- Department of Orthopedics, Beijing Chaoyang Hospital, Capital Medical University, Beijing, China
Background: This study aimed to identify radiological risk factors associated with reoperation after percutaneous transforaminal endoscopic decompression (PTED) for degenerative lumbar spinal stenosis (DLSS).
Methods: The preoperative clinical data of 527 consecutive patients with DLSS who underwent PTED were retrospectively reviewed. Overall, 44 patients who underwent reoperation were matched for age, sex, body mass index, and surgical segment to 132 control patients with excellent or good clinical outcomes. Radiological characteristics were compared between the groups using independent sample t-tests and Pearson's chi-square tests. A predictive model was established based on multivariate logistic regression analysis.
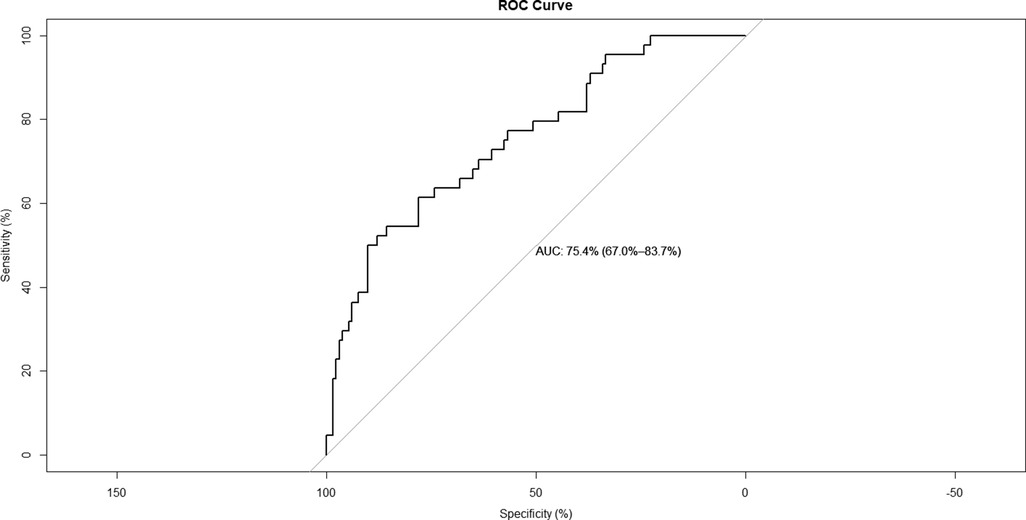
Results: The analyses revealed significant differences in the presence of lumbosacral transitional vertebra (LSTV, 43.2% vs. 17.4%, p = 0.001), the number of levels with senior-grade disc degeneration (2.57 vs. 1.96, p = 0.018) and facet degeneration (1.91 vs. 1.25 p = 0.002), and the skeletal muscle index (SMI, 849.7 mm2/m2 vs. 1008.7 mm2/m2, p < 0.001) between patients in the reoperation and control groups. The results of the logistic analysis demonstrated that LSTV (odds ratio [OR] = 2.734, 95% confidence interval [CI]:1.222–6.117, p < 0.014), number of levels with senior-grade facet degeneration (OR = 1.622, 95% CI:1.137–2.315, p = 0.008), and SMI (OR = 0.997, 95% CI:0.995–0.999, p = 0.001) were associated with reoperation after PTED. The application of the nomogram based on these three factors showed good discrimination (area under the receiver operating characteristic curve 0.754, 95% CI 0.670–0.837) and good calibration.
Conclusion: LSTV, more levels with senior-grade facet degeneration, and severe paraspinal muscle atrophy are independent risk factors for reoperation after PTED. These factors can thus be used to predict reoperation risk and to help tailor treatment plans for patients with DLSS.
Introduction
Degenerative lumbar spinal stenosis (DLSS) is one of the most diagnosed and treated pathologies of the spine (1). In the last two decades, as the use of minimally invasive techniques has become widespread, percutaneous transforaminal endoscopic decompression (PTED) has become a routine procedure for treating foraminal and lateral recess stenosis, and even central canal stenosis. In addition to herniated discs, PTED can be used to remove the hyperplastic ligament flavum and facet processes. This technique has been proven to be safe, clinically feasible, and effective (2).
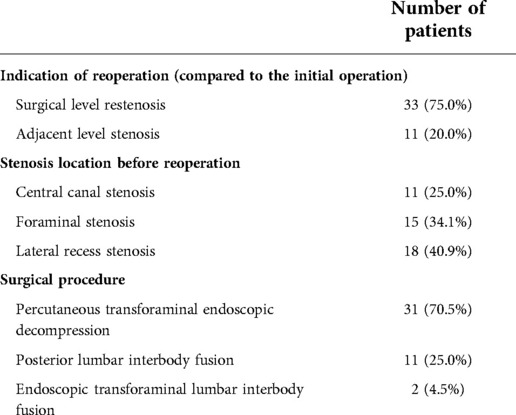
However, unsatisfactory clinical outcomes are common in DLSS patients. Previous studies have shown that 3.5%–17.7% of patients with DLSS required reoperation after minimally invasive decompression (1, 3–6). Reoperation is commonly defined as an additional lumbar operation in a patient who has experienced a pain-free interval of at least one month after the initial PTED. After excluding complications related to the surgical technique, the main reasons for reoperation were restenosis or adjacent segment stenosis due to the progression of lumbar degeneration (4). Risk factors included age, obesity, decompression level, and spondylolisthesis (7, 8). However, we speculated that, in addition to these factors, some radiological parameters may also be helpful in predicting postoperative degeneration and reoperation.
Radiological evaluation included intervertebral disc, ligament flavum, facet joint, paraspinal muscle, and range of motion. Disc degeneration is considered the initial factor of segmental degeneration (9), while hypertrophy of the ligamentum flavum and facet joint is an important cause of nerve root compression. In recent years, the paraspinal muscles are gaining increasing attention. A decrease in paraspinal muscle function can lead to vertebral instability (10). Thus, degeneration of the above-mentioned structures has been considered significantly associated with poor surgical outcomes and revisions after traditional spinal fusion (11, 12). However, to the best of our knowledge, few studies have focused on the radiological characteristics of patients who underwent reoperation after PTED for DLSS.
In the present study, we performed a retrospective, matched case-control study to investigate the association between radiological parameters and reoperation after PTED and to build a model to predict reoperation risk based on the verified risk factors.
Methods
Patient population
We retrospectively reviewed the clinical and imaging data of patients with lateral recess or foraminal stenosis who underwent single-level PTED at our institution between January 2016 and July 2020. The inclusion criteria were as follows: (1) age >40 years, (2) unilateral symptoms of lateral recess or foraminal stenosis, radiological findings consistent with clinical symptoms in terms of pain location. (3) failure of conservative treatment for at least three months. The corresponding exclusion criteria were as follows: (1) spondylolisthesis greater than grade-1, (2) multilevel symptomatic lumbar stenosis; (3) symptoms caused only by disc herniation; (4) significant residual pain or other short-term complications after PTED; (5) a follow-up time of less than 24 months or loss to follow-up; (6) a history of lumbar surgery; (7) nondegenerative lumbar diseases such as tumor, infection, and trauma; and (8) insufficient clinical or imaging data.
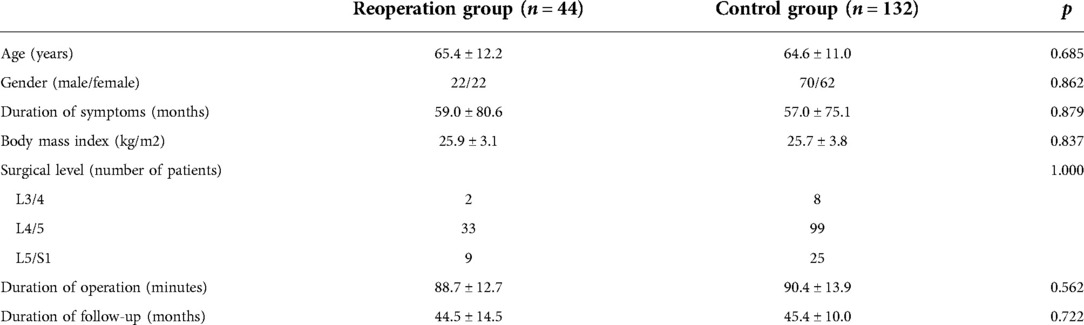
Overall, we identified 527 eligible patients, of whom 44 underwent additional PTED or spinal fusion at the same or adjacent level. Information on patients who underwent reoperation is summarized in Table 1. A control group of patients with excellent or good clinical outcomes were propensity score-matched to the reoperation group in terms of age, sex, body mass index (BMI), and surgical segment in a 1:3 manner. The demographic data of the patients in the two groups are summarized in Table 2. This study was performed in accordance with the Declaration of Helsinki, and approval was obtained from the institutional ethics committee.
Surgical methods
All PTED procedures were performed by a senior surgeon with experience of more than 100 percutaneous endoscopic procedures. In patients with multilevel radiographic stenosis, nerve root blocking was performed to determine the level of responsibility. PTED was further performed as follows: The entire procedure was performed with the patient in the prone position, and under local anesthesia. The entry point was set at 10–14 cm lateral to the spinal midline at the index intervertebral level. A puncture needle was inserted into the superior articular process (SAP). An 8 mm working cannula was placed in contact with the surface of the SAP after expending the surgical approach using serial hollow cannulas. A trephine was then used to remove the capsule and the ventral side of the SAP. Decompression was performed using continuous irrigation under direct vision. The osteophyte, thickened ligament flavum, perineural fat, degenerated annulus fibrosus, and nucleus pulposus were removed to ensure complete decompression.
Data collection and assessment
The demographic and clinical data, including age, sex, body mass index (BMI), smoking status, duration of symptoms, surgical level, duration of surgery, and follow-up duration, were recorded for all enrolled patients. Radiological data included disc degeneration grade, spinal stenosis grade, facet joint degeneration grade, lumbar lordosis, disc height index (DHI), disc wedging angle, facet orientation, facet tropism, paraspinal muscle degeneration, sagittal range of motion (sROM), Modic changes, and lumbosacral transitional vertebra (LSTV).
The disc degeneration grade was evaluated using sagittal T2-weighted magnetic resonance imaging (MRI) according to Pfirrmann (13). Spinal stenosis grade was evaluated using axial T2-weighted MRI according to Schizas (14). Facet joint degeneration was evaluated using axial computerized tomography images according to Weishaupt (15). The grade of surgical-level degeneration of the aforementioned structures and the number of lumbar levels with senior-grade degeneration were recorded. The skeletal muscle index (SMI) (16) was used to assess paraspinal muscle degeneration as body height has been proven to be related to paraspinal muscle mass. SMI was calculated as the bilateral functional cross-sectional area (Figure 1) of the paraspinal muscle at the mid-disk of the L4/5 level (expressed as millimeters squared)/the square of the patient's height (expressed as meters squared). Endplate degeneration was evaluated according to Modic changes. The measurements of disc wedging angle, DHI, sROM, lumbar lordosis, facet orientation, and facet tropism are shown in Figure 2.
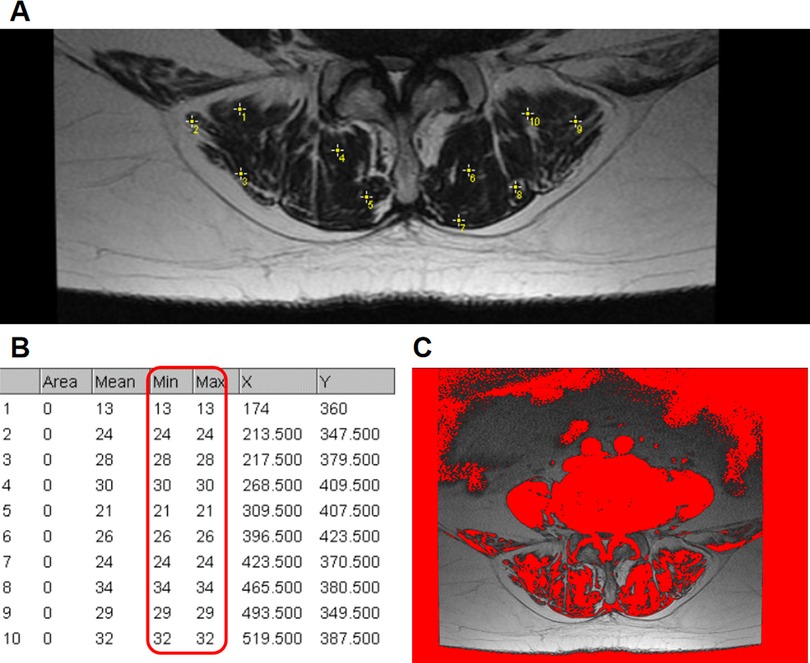
Figure 1. Use of the “Multi-point” tool to determine the threshold to distinguish lean muscle tissue from fat tissue. (A) Ten sample points without any visible pixel of fat tissue within the bilateral paraspinal muscle were selected. (B) The maximum signal intensity of the ten points was determined as the threshold. (C) An example axial T2 weighted MR image of the functional cross-sectional area of the muscles (red area), using the threshold method.
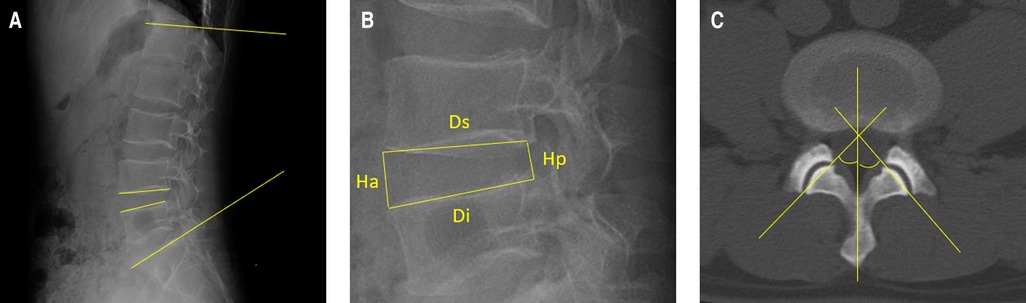
Figure 2. Illustration of the measurements of the radiological parameters. (A) Lumbar lordosis is defined as the angle between the superior endplates of L1 and S1. The disc wedging angle is defined as the angle between the lower endplate of the upper vertebra and the upper endplate of the lower vertebra. Additionally, the sagittal range of motion is defined as the absolute difference between the disc wedging angles in flexion and extension positions. (B) Disc height index is calculated with the equation: [(anterior disc height + posterior disc height)/(superior disc depth + inferior disc depth)]×100. (C) A line is drawn to connect the anterior and posterior margins of the superior articular process. The other line represents the midsagittal line. The facet orientation is calculated as the mean of the facet angle between the right and left sides. The facet tropism is the absolute difference between the two sides.
Statistical analysis
For statistical analyses, data were analyzed using statistical software (SPSS version 20.0, for Windows, IBM) and R 4.1.3 (The R Foundation for Statistical Computing, Vienna, Austria). All demographic, clinical, and radiological data were compared between the two groups using independent sample t-tests and Pearson's chi-square tests. Variables with p-values less than 0.10 were further included in the multivariable logistic regression analysis. Variance inflation factor (VIF) was used to diagnose collinearity. Based on the results of the regression analysis, a nomogram for reoperation probability was constructed, and its performance was assessed using the area under the receiver operating characteristic curve and a visual calibration plot. All continuous values are presented as the mean ± standard deviation. Statistical significance was set at p < 0.05.
Results
A total of 176 patients were included in this study which aimed to identify radiological predictors for reoperation after PTED (44 in the reoperation group and 132 in the control group). Demographic and clinical characteristics of the enrolled patients are shown in Table 2. There were no significant differences in age, sex, duration of symptoms, BMI, surgical level, duration of operation, or duration of follow-up between the two matched groups. Among the patients in the reoperation group, the mean reoperation time was 16.8 months.
The results of the univariate analyses of radiological parameters are shown in Table 3. Compared with patients in the control group, patients in the reoperation group had a higher risk of LSTV (43.2% vs. 17.4%, p = 0.001), greater number of levels with senior-grade disc degeneration (2.57 vs. 1.96, p = 0.018) and facet degeneration (1.91 vs. 1.25, p = 0.002), and a smaller SMI (849.7 mm2/m2 vs. 1008.7 mm2/m2, p < 0.001). There were no significant differences in other radiological characteristics between the two groups (p > 0.05).
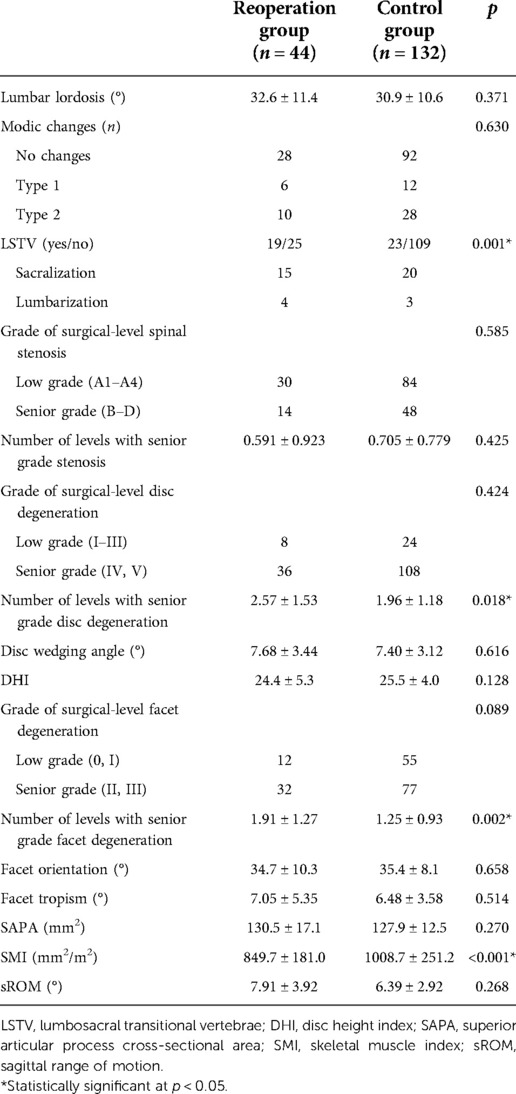
Table 3. Univariate analyses of the radiological parameters between patients in the reoperation and control groups.
The grade of surgical-level disc degeneration, LSTV, number of levels with senior-grade disc degeneration, number of levels with senior-grade facet degeneration, and SMI were included in further multivariate logistic regression analyses. The collinearity test revealed no collinearity among the variables (VIFs < 10). Multivariate logistic regression analysis demonstrated that LSTV (odds ratio [OR] = 2.734, 95% confidence interval [CI]:1.222–6.117, p < 0.014) and number of levels with senior-grade facet degeneration (OR = 1.622, 95% CI:1.137–2.315, p = 0.008) were independent risk factors for reoperation after PTED. SMI (OR = 0.997, 95% CI:0.995–0.999; p = 0.001) was a protective factor for reoperation after PTED (Table 4).
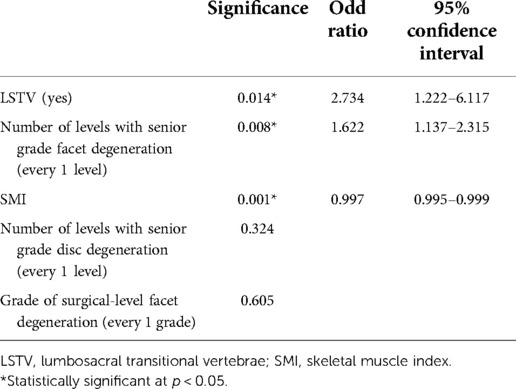
Table 4. Multivariate regression model of the predictors for reoperation following percutaneous transforaminal endoscopic decompression.
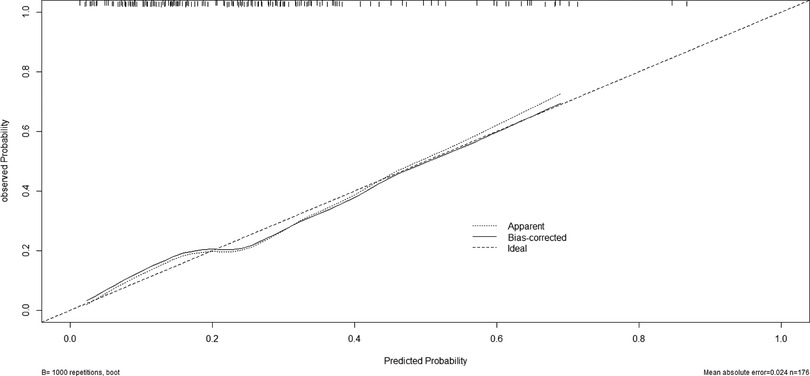
A nomogram for predicting reoperation after PTED was constructed based on radiological factors selected by logistic regression (Figure 3). The calibration curve of the nomogram indicated that the predicted probability agreed well with the actual recurrence (Figure 4). The area under the receiver operating characteristic curve of the model was 0.754 (95% CI,0.670–0.837), which shows the reliability of this model (Figure 5).
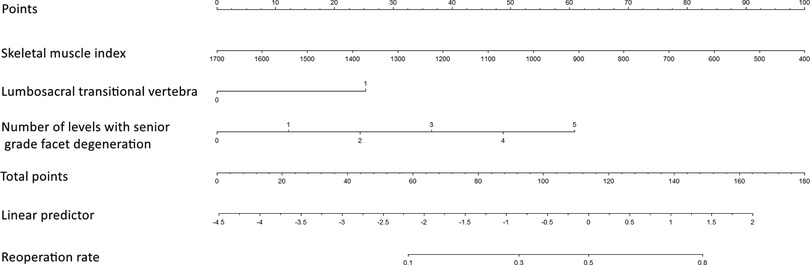
Figure 3. Nomogram for predicting reoperation risk in patients who underwent percutaneous transforaminal endoscopic lumbar decompression.
Discussion
PTED is a widely used surgical technique for DLSS treatment. However, recurrent postoperative symptoms and reoperations are disturbing problems, and the risk factors for reoperation after PTED have not been fully investigated. In this retrospective case-control study, we compared the radiological characteristics between patients who underwent reoperation and those with satisfactory outcomes. Patients in the reoperation group achieved remission immediately after primary surgery. Thus, the indication for reoperation was the progression or recurrence of lumbar stenosis, rather than surgical complications. The results of multivariate logistic regression analyses showed that the presence of LSTV, the number of levels with senior-grade facet degeneration, and a smaller SMI were independent risk factors for reoperation after PTED. A nomogram based on these radiological parameters could predict the risks of reoperation occurrence after PTED, with optimal discrimination and excellent calibration.
Urakawa (17) previously investigated factors associated with reoperations following posterior lumbar decompression and found that age <70 years and symptomatic neurogenic claudication were significantly associated with secondary fusions. Cummins (18) further reported that female sex and history of lumbar decompression were risk factors for revision surgery. Yin (7) concluded that age and BMI could influence recurrence rate. Our previous study also confirmed that age was a risk factor for reoperation after PTED (19). However, we found that the associations between age, sex, and reoperation were ambiguous in the existing literature. The conflicting results among previous studies may reflect differences in cultural and socioeconomic situations. Therefore, in this study we focused on the radiological characteristics, which are relatively objective indicators, and the influence of demographic characteristics on reoperation was decreased by matching. However, previous studies have proposed that risk factors might differ among subgroups (20). The main objects of this study were geriatric patients, and further studies stratified by factors such as age and sex based on a larger sample size, are required in the future.
In this study, the number of levels with senior-grade facet degeneration and the presence of LSTV were found to be risk factors for reoperation after PTED. Facet degeneration is considered an adaptive change of increased compression (21). Articular processes tend to maintain lumbar stability through hyperplasia and lengthening of the articular surface (22). Therefore, multilevel facet degeneration reflects severe lumbar aging and abrasion. In addition, hypertrophy of the superior articular process itself is a significant cause of nerve root compression (9), increasing the risk of progression of foraminal and lateral recess stenosis.
LSTV is a common anatomical variant, with a prevalence of 7%–36% (23). Although the biomechanical influence of the LSTV has not been fully explained, many scholars believe that individuals with LSTV are at a higher risk of degenerative diseases in each lumbar level, especially at the adjacent cephalad level (23–25). One potential explanation for this is that LSTV leads to uneven loads on the lumbar spine, resulting in hypermobility and an increase in segmental stress (26). Additionally, the enlarged transverse process and sacral ala are more likely to impinge on nerve roots and cause relative symptoms (27). Furthermore, there are several subtypes of LSTV. Some researchers believe that sacralization can lead to a more abnormal increase in segmental stress, just like the pathological changes after a spinal fusion (24, 28). Besides, unilateral LSTV may lead to asymmetric stress on the lumbar spine and thus more severe degeneration compared with bilateral LSTV (28). However, the influence of these subtypes is still under controversial and most of the previous studies tended to regard LSTV as a unified feature (24, 25). In this study, the small sample size limits our further analysis of LSTV. Larger studies are needed to investigate the effect of various types of LSTV on the prognosis of lumbar spinal stenosis.
Additionally, we found that patients who underwent reoperation had higher levels of senior-grade disc degeneration, which may have a similar effect on the lumbar spine as facet degeneration and LSTV (29). However, this is not a risk factor for reoperation, which may be because disc degeneration is common in geriatric patients.
Paraspinal muscle degeneration is a complex process which is significantly associated with spinal degeneration, deformity, and dysfunction, which has been a hot topic in the past decade. In many previous studies, the paraspinal muscle was evaluated as the sum of the multifidus muscle and erector spinae (30), since these act synergistically in most instances, and the boundaries between them are unclear in some cases. The paraspinal muscles attach directly to the lumbar vertebrae and play a significant role in the rotation, flexion, extension, and maintenance of lordosis of the lumbar spine (31, 32). In patients with paraspinal muscle atrophy, the role of muscle fibers as stabilizers is weakened, further causing increased spinal axial loading (10, 33). Additionally, the loss of muscle function may lead to a degree of sagittal and coronal misalignment. These changes can lead to disc degeneration, ligament flavum hypertrophy, and facet joint osteoarthritis, all of which cause lumbar stenosis (34, 35). Thus, paraspinal muscle atrophy may accelerate the progression of lumbar degeneration (36), which could explain the difference in paraspinal muscle mass between the groups (Figure 6).
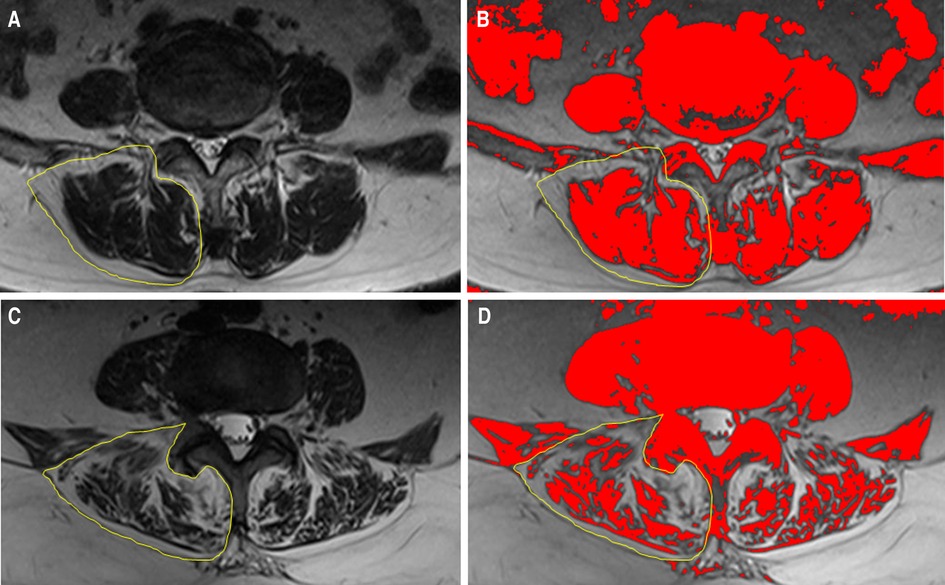
Figure 6. Illustration of the comparison between patients who underwent reoperation after PTED and controls. The circled area indicates the unilateral paraspinal muscle. (A,B)Preoperative paraspinal muscle mass of a 62-year-old woman in the control group. (C,D)Preoperative paraspinal muscle mass of a 65-year-old woman in the reoperation group.
In general, all the risk factors for reoperation identified in this study were associated with weakening of the support structure and an increase in axial loading. Although these changes are difficult to reverse, our results nevertheless point to several tactics that can be implemented in clinical practice. First, for high-risk patients, cautious selection of the appropriate treatment modality and informing of the possibility of relapse of the disease in advance are necessary. Second, it has been demonstrated that increased mechanical loading due to strenuous exercise and overstrain can lead to poor clinical outcome (37). The high-risk patients identified in this study were less capable of withstanding lumbar stress. Therefore, such individuals should pay special attention to avoiding overwork in their daily lives. Third, among the risk factors for reoperation in this study, paraspinal muscle degeneration was the only factor that could be ameliorated by intervention. Previous studies have shown that physical therapy such as trunk exercises and cupping can increase multifidus muscle thickness and improve surgical outcomes (38, 39).
Interestingly, no radiological characteristics at the surgical level, such as the grade of disc degeneration and presence of central canal stenosis, were found to be risk factors for reoperation after PTED in this study. This result differs from some previous findings (40). We speculate that this difference may be explained by the following points. First, with the development of a minimally invasive technique, PTED can achieve satisfactory results in the treatment of various lumbar degenerative diseases (2). After a detailed preoperative evaluation, a surgeon who has surpassed the learning curve can adequately decompress the spinal canal and nerve root in most cases. Second, spondylolisthesis is considered a risk factor for reoperation (41); however, it was excluded from the study. For geriatric DLSS patients without severe instability, lumbar discs and facet joints can achieve an instability-to-restabilization process with degeneration and reconstruction of the lumbar spine (42). Thus, segmental stability can be maintained even with severe degeneration, and segmental factors may have a limited effect on the progression of lumbar degeneration. Similarly, the level of Modic changes is not significantly associated with reoperation in this study. The presence of Modic changes has been considered to indicate local instability and therefore a risk factor for recurrent disc herniation postoperatively (20, 43). However, as is discussed above, this influence may not be as significant in geriatric DLSS patients. This conclusion is consistent with other previous findings (20, 44). Notably, due to the mechanical defect, patients with Modic changes, especially type 1, have a trend of deterioration in postoperative back pain, in which endoscopic procedures may be less effective (45).
This study had several limitations. First, the study was retrospective, based on a relatively small sample size, and the control group was established using propensity score matching. Second, the decision to reoperate was influenced by many factors, including osteoporosis, sociopsychological conditions, and personal choices. However, these confounders were not considered in the present study. Third, we identified several risk factors associated with reoperation after PTED; however, we could not determine the causal relationship between them. Therefore, long-term longitudinal studies are warranted. Fourth, although SMI has been recognized as the most accurate indicator of paraspinal muscle atrophy, it is a complicated continuous variable that results in a small odds ratio. As such, there is a need for a scientific grading system for paraspinal degeneration.
Conclusion
This study confirmed that the presence of LSTV, more levels with senior-grade facet degeneration, and severe paraspinal muscle atrophy were independent risk factors for reoperation after PTED. A nomogram based on these factors could be applied in clinical practice to predict the need for reoperation and help improve individualized treatment planning.
Data availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics statement
The research conducted has been performed in accordance with the Declaration of Helsinki. Approval for the study was obtained from the ethics committees of the Beijing Chaoyang Hospital (Registration number: 2021-8-5-5).
Author contributions
AW and TW: conceived the idea and wrote the manuscript; SY and NF: conducted manuscript review and editing; FS and PD: participated in data collection; LZ: supervised. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Alimi M, Hofstetter CP, Pyo SY, Paulo D, Härtl R. Minimally invasive laminectomy for lumbar spinal stenosis in patients with and without preoperative spondylolisthesis: clinical outcome and reoperation rates. J Neurosurg Spine. (2015) 22:339. doi: 10.3171/2014.11.SPINE13597
2. Lee C, Yoon K, Kim S. Percutaneous endoscopic decompression in lumbar canal and lateral recess stenosis—the surgical learning curve. Neurospine. (2019) 16:63–71. doi: 10.14245/ns.1938048.024
3. Bao BX, Zhou JW, Yu PF, Chi C, Qiang H, Yan H. Transforaminal endoscopic discectomy and foraminoplasty for treating central lumbar stenosis. Orthop Surg. (2019) 11:1093–100. doi: 10.1111/os.12559
4. Schöller K, Steingrüber T, Stein M, Vogt N, Müller T, Pons-Kühnemann J, et al. Microsurgical unilateral laminotomy for decompression of lumbar spinal stenosis: long-term results and predictive factors. Acta Neurochir. (2016) 158:1103–13. doi: 10.1007/s00701-016-2804-6
5. Rihn JA, Radcliff K, Hilibrand AS, Anderson DT, Zhao W, Lurie J, et al. Does obesity affect outcomes of treatment for lumbar stenosis and degenerative spondylolisthesis? Analysis of the spine patient outcomes research trial (sport). Spine. (2012) 37:1933–46. doi: 10.1097/BRS.0b013e31825e21b2
6. Xie P, Feng F, Chen Z, He L, Yang B, Chen R, et al. Percutaneous transforaminal full endoscopic decompression for the treatment of lumbar spinal stenosis. Bmc Musculoskel Dis. (2020) 21:546. doi: 10.1186/s12891-020-03566-x
7. Yin S, Du H, Yang W, Duan C, Feng C, Tao H. Prevalence of recurrent herniation following percutaneous endoscopic lumbar discectomy: a meta-analysis. Pain Physician. (2018) 21:337–50.30045591
8. Schar RT, Kiebach S, Raabe A, Ulrich CT. Reoperation rate after microsurgical uni- or bilateral laminotomy for lumbar spinal stenosis with and without low-grade spondylolisthesis: what do preoperative radiographic parameters tell us? Spine. (2019) 44:E245–51. doi: 10.1097/BRS.0000000000002798
9. Jenis LG, An HS. Spine update. Lumbar foraminal stenosis. Spine. (2000) 25:389–94. doi: 10.1097/00007632-200002010-00022
10. Ozcan-Eksi EE, Yagci I, Erkal H, Demir-Deviren S. Paraspinal muscle denervation and balance impairment in lumbar spinal stenosis. Muscle Nerve. (2016) 53:422–30. doi: 10.1002/mus.24759
11. Zotti MGT, Boas FV, Clifton T, Piche M, Yoon WW, Freeman BJC. Does pre-operative magnetic resonance imaging of the lumbar multifidus muscle predict clinical outcomes following lumbar spinal decompression for symptomatic spinal stenosis? Eur Spine J. (2017) 26:2589–97. doi: 10.1007/s00586-017-4986-x
12. Fortin M, Lazáry À, Varga PP, Battié MC. Association between paraspinal muscle morphology, clinical symptoms and functional status in patients with lumbar spinal stenosis. Eur Spine J. (2017) 26:2543–51. doi: 10.1007/s00586-017-5228-y
13. Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. (2001) 26:1873–8. doi: 10.1097/00007632-200109010-00011
14. Schizas C, Theumann N, Burn A, Tansey R, Wardlaw D, Smith FW, et al. Qualitative grading of severity of lumbar spinal stenosis based on the morphology of the dural sac on magnetic resonance images. Spine. (2010) 35:1919–24. doi: 10.1097/BRS.0b013e3181d359bd
15. Weishaupt D, Zanetti M, Boos N, Hodler J. Mr imaging and ct in osteoarthritis of the lumbar facet joints. Skeletal Radiol. (1999) 28:215–9. doi: 10.1007/s002560050503
16. Chang MY, Park Y, Ha JW, Zhang HY, Lee SH, Hong TH, et al. Paraspinal lean muscle mass measurement using spine mri as a predictor of adjacent segment disease after lumbar fusion: a propensity score-matched case-control analysis. AJR Am J Roentgenol. (2019):1–8. doi: 10.2214/AJR.18.20441. [Epub ahead of print]
17. Urakawa H, Jones T, Samuel A, Vaishnav AS, Othman Y, Virk S, et al. The necessity and risk factors of subsequent fusion after decompression alone for lumbar spinal stenosis with lumbar spondylolisthesis: 5 years follow-up in two different large populations. Spine J. (2020) 20:1566–72. doi: 10.1016/j.spinee.2020.04.026
18. Cummins DD, Callahan M, Scheffler A, Theologis AA. 5-year Revision rates after elective multilevel lumbar/thoracolumbar instrumented fusions in older patients: an analysis of state databases. J Am Acad Orthop Surg. (2022) 30:476–83. doi: 10.5435/JAAOS-D-21-00643
19. Wang T, Wang A, Zang L, Fan N, Wu Q, Lu X, et al. Reoperations after percutaneous endoscopic transforaminal decompression for treating lumbar spinal stenosis: incidence and predictors. Global Spine J. (2022):21925682221081030. doi: 10.1177/21925682221081030. [Epub ahead of print]35225015
20. Zhao C, Zhang H, Wang Y, Xu D, Han S, Meng S, et al. Nomograms for predicting recurrent herniation in petd with preoperative radiological factors. J Pain Res. (2021) 14:2095–109. doi: 10.2147/JPR.S312224
21. Kalichman L, Suri P, Guermazi A, Li L, Hunter DJ. Facet orientation and tropism: associations with facet joint osteoarthritis and degeneratives. Spine. (2009) 34:E579–85. doi: 10.1097/BRS.0b013e3181aa2acb
22. Liu X, Zhao X, Long Y, Huang K, Xie D, Wang F, et al. Facet sagittal orientation: possible role in the pathology of degenerative lumbar spinal stenosis. Spine. (2018) 43:955–8. doi: 10.1097/BRS.0000000000002493
23. Farshad-Amacker NA, Herzog RJ, Hughes AP, Aichmair A, Farshad M. Associations between lumbosacral transitional anatomy types and degeneration at the transitional and adjacent segments. Spine J. (2015) 15:1210–6. doi: 10.1016/j.spinee.2013.10.029
24. Abbas J, Peled N, Hershkovitz I, Hamoud K. Is lumbosacral transitional vertebra associated with degenerative lumbar spinal stenosis? Biomed Res Int. (2019) 2019:3871819. doi: 10.1155/2019/3871819
25. Griffith JF, Xiao F, Hilkens A, Griffith I, Leung J. Increased vertebral body area, disc and facet joint degeneration throughout the lumbar spine in patients with lumbosacral transitional vertebrae. Eur Radiol. (2022) 32:6238–46. doi: 10.1007/s00330-022-08736-0
26. Cheng L, Jiang C, Huang J, Jin J, Guan M, Wang Y. Lumbosacral transitional vertebra contributed to lumbar spine degeneration: an mr study of clinical patients. J Clin Med. (2022) 11(9):2339. doi: 10.3390/jcm11092339
27. Otani K, Konno S, Kikuchi S. Lumbosacral transitional vertebrae and nerve-root symptoms. J Bone Joint Surg Br. (2001) 83:1137–40. doi: 10.1302/0301-620x.83b8.11736
28. Fidan F, Balaban M, Hatipoglu SC, Veizi E. Is lumbosacral transitional vertebra associated with lumbar disc herniation in patients with low back pain? Eur Spine J. (2022) 31:2907–12. doi: 10.1007/s00586-022-07372-y
29. Desmoulin GT, Pradhan V, Milner TE. Mechanical aspects of intervertebral disc injury and implications on biomechanics. Spine. (2020) 45:E457–64. doi: 10.1097/BRS.0000000000003291
30. Kim JY, Ryu DS, Paik HK, Ahn SS, Kang MS, Kim KH, et al. Paraspinal muscle, facet joint, and disc problems: risk factors for adjacent segment degeneration after lumbar fusion. Spine J. (2016) 16:867–75. doi: 10.1016/j.spinee.2016.03.010
31. Ng JK, Richardson CA, Jull GA. Electromyographic amplitude and frequency changes in the iliocostalis lumborum and multifidus muscles during a trunk holding test. Phys Ther. (1997) 77:954–61. doi: 10.1093/ptj/77.9.954
32. Andersson EA, Grundstrom H, Thorstensson A. Diverging intramuscular activity patterns in back and abdominal muscles during trunk rotation. Spine. (2002) 27:E152–60. doi: 10.1097/00007632-200203150-00014
33. Malakoutian M, Street J, Wilke H, Stavness I, Dvorak M, Fels S, et al. Role of muscle damage on loading at the level adjacent to a lumbar spine fusion: a biomechanical analysis. Eur Spine J. (2016) 25:2929–37. doi: 10.1007/s00586-016-4686-y
34. Lao YJ, Xu TT, Jin HT, Ruan HF, Wang JT, Zhou L, et al. Accumulated spinal axial biomechanical loading induces degeneration in intervertebral disc of mice lumbar spine. Orthop Surg. (2018) 10:56–63. doi: 10.1111/os.12365
35. Adams MA, Dolan P. Recent advances in lumbar spinal mechanics and their clinical significance. Clin Biomech. (1995) 10:3–19. doi: 10.1016/0268-0033(95)90432-9
36. Kalichman L, Hunter DJ. Diagnosis and conservative management of degenerative lumbar spondylolisthesis. Eur Spine J. (2008) 17:327–35. doi: 10.1007/s00586-007-0543-3
37. Kong M, Xu D, Gao C, Zhu K, Han S, Zhang H, et al. Risk factors for recurrent l4-5 disc herniation after percutaneous endoscopic transforaminal discectomy: a retrospective analysis of 654 cases. Risk Manag Healthc Policy. (2020) 13:3051–65. doi: 10.2147/RMHP.S287976
38. Hebert JJ, Fritz JM, Thackeray A, Koppenhaver SL, Teyhen D. Early multimodal rehabilitation following lumbar disc surgery: a randomised clinical trial comparing the effects of two exercise programmes on clinical outcome and lumbar multifidus muscle function. Brit J Sport Med. (2014) 49:100–6. doi: 10.1136/bjsports-2013-092402
39. Markowski A, Sanford S, Pikowski J, Fauvell D, Cimino D, Caplan S. A pilot study analyzing the effects of chinese cupping as an adjunct treatment for patients with subacute low back pain on relieving pain, improving range of motion, and improving function. J Altern Complement Med. (2014) 20:113–7. doi: 10.1089/acm.2012.0769
40. Shi H, Zhu L, Jiang Z, Wu X. Radiological risk factors for recurrent lumbar disc herniation after percutaneous transforaminal endoscopic discectomy: a retrospective matched case-control study. Eur Spine J. (2021) 30:886–92. doi: 10.1007/s00586-020-06674-3
41. Ikegami D, Hosono N, Mukai Y, Tateishi K, Fuji T. Preoperative retrolisthesis as a predictive risk factor of reoperation due to delayed-onset symptomatic foraminal stenosis after central decompression for lumbar canal stenosis without fusion. Spine J. (2017) 17:1066–73. doi: 10.1016/j.spinee.2017.03.006
42. Kirkaldy-Willis WH, Farfan HF. Instability of the lumbar spine. Clin Orthop Relat Res. (1982) 165:110–23.
43. Hao L, Li S, Liu J, Shan Z, Fan S, Zhao F. Recurrent disc herniation following percutaneous endoscopic lumbar discectomy preferentially occurs when modic changes are present. J Orthop Surg Res. (2020) 15:176. doi: 10.1186/s13018-020-01695-6
44. Ulrich NH, Burgstaller JM, Gravestock I, Winklhofer S, Porchet F, Pichierri G, et al. The influence of endplate (modic) changes on clinical outcomes in lumbar spinal stenosis surgery: a Swiss prospective multicenter cohort study. Eur Spine J. (2020) 29:2205–14. doi: 10.1007/s00586-020-06364-0
Keywords: spinal stenosis, clinical outcome, minimally invasive surgery, endoscopy, paraspinal muscle, nomogram
Citation: Wang A, Wang T, Zang L, Fan N, Yuan S, Si F and Du P (2023) Identification of preoperative radiological risk factors for reoperation following percutaneous endoscopic lumbar decompression to treat degenerative lumbar spinal stenosis. Front. Surg. 9:1054760. doi: 10.3389/fsurg.2022.1054760
Received: 27 September 2022; Accepted: 24 November 2022;
Published: 6 January 2023.
Edited by:
Yang Lv, Peking University Third Hospital, ChinaReviewed by:
Zhuo Ran Sun, Peking University Third Hospital, ChinaShuai Jiang, Peking University Third Hospital, China
© 2023 Wang, Wang, Zang, Fan, Yuan, Si and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Zang emFuZ2xlaUBjY211LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Specialty Section: This article was submitted to Orthopedic Surgery, a section of the journal Frontiers in Surgery
 Aobo Wang†
Aobo Wang† Lei Zang
Lei Zang