- 1Department of Neurosurgery, University Hospital Carl Gustav Carus, Technische Universität Dresden, Dresden, Germany
- 2Department of Neurology, University Hospital Carl Gustav Carus, Technische Universität Dresden, Dresden, Germany
- 3Department of Cardiology, Bergmannsheil University Hospitals Bergmannsheil, Ruhr University Bochum, Bochum, Germany
Background: The incidence of spondylodiscitis (SD) and isolated spinal epidural empyema (ISEE) has been increasing in the last decades, but the distinct differences between both entities are poorly understood. We aimed to evaluate the clinical phenotypes and long-term outcomes of SD and ISEE in depth.
Methods: We performed a chart review and analyzed data from our cohorts of consecutive SD and ISEE patients who were treated and assessed in detail for demographic, clinical, imaging, laboratory, and microbiologic characteristics at a university neurosurgical center in Germany from 2002 to 2021. Between-group comparisons were performed to identify meaningful differences in both entities.
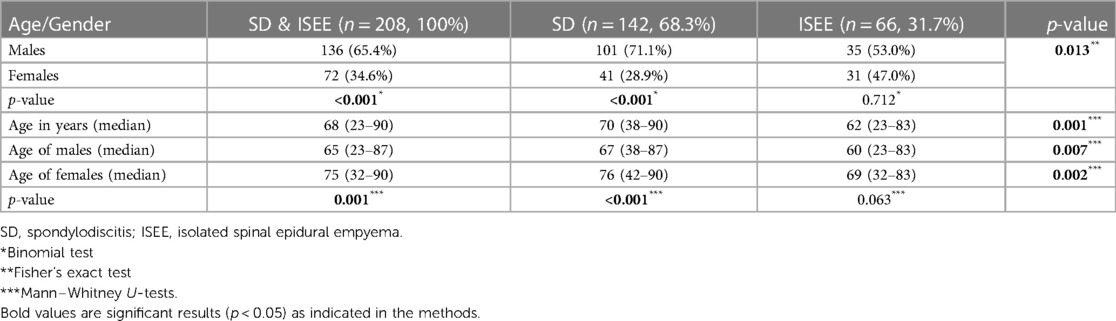
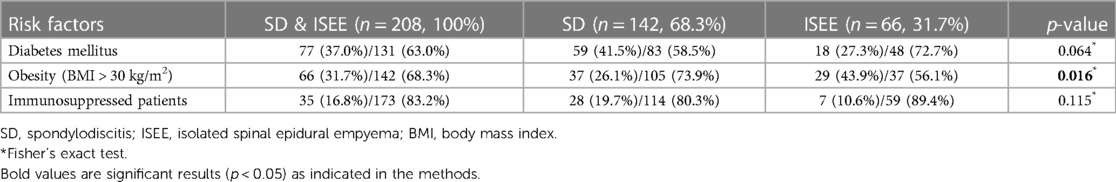
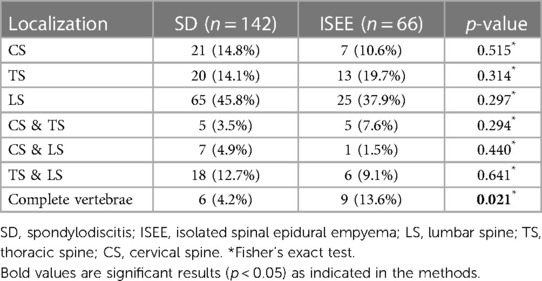
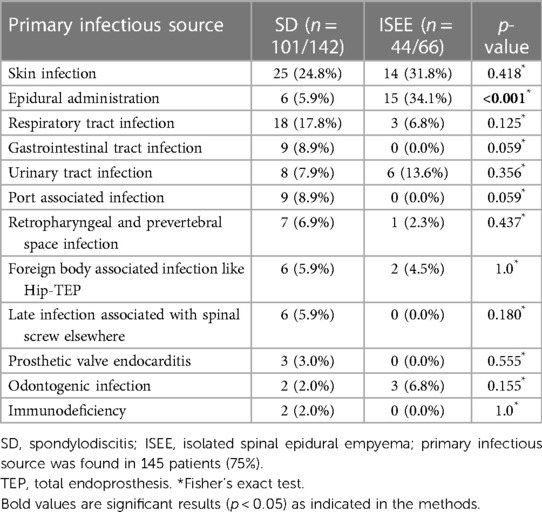
Results: We included 208 patients (72 females: age 75 [75 32–90] y vs. 136 males: 65 [23–87] y, median [interquartile range], p < 0.001), of which 142 (68.3%) had SD and 66 (31.7%) had ISEE. Patients with SD were older than ISEE (ISEE: 62 y vs. SD: 70 y, p = 0.001). While SD was more common in males than females (males: n = 101, 71.1% vs. females: n = 41, 28.9%, p < 0.001), there was no sex-related difference in ISEE (males: n = 35, 53.0% vs. females: n = 31, 47.0%, p = 0.71). Obesity was more frequent in ISEE than in SD (ISEE: n = 29, 43.9% vs. SD: n = 37, 26.1%, p = 0.016). However, there were no between-group differences in rates of diabetes and immunodeficiency. In the entire study population, a causative pathogen was identified in 192 (92.3%) patients, with methicillin-susceptible staphylococcus aureus being most frequent (n = 100, 52.1%) and being more frequent in ISEE than SD (ISEE: n = 43, 65.2% vs. SD: n = 57, 40.1%, p = 0.003). SD and ISEE occurred most frequently in the lumbar spine, with no between-group differences (ISEE: n = 25, 37.9% vs. SD: n = 65, 45.8%, p = 0.297). Primary infectious sources were identified in 145 patients (69.7%) and among this skin infection was most common in both entities (ISEE: n = 14, 31.8% vs. SD: n = 25, 24.8%, p = 0.418). Furthermore, epidural administration was more frequent the primary cause of infection in ISEE than SD (ISEE: n = 12, 27.3% vs. SD: n = 5, 4.9%, p < 0.001). The most common surgical procedure in SD was instrumentation (n = 87, 61%) and in ISEE abscess evacuation (n = 63, 95%). Patients with ISEE displayed lower in-hospital complication rates compared to SD for sepsis (ISEE: n = 12, 18.2% vs. SD: n = 94, 66.2%, p < 0.001), septic embolism (ISEE: n = 4/48 cases, 8.3% vs. SD: n = 52/117 cases, 44.4%, p < 0.001), endocarditis (ISEE: n = 1/52 cases, 1.9% vs. SD: n = 23/125 cases, 18.4%, p = 0.003), relapse rate (ISEE: n = 4/46, 8.7% vs. SD: n = 27/92, 29.3%, p = 0.004), and disease-related mortality (ISEE: n = 1, 1.5% vs. SD: n = 11, 7.7%, p = 0.108). Patients with SD showed prolonged length of hospital stay (ISEE: 22 [15, 30] d vs. SD: 38 [29, 53] d, p < 0.001) and extended intensive care unit stay (ISEE: 0 [0, 4] d vs. SD: 3 [0, 12] d, p < 0.002).
Conclusions: Our 20-year experience and cohort analysis on the clinical management of SD and ISEE unveiled distinct clinical phenotypes and outcomes in both entities, with ISEE displaying a more favorable disease course with respect to complications and relapse rates as well as disease-related mortality.
1. Introduction
Spondylodiscitis (SD) affects the vertebral body, intervertebral disc, and/or adjacent paraspinal tissues, due to an adjacent source (trauma, surgery, intervention) or hematogenous spread (1–3). However, isolated spinal epidural empyema (ISEE) is an infection of the epidural space with accumulation of purulent substance in the cavity between the dura and the osseo-ligamentous boundaries of the spinal canal, without findings of spondylodiscitis on magnetic resonance imaging (MRI) or intraoperatively (4). On the other hand, epidural empyema/abscess can be a secondary complication of a primary SD, spreading hematogeneously via septic thrombosis of epidural veins (5).
The incidence of SD and ISEE has been increasing over the last decades, due to an aging population with serious comorbidities and increasing spinal interventions, advanced imaging, and a growing number of drug abusers (6, 7). It ranges from 1:20,000 to 1:100,000, and the mortality rate varies between 2% and 20% in industrialized countries (2, 8–11). Diabetes mellitus, immunosuppression, and human immunodeficiency virus (HIV) infection are additional risk factors associated with SD and ISEE (12).
SD is commonly (55%–80%) caused by methicillin-sensitive staphylococcus aureus (MSSA) and spreads hematogeneously (13). MSSA is also the most common responsible pathogen in ISEE (14–17). Primary SD is associated with a more severe course and significantly higher mortality rate than secondary acquired SD, which is triggered by postoperative infections (18). The majority of ISEE occurs iatrogenically, following spinal surgical procedures, epidural anaesthesia, or spinal injections (19).
Although both pathologies represent clinically distinct etiologic and anatomic entities that require different treatments, only a few studies have addressed detailed differences between SD and ISEE. Therefore, here we aimed to evaluate and compare the clinical features, complications, treatments, and outcomes of both pathologic entities over 20 years.
2. Materials and methods
2.1. Study design
We performed a retrospective observational study of consecutive patients with SD or ISEE who underwent surgical treatment from 2002 to 2021 at our university neurosurgical spine centre in Dresden/Germany. Patients with late or early postoperative spinal infections were not considered in this study as the focus was on patients with primary spinal infections and not on secondary spinal infections resulting after surgery.
All patients who underwent surgery and had no intradural infection on admission between 2002 and 2021 were included. Twenty patients were excluded due to one of the following criteria:
- only conservative treatment
- intradural infection (subdural abscess or spinal cord abscess)
Two hundred twenty-eight patients with SD and ISEE were identified, whereas SD was diagnosed in 142 patients (68.3%) and ISEE in 66 patients (31.7%) (Figure 1).
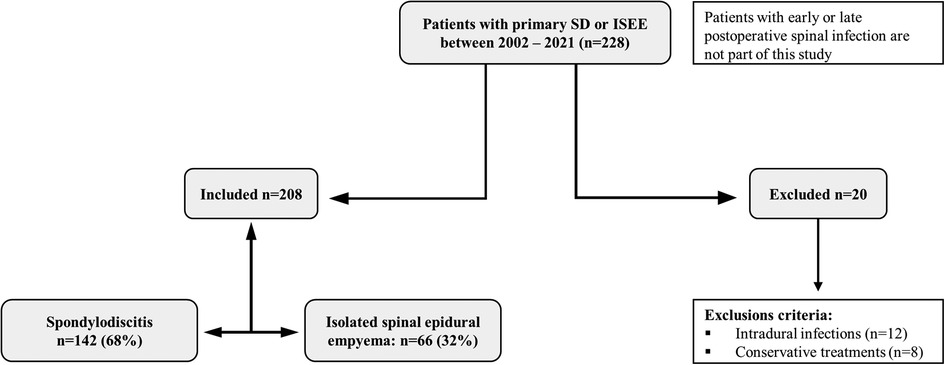
Figure 1. Study design. Study design: Two hundred twenty-eight patients had spondylodiscitis (SD: 142) or isolated spinal epidural empyema (ISEE: 66) without early or late postoperative spinal infection. Twenty patients were excluded due to exclusion criteria.
2.2. Patient data
The study was approved by our local ethics committee (Reference number BO-EK-17012022). After case identification, patient data were extracted by reviewing electronic medical records using the ORBIS system (ORBIS, Dedalus, Bonn, Germany). Radiological data, including gadolinium contrast-enhancing MRI, computer tomography (CT) and/or x-ray were available for review in all cases. Collected data included demographic information, risk factors, causative pathogens, spinal localization of infection, primary infectious source, surgical and antibiotic treatment, type of surgical procedure, length of hospital and intensive care stay, and complications such as sepsis, septic embolisms, endocarditis, relapse rates, and disease-related mortality.
2.3. Clinical management
SD or ISEE were diagnosed based on clinical history, fever, leukocyte count, C-reactive protein (CrP), typical radiological changes on MRI, and pathogen detection in blood cultures, intraoperative specimens, or CT-guided biopsies (20). Transthoracic echocardiogram (TTE) was performed in all patients, but transoesophageal echocardiogram (TEE) was obtained in patients with suspected endocarditis according to the modified DUKE criteria (21). First-line treatment was usually conservative with intravenous antibiotics, although surgical treatment was indicated in cases of source control, epidural abscess, neurologic deficits, or spinal instability. The type of surgical intervention was determined in the interdisciplinary spine conference or the neurosurgical-neuroradiological conference depending on the clinical, laboratory, and radiological findings. Between 2002 and 2015, cases were discussed in our neurosurgical-neuroradiology conference and treated in collaboration with infectious disease physicians. Since 2015, a multidisciplinary spine conference has been conducted involving neuroradiologists, neurosurgeons, trauma surgeons, orthopaedic surgeons, and infectious disease physicians to determine a treatment strategy for patients. Patients with ISEE underwent abscess evacuation with/without drainage or anterior cervical discectomy and fusion (ACDF) for abscess ventral to the spinal cord in the cervical spine, whereas patients with SD were treated with either abscess evacuation or instrumentation in cases of instability, deformity, and pain-related immobility. Therefore, surgical decision making depends on clinical experience and various defined radiological features. Preoperative CT scans were performed in all patients undergoing spinal instrumentation to assess bony integrity.
All patients received either a targeted antibiotic or empirical antibiotic treatment depending on the clinical condition at admission and following the recommendations of the local department of infectious diseases. The empirical antibiotic therapy was switched to targeted after detection of the causative pathogens. In SD patients, intravenous antibiotic therapy was switched to oral antibiotics after approximately 4–6 weeks, and the total duration of antibiotic therapy ranged from 10 to 12 weeks. On the other hand, ISEE patients received a 2-weeks intravenous antibiotic treatment, which was changed to oral administration with a total duration that ranged from 4 to 6 weeks. Clinical and radiological follow-up was performed on all patients who complied with our recommendation at 3, 6, and 12 months after hospital discharge.
2.4. Statistical analysis
All statistical analyses were carried out using the software package SPSS (SPSS Statistics 28, IBM, Armonk, New York, USA). Descriptive statistics were used, and categorical variables were compared between ISEE and SD using Fisher's exact tests when appropriate or Chi-squared test. Numeric variables were compared using Mann–Whitney U-tests when appropriate. A binomial test was used to analyse the distribution of gender. All statistical tests were two-tailed, and a p-value p < 0.05 was considered statistically significant. Univariate and multivariate analyses were performed to identify independent risk factors.
3. Results
3.1. Clinical characteristics
3.1.1. Demographic distribution
We enrolled 136 male patients (65.4%) and 72 female patients (34.6%), with males being significantly younger than females at the time of diagnosis (males: 65 y vs. females: 75 y, p < 0.001). SD patients were significantly older than ISEE (ISEE: 62 y vs. SD: 70 y, p = 0.001). In addition, SD occurred predominantly in male patients (males: 71.1% vs. females: 28.9%, p < 0.001), and male patients with SD were younger than females at the time of diagnosis (males: 67 y vs. females: 76 y, p < 0.001). In ISEE, sex (males: 53% vs. females: 47%, p = 0.712) and age (males: 60 y vs. females: 69 y, p = 0.063) were equally distributed (Table 1).
3.1.2. Risk factors
Age and sex are counted among the risk factors and are presented in the upper paragraph. Diabetes mellitus was observed in 77 patients (37%) and showed no difference between both groups (ISEE: n = 18, 27.3% vs. SD: n = 59, 41.5%, p = 0.064). A total of 66 patients with BMI (body mass index) higher than 30 kg/m2 (31.7%) were identified, whereas obesity was more frequent in ISEE than in SD (ISEE: n = 29, 43.9% vs. SD: n = 37, 26.1%, p = 0.016). We found 35 immunocompromised patients (16.8%) with no differences between the two entities (ISEE: n = 7, 10.6% vs. SD: n = 28, 19.7%, p = 0.115) (Table 2).
3.1.3. Causative pathogens
A total of 192 pathogens (92.3%) were identified by blood cultures, intraoperative specimens, and/or CT-guided biopsies. In 16 patients (7.8%), the causative pathogens could not be isolated, presumably due to prior empiric antibiotic treatment. MSSA was the most common pathogen in both groups together (n = 100, 52.1%), followed by streptococci and enterococci (n = 31, 16.1%), enterobacteriaceae (n = 20, 10.4%), coagulase-negative staphylococci (n = 20, 10.4%), anaerobic bacteria (n = 6, 3.1%), methicillin-resistant staphylococcus aureus (n = 5, 2.6%), pseudomonas aeruginosa (n = 4, 2.1%), fungi (n = 4, 2.1%), mycobacterium tuberculosis (n = 1, 0.5%), and bacillus pumilus (n = 1, 0.5%) (Figure 2). MSSA was more common in ISEE than SD (ISEE: n = 43, 65.2% vs. SD: n = 57, 40.1%, p = 0.003).
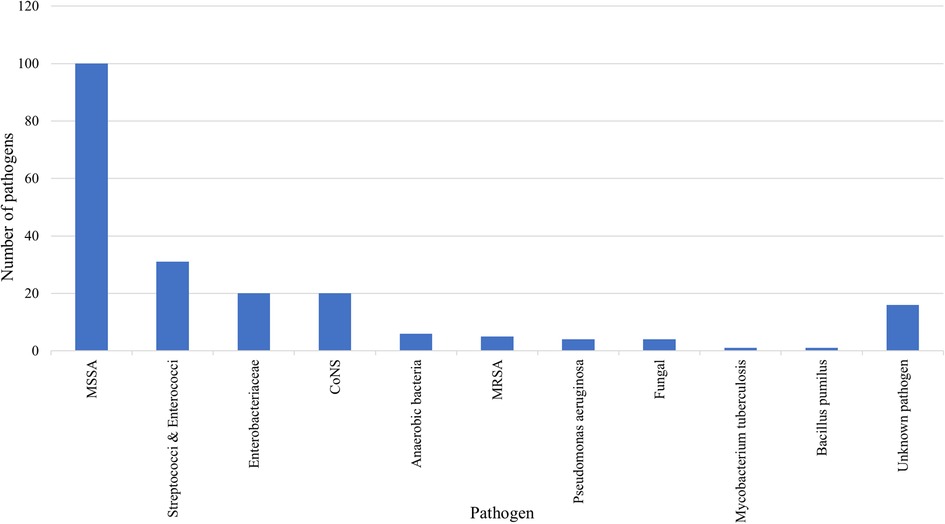
Figure 2. Causative pathogens of SD and ISEE. This figure presents the pathogens identified in spondylodiscitis (SD) and isolated spinal epidural empyema (ISEE). Methicillin-susceptible Staphylococcus aureus (MSSA): n = 100, 52.1%; streptococci and enterococci: n = 31, 16.1%; enterobacteriaceae: n = 20, 10.4%; coagulase-negative staphylococci (CoNS): n = 20, 10.4%; anaerobic bacteria: n = 6, 3.1%; methicillin-resistant staphylococcus aureus (MRSA): n = 5, 2.6%; pseudomonas aeruginosa: n = 4, 2.1%; fungi: n = 4, 2.1%; mycobacterium tuberculosis: n = 1, 0.5%; bacillus pumilus: n = 1, 0.5%.
3.1.4. Localization in the vertebral column
The lumbar spine (LS) was the most affected part of the vertebral column (VC) in both entities (ISEE: n = 41, 62.1% vs. SD: n = 96, 67.6%). The presence in the LS alone was not significantly different between both groups (ISEE: n = 25, 37.9% vs. SD: n = 65, 45.8%, p = 0.297). The thoracic spine (TS) was less affected than the LS in both entities (ISEE: n = 33, 50% vs. SD: n = 49, 34.5%) and manifestation in the TS alone was also not significant between both groups (ISEE: n = 13, 19.7% vs. SD: n = 20, 14.1%, p = 0.314). The cervical spine (CS) was least affected in both entities (ISEE: n = 22, 33.3% vs. SD: n = 39, 27.5%), and infection appeared in the CS alone without significant difference between the two groups (ISEE: n = 7, 10.6% vs. SD: n = 21, 14.8%, p = 0.515). Infection was noted more frequently in ISEE than in SD in all three spinal segments combined (CS, TS, and LS). (ISEE: n = 9, 13.6% vs. SD: n = 6, 4.2%, p = 0.021) (Table 3).
3.1.5. Primary infectious source
If primary spinal infection is present as SD or ISEE, a primary infectious source can usually be identified. Infections that resulted directly from surgical spinal procedures were not addressed in this study. Primary infectious sources were identified in 145 patients (69.7%). Skin infections were the most common cause in both groups, with no significant difference between groups (ISEE: n = 14, 32.8% vs. SD: n = 25, 24.8%, p = 0.418). Furthermore, epidural administration was significantly more common in the ISEE group than in the SD (ISEE: n = 12, 27.3% vs. SD: n = 5, 5%, p < 0.001). However, no significant difference was found between both groups in respiratory tract infections (ISEE: n = 3, 6.8% vs. SD: n = 18, 17.8%, p = 0.125), gastrointestinal tract infections (ISEE: n = 0, 0.0% vs. SD: n = 9, 8.9%, p = 0.059), urinary tract infections (ISEE: n = 6, 13.6% vs. SD: n = 8, 7.9%, p = 0.356), port-associated infections (ISEE: n = 0, 0.0% vs. SD: n = 9, 8.9%, p = 0.059), retropharyngeal and prevertebral infections (ISEE: n = 1, 2.3% vs. SD: n = 7, 6.9%, p = 0.437), foreign body-associated infections like Hip-total endoprosthesis (ISEE: n = 2, 4.5% vs. SD: n = 6, 5.9%, p = 1.0), late infection associated with spinal screw elsewhere (ISEE: n = 0, 0.0% vs. SD: n = 6, 5.9%, p = 0.180), prosthetic valve endocarditis (ISEE: n = 0, 0.0% vs. SD: n = 3, 3.0%, p = 0.555), odontogenic infections (ISEE: n = 3, 6.8% vs. SD: n = 2, 2.0%, p = 0.155), immunodeficiency (ISEE: n = 0, 0.0% vs. SD: n = 2, 2.0%, p = 1.0). Root causes of infection remained undetermined in 63 patients (ISEE: n = 22, 33.3% vs. SD: n = 41, 28.9%) (Table 4).
3.2. Treatment
3.2.1. Surgical procedures
Patients with ISEE received abscess evacuation with/without drainage in 95% (63 cases), whereas in only 5% (3 cases) abscess evacuation was managed by ACDF. No instrumentation was performed in any patient with ISEE. In contrast, instrumentation (ventral, dorsal, or ventrodorsal) was performed in 61% of SD (87 patients), whereas only 30 patients (21%) underwent abscess evacuation with/without drainage and 25 patients (18%) received ACDF (Figure 3).
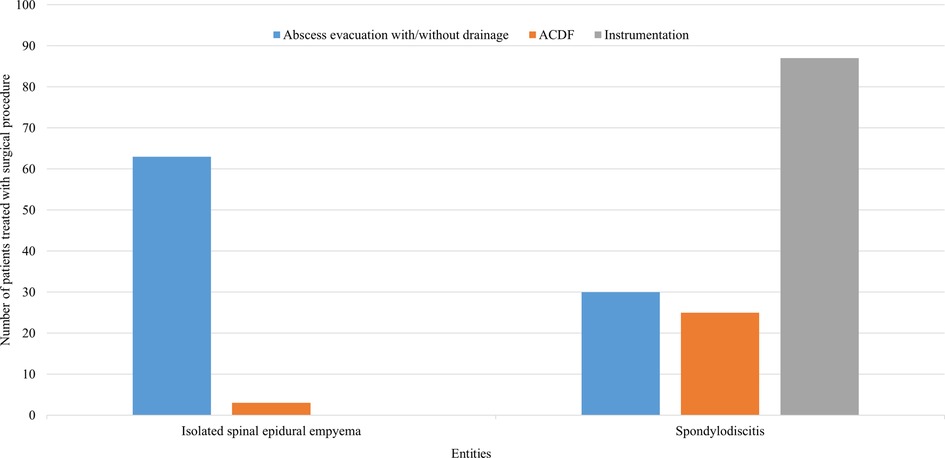
Figure 3. Surgical procedures used in SD and ISEE. This figure represents the surgical procedures for spondylodiscitis (SD) and isolated spinal epidural empyema (ISEE). ISEE were mainly addressed using abscess evacuation with/without drainage (n = 63, 95%), whereas SD were mostly managed with ventral, dorsal, or ventrodorsal instrumentation (n = 87, 61%). ACDF, anterior cervical discectomy with fusion.
3.2.2. CT-guided biopsy in case of psoas abscess
A psoas abscess was found in 128 patients (61.5%), whereas 33.7% (70/208) of our cohort (SD and ISEE) underwent CT-guided biopsy. Psoas abscess was detected in 66.9% of SD patients (95/142), and 35.2% (50/142) of those underwent CT-guided biopsy with abscess drainage. Half (33/66) of the ISEE patients had developed a psoas abscess, and CT-guided biopsy with abscess drainage was performed in 30.0% (20/66).
3.2.3. Antibiotic treatment
A total of 140 patients (67.3%) received empiric antibiotic therapy (ISEE: n = 51, 77.3% vs. SD: n = 89, 62.7%), which was switched to targeted antibiotic therapy based on pathogen identification. A causative pathogen was isolated in 68 patients (32.7%) before targeted antibiotic therapy began (ISEE: n = 15, 22.7% vs. SD: n = 53, 37.3%). Intravenous antibiotic therapy was switched to oral antibiotics in SD patients after 4 weeks (range: 0–19), and the total duration of antibiotic therapy was 10 weeks (range: 0–52). By contrast, ISEE patients received 2 week (range: 1–3) intravenous antibiotic treatment, which was then switched to oral administration for a total duration of 6 weeks (rage: 2–15).
3.3. Complications
Patients with ISEE were less likely to develop sepsis during the disease course than patients with SD (ISEE: n = 12, 18.2% vs. SD: n = 94, 66.2%, p < 0.001). Septic embolism occurred similarly less in ISEE than SD (ISEE: n = 4/48 cases, 8.3% vs. SD: n = 52/117 cases, 44.4%, p < 0.001). Required examinations to exclude septic embolism during disease course were lacking in 18 ISEE-patients and 25 SD-patients. We identified less infective endocarditis with proven vegetation in TTE in ISEE than in SD (ISEE: n = 1/52 cases, 1.9% vs. SD: n = 23/125 cases, 18.4%, p = 0.003). TEE was not performed in 14 ISEE-patients and in 17 SD-patients. The relapse rate was higher in SD-patients (ISEE: n = 4/46, 8.7% vs. SD: n = 27/92, 29.3%, p = 0.004). Relapse was defined as clinical, microbiological, and/or radiological progression during or after treatment up to the final follow-up of one year. Of note, 70 patients (50 SD, 20 ISEE, 33.7%) were lost to follow-up. Disease-related mortality was not significantly different between both entities (ISEE: n = 1, 1.5% vs. SD: n = 11, 7.7%, p = 0.108) (Figure 4).
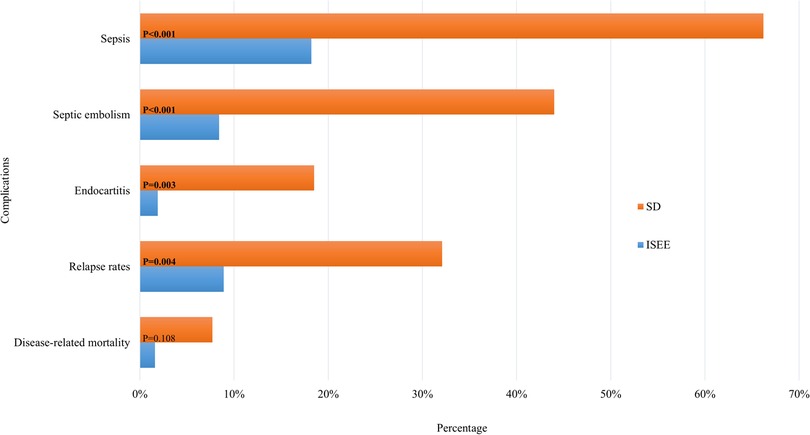
Figure 4. Complications in SD and ISEE. This figure shows the complications of spondylodiscitis (SD) and isolated spinal epidural empyema (ISEE). Sepsis, septic embolism, endocarditis, relapse rate and mortality rate demonstrate the contrast between the two pathologies. Required examinations to exclude septic embolism during disease course were lacking in 18 ISEE-patients and 25 SD-patients. TEE was not performed in 14 ISEE-patients and in 17 SD-patients. Relapse was defined as clinical, microbiological, and/or radiological progression during or after treatment up to the final follow-up of one year. Of note, 70 patients (50 SD, 20 ISEE, 33.7%) were lost to follow-up. * Indicates p-value in fisher's exact test.
3.4. Length of stay in hospital and intensive care unit
The length of hospital stay was longer in SD than ISEE (ISEE: 22 [15, 30] d vs. SD: 38 [29, 53] d, median [interquartile range], p < 0.001) as well as the length of ICU stay (ISEE: 0 [0, 4] d vs. SD: 3 [0, 12] d, p < 0.002) (Figure 5).
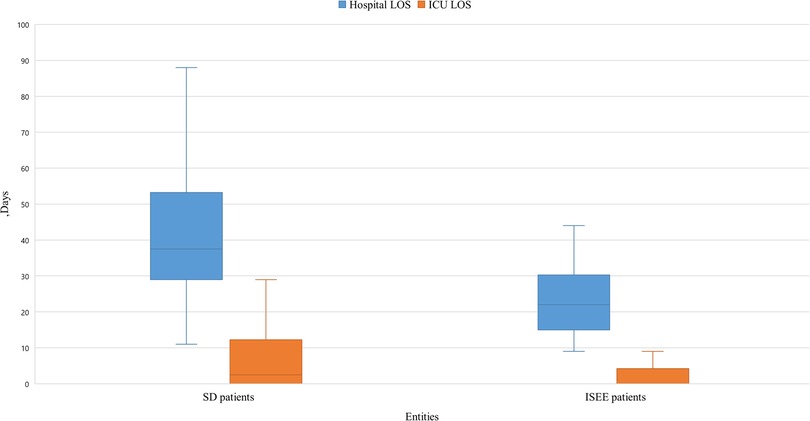
Figure 5. Length of stay in hospital and ICU of SD and ISEE. This figure displays the length of stay (LOS) in hospital and on the intensive care unit (ICU) in isolated spinal epidural empyema (ISEE) and spondylodiscitis (SD), which was significantly longer in the SD cohort than in the ISEE cohort (ISEE: 22 d vs. SD: 37 d, p < 0.001). LOS on the ICU differed significantly for both groups (median: ISEE 0 d vs. SD 2.5 d, p < 0.002). Box-and-whisker plot display sample mean (horizontal bar) and interquartile range from first to third quartiles (shadowed area). Whiskers indicate adjacent values (defined as ±1.5xIQR).
3.5. Occurrence rates of SD and ISEE in our centre between 2002 and 2021
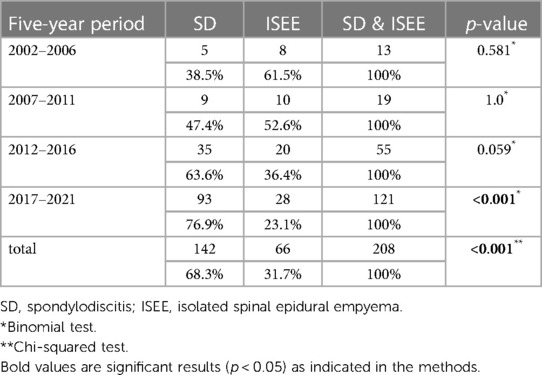
We observed a constantly increasing number of SD and ISEE cases over the last 20 years in our centre. SD cases occurred in a significantly higher range than ISEE cases (p < 0.001). We identified 13 cases between 2002 and 2006 (ISEE: 8 vs. SD: 5, p = 0.581), 19 cases between 2007 and 2011 (ISEE: 10 vs. SD: 9, p = 1.0), 55 cases between 2012 and 2016 (ISEE: 20 vs. SD: 35, p = 0.059), and 121 cases between 2017 and 2021 (ISEE: 28 vs. SD: 93, p < 0.001) (Table 5 and Figure 6).
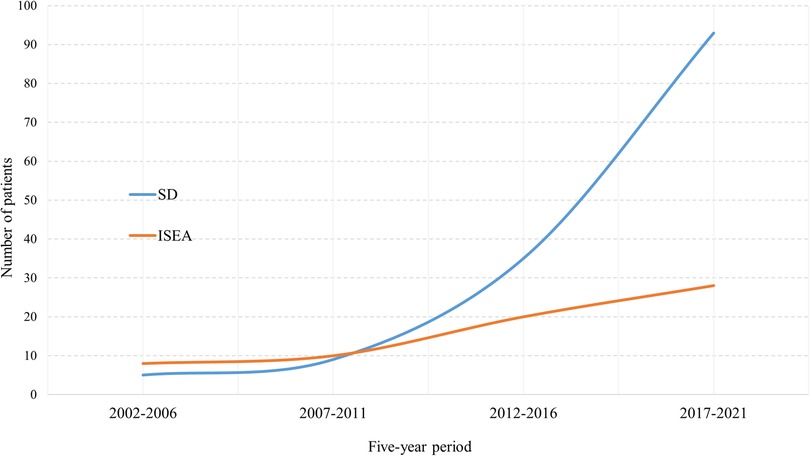
Figure 6. Five-year occurrence rates of SD and ISEE. This figure demonstrates the 5-year occurrence rate of spondylodiscitis (SD) and isolated spinal epidural empyema (ISEE) between 2002 and 2021 at our centre.
3.6. Risk factor associations of complications
Univariate and multivariate analyses are summarized in (Table 6). Sex (p = 0.036; OR: 0.430; 95% CI: 0.195–0.948) and entities (p < 0.001; OR: 0.151; 95% CI: 0.066–0.346) were identified as independent risk factors for development of complications such as sepsis, septic embolism, endocarditis, relapse, and disease-related mortality. Univariate analysis revealed that sex, age over 65 years, and entities were associated with complications, relapse, and disease-related mortality.
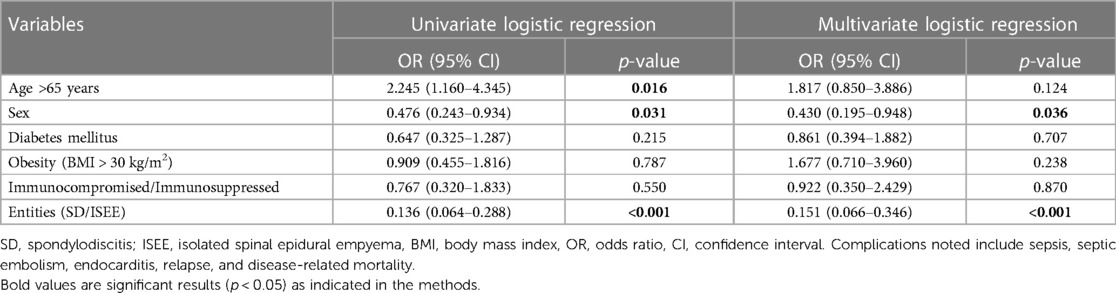
Table 6. Univariate and multivariate logistic regression analysis to identify independent risk factors for the development of complications.
4. Discussion
Our study provides insights into the management and clinical course of SD and ISEE and identifies distinct entity-related patterns of the characteristics and outcomes. In previous studies, SD and ISEE were diagnosed in male patients twice as often as females (22–25). In our cohort, SD occurred predominantly in male patients and was associated with a significantly higher age at diagnosis than patients in the ISEE group, in which the gender distribution was more balanced.
Diabetes mellitus, obesity, immunosuppression, age, and gender are the most known risk factors (12). SD showed significantly more obese elderly male patients than SD. Patients with diabetes mellitus and immunosuppression were equally distributed in both groups.
The pathogen detection rate in our study is higher than in previous reports (26–28). We detected pathogens in 92.3% using blood cultures, intraoperative specimens, and/or CT-guided biopsies, with MSSA as the most common pathogen. MSSA is the predominant bacterial aetiology in ISEE and SD (5, 23, 24, 27, 29–38).
Current studies demonstrate that the lumbar spine is the most involved region in ISEE and SD patients, followed by the thoracic spine (12, 16, 17, 23, 38–42). 67.6% of our SD and 62.1% of ISEE cases occurred in the lumbar spine, while the cervical spine was the least affected.
It is difficult to determine whether an infected organ is the primary infectious source causing secondary bacteraemia or if the organ was infected secondarily due to a primary bacteraemia. Indeed, from a therapeutic perspective, this differentiation is less relevant since both the infected focus and the bacteraemia must be simultaneously treated (43). A primary infectious source can be identified in 60% of ISEE cases and in 50%–68% of SD cases (23, 44). We found a primary infectious source in 71.1% of SD cases and in 66.7% of ISEE cases.
Skin infections were reported to present the most common focus in patients with SD (32, 34, 35). Similarly, Krishnamohan et al. reported that skin infections were the most frequent infectious source for ISEE. This is concurrent with our study, in which skin infections demonstrated the major primary source in the SD and ISEE groups. Chae et al. identified in 26% of ISEE cases epidural infiltration or acupuncture as the infectious source, which is consistent with our study in ISEE patients (34.1%) (44, 45).
Consistent with previous studies, we treated our ISEE patients almost exclusively with abscess evacuation with/without drainage (46, 47). SD patients were mainly instrumented (ventral, dorsal, or ventrodorsal) which has been shown to be an effective procedure (23, 48).
A paraspinal abscess was found in approximately 47% of SD cases with/without epidural empyema (26, 27, 35, 49, 50). However, Chae, H. J. et al. found a psoas abscess in 65% of ISEE patients (45). A paraspinal or psoas abscess was identified in 61.5% of our cases (SD and ISEE combined), and CT-guided biopsy with abscess drainage was performed in 33.7% of cases. The occurrence of psoas or paraspinal abscess was higher in the SD than in our ISEE cohort (66.9% vs. 50%).
Our medical management includes initially empiric or targeted antibiotic therapy, depending on the clinical severity of the disease, according to the recommendations of the literature and guidelines of the Infectious Diseases Society of America (IDSA) with an individually adapted therapy (12, 31, 38, 51). Two weeks of intravenous and four weeks of oral antibiotic therapy were performed in the ISEE patients, while the SD patients received four weeks of intravenous and 6 weeks of oral antibiotics.
Complications are common in SD and ISEE patients. Severe sepsis with multiple organ failures in SD patients with/without epidural empyema were reported in 14%–20% of cases (36, 52). Similarly, approximately 18% of SD patients with/without epidural empyema had a septic embolism during the course of the disease (52). We found that SD patients were significantly more likely to develop sepsis or septic embolism during the disease course than their ISEE counterparts. The pathogenesis of spondylodiscitis is thought to be septic embolic spread in the endplates of the vertebral body, where the vessels terminate, due to bacteraemia. This may explain the frequency of further septic embolism compared to local infection as in ISEE.
Endocarditis in SD patients with/without epidural empyema was widely described, for instance Patzakis et al. reported on 2% of endocarditis cases in his cohort (53), Pojskic et al. 4.2% (36), Nolla et al. 6% (27), Chae et al. 9% (45), Chelsom et al. 10% (54), Geisler et al. 12% (52), Mylona also et al. 12% (23), Osenbach et al. 15% (34), Ledermann et al. 24% (55), and Pigrau et al. 31% (35). Endocarditis was observed significantly more frequent in the SD than in the ISEE group (18.4% vs. 1.9%), which may be due to the haematogenous dissemination of SD.
Clinical, microbiologic, and radiological relapse rates following completed antibiotic treatment has been used in numerous studies to evaluate the success of SI treatment (56–58). Relapse rate in SD and ISEE patients was reported in 8%–15% (36, 59, 60). We noted a higher rate of relapses in the SD group than in the ISEE group (29.3% vs. 8.7%). Mylona et al. reported a mortality rate of 6% in a systematic review of 14 previous studies (23), which was comparable to the mortality rate in our study. However, the mortality rate was higher in SD patients than in ISEE patients.
Patients with SD or ISEE spent an average of 31.5–34 days in the hospital (41, 61). We found that SD patients stayed significantly longer in the hospital compared with ISEE patients (38 vs. 22 days). In addition, SD and ISEE patients stayed in the ICU for an average of 4 days (±11.0, range 1–68) (61). Thus, SD patients in our cohort spent more time in the ICU than ISEE patients (median: 3 vs. 0 days).
The incidences of SD and ISEE in western countries are estimated between 0.2–2.4/100,000 and 2.5–3.0/100,000, respectively (14, 40, 62, 63), with an increasing incidence in the last decades (7, 16, 24, 30, 37, 39, 64). Indeed, at our institution we observed a significant increase in SD and ISEE cases in the last 20 years. However, SD was more common than ISEE (68.3% vs. 31.7%).
4.1. Limitations and strengths of this study
The limitations of our retrospective study include a possible selection bias toward more severe cases because of the high degree of specialization of our university centre. The single-centric nature of our analysis might reduce the external validity of our observations. However, our cohort analysis is based at 20-year period of SD treatment in a large university neurosurgery centre, suggesting high internal validity of our study. Therefore, our observations might be useful to understand the clinical outcomes in patients with ISEE and SD. Furthermore, our data provide a basis for the design of prospective observational and interventional research.
5. Conclusions
Patients with ISEE show a more favourable disease course (sepsis, septic embolism, endocarditis, relapse rate, disease-related mortality, and LOS in hospital/ICU) than SD patients. Gender, age, occurrence rate of disease over the last 20 years, primary infection source, causative pathogens, localization, type of surgical procedure, and duration of antibiotic therapy differ between both pathologies. Therefore, it appears probable that both pathologies constitute two separate entities with different requirements on clinical management. The differences between both entities warrant prospective, multicentre follow-up research to further elucidate clinical and pathophysiologic patterns in these diseases.
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics committee of the university hospital Dresden (Reference number BO-EK-17012022). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
Conceptualization, data curation, investigation, methodology, project administration, resources, software, validation, visualization and writing original draft by HMM. Supervision by TJ and TS. Writing review and editing by TJ, TS, IE, DP, PG and GS. Drafting of figures and tables displayed in this article by HMM. All authors have read and agreed to the published version of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Calhoun JH, Manring MM. Adult osteomyelitis. Infect Dis Clin North Am. (2005) 19(4):765–86. doi: 10.1016/j.idc.2005.07.009
2. Lener S, Hartmann S, Barbagallo GMV, Certo F, Thome C, Tschugg A. Management of spinal infection: a review of the literature. Acta Neurochir (Wien). (2018) 160(3):487–96. doi: 10.1007/s00701-018-3467-2
3. Nickerson EK, Sinha R. Vertebral osteomyelitis in adults: an update. Br Med Bull. (2016) 117(1):121–38. doi: 10.1093/bmb/ldw003
4. Rosc-Bereza K, Arkuszewski M, Ciach-Wysocka E, Boczarska-Jedynak M. Spinal epidural abscess: common symptoms of an emergency condition. A case report. Neuroradiol J. (2013) 26(4):464–8. doi: 10.1177/197140091302600411
5. Hadjipavlou AG, Mader JT, Necessary JT, Muffoletto AJ. Hematogenous pyogenic spinal infections and their surgical management. Spine (Phila Pa 1976). (2000) 25(13):1668–79. doi: 10.1097/00007632-200007010-00010
6. Corrah TW, Enoch DA, Aliyu SH, Lever AM. Bacteraemia and subsequent vertebral osteomyelitis: a retrospective review of 125 patients. QJM. (2011) 104(3):201–7. doi: 10.1093/qjmed/hcq178
7. Stuer C, Stoffel M, Hecker J, Ringel F, Meyer B. A staged treatment algorithm for spinal infections. J Neurol Surg A Cent Eur Neurosurg. (2013) 74(2):87–95. doi: 10.1055/s-0032-1320022
8. Aagaard T, Roed C, Dahl B, Obel N. Long-term prognosis and causes of death after spondylodiscitis: a Danish nationwide cohort study. Infect Dis (Lond). (2016) 48(3):201–8. doi: 10.3109/23744235.2015.1103897
9. Artenstein AW, Friderici J, Holers A, Lewis D, Fitzgerald J, Visintainer P. Spinal epidural abscess in adults: a 10-year clinical experience at a tertiary care academic medical center. Open Forum Infect Dis. (2016) 3(4):ofw191. doi: 10.1093/ofid/ofw191
10. Kehrer M, Pedersen C, Jensen TG, Lassen AT. Increasing incidence of pyogenic spondylodiscitis: a 14-year population-based study. J Infect. (2014) 68(4):313–20. doi: 10.1016/j.jinf.2013.11.011
11. Schoenfeld AJ, Wahlquist TC. Mortality, complication risk, and total charges after the treatment of epidural abscess. Spine J. (2015) 15(2):249–55. doi: 10.1016/j.spinee.2014.09.003
12. Herren C, Jung N, Pishnamaz M, Breuninger M, Siewe J, Sobottke R. Spondylodiscitis: diagnosis and treatment options. Dtsch Arztebl Int. (2017) 114(51–52):875–82. doi: 10.3238/arztebl.2017.0875
13. de Lucas EM, Gonzalez Mandly A, Gutierrez A, Pellon R, Martin-Cuesta L, Izquierdo J, et al. CT-guided fine-needle aspiration in vertebral osteomyelitis: true usefulness of a common practice. Clin Rheumatol. (2009) 28(3):315–20. doi: 10.1007/s10067-008-1051-5
14. Arko L 4th, Quach E, Nguyen V, Chang D, Sukul V, Kim BS. Medical and surgical management of spinal epidural abscess: a systematic review. Neurosurg Focus. (2014) 37(2):E4. doi: 10.3171/2014.6.FOCUS14127
15. Ju KL, Kim SD, Melikian R, Bono CM, Harris MB. Predicting patients with concurrent noncontiguous spinal epidural abscess lesions. Spine J. (2015) 15(1):95–101. doi: 10.1016/j.spinee.2014.06.008
16. Schwab JH, Shah AA. Spinal epidural abscess: diagnosis, management, and outcomes. J Am Acad Orthop Surg. (2020) 28(21):e929–38. doi: 10.5435/JAAOS-D-19-00685
17. Shah AA, Ogink PT, Harris MB, Schwab JH. Development of predictive algorithms for pre-treatment motor deficit and 90-day mortality in spinal epidural abscess. J Bone Joint Surg Am. (2018) 100(12):1030–8. doi: 10.2106/JBJS.17.00630
18. Tschugg A, Lener S, Hartmann S, Rietzler A, Neururer S, Thome C. Primary acquired spondylodiscitis shows a more severe course than spondylodiscitis following spine surgery: a single-center retrospective study of 159 cases. Neurosurg Rev. (2018) 41(1):141–7. doi: 10.1007/s10143-017-0829-9
19. Zhou B, Kang YJ, Chen WH. Continuous epidural irrigation and drainage combined with posterior debridement and posterior lumbar inter-body fusion for the management of single-segment lumbar pyogenic spondylodiscitis. Surg Infect (Larchmt). (2020) 21(3):262–7. doi: 10.1089/sur.2019.038
20. Berbari EF, Kanj SS, Kowalski TJ, Darouiche RO, Widmer AF, Schmitt SK, et al. Executive summary: 2015 infectious diseases society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis. (2015) 61(6):859–63. doi: 10.1093/cid/civ633
21. Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG Jr, Ryan T, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. (2000) 30(4):633–8. doi: 10.1086/313753
22. Grammatico L, Baron S, Rusch E, Lepage B, Surer N, Desenclos JC, et al. Epidemiology of vertebral osteomyelitis (VO) in France: analysis of hospital-discharge data 2002–2003. Epidemiol Infect. (2008) 136(5):653–60. doi: 10.1017/S0950268807008850
23. Mylona E, Samarkos M, Kakalou E, Fanourgiakis P, Skoutelis A. Pyogenic vertebral osteomyelitis: a systematic review of clinical characteristics. Semin Arthritis Rheum. (2009) 39(1):10–7. doi: 10.1016/j.semarthrit.2008.03.002
24. Reihsaus E, Waldbaur H, Seeling W. Spinal epidural abscess: a meta-analysis of 915 patients. Neurosurg Rev. (2000) 23(4):175–204; discussion 205. doi: 10.1007/PL00011954
25. Zarghooni K, Rollinghoff M, Sobottke R, Eysel P. Treatment of spondylodiscitis. Int Orthop. (2012) 36(2):405–11. doi: 10.1007/s00264-011-1425-1
26. McHenry MC, Easley KA, Locker GA. Vertebral osteomyelitis: long-term outcome for 253 patients from 7 Cleveland-area hospitals. Clin Infect Dis. (2002) 34(10):1342–50. doi: 10.1086/340102
27. Nolla JM, Ariza J, Gomez-Vaquero C, Fiter J, Bermejo J, Valverde J, et al. Spontaneous pyogenic vertebral osteomyelitis in nondrug users. Semin Arthritis Rheum. (2002) 31(4):271–8. doi: 10.1053/sarh.2002.29492
28. Stangenberg M, Mende KC, Mohme M, Kratzig T, Viezens L, Both A, et al. Influence of microbiological diagnosis on the clinical course of spondylodiscitis. Infection. (2021) 49(5):1017–27. doi: 10.1007/s15010-021-01642-5
29. Adogwa O, Karikari IO, Carr KR, Krucoff M, Ajay D, Fatemi P, et al. Spontaneous spinal epidural abscess in patients 50 years of age and older: a 15-year institutional perspective and review of the literature: clinical article. J Neurosurg Spine. (2014) 20(3):344–9. doi: 10.3171/2013.11.SPINE13527
30. Baker AS, Ojemann RG, Swartz MN, Richardson EP Jr. Spinal epidural abscess. N Engl J Med. (1975) 293(10):463–8. doi: 10.1056/NEJM197509042931001
31. Bernard L, Dinh A, Ghout I, Simo D, Zeller V, Issartel B, et al. Antibiotic treatment for 6 weeks versus 12 weeks in patients with pyogenic vertebral osteomyelitis: an open-label, non-inferiority, randomised, controlled trial. Lancet. (2015) 385(9971):875–82. doi: 10.1016/S0140-6736(14)61233-2
32. Colmenero JD, Jimenez-Mejias ME, Sanchez-Lora FJ, Reguera JM, Palomino-Nicas J, Martos F, et al. Pyogenic, tuberculous, and brucellar vertebral osteomyelitis: a descriptive and comparative study of 219 cases. Ann Rheum Dis. (1997) 56(12):709–15. doi: 10.1136/ard.56.12.709
33. Hlavin ML, Kaminski HJ, Ross JS, Ganz E. Spinal epidural abscess: a ten-year perspective. Neurosurgery. (1990) 27(2):177–84. doi: 10.1227/00006123-199008000-00001
34. Osenbach RK, Hitchon PW, Menezes AH. Diagnosis and management of pyogenic vertebral osteomyelitis in adults. Surg Neurol. (1990) 33(4):266–75. doi: 10.1016/0090-3019(90)90047-S
35. Pigrau C, Almirante B, Flores X, Falco V, Rodriguez D, Gasser I, et al. Spontaneous pyogenic vertebral osteomyelitis and endocarditis: incidence, risk factors, and outcome. Am J Med. (2005) 118(11):1287. doi: 10.1016/j.amjmed.2005.02.027
36. Pojskic M, Carl B, Schmockel V, Vollger B, Nimsky C, Sabeta B. Neurosurgical management and outcome parameters in 237 patients with spondylodiscitis. Brain Sci. (2021) 11(8):1019. doi: 10.3390/brainsci11081019
37. Rigamonti D, Liem L, Sampath P, Knoller N, Namaguchi Y, Schreibman DL, et al. Spinal epidural abscess: contemporary trends in etiology, evaluation, and management. Surg Neurol. (1999) 52(2):189–96.doi: 10.1016/S0090-3019(99)00055-5
38. Ryang YM, Akbar M. [Pyogenic spondylodiscitis: symptoms, diagnostics and therapeutic strategies]. Orthopade. (2020) 49(8):691–701. doi: 10.1007/s00132-020-03945-1
39. Darouiche RO. Spinal epidural abscess. N Engl J Med. (2006) 355(19):2012–20. doi: 10.1056/NEJMra055111
40. Gouliouris T, Aliyu SH, Brown NM. Spondylodiscitis: update on diagnosis and management. J Antimicrob Chemother. (2010) 65(Suppl 3):iii11–24. doi: 10.1093/jac/dkq303
41. Loibl M, Stoyanov L, Doenitz C, Brawanski A, Wiggermann P, Krutsch W, et al. Outcome-related co-factors in 105 cases of vertebral osteomyelitis in a tertiary care hospital. Infection. (2014) 42(3):503–10. doi: 10.1007/s15010-013-0582-0
42. Patel AR, Alton TB, Bransford RJ, Lee MJ, Bellabarba CB, Chapman JR. Spinal epidural abscesses: risk factors, medical versus surgical management, a retrospective review of 128 cases. Spine J. (2014) 14(2):326–30. doi: 10.1016/j.spinee.2013.10.046
43. Rubinstein E. Staphylococcus aureus bacteraemia with known sources. Int J Antimicrob Agents. (2008) 32(Suppl 1):S18–20. doi: 10.1016/j.ijantimicag.2008.06.006
44. Krishnamohan P, Berger JR. Spinal epidural abscess. Curr Infect Dis Rep. (2014) 16(11):436. doi: 10.1007/s11908-014-0436-7
45. Chae HJ, Kim J, Kim C. Clinical characteristics of spinal epidural abscess accompanied by bacteremia. J Korean Neurosurg Soc. (2021) 64(1):88–99. doi: 10.3340/jkns.2020.0278
46. Curry WT Jr, Hoh BL, Amin-Hanjani S, Eskandar EN. Spinal epidural abscess: clinical presentation, management, and outcome. Surg Neurol. (2005) 63(4):364–71. doi: 10.1016/j.surneu.2004.08.081
47. Sorensen P. Spinal epidural abscesses: conservative treatment for selected subgroups of patients. Br J Neurosurg. (2003) 17(6):513–8. doi: 10.1080/02688690310001627731
48. Tschugg A, Hartmann S, Lener S, Rietzler A, Sabrina N, Thome C. Minimally invasive spine surgery in lumbar spondylodiscitis: a retrospective single-center analysis of 67 cases. Eur Spine J. (2017) 26(12):3141–6. doi: 10.1007/s00586-017-5180-x
49. Kaya S, Kaya S, Kavak S, Comoglu S. A disease that is difficult to diagnose and treat: evaluation of 343 spondylodiscitis cases. J Int Med Res. (2021) 49(11):3000605211060197. doi: 10.1177/03000605211060197
50. Zarrouk V, Feydy A, Salles F, Dufour V, Guigui P, Redondo A, et al. Imaging does not predict the clinical outcome of bacterial vertebral osteomyelitis. Rheumatology (Oxford). (2007) 46(2):292–5. doi: 10.1093/rheumatology/kel228
51. Mann S, Schutze M, Sola S, Piek J. Nonspecific pyogenic spondylodiscitis: clinical manifestations, surgical treatment, and outcome in 24 patients. Neurosurg Focus. (2004) 17(6):E3. doi: 10.3171/foc.2004.17.6.3
52. Geisler Crone C, Mose Tetens M, Bengaard Andersen A, Obel N, Lebech AM. Clinical characteristics of pyogenic vertebral osteomyelitis, and factors associated with inadequate treatment response. Int J Infect Dis. (2021) 108:487–93. doi: 10.1016/j.ijid.2021.05.078
53. Patzakis MJ, Rao S, Wilkins J, Moore TM, Harvey PJ. Analysis of 61 cases of vertebral osteomyelitis. Clin Orthop Relat Res. (1991) (264):178–83. PMID:19972331997233
54. Chelsom J, Solberg CO. Vertebral osteomyelitis at a Norwegian university hospital 1987–97: clinical features, laboratory findings and outcome. Scand J Infect Dis. (1998) 30(2):147–51. doi: 10.1080/003655498750003537
55. Ledermann HP, Schweitzer ME, Morrison WB, Carrino JA. MR Imaging findings in spinal infections: rules or myths? Radiology. (2003) 228(2):506–14. doi: 10.1148/radiol.2282020752
56. Chang WS, Ho MW, Lin PC, Ho CM, Chou CH, Lu MC, et al. Clinical characteristics, treatments, and outcomes of hematogenous pyogenic vertebral osteomyelitis, 12-year experience from a tertiary hospital in central Taiwan. J Microbiol Immunol Infect. (2018) 51(2):235–42. doi: 10.1016/j.jmii.2017.08.002
57. Lee YM, Cho OH, Park SY, Moon C, Chong YP, Kim SH, et al. Factors associated with sequelae after treatment of hematogenous pyogenic vertebral osteomyelitis. Diagn Microbiol Infect Dis. (2019) 94(1):66–72. doi: 10.1016/j.diagmicrobio.2018.11.024
58. Park KH, Cho OH, Lee JH, Park JS, Ryu KN, Park SY, et al. Optimal duration of antibiotic therapy in patients with hematogenous vertebral osteomyelitis at low risk and high risk of recurrence. Clin Infect Dis. (2016) 62(10):1262–9. doi: 10.1093/cid/ciw098
59. Pola E, Taccari F, Autore G, Giovannenze F, Pambianco V, Cauda R, et al. Multidisciplinary management of pyogenic spondylodiscitis: epidemiological and clinical features, prognostic factors and long-term outcomes in 207 patients. Eur Spine J. (2018) 27(Suppl 2):229–36. doi: 10.1007/s00586-018-5598-9
60. Torda AJ, Gottlieb T, Bradbury R. Pyogenic vertebral osteomyelitis: analysis of 20 cases and review. Clin Infect Dis. (1995) 20(2):320–8. doi: 10.1093/clinids/20.2.320
61. Spiegl UJA, Kilper A, Glasmacher S, Heyde CE, Josten C. [Which factors influence the inpatient course for patients with spondylodiscitis?]. Unfallchirurg. (2020) 123(9):724–30. doi: 10.1007/s00113-020-00781-y
62. Cheung WY, Luk KD. Pyogenic spondylitis. Int Orthop. (2012) 36(2):397–404. doi: 10.1007/s00264-011-1384-6
63. Sendi P, Bregenzer T, Zimmerli W. Spinal epidural abscess in clinical practice. QJM. (2008) 101(1):1–12. doi: 10.1093/qjmed/hcm100
Keywords: spondylodiscitis, vertebral ostemyelitis, sepsis, endocarditis, septic embolism, primary spinal infection, isolated spinal epidural empyema
Citation: Hijazi MM, Siepmann T, El-Battrawy I, Glatte P, Eyüpoglu I, Schackert G, Juratli TA and Podlesek D (2023) Clinical phenotyping of spondylodiscitis and isolated spinal epidural empyema: a 20-year experience and cohort study. Front. Surg. 10:1200432. doi: 10.3389/fsurg.2023.1200432
Received: 4 April 2023; Accepted: 2 May 2023;
Published: 18 May 2023.
Edited by:
Christoph Hohenberger, Departement of Neurosurgery Universitätsklinikum Regensburg, GermanyReviewed by:
Ioannis Siasios, Papageorgiou General Hospital, GreeceBasem Ishak, Heidelberg University, Germany
© 2023 Hijazi, Siepmann, El-Battrawy, Glatte, Eyüpoglu, Schackert, Juratli and Podlesek. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mido Max Hijazi bWlkby5oaWphemlAdWtkZC5kZQ==
 Mido Max Hijazi
Mido Max Hijazi Timo Siepmann
Timo Siepmann Ibrahim El-Battrawy
Ibrahim El-Battrawy Patrick Glatte
Patrick Glatte Ilker Eyüpoglu1
Ilker Eyüpoglu1 Tareq A. Juratli
Tareq A. Juratli Dino Podlesek
Dino Podlesek