- 1Department of Anesthesiology, Second Affiliated Hospital of Army Medical University, Chongqing, China
- 2Department of Anesthesiology, the Affiliated Hospital, School of Medicine, UESTC Chengdu Women’s & Children’s Central Hospital, Chengdu, China
- 3Department of Gynaecology and Obstetrics, Second Affiliated Hospital of Army Medical University, Chongqing, China
Objectives: To explore the effect of glucose-insulin-potassium (GIK) therapy on uterine cramping pain (UCP) following cesarean delivery (CD).
Design: Single-center, randomized controlled study.
Setting: Second Affiliated Hospital of Army Medical University, Chongqing, China.
Participants: A total of 140 women, aged 20–40 years, who underwent CD with a transverse incision were randomly assigned to the GIK (P) or control (C) groups in a 1:1 ratio.
Interventions: GIK was intravenously administered to patients in Group P. Patients in Group C received normal saline (NS). After umbilical cord clamping, oxytocin was administered intravenously. The same GIK and NS regimens were administered on postoperative days 1 and 2, followed by oxytocin 10 min later.
Primary and secondary outcome measures: Following oxytocin administration, UCP was assessed using the visual analog scale (VAS), and the maximum VAS score (primary outcome) was recorded.
Results: Patients in Group P had significantly lower maximum VAS scores than those in Group C on postoperative days 1 (38.4 ± 21.1 vs. 52.3 ± 20.8, p < 0.001) and 2 (10 [0,30] vs. 30.5 [8.75,50], p < 0.001). Group P patients also had shorter pain duration on postoperative day 1 (39.6 ± 19.5 min vs. 50.6 ± 18.2 min, p = 0.001). Group P patients had a lower incidence of inadequate analgesia of UCP than Group C on days 1 (45.5% vs. 74.2%, p < 0.001) and 2 (10.6% vs. 47.0%, p < 0.001); the RRs for experiencing inadequate analgesia for UCP postpartum in Group P patients was 0.612 (95% CI: 0.454–0.826, p < 0.001) on day 1 and 0.226 (95% CI: 0.107–0.476, p < 0.001) on day 2. The absolute risk reduction (ARR) was 28.7%; thus number needed to treat (NNT) was 3 after rounding up. A subgroup analysis demonstrated that Group P patients undergoing repeat CD had lower maximum VAS scores for UCP on both postoperative days 1 and 2.
Conclusion: Our findings suggest that GIK can relieve UCP and shorten its duration. Our results provide information to facilitate the development of novel approaches for managing UCP.
Clinical Trial Registration: This study was approved by the Medical Ethics Committee of Second Affiliated Hospital of Army Medical University (2020-109-01, 19/11/2020) and registered in the Chinese Clinical Trial Registry (http://www.chictr.org.cn, ChiCTR2100041607,01/01/2021).
Introduction
Cesarean delivery (CD) is the most common inpatient surgery worldwide (1) but remains associated with severe postoperative pain (2). A major cause of analgesic infectivity postoperatively is uterine cramping pain (UCP) (3). UCP is exacerbated by oxytocin, which is routinely used to reduce uterine bleeding following CD.
In China, it is routine to use oxytocin for more than 2 days following CD which aggravates UCP in puerperas (4, 5). The Society for Obstetric Anesthesia and Perinatology recommends a multimodal pain management plan for CD patients consisting of long-acting neuraxial opioid analgesia and scheduled nonsteroidal anti-inflammatory drugs (NSAIDs) or acetaminophen. Peripheral regional nerve block is reserved only for unique cases (6). Patient-controlled epidural analgesia provides effective but suboptimal outcomes due to the side effects which include motor block (7) and urinary retention (8). Patient-controlled opioid intravenous analgesia (PCIA) following CD allows pain control without these complications. Intravenous opioid analgesics, including sufentanil which is used in PCIA, however, have a poor effect on UCP (9) and intrinsic side effects associated with opioids (10). It is, therefore, imperative to identify novel therapies for UCP which can provide optimal pain relief with limited side effects.
Uterine ischemia/hypoxia is one of the mechanisms underlying UCP (11–13). A study in mice demonstrated that the uterine visceral pain pathways include altered muscle contractility and impaired perfusion (14). An oxytocin-induced increase in uterine pressure reduced uterine oxygenation by 38% in the absence of inflammatory molecules. Further, the use of platelet-activating factors which induce uterine contractions can reduce uterine perfusion by 40 ± 8%, elicit oxygen desaturation levels close to hypoxia (9.4 ± 3.4 mm Hg PaO2), and produce visceral pain. By improving tolerance to uterine ischemia/hypoxia and metabolism, UCP may be relieved and, therefore, physiological treatment strategies for UCP warrant studying.
Physiological treatments have been successful in the treatment of cardiac-related pain or angina. Angina is caused by cardiac ischemia/hypoxia resulting in the accumulation of lactate, 5-hydroxytryptamine, bradykinin, and prostaglandin (15). Similarly, uterine contraction causes ischemia/hypoxia and lactate accumulation (16), which are crucial mechanisms of pain. Angina and UCP may have similar mechanisms of pain and respond to similar treatments. In this regard, Dmitrovic et al. (17) reported that dysmenorrhea may be alleviated by increasing uterine blood flow using the vasodilator nitric oxide.
One treatment for angina is glucose-insulin-potassium (GIK). This is used clinically to increase myocardial metabolism (18), protect against acute myocardial ischemia, improve cardiac function (19), and relieve or delay angina (20, 21): It does so by promoting glucose uptake and utilization (22), increasing glycolysis, and effectively clearing lactate (23, 24). We hypothesized that GIK may improve uterine energy metabolism, reducing the accumulation of pain-causing molecules and helping relieve UCP symptoms. This randomized clinical trial aimed to explore the effect of GIK therapy on postpartum UCP following CD.
Materials and methods
Patients
This single-center, randomized controlled study was approved by the Institutional Ethics Committee of the Second Affiliated Hospital of Army Medical University (ID:2020-109-01). The study has been registered in the Chinese Clinical Trial Registry (http://www.chictr.org.cn, ChiCTR2100041607). All patients provided written informed consent before enrollment.
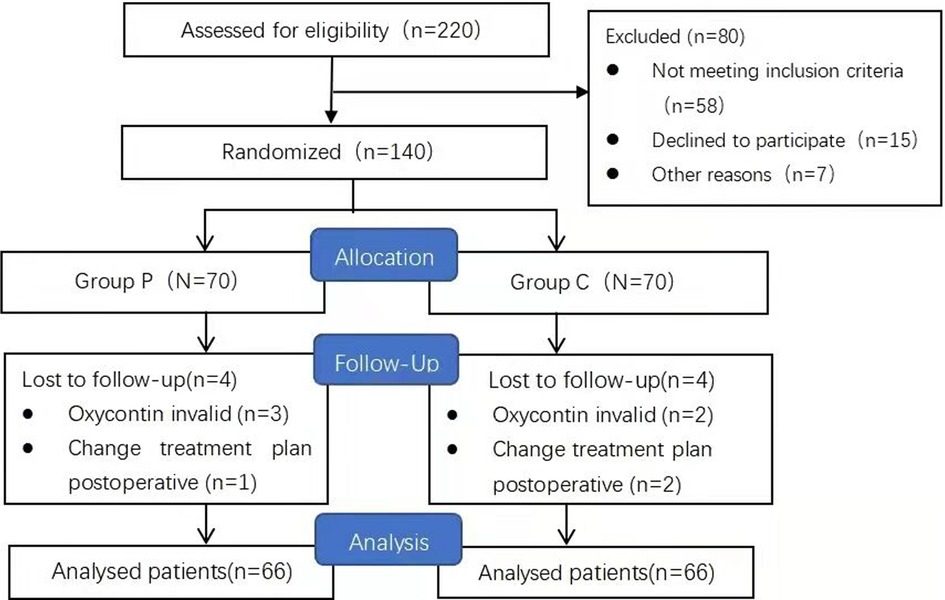
Between January and March 2021, we enrolled 140 women aged 20–40 years who were scheduled to undergo CD with a transverse incision (Figure 1). The inclusion criteria were as follows: a full-term gestation of 37–40 weeks, single pregnancy, an American Society of Anesthesiologists physical status scale of I–II, acceptance of the administration of subarachnoid anesthesia, and voluntary reception of intravenous PCIA. The exclusion criteria included: moderate or worse anxiety [Generalized Anxiety Disorder Questionnaire (GAD-7) score >9], depression [Patient Health Questionnaire-9 (PHQ-9) score >9], gestational diabetes mellitus, severe hypertension or eclampsia, placenta previa, chronic pain, long-term use of analgesics, and renal insufficiency. Withdrawal criteria included participants who did not experience uterine contraction and UCP (VAS score consistently 0 at all assessments) following oxytocin administration, postoperative changes in the treatment plan, and lactation during oxytocin effect after oxytocin administration.
Anesthesia and analgesia management
All patients agreed to receive subarachnoid anesthesia [Ropivacaine (AstraZeneca AB, 20–25 mg)] by an experienced anesthesiologist. This was initiated immediately following CD. PCIA was administered using an electronic infusion pump containing 800 mg tramadol (Sandoz Pharmaceutical Co. Ltd., China) in 0.9% normal saline (NS, 300 ml). The background volume was 6.0 ml/h, and the patient-controlled analgesia (PCA) dose was 2 ml, with a locking time of 15 min. Acetaminophen and NSAIDs were not administered, and tramadol was the only rescue analgesic for inadequate analgesia.
Drug intervention program
Participants were randomly assigned to the GIK (Group P) or control (Group C) groups. For Group P patients, GIK (250 ml of 10% glucose, 8 IU of insulin, and 0.8 g of KCl) was intravenously (IV) administered at a rate of 1.5 ml/kg/h when patients entered the operating room. For patients who did not complete GIK infusion intraoperatively, infusion was continued postoperatively. Group C received NS at the same dose and rate. Per the hospital's protocol, oxytocin (20 U in 100 ml of 0.9% normal saline) was administered as an IV continuous infusion immediately after umbilical cord clamping. Blood potassium and glucose levels were measured via arterial blood gas analysis before and after GIK or NS infusion on the day of surgery. On the morning of days 1 and 2 following CD, GIK or NS was infused using the described regimen, and IM oxytocin (10 U) was injected 10 min after infusion. The GIK composition and injection rate were based on prior studies (25, 26). The onset of insulin IV is approximately 15–30 min post-injection, whereas the onset of oxytocin IM is approximately 5 min. IM oxytocin was administered 10 min following GIK infusion with the expectation that the two drugs would take effect simultaneously.
Randomization and masking
We performed stratified randomization based on primary or repeat CD (the main factors affecting UCP) using the sealed envelope method and a ratio of 1:1. A biostatistician generated random numbers using IBM SPSS Statistics for Windows, version 26.0 (IBM Corp., Armonk, NY). The pain anesthesiologist prepared the distribution sequence and placed the numbers in sealed envelopes. Participants were randomly allocated into two groups with different intervention strategies (GIK or NS infusion) in the operating room before CD. The envelopes were re-sealed and kept blinded until the end of the study. GIK or NS were administered using the same infusion bag to ensure that patients and researchers could not distinguish between the medications. Although pain associated with injection might have led to unblinding for researchers, we attempted to ensure blinding to the best of our ability in this study. The pain management anesthesiologist was not involved in data collection or analysis. Preoperative and intraoperative data were collected by one anesthesiologist. A nurse anesthetist collected postoperative follow-up data during the hospitalization period and 1-week postoperatively through telephone interviews.
Outcome measurements
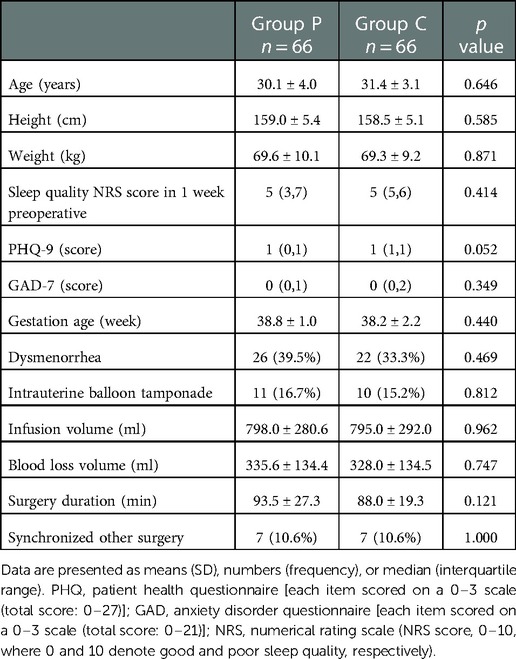
All data were collected from the medical records of the Second Affiliated Hospital of Army Medical University. Demographic characteristics including height, weight, age, preoperative complications such as anemia and subclinical thyroid dysfunction, dysmenorrhea, gestational age, and sleep quality in the week before the operation were collected. All participants completed the GAD-7 [each item scored on a 0–3 scale (total score, 0–21)] and PHQ-9 [each item scored on a 0–3 scale (total score, 0–27)] questionnaires to assess their anxiety and depression status, respectively. Information regarding operation time, fluid infusion volume, concomitant surgeries such as tubal ligation, uterine fibroids or ovarian cyst surgery, intrauterine balloon for uterine inertia, and intraoperative bleeding volume were recorded.
Primary outcomes
The primary outcome was the maximum UCP visual analog scale (VAS) scores measured during oxytocin use on the morning of postoperative days 1 and 2. VAS scores (0–100, where 0 and 100 were defined as no and maximum pain, respectively) were used to assess UCP following IM oxytocin injection at 10-min intervals. The nurse anesthetist remained with the patients at all times, verbally used the VAS scale to assess their pain level, assessed the maximum UCP every 2 min, and recorded their maximum VAS score as the maximum UCP over 10 min.
Secondary outcomes
The secondary outcomes included UCP and incisional pain before oxytocin administration, time of onset time, and duration of oxytocin use. We also assessed the period of maximum incision pain, UCP VAS following oxytocin initiation, and number of PCA applications during oxytocin treatment on postoperative days 1 and 2. Postoperative blood loss (12 h) and height of the uterine fundus (distance from the uterine fundus to the lower edge of the umbilicus assessed by a nurse anesthetist) were also recorded (27). We recorded the time of lactation commencement, i.e., the time until the first drop of breast milk was expressed. UCP was also assessed on postoperative day 7 using the numerical rating scale (NRS, 0–10, where 0 was defined as no pain and 10 as maximum pain) through a telephone interview.
The specific definitions for pain measurement were as follows:
• The onset time was defined as the time between IM oxytocin injection and the onset of UCP. If a patient already had symptoms of UCP, the onset time was defined as the onset of increased intensity or frequency of UCP.
• The duration time was defined as the time between IM oxytocin injection and the resolution of UCP. If a patient had UCP before oxytocin administration, the duration end-point was defined as the point at which the UCP intensity and frequency returned to pre-administration levels.
• The periods of maximum incisional pain and UCP on postoperative day 1 were the maximum VAS scores for incisional pain and UCP from the end of surgery to oxytocin injection on postoperative day 1.
• The periods of maximum incision pain and UCP on postoperative day 2 were the maximum VAS scores for incision pain and UCP from the resolution of UCP on postoperative day 1 to the time of oxytocin injection on postoperative day 2.
• The maximum VAS score for UCP referred to the maximum VAS score from the first oxytocin injection to the resolution of UCP after postoperative day 2.
• Inadequate analgesia for UCP was defined as a VAS score ≥40 during oxytocin treatment.
• The number of PCA applications referred to the number of PCA button presses between the first oxytocin injection and the resolution of UCP after postoperative day 2.
Sample size calculation
Based on our preliminary study (n = 20) of patients receiving routine clinical treatment, the main outcome measured was the maximum UCP VAS score during oxytocin use on postoperative day 1. The mean maximum UCP VAS score was 35.9 ± 22.9. The maximum UCP VAS score was expected to decrease with GIK therapy, and the minimum clinical between-group difference was 0.5 standard deviations (11.45). With the significance level and power set at 0.05 and 0.9, respectively, the minimum sample size required was 128. The sample size was calculated using PASS version 11.0 (NCSS, LLC. UT, USA). Approximating a loss of follow-up rate of 10%, we eventually included 140 patients.
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics for Windows, version 26.0. Statistical significance was set at p < 0.05. Continuous variables are presented as the mean (SD), number (frequency), and median (interquartile range).
A stepwise logistic regression analysis was used to evaluate the role of demographic, preoperative, intraoperative data to predict factors associated with inadequate analgesia of UCP. All variables in Table 1, primiparas or multiparas status, and the use of GIK were included in this model. Odds ratio (OR) with a 95% confidence interval (CI) was calculated.
An independent single-sample t-test was used for the between-group comparisons of normally distributed demographic and perioperative data. The Mann–Whitney U test was used for between-group comparisons of non-normally distributed data, including PHQ-9 score, GAD-7 score, onset time, height of the uterine fundus, number of PCA applications, VAS score for incisional pain and UCP before oxytocin, maximum VAS score for UCP during oxytocin administration, duration time on postoperative day 2, and UCP NRS on postoperative day 7. Pearson's chi-square test or Fisher's exact test were used for between-group comparisons of categorical data, including preoperative complications, dysmenorrhea, other concomitant surgeries, intrauterine balloon tamponade, inadequate analgesia for UCP during oxytocin treatment, and incisional pain. We calculated the relative risk (RRs) and 95% CI for inadequate analgesia of UCP during oxytocin treatment, and absolute risk reduction (ARR) and number needed to treat (NNT) were also calculated. A two-way repeated analysis of variance (ANOVA) was used to analyze between-group differences in blood glucose and blood potassium levels.
Results
We initially recruited 140 patients of which eight were excluded for deviation from the study protocol (Figure 1). At the end of the study, 132 patients were included in the analyses. Since a stratified randomization strategy depending on primary or repeat CD was utilized, 33 primary and 33 repeat CD patients were included in both Groups C and P. No significant between-group differences were found in the preoperative and intraoperative parameters (p > 0.05) (Table 1).
Risk factor analysis
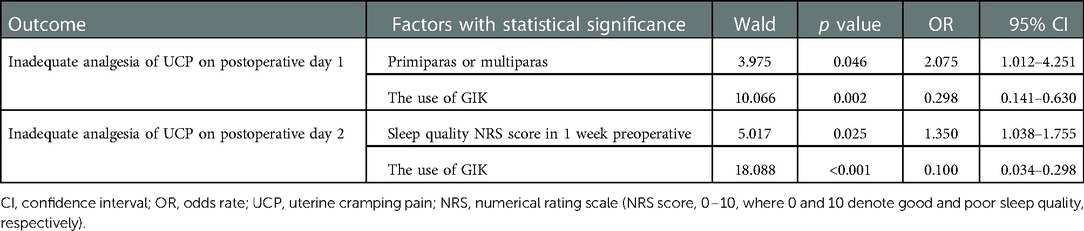
As summarized in Table 2, for all included participants, the results of stepwise logistic regression analysis showed that multiparas status was a risk factor for inadequate analgesia of UCP on postoperative 1 day (OR: 2.075, 95% CI: 1.012–4.215, p = 0.046). Sleep quality NRS score 1 week preoperative was also a risk factor for inadequate analgesia of UCP on postoperative 2 day (OR: 1.345, 95% CI: 1.038–1.755 p = 0.025). The use of GIK was identified as a protective factor for inadequate analgesia of UCP on postoperative days 1 and 2 (OR: 0.298, 95% CI: 0.141–0.630, p = 0.002 and OR: 0.100, 95% CI: 0.034–0.298, p < 0.001, respectively).
VAS scores on postoperative days 1 and 2
Compared to patients in Group C, Group P patients had a lower maximum VAS score for UCP during oxytocin use on days 1 (38.4 ± 21.1 vs. 52.3 ± 20.8, p < 0.001) and 2 (10 [0,30] vs. 30.5 [8.75,50], p < 0.001) (Figure 2).
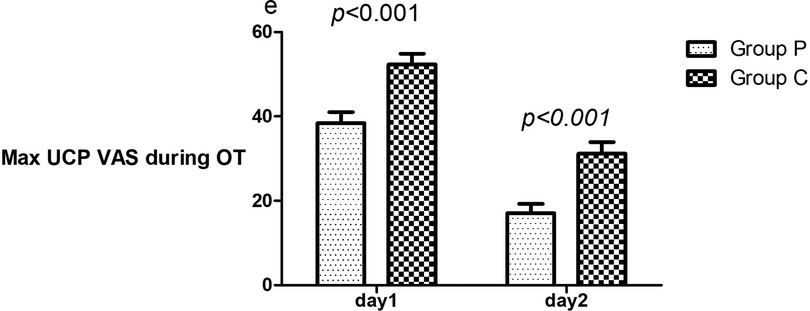
Figure 2. Comparison of maximum UCP VAS during oxytocin on days 1 and 2. The mean scores of patients in Groups P and C who underwent GIK and NS. VAS, visual analog scale (0–100, where 0 and 100 denote no and maximum pain, respectively); UCP, uterine cramping pain; OT, oxytocin; Max, maximum; GIK, glucose–insulin–potassium; VAS, visual analog scale.
Secondary outcomes on postoperative days 1 and 2
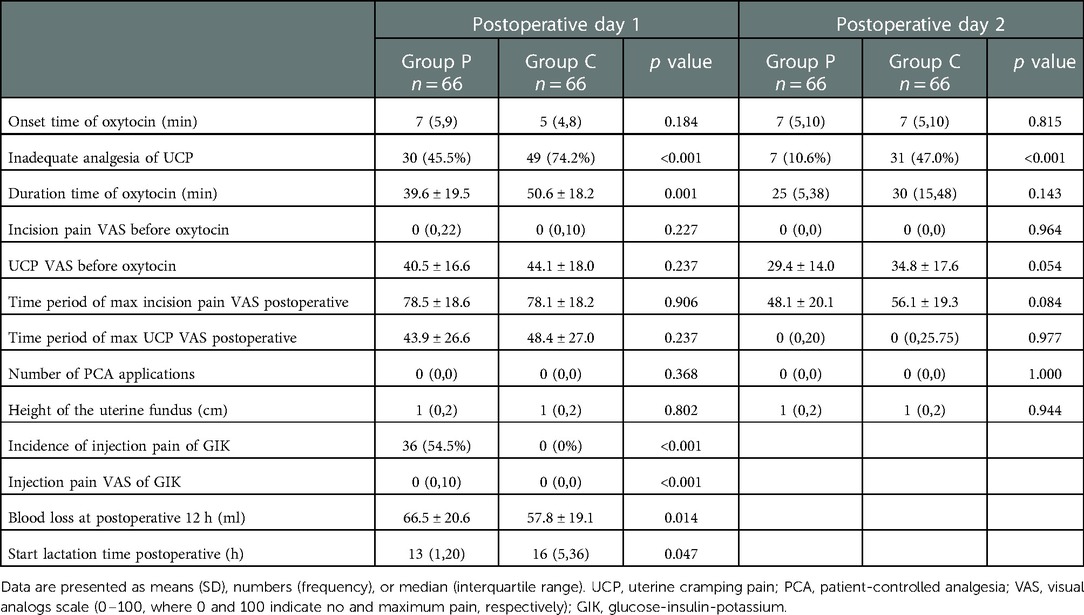
Group P patients had a lower incidence of inadequate analgesia of UCP than Group C on days 1 (45.5% vs. 74.2%, p < 0.001) and 2 (10.6% vs. 47.0%, p < 0.001); the RRs for experiencing inadequate analgesia for UCP postpartum in Group P patients was 0.612 (95% CI: 0.454–0.826, p < 0.001) on day 1 and 0.226 (95% CI: 0.107–0.476, p < 0.001) on day 2. The ARR was 28.7% (incidence of inadequate analgesia was 45.5% in group P and 74.2% in group C); thus NNT was 3 after rounding up. Additionally, Group P patients had a shorter duration of UCP than group C patients (39.6 ± 19.5 min vs. 50.6 ± 18.2 min, p = 0.001) on day 1 (Table 3).
Group P patients had a higher incidence of injection pain than Group C patients (54.5% vs. 0.0%, p < 0.001) (Table 2). Patients in Group P had less blood loss 12 h after CD than patients in Group C (57.7 ± 19.1 ml vs. 66.4 ± 20.6 ml, p = 0.014). Group P patients also had a shorter time to lactation than Group C (patients 13[1,20] h vs.16[5,36] h, p = 0.047). No significant between-group differences were found in the uterine fundus height (p > 0.05) and UCP NRS on postoperative day 7 (0[0,0] vs. 0[0,0], p = 0.664).
Subgroup analysis for primary and repeat CD patients
Subgroup analysis revealed that Group P patients had a lower maximum UCP VAS score on postoperative days 1 and 2 than Group C patients in repeat CD patients (39.8 ± 18.8 vs. 59.2 ± 17.9; 10 [0,29] vs. 23 [0,36], respectively, p < 0.001). No significant difference was noted in the maximum UCP VAS score in primary CD patients (Table 4).
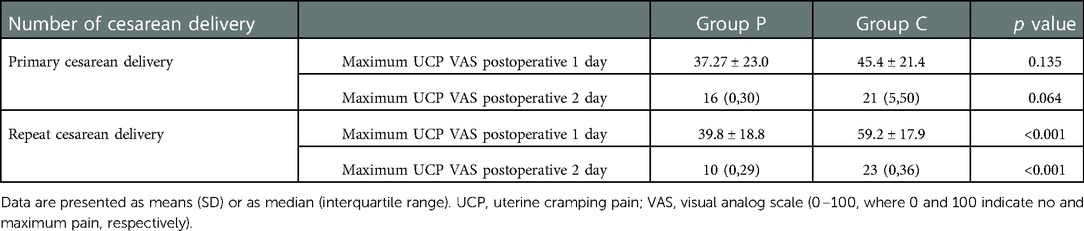
Table 4. Subgroup analysis for primary and repeat cesarean delivery on maximum UCP on postoperative days 1 and 2.
Effect of blood glucose and potassium levels on Maximum UCP
Blood glucose and potassium levels were measured before and after administration of GIK or NS on postoperative days 1 and 2. A two-way repeated ANOVA revealed no significant time, group, or interaction effects on potassium levels (p > 0.05). Blood glucose levels showed a significant time effect (p < 0.001); however, no significant group or time and group interaction effects were noted (p > 0.05). Blood glucose levels in Group P patients increased after medication (5.6 ± 1.0 vs.4.5 ± 0.6, p = 0.018), but no significant difference was observed in Group C patients.
Discussion
Our results show that GIK reduced patients’ UCP VAS score and pain duration during oxytocin treatment following CD. We also found GIK-related pain improvement to be significantly better in patients undergoing repeat CD and GIK to promote early lactation. Compared to controls, group P patients displayed a higher incidence of injection pain. Our findings suggest that GIK treatment improves pain in patients with CD but remains associated with slight injection pain.
Anai et al. (28) reported that lactate dehydrogenase deficiency in pregnant women decreased adenosine triphosphate production during anaerobic glycolysis in the myometrium and increased UCP. Glycolysis is an important energy supply in the uterine contraction process. Its metabolic disorders cause uterine inertia and UCP, suggesting that glycolysis can improve uterine energy metabolism and relieve UCP. A study on rats in the late trimester of pregnancy reported an increase in myometrial glycogen levels, an enhancement in glycogen decomposition for high-energy supply during labor, greater myometrial sensitivity to insulin, and increased glucose uptake in the presence of insulin (29). We observed that GIK decreased patients’ UCP VAS score and duration after CD, suggesting that glucose and insulin are critically involved in supplying energy for uterine contraction. As mentioned, GIK can stimulate myocardial glycolysis and clear lactate to relieve angina. The mechanisms underlying UCP and angina relief by GIK may be similar. We suspect that glucose in GIK may provide energy for uterine contraction and insulin can increase myometrial glucose and substrate metabolism. Additionally, GIK may stimulate glycolysis, increase high-energy phosphate levels, and effectively clear pain metabolites.
We found the pain treatment effect of GIK to be most apparent in patients undergoing repeat CD. A previous study has shown that multiparas experience more severe UCP (30), and other studies have suggested that UCP intensity is related to ischemia of the uterus and myometrial blood flow (11, 12). We speculate that GIK is more effective for patients with severe uterine contractions and severe ischemia. Nevertheless, the effects of GIK on glycolysis, local metabolites of the myometrium, and the relationship between the GIK analgesic effect and uterine contraction intensity need further investigation.
Pain inhibits the secretion of prolactin and oxytocin, delaying the start of lactation and reducing output (31). Glucose is an important energy substrate that is required for the initiation of lactation (32). Patients treated with GIK had increased blood glucose levels and an earlier lactation onset time than patients given saline. GIK-induced acceleration of lactation may contribute to UCP relief and increased blood glucose levels.
This study has several limitations. First, to minimize trauma to patients without diabetes, changes in blood sugar and potassium levels were monitored only before and after GIK or NS infusion on the day of surgery. Second, we did not monitor uterine local blood flow and metabolism, since there is no non-invasive method to accurately do so. Currently available methods are unable to penetrate the abdominal wall tissue because of the excessive thickness of maternal abdominal fat. Third, the optimal proportion, dosage, and rate for GIK infusion could not be established in this study and further studies are necessary. Fourth, this study may have lacked total blinding due to injection pain experienced by 54% of GIK recipients compared to 0% of control recipients. Finally, this study may lack generalizability to some postpartum populations as the experimental conditions implemented in this study were artificial due to IM oxytocin on postpartum days 1 and 2 to generate UCP, and these patients received no intrathecal opioids, acetaminophen, or NSAIDs for pain control.
Conclusion
In conclusion, our findings suggest that GIK can alleviate UCP and shorten its duration. Treatments that improve uterine tolerance to ischemia/hypoxia may effectively treat UCP. Our findings may provide a novel approach to UCP treatment. Multimodal pain management, however, remains the mainstay for CD analgesia. Scheduled acetaminophen and non-steroidal drugs were the basic drugs, and long-acting neuraxial opioid analgesia or other intravenous analgesia should be also used. When UCP following CD is not effectively controlled using standard multimodal analgesia regimens, GIK may be used as an auxiliary method for UCP control.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by The Institutional Ethics Committee of the Second Affiliated Hospital of Army Medical University, Chongqing, China (number:2020-109-01). The patients/participants provided their written informed consent to participate in this study.
Author contributions
Conceptualization: GY and HL. Data curation: ZW. Formal analysis: ZW. Funding acquisition: GY and HL. Investigation: QC. Methodology: GY, HL, FC, WL, and LL. Project administration: HL and XB. Resources: MW. Software: ZW. Supervision: XB. Validation and visualization: HL. Writing—original draft: GY. Writing—review and editing: HL and YC. All authors contributed to the article and approved the submitted version.
Funding
The study was supported by the Science Natural Foundation of Chongqing (No. cstc-2020jcyj-msxmX0453) and the Key projects of Science Natural Foundation of Chongqing (No. cstc-2019jcyj-zdxmX0001).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lester SA, Kim B, Tubinis MD, Morgan CJ, Powell MF. Impact of an enhanced recovery program for cesarean delivery on postoperative opioid use. Int J Obstet Anesth. (2020) 43:47–55. doi: 10.1016/j.ijoa.
2. van Wijck AJM, Aduckathil S, Kalkman CJ, Gerbershagen HJ, Peelen LM. Pain intensity on the first day after surgery A prospective cohort study comparing 179 surgical procedures. Anesthesiology. (2013) 118:934–44. doi: 10.1097/ALN.0b013e31828866b3
3. Yeh YC, Chen SY, Lin CJ, Yeh HM, Sun WZ. Differential analgesic effect of tenoxicam on post-cesarean uterine cramping pain between primiparous and multiparous women. J Formos Med Assoc. (2005) 104:647–51. doi: 10.1016/j.jpainsymman.2005.03.015
4. Liu Q, Zhao JN, Chen HX, Zhong TD. Oral controlled-release oxycodone for uterine cramping pain after cesarean section. Zhonghua Yi Xue Za Zhi. (2011) 91:2132–4. doi: 10.1007/s10570-010-9464-0
5. Duan G, Yang G, Peng J, Duan Z, Li J, Tang X, et al. Comparison of postoperative pain between patients who underwent primary and repeated cesarean section: a prospective cohort study. BMC Anesthesiol. (2019) 19:189. doi: 10.1186/s12871-019-0865-9
6. Bauchat JR, Weiniger CF, Sultan P, Habib AS, Ando K, Kowalczyk JJ, et al. Society for obstetric anesthesia and perinatology consensus statement: monitoring recommendations for prevention and detection of respiratory depression associated with administration of neuraxial morphine for cesarean delivery analgesia. Anesth Analg. (2019) 129(2):458–74. doi: 10.1213/ANE.0000000000004195
7. Bourgès J, Gakuba C, Plass F, Gérard JL, Simonet T, Hanouz JL. Effect of patient-controlled epidural analgesia with and without automatic intermittent bolus on levobupivacaine consumption during labour: a single-centre prospective double-blinded randomised controlled study. Anaesth Crit Care Pain Med. (2021) 40:100936. doi: 10.1016/j.accpm.2021.100936
8. Koh JC, Song Y, Kim SY, Park S, Ko SH, Han DW. Postoperative pain and patient-controlled epidural analgesia-related adverse effects in young and elderly patients: a retrospective analysis of 2,435 patients. J Pain Res. (2017) 10:897–904. doi: 10.2147/JPR.S133235
9. Nie JJ, Sun S, Huang SQ. Effect of oxycodone patient-controlled intravenous analgesia after cesarean section: a randomized controlled study. J Pain Res. (2017) 10:2649–55. doi: 10.2147/JPR.S142896
10. Chow CK, Koren G. Sedating drugs and breastfeeding. Can Fam Physician. (2015) 61:241–3. PMID: 25927109
11. Jurna I. Geburtsschmerz-Entstehung, erregungsleitung und folgen [Labor pain-causes, pathways and issues]. Schmerz. (1993) 7:79–84. doi: 10.1007/BF02527864
12. Shnol H, Paul N, Belfer I. Labor pain mechanisms. Int Anesthesiol Clin. (2014) 52:1–17. doi: 10.1097/AIA.0000000000000019
13. Irawati A, Susanti S, Haryono I. Mengurangi nyeri persalinan dengan teknik birthing ball. J Bidan Cerdas. (2019) 2:129. doi: 10.33860/jbc.v2i3.282
14. Hellman KM, Yu PY, Oladosu FA, Segel C, Han A, Prasad PV, et al. The effects of platelet-activating factor on uterine contractility, perfusion, hypoxia, and pain in mice. Reprod Sci. (2018) 25:384–94. doi: 10.1177/1933719117715122
15. Longhurst JC, Tjen-A-Looi SC, Fu LW. Cardiac sympathetic afferent activation provoked by myocardial ischemia and reperfusion. Mechanisms and reflexes. Ann N Y Acad Sci. (2001) 940:74–95. doi: 10.1111/j.1749-6632.2001.tb03668.x
16. Madaan A, Nadeau-Vallée M, Rivera JC, Obari D, Hou X, Sierra EM, et al. Lactate produced during labor modulates uterine inflammation via GPR81 (HCA1). Am J Obstet Gynecol. (2017) 60:e1–e17. doi: 10.1016/j.ajog.2016.09.072
17. Dmitrovic R, Kunselman AR, Legro RS. Sildenafil citrate in the treatment of pain in primary dysmenorrhea: a randomized controlled trial. Hum Reprod. (2013) 28:2958–65. doi: 10.1093/humrep/det324
18. Zhao K, Zhang Y, Li J, Cui Q, Zhao R, Chen W, et al. Modified glucose-insulin-potassium regimen provides cardioprotection with improved tissue perfusion in patients undergoing cardiopulmonary bypass surgery. J Am Heart Assoc. (2020) 9:e012376. doi: 10.1161/JAHA.119.012376
19. Ellenberger C, Sologashvili T, Kreienbühl L, Cikirikcioglu M, Diaper J, Licker M. Myocardial protection by glucose-insulin-potassium in moderate- to high-risk patients undergoing elective on-pump cardiac surgery: a randomized controlled trial. Anesth Analg. (2018) 126:1133–41. doi: 10.1213/ANE.0000000000002777
20. Chiong MA, West R, Parker JO. The protective effect of glucose-insulin-potassium on the response to atrial pacing. Circulation. (1976) 54:37–46. doi: 10.1161/01.cir.54.1.37
21. Thadani U, Chiong MA, Parker JO. Effects of glucose-insulin-potassium infusion on the angina response during treadmill exercise. Cardiology. (1979) 64:333–49. doi: 10.1159/000170632
22. Doenst T, Richwine RT, Bray MS, Goodwin GW, Frazier OH, Taegtmeyer H. Insulin improves functional and metabolic recovery of reperfused working rat heart. Ann Thorac Surg. (1999) 67:1682–8. doi: 10.1016/s0003-4975(99)00326-4
23. Kjellman UW, Björk K, Dahlin A, Ekroth R, Kirnö K, Svensson G, et al. Insulin(GIK) improves myocardial metabolism in patients during blood cardioplegia. Scand Cardiovasc J. (2000) 34:321–30. doi: 10.1080/713783123
24. Slob EMA, Shulman R, Singer M. Experience using high-dose glucose-insulin-potassium (GIK) in critically ill patients. J Crit Care. (2017) 41:72–7. doi: 10.1016/j.jcrc.2017.04.039
25. LaDisa JF Jr, Krolikowski JG, Pagel PS, Warltier DC, Kersten JR. Cardioprotection by glucose-insulinpotassium: dependence on KATP channel opening and blood glucose concentration before ischemia. Am J Physiol Heart Circ Physiol. (2004) 287:H601–7. doi: 10.1152/ajpheart.00122.2004
26. Ramanathan T, Morita S, Huang Y, Shirota K, Nishimura T, Zheng X, et al. Glucose-insulin-potassium solution improves left ventricular energetics in chronic ovine diabetes. Ann Thorac Surg. (2004) 77:1408–14. doi: 10.1016/j.athoracsur.2003.10.016
27. Huang L, Wang W. Clinical observation on analgesic effect and rehabilitation promotion of PCIA after cesarean section by nursing intervention of traditional Chinese medicine. J Sichuan Tradit Chin Med. (2019) 37:214–7. 10.2019-08-071
28. Anai T, Urata K, Tanaka Y, Miyakawa I. Pregnancy complicated with lactate dehydrogenase M-subunit deficiency: the first case report. J Obstet Gynaecol Res. (2002) 28:108–11. doi: 10.1046/j.1341-8076.2002.00015.x
29. Sakamoto H, Leranth C, MacLusky NJ, Saito Y, Naftolin F. Insulin specific binding sites in the myometrium of pregnant rats. Endocrinology. (1987) 120:1951–5. doi: 10.1210/endo-120-5-1951
30. Holdcroft A, Snidvongs S, Cason A, Doré CJ, Berkley KJ. Pain and uterine contractions during breast feeding in the immediate post-partum period increase with parity. Pain. (2003) 104:589–96. doi: 10.1016/S0304-3959(03)00116-7
31. Wang Y, Fang X, Liu C, Ma X, Song Y, Yan M. Impact of intraoperative infusion and postoperative PCIA of dexmedetomidine on early breastfeeding after elective cesarean section: a randomized double-blind controlled trial. Drug Des Devel Ther. (2020) 14:1083–93. doi: 10.2147/DDDT.S241153
Keywords: analgesia, cesarean delivery, glucose-insulin-potassium, postpartum, uterine cramping pain single-center, randomized controlled study
Citation: Yang G, Cui Y, Bao X, Wu Z, Chen Q, Chen F, Liu W, Wang M, Luo L and Li H (2023) Glucose-insulin-potassium alleviates uterine cramping pain following cesarean delivery: A randomized, controlled trial. Front. Surg. 9:1068993. doi: 10.3389/fsurg.2022.1068993
Received: 13 October 2022; Accepted: 12 December 2022;
Published: 9 January 2023.
Edited by:
Piotr Sieroszewski, Medical University of Lodz, PolandReviewed by:
Antonio Simone Laganà, University of Palermo, ItalySufyan Ibrahim, Mayo Clinic, United States
© 2023 Yang, Cui, Bao, Wu, Chen, Chen, Liu, Wang, Luo and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Li bGg3ODU1M0AxNjMuY29t
Specialty Section: This article was submitted to Obstetrics and Gynecological Surgery, a section of the journal Frontiers in Surgery
 Guiying Yang1
Guiying Yang1 Yu Cui
Yu Cui Hong Li
Hong Li