- Department of Otorhinolaryngology-Head and Neck Surgery, College of Medicine, Hanyang University, Seoul, Republic of Korea
Recently, lymph node metastasis to the suprasternal space (SSLN) and lymph nodes between the sternocleidomastoid and sternohyoid muscles (LNSS) have received attention. This article reports two cases of SSLN and LNSS recurrence and emphasizes the need for a thorough evaluation and consideration of the possibility of recurrence in this region. The clinical significance of the prophylactic dissection of SSLN and LNSS remains unclear, and further studies are required to determine its value. Regular follow-up checks of suspicious lymph nodes at SSLN and LNSS, as well as the central and lateral compartments, are recommended after thyroidectomy to detect recurrences and ensure appropriate management.
Introduction
The rate of pathologically proven lymph node metastasis in papillary thyroid carcinoma (PTC) can reach up to 80%–90%, especially in the central compartment (1–3). Although most patients with node-positive PTC have a good prognosis with low mortality rates, the presence of node metastasis is known to increase the risk of locoregional recurrence and hence requires reoperation (4). Therapeutic neck dissection is recommended for patients with clinically node-positive PTC in the central and lateral compartments (5). Prophylactic central neck dissection (CND) remains controversial, although it is not usually recommended (6, 7).
Central compartment neck dissection incorporates levels VI and VII, consisting of the region bounded superiorly by the hyoid bone, laterally by the carotid arteries, anteriorly by the investing layer of the deep cervical fascia, posteriorly by the prevertebral layer of the deep cervical fascia, and inferiorly by the innominate artery. However, in the central neck area, there are lymph nodes at the suprasternal space (SSLN) and between the sternocleidomastoid and sternohyoid muscles (LNSS).
Preoperative evaluation of the SSLN and LNSS is usually overlooked and is not commonly considered for dissection, especially because this space does not fall under the general classification of the central or lateral compartments of the neck (8).
Lymph node metastases to SSLN and LNSS are generally rare in PTC, and they occur occasionally in patients with PTC with lateral compartment lymph node metastases, especially metastases at levels III and IV (9–11). Recurrences do occur at SSLN and LNSS despite the very low rate of incidence.
We encountered two cases of lymph node recurrence at the SSLN and LNSS in PTC, including one case of recurrence at the SSLN and one case at the LNSS. Herein, we report two cases of recurrence in this region and discuss its clinical significance. Written informed consent was obtained from the patients for publication.
Case profiles
Case 1: A 62-year-old female was diagnosed with PTC arising in the left thyroid lobe and a thyroglossal duct cyst by fine-needle aspiration cytology (FNAC) and imaging studies, such as ultrasonography (US) and computed tomography (CT). US and CT examinations revealed two suspicious thyroid nodules, 8 and 7 mm in size, in the mid-portion of the left lobe and a 2-cm thyroglossal duct cyst, without any suspicious nodes in the central or lateral compartments. The patient underwent total thyroidectomy and prophylactic CND along with a Sistrunk operation for a suspected PTC of a thyroglossal duct cyst. The final pathological examination revealed PTCs in the left thyroid lobe and thyroglossal duct cyst. In addition, an occult metastatic node was identified in the central compartment. The patient underwent radioactive iodine (RAI) ablation (150 mCi of 131I) 1 month postoperatively. There was no evidence of regional or distant metastasis or remnant thyroid tissue in the thyroid bed on the follow-up RAI scan. The postoperative course was uneventful, and there has been no recurrence for up to 5 years after surgery. However, routine check-up US and CT revealed a suspicious lymph node in the suprasternal space 5 years postoperatively (Figure 1), and FNAC confirmed metastatic PTC. The patient underwent selective neck dissection (SND), including SSLN and LNSS and levels III–V (Figure 2). The final pathology confirmed metastatic PTC in one lymph node of the suprasternal space, whereas 48 lymph nodes from levels III–V showed no metastasis. Eleven years after the revision surgery, the patient is doing well without recurrence.
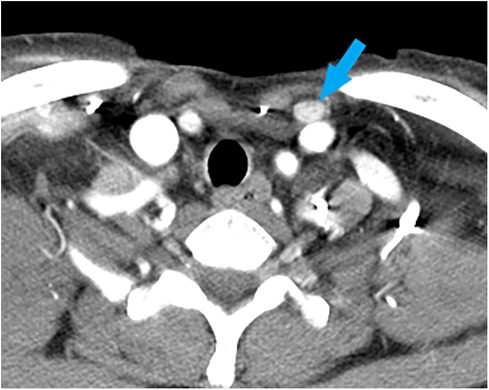
Figure 1. A computed tomography image of a metastatic lymph node (blue arrow), measuring 1.2 cm × 0.8 cm in size, at the left suprasternal space (SSLN).
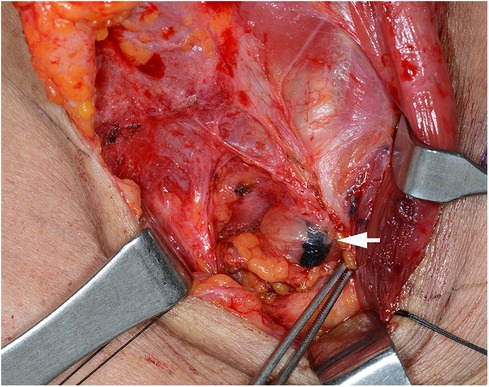
Figure 2. Surgical view of the metastatic lymph node, after tattooing (white arrow), at the left suprasternal space (SSLN).
Case 2: A 58-year-old female was referred to our hospital for managing bilateral vocal cord palsy after thyroidectomy 6 years before she visited our hospital. According to her medical records, the patient had already undergone total thyroidectomy, CND, left SND including levels II–V, and postoperative RAI therapy (150 mCi of 131I). She also underwent a third reoperation on both thyroid beds: CND, SND of the right level IV, and left levels II–IV. In addition, the patient received a second round of RAI therapy (150 mCi of 131I). During her initial visit to our hospital, imaging revealed no definite evidence of local or regional lymph node recurrence. Posterior cordotomy was performed using a transoral CO2 laser for bilateral vocal cord palsy, and her airway problems improved. At the 1-year follow-up, 7 years after the initial surgery, a newly developed hypoechoic nodule, measuring 0.9 cm × 1.2 cm in size, was found in an LNSS (Figure 3), and FNAC proved metastatic PTC. The patient underwent neck dissection at the right SSLN and LNSS, right CND, and right SND at levels II–V (Figure 4). Pathology revealed three metastatic lymph nodes at LNSS. The patient underwent another round of RAI therapy (180 mCi of 131I). Subsequently, the patient was lost to follow-up after a 2-year disease-free period.
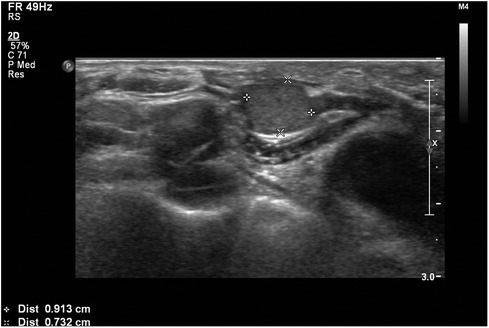
Figure 3. An ultrasound transverse view of a metastatic hypoechoic nodule, measuring 0.9 cm × 1.2 cm in size, within a lymph node between the right sternocleidomastoid and sternohyoid muscles (LNSS).
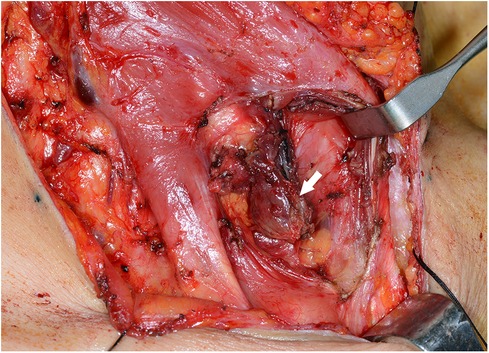
Figure 4. Surgical view of the metastatic lymph node (white arrow) between the right sternocleidomastoid and sternohyoid muscles (LNSS).
Discussion
Several previous studies have attempted to define SSLN and LNSS in patients with PTC to determine their significance (10–13). Sun et al. initially defined lymph nodes in the area between the sternocleidomastoid and sternohyoid muscles, hence its name LNSS, bounded by the intersection of the sternocleidomastoid and sternohyoid muscles superiorly, the suprasternal fossa and clavicle inferiorly, and the sternohyoid muscle as its medial and lateral boundaries (10). The suprasternal space is composed of superficial and deep layers of the investing deep cervical fascia above the manubrium of the sternum, also known as the burn space (11). Lee et al. defined the superficial level VI, and the boundaries of superficial level VI are superior to the lower margin of the cricoid cartilage, inferior to the sternal notch and clavicle, lateral to the lateral border of the sternohyoid muscle, medial to the medial border of the sternohyoid muscle, anterior to the investing layer of the deep cervical fascia, and posterior to the sternohyoid muscle (8).
Although it has been suggested that SSLN and LNSS metastases may be associated with preoperative lateral lymph node metastasis, primary tumors located in the inferior pole, strap muscle invasion, extrathyroidal extension, and the strength of correlation vary considerably among studies (10–13). Sun et al. demonstrated an LNSS metastasis rate of 22.6% in patients with PTC and clinically positive lateral lymph nodes (10). Yu et al. reported an SSLN metastasis rate of 20.7%, with the primary tumor at the inferior pole, strap muscle invasion, and level IV metastasis as independent risk factors (11). Similarly, Kwon et al. showed a positive rate for SSLN to be 12.9% and a correlation between a primary site in the inferior pole and level IV metastasis (12).
Several routes of metastasis to superficial-level lymph nodes have been proposed. Sun et al. speculated that metastasis to the LNSS may result from an increasing tumor load after lateral neck metastasis or communication between the superficial or deep anterior cervical chain and the deep lateral cervical chain (10). Homma et al. suggested that fibro-fatty tissues, including metastases from level IV, may gradually spread into the suprasternal space due to the constant movement of the neck (9). Similarly, Kwon et al. suggested that metastatic lymph nodes from level IV migrate medially, and those from level VI migrate laterally into the suprasternal space. Despite these efforts, in PTC patients, general patterns of lymph node metastasis to SSLN and LNSS are still not completely understood, and controversies remain.
The risk of recurrence is substantially higher in patients with PTC with initial clinically apparent metastases to the cervical lymph nodes. While the reported overall rate of nodal recurrence in patients with PTC ranges from 1% to 6%, the risk of recurrence in clinical N0 and N1 necks showed significant differences, with average rates of 4% and 22%, respectively (14). Moreover, nodal recurrence in patients with PTC preferentially occurs in the ipsilateral mid-lower jugular nodes, while posterior triangle and skipped metastases are uncommon (15). Although speculated to be even rarer, the incidence of recurrence at SSLN and LNSS is unknown because the lymph nodes of this area have received attention only recently.
In the two cases reported here, SSLN and LNSS recurrences were discovered on routine postoperative follow-up US or CT scans. There were no suspicious lymph nodes at SSLN and LNSS before the primary thyroidectomy. Therefore, after the initial thyroidectomy, it is essential to perform regular follow-ups for any suspicious lymph nodes at SSLN and LNSS and in the central and lateral compartments.
It is unclear whether prophylactic dissection of SSLN and LNSS can prevent recurrence in this area. However, we disagree with prophylactic dissection of SSLN and LNSS because the rate of occult metastasis is low. No clear evidence for the benefits of prophylactic dissection of SSLN and LNSS has been established. Lee et al. advised against prophylactic dissection of superficial level VI lymph nodes, including SSLN and LNSS, in the absence of suspicious findings on preoperative imaging, based on the low rate (3.1%) of superficial level VI occult metastases in their study (8). Yuan et al. suggested that greater attention should be paid to the lymph nodes between the investing layer of cervical fascia and deep fascia of infrahyoid strap muscles in a selected number of PTC patients based on the presence of suspected metastasis (13). Dissection of SSLN and LNSS should be carefully considered in cases with suspicious lymph nodes or confirmed metastasis in primary or recurrent cases. Further prospective studies with more cases and longer follow-ups are necessary to determine the predictive value and clinical significance of SSLN and LNSS metastasis.
Conclusion
Lymph node recurrence at the superficial level VI, including the SSLN and LNSS, can occasionally occur in patients with PTC. Therefore, careful evaluation and consideration should be given to the possibility of lymph node recurrence in this region.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Institutional Review Board (IRB) of Hanyang University Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
HC: Data curation, Formal analysis, Investigation, Methodology, Resources, Writing – original draft. CS: Formal analysis, Investigation, Methodology, Resources, Validation, Writing – review & editing. YJ: Data curation, Formal Analysis, Investigation, Validation, Writing – review & editing. KT: Conceptualization, Data curation, Supervision, Validation, Writing – review & editing.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Jin SH, Kim IS, Ji YB, Song CM, Chung MS, Tae K. Efficacy of prophylactic central neck dissection in hemithyroidectomy for papillary thyroid carcinoma. Eur Arch Otorhinolaryngol. (2020) 277:873–9. doi: 10.1007/s00405-019-05744-7
2. Zheng CM, Ji YB, Song CM, Ge MH, Tae K. Number of metastatic lymph nodes and ratio of metastatic lymph nodes to total number of retrieved lymph nodes are risk factors for recurrence in patients with clinically node negative papillary thyroid carcinoma. Clin Exp Otorhinolaryngol. (2018) 11(1):58–64. doi: 10.21053/ceo.2017.00472
3. Yoo BJ, Song CM, Ji YB, Lee JY, Park HJ, Tae K. Efficacy of central neck dissection for clinically node-negative papillary thyroid carcinoma: propensity scoring matching. Front Endocrinol. (2019) 10:172. doi: 10.3389/fendo.2019.00172
4. Lundgren CI, Hall P, Dickman PW, Zedenius J. Clinically significant prognostic factors for differentiated thyroid carcinoma: a population-based, nested case–control study. Cancer. (2006) 106(3):524–31. doi: 10.1002/cncr.21653
5. Moo TA, Fahey TJ III. Lymph node dissection in papillary thyroid carcinoma. Semin Nucl Med. (2011) 41(2):84–8. doi: 10.1053/j.semnuclmed.2010.10.003
6. Grant CS. Recurrence of papillary thyroid cancer after optimized surgery. Gland Surg. (2015) 4(1):52. doi: 10.3978/j.issn.2227-684X.2014.12.06
7. Haugen BR, Alexander EK, Bible KC, Doherty CM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. (2016) 26(1):1–133. doi: 10.1089/thy.2015.0020
8. Lee HN, Song CM, Ji YB, Myung JK, Lee YJ, Tae K. Occult metastasis to the superficial level VI lymph nodes in papillary thyroid carcinoma. Head Neck. (2022) 44(10):2796–802. doi: 10.1002/hed.27191
9. Homma A, Hatakeyama H, Mizumachi T, Furusawa J, Kano S, Sakashita T, et al. Lymph node metastasis in the suprasternal space from thyroid papillary cancer. Int Canc Conf J. (2015) 4(1):57–60. doi: 10.1007/s13691-014-0171-9
10. Sun G, Wang Y, Zhu Y, Wang Y, Xu K, Wei W, et al. Lymph node metastasis between sternocleidomastoid and sternohyoid muscle in clinically node-positive papillary thyroid carcinoma. Head Neck. (2013) 35(8):1168–70. doi: 10.1002/hed.23099
11. Yu ST, Ge JN, Sun BH, Wei ZG, Lei ST. Lymph node metastasis in suprasternal space in pathological node–positive papillary thyroid carcinoma. Eur J Surg Oncol. (2019) 45(11):2086–9. doi: 10.1016/j.ejso.2019.07.034
12. Kwon HK, Cheon YI, Shin SC, Sung ES, Lee JC, Kim IJ, et al. Risk factors of suprasternal lymph node metastasis in papillary thyroid carcinoma with clinical lateral cervical lymph node metastasis. Gland Surg. (2021) 10(2):512. doi: 10.21037/gs-20-368
13. Yuan Q, Hou J, Liao Y, Zheng L, Lu F, Wang K, et al. Lymph node metastasis in suprasternal space and intra-infrahyoid strap muscle space from papillary thyroid carcinoma. J Otolaryngol Head Neck Surg. (2020) 49(1):1–7. doi: 10.1186/s40463-020-00461-2
14. Randolph GW, Duh QY, Heller KS, LiVolsi VA, Mandel SJ, Steward DL, et al. The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as the presence of extranodal extension. Thyroid. (2012) 22(11):1144–52. doi: 10.1089/thy.2012.0043
Keywords: papillary thyroid carcinoma, lymph node metastasis, central neck dissection, suprasternal space, recurrence, thyroid cancer
Citation: Choi HW, Song CM, Ji YB and Tae K (2024) Case Report: Two cases of recurrences at the suprasternal space and lymph nodes between the sternocleidomastoid and sternohyoid muscles in papillary thyroid carcinoma. Front. Surg. 10:1258259. doi: 10.3389/fsurg.2023.1258259
Received: 13 July 2023; Accepted: 24 November 2023;
Published: 4 January 2024.
Edited by:
Fausto Famá, University of Messina, ItalyReviewed by:
Shi-Tong Yu, Southern Medical University, ChinaJoanna Jackowska, Poznan University of Medical Sciences, Poland
© 2024 Choi, Song, Ji and Tae. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kyung Tae a3l0YWVAaGFueWFuZy5hYy5rcg==
 Hae Won Choi
Hae Won Choi Chang Myeon Song
Chang Myeon Song Yong Bae Ji
Yong Bae Ji Kyung Tae
Kyung Tae