- 1Department of Hepatobiliary and Pancreatic Surgery, Hospital Universitario Mayor Méderi, Bogotá, Colombia
- 2School of Medicine, Universidad el Rosario, Bogotá, Colombia
- 3Chief and Chairman of Hepatobiliary and Pancreatic Surgery Department, Hospital Universitario Mayor Méderi, Bogotá, Colombia
Background: The Periampullary area comprehends a heterogeneous and complex structure with different histological tissues. Surgical standards include the peripancreatic regional lymphadenectomy, and during pancreatoduodenectomy (PD) the hepatic artery lymph node HALN(8a) is dissected. We aimed to describe the prognostic significance of the HALN(8a) lymph node metastasis in terms of disease-free survival (DFS) and overall survival (OS) in a specific cohort of patients in limited economic and social conditions.
Methods: A retrospective study was conducted based on a prospective database from the HPB department of patients who underwent pancreaticoduodenectomy (PD) due to periampullary tumors during 2014–2021. Overall survival (OS) and disease-free survival (DFS) were estimated to be associated with positive HALN(8a) using Kaplan-Meier analysis. Log Rank test and Cox proportional hazards regression analysis was used.
Results: 111 patients were included, 55,4% female. The most frequent pathology was ductal adenocarcinoma (60.3%). The positive rate of the HALN(8a) node was 21.62%. The Median OS time was 25.5 months, and the median DFS time was 13,8 months. Positive HLAN(8a) node, the cutoff of lymph node ratio resection (LNRR), and vascular invasion showed a strong association with OS. (CoxRegression p = 0.03 HR 0.5, p 0.003 HR = 1.8, p = 0.02 HR 0.4 CI 95%). In terms of DFS, lymph node ratio cutoff, tumoral size, and vascular invasion showed a statistically significant association with the outcome (p = 0.008, HR = 1.5; p = 0.04 HR = 2.1; p = 0.02 HR = 0.4 CI 95%).
Conclusion: In this series of PD, OS was reduced in patients with HALN(8a) compromise in patients with pancreatic cancer, however without statistical significance in DFS. In multivariate analysis, lymph node status remains an independent predictor of OS and DFS. Further studies are needed.
Introduction
The periampullary region is a complex area, composed of different histologic tissues, namely, biliary, duodenal, pancreatic, and ampullary. It is a frequent site of tumor growth; they correspond approximately to 5% of all gastrointestinal malignancies. Among the different types, pancreatic adenocarcinoma is the most frequent (70%), followed by ampullary cancers (15%–25%). Duodenal and distal common bile duct tumors (CBD) are present in approximately 10% (1). In rare cases, metastases from another primary cancer may be located in the periampullary region. The most frequent cancer type is renal cell carcinoma (3). Periampullary cancers arising from the duodenum, ampulla, and CBD are associated with better survival than pancreatic cancer and this may be related to tumor biology and more aggressive behavior (2).
Differentiation among histologic types preoperatively is difficult due to the proximity of the structures and their similar immunophenotype. Molecular characterization can be used for objective classification and targeted therapy (3, 4–6) However, despite their anatomical proximity and similar operative approach, these cancers have demonstrated a large disparity in outcomes. Currently, the most studied prognostic factors associated with disease-free survival (DFS) and overall survival (OS) are the resection margins, tumoral biology, vascular invasion, and lymph node metastasis (4–6).
The gold standard of treatment is pancreatoduodenectomy in all suspected or confirmed malignancy cases. During this procedure, resection of the hepatic artery node is performed routinely in most places to dissect and visualize the emergency of the gastroduodenal artery but is not mandatory in lymph node dissection (4, 8–10). The 8th edition of the TNM classification recommends pathologic examination of at least 12 nodes for reliable assessment of the lymphatic status (3). It has been identified in previous pancreatic cancer studies that the compromise of the hepatic artery lymph node (HALN), also called station 8a, a second-echelon node according to the Japan Pancreas Society classification (7) can be related to a reduction of OS (11). In fact, some case series report that the prognosis of HALN(8a) metastases can be compared with peritoneal carcinomatosis or liver metastases (10).
However, to our knowledge, studies evaluating the relevance of this node in other periampullary tumors are scarce. Additionally, no research studies have been published on patients from central and South America. The public health system in Colombia, like in the majority of countries in south and central America, is mixed with a significant private component with intermediation. This fact causes an important limitation to access to the health system and in this particular scenario, it is one of the biggest challenges to face with patients suffering from periampullary cancer who are not able to access adequate care such as neoadjuvant therapy at the right time. This is an interesting opportunity not only to understand but also to show some accurate data that will help to develop our own understanding of the specific social and economic conditions that are far from those in North America and Europe.
Given the different perspectives on the relation of HALN(8a) positive and poor prognosis, this retrospective study is proposed to assess the oncologic impact of HALN(8a) in our series of periampullary tumors taken to pancreaticoduodenectomy in Bogotá—Colombia.
Materials and methods
Patients
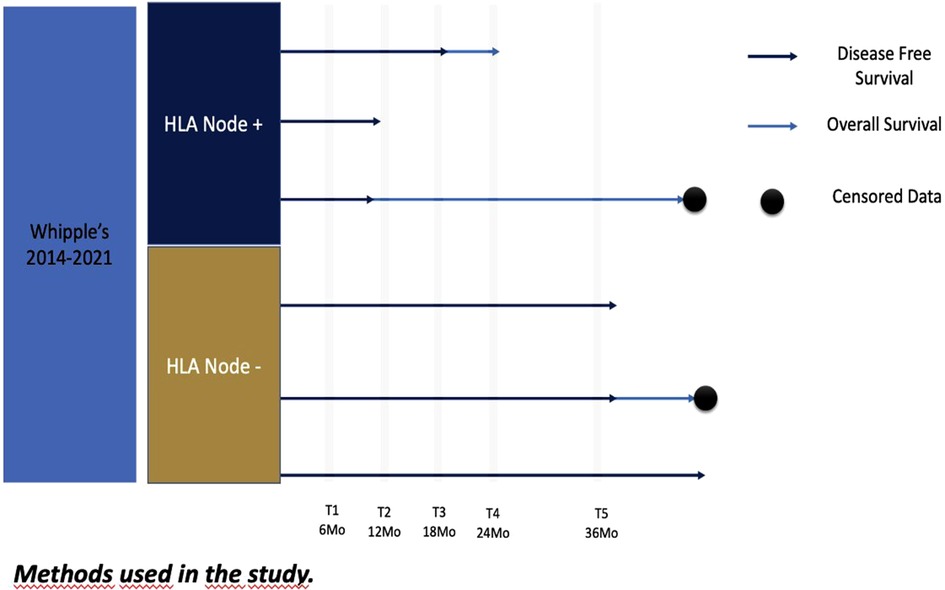
After the institutional review board and Ethical Committee approval, a retrospective cross-sectional review of patients of the single institution of the hepatic-pancreato-biliary database was performed. Patients who underwent pancreatoduodenectomy for periampullary tumors during 2014–2021 were included in this study (Figure 1). All these cases were treated with upfront surgery given its resectability or in some selected cases (by a multidisciplinary board) of borderline tumors according to the social and health care conditions. None of these borderline patients received neoadjuvant therapy because of limited healthcare accessibility or board institutional decisions. Pathologic reports were reviewed to identify hepatic node resection, oncological margins, and lymph nodes harvested.
Cases with incomplete data, positive macroscopic margins (R2), and not-reported hepatic nodes in pathologic specimens were excluded from the analysis. A descriptive analysis was made by calculating the proportions and frequencies of qualitative variables and central tendency measures for quantitative variables.
Variables were analyzed according to factors that have been shown relevance in previous studies for periampullary tumors: tumor size, histologic grade, vascular invasion, and completeness of resection.
Statistical analysis
Overall survival and disease-free survival
OS was defined from the date of the surgery until June 2021. Disease-free survival was defined as the time from the postoperative period, to the first documentation of tumoral relapse such as clinical (ascites), biomarkers analysis (Ca 19-9 antigen elevation), or radiographic findings (tomographic evidence of local or distance relapse) until June 2021. Survival analysis was performed using the Kaplan-Meier method for OS and DFS. LogRank tests were used to examine the association of clinical and pathological variables with the OS and DFS. Differential analysis was performed: patients that receive adjuvant therapy vs. HALN(8a) node compromise or not. Significant characteristics (p-value < 0.20) in LogRank tests were included in a multivariate cox proportional hazard regression model. Data with a minor or equal p-value of 0.05 were considered statistically significant. Statistical analysis for the comparison of mean values will be performed using t-tests or Wilcoxon-Mann-Whitney tests as appropriate. Assumptions regarding normal distribution will be checked by graphic representation, Shapiro-Wilk-Tests, and Q-Plots, respectively. The Chi-squared and Fisher's exact tests were used to compare categorical variables, as appropriate. Statistical analysis will be carried out using the Statistical Package of STATA Version 17.0 BE-Basic Edition (StataCorp LLC StataCorp 4,905 Lakeway Drive College Station, Texas 77,845 USA). A two-tailed p-value of < 0.05 will be considered statistically significant.
Lymph node ratio resection (LNRR)
A lymph node ratio resection (LNRR) was established as the main variable. LNRR is defined as the ratio between the total lymph node resected and the positive ones in the pathology report. A cutoff value was generated for this variable with logarithmic regression after Kaplan-Meier estimates, and a ROC value of 0.6 was estimated for the LNRR cutoff for the study population.
Follow-up
By institutional protocol, follow-up was performed at 6, 12, 18, 24, 30, and 36 months to determine the time of tumoral relapse, using tumoral biomarkers, clinical assessment, and tomographic studies. Mortality was determined by the time of deaths reported in the national database.
Lymph node subgroup analysis
Lymph node retrieval was classified according to peripancreatic and HALN positivity: Peripancreatic (+)/HALN (+) = 18.92% (n = 21/111), Peripancreatic (+), HALN (−) = 29.73% (n = 33/111), and Peripancreatic (−), HALN (−) = 48.65% (n = 54/111).
Results
Patients and tumor characteristics
A total of 136 patients underwent PD resection for pancreatic cancer during 2014–2020. Twenty-five patients were excluded from the study because of incomplete data. A total of 111 patients were included. The overall positive rate of lymph node resection, HALN(8a) positive rate, and the LNRR were analyzed for each patient.
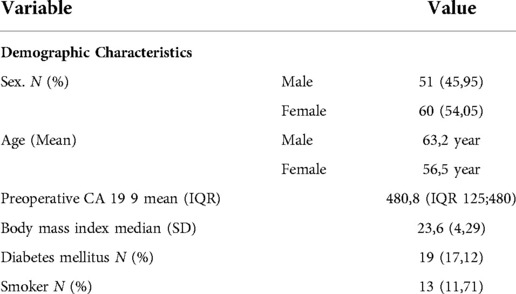
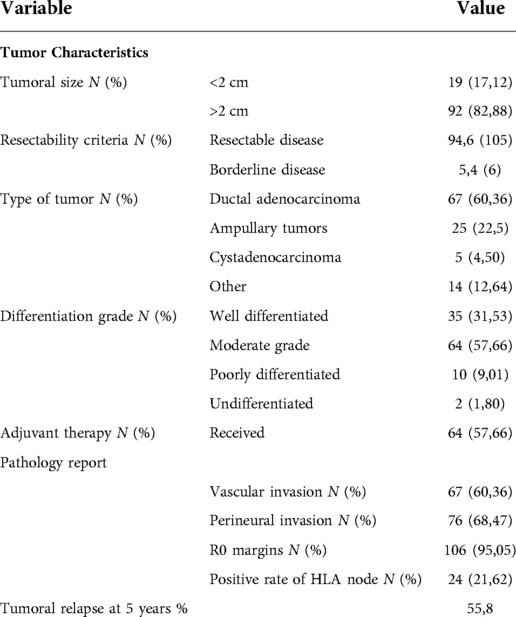
Females constituted 54,05% (n = 60) of cases. The mean age at the time of resection was 63.11y (SD 10.7). Pre-operative biomarker CA—19 9 was measured with a mean of 480 ng/dl (IQR 125;480). Other patient characteristics are summarized in Table 1. All patients underwent standard PD without pylorus preservation. The pathology in most of the patients was ductal adenocarcinoma at 60.36% (n = 67) followed by 22.52% for tumors of the ampulla of Vater. A moderate grade of differentiation of the tumor was found in 57% (n = 64) of the patients and a well-differentiated pathology (31.5%, n = 35).
Patients were classified using NCCN criteria. From the population of the study, 94.6% (n = 105) presented a resectable disease, the remaining patients were classified as borderline tumors. No locally advanced patients were included. R0 resections were achieved in 95,05% of the patients (n = 106); The majority of the patients have a vascular and perineural invasion in the pathology report (60,36% and 68,47% respectively). (See Table 2). The mean number of harvested lymph nodes was 14.53 (SD 5.8). The overall positive rate of the HALN(8a) node was 21.62% (n = 24). The overall positive nodes harvested was 9,01% (179 nodes out of 1613). The LNRR mean was 0.45 (SD 0.05). In the postoperative period, 42.34% (n = 47) do not receive adjuvant therapy due to both health insurance issues and personal decisions. During the analysis, the maximum time that patients receive chemotherapy after surgery was 24 months. 62,5% (n = 15) of the patients with positive HALN(8a) receive postoperative chemotherapy. Tumoral relapse was documented in 53.15% of the patients at 5 years of follow-up.
Survival analysis
The median follow-up for all the patients was 19.98 months. The median OS time was 25,5 months (IQR 12,5–84), and the median DFS time was 13.58 months (IQR 7,5–57). For patients that received adjuvant chemotherapy (57,66% n = 64/111), the median OS time was 16,3 months (IQR 5,5–84,6) and the median DFS time was 16 months (IQR 7,5–57), with a maximum of 57 months. The relationship between preoperative CA 19-9 value and hepatic artery lymph node positivity was evaluated, and a mean comparison was performed, there is a slight difference between the groups (HALN(8a)—515,1; HALN(8a) + = 354,3); two-way t-test was performed, however, there is any statistical relationship between CA 19-9 and HALN(8a) positivity (p-value = 0.42 CI 95%). The 5-year mortality and disease recurrence were analyzed in the most frequent pathology reports; ductal adenocarcinoma was related to an increased risk of disease recurrence and overall survival with statistical significance compared with ampullary tumors. (DFS: p = 0.008 CI 95% 1.21–3.70 HR 2.1; OS: p = 0.000 CI 95% 0.2–1.2 HR 1.5). The median overall survival in patients with ductal adenocarcinoma was 6.39 (IQR 6–7.1), and median disease-free survival in this group of patients was 9.97 months (IQR 7.6–11.2). For the ampulla of Vater tumors, the median OS was 7.7 (IQR 5.6–9.5); in terms of DFS, the median was 19,75 (IQR 12,5–24.4).
As well, pathology groups were classified according to the HALN(8a) positivity. In 26.8% (n = 18) of cases with ductal adenocarcinoma, hepatic artery lymph nodes were evidenced positive; in contrast patients with ampulla of Vater carcinoma, the HALN(8a) rate of positivity was 8% (n = 2). Median overall survival in patients with ductal adenocarcinoma with HALN(8a) + was slightly minor compared with HALN(8a)—group (Median 5.96 (IQR 4.5–7.8) vs. Median 6.5 (IQR 5.2–7.9) respectively). In terms of DFS, there is a difference as well comparing these two groups, in patients with HALN(8a) + median was 9.08 (IQR 7.4–11.2) and for patients with negative HALN(8a) median DFS was 10.34 (IQR 9.1–11.9).
In the group of the ampulla of Vater carcinoma with positive HALN(8a), the overall survival median was 5.56 (IQR 3.2–8.9) compared with the negative node group with a median of 7.96 (IQR 5.2–11.23). For disease-free survival in cases with positive HALN(8a) median was 4.5 (IQR 3.5–8.3), in the negative group, there is a greater difference with a median of 22.8 (IQR 17.2–27.5).
Overall survival
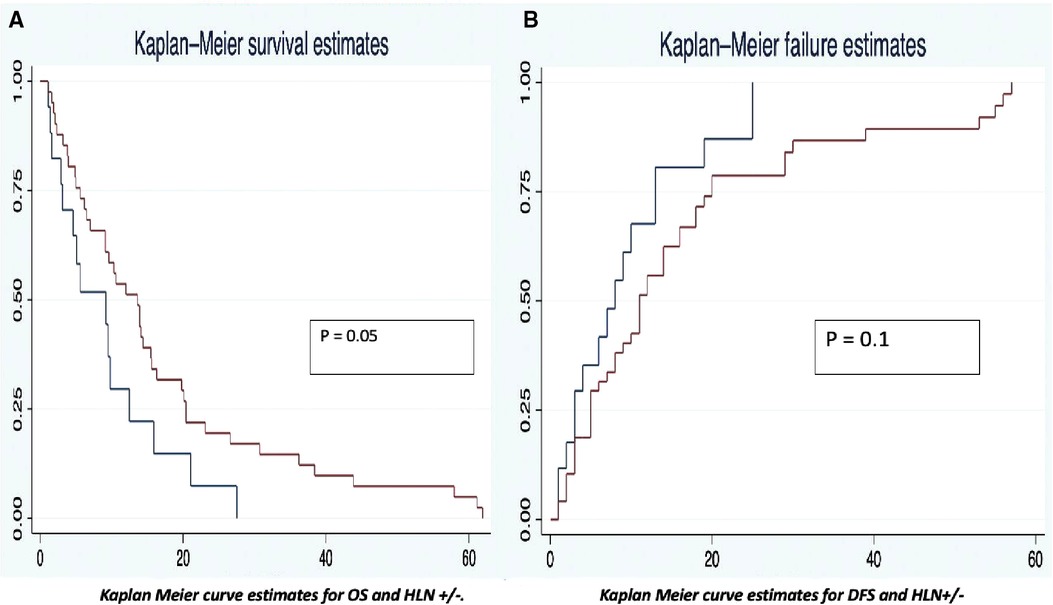
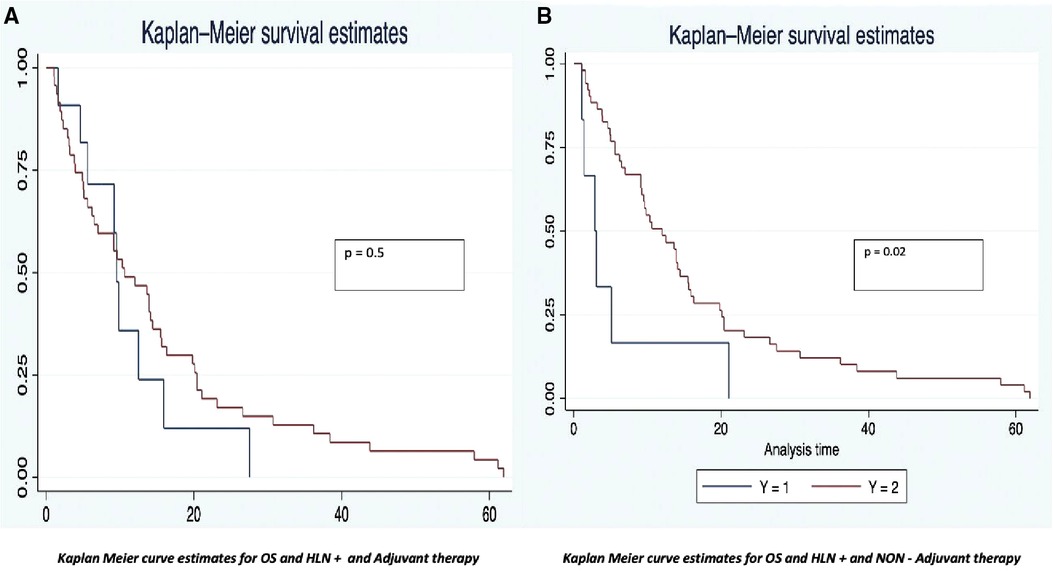
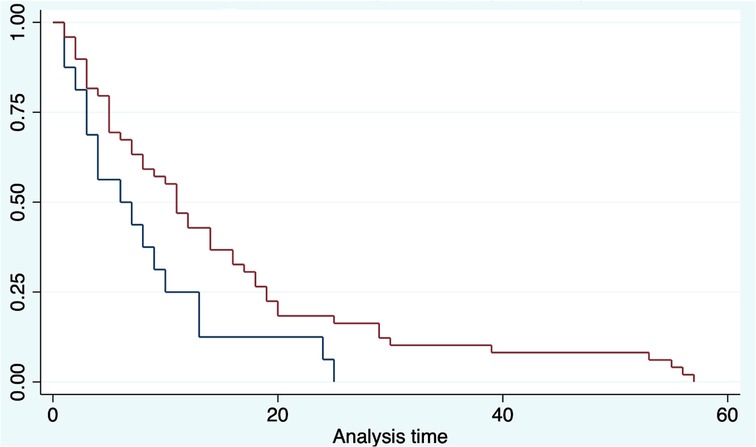
The OS was analyzed taking into consideration the HALN(8a) positive rate and its relation to the most relevant prognostic factors (vascular, perineural invasion, and margin of resection). For HALN(8a) positive cases (21,62% n = 24/111) the OS time was 12.5 months, vs. 20.4 in the negative node group (CI 95% 9–26). Figure 2 OS was significantly different in the adjuvant chemotherapy group analysis: for patients that did not receive adjuvant chemotherapy with positive HALN(8a) (62,5% n = 15/24), the median OS time was 12,5 months compared with 20,5 months in those who did receive adjuvant chemotherapy (36,5% n = 9/24). (Cox regression p = 0.02, HR 0.3, CI 95%) Kaplan Meier curves are displayed in Figure 3.
Overall survival was analyzed depending on the pathology report. In this analysis, pancreatic ductal adenocarcinoma with HALN(8a) compromise shows a statistical relationship with overall survival (p 0.00 CI 95% 0.0–0.99 HR 9.1). Ampulla of Vater tumors were excluded from this analysis due to the small sample size.
Regarding other prognostic variables, the LogRank test showed, for positive vascular invasion an OS time of 15.5 months, compared to 27.5 months (CI 95% 12–36) in the negative group. For perineural invasion, the positive group had an OS time of 15.9 months vs. the negative one of 30,7 months (CI 95% 15–61).
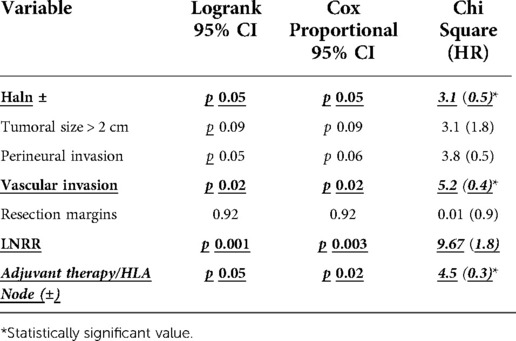
In the Cox regression model including relevant variables [HALN(8a) +/−, Vascular invasion +/−, perineural invasion +/−, resection margins +/−, and our new cutoff value for LNRR] statistical association was present between the positive HLA(8a), the cutoff of lymph node ratio resection, and vascular invasion (CoxRegression p = 0.05 HR 0.5; 0.003 HR1.8; p = 0.02 HR 0.4 CI 95%, respectively). LogRank tests and cox regression results are displayed in Table 3.
– Lymph node subgroup analysis.
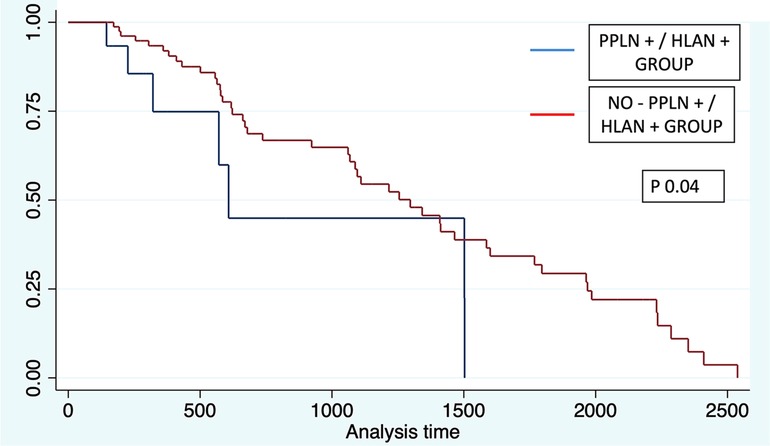
Evaluation of OS was performed according to each lymph node group. Significant differences were found for each group. For patients in peripancreatic (+) and HALN (+), the median overall survival time was 20 months (IQR 10;50), compared with peripancreatic (+) and HALN (−) with a median OS of 35 months (IQR 20;47). In patients with peripancreatic (−) and HALN (−), the median OS was 46 months (IQR 20;74).
Log-rank tests were performed for each group in order to evaluate the association with OS. Peripancreatic (+)/HALN (+) and Peripancreatic (−)/HALN (−) were related with significant statistical value with OS (Chi2 = 3.46; p 0.06 and Chi2 = 4.41; p 0.03 respectively). Further Cox-Proportional evidence that patients with peripancreatic (+)/HALN (+) have an increased 2.13-fold risk to have lesser OS with statistically significant value (HR 2.13 p 0.04 95% CI 0.94–4.84). (See Figure 4).
Disease-Free survival time
The disease-free survival time also had differences between the analysis groups. The positive HALN(8a) group patients have a DFS time of 13 months, compared with 20 months (CI 95% 12–22) in the negative HALN(8a) group, with a value as an independent factor to DFS (p = 0.09), but failed to be significative in the Cox proportional regression model. DFS was significantly different in the adjuvant chemotherapy group analysis: HALN(8a) + and adjuvant chemotherapy + patients (36,5% n = 9/24) have had a prolonged DFS time: (29 months), compared with the non-chemotherapy (62,5% n = 15/24) group: (13 months). In the Cox proportional regression model, DFS in HALN(8a) negative patients and adjuvant chemotherapy + did not differ significantly compared to HLAN(8a) negative and non-chemotherapy group (18 months CI 95% 17–20 vs. 19 months CI 95% 6–21, respectively).
Pancreatic ductal adenocarcinoma with HALN(8a) compromise was not related to the DFS period according to cox proportional analysis (p 0.5 CI 95% 0.6–2.4 HR 1.1). However; ampulla of Vater carcinoma with HALN compromise was related with statistical significance with DFS period (p 0.04 CI 95% 1.07–56 HR 7,7).
Regarding other relevant prognostic factors, positive vascular invasion presented a DFS of 14 months vs. 53 months in the negative group (CI 95%, 14–60). In the positive perineural invasion group, the DFS was 18 months, and in the negative one, the DFS was 53 months (CI 95%, 15–55). Kaplan Meier estimates curves for OS and DFS are displayed in Figure 3.
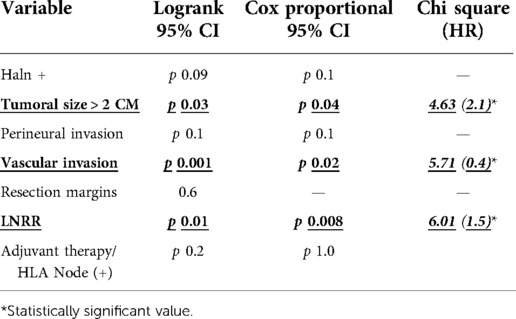
Cox proportional regression showed as relevant variables the lymph node ratio cutoff (p = 0.008, HR = 1.5 CI 95%), tumoral size > 2 cm (p = 0.04 HR = 2.1 CI 95%), and vascular invasion (p = 0.02 HR = 0.4, CI 95%). In this analysis, HALN(8a) + does not show a significant relationship with DFS. Log-Rank tests and cox regression results are displayed in Table 4.
– Lymph node subgroup analysis.
Evaluation of DFS was performed according to each lymph node group. For patients in peripancreatic (+) and HALN (+), the median disease-free survival time was 6 months (IQR 3;10), compared with peripancreatic (+) and HALN (−) with a median DFS of 8 months (IQR 5;17). In patients with peripancreatic (−) and HALN (−), the median DFS was 14 months (IQR 6;25).
LogRank tests were performed for each group in order to evaluate the association with DFS. Peripancreatic (+)/HALN (+) and Peripancreatic (−)/HALN (−) were related with significant statistical value with DFS (Chi2 = 4.81; p 0.02 and Chi2 = 5.77; p 0.01 respectively). Further Cox-Proportional evidence that patients with peripancreatic (+)/HALN (+) have an increased 1.82-fold risk to have lesser DFS with statistically significant value (HR 1.86; p 0.03 95% CI 1.04–3.34) (See Figure 5).
Discussion
Given the ominous prognosis in periampullary cancers, many prognostic factors are identified as relevant in the survival outcomes (11–24). Adequate Lymph node dissection is one of the most relevant prognostic factors, however, these cancers have multiple routes for lymphatic dissemination and periampullary tumors lack a single draining or sentinel lymph node that can be biopsied preoperatively to detect early metastasis (25). In fact, a study in 2007 performed on 28 patients discarded the use of the 8a node as a sentinel node (26). However, Recently, HALN(8a) study has gained interest and multiple retrospective studies describe the relevance as a prognosis factor (12–14). MicroRNAs panel assay in HALN(8a) biopsies found shorter recurrence-free survival when positive for MicroRNAs-10b in periampullary carcinomas (25).
This study found a reduction of OS in patients with positive HALN(8a). OS was 20.4 months in negative HALN(8a) compromise vs. 12.5 months in the positive group, nonetheless with no statistical difference (p = 0.05). These results are similar to those reported by Cordera et al. who described in 175 pancreatic cancer patients a median overall survival time of 14.7 months in a positive HALN(8a) compared with 16.1 months in peripancreatic node involvement and 22.9 months in all negative nodes (15). In addition, some authors described in a retrospective cohort of 147 patients with PC, a difference in OS in a group of patients divided according to the peripancreatic lymph node compromise in relation to HALN compromise [OS: 26 months PPLN−/HALN−; 17 months in the PPLN+/HALN−, and 13 months in the setting of HALN + (p = 0.017)]. HALN(8a) + has a 2.94-fold reduction in OS and 2.66-fold reduction in DFS (14). These results are similar to those observed in our population. (In HLA node-positive a 2.0-fold OS reduction and 1.77-fold in DFS); Our subgroup analysis demonstrates significant differences in overall survival comparing PPLN (+)/HALN (+) vs. PPLN (+)/HALN (−) (20 vs. 25 months) (p 0.04), thus reflecting the negative influence of HLAN positivity in patients who underwent Whipple procedure for malignant conditions.
Among relevant prognosis factors in OS included in the primary analysis (HALN positivity, tumoral size, perineural and vascular invasion, resection margins, LNRR, and HALN compromise with adjuvant therapy), the log-rank test showed statistical relationship as independent factors of vascular invasion, LNRR and the positivity of HALN Node in patients that receive adjuvant therapy; nonetheless, in Cox proportional analysis, HALN node positivity alone, failed to reach statistical significance in relationship with OS, however, vascular invasion, LNRR, and HALN in patients with adjuvant therapy show statistical relationship with overall survival increasing the risk of mortality in 5.2, 9.67, and 4.5 times respectively.
In the primary evaluation of DFS, significant variables were LNRR + (p = 0.01, chi2 6.01), HALN + (p = 0.09, chi2 3.32), tumoral size > 2 cm (p = 0.03 chi2 4.6), vascular invasion (p = 0.002 chi2 5.71), perineural invasion (p = 0.1 chi2 3.36), and resection margins (p-value 0.6 chi2 0.01). The multivariate analysis found a strong association of the lymph node ratio cutoff with a significant statistical value (p = 0.008, CI 95%), tumoral size > 2 cm (p = 0.02 CI 95%), and vascular invasion (p = 0.02 CI 95%). HALN(8a) compromise failed to confirm relevance in DFS Cox proportional hazard model. Data reported by Phillips et al. show that patients with lymphatic involvement (peripancreatic) had an OS of 19,5 months. However, patients without lymph node involvement had a median of 40,2 months (p < 0,001) (5) They didn't find a difference between OS and DFS of patients with positive HALN(8a) (mean 18,4 months and 10,6 months) and negative HALN(8a) patients (mean 19,7 months and 11,6 months) (5). One of the limitations of that study is the lack of evidence in pathology reports of HALN, and this could impact the final results.
Taking into consideration that patients with positive HALN(8a) have a significantly greater tumoral load and worse tumoral biology, some authors have compared the prognosis with peritoneal carcinomatosis (11, 12). Connor et al., found that the OS for patients with positive HALN(8a) was poor when compared to those with negative HALN(8a) (mean of 197 vs. 470 days, p = 0,003), which is why it could be compared with patients with unresectable metastatic disease (mean of 98 days, p = 0,072), thus considering the compromise of HALN(8a) an important predictor of occult metastatic disease. Nonetheless, current data does not support the involvement of HALN(8a) as a non-resectability criterion (12, 24).
Tumoral biomarkers' relevance in diagnosis, prognosis, and surgical decision-making processes is still a matter of debate (29, 30). Ca 19-9 it's one of the most studied; Santucci et al. (31, 32), found that major levels of CA 19-9 in patients with locally advanced pancreatic cancer or metastatic disease compared with those with resectable disease; however, limitations among the use of CA 19-9 in clinical practice includes that almost 50% of patients with tumors < 2 cm do not elevate any tumoral biomarker and for that reason, currently there is still a lack of evidence in terms of prognosis (30). Our study did not find any statistical difference between CA 19-9 levels in patients with positive HLA compared with the negative group.
As is known, multimodal treatment including surgery and chemotherapy is a cornerstone in the management of pancreatic cancer, Liu et al. (30) described the well-known impact on the prognosis of patients that receive chemotherapy after an R0 surgery, with an OS of 20 months compared with the non-chemotherapy group with 11.6 months. A large proportion of patients from this study did not receive adjuvant chemotherapy 42.34% (n = 47) because of health insurance issues or personal decisions that might cause a negative impact on the results. Therefore, a differential analysis of patients was performed in separate groups of patients who receive or do not adjuvant chemotherapy. Patients with HALN(8a) compromised plus chemotherapy have an OS of 20.5 months vs. non-chemotherapy with 12.5 months (p = 0.02) (26, 33, 34). The relevance of HALN(8a) is therefore highly influenced by other prognostic factors, like chemotherapy which is a cornerstone in periampullary cancer treatment.
There is no doubt about the importance of lymph node dissection, until now, a dissection of between 10 and 16 nodes is recommended in PD. A large retrospective cohort from the Surveillance, Epidemiology, and End Results (SEER) database, described an improved median overall survival from patients with more than 16 nodes harvested in comparison to the subgroup with less than 10 nodes harvested (117 months vs. 40 months, p < 0.001) (34–36). However, the dissection of the HALN(8a) is under debate as a preoperative or intraoperative tumoral progress. pancreatic cancer seems to be most affected by this node positivity. Few studies discuss the impact of lymph node retrieval in non-pancreatic periampullary cancers. However, given the histologic uncertainty in some cases, and the increasing evidence regarding the relevance of this node, we recommend sending it to pathology in all cases.
Limitations of our study include the retrospective nature of the study and the small sample size of patients receiving chemotherapy. None of our patients received neoadjuvant therapy and this can also influence the results. However, subgroup analysis was performed to decrease bias. To the best of our knowledge, this is the first report in Colombia evaluating survival analysis in the context of pancreatic cancer, reports in Latin American countries are limited to some reviews (27, 28).
Conclusion
This retrospective study with prospective data from an HPB center shows data and statistical analysis suggesting that HALN(8a) compromise in properly treated patients should be considered as an impact factor in survival. We cannot recommend stopping surgery in patients with positive HALN(8a). However, it could suggest the aggressive biology of the tumor based on the reduced OS. HALN(8a) metastases and an LNRR bigger than 0.6 are relevant prognostic factors in periampullary cancer to predict reduced OS and DFS respectively. Notwithstanding the worldwide literature, our study does not find any relationship between the preoperative value of CA 19-9 and HALN positivity. Further prospective studies are needed to confirm these results.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Universidad el Rosario. The patients/participants provided their written informed consent to participate in this study.
Author contributions
DC, CR, JCS: Conception of the idea, manuscript writing, edition, and critical revision of the manuscript. CR, DC: Data analysis. MP, AR: Manuscript writing, data recollection. All authors contributed to the article and approved the submitted version.
Acknowledgments
To our HPB patients.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Komine R, Kojima M, Ishi G, Kudo M, Sugimoto M, Kobayashi S, et al. Recognition and pathological features of periampullary region adenocarcinoma with an indeterminable origin. Cancer Med. (2021) 10(11):3499–510. doi: 10.1002/cam4.3809
2. van Geenen RC, van Gulik TM, Offerhaus GJ, de Wit LT, Busch OR, Obertop H, et al. Survival after pancreaticoduodenectomy for periampullary adenocarcinoma: an update. Eur J Surg Oncol. (2001) 27(6):549–57. doi: 10.1053/ejso.2001.1162
3. Skórzewska M, Kurzawa P, Ciszewski T, Pelc Z, Polkowski WP. Controversies in the diagnosis and treatment of periampullary tumours. Surg Oncol. (2022) 44:101853. doi: 10.1016/j.suronc.2022.101853
4. Fogel EL, Shahda S, Sandrasegaran K, DeWitt J, Easler JJ, Agarwal DM, et al. A multidisciplinary approach to pancreas cancer in 2016: a review. Am J Gastroenterol. (2017) 112(4):537–54. doi: 10.1038/ajg.2016.610
5. Philips P, Dunki-Jacobs E, Agle SC, Scoggins C, McMasters KM, Martin RC. The role of hepatic artery lymph node in pancreatic adenocarcinoma: prognostic factor or a selection criterion for surgery. HPB. (2014) 16(12):1051–5. doi: 10.1111/hpb.12306
6. Lee SR, Kim HO, Son BH, Yoo CH, Shin JH. Prognostic factors associated with long term survival and recurrence in pancreatic adeno- carcinoma. Hepatogastroenterology. (2013) 60:358–62. doi: 10.5754/hge12727
7. Breslin TM, Hess KR, Harbison DB, Jean ME, Cleary KR, Dackiw AP, et al. Neoadjuvant chemoradiotherapy for adenocarcinoma of the pancreas: treatment variables and survival duration. Ann Surg Oncol. (2001) 8:123–32. doi: 10.1007/s10434-001-0123-4
8. Kawarada Y. New classification of pancreatic carcinoma—Japan pancreas society. Nihon Shokakibyo Gakkai Zasshi. (2003) 100:974–80.12934535
9. Perlmutter BC, Hossain MS, Naples R, Tu C, Vilchez V, McMichael J, et al. Survival impact based on hepatic artery lymph node status in pancreatic adenocarcinoma: a study of patients receiving modern chemotherapy. J Surg Oncol. (2021) 123(2):399–406. doi: 10.1002/jso.26281
10. Brennan MF, Kattan MW, Klimstra D, Conlon K. Prognostic nomo-gram for patients undergoing resection for adenocarcinoma of the pancreas. Ann Surg. (2004) 240:293–8. doi: 10.1097/01.sla.0000133125.85489.07
11. Kayahara M, Nagakawa T, Kobayashi H, Mori K, Nakano T, Kadoya N, et al. Lymphatic flow in carcinoma of the head of the pancreas. Cancer. (1992) 70:2061–6. doi: 10.1002/1097-0142(19921015)70:8%3C2061::AID-CNCR2820700808%3E3.0.CO;2-V
12. Connor S, Bosonnet L, Ghaneh P, Alexakis N, Hartley M, Campbell F, et al. Survival of patients with periampullary carcinoma is pre- dicted by lymph node 8a but not by lymph node 16b1 status. Br J Surg. (2004) 91:1592–9. doi: 10.1002/bjs.4761
13. Maithel SK, Khalili K, Dixon E, Guindi M, Callery MP, Cattral MS, et al. Impact of regional lymph node evaluation in staging patients with periampullary tumours. Ann Surg Oncol. (2007) 14:202–10. doi: 10.1245/s10434-006-9041-9
14. LaFemina J, Chou JF, Gönen M, Rocha FG, Correa-Gallego C, Kingham TP, et al. Hepatic arterial nodal metastases in pancreatic cancer: is this the node of importance? J Gastrointest Surg. (2013) 17(6):1092–7. doi: 10.1007/s11605-012-2071-7
15. Cordera F, Arciero CA, Li T, Watson JC, Hoffman JP. Significance of common hepatic artery lymph node metastases during pancreaticoduodenectomy for pancreatic head adenocarcinoma. Ann Surg Oncol. (2007) 14(8):2330–6. doi: 10.1245/s10434-006-9339-7
16. Lin QJ, Yang F, Jin C, Fu DL. Current status and progress of pancreatic cancer in China. World J Gastroenterol. (2015) 21(26):7988. doi: 10.3748/wjg.v21.i26.7988
17. Wang Y, Duan H, Yang X, Guo J. Cigarette smoking and the risk of pancreatic cancer: a case–control study. Medical Oncology. (2014) 31(10):184. doi: 10.1007/s12032-014-0184-4
18. Ansari D, Tingstedt B, Andersson B, Holmquist F, Sturesson C, Williamsson C, et al. Pancreatic cancer: yesterday, today and tomorrow. Future Oncol. (2016) 12(16):1929–46. doi: 10.2217/fon-2016-0010
19. Ferrone CR, Brennan MF, Gonen M, Coit DG, Fong Y, Chung S, et al. Pancreatic adenocarcinoma: the actual 5-year survivors. J Gastrointest Surg. (2008) 12(4):701–6. doi: 10.1007/s11605-007-0384-8
20. Fesinmeyer MD. Differences in survival by histologic type of pancreatic cancer. Cancer Epidemiol Biomarkers Prev. (2005) 14(7):1766–73. doi: 10.1158/1055-9965.EPI-05-0120
21. Bachmann J, Michalski CW, Martignoni ME, Büchler MW, Friess H. Pancreatic resection for pancreatic cancer. HPB. (2006) 8(5):346–51. doi: 10.1080/13651820600803981
22. Izumo W, Higuchi R, Furukawa T, Yazawa T, Uemura S, Shiihara M, et al. Evaluation of preoperative prognostic factors in patients with resectable pancreatic ductal adenocarcinoma. Scand J Gastroenterol. (2019) 54(6):780–6. doi: 10.1080/00365521.2019.1624816
23. Mirkin KA, Hollenbeak CS, Wong J. Greater lymph node retrieval and lymph node ratio impacts survival in resected pancreatic cancer. J Surg Res. (2017) 220:12–24. doi: 10.1016/j.jss.2017.06.076
24. Riediger H, Keck T, Wellner U, zur Hausen A, Adam U, Hopt UT, et al. The lymph node ratio is the strongest prognostic factor after resection of pancreatic cancer. J Gastrointest Surg. (2009) 13(7):1337–44. doi: 10.1007/s11605-009-0919-2
25. Nguyen HV, Gore J, Zhong X, Savant SS, Deitz-McElyea S, Schmidt CM, et al. MicroRNA expression in a readily accessible common hepatic artery lymph node predicts time to pancreatic cancer recurrence postresection. J Gastrointest Surg. (2016) 20(10):1699–706. doi: 10.1007/s11605-016-3208-x
26. Rassadi R, Tarnasky PR, Linder JD, Saad AJ, Jeyarajah DR. Nodal sampling in pancreaticoduodenectomy: does it change our management? HPB. (2007) 9(6):461–5. doi: 10.1080/13651820701713733
27. Zheng Z-J, Wang M-J, Tan C-L, Chen Y-H, Ping J, Liu X-B. Prognostic impact of lymph node status in patients after total pancreatectomy for pancreatic ductal adenocarcinoma: a strobe-compliant study. Medicine. (2020) 99(8):e19327. doi: 10.1097/MD.0000000000019327
28. Bravo LE, García LS, Collazos P, Carrascal E, Ramírez O, Collazos T, et al. Reliable information for cancer control in Cali, Colombia. Colomb Med. (2018) 49(1):23–34. doi: 10.25100/cm.v49i1.3689
29. Chan C, Franssen B, Rubio A, Uscanga L. Pancreaticoduodenectomy in a latin American country: the transition to a high-volume center. J Gastrointest Surg. (2008) 12(3):527–33. doi: 10.1007/s11605-007-0274-0.17763915
30. Tempero MA, Malafa MP, Chiorean EG, Czito B, Scaife C, Narang AK, et al. Pancreatic adenocarcinoma, version 1.2019. J Natl Compr Canc Netw. (2019) 17(3):202–10. doi: 10.6004/jnccn.2019.0014
31. Liu C, Cheng H, Jin K, Fan Z, Gong Y, Qian Y, et al. Resected pancreatic cancer with N2 node involvement is refractory to gemcitabine-based adjuvant chemotherapy. Cancer Control. (2020) 27(1):1073274820915947. doi: 10.1177/1073274820915947
32. Salleh S, Thyagarajan A, Sahu RP. Exploiting the relevance of CA 19-9 in pancreatic cancer. J Cancer Metastasis Treat. (2020) 6:31. doi: 10.20517/2394-4722.2020.70
33. Santucci N, Facy O, Ortega-Deballon P, Lequeu JB, Rat P, Rat P. CA 19-9 Predicts resectability of pancreatic cancer even in jaundiced patients. Pancreatology. (2018) 18(6):666–70. doi: 10.1016/j.pan.2018.07.001
34. Asano D, Nara S, Kishi Y, Esaki M, Hiraoka N, Tanabe M, et al. A single-institution validation study of lymph node staging by the AJCC 8th edition for patients with pancreatic head cancer: a proposal to subdivide the N2 category. Ann Surg Oncol. (2019) 26(7):2112–20. doi: 10.1245/s10434-019-07390-z
35. Basturk O, Saka B, Balci S, Postlewait LM, Knight J, Goodman M, et al. Substaging of lymph node Status in resected pancreatic ductal adenocarcinoma has strong prognostic correlations: proposal for a revised N classification for TNM staging. Ann Surg Oncol. (2015) 22(Suppl 3):S1187–95. doi: 10.1245/s10434-015-4861-0
Keywords: periampullary tumors, hepatic artery lymph node, prognostic factor, disease-Free survival, pancreaticoduodenectomy, pancreatic adenocarcinoma.
Citation: Conde D, Rey C, Pardo M, Recaman A and Sabogal Olarte JC (2022) Hepatic artery lymph node relevance in periampullary tumors: A retrospective analysis of survival outcomes. Front. Surg. 9:963855. doi: 10.3389/fsurg.2022.963855
Received: 8 June 2022; Accepted: 18 November 2022;
Published: 6 December 2022.
Edited by:
Mark Girgis, University of California, United StatesReviewed by:
Alessio Vagliasindi, Santa Maria delle Croci Hospital, ItalyStefania Brozzetti, Sapienza University of Rome, Italy
© 2022 Conde, Rey, Pardo, Recaman and Sabogal Olarte. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carlos Rey Y2FybG9zcmV5OTkxQGdtYWlsLmNvbQ==
Specialty Section: This article was submitted to Visceral Surgery, a section of the journal Frontiers in Surgery
 Danny Conde
Danny Conde Carlos Rey
Carlos Rey Manuel Pardo2
Manuel Pardo2