- 1Department of Radiation Oncology, The First Hospital of Quanzhou Affiliated to Fujian Medical University, Quanzhou, China
- 2Department of Gastrointestinal Surgery, The First Hospital of Quanzhou Affiliated to Fujian Medical University, Quanzhou, China
Objective: Neoadjuvant chemoradiotherapy (nCRT) is the recommended standard treatment for locally advanced esophageal cancer (LA-EC). This study aimed to determine whether sex makes a difference in cancer-specific survival (CSS) and construct a novel nomogram model to predict CSS for LA-EC after nCRT based on the SEER database.
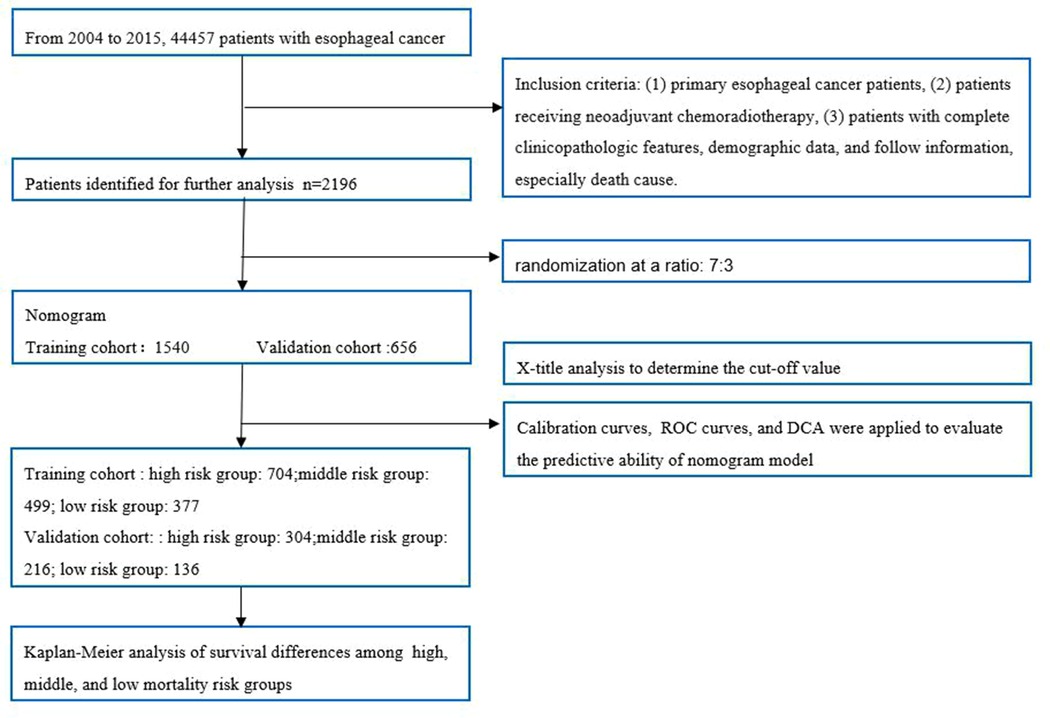
Methods: Patients coded by 04–15 were identified from the SEER database. Patients with systemic treatment and radiotherapy before surgery were defined as nCRT. We further divided this population into a training group and a verification group at a ratio of 7:3. Univariate and multivariate cox analyses were applied to determine the prognostic risk factors based on the training cohort, and then the Nomogram model was established. The area under the curve (AUC) was used to evaluate the predictive ability of the model. We used the calibration curve to evaluate the consistency between the predicted status and actual status and decision curve analysis (DCA) to evaluate the clinical value. We used X-tile software to determine the best cut-off value of nomogram scores and divided the population into low-risk, medium-risk, and high-risk groups, and Kaplan-Meier analysis was applied to compare the CSS.
Results: A total of 2096 LA-EC patients were included for further analysis, with 1,540 in the training cohort and 656 in the validation group. Male (HR: 1.29, 95% CI, 1.04 −1.58), T stage, N stage, and M stage were identified as independent risk factors of CSS based on the training cohort. A Nomogram model was constructed to predict the 3-, 5- and 7-years CSS. ROC curve and AUC confirmed that this nomogram has median discrimination ability. The calibration curve showed good agreement between predicted status and actual status. The DCA curves confirmed the clinical value. Kaplan-Meier analysis indicated that patients in the high-risk subgroup had poorer CSS in both the training cohort and validation cohort (P < 0.001).
Conclusion: Male patients had poorer CSS in LA-EC patients after nCRT. A nomogram model composed of sex, T stage, N stage, and M stage was constructed to identify the high-risk population and provide a personalized follow-up plan.
Introduction
Esophageal cancer is a highly aggressive malignancy, with a 5-year overall survival (OS) of only 10% to 20% in patients with advanced-stage (1). Compared with the surgery alone group, neoadjuvant chemoradiotherapy (nCRT) could significantly improve overall survival (OS) (100.1 months vs. 66.5 months) and disease-free survival (100.1 months vs. 41.7 months) for locally advanced esophageal cancer (LA-EC) (2). The 10-year OS of the CROSS trial indicated that the absolute benefit of nCRT was 13% (38% vs. 25%) (3). Based on current evidence, nCRT is still the first choice of treatment for LA-EC. Sex is reported to be a clinicopathological feature that could affect long-term survival (4, 5). However, at present, whether sex could affect the survival of LA-EC receiving nCRT is still unclear.
The Union for International Cancer Control tumor/node/Metastasis (TNM) staging system is widely used to predict long-term survival and guide adjuvant therapy, but its identification ability is limited. Sometimes, patients diagnosed with EC have different survival, even with the same TNM stage (4, 5). Nomograms are widely used to effectively predict survival in patients with all types of cancer-based on clinicopathological features (6). Nomogram is a new visualization tool that combines risk factors with other predictors to assess the absolute risk of an individual patient and is widely used to help doctors make decisions. The sample size is an important factor in constructing a reliable nomogram model. Surveillance, Epidemiology, and End Results (SEER) database population is a public population, which contains approximately 35% population of Americans, and could provide enough sample size for model development.
This study aimed to determine whether sex makes a difference in cancer-specific survival (CSS) and construct a novel nomogram model to predict CSS for LA-EC receiving nCRT based on the SEER database population, which could help in risk stratification and provide individualized therapy.
Methods
We downloaded data from SEER * stat software (version 8.3.6). The study included EC patients who underwent nCRT after esophagectomy between 2004 and 2015. Inclusion criteria: (1) primary EC, (2) preoperative nCRT, (3) sufficient clinicopathological features, demographic data, cause of death, and follow-up information. Exclusion criteria: (1) lack of basic clinical information such as age, sex, and marital status; (2) Lack of pathological information, T stage, N stage, pathological type, histological grade, and cause of death.
The demographic characteristics (age, sex, race, insurance status, and marital status), disease characteristics (histology, primary location, tumor size, grade, t, N, M stage), treatment methods (radiotherapy, chemotherapy), survival time and living status of patients were analyzed. We divided patients into three groups according to tumor size (<51, 51–76, and >76 mm). We divided the patients into three groups (>65 years old, 50 −65 years old, <50 years old). The primary site was defined according to the international classification of tumor diseases Code: lower esophagus 1 / 3 (15.5), middle esophagus 1 / 3 (15.4), upper esophagus 1 / 3 (15.3), and others. The histological types included adenocarcinoma, squamous cell carcinoma, and others. Tumor differentiation was divided into four groups: grade I, grade II, grade III, and grade IV. The population included in this study was staged by the 7th edition TNM stage system.
Statistical analysis
Univariate and multivariate Cox proportional hazards regression analyses were used to identify independent prognostic factors of CSS. Variables with P < 0.05 in univariate analysis were included in multivariate Cox regression for further analysis.We used backward likelihood ratio to select variables in the multivariate Cox regression analysis. The Nomogram model was constructed based on the identified independent risk factors. We used the area under the curve (AUC) to evaluate the predictive ability. Calibration curves were drawn for the prediction of 3-,5-, and 7-year CSS, respectively, and decision curve analysis (DCA) curves were drawn to evaluate the clinical value. Based on the nomogram score, patients were divided into low-, medium-, and high-risk groups in X-tile software. The nomogram model was constructed based on the training cohort and evaluated in both the training cohort and validation cohort. We conducted analysis in R software (version 3.6.1). A two-sided P value < 0.05 was defined as statistically significant. The primary endpoint of the study was CSS, defined as the time between the date of diagnosis and the date of cancer death or the date of the last follow-up.
Results
Baseline characteristics in the training cohort and validation group
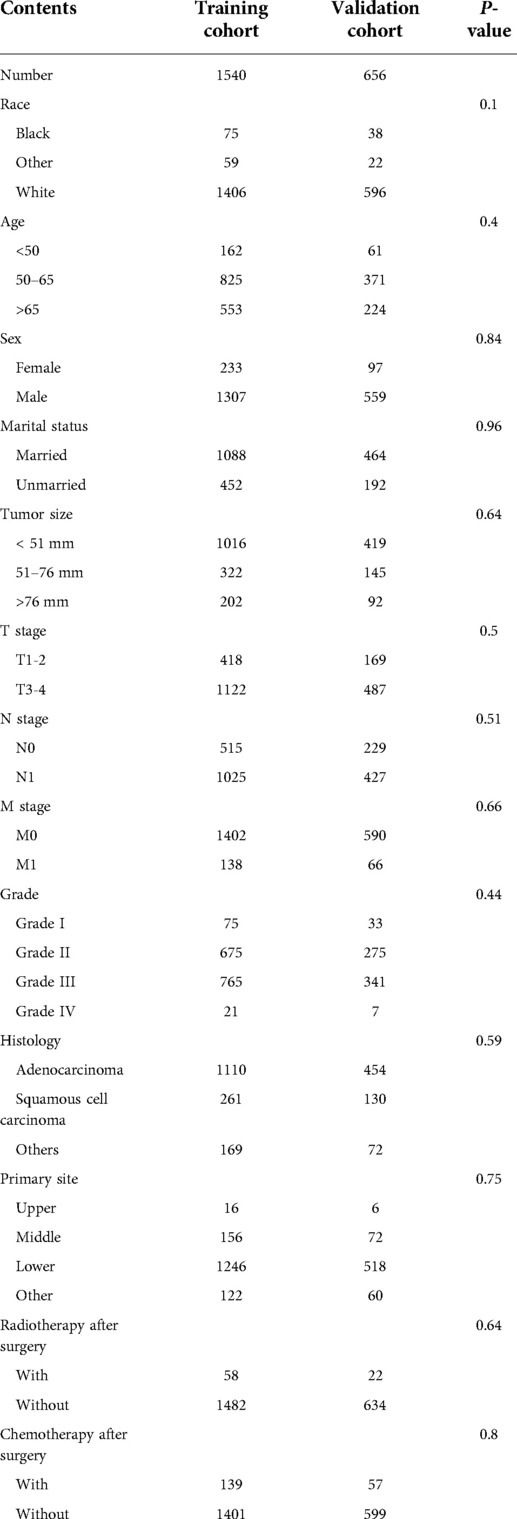
A total of 2,096 patients were identified from the SEER database using SEER*Stat Version 8.3.6 software. The details of patient selection were summarized in Figure 1. The total population was divided into a training cohort of 1,540 patients and a validation cohort of 656 patients. The training group and validation group were comparable in baseline characteristics (P > 0.05). The comparisons are summarized in Table 1.
Development and validation of nomogram model
We used the training cohort to find prognostic risk factors. Univariate analysis indicated that tumor size, M stage, N stage, T stage, grade, and sex were prognostic factors. Multivariate COX analysis determined that M stage (HR = 1.41, 95% CI, 1.14–1.75, P = 0.002), N stage (HR = 1.62, 95% CI, 1.39–1.89, P < 0.001), T stage (HR = 1.25, 1.06–1.46, P = 0.01), and sex (HR = 1.29, 95% CI, 1.04–1.58, P = 0.02) were independent prognostic factors. The details of univariate and multivariate Cox analysis were summarized in Table 2.
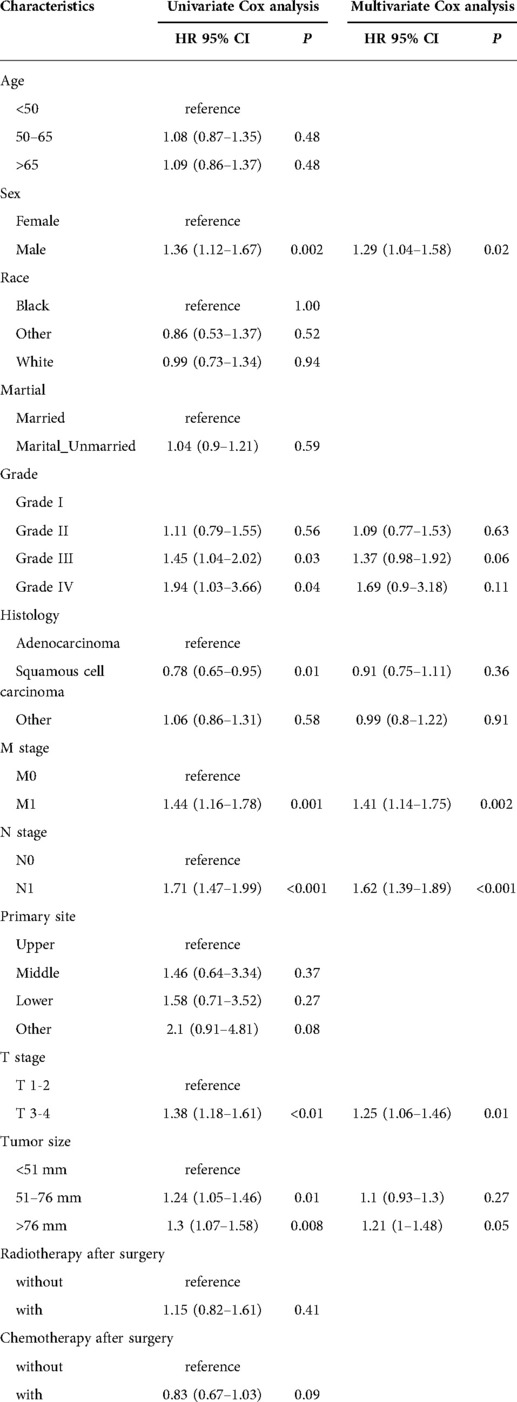
Table 2. Univariate and multivariate Cox analysis of cancer-specific survival for esophageal cancer patients receiving neoadjuvant chemoradiotherapy in the training cohort.
A Nomogram model was developed to predict 3-, 5-, and 7-years CSS (Figure 2). The AUC for 3-,5-, and 7-years CSS was 0.612,0.638, and 0.628 respectively in the training cohort, and 0.597,0.60, and 0.602 respectively in the validation cohort. Time-dependent ROCs noted that this model performed well in predicting CSS in both the training cohort and validation cohort (Figure 3) and also had a higher prediction accuracy than individual prognostic factors included in the model (Figure 4). The calibration curves indicated that the predicted survival status was highly consistent with the actual status in both training and validation cohort (Figure 5). DCA indicated that this nomogram model had strong clinical applicability (Figure 6).
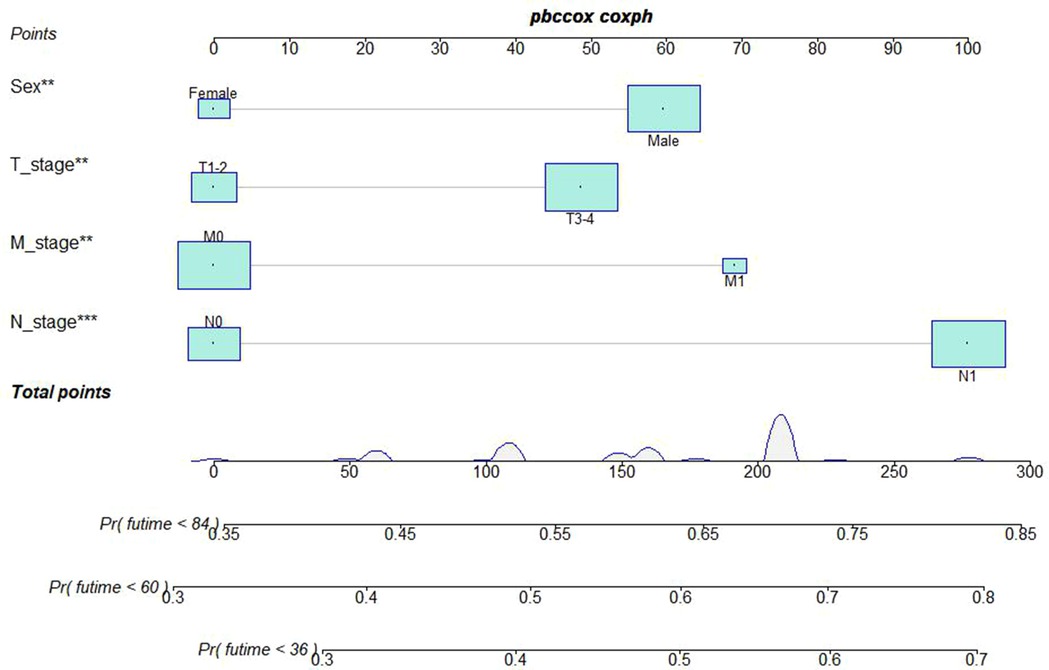
Figure 2. Nomogram model to predict the cancer-specific survival (CSS) at 3-,5-, and 7- years in patients receiving neoadjuvant chemoradiotherapy.
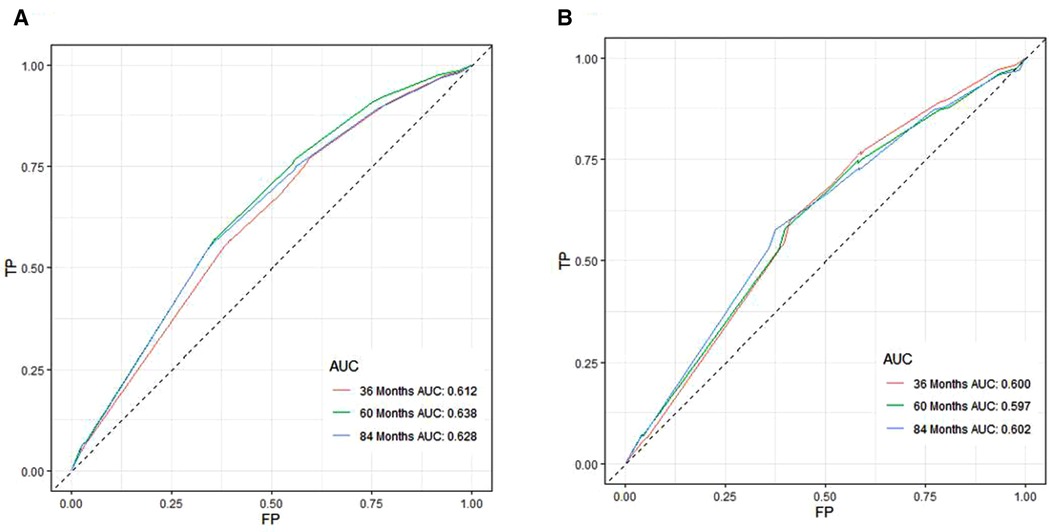
Figure 3. ROC curves for CSS prediction of patients receiving neoadjuvant chmoradiotherapy. (A) ROC curves of 3-, 5-, and 7-years in the training cohort, (B) ROC curves of 3-, 5-, and 7-years in the validation cohort. TP, True positive rate; FP, false positive rate; ROC, Receiver operating characteristic; CSS, cancer-specific survival.
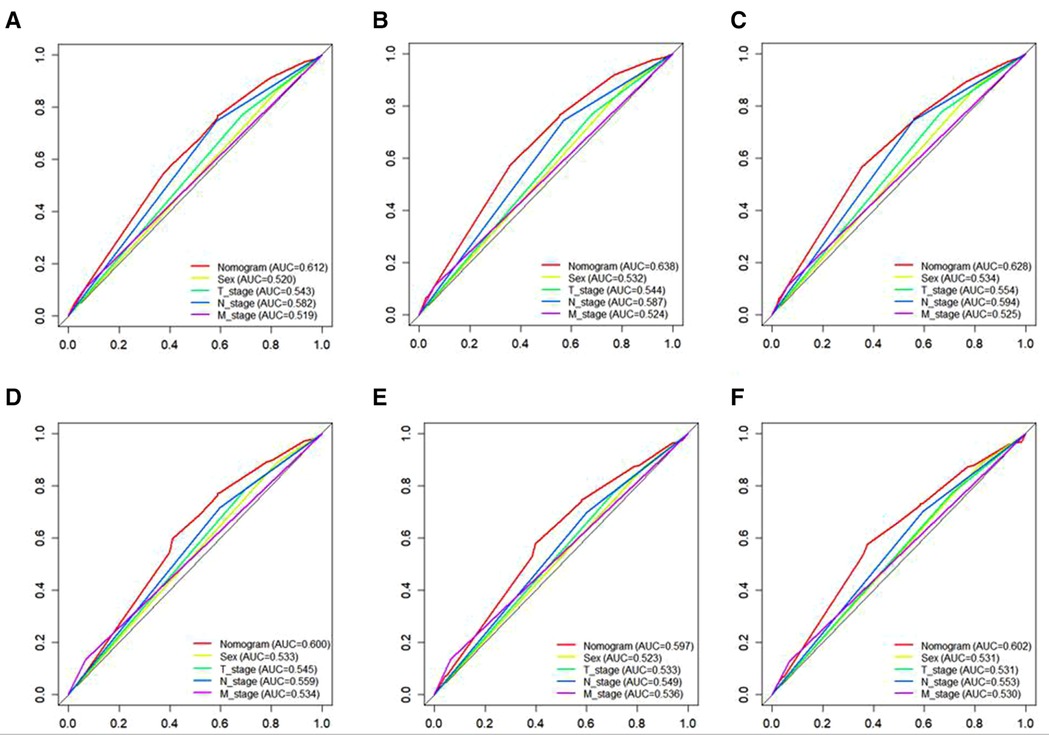
Figure 4. The ROC curves for CSS, including the nomogram model and all independent predictors at 3- (A), 5- (B), and 7-years (C) in the training cohort and at 3- (D), 5- (E), and 7-years (F) in the validation cohort. ROC, Receiver operating characteristic; CSS, cancer specific survival.
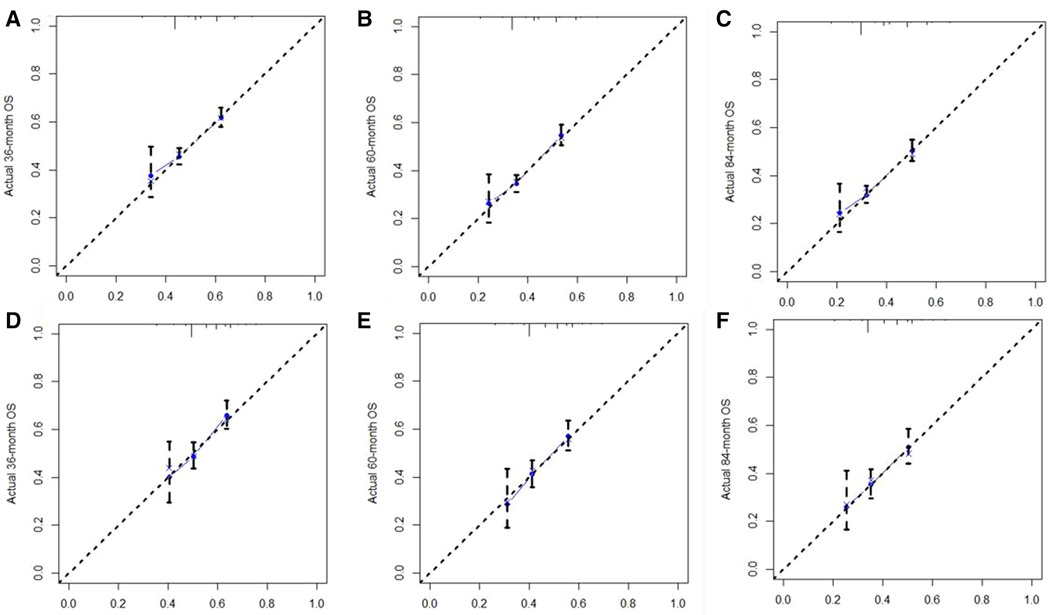
Figure 5. The calibration curve for predicting CSS at (A) 3-years, (B) 5-years, (C) 7-years in the training cohort, and at (D) 3-years, (E) 5-years, (F) 7-years in validation cohort. The nomogram-predicted probability of CSS is plotted on the X-axis, and the actual CSS is plotted on the Y-axis. CSS: cancer-specific survival.
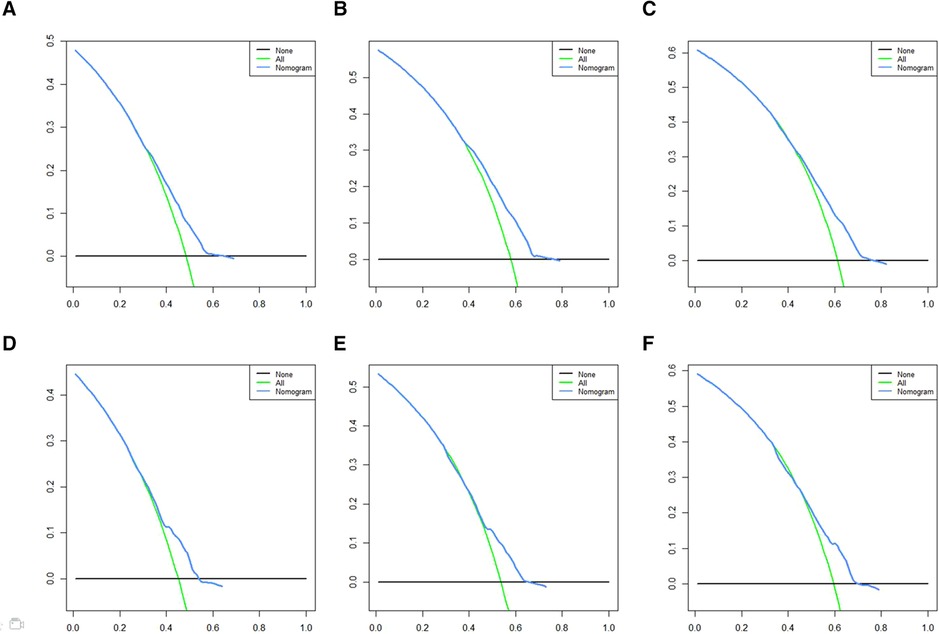
Figure 6. DCA for CSS prediction. (A) DCA of 3-years CSS in the training cohort, (B) DCA of 5-years CSS in the training cohort, (C) DCA of 7-years CSS in the training cohort, (D) DCA of 3-years CSS in the validation cohort, (E) DCA of 5-years CSS in the validation cohort, (F) DCA of 7-years CSS in the validation cohort. DCA, Decision curve analysis; CSS, cancer-specific survival.
Kaplan-Meier analysis and risk stratification
Using X-tile software, patients were divided into low-risk, medium-risk subgroups, and high-risk subgroups according to nomogram scores. Nomogram scores of 0–5 are defined as a low-risk group, 6–10 as a medium-risk group, and 11–14 as a high-risk group. Compared with the low-risk group, the relative risk of the medium-risk group and high-risk group were 1.28 and 1.51, respectively. In the validation cohort and training cohort, patients in the low-risk group had significantly better CSS (P < 0.001) (Figure 7).

Figure 7. Risk stratification based on nomogram score and Kaplan-Meier curves for cancer-specific survival in training cohort (A) and validation cohort (B).
Discussion
To our knowledge, there is significant heterogeneity in the individual survival rate of EC, and the prediction of cancer-specific survival rate using the AJCC staging system alone seems to be inaccurate and inadequate. Although the AJCC staging system is the most widely used system for prognostic assessment and clinical treatment of cancer patients (7). However, due to a lack of demographic information, the AJCC system is not a perfect predictor of CSS in EC patients. Previous studies have confirmed that age at diagnosis, gender, race, marital status, and occupation are significantly associated with cancer survival (8–10). In the establishment of prognostic models for patients with EC, the prognostic value is limited due to the relatively limited sample size (11, 12). We found that sex also played an important role in CSS of EC patients receiving nCRT, and we further developed a richer and more accurate prognostic model (including T stage, N stage, M stage, and sex) to predict CSS. The nomogram could be used to calculate individual CSS predictions and provide better treatment allocation. Based on the nomogram, we could divide patients into low-, medium-, and high-risk, and a personalized follow-up plan could be conducted.
Male was an independent risk factor for poor CSS in EC receiving nCRT. Whether there is a sex difference in survival is still conflicting. Nobel TB et al. reported that postoperative mortality and overall survival (OS) were similar between sexes. In patients with clinical stage II/III, females received neoadjuvant therapy less frequently than males and had worse survival (13). Recently, Ji Zhang et al. found that women had a lower excess mortality rate ratio of 0.76 in EAC subtypes and 0.52 in ESCC based on 1,301 patients from Sweden nationwide. In patients with neoadjuvant therapy, the sex difference benefits still persisted (14). Kauppila JH et al. found that the women had better long-term survival than men in the ESCC subtype but not in the EAC subtype (15). Rowse PG et al. found that after induction chemoradiotherapy, the male sex had an 80% increased risk of recurrence (hazard ratio 1.80, P = 0.008) (16). Estrogen receptors (ERs) are highly expressed in ESCC, and estrogens were reported to inhibit squamous cell tumor growth (17, 18). However, age-stratified studies did not show better survival in younger women with higher sex hormone levels. Other key prognostic factors for EC involve alcohol consumption, smoking consumption, obesity, lifestyle, and oncogenic types of HPV (19). The female sex could respond better to induction chemoradiotherapy. The response difference may be due to sex-related differences in pharmacokinetics and pharmacodynamics (20). The mechanism is still unclear and should be further explored.
Based on the 7th AJCC staging system, differentiation grade is a staging factor for EC. He W et al. also reported that although patients with poorly differentiated EC respond better to nCRT than those with well-differentiated or moderately differentiated EC, however, resulted in poorer survival (21). For EC patients with the same pathological stage, a worse pathological grade often indicates a worse prognosis and a higher postoperative recurrence rate (22). However, we found that pathological grade wasn't an independent risk factor of CSS for EC receiving nCRT, which was consistent with the 8th AJCC staging system. One possible reason is that the cell redistribution or loss of original morphology after neoadjuvant therapy affects the judgment of pathological grade, which would reduce the value of pathological grade in predicting survival.
At present, the number of population-based EC patients after NCRT is still relatively limited. This study clarified the value of gender differences in CSS for EC patients after nCRT and established a new model to predict the CSS of EC patients after nCRT at 3, 5, and 7 years. However, this study had the following three limitations: first, this study is based on the SEER database. Due to the differences in demographic characteristics and pathological subtypes, it was uncertain whether the conclusions of this study are applicable to the Asian population. Secondly, there was no record of a chemoradiotherapy regimen in the SEER database. The radiotherapy or chemotherapy dose described in SEER data was yes or no/unknown. We defined a combination of preoperative systemic therapy and preoperative radiotherapy as nCRT. There weren’t surgical method, R0 removal rate, and number of lymph nodes removed in the SEER database. Third, this novel model was only verified internally, not externally. The findings of this study should be further verified in later research.
Conclusions
Male patients had poorer CSS in LA-EC patients after nCRT. A nomogram model composed of sex, T stage, N stage, and M stage was constructed to identify the high-risk population and provide a personalized follow-up plan.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author contributions
JW and ZC conceived the concept and coordinated the design. JW and CY drafted the manuscript. All authors revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lu L, Mullins CS, Schafmayer C, Zeißig S, Linnebacher M. A global assessment of recent trends in gastrointestinal cancer and lifestyle-associated risk factors. Cancer Commun (Lond). (2021) 41(11):1137–51. doi: 10.1002/cac2.12220
2. Yang H, Liu H, Chen Y, Zhu C, Fang W, et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): a phase III multicenter, randomized, open-label clinical trial. J Clin Oncol. (2018) 36(27):2796–803. doi: 10.1200/JCO.2018.79.1483
3. Eyck BM, van Lanschot JJB, Hulshof MCCM, van der Wilk BJ, Shapiro J, et al. Ten-Year outcome of neoadjuvant chemoradiotherapy plus surgery for esophageal cancer: the randomized controlled CROSS trial. J Clin Oncol. (2021) 39(18):1995–2004. doi: 10.1200/JCO.20.03614
4. Zhang D, Ku J, Liu R, Wu H, Liang G, Wei Y, et al. Characterization of serum estradiol level and tissue estrogen receptor immunostaining with clinical response and reproductive factor changes in Chinese female patients with esophageal squamous cell carcinoma. Biomed Pharmacother. (2017) 93:879–84. doi: 10.1016/j.biopha.2017.07.020
5. Litle VR, Rice TW. The esophagus: do sex and gender matter? Semin Thorac Cardiovasc Surg Summer. (2011) 23(2):131–6. doi: 10.1053/j.semtcvs.2011.08.008
6. Zhang DY, Ku JW, Zhao XK, Zhang HY, Song X, Wu HF, et al. Increased prognostic value of clinical-reproductive model in Chinese female patients with esophageal squamous cell carcinoma. World J Gastroenterol. (2022) 28(13):1347–61. doi: 10.3748/wjg.v28.i13.1347
7. Inada M, Nishimura Y, Ishikawa K, Nakamatsu K, Wada Y, Uehara T, et al. Comparing the 7th and 8th editions of the American joint committee on cancer/union for international cancer control TNM staging system for esophageal squamous cell carcinoma treated by definitive radiotherapy. Esophagus. (2019) 16(4):371–6. doi: 10.1007/s10388-019-00675-y
8. Lan T, Liu W, Lu Y, Zheng R, Luo H, Shao X, et al. Role of marital status on the prognosis in esophagus adenocarcinoma: a real-world competing risk analysis. Future Oncol. (2020) 16(35):2923–37. doi: 10.2217/fon-2020-0613
9. Zhou YJ, Lu XF, Zheng KI, Wang QW, Chen JN, Zhang QW, et al. Marital status, an independent predictor for survival of gastric neuroendocrine neoplasm patients: a SEER database analysis. BMC Endocr Disord. (2020) 20(1):111. doi: 10.1186/s12902-020-00565-w
10. Pudło K, Błotniak A, Skoczylas T, Dąbrowski A, Szawłowski A, Kozłowski M, et al. The influence of patient-related constutional and environmental factors on early results of a combined modality therapy of esophageal cancer. Pol Przegl Chir. (2016) 88(5):254–63. doi: 10.1515/pjs-2016-0061
11. Du R, Ming J, Geng J, Zhu X, Zhang Y, Li S, et al. Establishment of prognostic models for adenocarcinoma of oesophagogastric junction patients with neoadjuvant chemoradiotherapy: a real-world study. Radiat Oncol. (2022) 17(1):45. doi: 10.1186/s13014-022-02016-3
12. Thota PN, Alkhayyat M, Gomez Cifuentes JD, Haider M, Bena J, McMichael J, et al. Clinical risk prediction model for neoadjuvant therapy in resectable esophageal adenocarcinoma. J Clin Gastroenterol. (2022) 56(2):125–32. doi: 10.1097/MCG.0000000000001489
13. Nobel TB, Livschitz J, Eljalby M, Janjigian YY, Bains MS, Adusumilli PS, et al. Unique considerations for females undergoing esophagectomy. Ann Surg. (2020) 272(1):113–7. doi: 10.1097/SLA.0000000000003202
14. Zhang J, Bellocco R, Ye W, Johansson J, Nilsson M, Lindblad M. Effect of sex on survival after resection of oesophageal cancer: nationwide cohort study. BJS Open. (2022) 6(3):zrac035. doi: 10.1093/bjsopen/zrac035
15. Kauppila JH, Wahlin K, Lagergren P, Lagergren J. Sex differences in the prognosis after surgery for esophageal squamous cell carcinoma and adenocarcinoma. Int J Cancer. (2019) 144(6):1284–91. doi: 10.1002/ijc.31840
16. Rowse PG, Jaroszewski DE, Thomas M, Harold K, Harmsen WS, Shen KR. Sex disparities after induction chemoradiotherapy and esophagogastrectomy for esophageal cancer. Ann Thorac Surg. (2017) 104(4):1147–52. doi: 10.1016/j.athoracsur.2017.05.030
17. Xie SH, Santoni G, Lagergren J. Menopausal hormone therapy and risk of oesophageal adenocarcinoma in a population-based cohort study. Br J Cancer. (2022) 126(1):129–33. doi: 10.1038/s41416-021-01575-8
18. Pinton G, Manzotti B, Balzano C, Moro L. Expression and clinical implications of estrogen receptors in thoracic malignancies: a narrative review. J Thorac Dis. (2021) 13(3):1851–63. doi: 10.21037/jtd-20-2277
19. Tribius S, Ihloff AS, Rieckmann T, Petersen C, Hoffmann M. Impact of HPV status on treatment of squamous cell cancer of the oropharynx: what we know and what we need to know. Cancer Lett. (2011) 304(2):71–9. doi: 10.1016/j.canlet.2011.02.002
20. Özdemir BC, Csajka C, Dotto GP, Wagner AD. Sex differences in efficacy and toxicity of systemic treatments: an undervalued issue in the era of precision oncology. J Clin Oncol. (2018) 36(26):2680–3. doi: 10.1200/JCO.2018.78.3290
21. He W, Mao T, Yan J, Leng X, Deng X, et al. Moderately differentiated esophageal squamous cell carcinoma has a poor prognosis after neoadjuvant chemoradiotherapy. Ann Transl Med. (2021) 9(8):706. doi: 10.21037/atm-21-1815
Keywords: neoadjuvant chemoradiotherapy, cancer-specific survival, SEER database, esophageal cancer, nomogram model, male, sex difference
Citation: Wang J, Ye C, Zhang C, Wang K, Hong F, Peng Q and Chen Z (2022) Sex differences in cancer-specific survival for locally advanced esophageal cancer after neoadjuvant chemoradiotherapy: A population-based analysis. Front. Surg. 9:989204. doi: 10.3389/fsurg.2022.989204
Received: 8 July 2022; Accepted: 18 July 2022;
Published: 29 July 2022.
Edited by:
Mingqiang Kang, Fujian Medical University Union Hospital, ChinaReviewed by:
Qiaojuan Guo, Fujian Provincial Cancer Hospital, ChinaPeiyuan Wang, Fujian Provincial Cancer Hospital, China
© 2022 Wang, Ye, Zhang, Wang, Hong, Peng and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zilong Chen Y2hlbnppbG9uZzE5NzFAMTI2LmNvbQ==
†These authors have contributed equally to this work.
Specialty Section: This article was submitted to Thoracic Surgery, a section of the journal Frontiers in Surgery
 Jiaqiang Wang1,†
Jiaqiang Wang1,† Zilong Chen
Zilong Chen