- 1Department of Isotope Biogeochemistry, Helmholtz Centre for Environmental Research – UFZ, Leipzig, Germany
- 2Department of Biological, Geological, and Environmental Sciences, Alma Mater Studiorum - Università di Bologna, Bologna, Italy
Background: Aquifer thermal energy storage (ATES) is a subsurface technology for urban heating and cooling. However, ATES systems may intersect with legacy groundwater contaminants from past anthropogenic activities. Chlorinated ethenes, particularly tetrachloroethene (PCE) and trichloroethene (TCE), are common pollutants that can undergo microbial reductive dechlorination to cis-dichloroethene (cis-DCE), vinyl chloride (VC), and ultimately ethene. Since microbial activity is temperature dependent, heat storage in ATES systems may influence dechlorination efficiency.
Methods: The study assessed the effect of temperature on microbial reductive dechlorination and community composition using sediment from a contaminated aquifer in Ferrara, Italy, where VC accumulation is of concern. Laboratory microcosms were amended with TCE and lactate, incubated at 10–60°C, and monitored for 105 days.
Results: Complete dechlorination to ethene occurred at 10–20°C and was linked to Dehalogenimonas spp. cis-DCE and VC accumulated at 30°C and 40°C, respectively, while no dechlorination activity was observed at 50°C and 60°C, suggesting temperature-related inhibition. Methanogenesis occurred between 10 and 40°C and was associated with Methanosarcina, Methanothrix (mainly in non-TCE-amended controls), and Methanomicrobia (10–30°C). Methanogenic activity was absent above 40°C and delayed at 10°C.
Conclusion: These results suggest that microbial dechlorination of chlorinated ethenes is impaired at temperatures exceeding 40°C. Therefore, integrating low- or medium-temperature (< 40°C) ATES with enhanced natural attenuation may offer a viable strategy for simultaneous energy storage and bioremediation in chlorinated solvent-contaminated aquifers.
Introduction
Aquifer thermal energy storage (ATES) is a subsurface technology for urban heating and cooling, offering a promising solution to reduce dependence on fossil fuels (Mathiesen, 2019), especially in densely populated areas with high energy demands (Elsland et al., 2017; Menberg, 2014). However, many urban aquifers are contaminated due to anthropogenic activities, including the release of chlorinated ethenes (CEs) through leakages and improper disposal practices (Bishop et al., 1993; Rittmann et al., 2000; Ruden, 2006).
Chlorinated ethenes (CEs), i.e. tetrachloroethene (perchloroethene, PCE), trichloroethene (TCE), the dichloroethene isomers (DCEs), and vinyl chloride (VC), are widely used in commercial and industrial applications, including as solvents, cleaning agents and plastics manufacturing. Due to their environmental persistence, toxicity, and frequent occurrence in urban groundwater systems, these compounds are classified as priority pollutants, subject to strict regulatory control to safeguard surface and drinking water quality (Kalnins et al., 2019; Lepom et al., 2009; Rivett et al., 2012; Squillace et al., 2004).
Under anoxic conditions, PCE and TCE can undergo microbial reductive dechlorination to cis-DCE and VC, and ultimately to the non-toxic end product ethene (Adrian and Löffler, 2016). Ensuring complete conversion to ethene is critical for effective bioremediation (Figure 1) (Huang et al., 2014; National Research Council, 2000).
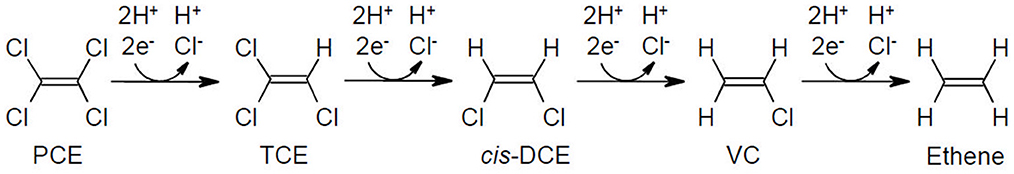
Figure 1. Reductive dechlorination pathway of PCE to ethene via TCE, cis-DCE, and VC. Each step involves microbial replacement of a chlorine atom with hydrogen under anoxic conditions.
In the subsurface, microbial dechlorination activity is influenced by environmental conditions, notably temperature (Badin et al., 2016; Wiedemeier et al., 1998). Temperature shifts can alter the composition of native microbial consortia composition and affect their reductive dechlorination ability (Beyer et al., 2016; Garcia et al., 2018; Yamazaki et al., 2022). Microbial reductive dehalogenation (RDH) to ethene has been observed up to 30°C; above this, this process often becomes incomplete—resulting in accumulation of DCE or VC—or is entirely inhibited (for a review, see Bin Hudari et al., 2022a). Temperature also influences microbial competition for electron donors and carbon sources. For instance, hydrogenotrophic methanogenesis may compete with dehalogenators and affect overall dechlorination potential (Smatlak et al., 1996; Wei et al., 2016). Methanogenesis can occur concurrently and typically spans a broader and higher temperature range (Jones et al., 1987; Prondzinsky et al., 2023).
Thus, moderately increasing subsurface temperatures (e.g., up to ~30°C) may support both ATES and enhanced contaminant bioremediation, while avoiding the inhibitory effects observed at higher temperatures (Badin et al., 2016; Bonte et al., 2013; Delille et al., 2004; Ni et al., 2018, 2015, 2016). However, for reasons of energy efficiency, there is growing interest in increasing groundwater reinjection temperatures in low-temperature (LT)-ATES (typically ≤25°C) to >50°C for high-temperature (HT)-ATES applications. HT-ATES systems offer greater sustainability for heating and cooling due to their higher energy storage capacity (Daniilidis et al., 2022; Drijver et al., 2012; Kallesøe and Vangkilde-Pedersen, 2019). This creates a trade-off between thermal energy optimization and maintaining favorable conditions for microbial contaminant degradation.
Despite its importance, few systematic studies have investigated the effects of temperature on microbial reductive dehalogenation, and even fewer that address shifts in the associated microbial communities. In particular, data on how elevated temperatures affect bioremediation in situ remain limited (for a comprehensive review, please see Bin Hudari et al., 2022a).
This study aimed to investigate the impact of increasing temperature on microbial reductive dehalogenation activity and microbial community composition using contaminated sediments in a controlled laboratory setting across a broad temperature range.
Materials and methods
Chemicals
All chemicals used in the experiments were purchased from Merck (Darmstadt, Germany), AppliChem (Darmstadt, Germany), Fluka (Buchs, Switzerland), or Sigma-Aldrich (Deisenhofen, Germany) and were of analytical grade at the highest purity.
Laboratory microcosm preparation
Sediment samples were collected from cores drilled at a chlorinated ethenes-contaminated site in northern Italy, near a former disposal area where chlorinated pitches from chloromethane production were illegally dumped between the 1950s and 1970s (Ghezzi et al., 2021; Nijenhuis et al., 2013). Field observations have indicated ongoing dechlorination of PCE and TCE at this site—where natural groundwater temperatures range from 16 to 20°C—with vinyl chloride (VC) often accumulating as the primary or sole detectable intermediate (Filippini et al., 2016). For the microcosm study, TCE was selected as the model compound and its reductive dehalogenation was investigated at six different temperatures (10, 20, 30, 40, 50, and 60°C). Product formation was monitored over 105 days, after which the bacterial and archaeal community structures were analyzed.
The sediment samples used in this study were obtained from a well-characterized hydrogeological setting comprising vertically stacked sandy aquifers and clayey aquitards (Filippini et al., 2016, 2020). PCE and TCE, originally contained in DNAPL (dense non-aqueous phase liquids) wastes, have migrated downward into the subsurface, resulting in chlorinated ethene contamination detected in groundwater and sediments to depths of up to 50 meters below ground surface (bgs) reaching hundreds of mg L−1. Sediments for the microcosms were collected from two core sections: one aquifer layer between 15 and 25 m bgs (corresponding to the “Upper A1” aquifer) and one aquitard layer between 25 and 30 m bgs (part of the “Lower Q1” facies). Sediments were obtained from cores collected during previous site investigations described by Nijenhuis et al. (2013) and Filippini et al. (2016); details of the drilling and sampling procedures can be found in those studies. These cores were recovered during a detailed site investigation at the Caretti site in 2013 (boreholes MC1-2, MC3, and MC4-5), which also included stratigraphic reconstruction using direct-push drillings, multilevel monitoring wells, and piezocone penetration testing (Filippini et al., 2016; Nijenhuis et al., 2013).
Microcosms were set up under anoxic conditions in a glove box (COY Laboratory Products Inc., Michigan, USA), maintained with an N2/H2 gas mixture (95:5%). This atmosphere ensured strict anoxic conditions; while H2 can act as an electron donor, lactate (3 mM) was provided as the primary electron donor and carbon source to support microbial reductive dechlorination. The intent was to ensure that electron donor availability was sufficient, though lactate was not added in large excess. All materials—including serum bottles, septa, and crimps—were autoclaved, dried, and placed inside the glove box several hours before use to prevent oxygen intrusion. Sediments were consolidated and homogenized inside the glove box. A total of 48 serum bottles (120 mL) were prepared, each comprising 20 g of sediment and 50 mL of mineral salts medium (composition in Supplementary Table S1) (Zinder, 1998). Bottles were sealed with Teflon coated rubber septa and aluminum crimps. Bottles were incubated lying on their sides to ensure that the liquid medium covered the septum, minimizing the risk of air intrusion through the septum after perforation.
Eight bottles were assigned to each of six different temperatures (10°C, 20°C, 30°C, 40°C, 50°C, and 60°C): five active replicates (A, B, C, F, G), two sterile controls (D and E), and one anaerobic, non-amended control (ANA) to monitor background activity such as methanogenesis or reductive dechlorination. Sterile replicates were autoclaved at 121°C for 40 min on three consecutive days (see Supplementary Table S2). All bottles were pre-acclimatized at the respective temperatures for at least 48 h before initiating the experiment. The experiment began with the addition of lactate (3 mM) and ~5 μL of neat TCE, resulting in an estimated starting concentration of 100 μmol L−1. ANA controls received no TCE. After amendment, bottles were equilibrated for 3 h at their target temperature prior to sampling. For sampling, 0.5 mL of the headspace was taken from each bottle using a Hamilton gas syringe, with gas transferred to a helium-flushed 10 mL gas chromatography (GC) headspace vial. Following TCE depletion, additional doses (100 μmol L−1) were added. The 20 and 30°C replicates received a total of four doses of TCE (0.4 mmol L−1 total).
Chemical analysis
Samples were analyzed via gas chromatography coupled with a flame ionization detector (GC-FID; Varian Chrompack CP-3800), with a GS-Q column (J&W Scientific, Waldbronn, Germany) and injected via a headspace autosampler HP 7694 (Hewlett Packard, Palo Alto, USA). The chromatographic separation program was adapted from Nijenhuis et al. (2007) and initially set to 100°C (held for 1 min), followed by a temperature ramp of 50°C min−1 to 225°C (held for 6 min). To improve separation of overlapping peaks—particularly as ethene and methane concentrations increased during incubation—the program was further modified after 63 days to start at 80°C (1 min), with the same ramp to 225°C (6 min hold). Data was analyzed by the Varian STAR software for the respective target compounds to obtain the area counts and concentrations were calculated using independent calibration curves for TCE, cis-DCE, VC, ethene, ethane, and methane (not shown).
Statistical analyses were performed in R (version 2024.12.1) using pairwise Wilcoxon rank-sum tests with Benjamini–Hochberg correction for multiple comparisons, based on the highest accumulated concentrations of ethene and methane across temperature treatments. Significant differences between treatments are indicated in the boxplots.
Microbial community analysis
The microcosms were sacrificed after 397 days of incubation. While the relevant results shown and discussed in this manuscript reflect observations up to day 105, active microcosms were maintained until day 397 through periodic amendment with TCE as the electron acceptor. TCE was re-supplied only when measurements indicated it had been fully consumed. Two to four mL of sediment slurry were collected from 36 of the sample bottles, except for the 12 sterile controls, into 2 mL Eppendorf tubes (see Supplementary Table S3). The slurry was centrifuged at 13 000 rpm for 2 min, the supernatant was discarded, and the pellet was stored at −20°C until extraction. At each temperature, one non-amended replicate (i.e., without TCE) was included to serve as background control, allowing qualitative assessment of microbial community composition in the absence of TCE. These background samples were not included in statistical comparisons but supported interpretation of temperature-related shifts in microbial profiles. DNA extraction was carried out with the DNeasy Powersoil Kit (Qiagen, Hilden, Germany) following the manufacturer's instructions with minor modifications.
For each replicate, sediment slurries from a single microcosm bottle were distributed into multiple Eppendorf tubes and processed in parallel. Prior to the elution step, lysates from the same replicate were sequentially loaded onto a single spin column during centrifugation, allowing DNA to be pooled and concentrated. The DNA was eluted in a final volume of ~20–30 μL. Specifically, the elution step was performed by first adding 20 μL of elution buffer onto the column membrane, letting it stand for 1 min before centrifugation. This step was repeated using the collected eluent (15 μL) combined with an additional 15 μL of buffer to maximize DNA recovery. DNA concentration was measured with the Qubit HS (High Sensitivity) Assay Kit (Thermo Fisher Scientific, USA) on the Qubit 3.0 Fluorometer (Life Technologies, Malaysia). MiSeq sequencing procedures were similar to previous studies prescribed elsewhere (Bin Hudari et al., 2020, 2022b) using the Klindworth primer pair (S-D-Bact-0341-b-S-17/S-D-Bact-0785-a-A-21). Briefly, sequencing libraries were assembled with Illumina MiSeq Reagent Kit v3 (2 x 300 bp) following protocols recommended by the manufacturer on 16S Metagenomic Sequencing Library Preparations (Illumina, 2013). Sequencing was done on the Illumina Miseq platform at the former Department of Environmental Microbiology (currently, Applied Microbial Ecology) of the Helmholtz Centre for Environmental Research—UFZ. Sequences were analyzed on a QIIME 2 v2019.1 platform using a pipeline as described previously (Bolyen et al., 2019). This pipeline removes primer sequences and adapters from the de-multiplexed sequences, then trimming and denoising them to remove low quality reads and chimeras, before merging. Amplicon sequence variants (ASVs) were then assigned to the bacterial DNA using the Silva132 database (Quast et al., 2013; Yilmaz et al., 2014). Sequences were deposited at the European Nucleotide Archive (ENA) under the primary accession number PRJEB73360 (https://www.ebi.ac.uk/ena/browser/view/PRJEB73360).
Results
Reductive dechlorination and methanogenesis at different temperatures
TCE dechlorination product formation was monitored for up to 105 days to highlight treatment-specific differences in activity. Product profiles varied with temperature: ethene was detected at 10–30°C, with ethane appearing at 20°C and 30°C, indicating more complete dechlorination at these temperatures. VC was observed only at 30°C, while cis-DCE accumulated at 30°C and 40°C, suggesting incomplete dechlorination at higher temperatures. No dechlorination products were detected at 50°C and 60°C.
Figure 2 presents representative data from 20, 30, and 40°C to illustrate the temperature-dependent transition from complete to incomplete dechlorination. These temperatures were selected to highlight the effects of thermal elevation on dechlorination and methanogenesis. The 10°C condition, which reflects the native groundwater temperature and exhibited complete dechlorination, is shown in Supplementary Figures S1A–C due to space limitations. Additional replicate data for all conditions, including full triplicate datasets at 10°C and 60°C, are provided in Supplementary Figures S1–S3.
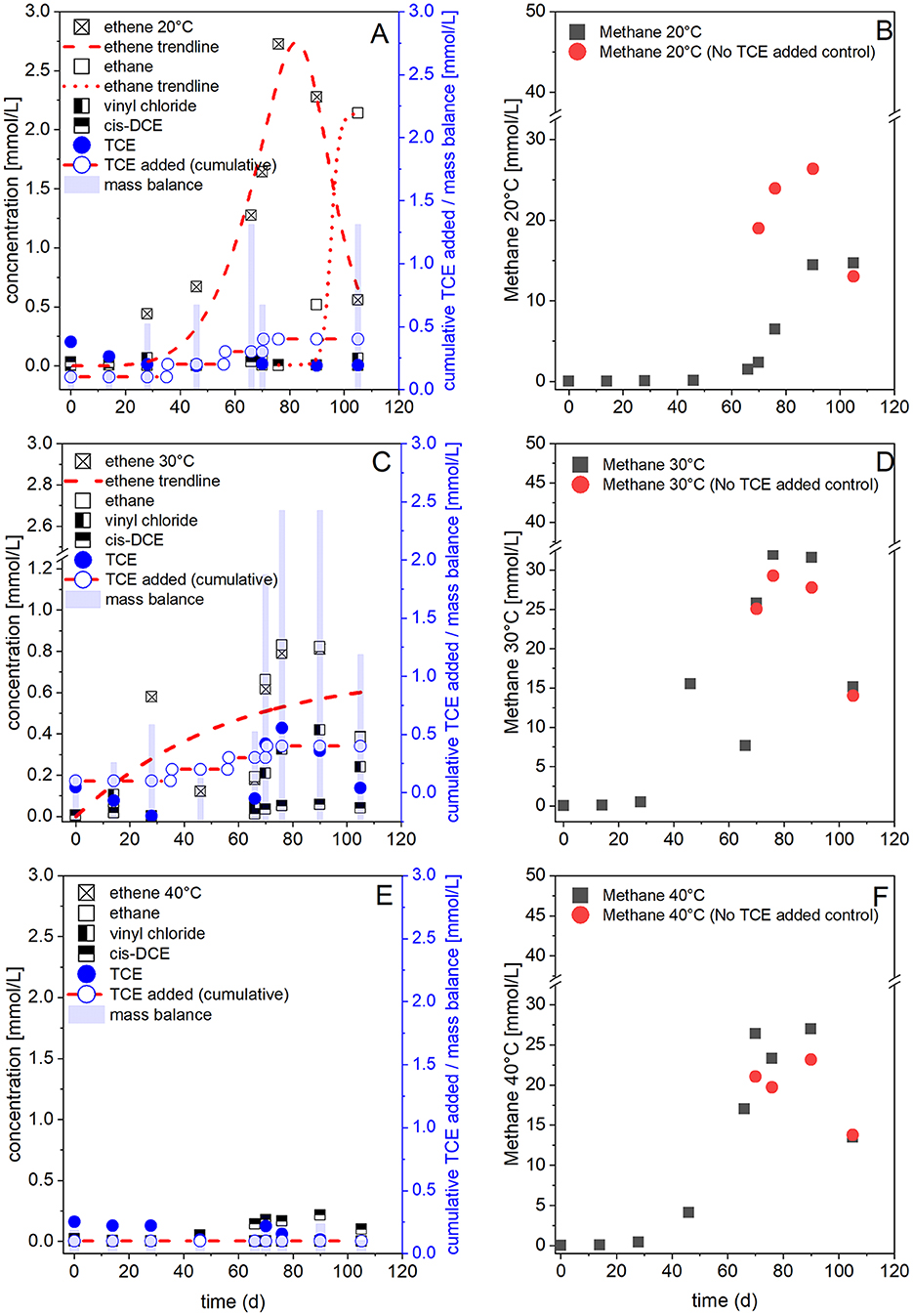
Figure 2. Representative profiles of trichloroethene (TCE) dechlorination and methanogenesis in microcosms incubated at 20°C (A, B), 30°C (C, D), and 40°C (E, F). (A, C, E) show concentrations of TCE, its dechlorination products (cis-DCE, vinyl chloride, ethene, ethane), and the cumulative TCE mass balance over 105 days. “Mass balance” represents the total molar sum of TCE and its dechlorination products. (B, D, F) show methane production in TCE-amended microcosms (black) and in the corresponding non-amended anaerobic controls (red). These replicates are representative; additional replicate data are provided in Supplementary Figures S1–S3.
Methanogenesis and reductive dechlorination were temperature dependent and inhibited at higher temperatures (≥50°C). Dechlorination occured at 10–40°C, though the extent decreased at 30 and 40°C. Complete TCE dechlorination to ethene was observed at 10°C (Supplementary Figures S1A–C), 20°C (Figure 2A; Supplementary Figures S1E–G), and 30°C (Figure 2C; Supplementary Figures S2A–C), with ethene concentrations increasing over time. No activity was observed in sterile controls (data not shown).
In setups at 10°C, following a lag phase, ethene accumulated after 50 days without detectable cis-DCE or VC accumulation (Supplementary Figures S1A–C). Methanogenesis was not significant during the early phase (<0.1 mmol L−1 of methane; Supplementary Figure S1D) but was observed after 130 d of incubation, including in the non-amended controls (data not shown). At 20°C and 30°C, ethene was observed after 10–20 days and increased before declining after 80 days, accompanied by rising ethane concentrations (Figures 2A, C; Supplementary Figures S1E–G, S2A–C). Methane accumulation in the TCE-amended replicates ranged between 11 and 18 mmol L−1 at 20°C (Figure 2B, Supplementary Figure S1H) and 16–31 mmol L−1 at 30°C (Figure 2D; Supplementary Figure S2D).
At 30°C, VC began to accumulate after 63 days, reaching stable levels (~0.4 mmol L−1), while ethene remained the primary product until 42 days, after which both ethene and ethane were detected (Supplementary Figures S2A–C). At 40°C, dechlorination stalled at cis-DCE by day 105 (Figure 2E; Supplementary Figures S2E–G), and no further transformation occurred even after extended incubations (397 days; data not shown). Methane accumulation in 40°C TCE-amended replicates was comparable to that at 20°C, ranging between 11 and 25 mmol L−1 (Figure 2F; Supplementary Figure S2H). Notably, methane production occurred to a similar extent in the non-amended controls, indicating that it was not directly coupled to TCE amendment.
In general, the total measured concentrations of chlorinated ethenes in microcosms incubated at 10–40°C exceeded the initial TCE input. This discrepancy is likely due to residual CEs in the sediment and potential sampling artifacts, such as increased partial pressures at higher temperatures or elevated headspace pressure resulting from methane production.
To summarize treatment effects across all conditions, ethene and methane concentrations were compared using boxplots and Wilcoxon rank-sum tests (Figure 3). Ethene production peaked at 20°C and 30°C, with statistically significant differences (p ≤ 0.05) compared to all other treatments. Methane showed a similar trend, with the highest concentrations at 20–30°C, moderate levels at 40°C, and little or no production at 10°C, 50°C, or 60°C. These results confirm a temperature optimum between 20 and 30°C for both reductive dechlorination and methanogenesis, while no significant product formation was observed at ≥50°C.
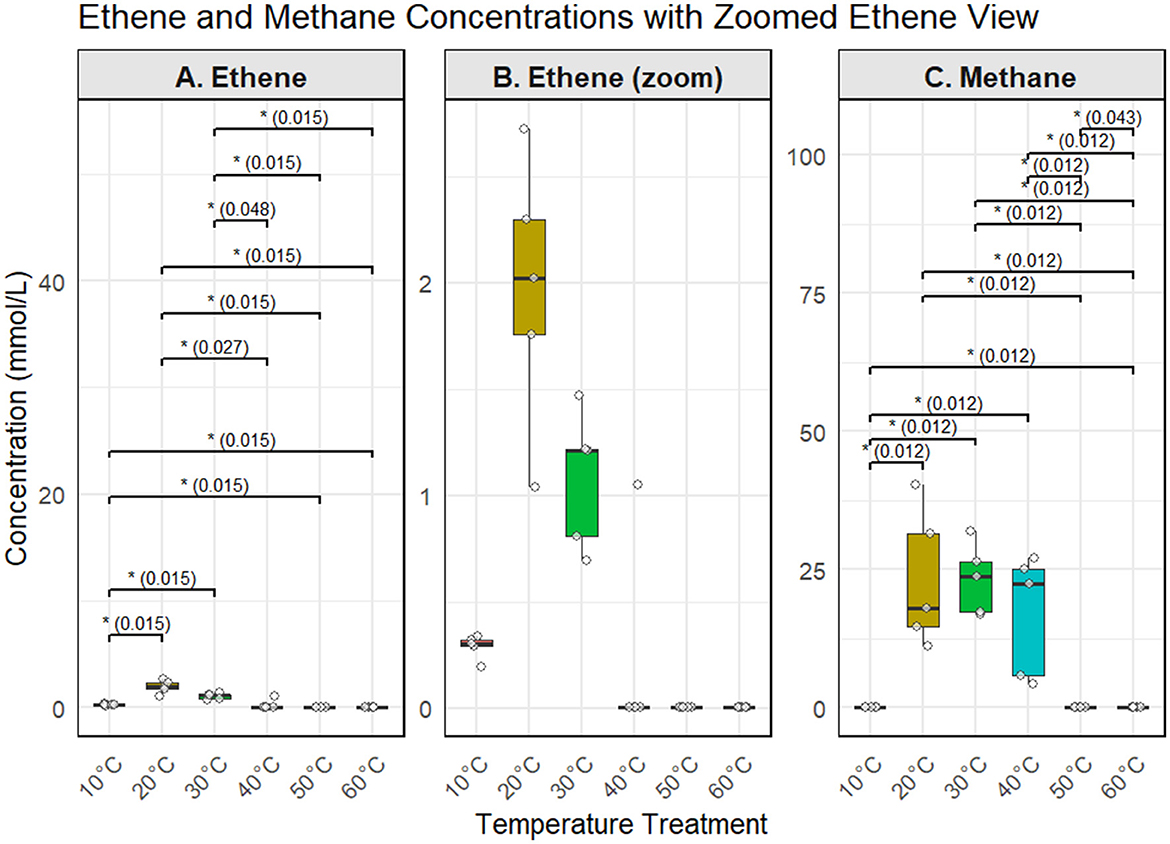
Figure 3. Maximum observed concentrations of ethene and methane in microcosms across temperature treatments. (A) Ethene; (B) Zoomed view of ethene (≤3 mmol L−1); (C) Methane. Boxplots represent the highest concentration measured in each replicate. Individual replicate values are overlaid as jittered points. Statistically significant differences between treatments (Wilcoxon test, Benjamini–Hochberg corrected) are indicated by asterisks with corresponding adjusted p-values.
The microcosms were monitored until day 397 to evaluate possible delayed activity in the 50°C and 60°C treatments. However, no dechlorination or methanogenesis was observed (Supplementary Figures S3A–H). A slight decrease in TCE concentration was noted, but no intermediate or end products accumulated, and these trends mirrored those of sterile controls. Meanwhile, active microcosms (e.g., 20°C and 30°C) were maintained with occasional TCE supplementation until the end of the experiment and were later sacrificed for microbial community analysis. While results up to 105 days and microbial community profiles are shown, data from the extended incubation period (105–397 days) are not shown.
Microbial community analysis
A total of 36 samples—comprising five TCE-amended biological replicates and one non-amended active control per temperature—were sequenced using 16S rRNA gene primers targeting bacteria. This approach generated an average of 72,867 reads per sample (±16,160), with read counts ranging from 42,205 to 104,396 reads. To assess methanogenic community composition, an additional eight samples (one replicate with and one without TCE amendment per temperature, for 10–40°C) were sequenced with the methanogen specific mcrA-targeted primers, targeting the methyl coenzyme M reductase gene (Supplementary Table S3). This mcrA sequencing generated an average of 75,749 reads per sample (±20,636), with read counts between 51,018 and 113,905. While the mcrA-based dataset provided valuable insight into methanogen diversity, we acknowledge that the use of single replicates precludes statistical analysis. Community composition profiles from one representative replicate per active temperature treatment (10–40°C) are shown in Figures 4, 5. Additional replicate data are provided in Supplementary Figures S4, S5 at both family and genus levels.
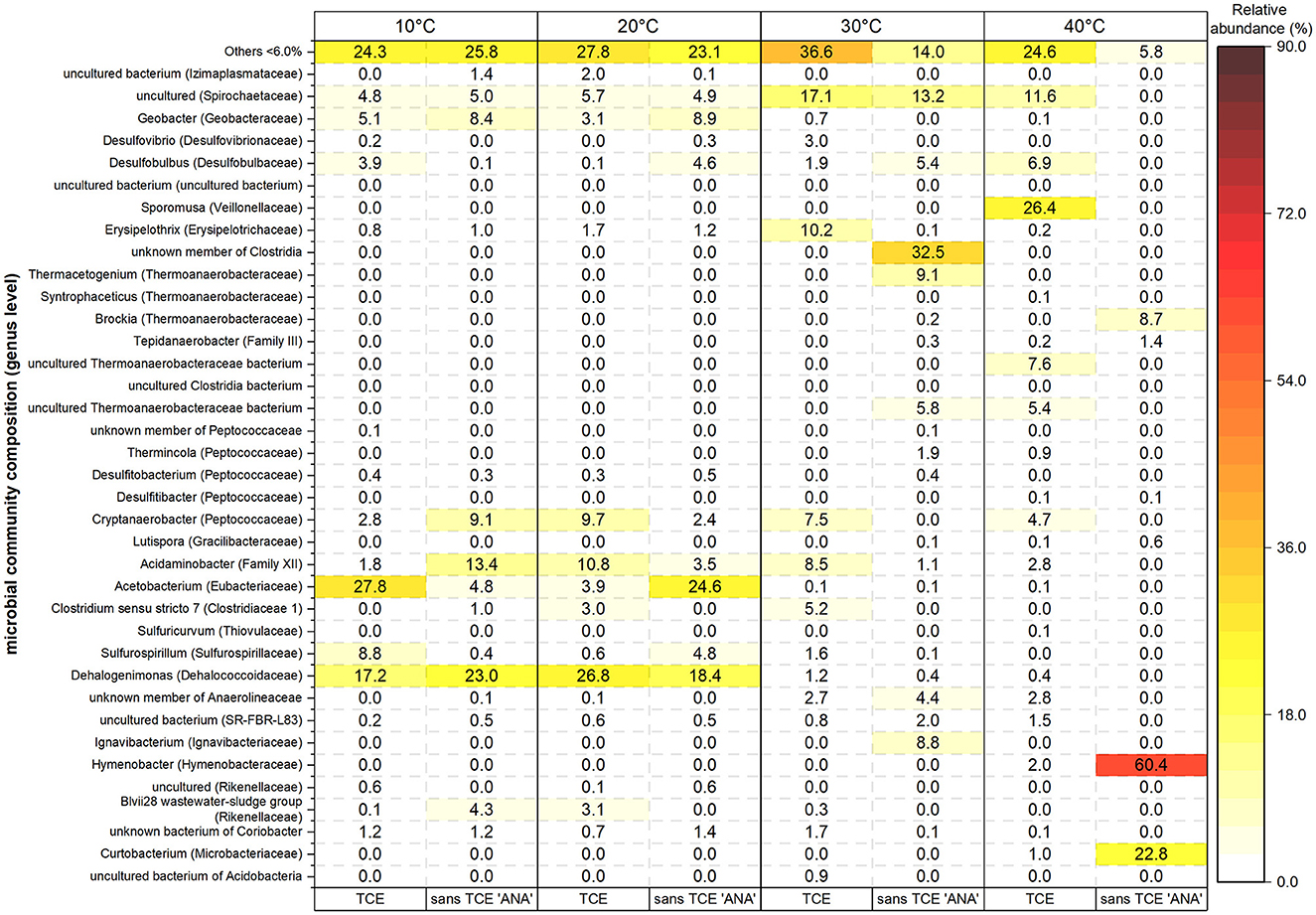
Figure 4. Relative abundance (%) of microbial genera (≥6% in at least one condition) across temperature treatments (10–40°C) under anaerobic conditions, with and without trichloroethene (TCE). Warmer heatmap colors indicate higher abundance. Conditions are labeled as “TCE” (with TCE) and “sans TCE ‘ANA”' (anaerobic control). Genera below 6% are grouped as “Others <6.0%.”
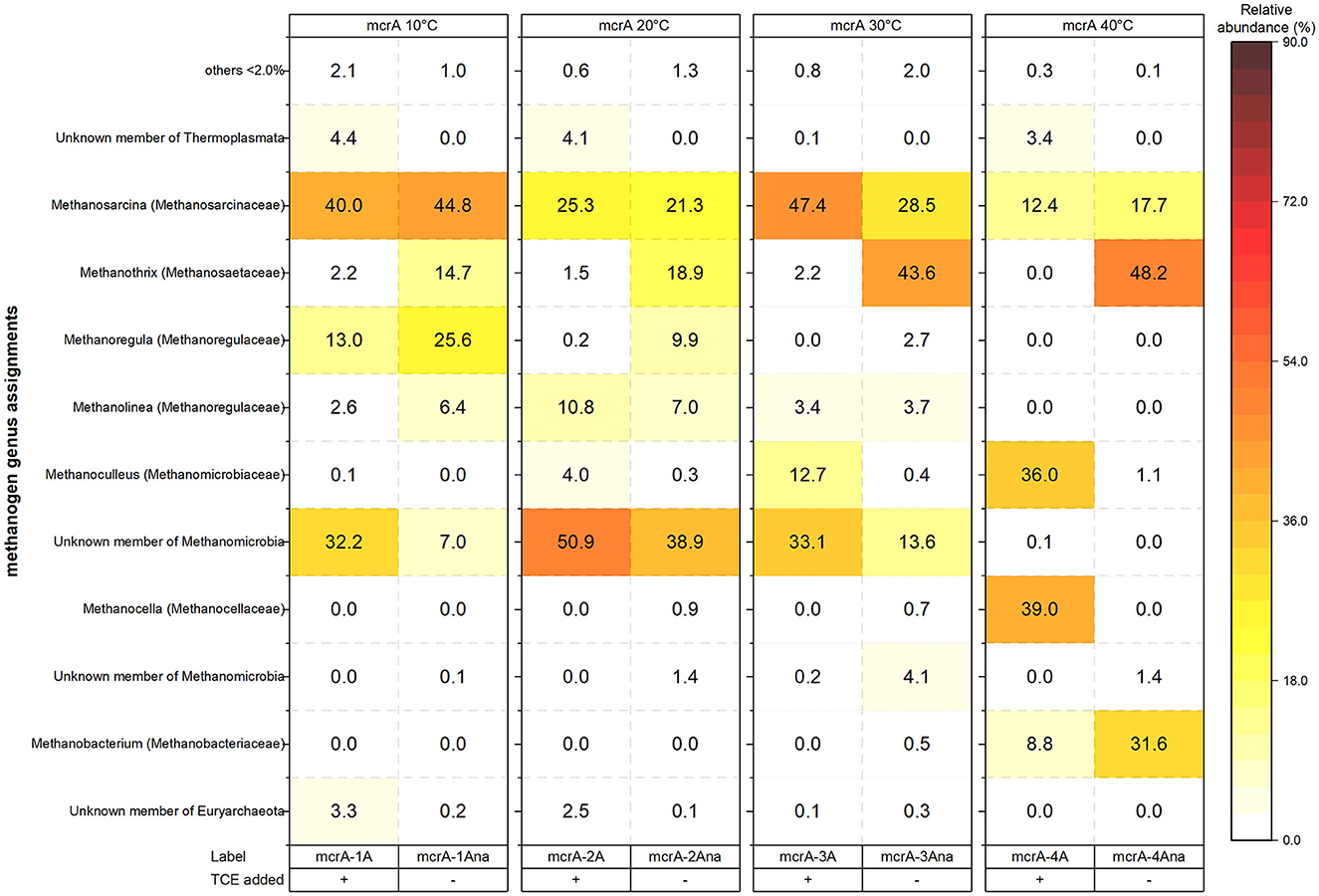
Figure 5. Methanogenic community composition (genus level) across temperature treatments (10–40°C) in TCE-amended (+) and unamended (–) anaerobic microcosms. Relative abundances (%) are based on mcrA gene sequencing. Genera ≥2% in at least one condition are shown; others are grouped as “<2%.” Color intensity reflects relative abundance.
Phylotypes observed in 10°C and 20°C replicates (with and without TCE) were relatively similar in composition (Figure 4 and Supplementary Figures S4, S5). Dehalogenimonas was consistently present at 17–27% relative abundance in all four microcosms (Figure 4). Acetobacterium was more abundant in the 10°C TCE-amended (28%) and 20°C non-amended (ANA) replicates (25%) but was less dominant (4–5%) in the corresponding reciprocal treatments. In contrast, phylotypes such as Acidaminobacter and Cryptanaerobacter showed higher relative abundances specifically in the TCE-amended 20°C microcosms. Other phylotypes detected at lower abundance (3–9%) included uncultured Spirochaetaceae members and Geobacter.
At 30°C, Cryptanaerobacter reached 8% in the 30°C TCE-amended replicate shown in Figure 4 and was also detected in the other replicates (Supplementary Figure S5). Dehalogenimonas remained present, albeit at lower levels (0.2–1.2%), while uncultured Spirochaetaceae (17%), Acidaminobacter (8%), Desulfovibrio (3%), and Desulfobulbus (2%) were also identified. At 40°C, the TCE amended replicate was dominated by Sporomusa (26%), uncultured Spirochaetaceae (12%) and Desulfobulbus (7%) while Dehalogenimonas was nearly absent (0.4%), indicating a potential temperature threshold for its activity or survival.
Methanogen community composition (10–40°C) is shown at the genus level in Figure 5. One TCE-amendment and one non-amended replicate was analyzed per temperature. At 10°C—despite the absence of methane production during the first 105 days (Supplementary Figure S1D), methane was detected after 130 days (data not shown), justifying the inclusion of this sample in the analysis. At 10°C, Methanosarcina was the dominant genus in both amended (40%) and non-amended (45%) microcosms (Figure 5). The amended setup also contained Methanomicrobia (32%) and Methanoregula (13%), whereas the non-amended replicate had higher Methanoregula (26%) and Methanothrix (15%).
At 20°C, unknown Methanomicrobia members were abundant in both the amended (51%) and non-amended (39%) setups, followed by Methanosarcina (25% and 21%, respectively). Methanolinea (11% and 7%) and Methanothrix (2% and 19%) were also present. At 30°C, Methanosarcina (47% and 29%) and an unknown member of Methanomicrobia (33% and 14%) dominated both treatments. Methanoculleus was more abundant in the TCE-amended replicate (13%), while Methanothrix (44%) was predominant in the non-amended replicate.
At 40°C, Methanosarcina abundance decreased in the TCE-amended setup (12%), while Methanoregula and Methanolinea —previously detected at lower temperatures—were absent. In contrast, Methanocella (39%) and Methanoculleus (36%) became more prominent, suggesting a shift toward thermotolerant methanogens. In the corresponding non-amended replicate, Methanosarcina (18%) and Methanothrix (48%) remained abundant, consistent with patterns observed in non-amended replicates at lower temperatures. Notably, Methanobacterium reached 32% in the 40°C non-amended replicate, indicating a possible temperature- and treatment-specific niche for this genus.
Discussion
Temperature significantly influenced both microbial community composition and the extent of TCE dechlorination, leading to cis-DCE and VC accumulation at 40°C and 30°C, respectively. Dechlorination rates were highest at 20–30°C, while temperatures >40°C were inhibitory to both reductive dechlorination and methanogenesis. Complete dechlorination to ethene occurred only in microcosms incubated between 10 and 30°C, confirming the temperature sensitivity of this process, as shown in earlier studies (Friis et al., 2007b; Heimann et al., 2007). A 10°C temperature increment (e.g., from 30 to 40°C) affected the transformation sequence, leading to VC accumulation at 30°C and cis-DCE at 40°C after 60 days. At higher temperatures (50–60°C), neither dechlorination nor methanogenesis occurred, consistent with previous reports (Friis et al., 2007b; Zhuang and Pavlostathis, 1995). This likely reflects the inability of key dechlorinating microbes to remain viable or competitive at these elevated temperatures (Fletcher et al., 2011; Magnuson et al., 1998).
Despite a lag phase at 10°C, complete TCE dechlorination to ethene was eventually observed, similar to findings at low temperatures—including 4, 10, and 15°C—in other studies (De Bruin et al., 1992; Heimann et al., 2007). In our study, ethene formation began after ~50 days, indicating delayed microbial activity, likely due to slower metabolism or growth rates at suboptimal temperatures.
Community analysis suggests that Dehalogenimonas (Dhgm.) is a likely candidate for TCE dechlorination to ethene at 10–20°C. For example, isolate Dehalogenimonas etheniformans has been reported to dechlorinate TCE, DCE, and VC to ethene at 15–34°C (Chen et al., 2022; Cui et al., 2023). Here, Dehalogenimonas reached 17–27 % relative abundance at 10–20°C in line with complete dechlorination (Figure 2). Conversely, its low abundance (~1%) at ≥30°C corresponded to partial TCE dechlorination, suggesting thermal inhibition. Although some Dehalogenimonas phylotypes were present at 60°C, no dechlorination occurred, reinforcing their temperature sensitivity. At 40°C, Dehalogenimonas was absent; instead, phylotypes such as Peptococcaceae (e.g., Desulfitobacterium) or Geobacter, known to reduce PCE to cis-DCE, may have contributed to TCE transformation (Röling, 2014; Villemur et al., 2006).
Although lactate degradation was not directly measured, the occurrence of reductive dechlorination and methanogenesis at 10–40°C suggests that fermentation likely occurred, providing the electron donors such as acetate and hydrogen. Lactate, added as both an electron donor and carbon source, is typically converted by a metabolically diverse microbial consortium into substrates used by organohalide-respiring bacteria (e.g., Dehalogenimonas) (summarized in Supplementary Figure S6) (McInerney et al., 2009; Schink and Stams, 2006; Stams and Plugge, 2009). Acetate is essential for biomass synthesis in dehalogenators (He et al., 2002; Robles et al., 2021; Rosell et al., 2019), while acetate and hydrogen can also be consumed by hydrogenotrophic and acetotrophic methanogens (Conrad, 2020; Jones et al., 1987).
Community composition analysis revealed temperature- and treatment-dependent trends among key microbial taxa. Acetobacterium showed notably contrasting patterns: it was abundant in the 10°C TCE-amended (27.8%) and 20°C non-amended (24.6%) replicates, but nearly absent (<5%) in the reciprocal conditions. This suggests a potential interaction between TCE exposure and temperature on Acetobacterium abundance or activity. Given its roles in lactate fermentation, hydrogen production, and corrinoid biosynthesis, Acetobacterium may have supported syntrophic partners such as dehalogenators under selective conditions (Puentes Jacome et al., 2019; Wen et al., 2015).
At 40°C, Sporomusa was more abundant (26.4%) in the TCE-amended setup, possibly serving as a temperature-adapted fermenter. In contrast, Dehalogenimonas, a key organohalide-respiring bacterium, was prominent at 10–30°C but declined at 40°C. Sulfate-reducing genera such as Desulfovibrio and Desulfobulbus, capable of incomplete lactate oxidation, were detected at 10–30°C and may have contributed to hydrogen cycling in both amended and non-amended systems.
Hymenobacter dominated one of the 40°C non-amended replicates (>60%), despite being primarily aerobic or facultatively anaerobic and not typically associated with anaerobic degradation. Its prevalence may reflect reduced microbial competition, thermal stress tolerance, or sequencing variability.
Spirochaetaceae were detected across treatments, with higher abundance at 30°C and 40°C. Species such as Rectinema cohabitans are known necromass feeders or acetate producers (Dollhopf et al., 2001; Dong et al., 2018; Koelschbach et al., 2017; Ritalahti et al., 2012). Some Spirochaetes can also oxidize acetate to produce hydrogen and carbon dioxide (Cheng et al., 2022; Si et al., 2016; Wang et al., 2019; Yi et al., 2020), and may be able to utilize lactate anaerobically (Troshina et al., 2015), suggesting a role in carbon and electron donor cycling.
These observations underscore the interplay between temperature and TCE in shaping microbial community structure and function, with implications for fermentation, methanogenesis, and reductive dechlorination dynamics.
The temperature range for ethene-to-ethane formation was narrower (20–30°C) than that observed for complete reductive dechlorination (10–30°C) or methanogenesis (20–40°C), suggesting that ethane formation may be coupled to other biological processes (Belay and Daniels, 1987; Fullerton et al., 2013; Koene-Cottaar and Schraa, 1998; Xie et al., 2013). For example, in a methanogenic consortia, members of the Methanomicrobiales have been postulated to reduce ethene to ethane, and this conversion was shown to be inhibited by the methanogenesis inhibitor BES (Koene-Cottaar and Schraa, 1998; Xie et al., 2013). In our study, ethene-to-ethane conversion coincided with complete dechlorination and methanogenesis at 20–30°C, supporting ethanogenesis within this temperature range.
Methanogenesis occurred at 20–40°C within 95 days and at 10°C after 130 days, but not at 50–60°C, even after extended incubation (up to 397 days). This indicates that the indigenous microbial community is adapted to mesophilic conditions (15°C to 40°C). Notably, methane production occurred in both TCE-amended and non-amended microcosms, indicating that methanogenesis was independent of TCE presence and likely fueled by fermentation-derived substrates such as hydrogen and acetate. Although methanogenesis can occur between −2.5 and 122°C in other systems (Jones et al., 1987; Mancini et al., 2002; Pannekens et al., 2019; Schupp et al., 2020; Zeman et al., 2014), the community in our study appears more temperature restricted.
Some methanogenic phylotypes exhibited broader tolerance. For example, Methanosarcina was present at 10–40°C and is known to grow under both mesophilic (25–40°C) and thermophilic (50–55°C) conditions, utilizing either acetate or H2/CO2 (Jetten et al., 1992; Wagner, 2020). Other phylotypes showed more restricted temperature distributions—for instance, an unclassified member of Methanomicrobia lineage was only detected between 10°C and 30°C, while Methanobacterium was predominant at 40°C, indicating a possible temperature-specific niche. The absence of methanogenesis above 40°C reinforces the idea that the native microbial community from this contaminated site is not adapted to higher temperatures.
Implications for the ATES-bioremediation combination and outlook
In this study, we systematically assessed the effect of temperature on microbial reductive dechlorination of TCE, relevant to combining enhanced natural attenuation with ATES. At the study site, TCE and its degradation products were detected in both the aquifer (particularly in groundwater) and the surrounding sediment matrix, down to a depth of 50 meters below ground surface.
Based on our findings, within LT- ATES systems (≤25°C), reductive dechlorination of TCE to ethene is unlikely to be inhibited, as we observed dechlorination to ethene up to 30°C. However, the VC accumulation at 30°C and cis-DCE at 40°C deserves more attention, specifically in the context of medium temperature ATES (MT-ATES; 25–40°C), given their health and environmental impacts (Benedict et al., 2024; Williams et al., 2022).
In HT-ATES systems, where injection temperatures exceed 60°C, microbial dechlorination is likely to be inhibited. Nevertheless, in situ temperature gradients are expected, ranging from elevated temperatures near the injection point to cooler conditions farther from the heat source (Kallesøe and Vangkilde-Pedersen, 2019; Lerm et al., 2013). This could create subsurface zones where dechlorination is enhanced (25–30°C) and others —particularly near the hotter core—where it may stall at cis-DCE. Importantly, such thermal effects would primarily occur in the aquifer, while surrounding low-permeability sediments (e.g., aquitards) may remain largely unaffected and continue to act as long-term sources or microbial reservoirs. These findings underscore the importance of accounting for spatial heterogeneity in thermal effects when designing ATES–bioremediation systems.
Beyond considering an upper temperature threshold (e.g., 40°C) or modulating injection and extraction temperatures, further in situ investigations are essential to evaluate the long-term feasibility of the combined approach. Future studies should also evaluate seasonal temperature fluctuations via heat cycle experiments and field validations in diverse geological settings.
Applying microbiological tools such as quantitative PCR (qPCR) and metagenomics could provide deeper insights into temperature-driven shifts in community structure and function, thereby improving our understanding of subsurface microbial interactions and bioremediations under dynamic thermal conditions. Moreover, studies should assess recolonisation rates—whether from surviving consortia in sediment or from microbial inflow following heat-affected phases (Friis, 2006; Friis et al., 2007a).
If complete reductive dechlorination cannot be re-established after high-temperature exposure, biostimulation or bioaugmentation with temperature-adapted consortia could be viable options (Delgado et al., 2014; Ni et al., 2018; Sewell and Gibson, 1991; Xiao et al., 2020).
Additionally, temperature effects on microbial partners supplying essential cofactors, electron donors (e.g., hydrogen) and carbon source (e.g., acetate) must be considered, as inhibition of these auxiliary processes may limit dechlorination rates.
In summary, our results show that lower temperatures (e.g., 10°C) delayed the onset of both dechlorination and methanogenesis (Supplementary Figures S1A–D). Temperature significantly influenced microbial community composition—promoting syntrophic organisms such as fermenters and acetogens that convert lactate into key metabolites. These, in turn, are utilized by reductive dechlorinators (e.g., Dehalogenimonas), and competitors such as hydrogenotrophic methanogens, which compete for shared resources. The 20°C and 30°C incubations supported more favorable conditions for lactate degraders and the dechlorinators, resulting in earlier and more complete product accumulation.
Conversely, unfavorable conditions (e.g., 40°C) could inhibit or eliminate reductive dechlorinators, leading to necromass scavenging and accumulation of intermediates like cis-DCE or VC. Accumulation may result from competition for hydrogen among processes such as reductive dechlorination, ethene-to-ethane reduction, and hydrogenotrophic methanogenesis, or from toxic effects of accumulated intermediates on the native community (Garcia et al., 2018; Lee and Lee, 2016; Smatlak et al., 1996).
Thus, the observed VC accumulation at 30°C and cis-DCE at 40°C remains a key challenge for bioremediation. In conclusion, combining LT-ATES with enhanced natural attenuation is feasible based on our results. However, maintaining a suitable operational temperature range is crucial when implementing MT- or HT-ATES at chlorinated ethene-contaminated sites to ensure a sustainable, continuous natural attenuation.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Author contributions
MB: Writing – original draft, Writing – review & editing, Data curation, Formal analysis, Investigation, Methodology. SD: Formal analysis, Investigation, Methodology, Writing – review & editing. CV: Writing – review & editing, Supervision. MF: Writing – review & editing, Resources. IN: Writing – review & editing, Conceptualization, Supervision, Writing – original draft.
Funding
The authors declare that financial support was received for the research and/or publication of this article. The authors would like to thank the support of the German Federal Ministry for Economic Affairs and Energy (BMWi) within the funding initiative Energiespeicher, project ANGUS II, Grant Number 03ET6122B.
Acknowledgments
The authors would like to thank Denny Popp for providing the structure of the QIIME 2 pipeline, Steffen Kummel, and Florian Tschernikl for GC support, Stephan Krantz for DNA extraction support and Nicole Steinbach and Ute Lohse for sequencing support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frwa.2025.1566161/full#supplementary-material
References
Adrian, L., and Löffler, F. E. (2016). Organohalide-Respiring Bacteria. Heidelberg: Springer. doi: 10.1007/978-3-662-49875-0
Badin, A., Broholm, M. M., Jacobsen, C. S., Palau, J., Dennis, P., and Hunkeler, D. (2016). Identification of abiotic and biotic reductive dechlorination in a chlorinated ethene plume after thermal source remediation by means of isotopic and molecular biology tools. J. Contam. Hydrol. 192, 1–19. doi: 10.1016/j.jconhyd.2016.05.003
Belay, N., and Daniels, L. (1987). Production of ethane, ethylene, and acetylene from halogenated hydrocarbons by methanogenic bacteria. Appl. Environ. Microbiol. 53, 1604–1610. doi: 10.1128/aem.53.7.1604-1610.1987
Benedict, R. T., Szafran, B., Melia, J., Herber, D., Crisman, J. S., Honey, P., et al. (2024). Toxicological Profile for Vinyl Chloride. Atlanta, GA: Agency for Toxic Substances and Disease Registry. Available online at: https://www.atsdr.cdc.gov/toxprofiles/tp20.pdf (Accessed April 29, 2024).
Beyer, C., Popp, S., and Bauer, S. (2016). Simulation of temperature effects on groundwater flow, contaminant dissolution, transport and biodegradation due to shallow geothermal use. Environ. Earth Sci. 75:1244. doi: 10.1007/s12665-016-5976-8
Bin Hudari, M. S., Richnow, H., Vogt, C., and Nijenhuis, I. (2022a). Effect of temperature on microbial reductive dehalogenation of chlorinated ethenes: a review. FEMS Microbiol. Ecol. 98:fiac081. doi: 10.1093/femsec/fiac081
Bin Hudari, M. S., Vogt, C., and Richnow, H. H. (2020). Effect of temperature on acetate mineralization kinetics and microbial community composition in a hydrocarbon-affected microbial community during a shift from oxic to sulfidogenic conditions. Front. Microbiol. 11:606565. doi: 10.3389/fmicb.2020.606565
Bin Hudari, M. S., Vogt, C., and Richnow, H. H. (2022b). Sulfidic acetate mineralization at 45°C by an aquifer microbial community: key players and effects of heat changes on activity and community structure. Environ. Microbiol. 24, 370–389. doi: 10.1111/1462-2920.15852
Bishop, P. K., Lerner, D. N., Jakobsen, R., Gosk, E., Burston, M. W., and Chen, T. (1993). Investigation of a solvent polluted industrial site on a deep sandstone-mudstone sequence in the UK. Part 2. Contaminant sources, distributions, transport and retardation. J. Hydrol. 149, 231–256. doi: 10.1016/0022-1694(93)90108-L
Bolyen, E., Rideout, J. R., Dillon, M. R., Bokulich, N. A., Abnet, C. C., Al-Ghalith, G. A., et al. (2019). Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37, 852–857. doi: 10.1038/s41587-019-0209-9
Bonte, M., Van Breukelen, B. M., and Stuyfzand, P. J. (2013). Environmental impacts of aquifer thermal energy storage investigated by field and laboratory experiments. J. Water Clim. Change 4, 77–89. doi: 10.2166/wcc.2013.061
Chen, G., Kara Murdoch, F., Xie, Y., Murdoch, R. W., Cui, Y., Yang, Y., et al. (2022). Dehalogenation of chlorinated ethenes to ethene by a novel isolate, “Candidatus Dehalogenimonas etheniformans”. Appl. Environ. Microbiol. 88:e00422. doi: 10.1128/aem.00443-22
Cheng, G., Gabler, F., Pizzul, L., Olsson, H., Nordberg, A., and Schnurer, A. (2022). Microbial community development during syngas methanation in a trickle bed reactor with various nutrient sources. Appl. Microbiol. Biotechnol. 106, 5317–5333. doi: 10.1007/s00253-022-12035-5
Conrad, R. (2020). Importance of hydrogenotrophic, aceticlastic and methylotrophic methanogenesis for methane production in terrestrial, aquatic and other anoxic environments: a mini review. Pedosphere 30, 25–39. doi: 10.1016/S1002-0160(18)60052-9
Cui, Y., Li, X., Yan, J., Lv, Y., Jin, H., Wang, J., et al. (2023). Dehalogenimonas etheniformans sp. nov., a formate-oxidizing, organohalide-respiring bacterium isolated from grape pomace. Int. J. Syst. Evol. Microbiol. 73. doi: 10.1099/ijsem.0.005881
Daniilidis, A., Mindel, J. E., De Oliveira Filho, F., and Guglielmetti, L. (2022). Techno-economic assessment and operational CO2 emissions of High-Temperature Aquifer Thermal Energy Storage (HT-ATES) using demand-driven and subsurface-constrained dimensioning. Energy 249:123682. doi: 10.1016/j.energy.2022.123682
De Bruin, W. P., Kotterman, M. J., Posthumus, M. A., Schraa, G., and Zehnder, A. J. (1992). Complete biological reductive transformation of tetrachloroethene to ethane. Appl. Environ. Microbiol. 58, 1996–2000. doi: 10.1128/aem.58.6.1996-2000.1992
Delgado, A. G., Kang, D. W., Nelson, K. G., Fajardo-Williams, D., Miceli, J. F. III., Done, H. Y., et al. (2014). Selective enrichment yields robust ethene-producing dechlorinating cultures from microcosms stalled at cis-dichloroethene. PLoS ONE 9:e100654. doi: 10.1371/journal.pone.0100654
Delille, D., Coulon, F., and Pelletier, E. (2004). Effects of temperature warming during a bioremediation study of natural and nutrient-amended hydrocarbon-contaminated sub-Antarctic soils. Cold Reg. Sci. Technol. 40, 61–70. doi: 10.1016/j.coldregions.2004.05.005
Dollhopf, S. L., Hashsham, S. A., Dazzo, F. B., Hickey, R. F., Criddle, C. S., and Tiedje, J. M. (2001). The impact of fermentative organisms on carbon flow in methanogenic systems under constant low-substrate conditions. Appl. Microbiol. Biotechnol. 56, 531–538. doi: 10.1007/s002530100612
Dong, X., Greening, C., Bruls, T., Conrad, R., Guo, K., Blaskowski, S., et al. (2018). Fermentative Spirochaetes mediate necromass recycling in anoxic hydrocarbon-contaminated habitats. ISME J. 12, 2039–2050. doi: 10.1038/s41396-018-0148-3
Drijver, B., van Aarssen, M., and Zwart, B. D. (2012). “High-temperature aquifer thermal energy storage (HT-ATES): sustainable and multi-usable,” in Proceedings of the Innostock, The 12th International Conference on Energy Storage (Lleida: Energy Conservation through Energy Storage/International Energy Agency), 1–10.
Elsland, R., Fleiter, T., Jakob, M., Reiter, U., Harmsen, R., Mines, P., et al. (2017). Heating and Cooling (Facts and Figures) - The Transformation Towards a Low-Carbon Heating and Cooling Sector. Available online at: https://www.isi.fraunhofer.de/content/dam/isi/dokumente/cce/2017/29882_Brochure_Heating-and-Cooling_web.pdf (Accessed February 4, 2022).
Filippini, M., Amorosi, A., Campo, B., Herrero-Martin, S., Nijenhuis, I., Parker, B. L., et al. (2016). Origin of VC-only plumes from naturally enhanced dechlorination in a peat-rich hydrogeologic setting. J. Contam. Hydrol. 192, 129–139. doi: 10.1016/j.jconhyd.2016.07.003
Filippini, M., Parker, B. L., Dinelli, E., Wanner, P., Chapman, S. W., and Gargini, A. (2020). Assessing aquitard integrity in a complex aquifer - aquitard system contaminated by chlorinated hydrocarbons. Water Res. 171:115388. doi: 10.1016/j.watres.2019.115388
Fletcher, K. E., Costanza, J., Cruz-Garcia, C., Ramaswamy, N. S., Pennell, K. D., and Löffler, F. E. (2011). Effects of elevated temperature on Dehalococcoides dechlorination performance and DNA and RNA biomarker abundance. Environ. Sci. Technol. 45, 712–718. doi: 10.1021/es1023477
Friis, A. K. (2006). The Potential for Reductive Dechlorination After Thermal Treatment of TCE-Contaminated Aquifers. DTU Environment. Available online at: https://backend.orbit.dtu.dk/ws/files/127446984/MR2006_007.pdf (Accessed May 10, 2022).
Friis, A. K., Edwards, E. A., Albrechtsen, H. J., Udell, K. S., Duhamel, M., and Bjerg, P. L. (2007a). Dechlorination after thermal treatment of a TCE-contaminated aquifer: laboratory experiments. Chemosphere 67, 816–825. doi: 10.1016/j.chemosphere.2006.10.012
Friis, A. K., Heimann, A. C., Jakobsen, R., Albrechtsen, H. J., Cox, E., and Bjerg, P. L. (2007b). Temperature dependence of anaerobic TCE-dechlorination in a highly enriched Dehalococcoides-containing culture. Water Res. 41, 355–364. doi: 10.1016/j.watres.2006.09.026
Fullerton, H., Crawford, M., Bakenne, A., Freedman, D. L., and Zinder, S. H. (2013). Anaerobic oxidation of ethene coupled to sulfate reduction in microcosms and enrichment cultures. Environ. Sci. Technol. 47, 12374–12381. doi: 10.1021/es4029765
Garcia, F. C., Bestion, E., Warfield, R., and Yvon-Durocher, G. (2018). Changes in temperature alter the relationship between biodiversity and ecosystem functioning. Proc. Natl. Acad. Sci. U. S. A. 115, 10989–10994. doi: 10.1073/pnas.1805518115
Ghezzi, D., Filippini, M., Cappelletti, M., Firrincieli, A., Zannoni, D., Gargini, A., et al. (2021). Molecular characterization of microbial communities in a peat-rich aquifer system contaminated with chlorinated aliphatic compounds. Environ. Sci. Pollut. Res. Int. 28, 23017–23035. doi: 10.1007/s11356-020-12236-3
He, J., Sung, Y., Dollhopf, M. E., Fathepure, B. Z., Tiedje, J. M., and Löffler, F. E. (2002). Acetate versus hydrogen as direct electron donors to stimulate the microbial reductive dechlorination process at chloroethene-contaminated sites. Environ. Sci. Technol. 36, 3945–3952. doi: 10.1021/es025528d
Heimann, A. C., Friis, A. K., Scheutz, C., and Jakobsen, R. (2007). Dynamics of reductive TCE dechlorination in two distinct H2 supply scenarios and at various temperatures. Biodegradation 18, 167–179. doi: 10.1007/s10532-006-9052-z
Huang, B., Lei, C., Wei, C., and Zeng, G. (2014). Chlorinated volatile organic compounds (Cl-VOCs) in environment - sources, potential human health impacts, and current remediation technologies. Environ. Int. 71, 118–138. doi: 10.1016/j.envint.2014.06.013
Illumina. (2013). 16S Metagenomic Sequencing Library Preparation. Available online at: https://emea.support.illumina.com/content/dam/illumina-support/documents/documentation/chemistry_documentation/16s/16s-metagenomic-library-prep-guide-15044223-b.pdf (Accessed February 11, 2022).
Jetten, M. S., Stams, A. J., and Zehnder, A. J. (1992). Methanogenesis from acetate: a comparison of the acetate metabolism in Methanothrix soehngenii and Methanosarcina spp. FEMS Microbiol. Rev. 8, 181–197. doi: 10.1111/j.1574-6968.1992.tb04987.x
Jones, W. J., Nagle Jr, D. P., and Whitman, W. B. (1987). Methanogens and the diversity of archaebacteria. Microbiol. Rev. 51, 135–177. doi: 10.1128/mr.51.1.135-177.1987
Kallesøe, A. J., and Vangkilde-Pedersen, T. (2019). Underground Thermal Energy Storage (UTES) – State-of-the-Art, Example Cases and Lessons Learned. HEATSTORE project report, GEOTHERMICA – ERA NET Cofund Geothermal.
Kalnins, S. N., Tunis, S. A., Parsaei, S. M., Gwayi, N., Yousif, Y. M., Romanov, A., et al. (2019). Stockholm Convention on Persistent Organic Pollutants (POPs) Text and Annexes. Geneva: The Secretariat of the Stockholm Convention. Available online at: http://chm.pops.int/theconvention/overview/textoftheconvention/tabid/2232/default.aspx (Accessed May 10, 2022).
Koelschbach, J. S., Mouttaki, H., Pickl, C., Heipieper, H. J., Rachel, R., Lawson, P. A., et al. (2017). Rectinema cohabitans gen. nov., sp. nov., a rod-shaped spirochaete isolated from an anaerobic naphthalene-degrading enrichment culture. Int. J. Syst. Evol. Microbiol. 67, 1288–1295. doi: 10.1099/ijsem.0.001799
Koene-Cottaar, F. H., and Schraa, G. (1998). Anaerobic reduction of ethene to ethane in an enrichment culture. FEMS Microbiol. Ecol. 25, 251–256. doi: 10.1111/j.1574-6941.1998.tb00477.x
Lee, J., and Lee, T. K. (2016). Development and characterization of PCE-to-ethene dechlorinating microcosms with contaminated river sediment. J. Microbiol. Biotechnol. 26, 120–129. doi: 10.4014/jmb.1510.10026
Lepom, P., Brown, B., Hanke, G., Loos, R., Quevauviller, P., and Wollgast, J. (2009). Needs for reliable analytical methods for monitoring chemical pollutants in surface water under the European Water Framework Directive. J. Chromatogr. A 1216, 302–315. doi: 10.1016/j.chroma.2008.06.017
Lerm, S., Westphal, A., Miethling-Graff, R., Alawi, M., Seibt, A., Wolfgramm, M., et al. (2013). Thermal effects on microbial composition and microbiologically induced corrosion and mineral precipitation affecting operation of a geothermal plant in a deep saline aquifer. Extremophiles 17, 311–327. doi: 10.1007/s00792-013-0518-8
Magnuson, J. K., Stern, R. V., Gossett, J. M., Zinder, S. H., and Burris, D. R. (1998). Reductive dechlorination of tetrachloroethene to ethene by a two-component enzyme pathway. Appl. Environ. Microbiol. 64, 1270–1275. doi: 10.1128/AEM.64.4.1270-1275.1998
Mancini, S. A., Lacrampe-Couloume, G., Jonker, H., van Breukelen, B. M., Groen, J., Volkering, F., et al. (2002). Hydrogen isotopic enrichment: an indicator of biodegradation at a petroleum hydrocarbon contaminated field site. Environ. Sci. Technol. 36, 2464–2470. doi: 10.1021/es011253a
Mathiesen, B. V. (2019). The Legacy of Heat Roadmap Europe Scenarios, Recommendations and Resources for Decarbonising the Heating and Cooling Sector in Europe and Complementing the Strategic Long-Term Vision of the EU. H. R. E. 4. Available online at: https://heatroadmap.eu/wp-content/uploads/2019/02/HRE_Final-Brochure_web.pdf (Accessed February 4, 2022).
McInerney, M. J., Sieber, J. R., and Gunsalus, R. P. (2009). Syntrophy in anaerobic global carbon cycles. Curr. Opin. Biotechnol. 20, 623–632. doi: 10.1016/j.copbio.2009.10.001
Menberg, K. (2014). Anthropogenic and Natural Alterations of Shallow Groundwater Temperatures (Doctoral dissertation). Universität Karlsruhe, Karlsruhe, Germany.
National Research Council. (2000). Natural Attenuation for Groundwater Remediation. Washington, DC: The National Academies Press. doi: 10.17226/9792
Ni, Z., van Gaans, P., Rijnaarts, H., and Grotenhuis, T. (2018). Combination of aquifer thermal energy storage and enhanced bioremediation: biological and chemical clogging. Sci. Total Environ. 613–614, 707–713. doi: 10.1016/j.scitotenv.2017.09.087
Ni, Z., van Gaans, P., Smit, M., Rijnaarts, H., and Grotenhuis, T. (2015). Biodegradation of cis-1,2-dichloroethene in simulated underground thermal energy storage systems. Environ. Sci. Technol. 49, 13519–13527. doi: 10.1021/acs.est.5b03068
Ni, Z., van Gaans, P., Smit, M., Rijnaarts, H., and Grotenhuis, T. (2016). Combination of aquifer thermal energy storage and enhanced bioremediation: resilience of reductive dechlorination to redox changes. Appl. Microbiol. Biotechnol. 100, 3767–3780. doi: 10.1007/s00253-015-7241-6
Nijenhuis, I., Nikolausz, M., Koth, A., Felfoldi, T., Weiss, H., Drangmeister, J., et al. (2007). Assessment of the natural attenuation of chlorinated ethenes in an anaerobic contaminated aquifer in the Bitterfeld/Wolfen area using stable isotope techniques, microcosm studies and molecular biomarkers. Chemosphere 67, 300–311. doi: 10.1016/j.chemosphere.2006.09.084
Nijenhuis, I., Schmidt, M., Pellegatti, E., Paramatti, E., Richnow, H. H., and Gargini, A. (2013). A stable isotope approach for source apportionment of chlorinated ethene plumes at a complex multi-contamination events urban site. J. Contam. Hydrol. 153, 92–105. doi: 10.1016/j.jconhyd.2013.06.004
Pannekens, M., Kroll, L., Muller, H., Mbow, F. T., and Meckenstock, R. U. (2019). Oil reservoirs, an exceptional habitat for microorganisms. N. Biotechnol. 49, 1–9. doi: 10.1016/j.nbt.2018.11.006
Prondzinsky, P., Toyoda, S., and McGlynn, S. E. (2023). The methanogen core and pangenome: conservation and variability across biology's growth temperature extremes. DNA Res. 30:dsac048. doi: 10.1093/dnares/dsac048
Puentes Jacome, L. A., Wang, P. H., Molenda, O., Li, Y. X. J., Islam, M. A., and Edwards, E. A. (2019). Sustained dechlorination of vinyl chloride to ethene in dehalococcoides-enriched cultures grown without addition of exogenous vitamins and at low pH. Environ. Sci. Technol. 53, 11364–11374. doi: 10.1021/acs.est.9b02339
Quast, C., Pruesse, E., Yilmaz, P., Gerken, J., Schweer, T., Yarza, P., et al. (2013). The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41, D590–D596. doi: 10.1093/nar/gks1219
Ritalahti, K. M., Justicia-Leon, S. D., Cusick, K. D., Ramos-Hernandez, N., Rubin, M., Dornbush, J., et al. (2012). Sphaerochaeta globosa gen. nov., sp. nov. and Sphaerochaeta pleomorpha sp. nov., free-living, spherical spirochaetes. Int. J. Syst. Evol. Microbiol. 62(Pt. 1), 210–216. doi: 10.1099/ijs.0.023986-0
Rittmann, B. E., Barden, M. J., Bekins, B. A., Ellis, D. E., Firestone, M. K., Lester, S., et al. (2000). Natural Attenuation for Groundwater Remediation. Washington, DC: The National Academies Press.
Rivett, M. O., Turner, R. J., Glibbery Nee Murcott, P., and Cuthbert, M. O. (2012). The legacy of chlorinated solvents in the Birmingham aquifer, UK: observations spanning three decades and the challenge of future urban groundwater development. J. Contam. Hydrol. 140–141, 107–123. doi: 10.1016/j.jconhyd.2012.08.006
Robles, A., Yellowman, T. L., Joshi, S., Mohana Rangan, S., and Delgado, A. G. (2021). Microbial chain elongation and subsequent fermentation of elongated carboxylates as H2-producing processes for sustained reductive dechlorination of chlorinated ethenes. Environ. Sci. Technol. 55, 10398–10410. doi: 10.1021/acs.est.1c01319
Röling, W. F. M. (2014). “The family Geobacteraceae,” in The Prokaryotes, eds. E. Rosenberg, E. F. DeLong, S. Lory, E. Stackebrandt, and F. Thompson (Berlin, Heidelberg: Springer), 157–172. doi: 10.1007/978-3-642-39044-9_381
Rosell, M., Palau, J., Mortan, S. H., Caminal, G., Soler, A., Shouakar-Stash, O., et al. (2019). Dual carbon - chlorine isotope fractionation during dichloroelimination of 1,1,2-trichloroethane by an enrichment culture containing Dehalogenimonas sp. Sci. Total Environ. 648, 422–429. doi: 10.1016/j.scitotenv.2018.08.071
Ruden, C. (2006). Science and policy in risk assessments of chlorinated ethenes. Ann. N. Y. Acad. Sci. 1076, 191–206. doi: 10.1196/annals.1371.046
Schink, B., and Stams, A. J. (2006). Syntrophism among prokaryotes. Prokaryotes 2, 309–335. doi: 10.1007/0-387-30742-7_11
Schupp, S., De la Cruz, F. B., Cheng, Q., Call, D. F., and Barlaz, M. A. (2020). Evaluation of the temperature range for biological activity in landfills experiencing elevated temperatures. ACS ES&T Eng. 1, 216–227. doi: 10.1021/acsestengg.0c00064
Sewell, G. W., and Gibson, S. A. (1991). Stimulation of the reductive dechlorination of tetrachloroethene in anaerobic aquifer microcosms by the addition of toluene. Environ. Sci. Technol. 25, 982–984. doi: 10.1021/es00017a024
Si, B., Liu, Z., Zhang, Y., Li, J., Shen, R., Zhu, Z., et al. (2016). Towards biohythane production from biomass: influence of operational stage on anaerobic fermentation and microbial community. Int. J. Hydrog. Energy 41, 4429–4438. doi: 10.1016/j.ijhydene.2015.06.045
Smatlak, C. R., Gossett, J. M., and Zinder, S. H. (1996). Comparative kinetics of hydrogen utilization for reductive dechlorination of tetrachloroethene and methanogenesis in an anaerobic enrichment culture. Environ. Sci. Technol. 30, 2850–2858. doi: 10.1021/es9602455
Squillace, P. J., Moran, M. J., and Price, C. V. (2004). VOCs in shallow groundwater in new residential/commercial areas of the United States. Environ. Sci. Technol. 38, 5327–5338. doi: 10.1021/es0349756
Stams, A. J., and Plugge, C. M. (2009). Electron transfer in syntrophic communities of anaerobic bacteria and archaea. Nat. Rev. Microbiol. 7, 568–577. doi: 10.1038/nrmicro2166
Troshina, O., Oshurkova, V., Suzina, N., Machulin, A., Ariskina, E., Vinokurova, N., et al. (2015). Sphaerochaeta associata sp. nov., a spherical spirochaete isolated from cultures of Methanosarcina mazei JL01. Int. J. Syst. Evol. Microbiol. 65, 4315–4322. doi: 10.1099/ijsem.0.000575
Villemur, R., Lanthier, M., Beaudet, R., and Lepine, F. (2006). The Desulfitobacterium genus. FEMS Microbiol. Rev. 30, 706–733. doi: 10.1111/j.1574-6976.2006.00029.x
Wagner, D. (2020). “Methanosarcina,” in Bergey's Manual of Systematics of Archaea and Bacteria, ed. W. B. Whitman (Hoboken, NJ: Wiley), 1–23. doi: 10.1002/9781118960608.gbm00519.pub2
Wang, H. Z., Lv, X. M., Yi, Y., Zheng, D., Gou, M., Nie, Y., et al. (2019). Using DNA-based stable isotope probing to reveal novel propionate- and acetate-oxidizing bacteria in propionate-fed mesophilic anaerobic chemostats. Sci. Rep. 9:17396. doi: 10.1038/s41598-019-53849-0
Wei, K., Grostern, A., Chan, W. W. M., Richardson, R. E., and Edwards, E. A. (2016). “Electron acceptor interactions between organohalide-respiring bacteria: cross-feeding, competition, and inhibition,” in Organohalide-Respiring Bacteria (Berlin, Heidelberg: Springer), 283–308. doi: 10.1007/978-3-662-49875-0_13
Wen, L. L., Zhang, Y., Pan, Y. W., Wu, W. Q., Meng, S. H., Zhou, C., et al. (2015). The roles of methanogens and acetogens in dechlorination of trichloroethene using different electron donors. Environ. Sci. Pollut. Res. Int. 22, 19039–19047. doi: 10.1007/s11356-015-5117-z
Wiedemeier, T. H., Swanson, M. A., Moutoux, D. E., Gordon, E. K., Wilson, J. T., Wilson, B. H., et al. (1998). Technical protocol for evaluating natural attenuation of chlorinated solvents in ground water. Natl. Risk Manag. Res. Lab. Office Res. Dev. 14:154.
Williams, M., Fay, M., Ingber, S. Z., Wohlers, D. W., Ingerman, L., Chrisman, J. S., et al. (2022). Toxicological Profile for 1,1-Dichloroethene. Atlanta, GA: Agency for Toxic Substances and Disease Registry. Available online at: https://www.atsdr.cdc.gov/toxprofiles/tp39.pdf (Accessed 29 April 2024).
Xiao, Z., Jiang, W., Chen, D., and Xu, Y. (2020). Bioremediation of typical chlorinated hydrocarbons by microbial reductive dechlorination and its key players: a review. Ecotoxicol. Environ. Saf. 202:110925. doi: 10.1016/j.ecoenv.2020.110925
Xie, S., Lazar, C. S., Lin, Y. S., Teske, A., and Hinrichs, K. U. (2013). Ethane- and propane-producing potential and molecular characterization of an ethanogenic enrichment in an anoxic estuarine sediment. Org. Geochem. 59, 37–48. doi: 10.1016/j.orggeochem.2013.03.001
Yamazaki, Y., Kitamura, G., Tian, X., Suzuki, I., Kobayashi, T., Shimizu, T., et al. (2022). Temperature dependence of sequential chlorinated ethenes dechlorination and the dynamics of dechlorinating microorganisms. Chemosphere 287(Pt. 1):131989. doi: 10.1016/j.chemosphere.2021.131989
Yi, Y., Wang, H., Chen, Y., Gou, M., Xia, Z., Hu, B., et al. (2020). Identification of novel butyrate- and acetate-oxidizing bacteria in butyrate-fed mesophilic anaerobic chemostats by DNA-based stable isotope probing. Microb. Ecol. 79, 285–298. doi: 10.1007/s00248-019-01400-z
Yilmaz, P., Parfrey, L. W., Yarza, P., Gerken, J., Pruesse, E., Quast, C., et al. (2014). The SILVA and “All-species Living Tree Project (LTP)” taxonomic frameworks. Nucleic Acids Res. 42:D643–D648. doi: 10.1093/nar/gkt1209
Zeman, N. R., Irianni Renno, M., Olson, M. R., Wilson, L. P., Sale, T. C., and De Long, S. K. (2014). Temperature impacts on anaerobic biotransformation of LNAPL and concurrent shifts in microbial community structure. Biodegradation 25, 569–585. doi: 10.1007/s10532-014-9682-5
Zhuang, P., and Pavlostathis, S. G. (1995). Effect of temperature, pH and electron donor on the microbial reductive dechlorination of chloroalkenes. Chemosphere 31, 3537–3548. doi: 10.1016/0045-6535(95)00204-L
Keywords: ATES, reductive dechlorination, methanogenesis, temperature, chlorinated ethenes, bioremediation
Citation: Bin Hudari MS, Deb S, Vogt C, Filippini M and Nijenhuis I (2025) Temperature-associated effects on methanogenesis and microbial reductive dechlorination of trichloroethene in contaminated aquifer sediments. Front. Water 7:1566161. doi: 10.3389/frwa.2025.1566161
Received: 24 January 2025; Accepted: 23 June 2025;
Published: 18 July 2025.
Edited by:
Giuseppe Oliveto, University of Basilicata, ItalyReviewed by:
Lucia Cavalca, University of Milan, ItalySeung Gu Shin, Gyeongsang National University, South Korea
Copyright © 2025 Bin Hudari, Deb, Vogt, Filippini and Nijenhuis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ivonne Nijenhuis, aXZvbm5lLm5pamVuaHVpc0B1ZnouZGU=
†Present Address: Sushmita Deb, Department of Applied Geology, Geochemistry and Environmental, University of Wroclaw, Wroclaw, Poland
 Mohammad Sufian Bin Hudari
Mohammad Sufian Bin Hudari Sushmita Deb
Sushmita Deb Carsten Vogt
Carsten Vogt Maria Filippini
Maria Filippini Ivonne Nijenhuis
Ivonne Nijenhuis