- 1Reconstructive Surgery and Regenerative Medicine Research Group (ReconRegen), Institute of Life Science, Swansea University Medical School, Swansea, United Kingdom
- 2The Welsh Centre for Burns and Plastic Surgery, Morriston Hospital, Swansea, United Kingdom
- 3Oxford University Medical School, Oxford, United Kingdom
- 4Department of Plastic Surgery, Birmingham Children’s Hospital, Birmingham, United Kingdom
Background: The use of robots in surgery has become commonplace in many specialties. In this systematic review, we report on the current uses of robotics in plastic and reconstructive surgery and looks to future roles for robotics in this arena.
Methods: A systematic literature search of Medline, EMBASE, and Scopus was performed using appropriate search terms in order to identify all applications of robot-assistance in plastic and reconstructive surgery. All articles were reviewed by two authors and a qualitative synthesis performed of those articles that met the inclusion criteria. The systematic review and results were conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta Analysis (PRISMA) guidelines.
Results: A total of 7,904 articles were identified for title and abstract review. Sixty-eight studies met the inclusion criteria. Robotic assistance in plastic and reconstructive surgery is still in its infancy, with areas such as trans-oral robotic surgery and microvascular procedures the dominant areas of interest currently. A number of benefits have been shown over conventional open surgery, such as improved access and greater dexterity; however, these must be balanced against disadvantages such as the lack of haptic feedback and cost implications.
Conclusion: The feasibility of robotic plastic surgery has been demonstrated in several specific indications. As technology, knowledge, and skills in this area improve, these techniques have the potential to contribute positively to patient and provider experience and outcomes.
Introduction
The use of robotics in surgery has captured the imagination of many. It is a growth area across the breadth of surgical specialties, with many procedures becoming routinely classed as “robot-assisted.” The rapid increase in surgical research involving robotic assistance can be witnessed by the rising number of articles published in consecutive years related to the subject (Figure 1).
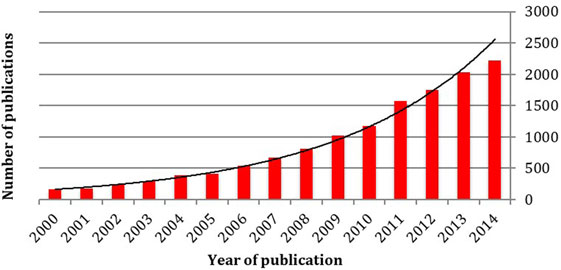
Figure 1. A 15-year literature review of the number of publications relating to robotic surgery demonstrating a highly significant exponential increase. Each column represents the number of papers published in that year, rising from 168 in 2000 to over 2,000 in the year 2014 (Source; Pubmed, searched using the terms “robot” and “surgery” from January 2000 to December 2014).
Since the first reported use of the daVinci® Surgical Robotic System (Intuitive Surgical, Sunnyvale, CA, USA) in a robotic-assisted laparoscopic cholecystectomy (1), Intuitive Surgical has become the leading force in surgical robotics. The daVinci® robot has been widely implemented in many surgical specialties, from cardiac surgery (2, 3) to gynecology (4, 5). In the USA, 80% of radical prostatectomies are now being performed robotically (6). With updates to the daVinci® robot including a fourth instrument arm, its application is broadening to other specialties such as colorectal surgery (7). The dominance of the daVinci® system is, however, beginning to be challenged with new competitors entering the market.
Plastic and reconstructive surgery is an innovative specialty, often at the forefront of technical innovation within surgery. It is also a specialty that works collaboratively with many other surgical disciplines and, therefore, those practicing it will likely come across advances in robotic surgery in these other specialties. It is, therefore, important for plastic surgeons to embrace this new surgical platform, explore potential uses for it, and learn from those who have already incorporated robotics into their surgical armamentarium.
This systematic review aimed to identify all current reported uses of robotic assistance in plastic and reconstructive surgery, from cadaveric to clinical examples. We have provided and up-to-date list of all areas of interest to the plastic and reconstructive surgeon, evaluating the relevant advantages and disadvantages of the use of robotics in these areas.
Methods
A database search was performed to identify all articles describing the use of robotic assistance in plastic and reconstructive surgery. The search strategy was constructed in line with the Preferred Reporting Items for Systematic Reviews and Meta Analysis (PRISMA) guidelines (8) and the Cochrane handbook (9). Key words and Medical Subject Heading terms were combined using Boolean logic and refined with the help of an information specialist (see Figure 2 for an example of the full search strategy). Medline (1946-present), EMBASE (1980-present), and Scopus electronic databases were all searched using the developed search strategy up to May 2017.
All studies identified were downloaded into EndNote V8 for Mac (Clarivate Analytics) and duplicates removed. De-duplicated results were then uploaded to Covidence (www.covidence.org) for screening. Titles and abstracts were reviewed by two independent reviewers (OC and HS) against the inclusion and exclusion criteria and discrepancies resolved through discussion with a third, independent reviewer (TD). Studies were considered eligible for qualitative synthesis if they met the following inclusion criteria:
• the study was published in English
• the study design was one of the following: case reports, case cohorts, case–control and randomized controlled studies. Both prospective and retrospectively designed studies were included.
• the study reports the use of a robotic surgical system for a potential plastic surgery-related operation, with both preclinical and clinical applications included.
Full-text articles of those included studies were subsequently reviewed independently for final inclusion. References were checked for further, un-identified articles, and these were added in if appropriate.
A data extraction sheet was developed to extract the following data from studies: Author, date of publication, location of study, study design, number of operations performed, operations/techniques, outcomes measured. This was piloted on a random sample of papers and subsequently refined. All data were extracted and tabulated using Microsoft Word and Excel (Redmond, Washington, DC, USA).
Results
Figure 3 illustrates the PRISMA flow diagram demonstrating the process of article retrieval and screening. A total of 7,904 articles were identified after de-duplication for screening. Of these 213 made it to full-text review. A total of 68 studies met the inclusion criteria and were eligible for inclusion in this systematic review. Included papers were divided into groups based on operative type or body location and a qualitative synthesis of the outcomes reported performed.
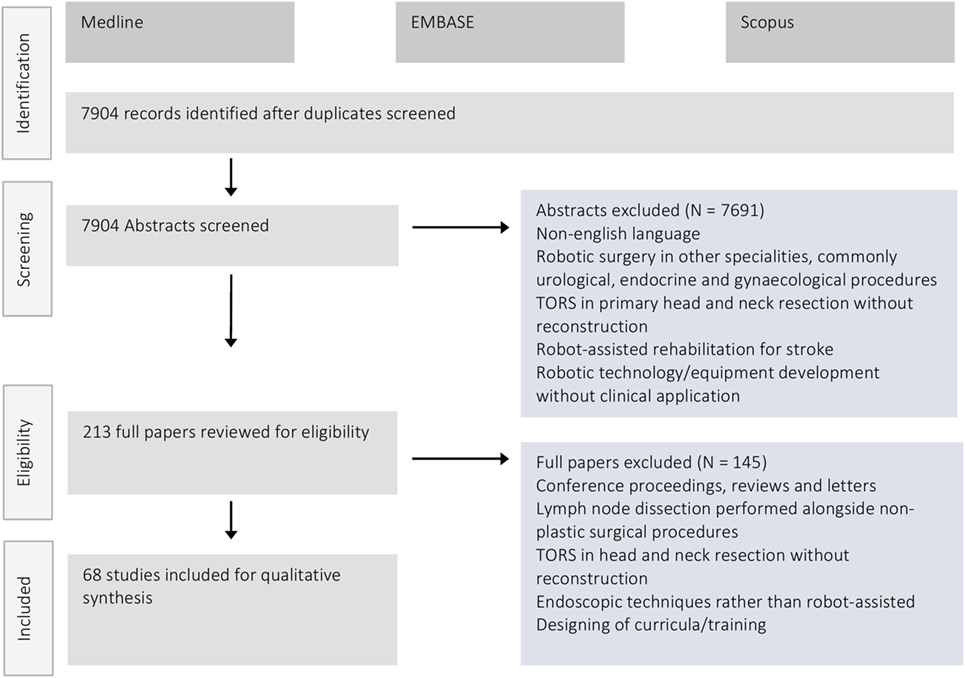
Figure 3. PRISMA flow diagram demonstrating the number of retrieved articles, those screened and final number included in the systematic review after full-text review.
Microsurgery
A total of 13 studies were identified discussing the use of robotics for a microsurgery application (Table 1). Eleven of these were preclinical studies in synthetic, animal, and cadaveric models (10–20) while two were clinical studies (21, 22). Katz et al. performed the first daVinci® system assisted anastomosis in a porcine model in 2005 (10), closely followed by work in canine tarsal and femoral vessels (13). In these studies, they concluded significant advantages such as the elimination of tremor at a microsurgical level, but that the lack of purpose-built microsurgical instruments was an important limitation. Further animal and human cadaveric work cemented the idea that robotically assisted microvascular surgery is both feasible and in some instances potentially beneficial, such as when working at depth and for surgeon comfort (20).
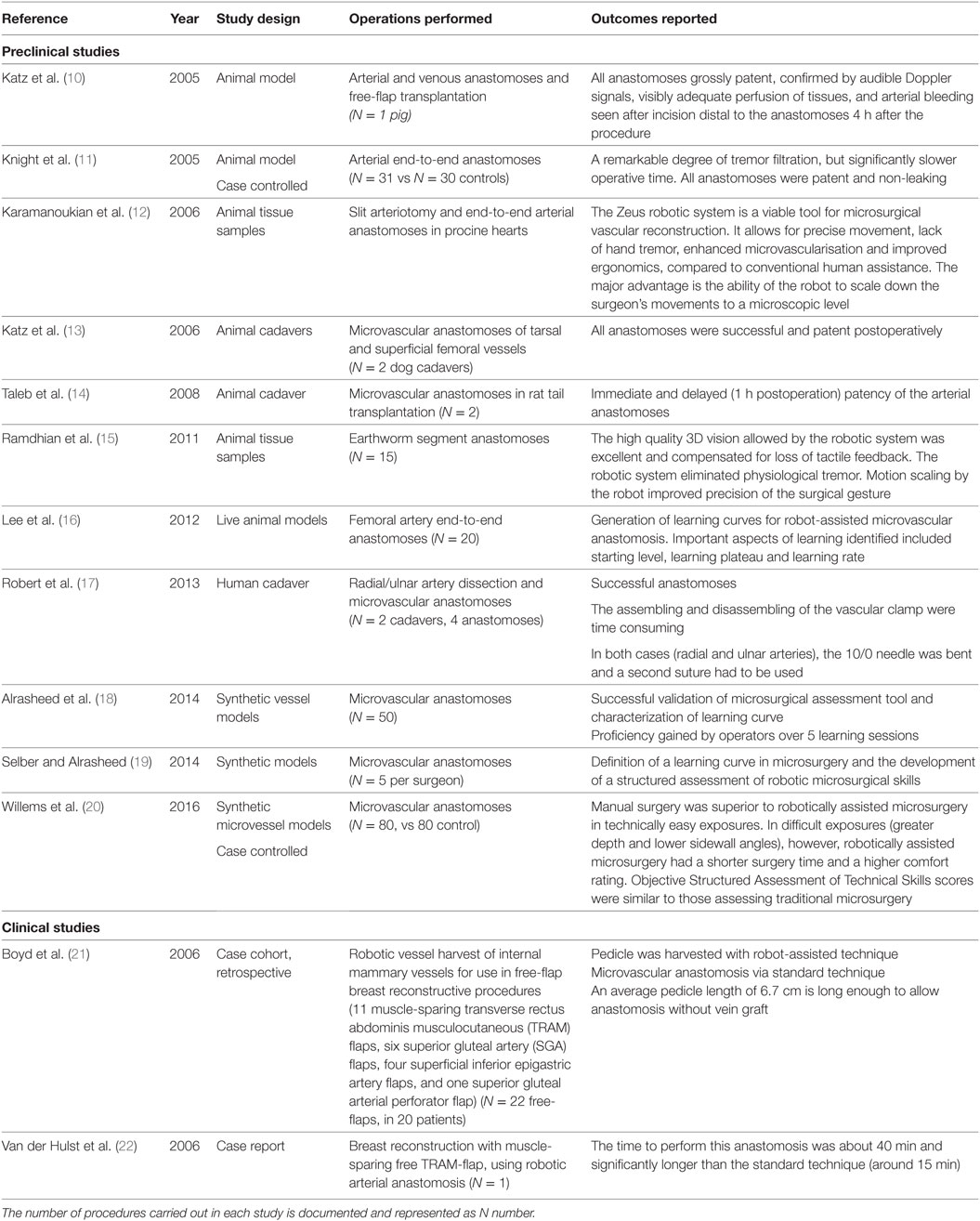
Table 1. Preclinical and clinical studies relating to the use of robotics in microvascular procedures.
Two clinical examples were identified, with one cohort study by Boyd et al. including 22-patients where the robot was used for harvesting the internal mammary vessels in free breast reconstruction (21). Van der Hulst et al. used the robot to perform the anastomosis, commenting on the increased time taken for this over traditional methods, as would be expected early on in the learning curve (22). As in preclinical studies, the benefits of using the daVinci® robot for performing the microvascular anastomosis include elimination of tremor and motion scaling.
Muscle Flap Harvest
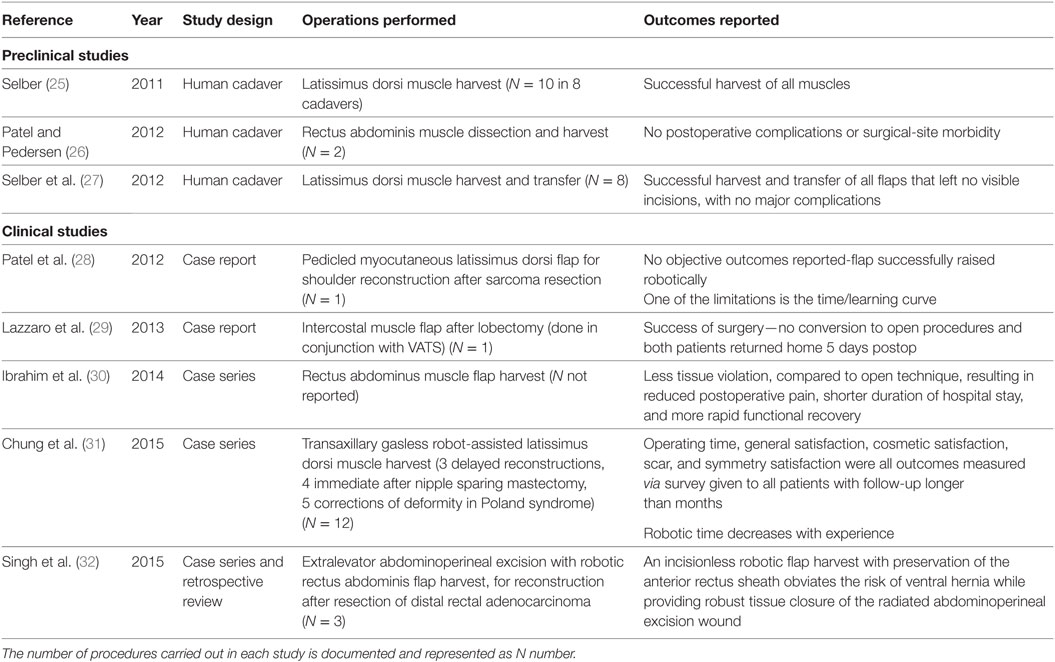
Traditionally muscle free-flaps are raised through a large incision overlying the muscle belly and are, therefore, a perfect example of where the robot can have marked benefit as minimally invasive harvesting can significantly reduce the size of externally visible scarring. Laparoscopic harvesting has been attempted, but with poor uptake due to difficulties with visualization of the operative field and the inherent limitations of laparoscopic instruments (23, 24). Three human cadaveric studies (25–27) and five clinical reports (28–32) were identified describing the use of the robot for muscle flap harvest (Table 2). In those clinical studies, it is clear that the robot improves visualization, reduces the scar burden and resulted in reduced postoperative pain and hospital stay.
The traditional approach to rectus muscle harvest is with a large abdominal skin incision. Not only is this cosmetically unappealing but also, in combination with division of the anterior abdominal wall fascia, can result in incisional hernia formation. As robot-assisted colorectal surgery becomes increasingly routine, with the advantages of minimal scarring, reduced conversion to open procedure, reduced time to intestinal motility, and reduced postoperative sexual dysfunction reported (33), it would seem a retrograde step to then introduce a large abdominal wound when harvesting the rectus abdominis muscle for perineal reconstruction. In a case series by Singh et al. the robot was used in tandem with a robotically performed abdominoperineal resection for adenocarcinoma to raise the rectus abdominis flap for reconstruction (32). This produced satisfactory closure of the defect without the risk of a ventral hernia. In these combined procedures the risks associated with entering the abdominal cavity are already present from the colorectal resection and, therefore, one of the major disadvantages of robotically assisted rectus abdominis muscle harvest is not a risk purely implicated through the use of this novel muscle harvest technique.
Nerve Surgery
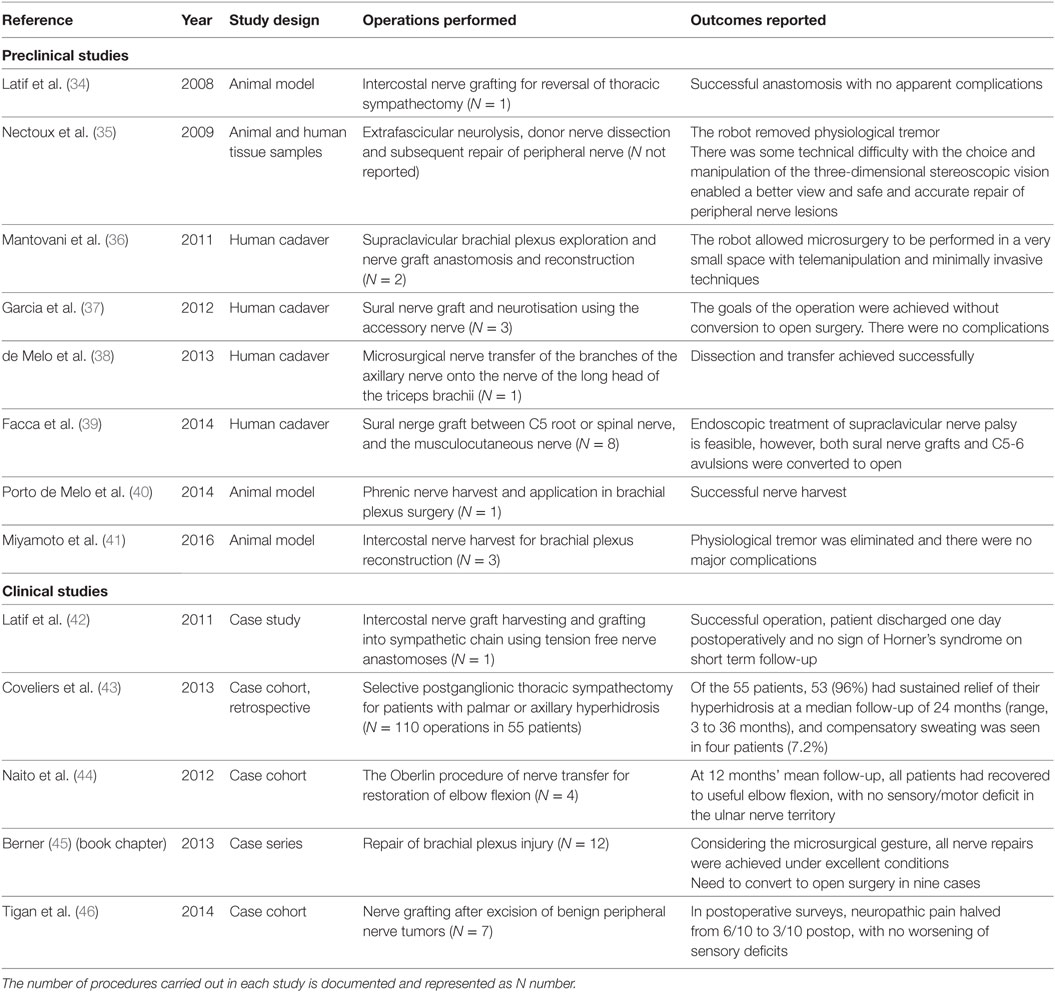
A total of eight preclinical studies and five clinical studies were identified, with the majority investigating the role of robotics in brachial plexus work (Table 3) (34–46). Epineural nerve repair using robotic assistance has been shown to be technically feasible in animal models, with the benefits of reduced physiological tremor and improved vision of the surgical field noted (35). Nerve harvest has also been demonstrated to be feasible in cadaveric and animal models (35, 37).
In those clinical studies identified, robotic assistance was successfully used to repair a brachial plexus (45), repair the sympathetic chain to treat Horner’s syndrome (42), perform a thoracic sympathectomy for palmar hyperhidrosis (43), repair a peripheral nerve following tumor excision (46), and undertake an Oberlin procedure (44).
Upper Limb
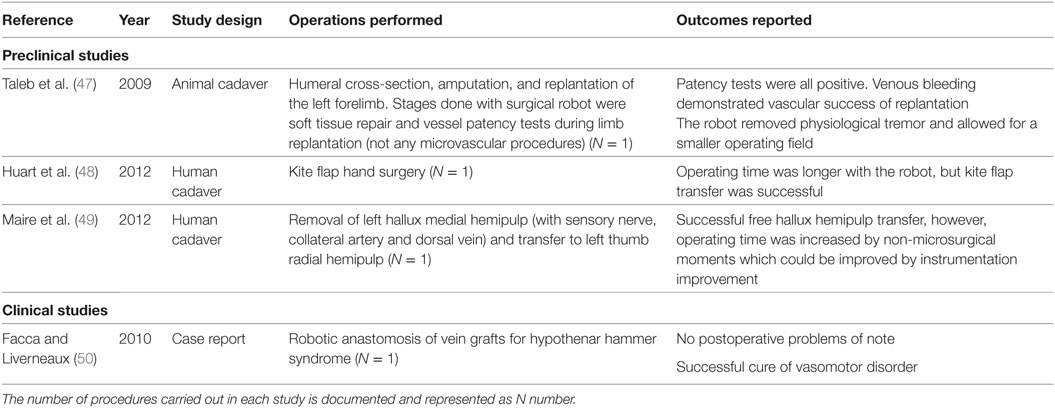
Table 4 illustrates those articles relating to procedures in the upper limb, with three preclinical (47–49) and one clinical study identified (50). As with a number of other areas of the body the use of the robot has so far only been for proof of concept and there has yet to be any concrete studies demonstrating a benefit.
Trans-Oral Robotic Surgery (TORS)
Trans-oral robotic surgery has allowed head and neck surgeons to treat benign and malignant conditions of the oral cavity and oropharynx avoiding more traditional jaw and lip split approaches, facilitated by the improved access and visualization afforded by the robotic instruments (51, 52). If there is no communication between the oral cavity or oropharynx and neck dissection then the defect could be left to heal by secondary intention; however, in more complex or advanced stages of disease, reconstruction using local flaps or free tissue transfer is required (53). If a jaw split has not been performed, access for satisfactory reconstruction can be almost impossible and thus developing reconstructive techniques using the robot in order to capitalize on the minimized morbidity associated with a TORS resection is of paramount importance.
Trans-oral robotic surgery has become the biggest area for robotic-assisted plastic surgery procedures, with 2 preclinical (54, 55) and 21 clinical studies identified (56–76) (Table 5). Local reconstructive options include the use of the Facial Artery Musculomucosal flap, commonly used in reconstruction of the floor of the mouth and soft palate. Bonawitz and Duvvuri have described using the robot for raising and in-setting the flap with good results (64, 65). Others demonstrated that the use of the robot to perform a musculomucosal advancement flap pharyngoplasty gives good results, both in terms of orocutaneous fistula risk and functional outcomes (60, 61).
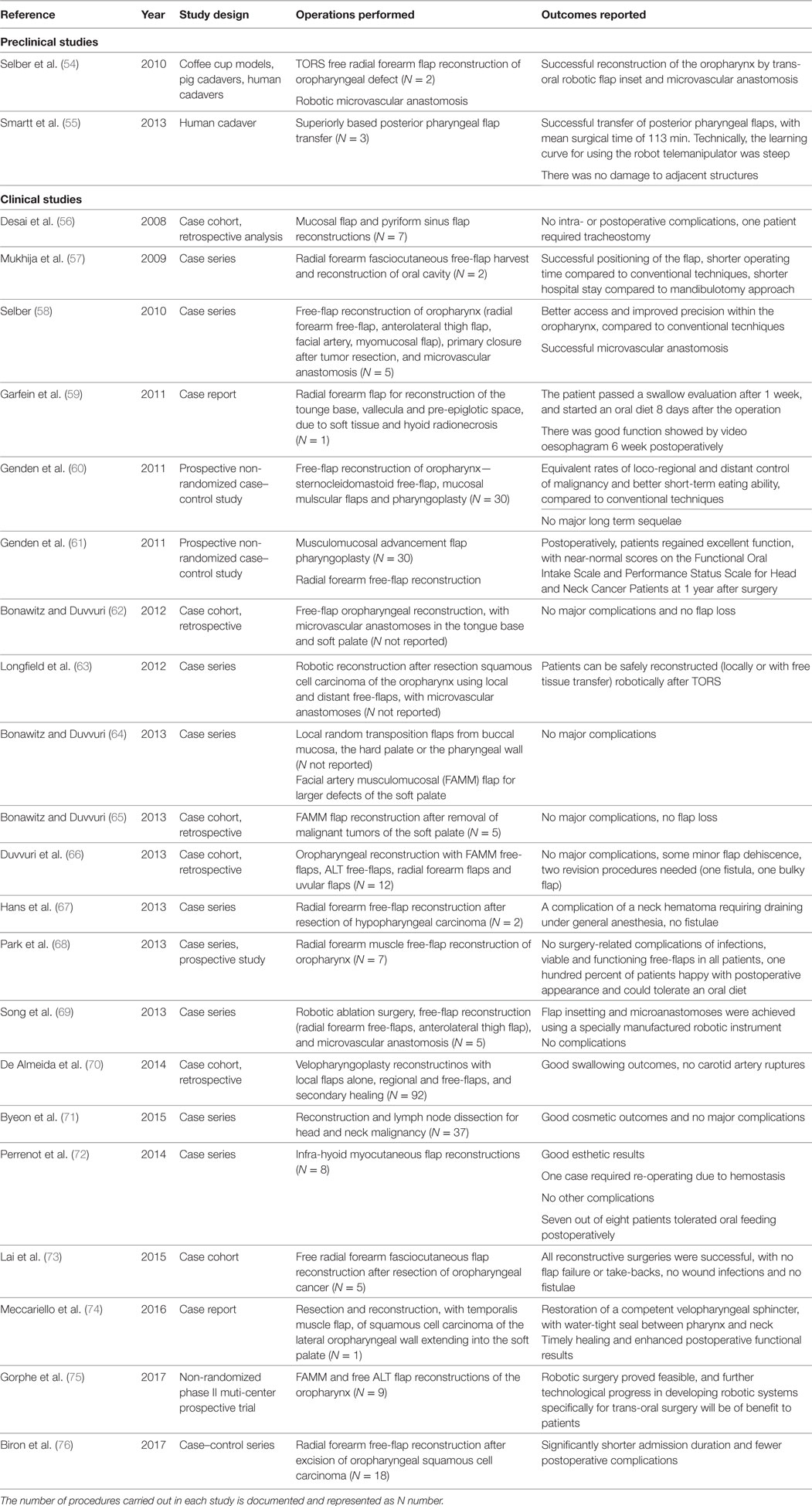
Table 5. Preclinical and clinical studies relating to the use of robotics in trans-oral robotic surgery (TORS) for a plastic surgery application.
In larger or more complex composite defects there is often the requirement for free-flap reconstruction, with specific indications including exposure of the carotid artery, large base-of-tongue defects and defects of the soft palate and tonsillar fossa which cannot be closed with local flap options. The commonest reported free-flap used following TORS resection is the radial forearm flap; however, others such as the anterolateral thigh flap are also described. In the majority of cases the robot was used for flap inset, with authors reporting good access and visualization that allowed a water-tight inset to be achieved and no flap complications despite the lack of a traditional jaw spilled. The robot was also used in a number of studies to perform the vascular anastomosis (58, 62, 63, 69).
Trans-Oral Robotic Cleft Surgery (TORCS)
Trans-oral robotic cleft surgery is still in its infancy with only three articles identified (77–79) (Table 6); however, it builds upon the same benefit profile achieved by TORS that has been outlined previously for access to the oral cavity and oropharynx in cleft lip and palate patients.
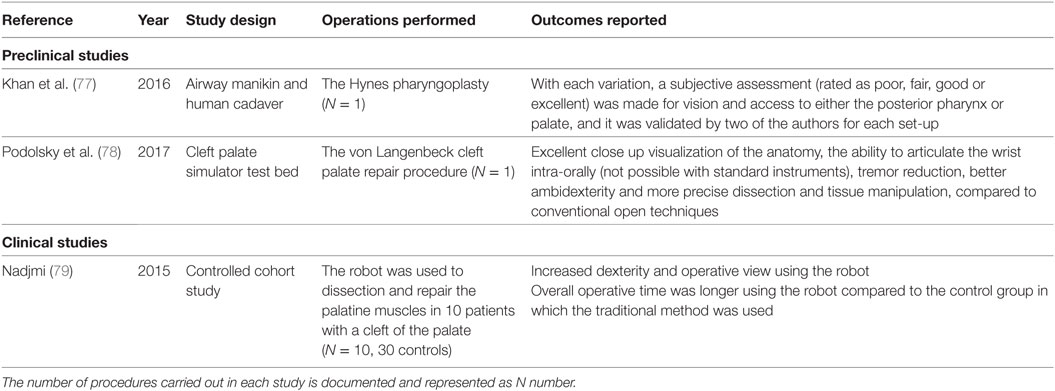
Table 6. Preclinical and clinical studies relating to the use of robotics in trans-oral robotic cleft surgery.
Other Indications
Table 7 demonstrates four other studies identified in the systematic review, which do not fit into the categories above (80–83). Of these indications, it is likely that only lymph node based procedures are likely to progress in the future, with some benefits such as the ability to perform supermicro-surgery an obvious advantage in lymph node transfer.
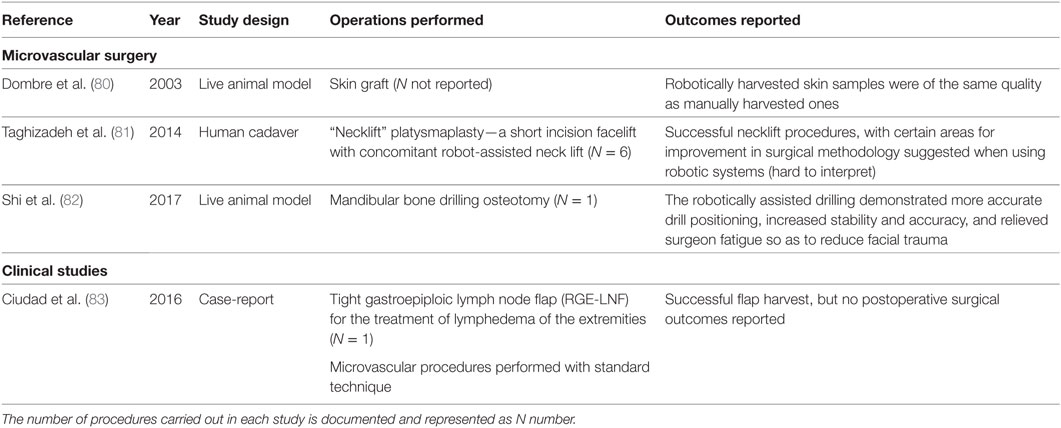
Table 7. Preclinical and clinical studies relating to the use of robotics in other, miscellaneous areas of plastic and reconstructive surgery.
Discussion
In the 30 years since the first robot was used in a surgical procedure the arena of robotic surgery has changed at a breathtaking pace, with the use of the daVinci® robot now common place in some specialties. This revolution has taken longer to impact on the plastic surgery community. It is, therefore, somewhat ironic that it was a plastic surgeon who was at the forefront of robotic and tele-surgery at its inception (84). However, this systematic review has shown that significant developments have been made in the last few years.
The benefits of robotic surgery have been well documented, albeit with no large scale studies, and include reduced blood loss, reduced postoperative pain, faster recovery, and improved cosmesis (85). In relation to plastic and reconstructive surgery the elimination of tremor, greater degree of freedom of the instrument and motion scaling all have the potential to improve the accuracy and reproducibility of microsurgery. The evidence suggests that while the initial learning curve is steep, proficiency in microsurgical skills using the robot can be gained in a short number of sessions (18).
Of the areas identified in this systematic review there are some that are further down the development road than others and some, where the advantages of robotic assistance are greater. For example, with the recent uptake of free-perforator flaps in the field of reconstructive surgery we are beginning to approach the limits of human dexterity at which point the robot may prove to be advantageous. However, to fully exploit this there needs to be focused development in the field of robotic instrument design, expanding the portfolio of micro-instruments. It is our opinion that the potential for robotic head and neck reconstruction is huge and is one of the areas that will most definitely see growth due to the obvious benefits it offers. This will be especially true as the indications for TORS resection continue to widen, resulting in larger and more complex defects. The current limitation to more widespread utilization is instrument design in order to perform microvascular anastomoses and easier inset and it is this area that research should focus. This may also be the case with TORCS. As with cancer resection, there are many circumstances where adequate access to the pharynx and palate in the pediatric cleft patient can pose a significant challenge. The space in which to operate, as well as access for instrumentation, can be severely restricted, especially in cases with abnormal anatomy, poor jaw opening, or anomalies of the nasopharyngeal space. Adequate illumination and visualization can also be difficult. Early work has shown that performing posterior pharyngeal wall surgery using the daVinci® robot is feasible, with benefits such as an improved view, easier dissection, reduced secondary surgical insult and preferential ergonomics for the operating surgeon (77). Its use may also open up avenues of new surgical interventions to areas of the oropharynx that were previously inaccessible.
There is currently less convincing evidence for the use of robotics in areas such as nerve and upper limb surgery. In brachial plexus reconstruction nerve harvest is often required and, therefore, reduced donor site morbidity through robotic harvest, such as with trans-thoracic harvest of intercostal and phrenic nerves, is an area that has the potential for future advancement. It will be important, however, to also demonstrate its safety and cost-effectiveness in order to justify the marginal reductions in scarring when compared to more traditional harvest sites. To date all of the preclinical and clinical studies investigating robotic nerve surgery have demonstrated that it is technically feasible. However, it is still mostly at a proof of concept level and while does have benefits in terms of reduced tremor, it is most likely to be of benefit in difficult to assess areas or when the robot is already being used to perform other parts of the procedure. Finally, at present the indications for the use of robotics in hand surgery are probably more limited than other areas discussed, especially as access is not normally a problem in hand surgery. However, the benefits as discussed for microsurgical anastomosis may prove to be useful in specific indications such as traumatic replantation or congenital reconstruction.
Robotic surgery’s main disadvantage remains the high cost of purchasing and maintaining the equipment. This will undoubtedly improve with time as a greater number of procedures are performed using the robot and the unit cost per operation reduces. A recent comparison of the cost of TORS compared to radiotherapy demonstrated that TORS is currently more expensive; however, this is likely to reduce through the creation of high-volume centers performing TORS (86). It has also been shown that in a center where the learning curve had already been overcome, robotic surgery was cheaper than equivalent open surgery for the surgical treatment of endometrial cancer (87).
Lack of haptic feedback is also often cited as another disadvantage of robotic surgery, with studies demonstrating that operators of augmented robotic surgical systems prefer those with haptic feedback (88). However, other studies such as by Hagen and colleagues who looked at 52 individuals and their perception of haptic feedback while performing robotic surgery demonstrated that visual cues are able to give the perception of haptic feedback, even when true haptic feedback is not present (89). Despite this evidence there is still a tremendous amount of working looking at ways to incorporate haptic feedback into robotic systems, summarized in a review by Okamura (90).
Finally, robotic surgery often results in longer operative times, although this improves with proficiency and in some cases is now comparable to traditional techniques.
The future of robotics in plastic surgery is clearly exciting. Over the last 5 years the range of procedures using the daVinci® robot being attempted by the plastic surgery community has increased significantly and, as technology continues to improve, this will gain further momentum. Of the 68 studies included in this review, only three used a robotic system other than the daVinci®. This dominance is beginning to be challenged and while equipment additions such as a micro-forcep is currently available for the daVinci® robot and external companies have developed micro-doppler probes and hydrojet dissectors (91) it will be the development of further microsurgical instruments that will allow greater use of the robot in the field of plastic and reconstructive surgery. The combination of motion scaling and tremor-free instrument manipulation with new instrument design will also allow new avenues in microsurgery that have to date been too technically demanding to be explored. Furthermore, the introduction of a new single port addition to the daVinci® system will allow greater access in trans-oral surgery, improving instrument maneuverability within the tight confines of the intra-oral cavity.
Conclusion
The potential value of robotic plastic surgery has already been investigated in several specific indications. It is still early days for the field and only time will tell if the use of robotics in plastic surgery is truly of benefit. As the technology, knowledge, and skills in this area improve, it is likely that in specific indications the use of robotic surgery will further contribute positively to patient and provider experience and outcomes. It is, therefore, imperative that the plastic surgery community embraces this new technology platform, but in doing so conducts well-designed, patient-focused research to ensure that it is only being used when there is true benefit to our patients.
Author Contributions
TD, KK, and IW developed the idea for this paper. TD, OC, and HS developed the search strategy and performed the systematic review and data extraction. TD, KK, IW, OC, and HS wrote the manuscript and all authors edited and agreed the final version.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This work has received no specific funding. TD is funded by the Welsh Clinical Academic Training Fellowship.
References
1. Ballantyne GH. Robotic surgery, telerobotic surgery, telepresence, and telementoring. Review of early clinical results. Surg Endosc (2002) 16(10):1389–402. doi:10.1007/s00464-001-8283-7
2. Kappert U, Cichon R, Schneider J, Gulielmos V, Tugtekin SM, Matschke K, et al. Robotic coronary artery surgery – the evolution of a new minimally invasive approach in coronary artery surgery. Thorac Cardiovasc Surg (2000) 48(4):193–7. doi:10.1055/s-2000-6904
3. Ishikawa N, Watanabe G, Iino K, Tomita S, Yamaguchi S, Higashidani K, et al. Robotic internal thoracic artery harvesting. Surg Today (2007) 37(11):944–6. doi:10.1007/s00595-007-3542-4
4. Cadière GB, Himpens J, Germay O, Izizaw R, Degueldre M, Vandromme J, et al. Feasibility of robotic laparoscopic surgery: 146 cases. World J Surg (2001) 25(11):1467–77. doi:10.1007/s00268-001-0132-2
5. Bush SH, Apte SM. Robotic-assisted surgery in gynecological oncology. Cancer Control (2015) 22(3):307–13. doi:10.1177/107327481502200308
6. Barbash GI, Glied SA. New technology and health care costs – the case of robot-assisted surgery. N Engl J Med (2010) 363(8):701–4. doi:10.1056/NEJMp1006602
7. Pappou EP, Weiser MR. Robotic colonic resection. J Surg Oncol (2015) 112(3):315–20. doi:10.1002/jso.23953
8. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med (2009) 151(4):264–9. doi:10.1016/j.jclinepi.2009.06.005
9. Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration. (2011).
10. Katz RD, Rosson GD, Taylor JA, Singh NK. Robotics in microsurgery: use of a surgical robot to perform a free flap in a pig. Microsurgery (2005) 25:566–9. doi:10.1002/micr.20160
11. Knight CG, Lorincz A, Cao A, Gidell K, Klein MD, Langenburg SE. Computer-assisted, robot-enhanced open microsurgery in an animal model. J Laparoendosc Adv Surg Tech A (2005) 15(2):182–5. doi:10.1089/lap.2005.15.182
12. Karamanoukian RL, Finley DS, Evans GR, Karamanoukian HL. Feasibility of robotic-assisted microvascular anastomoses in plastic surgery. J Reconstr Microsurg (2006) 22(06):429–32. doi:10.1055/s-2006-947697
13. Katz RD, Taylor JA, Rosson GD, Brown PR, Singh NK. Robotics in plastic and reconstructive surgery: use of a telemanipulator slave robot to perform microvascular anastomoses. J Reconstr Microsurg (2006) 22:53–7. doi:10.1055/s-2006-931908
14. Taleb C, Nectoux E, Liverneaux PA. Telemicrosurgery: a feasibility study in a rat model. Chir Main (2008) 27(2–3):104–8. doi:10.1016/j.main.2008.04.001
15. Ramdhian RM, Bednar M, Mantovani GR, Facca SA, Liverneaux PA. Microsurgery and telemicrosurgery training: a comparative study. J Reconstr Microsurg (2011) 27(09):537–42. doi:10.1055/s-0031-1285985
16. Lee JY, Mattar T, Parisi TJ, Carlsen BT, Bishop AT, Shin AY. Learning curve of robotic-assisted microvascular anastomosis in the rat. J Reconstr Microsurg (2012) 28(07):451–6. doi:10.1055/s-0031-1289166
17. Robert E, Facca S, Atik T, Bodin F, Bruant-Rodier C, Liverneaux P. Vascular microanastomosis through an endoscopic approach: feasibility study on two cadaver forearms. Chir Main (2013) 32(3):136–40. doi:10.1016/j.main.2013.01.002
18. Alrasheed T, Liu J, Hanasono MM, Butler CE, Selber JC. Robotic microsurgery: validating an assessment tool and plotting the learning curve. Plast Reconstr Surg (2014) 134:794–803. doi:10.1097/PRS.0000000000000550
19. Selber JC, Alrasheed T. Robotic microsurgical training and evaluation. Semin Plast Surg (2014) 28(1):005–010. doi:10.1055/s-0034-1368161
20. Willems JI, Shin AM, Shin DM, Bishop AT, Shin AY. A comparison of robotically assisted microsurgery versus manual microsurgery in challenging situations. Plast Reconstr Surg (2016) 137(4):1317–24. doi:10.1097/PRS.0000000000002030
21. Boyd B, Umansky J, Samson M, Boyd D, Stahl K. Robotic harvest of internal mammary vessels in breast reconstruction. J Reconstr Microsurg (2006) 22(4):261–6. doi:10.1055/s-2006-939931
22. van der Hulst R, Sawor J, Bouvy N. Microvascular anastomosis: is there a role for robotic surgery? J Plast Reconstr Aesthetic Surg (2007) 60(1):101–2. doi:10.1016/j.bjps.2006.05.011
23. Greensmith A, Januszkiewicz J, Poole G. Rectus abdominis muscle free flap by laparoscopic sheath-sparing technique. Plast Reconstr Surg (2000) 105(4):1438–41. doi:10.1097/00006534-200004040-00026
24. Aijaz T, Singhal D, Tan SA, Iqbal A. A novel method of minimally invasive rectus abdominis muscle flap harvest: laparoscopic surgeons take note. J Minim Access Surg (2017) 13(2):146–7. doi:10.4103/0972-9941.186688
25. Selber JC. Robotic latissimus dorsi muscle harvest. Plast Reconstr Surg (2011) 128(2):88.e–90.e. doi:10.1097/PRS.0b013e31821ef25d
26. Patel NV, Pedersen JC. Robotic harvest of the rectus abdominis muscle: a preclinical investigation and case report. J Reconstr Microsurg (2012) 28:477–80. doi:10.1055/s-0031-1287674
27. Selber JC, Baumann DP, Holsinger CF. Robotic harvest of the latissimus dorsi muscle: laboratory and clinical experience. J Reconstr Microsurg (2012) 28:457–64. doi:10.1055/s-0032-1315789
28. Patel NP, Van Meeteren J, Pedersen J. A new dimension: robotic reconstruction in plastic surgery. J Robot Surg (2012) 6(1):77–80. doi:10.1007/s11701-011-0300-9
29. Lazzaro RS, Guerges M, Kadosh B, Gulkarov I. Robotic harvest of intercostal muscle flap. J Thorac Cardiovasc Surg (2013) 146(2):486–7. doi:10.1016/j.jtcvs.2013.03.037
30. Ibrahim AE, Sarhane KA, Pederson JC, Selber JC. Robotic harvest of the rectus abdominis muscle: principles and clinical applications. Semin Plast Surg (2014) 28(1):26–31. doi:10.1055/s-0034-1368164
31. Chung JH, You HJ, Kim HS, Lee BI, Park SH, Yoon ES. A novel technique for robot assisted latissimus dorsi flap harvest. J Plast Reconstr Aesthet Surg (2015) 68:966–72. doi:10.1016/j.bjps.2015.03.021
32. Singh P, Teng E, Cannon LM, Bello BL, Song DH, Umanskiy K. Dynamic article: tandem robotic technique of extralevator abdominoperineal excision and rectus abdominis muscle harvest for immediate closure of the pelvic floor defect. Dis Colon Rectum (2015) 58(9):885–91. doi:10.1097/DCR.0000000000000419
33. Lee SH, Lim S, Kim JH, Lee KY. Robotic versus conventional laparoscopic surgery for rectal cancer: systematic review and meta-analysis. Ann Surg Treat Res (2015) 89:190–201. doi:10.4174/astr.2015.89.4.190
34. Latif MJ, Afthinos JN, Connery CP, Perin N, Bhora FY, Chwajol M, et al. Robotic intercostal nerve graft for reversal of thoracic sympathectomy: a large animal feasibility model. Int J Med Robot (2008) 4(3):258–62. doi:10.1002/rcs.205
35. Nectoux E, Taleb C, Liverneaux P. Nerve repair in telemicrosurgery: an experimental study. J Reconstr Microsurg (2009) 25:261–5. doi:10.1055/s-0028-1104562
36. Mantovani G, Liverneaux P, Garcia JC Jr, Berner SH, Bednar MS, Mohr CJ. Endoscopic exploration and repair of brachial plexus with telerobotic manipulation: a cadaver trial. J Neurosurg (2011) 115(3):659–64. doi:10.3171/2011.3.JNS10931
37. Garcia JC Jr, Lebailly F, Mantovani G, Mendonca LA, Garcia J, Liverneaux P. Telerobotic manipulation of the brachial plexus. J Reconstr Microsurg (2012) 28:491–4. doi:10.1055/s-0032-1313761
38. de Melo PP, Garcia JC, de Souza Montero EF, Atik T, Robert EG, Facca S, et al. Feasibility of an endoscopic approach to the axillary nerve and the nerve to the long head of the triceps brachii with the help of the Da Vinci Robot. Chir Main (2013) 32(4):206–9. doi:10.1016/j.main.2013.05.003
39. Facca S, Hendriks S, Mantovani G, Selber JC, Liverneaux P. Robot- assisted surgery of the shoulder girdle and brachial plexus. Semin Plast Surg (2014) 28:39–44. doi:10.1055/s-0034-1368167
40. Porto de Melo P, Miyamoto H, Serradori T, Ruggiero Mantovani G, Selber J, Facca S, et al. Robotic phrenic nerve harvest: a feasibility study in a pig model. Chir Main (2014) 33:356–60. doi:10.1016/j.main.2014.07.006
41. Miyamoto H, Serradori T, Mikami Y, Selber J, Santelmo N, Facca S, et al. Robotic intercostal nerve harvest: a feasibility study in a pig model. J Neurosurg (2016) 124(1):264–8. doi:10.3171/2015.1.JNS14603
42. Latif MJ, Park K, Razi SS, Bhora F, Todd G, Perin N, et al. Robotic microsurgical nerve grafting for sympathectomy reversal: technique and feasibility for first human case. Int J Med Robot Comput Assist Surg (2011) 7:27.
43. Coveliers H, Meyer M, Gharagozloo F, Wisselink W, Rauwerda JM, Tempesta B, et al. Robotic selective postganglionic thoracic sympathectomy for the treatment of hyperhidrosis. Ann Thorac Surg (2013) 95(1):269–74. doi:10.1016/j.athoracsur.2012.08.013
44. Naito K, Facca S, Lequint T, Liverneaux PA. The Oberlin procedure for restoration of elbow flexion with the da Vinci robot: four cases. Plast Reconstr Surg (2012) 129(3):707–11. doi:10.1097/PRS.0b013e318241287f
45. Berner S. Nerve repair. In: Liverneaux P, Berner S, Bednar M, Parekattil S, Mantovani Ruggiero G, Selber J, editors. Telemicrosurgery. Paris: Springer (2013) p. 119–22.
46. Tigan L, Miyamoto H, Hendriks S, Facca S, Liverneaux P. Interest of telemicrosurgery in peripheral nerve tumors: about a series of seven cases. Chir Main (2014) 33(1):13–6. doi:10.1016/j.main.2013.10.177
47. Taleb C, Nectoux E, Liverneaux P. Limb replantation with two robots: a feasibility study in a pig model. Microsurgery (2009) 29(3):232–5. doi:10.1002/micr.20602
48. Huart A, Facca S, Lebailly F, Garcia JC, Liverneaux PA. Are pedicled flaps feasible in robotic surgery? Report of an anatomical study of the kite flap in conventional surgery versus robotic surgery. Surg Innov (2012) 19(1):89–92. doi:10.1177/1553350611415869
49. Maire N, Naito K, Lequint T, Facca S, Berner S, Liverneaux P. Robot-assisted free toe pulp transfer: feasibility study. J Reconstr Microsurg (2012) 28:481–4. doi:10.1055/s-0032-1313760
50. Facca S, Liverneaux P. Robotic assisted microsurgery in hypothenar hammer syndrome: a case report. Comput Aided Surg (2010) 15(4–6):110–4. doi:10.3109/10929088.2010.507942
51. Hockstein NG, Nolan JP, O’Malley BW, Woo YJ. Robotic microlaryngeal surgery: a technical feasibility study using the daVinci surgical robot and an airway mannequin. Laryngoscope (2005) 115:780–5. doi:10.1097/01.MLG.0000159202.04941.67
52. Weinstein GS, O’Malley BW, Desai SC, Quon H. Transoral robotic surgery: does the ends justify the means? Curr Opin Otolaryngol Head Neck Surg (2009) 17:126–31. doi:10.1097/MOO.0b013e32832924f5
53. Weinstein GS, O’Malley BW, Snyder W, Sherman E, Quon H. Transoral robotic surgery: radical tonsillectomy. Arch Otolaryngol Head Neck Surg (2007) 133:1220–6. doi:10.1001/archotol.133.12.1220
54. Selber JC, Robb G, Serletti JM, Weinstein G, Weber R, Holsinger FC. Transoral robotic free flap reconstruction of oropharyngeal defects: a preclinical investigation. Plast Reconstr Surg (2010) 125(3):896–900. doi:10.1097/01.prs.0000371802.58729.30
55. Smartt JM Jr, Gerety P, Serletti JM, Taylor JA. Application of a robotic telemanipulator to perform posterior pharyngeal flap surgery: a feasibility study. Plast Reconstr Surg (2013) 131(4):841–5. doi:10.1097/PRS.0b013e318282761b
56. Desai SC, Sung CK, Jang DW, Genden EM. Transoral robotic surgery using a carbon dioxide flexible laser for tumors of the upper aerodigestive tract. Laryngoscope (2008) 118(12):2187–9. doi:10.1097/MLG.0b013e31818379e4
57. Mukhija VK, Sung CK, Desai SC, Wanna G, Genden EM. Transoral robotic assisted free flap reconstruction. Otolaryngol Head Neck Surg (2009) 140(1):124–5. doi:10.1016/j.otohns.2008.09.024
58. Selber JC. Transoral robotic reconstruction of oropharyngeal defects: a case series. Plast Reconstr Surg (2010) 126:1978–87. doi:10.1097/PRS.0b013e3181f448e3
59. Garfein ES, Greaney PJ, Easterlin B, Schiff B, Smith RV. Transoral robotic reconstructive surgery reconstruction of a tongue base defect with a radial forearm flap. Plast Reconstr Surg (2011) 127:2352–4. doi:10.1097/PRS.0b013e318213a0e4
60. Genden EM, Kotz T, Tong CC, Smith C, Sikora AG, Teng MS, et al. Transoral robotic resection and reconstruction for head and neck cancer. Laryngoscope (2011) 121(8):1668–74. doi:10.1002/lary.21845
61. Genden EM, Park R, Smith C, Kotz T. The role of reconstruction for transoral robotic pharyngectomy and concomitant neck dissection. Arch Otolaryngol Head Neck Surg (2011) 137:151–6. doi:10.1001/archoto.2010.250
62. Bonawitz SC, Duvvuri U. Robot-assisted oropharyngeal reconstruction with free tissue transfer. J Reconstr Microsurg (2012) 28:485–90. doi:10.1055/s-0032-1313758
63. Longfield EA, Holsinger FC, Selber JC. Reconstruction after robotic head and neck surgery: when and why. J Reconstr Microsurg (2012) 28(7):445–50. doi:10.1055/s-0032-1306376
64. Bonawitz SC, Duvvuri U. Robotic-assisted oropharyngeal reconstruction with local flaps. Oper Tech Otolaryngol (2013) 24:115–9. doi:10.1016/j.otot.2013.04.001
65. Bonawitz SC, Duvvuri U. Robotic-assisted FAMM flap for soft palate reconstruction. Laryngoscope (2013) 123:870–4. doi:10.1002/lary.23578
66. Duvvuri U, Bonawitz SC, Kim S. Robotic-assisted oropharyngeal reconstruction. J Robot Surg (2013) 7:9–14. doi:10.1007/s11701-011-0326-z
67. Hans S, Jouffroy T, Veivers D, Hoffman C, Girod A, Badoual C, et al. Transoral robotic-assisted free flap reconstruction after radiation therapy in hypopharyngeal carcinoma: report of two cases. Eur Arch Otorhinolaryngol (2013) 270(8):2359–64. doi:10.1007/s00405-013-2566-1
68. Park YM, Lee WJ, Yun IS, Lee DW, Lew DH, Lee JM, et al. Free flap reconstruction after robot-assisted neck dissection via a modified face-lift or retroauricular approach. Ann Surg Oncol (2013) 20(3):891–8. doi:10.1245/s10434-012-2731-6
69. Song HG, Yun IS, Lee WJ, Lew DH, Rah DK. Robot-assisted free flap in head and neck reconstruction. Arch Plast Surg (2013) 40:353–8. doi:10.5999/aps.2013.40.4.353
70. de Almeida JR, Park RC, Villanueva NL, Miles BA, Teng MS, Genden EM. Reconstructive algorithm and classification system for transoral oropharyngeal defects. Head Neck (2014) 36(7):934–41. doi:10.1002/hed.23353
71. Byeon HK, Holsinger FC, Kim DH, Kim JW, Park JH, Koh YW, et al. Feasibility of robot-assisted neck dissection followed by transoral robotic surgery. Br J Oral Maxillofac Surg (2015) 53:68–73. doi:10.1016/j.bjoms.2014.09.024
72. Perrenot C, Berengère P, Mastronicola R, Gangloff P, Dolivet G. Infrahyoid myocutaneous flap for reconstruction after robotic transoral surgery for oropharyngeal tumors. Plast Reconstr Surg (2014) 133(2):236.e–7.e. doi:10.1097/01.prs.0000437236.07930.fd
73. Lai CS, Chen IC, Liu SA, Lu CT, Yen JH, Song DY. Robot-assisted free flap reconstruction of oropharyngeal cancer—a preliminary report. Ann Plast Surg (2015) 74:S105–8. doi:10.1097/SAP.0000000000000464
74. Meccariello G, Montevecchi F, Deganello A, D’Agostino G, Bellini C, Zeccardo E, et al. The temporalis muscle flap for reconstruction of soft palate and lateral oropharyngeal wall after transoral robotic surgery. Auris Nasus Larynx (2016). doi:10.1016/j.anl.2016.11.011
75. Gorphe P, Von Tan J, El Bedoui S, Hartl DM, Auperin A, Qassemyar Q, et al. Early assessment of feasibility and technical specificities of transoral robotic surgery using the da Vinci Xi. J Robotic Surg (2017). doi:10.1007/s11701-017-0679-z
76. Biron VL, O’Connell DA, Barber B, Clark JM, Andrews C, Jeffery CC, et al. Transoral robotic surgery with radial forearm free flap reconstruction: case control analysis. J Otolaryngol Head Neck Surg (2017) 46(1):20. doi:10.1186/s40463-017-0196-0
77. Khan K, Dobbs T, Swan MC, Weinstein GS, Goodacre TEE. Trans-oral robotic cleft surgery (TORCS) for palate and posterior pharyngeal wall reconstruction: a feasibility study. J Plast Reconstr Aesthet Surg (2016) 69(1):97–100. doi:10.1016/j.bjps.2015.08.020
78. Podolsky DJ, Fisher DM, Riff KWW, Looi T, Drake JM, Forrest CR. Infant robotic cleft palate surgery: a feasibility assessment using a realistic cleft palate simulator. Plast Reconstr Surg (2017) 139(2):455.e–65.e. doi:10.1097/PRS.0000000000003010
79. Nadjmi N. Transoral robotic cleft palate surgery. Cleft Palate Craniofac J (2015) 53(3):326–31. doi:10.1597/14-077
80. Dombre E, Duchemin G, Poignet P. Dermarob: a safe robot for reconstructive surgery. IEEE Trans Robot Autom (2003) 19:876–84. doi:10.1109/TRA.2003.817067
81. Taghizadeh F, Reiley C, Mohr C, Paul M. Evaluation of robotic- assisted platysmaplasty procedures in a cadaveric model using the da Vinci Surgical System. J Robot Surg (2014) 8(1):63–71. doi:10.1007/s11701-013-0431-2
82. Shi Y, Lin L, Zhou C, Zhu M, Xie L, Chai G. A study of an assisting robot for mandible plastic surgery based on augmented reality. Minim Invasive Ther Allied Technol (2017) 26(1):23–30. doi:10.1080/13645706.2016.1216864
83. Ciudad P, Date S, Lee MH, Lo Torto F, Nicoli F, Araki J, et al. Robotic harvest of a right gastroepiploic lymph node flap. Arch Plast Surg (2016) 43(2):210–2. doi:10.5999/aps.2016.43.2.210
85. Ponnusamy K, Mohr C, Curet MJ. Clinical outcomes with robotic surgery. Curr Probl Surg (2011) 48(9):577–656. doi:10.1067/j.cpsurg.2011.05.002
86. Rudmik L, An W, Livingstone D, Matthews W, Seikaly H, Scrimger R, et al. Making a case for high-volume robotic surgery centers: a cost-effectiveness analysis of transoral robotic surgery. J Surg Oncol (2015) 112(2):155–63. doi:10.1002/jso.23974
87. Ind TEJ, Marshall C, Hacking M, Harris M, Bishop L, Barton D, et al. Introducing robotic surgery into an endometrial cancer service–a prospective evaluation of clinical and economic outcomes in a UK institution. Int J Med Robot Comp (2016) 12(1):137–44. doi:10.1002/rcs.1651
88. Koehn JK, Kuchenbecker KJ. Surgeons and non-surgeons prefer haptic feedback of instrument vibrations during robotic surgery. Surg Endosc (2015) 29(10):2970–83. doi:10.1007/s00464-014-4030-8
89. Hagen ME, Meehan JJ, Inan I, Morel P. Visual clues act as a substitute for haptic feedback in robotic surgery. Surg Endosc (2008) 22(6):1505–8. doi:10.1007/s00464-007-9683-0
90. Okamura AM. Haptic feedback in robot-assisted minimally invasive surgery. Curr Opin Urol (2009) 19(1):102–7. doi:10.1097/MOU.0b013e32831a478c
Keywords: robotic surgery, plastic surgery, microsurgery, head and neck, technology, innovation
Citation: Dobbs TD, Cundy O, Samarendra H, Khan K and Whitaker IS (2017) A Systematic Review of the Role of Robotics in Plastic and Reconstructive Surgery—From Inception to the Future. Front. Surg. 4:66. doi: 10.3389/fsurg.2017.00066
Received: 29 September 2017; Accepted: 01 November 2017;
Published: 15 November 2017
Edited by:
Vincenzo Neri, University of Foggia, ItalyReviewed by:
David J. Hunter-Smith, Monash University Plastic and Reconstructive Surgery Group, AustraliaOren Lapid, Academic Medical Center (AMC), Netherlands
Jeffrey B. Friedrich, University of Washington Tacoma, United States
Copyright: © 2017 Dobbs, Cundy, Samarendra, Khan and Whitaker. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thomas D. Dobbs, dG9tZG9iYnNAZG9jdG9ycy5vcmcudWs=
 Thomas D. Dobbs
Thomas D. Dobbs Olivia Cundy3
Olivia Cundy3 Harsh Samarendra
Harsh Samarendra Iain Stuart Whitaker
Iain Stuart Whitaker