- 1Department of Cardiothoracic Surgery, Neijiang Hospital of Traditional Chinese Medicine, Neijiang, China
- 2Department of Thoracic Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing, China
- 3Department of Thoracic Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital Hebei Hospital, Chinese Academy of Medical Sciences, Langfang, China
- 4Department of Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing, China
Objective: To investigate the feasibility of laparoscopic abdominal mobilization in patients with cancers of the esophagus or gastroesophageal junction who have a history of abdominal surgery.
Methods: A total of 132 patients who underwent resection for cancers of the esophagus or gastroesophageal junction from August 2018 to March 2022 in the Department of Thoracic Surgery, Cancer Hospital, Chinese Academy of Medical Sciences, were selected (66 patients with a history of abdominal surgery (observation group) and 66 patients without a history of abdominal surgery (control group)). All patients were treated with preoperative neoadjuvant therapy, based on the clinical stage. Thoracoscopic and laparoscopic resection was performed under general anesthesia. The intraoperative and postoperative conditions and surgical complications were compared between the two groups.
Results: No significant differences were found in baseline data between the observation group and the control group (p > 0.05). Laparoscopic abdominal mobilization was completed in both groups, and there were no significant differences between the two groups in the total operation time [(272.50 ± 86.45) min vs. (257.55 ± 67.96) min], abdominal mobilization time [(25.03 ± 9.82) min vs. (22.53 ± 3.88) min], blood loss [(119.09 ± 72.17) ml vs. (104.39 ± 43.82) ml], and postoperative time to first flatus [(3.44 ± 0.73) d vs. (3.29 ± 0.60) d] (p > 0.05). The abdominal mobilization time was longer in observation group than that in control group (p = 0.057). After excluding the patients (31/66) with a history of simple appendectomy from the observation group, the abdominal mobilization time was significantly longer in observation group than that in control group [(27.97 ± 12.16) min vs. (22.53 ± 3.88) min] (p < 0.05). There were significantly fewer dissected abdominal lymph nodes in the observation group than in the control group [(18.44 ± 10.87) vs. (23.09 ± 10.95), p < 0.05]. After excluding the patients (15/66) with a history of abdominal tumor surgery from the observation group, there was no significant difference in the number of dissected abdominal lymph nodes between the two groups [(20.62 ± 10.81) vs. (23.09 ± 10.95)] (p > 0.05).In addition, no postoperative complications, such as intestinal obstruction, abdominal infection and bleeding, occurred in either group.
Conclusion: Patients with cancers of the esophagus or gastroesophageal junction who have a history of abdominal surgery are suitable for minimally invasive laparoscopic mobilization.
1. Introduction
Cancers of the esophagus and gastroesophageal junction are common upper gastrointestinal malignancies. In 2016, the morbidity and mortality rates of cancer of the esophagus were 8.2/100,000 and 12.37/100,000 in China, ranking sixth and fifth among malignancies, respectively (1). Surgery is the main treatment means for cancers of the esophagus and gastroesophageal junction. In particular, thoracoscopic and laparoscopic surgery has been widely used in clinical treatment because it is minimally invasive, with a rapid postoperative recovery, a short length of stay, and few pulmonary complications (2, 3). With the rising incidence of cancer, there have been increasingly more patients with secondary cancer or a history of abdominal surgery. Studies have shown that intestinal adhesions occur in approximately 90% of patients following abdominal surgery (4) and that isolating the adhesion produces a risk of injury to the intestinal tract, making surgical operations complex and secondary tumor resection difficult. At present, minimally invasive laparoscopic surgery has advantages with regard to the treatment of lower gastrointestinal tumor patients with a history of abdominal surgery. However, the effect of laparoscopic abdominal mobilization in patients with cancers of the esophagus or gastroesophageal junction who have a history of abdominal surgery remains unclear. In this study, 66 patients with cancers of the esophagus or gastroesophageal junction who had abdominal surgery from August 2018 to March 2022 were selected, and they underwent thoracoscopic and laparoscopic surgery and laparoscopic abdominal mobilization.
2. Materials and methods
2.1. General data
Patients in the Esophageal Ward of the Cancer Hospital, Chinese Academy of Medical Sciences, from August 2018 to March 2022, with resectable cancers of the esophagus or gastroesophageal junction and a history of abdominal surgery were selected as the observation group. Patients with a history of abdominal surgery at least once were enrolled, and those with a history of inguinal hernia repair, simple laparoscopic exploration, endoscopic therapy or planned open surgery were excluded. Finally, a total of 66 patients were included. Additionally, 66 patients during the same period with resectable cancers of the esophagus or gastroesophageal junction who had no history of abdominal surgery were randomly selected as the control group. The general data of patients in the observation group and control group are shown in Table 1.
2.2. Methods
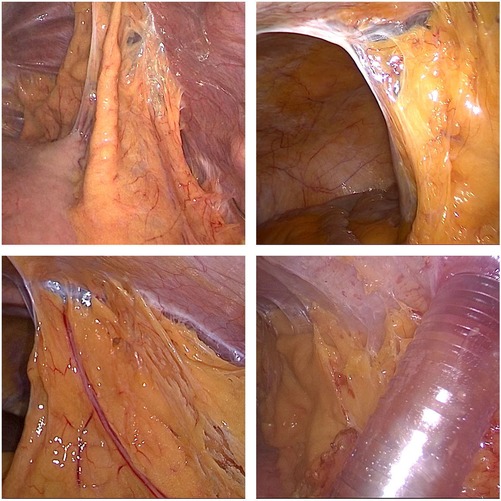
All patients were treated with neoadjuvant therapies, including chemoradiotherapy, chemotherapy, chemoradiotherapy + immunotherapy or chemotherapy + immunotherapy, based on the clinical stage. Thoracoscopic and laparoscopic resection was performed under general anesthesia. Specifically, McKeown surgery was performed for most cases of cancer of the esophagus, and Ivor-Lewis surgery was performed for some cases of cancer of the lower esophagus and most cases of cancer of the gastroesophageal junction. During abdominal mobilization, the chief surgeon standing on the left side of the patient made five semicircular incisions from the umbilicus to the bilateral subcostal arch and a small median incision in the abdomen, laparoscopically dissociated the stomach, omentum and blood vessels, and dissected abdominal lymph nodes. Finally, the stomach was taken out through the small median incision, and a tubular stomach was prepared under direct vision in vitro (Figure 1). For patients who required esophageal replacement with jejunum or colon, abdominal adhesions were laparoscopically released as far as possible, and the upper abdominal median incision was extended. The blood supply of the intestinal tract was determined under direct vision, and the appropriate intestinal segment was selected.
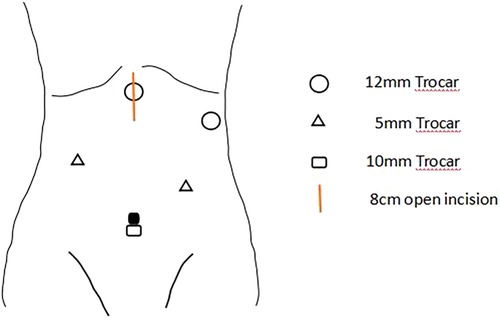
Figure 1. A 10 mm trocar will be placed at the navel for observation, and two 12 mm trocars will be placed at 2 cm below the rib edge on the left side and 2 cm below the xiphoid process, respectively. Two 5 mm Trocars will also be placed at the midpoint between the observation port and the Trocar on the left rib edge, and 6 cm below the right clavicle midline. After the stomach is freed, the incision below the xiphoid process will be extended to 8 cm, and the incision will be opened for gastric tube creation. The chief surgeon stands on the left side of the patient.
During abdominal mobilization in the observation group, a small incision far from the original surgical incision was first made. The first puncture point was as far as possible away from the original incision, by more than 5 cm, and the laparoscope was placed after finger exploration confirmed no abdominal adhesions. The second puncture point facilitated the separation of abdominal adhesions and provided conditions for the treatment of primary lesions; the puncture holes could be switched to facilitate adhesion separation and operation. There incisions were made at the non-adhesion site under the laparoscope, from which dissociation was completed. The abdominal mobilization time was calculated from the entry of the first trocar into the abdomen to the end of stomach dissociation and treatment with a tubular stomach, or to the end of treatment of intraoperative complications caused by dissociation, or to the end of exposure of the vascular arch and selection of an appropriate intestinal segment for esophageal replacement with jejunum or colon. Abdominal mobilization time, blood loss, number of dissected abdominal lymph nodes and perioperative complications were compared between the two groups.
2.3. Statistical analysis
SPSS 25.0 software was used for data analysis. Normally distributed data are presented as and were compared using Student’s t-test. Non-normally distributed data are presented as medians (ranges) and were compared using the Mann-Whitney U test. Categorical variables are presented as [n(%)] and were compared using the chi-square test or Fisher’s exact probability test. p ≤ 0.05 was considered statistically significant.
3. Results
In the observation group, there were 57 males (86.4%) and 9 females (13.6%) with a median age of 65 (30–83) years. In control group, there were 55 males (83.3%) and 11 females (16.7%) with a median age of 62.5 (32–86) years. No significant differences were found in baseline data such as body mass index (BMI), tumor type, pathological stage, preoperative neoadjuvant therapy, and ASA classification between the two groups (p > 0.05) (Table 2).
In the observation group, 52 patients (78.8%) had a history of abdominal surgery only once, and the remaining 14 patients (21.2%) had a history of more than one abdominal surgery. Specifically, 31 patients (47.0%) had undergone simple appendectomy, 15 patients (22.7%) had undergone cholecystectomy, 8 patients (12.1%) had undergone colorectal surgery, 6 patients (9.1%) had undergone gastric surgery, and 6 patients (9.1%) had undergone other surgeries (Table 2).
Laparoscopic abdominal mobilization was completed in both groups, and there were no significant differences between the two groups in the total operation time [(272.50 ± 86.45) min vs. (257.55 ± 67.96) min], blood loss [(119.09 ± 72.17) ml vs. (104.39 ± 43.82) ml], and postoperative time to first flatus [(3.44 ± 0.73) d vs. (3.29 ± 0.60) d] (p > 0.05). The abdominal mobilization time was longer in observation group than that in control group [(25.03 ± 9.82) min vs. (22.53 ± 3.88) min], but the difference was not significant (p = 0.057). After excluding the patients (31/66) with a history of simple appendectomy from the observation group, the abdominal mobilization time was significantly longer in the observation group than in the control group [(27.97 ± 12.16) min vs. (22.53 ± 3.88) min] (p < 0.05). There were significantly fewer dissected abdominal lymph nodes in the observation group than in the control group [(18.44 ± 10.87) vs. (23.09 ± 10.95), p < 0.05]. After excluding the patients (15/66) with a history of abdominal tumor surgery from the observation group, there was no significant difference in the number of dissected abdominal lymph nodes between the two groups [(20.62 ± 10.81) vs. (23.09 ± 10.95)] (p > 0.05) (Table 3).
No postoperative complications, such as intestinal obstruction, abdominal infection or bleeding, occurred in either group. In the observation group, one patient who suffered adhesive intestinal obstruction secondary to past open cholecystectomy underwent vascular anastomosis due to damage to the right gastroepiploic vessel during mobilization and recovered well postoperatively. One patient with a jejunal injury recovered smoothly after repair.
4. Discussion
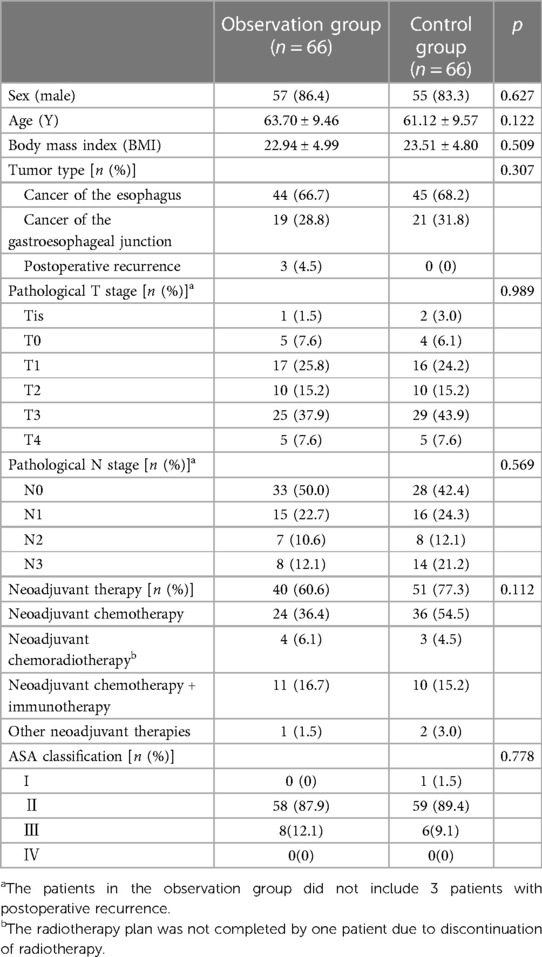
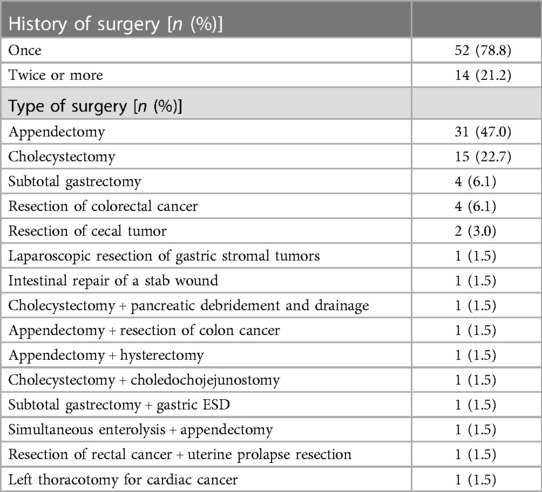
Abdominal adhesion is a common complication following abdominal surgery (Figure 2) and can lead to variations in the anatomical position of abdominal organs, reduce the surgical space due to insufficient pneumoperitoneum pressure, increase the difficulty of reoperation and the risk of intestinal or vascular injury during reoperation, and prolong the operation time (5). Therefore, a history of abdominal surgery has been listed as a relative contraindication to laparoscopic surgery (6). With the increase in life expectancy and tumors, however, patients with a history of abdominal surgery have become increasingly common, and surgeons have also been faced with challenges pertaining to reoperation.
With developments and improvements in technology and equipment as well as the continuous accumulation of operator experience, laparoscopic lysis of abdominal adhesions caused in previous abdominal surgery has become the first choice approach of increasingly more surgeons (7). The safety and effectiveness of thoracoscopic esophagectomy have been verified in previous studies (8, 9), and laparoscopic gastric mobilization has become a routine surgical procedure. Under magnification of the laparoscope system, the adjacent relationship of adhesions can be clearly exposed in the laparoscopic mobilization of abdominal adhesions, thus helping to avoid injury. However, there are few studies on laparoscopic abdominal mobilization and lymph node dissection in patients with cancers of the esophagus or gastroesophageal junction who have a history of abdominal surgery.
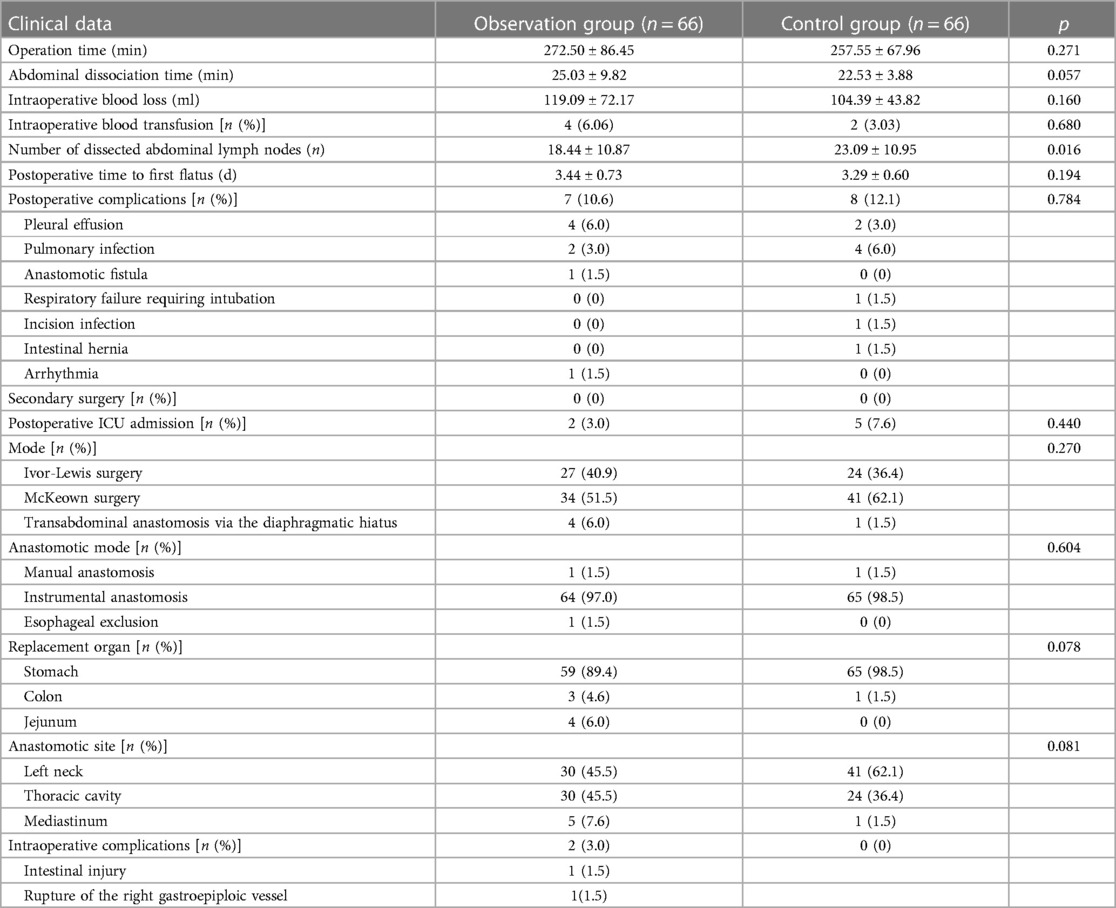
In this study, patients with cancers of the esophagus or gastroesophageal junction and a history of abdominal surgery underwent laparoscopic lysis of abdominal adhesions and stomach and abdominal lymph node dissection (Figure 3). The puncture point was selected to avoid injury to the abdominal viscera and blood vessels as much as possible. Additionally, a clear view under the laparoscope is required for the separation of adhesions. Adhesions were separated from intestinal vessels, and the spleen was protected. Ultrasonic scalpels were used for hemostasis. If the greater omentum was adhered to the anterior abdominal wall, especially when the pneumoperitoneum was perpendicular to the visual angle level, care was taken to avoid injury to the gastroepiploic vascular arch during mobilization. In the observation group, one patient underwent vascular repair and anastomosis due to damage to the right gastroepiploic vessel during mobilization and recovered well postoperatively. One patient with a jejunal injury recovered smoothly after repair. The two patients both had a history of upper abdominal surgery, illustrating again that abdominal adhesions caused by previous abdominal surgery increase the difficulty and risk of surgery. In particular, patients with a history of upper abdominal surgery should be closely monitored during abdominal adhesion lysis.
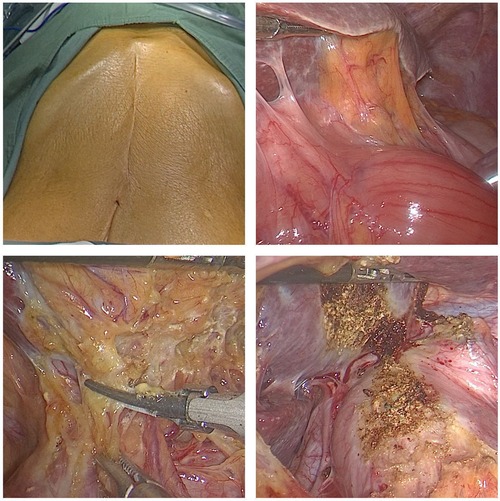
Figure 3. Reoperation for recurrence in patients with a history of transabdominal surgery for cardiac cancer. Operation time, 360 min; abdominal mobilization time, 35 min.
According to previous reports (8–13), thoracoscopic and laparoscopic esophagectomy can achieve the same effect as traditional open surgery, but it has obvious advantages such as less bleeding, mild postoperative pain, rapid recovery of gastrointestinal function and short length of stay. In this study, intraoperative blood loss was significantly lower in both groups than that previously reported for open surgery (14–16), but there was no significant difference in blood loss between the observation group and control group. In addition, postoperative recovery is faster for patients who undergo laparoscopic surgery than for those who undergo traditional open surgery, without increasing the incidence of postoperative complications (17–19). In this study, there were no significant differences between the observation group and control group in total operation time, abdominal mobilization time, blood loss, and postoperative time to first flatus, and neither surgical safety nor postoperative recovery were significantly different between the two groups. In this study, there were fewer abdominal lymph nodes in the observation group than in the control group. After excluding the patients (15/66) with a history of abdominal surgery from the observation group, there was no significant difference in the number of dissected abdominal lymph nodes between the two groups. A possible explanation for this result is that there were 13 patients (19.7%) with abdominal tumors in the observation group, and abdominal lymph node dissection during a previous surgery may affect the number of dissected abdominal lymph nodes in this study.
In this study, the mean operation time and abdominal mobilization time were longer in the observation group than in the control group, especially the latter, but there was no significant difference between the two groups. The possible reason is that the proportion of patients with a history of simple appendectomy was high (31/66) in the observation group. After excluding the patients with a history of simple appendectomy from the observation group, the abdominal mobilization time was significantly longer in the observation group than in the control group. As shown in previous studies, using McBurney’s point for laparoscopy or appendectomy may reduce the potential impact of adhesions following abdominal surgery (20). In this study, the degree of abdominal adhesions after simple appendectomy was significantly lower than that after other abdominal surgeries. Therefore, for patients with a history of abdominal surgery who undergo secondary surgery, the degree and impact of abdominal adhesions should be carefully determined before surgery based on the type of previous surgery, and the procedures and precautions for surgery should be planned to avoid secondary injuries as much as possible.
Certainly, there are some limitations in our study. Firstly, this is a retrospective cohort study, which may introduce selection bias. Secondly, we did not conduct further stratified analysis on cases with a history of previous abdominal surgery. The surgical approach for previous abdominal surgery, such as laparoscopic surgery and open surgery, can have a significant impact on postoperative abdominal adhesions.
In conclusion, previous surgery is not a contraindication to minimally invasive laparoscopic surgery for patients with cancers of the esophagus or gastroesophageal junction. A detailed preoperative inquiry of the surgical history, reasonable surgical planning, and care during the operation to avoid injury are necessary for laparoscopic lysis of abdominal adhesions to be an appropriate choice.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Cancer Hospital, Chinese Academy of Medical Sciences. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
All authors: Manuscript writing and final approval of manuscript. FL, FZ, WL, QZ, ZW, XZ, and LQ: Provision of study materials and patients. FL: Collection and assembly of data, data analysis and interpretation. YL: Conception and design; Data analysis and interpretation. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the Medical and Health Science and Technology Innovation Project of the Chinese Academy of Medical Sciences (2021-I2M-C&T-B-047), the Capital Health Research and Development Special Project (2022-2Z-40212), the China Cancer Foundation Beijing Hope Marathon Special Fund (LC2021A06), and the Beijing Natural Science Foundation of China (7222153).
Acknowledgments
The authors would like to thank AJE (china.aje.com) for editing this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zheng R, Zhang S, Zeng H, Wang S, Sun K, Chen R, et al. Cancer incidence and mortality in China, 2016. J Natl Cancer Cent. (2022) 2:1–9. doi: 10.1016/j.jncc.2022.02.002
2. Mariette C, Markar SR, Dabakuyo-Yonli TS, Meunier B, Pezet D, Collet D, et al. Hybrid minimally invasive esophagectomy for esophageal cancer. N Engl J Med. (2019) 380:152–62. doi: 10.1056/NEJMoa1805101
3. Nuytens F, Dabakuyo-Yonli TS, Meunier B, Gagnière J, Collet D, D'Journo XB, et al. Five-year survival outcomes of hybrid minimally invasive esophagectomy in esophageal cancer: results of the MIRO randomized clinical trial. JAMA Surg. (2021) 156:323–32. doi: 10.1001/jamasurg.2020.7081
4. Kamer E, Acar T, Cengiz F, Durak E, Haciyanli M. Laparoscopic colorectal surgery in patients with previous abdominal surgery: a single-center experience and literature review. Surg Laparosc Endosc Percutan Tech. (2017) 27:434–9. doi: 10.1097/sle.0000000000000470
5. Zeng WG, Liu MJ, Zhou ZX, Hou HR, Liang JW, Wang Z, et al. Impact of previous abdominal surgery on the outcome of laparoscopic resection for colorectal cancer: a case-control study in 756 patients. J Surg Res. (2015) 199:345–50. doi: 10.1016/j.jss.2015.05.022
6. Rahnemai-Azar AA, Rahnemaiazar AA, Naghshizadian R, Kurtz A, Farkas DT. Percutaneous endoscopic gastrostomy: indications, technique, complications and management. World J Gastroenterol. (2014) 20:7739–51. doi: 10.3748/wjg.v20.i24.7739
7. Sallinen V, Di Saverio S, Haukijärvi E, Juusela R, Wikström H, Koivukangas V, et al. Laparoscopic versus open adhesiolysis for adhesive small bowel obstruction (LASSO): an international, multicentre, randomised, open-label trial. Lancet Gastroenterol Hepatol. (2019) 4:278–86. doi: 10.1016/s2468-1253(19)30016-0
8. Luketich JD, Nguyen NT, Weigel T, Ferson P, Keenan R, Schauer P. Minimally invasive approach to esophagectomy. JSLS. (1998) 2:243–7.9876747
9. Dantoc M, Cox MR, Eslick GD. Evidence to support the use of minimally invasive esophagectomy for esophageal cancer: a meta-analysis. Arch Surg. (2012) 147:768–76. doi: 10.1001/archsurg.2012.1326
10. Palazzo F, Rosato EL, Chaudhary A, Evans NR, Sendecki JA, Keith S, et al. Minimally invasive esophagectomy provides significant survival advantage compared with open or hybrid esophagectomy for patients with cancers of the esophagus and gastroesophageal junction. J Am Coll Surg. (2015) 220:672–9. doi: 10.1016/j.jamcollsurg.2014.12.023
11. Petri R, Zuccolo M, Brizzolari M, Rossit L, Rosignoli A, Durastante V, et al. Minimally invasive esophagectomy: thoracoscopic esophageal mobilization for esophageal cancer with the patient in prone position. Surg Endosc. (2012) 26:1102–7. doi: 10.1007/s00464-011-2006-5
12. Safranek PM, Cubitt J, Booth MI, Dehn TC. Review of open and minimal access approaches to oesophagectomy for cancer. Br J Surg. (2010) 97:1845–53. doi: 10.1002/bjs.7231
13. Seesing MFJ, Gisbertz SS, Goense L, Van Hillegersberg R, Kroon HM, Lagarde SM, et al. A propensity score matched analysis of open versus minimally invasive transthoracic esophagectomy in the Netherlands. Ann Surg. (2017) 266:839–46. doi: 10.1097/sla.0000000000002393
14. Yibulayin W, Abulizi S, Lv H, Sun W. Minimally invasive oesophagectomy versus open esophagectomy for resectable esophageal cancer: a meta-analysis. World J Surg Oncol. (2016) 14(1):304. doi: 10.1186/s12957-016-1062-7
15. Li Z, Liu C, Liu Y, Yao S, Xu B, Dong G. Comparisons between minimally invasive and open esophagectomy for esophageal cancer with cervical anastomosis: a retrospective study. J Cardiothorac Surg. (2020) 15(1):128. doi: 10.1186/s13019-020-01182-3
16. Wang B, Zuo Z, Chen H, Qiu B, Du M, Gao Y. The comparison of thoracoscopic-laparoscopic esophagectomy and open esophagectomy: a meta-analysis. Indian J Cancer. (2017) 54(1):115–9. doi: 10.4103/0019-509X.219585
17. Di Martino N, Izzo G, Cosenza A, Cerullo G, Torelli F, Brillantino A, et al. Adenocarcinoma of gastric cardia in the elderly: surgical problems and prognostic factors. World J Gastroenterol. (2005) 11(33):5123–8. doi: 10.3748/wjg.v11.i33.5123
18. Maas KW, Biere SS, Scheepers JJ, Gisbertz SS, van-der-Peet DL, Cuesta MA. Laparoscopic versus open transhiatal esophagectomy for distal and junction cancer. Rev Esp Enferm Dig. (2012) 104(4):197–202. doi: 10.4321/S1130-01082012000400005
19. Petropoulos K, Macheras A, Liakakos T, Misiakos EP. Minimally invasive esophagectomy for esophageal cancer: techniques and outcomes. Chirurgia (Bucur). (2015) 110(2):99–108.26011830
Keywords: cancer of the esophagus, cancer of the gastroesophageal junction, history of abdominal surgery, laparoscopic secondary surgery, complications
Citation: Li F, Zhang F, Liu W, Zheng Q, Zhang M, Wang Z, Zhang X, Qi L and Li Y (2023) Is laparoscope surgery feasible for upper gastrointestinal cancer patients with a history of abdominal surgery?. Front. Surg. 10:1214175. doi: 10.3389/fsurg.2023.1214175
Received: 16 June 2023; Accepted: 25 September 2023;
Published: 9 October 2023.
Edited by:
Luigi Marano, University of Siena, ItalyReviewed by:
Antonio Brillantino, Hospital Antonio Cardarelli, ItalyYusef Moulla, University Hospital Leipzig, Germany
© 2023 Li, Zhang, Liu, Zheng, Zhang, Wang, Zhang, Qi and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Li bGl5b25nZG9jdG9yQDEyNi5jb20=
 Feng Li
Feng Li Fan Zhang
Fan Zhang Weixin Liu3
Weixin Liu3 Ling Qi
Ling Qi Yong Li
Yong Li