- 1Department of Anesthesiology, National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University, Chengdu China
- 2The Research Units of West China (2018RU012) -Chinese Academy of Medical Sciences, West China Hospital, Sichuan University, Chengdu China
- 3College of Software Engineering, Chengdu University of Information Technology, Chengdu China
Aim: Postoperative pulmonary complications (PPCs) can increase the risk of postoperative mortality, and the geriatric population has high incidence of PPCs. Early identification of high-risk geriatric patients is of great value for clinical decision making and prognosis improvement. Existing prediction models are based purely on structured data, and they lack predictive accuracy in geriatric patients. We aimed to develop and validate a deep neural network model based on combined natural language data and structured data for improving the prediction of PPCs in geriatric patients.
Methods: We consecutively enrolled patients aged ≥65 years who underwent surgery under general anesthesia at seven hospitals in China. Data from the West China Hospital of Sichuan University were used as the derivation dataset, and a deep neural network model was developed based on combined natural language data and structured data. Data from the six other hospitals were combined for external validation.
Results: The derivation dataset included 12,240 geriatric patients, and 1949(15.9%) patients developed PPCs. Our deep neural network model outperformed other machine learning models with an area under the precision-recall curve (AUPRC) of 0.657(95% confidence interval [CI], 0.655–0.658) and an area under the receiver operating characteristic curve (AUROC) of 0.884(95% CI, 0.883–0.885). The external dataset included 7579 patients, and 776(10.2%) patients developed PPCs. In external validation, the AUPRC was 0.632(95%CI, 0.632–0.633) and the AUROC was 0.889(95%CI, 0.888–0.889).
Conclusions: This study indicated that the deep neural network model based on combined natural language data and structured data could improve the prediction of PPCs in geriatric patients.
Introduction
More than 300 million surgeries are performed worldwide each year (1). Around one-third of elective surgeries are performed on patients aged over 65 years (2). Compared with younger adults, older individuals are more prone to postoperative complications because of age-related degenerative physiological characteristics (3).
Postoperative pulmonary complications (PPCs), including respiratory infection, atelectasis, and respiratory failure, are common, and even mild PPCs are associated with a prolonged hospital stay and increased postoperative mortality (4–6). The incidence of PPCs in major surgery ranges from 1% to 23% depending on different PPCs definitions and surgical specialties (7), and the postoperative mortality rate of patients with PPCs varies from 14% to 48% (8–10). Hospital stay is prolonged by 13–17 days in patients with PPCs (7). For the management of PPCs, preventive strategies may be more effective than treating established PPCs (11). Preoperatively identifying the risk of PPCs is critical for guiding preventive interventions to reduce the risk and incidence of PPCs (12).
Most risk assessment tools for PPCs were developed using traditional logistic regression (13), such as the Assess Respiratory Risk in Surgical Patients in Catalonia (ARISCAT) risk score (14). Traditional logistic regression constrains the number of input risk factors, which may omit potential predictors and limit the predictive accuracy (15). Machine learning algorithms are advantageous in that they can identify hidden insights from large datasets (16), which can help to build more accurate prediction models for PPCs (13).
Recent studies (17–19) have demonstrated the superiority of machine learning algorithms in predicting PPCs. For example, Xue et al. (19) used structured perioperative data to predict five postoperative complications, including pneumonia. To further improve predictive performance, overcoming several methodological deficiencies may be effective. First, most models are based purely on structured data, and natural language data are not generally included. Underutilizing natural language data in model development may cause loss of clinical information and limit predictive accuracy (20). Second, few predictive models have been developed specifically for geriatric patients. Geriatric patients are at a high risk of developing PPCs (9). Considering age-related physiological characteristics, predictive models based on data from the general patient population may be unsuitable for geriatric patients (21). Third, most studies lack external validation; thus, it is uncertain whether existing models could achieve comparable predictive performance at other institutions (13).
In this study, we aimed to develop and validate a deep neural network model to predict PPCs in geriatric patients based on combined natural language data and structured data. We hypothesized that this model could accurately predict patients who are at a high risk of developing PPCs.
Method
Data source
This study followed the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) guidelines (22). The study protocol was approved by the ethics committee of the West China Hospital of Sichuan University (2019-473) with a waiver of informed consent. The study is registered at www.chictr.org.cn (ChiCTR1900025160). In this study, we prospectively collected data from seven hospitals in China, including the West China Hospital of Sichuan University, the Second Affiliated Hospital of Chongqing Medical University, the Wuhan Union Hospital, the Guangdong Provincial People's Hospital, the First Affiliated Hospital of Kunming Medical University, the First People's Hospital of Zhaoqing, and the Qingyuan People's Hospital. Patients aged ≥65 years who underwent surgery under general anesthesia between 25th June 2019 and 31st December 2021 were enrolled. If patients underwent multiple surgeries during the study period, only the first surgery was included in the analysis. Related patient data were collected by trained residents on the day before surgery. The attending physician and the resident would re-check the collected information before surgery. If any errors or omissions existed, the clinician would make corrections or supplement the information. Preoperative laboratory tests were automatically retrieved from the laboratory information system. All laboratory tests were performed within 7 days before surgery. If a patient had more than one result for the same test, the most recent preoperative result was used in the analysis. Preoperative clinical data included demographic characteristics, preoperative vital signs, laboratory tests and comorbidities. Supplementary Table S1 shows the 127 variables included in our study.
Postoperative follow-up
To ascertain the presence of PPCs, we conducted prospective patient follow-up. Research personnel performed follow-up with patients at different time points, including 24 h after surgery, 48 h after surgery, before hospital discharge, and on the 30th day after surgery. If a patient developed PPCs, we stayed in contact with the patient until recovery or death. Throughout each patient's hospital stay, the research personnel conducted bedside follow-up visits, and after hospital discharge, patients were contacted via telephone.
Outcome definition
The outcome was the incidence of any PPC within 30 days after surgery. PPCs included unplanned mechanical ventilation, atelectasis, pulmonary congestion, respiratory infection, pleural effusion, pneumothorax, and respiratory failure. Supplementary Table S2 shows the definition of each PPC.
Data preprocessing and model development
Variables are presented as numerical, categorical, or free-text variables. Free-text data contain the descriptions of principal diagnoses and comorbidities. Missing values were imputed by 0 s, with indicators representing missingness, which regarded missing values as a separate group. Numerical variables were transformed to categorical variables using 5-bins equal-width scaling. Data from patients admitted to the West China Hospital of Sichuan University were used as the derivation dataset, and a deep neural network model was trained. Five random shuffles of five-fold cross-validation were performed to divide the training set and validation set. In each iteration, a different stratified fold was used for model evaluation, and model training was performed on the remaining folds.
The number of patients without PPCs was much higher than the number of patients with PPCs, which led to class imbalance. Cross-entropy loss function was used to overcome this issue by enhancing the accurate prediction of positive examples. Early stopping and dropout were used to avoid overfitting. Early stopping refers to ceasing model training when the validation loss starts to increase. A patience of 40 epochs was set for early stopping. Dropout is a method to prevent co-adapting by removing neurons from the network (23). In our study, dropout with a probability of 0.1 was applied to all layers.
Model comparison
Our deep neural network model was compared with several extensively used classifiers, including Elastic Net logistic regression, support vector machine, random forest, gradient boosting machine, and extreme gradient boosting.
To evaluate and compare the different models, we calculated performance metrics using the validation fold in each iteration and took the average over all repetitions. Performance metrics included sensitivity (recall), precision, F1 score, specificity, accuracy, area under the precision-recall curve (AUPRC), and area under the receiver operating characteristic curve (AUROC). Sensitivity reflects the ability to capture positive examples (24). In circumstances with an imbalanced class distribution, the precision and sensitivity can provide more direct insight into predictive performance (25). The F1 score is the harmonic mean of the precision and sensitivity. The AUROC is widely used to estimate the performance of binary classifiers. However, the AUROC can generate misleading conclusions about model performance for classifiers established on imbalanced datasets (26). The AUPRC gives no credit for predicting true negatives, and can provide a more accurate interpretation of a model's actual performance (25). In this study, we chose the F1 score and the AUPRC as the main evaluation metrics for model comparison. The calibration ability was measured using the Hosmer–Lemeshow calibration plot.
The overall architecture of our deep neural network model is depicted in Supplementary Figure S1. To indicate the risk level, we divided patients into three groups with a low, intermediate, and high risk of PPCs based on the predicted probability. The optimal cutoff values were confirmed using the minimum description length principle (MDLP) (27). The chi-square test was performed to compare the incidence of PPCs between the three groups.
The deep neural network model was implemented using PyTorch. Machine learning models were developed in Python 3.8.3 using the scikit-learn library. A P value of <0.05 was considered statistically significant.
Feature importance
In our model, Multi-Head Attention (28) in the Transformer layer could set the weight for each variable. The magnitude of the weight indicates the degree to which the input variable affects the prediction. To gain insight into the workings of our model, we calculated the feature weights for all patients in the derivation dataset using Multi-Head Attention. To illustrate individual risk prediction, we presented two examples and visualized the variable importance in these individual predictions.
External validation
Data from the other six participating hospitals were combined and used for external validation. These hospitals adopted the same preoperative interview and postoperative follow-up system as the West China Hospital of Sichuan University. We extracted the same features as mentioned above with the exception of some laboratory tests, because we could not retrieve these results from their laboratory information systems. Supplementary Table S1 shows the variables included in the external dataset.
Previous studies indicated the importance of local calibration considering institution-specific differences in patient populations and surgical practices (26, 29, 30). Demonstrating a generalizable method may be more reasonable than developing a globally used predictive model (26). To validate our overall methodology, we applied the same training method to recalibrate the deep neural network model based on the combined external dataset.
Results
The derivation dataset included 12,240 geriatric patients at the West China Hospital of Sichuan University between 25th June 2019 and 30th April 2021, the majority of whom were men (56.4%). Supplementary Table S3 shows summary statistics for patients' characteristics. Of these patients, 1949(15.9%) patients developed PPCs, including 533(4.4%) with unplanned mechanical ventilation, 526(4.3%) with atelectasis, 32(0.3%) with pulmonary congestion, 1009(8.2%) with respiratory infection, 1267(10.4%) with pleural effusion, 217(1.8%) with pneumothorax, and 163(1.3%) with respiratory failure.
Comparison of the deep neural network model with other models
Compared with other widely used classifiers, the deep neural network model achieved the greatest sensitivity of 0.603(95% confidence interval [CI], 0.602–0.604), the highest F1 score of 0.641(95%CI, 0.640–0.642), the greatest AUPRC value of 0.657(95%CI, 0.655–0.658), and the greatest AUROC value of 0.884(95%CI, 0.883–0.885) (Table 1). Hosmer-Lemeshow calibration plot (P = 0.80) showed good agreement between the deep neural network model-based prediction and observed outcome (Figure 1).
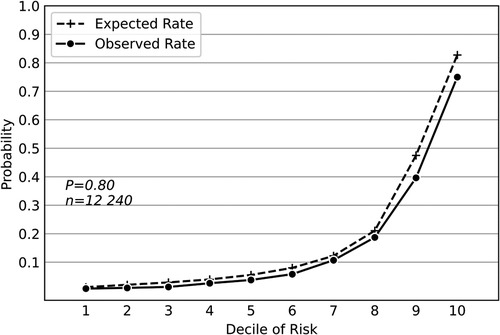
Figure 1. Hosmer-Lemeshow calibration plot of the deep neural network model based on the derivation dataset. Values on the x-axis are deciles of predicted risk of postoperative pulmonary complications and values on the y-axis are rates of postoperative pulmonary complications for each decile. The result of Hosmer–Lemeshow test (P = 0.80) showed good agreement between the deep neural network model-based prediction and observed outcome.
To indicate the risk level, patients were stratified into three groups with a low, intermediate, and high risk of PPCs based on the risk predicted probability of the deep neural network model. The optimal cutoff values of the risk predicted probability were confirmed by MDLP (27) (low risk: ≤0.3; intermediate risk: 0.3 < risk predicted probability ≤0.7; high risk: >0.7).
The incidence of PPCs was significantly different between the three groups (low-risk group: 5.5%; intermediate-risk group: 42.4%; high-risk group: 76.6%; P < 0.001 for all, Supplementary Table S4).
Feature importance
For patients in the derivation dataset, the top 10 most important variables in the deep neural network model were acidophil count, triglyceride, fibrinogen, functional capacity, platelet count, acidophil percentage, neck movement test, hydroxybutyrate dehydrogenase, mean corpuscular hemoglobin concentration, and mean corpuscular hemoglobin (Table 2).

Table 2. Top ten most important variables in the deep neural network model for patients in the derivation dataset and the two case examples.
We present two examples to illustrate individual risk prediction. These two patients were in the high-risk group and actually developed PPCs. Table 2 shows the variables that greatly contributed to individual prediction in these two patients. For patient A, the acidophil count contributed the most to risk prediction, with patient A having a high acidophil count (2.10 × 109/L; normal range: 0.02–0.52 × 109/L). For patient B, triglyceride was the most important variable in risk prediction, with patient B having a high triglyceride concentration of 7.35 mmol/L (normal range: 0.29–1.83 mmol/L). Patient B's acidophil count was within the normal range (0.02 × 109/L) and was not important in this prediction, ranking 105th, while patient A's triglyceride concentration was normal (0.94 mmol/L) and was not important in this prediction, ranking 123rd.
External validation
The combined external dataset included 7579 geriatric patients from 23rd April 2021 to 31st December 2021. A total of 776 patients (10.2%) developed PPCs, including 302 (4.0%) with unplanned mechanical ventilation, 240 (3.2%) with atelectasis, 2 (0.03%) with pulmonary congestion, 513 (6.8%) with respiratory infection, 506 (6.7%) with pleural effusion, 4 (0.05%) with pneumothorax, and 112 (1.5%) with respiratory failure. Supplementary Table S5 shows the summary statistics for patients' characteristics in the combined dataset. We applied the same methodology to retrain the deep neural network model based on the external dataset. Although some laboratory tests were not included in the external dataset, the deep neural network model maintained good predictive performance, with an F1 score of 0.602(95% CI, 0.602–0.603), an AUPRC of 0.632(95% CI, 0.632–0.633), and an AUROC of 0.889(95% CI, 0.888–0.889) (Table 1). Hosmer-Lemeshow calibration plot (P = 0.78) showed good agreement between the deep neural network model-based prediction and observed outcome (Figure 2).
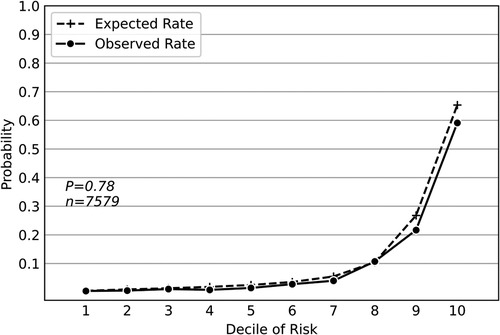
Figure 2. Hosmer-Lemeshow calibration plot of the deep neural network model based on the external dataset. Values on the x-axis are deciles of predicted risk of postoperative pulmonary complications and values on the y-axis are rates of postoperative pulmonary complications for each decile. The result of Hosmer–Lemeshow test (P = 0.78) showed good agreement between the deep neural network model-based prediction and observed outcome.
Discussion
PPCs are associated with a prolonged hospital stay and increased postoperative mortality (31). Early identification of high-risk patients could help to guide preventive interventions to improve prognosis. This study showed that the deep neural network model based on combined natural language data and structured data could improve the prediction of PPCs in geriatric patients. Patients were stratified into three risk groups to indicate the risk level, and the incidence of PPCs was significantly different among the three groups.
Geriatric patients are at a high risk of developing PPCs (9). In other studies (17–19), the data of older and younger patients have often been pooled together. Considering age-related physiological characteristics, ignoring age categories can cause inaccurate parameter estimation (21) and may decrease the discrimination ability in geriatric patients. Current assessment tools that are based on pooled data often underestimate risk in geriatric patients (21). In this study, we specifically focused on the geriatric population to improve the predictive accuracy in this population specifically.
Deep learning has the advantage of learning directly from natural language data without the need for manual processing (32, 33). In our study, natural language data contained descriptions about principal diagnoses and comorbidities. Considering that International Classification of Diseases codes can only be acquired at discharge, they are not available for preoperative prediction (24), and natural language data could supply this clinical information. In a clinical setting, correctly identifying patients who are at risk of PPCs is critical, so a model with high sensitivity is appropriate (34). Previous studies on predicting PPCs have achieved sensitivities in the range of 0.321–0.526 (4, 18, 19). Compared with previous studies and other models in our study, the deep neural network model achieved the highest sensitivity of 0.603, which indicated that the deep neural network model based on combined natural language data and structured data could more accurately identify patients with PPCs. To process natural language data, we performed embedding using MedBERT and mean-pooling, instead of traditional one-hot encoding. With one-hot encoding, natural language data are actually transformed into binary variables according to the presence or absence of particular words (26), which may hinder the learning of potential relationships between descriptions (32). Embedding does not regard two principal diagnoses as completely different categories. Instead, embedding enable all variables to be present in a multi-dimensional space, and similar features could be mapped next to each other. For example, a cholecystolithiasis is closer to a cholecystolithiasis with chronic cholecystitis than an acute cholecystitis in the embedding space.
In terms of individual predictions, Multi-Head Attention (28) in the deep neural network model was used to set the corresponding weight for each variable according to the patient-specific input value, instead of setting a fixed weight for each variable, as is the case with logistic regression. In the case example, patient A had a high acidophil count, and acidophil count contributed the most to the high risk of PPCs in patient A; thus, it was set the heaviest weight in this prediction. Patient B had a normal acidophil count, but the patient's triglyceride concentration was high and contributed the most to the high risk of PPCs; thus, triglyceride was set the heaviest weight in this individual prediction. Patient-specific characteristics may lead to more accurate individual predictions. After calculating the predictive probability and corresponding risk level, the model could output the variables that contributed to individual prediction. Recognizing these important variables may assist clinicians in early identification of potential factors, which could help to decide the treatment protocol to prevent PPCs and mitigate risk (35).
With ordinary external validation, the model is directly applied to a different dataset. In fact, local-specific parameters in certain models may not be generalizable to other populations considering hospital-specific patient populations and surgical practices (29, 30). Previous studies have emphasized the importance of recalibration to overcome this limitation (26, 29, 30). In our study, we validated the overall modeling methodology by retraining the deep neural network model based on the external dataset. Although some laboratory tests were not included in the external dataset, the recalibrated deep neural network model maintained good predictive performance, which indicated that our methodology was generalizable to other institutions, even if they could not collect complete features.
Our study has several limitations that should be noted. First, although the model could output variables that contributed greatly to individual predictions, the association between important features and outcome was not necessarily causation. We cannot conclude whether guiding the treatment protocol according to these variables could improve prognosis. Further research is necessary to quantify the benefit of this model in guiding interventions and improving patients' outcomes. Second, limited by the small number of patients in each group (divided by surgery type), a subgroup analysis based on the specific surgery type was not conducted. Thus, the model's predictive ability may be limited in some subspecialties.
In conclusion, this study indicated that the prediction of PPCs in geriatric patients could be improved by deep neural network model based on combined natural language data and structured data. The overall modeling methodology was generalizable to other institutions; thus, it could be used to construct their own predictive models.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Committee of Ethics from West China Hospital of Sichuan University (2019-473), and registered at www.chictr.org.cn (ChiCTR1900025160). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
XP, TZ and XH are responsible for the design of the study. TZ, GC, and XH contributed to data acquisition. XP, YW and XH contributed to data analysis and interpretation. XP contributed to initial drafting of manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Key R&D Program of China (No. 2018YFC2001800) to Xuechao Hao and Tao Zhu; National Clinical Research Center for Geriatrics, West China Hospital of Sichuan University (No. Z2018A02) to Tao Zhu; 1·3·5 project for disciplines of excellence, West China Hospital, Sichuan University (No. ZYJC18010) to Tao Zhu; CAMS Innovation Fund for Medical Sciences (No. 2019-I2M-5-011) to Tao Zhu; and Sichuan Provincial Science and Technology Key R&D Projects (No. 2019YFG0491) to Tao Zhu.
Acknowledgments
We thank Emily Woodhouse, PhD, from Liwen Bianji (Edanz) (www.liwenbianji.cn) for editing the English text of a draft of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.976536/full#supplementary-material.
References
1. Nepogodiev D, Martin J, Biccard B, Makupe A, Bhangu A, Nepogodiev D, et al. Global burden of postoperative death. The Lancet. (2019) 393:401. doi: 10.1016/s0140-6736(18)33139-8.
2. Kahli Z, Shelley RM, Richard S, Jeffrey B, Sandhya LD, Mitchell TH. Preoperative cognitive impairment as a predictor of postoperative outcomes in a collaborative care model. JAGS. (2018) 66:584–9. doi: 10.1111/jgs.15261.
3. Oresanya LB, Lyons WL, Finlayson E. Preoperative assessment of the older patient: a narrative review. JAMA. (2014) 311:2110–20. doi: 10.1001/jama.2014.4573.24867014
4. Chen C, Yang D, Gao S, Zhang Y, Chen L, Wang B, et al. Development and performance assessment of novel machine learning models to predict pneumonia after liver transplantation. Respir Res. (2021) 22:94. doi: 10.1186/s12931-021-01690-3.33789673
5. Neto AS, Hemmes SNT, Barbas CSV, Beiderlinden M, Fernandez-Bustamante A, Futier E, et al. Incidence of mortality and morbidity related to postoperative lung injury in patients who have undergone abdominal or thoracic surgery: a systematic review and meta-analysis. Lancet Respir Med. (2014) 2:1007–15. doi: 10.1016/s2213-2600(14)70228-0.25466352
6. Fernandez-Bustamante A, Frendl G, Sprung J, Kor DJ, Subramaniam B, Martinez Ruiz R, et al. Postoperative pulmonary complications, early mortality, and hospital stay following noncardiothoracic surgery: a multicenter stud by the perioperative research network investigators. JAMA Surg. (2017) 152:157–66. doi: 10.1001/jamasurg.2016.4065.27829093
7. Miskovic A, Lumb AB. Postoperative pulmonary complications. Br J Anaesth. (2017) 118:317–34. doi: 10.1093/bja/aex002.28186222
8. Sabate S, Mazo V, Canet J. Predicting postoperative pulmonary complications: implications for outcomes and costs. Curr Opin Anaesthesiol. (2014) 27:201–9. doi: 10.1097/ACO.0000000000000045.24419159
9. Yang CK, Teng A, Lee DY, Rose K. Pulmonary complications after major abdominal surgery: national surgical quality improvement program analysis. J Surg Res. (2015) 198:441–9. doi: 10.1016/j.jss.2015.03.028.25930169
10. Jammer I, Wickboldt N, Sander M, Smith A, Schultz MJ, Pelosi P, et al. Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine: european perioperative clinical outcome (EPCO) definitions: a statement from the ESA-ESICM joint taskforce on perioperative outcome measures. Eur J Anaesthesiol. (2015) 32:88–105. doi: 10.1097/EJA.0000000000000118.25058504
11. Gupta H, Gupta PK, Fang X, Miller WJ, Cemaj S, Forse RA, et al. Development and validation of a risk calculator predicting postoperative respiratory failure. Chest. (2011) 140:1207–15. doi: 10.1378/chest.11-0466.21757571
12. Qaseem A, Snow V, Fitterman N, Hornbake ER, Lawrence VA, Smetana GW, et al. Risk assessment for and strategies to reduce perioperative pulmonary complications for patients undergoing noncardiothoracic surgery: a guideline from the American college of physicians. Ann Intern Med. (2006) 144:575–80. doi: 10.7326/0003-4819-144-8-200604180-00008.16618955
13. Nijbroeka SG, Schultzb MJ, Hemmes SNT. Prediction of postoperative pulmonary complications. Curr Opin Anaesthesiol. (2019) 32:443–51. doi: 10.1097/ACO.0000000000000730.30893115
14. Canet J, Gallart L, Gomar C, Paluzie G, Vallè s J, Castillo J, et al. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology. (2010) 113:1338–50. doi: 10.1097/ALN.0b013e3181fc6e0a.21045639
15. Wijeysundera DN, Pearse RM, Shulman MA, Abbott TEF, Torres E, Ambosta A, et al. Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. The Lancet. (2018) 391:2631–40. doi: 10.1016/s0140-6736(18)31131-0.
16. Feng Z, Bhat RR, Yuan X, Freeman D, Baslanti T, Bihorac A, et al. Intelligent perioperative system: towards real-time big data analytics in surgery risk assessment. DASC PICom DataCom CyberSciTech. (2017) 2017:1254–9. doi: 10.1109/DASC-PICom-DataCom-CyberSciTec.2017.201.
17. Hyer JM, White S, Cloyd J, Dillhoff M, Tsung A, Pawlik TM, et al. Can we improve prediction of adverse surgical outcomes? Development of a surgical complexity score using a novel machine learning technique. J Am Coll Surg. (2020) 230:43–52. doi: 10.1016/j.jamcollsurg.2019.09.015.31672674
18. Xue Q, Wen D, Ji MH, Tong J, Yang JJ, Zhou CM. Developing machine learning algorithms to predict pulmonary complications after emergency gastrointestinal surgery. Front Med (Lausanne). (2021) 8:655686. doi: 10.3389/fmed.2021.655686.34409047
19. Xue B, Li D, Lu C, King CR, Wildes T, Avidan MS, et al. Use of machine learning to develop and evaluate models using preoperative and intraoperative data to identify risks of postoperative complications. JAMA Network Open. (2021) 4:e212240. doi: 10.1001/jamanetworkopen.2021.2240.33783520
20. Marafino BJ, Park M, Davies JM, Thombley R, Luft HS, Sing DC, et al. Validation of prediction models for critical care outcomes using natural language processing of electronic health record data. JAMA Network Open. (2018) 1:e185097. doi: 10.1001/jamanetworkopen.2018.5097.30646310
21. Alrezk R, Jackson N, Al Rezk M, Elashoff R, Weintraub N, Elashoff D, et al. Derivation and validation of a geriatric-sensitive perioperative cardiac risk Index. J Am Heart Assoc. (2017) 6:1–10. doi: 10.1161/JAHA.117.006648.
22. Moons KG, Altman DG, Reitsma JB, Ioannidis JP, Macaskill P, Steyerberg EW, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. (2015) 162:W1–73. doi: 10.7326/M14-0698.25560730
23. Baldi P, Sadowski P. The dropout learning algorithm. Artif Intell. (2014) 210:78–122. doi: 10.1016/j.artint.2014.02.004.24771879
24. Lee CK, Hofer I, Gabel E, Baldi P, Cannesson M. Development and validation of a deep neural network model for prediction of postoperative in-hospital mortality. Anesthesiology. (2018) 129:649–62. doi: 10.1097/ALN.0000000000002186.29664888
25. Saito T, Rehmsmeier M. The precision-recall plot is more informative than the ROC plot when evaluating binary classifiers on imbalanced datasets. PLoS One. (2015) 10:1–21. doi: 10.1371/journal.pone.0118432.
26. Chiew CJ, Liu N, Wong TH, Sim YE, Abdullah HR. Utilizing machine learning methods for preoperative prediction of postsurgical mortality and intensive care unit admission. Ann Surg. (2020) 272:1133–9. doi: 10.1097/SLA.0000000000003297.30973386
27. Liu H, Hussain F, Tan CL, Dash M. Discretization: an enabling technique. Data Min Knowl Discov. (2002) 6:393–423. doi: 10.1023/A:1016304305535.
28. Huang X, Khetan A, Cvitkovic M, Karnin Z. Tabtransformer: tabular data modeling using contextual embeddings. Computing Research Repository (2020). Available at: https://arxiv.org/pdf/2012.06678.pdf (Accessed December 11, 2020).
29. Misic VV, Gabel E, Hofer I, Rajaram K, Mahajan A. Machine learning prediction of postoperative emergency department hospital readmission. Anesthesiology. (2020) 132:968–80. doi: 10.1097/ALN.0000000000003140.32011336
30. He J, Baxter SL, Xu J, Xu J, Zhou X, Zhang K. The practical implementation of artificial intelligence technologies in medicine. Nat Med. (2019) 25:30–6. doi: 10.1038/s41591-018-0307-0.30617336
31. investigators LV. Epidemiology, practice of ventilation and outcome for patients at increased risk of postoperative pulmonary complications: LAS VEGAS - an observational study in 29 countries. Eur J Anaesthesiol. (2017) 34:492–507. doi: 10.1097/EJA.0000000000000646.28633157
32. Bonde A, Varadarajan KM, Bonde N, Troelsen A, Muratoglu OK, Malchau H, et al. Assessing the utility of deep neural networks in predicting postoperative surgical complications: a retrospective study. The Lancet Digital Health. (2021) 3:e471–e85. doi: 10.1016/s2589-7500(21)00084-4.34215564
33. Cosgriff CV, Celi LA. Deep learning for risk assessment: all about automatic feature extraction. Br J Anaesth. (2020) 124:131–3. doi: 10.1016/j.bja.2019.10.017.31813571
34. Bolourani S, Tayebi MA, Diao L, Wang P, Patel V, Manetta F, et al. Using machine learning to predict early readmission following esophagectomy. J Thorac Cardiovasc Surg. (2021) 161:1926–39.e8. doi: 10.1016/j.jtcvs.2020.04.172.32711985
Keywords: postoperative pulmonary complications, deep neural network model, geriatric assessment (MeSH), risk assessment, electronic health records
Citation: Peng X, Zhu T, Chen G, Wang Y and Hao X (2022) A multicenter prospective study on postoperative pulmonary complications prediction in geriatric patients with deep neural network model. Front. Surg. 9:976536. doi: 10.3389/fsurg.2022.976536
Received: 23 June 2022; Accepted: 26 July 2022;
Published: 9 August 2022.
Edited by:
Gabriel Sandblom, Karolinska Institutet (KI), SwedenReviewed by:
Jingxiang Wu, Shanghai Jiao Tong University, ChinaEbba Hillstedt, Karolinska Institutet (KI), Sweden
© 2022 Peng, Zhu, Chen, Wang and Hao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuechao Hao YW5lc2h4Y0AxNjMuY29t
Speciality Section: This article was submitted to Visceral Surgery, a section of the journal Frontiers in Surgery
Abbreviations PPCs, Postoperative pulmonary complications; AUPRC, area under the precision-recall curve; AUROC, area under the receiver operating characteristic curve; MDLP, minimum description length principle; CI, confidence interval.
 Xiran Peng
Xiran Peng Tao Zhu
Tao Zhu Guo Chen
Guo Chen Yaqiang Wang
Yaqiang Wang Xuechao Hao
Xuechao Hao