- 1Department of Burns and Plastic Surgery, The Second Hospital, Cheeloo College of Medicine, Shandong University, Jinan, China
- 2Department of Emergency Medical Center, The Second Hospital, Cheeloo College of Medicine, Shandong University, Jinan, China
The burn wound is a dynamic living environment that is affected by many factors. It may present a progressive expansion of necrosis into the initially viable zone of stasis within a short time postburn. Therefore, how to salvage of the zone of stasis is of crucial importance in prevention and treatment strategies of burn wound progressive deepening. This review focuses on the cellular basis of tissue injury and the current progress of prevention and treatment strategies of burn wound progressive deepening, in order to provide references for the treatment of burn wounds in the early phase.
Introduction
Burns wound surface is the source of dynamic local and systemic responses postburn that strongly affect clinical outcome. Meanwhile, the wound changes rapidly in the early phase of burns. In the first fews days, the secondary tissue damage may expand to the initially viable tissues nearby after the primary burn injury both in area and depth (1). Also, a better understanding of the mechanisms that lead to burn wound conversion may lead to more novel therapies that limit burn wound progression in the early stage, and ultimately lead to better healing.
Pathophysiological changes of early burn wounds
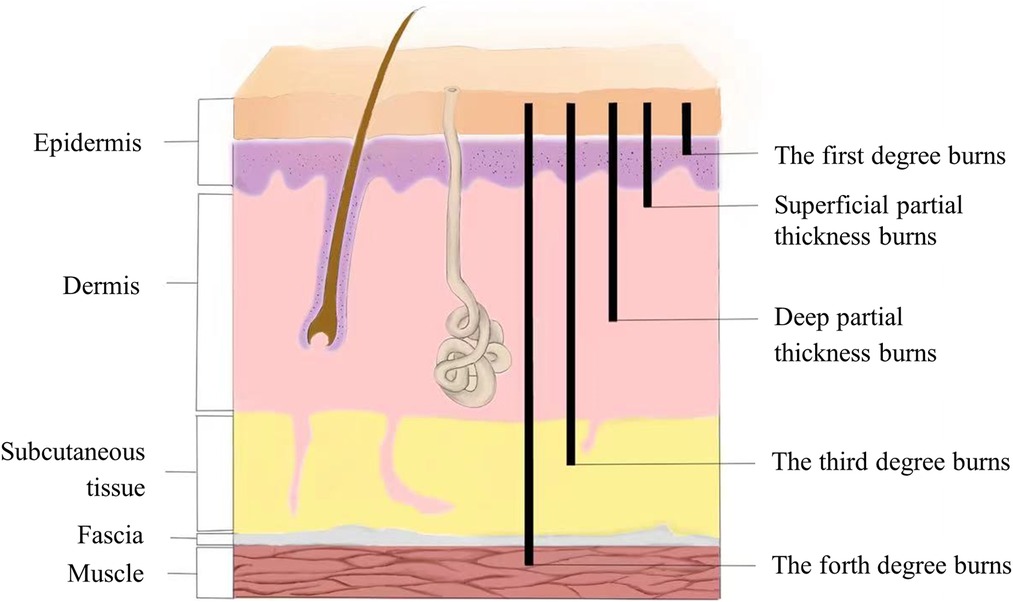
In addition to the immediate coagulation and necrosis of the tissue in the coagulation zone of the skin wound after the burn, the zone of stasis also has a progressive damaging effect in the early stages, especially in the 12–24 h after the injury (2). A range of pathophysiological changes will appear in the zone of stasis: ① Dilation of capillaries and venules, swelling and loose arrangement of vascular endothelial cells, cracks appear between cells, and vascular endothelial cell permeability increases. At the same time, vascular endothelial cells can release a variety of inflammatory mediators and aggravate the inflammatory response. ② During the early phase of burn injury, neutrophils detach from the blood vessels and reach the interstitial space releasing oxygen free radicals, proteolytic enzymes, etc., causing new damage to the tissue and causing the progression of the wound. ③ During the burn, microthrombosis gradually develops due to the accumulation of large numbers of dissolved red blood cells in the lumen (3). The more microthrombosis is formed, the worse the microcirculation state of the wound is, and the degree of tissue necrosis is also deepened, which is one of the reasons for the progressive deepening of the wound after burn (Figure 1).
Treatment of the wound progression
Progression of burn wounds, or the conversion of superficial burns to deep burns, is characteristic of many burns and leads to worse outcomes. Appropriate treatment of the wound and enhanced protection of the zone of stasis are very important to prevent the deepening of the wound. This section will summarize some of the findings of past studies and highlight the lastest promising developments in various fields.
Early debridement
There are a lot of inflammatory mediators and endotoxin in eschar and subeschar edema fluid. Improper treatment of early burn wounds makes the wound become the source of infection in burn patients (4). In the early stage of burn, the systemic inflammatory reaction of the patient is not obvious. Debridement in good physical condition can effectively reduce the occurrence of visceral complications and systemic infection after burn, improve the long-term prognosis, and improve the quality of life of the patient. The common methods of debridement used in burn surgery include the sharp debridement and the blunt debridement.
The most common means of removing eschar is escharectomy. The debridement effect of escharectomy is undeniable, but at the same time, excessive removal of healthy tissue is unavoidable. Moreover, hemorrhagic shock is often caused by insufficient hemostasis after extensive excision of eschar. In case of that, there are some precise debridement techniques could be chosen, such as hydrodynamic debridement system (5) and enzymatic debridement (6). These alternative methods of eschar removal that are less traumatic and more selective than escharectomy. Bromelain-based enzymatic debridement and hydrotherapy using pressurized saline flow can effectively remove necrotic tissue with less pain. And the preservation of dermal tissue could reduce surgical burden and improve long-term prognosis (7). Apart of these methods, blunt debridement techniques are promising as well. Dermabrasion and decompression in the initial debridement in the early stage of burns can dredge the blood circulation of the wound, reduce the burn damage, improve the revival of the zone of stasis (8).
Tissue engineering and stem cell therapy
Tissue engineering has begun to provide cellular therapies for burns and many other tissue injuries in the human body. Among which, stem cell biology has been an important part of the equation when it comes to salvaging the zone of stasis. It is particularly important to preserve the initially unaffected and salvageable stasis area (9) during the treatment process. In recent years, with the development of stem cell technology, its application in wound treatment has also been further developed. Many types of stem cells have been used in clinical management to prevent the delayed necrosis of initially viable tissues surrounding the zone of stasis, such as embryonic stem cell (ESC), somatic stem cell (SSC), induced pluripotent stem cell (iPSC) and mesenchymal stem cell (MSC) (10). In this part, we will focus on the MSCs and describe their usages and functional mechanisms. MSCs can be isolated from different tissues, first from bone marrow, but also from different tissues (11, 12). MSCs represent a type of pluripotent stem cells that can differentiate into various mesenchymal lineages, and are one of the most widely used stem cells in the field of skin injury and repair (13), and play a certain role in protecting the stasis area of burns (14). The mechanisms by which MSCs limit wound deepening and promote wound healing are mainly as follows.
Paracrine impact of MSCs
Studies have shown that MSCs can participate in tissue repair through paracrine exosomes to regulate the wound microenvironment (15, 16). Exosomes are nano-sized extracellular vesicles (EVs) secreted by cells, which carry biologically active substances such as nucleic acids, proteins, and lipids, and function in various physiological and pathological processes in the body. For example, mesenchymal stem cell-derived exosomes (MSC-exosomes) are enriched with various miRNAs and proteins that mediate multiple intercellular signaling pathways and reduce inflammation by regulating the levels of various cytokines, including transforming growth factor-β1 (TGF-β1), hepatocyte growth factor (HGF), nitric oxide (NO), and interleukin-4 (IL-4) (17). MSCs can induce regeneration of the epidermal skin barrier by increasing the synthesis of ceramide and dihydroceramides (18). In addition to these functions, MSCs can also support angiogenesis through paracrine influences. Pro-angiogenic factors released by MSCs involve vascular endothelial growth factor (VEGF), placental growth factor (PGF), transforming growth factor-β (TGF-β), platelet derived growth factor (PDGF), angiopoietin-1 (Ang-1), interleukin-6 (IL-6), and monocyte chemotactic protein-1 (MCP-1), which can stimulate the inducing multiple signaling axes to promote therapeutic angiogenesis in wound tissue (19, 20).
Multi-directional differentiation of MSCs
Apart from the paracrine pathway, human MSCs are largely thought to differentiate directly into skin cells and participate in wound healing. MSCs are pluripotent stem cells capable of self-renewal and multi-directional differentiation. When it is implanted in the wound, it undergoes spontaneous differentiation under the influence of the wound microenvironment (12). Under suitable conditions, MSCs can differentiate into various types of cells such as nerve cells, vascular endothelial cells, sweat gland cells, and epidermal cells (21–23).
Immunomodulatory and anti-inflammatory functions of MSCs
MSCs transplantation can alleviate the inflammatory response in the early stage of burns. After MSCs transplantation in burn wounds, the number of neutrophils infiltrating into the zone of stasis decreased and the activity of myeloperoxidase, which represents tissue neutrophil accumulation, decreased, and pro-inflammatory cytokines such as TNF-α, IL-6, IL-1β and IL-10 expression levels were significantly reduced (14, 24). In addition, MSCs can alleviate burn-induced oxidative stress in the stasis area, which may also be associated with the reduction of inflammatory response (3).
MSCs need to be expanded in vitro to perform their regenerative and immunosuppressive functions for various clinical applications. Rombounts (25) found that there was a problem of reduced activity of stem cells after passage: the homing rate of fresh uncultured MSCs after transplantation was 55%–65%, while that of cultured MSCs was 55%–65%. When transplanted after 24 h, the homing rate dropped to 10%. The conventional stem cell transplantation method is to increase the number of stem cells by in vitro expansion after the isolated stem cells are obtained. So in stem cell therapy, there is also a trade-off between transplant time and cell viability.
Measures to reduce local inflammatory response
NLRP3 inhibitor
Burn wound infection is one of the important factors leading to early burn wound deepening. The activity of NLRP3 inflammasome is significantly enhanced in macrophages in burn stasis area after scald (26, 27), and inhibiting its activity can improve early burn wound progression and promote wound healing. For example, the NLRP3 inflammasome-specific inhibitor MNS (3,4-Methylenedioxy-β-N) can significantly inhibit the activation of NLRP3 inflammasome and the production of inflammatory factors in burn wounds, and improve the progress of burn wounds. At the same time, increasing the level of autophagy in the wound also inhibited the NLRP3 inflammasome activity in the stasis area. Far-infrared (FIR) irradiation can increase the level of autophagy in the wound, improve the inflammatory infiltration of the wound, and reduce the deepening of the trauma (28).
Hyperbaric oxygen therapy (HBO)
HBO improves tissue hypoxia, ischemia-reperfusion injury and reduces pathological inflammation in various clinical settings. It can also shorten the healing time and improve outcomes of patients (29). The skin near II° and III° burns is more hypoxic than normal skin, and the hypoxic tissue around the burn site can be restored to normal oxygen levels by giving oxygen under pressure. HBO can help vasoconstriction to reduce edema, and maintain microcirculation through direct infiltration, enhancing oxygen delivery. HBO also helps inactivate leukocyte adhesion (2) and has a potential broad-spectrum antimicrobial effect (30), so the use of hyperbaric oxygen therapy in the treatment of burns can lead to faster wound healing and reduced morbidity and mortality from complications (31).
Measures to protect the wound microenvironment
Negative pressure wound therapy (NPWT)
NPWT consists of two key techniques: vacuum sealing drainage (VSD) and vacuum assisted closure (VAC). In the early stage of burns, the systemic inflammatory response of patients is not yet obvious. Timely debridement and escharectomy when patients are in good physical condition can effectively reduce the incidence of visceral complications and systemic infections after burns, and improve long-term prognosis (32). Studies have shown that the treatment with VSD after scab grinding in the early stage can increase wound perfusion, shorten wound healing time, reduce redness and swelling of the wound edges, promote drainage of wound secretions, and reduce the incidence of wound infection, effectively prevent the progressive necrosis of the stasis area (33–35). The traditional mode of continuous negative pressure suction mode has shown good results in clinical work, but the continuous negative pressure state can cause the local tissue to adapt to the wound, and the blood perfusion will be insufficient. Under the treatment mode of intermittent negative pressure, the blood flow in the hypoperfusion area near the wound edge is guaranteed, which is conducive to the transport of nutrients and the discharge of metabolites (36), thereby shortening the wound healing time. At present, clinicians are constantly improving the VSD technique in order to achieve better clinical results.
Topical dressings
When we treating burn wounds, blister skin should be retained as a biofilm to protect the wound surface, create a moist wound microenvironment, and at the same time reduce the probability of infection, thereby limiting the progressive deepening of the wound surface and promoting wound recovery. For those who cannot retain the blister skin, we also have many artificial dressings to choose from. A variety of wound dressings have been developed, including biosynthetic (skin substitute) dressings, silver-containing dressings, and silicon-coated dressingsgauze, hydrocolloids, and hydrogels (37). The hydrogels are wildly used because of their similarity in structure and composition with natural extracellular matrix. Appropriate pore size helps the hydrogels to retain a moist healing enviroment for wound cell proliferation and angiogen (38). In recent years, researchers have also used xenograft tilapia. The skin covers the burn wound. Tilapia skin has a higher adhesion to wound skin, which can reduce the frequency of dressing changes and the use of analgesics, shorten the time of wound re-epithelialization, and may be a low-cost alternative to accelerate the healing of burn patients and reduce patient pain (39, 40).
Measures to improve the microcirculation of burn wounds
Moistened exposure burn therapy (MEBT)
Moistened exposure burn therapy (MEBT) is a local treatment method that treats burn wounds with moist exposed burn ointment(MEBO) and exposes burn tissue to repair and regeneration in a physiologically moist environment. MEBO applied to the wound surface can effectively improve the microcirculation in the zone of stasis and prevent the progressive necrosis of the tissue nearby (41). The advantages of MEBT are as follows: First, MEBT can effectively improve the microcirculation of the wound, avoiding the formation of a large number of microthrombosis and reducing the degree of ischemia and hypoxia in the zone of stasis. Secondly, MEBT can effectively reduce the capillary permeability and prevent the wound surface from massive extravasation. It relieves wound edema and reduce the possibility of exudative shock in patients after injury (42). Finally, MEBT can effectively reduce the generation of free radicals, inhibit the progressive deepening of the wound caused by lipid peroxidation damage, and play a role in the tissue protection in the stasis area.
Cold therapy
Appropriate cold therapy can reduce local thermal damage, decrease edema, inhibit the release of oxygen free radicals, reduce inflammation, and effectively improve local wound microcirculation. Treatment with moderate hypothermia at 31°C–33°C for 4 h, starting from 2 h after the burn resulted in a 23% reduction in burn depth compared to the control group at 24 h postburn. Simultaneous hypothermia-induced upregulation of skin protective genes such as CCL4, CCL6, and CXCL13 and downregulation of deleterious tissue remodeling genes such as MMP-9 may contribute to improved burn depth progression (43). However, perfusion in the zone of stasis may be further affected by vasoconstriction caused by supercooling, leading to further wound deterioration. The perfusion of the zone of stasis was somewhat improved under warm water (37°C), and the tissue viability was enhanced (44).
Early anticoagulation therapy
The blood in the early stage of burn is hypercoagulable, leading to stasis of microcirculation and microthrombosis, which is one of the important mechanisms for the development of stasis zone into coagulation zone (45). In recent years, anticoagulant and thrombolytic drugs have emerged to improve microcirculation thrombosis and vascular occlusion. For example, the well-known human erythropoietin (EPO) is a multifunctional cytoprotective cytokine in addition to promoting erythropoiesis, with anti-apoptotic, anti-inflammatory and immunomodulatory properties. Bohr et al. (46, 47) showed that systemic administration of the EPO derivative helical β-surface peptide (ARA290) within 24 h postburn prevents secondary microvascular thrombosis and inflammatory response in skin burns, and improves local microcirculation, while reducing the cellular stress response mediated by inflammatory factors such as TNF-α, thereby reducing the further deepening of the depth and area of burn wounds. Secondly, increased plasma endothelin levels following burns lead to thrombosis and occlusion of vessels in the dermis and vascular responses in the adjacent uninjured dermis. Previous studies have demonstrated that non-selective endothelin receptor antagonists [TAK-044 (48), PD145065 (49), etc.] can improve local microcirculation.
Conclusion and prospect
Burns are one of the most common and devastating forms of trauma. In the clinical environment, early debridement, early wound coverage, and the application of various therapies to promote healing can improve the progressive deepening and aggravation of early burn wounds, avoid the pain caused by large-scale surgical operations to a certain extent, and improve long-term outcomes. Given the complexity and diversity of the principles of each treatment, the categories mentioned above do not encompass the full range of functions of the measures mentioned. Combining multiple therapies may lead to better clinical benefits. We can also see that many therapies have their shortcomings, such as the issue of stem cell homing rates, and many drugs are still in clinical trials. At the same time, there are few studies on exosomes derived from mesenchymal stem cells. Their mechanisms of action overlap and need further research study.
Author contributions
ML drafted and proofed the manuscript. JZ and JJZ helped in critical review and revision. XW and FS edited the manuscript. DJ provided the financial support. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (grant no. 81372074, 81873934), Wang Zhengguo Foundation for Traumatic Medicine (growth factor rejuvenation project), and Technology Innovation Program of Jinan (grant no. 201704129).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bohr S, Patel SJ, Sarin D, Irimia D, Yarmush ML, Berthiaume F. Resolvin D2 prevents secondary thrombosis and necrosis in a mouse burn wound model. Wound Repair Regen. (2013) 21(1):35–43. doi: 10.1111/j.1524-475X.2012.00853.x
2. Edwards M, Cooper JS. Hyperbaric treatment of thermal burns. In: StatPearls. Treasure Island (FL): StatPearls Publishing (2022). https://pubmed.ncbi.nlm.nih.gov/29262193/
3. Li HS, Luo GX, Yuan ZQ. Research advances on the prevention and treatment strategies of burn wound progressive deepening. Chin J Burns. (2021) 37(12):1199–204. doi: 10.3760/cma.j.cn501120-20200828-00396
4. Shen CA. Preliminary discussion on the prevention and treatment of shock after severe burns. Chin J Burns Wounds. (2022) 38(1):9–12. doi: 10.3760/cma.j.cn501120-20211130-00402
5. Ferrer-Sola M, Sureda-Vidal H, Altimiras-Roset J, Fontsere-Candell E, Gonzalez-Martinez V, Espaulella-Panicot J, et al. Hydrosurgery as a safe and efficient debridement method in a clinical wound unit. J Wound Care. (2017) 26(10):593–9. doi: 10.12968/jowc.2017.26.10.593
6. Hirche C, Kreken Almeland S, Dheansa B, Fuchs P, Governa M, Hoeksema H, et al. Eschar removal by bromelain based enzymatic debridement (Nexobrid®) in burns: European consensus guidelines update. Burns. (2020) 46(4):782–96. doi: 10.1016/j.burns.2020.03.002
7. Arkoulis N, Mabvuure NT, Smith A, Barnes DE. Early experiences using bromelain-based enzymatic debridement in a tertiary burns centre in the United Kingdom: a retrospective case series review. J Plast Reconstr Aesthet Surg. (2021) 74(6):1402–7. doi: 10.1016/j.bjps.2020.12.028
8. Zhao R, Cao YQ, Zang CY, Wang YB. Advances in the research of dermabrasion in burn wounds. Zhonghua Shao Shang Za Zhi. (2018) 34(3):187–9. doi: 10.3760/cma.j.issn.1009-2587.2018.03.016
9. Hamblin MR. Novel pharmacotherapy for burn wounds: what are the advancements. Expert Opin Pharmacother. (2019) 20:305–21. doi: 10.1080/14656566.2018.1551880
10. Li Z, Yue M, Liu Y, Zhang P, Qing J, Liu H, et al. Advances of engineered hydrogel organoids within the stem cell field: a systematic review. Gels. (2022) 8(6):379. doi: 10.3390/gels8060379
11. Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. (1968) 6:230–47. doi: 10.1097/00007890-196803000-00009
12. da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. (2006) 119:2204–13. doi: 10.1242/jcs.02932
13. Yang Q, Zeng YN, Xu YA. Advances in researches on mesenchymal stem cell-derived exosomes involved in skin repairing and regeneration. Chinese Journal of Experimental Surgery. (2020) 37:1362–5. doi: 10.3760/cma.j.cn.421213-20190731-00571
14. Abbas OL, Özatik O, Gönen ZB, Öğüt S, Entok E, Özatik FY, et al. Prevention of burn wound progression by mesenchymal stem cell transplantation: deeper insights into underlying mechanisms. Ann Plast Surg. (2018) 81(6):715–24. doi: 10.1097/SAP.0000000000001620
15. Cornelissen AS, Maijenburg MW, Nolte MA, Voermans C. Organ-specific migration of mesenchymal stromal cells: who, when, where and why? Immunol Lett. (2015) 168:159–69. doi: 10.1016/j.imlet.2015.06.019
16. Zhang Y, Bi J, Huang J, Tang Y, Du S, Li P. Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int J Nanomedicine. (2020) 15:6917–34. doi: 10.2147/IJN.S264498
17. Bian D, Wu Y, Song G, Azizi R, Zamani A. The application of mesenchymal stromal cells (MSCs) and their derivative exosome in skin wound healing: a comprehensive review. Stem Cell Res Ther. (2022) 13(1):24. doi: 10.1186/s13287-021-02697-9
18. Shin KO, Ha DH, Kim JO, Crumrine DA, Meyer JM, Wakefield JS, et al. Exosomes from human adipose tissue-derived mesenchymal stem cells promote epidermal barrier repair by inducing de novo synthesis of ceramides in atopic dermatitis. Cells. (2020) 9(3):680. doi: 10.3390/cells9030680
19. Hu S, Li Z, Cores J, Huang K, Su T, Dinh PU, et al. Needle-free injection of exosomes derived from human dermal fibroblast spheroids ameliorates skin photoaging. ACS Nano. (2019) 13(10):11273–82. doi: 10.1021/acsnano.9b04384
20. Roşca AM, Ţuţuianu R, Titorencu ID. Mesenchymal stromal cells derived exosomes as tools for chronic wound healing therapy. Rom J Morphol Embryol. (2018) 59:655–62. PMID: 30534802
21. Ladak A, Olson J, Tredget EE, Gordon T. Differentiation of mesenchymal stem cells to support peripheral nerve regeneration in a rat model. Exp Neurol. (2011) 228:242–52. doi: 10.1016/j.expneurol.2011.01.013
22. Zeng YN, Kang YB, Xu YA. Research advances on skin sweat gland regeneration induced by stem cells and tissue engineering. Chin J Burns. (2021) 37(9):900–4. doi: 10.3760/cma.j.cn501120-20200624-00328
23. Li D, Chai J, Shen C, Han Y, Sun T. Human umbilical cord-derived mesenchymal stem cells differentiate into epidermal-like cells using a novel co-culture technique. Cytotechnology. (2014) 66:699–708. doi: 10.1007/s10616-013-9569-z
24. Yeganeh PM, Tahmasebi S, Esmaeilzadeh A. Cellular and biological factors involved in healing wounds and burns and treatment options in tissue engineering. Regen Med. (2022) 17(6):401–18. doi: 10.2217/rme-2022-0029
25. Rombouts WJ, Ploemacher RE. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia. (2003) 17:160–70. doi: 10.1038/sj.leu.2402763
26. Xiao MJ. Role of NLRP3inflammasome in burn wound progressionand its regulation by autophagy [Doctoral dissertation]. Beijing: Chinese PLA general hospital / medical school (2016).
27. Xiao M, Li L, Li C, Liu L, Yu Y, Ma L. 3,4-Methylenedioxy-β-nitrostyrene ameliorates experimental burn wound progression by inhibiting the NLRP3 inflammasome activation. Plast Reconstr Surg. (2016) 137(3):566e–75e. doi: 10.1097/01.prs.0000479972.06934.83
28. Chiu HW, Chen CH, Chang JN, Chen CH, Hsu YH. Far-infrared promotes burn wound healing by suppressing NLRP3 inflammasome caused by enhanced autophagy. J Mol Med. (2016) 94:809–19. doi: 10.1007/s00109-016-1389-0
29. Smolle C, Lindenmann J, Kamolz L, Smolle-Juettner FM. The history and development of hyperbaric oxygenation (HBO) in thermal burn injury. Medicina. (2021) 57(1):49. doi: 10.3390/medicina57010049
30. Chong SJ, Kan EM, Song C, Soh CR, Lu J. Characterization of early thermal burns and the effects of hyperbaric oxygen treatment: a pilot study. Diving Hyperb Med. (2013) 43:157–61. PMID: 24122191
31. Weitgasser L, Ihra G, Schäfer B, Markstaller K, Radtke C. Update on hyperbaric oxygen therapy in burn treatment. Wien Klin Wochenschr. (2021) 133:137–43. doi: 10.1007/s00508-019-01569-w
32. Singer AJ, Boyce ST. Burn wound healing and tissue engineering. J Burn Care Res. (2017) 38:e605–13. doi: 10.1097/bcr.0000000000000538
33. Morykwas MJ, David LR, Schneider AM, Whang C, Jennings DA, Canty C, et al. Use of subatmospheric pressure to prevent progression of partial-thickness burns in a swine model. J Burn Care Rehabil. (1999) 20(1 Pt 1):15–21. doi: 10.1097/00004630-199901001-00003
34. Kamolz LP, Andel H, Haslik W, Winter W, Meissl G, Frey M. Use of subatmospheric pressure therapy to prevent burn wound progression in human: first experiences. Burns. (2004) 30(3):253–8. doi: 10.1016/j.burns.2003.12.003
35. Xu JJ, Li LH, Zhang P, Lin C. The clinical effectiveness of treatment combining thinning tangential excision of eschar and gridding scratch operation and vacuum scaling drainage in patients with deep II degree burn of medium and small area. China Modern Doctor. (2018) 56(08):7–11.
36. Yang B, Liu K, Wang DX, Liang CY. Application of intermittent negative pressure attraction technique in complex wounds. World Latest Med Inf. (2018) 18:196–7. doi: 10.19613/j.cnki.1671-3141.2018.88.091
37. Qi L, Zhang C, Wang B, Yin J, Yan S. Progress in hydrogels for skin wound repair. Macromol Biosci. (2022) 22(7):e2100475. doi: 10.1002/mabi.202100475
38. Chen G, Zhou Y, Dai J, Yan S, Miao W, Ren L. Calcium alginate/PNIPAAm hydrogel with body temperature response and great biocompatibility: application as burn wound dressing. Int J Biol Macromol. (2022) 216:686–97. doi: 10.1016/j.ijbiomac.2022.07.019
39. Lima Júnior EM, De Moraes Filho MO, Costa BA, Rohleder AVP, Sales Rocha MB, Fechine FV, et al. Innovative burn treatment using tilapia skin as a xenograft: a phase II randomized controlled trial. J Burn Care Res. (2020) 41(3):585–92. doi: 10.1093/jbcr/irz205
40. Lima Júnior EM, de Moraes Filho MO, Costa BA, Fechine FV, Vale ML, Diógenes AKL, et al. Nile tilapia fish skin-based wound dressing improves pain and treatment-related costs of superficial partial-thickness burns: a phase III randomized controlled trial. Plast Reconstr Surg. (2021) 147(5):1189–98. doi: 10.1097/PRS.0000000000007895
41. Zhang Y, Cui GH, Liu FF, Huang XZ, Dai SM, Li QY, et al. Research progress in the application of MEBO in wound healing. Int Med Health Guidance News. (2022) 28(8):1134–7. doi: 10.3760/cma.j.issn.1007-1245.2022.08.023
42. Zheng A, Ma H, Liu X, Huang Q, Li Z, Wang L, et al. Effects of moist exposed burn therapy and ointment (MEBT/MEBO) on the autophagy mTOR signalling pathway in diabetic ulcer wounds. Pharm Biol. (2020) 58(1):124–30. doi: 10.1080/13880209.2019.1711430
43. Rizzo JA, Burgess P, Cartie RJ, Prasad BM. Moderate systemic hypothermia decreases burn depth progression. Burns. (2013) 39(3):436–44. doi: 10.1016/j.burns.2012.07.022
44. Tobalem M, Harder Y, Tschanz E, Speidel V, Pittet-Cuénod B, Wettstein R. First-aid with warm water delays burn progression and increases skin survival. J Plast Reconstr Aesthet Surg. (2013) 66(2):260–6. doi: 10.1016/j.bjps.2012.09.014
45. Yucel B, Coruh A, Deniz K. Salvaging the zone of stasis in burns by pentoxifylline: an experimental study in rats. J Burn Care Res. (2019) 40(2):211–9. doi: 10.1093/jbcr/irz005
46. Bohr S, Patel SJ, Shen K, Vitalo AG, Brines M, Cerami A, et al. Alternative erythropoietin-mediated signaling prevents secondary microvascular thrombosis and inflammation within cutaneous burns. Proc Natl Acad Sci U S A. (2013) 110(9):3513–8. doi: 10.1073/pnas.1214099110
47. Bohr S, Patel SJ, Vasko R, Shen K, Iracheta-Vellve A, Lee J, et al. Modulation of cellular stress response via the erythropoietin/CD131 heteroreceptor complex in mouse mesenchymal-derived cells. J Mol Med. (2015) 93(2):199–210. doi: 10.1007/s00109-014-1218-2
48. Battal MN, Hata Y, Matsuka K, Ito O, Matsuda H, Yoshida Y, et al. Reduction of progressive burn injury by using a new nonselective endothelin-A and endothelin-B receptor antagonist, TAK-044: an experimental study in rats. Plast Reconstr Surg. (1997) 99(6):1610–9. doi: 10.1097/00006534-199705010-00022
Keywords: burns, mechanism, wound progression, mesenchymal stem cells, wound healing
Citation: Lu M, Zhao J, Wang X, Zhang J, Shan F and Jiang D (2022) Research advances in prevention and treatment of burn wound deepening in early stage. Front. Surg. 9:1015411. doi: 10.3389/fsurg.2022.1015411
Received: 9 August 2022; Accepted: 28 September 2022;
Published: 21 October 2022.
Edited by:
Biao Cheng, General Hospital of Southern Theater Command of PLA, China© 2022 Lu, Zhao, Wang, Zhang, Shan and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Duyin Jiang amR5YnMyQHZpcC4xNjMuY29t
Specialty Section: This article was submitted to Reconstructive and Plastic Surgery, a section of the journal Frontiers in Surgery
 Meiqi Lu
Meiqi Lu Jie Zhao2
Jie Zhao2 Duyin Jiang
Duyin Jiang