- 1Department of Pathology, Huzhou Central Hospital, Affiliated Central Hospital Huzhou University, Huzhou, China
- 2Department of Orthopaedic Surgery, Dianjiang People’s Hospital of Chongqing, Chongqing, China
- 3Department of Orthopaedic Surgery, The Second People’s Hospital of Yibin, Yibin, China
- 4Department of Orthopaedic Surgery, Ningbo No.6 Hospital, Ningbo, China
Background: Cryotherapy is widely applied to relieve pain and improve functional outcomes after total knee arthroplasty (TKA). New cryotherapy devices have recently been developed to guarantee a fixed temperature for a prolonged time. Therefore, we conducted a systematic review and meta-analysis to compare continuous cryotherapy and traditional cryotherapy (ice bag or gel pack) for patients after TKA.
Methods: This study was conducted according to a predefined protocol registered on PROSPERO. Two independent reviewers performed an electronic database search of PubMed, Embase, Cochrane, Web of Science, Google Scholar, and ClinicalTrials.gov. Dichotomous outcomes were reported as risk difference (RD) with 95% confidence intervals (CIs), and continuous outcomes were reported as mean difference (MD), or standardized mean difference (SMD) with 95% CIs.
Results: Seven trials enrolling a total of 519 patients were included. There were no differences in pain intensity (MD: −0.54, 95% CI: −1.55 to 0.47; P = 0.30), analgesics consumption (MD: −0.37, 95% CI: −1.28 to 0.55; P = 0.43), postoperative range of motion (MD: 0.47, 95% CI: −4.09 to 5.03; P = 0.84), swelling of the knee joint, blood loss, change in hemoglobin, or transfusion rate. Meanwhile, there were no differences in length of hospital stay (MD: −0.77, 95% CI: −1.62 to 0.08; P = 0.07) and adverse events (RD: 0, 95% CI: −0.02 to 0.03; P = 0.74). In addition, continuous cryotherapy leads to extra costs and resources than traditional cryotherapy.
Conclusions: Continuous cryotherapy does not appear to offer significant benefits for TKA when compared with traditional cryotherapy. Based on currently available evidence, traditional cryotherapy is still recommended as continuous cryotherapy is not cost-effective. Further well-designed studies with larger sample sizes are warranted to further confirm these preliminary results.
PROSPERO Registration: Identifier [CRD42022308217].
1. Introduction
Total knee arthroplasty (TKA) is an effective surgical intervention for end-stage arthritis of the knee joint, which could provide better overall improvements in function, mobility, pain, and health-related quality of life (1, 2). Despite several studies with short- to mid-term follow-up have reported excellent results with high rates of satisfaction, the postoperative period after TKA may be pretty challenging: patients may experience acute pain, potential blood loss, local swelling, and edema resulting from tissue damage and acute inflammatory responses, restricted motion, and stiffness of the knee joint, reduced quadriceps strength, and finally lead to delayed recovery and prolonged hospital stay (3–5). Thus, even with the latest advances in multimodal pain management protocols, surgical and anesthetic techniques, TKA remains a difficult procedure for most patients. It is, therefore, a pressing need for the introduction and implementation of the enhanced recovery after surgery (ERAS) principles, which aim to optimize perioperative care, reduce complications, shorten the length of hospital stay, and reduce readmission rates and costs (6–8). Cryotherapy, as a nonpharmaceutical treatment, plays a vital role in addressing immediate postoperative complications, mainly for severe pain and significant swelling (9, 10).
Cryotherapy, also known as cold therapy, was utilized for inflammation and infection treatment as early as 3,000 BC, and was utilized for anesthesia before operations and amputations for its analgesic and numbing effects in the 1800s (11, 12). At present, cryotherapy is still commonly recommended and widely applicated following orthopaedic procedures, which is also utilized to enhance recovery and outcomes after TKA (13). Despite many advances in postoperative rehabilitation, cryotherapy remains popular and is universally considered appealing for its minimal disadvantages compared with the possible benefits. External application of cryotherapy in TKA is the application of external cold mediums to the skin around the knee joint and is supposed to reduce the intra-articular temperature, which on the one hand, could slow the conduction velocity of nerve fibers and potentially reduce pain transmission, and on the other hand, could reduce peripheral blood flow due to circulating vasoconstriction and therefore decrease the local inflammation and swelling (13). Traditionally, ice bag or gel pack is the most common and economical cryotherapy method, which is typically discontinuous with unregulated cold temperature and demands a manual replacement by the staff nurses (14). Therefore, continuous cryotherapy devices have been developed to deliver a steady cooling temperature for a prolonged time (15). However, it remains unclear whether the newly developed continuous cryotherapy devices were superior to traditional ice/gel pack for TKA.
A broad scope of the literature has suggested that the volume of randomized controlled trials (RCTs) specifically focusing on continuous cryotherapy vs. traditional cryotherapy has increased, and findings are conflicting (16–22). The aim of this study was to perform a comprehensive systematic review and use a meta-analytic approach to pool outcomes to compare the efficacy, safety, and cost-effectiveness of continuous cryotherapy to traditional cryotherapy for TKA.
2. Methods
The present systematic review and meta-analysis was designed in accordance with the guidelines proposed by the Cochrane Collaboration in the Cochrane Handbook for Systematic Reviews of Interventions (http://www.cochrane-handbook.org) and completed according to a predefined protocol, which has been listed on the International Prospective Register of Systematic Reviews (PROSPERO; registration number CRD42022308217) (23). The study was completed in adherence with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement (24).
2.1 Literature search
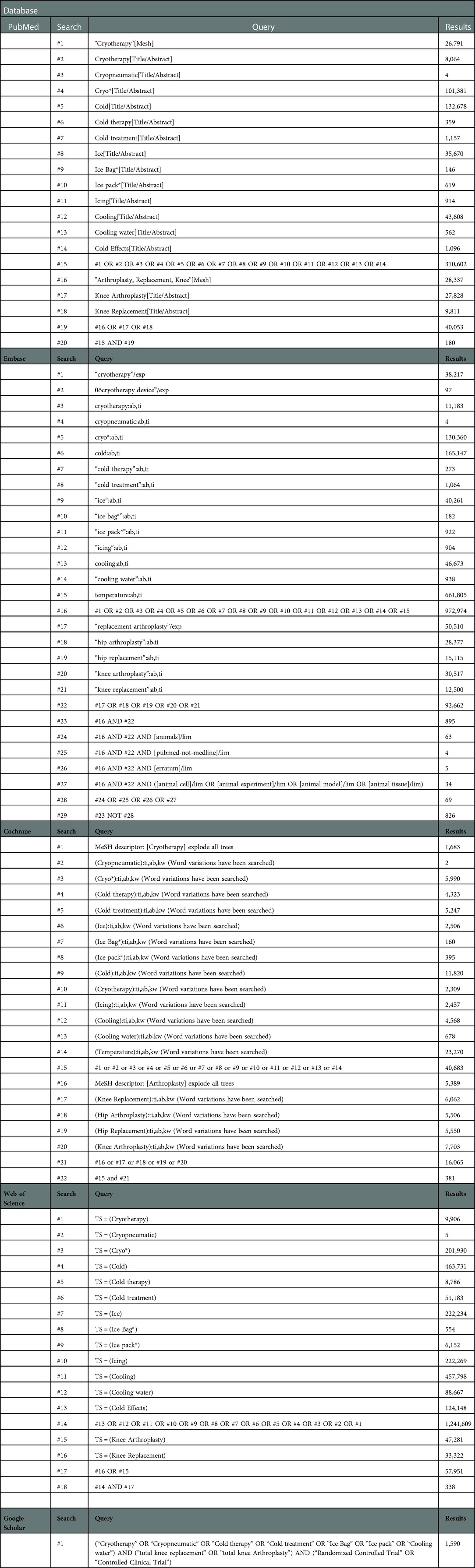
We searched the following electronic bibliographic databases from inception to March 2,022 to capture all recent relevant studies: PubMed, Embase, The Cochrane Library (Cochrane Database of Systematic Reviews), Web of Science, and Google Scholar,. We performed electronic searches using exploded Medical Subject Headings (MeSH) terms with corresponding keywords. The search was broad and applied no language restriction. A detailed description can be found in Appendix 1. In addition, we further searched the ClinicalTrials.gov registry (https://clinicaltrials.gov/) and checked the reference lists of all included full-text articles and previous systematic reviews to identify any additional eligible studies. Corresponding authors of included articles were contacted, where possible, to obtain detailed information or numerical data.
2.2 Study eligibility and selection
Two investigators independently conducted the initial electronic databases search and carefully reviewed all yielded records for inclusion using pre-determined eligibility criteria. All records were screened by title, abstract, and keywords for possible inclusion, and subsequently, identified as “included”, “excluded”, or “required further retrieval” to identify eligibility. No language or publication database filter was applied. Any discrepancies were resolved through discussion by the review team.
The inclusion criteria were:
(i) Population: adult patients undergoing TKA;
(ii) Intervention: received continuous cryotherapy (without compression) after TKA;
(iii) Comparison: received traditional cryotherapy after TKA;
(iv) Outcomes: reporting at least one of the outcomes of interest listed below;
(v) Study type: RCT.
Exclusion criteria were non-RCT interventional studies, observational studies, conference abstracts, editorials, correspondence, expert opinions, case series or reports, and unavailable full texts.
2.3 Data review and extraction
Two independent reviewers extracted details pertaining to the participants from each included trial. The following data were extracted from each included study: first author; year of publication; study location; publication journal; study design; clinical settings; study population; demographic data; intervention management; control management; outcomes of interest. These extracted data were entered into a standardized data extraction form. When the information was unclear or missing, we attempted to contact the corresponding authors of the original studies. The differences in the extracted data were discussed and resolved by referring to the original article by the panel of all the reviewers. The main outcomes of interest were the efficacy, safety, and cost-effectiveness of continuous cryotherapy when compared with traditional cryotherapy. In detail, the primary outcomes include pain intensity, analgesics consumption, postoperative range of motion (PROM), and swelling of the knee joint; while the secondary outcomes include blood loss, change in hemoglobin, transfusion rate, adverse events, length of hospital stay, and cryotherapy costs.
2.4 Quality assessment
Two reviewers independently evaluated the risk of bias of each study using the assessment tool from the Cochrane Handbook (25). The major domains of bias (random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias) in each trial were reviewed. Each study was graded as “low risk of bias”, “unclear risk of bias”, or “high risk of bias”. The highest risk score from any one domain was used to inform the overall risk. If the highest risk score was “unclear risk of bias” but occurred across multiple domains, it was classed as high risk of bias. Therefore, to be of low risk of bias overall, the trial had to be at low risk of bias across all domains. The disagreements between the two reviewers were resolved via discussion and consensus.
2.5 Statistical analysis
This meta-analysis was performed using Review Manager version 5.3 (Nordic Cochrane Center) for all prespecified outcomes if three or more studies reported the outcome (23). The risk differences (RDs) with 95% confidence intervals (CIs) were calculated for dichotomous data; and the mean differences (MDs) or standardized mean difference (SMD) with 95% CIs were calculated for continuous data. When the mean values are not available for continuous outcomes, the median values was utilized for estimation; other potential missing data will be estimated using the methods described in the Cochrane Handbook (23). A random-effects model was used due to anticipated heterogeneity. Results were reported in a Forest plot with 95% CIs. Heterogeneity was assessed via three means: visual inspection of overlapping confidence intervals, the statistical heterogeneity across studies quantified using I2 statistics, with P ≥ 0.05 considered statistically non-significant. Heterogeneity will be considered to be substantial if the I2 value > 50%. All P values were two-sided, and a P value < 0.05 was considered to be statistically significant evidence.
3. Results
3.1 Study selection
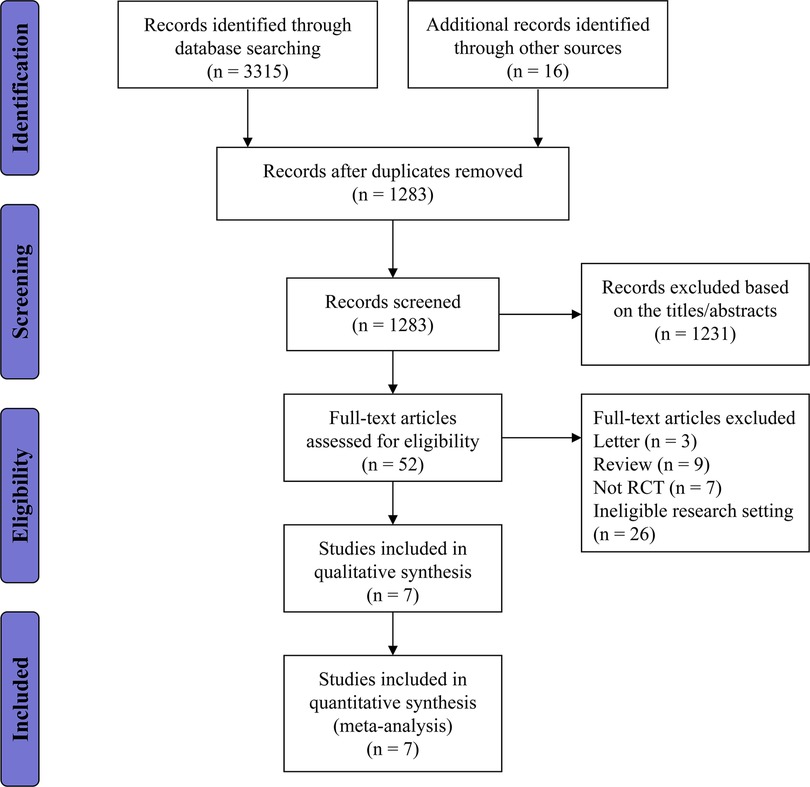
In total, 1,387 articles were obtained from the electronic search strategy, with an additional 16 articles identified through other resources. After the removal of duplicates and irrelevant references, 35 publications were thought to be potentially eligible for inclusion and further assessed for eligibility. Overall we excluded 28 publications for not meeting the inclusion criteria, and seven RCTs were included. The flow diagram with the number of and reasons for exclusions at each stage is provided in Figure 1.
3.2 Characteristics of included studies
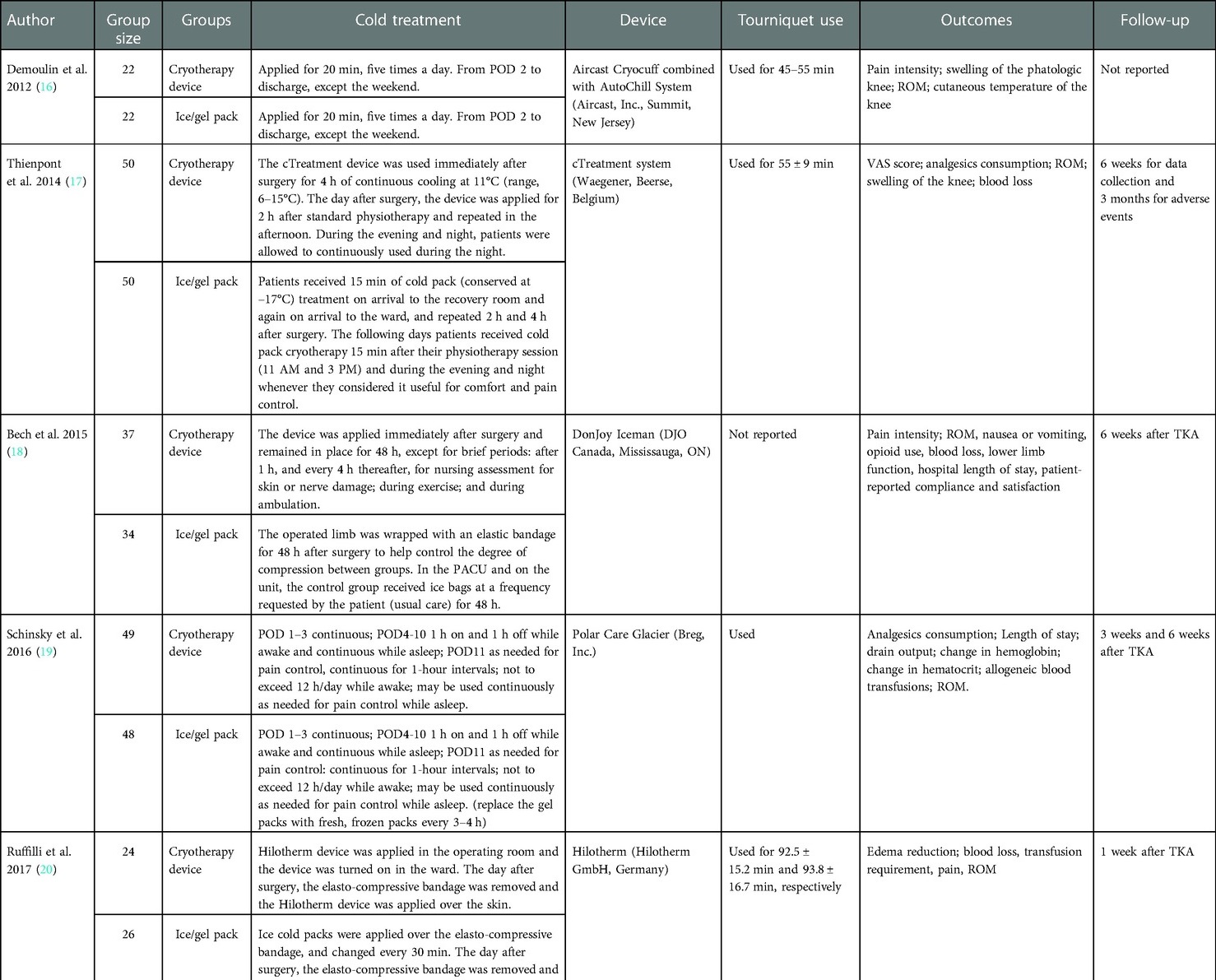
The characteristics of included studies can be found in the study characteristics tables (Tables 1, 2). Seven trials were included in our meta-analysis, which randomized 519 patients into continuous cryotherapy group (n = 263) and traditional cryotherapy (n = 256). These studies were published between 2012 and 2019, with a sample size ranging from 44 to 100. Notably, the application protocols between the continuous and traditional cryotherapy groups differed significantly with respect to the applied time and intervals, and the difference is even more significant among studies.
3.3 Risk of bias in included studies
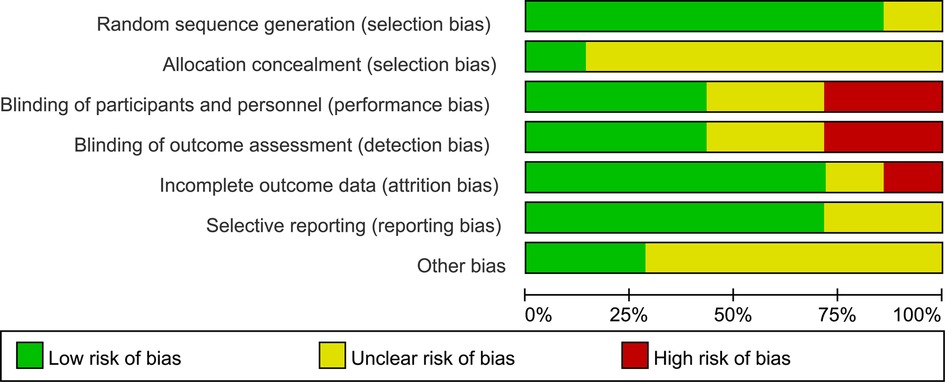
No trials were considered to be at low risk of bias. Three studies were judged to be at high risk of bias, and four studies were felt to be at unclear risk of bias (Figures 2, 3). More specifically, adequate randomized sequence generation was reported in six trials, while appropriate allocation concealment was conducted in one trial. Blinding of outcome assessments was achieved by three trials, thus, the primary efficacy outcome and other outcomes assessment may have been affected by lack of blinding to some extent.
4. Outcomes
4.1 Primary outcomes
4.1.1 Pain
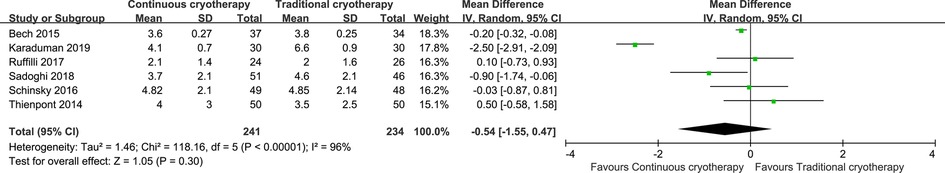
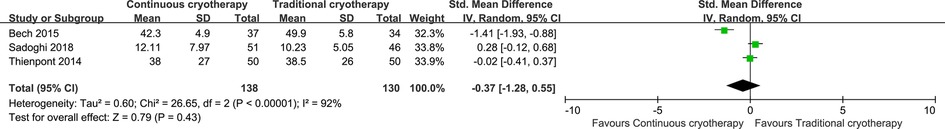
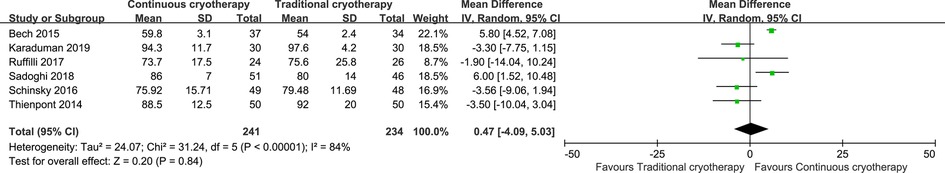
Data for pain intensity were reported by six trials that recruited 475 patients (n = 241 vs. n = 234 in the continuous cryotherapy group and traditional cryotherapy group, respectively). Meta-analysis was performed on studies that reported a pain score for participants at 48 h postoperatively (Figure 4). There was no statistically significant difference in the pain score at 48 h between the continuous cryotherapy group and the traditional cryotherapy group (MD: −0.54, 95% CI: −1.55 to 0.47; P = 0.30). A high level of heterogeneity was observed (I2 = 96%). Data for analgesics consumption were reported by three trials that recruited 268 patients (n = 138 vs. n = 130 in the continuous cryotherapy group and traditional cryotherapy group, respectively). SMD was used as there were differences in the calculating conversations of analgesics consumption. There was no statistically significant difference in analgesics consumption between the continuous cryotherapy group and the traditional cryotherapy group (SMD: −0.37, 95% CI: −1.28 to 0.55; P = 0.43) (Figure 5). A high level of heterogeneity was observed (I2 = 92%).
4.1.2 Swelling
Data for PROM were reported by six trials that recruited 475 patients (n = 241 vs. n = 234 in the continuous cryotherapy group and traditional cryotherapy group, respectively). There was no statistically significant difference in the PROM between the continuous cryotherapy group and the traditional cryotherapy group (MD: 0.47, 95% CI: −4.09 to 5.03; P = 0.84) (Figure 6). A high level of heterogeneity was observed (I2 = 84%).
Knee circumference is another parameter that reflects swelling of the knee joint and was reported in three trials. Meta-analysis was not performed as two trials reported the postoperative knee circumference while one trial reported the difference in knee circumference. Overall, all three trials found no statistically significant difference in knee circumference between the continuous cryotherapy group and the traditional cryotherapy group.
4.2 Secondary outcomes
4.2.1 Blood loss
Only one study reported blood loss and there was no statistically significant difference between the continuous cryotherapy group and the traditional cryotherapy group.
4.2.2 Change in hemoglobin
Three trials reported data for hemoglobin changes, and meta-analysis was not performed because two trials reported the preoperative and postoperative hemoglobin while one trial reported the change in hemoglobin. Only one study detected a statistically significant difference between the continuous cryotherapy group and the traditional cryotherapy group (22).
4.2.3 Transfusion rate
Data for transfusion rate was reported by three trials, and meta-analysis was not performed as two trials reported the number of transfusions while one study reported the number of units of allogeneic blood transfusions. Overall, all three trials found no statistically significant difference in transfusion rate between the continuous cryotherapy group and the traditional cryotherapy group.
4.2.4 Length of hospital stay
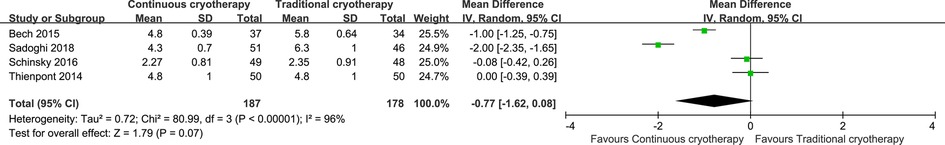
Data for the length of hospital stay were reported by four trials that recruited 265 patients (n = 187 vs. n = 178 in the continuous cryotherapy group and traditional cryotherapy group, respectively). There was no statistically significant difference in the length of hospital stay between the continuous cryotherapy group and the traditional cryotherapy group (MD: −0.77, 95% CI: −1.62 to 0.08; P = 0.07) (Figure 7). A high level of heterogeneity was observed (I2 = 84%).
4.2.4 Safety
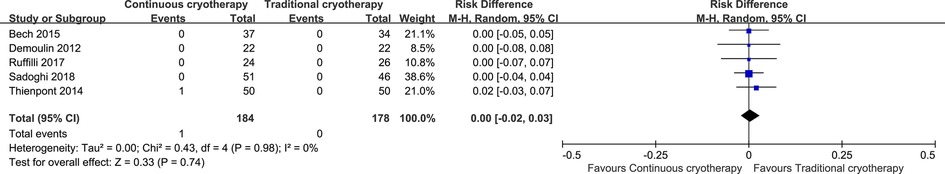
Data for adverse events were reported by six trials that recruited 362 patients (n = 184 vs. n = 178 in the continuous cryotherapy group and traditional cryotherapy group, respectively). There was no statistically significant difference in the incidence of adverse events between the continuous cryotherapy group and the traditional cryotherapy group (RD: 0, 95% CI: −0.02 to 0.03; P = 0.74) (Figure 8).
4.2.4 Cost
Data for cryotherapy costs were reported by three trials. Thienpont et al. reported that the cost of continuous cryotherapy is $ 520, Schinsky et al. reported that continuous cryotherapy costs $97.34 than traditional cryotherapy per patient, while Karaduman reported that they found no significant additional costs associated with the use of continuous cryotherapy.
5. Discussion
5.1 Main findings
Our meta-analysis comprehensively and systematically reviewed the currently available literature, and the study results suggest that continuous cryotherapy does not appear to offer significant clinical benefits for TKA compared with traditional cryotherapy. There were no significant differences in pain intensity, analgesics consumption, postoperative range of motion, swelling of the knee joint, blood loss, change in hemoglobin, transfusion rate, length of hospital stay, and adverse events. In addition, continuous cryotherapy may lead to extra costs and resources than traditional cryotherapy.
5.2 Implication for clinical practice
Although TKA shows long-lasting clinical and structural improvement for the management of severe osteoarthritis, patients in the immediate postoperative period are often associated with acute pain, hidden bleeding, severe edema, and reduced range of motion. Cryotherapy has been shown to appreciably reduce the intraarticular temperature, especially in the knee, blood flow by vasoconstriction, the local inflammatory reaction, postoperative bleeding and swelling, pain transmission, and the length of hospital stay (26–29). In the clinic, several cryotherapy options are available, including: (i) the first-generation cold therapy such as ice bag or gel pack; (ii) second-generation cryotherapy devices with circulating ice water with or without compression; (iii) third-generation devices with advanced computer-assisted devices to provide continuous controlled cold therapy (17). Compared with ice/gel pack, advanced cryotherapy devices are developed and are expected to be even more efficient as they maintain a steady low temperature for an extended time. Thus, in theory, continuous cryotherapy could play a better role in fast-track rehabilitation after TKA by reducing inflammation, pain, and swelling. However, this meta-analysis observed no differences in clinical outcomes between continuous cryotherapy and traditional cryotherapy, which could be caused by several factors such as the level of tissue penetration of cold therapy, method of cryotherapy, time of application, and types of outcome measurement. TKA-induced inflammation leads to a significant increase in temperature deep inside the knee joint, and the effect of cryotherapy after TKA is closely related to the temperature-dependent mechanism (13). After the cold temperature penetrates tissues and reaches the intended area, which reduces inflammation, reduces nerve conduction velocity, induces local vasoconstriction, and reduces blood flow to muscles (30–35). Although continuous cryotherapy is a more effective treatment to consistently maintain the temperature of the knee joint below the body temperature, the findings of this study suggested that traditional cryotherapy using ice/gel pack could achieve a similar decrease in temperature and reach similar clinical effects. However, a significant weakness of these trials is that neither the skin temperature nor the intraarticular temperature was persistently measured and monitored to confirm effective cooling (17–22). Currently, the optimal cold treatment protocol remains unclear, including the cold temperature, application time and interval, and whether it needs relevant adjustment for different joints. Therefore, further exploration of the application of cryotherapy should be considered, and attention should also be paid that statistically significant findings may not translate into clinically significant results.
On the other hand, as healthcare providers, it is our duty to appropriately allocate finite resources to evidence-based approaches that are efficacious in an era of increasing expenses. Therefore, apart from the convenience that continuous cryotherapy devices provide prolonged continuous cooling and do not need to change the ice/gel pack, which does not offer any extra clinical advantage for patients undergoing TKA when compared with traditional cryotherapy (13). However, continuous cryotherapy warrants additional costs and resources associated with providing the cooling devices, which may not be covered by insurance (13, 17, 19, 22). In comparison, the cost of traditional cryotherapy is almost neglectable but achieves similar clinical effects. Thus, the currently available evidence does not support the theoretical cost-effectiveness of utilizing continuous cryotherapy after TKA, and future high-level prospective studies are needed to verify these findings.
In addition, the current available RCTs only applied continuous cryotherapy and traditional cryotherapy during hospitalization and not after discharge, which is a relatively short duration, and the minimal difference between continuous cryotherapy and traditional cryotherapy may not be detected (17, 29). Therefore, the extended application of cryotherapy at home could also be explored in future studies.
5.3 Strengths and limitations
To the best of our knowledge, this is the first systematic review and meta-analysis that systemically and comprehensively reviewed currently available evidence to compare the efficacy, safety, and cost-effectiveness of continuous cryotherapy vs. traditional cryotherapy for TKA.
Our study also has several potential limitations. First of all, in more than half of included studies, neither patients nor healthcare providers were blinded to group allocation and outcome assessment, hence, subjective assessments such as pain level are subject to potential bias. Second, the comparison of continuous cryotherapy vs. traditional cryotherapy was specialized to the TKA procedure, which may not be generalizable to other surgical procedures, such as arthroscopic surgery. Third, substantial heterogeneity across studies was noticed, which may be explained by the considerable difference in cryotherapy protocols. Lastly, almost all eligible trials included in the meta-analysis had relatively modest sample sizes (<100 patients), and overestimation of the treatment effect is more likely than in larger trials.
6. Conclusion
Our systematic review and meta-analysis suggested that continuous cryotherapy showed no superiority in reducing pain intensity, analgesics consumption, swelling, blood loss, length of hospital stay, and improving ROM compared with traditional cryotherapy in the acute postoperative setting after TKA. Continuous cryotherapy may further lead to extra costs and resources, so the currently available evidence does not support continuous cryotherapy could be added as an adjunct therapy. Additional well-designed studies with larger sample sizes are needed to confirm these preliminary results.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
Conceived and designed the study: M-ML, MT, SW, LS. Acquired, analyzed, and interpreted the data: M-ML, MT, CL, SW, LS. Drafted or revised the article: M-ML, MT, CL, SW, LS. Final approval of the version to be published: M-ML, MT, CL, SW, LS. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Medical and Health Technology Plan of Zhejiang Province (No. 2022RC068).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. (2007) 89(4):780–5. doi: 10.2106/JBJS.F.00222
2. Patel A, Pavlou G, Mújica-Mota RE, Toms AD. The epidemiology of revision total knee and hip arthroplasty in England and Wales: a comparative analysis with projections for the United States. A study using the national joint registry dataset. Bone Joint J. (2015) 97-B(8):1076–81. doi: 10.1302/0301-620X.97B8.35170
3. Matsen FA 3rd, Questad K, Matsen AL. The effect of local cooling on postfracture swelling. A controlled study. Clin Orthop Relat Res. (1975) 109:201–6. doi: 10.1097/00003086-197506000-00029
4. Hecht PJ, Bachmann S, Booth RE Jr, Rothman RH. Effects of thermal therapy on rehabilitation after total knee arthroplasty. A prospective randomized study. Clin Orthop Relat Res. (1983) 178:198–201. doi: 10.1097/00003086-198309000-00023
5. Grosu I, Lavand’homme P, Thienpont E. Pain after knee arthroplasty: an unresolved issue. Knee Surg Sports Traumatol Arthrosc. (2014) 22(8):1744–58. doi: 10.1007/s00167-013-2750-2
6. Andersen LØ, Gaarn-Larsen L, Kristensen BB, Husted H, Otte KS, Kehlet H. Subacute pain and function after fast-track hip and knee arthroplasty. Anaesthesia. (2009) 64(5):508–13. doi: 10.1111/j.1365-2044.2008.05831.x
7. Kehlet H. Fast-track hip and knee arthroplasty. Lancet. (2013) 381(9878):1600–2. doi: 10.1016/S0140-6736(13)61003-X
8. Hansen TB. Fast track in hip arthroplasty. EFORT Open Rev. (2017) 2(5):179–88. doi: 10.1302/2058-5241.2.160060
9. Martin SS, Spindler KP, Tarter JW, Detwiler KB. Does cryotherapy affect intraarticular temperature after knee arthroscopy? Clin Orthop Relat Res. (2002) 400:184–9. doi: 10.1097/00003086-200207000-00023
10. Zhong Y, Zheng C, Du W, Zheng J, Xu S, Tong P. Mirabilite with ice pack after total knee arthroplasty: a randomized controlled trial study. Evid Based Complement Alternat Med. (2021) 2021:6611614. doi: 10.1155/2021/6611614
11. Maranda E, Simmons BJ, Romanelli P. Cryotherapy-As ancient as the pharaohs. JAMA Dermatol. (2016) 152(6):730. doi: 10.1001/jamadermatol.2015.1616
12. Wang H, Olivero W, Wang D, Lanzino G. Cold as a therapeutic agent. Acta Neurochir (Wien). (2006) 148(5):565–70; discussion 569–70. doi: 10.1007/s00701-006-0747-z
13. Kunkle BF, Kothandaraman V, Goodloe JB, Curry EJ, Friedman RJ, Li X, et al. Orthopaedic application of cryotherapy: a comprehensive review of the history, basic science, methods, and clinical effectiveness. JBJS Rev. (2021) 9(1):e20.00016. doi: 10.2106/JBJS.RVW.20.00016
14. Ruffilli A, Buda R, Castagnini F, Di Nicolantonio D, Evangelisti G, Giannini S, et al. Temperature-controlled continuous cold flow device versus traditional icing regimen following anterior cruciate ligament reconstruction: a prospective randomized comparative trial. Arch Orthop Trauma Surg. (2015) 135(10):1405–10. doi: 10.1007/s00402-015-2273-z
15. Chughtai M, Sodhi N, Jawad M, Newman JM, Khlopas A, Bhave A, et al. Cryotherapy treatment after unicompartmental and total knee arthroplasty: a review. J Arthroplasty. (2017) 32(12):3822–32. doi: 10.1016/j.arth.2017.07.016
16. Demoulin C, Brouwers M, Darot S, Gillet P, Crielaard JM, Vanderthommen M. Comparison of gaseous cryotherapy with more traditional forms of cryotherapy following total knee arthroplasty. Ann Phys Rehabil Med. (2012) 55(4):229–40 (English, French). doi: 10.1016/j.rehab.2012.03.004
17. Thienpont E. Does advanced cryotherapy reduce pain and narcotic consumption after knee arthroplasty? Clin Orthop Relat Res. (2014) 472(11):3417–23. doi: 10.1007/s11999-014-3810-8
18. Bech M, Moorhen J, Cho M, Lavergne MR, Stothers K, Hoens AM. Device or ice: the effect of consistent cooling using a device compared with intermittent cooling using an ice bag after total knee arthroplasty. Physiother Can. (2015) 67(1):48–55. doi: 10.3138/ptc.2013-78
19. Schinsky MF, McCune C, Bonomi J. Multifaceted comparison of two cryotherapy devices used after total knee arthroplasty: cryotherapy device comparison. Orthop Nurs. (2016) 35(5):309–16. doi: 10.1097/NOR.0000000000000276
20. Ruffilli A, Castagnini F, Traina F, Corneti I, Fenga D, Giannini S, et al. Temperature-Controlled continuous cold flow device after total knee arthroplasty: a randomized controlled trial study. J Knee Surg. (2017) 30(7):675–81. doi: 10.1055/s-0036-1593874
21. Sadoghi P, Hasenhütl S, Gruber G, Leitner L, Leithner A, Rumpold-Seitlinger G, et al. Impact of a new cryotherapy device on early rehabilitation after primary total knee arthroplasty (TKA): a prospective randomised controlled trial. Int Orthop. (2018) 42(6):1265–73. doi: 10.1007/s00264-018-3766-5
22. Karaduman ZO, Turhal O, Turhan Y, Orhan Z, Arican M, Uslu M, et al. Evaluation of the clinical efficacy of using thermal camera for cryotherapy in patients with total knee arthroplasty: a prospective study. Medicina (Kaunas). (2019) 55(10):661. doi: 10.3390/medicina55100661
23. Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0. (updated March 2011). Available at: http://www.cochrane.org/handbook
24. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6(7):e1000097. doi: 10.1371/journal
25. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. Br Med J. (2011) 343:d5928. doi: 10.1136/bmj.d5928
26. Markert SE. The use of cryotherapy after a total knee replacement: a literature review. Orthop Nurs. (2011) 30(1):29–36. doi: 10.1097/NOR.0b013e318205749a
27. Ewell M, Griffin C, Hull J. The use of focal knee joint cryotherapy to improve functional outcomes after total knee arthroplasty: review article. PM&R. (2014) 6(8):729–38. doi: 10.1016/j.pmrj.2014.02.004
28. Ni SH, Jiang WT, Guo L, Jin YH, Jiang TL, Zhao Y, et al. Cryotherapy on postoperative rehabilitation of joint arthroplasty. Knee Surg Sports Traumatol Arthrosc. (2015) 23(11):3354–61. doi: 10.1007/s00167-014-3135-x
29. Thacoor A, Sandiford NA. Cryotherapy following total knee arthroplasty: what is the evidence? J Orthop Surg (Hong Kong). (2019) 27(1):2309499019832752. doi: 10.1177/2309499019832752
30. Lindsay A, Carr S, Cross S, Petersen C, Lewis JG, Gieseg SP. The physiological response to cold-water immersion following a mixed martial arts training session. Appl Physiol Nutr Metab. (2017) 42(5):529–36. doi: 10.1139/apnm-2016-0582
31. White GE, Wells GD. Cold-water immersion and other forms of cryotherapy: physiological changes potentially affecting recovery from high-intensity exercise. Extrem Physiol Med. (2013) 2(1):26. doi: 10.1186/2046-7648-2-26
32. Kang JI, Jeong DK, Choi H. Effects of microcurrent and cryotherapy on C-reactive protein levels and muscle tone of patients with rotator cuff reconstruction. J Phys Ther Sci. (2018) 30(1):37–41. doi: 10.1589/jpts.30.37
33. Algafly AA, George KP. The effect of cryotherapy on nerve conduction velocity, pain threshold and pain tolerance. Br J Sports Med. (2007) 41(6):365–9; discussion 369. doi: 10.1136/bjsm.2006.031237
34. Stålman A, Berglund L, Dungnerc E, Arner P, Felländer-Tsai L. Temperature-sensitive release of prostaglandin E₂and diminished energy requirements in synovial tissue with postoperative cryotherapy: a prospective randomized study after knee arthroscopy. J Bone Joint Surg Am. (2011) 93(21):1961–8. doi: 10.2106/JBJS.J.01790
35. Vieira Ramos G, Pinheiro CM, Messa SP, Delfino GB, Marqueti Rde C, Salvini Tde F, et al., Cryotherapy reduces inflammatory response without altering muscle regeneration process and extracellular matrix remodeling of rat muscle. Sci Rep. (2016) 6:18525. doi: 10.1038/srep18525
Appendix 1. Literature search strategy.
Keywords: cryotherapy, total knee arthroplasty, postoperative pain, analgesics consumption, swelling, range of motion, cost
Citation: Liu M, Tian M, Luo C, Wang S and Shao L (2023) Continuous cryotherapy vs. traditional cryotherapy after total knee arthroplasty: A systematic review and meta-analysis of randomized controlled trials. Front. Surg. 9:1073288. doi: 10.3389/fsurg.2022.1073288
Received: 18 October 2022; Accepted: 5 December 2022;
Published: 11 January 2023.
Edited by:
Xisheng Weng, Peking Union Medical College Hospital (CAMS), ChinaReviewed by:
Alfonso Javier Ibáñez-Vera, University of Jaén, SpainYaying Sun, Fudan University, China
© 2023 Liu, Tian, Luo, Wang and Shao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meng-Meng Liu bGl1bWVuZ21lbmcyMDg1QDEyNi5jb20= Long Shao aGlwa25lZWRvY0AxNjMuY29t
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Orthopedic Surgery, a section of the journal Frontiers in Surgery
 Meng-Meng Liu1†
Meng-Meng Liu1† Shicheng Wang
Shicheng Wang Long Shao
Long Shao