- 1Department of Cardiology, The First Hospital of Putian, Putian, China
- 2Department of Emergency, The First Affiliated Hospital of Fujian Medical University, Fuzhou, China
Aortoesophageal fistula (AEF), secondary to thoracic pseudoaneurysm as a result of upper gastrointestinal bleeding, is a rare condition and will be undoubtedly lethal without prompt surgical intervention. The estimated annual incidence of primary AEFs and secondary AEFs is about 0.0015% and 0.6%–2%, respectively. The challenges of the therapy posed by AEF are control of the hemorrhage, arterial reconstruction in an infection field, control of sepsis, and re-establishment of the alimentary tract. We present a case of a 58-year-old man who suffered from chest pain and hematemesis and was finally diagnosed with pAEF caused by descending thoracic pseudoaneurysm. Our team successfully deployed an endovascular stent graft and esophageal stent to seal ruptured thoracic aorta and esophageal defects, which provided a new surgical strategy for aortoesophageal fistula in the endovascular era.
Introduction
Aortoesophageal fistula (AEF) was first described by Dubreuil (1) in 1818. The classical aortoesophageal syndromes of AEF, which were reported by Chiari (2), include a sequence of mid-thoracic pain or dysphagia, sentinel minor bleeding, and exsanguination after a symptom-free interval (3). An early careful evaluation and a high index of suspicion for the atypical hematemesis are crucial. In spite of various surgical interventions for AEF, few survivors have been reported. Therefore, there is no consensus or guidelines at present.
Case Report
A 58-year-old male with a history of poorly controlled hypertension and hyperuricemia presented to a local hospital with complaints of chest pain for the past 1 month; then, aspirin was prescribed due to the change in the ST–T segment. The symptoms were still persistent, and he was admitted to the first-aid room of our institution with an episode of massive hematemesis from the last 12 h. He denied a history of abdominal pain, alcoholism, past history of gastrointestinal (GI) bleeding, or any significant past medical history. On physical examination, his heart rate (HR) was 86 beats/min with blood pressure (BP) of 138/81 mmHg, and the other laboratory indexes were unremarkable. Workup showed decreased hemoglobin (100 g/L) and hematocrit (0.295L/L), C-reactive protein (CRP) of 49 mg/L (normal range: <10.00 mg/L), and without coagulopathy. Surprisingly, plasma ammonia was normal. There was no history of melaena but persistent chest pain and normal plasma ammonia, which aroused our strong suspicion of an AEF. So, esophagigastroduodenoscopy (EGD) was canceled, and emergent computed tomography (CT) and angiography of the thorax (ACT) were performed. The aortogram revealed the presence of pseudoaneurysm (size 40 × 60 mm) in the descending thoracic aorta (Figure 1A). Urgently, the vascular surgical team was consulted, and thoracic endovascular aneurysm repair was done with a stent graft (Ankura) (Figure 2A–C); the outcome was favorable. Meanwhile, oral empirical treatment with moxifloxacin was initiated despite negative blood cultures. The patient was completely stable and underwent the treatment of esophageal lesion with the deployment of an esophageal stent on the fourth postoperative day (Figure 3). In addition, a gastrostomy tube was placed for postoperative transenteral nutrition. He received an oral intake of antibiotic therapy for 2 weeks until inflammation findings were negative. Postoperation computed tomographic investigation conducted on the first day and 14 days after AEF repairment suggested no esophageal leakage or grant infection (Figure 2D, E). He recovered well and was discharged 14 days after surgery. Two months after the discharge, the patient underwent the gastroscopy again, and the results showed that the fistula healed, the esophageal stent was removed, and the gastrostomy was closed. Gastroscope and chest CT were re-examined regularly every 3–6 months. There is no fever, chest pain, and other symptoms of infection. Now, the patient has recovered well and is back to normal life.
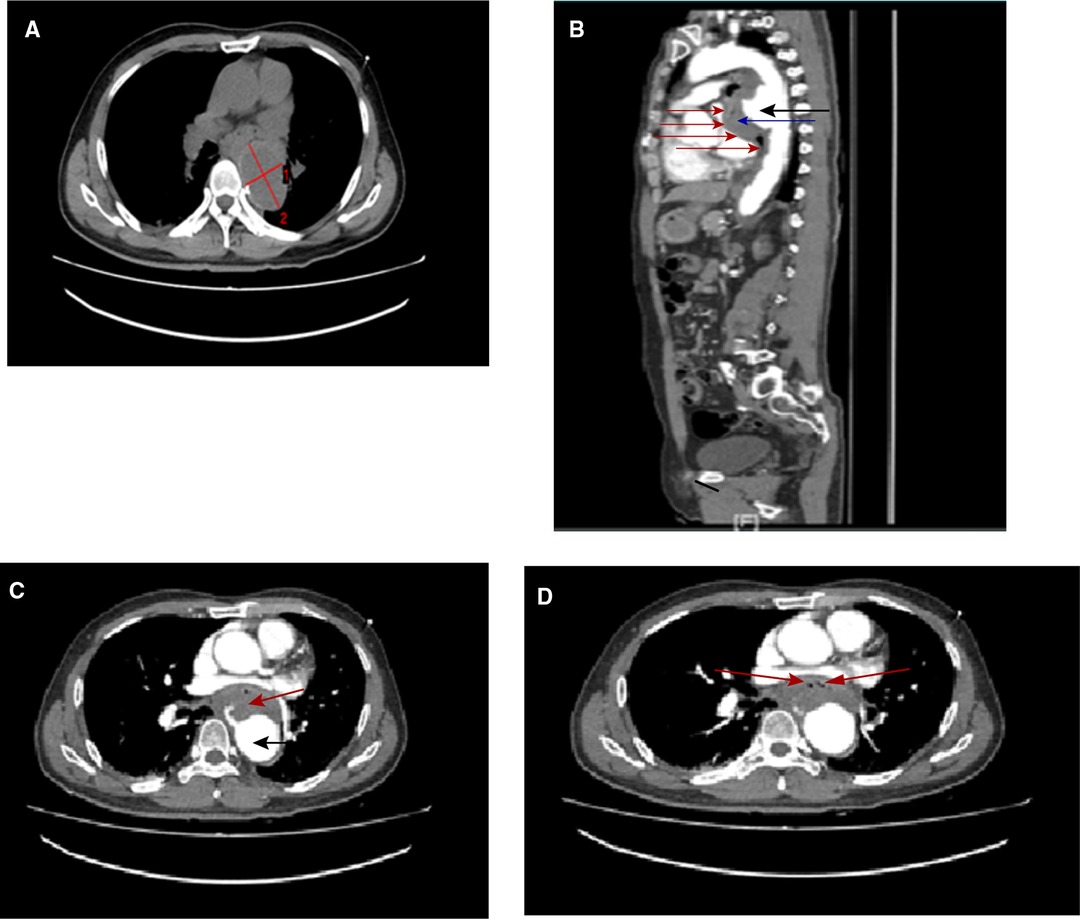
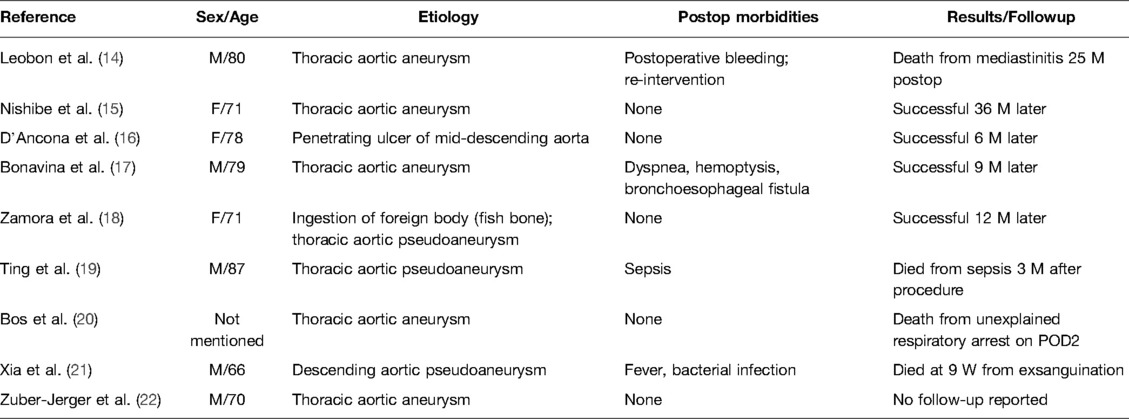
Figure 1. Computed tomography (CT) images showing (A) dilatation of descending aorta; the widest place reaches 4×6 cm (red line). Multidetector CT angiography: (B) sagittal reformatted images through the thorax showing active aortic bleeding generating an anterior hematoma (black arrow head) and exudate or rupture to form a large thrombus (blue arrow head), which is compressing the posterior wall of the esophagus (red arrow head), resulting in the abnormal course of the esophagus and is not recognizable in the picture. Multidetector CT angiography: (C) axial reformatted images through the thorax revealing active aortic bleeding generating an anterior hematoma (black arrow head) and exudate or rupture to form a large thrombus (red arrow head). (D) air bubbles (red arrow head) inside the aneurysm’s thrombus that are suggestive of esophageal erosion.
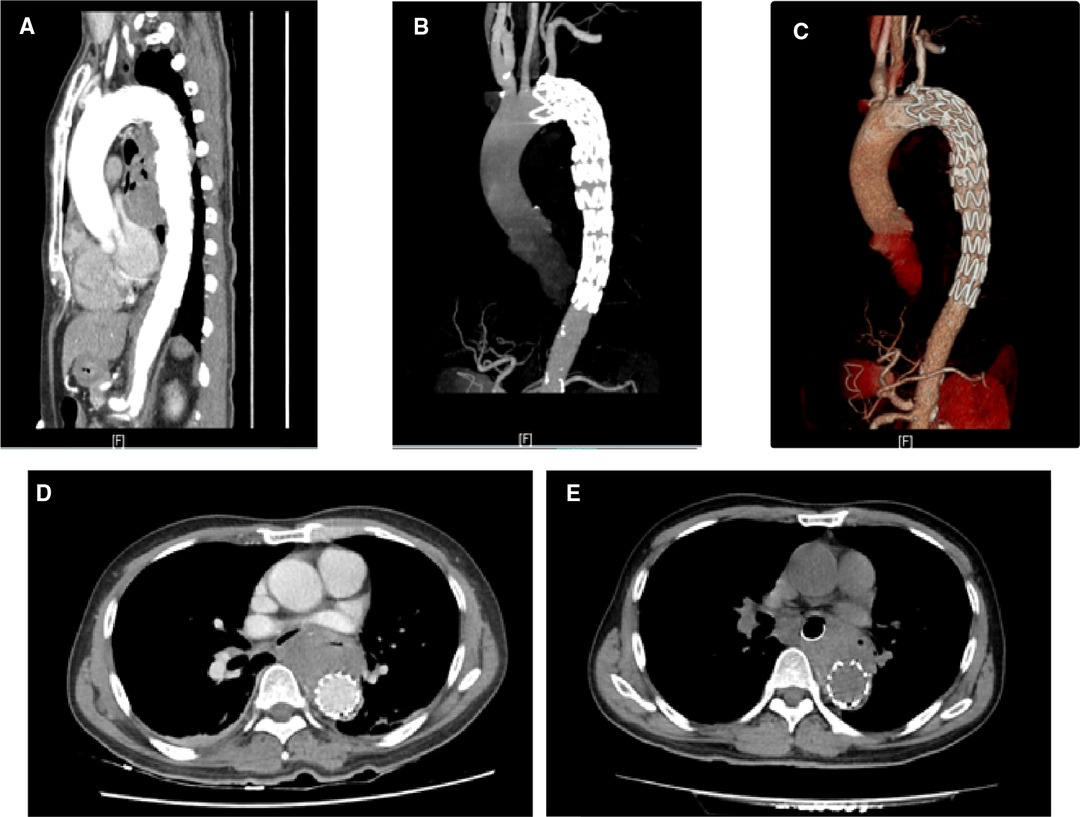
Figure 2. Postoperation computed tomographic angiography (A–C) and computed tomography (D) 1 day and (E) 14 days after AEF repair showing that the aortic lesions have been completely excluded, with no signs of endoleak, mediastinal infection, or stent-graft contamination.
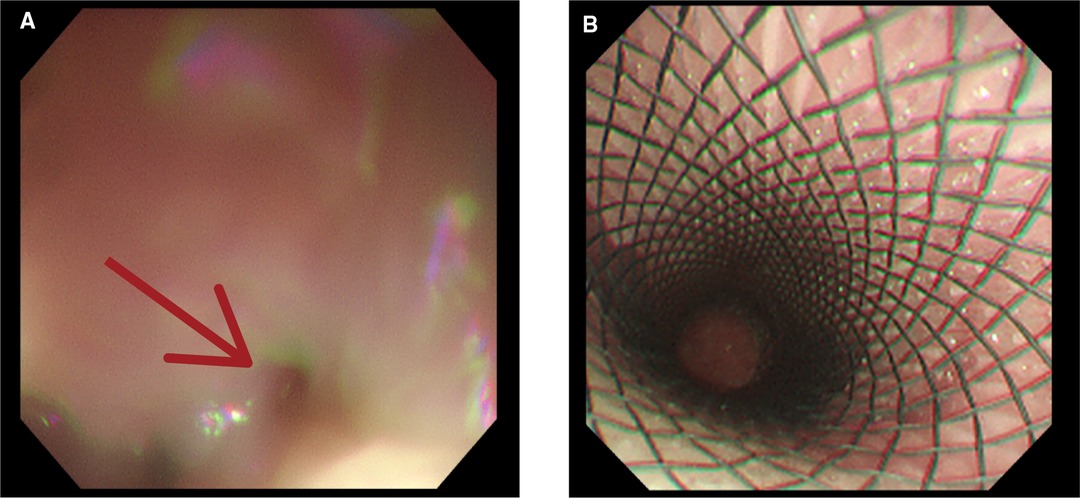
Figure 3. Upper gastrointestinal endoscopy revealing (A) an esophageal lesion (red arrow head) and (B) installation of an esophageal stent.
Discussion
AEF is a subtype of aortoenteric fistula since the lesion primarily involves the thorax rather than the abdomen, which constitutes approximately 10% of all aortoenteric fistulas (4). pAEF most frequently occurs between the duodenum and abdominal aorta. Sears and Scheltinga (5) received 81 published cases of aortoenteric fistulas and reported that it was most commonly located in the duodenum (54%), followed by the esophagus (28%). pAEF is rare and mostly secondary to a true aneurysm but less frequent to a pseudoaneurysm (6, 7). An additional rare cause of pAEF is penetrating aortic ulcer (PAU), which was first described in 1934 by Shennan (8, 9). To our best knowledge, this is the third case of AEF, which was caused by PAU. In our patient, there was no significant previous medical or surgical history, and the scan of computed tomography angiography showed a tortuous, atheromatous aorta with calcified plaques. Multiple parietal ulcers were observed on the descending aorta (Figure 4), which resulted from the ulceration of a previous atherosclerotic plaque. PAUs penetrate the aortic wall from the internal elastic lamina to the arterial media; the hematoma is initially contained by the tunica adventitia, leading to a pseudoaneurysm formation (10). Therefore, we can see a hematoma of 40 × 60 mm in the CTA due to active contrast media extravasation. According to Laplace’s equation, as the diameter of the aneurysm increases, the wall tension at any given intraluminal pressure increases and slowly weakens the aorta wall or ruptures the adventitia (11). These factors contribute to persistent extravasation of blood to form a large thrombus outside the pseudoaneurysm, which compresses the esophagus obviously (Figure 1B, C). In the thrombus, we also observed some free air bubbles, which suggested evidence of esophageal rupture (Figure 1D). The cause of esophageal rupture is that the esophageal wall is in contact with the high-pressure pulsating aorta for a long time, resulting in esophageal wall injury and ischemic necrosis of the esophageal wall. If AEF is suspected on medical history, symptoms, and work-up, the EGD must be avoided because of the high risk of fatal bleeding under the reduced intraesophageal pressure. On the contrary, angiography of the thorax should be performed immediately to confirm the diagnosis of AEF. Tokeno et al. (12) proposed the theory that AEF is essentially an infection in which the bacteria-free aorta becomes contaminated by material from the gastrointestinal tract. Urgent operation and strong, broad-spectrum antibiotic therapy offer the only chance for survival in these patients with AEFs. However, no consensus has been reached on therapeutic strategy because of the rarity of the disease and the changing therapeutic trends. Tokeno et al. (12) also reviewed articles published in the last decade concerning the trends in AEFs and showed that a treatment strategy combining graft replacement with esophagectomy has the most favorable prognosis among all the possible options.
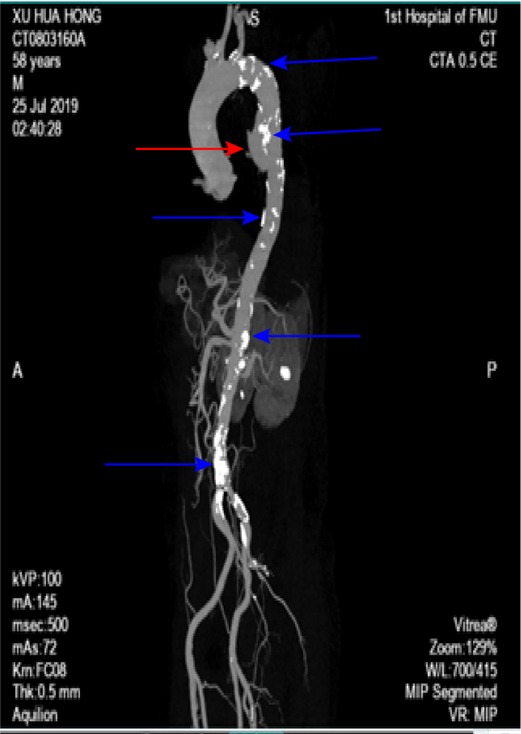
Figure 4. Three-dimensional reconstruction showing a descending thoracic aortic pseudoaneurysm (redarrow head) on the anterior aortic wall and multiple atheromatous plaques (blue arrow head).
A review of the literature in PubMed reveals nine published reports of nine primary AEFs caused by pseudoaneurysm or aneurysm who underwent the endovascular stent-graft repair (Table 1). Of these nine patients, one patient did not have any follow-up, four patients were doing well up to 36 months after the procedure (maximum follow-up time of 36 months, minimum follow-up time of 6 months), two patients died from an infection related to their stent graft (one from mediastinitis and one from sepsis), and two patients died from causes not related to infection of their stent graft (one patient from respiratory arrest and one from GI bleeding). The four patients who were reported to be alive did not suffer infections. Based on the published cases, first-stage graft replacement also faces a high risk of endograft infection, especially if there has been mediastinal contamination before operation (13). Thus, it has been suggested that thoracic endovascular aortic repair (TEAR) may be used as a damage control surgery for hemostasis as a bridge to graft replacement after an improvement in the patient’s general condition has been achieved (3). Moreover, Nishibe et al. (15) have reported a case in which the endurance of endovascular stents exceeded more than 3 years without any serious local or systemic complications. The strategy of repairment should be highly individualized and according to the capacity of the surgical team of the institution.
The patient, who was in the first-aid room at that moment, said only his wife was with him and his children were abroad. He experienced fear and anxiety from vomiting blood and chest pain, which would have been worse for him if he had undergone conventional general anesthesia. The patient was conscious during the procedure, and our staff were always comforting and encouraging, making him feel relaxed during the procedure.
In this case, our successful experience demonstrates the feasibility of the operation of pAEF using an endovascular stent graft and an esophageal stent. Compared to the traditional surgical treatment that includes resection or exclusion of aneurysms combined with external anatomical bypass surgery or in situ aneurysm repair, stent implantation is less invasive, especially in the acute phase of the disease. There are a variety of tubes for in situ replacement, including autologous veins, polytetrafluoroethylene grafts, cryopreserved allografts, and antibiotic-bound grafts. So, stent implantation showed its convenience. However, the mediastinum is a potential infection area and may cause a severe infection of vascular stents, which is the biggest limitation we are most concerned about. To our knowledge, there was no case reported to date about this scheme of operation. Large series cases and long-term follow-ups will be necessary to further establish the prospect of the proposed combined treatment of pAEF.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material; further inquiries can be directed to the corresponding author/s.
Ethics Statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
Z-WW and Y-DY: case collection. Z-WW and Y-DY, and Y-ML: analysis of the case. Z-WW and Y-ML: manuscript draft preparation. All authors reviewed the results and approved the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Dubrueil O. Observation sur la perforation de l’esophague et de l’aorte thoracique par une portion d’os oval: avec des réflexions. J Univ Sci Med. (1818) 9:357–63.
2. Chiari H. Ueber Fremdkorpeverletzung des oesophagus mit aortenperforation. Ber Klin Wochenschr. (1914) 51:7–9.
3. Prokakis C, Koletsis E, Apostolakis E, Dedeilias P, Dougenis D. Aortoesophageal fistulas due to thoracic aorta aneurysm: surgical versus endovascular repair. Is there a role for combined aortic management? Med. Sci. Monit. (2008) 14(4):Ra48–54. PMID: 18376359
4. Contini S, Corrente V, Nervi G, Franzè A, Scarpignato C. Dysphagia aortica: a neglected symptom of aortoesophageal fistula. Dig Liver Dis. (2006) 38(1):51–4. doi: 10.1016/j.dld.2005.03.015
5. Saers SJF, Scheltinga MRM. Primary aortoenteric fistula. J Br Surg. (2005) 92(2):143–52. doi: 10.1002/bjs.4928
6. Lee OJ, Kim SH. Aortoesophageal fistula associated with tuberculous mediastinitis, mimicking esophageal Dieulafoy’s disease. J Korean Med Sci. (2002) 17(2):266–9. doi: 10.3346/jkms.2002.17.2.266
7. Shindo S, Suzuki O, Kamiya K, Tada Y. Primary aortoduodenal fistula treated successfully with surgery in a patient with Takayasu’s arteritis: case report. International. (1999) 18(3):244. PMID: 10688426
8. Van Leerdam ME. Epidemiology of acute upper gastrointestinal bleeding. Best Pract Res Clin Gastroenterol. (2008) 22(2):209–24. doi: 10.1016/j.bpg.2007.10.011
9. Blatchford O, Davidson LA, Murray WR, Blatchford M, Pell J. Acute upper gastrointestinal haemorrhage in west of Scotland: case ascertainment study. BMJ. (1997) 315(7107):510–4. doi: 10.1136/bmj.315.7107.510
10. Gomes SIM, de Campos FPF, Martines BMR, dos Santos Martines JA, Tafner E, Maruta LM. Primary aortoesophageal fistula: a rare cause of acute upper gastrointestinal bleeding. Autopsy Case Rep. (2011) 1(4):57. doi: 10.4322/acr.2011.018
11. Amin S, Luketich J, Wald A. Aortoesophageal fistula: case report and review of the literature. Dig Dis Sci. (1998) 43(8):1665–71. doi: 10.1023/A:1018850728928
12. Takeno S, Ishii H, Nanashima A, Nakamura K. Aortoesophageal fistula: review of trends in the last decade. Surg Today. (2020) 50(12):1551–9. doi: 10.1007/s00595-019-01937-z
13. Prokakis C, Charoulis N, Tselikos D, Koletsis EN, Apostolakis E, Dougenis D. Primary aortoesophageal fistula due to thoracic aortic aneurysm: successful surgical treatment. Tex Heart Inst J. (2009) 36(6):607. PMID: 20069092
14. Leobon B, Roux D, Mugniot A, Rousseau H, Cerene A, Glock Y, et al. Endovascular treatment of thoracic aortic fistulas. Ann Thorac Surg. (2002) 74(1):247–9. doi: 10.1016/S0003-4975(02)03466-5
15. Nishibe T, Koizumi J, Kudo F, Miyazaki K, Nishibe M, Yasuda K. Successful endovascular stent-graft treatment for an aortoesophageal fistula caused by a descending thoracic aortic aneurysm: report of a case. Surg Today. (2004) 34(6):529–31. doi: 10.1007/s00595-004-2748-y
16. D’Ancona G, Dagenais F, Bauset R. Endoluminal stenting of the aorta as treatment of aortoesophageal fistula due to primary aortic disease. Tex Heart Inst J. (2002) 29(3):216–7. PMID: 12224728
17. Bonavina L, Lupattelli T, Bona D, Trimarchi S, Nano G, Rampoldi V, et al. Bronchoesophageal fistula after endovascular repair of ruptured aneurysm of the descending thoracic aorta. J Vasc Surg. (2005) 41(4):712–4. doi: 10.1016/j.jvs.2005.01.041
18. Zamora CA, Sugimoto K, Tsuji Y, Matsumori M, Taniguchi T, Sugimura K, et al. Stent-grafting of an infected aortoesophageal fistula following ingestion of a fish bone. J Endovasc Ther. (2005) 12(4):522–3. doi: 10.1583/05-1556.1
19. Ting AC, Cheng SW, Ho P, Poon JT. Endovascular stent graft repair for infected thoracic aortic pseudoaneurysms – a durable option? J Vasc Surg. (2006) 44(4):701–5. doi: 10.1016/j.jvs.2006.05.055
20. Bos WT, Verhoeven EL, Zeebregts CJ, Tielliu IF, Prins TR, Oranen BI, et al. Emergency endovascular stent grafting for thoracic aortic pathology. Vascular. (2007) 15(1):12–7. doi: 10.2310/6670.2007.00002
21. Xia M, Guo JZ, Zhan Q, Yan J. Aortoesophageal fistula caused by descending aortic pseudoaneurysm: one case report. Chin Med J (Engl). (2007) 120(23):2149–50. doi: 10.1097/00029330-200712010-00017
Keywords: aortoesophageal fistula, endovascular stent, esophageal stent, case report, treatment
Citation: Wu Z, Yao Y and Li Y (2022) Case Report: Successful Repair of Primary Aortoesophageal Fistula With an Endovascular Stent Graft and an Esophageal Stent. Front. Surg. 9:868663. doi: 10.3389/fsurg.2022.868663
Received: 22 February 2022; Accepted: 20 May 2022;
Published: 14 June 2022.
Edited by:
Alberto Aiolfi, Università degli Studi di Milano, ItalyReviewed by:
Luigi Bonavina, University of Milan, ItalySavvas Lampridis, Guy’s and St Thomas’ NHS Foundation Trust, United Kingdom
Copyright © 2022 Wu, Yao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi-Ming Li NTEyNzMxMjJAcXEuY29t
†These authors have contributed equally to this work
Specialty section: This article was submitted to Vascular Surgery, a section of the journal Frontiers in Surgery
 Zhi-Wei Wu1†
Zhi-Wei Wu1† Yi-Ming Li
Yi-Ming Li