- Department of Cardiology, Jiangsu Province Hospital, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China
Aims: The present study aimed to compare the effects of left bundle branch area pacing (LBBAP) on cardiac function and clinical outcomes in patients with left bundle branch block (LBBB) and left ventricular ejection fraction (LVEF) >35 vs. ≤35%.
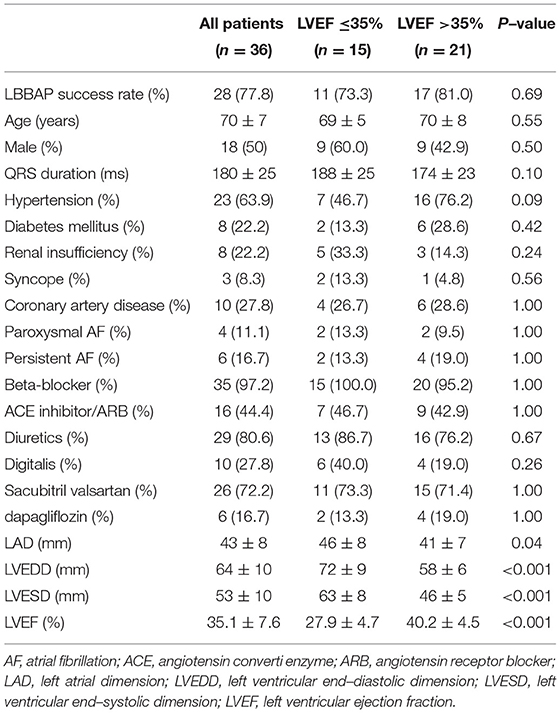
Methods and Results: Thirty-six consecutive patients with LBBB and LVEF <50% were enrolled. All patients were followed up for a mean of 6 months. The successful LBBAP was defined as a paced QRS complex presented as right bundle branch block (RBBB) morphology and QRSd < 130 ms. Echocardiography parameters, pacing parameters and clinical outcomes were collected. The successful LBBAP was achieved in 77.8% of all cases (28/36). In LVEF > 35% group (70 ± 8 years, 9 male), the success rate was 81.0% (17/21). QRSd significantly decreased from 174 ± 23 ms to 108 ± 13 ms (P < 0.001). The pacing threshold and R-wave amplitude were 0.6 ± 0.2 V @ 0.5 ms and 12 ± 7 mV, respectively. In LVEF ≤ 35% group (69 ± 5 years, 9 male), the success rate was 73.3% (11/15) with QRSd decreasing from 188 ± 25 ms to 107 ± 11 ms (P < 0.001). The hyperresponders to LBBAP (functional recovery and LVEF ≥ 50%) in LVEF > 35% group was 52.9%, which were almost twice of that in LVEF ≤ 35% group (33.3%). Whether patients had LBBAP or left ventricular septal pacing (LVSP), patients in the LVEF > 35% group showed significantly lower incidence of heart failure hospitalizations or death from any cause (hazard ratio in LVEF > 35% group, 0.22; 95%CI, 0.06 to 0.75, P = 0.011).
Conclusions: LBBAP can significantly shorten the QRSd and improve cardiac function in LBBB patients with either LVEF > 35 or ≤ 35%. LBBAP should be considered as an effective therapy for preventing the deterioration of cardiac function in early-stage heart failure patients with LBBB and LVEF > 35%.
Introduction
It is well established that left bundle branch block (LBBB) has bad effect on left ventricular (LV) function independent of coexisting heart disease. The electromechanical dyssynchrony of the ventricular contractions can contribute to adverse remodeling, reduction of left ventricular ejection fraction (LVEF), and mitral regurgitation in the long term. Cardiac resynchronization therapy (CRT), which involves simultaneous pacing of both right and left ventricles is beneficial and widely used around the world. Major US (ACC/AHA/HRS) (1) and European Society of Cardiology (ESC) guidelines (2, 3) were consistent in issuing Class I and IIA recommendations for CRT in patients who have LVEF ≤ 35% and LBBB with a QRS duration (QRSd) ≥ 150 ms, and New York Heart Association (NYHA) class II, III, or ambulatory IV symptoms. However, when LVEF is more than 35%, the recommendation level degrades, which seems to be arbitrary. The LVEF cut-off of ≤ 35% is adopted by heart failure (HF) major clinical trials of CRT, such as COMPANION (4), because people with LVEF ≤ 35% have higher incidence of adverse events, both in terms of sensitivity and specificity of incidence. However, LVEF or LV systolic dysfunction are continuous variables. And LVEF measured by echocardiography is not highly precise compared to magnetic resonance imaging (MRI). In addition, patients with LVEF > 35% are being neglected and the proportion of them is increasing. And they have similar characteristics and treatment patterns to those with LVEF <35%. In eraly-stage HF patients, those with LBBB have significantly worse clinical outcomes than patients without conduction system disease. Although common practice indicates that we implant CRT outside of guideline recommendations, randomized, multicenter studies in this population have not been conducted yet.
What's more, up to 30% of patients do not respond to CRT and the published data may be underestimated (5). Significant scar burden related to lead position (6), QRSd <150 ms (7), right ventricular failure (8), right bundle branch block (RBBB) morphology (9) have been demonstrated to be associated with lack of response. And in combination with national conditions of China, the price of CRT may be too high to be accepted in many patients. In 2017, Huang et al. (10) first reported a novel pacing method to correct the LBBB in the site of the left bundle branch (LBB) area with low and stable output; clinical outcome significantly improved over one year of follow-up. A large single center study (11), which included 632 patients who underwent left bundle branch area pacing (LBBAP), demonstrated that LBBAP was feasible and safe with high success rate in bradycardia or HF patients during long-term follow-up. And several studies (12, 13) have proved that LBBAP could achieve narrowing of QRS duration and improvement of clinical and echocardiographic outcomes in HF patients with LBBB, which means that LBBAP could be a promising resynchronization therapy alternative to biventricular pacing (BVP) for patients with CRT-indications. Since LBBAP is more convenient and cheaper compared to CRT, it would be of clinical interest whether LBBAP could benefit for the HF patients with LBBB and LVEF > 35%. Consequently, this study was undertaken to compare the clinical outcomes of LBBAP in patients with LBBB and LVEF > 35 vs. ≤ 35%.
Methods
Study Population
This was a single-center retrospective study. Consecutive patients underwent LBBAP were enrolled from the First Affiliated Hospital of Nanjing Medical University between May 2017 and December 2020. Patients who met the following criteria were included: (1) complete LBBB morphology that met Strauss criteria (14); (2)echocardiographic evidence of LVEF < 50%; (3) follow-up period over 6 months. All the patients included were provided written informed consent to the study protocol, and were approved by the Institutional Review Board.
Implantation Procedure
The technique of LBBAP procedure has been described in previous reports (15–19). Briefly, a ventricular pacing electrode (Medtronic 3830 electrode) with a 7-Fr guiding catheter (Model C315-S10; Medtronic Inc) was introduced into right ventricle via left subclavian or axillary vein, from His bundle area advanced 1–2 cm toward the right ventricle apex against the ventricular septum, then screwed through the interventricular septum (IVS) to the LBB area. When unipolar paced QRS complex presented as right bundle branch block morphology (qR or rSR' morphology in V1), and pacing parameters were satisfied, lead position and no perforation were assessed by angiogram through C315 sheath under left anterior oblique (LAO) 40°, the sheath was removed and lead was fixed. Successful LBBAP was defined as unipolar paced QRS morphology present as RBBB pattern and QRSd < 130 ms (15). If successful LBBAP could not be achieved after 5 attempts of lead positioning or fluoroscopy duration exceeded 30 min, the left ventricular septum pacing (LVSP) was also accepted, placing the 3,830 lead in the LV mid-septum to achieve a relatively narrow QRSd (17).
Data Collection
The baseline characteristics and medical history of participants were collected at enrollment. The LBBAP paced electrocardiogram (ECG) were interpreted by two cardiologists. The stimulus to peak LV activation time (SPLVAT), defined as the duration between the ventricular stimulation signal and R peak in lead V5, was measured, which meant LBBAP indirectly captured either the main LBB or its branches as previously described (15, 17, 18). Other electrocardiographic parameters such as the intrinsic QRSd, paced QRSd (pQRSd) were also measured. Pacing parameters like pacing thresholds, pacing impedance, R-wave amplitude were recorded. Echocardiographic parameters including left atrial dimension (LAD), left ventricular end diastolic diameter (LVEDD), left ventricular end systolic diameter (LVESD) and LVEF were also recorded.
Follow Up
Patients were followed up in the clinic or in hospital at baseline, 3, 6 and 12 months. Clinical characteristics, echocardiographic parameters and lead-related complications were recorded. LBBAP responder was defined as a patient who had an LVEF improvement of at least 5% at the 6-month follow-up. Patients were considered to be “hyperresponders” (20), if they met two following criteria: functional recovery and LVEF ≥ 50%. The primary composite endpoint included death from any cause or hospitalizations for HF. The diagnosis of HF hospitalization was made by professional physicians, if patients were developing symptoms that current treatments could not control and have to be hospitalized again due to the congestive HF.
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation (SD). Categorical variables were expressed as numbers and percentage values and compared using chi-square or Fisher's exact test. Comparisons between continuous variables were tested using Student's t-test. Kaplan–Meier survival curves were used to estimate for the combined endpoint of time to death or first HF hospitalization. The log rank test compared survival curves between two groups. Statistical analysis was performed using SPSS version 20.0 software. All P-values were two-tailed and P-values < 0.05 were considered significant.
Results
Baseline Characteristics
From May 2017 to December 2020, 496 patients underwent LBBAP. Of the 69 patients who had complete LBBB morphology, 33 patients with normal LVEF were excluded. Finally, 36 LBBB patients (age: 70 ± 7 years, male = 18) underwent an attempted LBBAP. There were 15 LBBB patients with LVEF ≤ 35% (27.9 ± 4.7), 21 LBBB patients with LVEF > 35% (40.2 ± 4.5). Successful LBBAP was achieved in 77.8% (28/36) of cases. The baseline characteristics were summarized in Table 1. There was no difference in age, sex, QRSd, drug utilization and complications including hypertension, diabetes mellitus, renal insufficiency, syncope, coronary artery disease and atrial fibrillation (AF) between the two groups. The LAD, LVEDD and LVESD were significantly higher in LVEF ≤ 35% group (46 ± 8 vs. 41 ± 7, P < 0.05; 72 ± 9 vs. 58 ± 6, P < 0.001; 63 ± 8 vs. 46 ± 5, P < 0.001).
Pacing Parameters
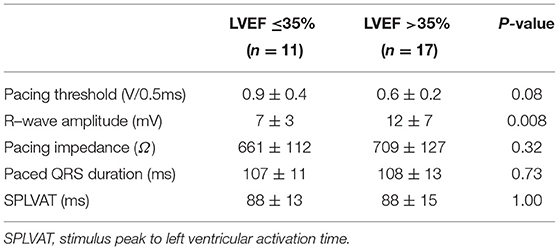
In LVEF ≤ 35% group, the QRSd significantly decreased from 188 ± 25 ms to 107 ± 11 ms (P < 0.001) and the SPLVAT was 88 ± 13 ms. In LVEF > 35% group, the QRSd also decreased from 174 ± 23 ms to 108 ± 13 ms (P < 0.001), and the SPLVAT was 88 ± 15 ms. In Table 2, during the LBBAP procedure, R-wave amplitude of LVEF > 35% group was significantly higher than LVEF ≤ 35% group (12 ± 7 mV vs. 7 ± 3 mV, P < 0.01). The pacing threshold, pacing impedance, paced QRSd and SPLVAT between the two groups were of no significance.
Clinical Outcomes
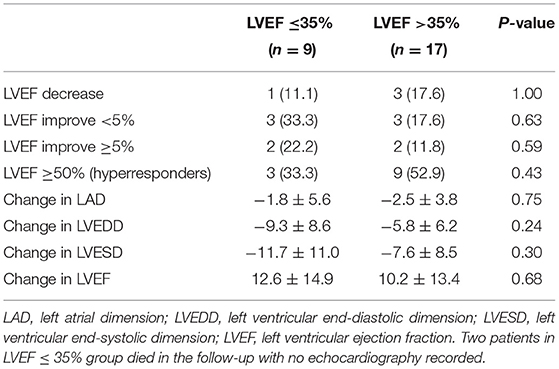
During the follow-up of a mean of 6 months, no complications associated with LBBAP such as lead perforation and dislodgement, pericardial effusion, pneumothorax, and thromboembolism were observed. There was one person in each group who had pocket infection and underwent incision and drainage of pocket. Clinical endpoint in successful LBBAP patients at the 12-month follow-up was shown in Table 3. The primary outcome occurred in 3 of 17 patients (17.6%) in LVEF > 35% group and 5 of 11 patients (45.5%) in LVEF ≤ 35% group. In addition, as shown in Figure 1, in all 36 patients recruited, the Kaplan-Meier survival curve of the primary endpoint of LVEF > 35% group including hospitalization for HF or death from any cause was significantly higher than of LVEF ≤ 35% group (hazard ratio in LVEF > 35% group, 0.22; 95%CI, 0.06 to 0.74, P = 0.011). And so did the Kaplan-Meier survival curve of death from any cause with P-value < 0.05.
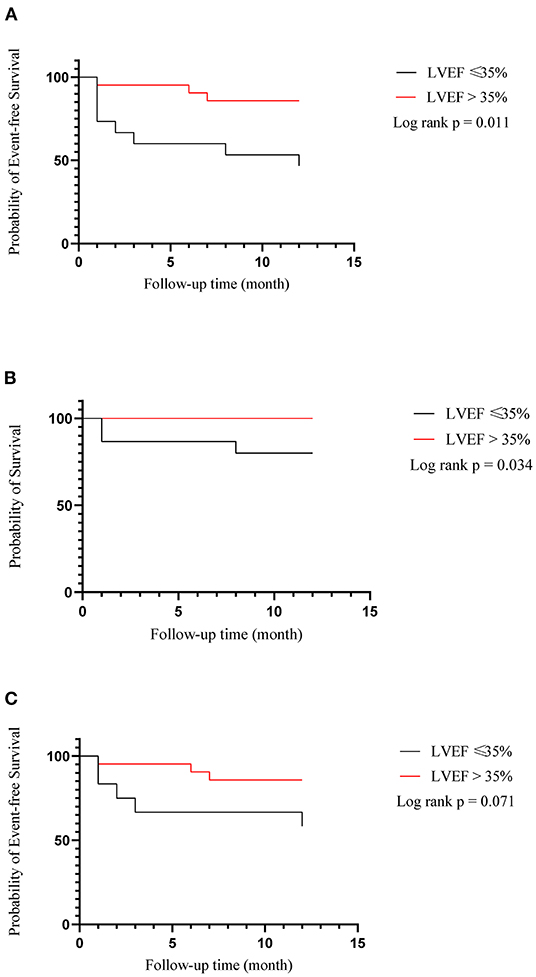
Figure 1. Kaplan–meier estimates of death or hospitalization for heart failure (composite primary outcome), death from any cause and hospitalization for heart failure among all of 36 patients recruited. (A) Heart failure hospitalization or death from any cause; (B) Death from any cause; (C) Heart failure hospitalization.
As it was shown in Table 4 and Figure 2, LAD, LVEDD, LVESD had shortened and LVEF had improved in both groups, but there was no difference in ΔLAD, ΔLVEDD, ΔLVESD and ΔLVEF between the two groups. However, the number of LBBAP hyperresponders in LVEF > 35% group was 9 (52.9%), which was almost twice of that in LVEF ≤ 35% group (33.3%).
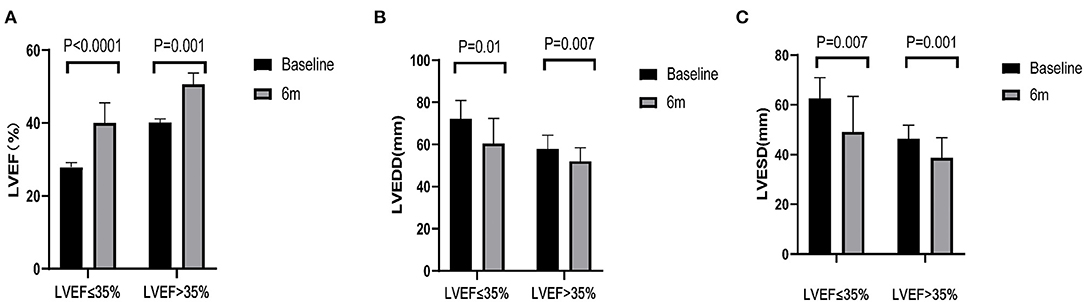
Figure 2. Echocardiographic parameters. (A–C) Left ventricular ejection fraction (LVEF), left ventricular end-diastolic dimension (LVEDD) and left ventricular end-systolic dimension (LVESD) of patients pre-left bundle branch area pacing (LBBAP) and 6 months after LBBAP.
In our study, a total of 3 patients died (one at 8 month, two at 1 month) after LBBAP due to progressive HF. The baseline LVEF of these patients were below 35%. Of them, 2 accepted LBBAP and 1 accepted LVSP. They were over 70 years old and had HF for many years with N-terminal pro-B-type natriuretic peptide (NT-proBNP) over 7000 pg/ml before LBBAP. Two of them had chronic kidney disease (21), which is a major contributor to mortality and HF exacerbations. One patient with LVEF below 25% died soon after LVSP due to ventricular fibrillation.
Discussion
In this study, we found that LBBAP could significantly shorten the QRSd and improve the cardiac function in LBBB patients with LVEF > 35%. Compared with LVEF ≤ 35%, patients with LVEF > 35% showed lower risk of combined endpoint of death from any cause or hospitalizations for HF and better echocardiographic response to LBBAP.
Active measures have been taken on those patients with LVEF ≤ 35% and the mortality and hospitalization have been decreasing in recent years. However, patients with higher LVEF are not being treated positively and promptly at the same time. The data from the American Heart Association's Get With The Guidelines (GWTG)(22) which included 110,621 patients showed that preserved and borderline LVEF (>40%) accounted for about half of all HF hospitalizations and the number was on increase. And LVEF is recognized to be an independent predictor of mortality and morbility in HF patients (23). Patients with LVEF ranging from 36 to 45% still have higher risk of adverse outcomes. Further, Witt et al. (24) proved that in patients with LVEF between 35 and 50%, those with LBBB had poorer clinical outcomes than those without conduction disease in the long-term follow-up.
Recently, there have been some studies which aim to prove the effect of CRT in patients with LVEF > 35%. Fung et al. (25) and Foley et al. (26) both reported that CRT could improve cardiac function and reverse LV remodeling in small groups of HF patients with LVEF > 35%. And in PROSPECT trial (27), CRT improved the clinical composite score (CCS) and decreased left ventricular end-systolic volume (LVESV) similarly in patients with LVEF ≤ 35 and >35%. However, In REVERSE (28), the study discovered that in patients with LVEF > 30%, CCS was improving by CRT but of no significance. Besides, a statistically significant decrease of LV end diastolic volume index (LVEDVi) was only seen in patients with LVEF <30%. Reasons why REVERSE showed lower LV reverse remodeling than the other studies are unclear. Interestingly, a prospective, randomized, controlled, double-blinded study called MIRACLE EF study (29) which aimed to prove that CRT could achieve clinical benefit in patients combined with moderately reduced LVEF (36–50%) and LBBB with the minimum 24-month follow-up. However the study was stopped after 13 months due to poor recruitment and enrolling only 44 patients. Reasons are complicated but one reason may be in short of understanding the feasibility and necessity of preventive treatment in this population. Current studies show contradictory results and the sample size is too small to be convincing. Besides, there is a lack of non-CRT group comparison and multicenter, randomized study to reflect the clinical effect of CRT in HF patients with LVEF > 35%, especially in the presence of LBBB.
Since the population of patients with LBBB and LVEF from 36 to 50% has been on the increase and the prognosis of them is quite poor if any proper measure is taken, there exists the need to take effective interventions ahead of time. Except for CRT, LBBAP is another appropriate choice as a new strategy for physiological pacing to achieve electrical synchrony of LV with high success rate.
In recent years, there has been many articles to prove the safety and feasibility of LBBAP in LBBB patients. Zhang et al. (30) used to demonstrate that QRSd was significantly shortened with shorter interventricular mechanical delay by LBBAP. And in 2020, Guo et al. (12) made a comparison between LBBAP and biventricular pacing (BIV) and the study showed that LBBAP could restore electrical synchrony better and achieve greater improvement in echocardiographic and clinical outcomes. We can take LBBAP to be a feasible treatment as a rescue pacing method or as the primary pacing strategy for HF patients with CRT indications (13, 31).
Our research and previous studies have yielded similar results. Furthermore, in LVEF >35% group, the number of hyperresponders is more than that in LVEF ≤ 35% group. Meanwhile, in this group, more than half of the patients had LV restored [defined as return to NYHA I and LVEF > 50% (32)]. And LVEF > 35% group has higher R-wave amplitude, possibly because fewer people in this group have myocardial injury, fibrosis, or infarction, which contributes to better response to LBBAP. Besides, there may be a “sweet spot” (33) for LBBAP as well, just like CRT. If the ventricular function gets worse to a certain level, the myocardium is too “sick” to respond to any therapy. As a result of the decline in LVEF, adverse remodeling also progressed so that the cardiac function of patients is hard to return to normal.
In all the 36 patients recruited in our study, whether patients have LBBAP or LVSP, compared with LVEF ≤ 35% group, patients in LVEF > 35% group show significantly lower incidence of death from any cause or hospitalization for HF via LBBAP (P = 0.011). Besides, all-cause mortality is significantly lower in the LVEF > 35% group as well (P = 0.034). In our study, many of patients with primary endpoints had chronic kidney disease or persistent AF before procedure, both of which can accelerate the overall progression of HF independently. And in EAARN score (34), renal failure with GRF <60 mL/min/1.73 m2 was predictive of poor outcomes in patients treated with CRT. Besides, AF was associated with poorer survival in CRT patients despite the benefits of the therapy.
Limitation
First, our study did not directly verify that LBBAP captured the cardiac conduction system by recording left bundle branch potential. Due to LBBB in most of patients, the potential could not be recorded in the conventional way; it could be achieved by double leads method, but this is not practical in regular clinical practice. In any event, our results of degree of narrowing QRSd and SPLVAT were comparable with other studies using direct left bundle branch potential recording (17–19). Thus the definition of LBBAP used in our study may include both left bundle branch pacing (LBBP) and LVSP. Nonetheless, LBBAP was supposed to have the same effect as LBBP. Second, the sample size was relatively small and follow-up was short-term. A large-scale randomized study with longer follow-up is necessary to clarify the role of LBBAP in these patients.
Conclusion
LBBAP could significantly shorten QRS duration and improve cardiac function during medium-and-short term follow up in patients with LBBB and LVEF between 35 and 50%. The degree of echocardiographic and clinical improvement by LBBAP in these patients was better than those with LVEF ≤ 35%. Thus, LBBAP is a promising physiological ventricular pacing which could be an effective therapy for preventing the deterioration of cardiac function in early-stage HF patients.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by the First Affiliated Hospital of Nanjing Medical University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was supported by the First Affiliated Hospital of Nanjing Medical University (JSPH-511C-2018-5).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are thankful for all the volunteers who participated in this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.838708/full#supplementary-material
References
1. Writing Committee M, Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. (2013) 128:e240–327. doi: 10.1161/CIR.0b013e31829e8776
2. European European Society of C, European Heart Rhythm A, Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, et al. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the task force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Europace. (2013) 15:1070–118. doi: 10.1093/europace/eut206
3. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. (2016) 37:2129–200. doi: 10.1093/eurheartj/ehw128
4. Bristow MR, Feldman AM, Saxon LA. Heart failure management using implantable devices for ventricular resynchronization: Comparison of Medical Therapy, Pacing, and Defibrillation in Chronic Heart Failure (COMPANION) trial. COMPANION Steering Committee and COMPANION Clinical Investigators. J Card Fail. (2000) 6:276–85. doi: 10.1054/jcaf.2000.9501
5. Birnie DH, Tang AS. The problem of non-response to cardiac resynchronization therapy. Curr Opin Cardiol. (2006) 21:20–6. doi: 10.1097/01.hco.0000198983.93755.99
6. Adelstein EC, Saba S. Scar burden by myocardial perfusion imaging predicts echocardiographic response to cardiac resynchronization therapy in ischemic cardiomyopathy. Am Heart J. (2007) 153:105–12. doi: 10.1016/j.ahj.2006.10.015
7. Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. (2009) 361:1329–38. doi: 10.1056/NEJMoa0906431
8. Haddad F, Doyle R, Murphy DJ, Hunt SA. Right ventricular function in cardiovascular disease, part II: pathophysiology, clinical importance, and management of right ventricular failure. Circulation. (2008) 117:1717–31. doi: 10.1161/CIRCULATIONAHA.107.653584
9. Bilchick KC, Kamath S, DiMarco JP, Stukenborg GJ. Bundle-branch block morphology and other predictors of outcome after cardiac resynchronization therapy in Medicare patients. Circulation. (2010) 122:2022–30. doi: 10.1161/CIRCULATIONAHA.110.956011
10. Huang W, Chen X, Su L, Wu S, Xia X, Vijayaraman P. A beginner's guide to permanent left bundle branch pacing. Heart Rhythm. (2019) 16:1791–6. doi: 10.1016/j.hrthm.2019.06.016
11. Su L, Wang S, Wu S, Xu L, Huang Z, Chen X, et al. Long-term safety and feasibility of left bundle branch pacing in a large single-center study. Circ Arrhythm Electrophysiol. (2021) 14:e009261. doi: 10.1161/CIRCEP.120.009261
12. Guo J, Li L, Xiao G, Ye T, Huang X, Meng F, et al. Remarkable response to cardiac resynchronization therapy via left bundle branch pacing in patients with true left bundle branch block. Clin Cardiol. (2020) 43:1460–8. doi: 10.1002/clc.23462
13. Vijayaraman P, Ponnusamy S, Cano O, Sharma PS, Naperkowski A, Subsposh FA, et al. Left bundle branch area pacing for cardiac resynchronization therapy: results from the international LBBAP collaborative study group. JACC Clin Electrophysiol. (2021) 7:135–47. doi: 10.1016/j.jacep.2020.08.015
14. Strauss DG, Selvester RH, Wagner GS. Defining left bundle branch block in the era of cardiac resynchronization therapy. Am J Cardiol. (2011) 107:927–34. doi: 10.1016/j.amjcard.2010.11.010
15. Chen K, Li Y, Dai Y, Sun Q, Luo B, Li C, et al. Comparison of electrocardiogram characteristics and pacing parameters between left bundle branch pacing and right ventricular pacing in patients receiving pacemaker therapy. Europace. (2019) 21:673–80. doi: 10.1093/europace/euy252
16. Chen X, Wu S, Su L, Su Y, Huang W. The characteristics of the electrocardiogram and the intracardiac electrogram in left bundle branch pacing. J Cardiovasc Electrophysiol. (2019) 30:1096–101. doi: 10.1111/jce.13956
17. Li X, Li H, Ma W, Ning X, Liang E, Pang K, et al. Permanent left bundle branch area pacing for atrioventricular block: Feasibility, safety, and acute effect. Heart Rhythm. (2019) 16:1766–73. doi: 10.1016/j.hrthm.2019.04.043
18. Li Y, Chen K, Dai Y, Li C, Sun Q, Chen R, et al. Left bundle branch pacing for symptomatic bradycardia: Implant success rate, safety, and pacing characteristics. Heart Rhythm. (2019) 16:1758–65. doi: 10.1016/j.hrthm.2019.05.014
19. Vijayaraman P, Subzposh FA, Naperkowski A, Panikkath R, John K, Mascarenhas V, et al. Prospective evaluation of feasibility and electrophysiologic and echocardiographic characteristics of left bundle branch area pacing. Heart Rhythm. (2019) 16:1774–82. doi: 10.1016/j.hrthm.2019.05.011
20. Castellant P, Fatemi M, Bertault-Valls V, Etienne Y, Blanc JJ. Cardiac resynchronization therapy: “nonresponders” and “hyperresponders”. Heart Rhythm. (2008) 5:193–7. doi: 10.1016/j.hrthm.2007.09.023
21. Schefold JC, Filippatos G, Hasenfuss G, Anker SD, von Haehling S. Heart failure and kidney dysfunction: epidemiology, mechanisms and management. Nat Rev Nephrol. (2016) 12:610–23. doi: 10.1038/nrneph.2016.113
22. Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP, et al. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation. (2012) 126:65–75. doi: 10.1161/CIRCULATIONAHA.111.080770
23. Solomon SD, Claggett B, Lewis EF, Desai A, Anand I, Sweitzer NK, et al. Influence of ejection fraction on outcomes and efficacy of spironolactone in patients with heart failure with preserved ejection fraction. Eur Heart J. (2016) 37:455–62. doi: 10.1093/eurheartj/ehv464
24. Witt CM, Wu G, Yang D, Hodge DO, Roger VL, Cha YM. Outcomes with left bundle branch block and mildly to moderately reduced left ventricular function. JACC Heart Fail. (2016) 4:897–903. doi: 10.1016/j.jchf.2016.07.002
25. Fung JW, Zhang Q, Yip GW, Chan JY, Chan HC, Yu CM. Effect of cardiac resynchronization therapy in patients with moderate left ventricular systolic dysfunction and wide QRS complex: a prospective study. J Cardiovasc Electrophysiol. (2006) 17:1288–92. doi: 10.1111/j.1540-8167.2006.00612.x
26. Foley PW, Stegemann B, Smith RE, Sanderson JE, Leyva F. Cardiac resynchronization therapy in patients with mildly impaired left ventricular function. Pacing Clin Electrophysiol. (2009) 32:S186–189. doi: 10.1111/j.1540-8159.2008.02280.x
27. Chung ES, Katra RP, Ghio S, Bax J, Gerritse B, Hilpisch K, et al. Cardiac resynchronization therapy may benefit patients with left ventricular ejection fraction >35%: a PROSPECT trial substudy. Eur J Heart Fail. (2010) 12:581–7. doi: 10.1093/eurjhf/hfq009
28. Linde C, Daubert C, Abraham WT, St John Sutton M, Ghio S, Hassager C, et al. Impact of ejection fraction on the clinical response to cardiac resynchronization therapy in mild heart failure. Circ Heart Fail. (2013) 6:1180–9. doi: 10.1161/CIRCHEARTFAILURE.113.000326
29. Linde C, Curtis AB, Fonarow GC, Lee K, Little W, Tang A, et al. Cardiac resynchronization therapy in chronic heart failure with moderately reduced left ventricular ejection fraction: Lessons from the Multicenter InSync Randomized Clinical Evaluation MIRACLE EF study. Int J Cardiol. (2016) 202:349–55. doi: 10.1016/j.ijcard.2015.09.023
30. Zhang W, Huang J, Qi Y, Wang F, Guo L, Shi X, et al. Cardiac resynchronization therapy by left bundle branch area pacing in patients with heart failure and left bundle branch block. Heart Rhythm. (2019) 16:1783–90. doi: 10.1016/j.hrthm.2019.09.006
31. Jastrzebski M, Moskal P, Huybrechts W, Curila K, Sreekumar P, Rademakers LM, et al. Left bundle branch-optimized cardiac resynchronization therapy (LOT-CRT): results from an international LBBAP collaborative study group. Heart Rhythm. (2022) 19:13–21. doi: 10.1016/j.hrthm.2021.07.057
32. Kutyifa V, Kloppe A, Zareba W, Solomon SD, McNitt S, Polonsky S, et al. The influence of left ventricular ejection fraction on the effectiveness of cardiac resynchronization therapy: MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy). J Am Coll Cardiol. (2013) 61:936–44.
33. Gasparini M, Galimberti P. Device therapy in heart failure: has CRT changed “the sickest benefit the most” to “the healthiest benefit the most?” J Am Coll Cardiol. (2013) 61:945–7. doi: 10.1016/j.jacc.2012.11.048
Keywords: left bundle branch block, left bundle branch area pacing, cardiac resynchronization therapy, QRS duration, heart failure
Citation: Jiang Z, Wu T, Wu Y, Chen Z, Yang W, Chen C, Zhou X and Shan Q (2022) Clinical Outcomes of Permanent Left Bundle Branch Area Pacing in Patients With Left Bundle Branch Block and Left Ventricular Ejection Fraction >35 vs. ≤35%. Front. Cardiovasc. Med. 9:838708. doi: 10.3389/fcvm.2022.838708
Received: 18 December 2021; Accepted: 21 February 2022;
Published: 17 March 2022.
Edited by:
Carlo de Asmundis, University Hospital Brussels, BelgiumReviewed by:
Alexandre Almorad, University Hospital Brussels, BelgiumWeijian Huang, First Affiliated Hospital of Wenzhou Medical University, China
Copyright © 2022 Jiang, Wu, Wu, Chen, Yang, Chen, Zhou and Shan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qijun Shan, cWpzaGFuQG5qbXUuZWR1LmNu; Xiujuan Zhou, enhqaGVhcnRAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Zhixin Jiang†
Zhixin Jiang† Wen Yang
Wen Yang Xiujuan Zhou
Xiujuan Zhou Qijun Shan
Qijun Shan