- 1Department of Gastroenterology, Sir Run Run Shaw Hospital, Medical School, Zhejiang University, Hangzhou, China
- 2Institute of Gastroenterology, Zhejiang University (IGZJU), Hangzhou, China
- 3Zhejiang University Cancer Center, Hangzhou, China
As one of the most common mesenchymal malignancies in the digestive system, gastrointestinal stromal tumors (GISTs) occur throughout the alimentary tract with diversified oncological characteristics. With the advent of the tyrosine kinase inhibitor era, the treatment regimens of patients with GISTs have been revolutionized and GISTs have become the paradigm of multidisciplinary therapy. However, surgery resection remains recognized as the potentially curative management for the radical resection and provided with favorable oncological outcomes. The existing available surgery algorithms in clinical practice primarily incorporate open procedure, and endoscopic and laparoscopic surgery together with combined operation techniques. The performance of various surgery methods often refers to the consideration of risk evaluation of recurrence and metastases; the degree of disease progression; size, location, and growth pattern of tumor; general conditions of selected patients; and indications and safety profile of various techniques. In the present review, we summarize the fundamental principle of surgery of GISTs based on risk assessment as well as tumor size, location, and degree of progress with an emphasis on the indications, strengths, and limitations of current surgery techniques.
1. Introduction
Gastrointestinal stromal tumors (GISTs) are rare mesenchymal subepithelial tumors in the alimentary canal with an estimated incidence worldwide of 1–2 per 100,000 (1, 2). As an oncological clinical entity, they were characterized as a virtually indolent property and could progress to highly aggressive malignancies (3). The anatomical position of GISTs nearly covers the complete gastrointestinal tract, primarily the stomach (50%–60%) followed by the small intestine (20%–30%), while they are infrequent in the colorectum and esophagus and rare in extragastrointestinal sites (mesentery and omentum) (4). GISTs are supposed to arise from the spindle-shaped interstitial cells of Cajal (ICCs) situated in the muscularis propria (MP) layer, known as pacemaker cells responsible for peristalsis, or their precursors (4, 5), with potential tumorigenesis referring to gain-of-function oncogenic activating mutation of receptor tyrosine protein kinase encoded by KIT or platelet-derived growth factor receptor alpha (PDGFRA) gene (6). Clinical manifestation of GISTs vary from asymptomatic incidental findings to palpable presentation including bleeding, obstruction, perforation, or abdominal mass (7). Histopathological biopsy and immunohistochemical analysis are of great significance to the diagnosis of GISTs (8). Therapeutic algorithms of GISTs incorporate surgical operation, endoscopy, interventional therapy, and medication, of which surgical resection of the entire tumor is considered to be the exclusive effective way to be completely curative for resectable primaries (9, 10). After the approval of imatinib mesylate by the Food and Drug Administration (FDA) of the United States in 2002 for molecular targeted medication, the availability of tyrosine kinase inhibitor (TKI) revolutionized the therapeutic management of GISTs, and GISTs have become the paradigm of multidisciplinary and multimodal therapy with reference to gastroenterologists, oncologists, and pathology specialists (11–13). Unfortunately, recurrence and metastasis remain common despite the remarkable efficacy of TKI therapy. (14). A growing number of investigations have demonstrated the safety and prognosis-improving benefits of surgery even in the metastatic scenario and updated original operative techniques (15). The present review aims to give an overview of updated surgical management in GISTs based on risk evaluation, progress degree, tumor size, and tumor location with introduction of modalities in the process of exploration.
2. Risk evaluation
The management modality of GISTs is dependent on preoperative risk evaluation with diversified regimens varying from procedures to chemotherapy being based on the location, size, and aggressiveness of GISTs. There are noteworthy limitations of the TNM staging system for assessing progression risk of GISTs, which is not regularly indicated in clinical practice (7). Tumor size and mitotic index were well-recognized prognosis indicators for resectable primaries of which tumor size in dimension <2 cm and mitotic count <5 per 50 high power fields (HPFs) are considered very low risk and tumor size in dimension >10 cm or mitotic count >10 per 50 HPFs are considered high risk in 2001 National Institutes of Health (NIH) taxonomies based on expert consensus (16). Considering patients with GISTs located in extragastric sites present with considerably higher risk of disease recurrence in comparison with those with gastric GISTs, another risk criterion for localized GISTs was developed by the Armed Forces Institute of Pathology (AFIP) with an additional incorporation of tumor location (Table 1) (17). Subsequently, modified NIH criteria, including mitotic count, tumor size, and location, especially tumor rupture, came into being as demanded under the situation where preoperative tumor rupture was found to be independently correlated with dismal recurrence-free survival (RFS) of GIST patients (Table 2) (18). Considering that tumor size and mitotic count are nonlinear continuous indices, precluding the accurate calibration of cutoff criteria, several prognostic contour maps, and nomogram, where these variables included in nonlinear modeling, have been proposed for the optimization of risk classification, which were validated to be more applicable for risk assessment of progressive aggravation (19, 20). Considering convenience and feasibility for clinical application in the Asian population, Chinese consensus guidelines for diagnosis and management of GISTs recommend modified NIH criteria for risk evaluation (21). However, above-mentioned AFIP criteria were suggested by the National Comprehensive Cancer Network of the United States (NCCN) and the European Society for Medical Oncology (ESMO) guidelines due to wide availability and prediction accuracy (22, 23). There are several limitations of existent risk classification criteria, which fail to perfectly predict metastasis and recurrence hazard for GISTs. Take SDH-deficient GISTs as an example, mitotic index was negatively associated with the risk of liver metastases but with a relatively extended period to develop metastases (21). Collectively, the establishment of new risk criteria-based prospective multicenter series is still warranted and the effective selection of multiple classifications for individualized patients under specific clinical circumstances is recommended for further formulation of treatment regimens.
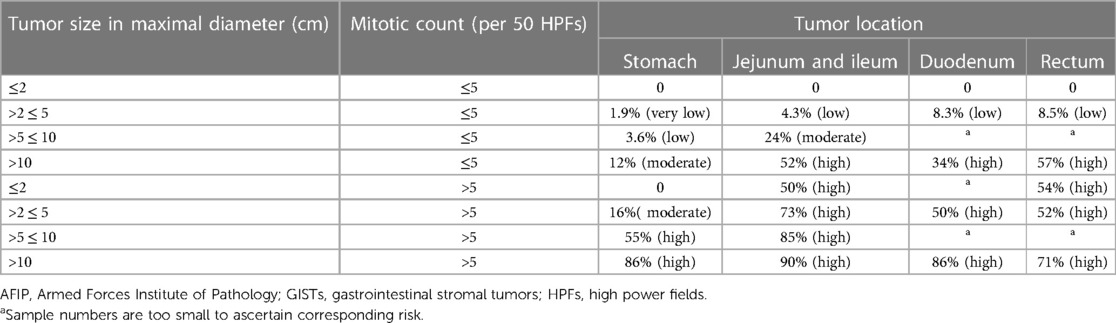
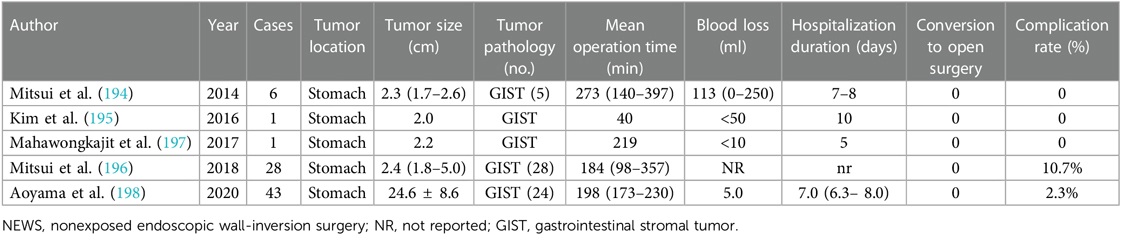
Table 1. AFIP criteria for risk evaluation of metastasis or recurrence or tumor-related death for patients with primary GISTs (17).
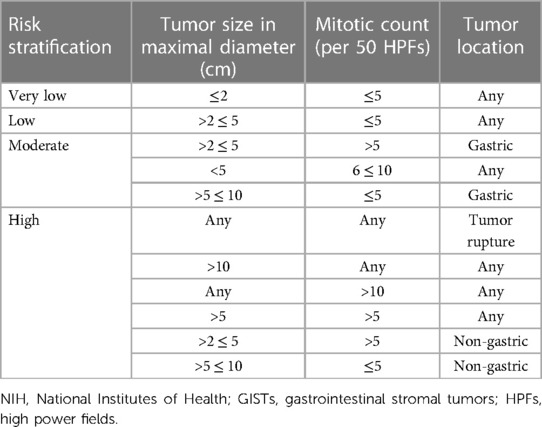
Table 2. Modified NIH criteria for risk evaluation of metastasis or recurrence or tumor-related death for patients with primary GISTs (18).
3. Surgical management in gastrointestinal stromal tumors
3.1. Surgical management stratified by tumor stages
An overview of the management algorithm of primary and advanced/metastatic GISTs is summarized in Figure 1. The detailed surgical managements based on different tumor stages are as follows.
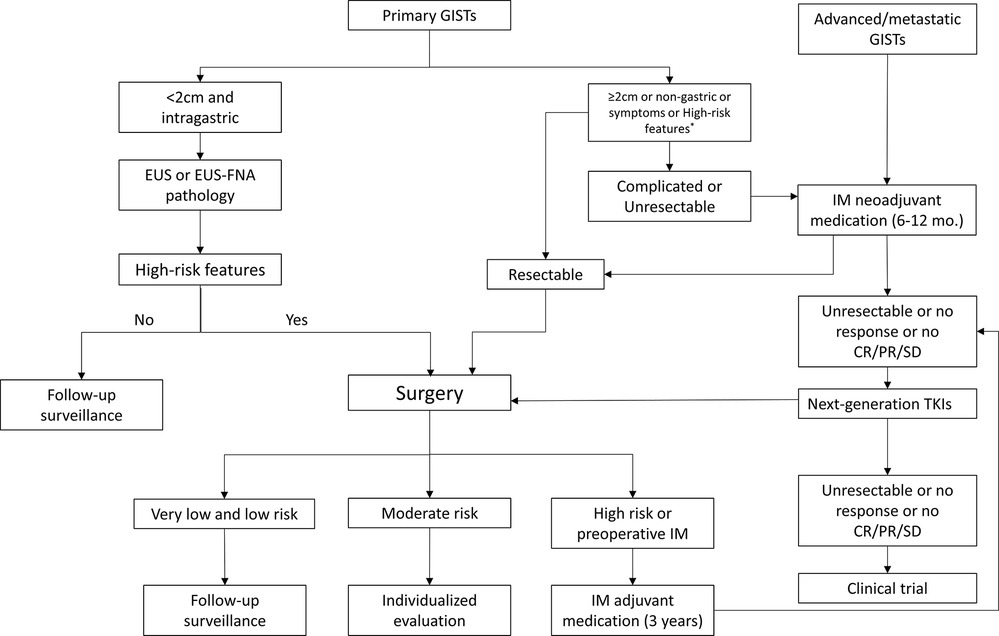
Figure 1. Management algorithm of primary and advanced/metastatic GISTs. GISTs, gastrointestinal stromal tumors; EUS, endoscopic ultrasonography; EUS-FNA, EUS-guided fine needle aspiration; IM, imatinib mesylate; TKIs, tyrosine kinase inhibitors; CR, Complete remission; PR, partial response; SD, stable disease; high-risk features refer to irregular and unclear border, echogenic foci, high mitotic rate, cystic degeneration, strong echo, ulcer, hemorrhage, and heterogeneity.
3.1.1. Localized primary GISTs
Preoperative or intraoperative biopsy for pathological identification is not a prerequisite unless presurgical molecular diagnosis is necessary for targeted medication, which is not routinely recommended in cases with removable primaries to avoid the potential possibility of tumor rupture, bleeding, and diffusion (11). Among multiple biopsy approaches, endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) is mostly used in clinical practice with distinct advantages of accuracy, safety, and relatively low possibility of dissemination. Potential limitations are inevitable considered that it is only applicable for GISTs situated in the lumen and fewer samples often lead to uncertainty of pathological diagnosis (24–26). Postoperative pathology report is of great value for the confirmation of diagnosis (27).
Surgery indications often refer to non-gastric GISTs, tumor size larger than or equal to 2 cm, or symptoms presented such as abdominalgia and gastrointestinal bleeding without regard to tumor size and location. Even though GISTs are located in the stomach with size less than 2 cm, attention should be paid on the risk under EUS or pathology for further determination of the implementation of operation. Higher risk tends to include irregular and unclear border, echogenic foci, high mitotic rate, cystic degeneration, strong echo, ulcer, hemorrhage, and heterogeneity (21, 28, 29). Without the presentation of these manifestations, regular follow-up surveillance is recommended.
It has been well-recognized that radical surgical resection is the first-line cornerstone and mainstay for the treatment of localized primary GISTs, which is the exclusive way for potentially thorough cure (30, 31). Surgery should be performed by experienced surgical specialists when open surgery or laparoscopy management was planned (7). The fundamental principle is to carefully complete en bloc removal of the tumor entity securing a sufficient histopathologically negative margin (R0 resection) achieving minor complications without damage of organ function and violation of tumor pseudocapsule prior to or during procedure as much as possible, which might contribute to accidental tumor perforation and rupture with tremendous risk of intraperitoneal implantation and spreading of tumor cells. To this end, “no touch, less compression” code and “extract bag” approach are available (21). Tumor excision incorporating peripheral tissues, whenever and wherever necessary and possible, in the case surrounding tissues or organs, is involved so as to realize the principle of R0 resection (7, 28). R0 resection is considered as the well-recognized favorable prognosis indicator for patients with localized primary GISTs (32). R1 resection (microscopically positive margins) is inclined to the development of tumor relapse, which occurs practically in all cases with tumor rupture (33). With respect to the situation where preoperative medication is unavailable or shows obscure benefits in patients with R0 resection that implicated major sequelae, R1 resection is recommended to be taken into consideration with no demonstration of correlation with dismal outcome especially in low-risk cases (23, 34). In addition, reoperation is a substitute for discussion as an individualized management after unexpected R1 resection and R2 excision (macroscopic incomplete excision) (7, 35). Inconsistent with surgical treatment of other malignancies, the proportion of lymphatic dissemination is comparatively diminutive and extensive lymphadenectomy is not regularly indicated with an exception of proof-positive lymphadenopathy, which implicated SDH-deficient genotyping especially in pediatric GISTs (27, 36, 37).
3.1.2. Localized advanced GISTs
Preoperative neoadjuvant molecular targeted chemotherapy with TKIs like imatinib was indicated in situations where R0 resection is laborious to perform accompanied by alarmingly significant surgery risk and morbidity and increased possibility of perioperative complication like tumor rupture and bleeding or common postoperative functional sequelae, which will seriously affect organ function (23, 38). More often than not, potential occasion refers to large tumor size (>10 cm in maximal diameter) or difficult and unfavorable tumor sites located in operatively challenging areas such as the esophagus, esophagogastric junction (EGJ), duodenum, and rectum (39–41).
The rationale is that relatively large tumor size is reported to be positively correlated with the decreased rate of radical removal and increased risk of tumor recurrence (42). Consequently, shrinkage of tumor volume by neoadjuvant administration is of great significance for subsequent surgery, which not only contributes to improving the success rate of R0 resection and function preservation of involved organ and normal tissues to the greatest extent, as the avoidance of extended total gastrectomy and preservation of the anal sphincter, but also improve the 5-year overall survival (OS) and progression-free survival (PFS) (43–45). Simultaneously, genotyping tests prior to TKI therapy are essential for the identification of types of targeted drugs, and vigilant focus should be paid on imatinib-insensitive tumors such as PDGFRA D842 V mutations (46). Prior to the operation, neoadjuvant therapy should be conducted for 6–12 months so as to realize maximal chemotherapy outcome (29, 35). With respect to the embarking moment of surgery, the ESMO–EURACAN guidelines recommend to stop neoadjuvant therapy just before surgery and resume immediately after patients’ postoperative recovery (23). Further potent evidence based on large-scale multicenter clinical trials for the consolidation of efficaciousness of preoperative neoadjuvant medication remains to be developed.
Apart from aforementioned GISTs population with preoperative neoadjuvant chemotherapy with TKI, postoperative adjuvant therapy is often indicated as an indispensable part of standard treatment. Pertinent scenarios mainly refer to those categorized as “high risk” of recurrence and metastasis according to various classification criteria and those with R1 resection (11). According to a long-term randomized multicenter clinical trial, the postsurgical administration of TKI adjuvant chemotherapy was recommended for at least 3 years (47). Individualized evaluation is often recommended for moderate-risk patients to discuss whether to carry out adjuvant chemotherapy or follow-up surveillance (35).
3.1.3. Recurrent, metastatic, or unresectable GISTs
Even though the tumor is completely removed through operation and TKI therapy presents amenable effectiveness, recurrence and metastasis are fairly commonplace, especially in high-risk patients with adverse 5-year OS and relapse-free survival (RFS) (48). Systemic therapy with TKI targeted medication based on genotyping is the first therapy and gold standard in recurrent, metastatic, or inoperable circumstances, and surgery is not generally recommended as a primary choice but considered as an available alternative and non-contraindication (28, 41, 49). As gross tumor volume (GTV) of GISTs is investigated to be positively correlated with tumor progression and negatively associated with the survival outcome of patients, debulking surgery and cytoreduction potentially decreased the likelihood of imatinib-resistant clones emerging, and the risk of secondary mutations has become a significant component of management in patients with recurrence or metastasis scenario (50, 51). Of note, debulking surgery is found to be efficacious in outcome improvement for most TKI-respondent patients and should be conducted before tumor progression (51, 52). Postoperatively, complete removal of tumor along with TKI therapy provides preferable outcomes for patients with more effectiveness and minimal complications and side effects compared with TKI alone (49, 53). As mentioned above, for those administrated with preprocedural TKI neoadjuvant regimens, adjuvant chemotherapy after surgery needs to be resumed as soon as possible (35). Specifically, patients who develop disease progression with TKI resistance will not benefit from identical TKI regimen used preoperatively, and postsurgical genetic test that is dedicated to identifying further therapeutic target is of vital value under this circumstance (41).
The indication of surgical administration in selected patients is as follows: better responding with imatinib therapy and tumor transfer from unresectable to resectable entity with minor operation-associated risk in residual lesions; localized progression with TKI resistance after targeted therapy including both sunitinib second-line therapy (54) and regorafenib third-line therapy (55) in addition to first-line treatment with imatinib (56); advanced tumor with a relatively small gross tumor volume of which lesion foci is evaluated to be completely removed through procedure; relatively limited and isolated metastatic foci or superficial lesions with operative feasibility; excision in cases with high risk of rupture necrotic metastases foci; patients in generally good condition and surgical tolerability; symptoms such as uncontrollable hemorrhage, perforation, and obstruction or acute pain with palliative emergency operation being necessary (28, 35). Surgical management for patients with general and disseminated progression following TKI medication is absolutely inadvisable, which could not present favorable survival benefits (21, 35).
Liver is one of the most common metastatic sites of GISTs; transcatheter arterial chemoembolization (TACE) and radiofrequency ablation (RFA) play a palliative adjunctive role in tumor control with feasibility and safety in addition to surgery (57–59). However, RFA is inclined to be suitable for tumors with a maximum diameter of 3 cm and is contraindicated in patients in whom tumor is contiguous with large vessels or supersedes liver capsule (7). Efforts has been made for the establishment of successful cases of liver transplantation but prospective research are still limited, and this management regimen is not regularly considered in clinical practice with scrupulous discussion processing required by multidisciplinary specialists for comprehensive prognosis evaluation of patients and conducted in experienced transplant centers (60, 61).
Taken together, multidisciplinary cooperation should be taken into consideration for the evaluation of feasibility of debulking operation and metastasectomy for patients with recurrent or metastatic GISTs and provide them with individualized treatment algorithm-integrated systemic medication with localized palliative procedure for further outcome improvement. If surgery is planned to be conducted, with the premise of ensuring R0 or R1 excision as much as possible, scrupulous consideration of patient selection refers to feasibility and complexity of operation, the degree of disease progression, the involvement of adjacent organs, the patient's age, physical condition, possible complications, postoperative recovery time, benefits, and risks.
3.2. Surgical management in tumor location subgroups
3.2.1. Esophageal GISTs
Esophageal GISTs are comparably rare and ordinarily occur in the esophagogastric junction (62). Patients with relatively larger GISTs that are situated in the esophagogastric junction could benefit from TKI therapy followed by localized resection with avoidance of traumatic total gastrectomy, realizing organ function preservation to the greatest extent. Once the diagnosis of GISTs is established, surgery is suggested to be conducted immediately for such special anatomic location with operation difficulty (21). Choosing either complete esophagectomy or enucleation procedure is primarily based on the tumor size, location, and surgery-related risk (63). The enucleation technique could be a feasible alternative for relatively smaller and posterior wall GISTs, while radical esophagectomy is indicated for large tumors (4).
3.2.2. Gastric GISTs
The gastric subgroup constitutes a majority of occurrence sites for GISTs. As mentioned above, on the premise of the absence of risk presentation under EUS, gastric GISTs with GTV of less than 2 cm without tumor-related symptom is indicated for follow-up surveillance. Surgery is recommended for cases inconsistent with these scenarios such as those in extragastric sites.
Due to the possibility of associated risk of local recurrences, enucleation is not generally recommended (35). However, in order to preserve normal organ function, enucleation approach could be tried with regard to small GISTs situated in the posterior wall (4). GISTs with a tumor size ≤4 cm located in the stomach are reported to benefit from resection by endoscopic techniques with adjuvant therapy or additional operation, when necessary, based on risk evaluation while superiority is not shown in cases with tumor size >4 cm in which surgery is often necessary (64). Surgical resection approaches of gastric GISTs primarily incorporate wedge resection (WR) or larger resection securing a margin of 1–2 cm (65). In the case where wedge resection is not feasible, segmental resection is an appropriate option referring Biliroth I, Biliroth II, and total gastrectomy with gastroduodenostomy, gastrojejunostomy, or Roux-en-Y reconstruction (4). Partial gastrectomy or total gastrectomy rather than proximal gastrectomy is recommended in GISTs located near the cardia or its extension (28, 35). Located in relatively favorable anatomic sites, greater curvature, or anterior wall of gastric body with tumor size of <5 cm is indicated for the application of laparoscopic procedure with safety, feasibility, less invasiveness, and comparable outcome compared to open surgery (21, 66, 67), and laparoscopic–endoscopic hybrid partial gastrectomy is a successful surgical method that secures complete resection without excessive violation of the normal anatomical structure (68). Simultaneously, this endoscopy-assisted laparoscopic operation is a recommendable alternative of open gastrectomy for gastric GISTs with feasibility and safety profile (69).
3.2.3. Duodenum GISTs
Duodenum GISTs account for a practically small proportion of all GISTs with its second part, descending part, being the most preferred site (70). Operative procedure to completely remove tumor securing an adequate margin remains to be the optimal choice for radical cure in localized primaries with resectable feasibility. However, the complex and special anatomical position of the duodenum itself, which is contiguous with the head of pancreas, common bile duct, pancreatic duct, mesenteric blood vessels, and ampulla of Vater combined with relatively insufficient procedural experience owing to its rarity, brings about enormous challenge for performing the surgery (71, 72). The available operative approaches based on diverse instruments primarily consist of open surgery, endoscopy, laparoscopy, and hybrid surgery such as endo–laparoscopic cooperative procedure. Open surgery is applicable in most cases after feasibility and safety evaluation especially in ampulla of Vater and pancreas involved complex cases in which pancreaticoduodenectomy (PD), known as Whipple resection, is ordinarily necessary but with enormous invasiveness and damage to normal organ function (73, 74). Segmental intestinal resection can be performed when the tumor size is small and lesion is remote from the essential ampulla location. Followed by presurgical neoadjuvant TKI administration, limited resection instead of PD is often accessible, which decreases compromising the proximal structures due to large invasiveness. Limited resection approaches often incorporate WR and segmental duodenum resection with intestinal anastomosis according to the lesion site and extension (75). Endoscopic resection remains challenging and is intractable to securely perform with a comparable risk of nonradical resection and tumor rupture although there are advances in endoscopy techniques identically due to anatomic characteristics of the duodenum (76). In recent years, laparoscopy and endo–laparoscopic approaches combined hybrid surgery have been explored in the operative management of GISTs, with predominant advantages of minimal invasiveness, decreased perioperative complications, and long-term survival (77–80). Consequently, meticulous consideration should be taken referring to size, location, and invasiveness to adjacent structures for the individualized evaluation of optimal procedure option.
3.2.4. Jejunum and ileum GISTs
The small intestine is the second most common site of gastrointestinal stromal tumors consisting of nearly a third of all GISTs (81). Open operation is ordinary practice while laparoscopic surgery should be taken into consideration for exploring and locating the corresponding lesion (21). In comparison with laparotomy, however, laparoscopic surgery is reported to assume less operation duration and less postsurgical complications as there was no significant between-group difference in oncological outcome according to a meta-analysis (82). Similarly, a retrospective review found that laparoscopic operation is safe and effective comparable to open operation in oncological prognosis and preferable in perioperative indicators (83). As for small intestine GISTs with size <10 cm in diameter, laparoscopic surgery can be recommended based on subgroup RFS analysis (84). Under normal circumstances, segmental resection with end-to-end intestinal anastomosis is recommended according to French clinical practice guidelines (35).
3.2.5. Colon GISTs
Consistent with their incidence in the esophagus and the duodenum, GISTs situated in the colorectum are similarly rare. Compared with the left colon, GISTs tend to occur in the right side (85). All colon GISTs are recommended to be surgically resected regardless of tumor size and malignancy degree (86). Segmental resection with end anastomosis is the frequently used operative approach parallel with GISTs in the small intestine (35). Unsophisticated laparoscopic appendectomy is often applicable for particularly rare appendiceal GISTs (4).
3.2.6. Rectum GISTs
GISTs located in rectum together with rectal vaginal space are strongly recommended for complete resection regardless of the tumor size since there are comparable risks for postsurgical recurrence and metastasis once diagnosis of GISTs is established (7) and enucleation is not regularly recommended (35). Considering that surgical difficulty and possibility of multivisceral resection will significantly increase with the enlargement of tumor size, operation should perform as early as possible (21).
There are different operative methods based on corresponding tumor location with respect to rectum GISTs. Low anterior resection and end-to-end intestinal anastomosis along with temporary colostomy are applicable for the upper section of the rectum with adequate distance to the anal sphincter. As for GISTs located in the lower rectum adjacent to the anal sphincter, abdominoperineal combined resection, known as Miles’ operation, with permanent sigmoid colostomy are commonly performed (4). Research reported that transanal full-thickness resection (FTR) presents more benefits concerning lower rectum GISTs and should be discussed when referring to minor tumor size (65). Original sphincter-sparing operation approaches with less invasiveness have been explored such as transanal endoscopic operation (TEO) (87) and transanal minimally invasive surgery (TAMIS) (88). Laparoscopic resection is another applicable option for lesions located in the upper rectum of <2 cm but not regularly recommended when the tumor increases in size (21). Preoperative neoadjuvant chemotherapy is necessary for the preservation of sphincter function as much as possible by tumor shrinkage with the improvement of outcomes (89). A multicenter cohort research performed by Wang et al. (90) was investigated to the compare oncologic outcomes in patients with low rectum GISTs between a local resection cohort and a radical resection cohort; they found that local resection presented a significant superiority in sphincter preservation, minimizing operation time. and postoperative complications compared to radical resection. However, in terms of tumor size >2 cm, there was a preferable survival outcome in the radical resection cohort.
3.2.7. Extragastrointestinal GISTs
Actually, a great number of extragastrointestinal GISTs (EGISTs) reported are metastases foci of primary GISTs. Mesentery and omentum are common tumor sites of genuine primary EGISTs (91, 92). Other rare and unusual locations are pancreas (93), prostate (94), and pleura (95). With rare and aggressive malignancy, patients with EGISTs often have comparatively poor survival outcome, classified as high-risk lesions (96). Early identification and en bloc surgical excision as complete as possible remain the preferred treatment (97). Risk and cost-effectiveness of treatment regimens should be taken into account, and surgery combined with systemic neoadjuvant or adjuvant medication are often necessary (98) Cytoreductive debulking surgery might act as a potential palliative therapeutic strategy for symptomatic remission (99). Laparoscopy has been used for the surgical practice in EGISTs and achieved successful resection of tumor mass and oncological outcome as an available procedure option (100).
3.3. Surgical management in tumor size subgroups
GISTs with tumor size <2 cm are classified as small GISTs (101). More detailed subgroup categories include micro-GIST defined as GISTs <1 cm and mini GIST with size between 1 and 2 cm (102). Unless there are manifestations of high-risk characteristics based on EUS and biopsy, which warrant surgical management, the routine treatment for small GISTs is periodical follow-up surveillance with endoscopy and radiography (102). GISTs with size measuring equal to or larger than 2 cm (non-small GISTs) are recommended for surgical administration. With respect to those measuring over 5 cm accompanied with the presence or of symptoms or not, surgery is strongly indicated irrespective of whether the pathological diagnosis of GISTs is established or not (28).
Endoscopic resection is especially useful and safe for <2 cm GISTs. As for GISTs with 2–5 cm in size, enucleation, such as endoscopic submucosal dissection (ESD), is an available option ensuring complete excision but with risk of recurrence (28). As mentioned above, based on a long-term follow-up evaluation by Zhang et al., 4.0 cm might act as a threshold for choosing endoscopic resection in gastric GISTs stemmed from the MP layer with lower risk; however, surgery is still recommended for the population with increased risk (64). Laparoscopic resection also shows preferable outcomes and is recommended for GISTs between 2 and 5 cm, especially in easily accessible anatomic sites according to Chinese consensus guidelines (21, 103). A single-center long-term retrospective study was conducted for the comparison between surgery and endoscopy in the treatment of 2–5 cm GISTs. There were more complications and reoperation rates found in the endoscopic group compared to the surgery group with parallel outcome, and surgery, especially laparoscopic resection, is recommended more often (104). A size-matched comparison between laparoscopy and laparotomy found that gastric GISTs with size ≤8 cm might benefit more from laparoscopy based on oncologic outcomes (105). However, concerning relatively lager tumors (>5 cm) with excision needing larger incision, laparoscopic operation is not advocated due to pertinent tumor dissemination and open surgery is often encouraged (21). Patients with high risk, such as tumor size >10 cm in diameter or tumor size >5 cm plus mitotic count >5 per 50 HPFs, will benefit from neoadjuvant and adjuvant chemotherapy and achieve sound postsurgical oncological outcome.
4. Advances in laparoscopic operation
Under the premise of technical feasibility and the inexistence of operation contraindications, laparoscopy presents safe, efficient, and comparable postoperative oncological outcomes in selected patients with GISTs located in operationally facile sites like gastric and small bowel and with small size compared with open surgery (81, 106).
Recently, robotic surgical systems have been rapidly developed and received incremental interests. With kinetic stability, ergonomic design, and operation accuracy, they provide clinical surgeons with three-dimensional views and minimize the occurrence of tremors, thus reducing unnecessary tissue trauma as well as tumor manipulation and realizing the principle of minimally invasive surgery (107). Several reported research studies have confirmed the technical feasibility and safety of robot-assisted laparoscopic resection and suture reconstruction for the management of GISTs located in the upper gastrointestinal tract, especially for unfavorably positioned and relatively large series, which require more professional skills to avoid the risk of tumor rupture (108). Additionally, the Da Vinci Robot System has been introduced for optimization in laparoscopic operation, which is often preferred in complex and technically demanding cases (109–111). Researches of robot-assisted laparoscopic surgery are summarized in Table 3.
5. Advances in endoscopy techniques
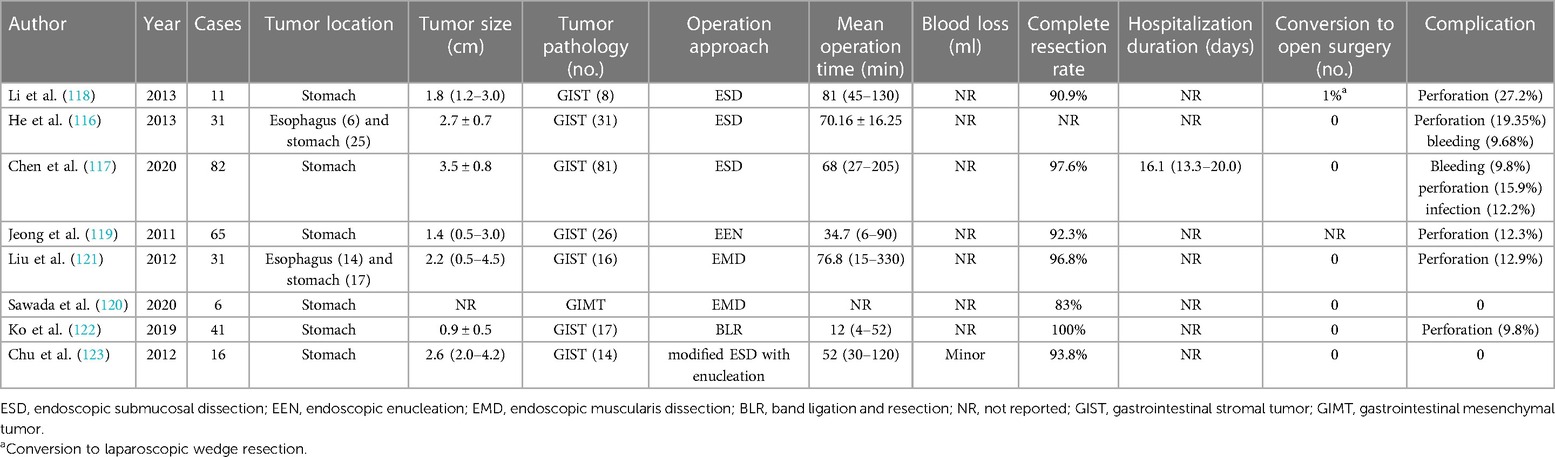
Different from gastrointestinal epithelial tumors, it is the occurrence site of GISTs that confine the application of endoscopy for the evaluation of tumor features and further resection. Of note, there are comparatively high perforation and incomplete excision rates during endoscopic operation especially in GISTs with larger size or extraluminal involvement (115). However, with the increasing maturity of endoscopy, several original endoscopic approaches have been investigated for the treatment of GISTs. According to research exploring conventional ESD for esophagus and stomach GISTs with 2–5 cm in size, the perforation rate was around 20%, and no recurrence or metastasis was observed (116). The high perforation rate remains challenging and fundus was identified as the risk location of complications (117, 118). Based on the consideration of limitation of conventional ESD, several modified techniques have been developed (Table 4). Endoscopic enucleation (EEN) is validated to be an effective method for resection (92.3%, 60 of 64) but without the avoidance of higher perforation in the fundus (119). Derived from ESD approach, the endoscopic muscularis dissection (EMD) procedure presents a sufficient complete resection rate for gastrointestinal mesenchymal tumors originating from the MP layer (120). Inconsistent with the circumferential incision in ESD, a longitudinal incision was performed followed by electrical or blunt dissection and clips closing. Although complete resection was achieved at 96.8%, there was a higher risk of perforation than that in ESD (121). Band ligation and resection (BLR) is another operation option of EEN assuming a comparably high resection rate (41 of 41) and nearly 10% (4 of 41) perforation (122). A modified ESD with enucleation was introduced for removing GISTs, and no serious complications were reported (123).
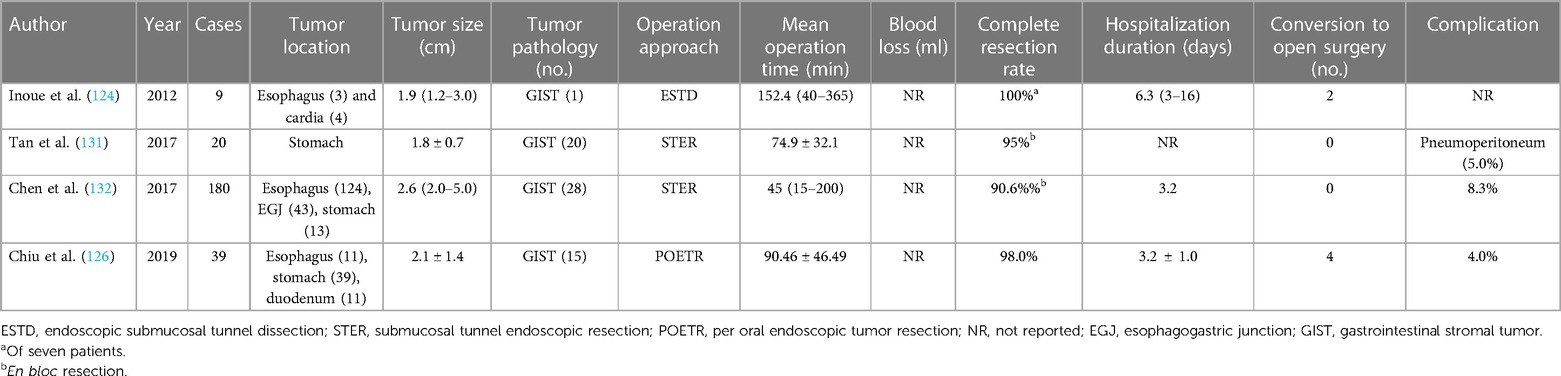
In order to preserve the integrity of the mucosa and avoid pertinent perforation, strictures, and scars induced by endoscopic procedure, endoscopic submucosal tunnel dissection (ESTD) (124), also known as submucosal tunnel endoscopic resection (STER) (125) or per oral endoscopic tumor resection (POETR) (126), has been introduced for the treatment of GISTs originating from the MP layer based on the peroral endoscopic myotomy (POEM) (127) approach (Table 5). Following the creation of mucosal entrance proximal to the tumor, approximately 5 cm, a tunnel between the submucosa and MP layer was developed and the tumor was completely removed. Endoclips were employed to seal off the entrance. ESTD is evaluated to be a curative treatment option for GISTs with less invasiveness and apparent postoperative complications (128). However, due to the instinct difficulty of tunnel development in thick stomach mucosa, the majority of ESTD were performed in the esophagus or esophagogastric junction with insufficient efficacy validation in the stomach (129, 130). Moreover, limited tunneling space will preclude the en bloc resection of large GISTs with the routine criterion of <4 cm (126, 130).
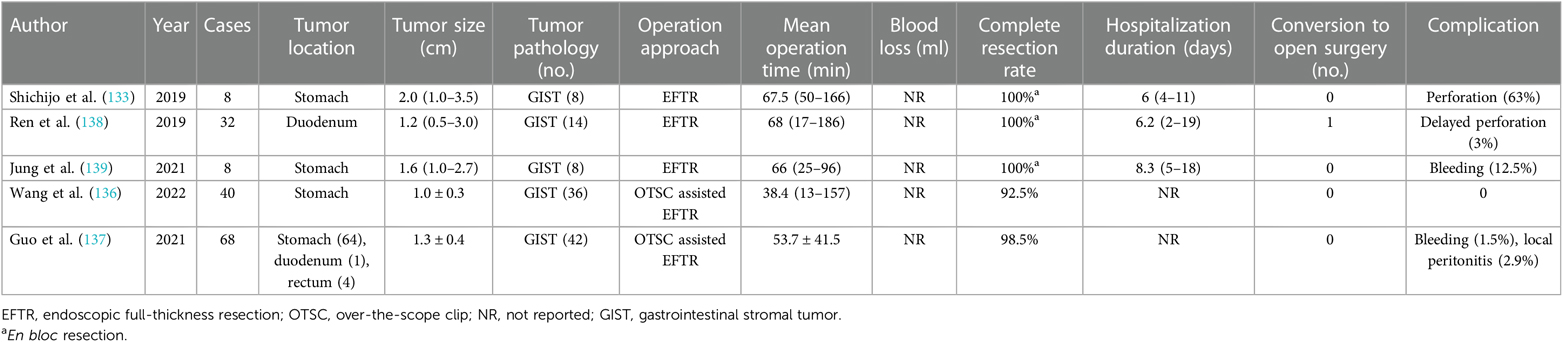
Endoscopic full-thickness resection (EFTR) cooperated with the full-thickness suturing technique will contribute to realizing radical resection of gastric GISTs located in the deep MP layer with endoscopically manageable complications and sound oncological outcomes, which is applicable to GISTs up to 4 cm and those located in all anatomical positions of the stomach (133). A dedicated full-thickness resection device for EFTR was applied in a 60-year-old patient with GIST with the advantage of protecting the peritoneal cavity from bowel contents (134). Another new technique called the clip-with-line traction-assisted preclosure assisted in EFTR was reported in a 47-year-old man with fundal GIST and was beneficial to preventing GIST falling into the abdominal cavity and the development of related complications such as peritonitis (135). Over-the-scope clip (OTSC) is an applicable device for the assistance of EFTR and verified with 100% excision success rate in treating GISTs with safety and effectiveness, which is especially recommended for a tumor size of <2 cm (136, 137). Researches of EFTR techniques are summarized in Table 6.
6. Advances in laparoscopy–endoscopy cooperative techniques
Since endoscopic operation alone is faced with certain limitations, such as demanding skills of experienced endoscopists and high risk of procedural complications, the application of laparoscopy performed in the process of endoscopy plays a significant role in decreasing the perforation rate and improving the complete resection rate especially in relatively large tumors in size and has been expanded into clinical practice. Based on the roles of the two kinds of procedures in the operation process, operation modalities of these cooperative techniques primarily incorporate laparoscopy-assisted endoscopic surgery (LAES), endoscope-assisted laparoscopic surgery (EALS), and integrated laparoscopy–endoscopy cooperative surgery (LECS). With respect to LAES and EALS, one technique presents the basic role with the assistance of the other. However, laparoscopy and endoscopy teams cooperate with each other for the resection of lesion in LECS with essential significance rather than assistance.
6.1. Laparoscopy-assisted endoscopic surgery
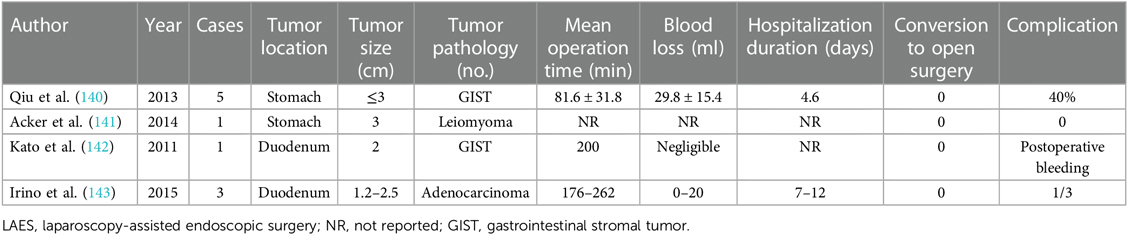
Endoscopy plays a substantial role in LAES while laparoscopy provides backup and real-time control (Table 7). In the research by Qiu et al. (140), LAES, reported as laparoscopy-assisted endoscopic resection (LAER), was applied for resecting GISTs ≤3 cm in diameter. EMR or ESD was performed under endoscopy. The laparoscopy team facilitates the exposure and localization of the lesion from the perspective of peritoneal cavity. Through providing traction, lesions could be easily removed by endoscopy, especially with technical difficulty such as lesion located near the EGJ (141). Simultaneously, any complications such as perforation and bleeding that occur perioperatively could be treated immediately by laparoscopy. Controllable complications and no recurrence were observed. Apart from the stomach, lesions situated in the duodenum could also benefit from LAES with feasibility (142, 143).
6.2. Endoscope-assisted laparoscopic surgery
The lesion is removed by laparoscopic surgery with an endoscope contributing to the orientation and exposure of the mass. According to the various lesion locations and access approaches of laparoscopy, EALS mainly consists of endoscope-assisted wedge resection (EAWR), endoscope-assisted laparoscopic trans-gastric resection (EATR), and endoscope-assisted laparoscopic intragastric surgery (LIGS).
6.2.1. Endoscope-assisted wedge resection
Table 8 summarizes corresponding researches of EAWR technique. With real-time monitoring, locating, and marking done by endoscopists, the lesion was removed by conventional laparoscopic wedge resection using a linear endoscopic gastrointestinal stapler. Subsequently, the mass resected was retrieved through the laparoscope followed by endoscopic examination of the existence of residual lesion and potential complications. EAWR is applicable for lesion not only in the anterior wall of the stomach but also in the posterior gastric wall, EGJ, and pyloric ring, which is more recommendable for an ultrasonic shear device or a vascular sealing system to avoid pertinent damage or stenosis (144, 145). More often than not, lesions situated in the posterior wall of the gastric body, limited to the central sections, were recommended for EAWR, which were easily accessible through the mentum or gastrocolic ligament by creating a small incision (146). In addition, the lesion with exophytic characteristic is another indication for EAWR.
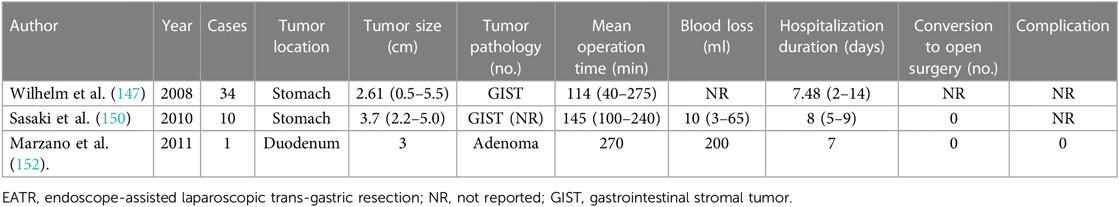
6.2.2. Endoscope-assisted laparoscopic trans-gastric resection
EATR is more applicable for lesions located in the posterior gastric wall with a relatively large size or with intraluminal growth or near the EGJ (150, 151) (Table 9). As mentioned above, lesions located in the posterior gastric wall of the fundus or antrum instead of the body were preferred for this approach (146). With the assistance of endoscopic identification of the lesion location by palpation and diaphanoscopy, gastrotomy of the anterior gastric wall was performed by a laparoscopic team and the corresponding lesion was exposed. Subsequently, routine inverted laparoscopic wedge resection or full-thickness resection was conducted. Finally, the defect in the anterior gastric wall was closed using endoscopic stapler devices or laparoscopic sutures. Lesions located in the posterior of the duodenum were also indicated for applying EATR with robotic assistance. Based on the comparison analyses of Marzano et al., EAWR was recommended as the first choice for most gastric submucosal tumors (SMTs) than EATR due to gastrotomy and related complications such as increased operative time and blood loss, digestive fluid leakage, and the risk of abdominal cavity spread (152).
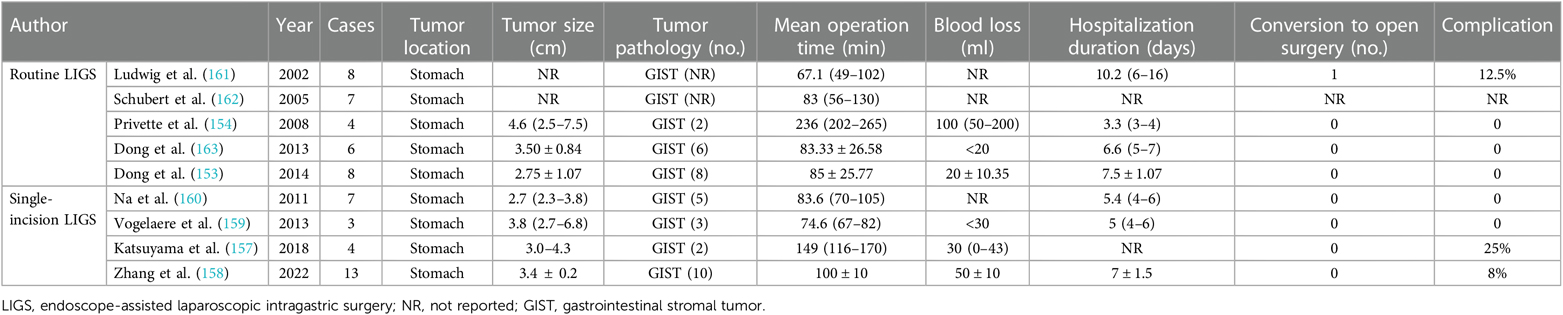
6.2.3. Endoscope-assisted laparoscopic intragastric surgery
Endoscopy played a significant role in identifying lesion location, and then several laparoscopic trocars penetrated both the abdominal wall and the anterior stomach wall into the gastric cavity. Stay sutures could facilitate the lift of the anterior gastric wall to the abdominal wall. With the real-time monitoring and assistance of endoscopy during the operation, the lesion was resected through wedge resection or full-thickness resection by the laparoscopic team. Finally, the lesion specimen resected was retrieved perorally, and perforation and defect were closed by endoscopists and the laparoscopic team. Gastrotomy was performed when necessary, concerning large specimen with difficulty to be retrieved though the mouth (153, 154).
LIGS was first reported by Ohashi in 1995 and often recommended in lesions situated in the posterior gastric wall with intraluminal growth and relatively small tumor size (155, 156). Compared to EATR, LIGS is safer and with decreased blood loss and postsurgical complications through gastric perforation instead of gastrotomy.
In recent years, a modified LIGS technique, known as single-incision LIGS (sLIGS), has been reported by researchers. Only a 3 cm longitudinal incision was made near the umbilicus and a wound-protecting device was placed. Following a mini-size gastrotomy, three to four ports were placed through a single port device in the single incision. The lesion resection was similar with conventional LIGS and defect of gastrotomy and abdominal wall were closed. The indications for sLIGS were parallel to those of the routine LIGS technique. Of note, single incisions often correlated with higher perioperative security and decreased complications (157–160). Researches of LIGS technique are summarized in Table 10.
6.3. Integrated LECS
6.3.1. Classical LECS
LECS has been reported to successfully resect gastric submucosal GISTs, which is known as classical LECS (Table 11). First, ESD was performed via intraluminal endoscopy for circumferentially dissecting three quarters of the tumor submucosa. Next, the seromuscular layer was dissected by laparoscopy along the corresponding cut line, and the tumor was laparoscopically resected by a stapling device. There are several advantages of this technique, according to researchers’ opinion, such as it is independent of the tumor location without excessive sections of healthy tissues (164, 165). Theoretically, the application of the LECS technique is not restricted by the size of the tumor as the lesion is retrieved through the abdominal wall. However, LECS is shown to be more applicable for relatively small GISTs (166) and not recommended in large (> 5 cm) or ulcerative cases due to the increased risk of peritoneal contamination and tumor dissemination (167). Based on the research by Ri et al., subepithelial tumor located in the esophagogastric junction has a risk of conversing operation procedure from LECS to proximal gastrectomy, which is safer (168).
6.3.2. Laparoscopy-assisted endoscopic full-thickness resection
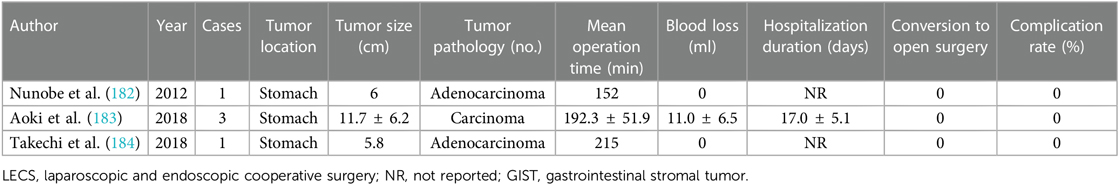
With the assistance of laparoscopy, the EFTR procedure achieved considerable improvement of avoiding excessive resection of normal tissues, and an original cooperative surgery based on the principles of LECS, called laparoscopy-assisted endoscopic full-thickness resection (LAEFR), has been developed by Abe et al. (175) (Table 12). Described as a hybrid natural orifice transluminal endoscopic surgery (NOTES), ESD followed by EFTR was endoscopically performed for the resection of 2/3 to 3/4 of tissue with the assistance of a laparoscopic team, which facilitates the exposure, and then the remaining tumor was completely resected and retrieved either perorally or through a laparoscope. The gastric wall defect was then hand-sewn and closed by laparoscopy. Apart from the accurate and complete removal of the tumor, the advantages of LAEFR incorporate minimal invasiveness, inexpensiveness in comparison with other laparoscopic surgery, and less perioperative adverse events managed via laparoscopic therapy. It is noteworthy that the closure of the artificial gastric wall perforation is easier and safer by means of laparoscopy compared to endoscopy (176). This technique is especially applicable for the resection of GISTs located in the MP layer with intraluminal growth modality (177).
6.3.3. Inverted LECS
In 2019, there was a case report in which a patient with remnant stomach GIST received inverted LECS for full-thickness resection with sound postoperative outcome (180) (Table 13). The brief operation modality of inverted LECS is described as follows. The first step is the identification of resection line and circumferential elevation of the gastric wall like a crown using stitches. Then, the seromuscular layer is dissected after artificial perforation followed by conducting EFTR. A .laparoscopic stapler is used to dissect the residual gastric wall, and tumor tissue is perorally retrieved. The final step is the defect suture by laparoscopic devices (181). In general, inverted LECS is performed with the traction inversion of tumor toward the intragastric cavity as a crown. There is, however, still relatively lower risk of gastric content spillage during this technique owing to gastric lumen exposure. Moreover, not all sites were feasible for inverted LECS such as the posterior wall.
6.3.4. “Nonexposure” LECS techniques
For the purpose of the reduction of tumor cells seeding into the abdominal cavity, several newer innovative “nonexposure” techniques, originated from the classical LECS procedure for full-thickness resection, named as a combination of laparoscopic and endoscopic approaches to neoplasia applying nonexposure technique (CLEAN-NET), nonexposed endoscopic wall-inversion surgery (NEWS), and closed-LECS, have been developed.
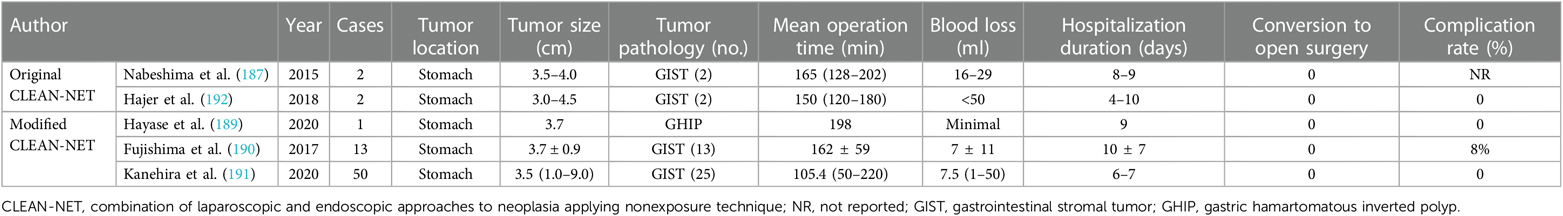
6.3.4.1. Combination of laparoscopic and endoscopic approaches to neoplasia applying nonexposure technique
Inoue et al. took initiative to report the CLEAN-NET technique for the resection of a gastric neoplasm in 2012 (185). The corresponding procedures incorporate mucosal marking by endoscopy followed by the fixation of the mucosal layer into the seromuscular layer by four full-thickness stay sutures via laparoscopy. The next step is seromuscular layer dissection using a laparoscopic electrocautery knife and full-layer tumor dissection with pulling-out performed by a laparoscopic linear stapler, sealing the specimen into a protective mucosal “net.” It is obvious that the CLEAN-NET procedure is less invasive and has the capability of completely preventing between-luminal communication and thus consequent tumor dissemination and bacterial contamination. However, potential limitations refer to the risk of incomplete resection with positive margin, mucosal laceration, and incision line determination (80, 186, 187). To ensure the normal operation of the mucosal mechanical barrier and prevent mucosa tear, a tumor <3 cm in size is recommended for the application of this technique (181). Moreover, tumors located in technically demanding and inaccessible sites with the risk of deformity of the stomach, such as EGJ, the pyloric ring, the lesser curvature, and posterior wall, restrict the operation of CLEAN-NET due to potential risk of postoperative stenosis (188). A modified CLEAN-NET technique was introduced for the improvement of resection of gastric submucosal tumor especially in technically demanding locations and large cases (>3 cm), which secure the surgical field with anchor sutures and decrease stomach deformation and pertinent complications (189–191). Researches of conventional and modified CLEAN-NET technique are summarized in Table 14.
6.3.4.2. Nonexposed endoscopic wall-inversion surgery
In 2011, the NEWS technique was first reported by Goto et al. (193) and developed as another novel nonexposure LECS, apart from CLEAN-NET, for full-thickness resection of gastric SMT without artificial perforation that avoids tumor seeding. Steps of NEWS primarily consist of marking mucosal and serosal surface around the tumor endoscopically and laparoscopically. Next, endoscopists inject sodium hyaluronate with an indigo carmine dye into the submucosal layer, which is beneficial to the following circumferential seromuscular dissection around the tumor by the laparoscopic team. Subsequently, tumor is inverted and suture closure of the seromuscular layer is performed laparoscopically. Finally, circumferential muco-submucosal incision of the intruded tumor is performed by endoscopists and tumor specimen is retrieved perorally followed by mucosal closure (80, 181, 194). Besides the benefit that precludes interluminal communication, NEWS could achieve an accurate determination of the resection line (195). However, it is obvious that the NEWS technique is restrained by tumor size as tumors with a relatively larger size (>3 cm) are laborious to be retrieved through the mouth. Simultaneously, tumor locations such as demanding EGJ and the pyloric ring also limit its application (181). NEWS is also found to be time-consuming; as reported by Mitsui et al., the median operation time in 28 patients with gastric GIST was 184 min (196). Researches of NEWS technique are summarized in Table 15.
6.3.4.3. Closed-LECS
Closed-LECS is also a completely non-open technique, which was reported by Kikuchi et al. for the resection of gastric SMTs (199). The detailed operation incorporates the following steps: routine ESD technique is performed after the submucosal injection. Subsequently, the incision line is marked by laparoscopy around the serosal surface followed by seromuscular suturing while inverting tumor into intragastric cavity. After the circumferential dissection of serosal muscular layer done by the endoscopic team, the lesion is removed perorally. Closed-LECS is more applicable for intraluminal and small GISTs with identical limitation of tumor size (<3 cm) for peroral approach (28). The operation process of the closed-LECS technique is roughly similar to NEWS with tumors all being retrieved perorally. Of note, endoscopic circumferential seromuscular incision is specifically performed in NEWS while not in closed-LECS (186). Researches of Closed-LECS technique are summarized in Table 16.
An overview and comparison of classical and modified LECS techniques are shown in Table 17. Collectively, a majority of newer innovated techniques are investigated in gastric neoplasm with little application reports in GISTs. The classical LECS technique will not cause mucosal defects and is independent of the size and location of the tumor, but there is a risk of abdominal spread. Modified LECS can prevent the tumor dissemination but is limited by the tumor size, location, and technical requirements. Individualized evaluation is necessary for selected patients for optimal operation approach. And further research studies in the GIST population are warranted to be investigated.
7. Conclusions
GISTs have been recognized as the paradigm of multidisciplinary and multimodal therapy integrating surgical resection with TKI neoadjuvant and adjuvant chemotherapy. Preoperative or postoperative molecularly targeted medication in a high-risk cohort could substantially contribute to the subsequent surgery resection and function preservation of the involved organ with improved survival outcome. Operation with complete resection is the mainstay for the management of patients with GISTs in clinical practice, which is often indicated in those with non-gastric GISTs, tumor size ≥2 cm, palpable symptoms, or EUS-related pathological risk. The advance of endoscopic and laparoscopic techniques, such as ESTD, EFTR, EAWR, and especially modified nonexposure LECS techniques have practically improved the success rate of operation, realized minimal invasiveness and more safety, and reduced perioperative complications. Robotic surgical systems are attractive treatment candidates for challenging cases. Tumor resection, to some extent, could be conducted and provide some earnings for properly selected patients with indications in recurrent and metastatic situation to improve their survival, but the benefits and risks should be considered comprehensively. Individualized evaluation from the multidisciplinary team and elaborative consideration of treatment algorithm for each patient are warranted. Further research studies in the GIST population are warranted.
Author contributions
LY and WH contributed to the conception and design. All authors contributed to the writing of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by a grant from the Zhejiang Medical Association (No. 2019ZYC-A88).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
GISTs, gastrointestinal stromal tumors; ICCs, interstitial cells of Cajal; TKI, tyrosine kinase inhibitor; NIH, National Institutes of Health; AFIP, Armed Forces Institute of Pathology; NCCN, National Comprehensive Cancer Network of the United States; ESMO, European Society for Medical Oncology; HPFs, high power fields; EUS-FNA, endoscopic ultrasound-guided fine needle aspiration; OS, overall survival; PFS, progression-free survival; RFS, recurrence-free survival; GTV, gross tumor volume; TACE, transcatheter arterial chemoembolization; PD, pancreaticoduodenectomy; WR, wedge resection; FTR, full-thickness resection; EGJ, esophagogastric junction; EGISTs, extragastrointestinal GISTs; ESD, endoscopic submucosal dissection; EEN, endoscopic enucleation; EMD, endoscopic muscularis dissection; BLR, band ligation and resection; ESTD, endoscopic submucosal tunnel dissection; STER, submucosal tunnel endoscopic resection; POETR, per oral endoscopic tumor resection; POEM, peroral endoscopic myotomy; NR, not reported; EFTR, endoscopic full-thickness resection; OTSC, over-the-scope clip; LAES, laparoscopy-assisted endoscopic surgery; EALS, endoscope-assisted laparoscopic surgery; LECS, laparoscopy–endoscopy cooperative surgery; EAWR, endoscope-assisted wedge resection; EATR, endoscope-assisted laparoscopic trans-gastric resection; LIGS, endoscope-assisted laparoscopic intragastric surgery; sLIGS, single-incision LIGS; LAEFR, laparoscopy-assisted endoscopic full-thickness resection; NOTES, natural orifice transluminal endoscopic surgery; CLEAN-NET, combination of laparoscopic and endoscopic approaches to neoplasia applying nonexposure technique; NEWS, nonexposed endoscopic wall-inversion surgery.
References
1. Mantese G. Gastrointestinal stromal tumor: epidemiology, diagnosis, and treatment. Curr Opin Gastroenterol. (2019) 35:555–9. doi: 10.1097/MOG.0000000000000584
2. Hemming ML, Heinrich MC, Bauer S, George S. Translational insights into gastrointestinal stromal tumor and current clinical advances. Ann Oncol. (2018) 29:2037–45. doi: 10.1093/annonc/mdy309
3. von Mehren M, Joensuu H. Gastrointestinal stromal tumors. J Clin Oncol. (2018) 36:136–43. doi: 10.1200/JCO.2017.74.9705
4. Ahmed M. Recent advances in the management of gastrointestinal stromal tumor. World J Clin Cases. (2020) 8:3142–55. doi: 10.12998/wjcc.v8.i15.3142
5. Hemming ML, Coy S, Lin JR, Andersen JL, Przybyl J, Mazzola E, et al. HAND1 and BARX1 act as transcriptional and anatomic determinants of malignancy in gastrointestinal stromal tumor. Clin Cancer Res. (2021) 27:1706–19. doi: 10.1158/1078-0432.CCR-20-3538
6. Joensuu H, Wardelmann E, Sihto H, Eriksson M, Sundby Hall K, Reichardt A, et al. Effect of KIT and PDGFRA mutations on survival in patients with gastrointestinal stromal tumors treated with adjuvant imatinib: an exploratory analysis of a randomized clinical trial. JAMA Oncol. (2017) 3:602–9. doi: 10.1001/jamaoncol.2016.5751
7. Judson I, Bulusu R, Seddon B, Dangoor A, Wong N, Mudan S. UK clinical practice guidelines for the management of gastrointestinal stromal tumours (GIST). Clin Sarcoma Res. (2017) 7:1–10. doi: 10.1186/s13569-017-0072-8
8. El-Menyar A, Mekkodathil A, Al-Thani H. Diagnosis and management of gastrointestinal stromal tumors: an up-to-date literature review. J Cancer Res Ther. (2017) 13:889–900. doi: 10.4103/0973-1482.177499
9. Callegaro D, Roland CL, Raut CP. Relevant trials update in sarcomas and gastrointestinal stromal tumors: what surgeons should know. Surg Oncol Clin N Am. (2022) 31:341–60. doi: 10.1016/j.soc.2022.03.002
10. Gheorghe G, Bacalbasa N, Ceobanu G, Ilie M, Enache V, Constantinescu G, et al. Gastrointestinal stromal tumors—a mini review. J Pers Med. (2021) 11:694. doi: 10.3390/jpm11080694
11. Tu L, Hohenberger P, Allgayer H, Cao H. Standard approach to gastrointestinal stromal tumors—differences between China and Europe. Visc Med. (2018) 34:353–8. doi: 10.1159/000494347
12. Kelly CM, Gutierrez Sainz L, Chi P. The management of metastatic GIST: current standard and investigational therapeutics. J Hematol Oncol. (2021) 14:1–12. doi: 10.1186/s13045-020-01026-6
13. Bauer S, George S, von Mehren M, Heinrich MC. Early and next-generation KIT/PDGFRA kinase inhibitors and the future of treatment for advanced gastrointestinal stromal tumor. Front Oncol. (2021) 11:672500. doi: 10.3389/fonc.2021.672500
14. Chen T, Ye LY, Feng XY, Qiu HB, Zhang P, Luo YX, et al. Performance of risk stratification systems for gastrointestinal stromal tumors: a multicenter study. World J Gastroenterol. (2019) 25:1238–47. doi: 10.3748/wjg.v25.i10.1238
15. Yonkus JA, Alva-Ruiz R, Grotz TE. Surgical management of metastatic gastrointestinal stromal tumors. Curr Treat Options Oncol. (2021) 22:37. doi: 10.1007/s11864-021-00837-0
16. Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol. (2002) 33:459–65. doi: 10.1053/hupa.2002.123545
17. Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. (2006) 23:70–83. doi: 10.1053/j.semdp.2006.09.001
18. Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. (2008) 39:1411–9. doi: 10.1016/j.humpath.2008.06.025
19. Joensuu H, Vehtari A, Riihimaki J, Nishida T, Steigen SE, Brabec P, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. (2012) 13:265–74. doi: 10.1016/S1470-2045(11)70299-6
20. Gold JS, Gonen M, Gutierrez A, Broto JM, Garcia-del-Muro X, Smyrk TC, et al. Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localised primary gastrointestinal stromal tumour: a retrospective analysis. Lancet Oncol. (2009) 10:1045–52. doi: 10.1016/S1470-2045(09)70242-6
21. Li J, Ye Y, Wang J, Zhang B, Qin S, Shi Y, et al. Chinese Consensus guidelines for diagnosis and management of gastrointestinal stromal tumor. Chin J Cancer Res. (2017) 29:281–93. doi: 10.21147/j.issn.1000-9604.2017.04.01
22. Demetri GD, Benjamin RS, Blanke CD, Blay JY, Casali P, Choi H, et al. NCCN task force report: management of patients with gastrointestinal stromal tumor (GIST)—update of the NCCN clinical practice guidelines. J Natl Compr Canc Netw. (2007) 5(S2):S1–29, quiz S30. doi: 10.6004/jnccn.2007.2002
23. Casali PG, Abecassis N, Aro HT, Bauer S, Biagini R, Bielack S, et al. Gastrointestinal stromal tumours: ESMO-EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2018) 29:iv68–78. doi: 10.1093/annonc/mdy095
24. Dumonceau JM, Polkowski M, Larghi A, Vilmann P, Giovannini M, Frossard JL, et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy. (2011) 43:897–912. doi: 10.1055/s-0030-1256754
25. Nishida T, Blay JY, Hirota S, Kitagawa Y, Kang YK. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer. (2016) 19:3–14. doi: 10.1007/s10120-015-0526-8
26. Akahoshi K, Sumida Y, Matsui N, Oya M, Akinaga R, Kubokawa M, et al. Preoperative diagnosis of gastrointestinal stromal tumor by endoscopic ultrasound-guided fine needle aspiration. World J Gastroenterol. (2007) 13:2077–82. doi: 10.3748/wjg.v13.i14.2077
27. Demetri GD, von Mehren M, Antonescu CR, DeMatteo RP, Ganjoo KN, Maki RG, et al. NCCN task force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw. (2010) 8(S2):S1–S41, quiz S42–44. doi: 10.6004/jnccn.2010.0116
28. Sugiyama Y, Sasaki M, Kouyama M, Tazaki T, Takahashi S, Nakamitsu A. Current treatment strategies and future perspectives for gastrointestinal stromal tumors. World J Gastrointest Pathophysiol. (2022) 13:15–33. doi: 10.4291/wjgp.v13.i1.15
29. Sharma AK, Kim TS, Bauer S, Sicklick JK. Gastrointestinal stromal tumor: new insights for a multimodal approach. Surg Oncol Clin N Am. (2022) 31:431–46. doi: 10.1016/j.soc.2022.03.007
30. Casali PG, Jost L, Reichardt P, Schlemmer M, Blay JY; ESMO Guidelines Working Group. Gastrointestinal stromal tumours: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. (2009) 20(S4):64–7. doi: 10.1093/annonc/mdp131
31. Schmidt T, Ghadimi M, Fuchs HF, Bruns CJ. Surgical and interdisciplinary treatment of gastrointestinal stromal tumors. Chirurg. (2022) 93:27–33. doi: 10.1007/s00104-021-01527-1
32. DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. (2000) 231:51–8. doi: 10.1097/00000658-200001000-00008
33. Hohenberger P, Ronellenfitsch U, Oladeji O, Pink D, Strobel P, Wardelmann E, et al. Pattern of recurrence in patients with ruptured primary gastrointestinal stromal tumour. Br J Surg. (2010) 97:1854–9. doi: 10.1002/bjs.7222
34. McCarter MD, Antonescu CR, Ballman KV, Maki RG, Pisters PW, Demetri GD, et al. Microscopically positive margins for primary gastrointestinal stromal tumors: analysis of risk factors and tumor recurrence. J Am Coll Surg. (2012) 215:53–9, discussion 59–60. doi: 10.1016/j.jamcollsurg.2012.05.008
35. Landi B, Blay JY, Bonvalot S, Brasseur M, Coindre JM, Emile JF, et al. Gastrointestinal stromal tumours (GISTs): French intergroup clinical practice guidelines for diagnosis, treatments and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO). Dig Liver Dis. (2019) 51:1223–31. doi: 10.1016/j.dld.2019.07.006
36. Rinelli M, Agolini E, Milano GM, Russo I, Crocoli A, De Vito R, et al. Pediatric gastrointestinal stromal tumor: report of two novel patients harboring germline variants in SDHB and SDHC genes. Cancer Genet. (2020) 241:61–5. doi: 10.1016/j.cancergen.2019.12.002
37. Boikos SA, Pappo AS, Killian JK, LaQuaglia MP, Weldon CB, George S, et al. Molecular subtypes of KIT/PDGFRA wild-type gastrointestinal stromal tumors: a report from the National Institutes of Health gastrointestinal stromal tumor clinic. JAMA Oncol. (2016) 2:922–8. doi: 10.1001/jamaoncol.2016.0256
38. Cananzi FC, Judson I, Lorenzi B, Benson C, Mudan S. Multidisciplinary care of gastrointestinal stromal tumour: a review and a proposal for a pre-treatment classification. Eur J Surg Oncol. (2013) 39:1171–8. doi: 10.1016/j.ejso.2013.08.030
39. Lee SY, Goh BK, Sadot E, Rajeev R, Balachandran VP, Gonen M, et al. Surgical strategy and outcomes in duodenal gastrointestinal stromal tumor. Ann Surg Oncol. (2017) 24:202–10. doi: 10.1245/s10434-016-5565-9
40. Cavnar MJ, Wang L, Balachandran VP, Antonescu CR, Tap WD, Keohan M, et al. Rectal gastrointestinal stromal tumor (GIST) in the era of imatinib: organ preservation and improved oncologic outcome. Ann Surg Oncol. (2017) 24:3972–80. doi: 10.1245/s10434-017-6087-9
41. Schaefer IM, DeMatteo RP, Serrano C. The GIST of advances in treatment of advanced gastrointestinal stromal tumor. Am Soc Clin Oncol Educ Book. (2022) 42:1–15. doi: 10.1200/EDBK_351231
42. Dematteo RP, Gold JS, Saran L, Gonen M, Liau KH, Maki RG, et al. Tumor mitotic rate, size, and location independently predict recurrence after resection of primary gastrointestinal stromal tumor (GIST). Cancer. (2008) 112:608–15. doi: 10.1002/cncr.23199
43. Tielen R, Verhoef C, van Coevorden F, Gelderblom H, Sleijfer S, Hartgrink HH, et al. Surgical treatment of locally advanced, non-metastatic, gastrointestinal stromal tumours after treatment with imatinib. Eur J Surg Oncol. (2013) 39:150–5. doi: 10.1016/j.ejso.2012.09.004
44. Tielen R, Verhoef C, van Coevorden F, Reyners AK, van der Graaf WT, Bonenkamp JJ, et al. Surgical management of rectal gastrointestinal stromal tumors. J Surg Oncol. (2013) 107:320–3. doi: 10.1002/jso.23223
45. Kurokawa Y, Yang HK, Cho H, Ryu MH, Masuzawa T, Park SR, et al. Phase II study of neoadjuvant imatinib in large gastrointestinal stromal tumours of the stomach. Br J Cancer. (2017) 117:25–32. doi: 10.1038/bjc.2017.144
46. Al-Share B, Alloghbi A, Al Hallak MN, Uddin H, Azmi A, Mohammad RM, et al. Gastrointestinal stromal tumor: a review of current and emerging therapies. Cancer Metastasis Rev. (2021) 40:625–41. doi: 10.1007/s10555-021-09961-7
47. Joensuu H, Eriksson M, Sundby Hall K, Reichardt A, Hermes B, Schutte J, et al. Survival outcomes associated with 3 years vs 1 year of adjuvant imatinib for patients with high-risk gastrointestinal stromal tumors: an analysis of a randomized clinical trial after 10-year follow-up. JAMA Oncol. (2020) 6:1241–6. doi: 10.1001/jamaoncol.2020.2091
48. Wang L, Xu W, Yao X, Yan C, Li C, Zhu Z, et al. Analysis of clinical features and prognostic factors on reoperation patients with postoperative recurrence or metastasis of gastrointestinal stromal tumor. Zhonghua Wei Chang Wai Ke Za Zhi. (2018) 21(11):1274–9. PMID: 30506539.30506539
49. Du CY, Zhou Y, Song C, Wang YP, Jie ZG, He YL, et al. Is there a role of surgery in patients with recurrent or metastatic gastrointestinal stromal tumours responding to imatinib: a prospective randomised trial in China. Eur J Cancer. (2014) 50:1772–8. doi: 10.1016/j.ejca.2014.03.280
50. Chang SC, Liao CH, Wang SY, Tsai CY, Chiang KC, Cheng CT, et al. Feasibility and timing of cytoreduction surgery in advanced (metastatic or recurrent) gastrointestinal stromal tumors during the era of imatinib. Medicine (Baltimore). (2015) 94:e1014. doi: 10.1097/MD.0000000000001014
51. Fairweather M, Balachandran VP, Li GZ, Bertagnolli MM, Antonescu C, Tap W, et al. Cytoreductive surgery for metastatic gastrointestinal stromal tumors treated with tyrosine kinase inhibitors: a 2-institutional analysis. Ann Surg. (2018) 268:296–302. doi: 10.1097/SLA.0000000000002281
52. Bauer S, Rutkowski P, Hohenberger P, Miceli R, Fumagalli E, Siedlecki JA, et al. Long-term follow-up of patients with GIST undergoing metastasectomy in the era of imatinib—analysis of prognostic factors (EORTC-STBSG collaborative study). Eur J Surg Oncol. (2014) 40:412–9. doi: 10.1016/j.ejso.2013.12.020
53. Xia L, Zhang MM, Ji L, Li X, Wu XT. Resection combined with imatinib therapy for liver metastases of gastrointestinal stromal tumors. Surg Today. (2010) 40:936–42. doi: 10.1007/s00595-009-4171-x
54. Yeh CN, Wang SY, Tsai CY, Chen YY, Liu CT, Chiang KC, et al. Surgical management of patients with progressing metastatic gastrointestinal stromal tumors receiving sunitinib treatment: a prospective cohort study. Int J Surg. (2017) 39:30–6. doi: 10.1016/j.ijsu.2017.01.045
55. Yeh CN, Hu CH, Wang SY, Wu CE, Chen JS, Tsai CY, et al. Cytoreductive surgery may be beneficial for highly selected patients with metastatic gastrointestinal stromal tumors receiving regorafenib facing local progression: a case controlled study. J Cancer. (2021) 12:3335–43. doi: 10.7150/jca.50324
56. Kikuchi H, Setoguchi T, Miyazaki S, Yamamoto M, Ohta M, Kamiya K, et al. Surgical intervention for imatinib and sunitinib-resistant gastrointestinal stromal tumors. Int J Clin Oncol. (2011) 16:741–5. doi: 10.1007/s10147-011-0208-4
57. Yoon IS, Shin JH, Han K, Kim PN, Kim KH, Kang YK, et al. Ultrasound-guided intraoperative radiofrequency ablation and surgical resection for liver metastasis from malignant gastrointestinal stromal tumors. Korean J Radiol. (2018) 19:54–62. doi: 10.3348/kjr.2018.19.1.54
58. Chen Q, Li C, Yang H, Zhao H, Zhao J, Bi X, et al. Radiofrequency ablation versus resection for resectable liver metastases of gastrointestinal stromal tumours: results from three national centres in China. Clin Res Hepatol Gastroenterol. (2019) 43:317–23. doi: 10.1016/j.clinre.2018.10.012
59. Cao G, Li J, Shen L, Zhu X. Transcatheter arterial chemoembolization for gastrointestinal stromal tumors with liver metastases. World J Gastroenterol. (2012) 18:6134–40. doi: 10.3748/wjg.v18.i42.6134
60. Fernandez JA, Alconchel F, Gomez B, Martinez J, Ramirez P. Unresectable GIST liver metastases and liver transplantation: a review and theoretical basis for a new indication. Int J Surg. (2021) 94:106126. doi: 10.1016/j.ijsu.2021.106126
61. Li H, Meng X, Zhang K, Tang H. Liver transplantation for metastatic non-resectable gastrointestinal stromal tumor after molecular targeted therapies: a case report. Int J Surg Case Rep. (2022) 95:107185. doi: 10.1016/j.ijscr.2022.107185
62. Lott S, Schmieder M, Mayer B, Henne-Bruns D, Knippschild U, Agaimy A, et al. Gastrointestinal stromal tumors of the esophagus: evaluation of a pooled case series regarding clinicopathological features and clinical outcome. Am J Cancer Res. (2015) 5:333–43. PMID: 25628942.25628942
63. Duffaud F, Meeus P, Bertucci F, Delhorme JB, Stoeckle E, Isambert N, et al. Patterns of care and clinical outcomes in primary oesophageal gastrointestinal stromal tumours (GIST): a retrospective study of the French sarcoma group (FSG). Eur J Surg Oncol. (2017) 43:1110–6. doi: 10.1016/j.ejso.2017.03.017
64. Zhang Y, Mao XL, Zhou XB, Yang H, Zhu LH, Chen G, et al. Long-term outcomes of endoscopic resection for small (</= 4.0 cm) gastric gastrointestinal stromal tumors originating from the muscularis propria layer. World J Gastroenterol. (2018) 24:3030–7. doi: 10.3748/wjg.v24.i27.3030
65. von Mehren M. Management of gastrointestinal stromal tumors. Surg Clin North Am. (2016) 96:1059–75. doi: 10.1016/j.suc.2016.05.011
66. Chen K, Zhou YC, Mou YP, Xu XW, Jin WW, Ajoodhea H. Systematic review and meta-analysis of safety and efficacy of laparoscopic resection for gastrointestinal stromal tumors of the stomach. Surg Endosc. (2015) 29:355–67. doi: 10.1007/s00464-014-3676-6
67. Koh YX, Chok AY, Zheng HL, Tan CS, Chow PK, Wong WK, et al. A systematic review and meta-analysis comparing laparoscopic versus open gastric resections for gastrointestinal stromal tumors of the stomach. Ann Surg Oncol. (2013) 20:3549–60. doi: 10.1245/s10434-013-3051-1
68. Ntourakis D, Michalinos A, Schizas D. Hybrid laparoscopic and endoscopic partial gastrectomy for ulcerated GIST: surgical technique with video. World J Surg. (2020) 44:202–6. doi: 10.1007/s00268-019-05192-8
69. Huang JL, Zheng ZH, Wei HB, Chen TF, Liu JP, Huang Y, et al. Endoscopy-assisted laparoscopic resections for gastric gastrointestinal stromal tumors: a retrospective study. J Laparoendosc Adv Surg Tech A. (2017) 27:110–4. doi: 10.1089/lap.2016.0068
70. El-Haddad HM, Kassem MI, Shehata GA, El-Sayes IA. Outcome after surgical treatment of gastrointestinal stromal tumors in the second part of duodenum: is localized resection appropriate? J Invest Surg. (2022) 35:814–20. doi: 10.1080/08941939.2021.1968081
71. Fu X, Wang X, Xiong J, Yao Y, Tan C, Liu X. Surgical strategies for duodenal gastrointestinal stromal tumors. Langenbecks Arch Surg. (2022) 407:835–44. doi: 10.1007/s00423-022-02460-5
72. Lim KT. Current surgical management of duodenal gastrointestinal stromal tumors. World J Gastrointest Surg. (2021) 13:1166–79. doi: 10.4240/wjgs.v13.i10.1166
73. Vassos N, Perrakis A, Hohenberger W, Croner RS. Surgical approaches and oncological outcomes in the management of duodenal gastrointestinal stromal tumors (GIST). J Clin Med. (2021) 10:4459. doi: 10.3390/jcm10194459
74. Tien YW, Lee CY, Huang CC, Hu RH, Lee PH. Surgery for gastrointestinal stromal tumors of the duodenum. Ann Surg Oncol. (2010) 17:109–14. doi: 10.1245/s10434-009-0761-5
75. El-Gendi A, El-Gendi S, El-Gendi M. Feasibility and oncological outcomes of limited duodenal resection in patients with primary nonmetastatic duodenal GIST. J Gastrointest Surg. (2012) 16:2197–202. doi: 10.1007/s11605-012-2034-z
76. Gaspar JP, Stelow EB, Wang AY. Approach to the endoscopic resection of duodenal lesions. World J Gastroenterol. (2016) 22:600–17. doi: 10.3748/wjg.v22.i2.600
77. Xiong W, Xu Y, Chen T, Feng X, Zhou R, Wan J, et al. Laparoscopic vs. open surgery for gastrointestinal stromal tumors of esophagogastric junction: a multicenter, retrospective cohort analysis with propensity score weighting. Chin J Cancer Res. (2021) 33:42–52. doi: 10.21147/j.issn.1000-9604.2021.01.05
78. Ojima T, Nakamura M, Hayata K, et al. Laparoscopic limited resection for duodenal gastrointestinal stromal tumors. J Gastrointest Surg. (2020) 24:2404–8. doi: 10.1007/s11605-020-04692-6
79. Ohata K, Murakami M, Yamazaki K, Nonaka K, Misumi N, Tashima T, et al. Feasibility of endoscopy-assisted laparoscopic full-thickness resection for superficial duodenal neoplasms. ScientificWorldJournal. (2014) 2014:239627. doi: 10.1155/2014/239627
80. Ntourakis D, Mavrogenis G. Cooperative laparoscopic endoscopic and hybrid laparoscopic surgery for upper gastrointestinal tumors: current status. World J Gastroenterol. (2015) 21:12482–97. doi: 10.3748/wjg.v21.i43.12482
81. Peng F, Liu Y. Gastrointestinal stromal tumors of the small intestine: progress in diagnosis and treatment research. Cancer Manag Res. (2020) 12:3877–89. doi: 10.2147/CMAR.S238227
82. Chen K, Zhang B, Liang YL, Ji L, Xia SJ, Pan Y, et al. Laparoscopic versus open resection of small bowel gastrointestinal stromal tumors: systematic review and meta-analysis. Chin Med J (Engl). (2017) 130:1595–603. doi: 10.4103/0366-6999.208249
83. Liao CH, Yeh CN, Wang SY, Fu CY, Tsai CY, Liu YY, et al. Surgical option for intestinal gastrointestinal stromal tumors—perioperative and oncological outcomes of laparoscopic surgery. Anticancer Res. (2015) 35:1033–40. PMID: 25667491.25667491
84. Ihn K, Hyung WJ, Kim HI, An JY, Kim JW, Cheong JH, et al. Treatment results of small intestinal gastrointestinal stromal tumors less than 10 cm in diameter: a comparison between laparoscopy and open surgery. J Gastric Cancer. (2012) 12:243–8. doi: 10.5230/jgc.2012.12.4.243
85. Roşulescu A, Pechianu N, Hortopan M, Mihai M, Dima S, Stroescu C, et al. Gastrointestinal stromal tumors of the colon and rectum. Pol J Pathol. (2020) 71:200–6. doi: 10.5114/pjp.2020.99786
86. Syllaios A, Schizas D, Davakis S, Koutras A, Vailas M, Machairas N, et al. GISTs of the large intestine: review of the literature. J Buon. (2020) 25:15–22. PMID: 32277610.32277610
87. Eldamshety O, Metwally IH, Ghoneem E, Elkashef WF. Resection of rectal GIST using a novel technique: a report of two cases. Ecancermedicalscience. (2017) 11:760. doi: 10.3332/ecancer.2017.760
88. Nepal P, Mori S, Kita Y, Tanabe K, Baba K, Uchikado Y, et al. Management of a case of high-risk gastrointestinal stromal tumor in rectum by transanal minimal invasive surgery. World J Surg Oncol. (2018) 16:165. doi: 10.1186/s12957-018-1463-x
89. Yang W, Liu Q, Lin G, Zhang B, Cao H, Zhao Y, et al. The effect of neoadjuvant imatinib therapy on outcome and survival in rectal gastrointestinal stromal tumors: a multiinstitutional study. J Surg Oncol. (2021) 124:1128–35. doi: 10.1002/jso.26628
90. Wang T, Zhao Y, Wang M, Zhang P, Lin G, Liu Q, et al. Radical resection versus local excision for low rectal gastrointestinal stromal tumor: a multicenter propensity score-matched analysis. Eur J Surg Oncol. (2021) 47:1668–74. doi: 10.1016/j.ejso.2021.01.027
91. Iqbal N, Sharma A, Iqbal N. Clinicopathological and treatment analysis of 13 extragastrointestinal stromal tumors of mesentery and retroperitoneum. Ann Gastroenterol. (2015) 28:105–8. PMID: 25608620.25608620
92. Kataoka M, Saitoh T, Kawashima K, Yazaki T, Sonoyama H, Okimoto E, et al. Primary extragastrointestinal stromal tumor of greater omentum with intraperitoneal bleeding. Intern Med. (2021) 60:3413–9. doi: 10.2169/internalmedicine.6519-20
93. Yol S, Polat E, Duman M, Uzun O, Yaşar NF, Peker KD, et al. Pancreatic extragastrointestinal stromal tumor invading the duodenum. Turk J Surg. (2018) 34:231–3. doi: 10.5152/turkjsurg.2017.2715
94. Almagharbi SA, Fayoumi YA, Abdel-Meguid TA, Abdelsalam A, Bokhary RY, Azhar RA. Extragastrointestinal stromal tumor of prostate. Urol Ann. (2018) 10:416–9. doi: 10.4103/UA.UA_26_18
95. Zhang CQ, Lu DG, Liu QF, Xiao W. Primary extragastrointestinal stromal tumor of the pleura: a case report. Oncol Lett. (2016) 11:3135–8. doi: 10.3892/ol.2016.4344
96. Hu W, Zheng C, Li R, Feng X, Zheng G, Zheng Z, et al. Retroperitoneal extragastrointestinal stromal tumors have a poor survival outcome: a multicenter observational study. Cancer Manag Res. (2020) 12:10491–504. doi: 10.2147/CMAR.S278612
97. Kadel D, Bhuju S, Thapa BR, Chalise S, Kumar Sah S. Curative intent treatment of late presented extragastrointestinal stromal tumor: two identical case reports with literature review. J Surg Case Rep. (2021) 2021:rjab220. doi: 10.1093/jscr/rjab220
98. Li H, Li J, Li X, Kang Y, Wei Q. An unexpected but interesting response to a novel therapy for malignant extragastrointestinal stromal tumor of the mesoileum: a case report and review of the literature. World J Surg Oncol. (2013) 11:174. doi: 10.1186/1477-7819-11-174
99. Fernandes MR, Ghezzi CLA, Grezzana-Filho TJ, Feier FH, Leipnitz I, Chedid AD, et al. Giant hepatic extra-gastrointestinal stromal tumor treated with cytoreductive surgery and adjuvant systemic therapy: a case report and review of literature. World J Gastrointest Surg. (2021) 13:315–22. doi: 10.4240/wjgs.v13.i3.315
100. Sezgin B, Camuzcuoglu A, Camuzcuoglu H. Laparoscopic resection of an extragastrointestinal stromal tumor in the presacral area. J Minim Invasive Gynecol. (2019) 26:812–3. doi: 10.1016/j.jmig.2018.10.023
101. Fernandez JA, Gomez-Ruiz AJ, Olivares V, Ferri B, Frutos MD, Soria T, et al. Clinical and pathological features of “small” GIST (</=2 cm). What is their prognostic value? Eur J Surg Oncol. (2018) 44:580–6. doi: 10.1016/j.ejso.2018.01.087
102. Nishida T, Goto O, Raut CP, Yahagi N. Diagnostic and treatment strategy for small gastrointestinal stromal tumors. Cancer. (2016) 122:3110–8. doi: 10.1002/cncr.30239
103. Otani Y, Furukawa T, Yoshida M, Saikawa Y, Wada N, Ueda M, et al. Operative indications for relatively small (2–5 cm) gastrointestinal stromal tumor of the stomach based on analysis of 60 operated cases. Surgery. (2006) 139:484–92. doi: 10.1016/j.surg.2005.08.011
104. Lei T, Tan F, Liu H, Ouyang M, Zhou H, Liu P, et al. Endoscopic or surgical resection for patients with 2–5 cm gastric gastrointestinal stromal tumors: a single-center 12-year experience from China. Cancer Manag Res. (2020) 12:7659–70. doi: 10.2147/CMAR.S266898
105. Karakousis GC, Singer S, Zheng J, Gonen M, Coit D, DeMatteo RP, et al. Laparoscopic versus open gastric resections for primary gastrointestinal stromal tumors (GISTs): a size-matched comparison. Ann Surg Oncol. (2011) 18:1599–605. doi: 10.1245/s10434-010-1517-y
106. Chen YH, Liu KH, Yeh CN, Hsu JT, Liu YY, Tsai CY, et al. Laparoscopic resection of gastrointestinal stromal tumors: safe, efficient, and comparable oncologic outcomes. J Laparoendosc Adv Surg Tech A. (2012) 22:758–63. doi: 10.1089/lap.2012.0115
107. Grimaldi SDM, Marano A, Pellegrino L, Geretto P, Palagi S, Borghi F. Robotic wedge resection for unfavorably located gastric gastrointestinal stromal tumors: perioperative and long-term oncological outcomes. J Laparoendosc Adv Surg Tech A. (2021) 31:772–8. doi: 10.1089/lap.2020.0660
108. Maggioni C, Shida A, Mancini R, Ioni L, Pernazza G. Safety profile and oncological outcomes of gastric gastrointestinal stromal tumors (GISTs) robotic resection: single center experience. Int J Med Robot. (2019) 15:e2031. doi: 10.1002/rcs.2031
109. Dreifuss NH, Schlottmann F, Cubisino A, Bianco FM. Novel surgical approach for gastric gastrointestinal stromal tumor (GIST): robotic single port partial gastrectomy. Surg Oncol. (2022) 40:101704. doi: 10.1016/j.suronc.2021.101704
110. Marano A, Allisiardi F, Perino E, Pellegrino L, Geretto P, Borghi F. Robotic treatment for large duodenal gastrointestinal stromal tumor. Ann Surg Oncol. (2020) 27:1101–2. doi: 10.1245/s10434-019-08041-z
111. Furbetta N, Palmeri M, Guadagni S, Di Franco G, Gianardi D, Latteri S, et al. Gastrointestinal stromal tumours of stomach: robot-assisted excision with the da Vinci surgical system regardless of size and location site. J Minim Access Surg. (2018) 15:142–7. doi: 10.4103/jmas.JMAS_260_17
112. Yamamoto H, Ebihara Y, Tanaka K, Matsui A, Nakanishi Y, Asano T, et al. Robot-assisted thoracoscopic esophagectomy for gastrointestinal stromal tumor of the esophagus: a case report. Int J Surg Case Rep. (2021) 86:106335. doi: 10.1016/j.ijscr.2021.106335
113. Vicente E, Quijano Y, Ielpo B, Duran H, Diaz E, Fabra I, et al. Robot-assisted resection of gastrointestinal stromal tumors (GIST): a single center case series and literature review. Int J Med Robot. (2016) 12:718–23. doi: 10.1002/rcs.1712
114. Hirata Y, Scally C, Badgwell BD, Ikoma N. Robotic excision of gastric and duodenal gastrointestinal stromal tumor. Updates Surg. (2022) 74:1483–4. doi: 10.1007/s13304-022-01261-1
115. Kim HH. Endoscopic treatment for gastrointestinal stromal tumor: advantages and hurdles. World J Gastrointest Endosc. (2015) 7:192–205. doi: 10.4253/wjge.v7.i3.192
116. He Z, Sun C, Zheng Z, Yu Q, Wang T, Chen X, et al. Endoscopic submucosal dissection of large gastrointestinal stromal tumors in the esophagus and stomach. J Gastroenterol Hepatol. (2013) 28:262–7. doi: 10.1111/jgh.12056
117. Chen Q, Yu M, Lei Y, Zhong C, Liu Z, Zhou X, et al. Efficacy and safety of endoscopic submucosal dissection for large gastric stromal tumors. Clin Res Hepatol Gastroenterol. (2020) 44:90–100. doi: 10.1016/j.clinre.2019.03.004
118. Li L, Wang F, Wu B, Wang Q, Wang C, Liu J. Endoscopic submucosal dissection of gastric fundus subepithelial tumors originating from the muscularis propria. Exp Ther Med. (2013) 6:391–5. doi: 10.3892/etm.2013.1181
119. Jeong ID, Jung SW, Bang SJ, Shin JW, Park NH, Kim DH. Endoscopic enucleation for gastric subepithelial tumors originating in the muscularis propria layer. Surg Endosc. (2011) 25:468–74. doi: 10.1007/s00464-010-1195-7
120. Sawada A, Hirasawa K, Maeda S. Endoscopic muscularis dissection for gastrointestinal mesenchymal tumor. Dig Endosc. (2020) 32:e106–8. doi: 10.1111/den.13707
121. Liu BR, Song JT, Qu B, Wen JF, Yin JB, Liu W. Endoscopic muscularis dissection for upper gastrointestinal subepithelial tumors originating from the muscularis propria. Surg Endosc. (2012) 26:3141–8. doi: 10.1007/s00464-012-2305-5
122. Ko EJ, Bang BW, Kwon KS, Shin YW, Kim HK. Endoscopic enucleation is effective and relatively safe in small gastric subepithelial tumors originating from muscularis propria. Dig Dis Sci. (2019) 64:524–31. doi: 10.1007/s10620-018-5348-1
123. Chu YY, Lien JM, Tsai MH, Chiu CT, Chen TC, Yang KC, et al. Modified endoscopic submucosal dissection with enucleation for treatment of gastric subepithelial tumors originating from the muscularis propria layer. BMC Gastroenterol. (2012) 12:124. doi: 10.1186/1471-230X-12-124
124. Inoue H, Ikeda H, Hosoya T, Onimaru M, Yoshida A, Eleftheriadis N, et al. Submucosal endoscopic tumor resection for subepithelial tumors in the esophagus and cardia. Endoscopy. (2012) 44:225–30. doi: 10.1055/s-0031-1291659
125. Du C, Linghu E. Submucosal tunneling endoscopic resection for the treatment of gastrointestinal submucosal tumors originating from the muscularis propria layer. J Gastrointest Surg. (2017) 21:2100–9. doi: 10.1007/s11605-017-3579-7
126. Chiu PWY, Yip HC, Teoh AYB, Wong VWY, Chan SM, Wong SKH, et al. Per oral endoscopic tumor (POET) resection for treatment of upper gastrointestinal subepithelial tumors. Surg Endosc. (2019) 33:1326–33. doi: 10.1007/s00464-018-06627-4
127. Ujiki MB, VanDruff VN. Peroral endoscopic myotomy for achalasia. World J Surg. (2022) 46:1542–6. doi: 10.1007/s00268-022-06477-1
128. Zhou DJ, Dai ZB, Wells MM, Yu DL, Zhang J, Zhang L. Submucosal tunneling and endoscopic resection of submucosal tumors at the esophagogastric junction. World J Gastroenterol. (2015) 21:578–83. doi: 10.3748/wjg.v21.i2.578
129. Tu S, Huang S, Li G, Tang X, Qing H, Gao Q, et al. Submucosal tunnel endoscopic resection for esophageal submucosal tumors: a multicenter study. Gastroenterol Res Pract. (2018) 2018:2149564. doi: 10.1155/2018/2149564
130. Gong W, Xiong Y, Zhi F, Liu S, Wang A, Jiang B. Preliminary experience of endoscopic submucosal tunnel dissection for upper gastrointestinal submucosal tumors. Endoscopy. (2012) 44:231–5. doi: 10.1055/s-0031-1291720
131. Tan Y, Tang X, Guo T, Peng D, Tang Y, Duan T, et al. Comparison between submucosal tunneling endoscopic resection and endoscopic full-thickness resection for gastric stromal tumors originating from the muscularis propria layer. Surg Endosc. (2017) 31:3376–82. doi: 10.1007/s00464-016-5350-7
132. Chen T, Zhou PH, Chu Y, Zhang YQ, Chen WF, Ji Y, et al. Long-term outcomes of submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors. Ann Surg. (2017) 265:363–9. doi: 10.1097/SLA.0000000000001650
133. Shichijo S, Uedo N, Yanagimoto Y, Yamamoto K, Kono M, Fukuda H, et al. Endoscopic full-thickness resection of gastric gastrointestinal stromal tumor: a Japanese case series. Ann Gastroenterol. (2019) 32:593–9. doi: 10.20524/aog.2019.0413
134. Perbtani Y, Gupte A, Draganov PV, Esnakula A, Yang D. Endoscopic full-thickness resection of a stomach gastrointestinal stromal tumor using a dedicated full-thickness resection device. VideoGIE. (2020) 5:470–2. doi: 10.1016/j.vgie.2020.05.024
135. Liu XG, Chen ZY, Yang YC, Zhang RY, Liu WH. Combined preclosure technique and traction method facilitating endoscopic full-thickness resection of a gastric fundal gastrointestinal stromal tumor. Endoscopy. (2020) 52:E293–4. doi: 10.1055/a-1104-5245
136. Wang W, Liu CX, Niu Q, Wang AL, Shi N, Ma FZ, et al. OTSC assisted EFTR for the treatment of GIST: 40 cases analysis. Minim Invasive Ther Allied Technol. (2022) 31:238–45. doi: 10.1080/13645706.2020.1781190
137. Guo JT, Zhang JJ, Wu YF, Liao Y, Wang YD, Zhang BZ, et al. Endoscopic full-thickness resection using an over-the-scope device: a prospective study. World J Gastroenterol. (2021) 27:725–36. doi: 10.3748/wjg.v27.i8.725
138. Ren Z, Lin SL, Zhou PH, Cai SL, Qi ZP, Li J, et al. Endoscopic full-thickness resection (EFTR) without laparoscopic assistance for nonampullary duodenal subepithelial lesions: our clinical experience of 32 cases. Surg Endosc. (2019) 33:3605–11. doi: 10.1007/s00464-018-06644-3
139. Jung AL, Park SW, Hong GY, Moon HC, Eun SJ. Endoscopic full-thickness resection for gastric subepithelial lesions arising from the muscularis propria. Clin Endosc. (2021) 54:131–5. doi: 10.5946/ce.2020.070
140. Qiu WQ, Zhuang J, Wang M, Liu H, Shen ZY, Xue HB, et al. Minimally invasive treatment of laparoscopic and endoscopic cooperative surgery for patients with gastric gastrointestinal stromal tumors. J Dig Dis. (2013) 14:469–73. doi: 10.1111/1751-2980.12076
141. Acker S, Dishop M, Kobak G, Vue P, Somme S. Laparoscopic-assisted endoscopic resection of a gastric leiomyoma. European J Pediatr Surg Rep. (2014) 2:003–6. doi: 10.1055/s-0034-1370773
142. Kato M, Nakajima K, Nishida T, Yamasaki M, Nishida T, Tsutsui S, et al. Local resection by combined laparoendoscopic surgery for duodenal gastrointestinal stromal tumor. Diagn Ther Endosc. (2011) 2011:645609. doi: 10.1155/2011/645609
143. Irino T, Nunobe S, Hiki N, Yamamoto Y, Hirasawa T, Ohashi M, et al. Laparoscopic-endoscopic cooperative surgery for duodenal tumors: a unique procedure that helps ensure the safety of endoscopic submucosal dissection. Endoscopy. (2015) 47:349–51. doi: 10.1055/s-0034-1390909
144. Kang WM, Yu JC, Ma ZQ, Zhao ZR, Meng QB, Ye X. Laparoscopic-endoscopic cooperative surgery for gastric submucosal tumors. World J Gastroenterol. (2013) 19:5720–6. doi: 10.3748/wjg.v19.i34.5720
145. Ismael H, Ragoza Y, Caccitolo J, Cox S. Optimal management of GIST tumors located near the gastroesophageal junction: case report and review of the literature. Int J Surg Case Rep. (2016) 25:91–6. doi: 10.1016/j.ijscr.2016.06.006
146. Basso N, Rosato P, De Leo A, Picconi T, Trentino P, Fantini A, et al. Laparoscopic treatment of gastric stromal tumors. Surg Endosc. (2000) 14:524–6. doi: 10.1007/s004640000021
147. Wilhelm D, von Delius S, Burian M, Schneider A, Frimberger E, Meining A, et al. Simultaneous use of laparoscopy and endoscopy for minimally invasive resection of gastric subepithelial masses—analysis of 93 interventions. World J Surg. (2008) 32:1021–8. doi: 10.1007/s00268-008-9492-1
148. Kiyozaki H, Saito M, Chiba H, Takata O, Rikiyama T. Laparoscopic wedge resection of the stomach for gastrointestinal stromal tumor (GIST): non-touch lesion lifting method. Gastric Cancer. (2014) 17:337–40. doi: 10.1007/s10120-013-0272-8
149. Davila JS, Momblan D, Gines A, Sanchez-Montes C, Araujo I, Saavedra-Perez D, et al. Endoscopic-assisted laparoscopic resection for gastric subepithelial tumors. Surg Endosc. (2016) 30:199–203. doi: 10.1007/s00464-015-4183-0
150. Sasaki A, Koeda K, Obuchi T, Nakajima J, Nishizuka S, Terashima M, et al. Tailored laparoscopic resection for suspected gastric gastrointestinal stromal tumors. Surgery. (2010) 147:516–20. doi: 10.1016/j.surg.2009.10.035
151. Ye X, Yu J, Kang W, Ma Z, Xue Z. Short- and long-term outcomes of endoscope-assisted laparoscopic wedge resection for gastric submucosal tumors adjacent to esophagogastric junction. J Gastrointest Surg. (2018) 22:402–13. doi: 10.1007/s11605-017-3628-2
152. Marzano E, Ntourakis D, Addeo P, Oussoultzoglou E, Jaeck D, Pessaux P. Robotic resection of duodenal adenoma. Int J Med Rob. (2011) 7:66–70. doi: 10.1002/rcs.371
153. Dong HY, Wang YL, Jia XY, Li J, Li GD, Li YQ. Modified laparoscopic intragastric surgery and endoscopic full-thickness resection for gastric stromal tumor originating from the muscularis propria. Surg Endosc. (2014) 28:1447–53. doi: 10.1007/s00464-013-3375-8
154. Privette A, McCahill L, Borrazzo E, Single RM, Zubarik R. Laparoscopic approaches to resection of suspected gastric gastrointestinal stromal tumors based on tumor location. Surg Endosc. (2008) 22:487–94. doi: 10.1007/s00464-007-9493-4
155. Ohashi S. Laparoscopic intraluminal (intragastric) surgery for early gastric cancer. A new concept in laparoscopic surgery. Surg Endosc. (1995) 9:169–71. doi: 10.1007/BF00191960
156. Novitsky YW, Kercher KW, Sing RF, Heniford BT. Long-term outcomes of laparoscopic resection of gastric gastrointestinal stromal tumors. Ann Surg. (2006) 243:738–45. doi: 10.1097/01.sla.0000219739.11758.27
157. Katsuyama S, Nakajima K, Kurokawa Y, Takahashi T, Miyazaki Y, Makino T, et al. Single-Incision laparoscopic intragastric surgery for gastric submucosal tumor located adjacent to esophagogastric junction: report of four cases. J Laparoendosc Adv Surg Tech A. (2018) 28:78–82. doi: 10.1089/lap.2017.0026
158. Zhang M, Cai X, Liang C, Weng Y, Yu W. Single-incision laparoscopic intragastric surgery for gastric submucosal tumors located near the esophagogastric junction. J Laparoendosc Adv Surg Tech A. (2022) 32:360–5. doi: 10.1089/lap.2021.0186
159. De Vogelaere K, Van De Winkel N, Simoens C, Delvaux G. Intragastric SILS for GIST, a new challenge in oncologic surgery: first experiences. Anticancer Res. (2013) 33:3359–63. PMID: 23898104.23898104
160. Na JU, Lee SI, Noh SM. The single incision laparoscopic intragastric wedge resection of gastric submucosal tumor. J Gastric Cancer. (2011) 11:225–9. doi: 10.5230/jgc.2011.11.4.225
161. Ludwig K, Wilhelm L, Scharlau U, Amtsberg G, Bernhardt J. Laparoscopic-endoscopic rendezvous resection of gastric tumors. Surg Endosc. (2002) 16:1561–5. doi: 10.1007/s00464-001-9224-1
162. Schubert D, Kuhn R, Nestler G, Kahl S, Ebert MP, Malfertheiner P, et al. Laparoscopic-endoscopic rendezvous resection of upper gastrointestinal tumors. Dig Dis. (2005) 23:106–12. doi: 10.1159/000088591
163. Dong HY, Wang YL, Li J, Pang QP, Li GD, Jia XY. New-style laparoscopic and endoscopic cooperative surgery for gastric stromal tumors. World J Gastroenterol. (2013) 19:2550–4. doi: 10.3748/wjg.v19.i16.2550
164. Tsujimoto H, Yaguchi Y, Kumano I, Takahata R, Ono S, Hase K. Successful gastric submucosal tumor resection using laparoscopic and endoscopic cooperative surgery. World J Surg. (2012) 36:327–30. doi: 10.1007/s00268-011-1387-x
165. Hiki N, Yamamoto Y, Fukunaga T, Yamaguchi T, Nunobe S, Tokunaga M, et al. Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor dissection. Surg Endosc. (2008) 22:1729–35. doi: 10.1007/s00464-007-9696-8
166. Cao L, Zheng K, Wang H, Zhao Y, Yang Z, Li W. Laparoscopic and endoscopic cooperative dissection for small gastric gastrointestinal stromal tumor without causing injury to the mucosa. Gastroenterol Res Pract. (2019) 2019:7376903. doi: 10.1155/2019/7376903
167. Tsuji R, Komatsu S, Kumano T, Ohta A, Furuke H, Tanaka S, et al. Laparoscopy and endoscopy cooperative surgery (LECS)-assisted open partial gastrectomy for a high-risk gastrointestinal stromal tumor. Gan to Kagaku Ryoho. (2019) 46:172–4. PMID: 30765678.30765678
168. Ri M, Nunobe S, Makuuchi R, Ida S, Kumagai K, Ohashi M, et al. Is laparoscopic and endoscopic cooperative surgery (LECS) for gastric subepithelial tumor at the esophagogastric junction safe? Asian J Endosc Surg. (2021) 14:223–31. doi: 10.1111/ases.12857
169. Hoteya S, Haruta S, Shinohara H, Yamada A, Furuhata T, Yamashita S, et al. Feasibility and safety of laparoscopic and endoscopic cooperative surgery for gastric submucosal tumors, including esophagogastric junction tumors. Dig Endosc. (2014) 26:538–44. doi: 10.1111/den.12215
170. Matsuda T, Hiki N, Nunobe S, Aikou S, Hirasawa T, Yamamoto Y, et al. Feasibility of laparoscopic and endoscopic cooperative surgery for gastric submucosal tumors (with video). Gastrointest Endosc. (2016) 84:47–52. doi: 10.1016/j.gie.2015.11.040
171. Obuchi T, Sasaki A, Baba S, Nitta H, Otsuka K, Wakabayashi G. Single-port laparoscopic and endoscopic cooperative surgery for a gastric gastrointestinal stromal tumor: report of a case. Surg Today. (2015) 45:641–6. doi: 10.1007/s00595-014-0870-z
172. Ohi M, Yasuda H, Ishino Y, Katsurahara M, Saigusa S, Tanaka K, et al. Single-incision laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor arising from the duodenum. Asian J Endosc Surg. (2013) 6:307–10. doi: 10.1111/ases.12059
173. Tsushimi T, Mori H, Harada T, Nagase T, Iked Y, Ohnishi H. Laparoscopic and endoscopic cooperative surgery for duodenal neuroendocrine tumor (NET) G1: report of a case. Int J Surg Case Rep. (2014) 5:1021–4. doi: 10.1016/j.ijscr.2014.10.051
174. Tamegai Y, Fukunaga Y, Suzuki S, Lim DNF, Chino A, Saito S, et al. Laparoscopic and endoscopic cooperative surgery (LECS) to overcome the limitations of endoscopic resection for colorectal tumors. Endosc Int Open. (2018) 6:E1477–85. doi: 10.1055/a-0761-9494
175. Abe N, Takeuchi H, Yanagida O, Masaki T, Mori T, Sugiyama M, et al. Endoscopic full-thickness resection with laparoscopic assistance as hybrid NOTES for gastric submucosal tumor. Surg Endosc. (2009) 23:1908–13. doi: 10.1007/s00464-008-0317-y
176. Kim HH, Uedo N. Hybrid NOTES: combined laparo-endoscopic full-thickness resection techniques. Gastrointest Endosc Clin N Am. (2016) 26:335–73. doi: 10.1016/j.giec.2015.12.011
177. Kim SY, Kim KO. Management of gastric subepithelial tumors: the role of endoscopy. World J Gastrointest Endosc. (2016) 8:418–24. doi: 10.4253/wjge.v8.i11.418
178. Mori H, Kobara H, Fujihara S, Nishiyama N, Ayagi M, Matsunaga T, et al. Establishment of the hybrid endoscopic full-thickness resection of gastric gastrointestinal stromal tumors. Mol Clin Oncol. (2015) 3:18–22. doi: 10.3892/mco.2014.412
179. Lim SG, Hur H, Han SU, Lee KM, Kang JK, Shin SJ, et al. Laparoscopy-assisted endoscopic full-thickness resection for gastric subepithelial tumors originated from the muscularis propria layer: a pilot study with literature review. Scand J Gastroenterol. (2017) 52:257–63. doi: 10.1080/00365521.2016.1230778
180. Watanabe M, Nishizaki M, Takenaka R, Takei K, Mikane Y, Shoji R, et al. A case of inverted LECS for GIST in the remnant stomach after distal gastrectomy. Gan to Kagaku Ryoho. (2019) 46:1954–6. PMID: 32157024.32157024
181. Hiki N, Nunobe S, Matsuda T, Hirasawa T, Yamamoto Y, Yamaguchi T. Laparoscopic endoscopic cooperative surgery. Dig Endosc. (2015) 27:197–204. doi: 10.1111/den.12404
182. Nunobe S, Hiki N, Gotoda T, Murao T, Haruma K, Matsumoto H, et al. Successful application of laparoscopic and endoscopic cooperative surgery (LECS) for a lateral-spreading mucosal gastric cancer. Gastric Cancer. (2012) 15:338–42. doi: 10.1007/s10120-012-0146-5
183. Aoki M, Tokioka S, Narabayashi K, Hakoda A, Inoue Y, Yorifuji N, et al. Laparoscopic and endoscopic cooperative surgery for intra-mucosal gastric carcinoma adjacent to the ulcer scars. World J Surg Oncol. (2018) 16:53. doi: 10.1186/s12957-018-1355-0
184. Takechi H, Fujikuni N, Takemoto Y, Tanabe K, Amano H, Noriyuki T, et al. Palliative surgery for advanced gastric cancer: partial gastrectomy using the inverted laparoscopic and endoscopic cooperative surgery method. Int J Surg Case Rep. (2018) 50:42–5. doi: 10.1016/j.ijscr.2018.06.042
185. Inoue H, Ikeda H, Hosoya T, Yoshida A, Onimaru M, Suzuki M, et al. Endoscopic mucosal resection, endoscopic submucosal dissection, and beyond: full-layer resection for gastric cancer with nonexposure technique (CLEAN-NET). Surg Oncol Clin N Am. (2012) 21:129–40. doi: 10.1016/j.soc.2011.09.012
186. Wang H, Cao L, Zheng K, Zhao Y. Laparoscopic endoscopic cooperative surgery for gastrointestinal stromal tumors. Surg Laparosc Endosc Percutan Tech. (2018) 28:354–8. doi: 10.1097/SLE.0000000000000591
187. Nabeshima K, Tomioku M, Nakamura K, Yasuda S. Combination of laparoscopic and endoscopic approaches to neoplasia with non-exposure technique (CLEAN-NET) for GIST with ulceration. Tokai J Exp Clin Med. (2015) 40:115–9. PMID: 26369265.26369265
188. Onimaru M, Inoue H, Ikeda H, Abad MRA, Quarta Colosso BM, Shimamura Y, et al. Combination of laparoscopic and endoscopic approaches for neoplasia with non-exposure technique (CLEAN-NET) for gastric submucosal tumors: updated advantages and limitations. Ann Transl Med. (2019) 7:582. doi: 10.21037/atm.2019.09.19
189. Hayase S, Sakuma M, Chida S, Saito M, Ami H, Koyama Y, et al. Diagnosis and treatment of gastric hamartomatous inverted polyp (GHIP) using a modified combination of laparoscopic and endoscopic approaches to neoplasia with a non-exposure technique (modified CLEAN-NET): a case report. Surg Case Rep. (2020) 6:200. doi: 10.1186/s40792-020-00951-5
190. Fujishima H, Etoh T, Hiratsuka T, Akagi T, Tajima M, Shibata T, et al. Serosal and muscular layers incision technique in laparoscopic surgery for gastric gastrointestinal stromal tumors. Asian J Endosc Surg. (2017) 10:92–5. doi: 10.1111/ases.12327
191. Kanehira E, Kanehira AK, Tanida T, Takahashi K, Obana Y, Sasaki K. CLEAN-NET: a modified laparoendoscopic wedge resection of the stomach to minimize the sacrifice of innocent gastric wall. Surg Endosc. (2020) 34:290–7. doi: 10.1007/s00464-019-06765-3
192. Hajer J, Havluj L, Whitley A, Gurlich R. Non-exposure endoscopic-laparoscopic cooperative surgery for stomach tumors: first experience from the Czech Republic. Clin Endosc. (2018) 51:167–73. doi: 10.5946/ce.2017.076
193. Goto O, Mitsui T, Fujishiro M, Wada I, Shimizu N, Seto Y, et al. New method of endoscopic full-thickness resection: a pilot study of non-exposed endoscopic wall-inversion surgery in an ex vivo porcine model. Gastric Cancer. (2011) 14:183–7. doi: 10.1007/s10120-011-0014-8
194. Mitsui T, Niimi K, Yamashita H, Goto O, Aikou S, Hatao F, et al. Non-exposed endoscopic wall-inversion surgery as a novel partial gastrectomy technique. Gastric Cancer. (2014) 17:594–9. doi: 10.1007/s10120-013-0291-5
195. Kim DW, Kim JS, Kim BW, Jung JY, Kim GJ, Kim JJ. Non-exposed endoscopic wall-inversion surgery for gastrointestinal stromal tumor of the stomach: first case report in Korea. Clin Endosc. (2016) 49:475–8. doi: 10.5946/ce.2016.002
196. Mitsui T, Yamashita H, Aikou S, Niimi K, Fujishiro M, Seto Y. Non-exposed endoscopic wall-inversion surgery for gastrointestinal stromal tumor. Transl Gastroenterol Hepatol. (2018) 3:17. doi: 10.21037/tgh.2018.03.02
197. Mahawongkajit P, Techagumpuch A, Suthiwartnarueput W. Non-exposed endoscopic wall-inversion surgery for a gastrointestinal stromal tumor of the stomach: a case report. Oncol Lett. (2017) 14:4746–50. doi: 10.3892/ol.2017.6787
198. Aoyama J, Goto O, Kawakubo H, Mayanagi S, Fukuda K, Irino T, et al. Clinical outcomes of non-exposed endoscopic wall-inversion surgery for gastric submucosal tumors: long-term follow-up and functional results. Gastric Cancer. (2020) 23:154–9. doi: 10.1007/s10120-019-00985-1
199. Kikuchi S, Nishizaki M, Kuroda S, Tanabe S, Noma K, Kagawa S, et al. Nonexposure laparoscopic and endoscopic cooperative surgery (closed laparoscopic and endoscopic cooperative surgery) for gastric submucosal tumor. Gastric Cancer. (2017) 20:553–7. doi: 10.1007/s10120-016-0641-1
Keywords: gastrointestinal stromal tumors (GISTs), surgery, risk evaluation (RE), endoscopy, LECs
Citation: Yue L, Sun Y, Wang X and Hu W (2023) Advances of endoscopic and surgical management in gastrointestinal stromal tumors. Front. Surg. 10:1092997. doi: 10.3389/fsurg.2023.1092997
Received: 8 November 2022; Accepted: 24 March 2023;
Published: 12 April 2023.
Edited by:
Matteo De Pastena, University of Verona, ItalyReviewed by:
Francesca Pecchini, Baggiovara Civil Hospital, ItalyMarco Massani, ULSS2 Marca Trevigiana, Italy
© 2023 Yue, Sun, Wang and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiling Hu aHV3ZWlsaW5nQHpqdS5lZHUuY24=
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
 Lei Yue
Lei Yue Yingchao Sun
Yingchao Sun Xinjie Wang
Xinjie Wang Weiling Hu
Weiling Hu