- 1Department of Surgery, Minimally Invasive Surgery Unit, University of Rome “Tor Vergata”, Rome, Italy
- 2Department of Surgery, Unit of Oncologic and Minimally Invasive Surgery, Sapienza University of Rome, Rome, Italy
Background: Traditionally, synchronous liver resection (LR), cytoreductive surgery (CRS), and hyperthermic intraperitoneal chemotherapy for colorectal liver and peritoneal metastases have been contraindicated. Nowadays, clinical practice has promoted this aggressive treatment in selected cases. This study aimed to review surgical and survival results of an extensive surgical approach including CRS with hyperthermic intraperitoneal chemotherapy (HIPEC) and LR.
Methods: PubMed, EMBASE, and Web of Science databases were matched to find the available literature on this topic. The search period was limited to 10 years (January 2010–January 2021). A threshold of case series of 10 patients or more was applied.
Results: In the search period, out of 114 studies found about liver and peritoneal metastases from colorectal cancer, we found 18 papers matching the inclusion criteria. Higher morbidity and mortality were reported for patients who underwent such an extensive surgical approach when compared with patients who underwent only cytoreductive surgery and HIPEC. Also, survival rates seem worse in the former than in the latter.
Conclusion: The role of combined surgical strategy in patients with synchronous liver and peritoneal metastases from colorectal cancer remains controversial. Survival rates and morbidity and mortality seem not in favor of this option. A more accurate selection of patients and more restrictive surgical indications could perhaps help improve results in this subgroup of patients with limited curative options.
Introduction
Colorectal cancer (CRC) is a major health problem and is the leading cause of death in developed countries (1).
Metastatic diseases are present in approximately 20%–25% of patients with advanced CRC (2).
In patients with metastatic diseases from colorectal cancer, the liver and peritoneum are the most frequently affected sites; liver metastases (LM) are present in up to 55% of patients, while secondary peritoneal involvement (PM) affects up to 25% of patients (3–5).
Peritoneal carcinomatosis is considered a negative prognostic factor in metastatic colorectal cancer (6). Peritoneal carcinomatosis occurs when the tumor invades the bowel serosa, allowing malignant cells to shed and circulate through the peritoneal fluid. During surgery, iatrogenic manipulation may lead to tumor cells seeding within the peritoneal cavity; these tumor cells implant in the peritoneal microenvironment with blood vessels and lymphatics. Due to gravity and physiologic peritoneal fluid circulation, anatomical sites of the peritoneum that are most frequently affected include the upper abdominal regions such as the subphrenic regions, the lesser sac, bowel surfaces, mesentery, and in the pelvis. Tumor cell implantation leads to tumor plaque formation that may then involve extending to peritoneal surfaces (7, 8). The National Comprehensive Cancer Network (NCCN) guidelines recommend, in high-volume centers and for patients with limited peritoneal metastases [i.e., peritoneal cancer index—peritoneal cancer index (PCI) not more than 16–20, depending on different experiences], cytoreductive surgery (CRS) in association with hyperthermic intraperitoneal chemotherapy (HIPEC) (9).
Metastatic spread from the primary tumor to the liver occurs through hematogenous dissemination. The production of tumor growth factors induces the secretion of vascular endothelial growth factor that stimulates the generation of new endothelial cells through angiogenesis. Malignant cell dissemination happens from microscopic vessels to the portal venous system and liver sinusoids, which represent the suitable microenvironment for tumor growth (10).
Oligometastatic diseases with combined hepatic and peritoneal metastatic spread affect approximately 8% of those with CRC (6), especially the presence of peritoneal metastases associated with shorter overall survival (OS) (11). The prognosis of patients with isolated LM or isolated peritoneal metastases (PM) has improved with the combination of systemic chemotherapy and complete resection, yielding a 5-year overall survival rate of 40%–50% (12, 13). CRS with intraperitoneal chemotherapy, including HIPEC, has been considered a potentially curative treatment for PM of CRC, reaching a median OS of 31 months and up to 41 months in highly selected patients (14–16).
The best strategy to treat advanced colorectal cancer with synchronous peritoneal and liver metastases (PMLM) is unclear; in the past, this was considered a terminal condition, and these patients were referred to palliative care with systemic chemotherapy with a median survival of 12–24 months (17).
A change in the trend started in 2008 when patients with CRC with up to three or fewer small resectable parenchymal hepatic metastases, good performance status, and no major comorbidities could be considered as candidates for complete R0 resection of all tumors with CRS, liver resection (LR), and hyperthermic intraperitoneal chemotherapy (HIPEC) (18).
In recent years, smaller pilot series have shown, in highly selected patients, excellent median survival beyond 40 months in resections of simultaneous liver and peritoneal metastases with CRS plus HIPEC (16, 19–23). However, to date, no standard management has been established.
Moreover, the resectability rate in patients with unresectable or multiple hepatic metastases can be increased by approaching these cases with advanced procedures such as portal vein embolization or two-stage hepatectomy (24).
Optimizing patient selection with good performance status or with minimal comorbidity and accurate perioperative management is crucial to maximizing patient outcomes while minimizing morbidity and mortality. Variations in outcomes depend on the severity of the disease represented by the PCI, tumor differentiation, histologic findings, liver extension, and the completeness of cytoreduction (25, 26). Currently, centers demonstrate large heterogeneity in whether combining CRS–HIPEC with liver resection can offer beneficial results.
Given the contradicting data and the lack of standardized management for patients with simultaneous peritoneal and hepatic metastases from CRC, a thorough evaluation of the current literature is warranted to guide the correct strategy for these patients.
This study aimed to review surgical and survival results of an extensive surgical approach including CRS + HIPEC combined with LR in patients affected by peritoneal and hepatic metastases from CRC.
Methods
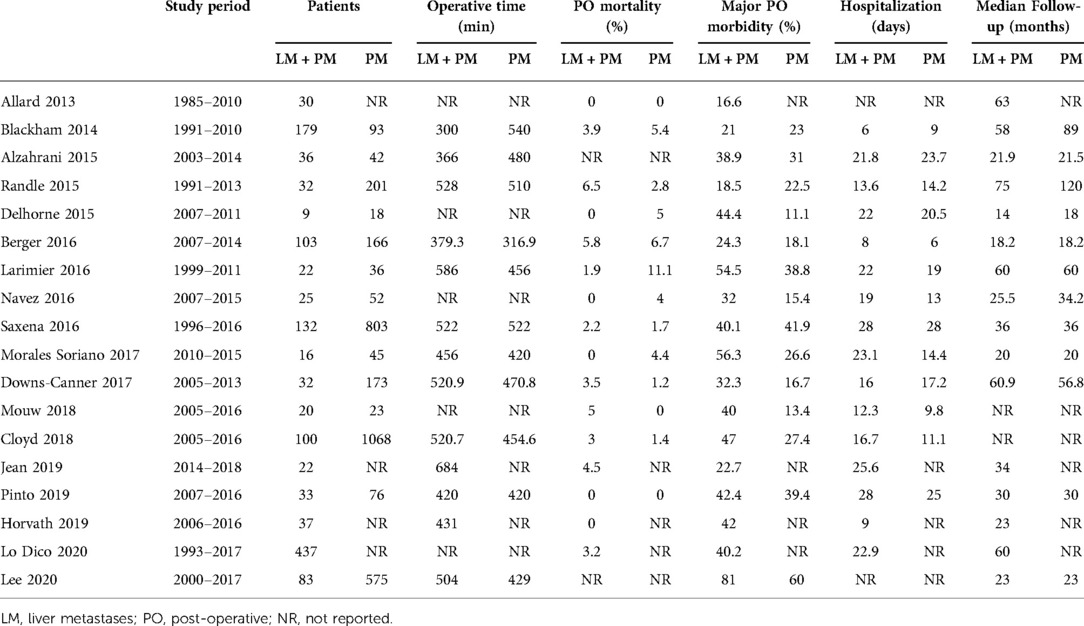
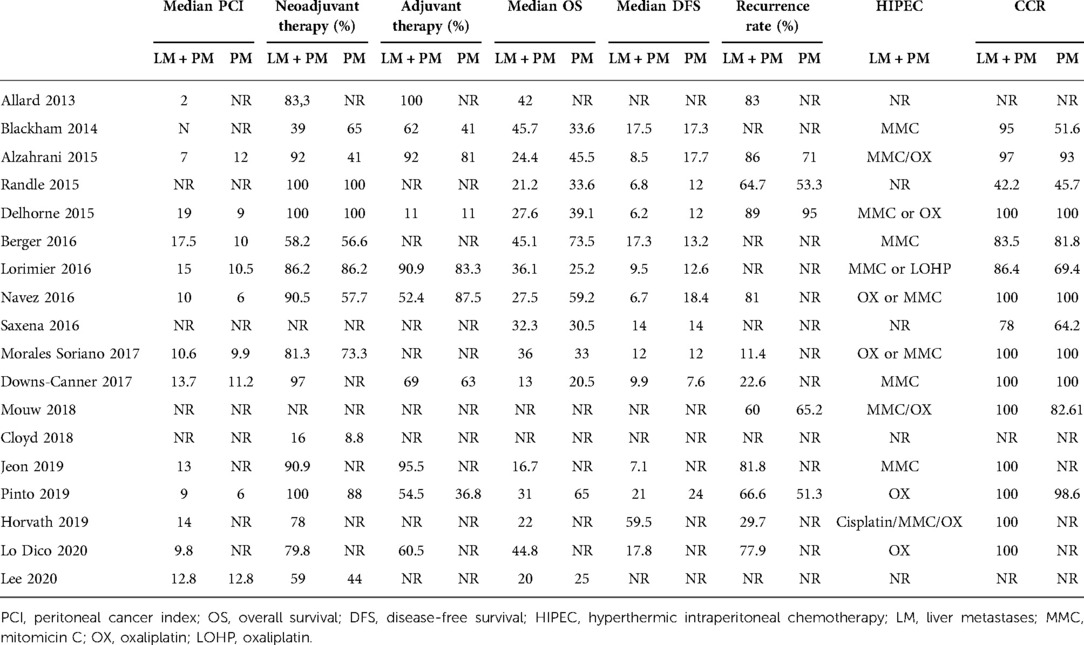
PubMed, EMBASE, Cochrane, and Web of Science databases were matched to find the available literature on this topic. The search period was limited to 10 years (2010–2021) to consider only up-to-date experiences in this relatively recent field of integrated treatments. Search terms including synonyms and keywords such as “metastatic colorectal cancer, HIPEC, intraperitoneal chemotherapy, liver metastases, liver resection, hepatectomy, peritoneal carcinomatosis, and peritoneal metastases” were used. Case reports, case series analyzing fewer than 10 patients, and duplicate articles were excluded. Two reviewers screened all potentially relevant titles and abstracts, selecting papers that described patients treated with CRS–HIPEC who had peritoneal and liver metastases. English-language articles were eligible for inclusion if they specified types of studies [randomized control studies (RCTs), cohort studies, case–control studies, and cross-sectional studies], types of participants (patients with colorectal cancer metastasized to the liver or peritoneum), and types of treatments (both CRS and HIPEC). The review excluded letters to the editor, case reports, reviews, and meta-analyses. Data were collected from the included studies. Patients were divided into two groups: a group of patients with PM only and a group of patients with PMLM. The primary endpoints were OS and disease-free survival (DFS) calculated from the date of CRS–HIPEC. The secondary endpoints were perioperative outcomes including morbidity and mortality. Major morbidity was defined as the presence of a complication classified as Clavien–Dindo grade 3 or higher. Data on length of stay, operative time, PCI, pre- and postoperative chemotherapy, and follow-up period were also recorded (Tables 1 and 2).
Results
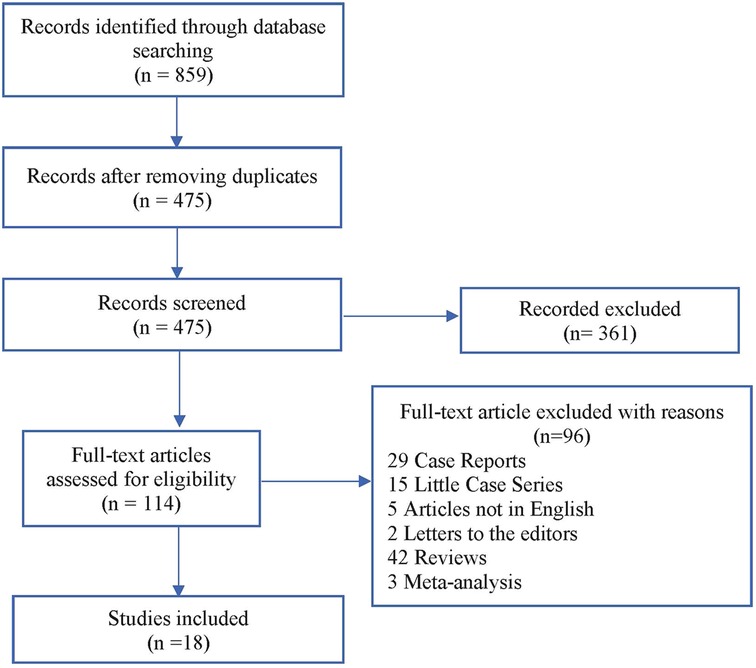
Our literature search identified 859 studies. After removing duplicates, 361 of the 475 remaining studies were excluded based on title and abstract assessment. Exclusion criteria are as follows: studies describing only peritoneal metastases patients, studies describing only colorectal liver metastases patients, articles reporting multiple types of malignancies, where differentiation between patients with colorectal cancer and those with other types of tumors was not possible, articles in which survival outcomes have not been clearly reported, article that failed to extract survival data comparing the peritoneal metastases + liver metastases group with the peritoneal metastases alone group, articles that failed to retrieve peritoneal metastases in combination with colorectal liver metastases data, or studies about debulking surgery alone or in combination with systemic chemotherapy. Out of 114 remaining studies, we found only 18 studies in which data on procedures and outcomes could be completely retrieved. A flow diagram of the literature search procedure according to the PRISMA guidelines is shown in Figure 1.
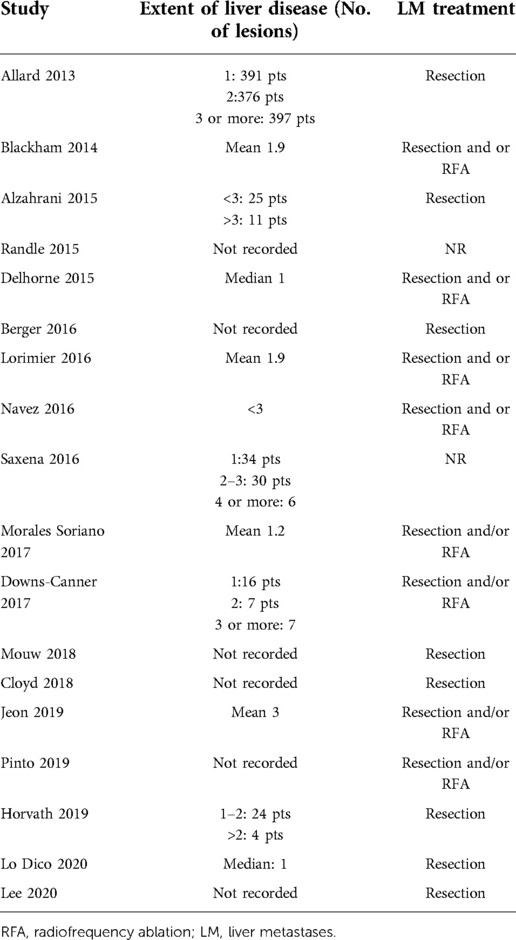
All 18 studies included in the review were published during the study period. In total, 4,719 patients were included in the study. Of these, 1,348 patients presented with synchronous PC + LM and had been treated with liver resection (or alternative therapy such as radiofrequency ablation—RFA) in combination with CRS and HIPEC. The remaining 3,371 patients presented with isolated PC and had been treated with CRS and HIPEC. In most of the studies, the PCI was comparable, and in all cases, it was below 20, which corresponds with clinical guidelines (Table 2). With the exception of the studies by Pinto et al. (28), Lo Dico et al. (40), and Jeon et al. (35), the studies in this review presented patients treated in a one-step procedure with CRS–HIPEC and liver resection/ablation performed during the same surgical procedure. Only a few studies reported the number of liver lesions. In most cases, liver resection was limited to small resection and RFA. Details of the liver treatment are presented in Table 3.
Discussion
This review shows that combined treatment of peritoneal and hepatic metastases for selected patients is feasible, resulting in a mean overall survival of 30 months. Combined CRS–HIPEC and liver resection can be an alternative for patients with limited diseases, leading to an improvement in terms of survival compared to patients who could receive only systemic therapy (42, 44). Despite the feasibility and safety of the combined LR and CRS–HIPEC in metastatic CRC reported from several studies (20, 22, 23, 27, 33, 36, 37, 39, 45), data on the matter show conflicting results, with updated studies and meta-analyses demonstrating evidence to the contrary (5, 21, 32, 34, 46). Razenberg et al. (47) reported a significantly lower median OS in patients with concomitant PC + LM treated with palliative chemotherapy compared to the patients treated with CRS and HIPEC (12.5 vs. 23.1 months). However, there could be a biased selection in interpreting this result as no data regarding the two groups (dissemination of the disease, history prior to treatment, and general conditions of the patients) were available. Lo Dico et al. (39), in their multicenter study, showed that extended surgical management with curative resection plus HIPEC in selected patients with PM + LM is feasible with acceptable morbidity and mortality rates (31% and 4%, respectively) and a better OS. These results are probably associated with a better selection of patients and with the choice of performing the combined procedure only if a minor LR was required. In fact, the study suggested performing a liver-first approach in the case of a two-step procedure and when a minor resection was not feasible. Our primary aim was to review the surgical and survival results of an extensive surgical approach including CRS + HIPEC and LR. Our updated literature review found worse perioperative outcomes (40% vs. 25%) among patients undergoing synchronous LR and CRS–HIPEC compared to the patients undergoing CRS–HIPEC alone. However, no data were available to clarify the risk factors to determine the difference in morbidity. Our results are in line with the findings of Cloyd et al. (35), who described that concomitant LR and CRS/HIPEC were associated with an increased number of postoperative complications and increased readmission compared to patients undergoing CRS/HIPEC alone. However, contradictory results of single-institution studies reporting no difference in postoperative morbidity have been published (3, 23, 27, 33). Lorimier et al. (27), in their monocentric retrospective study, showed better median OS in the PCLM group compared to the PC group only (36 and 25 months, respectively) but without significant statistical difference and with the same OS rate at 5 years (>40%). However, patients in the PCLM group had more hepatic and peritoneal recurrence than those in the PC group. Mortality linked to the surgical procedure was 6.8%, and global morbidity was 38%, without a significant difference between the two groups. In accordance with previous publications, the major postoperative complications occurred more frequently in patients with a PCI >20. Maggiori et al. also described a morbidity of 51% and mortality of 8% for patients undergoing the combined procedure, but almost half of the patients underwent major hepatectomy (48). Delhorme et al. (20) confirmed a significant morbidity rate (44%) when concomitant HIPEC and LS were performed compared with HIPEC alone (11%). Navez et al. (23) described a morbidity rate of 32% when the combined procedure was performed and a median OS of 27.5 months (25). Major postoperative complications were higher in the study by Down-Canner et al. (34) as well (32% vs. 17%). Furthermore, most studies showed a trend toward a shorter median survival time in the PC + LM group and the median OS reported was 29 months. These adverse clinical outcomes should be considered when selecting patients for such aggressive treatment, given that it may provide minimal benefit in terms of prognosis. Nevertheless, other additional factors should be considered in the selection of the patients. For example, survival is also associated with PCI, which is used to evaluate disease extent in peritoneal surface malignancies. Low PCI and the completeness of cytoreduction (CC-0 or -1) were demonstrably associated with a survival benefit with an inverse linear relationship present between PCI and OS; PCI is in fact recognized as an independent prognostic indicator in patients with metastatic peritoneal disease (49). Maggiori et al. (48) reported a median OS of 40 months in patients with a PCI <12 and ≤2 LM, and a higher PCI and more LM were associated with a lower OS (17). Alzahrani et al. showed that the median survival for patients with PCI ≤ 7 and ≤ 3 LM was longer than those with a PCI > 7 and >3 LM (31). Soriano et al. recommended not to perform completeness of cytoreduction rate (CCR) + HIPEC in patients with a PC index higher than 18 points because of its elevated morbidity and poor survival and limited the simultaneous hepatic and peritoneal resection to patients with three or fewer liver lesions (41). Further research is necessary to determine the prognostic effect of these two variables and the relationship with other variables such as tumor histology, performance status, and lymph node metastasis. In a recent review, Lo Dico et al. reviewed all the available major experiences in the combined treatment of liver and peritoneal metastases from colorectal cancer, and their results suggested that patients with limited peritoneal disease (mean PCI of all the reviewed studies was 9.8) and those who need minor liver resections (defined as fewer than three hepatic segments) are the most likely to have better prognostic outcomes (39).
In the past, the presence of synchronous liver and peritoneal metastatic disease was considered a contraindication to surgical resection, and palliative chemotherapy was considered the only possible option (21). Systemic chemotherapy can improve the prognosis, achieving a median OS of 12–16 months (43, 50). Compared to classical chemotherapy regimens, the FOLFOXIRI regimen has shown better in metastatic CRC patients (51). By performing CRS with hyperthermic intraperitoneal chemotherapy, median OS can be brought up to 31–40 months with complete macroscopic resection, which could be increased even more through accurate patient selection (20). Regarding HIPEC role and toxicity, a recent prospective randomized multicenter phase III French trial (PRODIGE 7) has raised concerns about the benefits of adding HIPEC to CRS on survival in patients who underwent CRS + HIPEC compared to those who underwent CRS only (39). Regarding the results of our review, only a few papers report LR + CRS without HIPEC, and mostly, this happens when a minimal peritoneal disease is discovered accidentally and thus resected (29, 52). In larger experiences, the association of HIPEC to CRS correlates with a survival advantage and only a little increase in morbidity. HIPEC should be avoided only in cases where the expected increase in morbidity could be high (for example, patients with multiple comorbidities, renal, hepatic, or bone marrow failure, representing common contraindications to HIPEC) (39).
Certainly, drugs, regimens, and intraperitoneal (IP) perfusion duration influence results. Currently, two regimens are widely used: open-abdomen oxaliplatin ± irinotecan with concurrent intravenous 5-fluorouracil and folinic acid and open- or close-abdomen mitomycin-C, alone or in combination with other drugs (52). In these specific settings of patients, IP regimens with oxaliplatin seem to provide the best improvement in outcomes. Whether this improvement depends on the use of a specific drug or the different duration of IP perfusion (30 vs. 90 min) remains debatable (53).
However, in the reported experiences considered for this review, no increased toxicity of chemotherapeutic agents has been observed in patients who underwent LR compared to those who did not. Pinto et al. reported a median OS of 31 months for patients who underwent HIPEC + LR and received neoadjuvant chemotherapy, highlighting how the response or nonprogression during neoadjuvant treatment can be beneficial in selecting patients. He also proposed a two-step procedure for patients with bilobar metastases to avoid major hepatic resection during HIPEC, reducing postoperative morbidity and mortality rates (28). In fact, in the presence of hepatic metastases, the resectability rate can be increased by several surgical techniques, such as two-stage hepatectomy or portal vein embolization, even in patients with initially unresectable, multiple secondary diseases. LM may require only minor liver surgery procedures, usually performed at the same time as CRS and HIPEC, or it may require complex liver resection surgery that could be performed by two-step procedures; hence, HM management could be adapted depending on the extension of the metastatic disease and even the need for aggressive liver surgery such as major hepatectomy that could be performed in the simultaneous, delayed, and liver-first approach (14). Commonly, liver surgery is limited to minor resections in most of the experiences because cytoreductive surgery associated with major liver surgery, such as two-stage hepatectomy followed by HIPEC, seems to be correlated to unacceptable morbidity rates in the few papers that considered this approach (28–30). Other major liver procedures such as associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) are not described in the papers considered for this review. This review shows that combined integrated local treatment of peritoneal and hepatic metastases for selected patients is feasible, although its outcomes remain controversial. The survival rates of these patients suggest an advantage compared with patients who only received systemic chemotherapy. On the other hand, major morbidity rates seem to be worsened by the association of two major surgical procedures like LR and CRS + HIPEC. From this point of view, a key role is played by the extension of hepatic and peritoneal surgical resections that should represent a cornerstone in the preoperative evaluation of these patients, as more aggressive surgical procedures have been demonstrated to link with a higher rate of postoperative complications, as clearly reported by major experiences in the field (40). Aside from the surgical extension, specific organs resection also seems to be linked to the morbidity rate such as rectal resection or organ resections associated with upper quadrant peritonectomy (i.e., resection of the diaphragm, spleen, or pancreas) (33, 36, 54). As operative and patient factors both contribute to morbidity and mortality, additional factors that should be considered are the number of hepatic metastases, liver function tests, low or intermediate PCI scores, types of drugs and perfusion's duration of intraperitoneal chemotherapy, patient's characteristics such as age, performance status, and comorbidities, and tumor characteristics including tumor histology and grading (advanced tumors or signet ring cell histology), neoadjuvant therapy, and response to systemic chemotherapy (RECIST criteria). The incidence of major complications represents one of the most determinant factors limiting the results of this combined approach and the most relevant in worsening prognosis. Another significant factor impacting morbidity and hence prognosis is the number of liver metastases. An attempt to preoperatively estimate the expected survival after the combined procedure has been proposed by Elias et al. by the development of a nomogram including criteria such as the number of liver metastases, PCI, and type of surgery (CRS/HIPEC alone, LR alone, or concomitant LR and CRS/HIPEC) (20, 55). Patient selection and risk stratification may also be carried out by the use of risk scores in which an increased number of factors detected has been associated with decreased OS; factors proposed to assess the risk score are patient's age, primary tumor histology, number of liver lesions (single vs. multiple), and pathways of recurrence (38, 56–58). The median follow-up in our review was 32 months (20–63 months), and recurrence rates were respectively 81% and 71% in the PM + LM group and the PM group regardless of the additional use of neoadjuvant or adjuvant therapy. The incidence of postoperative mortality was 2.6% in the PM + LM group and 2.8% in the PM group. No studies showed a significant difference in postoperative mortality between the two groups. To date, neither patient selection nor patient management criteria have been standardized for combined treatment; considering the aforementioned survival rates and morbidity and mortality data, extensive surgical approaches including CRS and hepatic LR should not be defined as safe and risk-free, as some studies previously reported. Nevertheless, accurate patient selection and an individualized preoperative decision-making process should be considered fundamental steps in the initial management of patients selected for combined treatment (59, 60).
Conclusion
The role of combined surgical strategy (CRS + HIPEC and LR) in patients with synchronous liver and peritoneal metastases from colorectal cancer remains controversial. Survival rates and morbidity and mortality seem not in favor of this option. A strict and homogeneous selection of patients and a “tailored” surgical strategy (one-step vs. two-step liver surgery, extent of cytoreduction, and increasing use of laparoscopic techniques) (61–64) are mandatory to obtain the best results without increasing morbidity, and it would perhaps help improve the misleading results in this subgroup of patients with limited curative options.
Author contributions
SDC, GC, SS, and LI provided substantial contributions to the conception and design of the work. VU, MF, PI, and FLR contributed to the acquisition, analysis, and interpretation of data for the work. LS, AF, and SD drafted the work. EF and PR revised it critically for important intellectual content. All authors provide their approval for publication of the content and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling Editor GG declared a shared parent affiliation with the authors, GC, FLR, PI, LI, AF, and EF, at the time of review.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424 [published correction appears in CA Cancer J Clin. 2020;70(4):313]. doi: 10.3322/caac.21492
2. van Rooijen KL, Shi Q, Goey KKH, Meyers J, Heinemann V, Diaz-Rubio E, et al. Prognostic value of primary tumour resection in synchronous metastatic colorectal cancer: individual patient data analysis of first-line randomised trials from the ARCAD database. Eur J Cancer. (2018) 91:99–106. doi: 10.1016/j.ejca.2017.12.014
3. van der Geest LG, Lam-Boer J, Koopman M, Verhoef C, Elferink MA, de Wilt JH. Nationwide trends in incidence, treatment and survival of colorectal cancer patients with synchronous metastases. Clin Exp Metastasis. (2015) 32(5):457–65. doi: 10.1007/s10585-015-9719-0
4. Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC intergroup trial 40983): a randomised controlled trial. Lancet. (2008) 371(9617):1007–16. doi: 10.1016/S0140-6736(08)60455-9
5. Segelman J, Granath F, Holm T, Machado M, Mahteme H, Martling A. Incidence, prevalence and risk factors for peritoneal carcinomatosis from colorectal cancer. Br J Surg. (2012) 99(5):699–705. doi: 10.1002/bjs.8679
6. Schuell B, Gruenberger T, Kornek GV, Dworan N, Depisch D, Lang F, et al. Side effects during chemotherapy predict tumour response in advanced colorectal cancer. Br J Cancer. (2005) 93(7):744–8. doi: 10.1038/sj.bjc.6602783
7. Di Giorgio A, Cardi M, Biacchi D, Sibio S, Accarpio F, Ciardi A, et al. Depth of colorectal-wall invasion and lymph-node involvement as major outcome factors influencing surgical strategy in patients with advanced and recurrent ovarian cancer with diffuse peritoneal metastases. World J Surg Oncol. (2013) 11:64. doi: 10.1186/1477-7819-11-64
8. Lemoine L, Sugarbaker P, Van der Speeten K. Pathophysiology of colorectal peritoneal carcinomatosis: role of the peritoneum. World J Gastroenterol. (2016) 22(34):7692–707. doi: 10.3748/wjg.v22.i34.7692
9. Segelman J. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology Colon Cancer (Version 4.2018). doi: 10.6004/jnccn.2018.0021. Available at: www.nccn.org/professionals/physician_gls/pdf/colon.pdf (Accessed June 18, 2022).
10. Kawada K, Hasegawa S, Murakami T, Itatani Y. Molecular mechanisms of liver metastasis. Int J Clin Oncol. (2011) 16(5):464–72. doi: 10.1007/s10147-011-0307-2
11. Franko J, Shi Q, Goldman CD, Pockaj BA, Nelson GD, Goldberg RM, et al. Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: a pooled analysis of north central cancer treatment group phase III trials N9741 and N9841. J Clin Oncol. (2012) 30(3):263–7. doi: 10.1200/JCO.2011.37.1039
12. Quénet F, Elias D, Roca L, Goéré D, Ghouti L, Pocard M, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. (2021) 22(2):256–66. doi: 10.1016/S1470-2045(20)30599-4
13. Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. (2013) 14(12):1208–15. doi: 10.1016/S1470-2045(13)70447-9
14. Baratti D, Kusamura S, Pietrantonio F, Guaglio M, Niger M, Deraco M. Progress in treatments for colorectal cancer peritoneal metastases during the years 2010-2015. A systematic review. Crit Rev Oncol Hematol. (2016) 100:209–22. doi: 10.1016/j.critrevonc.2016.01.017
15. Quenet F, Elias D, Roca L, Goere D, Ghouti L, Pocard M, et al. A UNICANCER phase III trial of hyperthermic intra-peritoneal chemotherapy (HIPEC) for colorectal peritoneal carcinomatosis (PC): PRODIGE 7. J Clin Oncol. (2018) 36:LBA3503. 190.1200/JCO.2018.36.18_suppl.LBA3503
16. Cardi M, Sammartino P, Mingarelli V, Sibio S, Accarpio F, Biacchi D, et al. Cytoreduction and HIPEC in the treatment of “unconventional” secondary peritoneal carcinomatosis. World J Surg Oncol. (2015) 13:305. doi: 10.1186/s12957-015-0703-6
17. Tanaka T, Ozawa H, Nakagawa Y, Hirata A, Fujita S, Sugihara K. Verifying the M1c category of CRC: analysis of the data from a Japanese multi-institutional database. Int J Colorectal Dis. (2020) 35(1):125–31. doi: 10.1007/s00384-019-03408-w
18. Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. (2003) 21(20):3737–43. doi: 10.1200/JCO.2003.04.187
19. Esquivel J, Elias D, Baratti D, Kusamura S, Deraco M. Consensus statement on the loco regional treatment of colorectal cancer with peritoneal dissemination. J Surg Oncol. (2008) 98(4):263–7. doi: 10.1002/jso.21053
20. Elias D, Faron M, Goéré D, Dumont F, Honoré C, Boige V, et al. A simple tumor load-based nomogram for surgery in patients with colorectal liver and peritoneal metastases. Ann Surg Oncol. (2014) 21(6):2052–8. doi: 10.1245/s10434-014-3506-z
21. Delhorme JB, Dupont-Kazma L, Addeo P, Lefebvre F, Triki E, Romain B, et al. Peritoneal carcinomatosis with synchronous liver metastases from colorectal cancer: who will benefit from complete cytoreductive surgery? Int J Surg. (2016) 25:98–105. doi: 10.1016/j.ijsu.2015.11.025
22. de Cuba EM, Kwakman R, Knol DL, Bonjer HJ, Meijer GA, Te Velde EA. Cytoreductive surgery and HIPEC for peritoneal metastases combined with curative treatment of colorectal liver metastases: systematic review of all literature and meta-analysis of observational studies. Cancer Treat Rev. (2013) 39(4):321–7. doi: 10.1016/j.ctrv.2012.11.003
23. Berger Y, Aycart S, Tabrizian P, Agmon Y, Mandeli J, Heskel M, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in patients with liver involvement. J Surg Oncol. (2016) 113(4):432–7. doi: 10.1002/jso.24153
24. Navez J, Remue C, Leonard D, Bachmann R, Kartheuser A, Hubert C, et al. Surgical treatment of colorectal cancer with peritoneal and liver metastases using combined liver and cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: report from a single-centre experience. Ann Surg Oncol. (2016) 23(Suppl 5):666–73. doi: 10.1245/s10434-016-5543-2
25. Selby K, Hernandez-Alejandro R. Two-stage hepatectomy for liver metastasis from colorectal cancer. CMAJ. (2014) 186(15):1163–6. doi: 10.1503/cmaj.131022
26. Hallam S, Tyler R, Price M, Beggs A, Youssef H. Meta-analysis of prognostic factors for patients with colorectal peritoneal metastasis undergoing cytoreductive surgery and heated intraperitoneal chemotherapy. BJS Open. (2019) 3(5):585–94. doi: 10.1002/bjs5.50179
27. Sibio S, Di Giorgio A, D'Ugo S, Palmieri G, Cinelli L, Formica V, et al. Histotype influences emergency presentation and prognosis in colon cancer surgery. Langenbecks Arch Surg. (2019) 404(7):841–51. doi: 10.1007/s00423-019-01826-6
28. Lorimier G, Linot B, Paillocher N, Dupoiron D, Verrièle V, Wernert R, et al. Curative cytoreductive surgery followed by hyperthermic intraperitoneal chemotherapy in patients with peritoneal carcinomatosis and synchronous resectable liver metastases arising from colorectal cancer. Eur J Surg Oncol. (2017) 43(1):150–8. doi: 10.1016/j.ejso.2016.09.010
29. Pinto A, Hobeika C, Philis A, Kirzin S, Carrère N, Ghouti L. Synchronous liver metastases and peritoneal carcinomatosis from colorectal cancer: different strategies for curative treatment? Langenbecks Arch Surg. (2019) 404(4):477–88. doi: 10.1007/s00423-019-01787-w
30. Allard MA, Adam R, Ruiz A, Vibert E, Paule B, Levi F, et al. Is unexpected peritoneal carcinomatosis still a contraindication for resection of colorectal liver metastases? Combined resection of colorectal liver metastases with peritoneal deposits discovered intra-operatively. Eur J Surg Oncol. (2013) 39(9):981–7. doi: 10.1016/j.ejso.2013.06.009
31. Blackham AU, Swett K, Eng C, Sirintrapun J, Bergman S, Geisinger KR, et al. Perioperative systemic chemotherapy for appendiceal mucinous carcinoma peritonei treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Surg Oncol. (2014) 109(7):740–5. doi: 10.1002/jso.23547
32. Alzahrani N, Ung L, Valle SJ, Liauw W, Morris DL. Synchronous liver resection with cytoreductive surgery for the treatment of liver and peritoneal metastases from colon cancer: results from an Australian centre. ANZ J Surg. (2017) 87(11):E167–72. doi: 10.1111/ans.13231
33. Randle RW, Doud AN, Levine EA, Clark CJ, Swett KR, Shen P, et al. Peritoneal surface disease with synchronous hepatic involvement treated with cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC). Ann Surg Oncol. (2015) 22(5):1634–8. doi: 10.1245/s10434-014-3987-9
34. Saxena A, Valle SJ, Liauw W, Morris DL. Limited synchronous hepatic resection does not compromise peri-operative outcomes or survival after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Surg Oncol. (2017) 115(4):417–24. doi: 10.1002/jso.24543
35. Jeon Y, Park EJ, Lim JH, Baik SH. Clinical outcomes of complete cytoreduction with concurrent liver resection followed by hyperthermic intraperitoneal chemotherapy for synchronous peritoneal and liver metastatic colorectal cancer. World J Surg Oncol. (2019) 17(1):214. doi: 10.1186/s12957-019-1746-x
36. Cloyd JM, Abdel-Misih S, Hays J, Dillhoff ME, Pawlik TM, Schmidt C. Impact of synchronous liver resection on the perioperative outcomes of patients undergoing CRS-HIPEC. J Gastrointest Surg. (2018) 22(9):1576–84. doi: 10.1007/s11605-018-3784-z
37. Horvath P, Beckert S, Königsrainer A, Nadalin S, Königsrainer I. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy combined with liver resection for concurrent peritoneal and hepatic metastases of gastrointestinal and gynecological primary tumors. J Visc Surg. (2019) 156(6):475–84. doi: 10.1016/j.jviscsurg.2019.06.007
38. Sileri P, Mele A, Stolfi VM, Grande M, Sica G, Gentileschi P, Di Carlo S, Gaspari AL. Medical and surgical treatment of chronic anal fissure: A prospective study. J Gastrointest Surg. (2007) 11(11):1541–48. doi: 10.1007/s11605-007-0255-3
39. Lee RM, Gamboa AC, Turgeon MK, Zaidi MY, Kimbrough C, Leiting J, et al. A novel preoperative risk score to optimize patient selection for performing concomitant liver resection with cytoreductive surgery/HIPEC. J Surg Oncol. (2021) 123(1):187–95. doi: 10.1002/jso.26239
40. Lo Dico M, Faron Y, Yonemura O, Glehen M, Pocard A, Sardi M, et al. Combined liver resection and cytoreductive surgery with HIPEC for metastatic colorectal cancer: results of a worldwide analysis of 565 patients from the peritoneal surface oncology group international (PSOGI). Eur J Surg Oncol. (2020) 47(1):89–100. doi: 10.1016/j.ejso.2020.07.038
41. Mouw TJ, Lu J, Woody-Fowler M, Ashcraft J, Valentino J, DiPasco P, et al. Morbidity and mortality of synchronous hepatectomy with cytoreductive surgery/hyperthermic intraperitoneal chemotherapy (CRS/HIPEC). J Gastrointest Oncol. (2018) 9(5):828–32. doi: 10.21037/jgo.2018.06.04
42. Soriano RM, Canis JMM, Romero XM, Celada JP, Gavela ST, Sampedro JJS, et al. Influence of simultaneous liver and peritoneal resection on postoperative morbi-mortality and survival in patients with colon cancer treated with surgical cytoreduction and intraperitoneal hyperthermic chemotherapy. Influencia de la resección hepática y peritoneal simultánea, en la morbimortalidad y supervivencia de los pacientes con cáncer de colon intervenidos mediante cirugía citorreductora con quimioterapia intraperitoneal hipertérmica. Cir Esp. (2017) 95(4):214–21. doi: 10.1016/j.ciresp.2017.03.003
43. Simkens GA, Razenberg LG, Lemmens VE, Rutten HJ, Creemers GJ, de Hingh IH. Histological subtype and systemic metastases strongly influence treatment and survival in patients with synchronous colorectal peritoneal metastases. Eur J Surg Oncol. (2016) 42(6):794–800. doi: 10.1016/j.ejso.2016.03.014
44. Franko J, Shi Q, Meyers JP, Maughan TS, Adams RA, Seymour MT, et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trials from the analysis and research in cancers of the digestive system (ARCAD) database. Lancet Oncol. (2016) 17(12):1709–19. doi: 10.1016/S1470-2045(16)30500-9
45. Razenberg LG, Lemmens VE, Verwaal VJ, Punt CJ, Tanis PJ, Creemers GJ, et al. Challenging the dogma of colorectal peritoneal metastases as an untreatable condition: results of a population-based study. Eur J Cancer. (2016) 65:113–20. doi: 10.1016/j.ejca.2016.07.002
46. Chua TC, Liauw W, Koong HN, Esquivel J. Surgical therapies in metastatic colorectal cancer with a potential for cure. Am J Clin Oncol. (2011) 34(3):326–31. doi: 10.1097/COC.0b013e3181dbb9ad
47. Zou Y, Chen X, Zhang X, Shen Z, Cai J, Tan Y, et al. Clinical outcomes of curative treatment for colorectal liver metastases combined with cytoreductive surgery and intraperitoneal chemotherapy for peritoneal metastases: a systematic review and meta-analysis of current evidence. Int J Hyperthermia. (2020) 37(1):944–54. doi: 10.1080/02656736.2020.1803424
48. Razenberg LG, van Gestel YR, Creemers GJ, Verwaal VJ, Lemmens VE, de Hingh IH. Trends in cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for the treatment of synchronous peritoneal carcinomatosis of colorectal origin in the Netherlands. Eur J Surg Oncol. (2015) 41(4):466–71. doi: 10.1016/j.ejso.2015.01.018
49. Maggiori L, Goéré D, Viana B, Zanis D, Dumont F, Honoré C, et al. Should patients with peritoneal carcinomatosis of colorectal origin with synchronous liver metastases be treated with a curative intent? A case-control study. Ann Surg. (2013) 258(1):116–21. doi: 10.1097/SLA.0b013e3182778089
50. Goéré D, Souadka A, Faron M, Cloutier AS, Viana B, Honoré C, et al. Extent of colorectal peritoneal carcinomatosis: attempt to define a threshold above which HIPEC does not offer survival benefit: a comparative study. Ann Surg Oncol. (2015) 22(9):2958–64. doi: 10.1245/s10434-015-4387-5
51. Koopman M, Antonini NF, Douma J, Wals J, Honkoop AH, Erdkamp FL, et al. Sequential versus combination chemotherapy with capecitabine, irinotecan, and oxaliplatin in advanced colorectal cancer (CAIRO): a phase III randomised controlled trial. Lancet. (2007) 370(9582):135–42. doi: 10.1016/S0140-6736(07)61086-1
52. Cremolini C, Loupakis F, Antoniotti C, Lupi C, Sensi E, Lonardi S, et al. FOLFOXIRI Plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. (2015) 16(13):1306–15. doi: 10.1016/S1470-2045(15)00122-9
53. Desolneux G, Maziere C, Vara J. Cytoreductive surgery of colorectal peritoneal metastases: outcomes after complete complete cytoreductive surgery and systemic chemotherapy only. PLoS One. (2015) 10(3):e0122816. doi: 10.1371/journal.pone.0122816
54. Gavriilidis P, Katsanos K, Sutcliffe RP, Simopoulos C, Azoulay D, Roberts KJ. Simultaneous, delayed and liver-first hepatic resections for synchronous colorectal liver metastases: a systematic review and network meta-analysis. J Clin Med Res. (2019) 11(8):572–82. doi: 10.14740/jocmr3887
55. Di Giorgio A, Cardini CL, Sammartino P, Sibio S, Naticchioni E. Dual-layer sandwich mesh repair in the treatment of major diaphragmatic eventration in an adult. J Thorac Cardiovasc Surg. (2006) 132(1):187–9. doi: 10.1016/j.jtcvs.2006.02.033
56. Sileri P, Sica G, Gentileschi P, Venza M, Manzelli A, Palmieri G, et al. Ischemic preconditioning protects intestine from prolonged ischemia. Transplant Proc. (2004) 36(2):283–5. doi: 10.1016/j.transproceed.2004.01.078
57. Sibio S, Fiorani C, Stolfi C, Divizia A, Pezzuto R, Montagnese F, et al. Detection methods and clinical significance of free peritoneal tumor cells found during colorectal cancer surgery. World J Gastrointest Surg. (2015) 7(9):178–84. doi: 10.4240/wjgs.v7.i9.178.
58. Sica GS, Fiorani C, Stolfi C, Monteleone G, Candi E, Amelio I, et al. Peritoneal expression of matrilysin helps identify early post-operative recurrence of colorectal cancer. Oncotarget. (2015) 6(15):13402–15. doi: 10.18632/oncotarget.2830
59. Downs-Canner S, Shuai Y, Ramalingam L, Pingpank JF, Holtzman MP, Zeh HJ, Bartlett DL, Choudry HA Safety and efficacy of combined resection of colorectal peritoneal and liver metastases. J Surg Res. (2017) 219:194–201. doi: 10.1016/j.jss.2017.05.126
60. Amelio I, Bertolo R, Bove P, Buonomo OC, Candi E, Chiocchi M, et al. Liquid biopsies and cancer omics. Cell Death Discov. (2020) 6(1):131. doi: 10.1038/s41420-020-00373-0
61. Rossi P, Sileri P, Gentileschi P, Sica G, Forlini A, Stolfi V, et al. Percutaneous liver biopsy using an ultrasound-guided subcostal route. Dig Dis Sci. (2001) 46(1):128–32. doi: 10.1023/a:1005571904713
62. Gentileschi P, Camperchioli I, Benavoli D, Di Lorenzo N, Sica G, Gaspari AL. Laparoscopic single-port sleeve gastrectomy for morbid obesity: preliminary series. Surg Obes Relat Dis. (2010) 6(6):665–9. doi: 10.1016/j.soard.2010.01.011
63. Sica GS, Iaculli E, Benavoli D, Biancone L, Calabrese E, Onali S, et al. Laparoscopic versus open ileo-colonic resection in Crohn's disease: short- and long-term results from a prospective longitudinal study. J Gastrointest Surg. (2008) 12(6):1094–102. doi: 10.1007/s11605-007-0394-6
Keywords: peritoneal metastases, cytoreductive surgery, liver resection, liver metastases, HIPEC, colorectal metastases.
Citation: Di Carlo S, Cavallaro G, La Rovere F, Usai V, Siragusa L, Izzo P, Izzo L, Fassari A, Izzo S, Franceschilli M, Rossi P, Dhimolea S, Fiori E and Sibio S (2022) Synchronous liver and peritoneal metastases from colorectal cancer: Is cytoreductive surgery and hyperthermic intraperitoneal chemotherapy combined with liver resection a feasible option?. Front. Surg. 9:1006591. doi: 10.3389/fsurg.2022.1006591
Received: 29 July 2022; Accepted: 31 October 2022;
Published: 15 December 2022.
Edited by:
Gaetano Gallo, Sapienza University of Rome, ItalyReviewed by:
Cihangir Akyol, Ankara University, TurkeyAlexander Reinisch, University of Giessen, Germany
Argyrios Ioannidis, Athens Medical Group, Greece
© 2022 Di Carlo, Cavallaro, La Rovere, Usai, Siragusa, Izzo, Izzo, Fassari, Izzo, Franceschilli, Rossi, Dhimolea, Fiori and Sibio. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Simone Sibio c2ltb25lLnNpYmlvQHVuaXJvbWExLml0
Specialty Section: This article was submitted to Visceral Surgery, a section of the journal Frontiers in Surgery
 Sara Di Carlo
Sara Di Carlo Giuseppe Cavallaro
Giuseppe Cavallaro Francesca La Rovere2
Francesca La Rovere2 Leandro Siragusa
Leandro Siragusa Luciano Izzo
Luciano Izzo Alessia Fassari
Alessia Fassari Marzia Franceschilli
Marzia Franceschilli Piero Rossi
Piero Rossi Enrico Fiori
Enrico Fiori Simone Sibio
Simone Sibio