema spp.
The second group of A. affine, A. pacificum (Group IV), and A. tamiyavanichii was not detected or was detected at low abundance in the upper layers of the core sample (Figure 1, URA01–08), whereas these species were detected in the deeper layers (under URA09 and URA10, whose years of occurrence were estimated to be 1980s).
The third group was composed of two HAB species, A. leei and A. ‘poporum’, which were not detected in the deep layers, unlike the URA10 and URA11 samples, respectively, but were detected in the upper layers (Figure 1, URA01–URA11).
4 Discussion
4.1 DNA of HABs found by metabarcoding
Among the detected HAB species, Alexandrium hiranoi is known to form resting cysts in the winter and can remain dormant until the environmental conditions become suitable for germination (Kita et al., 1985). A. leei (Anderson et al., 2012; Shikata et al., 2020), A. pseudogonyaulax (Anderson et al., 2012; Lassus et al., 2016; Montresor, 1995; Montresor and Marino, 1996), A. affine, A. pacificum (Group IV) and A. tamiyavanichii are also known to form cysts (Anderson et al., 2012; Nguyen-Ngoc, 2004). The following other HAB species are also known to form resting cysts in the sediment: A. poporum (Gu et al., 2013a; Luo et al., 2018), C. marina complex (Imai and Yamaguchi, 2012; Ishikawa et al., 2022; Katano et al., 2014), F. japonica (Cucchiari et al., 2010; Yoshimatsu, 1987), H. circularisquama (Shiraishi et al., 2008), H. akashiwo (Mehdizadeh Allaf, 2023; Mehdizadeh Allaf and Trick, 2023) and Skeletonema spp (Ellegaard and Ribeiro, 2018; McQuoid and Hobson, 1996; Stenow et al., 2020). Therefore, those DNAs detected in the sediment samples have the possibility of originating from cysts or from DNA adsorbed on humic acids (Lewis et al., 2023; Pedersen et al., 2015; Saeki et al., 2011) in the sediment samples.
4.2 Insights into the occurrence trends of HAB species group
From the vertical distribution of eighteen HAB species analyzed by heatmap, the first six HAB species group (A. hiranoi/pseudogonyaulax, C. marina complex, F. japonica, H. ‘circularisquama’, H. ‘akashiwo’ and Skeletonema spp.) which appeared in almost every sediment sample suggest that these six cyst-forming species occurred continuously throughout the ages, and DNA derived from the cysts or cells were deposited in the bottom sediment resulting in their detection in all sediment samples. Since 1984, when records began to be kept by the Kochi Prefectural Fisheries Experimental Station, four genera, Chattonella, Fibrocapsa, Heterocapsa, and Heterosigma, have repeatedly formed red tides and caused economic damage to aquaculture (~60 million JPY, Table 2). Although there are no records prior to 1984, these records after 1984 seem to correspond to the existence of those four genera in the sediments during that period (Figure 1, URA01–URA09).
According to Kochi Prefectural Fisheries Experimental Station, four Alexandrium species (A. affine, A. pacificum (Group IV), A. tamiyavanichii and A. leei) detected by metabarcoding have not been reported by direct cell counting, but three Raphidophyta (genera Chattonella, Fibrocapsa, and Heterosigma) have been reported (Table 2). The reason for this difference is the number of copies of 18S rDNA per cell in Alexandrium spp. and Raphidophyta. It is known that the copy number of 18S rDNA per cell in the genus Alexandrium is higher than that in Raphidophyta (Yarimizu et al., 2021). Therefore, it is likely that metabarcoding would have detected Raphidophyta but Alexandrium was not. This is a weakness of the occurrence analysis by metabarcoding and should be analyzed by quantitative metabarcoding in the future (Ushio et al., 2018; Sato et al., 2021; Tsuji et al., 2022).
The reason why A. ‘poporum’ was detected by metabarcoding but its occurrence has not been recorded may be because the size of this species is small and difficult to identify under microscopic observation (Takahashi et al., 2021b; Tillmann et al., 2011). Considering these issues, analysis of HAB species in bottom sediments by metabarcoding is useful for clarifying the occurrence history of HAB species.
In relation with the second HAB species group (A. affine, A. pacificum (Group IV), and A. tamiyavanichii) in the heatmap, Alexandrium spp. blooms occur on a large scale in Osaka Bay and Hiroshima Bay in the Seto Inland Sea, Japan, when nutrient concentrations such as DIN and PO4 are less than 12.8 μM and 0.4 μM, respectively (Itakura et al., 2002; Yamamoto, 2019). Considering these issues, it has been reported that conditions suitable for Alexandrium spp. proliferation may involve oligotrophic or mesotrophic waters rather than eutrophic waters (Itakura et al., 2002; Natsuike et al., 2018a, 2018b; Yamamoto, 2019). Therefore, the bloom formation of the Alexandrium species in Uranouchi Inlet may have been suppressed by eutrophication caused by aquaculture after 1977–1988 (Table 3), as discussed later.
There are two possibilities for why the third HAB species group (A. leei and A. ‘poporum’) in the heatmap were not detected in deeper samples than the URA11 and URA12 samples, whose years estimated by radiometric dating (Takahashi et al., 2021a) were 1977 and 1954. First, these two species did not occur in Uranouchi Inlet prior to 1977 or 1954 and appeared after those years; second, the cysts that formed prior to 1977 or 1954 might have decomposed since the cysts of these two HAB species are not highly durable. Regarding the first possibility, yellowtail aquaculture started in Uranouchi Inlet in 1959, and the aquaculture industry may have caused environmental changes such as eutrophication, as discussed later in the enclosed inlet, which may have enhanced bloom formation in these two species. The cyst durability of these two species should be investigated to clarify which possibility is conceivable.
4.3 Replacement of HAB species groups in the sediment of Uranouchi Inlet
Possible causes of the change in HAB species composition after 1977–1988 (URA09–11) include the following two possibilities: first, eutrophication of Uranouchi Inlet due to the start of aquaculture, and second, climate change, such as global warming. Regarding the first possibility, the total N (T-N) and total P (T-P) concentrations in Uranouchi Inlet in 1985 were 0.260 mg/L and 0.070 mg/L, respectively (Table 3). These values suggest that the seawaters in Uranouchi Inlet were eutrophicated or polluted at that time, based on the criterion of eutrophication (T-N: 0.220– 0.650 mg/L, T-P: 0.03–0.09 mg/L, Tavakoly Sany et al., 2014) and pollution in coastal waters (‘low-level’ T-N pollution: 0.252–0.308 mg/L; and ‘very high level’ T-P pollution: > 0.031 mg/L, Smith, 2003). It is possible that the eutrophication in Uranouchi Inlet may have occurred as a result of the proliferation of aquaculture in Uranouchi Inlet from the 1960s to the 1970s, which resulted in changes in HAB species composition from 1977–1988. However, information on nutrient concentrations in the years prior to the 1980s is not available. This hypothesis is supported by the finding that Skeletonema spp., a known indicator of eutrophication in the Seto Inland Sea of Japan (Itakura and Yamaguchi, 2007; Nishikawa et al., 2010; Yamada et al., 1980a, 1980b, 2011), have been detected in greater numbers in Uranouchi Inlet since 1977–1988 (Figure 1). On the other hand, there is a gap of more than a decade between the start of aquaculture in Uranouchi Inlet (1959) and the transition timing of the HAB species groups occurred (1977– 1988). This gap may be due to eutrophication caused by nutrients increasing beyond the environmental capacity while the aquaculture was gradually expanded since the start of aquaculture was not sudden on a large scale.
Second, the sea surface temperature (SST) around Japan has increased due to global warming, and the annual average SST in the northwestern Pacific around Japan has shown an increasing trend (1.24 °C/100 years, Japan Meteorological Agency, 2023). The annual average SST in Uranouchi Inlet increased by 0.19 °C/decade from the 1970s to the 2010s (Tanaka et al., 2012). Such SST trends in Uranouchi Inlet and nearby sea areas may increase blooms of HAB species such as A. leei, which have been reported to occur in tropical and subtropical areas, such as Malaysia (Usup et al., 2002), Singapore (Tang et al., 2007), Thailand (Kodama et al., 1982), Vietnam (Nguyen-Ngoc, 2004), and China (Gu et al., 2013b). This hypothesis was supported by the report that A. leei recently formed blooms and caused fish mortality in Nomi Bay in 2017 (Shikata et al., 2020), which is close to Uranouchi Inlet. Although it is difficult to directly link such changes in the bloom formation of HAB species with global warming, it may be important to consider global warming as at least one of the factors that caused the changes in the occurrence trends of HAB species in Uranouchi Inlet observed in this study.
In this study, we revealed the presence of eleven HAB species, with notable shifts from approximately 1977–1988 in Uranouchi Inlet, Kochi, Japan. This shift corresponded to two hypotheses: the development of aquaculture and the resulting eutrophication, or sea surface temperature rising due to global warming. Moreover, metabarcoding using vertical core sediment samples provides footprints of how HAB species composition has changed and maybe be affected by anthropogenic environmental changes.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
HF: Conceptualization, Writing – original draft, Methodology, Visualization, Data curation, Formal analysis, Investigation. CG: Methodology, Data curation, Writing – original draft. TN: Data curation, Writing – original draft. KT: Writing – original draft, Resources. KK: Formal analysis, Writing – original draft, Data curation. TK: Data curation, Writing – original draft, Formal analysis. KN: Writing – original draft, Funding acquisition. MA: Writing – review & editing, Writing – original draft, Conceptualization, Supervision, Funding acquisition, Visualization, Validation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Ministry of Agriculture, Forestry and Fisheries (Project Number: JP005317).
Acknowledgments
We thank Kohei Ohnishi for allowing us to use his facilities and for this technical support for MiSeq sequencing. We also thank Michiko Takahashi for sharing sediment samples of Uranouchi Inlet. We thank Kazuno Arai and Masafumi Murayama for providing radiometric measurements and estimated dating data of sediment samples.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frpro.2025.1612811/full#supplementary-material
References
Afgan E., Baker D., Batut B., van den Beek M., Bouvier D., Čech M., et al. (2018). The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res. 46, W537–W544. doi: 10.1093/nar/gky379
Anderson D. M., Alpermann T. J., Cembella A. D., Collos Y., Masseret E., and Montresor M. (2012). The globally distributed genus Alexandrium: Multifaceted roles in marine ecosystems and impacts on human health. Harmful Algae. 14, 10–35. doi: 10.1016/j.hal.2011.10.012
Armbrecht L., Bolch C. J. S., Paine B., Cooper A., McMinn A., Woodward C., and Hallegraeff G. (2024). Recovering sedimentary ancient DNA of harmful dinoflagellates accumulated over the last 9000 years off Eastern Tasmania, Australia. ISME Commun. 4. doi: 10.1093/ismeco/ycae098
Astuya-Villalón A., Ramírez A. E., Aballay A., Araya J., Silva J., Ulloa V., et al. (2015). Neurotoxin-like compounds from the ichthyotoxic red tide alga Heterosigma akashiwo induce a TTX-like synaptic silencing in mammalian neurons. Harmful Algae. 47, 1–8. doi: 10.1016/j.hal.2015.04.006
Astuya-Villalón A., Rivera A., Vega-Drake K., Aburto C., Cruzat F., Ulloa V., et al. (2018). Study of the ichthyotoxic microalga Heterosigma akashiwo by transcriptional activation of sublethal marker Hsp70b in Transwell co-culture assays. PloS One. 13, e0201438. doi: 10.1371/journal.pone.0201438
Astuya-Villalón A., López B., Avello V., Rivera A., Aballay-González A., Ulloa V., et al. (2023). In vitro evaluation of the potential allelopathic and ichthyotoxic effect of the raphidophyte Heterosigma akashiwo and the dinoflagellate Alexandrium catenella. Mar. Environ. Res. 183, 105800. doi: 10.1016/j.marenvres.2022.105800
Boere A. C., Abbas B., Rijpstra W. I. C., Versteegh G. J. M., Volkman J. K., Sinninghe DamstÉ J. S., et al. (2009). Late-Holocene succession of dinoflagellates in an Antarctic fjord using a multiproxy approach: Paleoenvironmental genomics, lipid biomarkers and palynomorphs. Geobiology. 7, 265–281. doi: 10.1111/j.1472-4669.2009.00202.x
Boere A. C., Sinninghe Damsté J. S., Rijpstra W. I. C., Volkman J. K., and Coolen M. J. L. (2011). Sourcespecific variability in post-depositional DNA preservation with potential implications for DNA based paleoecological records. Org. Geochem. 42, 1216–1225. doi: 10.1016/J.ORGGEOCHEM.2011.08.005
Borsato G. T., Salgueiro F., De’Carli G. A. L., Morais A. M., Goulart A. S., de Paula J. C., et al. (2023). Taxonomy and abundance of epibenthic Prorocentrum (Dinophyceae) species from the tropical and subtropical Southwest Atlantic Ocean including a review of their global diversity and distribution. Harmful Algae. 127, 102470. doi: 10.1016/j.hal.2023.102470
Brosnahan M. L., Fischer A. D., Lopez C. B., Moore S. K., and Anderson D. M. (2020). Cyst-forming dinoflagellates in a warming climate. Harmful Algae. 91, 101728. doi: 10.1016/j.hal.2019.101728
Brown E. R., Moore S. G., Gaul D. A., and Kubanek J. (2021). Differentiating toxic and nontoxic congeneric harmful algae using the non-polar metabolome. Harmful Algae. 110, 102129. doi: 10.1016/J.HAL.2021.102129
Camacho C., Coulouris G., Avagyan V., Ma N., Papadopoulos J., Bealer K., et al. (2009). BLAST+: architecture and applications. BMC Bioinformatics. 10, 421. doi: 10.1186/1471-2105-10-421
Coolen M. J. L., Orsi W. D., Balkema C., Quince C., Harris K., Sylva S. P., et al. (2013). Evolution of the plankton paleome in the Black Sea from the Deglacial to Anthropocene. Proc. Natl. Acad. Sci. U. S. A. 110, 8609–8614. doi: 10.1073/PNAS.1219283110/SUPPL_FILE/SAPP.PDF
Cucchiari E., Pistocchi R., Pezzolesi L., Penna A., Battocchi C., Cerino F., et al. (2010). Resting cysts of Fibrocapsa japonica (Raphidophyceae) from coastal sediments of the northern Adriatic Sea (Mediterranean Sea). Harmful Algae. 10, 81–87. doi: 10.1016/j.hal.2010.07.003
de Boer M. K., Tyl M. R., Fu M., Kulk G., Liebezeit G., Tomas C. R., et al. (2009). Haemolytic activity within the species Fibrocapsa japonica (Raphidophyceae). Harmful Algae. 8, 699–705. doi: 10.1016/J.HAL.2012.03.005
Dzhembekova N., Moncheva S., Ivanova P., Slabakova N., and Nagai S. (2018). Biodiversity of phytoplankton cyst assemblages in surface sediments of the Black Sea based on metabarcoding. Biotechnol. Biotechnol. Equip. 32, 1507–1513. doi: 10.1080/13102818.2018.1532816
Ellegaard M. and Ribeiro S. (2018). The long-term persistence of phytoplankton resting stages in aquatic ‘seed banks.’. Biol. Rev. 93, 166–183. doi: 10.1111/brv.12338
Engesmo A., Eikrem W., Seoane S., Smith K., Edvardsen B., Hofgaard A., et al. (2016). New insights into the morphology and phylogeny of Heterosigma akashiwo (Raphidophyceae), with the description of. Heterosigma minor sp. nov. Phycologia. 55, 279–294. doi: 10.2216/15-115.1
Feifel K. M., Fletcher S. J., Watson L. R., Moore S. K., and Lessard E. J. (2015). Alexandrium and Scrippsiella cyst viability and cytoplasmic fullness in a 60-cm sediment core from Sequim Bay, WA. Harmful Algae. 47, 56–65. doi: 10.1016/j.hal.2015.05.009
Feifel K. M., Moore S. K., and Horner R. A. (2012). An Alexandrium spp. cyst record from Sequim Bay, Washington State, USA, and its relation to past climate variability. J. Phycol. 48, 550–558. doi: 10.1111/j.1529-8817.2012.01175.x
Flores-Leñero A., Vargas-Torres V., Paredes-Mella J., Norambuena L., Fuenzalida G., Lee-Chang K., et al. (2022). Heterosigma akashiwo in patagonian fjords: genetics, growth, pigment signature and role of PUFA and ROS in ichthyotoxicity. Toxins 14, 577. doi: 10.3390/toxins14090577
Funaki H., Gaonkar C. C., Kataoka T., Nishimura T., Tanaka K., Yanagida I., et al. (2022). Horizontal and vertical distribution of Gambierdiscus spp. (Dinophyceae) including novel phylotypes in Japan identified by 18S rDNA metabarcoding. Harmful Algae. 111, 102163. doi: 10.1016/J.HAL.2021.102163
Gu H., Luo Z., Krock B., Witt M., and Tillmann U. (2013a). Morphology, phylogeny and azaspiracid profile of Azadinium poporum (Dinophyceae) from the China Sea. Harmful Algae. 21–22, 64–75. doi: 10.1016/J.HAL.2012.11.009
Gu H., Zeng N., Liu T., Yang W., Müller A., and Krock B. (2013b). Morphology, toxicity, and phylogeny of Alexandrium (Dinophyceae) species along the coast of China. Harmful Algae. 27, 68–81. doi: 10.1016/j.hal.2013.05.008
Guillou L., Bachar D., Audic S., Bass D., Berney C., Bittner L., et al. (2013). The Protist Ribosomal Reference database (PR2): A catalog of unicellular eukaryote Small Sub-Unit rRNA sequences with curated taxonomy. Nucleic Acids Res. 41, D597–D604. doi: 10.1093/nar/gks1160
Hallegraeff G. M., Anderson D. M., and Cembella A. D. (2003). “Manual on harmful marine microalgae,” in Monographs on oceanographic methodology, vol. 11. (UNESCO publishing, Paris, France).
Härnström K., Ellegaard M., Andersen T. J., and Godhe A. (2011). Hundred years of genetic structure in a sediment revived diatom population. Proc. Natl. Acad. Sci. U. S. A. 108, 4252–4257. doi: 10.1073/pnas.1013528108
Hashimoto T., Matsuoka S., Yoshimatsu S., Miki K., Nishibori N., Nishio S., et al. (2002). First paralytic shellfish poison (PSP) infestation of bivalves due to toxic dinoflagellate Alexandrium tamiyavanichii, in the southeast coasts of the Seto Inland Sea, Japan. J. Food Hyg. Soc Japan. 43, 1–5. doi: 10.3358/shokueishi.43.1
Hori K., Mine T., and Masuda Y. (2019). The population dynamic and environmental characteristic of Skeletonema spp. causing pyropia bleaching in the Ariake Sea off Saga Prefecture (In Japanese). Bull. Saga. Prefect. Ariake. Fish. Res. Dev. Cent. 3. 29, 25–29. Available at: https://www.pref.saga.lg.jp/kiji00371873/3_71873_152851_up_ng18m77n.pdf (Accessed April 14, 2024).
Horiguchi T. (1995). Heterocapsa circularisquama sp. nov. (Peridiniales, Dinophyceae): A new marine dinoflagellate causing mass mortality of bivalves in Japan. Phycol. Res. 43, 129–136. doi: 10.1111/j.1440-1835.1995.tb00016.x
Imai I., Yamaguchi M., and Hori Y. (2006). Eutrophication and occurrences of harmful algal blooms in the Seto Inland Sea, Japan. Plankton Benthos Res. 1, 71–84. doi: 10.3800/pbr.1.71
Imai I. and Yamaguchi M. (2012). Life cycle, physiology, ecology and red tide occurrences of the fishkilling raphidophyte Chattonella. Harmful Algae. 14, 46–70. doi: 10.1016/j.hal.2011.10.014
Imai I., Inaba N., and Yamamoto K. (2021). Harmful algal blooms and environmentally friendly control strategies in Japan. Fish. Sci. 87, 437–464. doi: 10.1007/s12562-021-01524-7
Ishikawa A., Takei Y., Ishii K., and Yamaguchi M. (2022). Population dynamics of Chattonella (Raphidophyceae), causative flagellates of noxious red tides in Ago Bay, central Japan, with an emphasis on cyst germination. Plankton Benthos Res. 17, 383–392. doi: 10.3800/pbr.17.383
Itakura S. and Yamaguchi M. (2007). Environmental change and occurrence mechanism of harmful algal blooms in the seto inland sea (in Japanese with english abstract). Japanese J. Benthol. 62, 57–61. doi: 10.5179/benthos.62.57
Itakura S., Yamaguchi M., Yoshida M., and Fukuyo Y. (2002). The seasonal occurrence of Alexandrium tamarense (Dinophyceae) vegetative cells in Hiroshima Bay, Japan. Fish. Sci. 68, 77–86. doi: 10.1046/J.1444-2906.2002.00392.X
Iwasaki H. (1971). Studies on the red tide flagellates—VI - On Eutreptiella sp. and Exuviaella sp. appeared in Bingo-Nada, the Seto Inland Sea, in 1970 (in Japanese with English abstract). J. Oceanogr. Soc Japan. 27, 152–157. doi: 10.1007/BF02109134
Japan Meteorological Agency (2023). Climate change monitoring report 2022 (Tokyo: Japan Meteorological Agency). Available at: https://www.jma.go.jp/jma/en/NMHS/ccmr/ccmr2022.pdf.
Katano T., Yoshino K., Ito Y., and Hayami Y. (2014). Relationship between the number of cysts that germinate and the occurrence of red tides in the inner part of the Ariake Sea (In Japanese with English abstract). Bull. Plankton Soc. Japan. 61, 8–14. doi: 10.24763/bpsj.61.1_8
Kent M. L., Whyte J. N. C., and LaTrace C. (1995). Gill lesions and mortality in seawater pen-reared Atlantic salmon Salmo salar associated with a dense bloom of Skeletonema costatum and Thalassiosira species. Dis. Aquat. Organ. 22, 77–81. doi: 10.3354/dao022077
Kita T., Fukuyo Y., Tokuda H., and Hirano R. (1985). Life history and ecology of Goniodoma pseudogoniaulax (Pyrrhophyta) in a rockpool. Bull. Mar. Sci. 37, 643–651.
Kodama M., Fukuyo Y., Ogata T., Igarashi T., Kamiya H., and Matsuura F. (1982). Comparison of toxicities of Protogonyaulax cells of various sizes. Bull. Japanese Soc. Sci. Fish. 48, 567–571. doi: 10.2331/suisan.48.567
Krock B., Tillmann U., Potvin É., Jeong H. J., Drebing W., Kilcoyne J., et al. (2015). Structure elucidation and in vitro toxicity of new azaspiracids isolated from the marine dinoflagellate Azadinium poporum. Mar. Drugs 13, 6687–6702. doi: 10.3390/md13116687
Landsberg J. H. (2002). The effects of harmful algal blooms on aquatic organisms. Rev. Fish. Sci. 10, 113–390. doi: 10.1080/20026491051695/ASSET//CMS/ASSET/3F871394-863B-45E1-B8CC-34518DF2398F/20026491051695.FP.PNG
Lassus P., Chomérat N., Hess P., and Nézan E. (2016). Toxic and harmful microalgae of the world ocean (Denmark: IOC/UNESCO).
Lewis D. A., Simpson R., Hermes A., Brown A., and Llamas B. (2023). More than dirt: Sedimentary ancient DNA and Indigenous Australia. Mol. Ecol. Resour. 25, 1–9. doi: 10.1111/1755-0998.13835
Li T. S., Yu R. C., and Zhou M. J. (2011). Short-term effects of different nitrogen substrates on growth and toxin production of dinoflagellate Alexandrium catenella Balech (strain ACDH). Harmful Algae. 12, 46–54. doi: 10.1016/J.HAL.2011.08.011
Lim P. T., Leaw C. P., Usup G., Kobiyama A., Koike K., and Ogata T. (2006). Effects of light and temperature on growth, nitrate uptake, and toxin production of two tropical dinoflagellates: Alexandrium tamiyavanichii and Alexandrium minutum (Dinophyceae). J. Phycol. 42, 786–799. doi: 10.1111/j.1529-8817.2006.00249.x
Liu M., Tillmann U., Ding G., Wang A., and Gu H. (2023). Metabarcoding revealed a high diversity of Amphidomataceae (Dinophyceae) and the seasonal distribution of their toxigenic species in the Taiwan Strait. Harmful Algae. 124, 102404. doi: 10.1016/J.HAL.2023.102404
Lundholm N., Bernard C., Churro C., Escalera L., Hoppenrath M., Iwataki M., et al. (2009). IOC-UNESCO taxonomic reference list of harmful micro algae. Available online at: https://www.marinespecies.org/hab (Accessed February 26, 2025).
Lundholm N., Ribeiro S., Andersen T. J., Koch T., Godhe A., Ekelund F., et al. (2011). Buried alive - Germination of up to a century-old marine protist resting stages. Phycologia. 50, 629–640. doi: 10.2216/11-16.1
Luo Z., Krock B., Mertens K. N., Nézan E., Chomérat N., Bilien G., et al. (2017). Adding new pieces to the Azadinium (Dinophyceae) diversity and biogeography puzzle: Nontoxigenic Azadinium zhuanum sp. nov. from China, toxigenic A. poporum from the Mediterranean, and a non-toxigenic A. dalianense from the French Atlantic. Harmful Algae. 66, 65–78. doi: 10.1016/J.HAL.2017.05.001
Luo Z., Krock B., Giannakourou A., Venetsanopoulou A., Pagou K., Tillmann U., et al. (2018). Sympatric occurrence of two Azadinium poporum ribotypes in the Eastern Mediterranean Sea. Harmful Algae. 78, 75–85. doi: 10.1016/J.HAL.2018.08.003
Matsuyama Y. (2012). Impacts of the harmful dinoflagellate Heterocapsa circularisquama bloom on shellfish aquaculture in Japan and some experimental studies on invertebrates. Harmful Algae. 14, 144–155. doi: 10.1016/j.hal.2011.10.019
McQuoid M. R. and Hobson L. A. (1996). Diatom resting stages. J. Phycol. 32, 889–902. doi: 10.1111/j.0022-3646.1996.00889.x
Mehdizadeh Allaf M. (2023). Heterosigma akashiwo, a fish-killing flagellate. Microbiol. Res. (Pavia). 14, 132–147. doi: 10.3390/microbiolres14010012
Mehdizadeh Allaf M. and Trick C. G. (2023). Insights into Cellular Localization and Environmental Influences on the Toxicity of Marine Fish-Killing Flagellate, Heterosigma akashiwo. Int. J. Mol. Sci. 24, 10333. doi: 10.3390/ijms241210333
Minamiura N. and Yamaguchi S. (2019). Relationship between physical environment and 3 phytoplankton species, Skeletonema spp., Eucampia zodiacus, Asteroplanus karianus causing color bleaching of cultured nori in the Ariake Sea during winter (In Japanese with English abstract). J. Jpn. Soc. Civil Eng., Ser. B2 (Coast. Eng.). 75, 991–996. doi: 10.2208/kaigan.75.I_991
Ministry of Agriculture and Forestry and Fisheries (Japan) (2018). Statistics on Marine Fishery Production (in Japanese). Available at: https://www.e-stat.go.jp/stat-search/files?page=1&layout=datalist&toukei=00500216&tstat=000001015174&cycle=7&year=20180&month=0&tclass1=000001015175&tclass2=000001136043&tclass3val=0 (Accessed April 14, 2024).
Miyazono A., Nagai S., Kudo I., and Tanizawa K. (2012). Viability of Alexandrium tamarense cysts in the sediment of Funka Bay, Hokkaido, Japan: Over a hundred year survival times for cysts. Harmful Algae. 16, 81–88. doi: 10.1016/j.hal.2012.02.001
Montresor M. (1995). The life history of Alexandrium pseudogonyaulax (Gonyaulacales, Dinophyceae). Phycologia. 34, 444–448. doi: 10.2216/i0031-8884-34-6-444.1
Montresor M. and Marino D. (1996). Modulating effect of cold-dark storage on excystment in Alexandrium pseudogonyaulax (Dinophyceae). Mar. Biol. 127, 55–60. doi: 10.1016/S0040-4039(00)86674-5
Murakami M., Makabe K., Yamaguchi K., Konosu S., and Wälchli M. R. (1988). Goniodomin a, a novel polyether macrolide from the dinoflagellate goniodoma pseudogoniaulax. Tetrahedron Lett. 29, 1149–1152. doi: 10.1016/S0040-4039(00)86674-5
Murakami M., Okita Y., Matsuda H., Okino T., and Yamaguchi K. (1998). From the dinoflagellate Alexandrium hiranoi. Phytochemistry. 48, 85–88. doi: 10.1016/S0031-9422(97)00756-5
Natsuike M., Shiraishi T., Ishii K.-I., Yamamoto K., Nakajima M., Sawayama S., et al. (2018a). Different Nutrient Availabilities of Surface and Bottom Water under Nutrient-depleted Conditions during Bloom Formation of the Toxic Dinoflagellate Alexandrium tamarense in Osaka Bay, Japan. Bull. Fish. Sci. Hokkaido Univ. 68, 7–16. doi: 10.14943/bull.fish.68.1.7
Natsuike M., Yamamoto K., Nakajima M., Sawayama S., and Imai I. (2018b). Comparison of nutrient availabilities between the toxic dinoflagellate Alexandrium tamarense and the non-toxic diatom Skeletonema sp. in Osaka Bay, Japan (In Japanese with English abstract). Bull. Fish. Sci. Hokkaido Univ. 68, 17–26. doi: 10.14943/bull.fish.68.1.17
Nguyen-Ngoc L. (2004). An autecological study of the potentially toxic dinoflagellate Alexandrium affine isolated from Vietnamese waters. Harmful Algae. 3, 117–129. doi: 10.1016/S1568-9883(03)00062-3
Nishikawa T., Hori Y., Nagai S., Miyahara K., Nakamura Y., Harada K., et al. (2010). Nutrient and phytoplankton dynamics in Harima-Nada, eastern Seto Inland Sea, Japan during a 35-year period from 1973 to 2007. Estuar. Coast. 33, 417–427. doi: 10.1007/s12237009-9198-0
Pedersen M. W., Overballe-Petersen S., Ermini L., Sarkissian C. D., Haile J., Hellstrom M., et al. (2015). Ancient and modern environmental DNA. Phil. Trans. R. Soc. B, Biol. Sci. 370, 1–11. doi: 10.1098/rstb.2013.0383
R Core Team (2023). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available at: https://www.R-project.org. (Accessed April 14, 2024).
RStudio Team (2023). RStudio: Integrated Development Environment for R. Posit Software, PBC, Boston, MA. Available at: http://www.posit.co/. (Accessed April 14, 2024).
Saeki K., Ihyo Y., Sakai M., and Kunito T. (2011). Strong adsorption of DNA molecules on humic acids. Environ. Chem. Lett. 9, 505–509. doi: 10.1007/s10311-011-0310-x
Sagara T., Taniyama S., Yoshimatsu S., Takatani T., Hashimoto T., Nishibori N., et al. (2010). Toxicity and toxin profile of the dinoflagellate Alexandrium tamiyavanichii and toxic mussels in Harima-Nada of Seto Inland sea, Japan (In Japanese with English abstract). J. Food Hyg. Soc. Japan. 51, 170–177. doi: 10.3358/shokueishi.51.170
Sakamoto S., Lim W. A., Lu D., Dai X., Orlova T., and Iwataki M. (2021). Harmful algal blooms and associated fisheries damage in East Asia: Current status and trends in China, Japan, Korea and Russia. Harmful Algae. 102, 101787. doi: 10.1016/J.HAL.2020.101787
Sato M., Inoue N., Nambu R., Furuichi N., Imaizumi T., and Ushio M. (2021). Quantitative assessment of multiple fish species around artificial reefs combining environmental DNA metabarcoding and acoustic survey. Sci. Rep. 11, 1–14. doi: 10.1038/S41598-021-98926-5
Schloss P. D., Westcott S. L., Ryabin T., Hall J. R., Hartmann M., Hollister E. B., et al. (2009). Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75, 7537–7541. doi: 10.1128/AEM.01541-09
Shikata T., Taniguchi E., Sakamoto S., Kitatsuji S., Yamasaki Y., Yoshida M., et al. (2020). Phylogeny, growth and toxicity of the noxious red-tide dinoflagellate Alexandrium leei in Japan. Reg. Stud. Mar. Sci. 36, 101265. doi: 10.1016/J.RSMA.2020.101265
Shikata T., Yuasa K., Kitatsuji S., Sakamoto S., Akita K., Fujinami Y., et al. (2021). Superoxide production by the red tide-producing Chattonella marina complex (Raphidophyceae) correlates with toxicity to aquacultured fishes. Antioxidants. 10, 1635. doi: 10.3390/antiox10101635
Shiraishi T., Hiroishi S., Taino S., Ishikawa T., Hayashi Y., Sakamoto S., et al. (2008). Identification of overwintering vegetative cells of the bivalve-killing dinoflagellate Heterocapsa circularisquama in Uranouchi Inlet, Kochi Prefecture, Japan. Fish. Sci. 74, 128–136. doi: 10.1111/j.1444-2906.2007.01502.x
Siano R., Lassudrie M., Cuzin P., Briant N., Loizeau V., Schmidt S., et al. (2021). Sediment archives reveal irreversible shifts in plankton communities after World War II and agricultural pollution. Curr. Biol. 31, 2682–2689.e7. doi: 10.1016/J.CUB.2021.03.079
Smith V. H. (2003). Eutrophication of freshwater and coastal marine ecosystems: A global problem. Environ. Sci. pollut. Res. 10, 126–139. doi: 10.1065/espr2002.12.142
Stenow R., Olofsson M., Robertson E. K., Kourtchenko O., Whitehouse M. J., Ploug H., et al. (2020). Resting stages of Skeletonema marinoi assimilate nitrogen from the ambient environment under dark, anoxic conditions. J. Phycol. 56, 699–708. doi: 10.1111/jpy.12975
Takahashi K., Lum W. M., Benico G., Uchida H., Ozawa M., Oikawa H., et al. (2021b). Toxigenic strains of Azadinium poporum (Amphidomataceae, Dinophyceae) from Japan and Vietnam, with first reports of A. poporum (ribotype A) and A. trinitatum in Asian Pacific. Phycol. Res. 69, 175–187. doi: 10.1111/pre.12455
Takahashi M., Wada K., Takano Y., Matsuno K., Masuda Y., Arai K., et al. (2021a). Chronological distribution of dinoflagellate-infecting RNA virus in marine sediment core. Sci. Total Environ. 770, 145220. doi: 10.1016/j.scitotenv.2021.145220
Tanaka K., Taino S., Haraguchi H., Prendergast G., and Hiraoka M. (2012). Warming off southwestern Japan linked to distributional shifts of subtidal canopy-forming seaweeds. Ecol. Evol. 2, 2854–2865. doi: 10.1002/ece3.391
Tang Y. Z., Kong L., and Holmes M. J. (2007). Dinoflagellate Alexandrium leei (Dinophyceae) from Singapore coastal waters produces a water-soluble ichthyotoxin. Mar. Biol. 150, 541–549. doi: 10.1007/S00227-006-0396-Z/FIGURES/3
Tavakoly Sany S. B., Hashim R., Rezayi M., Salleh A., and Safari O. (2014). A review of strategies to monitor water and sediment quality for a sustainability assessment of marine environment. Environ. Sci. pollut. Res. 21, 813–833. doi: 10.1007/s11356-013-2217-5
Thoha H., Muawanah, Bayu Intan M. D., Rachman A., Sianturi O. R., Sidabutar T., et al. (2019). Resting cyst distribution and molecular identification of the harmful dinoflagellate Margalefidinium polykrikoides (Gymnodiniales, dinophyceae) in Lampung Bay, Sumatra, Indonesia. Front. Microbiol. 10. doi: 10.3389/fmicb.2019.00306
Tillmann U., Edvardsen B., Krock B., Smith K. F., Paterson R. F., and Voß D. (2018). Diversity, distribution, and azaspiracids of Amphidomataceae (Dinophyceae) along the Norwegian coast. Harmful Algae. 80, 15–34. doi: 10.1016/J.HAL.2018.08.011
Tillmann U., Elbrächter M., John U., and Krock B. (2011). A new non-toxic species in the dinoflagellate genus Azadinium: A. poporum sp. nov. Eur. J. Phycol. 46, 74–87. doi: 10.1080/09670262.2011.556753
Triki H. z., Laabir M., Moeller P., Chomérat N., and Kéfi Daly-Yahia O. (2016). First report of goniodomin A production by the dinoflagellate Alexandrium pseudogonyaulax developing in southern Mediterranean (Bizerte Lagoon, Tunisia). Toxicon. 111, 91–99. doi: 10.1016/j.toxicon.2015.12.018
Tsuji S., Inui R., Nakao R., Miyazono S., Saito M., Kono T., et al. (2022). Quantitative environmental DNA metabarcoding shows high potential as a novel approach to quantitatively assess fish community. Sci. Rep. 12, 21524. doi: 10.1038/s41598-022-25274-3
Twiner M. J., Rehmann N., Hess P., and Doucette G. J. (2008). Azaspiracid shellfish poisoning: a review on the chemistry, ecology, and toxicology with an emphasis on human health impacts. Mar. Drugs 6, 39–72. doi: 10.3390/md20080004
Ushio M., Murakami H., Masuda R., Sado T., Miya M., Sakurai S., et al. (2018). Quantitative monitoring of multispecies fish environmental DNA using highthroughput sequencing. Metabarcoding and Metagenomics. 2, 1–15. doi: 10.3897/mbmg.2.23297
Usup G., Pin L. C., Ahmad A., and Teen L. P. (2002). Alexandrium (Dinophyceae) species in Malaysian waters. Harmful Algae. 1, 265–275. doi: 10.1016/S1568-9883(02)00044-6
Wang Z., Peng L., Xie C., Wang W., Zhang Y., Xiao L., et al. (2022a). Metabarcoding of harmful algal bloom species in sediments from four coastal areas of the southeast China. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.999886
Wang Z., Wang C., Wang M., Li W., Zhong W., Liu L., et al. (2022b). Diversity and community structure of eukaryotic microalgae in surface sediments in the central Bohai Sea, China, based on a metabarcoding approach. J. Oceanol. Limnol. 40, 2277–2291. doi: 10.1007/S00343-021-0481-7/METRICS
Wang L., Yan T., Yu R., and Zhou M. (2005). Experimental study on the impact of dinoflagellate Alexandrium species on populations of the rotifer Brachionus plicatilis. Harmful Algae. 4, 371–382. doi: 10.1016/j.hal.2004.06.014
Yamada M., Tsuruta A., and Yoshida Y. (1980a). A list of phytoplankton as eutrophic level indicator (in Japanese with English abstract). Bull. Japanese Soc. Sci. Fish. 46, 1435–1438. doi: 10.2331/suisan.46.1435
Yamada M., Tsuruta A., and Yoshida Y. (1980b). Classification of Eutrophic level in Several Marine Regions (Japanese with English abstract). Bull. Japanese Soc. Sci. Fish. 46, 1439–1444. doi: 10.2331/suisan.46.1439
Yamada M., Ueda N., and Hamada K.-I. (2011). Changes in red tide occurrence and organisms responsible for declining eutrophic level in hyper-eutrophic Dokai Bay, Japan. Nippon. Suisan. Gakkaishi. 77, 647–655. doi: 10.2331/suisan.77.647
Yamaguchi M., Itakura S., Imai I., and Ishida Y. (1995). A rapid and precise technique for enumeration of resting cysts of Alexandrium spp. (Dinophyceae) in natural sediments. Phycologia. 34, 207–214. doi: 10.2216/i0031-8884-34-3-207.1
Yamaguchi H., Tanimoto Y., Hayashi Y., Suzuki S., Yamaguchi M., and Adachi M. (2018). Bloom dynamics of noxious Chattonella spp. (Raphidophyceae) in contrastingly enclosed coastal environments: A comparative study of two coastal regions. J. Mar. Biol. Assoc. U. K. 98, 657–663. doi: 10.1017/S0025315417000017
Yamamoto K. (2019). Long-Term Fluctuations in Phytoplankton and Marked Bloom of the Toxic Dinoflagellate Alexandrium tamarense in Osaka Bay, Eastern Seto Inland Sea, Japan (In Japanese with English abstract). Bull. Coast. Oceanogr. 56, 63–72. doi: 10.32142/ENGANKAIYO.56.2_63
Yarimizu K., Sildever S., Hamamoto Y., Tazawa S., Oikawa H., Yamaguchi H., et al. (2021). Development of an absolute quantification method for ribosomal RNA gene copy numbers per eukaryotic single cell by digital PCR. Harmful Algae. 103, 102008. doi: 10.1016/j.hal.2021.102008
Yoshimatsu S. (1987). The Cysts of Fibrocapsa japonica(Raphidophyceae) found in bottom sediment in Harima-Nada, Eastern Inland Sea of Japan (Japanese with English abstract). Bull. Plankton Soc. Japan. 34, 25–31.
Keywords: metabarcoding, 18S rDNA, eutrophication, anthropogenic impact, HABs, core sample
Citation: Funaki H, Gaonkar CC, Nishimura T, Tanaka K, Kamimura K, Kaji T, Nagasaki K and Adachi M (2025) Vertical distribution of harmful algae in the sediment of Uranouchi Inlet by metabarcoding. Front. Protistol. 3:1612811. doi: 10.3389/frpro.2025.1612811
Received: 16 April 2025; Accepted: 30 May 2025;
Published: 30 June 2025.
Edited by:
Kirsty Smith, Cawthron Institute, New ZealandReviewed by:
Laura Biessy, Cawthron Institute, New ZealandTiago Pereira, University of São Paulo, Brazil
Copyright © 2025 Funaki, Gaonkar, Nishimura, Tanaka, Kamimura, Kaji, Nagasaki and Adachi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Masao Adachi, bWFkYWNoaUBrb2NoaS11LmFjLmpw
†Present addresses: Chetan Chandrakant Gaonkar, Department of Oceanography, Texas A&M University, College Station, TX, United States
Tomohiro Nishimura, Fisheries Technology Institute, Japan Fisheries Research and Education Agency, Hatsukaichi, Japan
 Hiroshi Funaki
Hiroshi Funaki Chetan Chandrakant Gaonkar1†
Chetan Chandrakant Gaonkar1† Keizo Nagasaki
Keizo Nagasaki Masao Adachi
Masao Adachi