- 1Department of Surgery, University at Buffalo Jacobs School of Medicine and Biomedical Sciences, The State University of New York, Buffalo, NY, United States
- 2Division of Thoracic Surgery, Buffalo General Medical Center, Buffalo, NY, United States
Background: Marijuana use has become more common since its legalization, as have reports of marijuana-associated spontaneous pneumomediastinum. Non-spontaneous causes such as esophageal perforation are often ruled out on presentation due to the severe consequences of untreated disease. Here we seek to characterize the presentation of marijuana-associated spontaneous pneumomediastinum and explore whether esophageal imaging is necessary in the setting of an often benign course and rising healthcare costs.
Materials and Methods: Retrospective review was performed for all 18–55 year old patients evaluated at a tertiary care hospital between 1/1/2008 and 12/31/2018 for pneumomediastinum. Iatrogenic and traumatic causes were excluded. Patients were divided into marijuana and control groups.
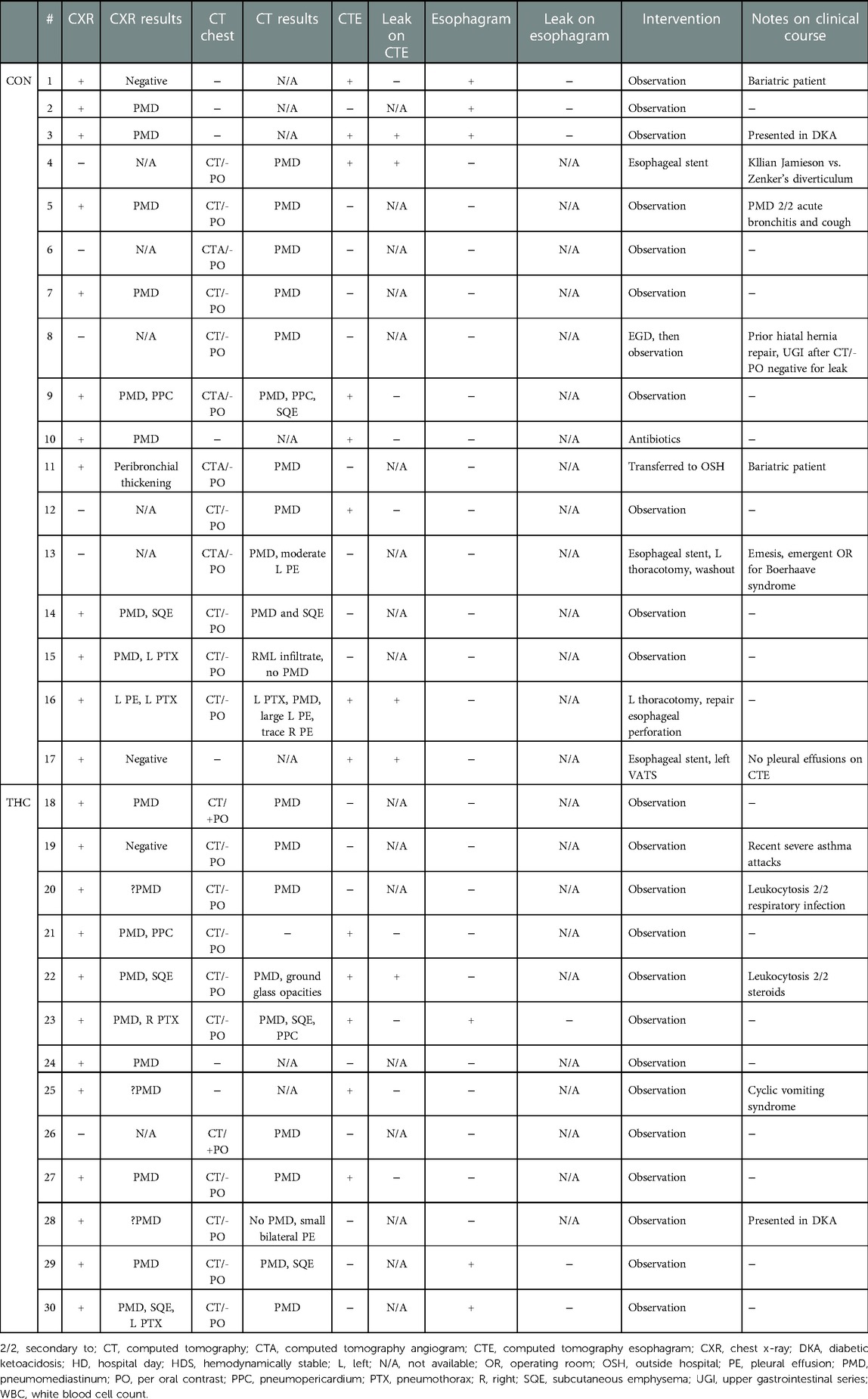
Results: 30 patients met criteria, with 13 patients in the marijuana group. The most common presenting symptoms were chest pain/discomfort and shortness of breath. Other symptoms included neck/throat pain, wheezing, and back pain. Emesis was more common in the control group but cough was equally prevalent. Leukocytosis was present in most patients. Four out of eight of computed tomography esophagarams in the control group showed a leak requiring intervention, while only one out of five in the marijuana group showed even a possible subtle extravasation of contrast but this patient ultimately was managed conservatively given the clinical picture. All standard esophagrams were negative. All marijuana patients were managed without intervention.
Discussion: Marijuana-associated spontaneous pneumomediastinum appears to have a more benign clinical course compared to non-spontaneous pneumomediastinum. Esophageal imaging did not change management for any marijuana cases. Perhaps such imaging could be deferred if clinical presentation of pneumomediastinum in the setting of marijuana use is not suggestive of esophageal perforation. Further research into this area is certainly worth pursuing.
Introduction
Cannabis, also known as marijuana, has a long history of use due to its intoxicating and medicinal properties (1), often cited as being relaxing and meditative (2), but now known to have various psychoactive effects (3). The spectrum of symptoms is related both to the concentration of one of its active components, tetrahydrocannabinol, as well as the method of administration, which most often includes inhalation or oral consumption (4). Since its legalization in various states, marijuana use has become more common (5).
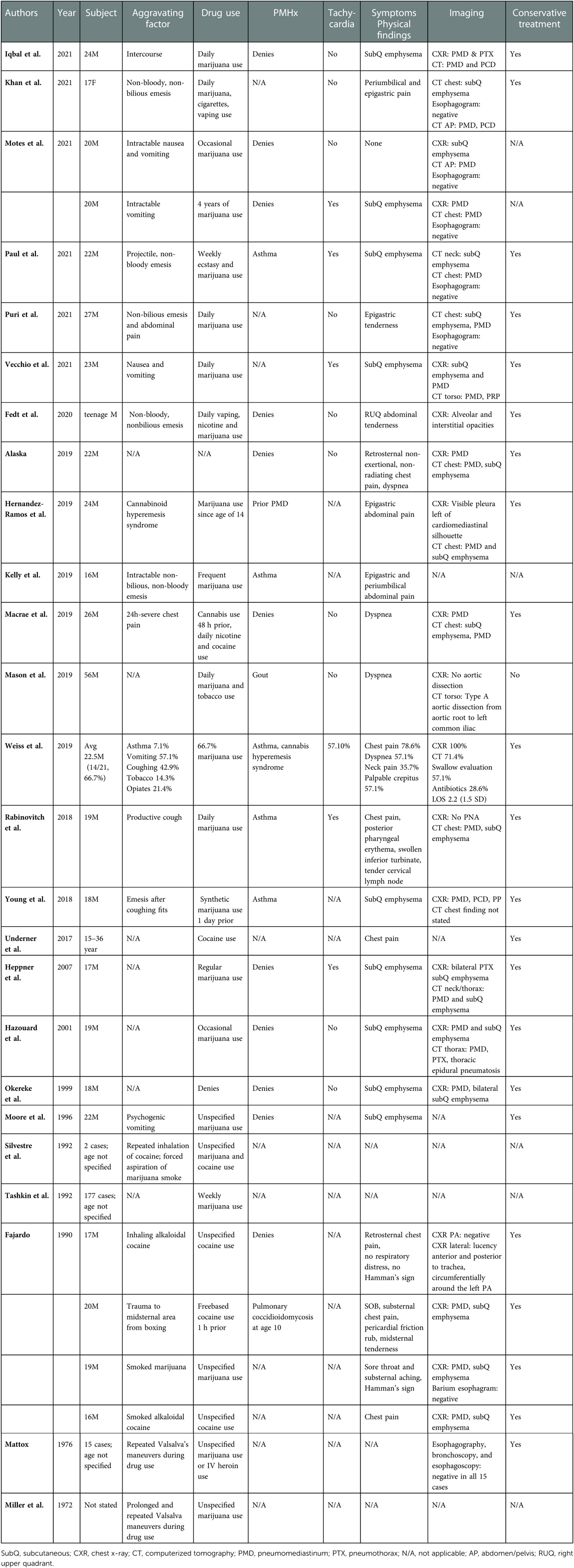
Since 1972, there have been at least 30 case reports (Table 1) of spontaneous pneumomediastinum (sPMD) associated with marijuana use. Pneumomediastinum (PMD) is the presence of free air within the mediastinum (6) and is termed spontaneous when it is secondary to rupture of lung alveoli (7). A causative relationship between inhalational marijuana and sPMD has been suggested, due to several known triggers and physiological changes that are associated with development of sPMD. These include episodes of coughing or emesis (8), inhalational drugs, valsalva maneuvers (7), and other maneuvers associated with inhalational drug use, such as use of a bong and Müller's maneuver (9), which is inspiration against a closed mouth and nose. Additionally, marijuana use has been associated with hyperemesis syndrome (10) and bullae formation (11), which can predispose to increased intrathoracic pressures and rupture of alveoli respectively. In marijuana-associated sPMD, much like in sPMD not associated with marijuana use, the mechanism behind development of mediastinal air is the Macklin effect (7, 9), in which rupture of the alveoli from increased alveolar pressure leads to dissection of air along the peribronchial sheaths. A causative effect has not been proven however, given the lack of large volume data that is free of confounding factors, such as tobacco and other recreational drug use, which can also produce barotrauma and other damage to the lungs (12–14).
While most cases of sPMD are benign and resolve without intervention (15), this is not often true for non-spontaneous PMD, which has a variety of other causes, including esophageal perforation, traumatic respiratory tract injury, and infection. Patients presenting with suspected sPMD are often worked up for esophageal perforation because such cases can rapidly lead to sepsis and death if not adequately treated in a timely manner. This workup is quite resource intensive, and often includes admission to the hospital for an average of 4.4 days, multiple imaging studies, and sometimes even invasive studies such as esophagogastroduodenoscopy (15). Similarly, the recommended workup for sPMD in the setting of marijuana use includes admission with esophagram or computed tomography esophagram (CTE) (16). These cases, however, are very rarely found to have esophageal perforations on imaging and often are managed conservatively (17).
At this time, the literature appears limited mostly to case reports and literature review. There is one case series that examined 14 patients with sPMD and marijuana use with a focus on safe use of smoking devices and inhalational techniques. This study concluded that inhaled marijuana could be a risk factor for sPMD, but that further research was needed (18). In this descriptive retrospective case series, we seek to characterize and define the clinical presentation of marijuana-associated sPMD and explore whether esophageal imaging is necessary in the setting of an often benign clinical course and rising healthcare costs (19, 20). To the best of our knowledge, this study would be the first to address this question in this particular disease process and serves as a gateway to determining whether further research into this topic is safe and worthwhile.
Materials and methods
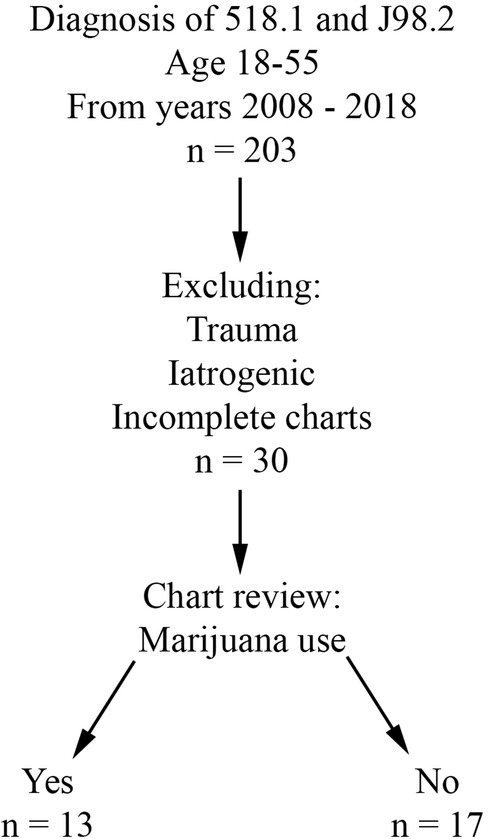
Retrospective review was performed for all patients evaluated at Buffalo General Hospital, a single academic and tertiary care hospital, between 1/1/2008 and 12/31/2018. Charts after 2018 were not included in the study due to the possibility of Covid-19 acting as a confounding factor. The University at Buffalo Institutional Review Board approval (assurance ID FWA00008824) and waiver of patient consent were obtained prior to the start of data collection. The electronic medical record in use at this institution is Cerner PowerChart. Inclusion criteria included age 18 to 55 years and a primary or secondary diagnosis of pneumomediastinum (ICD-9 518.1 and ICD-10 J98.2). Patients with an iatrogenic or traumatic cause of PMD were excluded from the study. Patient charts lacking subjective information (i.e., due to poor documentation or issues with the electronic medical record during that evaluation) were also excluded. Patients were divided into two groups based on presence (THC group) or absence (CON group) of marijuana use (Figure 1).
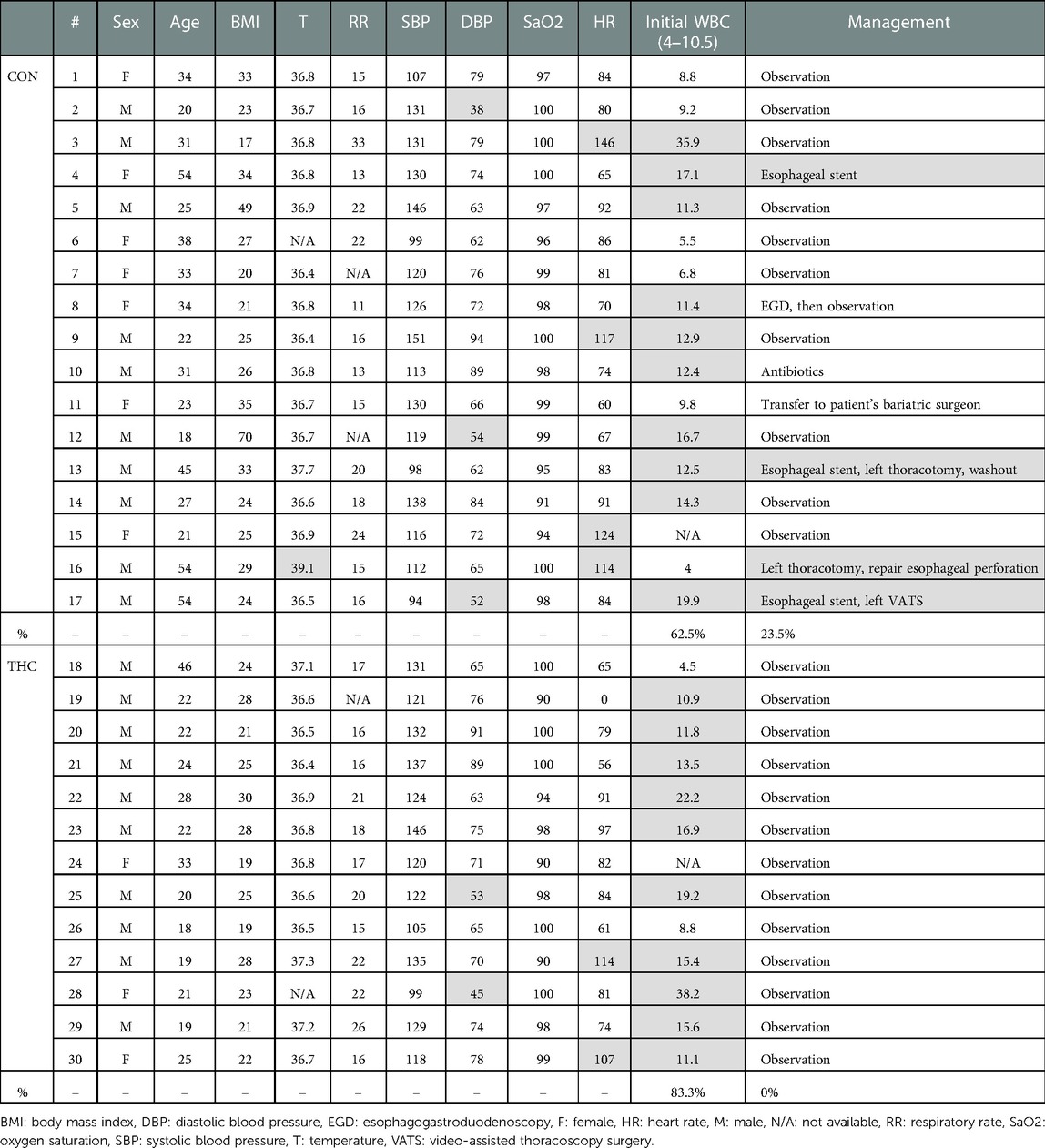
Demographic data, including age at presentation, sex, and body mass index were collected. Charts were reviewed to evaluate objective data at presentation, such as admitting vital signs and white blood cell count (WBC, designated as normal in Powerchart if between 4 and 10.5), as well as subjective data at presentation, such as reported symptoms and history of present illness. Diagnostic imaging studies, operative interventions, and other treatments were also reviewed.
Results
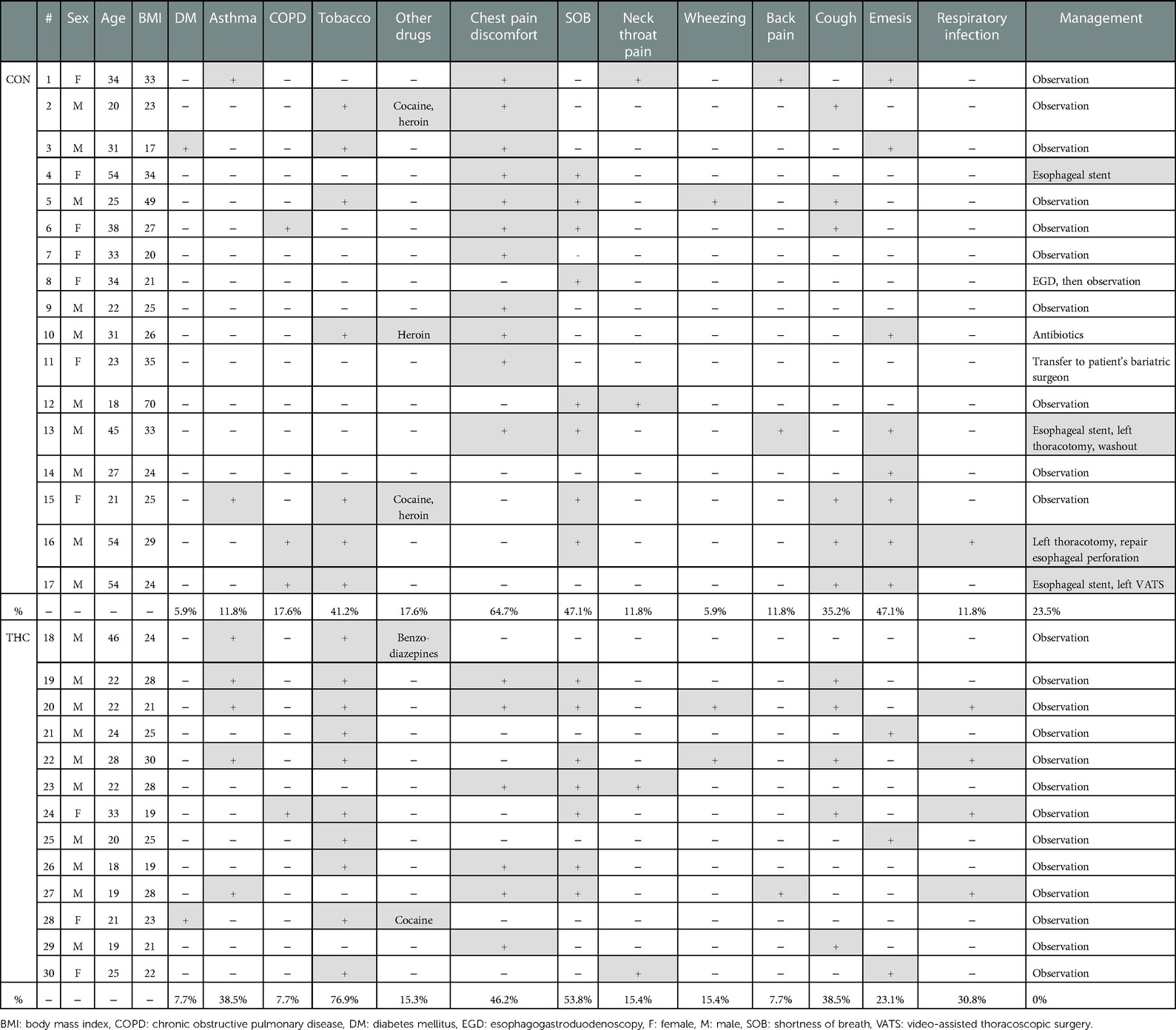
After chart review, 30 patients with sPMD met criteria, with NTHC = 13 (43.3%) and NCON = 17 (56.7%). Average age was 24.5 years in the THC group and 33.2 years in the CON group. In the CON group, there was a slight male predominance (CON male 58.8%, CON female 41.2%) compared with the drastic male predominance in the THC group (THC male 76.9%, THC female 23.1%). None of the patients had a history of sarcoidosis, interstitial lung disease, cystic fibrosis, or any other lung disease. One patient in each group had a history of diabetes, and both of these patients presented in diabetic ketoacidosis. Asthma was present in 2 CON patients compared to 5 THC patients. Chronic obstructive pulmonary disease was present in 3 CON patients compared to 1 THC patient. Demographic and symptom data are summarized in Table 2.
Some patients reported a history of preceding respiratory illness (THC 30.8%, CON 11.8%), smoking (THC 76.9%, CON 41.2%), and other drug use (THC 15.3%, CON 17.6%). Common presenting symptoms included chest pain or discomfort (THC 46.2%, CON 64.7%), shortness of breath (THC 53.8%, CON 47.1%), neck or throat pain (THC 15.4%, CON 11.8%), wheezing (THC 15.4%, CON 5.9%), and/or back pain (THC 7.7%, CON 11.8%). Ongoing or recent cough (THC 38.5%, CON 35.2%) and emesis (THC 23.1%, CON 47.1%) were also reported. Temperature of greater than 38.5°C was present in 1 patient in the CON group; all THC patients were afebrile at presentation. No patients in either THC or CON groups had a presenting systolic blood pressure of less than 90. Heart rate greater than 100 was present in 4 patients in the CON group and 2 patients in the THC group. Leukocytosis was present in 83.3% of THC patients and 62.5% of CON patients. Patients for whom WBC was not available were excluded from this portion of the analysis. Objective presenting data is summarized in Table 3.
Diagnostic images that were frequently obtained included chest x-ray (THC 92.3%, CON 70.6%), computed tomography chest imaging apart from CTE (THC 76.9%, CON 70.6%), CTE (THC 38.5%, CON 47.1%), and standard esophagram (THC 23.1%, CON 29.4%). Of the 8 CTEs obtained in the CON group, 4 found extraluminal contrast. Of the 5 CTEs obtained in the THC group, 4 were negative and one was read to have subtle extraluminal contrast, although the patient was clinically determined to be at low-risk for esophageal perforation and was managed conservatively. Of the 3 standard esophagrams obtained in the CON group, none demonstrated a leak. A total of 3 standard esophagrams were obtained in the THC group as well with no diagnosed leaks. Pneumothorax was present in 2 THC patients compared to 4 in the CON group.
A total of 4 patients required operative intervention, all of whom were in the CON group. Interventions included esophageal stenting (3/4), thoracotomy (2/4), and video-assisted thoracoscopic surgery (VATS) (1/4), all for esophageal perforation and, in some cases, sequelae of esophageal perforation such as empyema and persistent leak. All THC patients were managed conservatively without need for further intervention. Diagnostic imaging and intervention data are summarized in Table 4.
Discussion
In this study population, approximately 25% of PMD diagnosed in patients without marijuana use were found to be non-spontaneous in nature and secondary to esophageal disruption, requiring operative intervention. In contrast, sPMD in marijuana users were all managed non-operatively and with observation, which is consistent with the more benign nature of marijuana-associated sPMD.
Esophageal imaging was obtained in 9/17 CON patients. The four that were diagnosed with esophageal disruption underwent operative intervention, as previously noted. Esophageal imaging was obtained in 7/13 THC patients. None of the THC patients underwent operative intervention, including the one patient in whom there was question of possible extraluminal contrast. There were also several patients in whom further dedicated esophageal imaging was deferred due to lack of extraluminal contrast on a non-dedicated computed tomography scan with oral contrast. Therefore, it could be argued that in this study population, esophageal imaging in marijuana-associated sPMD did not change the ultimate management of the patient.
Unfortunately, our study did not reveal any particular symptoms, vital sign abnormalities, or WBC abnormality that was able to predict whether the patient would have a leak or require intervention. The most frequently observed derangement in presenting vital signs across both groups was tachycardia, but only one of the patients requiring intervention was tachycardic at presentation. Leukocytosis was encountered in 3 of the 4 patients requiring intervention, but it was also very common across patients of both groups who did not require intervention, and is frequently encountered in PMD, even in the absence of diagnosed infection (21). Interestingly, tobacco-related leukocytosis has also been observed (22) and could be contributing to the wide-spread elevation in WBC seen across both of these study groups.
Large and moderate pleural effusions (Figure 2), however, were only found in patients who required operative intervention, and is a radiographic finding that is commonly associated with esophageal perforation (23). In patients presenting with this finding, suspicion for esophageal perforation should remain high, and dedicated esophageal imaging should not be deferred in the stable patient who does not require emergent operative intervention. Similarly, forceful and/or repeated emesis can lead to full-thickness esophageal disruption, defined as Boerhaave syndrome. As mentioned previously, marijuana use can lead to cannabinoid hyperemesis syndrome, which is relatively rare but occurs most frequently in daily long-term users (10). Only three of our THC patients reported any form of emesis, of which only one was thought to have cannabinoid hyperemesis syndrome. This patient was worked up with a CTE that showed no leak and he was managed conservatively. As illustrated with this patient, any history of forceful or repeated bouts of emesis should prompt the provider to be concerned about Boerhaave syndrome, and the patient should be worked up appropriately, regardless of marijuana use status.
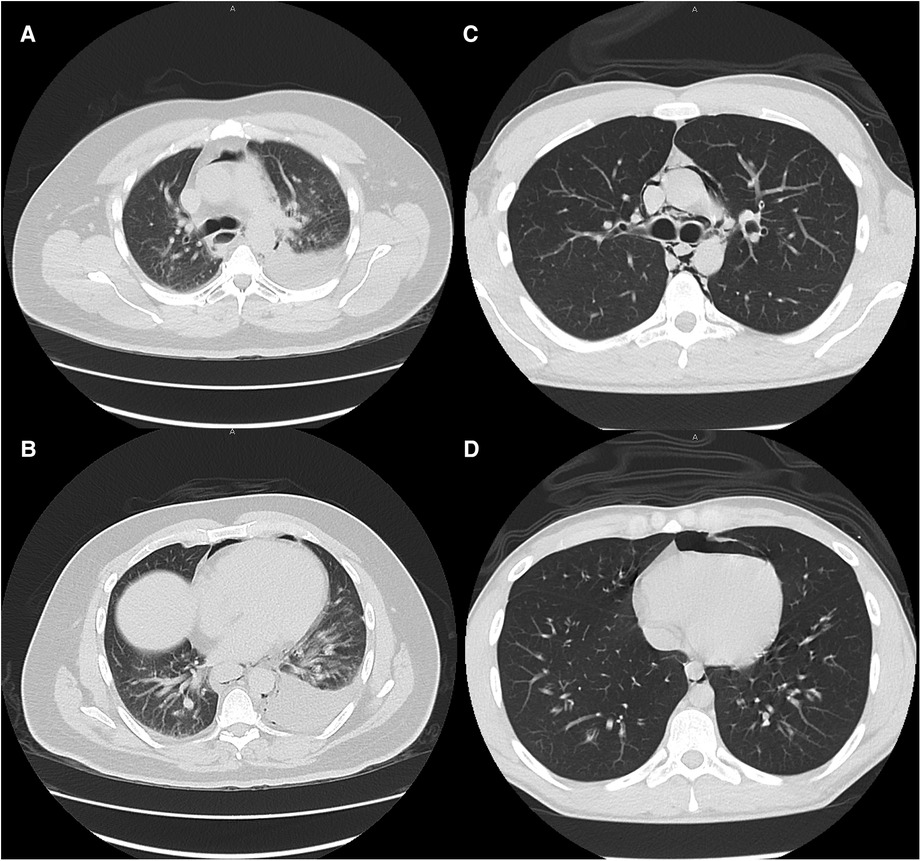
Figure 2. Typical CT scan findings of esophageal perforation (patient 13, images A and B) and marijuana-associated pneumomediastinum (patient 27, images C and D) at similar levels. Moderate to large pleural effusions are typically associated with esophageal perforation. None of the marijuana-associated sPMD patients presented with pleural effusions.
Given our results and the available literature to date, we propose that marijuana-associated sPMD be classified as a separate entity from non-marijuana associated sPMD and defined as isolated PMD occurring in patients who inhale marijuana that do not have an underlying history or presenting symptoms concerning for cannabinoid hyperemesis syndrome. Ebina et al. have already demonstrated that sPMD can be safely treated on an outpatient basis without dedicated esophageal imaging or antibiotics (24). Due to the proposed mechanism of alveolar rupture and the Macklin effect in sPMD, marijuana inhalation serves as an additional risk factor for the development of sPMD without increasing the likelihood of esophageal damage in the absence of cannabinoid hyperemesis syndrome. Overall, this makes marijuana-associated sPMD much more likely to be safely managed conservatively or on an outpatient basis. One of the key points in management of this condition is cessation counselling. Our providers provide cessation counselling both at the initial encounter and in the clinic during the follow up appointment. Providers who encounter patients that smoke marijuana should be aware of the possible ramifications and strive to encourage cessation of recreational use. This is the basis for classifying marijuana-associated sPMD as a separate disease. Adding dedicated esophageal imaging would unnecessarily increase the healthcare costs of managing a condition that often can be managed conservatively, and possibly without admission to the hospital. Our results are in-line with this theory.
This study has a number of limitations. The sample size is small, and by nature of a retrospective case series, broader conclusions about the epidemiology of PMD cannot be drawn. As with much of the current literature, there are many confounding factors present within our data. A subset of our patients presented with a separate disease process associated with PMD, such as asthma (25) and diabetic ketoacidosis (26), which could have contributed to lab and vital sign abnormalities unrelated to the presence of PMD. Concurrent use of recreational drugs and tobacco, which are known to have deleterious effects on the lung (27, 28), was present in many of our THC and CON patients. There did not appear to be an association between esophageal disruption and tobacco as 50% reported use and 50% did not, and none of the esophageal perforation patients had reported other recreational drug use. PMD associated with cocaine use is an uncommon but documented phenomenon (29), and cocaine use reported by the three patients in this study group may be a confounding factor. It is also important to note that the incidence of marijuana use and other recreational drug use may be underreported in this patient population because of the fear of legal repercussions. With the relatively recent legalization within New York State, we may see a more accurate reporting of marijuana use moving forward, but many patients may still be reluctant to disclose their habits. This makes collecting specific information such as joint-years, modality of inhalation, and time-relation to presentation more difficult. During the time period from which our data was collected, there were also few objective measures of marijuana use such as drug screens, as these tests were not frequently indicated for the patient's presenting symptoms. Future prospective studies utilizing drug testing and patient questionnaires would be helpful to better understand these important data points.
Nonetheless, these results suggest that with further research, it may be safe to initially observe patients with marijuana-associated sPMD in the hospital or via close outpatient follow up, perhaps deferring dedicated esophageal imaging in the absence of clinical signs or symptoms concerning for a more ominous underlying process, such as esophageal perforation. Higher-powered studies analyzing data with minimal confounding factors such as lung disease, smoking, and other drug use would allow for better characterization of marijuana as an isolated risk factor for sPMD. This, in turn, would allow for future retrospective and prospective studies, as well as randomized control trials, to better determine the role of esophageal imaging in the management of marijuana-associated sPMD.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors upon request, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by University at Buffalo Institutional Review Board. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
IY: contributed to data collection, study design, data analysis, data interpretation, and writing of the manuscript. KT: contributed to data interpretation and writing of the manuscript. RD: contributed to data collection and writing of the manuscript. RTQ: contributed to data interpretation and writing of the manuscript. YP: contributed to study design, data analysis, data interpretation, and writing of the manuscript. All authors contributed to the article and approved the submitted version
Funding
Funding for the publishing fee was provided by an internal University at Buffalo grant: University at Buffalo Foundation #9333-837625.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CTE, Computed tomography esophagram; PMD, Pneumomediastinum; sPMD, Spontaneous pneumomediastinum; WBC, White blood cell count.
References
1. Elsohly MA, Slade D. Chemical constituents of marijuana: the complex mixture of natural cannabinoids. Life Sci. (2005) 78:539–48. doi: 10.1016/j.lfs.2005.09.011
2. Leal-Galicia P, Betancourt D, Gonzalez-Gonzalez A, Romo-Parra H. A brief history of marijuana in the western world. Rev Neurol. (2018) 67:133–40. doi: 10.33588/RN.6704.2017522
4. Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet. (2003) 42:327–60. doi: 10.2165/00003088-200342040-00003
5. Randall K, Hayward K. Emergent medical illnesses related to cannabis use. Mo Med. (2019) 116:226–8.31527946
6. Alemu BN, Yeheyis ET, Tiruneh AG. Spontaneous primary pneumomediastinum: is it always benign? J Med Case Rep. (2021) 15:157. doi: 10.1186/s13256-021-02701-z
7. Murayama S, Gibo S. Spontaneous pneumomediastinum and macklin effect: overview and appearance on computed tomography. World J Radiol. (2014) 6:850–4. doi: 10.4329/wjr.v6.i11.850
8. Jougon JB, Ballester M, Delcambre F, Mac Bride T, Dromer CEH, Velly J-F. Assessment of spontaneous pneumomediastinum: experience with 12 patients. Ann Thorac Surg. (2003) 75:1711–4. doi: 10.1016/s0003-4975(03)00027-4
9. Hazouard E, Koninck JC, Attucci S, Fauchier-Rolland F, Brunereau L, Diot P. Pneumorachis and pneumomediastinum caused by repeated Müller's Maneuvers: complications of marijuana smoking. Ann Emerg Med. (2001) 38:694–7. doi: 10.1067/mem.2001.118016
11. Johnson MK, Smith RP, Morrison D, Laszlo G, White RJ. Large lung bullae in marijuana smokers. Thorax. (2000) 55:340–2. doi: 10.1136/thorax.55.4.340
12. Perper JA, Van Thiel DH. Respiratory complications of cocaine abuse. Recent Dev Alcohol. (1992) 10:363–77. doi: 10.1007/978-1-4899-1648-8_18
13. Seaman ME. Barotrauma related to inhalational drug abuse. J Emerg Med. (1990) 8:141–9. doi: 10.1016/0736-4679(90)90223-i
14. Olesen WH, Katballe N, Sindby JE, Titlestad IL, Andersen PE, Ekholm O, et al. Cannabis increased the risk of primary spontaneous pneumothorax in tobacco smokers: a case-control study. Eur J Cardiothorac Surg. (2017) 52:679–85. doi: 10.1093/ejcts/ezx160
15. Morgan CT, Maloney JD, Decamp MM, McCarthy DP. A narrative review of primary spontaneous pneumomediastinum: a poorly understood and resource-intensive problem. J Thorac Dis. (2021) 13:3721–30. doi: 10.21037/jtd-21-193
16. Paul M, Paul P, Dey D, Bhardwaj A, Paul K. A case of spontaneous pneumomediastinum following ecstasy and marijuana use. Cureus. (2021) 13:e15871. doi: 10.7759/cureus.15871
17. Motes A, Laoveeravat P, Thongtan T, Nugent K, Islam S, Islam E. Marijuana use-induced spontaneous pneumomediastinum. Proc (Bayl Univ Med Cent). (2020) 34:274–5. doi: 10.1080/08998280.2020.1849485
18. Weiss ZF, Gore S, Foderaro A. Pneumomediastinum in marijuana users: a retrospective review of 14 cases. BMJ Open Respir Res. (2019) 6:e000391. doi: 10.1136/bmjresp-2018-000391
19. Bush M. Addressing the root cause: rising health care costs and social determinants of health. N C Med J. (2018) 79:26–9. doi: 10.18043/ncm.79.1.26
20. Martin AB, Hartman M, Lassman D, Catlin A. National health care spending in 2019: steady growth for the fourth consecutive year. Health Aff. (2021) 40:14–24. doi: 10.1377/hlthaff.2020.02022
21. Munsell, W. P. Pneumomediastinum. A report of 28 cases and review of the literature. Jama. (1967) 202:689–93. doi: 10.1001/jama.202.8.689
22. Smith CJ, Kluck LA, Ruan GJ, Ashrani AA, Marshall AL, Pruthi RK, et al. Leukocytosis and tobacco use: an observational study of asymptomatic leukocytosis. Am J Med. (2021) 134:e31–5. doi: 10.1016/j.amjmed.2020.06.014
23. White CS, Templeton PA, Attar S. Esophageal perforation: cT findings. AJR Am J Roentgenol. (1993) 160:767–70. doi: 10.2214/ajr.160.4.8456662
24. Ebina M, Inoue A, Takaba A, Ariyoshi K. Management of spontaneous pneumomediastinum: are hospitalization and prophylactic antibiotics needed? Am J Emerg Med. (2017) 35:1150–3. doi: 10.1016/j.ajem.2017.03.017
25. Elhakim TS, Abdul HS, Pelaez Romero C, Rodriguez-Fuentes Y. Spontaneous pneumomediastinum, pneumothorax and subcutaneous emphysema in COVID-19 pneumonia: a rare case and literature review. BMJ Case Rep. (2020) 13:e239489. doi: 10.1136/bcr-2020-239489
26. Zhang W, Chen J, Wu X, Chen L, Wei J, Xue M, et al. Analysing the clinical features of pneumomediastinum associated with diabetic ketoacidosis in 79 cases. Diabetes Metab Syndr Obes. (2020) 13:405–12. doi: 10.2147/dmso.S230799
27. Wolff AJ, O'Donnell AE. Pulmonary effects of illicit drug use. Clin Chest Med. (2004) 25:203–16. doi: 10.1016/s0272-5231(03)00137-0
28. Bravo-Gutiérrez OA, Falfán-Valencia R, Ramírez-Venegas A, Sansores RH, Ponciano-Rodríguez G, Pérez-Rubio G. Lung damage caused by heated tobacco products and electronic nicotine delivery systems: a systematic review. Int J Environ Res Public Health. (2021) 18(8):4079. doi: 10.3390/ijerph18084079
Keywords: marijuana, pneumomediastinum, marijuana-associated pneumomediastinum, esophagram, barotrauma
Citation: Yu I, Tung K, Dugan R, Qaqish RT and Perry Y (2023) Dedicated esophageal imaging may be unnecessary in marijuana-associated spontaneous pneumomediastinum: Findings from a retrospective cohort study. Front. Surg. 10:1043729. doi: 10.3389/fsurg.2023.1043729
Received: 14 September 2022; Accepted: 24 January 2023;
Published: 16 February 2023.
Edited by:
Mohsen Ibrahim, Sapienza University of Rome, ItalyReviewed by:
Miao Zhang, Xuzhou Central Hospital, ChinaPhilip Ind, National Heart and Lung Institute, United Kingdom
© 2023 Yu, Tung, Dugan, Qaqish and Perry. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yaron Perry eXBlcnJ5QGJ1ZmZhbG8uZWR1
Specialty Section: This article was submitted to Thoracic Surgery, a section of the journal Frontiers in Surgery
 Irene Yu
Irene Yu Kaity Tung
Kaity Tung Ryanne Dugan2
Ryanne Dugan2 Yaron Perry
Yaron Perry