- 1Department of Colorectal Surgery, School of Medicine, Xinhua Hospital, Shanghai Jiaotong University, Shanghai, China
- 2Department of Colorectal and Anal Surgery, Hubei Key Laboratory of Intestinal and Colorectal Diseases, Zhongnan Hospital of Wuhan University, Wuhan, China
Background: Pouchitis is the most common long-term complication after ileal pouch–anal anastomosis (IPAA) in patients with ulcerative colitis (UC). Ulcerative colitis endoscopic index of severity (UCEIS) and Mayo endoscopic score (MES) are widely used indices to evaluate endoscopic activity. This study aimed to clarify the predictive value of preoperative endoscopic activity on the occurrence of pouchitis after IPAA.
Methods: Data of patients with UC who underwent IPAA from January 2008 to January 2020 were collected retrospectively. UCEIS and MES were based on the preoperative colonoscopy findings of two independent endoscopists.
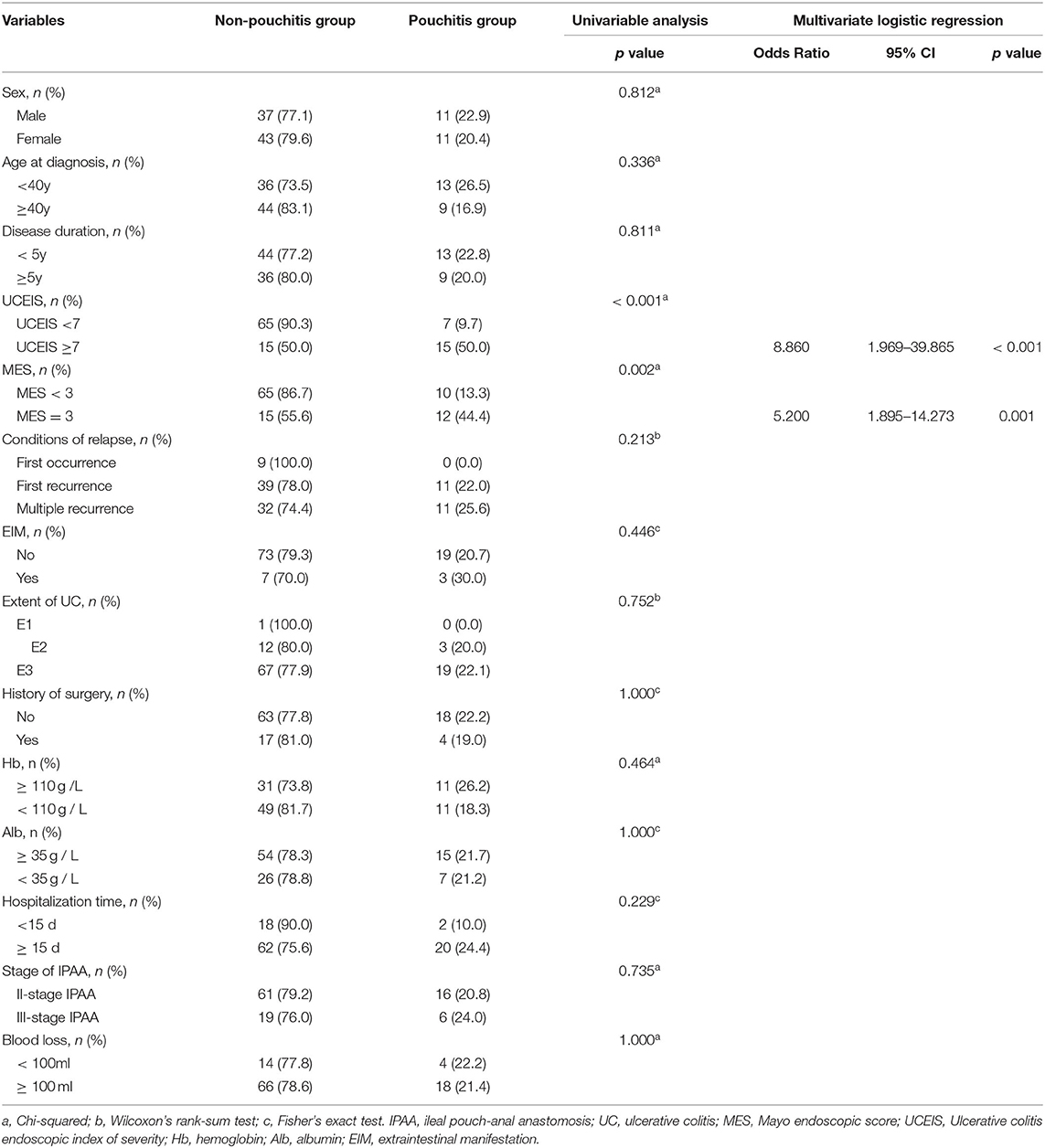
Results: A total of 102 patients with a median follow-up of 5 (interquartile range, 2–9) years were included in the study. Among them, 21.6% developed pouchitis. Compared with MES, UCEIS had a stronger correlation with pouchitis disease activity index. UCEIS ≥ 7 had the most significant receiver-operating characteristic (ROC) curve area of 0.747 with a sensitivity of 68.2% and specificity of 81.2% in predicting pouchitis, which outperformed MES of 3 with an ROC area of 0.679 with a sensitivity of 54.5% and specificity of 81.2%. Furthermore, we found that UCEIS ≥ 7 was an independent risk factor for post-IPAA pouchitis [odds ratio (OR), 8.860; 95% CI, 1.969–39.865, p < 0.001] with a higher risk than MES of 3 (OR, 5.200; 95% CI, 1.895–14.273; p = 0.001).
Conclusion: Ulcerative colitis endoscopic index of severity performed better in predicting pouchitis after IPAA than MES. Earlier and more frequent postoperative colonoscopic surveillance should be considered in patients with preoperative UCEIS ≥ 7 to detect the occurrence of pouchitis earlier.
Introduction
Ulcerative colitis (UC) is a chronic intestinal inflammatory disease, which is characterized by recurrent relapse and remission (1). Total proctocolectomy with ileal pouch–anal anastomosis (IPAA), first proposed in 1978 (2), involved the construction of a “J” pouch to maintain the continuity of the intestine and prevent permanent ileostomy. IPAA was considered as a radical surgery for patients with UC (2).
Although IPAA can significantly improve the long-term quality of life (3–5), the postoperative complications are inevitable. Pouchitis is the most common late complication of IPAA (6, 7), which seriously compromises the prognosis of the patients. Moreover, the etiology of pouchitis remains unclear (8). The pouchitis disease activity index (PDAI), revised in 1994 (9), is widely used in clinical practice to assess the clinical manifestation, endoscopic examination, and histologic findings of the pouch (10). Exploring appropriate indicators to predict the occurrence of post-IPAA pouchitis has become increasingly important.
Preoperative colonoscopy is necessary for each patient to evaluate endoscopic activity. However, data on the predictive value of the two most commonly used indices, namely, ulcerative colitis endoscopic index of severity (UCEIS) and the Mayo endoscopic score (MES) are scarce (11–13). Thus, we aimed to clarify the predictive value of preoperative endoscopic activity on the occurrence of pouchitis after IPAA. We performed this study to discover the incidence of post-IPAA pouchitis in our institute. Then, we compared the predictive values of UCEIS and MES for pouchitis to establish the best cut-off indicators for early colonoscopic surveillance after IPAA to predict the occurrence of pouchitis.
Methods
Patients
Some consecutive patients with UC who received long-term and regular medical treatment from January 2008 to January 2020 at our inflammatory bowel disease (IBD) surgery centers (Department of Colorectal Surgery, Xinhua Hospital, Shanghai Jiaotong University School of Medicine and Department of Colorectal and Anal Surgery, Zhongnan Hospital of Wuhan University) and IBD-medicine treatment center (Department of Gastroenterology and Rui-Jin Hospital, Shanghai Jiao Tong University School of Medicine) were considered to participate in this study. All eligible patients with UC who underwent IPAA in our IBD surgery centers or had regular follow-up after IPAA in our IBD medicine treatment center were ultimately enrolled in this study.
The baseline characteristics of patients, medical history, examination data, surgical information, preoperative endoscopic activity, and post-IPAA complications were retrospectively collected from medical records of hospital, outpatient examination, long-term regular follow-up, and the prospectively maintained, institutional review board-approved pouch database (3).
Inclusion and Exclusion Criteria
Patients older than 18 years who had received standard IPAA with pouch construction, had regular follow-up, and had complete clinical data were included in the study.
Patients who developed complications following symptomatic surgery such as subtotal colectomy with temporary or permanent ileostomy without pouch construction, those diagnosed with Crohn's disease and familial adenomatous polyposis, and those lost to follow-up or with incomplete clinical data were excluded.
UCEIS and MES Evaluation
In our institution, hospitalized patients with UC routinely undergo a colonoscopy before surgery. The UCEIS and MES were recorded by two independent and experienced endoscopists who received formal and appropriate training to evaluate endoscopic activity. When the endoscopic activity of the same affected area was inconsistent, the higher value was used in the subsequent analysis. All UCEIS and MES records were collected form the pre-IPAA colonoscopy. As reported in the previous studies, the UCEIS comprises three scoring criteria, namely, vascular pattern (0–2), bleeding (0–3), and erosions and ulcers (0–3) (13). Similarly, MES ranges from 0–3 based on the endoscopic findings (14).
Clinical Evaluation
The primary outcome was the occurrence of pouchitis after IPAA. The diagnosis post-IPAA was evaluated based on a PDAI score ≥7. UC and UC-associated malignant transformation were dependent on the final pathological results. The extent of UC was also divided into proctitis (E1), left-sided colitis (E2), and pancolitis (E3) according to the Montreal classification system (15). The use of mesalamine, biologics, steroids, and immunomodulators and the levels of hemoglobin (Hb) and albumin (Alb) were recorded preoperatively. In this study, early and late post-IPAA complications were defined by the cut-off of 1 month after pouch surgery.
Statistical Analysis
SPSS version 19.0 software (IBM 2010, Chicago, Illinois, USA) and GraphPad Prism 5 Software (San Diego, California, USA) were used for statistical analysis and creation of figures. Univariate analyses using the Chi-squared, Fisher's exact and Wilcoxon's rank-sum tests were performed for different variables. Multivariate logistic regression to determine the independent risk factor for post-IPAA pouchitis was performed. Pearson's correlation test was used to explore the relationship among UCEIS, MES, and PDAI. Receiver-operating characteristic (ROC) curve analysis and Kaplan–Meier method with the log-rank test were also performed to analyze the predictive value and pouchitis-free overall rate in UCEIS and MES, respectively. In this study, we considered a p < 0.05 significant with two-sided test and CI set at 95%.
Ethical Considerations
The Ethics Committee has reviewed the study protocol and process as well as the application form. We have certified that this study did not raise any issues of the risk of patients. Moreover, the study was conducted in accordance with the Declaration of Helsinki and was free of ethical problems. The Ethics Committee of Xinhua Hospital approved this study (Approval No. XHEC-D-2020-107).
Results
Main Characteristics of the Patients
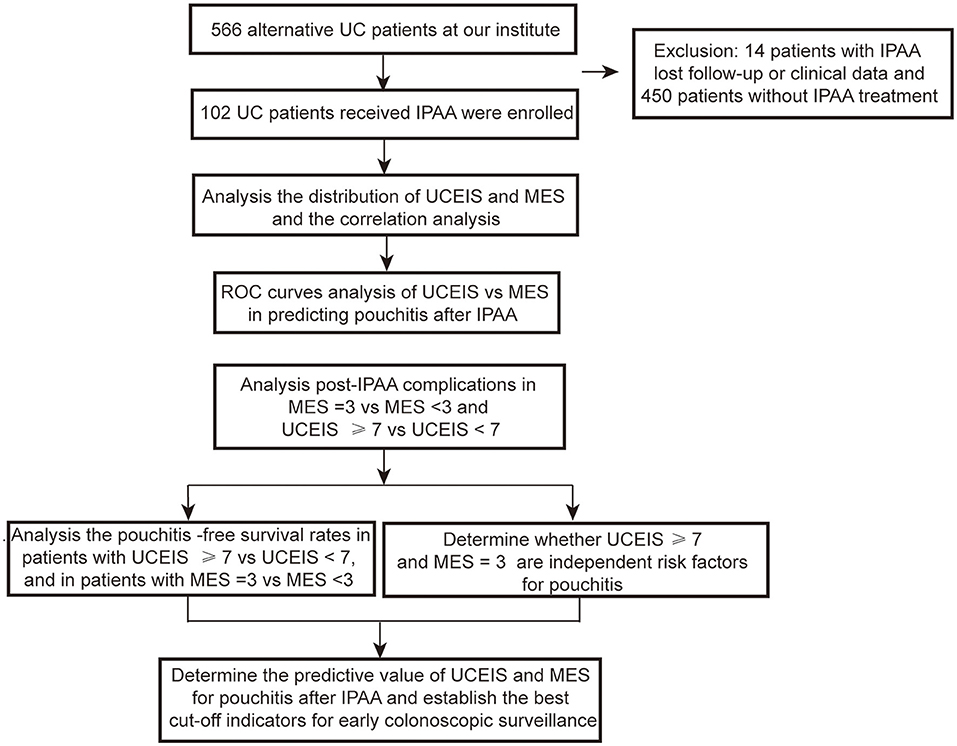
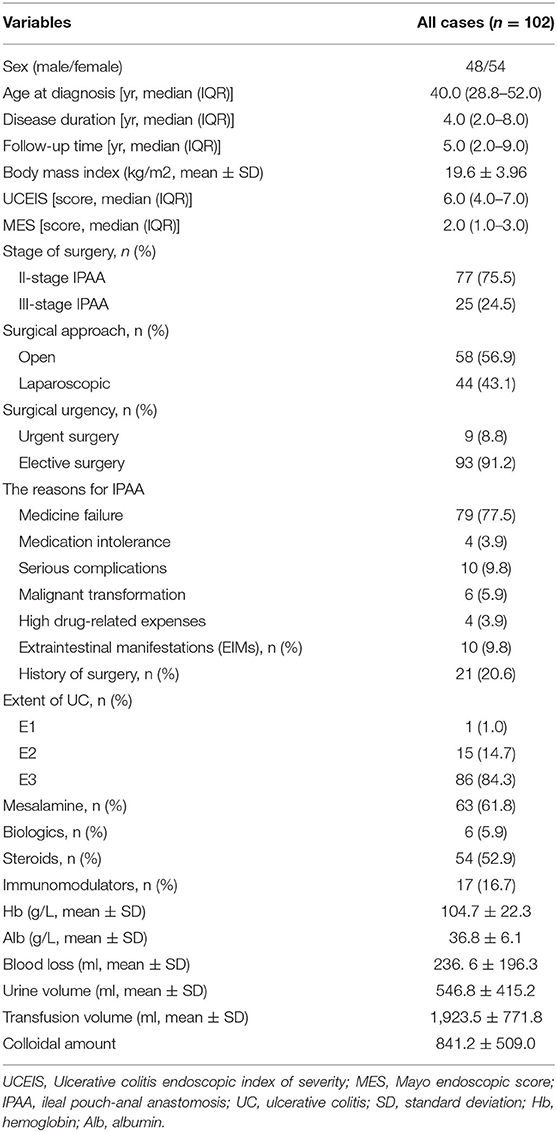
A total of 566 patients with UC were treated at our institute. Among these patients, 450 patients underwent medicine treatment and 14 patients had IPAA without complete follow-up or clinical data. Thus, 102 (18.2%) patients with UC who received IPAA intervene were ultimately enrolled in this study. The flow diagram is shown in Figure 1. The median age at diagnosis was 40.0 years [interquartile range (IQR): 28.8–52.0) years with a median follow-up time of 5.0 (IQR: 2.0–9.0) years]. Among the patients, 1 (1.0%) patient was diagnosed as proctitis, 15 (14.7%) experienced left-sided colitis, and 86 (84.3%) developed pancolitis. In addition, 10 (9.8%) patients developed extraintestinal manifestation (EIM) during the disease course. The median UCEIS and MES of the patients were 6.0 (IQR: 4.0–7.0) and 2.0 (IQR: 1.0–3.0), respectively (Table 1). We further analyzed the number of patients with differing UCEIS and MES (Figures 2A,B) and the relationship between UCEIS and MES. As shown in Figure 2C, the association of UCEIS with MES was significant (R = 0.7849, p < 0.0001).
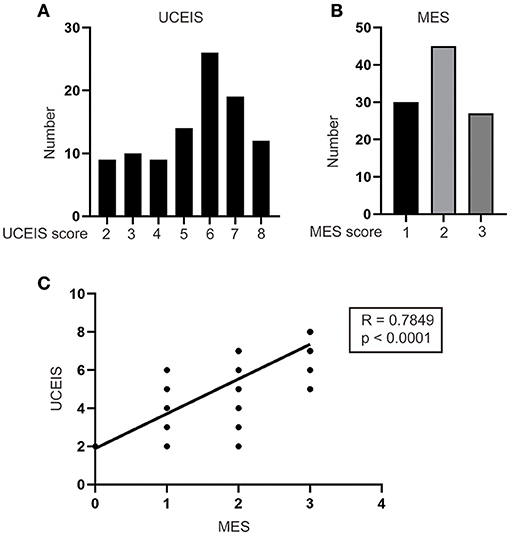
Figure 2. Analysis of the distribution and correlation of ulcerative colitis endoscopic index of severity (UCEIS) and Mayo endoscopic score (MES). The number of patients with different (A) UCEIS and (B) MES were analyzed. (C) Significant correlations existed between the UCEIS and MES (R = 0.7849, p < 0.0001).
Analysis of the Postoperative Complications of IPAA
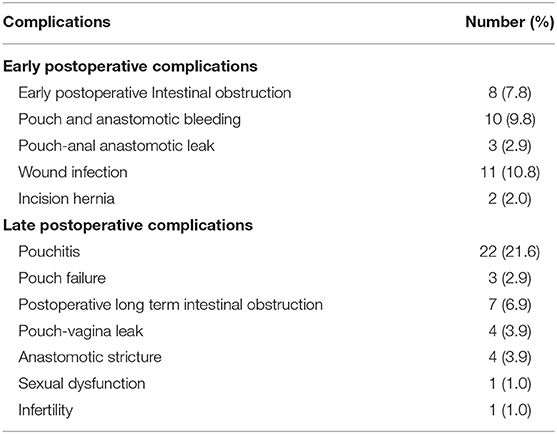
A total of 34 (33.3%) patients presented with late postoperative complications. Of these patients, 22 (21.6%) developed pouchitis, 3 (2.9%) developed pouch failure, 7 (6.9%) experienced postoperative long-term intestinal obstruction, 4 (3.9%) had pouch–vagina leak, 4 (3.9%) developed anastomotic stricture, 1 (1.0%) had sexual dysfunction and 1 (1.0%) was diagnosed with infertility (Table 2). This demonstrated that pouchitis was the most common late complication of IPAA in this study.
Analysis of the Relationship Among UCEIS, MES, and PDAI
To determine whether UCEIS or MES was associated with the PDAI, Pearson's correlation test was performed. As shown in Figures 3A,B, UCEIS demonstrated a stronger correlation with PDAI than MES (UCEIS: R = 0.6595, p = 0.0045 vs. MES: R = 0.5492, p = 0.0081).
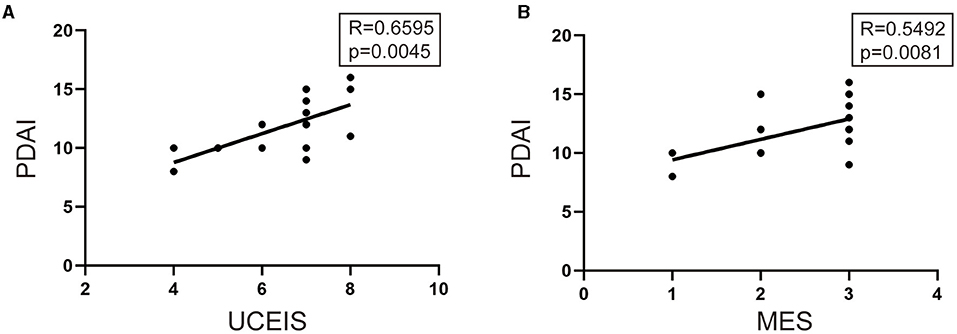
Figure 3. Pearson's correlation test was performed to analyze the relationship among UCEIS, MES, and pouchitis disease activity index (PDAI). (A) Significant correlations existed between the UCEIS and PDIA (R = 0.6595, p = 0.0045) and between (B) MES and PDAI (R = 0.5492, p = 0.0081).
UCEIS and MES Threshold Evaluation for Predicting Pouchitis After IPAA
To establish the best threshold value of UCEIS and MES for the indication of pouchitis, the ROC curve analysis was performed. As shown in Figure 4, UCEIS had the most significant area under the ROC curve (AUC) of 0.747 with a sensitivity of 68.2% and specificity of 81.2% at the cut-off value of 7 (p < 0.001), while MES of three had the biggest AUC of 0.679 with sensitivity of 54.5% and specificity 81.2% (p = 0.001). This indicates that UCEIS has a better predictive value for pouchitis than MES.
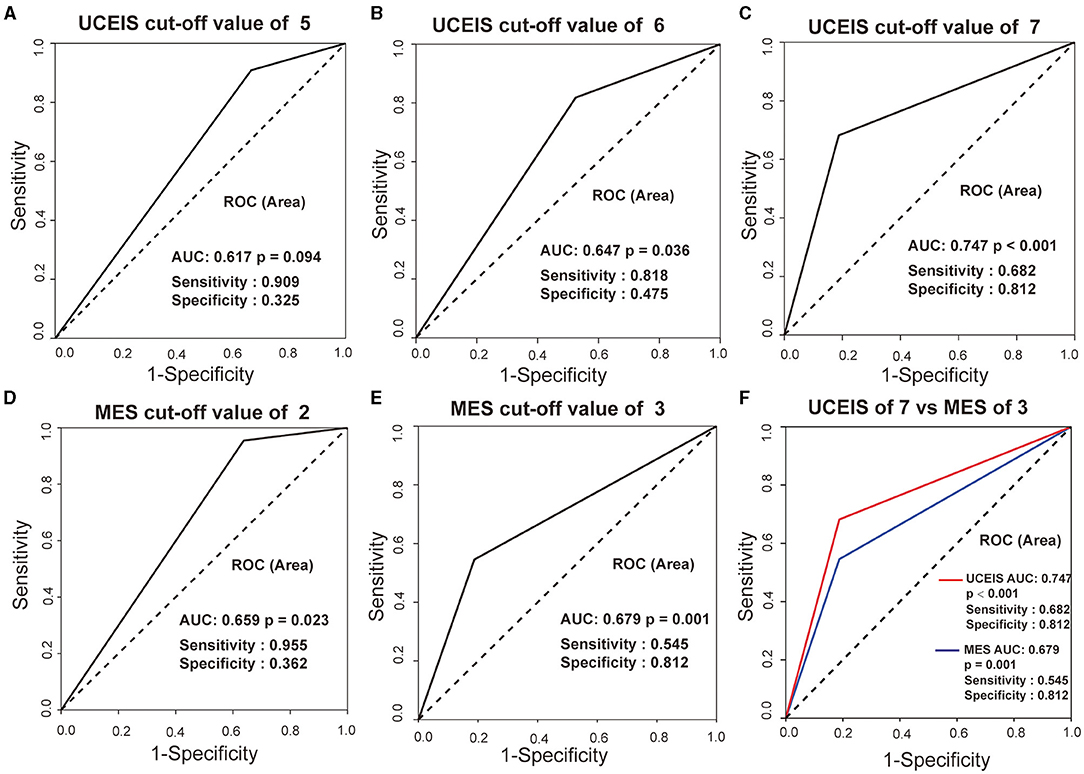
Figure 4. Receiver-operating characteristic (ROC) curves of UCEIS and MES in predicting pouchitis in patients with ulcerative colitis (UC). UCEIS had the most significant AUC of 0.747 with a sensitivity of 68.2%, and a specificity of 81.2% with a cut-off value of 7 (A–C, F), while MES = 3 had an AUC of 0.679 with a sensitivity of 54.5% and specificity of 81.2% (D, E, F).
Analysis of Post-IPAA Complications in Patients With Different MES and UCEIS
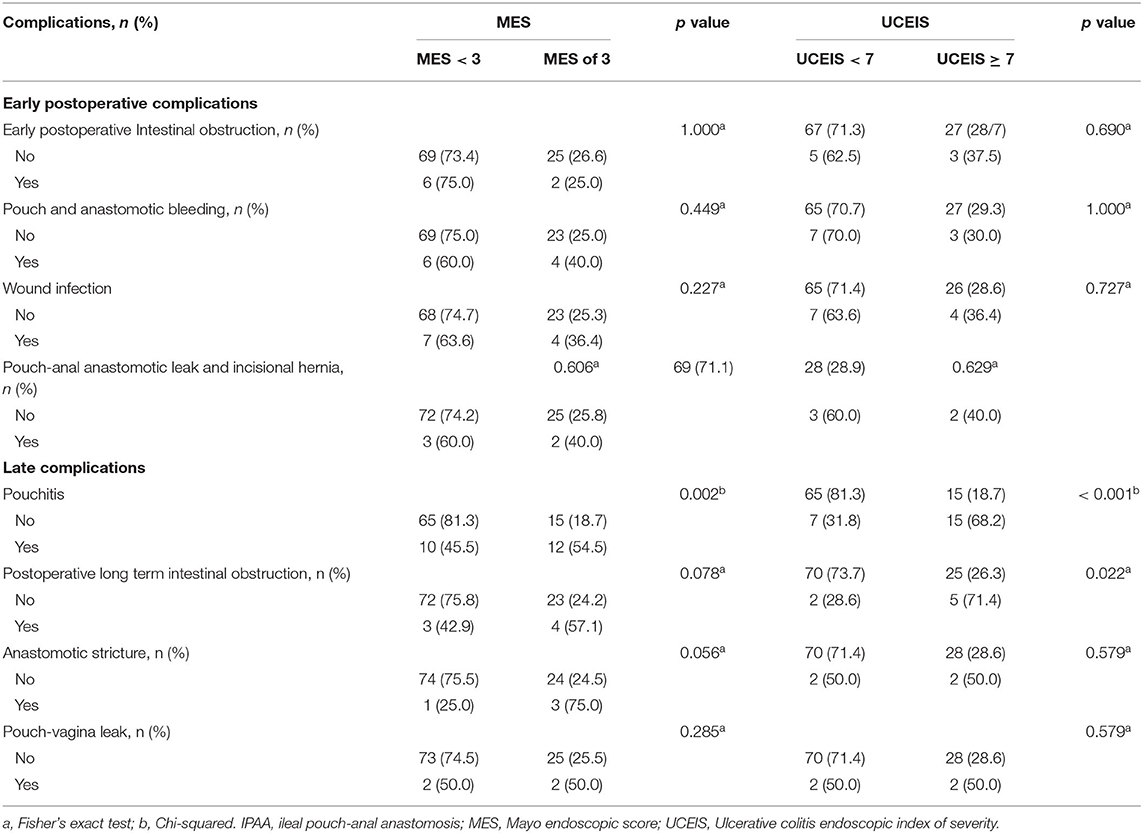
Based on the results of the ROC analysis, we have chosen UCEIS of seven and MES of three as cut-off values for further analysis of postoperative complications. We found that patients with MES = 3 were more likely to develop pouchitis than those with MES <3 (p = 0.002). We further found that patients with UCEIS ≥ 7 had a higher likelihood of developing postoperative pouchitis and long-term intestinal obstruction compared with those having UCEIS <7 (p < 0.001; p = 0.022) (Table 3).
UCEIS ≥ 7 and MES = 3 Were Independent Risk Factors for Pouchitis
Furthermore, we explored whether UCEIS ≥ 7 and MES = 3 were contributing factors to pouchitis. Univariate analysis revealed that UCEIS and MES were significantly associated with pouchitis (p < 0.001, p = 0.002) (Table 4). Multivariate logistic regression further demonstrated that UCEIS ≥ 7 and MES = 3 were both independent risk factors for pouchitis and patients with UCEIS ≥ 7 [odds ratio (OR), 8.860; 95% CI, 1.969–39.865, p < 0.001] had a higher risk of developing pouchitis than those with MES of 3 (OR, 5.200; 95% CI, 1.895–14.273; p = 0.001) (Table 4).
Moreover, we used the Kaplan–Meier method with the log-rank test to further compare the pouchitis-free survival rate in patients with different UCEIS and MES. As presented in Figures 5A,B, patients with UCEIS ≥ 7 and MES = 3 had significantly lower overall pouchitis-free survival than those with UCEIS <7 (p < 0.0001) and MES <3 (p = 0.002), respectively.
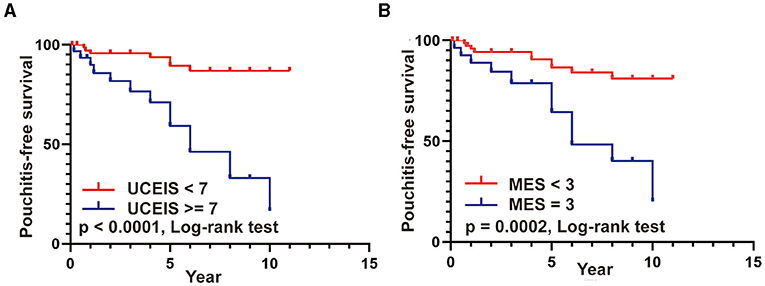
Figure 5. (A,B) Pouchitis-free survival rates in patients with UCEIS ≥ 7 vs. UCEIS <7 and MES = 3 vs. MES <3.
Discussion
The UCEIS and MES were widely used systems to assess the endoscopic severity of UC to allow for convenient and appropriate decision-making by clinicians. However, whether UCEIS and MES can predict the development of pouchitis after IPAA and the comparison between their predictive values are still unclear. First, our study indicated that pouchitis occurred in 21.6% of IPAA cases in our institute and then demonstrated the predictive value of preoperative endoscopic activity on the occurrence of pouchitis. Furthermore, we proved that pre-IPAA UCEIS ≥ 7 and MES = 3 were contributing factors to pouchitis and that UCEIS outperformed MES as a predictor of pouchitis. These findings indicated the need for earlier and more frequent colonoscopic surveillance of the pouch after IPAA when patients had preoperative UCEIS ≥ 7.
Many previous studies have reported the risk factors for pouchitis (16, 17). The most recent study reported that patients with longer disease duration were susceptible to development of pouchitis (18). Similarly, researchers found that the cumulative incidence rates of pouchitis at 1, 2, 3, 4, and 5 years after IPAA were 25, 32, 36, 40, and 45%, respectively (19). Moreover, researchers reported that patients with cholangitis are more likely to undergo pouchitis surgery, with an incidence rate of 79% in the 10th year after IPAA (20). In addition, pancolitis, EIM, use of non-steroidal anti-inflammatory drugs, and specific gut microbiota were contributing factors to pouchitis (21–23). However, whether UCEIS and MES were associated with pouchitis was not reported in the literature. To the best of our knowledge, this study is the first to demonstrate that patients with UCEIS ≥ 7 had a higher risk of developing pouchitis than those with MES = 3. Previous study reported that histological and asymptomatic pouchitis had higher preoperative white blood cell count (p < 0.001) (24). Kalkana et al. discovered the relationship between the preoperative severity and activity of the disease and subsequent pouchitis development. They found that a higher disease activity index (p = 0.02) was an independent risk factors for the development of pouchitis (25). Therefore, we first constructed the endoscopic prediction model of preoperative UCEIS ≥ 7, which could reflect degree of the preoperative inflammation to provide a more direct and convenient approach to predict post-IPAA pouchitis to some extent.
Exploring novel predictors for pouchitis to enable early intervention is important in enhancing the management of UC after IPAA. Several recent studies have focused on the predictive value of fecal calprotectin (FC) for pouchitis. Johnson et al. indicated that the concentration of FC in pouchitis was significantly higher than that in uninflamed pouches and was associated with PDAI. Moreover, an FC cut-off value of 92.5 μg/g demonstrated a satisfactory predictive value for pouchitis (26). In another prospective study in which the FC level had already been evaluated before the diagnosis of pouchitis, an FC cut-off value of 56 μg/g exhibited a sensitivity of 100% and a specificity of 84% in predicting pouchitis (27). Thus, FC appeared to be a non-invasive and effective predictor of post-IPAA (28). However, the optimal threshold value of FC in predicting pouchitis and the timing of retesting of FC after IPAA has not been unified. In addition, not all patients will be tested for FC after IPAA, but all patients who undergo IPAA surgery will be subjected to preoperative endoscopic assessment for UCEIS and MES. We herein first indicated that UCEIS ≥ 7 had a better predictive value than MES = 3. Taken together, UCEIS outperformed MES in predicting pouchitis. Preoperative evaluation of endoscopic severity to determine UCEIS score could help clinicians predict the occurrence of post-IPAA pouchitis and earlier and more frequent postoperative colonoscopic surveillance could be conducted to prevent the development of pouchitis.
Limitations of this study included the loss to follow-up, which is inevitable in a retrospective study. Our sample size was relatively small. Larger sample sizes are recommended for future research.
Conclusion
In this study, we discovered the incidence of post-IPAA pouchitis in our institute and demonstrated that the UCEIS performed better than MES in predicting post-IPAA pouchitis. We found that pre-IPAA UCEIS ≥ 7 had a significant association with PDAI and was an effective predictor of pouchitis. We further demonstrated that pre-IPAA UCEIS ≥ 7 was an independent risk factor for pouchitis with a higher risk than an MES = 3. Therefore, earlier and more frequent postoperative colonoscopic surveillance should be performed in patients with preoperative UCEIS ≥ 7 to prevent the occurrence of pouchitis and enhance its management.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by The Ethics Committee of Xinhua Hospital (Approval No. XHEC-D-2020-107). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
PD and ZD conceived the study. WX analyzed the data and wrote the manuscript. WT and WD assisted some analysis. HH, WC, QQ, and LC collaborated to collect the information of patients. All authors participated in revising the manuscript and approved the final version.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82000481 and 81873547), the Shanghai Sailing Program (No. 20YF1429400), and the Supporting Project of the Medical Science and Technology Innovation Platform of Health Commission of Hubei Province (PTXM2020011).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Marchioni Beery R, Kane S. Current approaches to the management of new-onset ulcerative colitis. Clin Exp Gastroenterol. (2014) 7:111–32. doi: 10.2147/CEG.S35942
2. Parks AG, Nicholls RJ. Proctocolectomy without ileostomy for ulcerative colitis. Br Med J. (1978) 2:85–8. doi: 10.1136/bmj.2.6130.85
3. Xu W, Ye H, Zhu Y, Ding W, Fu J, Cui L, et al. Long-term quality of life associated with early surgical complications in patients with ulcerative colitis after ileal pouch-anal anastomosis: a single-center retrospective study. Int J Surg. (2017) 48:174–9. doi: 10.1016/j.ijsu.2017.10.070
4. Fazio VW, O'Riordain MG, Lavery IC, Church JM, Lau P, Strong SA, et al. Long-term functional outcome and quality of life after stapled restorative proctocolectomy. Annals Surg. (1999) 230:575–84. doi: 10.1097/00000658-199910000-00013
5. Muir AJ, Edwards LJ, Sanders LL, Bollinger RR, Koruda MJ, Bachwich DR, et al. A prospective evaluation of health-related quality of life after ileal pouch anal anastomosis for ulcerative colitis. Am J Gastroenterol. (2001) 96:1480–5. doi: 10.1111/j.1572-0241.2001.03801.x
6. Mahadevan U, Sandborn WJ. Diagnosis and management of pouchitis. Gastroenterology. (2003) 124:1636–50. doi: 10.1016/S0016-5085(03)00325-1
7. Landy J, Al-Hassi HO, McLaughlin SD, Knight SC, Ciclitira PJ, Nicholls RJ, et al. Etiology of pouchitis. Inflamm Bowel Dis. (2012) 18:1146–55. doi: 10.1002/ibd.21911
8. Chowdhry S, Katz JA. Update on the pathogenesis and management of pouchitis. Curr Infect Dis Rep. (2014) 16:442. doi: 10.1007/s11908-014-0442-9
9. Sandborn WJ, Tremaine WJ, Batts KP, Pemberton JH, Phillips SF. Pouchitis after ileal pouch-anal anastomosis: a pouchitis disease activity index. Mayo Clinic proceedings. (1994) 69:409–15. doi: 10.1016/S0025-6196(12)61634-6
10. Shen B, Achkar JP, Lashner BA, Ormsby AH, Remzi FH, Bevins CL, et al. Endoscopic and histologic evaluation together with symptom assessment are required to diagnose pouchitis. Gastroenterology. (2001) 121:261–7. doi: 10.1053/gast.2001.26290
11. de Jong DC, Löwenberg M, Koumoutsos I, Ray S, Mawdsley J, Anderson S, et al. Validation and investigation of the operating characteristics of the ulcerative colitis endoscopic index of severity. Inflamm Bowel Dis. (2019) 25:937–44. doi: 10.1093/ibd/izy325
12. Travis SP, Schnell D, Krzeski P, Abreu MT, Altman DG, Colombel JF, et al. Developing an instrument to assess the endoscopic severity of ulcerative colitis: the ulcerative colitis endoscopic index of severity (UCEIS). Gut. (2012) 61:535–42. doi: 10.1136/gutjnl-2011-300486
13. Travis SP, Schnell D, Krzeski P, Abreu MT, Altman DG, Colombel JF, et al. Reliability and initial validation of the ulcerative colitis endoscopic index of severity. Gastroenterology. (2013) 145:987–95. doi: 10.1053/j.gastro.2013.07.024
14. Fukuda T, Naganuma M, Sugimoto S, Ono K, Nanki K, Mizuno S, et al. Efficacy of therapeutic intervention for patients with an ulcerative colitis mayo endoscopic score of 1. Inflamm Bowel Dis. (2019) 25:782–8. doi: 10.1093/ibd/izy300
15. Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a working party of the 2005 Montreal world congress of gastroenterology. Can J Gastroenterol. (2005) 19:5a−36a. doi: 10.1155/2005/269076
16. Gallo G, Kotze PG, Spinelli A. Surgery in ulcerative colitis: when? how? best practice & research. Clin Gastroenterol. 2018; 32-33: 71–8. doi: 10.1016/j.bpg.2018.05.017
17. Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. (2012) 380:1606–19. doi: 10.1016/S0140-6736(12)60150-0
18. Lavryk OA, Stocchi L, Hull TL, Lipman JM, Shawki S, Holubar SD, et al. Impact of preoperative duration of ulcerative colitis on long-term outcomes of restorative proctocolectomy. Int J Colorectal Dis. (2020) 35:41–9. doi: 10.1007/s00384-019-03449-1
19. Ferrante M, Declerck S, De Hertogh G, Van Assche G, Geboes K, Rutgeerts P, et al. Outcome after proctocolectomy with ileal pouch-anal anastomosis for ulcerative colitis. Inflamm Bowel Dis. (2008) 14:20–8. doi: 10.1002/ibd.20278
20. Penna C, Dozois R, Tremaine W, Sandborn W, LaRusso N, Schleck C, et al. Pouchitis after ileal pouch-anal anastomosis for ulcerative colitis occurs with increased frequency in patients with associated primary sclerosing cholangitis. Gut. (1996) 38:234–9. doi: 10.1136/gut.38.2.234
21. Shen B, Fazio VW, Remzi FH, Brzezinski A, Bennett AE, Lopez R, et al. Risk factors for diseases of ileal pouch-anal anastomosis after restorative proctocolectomy for ulcerative colitis. Clin Gastroenterol Hepatol. (2006) 4:81–9. doi: 10.1016/j.cgh.2005.10.004
22. van Assche G, Dignass A, Bokemeyer B, Danese S, Gionchetti P, Moser G, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis Part 3: special situations (Spanish version). Rev Gastroenterol Mex. (2015) 80:74–106. doi: 10.1016/j.rgmx.2014.10.008
23. Machiels K, Sabino J, Vandermosten L, Joossens M, Arijs I, de Bruyn M, et al. Specific members of the predominant gut microbiota predict pouchitis following colectomy and IPAA in UC. Gut. (2017) 66:79–88. doi: 10.1136/gutjnl-2015-309398
24. Lipman JM, Kiran RP, Shen B, Remzi F, Fazio VW. Perioperative factors during ileal pouch-anal anastomosis predict pouchitis. Dis Colon Rectum. (2011) 54:311–7. doi: 10.1007/DCR.0b013e3181fded4d
25. Kalkan IH, Dagli Ü, Önder FO, Tunç B, Öztaş E, Ülker A, et al. Evaluation of preoperative predictors of development of pouchitis after ileal-pouch-anastomosis in ulcerative colitis. Clin Res Hepatol Gastroenterol. (2012) 36:622–7. doi: 10.1016/j.clinre.2012.04.012
26. Johnson MW, Maestranzi S, Duffy AM, Dewar DH, Forbes A, Bjarnason I, et al. Faecal calprotectin: a noninvasive diagnostic tool and marker of severity in pouchitis. Eur J Gastroenterol Hepatol. (2008) 20:174–9. doi: 10.1097/MEG.0b013e3282f1c9a7
27. Yamamoto T, Shimoyama T, Bamba T, Matsumoto K. Consecutive monitoring of fecal calprotectin and lactoferrin for the early diagnosis and prediction of pouchitis after restorative proctocolectomy for ulcerative colitis. Am J Gastroenterol. (2015) 110:881–7. doi: 10.1038/ajg.2015.129
Keywords: ulcerative colitis, pouchitis, ulcerative colitis endoscopic index of severity, mayo endoscopic score, IPAA
Citation: Xu W, Tang W, Ding W, Hu H, Chen W, Qian Q, Cui L, Ding Z and Du P (2021) Preoperative Endoscopic Activity Predicts the Occurrence of Pouchitis After Ileal Pouch–Anal Anastomosis in Ulcerative Colitis: A Multicenter Retrospective Study in China. Front. Surg. 8:740349. doi: 10.3389/fsurg.2021.740349
Received: 12 July 2021; Accepted: 24 August 2021;
Published: 23 September 2021.
Edited by:
Gaetano Luglio, University of Naples Federico II, ItalyReviewed by:
Gianluca Pagano, University of Naples Federico II, ItalyGaetano Gallo, University of Catanzaro, Italy
Copyright © 2021 Xu, Tang, Ding, Hu, Chen, Qian, Cui, Ding and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhao Ding, ZGluZ3poYW9Ad2h1LmVkdS5jbg==; Peng Du, ZHVwZW5nQHhpbmh1YW1lZC5jb20uY24=
†These authors have contributed equally to this work
 Weimin Xu
Weimin Xu Wenbo Tang1†
Wenbo Tang1† Peng Du
Peng Du