- 1Department of Neurosurgery and Klinisches Neurozentrum Zurich ZH, Universität Zürich; Universitätsspital Zürich, Zurich, Switzerland
- 2Julius Center for Health Sciences and Primary Care, Medical Humanities, University Medical Center Utrecht, Utrecht, Netherlands
- 3Image Science Institute, University Medical Center Utrecht, Utrecht, Netherlands
Background: Precise preoperative anatomical visualization and understanding of an intracranial aneurysm (IA) are fundamental for surgical planning and increased intraoperative confidence. Application of virtual reality (VR) and mixed reality (MR), thus three-dimensional (3D) visualization of IAs could be significant in surgical planning. Authors provide an up-to-date overview of VR and MR applied to IA surgery, with specific focus on tailoring of the surgical treatment.
Methods: A systematic analysis of the literature was performed in accordance with the PRISMA guidelines. Pubmed, and Embase were searched to identify studies reporting use of MR and VR 3D visualization in IA surgery during the last 25 years. Type and number of IAs, category of input scan, visualization techniques (screen, glasses or head set), inclusion of haptic feedback, tested population (residents, fellows, attending neurosurgeons), and aim of the study (surgical planning/rehearsal, neurosurgical training, methodological validation) were noted.
Results: Twenty-eight studies were included. Eighteen studies (64.3%) applied VR, and 10 (35.7%) used MR. A positive impact on surgical planning was documented by 19 studies (67.9%): 17 studies (60.7%) chose the tailoring of the surgical approach as primary outcome of the analysis. A more precise anatomical visualization and understanding with VR and MR was endorsed by all included studies (100%).
Conclusion: Application of VR and MR to perioperative 3D visualization of IAs allowed an improved understanding of the patient-specific anatomy and surgical preparation. This review describes a tendency to utilize mostly VR-platforms, with the primary goals of a more accurate anatomical understanding, surgical planning and rehearsal.
Introduction
Intracranial aneurysms (IAs) are pathological dilatations of cerebral arteries. IAs are relatively commonly acquired lesions occurring with a frequency ranging between 0.5% and 3% in the general population, and accounting for about 80%–85% of non-traumatic subarachnoid hemorrhages (1). Upon detection of an IA, tailoring of the optimal treatment strategy is based on careful consideration of the patient history and specific aneurysm characteristics. Treatment approaches are surgical and/or endovascular. With advances in endovascular approaches, the indications for surgical clipping of IAs have been decreased. Currently, open IA clipping is generally reserved for complex aneurysms. Successful and safe surgery of these cases depends on accurate surgical planning, which implies precise pre-operative characterization of lesion-specific anatomical features. The current gold standard imaging modality for the preoperative study of IAs is digital subtraction angiography (DSA). DSA allows a comprehensive anatomical examination of the most relevant IAs' features (i.e.,: relation to the parent vessels, neck's width, dome's regularity and orientation) at the cost of invasiveness. The role of magnetic resonance flow (MR-flow) has indeed been increasing for the diagnosis and the preoperative analysis of IAs. Nonetheless, MR studies are mostly black-and-white and visualized on two-dimensional (2D) screens. When compared to two-dimensional images, three-dimensional (3D) anatomical visualization with virtual reality (VR) and mixed reality (MR) offers a more comprehensive anatomical visualization and understanding in the perioperative phase. In a VR environment, the user is fully immersed in a simulated world. To create an immersive environment, each eye is provided with a separate image by the displays in the VR device. The user's physical movement is registered by cameras in the VR device and matched to the digital world. An MR device enhances the user's physical environment with a digital overlay, a so-called hologram. MR provides the opportunity to interact with the digital objects in the physical world through (depth) cameras and a motion sensor in the device that map out the user's surroundings and track their movements (2).
Both VR and MR techniques are increasingly adopted in neurosurgical preparation to provide a safe environment to plan surgical procedures, rehearse and foresee possible technical difficulties, and make the intraoperative phase more efficient (3).
Despite their substantial promise, a systematic analysis of the literature examining the role of MR and VR applications and their benefits as perioperative adjuncts in open IA surgery has been lacking. Authors present a comprehensive review on the topic, with the primary goal to study the true measurable benefits of using 3D visualization with MR and VR in preparation of IA surgery. This analysis thereby provides an overview of the technology used, its drawbacks and the potential future improvements.
Materials and methods
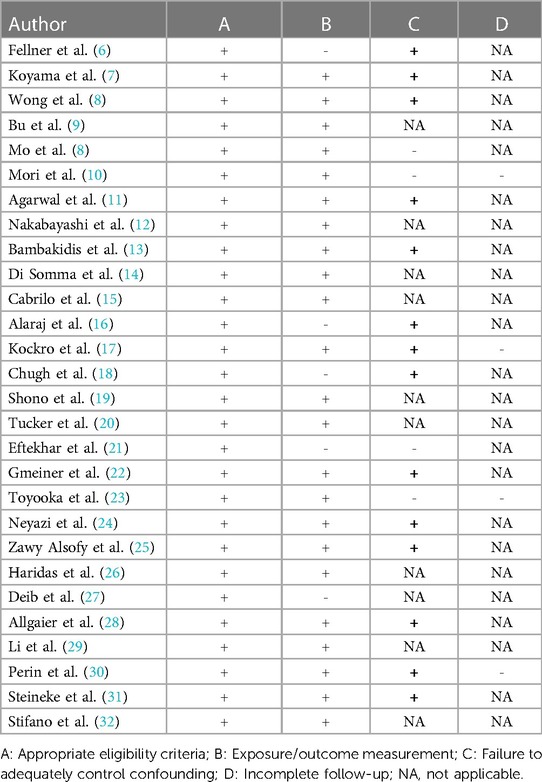
A systematic review was performed using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (4). Two reviewers (EC and TK) screened records independently, and disagreements at any stage were resolved by discussion and consensus. Two additional records were identified through reference search. The critical appraisal of the included studies was performed by means of a risk of bias score using a modified version of the Cochrane Risk of Bias tool as shown in Table 1 (5).
Search strategy
The PubMed and EMBASE databases were searched to identify eligible papers. The query was performed using the Boolean operators “AND” or “OR”, and database-related filters to maximize the chance to identify articles focusing on 3D visualization through MR and VR system applied to IA surgery. The following string was entered:
((“neurosurg*"[Title/Abstract] OR “Neurosurgery"[MeSH Terms] OR “Neurosurgical Procedures"[MeSH Terms] OR “ventriculostom*"[Title/Abstract] OR “lobectom*"[Title/Abstract] OR “craniotom*"[Title/Abstract] OR “neuro surg*"[Title/Abstract] OR “neurologic surg*"[Title/Abstract]) AND (“augmented realit*"[Title/Abstract] OR “Augmented Reality"[MeSH Terms] OR “mixed realit*"[Title/Abstract] OR “virtual realit*"[Title/Abstract] OR “extended realit*"[Title/Abstract] OR “hologra*"[Title/Abstract] OR “Holography"[MeSH Terms] OR “head mounted display*"[Title/Abstract] OR “head up display*"[Title/Abstract] OR “head worn display*"[Title/Abstract] OR “Smart Glasses"[MeSH Terms])).
The most recent search was performed on November 28th 2022.
Selection criteria
Articles were included if the following criteria were met: (1) Studies published after 1997; (2) Studies analyzing specifically the role of MR and VR in IA pre-surgical and intraoperative phases; (3) A specified 2D or 3D visualization technique as a mean to study angioarchitecture; (4) English, Italian, French or German language.
Data extraction
The following information was extracted from all included publications: (1) study group and year of publication; (2) type and number of IA included in the analysis; (3) imaging data source (computed tomography angiography (CTA), magnetic resonance angiography (MRA), digital subtraction angiography (DSA)); (4) category of visualization techniques (screen, glasses, head-mounted device (HMD); (5) inclusion of haptic feedback; (6) aim of the study (surgical planning/rehearsal, neurosurgical training, methodological validation); (7) study population (residents, fellows, attending neurosurgeons).
Statistical analysis
The descriptive statistical analyses were performed using R Studio. Data were presented as numbers and percentages.
Results
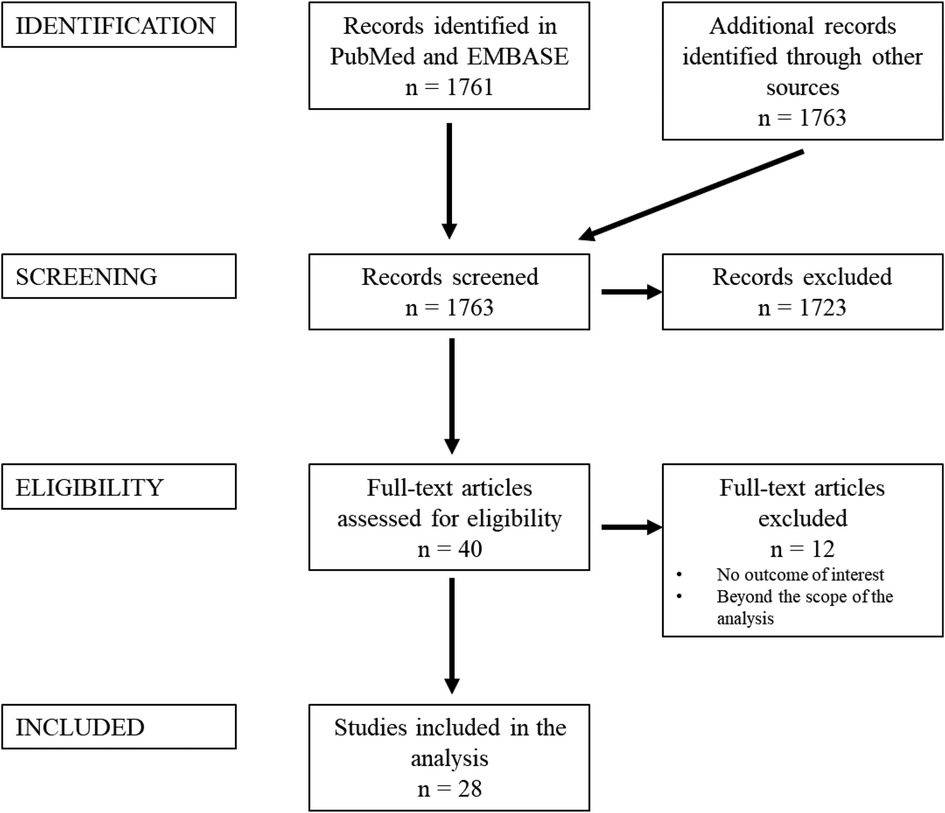
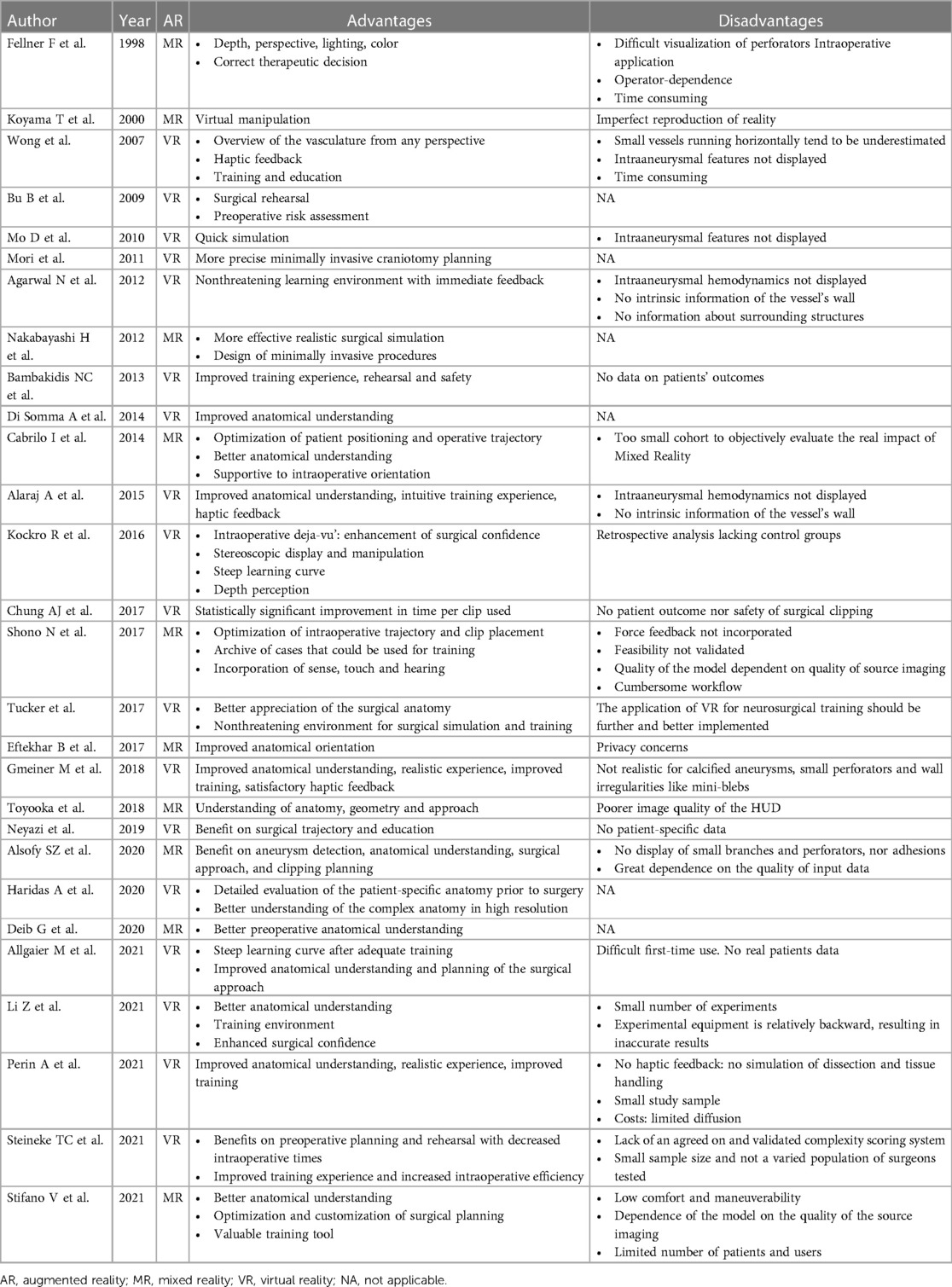
A PRISMA flowchart is displayed in Figure 1. A total of 1,763 publications were screened, 40 full-text articles were assessed for eligibility and 28 studies were included in this review. Studies were excluded when considered beyond the scope for the aims of the present analysis, and/or when their outcomes were not of interest. An overview of the included studies highlighting their major goals and advantages/disadvantages of augmented reality application as perceived by the authors of the publications is illustrated respectively in Table 2 and Table 3. In Table 3, where no data was specified, it means that the authors of the publication did not express it. Table 1 provides a visual summary of the quality review of the included studies.
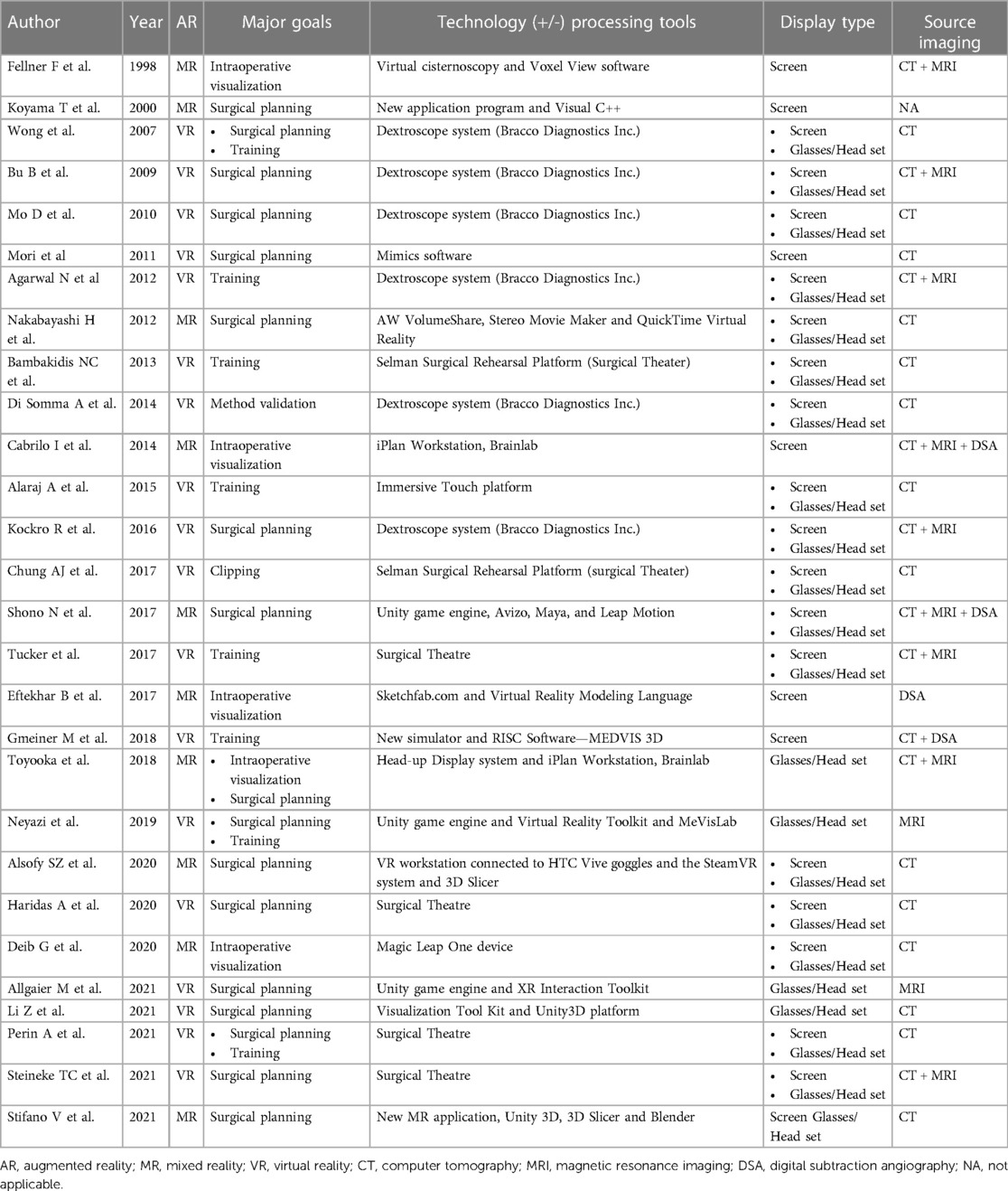
Table 2. Studies’ major goals, imaging elaboration techniques and display types, and source imaging.
Virtual reality
Virtual reality implies the use of a system which generates a complete immersion in a digital environment, that could provide a realistic simulation of the surgical approach (16). This type of technology was applied by 18 of the studies (64.3%) (8–11, 13, 14, 16–18, 20, 22, 24, 26, 28–31, 33), with a total of 321 aneurysms included in the studies. The VR systems that were mostly used were the Dextroscope system (Bracco Diagnostics Inc., Milan, Italy), documented by 6 studies (33%) (8, 9, 11, 14, 17, 33), and Surgical Theater (Surgical Theater Inc., Los Angeles, CA), utilized by 4 studies (22%) (20, 26, 30, 31). Thirteen studies (72%) chose the combination of screen and glasses/HMD as preferred visualization method (8, 9, 11, 13, 14, 16–18, 20, 26, 30, 31, 33). Exclusive use of a 2D visualization of the CT images represented the most relevant imaging source in 10 studies (56%) (8, 10, 13, 14, 16, 18, 26, 29, 30, 33).
• Preoperative planning:
None of the studies of this subgroup applied VR intraoperatively. 16 of the studies (89%) focused on the pre-operative planning of the surgical approach. The benefit of VR application for preoperative planning was qualitatively assessed using Likert scales and the Think Aloud Method, specifically for evaluation of anatomical understanding, depth perception and visualization of the surgical trajectory perceived by nine study groups (8, 14, 16, 17, 22, 24, 28, 30, 31).
• Benefit on training:
Eight studies (44%) assessed the impact of this technology on training neurosurgical residents, focusing on the benefits of VR with regard to realistic anatomical understanding, haptic feedback satisfaction and enhancement of surgical confidence (8, 11, 13, 16, 20, 24, 30). User satisfaction was assessed by means of Likert Scales and the Think-Aloud Method.
• Impact on patients:
Three studies (17%) evaluated the potential effect of 3D visualization in VR on clinical outcomes (10, 17, 30). None of the 18 studies aimed to evaluate the impact of surgical planning with VR on patient safety, and only one study (5.6%) aimed to assess the benefits of VR on patient education and understanding of the surgical procedure (8).
• Perceived disadvantages:
The lack of information on intra-aneurysmal hemodynamics and vessel wall characteristics was also reported as a disadvantage (8, 11, 16, 22). Furthermore, small vessels and perforating arteries tended to be underestimated or not displayed (8, 22).
Mixed reality
Conceptually, MR differs from VR in that it integrates a virtual environment with the real world, whereas the latter is a full immersion in a virtual environment. MR provides an interaction with digital objects in the real world. Ten of the collected studies applied this technology (6, 7, 12, 15, 19, 21, 23, 25, 27, 32), with a total of 183 analyzed IAs. In this subgroup, there was indeed no homogeneity among the MR systems used: each group utilized a center-specific system and different softwares for the segmentations and the post-processing of the images. 3D visualization occurred by means of a combination of screen and glasses/HMD in 6 out of 10 studies (60%) (7, 12, 19, 25, 27, 32). The remaining 4 studies (40%) utilized solely screen for image visualization (6, 7, 15, 21). CT as exclusive imaging input source was used by 3 studies (30%) (12, 25, 27), and 4 studies (40%) chose a multimodal imaging source (6, 15, 19, 23). Only one group (10%) used DSA only as the input source (21). None of the studies in this subgroup utilized MRI as the exclusive imaging source.
• Preoperative planning:
Four studies (40%) used this technology for both the surgical planning and intra-operative guidance (6, 15, 21, 27). The major goal documented in this subgroup was again planning of the best surgical approach, as documented by 5 studies (50%). Similarly to the VR-subgroup, the advantages perceived for the preoperative planning were based on an improved anatomical orientation, better depth perception and more adequate understanding of the surgical approach (6, 21, 25). The major outcomes of these studies were mostly evaluated through Likert Scales for a qualitative assessment. Only 2 studies (20%) performed a structured statistical analysis to examine the outcomes (23, 25).
• Benefit on training:
One of the studies in this subgroup aimed to assess the impact of MR visualization on neurosurgical training, testing the technology on residents neurosurgeons (32).
• Impact on patients:
None of the studies in this subgroup aimed to validate the impact of MR visualization on patient education/safety or clinical outcomes.
• Perceived disadvantages:
The most relevant drawback reported in the MR-subgroup was the difficult, if not impossible, visualization of small vessels and perforators, and the dependence of the segmentation on the quality of the input data (5/10 studies, 50%) (6, 7, 21, 23, 25).
Discussion
The present analysis represents an up-to-date systematic review of all published studies, which applied perioperative 3D visualization through MR and VR to IA microsurgery from 1997 to November 2022.
A relevant aspect emerging from the present analysis is the lack of measurable hardcore values to quantitatively examine the real added value of VR and MR applied to open IA surgery. While a qualitative assessment of the benefits of these 3D technologies is possible using Likert Scales and the Think Aloud Method, the absence of objective qualitative parameters makes the analysis partial and may hinder objective comparisons among the different 3D modalities, especially when a structured statistical analysis is not performed. Under this premise, this systematic review suggests an improved anatomical understanding, a better depth perception and a nonthreatening learning environment to be the most relevant perceived advantages of VR, MR applied for IA surgery planning, compared to conventional visualization strategies. The 3D and realistic replication of the cerebrovascular anatomy could help the acquisition of procedural motor skills, and enhance surgical orientation and confidence (34).
Most of the included studies used multimodal imaging input to create a more informative 3D vascular model, overcoming the disadvantages of exclusive use of one imaging modality. While CTA allows for a precise understanding of aneurysmal size and shape, provides detailed information on the parent vessel, and anatomical relationships with the skull base, combination with digital subtraction angiography (DSA) or MR-flow adds information on the flow patterns (22, 35). Nonetheless, none of the studies in the present cohort integrated hemodynamic information to the 3D visualization. Furthermore, the combination of CT and MR imaging provides important information on vessel/aneurysm spatial relationships with the parenchyma and the cisternal system, which allow a better surgical orientation (11).
As far as visualization techniques are concerned, merging glasses or HMD's with 2D visualization of 3D vascular models enhances the perception of spatial position and surgical orientation (8). Glasses and HDMs may also allow a more intuitive and immersive interaction with the 3D models (36).
The use of VR. MR and RV does not come without limitations. The studies published so far are mostly retrospective, with small sample sizes and no control groups. The analyzed studies examine almost exclusively aneurysms treated in elective settings, with specific focus on anterior circulation IAs, and rarely provide information on patient functional outcomes. The lack of an objective strategy to qualitatively assess the benefits of these technologies represents a major bias as well. While Likert-scales or Think-Aloud Method are mostly applied to evaluate the intuitiveness and the satisfaction of the users, no standardized, agreed on quantitative scores have been provided yet. To obviate this absence, objective parameters such as size of the aneurysmal dome, width of the neck, orientation of the dome, distance of the aneurysm from relevant anatomical structures should be noted, when using VR and/or MR, validated and combined into quantitative scores. The difficulty of these analyses may lie in the paucity of data and in the novelty of these technologies, which are still not available in every center. Their diffusion may also be limited by their often not affordable costs. Another relevant aspect resulting from the paucity and diversity of the available data is the lack of unified criteria to provide an objective appraisal of the current literature. The advantages and the disadvantages reported for each paper come mostly from the appraisal and experience of the original authors. With further implementation of these technologies and gathering of more extensive and unified data, this limitation could be obviated. Furthermore, segmentation of intracranial vessels and fine anatomical structures is still highly dependent on the quality of input data, which makes the integration of hemodynamic information, small vessels or intramural particularities difficult. The integration of hemodynamic information into a 3D preoperative study of IAs may help characterize their angioarchitecture more accurately. This information may provide major advantages on the tailoring of their treatment in a pathology-specific way. Such a tailoring could potentially increase intraoperative safety and therapeutic efficiency.
Conclusion
This analysis endorses the promising role of MR and VR to provide a more accurate aneurysm-specific anatomical visualization and understanding. The absence of a standardized set of quantitative parameters to provide an objective assessment of the real benefit of these technologies on training in IA surgery should be a major drive for future studies on the topic.
Furthermore, integration of hemodynamic analysis to the 3D visualization may also be a promising avenue for future research.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
EC conceptualized the study with TD; EC wrote the manuscript text, prepared all tables and the figure. BL and TK helped with the systematic review of the literature and the amendment of the manuscript. TD supported the gathering and the processing of the data. All authors contributed to the article and approved the submitted version.
Funding
This work is part of “SURGENT” under the auspices of University Medicine Zurich/Hochschulmedizin Zürich.
Conflict of interest
TD is Co-founder and CMO of Augmedit bv, an augmented reality company.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Brown RD, Broderick JP. Unruptured intracranial aneurysms: epidemiology, natural history, management options, and familial screening. Lancet Neurol. (2014) 13(4):393–404. doi: 10.1016/S1474-4422(14)70015-8
2. Andrews C, Southworth MK, Silva JNA, Silva JR. Extended reality in medical practice. Curr Treat Options Cardiovasc Med. (2019) 21(4):18. doi: 10.1007/s11936-019-0722-7
3. Zhang C, Gao H, Liu Z, Huang H. The potential value of mixed reality in neurosurgery. J Craniofac Surg. (2021) 32(3):940–3. doi: 10.1097/SCS.0000000000007317
4. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Br Med J. (2009) 339:b2535. doi: 10.1136/bmj.b2535
5. Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P, Montori V, Akl EA, Djulbegovic B, Falck-Ytter Y, Norris SL, Williams JW Jr, Atkins D, Meerpohl J, Schünemann HJ. GRADE guidelines: 4. Rating the quality of evidence–study limitations (risk of bias). J Clin Epidemiol. (2011) 64(4):407–15. doi: 10.1016/j.jclinepi.2010.07.017
6. Fellner F, Blank M, Fellner C, Böhm-Jurkovic H, Bautz W, Kalender WA. Virtual cisternoscopy of intracranial vessels: a novel visualization technique using virtual reality. Magn Reson Imaging. (1998) 16(9):1013–22. doi: 10.1016/s0730-725x(98)00113-1
7. Koyama T, Hongo K, Tanaka Y, Kobayashi S. Simulation of the surgical manipulation involved in clipping a basilar artery aneurysm: concepts of virtual clipping. Technical note. J Neurosurg. (2000) 93(2):355–60. doi: 10.3171/jns.2000.93.2.0355
8. Wong GKC, Zhu CXL, Ahuja AT, Poon WS. Craniotomy and clipping of intracranial aneurysm in a stereoscopic virtual reality environment. Neurosurgery. (2007) 61(3):564–8; discussion 568–569. doi: 10.1227/01.NEU.0000290904.46061.0D
9. Bu B, Zhou D-B, Xu B-N, Yu X-G, Zhang Y-Z, Wei S-B. Application of dextroscope operation planning workstation in intracranial lesion operations: preoperative images reconstruction, simulation and dissection. J Clin Rehab Tissue Eng Res. (2009) 13(4):789–92. doi: 10.1093/eurheartj/ehab777
10. Mori K, Esaki T, Yamamoto T, Nakao Y. Individualized pterional keyhole clipping surgery based on a preoperative three-dimensional virtual osteotomy technique for unruptured middle cerebral artery aneurysm. Minim Invasive Neurosurg. (2011) 54(5–6):207–13. doi: 10.1055/s-0031-1286335
11. Agarwal N, Schmitt PJ, Sukul V, Prestigiacomo CJ. Surgical approaches to complex vascular lesions: the use of virtual reality and stereoscopic analysis as a tool for resident and student education. BMJ Case Rep. (2012) 2012:bcr0220125859. doi: 10.1136/bcr.02.2012.5859
12. Nakabayashi H, Shimizu K. Stereoscopic virtual realistic surgical simulation in intracranial aneurysms. Neurol India. (2012) 60(2):191–7. doi: 10.4103/0028-3886.96399
13. Bambakidis NC, Selman WR, Sloan AE. Surgical rehearsal platform: potential uses in microsurgery. Neurosurgery. (2013) 73(Suppl 1):122–6. doi: 10.1227/NEU.0000000000000099
14. Somma AD, Bronzoni C, Guadagno E, Solari D, Dell'aversana GO, De Caro BS, et al. Extended endoscopic endonasal approaches for cerebral aneurysms: anatomical, virtual reality and morphometric study. Biomed Res Int. (2014) 2014:703792. doi: 10.1155/2014/703792
15. Cabrilo I, Bijlenga P, Schaller K. Augmented reality in the surgery of cerebral aneurysms: a technical report. Neurosurgery. (2014) 10(Suppl 2):252–60; discussion 260–261. doi: 10.1227/NEU.0000000000000328
16. Alaraj A, Luciano CJ, Bailey DP, Elsenousi A, Roitberg BZ, Bernardo A, et al. Virtual reality cerebral aneurysm clipping simulation with real-time haptic feedback. Neurosurgery. (2015) 11(Suppl 2):52–8. doi: 10.1227/NEU.0000000000000583
17. Kockro RA, Killeen T, Ayyad A, Glaser M, Stadie A, Reisch R, et al. Aneurysm surgery with preoperative three-dimensional planning in a virtual reality environment: technique and outcome analysis. World Neurosurg. (2016) 96:489–99. doi: 10.1016/j.wneu.2016.08.124
18. Chugh AJ, Pace JR, Singer J, Tatsuoka C, Hoffer A, Selman WR, et al. Use of a surgical rehearsal platform and improvement in aneurysm clipping measures: results of a prospective, randomized trial. J Neurosurg. (2017) 126(3):838–44. doi: 10.3171/2016.1.JNS152576
19. Shono N, Kin T, Nomura S, Miyawaki S, Saito T, Imai H, et al. Microsurgery simulator of cerebral aneurysm clipping with interactive cerebral deformation featuring a virtual arachnoid. Oper Neurosurg (Hagerstown). (2018) 14(5):579–89. doi: 10.1093/ons/opx155
20. Tucker AM, Beckett JS, Martin NA. Next generation case report: supraorbital craniotomy for anterior communicating artery aneurysm clipping in annotated virtual reality environment. Oper Neurosurg (Hagerstown). (2018) 15(5):E73–6. doi: 10.1093/ons/opy039
21. Eftekhar B. Smartphone as a remote touchpad to facilitate visualization of 3D cerebral angiograms during aneurysm surgery. J Neurol Surg A Cent Eur Neurosurg. (2017) 78(5):502–6. doi: 10.1055/s-0037-1598049
22. Gmeiner M, Dirnberger J, Fenz W, Gollwitzer M, Wurm G, Trenkler J, et al. Virtual cerebral aneurysm clipping with real-time haptic force feedback in neurosurgical education. World Neurosurg. (2018) 112:e313–23. doi: 10.1016/j.wneu.2018.01.042
23. Toyooka T, Otani N, Wada K, Tomiyama A, Takeuchi S, Fujii K, et al. Head-up display may facilitate safe keyhole surgery for cerebral aneurysm clipping. J Neurosurg. (2017) 129(4):883–9. doi: 10.3171/2017.5.JNS162692
24. Neyazi B, Saalfeld P, Berg P, Skalej M, Preim B, Erol İ, et al. VR Craniotomy for optimal intracranial aneurysm surgery planning. Reutlingen: Conference: Computer and Robotic Assisted Surgery (CURAC) (2019). p. 234–9.
25. Zawy Alsofy S, Sakellaropoulou I, Nakamura M, Ewelt C, Salma A, Lewitz M, et al. Impact of virtual reality in arterial anatomy detection and surgical planning in patients with unruptured anterior communicating artery aneurysms. Brain Sci. (2020) 10(12):E963. doi: 10.3390/brainsci10120963
26. Haridas A, Miller M. Middle cerebral artery aneurysm clipping with immersive 360° virtual reality model: 2-dimensional operative video. Oper Neurosurg (Hagerstown). (2021) 20(4):E314. doi: 10.1093/ons/opaa416
27. Deib G, Smith D, Chaudhary B, Boo S, Tarabishy AJ, Carpenter J, et al. E-195 A mixed reality spatial computing framework for preprocedural evaluation of cerebral aneurysms: approach and preliminary results. J Neurointervent Surg. (2020) 12(1):A134–5. doi: 10.1136/neurintsurg-2020-SNIS.226
28. Allgaier M, Amini A, Neyazi B, Sandalcioglu IE, Preim B, Saalfeld S. VR-based training of craniotomy for intracranial aneurysm surgery. Int J Comput Assist Radiol Surg. (2022) 17(3):449–56. doi: 10.1007/s11548-021-02538-3
29. Li Z, Huo G, Feng Y, Ma Z. Application of virtual reality based on 3D-CTA in intracranial aneurysm surgery. J Healthc Eng. (2021) 2021:9913949. doi: 10.1155/2021/9913949
30. Perin A, Gambatesa E, Galbiati TF, Fanizzi C, Carone G, Rui CB, et al. The “STARS-CASCADE” study: virtual reality simulation as a new training approach in vascular neurosurgery. World Neurosurg. (2021) 154:e130–46. doi: 10.1016/j.wneu.2021.06.145
31. Steineke TC, Barbery D. Microsurgical clipping of middle cerebral artery aneurysms: preoperative planning using virtual reality to reduce procedure time. Neurosurg Focus. (2021) 51(2):E12. doi: 10.3171/2021.5.FOCUS21238
32. Stifano V, Palumbo MC, Chidambaram S, Sturiale CL, Albanese A, Marchese E, et al. The use of mixed reality for the treatment planning of unruptured intracranial aneurysms. J Neurosurg Sci. (2023) 67(4):491–7. doi: 10.23736/S0390-5616.21.05356-X
33. Mo D, Bao S, Li L, Yi Z, Zhang J, Zhang Y. Virtual reality system for diagnosis and therapeutic planning of cerebral aneurysms. Chin Med J (Engl). (2010) 123(16):2206–10. doi: 10.1007/s00701-016-2892-3
34. Wurm G, Lehner M, Tomancok B, Kleiser R, Nussbaumer K. Cerebrovascular biomodeling for aneurysm surgery: simulation-based training by means of rapid prototyping technologies. Surg Innov. (2011) 18(3):294–306. doi: 10.1177/1553350610395031
35. Andereggen L, Gralla J, Andres RH, Weber S, Schroth G, Beck J, et al. Stereolithographic models in the interdisciplinary planning of treatment for complex intracranial aneurysms. Acta Neurochir (Wien). (2016) 158(9):1711–20. doi: 10.1007/s00701-016-2892-3
Keywords: cerebrovascular surgery, intracranial aneurysms, virtual reality, mixed reality, 3D visualization
Citation: Colombo E, Lutters B, Kos T and van Doormaal T (2023) Application of virtual and mixed reality for 3D visualization in intracranial aneurysm surgery planning: a systematic review. Front. Surg. 10:1227510. doi: 10.3389/fsurg.2023.1227510
Received: 31 May 2023; Accepted: 18 September 2023;
Published: 27 September 2023.
Edited by:
Mohammed Ali Alvi, University Health Network (UHN), CanadaReviewed by:
Cristian Luciano, University of Illinois Chicago, United StatesBernhard Preim, Otto von Guericke University Magdeburg, Germany
© 2023 Colombo, Lutters, Kos and van Doormaal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elisa Colombo RWxpc2EuY29sb21ib0B1c3ouY2g=
 Elisa Colombo
Elisa Colombo Bart Lutters2
Bart Lutters2 Tristan van Doormaal
Tristan van Doormaal