- 1Department of Clinical Studies, College of Veterinary Medicine and Agriculture, Addis Ababa University, Bishoftu, Ethiopia
- 2Faculty of Engineering Science and Technology, UiT The Arctic University of Norway, Narvik, Norway
- 3Department of Sociology, Addis Ababa University, Addis Ababa, Ethiopia
- 4Department of Veterinary Pharmacology, Jinka University, Jinka, Ethiopia
- 5Department of Biomedical Science, College of Veterinary Medicine and Agriculture, Addis Ababa University, Addis Ababa, Ethiopia
- 6Center for Food Security, Addis Ababa University, Addis Ababa, Ethiopia
This study examines the toxic effects of river pollution on cattle health in Akaki River Catchment, central Ethiopia. Water and blood samples were collected from ten sampling points and four clusters, kept the first sampling point and first clusters as control. Water samples were collected from the river and analyzed for physicochemical and heavy metal parameters, while blood samples were collected from cattle and tested for heavy metal accumulation and key hematological and biochemical health indicators. Based on these analyses, the Livestock Water Pollution Index (LWPI) and Livestock Health Index (LHI) were developed to assess water quality and cattle health, respectively. The study found that LWPI values ranged from 107.16 to 429.93, with a mean value of 299.26. The LWPI exceeded safe limit (LWPI = 100) at all ten sampling points, with pollution levels increasing progressively downstream. Among the measured parameters, turbidity, lead (Pb), zinc (Zn), and cadmium (Cd) were the most significant contributors to river pollution, in that order. Blood analysis results showed that the LHI ranged between 152.48 and 290.82, with a mean value of 232.81 across all clusters. Similarly, the LHI was above the normal threshold (LHI = 100) in all clusters studied, with elevated levels also observed downstream. Lead, blood urea nitrogen (BUN), and Cd emerged as key contributors to declining livestock health, highlighting heavy metal contamination and physiological stress as major risk factors. The relationship between LWPI and LHI revealed a strong positive correlation, suggesting that river pollution significantly contributed to livestock health risks. The findings highlight risks to cattle health, with health implications for human consuming milk, meat, and its products. The study calls for the implementation of integrated one-health strategies, focusing on enforcement of regulations to reduce waste discharges to the river, provide safe water alternatives for livestock, assess human health risks from contaminated cattle products, and engage communities in sustainable practices through river stewardship programs.
1 Introduction
Water resources including rivers, lakes, and streams are essential for human and livestock consumption, irrigation, industry, transportation, and recreation (Amenu et al., 2013). Clean water is crucial for maintaining healthy ecosystems, supporting biodiversity, and ensuring sustainable agricultural practices. Water quality significantly impacts livestock production, affecting productivity, milk yield, disease resistance, and reproductive success (Bekele and Engdawork, 2022; Doreau et al., 2012; Lardner et al., 2005). However, rapid urbanization and population growth in Ethiopia, particularly in Addis Ababa, have stressed the city’s wastewater treatment infrastructure. This imbalance has led to significant environmental challenges, particularly the degradation of local water bodies (Dessie et al., 2024). The situation is further exacerbated by rapid urbanization, weak pollution control measures, and inadequate sanitation infrastructure (Angello et al., 2021).
Water pollution, mainly driven by human activities, remains a major environmental concern in many countries (Habeeb et al., 2018). Similarly, the Ethiopian government has implemented various institutional and policy frameworks to prevent and control water pollution, aiming to reduce its harmful effects on ecosystems and human health [Federal Democratic Republic of Ethiopia National Water Policy and Strategy (FDRE), 2020]. These efforts include adopting the ‘polluter pays’ principle, which requires those responsible for pollution to bear the cost of reducing it, either based on the damage caused to society or the extent to which pollution exceeds acceptable standards [Ministry of Water, Irrigation, and Energy (MoWIE), 2020].
Despite these measures, there is evidence (Tadesse et al., 2018; Mekuria et al., 2021) that water quality degradation due to pollution is on the rise. The size and severity of water pollution are substantially higher in and around Addis Ababa, notably in the Akaki River Catchment. Unregulated household and industrial waste disposal, as well as waste disposal from the agricultural fields, livestock farms, and healthcare facilities have been contributing to the worsening of water quality (Tadesse et al., 2018). Both people and livestock residing in the downstream areas of the Akaki River Catchment in Addis Ababa are exposed to pollution originating from industrial, municipal, and healthcare waste sources (Abosse et al., 2024; Mekuria et al., 2021).
Approximately two-thirds of Ethiopia’s industries are concentrated in and around the Addis Ababa River Catchment, particularly in the Akaki River Catchment areas. The vast majority (90%) of these industries, along with some healthcare facilities, lack on-site wastewater treatment systems. As a result, large volumes of untreated industrial, commercial, and domestic waste are discharged into local agricultural and grazing lands, rivers, and streams (Eliku and Leta, 2016; Aschale et al., 2021; Dessie et al., 2022).
Heavy metal contamination of river water represents a serious environmental and public health risks (Zinabu et al., 2019; Kumar et al., 2024) because of its harmfulness, ingenuity, bioaccumulation, and bio-amplification highpoints. The recent reports on Frontiers nutrition showed that, the inspected industrial products have detrimental impacts on human health from increased concentrations of lead (Pb) and cadmium (Cd). Likewise, high concentrations of toxic metals that surpass allowed limits in river water might harm the convenience of river water for irrigation due to soil pollution and phytotoxicity to plants, which affects the quality of soil, grasses and crops and threatens livestock, aquatic life and human health through the food chain (Mekuria et al., 2021). However, many small-scale farmers use the river water to cultivate a variety of vegetables, use grasses for animal foods and cleaning purposes (Mengesha et al., 2023).
Livestock farming in peri-urban areas of Ethiopia mainly depend on the river for drinking water and graze on potentially contaminated grasslands (Mekonnen et al., 2020; Weldesilassie et al., 2011). Consequently, impressive degrees of toxic metals could be moved to animals straightforwardly or by implication from contaminated water sources, spreading through the order of things and representing a critical health hazard to individuals consuming livestock product (Gupta et al., 2021; Mengesha et al., 2023). Heavy metals can transfer from irrigation water to agricultural soils, posing a serious risk to livestock and human health. This risk arises from direct contact with contaminated soil and the bioaccumulation of metals in forages (grasses), which can, in turn, contaminate animal products. Increased toxicity of heavy metals in human and animal bodies can contribute to cancer and other non-cancerous diseases (Castro-González et al., 2017).
Since heavy metals are non-biodegradable, they tend to accumulate in the food chain. Their presence in animals can lead to adverse health effects by accumulating in internal organs and causing hematobiochemical and pathological alterations (Gashua et al., 2018). Ultimately, bioaccumulation and toxicopathological damage can compromise food security and pose significant public health risks (Jorge et al., 2013).
Studies on heavy metal contamination of water (Mekuria et al., 2021), soil (Kaczala and Blum, 2016), and vegetables (Yohannes and Elias, 2017) in the little Akaki River catchment have shown that the studied heavy metals were above recommended limits. Industries, commercial activities, health facilities, petrol stations, garages, public and domestic utilities release untreated wastes into nearby environments (Aschale et al., 2021; Olsson et al., 2022). Consequently, a huge amount of waste is generated every day from different point and non-point sources.
Several researchers (Khan et al., 2021; Luo et al., 2011; Waleed Makki et al., 2023), reported that serious health problems may develop as a result of excessive accumulation of heavy metals and even essential trace elements such as Cu and Zn in human body and animals consuming these wastewater and contaminated grasses by the wastewater (Teketel, 2023). In Addis Ababa, large volumes of untreated water are released to water bodies which farmers use for irrigation (Weldesilassie et al., 2011). Despite the health risks, many urban residents rely on irrigated farming for their livelihoods, using polluted river water to grow high-value crops. Notably, about 60% of the city’s leafy vegetables are produced by urban farmers using this contaminated water (Weldesilassie et al., 2011).
However, considering recent rapid dynamics in the river catchment, it is essential to comprehensively analyze the level of Akaki River pollution. Moreover, the effect that Akaki River contamination will have on cattle exposed to the polluted water has not been given attention in the existing literature. Particularly, there is lack of information on the heavy metal bioaccumulation in cattle bodies as no studies conducted in Ethiopia. This study were hypothesized with livestock with access to clean, uncontaminated river water show significantly lower blood heavy metal concentrations and better health indices than those exposed to polluted sources. Hence, this study is the first of its kind to examine relationship between wastewater and heavy metal bioaccumulation in animal body. This study aims to determine the level of Akaki River pollution and its effect on cattle health, with specific objectives of analyzing (1) physicochemical and heavy metal concentration in water samples, and (2) heavy metals bioaccumulation and its effect on hematological and biochemical parameters in cattle blood.
2 Materials and methods
2.1 Description of the study area
The study was carried out in the Akaki River Catchment, which drains the Shagar and Addis Ababa city administrations. It is situated at elevations ranging from 2,464 meter above sea level (a.s.l) at Gafarsa reservoir in the North to 2048 m.a.s.l at Aba Samuel reservoir in the South. The river originates from Mount Entoto in Shagar City, situated to the Northwest of Addis Ababa (Teketel, 2023). It subsequently merges with the Gafarsa reservoir, traverses the Southwestern part of Addis Ababa, and ultimately empties into the Aba Samuel reservoir after covering approximately 40 kilometers, as shown in Figure 1. The area of the catchment covers 403.2 km2 (Gonfa et al., 2023), with a temperate Afro-Alpine climate. The average annual rainfall is 1,254 mm, and the average daily temperature ranges between 9.9 and 24.6 °C. The lower course of river has particularly been the main source of drinking water for community and the livestock. There are varieties of vegetables, grasses and other plant species across the Akaki River Catchment.
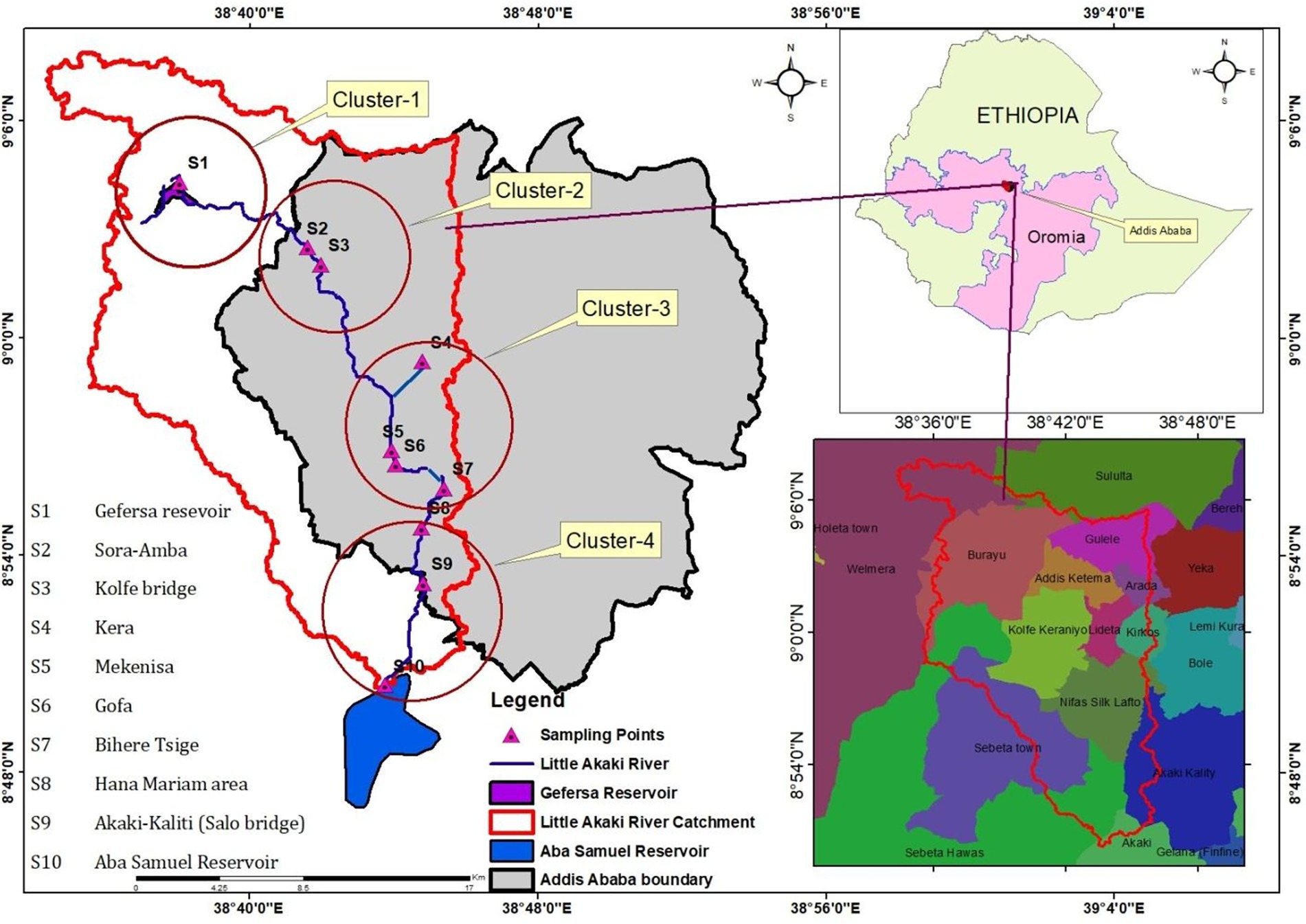
Figure 1. Map of the study area: Ethiopia, Akaki River Catchment (ARC), sampling points, and clusters.
The catchment includes eight Addis Ababa Sub-cities: Gulele, Kolfe keranyo, Addis ketema, Arada, Lideta, Kirkos, Nefas silk Lafto, and Akaki kality. The catchment also includes five Shagar Administrative sub-cities: Sululta, Burayu, Furi, Sabata, and Galan (Gonfa et al., 2023). Over the past few decades, there have been significant changes in land use and land cover within the catchment, characterized by a notable increase in settlement and built-up areas. These changes have had an impact on the quality of river water (Abi et al., 2025) and grasslands found around the river catchments. The data collection was preceded by ethical approval from the Research Ethics Committee of the College of Veterinary Medicine and Agriculture at Addis Ababa University.
2.2 Sampling design
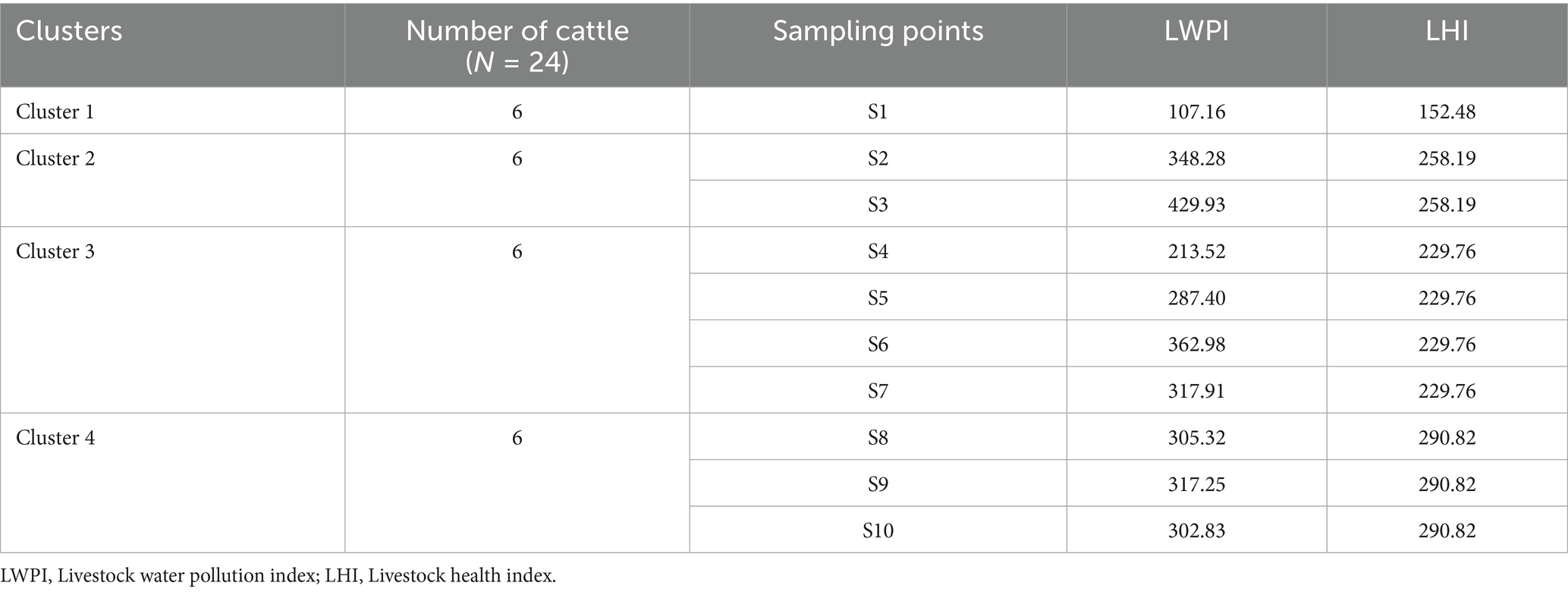
To assess river pollution levels, ten sampling points (S1-S10) were purposively selected, considering factors such as the presence of tributaries, flood occurrences, industrial, healthcare facilities, and the river’s various functions. Water samples were collected three times during the dry season (February and March 2024) from ten designated sampling points. The sampling locations were organized into four clusters, as illustrated in Figure 1. At each point, triplicate samples were obtained in pre-cleaned 500 mL high-density polyethylene (HDPE) bottles to minimize contamination. The samples were immediately transported to the laboratory under chilled conditions for subsequent physicochemical and heavy metal analyses. In total, 30 water samples were collected (three replicates from each of the ten sites). Sampling was conducted during the dry season to reduce the potential influence of non-point source pollution commonly associated with flooding or intense rainfall events. Blood samples were collected during February and March 2024 from 24 cattle inhabiting the Akaki River catchment, distributed across four clusters: Cluster 1 (Gafarsa Reservoir, S1), Cluster 2 (Qarsa, S2–S3), Cluster 3 (Ichu, S4–S7), and Cluster 4 (Galan-Gudda, S8–S10), as illustrated in Figure 1. These clusters were categorized as good, poor, very poor, and unsuitable for drinking purposes, following the classification criteria established by Gani et al. (2025).
A total of 24 animals were sampled across the four clusters. Six animals were selected from Cluster 1 and designated as the control group, while six animals were sampled from each of the remaining three clusters, which served as the treatment groups. The clustering framework was developed based on the spatial distribution of grazing sites, the presence and density of cattle, the occurrence of point and non-point pollution sources, and the degree of exposure to wastewater within the river catchments. Cluster 1 was deliberately designated as the control site, representing cattle with no documented history of contact with wastewater during grazing activities from the researcher’s observations and report of urban livestock agency. The animals were grouped according to the geographical boundaries of the town, which limited movement between clusters. All sampled animals were freely grazing around the river catchments at the study sites. Blood samples were collected in three replicates during each sampling period, in parallel with water sampling. After analysis, the mean concentrations of heavy metal residues were used for result interpretation.
Confounding factors such as breed, species, feed type, sex, and age were recorded and assessed in this study. To minimize variation and enhance internal validity, breed, feed type, and species were controlled across all study sites. Specifically, only local cattle breeds were included, and all animals had free access to the same type of grazing land. By restricting the sample to a single breed, species, and feed type, the study reduced potential confounding and ensured greater comparability among groups (Rothman et al., 2008).
Additionally, animals with identified exposure to river water, particularly those grazing near river catchments and exposed to grasses collected from the areas, were prioritized. Older animals were ranked for sampling, with the cattle in this study having an average age of seven years. Of the sampled animals, fourteen were female and ten were male. Animals sampled from the Gafarsa reservoir, an area free from known environmental pollutants were used as the control group.
Blood was obtained from the jugular vein in an Ethylene-di-amine-tetra-acetic-acid (EDTA) coated and plain vacutainer tube (10 mL), stored in an ice-packed cold box, and transported to Chemistry Laboratory for heavy metal examination. At the same time, the bloods sampled by the indicated procedures were transported to Biomedical Laboratory, for hematology and biochemical analysis. These samples underwent heavy metal, biochemical, and hematological analysis.
2.2.1 Physicochemical analysis of water sample
Physicochemical analysis of water samples was conducted immediately after delivery to the College of Natural and Computational Science of Addis Ababa University, Chemistry laboratory. The characteristics studied were temperature (°C), power of hydrogen (pH), dissolved oxygen (DO), total dissolved solid (TDS) (mg/l), salinity, and turbidity (NTU) in practical salinity unit (PSU). They were measured using a HANNA multi-parameter instrument (model Hl9829-01042) and EUTECH conductivity/Co/Fo meter. The NTU of the water sample was measured with equipment TL2360 LED Turbidimeter, ISO, 0–10,000 NTU.
2.2.2 Water sample digestion and analysis for heavy metals
The samples were preserved in 5 mL of nitric acid (HNO3) and kept in the refrigerator at 4 °C to avoid volatilization and biological deterioration between sampling and analysis as such procedures of Weldegebriel et al. (2012). To determine the heavy metals contents, the water samples were digested following standard protocols of American Public Health Association, American Water Works Association, Water Environment Federation (AAW), 2023. The preserved water samples were well mixed, and 100 mL of the combination was digested in a glass-covered beaker with 5 mL of strong HNO3, which is used to dissolve the sample and remove its complete organic matrix following the procedures developed by Mitra (2003). Water sample digestions was followed routine sample digestion procedures [Kingston and Jassie, 1986; United States Environmental Protection Authority (USEPA), 1992; Turek et al., 2019]. 50 mL of water sample and 5 mL of concentrated HNO₃ were mixed in the glass and teflon digestion vessel and heated at 95 °C on a hot plate or digestion block (to avoid boiling) for 30–60 min. Then the HNO₃ were added and the sample was cooled to room temperature followed the procedures used by Yong and Thomas (1999). Organic matter content was determined by loss-on-ignition (LOI) at 105 °C for 6 h followed by ignition at 60 °C for 6 h. Sludge pH was measured potentiometrically in a 1:2.5 (m/v) sample-to- potassium chloride (KCl) (1 mol/dm3) solution using a Mettler Toledo Delta 350 pH meter (Jakubus and Czekała, 2001).
After boiling and evaporating the sample, 5 mL of concentrated HNO3 and 2 mL of hydrogen peroxide (H2O2) were added to the digest and reheated until the digest became a light and clear solution. Following the digestion process, the wall of the volumetric flask was cleaned with deionized water and the digests were filtered through Whatman filter paper No. 42. The filtrate was then transferred to a glass cup that had been previously cleaned, filled to a capacity of 50 mL with deionized water, sealed, and refrigerated at 4 °C until analysis. Finally, the outcrop underwent cooling, filtration, and was measured using inductively coupled plasma optical emission spectrometry (ICP-OES; Arcos Spectrophotometer, Germany) analysis. The water samples were tested for heavy metals, including Pb, Cd, Cr, and Zn concentrations.
The instrument was calibrated and adjusted according to the manufacturer’s specifications. A continuous sample introduction system, incorporating an auto-sampler for injecting and analyzing both the acid-diluted sample solution and the reference sample, was used. Finally, the concentrations of each heavy metal were assessed using spike tests and reference samples.
2.2.3 Blood samples digestion and analysis
Blood samples were digested following the procedures used by Pompilio et al. (2021). From the digested blood, 2 mL of blood plasma and 2 mL of sulfuric acid (H2SO4) were combined and left in the laboratory overnight for digestion. The blood was digested by heating the sample solution to 120 °C. To aid digestion by breaking it down at this point, 2 mL of H2O2 was added. The excess acid mixture was evaporated and chilled to a semi-dry bulk. The digested samples were diluted with up to 50 mL of distilled water, placed in glass tubes, and refrigerated at 4 °C until they were analyzed using an ICP-OES according to Hussain et al. (2021) procedures.
Digestion parameters were followed the measurement unit of 1,600 W power, 15-min ramp, 1,600 psi pressure, and 20 °C temperature for 15 min. Heavy metal concentrations in the digested blood samples were determined by ICP-OES. All chemicals used were of analytical reagent grade and solutions were prepared using 18.2 MΩ cm deionized water. Calibration standards were prepared from a standard XVI multi-element inductively coupled plasma (ICP) standard solution (Merck KGaA, Darmstadt, Germany). Sample solution obtained by acid dilution as well as the reference sample was injected to the continuous sample introduction system using the auto-sampler. Finally, the concentration of each of the studied heavy metals was determined based on spike test and reference samples.
2.2.4 Hematological and biochemical analysis of blood sample
Hematological and biochemical analysis were performed on the collected blood. The measurement of Packed Cell Volume (PCV), Hemoglobin (Hb), White Blood Cell (WBC) count, and neutrophil count percentage (NCP). NCP were determined from a whole blood sample using hematological measurement methods. Blood biochemical markers such as Aspartate Aminotransferase (AST), Alkaline Phosphate (ALP), Total Protein (TP), Albumin (ALB), and blood urea nitrogen (BUN) were analyzed with an automated biochemical analyzer (Automatic Biochemical Analyzer, Emp-168), a commercially available blood auto analyzer at College of Veterinary Medicine and Agriculture (CVMA), Addis Ababa University. The blood heavy metal analyses were processed as the procedures used for water sample analysis by ICP-OES (Pompilio et al., 2021).
2.2.5 Preparation of standard reference of heavy metals in both water and blood samples
To prepare the calibration solutions, HNO3 and internal standards were used to dilute the multi-element standards. The validation measurements of ICP-OES were performed using a working calibration solution of the studied toxic heavy metals which were prepared by appropriate stepwise dilution of a certified standard stock solution of the elements (Ultra grade 1,000 g/mL, 5% HNO3, ULTRA scientific analysis solutions) following the procedures of De Luna et al. (2019).
A two-step process was used to prepare intermediate standard solutions. At first step, 1.000 mL of Cr, Cd, Zn, and Pb were pipetted from stock standards into a 100 mL volumetric flask (Nalgene®, Rochester, NY, United States), the solution was adjusted to a final volume of 100 mL with a synthetic matrix solution with alternatively, 1.000 mL of a four-metal custom stock standard was used to prepare 100 mL of the solution. In the second step, four levels of intermediate standards were prepared by aliquoting varying volumes of the step 1 solution into 15 mL centrifuge tubes (Falcon®, Becton Dickinson), the volumes were adjusted to 10.0 mL with the synthetic matrix solution and the first level contained only the diluent (adopted from Gajek et al., 2013).
2.2.6 Preparation of quality control
For precisions and accuracy of instrumentations, quality control (QC) samples were prepared by spiking defibrinated sheep blood with inorganic stock standard solutions at three levels (QCLow, QCMed, and QCHigh). The spiked blood was preserved with approximately 1.5 mg/mL Disodium dihydrogen ethylenediaminetetraacetate (Na₂H₂EDTA) (Fisher Scientific, Pittsburg, PA, United States) and stored in 15 mL Falcon® centrifuge tubes at −20 °C. To minimize freeze–thaw cycles, a set of three QC samples at different levels was thawed and divided into 1 mL aliquots in 2 mL cryogenic vials (Corning Inc., Corning, NY, United States) (Gajek et al., 2013).
Blood specimens, QC samples, and intermediate standards were diluted 50-fold with a diluent containing Cr, Cd, Pb, and Zn (20 μg/L each) as internal standards (ISTDs). A Digiflex CX dispenser (Titertek, Huntsville, AL, United States) was used to dispense 4,900 μL of diluent into 15 mL Falcon® centrifuge tubes. Then, 100 μL of blood specimen or QC sample was added using a manual Eppendorf pipette (Eppendorf AG, Hamburg, Germany). Any excess sample on the pipette tip was carefully wiped away with an absorbent wipe (Fisher Scientific, Pittsburg, PA, United States). Homogenizations between each sample analysis were done to minimize the micro-clots of blood.
2.3 Data analysis
The collected data was analysed using Statistical Package for the Social Sciences (SPSS) software. Descriptive statistics (mean, standard deviation) were used to present the levels of physicochemical parameters and heavy metal concentrations in water samples, as well as heavy metal concentrations and haematological and biochemical parameters in cattle blood. All parameters were compared against established normal acceptable values or ranges to assess the extent of the river pollution and cattle health.
To examine the impact of water pollution on cattle health, two indices are developed: the LWPI and the LHI. These indices are calculated using a structured approach that incorporates measurements from both water and cattle blood samples. LWPI was calculated for the ten sampling points, while the LHI was calculated for the four clusters. The calculation process considers three key components: (1) the measured values of selected water quality and blood parameters, (2) established standard normal limits of water safety for livestock consumption and livestock health parameters, and (3) the relative importance of each parameter, represented through weighted contributions. By integrating these factors, the LWPI reflects the level of water contamination affecting livestock, while the LHI provides an overall assessment of livestock health in response to water quality conditions.
2.3.1 Livestock water pollution index
Livestock Water Pollution Index was calculated using physicochemical (TDS, salinity, pH, turbidity, temperature, and DO) and heavy metal (Pb, Cd, Cr, Zn) analysis of water samples. The detailed procedures on heavy metal pollution indices, were derived from the procedure of Mohan et al. (1996) and Tiwari et al. (2015). The standard safe limit, weight, direction of risk, and justification for weight of the parameters are provided in Supplementary Table 1. LWPI is calculated as Equation 1:
1) If the safe values are < standard safe limit,
= Sub-index for parameter, calculated as Equation 2:
= Measured/observed concentration of parameter
= Standard safe limit for parameter
= Weight assigned to parameter
ii) If safe values lie in the mid of the parameter values, e.g., pH and Temperature, piecewise equation is used (Equation 3).
If the safe values are > standard safe limit, e.g., DO calculate as follows (Equation 4). If = 0, simply assign a higher value for, e.g., 500. However, this is an extremely rare situation.
The actual calculation of LWPI is provided in a spreadsheet in Supplementary Table 2. The index is interpreted based on a threshold value of 100. The LWPI value below 100 indicates that the water is within safe limits and is not contaminated, posing no significant risk to livestock health. Conversely, an LWPI value exceeding 100 suggests the presence of heavy metals and other pollutants at levels beyond acceptable standards (Badeenezhad et al., 2023). Such contamination renders the water unsafe for livestock consumption, potentially leading to adverse health effects.
2.3.2 Livestock health index
Livestock Health Index was calculated using heavy metals bioaccumulation (Pb, Cd, Cr, and Zn), and haematological and biochemical (PCV, Hb, WBC, NCP, AST, TP, BUN, ALP, and ALB) analysis of blood samples. Again, the standard safe limit, weight, direction of risk, and justification for weight of the parameters are provided in Supplementary Table 1. To calculate the LHI, the following equation was used (Equation 5):
i) If the safe values are < standard safe limit,
= Sub-index for parameter, calculated as (Equation 6):
= Measured/observed concentration of parameter
= Standard safe limit for parameter
= Weight assigned to parameter
ii) If safe values lie in the mid of the parameter values, piecewise equation is used (Equation 7).
Where: is the low bound of the safe limit for parameter.
is the upper bound of the safe limit for parameter .
= Measured/observed concentration of parameter
The LHI serves as an indicator of the overall health status of livestock (see Supplementary Table 2 for calculation of the index). The LHI value below 100 suggests the absence of toxicity, indicating that the measured health parameters fall within normal ranges. In contrast, an LHI value exceeding 100 signals the presence of severe deficiencies or toxicities, with higher values reflecting greater levels of stress or health deterioration.
2.3.3 Relationship between LAR pollution and cattle health
For both the LWPI and LHI, sampling point S1 was designated as the control site. Differences between S1 and the other sampling points (S2–S10) were assessed using a One-Sample T-Test to determine trends in river pollution and its potential impact on cattle health along the riverbank. Prior to conducting the T-test, the normality of both indices was evaluated using the Shapiro–Wilk test. The results indicated that LWPI (Statistic = 0.908, with df = 10 and p = 0.270) was approximately normally distributed. For LHI, the data points of the four clusters were attributed to the ten sampling points (S1-S10), since the clusters encompass the sampling points. The LHI of Cluster 1 was mapped to S1, Cluster 2 to S2 and S3, Cluster 3 to S4, S5, S6, and S7, and Cluster 4 to S8, S9, and S10 (see Table 1). The clustered division of sampling points are based on observed anthropogenic sources of pollution to ARC. This ensures that both LWPI and LHI have the same number of data points and the S1 and Cluster 1 was kept as control point of the analyzed results.
The resulting LHI distribution, hereafter adjusted LHI, was found to be approximately normal, as confirmed by Shapiro–Wilk test (statistic = 0.851, with df 10 and p = 0.06). To visualize the distribution of the indices and the relative contribution of different parameters used in calculating the indices, bar graphs were employed. Finally, a simple linear regression analysis was conducted, with LWPI as the predictor variable and the LHI as the outcome variable, to examine the effect of ARC pollution on cattle health across the sampling points.
3 Results
3.1 Water sample analysis
3.1.1 Physicochemical analysis of water samples
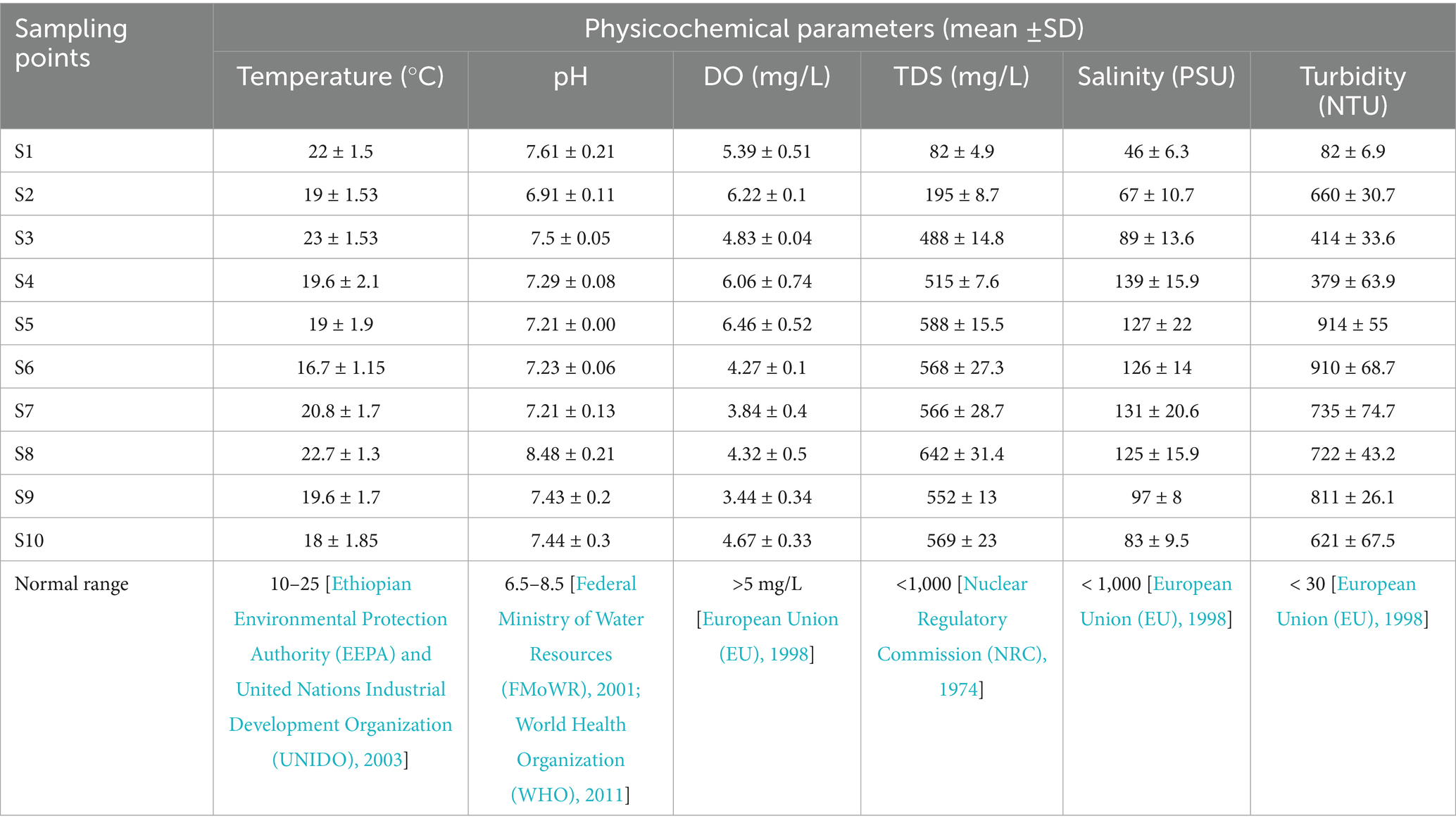
Physicochemical analyses of water samples were conducted in ten selected sampling points to determine water quality of the Akaki River Catchment (ARC). An analysis of physicochemical parameters provides vital information about the overall quality of water bodies. As shown in Table 1, the ARC water samples exhibited variations in several physiochemical parameters across sampling points (S1 to S10). The average temperature of the water samples ranged from 16.7 ± 1.15 to 23 ± 1.53 °C, with all sampling points falling within the normal range. The sampling points three (S3) recorded the highest temperature, while the sampling points six (S6) recorded the lowest.
The power of hydrogen (pH) level ranged from 6.91 ± 0.11 to 8.48 ± 0.21. A pH of seven would be neutral; anything over seven denotes alkalinity, and anything under seven denotes acidity. The pH value denotes a slight deviation from the normal range of 7, except at sampling points eight (S8), where the pH level was the highest. On the contrary, the lowest pH value was recorded at sampling points two (S2). Overall, the pH value for the water samples fell within acceptable ranges.
The average dissolved oxygen (DO) content in the Akaki River Catchment water samples varied from 3.44 ± 0.34 to 6.46 ± 0.52 mg/L, with the highest concentration observed at sampling points five (S5) and the lowest at sampling points nine (S9). The DO level exceeded the normal limit from sampling points one (S1) to S5 but fell below the normal limit from S6 to sampling points ten (S10). It appears that the level of DO was higher in the upper stream and lower in the downstream areas of the river. The lower the DO at the lower stream is associated with the highest pollution level of the river.
The total dissolved solid (TDS) showed a broad range, with average values ranging from 82 ± 4.9 to 642 ± 31.4 mg/L. The highest TDS concentration was observed at S8, while the lowest was recorded at S1. The TDS level was below the normal limit from S1 to S3 but exceeded the normal limit from sampling points four (S4) to S10.
Salinity levels, measured in PSU, ranged from 46 ± 6.3 to 139 ± 15.9 PSU, with the highest value observed at S4 and the lowest at S1. The level of salinity at all sampling points was higher than normal range. The turbidity measurements at the sampling points varied widely, ranging from 82 ± 6.8 to 914 ± 55 NTU. The highest turbidity level was observed at S6, while the lowest was recorded at S1. Again, the level of turbidity was higher than the normal limit at all sampling points. Overall, except temperature all physicochemical parameters were outside the normal range (Table 2). The pH and TDS levels of water samples appear to be higher in the downstream areas, while the level of DO was higher at the upper stream and lower in the downstream areas. Salinity and turbidity levels were higher than the normal ranges at all sampling points.
3.1.2 Heavy metal analysis of water samples
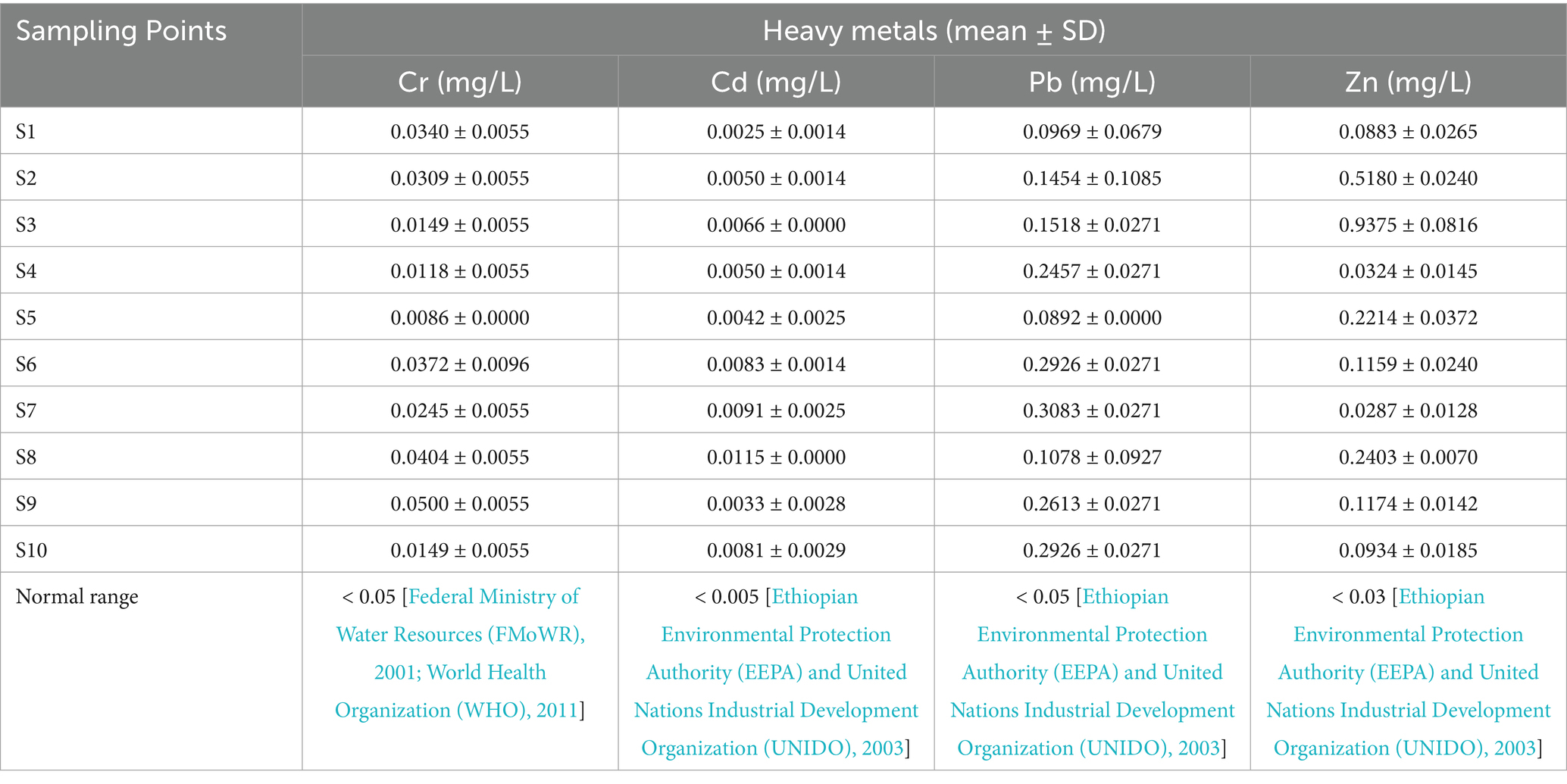
Due to its toxicity and capacity to build up and intensify in the food chain, heavy metal pollution in river water poses serious risks to the environment and public health (Mekuria et al., 2021). Table 3 presents the heavy metal concentrations in the ARC alongside their standardized normal ranges. The mean concentration of chromium (Cr) ranged from 0.0086 ± 0.0000 mg/L to 0.0500 ± 0.0055 mg/L, all within the normal range. The highest concentration was recorded at S9, while the lowest was recorded at sampling points seven (S7). Similarly, the concentration of cadmium (Cd) varied from 0.0025 ± 0.0014 mg/L to 0.0115 ± 0.0000 mg/L, also falling within the normal range across all sampling points. The highest concentration of Cd was observed at S8, while the lowest was observed at S1. Though the concentration of both Cr and Cd were within acceptable normal range, their concentration tends to increase towards the downstream areas of the river.
Conversely, the concentration of lead (Pb) ranged from 0.0892 ± 0.0000 mg/L to 0.3083 ± 0.0271 mg/L, with all sampling points exceeding the normal range. Similarly, the concentration of zinc (Zn) ranged from 0.0287 ± 0.0128 mg/L to 0.9375 ± 0.0816 mg/L, with all sampling points surpassing the normal range, except S7. The concentrations of Pb tends to increase towards the midstream and downstream areas of the river, while the concentration of Zn has shown a mixed result, with higher levels observed in the upper stream part of the river.
3.1.3 Summary of the water sample analysis
The distribution of the LWPI across the ten sampling points reveals significant variation in water quality. The LWPI values range from 107.16 to 429.93, with a mean LWPI of 299.26. All ten sampling points exceeded the safe limit of the water (LWPI = 100), indicating widespread water pollution, as shown in Figure 2. The highest pollution level was observed at S3 (LWPI = 429.93), followed closely by S6 (LWPI = 362.98). Conversely, the lowest pollution level was recorded at control point, i.e., S1 (LWPI = 107.16), though it still exceeds the safe limit. These results suggest considerable spatial variability in pollution severity along the river. A One-Sample T-Test was conducted to compare S1 with other sampling points (S2–S10) to assess the statistical significance of pollution differences. The results show a mean difference of 213.44, with a t-value of 10.892 (p < 0.05), confirming a significant increase in pollution levels at downstream sites.
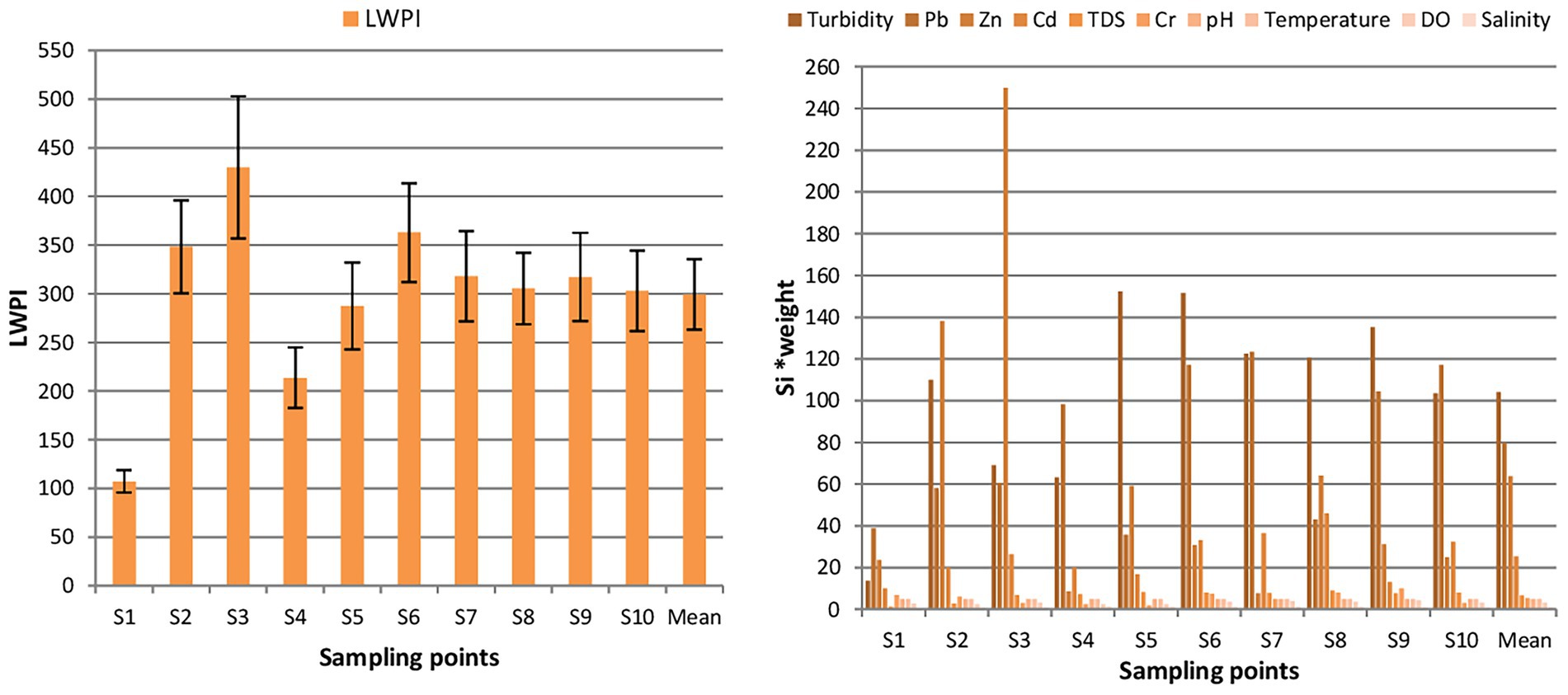
Figure 2. Distribution of LWPI (left) and its contributing parameters (right) across sampling points. Si*weight; standard safe limit for parameter i multiplying by weight.
Among the measured parameters, turbidity, Pb, Zn, and Cd were the most significant contributors to river pollution (see Figure 2). The mean turbidity across the sampling points was 104.1 NTU, with the highest values observed at S5 (152.3) and S6 (151.7), indicating excessive suspended particles that can affect water clarity and quality. Lead (Pb) levels averaged 79.7, with the highest concentrations recorded at S7 (123.3) and S6 (117.0), exceeding safe limits and posing toxicity risks to livestock. Zinc (Zn) exhibited a mean concentration of 63.8, with the highest value at S3 (250.0), significantly deviating from the control site (S1). Cadmium (Cd) also showed elevated levels, averaging 25.4, with peak concentrations at S8 (46.0) and S7 (36.4), indicating potential bioaccumulation hazards.
3.2 Blood sample analysis
3.2.1 Heavy metals bioaccumulation in blood samples
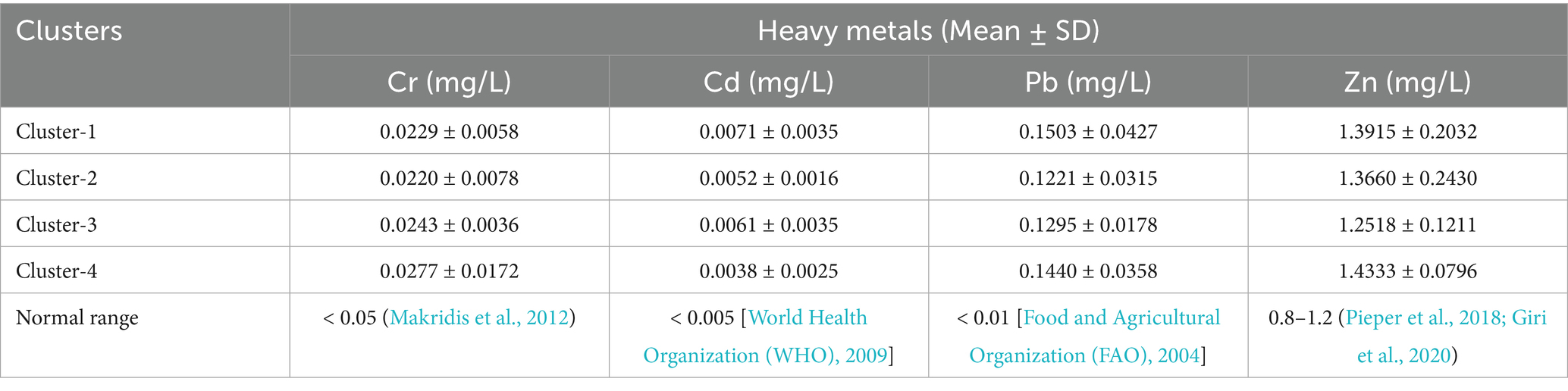
Chronic toxicity in animals could be caused by accumulation of heavy metals in their body (Tahir and Alkheraije, 2023). Heavy metal analysis was performed on the blood samples collected from cattle across four distinct clusters along ARC (Table 4). The concentrations of Cr ranged from 0.0220 ± 0.0078 mg/L in cluster 2 to 0.0277 ± 0.0172 mg/L in cluster 4. In general, there is a discernible trend of increasing Cr levels from upper stream to downstream areas of the river. Nevertheless, Cr levels did not exceed the recommended limit in any of the clusters. However, the concentrations of Cd varied from 0.0071 ± 0.0035 mg/L in cluster 1 to 0.0038 ± 0.0025 mg/L in cluster 4, indicating a declining trend of level of Cd from upper stream to downstream areas of the river. Again, the concentration of Cd was not above recommended normal range in all clusters.
The concentrations of Pb ranged from 0.1221 ± 0.0315 mg/L in cluster 2 to 0.1440 ± 0.0358 mg/L in cluster 4. It is crucial to note that these concentrations exceeded the permissible normal range in all clusters, suggesting higher accumulation of Pb in the blood of cattle along the river catchment. Similarly, Zn concentration varied from 1.2518 ± 0.1211 mg/L in Cluster 3 to 1.4333 ± 0.0796 mg/L in Cluster 4. However, the level of Zn concentration was below standardized normal range in all clusters. The female animals and older age groups were showed higher concentrations of all the analyzed heavy metals.
3.2.2 Hematological and biochemical analysis of blood samples
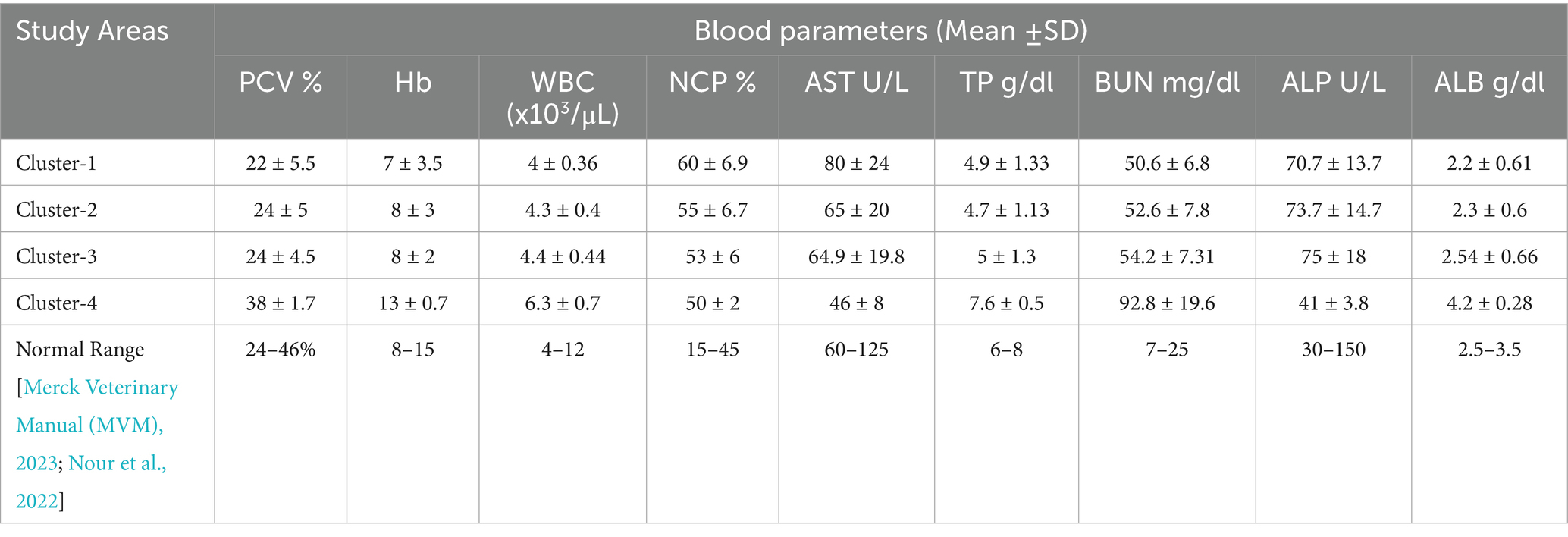
Studies have shown that the exposure of animals to heavy metals, particularly Cd and Pb, at concentrations above the permissible limit causes a significant change in their biochemical and hematological indicators such as Aspartate Aminotransferase (AST), Alkaline Phosphate (ALP), blood urea nitrogen (BUN), Total Protein (TP), Albumin (ALB), packed cell volume (PCV), and hemoglobin (Hb) (Nisha et al., 2009; Patra, 2011; Sato et al., 2019). An analysis of PCV reveals a rising trend from Cluster 1 to Cluster 4. However, with the exception of Cluster 1 where the PCV level was lower, PCV levels were within the normal range for the other Clusters (Table 5).
Hemoglobin levels remained consistent across the first three clusters, with a slight increase observed in Cluster 4. However, while Hb levels were within the normal range in all clusters, they were decreased in Cluster 1. WBC counts showed minimal variation across the clusters, with a relatively higher value observed in Cluster 4. However, white blood cell (WBC) levels remained within the recommended normal range in all clusters. In contrast, neutrophil count percent (NCP) levels exhibited a declining trend from Cluster 1 to Cluster 4, falling outside the recommended range in all clusters. Similarly, AST levels also showed a downward trend from Cluster 1 to Cluster 4, with AST values falling outside the normal range in Cluster 4.
The TP values were relatively consistent across the clusters, with increase in Cluster 4. However, the level of TP values was not within a recommended limit in the first three Clusters. The BUN values have also shown an increasing trend from Cluster 1 to Cluster 4, and above normal range in all Clusters. Conversely, the values of ALP have shown a declining trend from Cluster 1 to Cluster 4. The level of ALP was within a recommended normal range in all the clusters. The ALB values increased from Cluster 1 to Cluster 4, and outside normal range in all clusters.
3.2.3 Summary of blood sample analysis
The mean LHI across all clusters is 232.81, indicating an overall presence of toxicity in cattle. LHI is above 100 in all sampling points, suggesting potential health risks (see Figure 3). Among the four clusters, Cluster 1 has the lowest LHI value (152.48), suggesting minimal toxicity, while Cluster 4 has the highest, indicating severe toxicity levels. A One-Sample T-Test was conducted to compare Cluster 1 with other clusters (cluster 2 – cluster 4) to assess the statistical significance of livestock health across the clusters. The results show a mean difference of 107.95, with a t-value of 6.072 (p = 0.026), confirming a significant increase in cattle toxicity levels at downstream sites.
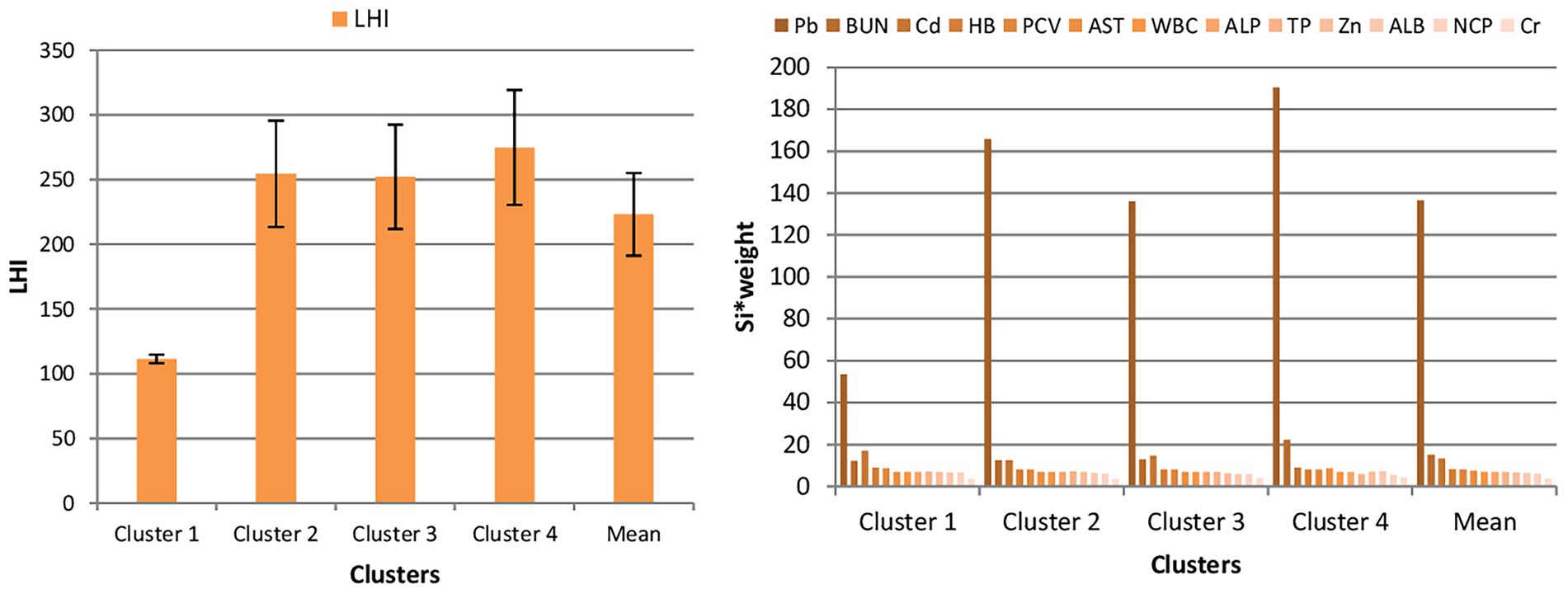
Figure 3. Distribution of LHI (left) and its contributing parameters (right) across clusters. Si*weight; standard safe limit for parameter i multiplying by weight.
Among the parameters contributing to LHI, Pb appears to be the most significant, with its highest concentration in Cluster 4 (190.44) and a mean value of 136.47. BUN also plays a crucial role, with a peak value in Cluster 4 (22.27) and an average of 15.01, indicating potential kidney stress. Cd, another heavy metal, shows high concentrations in Clusters 1 and 3, with a mean of 13.32, further contributing to toxicity. These findings emphasize the impact of heavy metal contamination and metabolic imbalances on livestock health across the sampling clusters.
3.3 Relationship between Akaki River Catchment pollution and cattle health
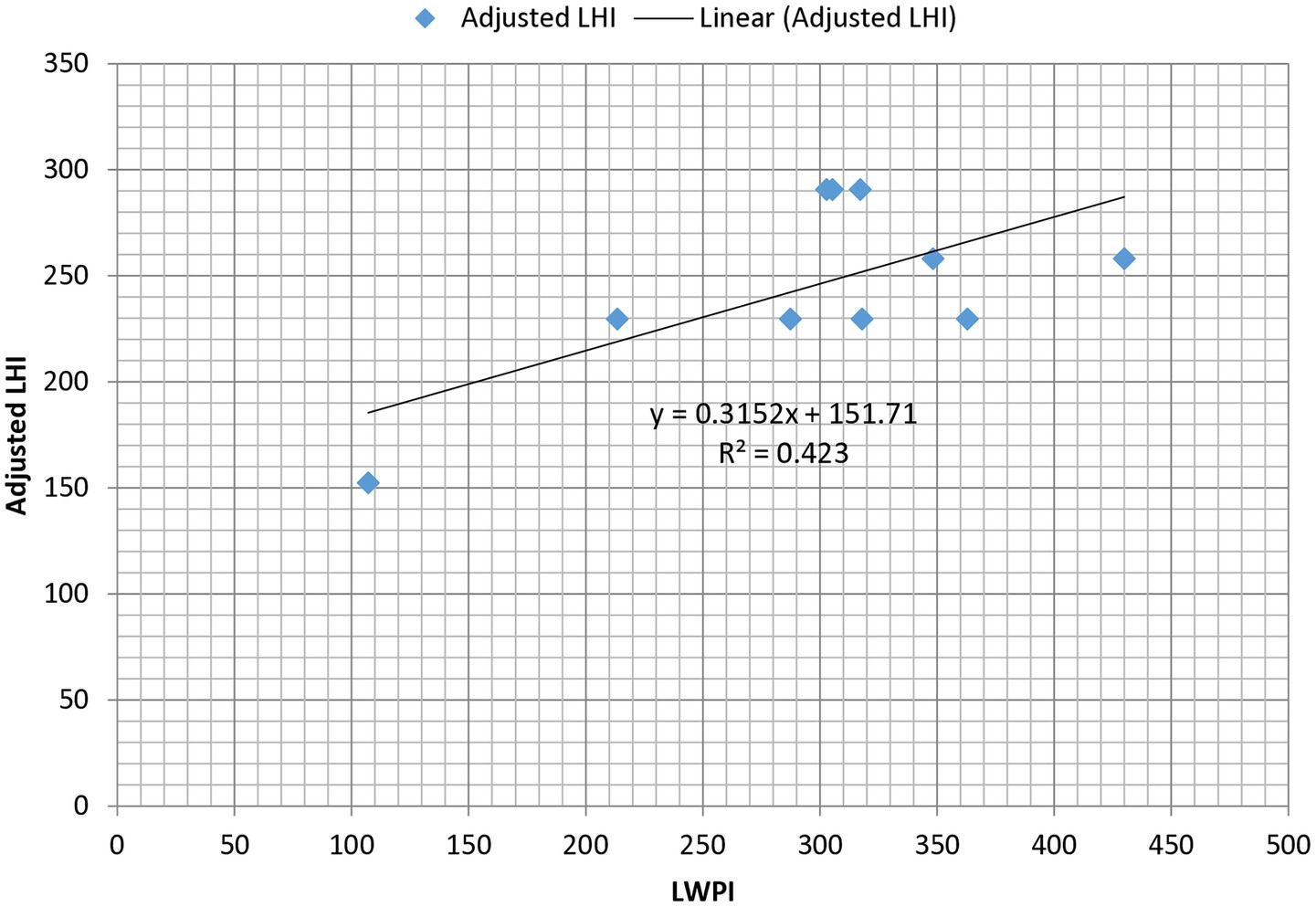
To assess the impact of Akaki River pollution on cattle health, the relationship between LWPI and the adjusted LHI was examined. Both LWPI and adjusted LHI were calculated for ten sampling points. The scatter-gram of the relationship between LWPI and the LHI shows a positive relationship between LWPI and the adjusted LHI (Figure 4). The slope of the regression equation shows (β = 0.3152) a positive association between LWPI and adjusted LHI. For every one-unit increase in LWPI, the predicted adjusted LHI increases by 0.423 units. Pearson correlation () shows a strong positive relationship between LWPI and LHI (= 0.6504, with p = 0.0417). The indicates that approximately 42.3% of the variation in adjusted LHI is explained by LWPI. This suggests a moderate explanatory power, suggesting that while LWPI has substantial influence on adjusted LHI, other factors are likely to contribute to adjusted LHI.
4 Discussion
This study examines river pollution and its impact on cattle health in the Akaki River Catchment (ARC), Central Ethiopia. It draws on an analysis of physicochemical parameters and heavy metal concentrations in water samples, as well as heavy metal bioaccumulation and hematological and biochemical parameters in cattle blood. Water analysis results were used to calculate the Livestock Water Pollution Index (LWPI) at ten sampling points along the river to assess water quality, while blood analysis results were used to compute the Livestock Health Index (LHI) across four clusters to indicate cattle health.
4.1 Level of Akaki River Catchment pollution
The LWPI values across ten sampling points showed significant variation, ranging from 107.16 to 429.93, with an average of 299.26, all above the safe limit of 100, indicating widespread pollution. A one-sample t-test comparing control point or sampling points one (S1) to downstream points or sampling points two to ten (S2–S10) confirmed a statistically significant increase in pollution downstream, highlighting spatial variability in water quality. The main contributors to river pollution were turbidity, lead (Pb), zinc (Zn), and cadmium (Cd). Turbidity averaged 104.1 NTU, with the highest levels at S5 and S6, indicating excessive suspended particles that can affect water clarity and quality. Elevated turbidity levels reduce sunlight penetration, and affecting aquatic biodiversity (Bayissa and Gebeyehu, 2021; Jessica and Delbert, 2020; Yilma et al., 2019).
Pb levels averaged 79.7, peaking at S7 and S6, exceeding safe limits and posing toxicity risks to livestock. The main source of Pb concentration in the river could be attributed to wastewater discharge from car-wash sites, garages, and fuel stations, which is also confirmed by the study of Mekuria et al. (2021), Singh et al. (2022), and Yilma et al. (2019). Pb is known to disrupt reproductive hormones and cause toxicity in livestock (Valente-Campos et al., 2019).
Zn had a mean of 63.8, with a notably high concentration at S3. High Zn levels in the river could be attributed to industrial effluents, fertilizers, and pesticides (Boateng et al., 2015; Wuana and Okieimen, 2011; Chetty and Pillay, 2019). Similarly, Chronic Zn exposure in livestock could result in adverse livestock health, including anemia, weight loss, renal disease, and gastrointestinal distress (Alharthi et al., 2025).
Cd levels averaged 25.4, with the highest at S8 and S7, consistent with previous studies (Mekuria et al., 2021). Common sources of Cd in the river include nickel-cadmium batteries, phosphate fertilizers, and industrial waste. Cd is highly toxic, accumulating in organs over time and causing kidney, liver, and bone damage (Alharthi et al., 2025; Dessie et al., 2022; Genchi et al., 2020). The study suggests continuous Cd exposure in cattle, reflecting daily intake and raising concerns about long-term health impacts.
4.2 Cattle health indicators
The study found that the average LHI across all clusters was 232.81, indicating widespread toxicity in cattle. All clusters had LHI values above the safe threshold (LHI = 100), with Cluster 1, located in areas with relatively lower water pollution in upper stream, shows the least toxicity, while Cluster 4 at the downstream exhibits the highest LHI.
The analysis identified Pb as the major contributor to LHI, with the highest concentration found in Cluster 4. The observed Pb levels were higher than those reported in previous studies (e.g., Rodríguez et al., 2015; Ahmad et al., 2016). In acute cases, Pb poisoning in cattle can lead to sudden death with no visible symptoms, while chronic exposure may cause stunted growth, impaired reproductive performance, and contamination of meat and dairy products (Alharthi et al., 2025). The elevated Pb levels observed in cattle from downstream areas indicate a potential health risk to consumers through the consumption of contaminated animal products.
Similarly, Blood Urea Nitrogen (BUN) levels were elevated, particularly in Cluster 4. Increased BUN concentrations may indicate compromised kidney function, potentially linked to heavy metal exposure, as noted by Godt et al. (2006). This finding suggests that livestock in downstream areas may be experiencing physiological stress or early signs of organ dysfunction, posing both animal welfare concerns and potential risks to food safety. Elevated AST levels, typically two to four times higher than normal, suggest damage to organs like the heart, liver, or kidneys. Even mild kidney impairment can alter BUN levels (Afzal and Mahreen, 2024; Godt et al., 2006).
Cd, another heavy metal, exhibited high concentrations in Clusters 1 and 3, further contributing to overall toxicity. Chronic exposure to elevated Cd levels has been associated with metabolic and reproductive disorders and may even contribute to cancer development in cattle (Kurdziel et al., 2023). These findings raise serious concerns about the long-term health of livestock in the affected areas and suggest that continued exposure could reduce productivity and pose risks to the safety of animal-derived food products.
4.3 Toxic effects of Akaki River Catchement pollution on cattle health
To assess the impact of Akaki River pollution on cattle health, the relationship between LWPI and the LHI was examined. The relationship between LWPI and LHI demonstrates a strong positive correlation, with water pollution explaining approximately 42.3% of the variation in cattle health. The regression analysis indicates that as LWPI increases, LHI also rises, suggesting that water contamination plays a crucial role in livestock health deterioration. However, the remaining unexplained variation implies that other factors, such as diet, disease, or additional environmental stressors, may also contribute to poor cattle health outcomes (Dessie et al., 2024; Mengesha et al., 2023).
Among the studied parameters, Pb, Cd, and Cr pose significant public health risks due to their high toxicity. As systemic toxicants, they can cause organ damage even at low exposure levels and are classified as human carcinogens by the U.S. Environmental Protection Agency and the International Agency for Research on Cancer (Chen et al., 2022; Tchounwou et al., 2012).
These results emphasize the importance of integrated water and livestock management strategies, ensuring that pollution control efforts are aligned with broader livestock health interventions to safeguard both water quality and animal well-being. In addition it emphasizes the impacts of increasing level of pollutions posing significant threat to human and animal health. This quest for effective and sustainable methods to purify water through different ways like photocatalytic water purification technology (Omar et al., 2024).
5 Limitations of the study
The study identified that, those cattle frequently exposed to wastewater are more prone to toxic heavy metals than cattle less exposed to the wastes. Despite its valuable contributions and insights into the impact of water pollution on livestock health and its consequences to human health, this study had some limitations. It did not assess point-source pollution from specific industrial, healthcare, or municipal discharge sites, nor did it include heavy metal analysis of forage, an important potential route of contamination. While confounding factors such as breed, feed type, and species were controlled, further research is recommended under expanded study conditions, including larger sample sizes, different breeds and species, various feed types, and additional sampling sites. Moreover, the absence of alternative clean river water sources limited the inclusion of appropriate control groups. Addressing these gaps in future studies is essential to generate more robust evidence to inform policy and intervention strategies.
6 Conclusion
This study investigates the toxic effects of river pollution on cattle health in the ARC, central Ethiopia. Water samples from ten sites were analyzed for physicochemical and heavy metal parameters, while cattle blood samples were tested for heavy metal accumulation and key hematological and biochemical health indicators. Using these data, the Livestock Water Pollution Index (LWPI) and Livestock Health Index (LHI) were developed to assess water quality and cattle health, respectively.
This study highlights high level of pollution in the ARC, with all ten sampling points recording LWPI values far exceeding the safe threshold. The results indicate spatial variation in water quality, with downstream areas experiencing significantly higher contamination. Key pollutants identified include turbidity, lead (Pb), zinc (Zn), and cadmium (Cd), all of which have known detrimental effects on water quality, ecosystem integrity, and livestock health. These findings suggest the urgent need for targeted pollution control measures to mitigate the impact of untreated waste discharge into the river system.
The analysis of cattle health revealed high LHI values across all clusters, indicating widespread physiological stress and toxicity. Cluster 4, located downstream, and exhibited the highest LHI, consistent with greater water pollution exposure. Pb emerged as the most critical contaminant affecting livestock health, with its presence linked to both acute and chronic health issues in cattle and potential contamination of meat and dairy products. Other biochemical markers, such as high Blood Urea Nitrogen (BUN), also signal possible kidney dysfunction associated with heavy metal exposure, underscoring the threat to both animal welfare and public health.
A strong positive relationship was observed between LWPI and LHI, with water pollution accounting for over 42% of the variation in cattle health outcomes. This finding demonstrates a clear link between environmental contamination and livestock well-being. However, the unexplained variation also points to the potential influence of other contributing factors such as nutrition, disease, breed, species or alternative contamination pathways. Improving outcomes therefore requires a multifaceted approach that includes pollution reduction, animal health interventions, and ecological management. Incorporating One Health principles into future interventions will help ensure that environmental, animal, and human health are addressed sustainably.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Ethics statement
The animal studies were approved by the Research Ethics Committee of the College of Veterinary Medicine and Agriculture, Addis Ababa University approved all aspects of this study, including the informed oral consent process. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
JA: Methodology, Conceptualization, Project administration, Supervision, Writing – original draft, Writing – review & editing, Funding acquisition. SA: Project administration, Methodology, Writing – review & editing, Funding acquisition, Conceptualization, Writing – original draft, Supervision. ZT: Writing – original draft, Formal analysis, Data curation. DA: Writing – original draft, Supervision, Data curation. BW: Visualization, Writing – original draft, Data curation. FE: Supervision, Writing – review & editing, Resources.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Addis Ababa University, Ethiopia, through its thematic research project titled “Integrated Water Management for Improved Livestock and Human Health in the Little Akaki River Catchment, Central Ethiopia (2022–2025)”.
Acknowledgments
We extend our deepest gratitude to the Vice President for Research and Technology Transfer at Addis Ababa University for funding this research. We also wish to thank the local farmers for their cooperation during blood sample collection. Additionally, our appreciation goes to the Akaki District Livestock Agency and the College of Veterinary Medicine and Agriculture at Addis Ababa University for facilitating field sample collection. Finally, we extend our gratitude to UiT the Arctic University of Norway and NORHED II One Health Project (Project No. 61720) titled “The Urban-Suburban Nexus towards One Health Approach,” for providing access to valuable resources and support during manuscript editing and publishing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frwa.2025.1642296/full#supplementary-material
References
Abi, M., Wolde, B., Abosse, J. S., and Assefa, S. (2025). Effect of land use and land cover changes on river water quality in the Akaki River catchment. Central Ethiopia.
Abosse, J. S., Megersa, B., Zewge, F., and Eregno, F. E. (2024). Healthcare waste management and antimicrobial resistance: a critical review. J. Water Health 22, 2076–2093. doi: 10.2166/wh.2024.232
Afzal, A., and Mahreen, N. (2024). Emerging insights into the impacts of heavy metals exposure on health, reproductive and productive performance of livestock. Front. Pharmacol. 15:1375137. doi: 10.3389/fphar.2024.1375137
Ahmad, M., Roy, S. P. K., Sarwar, N., Morshed, S., Alam, M. K., Matin, A., et al. (2016). Contamination of raw fresh milk, market pasteurized milk and powdered milk by toxic heavy metals in Bangladesh. Sci. Res. J.(SCIRJ) 4, 19–24.
Alharthi, S., Uguru, H., Akpokodje, O. I., Sami, R., Alqurashi, M., Aloufi, S., et al. (2025). The effect of pollution on the livestock management, microbial evaluation, health risks, and HPLC analysis of aflatoxins in animal meat and organs. Front. Sustain. Food Syst. 9. doi: 10.3389/fsufs.2025.1587783
Amenu, K., Markemann, A., and Valle Zárate, A. (2013). Water for human and livestock consumption in rural settings of Ethiopia: assessments of quality and health aspects. Environ. Monit. Assess. 185, 9571–9586. doi: 10.1007/s10661-013-3275-3
American Public Health Association, American Water Works Association, Water Environment Federation (AAW) (2023). Standard methods for the examination of water and wastewater. 20th Edn. Washington, D.C., USA.
Angello, Z., Behailu, B., and Tränckner, J. (2021). Selection of optimum pollution load reduction and water quality improvement approaches using scenario-based water quality modeling in little Akaki River, Ethiopia. Water 13:584. doi: 10.3390/w13050584
Aschale, M., Sileshi, Y., Kelly-Quinn, M., and Hailu, D. (2021). Multivariate analysis of potentially toxic elements in surface waters in Ethiopia. Appl Water Sci 11:80. doi: 10.1007/s13201-021-01412-6
Badeenezhad, A., Soleimani, H., Shahsavani, S., Parseh, I., Mohammadpour, A., Azadbakht, O., et al. (2023). Comprehensive health risk analysis of heavy metal pollution using water quality indices and Monte Carlo simulation in R software. Sci. Rep. 13:15817. doi: 10.1038/s41598-023-43161-3
Bayissa, L. D., and Gebeyehu, H. R. (2021). Vegetables contamination by heavy metals and associated health risk to the population in Koka area of Central Ethiopia. PLoS One 16:e0254236. doi: 10.1371/journal.pone.0254236
Bekele, A., and Engdawork, A. (2022). Liquid waste management practices and the role of communal treatment plant in the eastern Industrial Park of Dukem town, Ethiopia. Int. J. Water Wastewater Treat. 8. doi: 10.16966/2381-5299.184
Boateng, T. K., Opoku, F., Acquaah, S. O., and Akoto, O. (2015). Pollution evaluation, sources and risk assessment of heavy metals in hand-dug wells from Ejisu-Juaben municipality, Ghana. Environ. Syst. Res. 4:18. doi: 10.1186/s40068-015-0045-y
Castro-González, N. P., Calderón-Sánchez, F., Moreno-Rojas, R., Moreno-Ortega, A., and Tamariz-Flores, J. V. (2017). Health risks in rural populations due to heavy metals found in agricultural soils irrigated with wastewater in the alto balsas sub-basin in Tlaxcala and Puebla, Mexico. Int. J. Environ. Health Res. 27, 476–486. doi: 10.1080/09603123.2017.1386767
Chen, F., Muhammad, F. G., Khan, Z. I., Ahmad, K., Malik, I. S., Ashfaq, A., et al. (2022). Bioaccumulation and transfer of zinc in soil plant and animal system: a health risk assessment for the grazing animals. Environ. Sci. Pollut. Res. 29, 2718–2727. doi: 10.1007/s11356-021-15808-z
Chetty, S., and Pillay, L. (2019). Assessing the influence of human activities on river health: a case for two south African rivers with differing pollutant sources. Environ. Monit. Assess. 191:168. doi: 10.1007/s10661-019-7308-4
De Luna, P., Hahn, C., Higgins, D., Jaffer, S. A., Jaramillo, T. F., and Sargent, E. H. (2019). What would it take for renewably powered electrosynthesis to displace petrochemical processes? Science 364:eaav3506. doi: 10.1126/science.aav3506
Dessie, B. K., Aschale, M., Polaine, X., Melaku, S., Alamirew, T., Claire, L. W., et al. (2024). An integrated approach for water quality assessment in African catchments based on physico-chemical and biological indicators. Sci. Total Environ. 908:168326. doi: 10.1016/j.scitotenv.2023.168326
Dessie, B. K., Tessema, B., Asegide, E., Tibebe, D., Alamirew, T., Walsh, C. L., et al. (2022). Physicochemical characterization and heavy metals analysis from industrial discharges in upper Awash River basin, Ethiopia. Toxicol. Rep. 9, 1297–1307. doi: 10.1016/j.toxrep.2022.06.002
Doreau, M., Corson, M. S., and Wiedemann, S. G. (2012). Water use by livestock: a global perspective for a regional issue? Anim. Front. 2, 9–16. doi: 10.2527/af.2012-0036
Eliku, T., and Leta, S. (2016). Assessment of heavy metal contamination in vegetables grown using paper mill wastewater in Wonji Gefersa, Ethiopia. Bull. Environ. Contam. Toxicol. 97, 714–720. doi: 10.1007/s00128-016-1915-3
Ethiopian Environmental Protection Authority (EEPA) and United Nations Industrial Development Organization (UNIDO) (2003). Guideline ambient environment standards for Ethiopia. Addis Ababa, Ethiopia.
European Union (EU) (1998). Council directive 98/83/EC of 3 number 1998 on the quality of water intended for human consumption. Offic. J. Europ. Commun. 330, 32–54.
Federal Democratic Republic of Ethiopia National Water Policy and Strategy (FDRE). (2020). Ministry of Water, irrigation and energy, the Ministry of Water, irrigation and energy (MoWIE) is a federal institution established by proclamation no. 1097/2018, march, 2020. Addis Ababa, Ethiopia.
Federal Ministry of Water Resources (FMoWR) (2001). National drinking water guideline recommendations, draft report. Addis Ababa, Ethiopia: Ministry of Water Resources.
Food and Agricultural Organization (FAO) (2004). The state of food insecurity in the world. Monitoring progress towards the world food summit and millennium development goals. Vialedelle Terme di Caracalla, Rome.
Gajek, R., Barley, F., and She, J. (2013). Determination of essential and toxic metals in blood by ICP-MS with calibration in synthetic matrix. Anal. Methods 5:2193. doi: 10.1039/c3ay26036d
Gani, A., Hussain, A., Pathak, S., Ahmed, S., and Omar, P. J. (2025). Impact of pollutants on groundwater quality and health risk assessment of quaternary aquifers in northern India. J. Hazard. Toxic Radioact. Waste 29. doi: 10.1061/jhtrbp.hzeng-1401
Gashua, M. M., Kabir, J., Suleiman, M. M., and Abdulrahman, H. I. (2018). A baseline study for cadmium concentrations in blood of goats in some communities of bade, northern Yobe, Nigeria. Asian J. Res. Anim. Vet. Sci. 1, 205–214. doi: 10.9734/AJRAVS/2018/44244
Genchi, G., Sinicropi, M. S., Lauria, G., Carocci, A., and Catalano, A. (2020). The effects of cadmium toxicity. Int. J. Environ. Res. Public Health 17:3782. doi: 10.3390/ijerph17113782
Giri, A., Bharti, V. K., Kalia, S., Arora, A., Balaje, S. S., and Chaurasia, O. P. (2020). A review on water quality and dairy cattle health: a special emphasis on high-altitude region. Appl Water Sci 10:79. doi: 10.1007/s13201-020-1160-0
Godt, J., Scheidig, F., Grosse-Siestrup, C., Esche, V., Brandenburg, P., Reich, A., et al. (2006). The toxicity of cadmium and resulting hazards for human health. J. Occup. Med. Toxicol. 1:22. doi: 10.1186/1745-6673-1-22
Gonfa, Y. H., Tessema, F. B., Bachheti, A., Rai, N., Tadesse, M. G., Nasser Singab, A., et al. (2023). Anti-inflammatory activity of phytochemicals from medicinal plants and their nanoparticles: a review. Curr. Res. Biotechnol. 6:100152. doi: 10.1016/j.crbiot.2023.100152
Gupta, A. R., Bandhyopadhyay, S., Sultana, F., and Swarup, D. (2021). Heavy metal poisoning and its impact on livestock health and production system. Indian J. Anim. Health. 60. doi: 10.36062/ijah.2021.spl.00421
Habeeb, A. A., Gad, A. E., and Atta, M. A. (2018). Temperature-humidity indices as indicators to heat stress of climatic conditions with relation to production and reproduction of farm animals. Int. J. Biotechnol. Recent Adv. 1, 35–50. doi: 10.18689/ijbr-1000107
Hussain, S., Ulhassan, Z., Brestic, M., Zivcak, M., Zhou, W., Allakhverdiev, S. I., et al. (2021). Photosynthesis research under climate change. Photosynth. Res. 150, 5–19. doi: 10.1007/s11120-021-00861-z
Jakubus, M., and Czekała, J. (2001). Heavy metal speciation in sewage sludge. Pol. J. Environ. Stud. 10, 245–250. Available online at: http://www.pjoes.com/Heavy-metal-speciation-in-sewage-sludge,87379,0,2.html (accessed on 5 February 2019).
Jessica, L., and Delbert, L. S. (2020). Turbidity alters estuarine biodiversity and species composition. ICES J. Mar. Sci. 77, 379–387. doi: 10.1093/icesjms/fsz214
Jorge, C., de Romaña Daniel, L., Patricia, B., de Romaña Guillermo, L., and Doris, C. (2013). Lead and cadmium in maternal blood and placenta in pregnant women from a mining-smelting zone of Peru and transfer of these metals to their newborns. J. Toxicol. 5, 156–165. doi: 10.5897/JTEHS2013.0276
Kaczala, F., and Blum, E. (2016). The occurrence of veterinary pharmaceuticals in the environment: a review. Curr. Anal. Chem. 12, 169–182. doi: 10.2174/1573411012666151009193108
Khan, M. T., Shah, I. A., Ihsanullah, I., Naushad, M., Ali, S., Shah, S. H. A., et al. (2021). Hospital wastewater as a source of environmental contamination: an overview of management practices, environmental risks, and treatment processes. J Water Process Eng 41:101990. doi: 10.1016/j.jwpe.2021.101990
Kingston, H. M., and Jassie, L. B. (1986). Microwave energy for acid decomposition at elevated temperatures and pressures using biological and botanical samples. Anal. Chem. 58, 2534–2541. doi: 10.1021/ac00125a038
Kumar, S., Saxena, A., Srivastava, R. K., Singh, S. B., Ram, R. N., Ganie, P. A., et al. (2024). Composition of heavy metals in sediment, water, and fish of the ganga and Yamuna Rivers in two major cities of India. Environ. Monit. Assess. 196:612. doi: 10.1007/s10661-024-12777-x
Kurdziel, A., Sychta, K., Sliwinska, E., Miszczak, S., Szarek-Łukaszewska, G., Rostański, A., et al. (2023). Stable artificial autopolyploids of the Zn/cd accumulator Arabidopsis arenosa—a promising genetic resource for phytoremediation. Appl. Sci. 13:1617. doi: 10.3390/app13031617
Lardner, H. A., Kirychuk, B. D., Braul, L., Willms, W. D., and Yarotski, J. (2005). The effect of water quality on cattle performance on pasture. Aust. J. Agric. Res. 56:97. doi: 10.1071/AR04086
Luo, C., Liu, C., Wang, Y., Liu, X., Li, F., Zhang, G., et al. (2011). Heavy metal contamination in soils and vegetables near an e-waste processing site, South China. J. Hazard. Mater. 186, 481–490. doi: 10.1016/j.jhazmat.2010.11.024
Makridis, C., Svarnas, C., Rigas, N., Gougoulias, N., Roka, L., and Leontopoulos, S. L. (2012). Transfer of heavy metal contami nants from animal feed to animal products. J. Agric. Sci. Technol. 2:149.
Mekonnen, A., Gebreegziabher, Z., Beyene, A. D., and Hagos, F. (2020). Valuation of access to irrigation water in rural Ethiopia: application of choice experiment and contingent valuation methods. Water Econ. Policy 6:1950007. doi: 10.1142/S2382624X19500073
Mekuria, D. M., Kassegne, A. B., and Asfaw, S. L. (2021). Assessing pollution profiles along little Akaki River receiving municipal and industrial wastewaters, Central Ethiopia: implications for environmental and public health safety. Heliyon 7:e07526. doi: 10.1016/j.heliyon.2021.e07526
Mengesha, S. D., Asfaw, Y. B., Kidane, A. W., Teklu, K. T., Serte, M. G., Kenea, M. A., et al. (2023). Microbial risk assessment and health concern of vegetables irrigated with Akaki River in Addis Ababa, Ethiopia. Sci. Afr. 19:e01541. doi: 10.1016/j.sciaf.2022.e01541
Merck Veterinary Manual (MVM) (2023). The veterinary manual. 11th Edn. Rahway, NJ, USA: Merck & Co., Inc.
Ministry of Water, Irrigation, and Energy (MoWIE) (2020). National Water Policy and strategy. Addis Ababa, Ethiopia: Federal Democratic Republic of Ethiopia.
Mitra, S. (2003). Sample preparation techniques in analytical chemistry : A John Wiley & Sons, Inc., Publication. doi: 10.1002/0471457817
Mohan, S. V., Nithila, P., and Reddy, S. J. (1996). Estimation of heavy metals in drinking water and development of heavy metal pollution index. J. Environ. Sci. Health A 31, 283–289. doi: 10.1080/10934529609376357
Nisha, P., Abdul Nazar, P., and Jayamurthy, P. (2009). A comparative study on antioxidant activities of different varieties of Solanum melongena. Food Chem. Toxicol. 47, 2640–2644. doi: 10.1016/j.fct.2009.07.026
Nour, H. E., Alshehri, F., Sahour, H., and El-Sorogy, A. S. (2022). Evaluation of sediment and water quality of Ismailia Canal for heavy metal contamination, eastern Nile Delta, Egypt. Reg. Stud. Mar. Sci. 56:102714. doi: 10.1016/j.rsma.2022.102714
Nuclear Regulatory Commission (NRC) (1974). The U.S. Nuclear Regulatory Commission (NRC) was created as an independent agency by congress in 1974 to ensure the safe use of radioactive materials for beneficial civilian purposes while protecting people and the environment. United State Atomic Energy Commission, ESA.
Olsson, D., Gericke, N., and de Boeve-Pauw, J. (2022). The effectiveness of education for sustainable development revisited – a longitudinal study on secondary students’ action competence for sustainability. Environ. Educ. Res. 28, 405–429. doi: 10.1080/13504622.2022.2033170
Omar, P. J., Tripathi, R., and Azamathulla, H. (2024). Photocatalytic water purification Technology for Contaminated Water Treatment. Top. Catal. 67, 959–960. doi: 10.1007/s11244-024-01991-z
Patra, A. K. (2011). Effects of essential oils on rumen fermentation, microbial ecology and ruminant production. Asian J. Anim. Vet. Adv. 6, 416–428. doi: 10.3923/ajava.2011.416.428
Pieper, K. J., Martin, R., Tang, M., Walters, L., Parks, J., Roy, S., et al. (2018). Evaluating water lead levels during the Flint water crisis. Environ. Sci. Technol. 52, 8124–8132. doi: 10.1021/acs.est.8b00791
Pompilio, C. G. N., Francisco, C. S., Tulio, F. D. M. T. M., Samuel, S. M. S., and Elisa, G. J. F. (2021). Heavy metals in blood, milk and cow’s urine reared in irrigated areas with wastewater. Heliyon 7:e06693. doi: 10.1016/j.heliyon.2021.e06693
Rodríguez, J. M., Murphy, K., Stanton, C., Ross, R. P., Kober, O. I., Juge, N., et al. (2015). The composition of the gut microbiota throughout life, with an emphasis on early life. Microb. Ecol. Health Dis. 26:26050. doi: 10.3402/mehd.v26.26050
Rothman, K. J., Greenland, S., and Lash, T. (2008). Modern epidemiology. Third Edn. Philadelphia: Lippincott Williams & Wilkins, 303–327.
Sato, S., Basse, A. L., Schönke, M., Chen, S., Samad, M., Altıntaş, A., et al. (2019). Time of exercise specifies the impact on muscle metabolic pathways and systemic energy homeostasis. Cell Metab. 30, 92–110.e4. doi: 10.1016/j.cmet.2019.03.013
Singh, P., Berawala, N., and Patil, Y. (2022). Automobile service station waste assessment and promising biological treatment alternatives: a review. Environ. Monit. Assess. 194:753. doi: 10.1007/s10661-022-10387-z
Tadesse, S., Alemayehu, H., Tenna, A., Tadesse, G., Tessema, T. S., Shibeshi, W., et al. (2018). Antimicrobial resistance profile of Staphylococcus aureus isolated from patients with infection at Tikur Anbessa specialized hospital, Addis Ababa, Ethiopia. BMC Pharmacol. Toxicol. 19:24. doi: 10.1186/s40360-018-0210-9
Tahir, I., and Alkheraije, K. A. (2023). A review of important heavy metals toxicity with special emphasis on nephrotoxicity and its management in cattle. Front. Vet. Sci. 10:1149720. doi: 10.3389/fvets.2023.1149720
Tchounwou, P. B., Yedjou, C. G., Patlolla, A. K., and Sutton, D. J. (2012). Heavy metal toxicity and the environment. Exp Suppl. 101, 133–164. doi: 10.1007/978-3-7643-8340-4_6
Teketel, Z. (2023). Toxic effects of heavy metals on health and productivity of livestock in little Akaki river catchment, Central Ethiopia. Ethiopia: Addis Ababa University.
Tiwari, A. K., De Maio, M., Singh, P. K., and Mahato, M. K. (2015). Evaluation of surface water quality by using GIS and a heavy metal pollution index (HPI) model in a coal mining area, India. Bull. Environ. Contam. Toxicol. 95, 304–310. doi: 10.1007/s00128-015-1558-9
Turek, A., Wieczorek, K., and Wolf, W. M. (2019). Digestion procedure and determination of heavy metals in sewage sludge—an analytical problem. Sustainability 11:1753. doi: 10.3390/su11061753
United States Environmental Protection Authority (USEPA) (1992) Method 3005A: Acid digestion of waters for total recoverable or dissolved metals for analysis by flame atomic absorption (FLAA) or inductively coupled plasma (ICP) spectroscopy. Revision 1
Valente-Campos, S., Spry, D. J., Pascale Palhares, J. C., Jakomin Rudez, L. M., and Umbuzeiro, G. D. A. (2019). Critical issues and alternatives for the establishment of chemical water quality criteria for livestock. Regul. Toxicol. Pharmacol. 104, 108–114. doi: 10.1016/j.yrtph.2019.03.003
Waleed Makki, H., Waleed Makki, H., Mohamed, T. S. A., Omer Hamad Abd El-Raheem, G., Bashir Abdel Mahmoud, A.-Z., Mustafa Elfadul, M., et al. (2023). Health impact of household waste burning in Khartoum state, Sudan. Risk Manag. Healthc. Policy 16, 1297–1307. doi: 10.2147/RMHP.S395694
Weldegebriel, Y., Chandravanshi, B. S., and Wondimu, T. (2012). Concentration levels of metals in vegetables grown in soils irrigated with river water in Addis Ababa, Ethiopia. Ecotoxicol. Environ. Saf. 77, 57–63. doi: 10.1016/j.ecoenv.2011.10.011
Weldesilassie, A. B., Boelee, E., Drechsel, P., and Dabbert, S. (2011). Wastewater use in crop production in peri-urban areas of Addis Ababa: impacts on health in farm households. Environ. Dev. Econ. 16, 25–49. doi: 10.1017/S1355770X1000029X
World Health Organization. (2009). Global health risks: mortality and burden of disease attributable to selected major risks. World Health Organization. Available online at: https://iris.who.int/handle/10665/44203
World Health Organization (WHO) (2011). Guidelines for drinking water quality. 4th Edn. Geneva, Switzerland.
Wuana, R. A., and Okieimen, F. E. (2011). Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecol. 2011, 1–20. doi: 10.5402/2011/402647
Yilma, M., Kiflie, Z., Windsperger, A., and Gessese, N. (2019). Assessment and interpretation of river water quality in little Akaki River using multivariate statistical techniques. Int. J. Environ. Sci. Technol. 16, 3707–3720. doi: 10.1007/s13762-018-2000-8
Yohannes, H., and Elias, E. (2017). Contamination of rivers and water reservoirs in and around Addis Ababa city and actions to combat it. Environ. Poll. Clim. Change 1, 1–12. doi: 10.4172/2753-458X.1000116
Yong, R. N., and Thomas, H. R. (1999) Geoenvironmental engineering: Ground contamination: pollutant management and remediation: proceedings of the second conference organized by the British geotechnical society and the Cardiff School of Engineering, University of Wales, Cardiff, and held in London on 13–15 September 1999 Thomas Telford
Zinabu, E., Kelderman, P., van der Kwast, J., and Irvine, K. (2019). Monitoring river water and sediments within a changing Ethiopian catchment to support sustainable development. Environ. Monit. Assess. 191:455. doi: 10.1007/s10661-019-7545-6
Glossary
AAS - Atomic Absorption Spectrophotometry
LB - Albumin
ALP - Alkaline Phosphate
AAW - American Public Health Association, American Water Works Association, Water Environment Federation
ARC - Akaki River Catchment
AST - Aspartate Aminotransferase
BUN - Blood urea nitrogen
Cr - Chromium
Cd - Cadmium
CVMA - College of Veterinary Medicine and Agriculture
QC - Quality control
DO - dissolved oxygen
EDTA - Ethylene-di-amine-tetra-acetic-acid
FDRE - Federal Democratic Republic of Ethiopia
Hb - Hemoglobin
HNO3 - nitric acid
H2O2 - hydrogen peroxide
ICP-OES - Inductively Coupled Plasma Optical Emission Spectrometry
KCl - potassium chloride
LHI - Livestock Health Index
LOI - Loss-on-ignition
LWPI - Livestock Water Pollution Index
MoWIE - Ministry of Water Irrigation and Energy
Na₂H₂EDTA - Disodium dihydrogen ethylenediaminetetraacetate
NCP - neutrophil count percentage
Pb - Lead
PCV - Packed Cell Volume
pH - power of hydrogen
H2SO4 - sulfuric acid
TDS - total dissolved solid
TP - Total Protein
USEPA - United State Environmental Protection Agency
WBC - White Blood Cell
Zn - Zinc
Keywords: livestock, one-health, pollution, public health, water management, Ethiopia
Citation: Abosse JS, Assefa S, Teketel Z, Efa DA, Wolde B and Eregno FE (2025) Water pollution and cattle health risks in the Akaki River Catchment, Central Ethiopia: implications for one-health. Front. Water. 7:1642296. doi: 10.3389/frwa.2025.1642296
Edited by:
Teddie O. Rahube, Botswana International University of Science and Technology, BotswanaReviewed by:
Padam Jee Omar, Babasaheb Bhimrao Ambedkar University, IndiaSunitha Vangala, Yogi Vemana University, India
Copyright © 2025 Abosse, Assefa, Teketel, Efa, Wolde and Eregno. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jirata Shiferaw Abosse, amlyYXRhLnNoaWZlcmF3QGFhdS5lZHUuZXQ=; Samuel Assefa, c2FtdWVsLmFzc2VmYUBhYXUuZWR1LmV0
†ORCID: Jirata Shiferaw Abosse, orcid.org/0000-0001-7411-595X
Samuel Assefa, orcid.org/0000-0001-9557-3810
Zerihun Teketel, orcid.org/0009-0004-2773-7835
Debela Abdeta, orcid.org/0000-0001-6407-973X
Biruk Wolde, orcid.org/0009-0001-8210-7172
Fasil Ejigu Eregno, orcid.org/0000-0002-3829-9701
 Jirata Shiferaw Abosse
Jirata Shiferaw Abosse Samuel Assefa3*†
Samuel Assefa3*† Fasil Ejigu Eregno
Fasil Ejigu Eregno