- 1Department of Psychiatry, University of California San Diego, San Diego, CA, United States
- 2Department of Neurosciences, University of California San Diego, San Diego, CA, United States
- 3Center for Functional MRI, University of California San Diego, San Diego, CA, United States
- 4Department of Radiology, University of California San Diego, San Diego, CA, United States
Background: Despite declining use of traditional combustible cigarettes, the use of nicotine and tobacco-related products (NTPs) remains high among adolescents and emerging adults largely due to the use of e-cigarettes. Adolescents and emerging adults who initiate e-cigarette use reach comparable indices of nicotine dependence as traditional cigarette smokers and can report symptoms of dependence even before developing a pattern of daily use. Symptoms such as craving, positive and negative reinforcement, and biological markers of toxicity are closely linked to the development and persistence of substance use problems. Adolescents/emerging adults may transition to dependence more quickly than adults, and the age of onset of regular NTP use is a highly predictive risk factor for future use and problems. Within the brain, the hippocampus is particularly sensitive to the effects of nicotine and may play a role in the transition from NTP initiation to more habitual and even problematic use.
Methods: A cross-sectional sample of healthy, NTP-using late adolescents/emerging adults (N = 86) ages 16–22 completed a structural MRI to examine whether subjective nicotine craving, stronger positive and negative reinforcement, elevated cotinine levels, and earlier age of onset of regular nicotine use would be associated with hippocampal volumes.
Results: Across measures of nicotine addiction, linear regression models revealed an interaction between symptoms and age of onset of regular use. A general pattern emerged such that greater symptom severity and younger age of onset of regular use was associated with larger hippocampal volumes.
Conclusions: These findings provide potential insight into the relationship between late adolescent/emerging adult brain health and a risk factor for NTP initiation and symptoms of nicotine addiction. Greater understanding of these interactions is essential for informing prevention, intervention, and public health policy.
Introduction
While the use of combustible cigarettes has declined among late adolescents and emerging adults [AEAs, (1, 2)], the prevalence of nicotine and tobacco-related products (NTPs) remains high (1, 3, 4), largely due to the increased use of e-cigarettes (2, 5). Although e-cigarettes were initially marketed as tools for cigarette cessation (6, 7), AEAs have frequently been targeted with digital advertising by tobacco companies (8, 9), which may contribute to reductions in perceived risk and more favorable attitudes towards e-cigarette use (10–12). E-cigarettes provide similar or possibly greater nicotine delivery per puff as compared to combustible cigarettes (13), and vaping further allows for easy consumption across the day leading to increased use, intensity, and nicotine exposure (14, 15). AEAs who initiate use of e-cigarettes reach comparable indices of nicotine dependence as cigarette users (16–18) highlighting the highly addictive nature of nicotine for AEAs (19–21). Notably, use of e-cigarettes among AEAs has been associated with problematic substance use including alcohol and cannabis misuse (22–25). Therefore, understanding the associations between known risk factors for the initiation of e-cigarettes and other nicotine use, the progression to nicotine dependence, and their impact for brain health is crucial for informing prevention, intervention, and public health policy.
Craving, positive and negative reinforcement (e.g., pleasurable effects, escaping unpleasant states), and biological markers of toxicity (i.e., the accumulation of harmful metabolites in the body) are closely linked to the development and persistence of substance use problems. Substance dependence can generally be defined as intense cravings for the substance of choice, development of tolerance, and loss of autonomy over use despite potential negative consequences (26). Consistent with this definition, reports of initial symptoms of nicotine dependence among AEAs have included intense craving or desire to use, feelings of loss of control, withdrawal, and tolerance (19, 27–29). These symptoms have been associated with increased risk for continued and even escalated NTP use (19, 27–30). Initial pleasurable experiences with NTPs can similarly predict future use as well as severity of dependence symptoms among AEAs (28). This pattern maps on to models of addiction in which substances such as nicotine are often initiated for their hedonic effects and continued due to positive reinforcement of those experiences (26). Repeated use of nicotine can then lead to tolerance and the need for greater consumption (26). Consistent with more intense NTP use, higher levels of cotinine, the primary metabolite of nicotine, have been associated with greater symptoms of dependence in AEAs, potentially reflecting greater physiological dependence and thus neurobiological changes (31–33). This is particularly concerning as AEAs may report dependence symptoms even before developing a pattern of daily use (20, 34), suggesting heightened sensitivity to the effects of nicotine (35).
Age of onset of regular substance use is also a highly predictive risk factor for future use and dependence (36–40). Individuals who regularly engage in NTP use at younger ages are at increased risk of developing nicotine dependence (36, 39, 40) and may transition to dependence more quickly than adults and even older AEAs (37, 39). Indeed, AEAs may develop nicotine dependence even after minimal exposure (19–21, 27, 28, 34), and nicotine exposure during adolescence/emerging adulthood may uniquely impact brain health compared to older adults (35, 41–43), underscoring the heightened sensitivity of this developmental period to substance use (44). This may be related to nicotine's binding to nicotinic acetylcholine receptors (nAChRs), which can alter nACHRs expression and function (42). These receptors are distributed throughout the brain (42) and may play a role in the gray and white matter morphometric changes observed in association with AEA NTP use (45–51). In particular, the hippocampus is dense with nAChRs (42) and is involved with reinforcement learning and episodic memory of rewarding stimuli (52), which may be particularly heightened in AEAs (41). Nicotine may also enhance dopaminergic transmission within the nucleus accumbens and dorsal striatum, regions heavily implicated in reward processing and the development of substance dependence (41, 52).
We recently reported greater cumulative 6-month NTP use was associated with larger bilateral hippocampal volumes in a sample of AEAs (49). Given greater cumulative use is associated with more severe dependence (19, 27–30) as well as younger age of onset of use (36, 37, 39, 40), in this report we sought to examine these relationships with hippocampal volumes within the sample of AEAs who had initiated regular NTP use. More specifically, we hypothesized that indicators of problematic NTP use, including greater subjective nicotine craving, stronger positive and negative reinforcement, elevated cotinine levels, and earlier age of onset of regular NTP use would be associated with larger hippocampal volumes.
Methods
Participants and procedures
Eighty-six participants were selected for this analysis from a larger study on the effects of nicotine and cannabis co-use on brain structure and function during late adolescence/emerging adulthood. As previously reported (48, 53), participants were recruited via flyers posted physically and electronically at schools, community colleges, four-year universities, and social media sites targeting San Diego County. Recruitment was stratified based on use of NTPs, cannabis products, or both during the previous 6-month period to ensure variability in NTP and cannabis use.
Exclusion criteria included >10 lifetime episodes of illicit substance use; lifetime DSM-5 psychiatric diagnoses other than tobacco and/or cannabis use disorder; acute influence of cannabis or alcohol use at study visit; use of any psychoactive medications; major medical problems; MRI contraindications; or history of prenatal substance exposure or developmental disability.
Participants completed a single 4-hour session consisting of a battery of interviews, self-report assessments covering demographic information, mental health, substance use, and neurocognitive functioning, which was followed by an MRI session. Before beginning the study session, all participants gave written informed consent (≥18 years old) or parental consent and participant assent (<18 years old). Participants were asked to refrain from using cannabis and alcohol 12 h prior to the appointment, which was confirmed with oral fluid, urine, and breathalyzer. Urine samples were used to confirm abstinence from illicit substances. Participants abstained from caffeine for at least 30 minutes prior to MRI scanning. They were not required to abstain from NTP use to avoid nicotine withdrawal effects during testing. Time of last NTP use was documented. All procedures were approved by the University of California, San Diego Human Research Protections Program.
Measures
Demographic data (e.g., age, sex at birth, race/ethnicity, education) were obtained from a psychosocial interview. To assess quantity and frequency of NTP and cannabis use, the Customary Drinking and Drug Use Record structured interview (54) was used, including a modification to include additional nicotine and cannabis questions (55–57). Lifetime use of nicotine, cannabis and alcohol were estimated in terms of independent episodes, allowing for multiple uses to be reported within a single day (e.g., first thing in the morning, again before bed). Participants were asked to provide additional details related to each substance reported including age at first use and onset of regular (at least weekly) use.
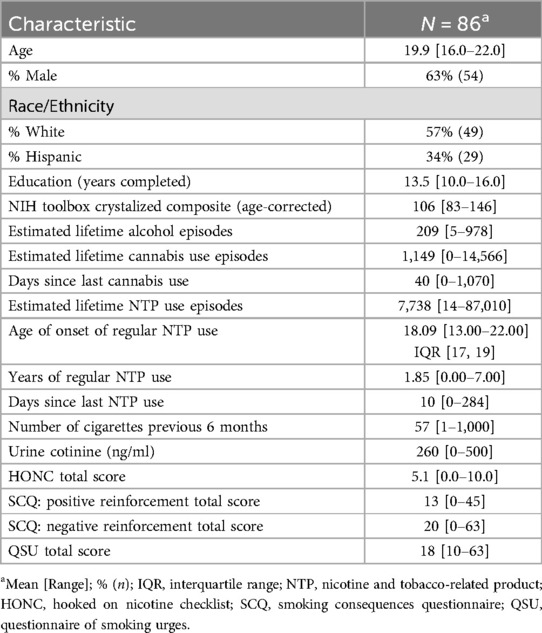
As part of the assessment, participants completed a range of self-report questionnaires related to their NTP use experiences. Severity of nicotine dependence was assessed using the Hooked on Nicotine Checklist [HONC, (58)]. They completed an adapted Smoking Consequences Questionnaire [SCQ, (59)] with questions specific to e-cigarette use. Four subscales can be calculated from the SCQ: negative consequences, positive reinforcement, negative reinforcement, and weight control. For the purposes of this study, only the positive and negative reinforcement subscales were included in analyses. To examine nicotine craving, participants completed the 10-item version of the Questionnaire on Smoking Urges, which was modified to reflect both cigarette and vaping urges, and a total score was computed [QSU, (60, 61)]. Acute nicotine exposure was examined through quantification of urine cotinine levels, which is nicotine's major metabolite (quantification conducted by Redwood Toxicology). Cotinine values were capped at 500 ng/ml per Redwood Toxicology's standard procedures. See Table 1 for a complete description of the sample demographics and substance use characteristics.
Imaging acquisition and processing
Participants were scanned on a 3.0 Tesla GE Discovery MR750 scanner with a 32-channel receive head coil at the UCSD Center for Functional MRI. A high-resolution T1-weighted anatomical fast spoiled gradient echo (FSPGR) scan was acquired with TI/TE/TR = 1,060/2/2,500 ms, 256 × 256 matrix, flip angle = 8˚, FOV = 256 mm, 1.0 mm3 voxels. Brain images for each participant were spatially normalized, field-bias corrected, and segmented using the Freesurfer pipeline [version 6.0, (62, 63)]. To identify errors made during the Freesurfer reconstruction process, one rater (QS), blind to participant characteristics, followed the reconstruction procedures to correct any errors made during the cortical and subcortical reconstruction process. This involved verification of the automated skull stripping and a slice-by-slice inspection of the gray/white and gray/cerebral spinal fluid surfaces. Modifications to the surfaces were made as necessary to correct for tissue misclassifications (e.g., residual dura mater classified as cortex). Right and left hippocampal volumes and an estimate of total brain volume (“BrainSegVolNotVent”) were extracted for analyses.
Data analyses
Data analyses were conducted using R (v4.3.2). Estimates of bilateral hippocampal volumes were examined using individual linear regressions that modeled the interaction between age of onset of regular NTP use and four indices of nicotine addiction severity including: (1) severity of acute nicotine exposure quantified in urine cotinine values; (2) the self-reported positive and negative reinforcing effects of nicotine (SCQ subscales); (3) the craving and urge to use NTPs (QSU total); and (4) nicotine dependence symptoms (HONC total). Total brain volume, current age, sex assigned at birth, and lifetime alcohol, cannabis, and NTP use episodes were included in the models as covariates.
Results
Cotinine
Regression models were used to examine the relationship between urine cotinine and age of onset of regular NTP use with bilateral hippocampal volumes, controlling for current age, sex, lifetime alcohol, cannabis, and NTP use, and estimated brain volume. Results indicated a significant age of regular use x cotinine interaction for both the left and right hippocampal volumes (Left: B = −0.17, t = −2.4, p = 0.021; Right: B = −0.22, t = −2.7, p = 0.010) (Figure 1A, left not shown). This inverse relationship suggests that as age of regular use of NTPs became younger, hippocampal volumes were larger as a function of increasing cotinine values. Current age, sex, and lifetime alcohol, cannabis, and NTP use were not significant covariates (ps > 0.1), though estimated brain volume was a significant covariate for both left and right volumes (ps < 0.0001).
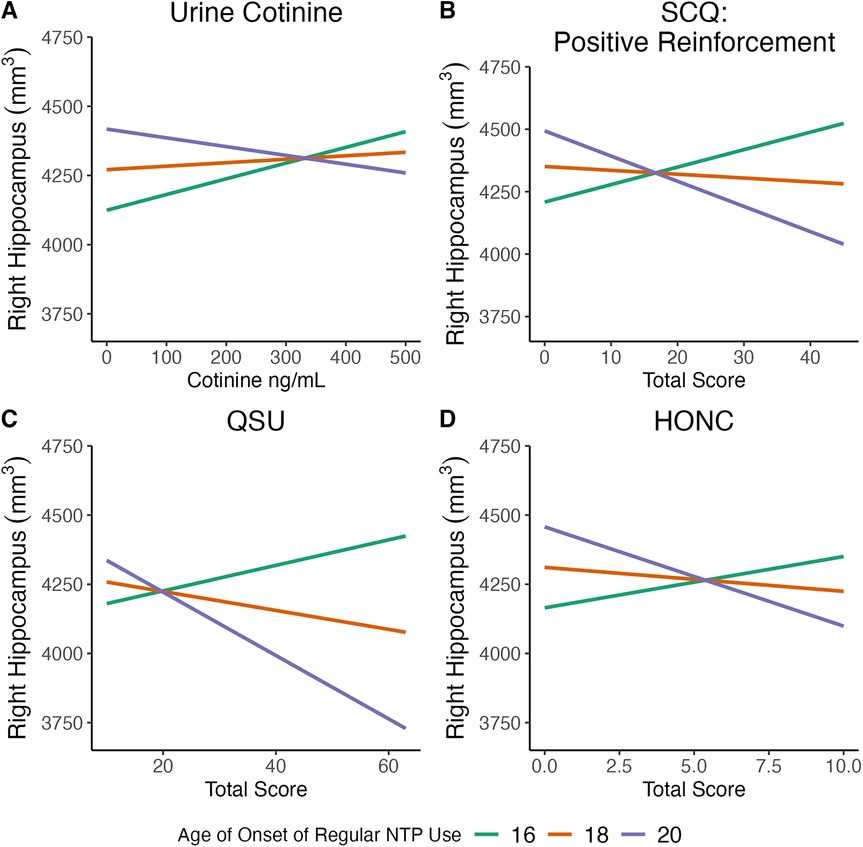
Figure 1. Significant relationships were observed between age of onset of regular use of nicotine and tobacco-related products (NTPs) and measures of nicotine addiction severity on (right) hippocampal volume. Measures of nicotine addiction severity included (A) urine cotinine; (B) Smoking Consequences Questionnaire (SCQ): positive reinforcement; (C) Questionnaire on Smoking Urges (QSU); and (D) Hooked on Nicotine Checklist (HONC). Data presented are for visualization purposes only and represent trend lines for age of onset of regular NTP use mean age (18 years old) ± 1 SD (20 and 16 years old, respectively).
Smoking consequences questionnaire: positive reinforcement
Regression models were used to examine the relationship between the self-reported positive reinforcing effects of nicotine as measured by the SCQ and age of onset of regular NTP use with bilateral hippocampal volumes, controlling for current age, sex, lifetime alcohol, cannabis, and NTP use, and estimated brain volume. Results indicated a significant age of regular use x positive reinforcement interaction for both the left and right hippocampal volumes (Left: B = −2.4, t = −2.2, p = 0.03; Right: B: −4.3, t = −3.4, p = 0.001) (Figure 1B, left not shown). The inverse relationship indicates that hippocampal volumes increased as a function of younger regular use of NTPs and higher positive reinforcement from NTP use. Current age, sex, and lifetime alcohol, cannabis, and NTP use were not significant covariates (ps > 0.2), though estimated brain volume was a significant covariate for both volumes (ps < 0.0001).
Smoking consequences questionnaire: negative reinforcement
Regression models were used to examine the relationship between the self-reported negative reinforcing effects of nicotine as measure by the SCQ and age of onset of regular NTP use with bilateral hippocampal volumes, controlling for current age, sex, lifetime alcohol, cannabis, and NTP use, and estimated brain volume. A trend was observed between age of first regular use and negative reinforcement for the right hippocampal volume (B = −2.3, t = −1.9, p = 0.06), though not for the left (p > 0.6). The inverse relationship, though not significant, indicates that hippocampal volumes increased as a function younger age of onset of regular use and higher negative reinforcement from NTP use. Current age, sex, and lifetime alcohol, cannabis, and NTP use were not significant covariates (ps > 0.4), though estimated brain volume was significant (p < 0.0001).
Questionnaire on smoking urges
Regression models were used to examine the relationship between self-reported smoking/vaping urge symptoms as measured by the QSU and age of onset of regular NTP use with bilateral hippocampal volumes, controlling for current age, sex, lifetime alcohol, cannabis, and NTP use, and estimated brain volume. Results indicated a significant interaction between age of regular use and smoking/vaping urges for the right hippocampal volume (B = −4.0, t = −2.1, p = 0.039) (Figure 1C), though no relationship was observed for the left (p > 0.3). The inverse relationship observed for the right hippocampus suggests that as age of regular use of NTPs became younger and smoking/vaping urge symptoms increased hippocampal volumes were larger. For the right hippocampus, current age, sex, and lifetime alcohol, cannabis, and NTP use were not significant covariates (ps > 0.4), though estimated brain volume was significant (p < 0.0001).
HONC dependence
Regression models were used to examine the relationship between nicotine dependence as measure by the HONC and age of onset of regular NTP use with bilateral hippocampal volumes, controlling for current age, sex, lifetime alcohol, cannabis, and NTP use, and estimated brain volume. Results indicated a significant age of regular use x HONC interaction for the right hippocampal volume (B = −0.02, t = −3.1, p = 0.003) (Figure 1D), though no relationship was observed for the left (p > 0.9). The inverse association for the right hippocampus indicates that as age of regular use of NTPs became younger and individuals currently exhibited symptoms of nicotine addiction, hippocampal volumes were larger. For the right hippocampus, current age, sex, and lifetime alcohol, cannabis, and NTP use were not significant covariates (ps > 0.1), though estimated brain volume was a significant covariate (p < 0.0001).
Discussion
We previously reported greater 6-month nicotine use was associated with larger bilateral hippocampal volumes in a sample of late adolescents and emerging adults (49). In this follow-up report, we sought to examine whether indicators of more problematic nicotine use, including greater subjective nicotine craving, stronger positive and negative reinforcement, elevated cotinine levels, and earlier age of onset of regular nicotine use would be associated with larger hippocampal volumes. Consistent with our hypotheses, the results revealed a general pattern and interaction such that as age of onset of regular use became younger and symptoms of nicotine addiction became more severe, hippocampal volumes increased. Notably, negative reinforcement, or the alleviation of unpleasant states, was not associated with hippocampal volume in this study, which is consistent with adolescents and emerging adults being less sensitive to the negative effects of nicotine but more sensitive to the rewarding aspects (41).
The hippocampus is implicated in the development and maintenance of substance use disorders (64, 65) by its involvement in modulating reinforcement learning and episodic memory of rewards (52). While few studies have examined the relationship between hippocampal volumes and indices of problematic nicotine use, larger bilateral hippocampal volumes have been associated with worse smoking cessation outcomes in a group of adult cigarette smokers (66). Functional MRI studies similarly suggest enhanced activation of the hippocampus in response to contextual smoking cues (67), while increased resting state functional connectivity between the hippocampus and striatum predicted greater substance use at follow-up in adolescents (68). Like the hippocampus, the dorsal striatum is heavily involved in habit formation (69, 70) and contributes to the development of substance dependence (65, 70). Differences in dorsal striatal regions have been observed to be associated with nicotine dependence symptoms such as craving. In a small sample of emerging adults, larger dorsal striatal volume and surface area was related to higher subjective cigarette craving and craving induced by exposure to smoking cues (71). Similarly, larger putamen volumes were associated with greater lifetime history of cigarette smoking as well as younger age of smoking initiation (72). In this context, the larger hippocampal volumes in the present study being associated with more severe symptoms of nicotine dependence, including craving, could reflect enhanced substance-related reinforcement learning, particularly in those who initiate regular use at younger ages. Overall, these processes may be heightened in adolescents and emerging adults (41) and represent a risk factor for the development of nicotine dependence.
Given the cross-sectional nature of the current study, the causal relationship between hippocampal volume and indices of nicotine dependence cannot be determined. Indeed, the larger hippocampal volumes reported here could be a pre-existing risk factor for initiating nicotine use and subsequent development of nicotine-related problems. However, despite the prevalance of NTP use among AEAs (1, 3, 4) and its addictive nature (19–21), few longitudinal studies have focused on identifying brain morphometry that can predict future use (73). One study reported smaller ventromedial prefrontal cortex gray matter volumes among adolescents predicting smoking initiation and maintenance of smoking behavior at follow-up five years later (74). Smaller amygdala volumes similarly predicted daily smoking as well as being associated with externalizing behaviors (75). Notably, these studies specifically examined traditional cigarette smoking initiation while participants in the present study were primarily e-cigarette users. Moreover, a majority of the present sample also used cannabis, at least minimally. Longitudinal studies suggest larger orbitofrontal cortex volumes may predict adolescents who initiate cannabis use as well as greater sensitivity to rewards at baseline (76). Likewise, adolescents who went on to initiate both heavier cannabis and alcohol use were noted to have increased thickness of the parahippocampal gyrus (77). Thus, while lifetime cannabis use was not a significant factor in the present study, our findings may not align with the few existing studies that focused on individuals who engaged primarily in smoking traditional cigarettes.
The results and conclusions of this study must be considered within its limitations. As noted, this study was cross-sectional in design which limits the ability to make causal interpretations. Longitudinal studies like the Adolescent Brain Cognitive Development (ABCD) Study (78) that have followed adolescents prior to and after initiation of nicotine use are needed to understand the relationships between symptoms of nicotine dependency, age of onset of use, and brain morphometry. Additionally, while participants were recruited for low levels of alcohol use and lifetime alcohol use episodes was a non-significant covariate, the total quantity of alcohol use could possibly have an impact on hippocampal volumes (77, 80, 81). Similarly, the sample size reported here is relatively small and, therefore, the results should be replicated in a larger sample size. Moreover, statistical analyses were not controlled for multiple comparisons, highlighting the somewhat preliminary and exploratory nature of these findings. However, initial findings from ABCD-derived data do suggest that larger hippocampal and parahippocampal morphometry may predict substance use initiation more generally (79).
Overall, the present study revealed a relationship between severity of nicotine dependence symptoms, age of onset of regular use, and hippocampal volumes in a sample of late adolescents and emerging adults. The overall pattern of results indicated that greater nicotine dependence symptom severity and younger age of onset is associated with larger hippocampal volumes. While these findings could be related to enhanced reinforcement and learning of NTP-related habits, they could also reflect a predisposing vulnerability. Greater understanding of these interactions between symptoms of nicotine dependence and age of onset of use as well as their relationship with brain health are essential for informing prevention, intervention, and public health policy.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
JH: Formal analysis, Writing – original draft, Writing – review & editing. KC: Writing – review & editing. RB: Investigation, Project administration, Writing – review & editing. GA: Investigation, Project administration, Writing – review & editing. QS: Data curation, Writing – review & editing. TL: Writing – review & editing. JJ: Funding acquisition, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Research was supported by the National Institute on Drug Abuse grants U01 DA041089, R21 DA047953, R01 DA054106, and T32 DA031098 and the California Tobacco-Related Disease Research Grants Program Office of the University of California grants 580264 and T30IP0962.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gentzke AS, Creamer M, Cullen KA, Ambrose BK, Willis G, Jamal A, et al. Tobacco product use among middle and high school students - United States, 2011–2018. Mmwr-Morbidity and Mortality Weekly Report. (2019) 68(6):157–64. doi: 10.15585/mmwr.mm6806e1
2. Foxon F, Selya AS. Electronic cigarettes, nicotine use trends and use initiation ages among US adolescents from 1999 to 2018. Addiction. (2020) 115(12):2369–78. doi: 10.1111/add.15099
3. Miech RA, Johnston LD, Patrick ME, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future National Survey Results on Drug Use, 1975–2022: Secondary School Students. Ann Arbor, MI: Institute for Social Research, University of Michigan (2023).
4. Patrick ME, Miech RA, Johnston LD, O'Malley PM. Monitoring the Future Panel Study Annual Report: National Data on Substance Use Among Adults Ages 19 to 60, 1976–2022. Ann Arbor, MI: Institute for Social Research, University of Michigan (2023).
5. Johnston L, Miech R, O'Malley P, Bachman J, Schulenberg J, Patrick M. Monitoring the Future National Survey Results on Drug Use, 1975–2019: Overview, Key Findings on Adolescent Drug Use. Ann Arbor, MI: Institute for Social Research, University of Michigan (2020).
6. Klein EG, Berman M, Hemmerich N, Carlson C, Htut S, Slater M. Online e-cigarette marketing claims: a systematic content and legal analysis. Tob Regul Sci. (2016) 2(3):252. doi: 10.18001/TRS.2.3.5
7. Wagoner KG, Berman M, Rose SW, Song E, Ross JC, Klein EG, et al. Health claims made in vape shops: an observational study and content analysis. Tob Control. (2019) 28(e2):e119–25. doi: 10.1136/tobaccocontrol-2018-054537
8. Hung M, Spencer A, Goh C, Hon ES, Cheever VJ, Licari FW, et al. The association of adolescent e-cigarette harm perception to advertising exposure and marketing type. Arch Public Health. (2022) 80(1):114. doi: 10.1186/s13690-022-00867-6
9. Venrick SJ, Kelley DE, O'Brien E, Margolis KA, Navarro MA, Alexander JP, et al. US Digital tobacco marketing and youth: a narrative review. Prev Med Rep. (2023) 31:102094. doi: 10.1016/j.pmedr.2022.102094
10. Bernat D, Gasquet N, Wilson KOD, Porter L, Choi K. Electronic cigarette harm and benefit perceptions and use among youth. Am J Prev Med. (2018) 55(3):361–7. doi: 10.1016/j.amepre.2018.04.043
11. Tsai J, Walton K, Coleman B, Sharapova S, Johnson S, Kennedy S, et al. Reasons for electronic cigarette use among middle and high school students-national youth tobacco survey, United States, 2016. MMWR Morb Mortal Wkly Rep. (2018) 67(6):196–200. doi: 10.15585/mmwr.mm6706a5
12. Wade NE, Courtney KE, Doran N, Baca R, Aguinaldo LD, Thompson C, et al. Young adult e-cigarette and combustible tobacco users attitudes, substance use behaviors, mental health, and neurocognitive performance. Brain Sci. (2022) 12(7):889. doi: 10.3390/brainsci12070889
13. Prochaska JJ, Vogel EA, Benowitz N. Nicotine delivery and cigarette equivalents from vaping a JUULpod. Tob Control. (2022) 31(e1):e88–93. doi: 10.1136/tobaccocontrol-2020-056367
14. Cerdá M, Mauro C, Hamilton A, Levy NS, Santaella-Tenorio J, Hasin D, et al. Association between recreational marijuana legalization in the United States and changes in marijuana use and Cannabis use disorder from 2008 to 2016. JAMA Psychiatry. (2020) 77(2):165–71. doi: 10.1001/jamapsychiatry.2019.3254
15. Vogel EA, Cho JH, McConnell RS, Barrington-Trimis JL, Leventhal AM. Prevalence of electronic cigarette dependence among youth and its association with future use. Jama Network Open. (2020) 3(2):e1921513–e1921513. doi: 10.1001/jamanetworkopen.2019.21513
16. Glantz S, Jeffers A, Winickoff JP. Nicotine addiction and intensity of e-cigarette use by adolescents in the US, 2014 to 2021. JAMA Netw Open. (2022) 5(11):e2240671–e2240671. doi: 10.1001/jamanetworkopen.2022.40671
17. Adjei A, Chen B, Mantey DS, Wilkinson AV, Harrell MB. Symptoms of nicotine dependence by e-cigarette and cigarette use behavior and brand: a population-based, nationally representative cross-sectional study. Drug Alcohol Depend. (2024) 255:111059. doi: 10.1016/j.drugalcdep.2023.111059
18. Gomes MN, Reid JL, Rynard VL, East KA, Goniewicz ML, Piper ME, et al. Comparison of indicators of dependence for vaping and smoking: trends between 2017 and 2022 among youth in Canada, England, and the United States. Nicotine Tob Res. (2024) 26(9):1192–200. doi: 10.1093/ntr/ntae060
19. DiFranza JR, Rigotti NA, McNeill AD, Ockene JK, Savageau JA, St Cyr D, et al. Initial symptoms of nicotine dependence in adolescents. Tob Control. (2000) 9(3):313–9. doi: 10.1136/tc.9.3.313
20. DiFranza JR, Savageau JA, Fletcher K, O'Loughlin J, Pbert L, Ockene JK, et al. Symptoms of tobacco dependence after brief intermittent use: the development and assessment of nicotine dependence in youth-2 study. Arch Pediatr Adolesc Med. (2007) 161(7):704–10. doi: 10.1001/archpedi.161.7.704
21. Lin C, Gaiha SM, Halpern-Felsher B. Nicotine dependence from different E-cigarette devices and combustible cigarettes among US adolescent and young adult users. Int J Environ Res Public Health. (2022) 19(10):5846. doi: 10.3390/ijerph19105846
22. Cobb CO, Soule EK, Rudy AK, Sutter ME, Cohn AM. Patterns and correlates of tobacco and cannabis co-use by tobacco product type: findings from the Virginia youth survey. Subst Use Misuse. (2018) 53(14):2310–9. doi: 10.1080/10826084.2018.1473437
23. Fadus MC, Smith TT, Squeglia LM. The rise of e-cigarettes, pod mod devices, and JUUL among youth: factors influencing use, health implications, and downstream effects. Drug Alcohol Depend. (2019) 201:85–93. doi: 10.1016/j.drugalcdep.2019.04.011
24. Gelino BW, Reed DD, Spindle TR, Amlung M, Strickland JC. Association of electronic nicotine delivery system (ENDS) and cigarette solo and dual use with alcohol-related consequences among US adults. Addict Behav. (2023) 146:107806. doi: 10.1016/j.addbeh.2023.107806
25. Han DH, Elam KK, Quinn PD, Huang C, Seo DC. Within-person associations of escalated electronic nicotine delivery systems use with cigarette, alcohol, marijuana and drug use behaviors among US young adults. Addiction. (2023) 118(3):509–19. doi: 10.1111/add.16082
26. Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. (2010) 35(1):217–38. doi: 10.1038/npp.2009.110
27. O'Loughlin J, DiFranza J, Tyndale RF, Meshefedjian G, McMillan-Davey E, Clarke PB, et al. Nicotine-dependence symptoms are associated with smoking frequency in adolescents. Am J Prev Med. (2003) 25(3):219–25. doi: 10.1016/S0749-3797(03)00198-3
28. Kandel DB, Hu M-C, Griesler PC, Schaffran C. On the development of nicotine dependence in adolescence. Drug Alcohol Depend. (2007) 91(1):26–39. doi: 10.1016/j.drugalcdep.2007.04.011
29. Doubeni CA, Reed G, DiFranza JR. Early course of nicotine dependence in adolescent smokers. Pediatrics. (2010) 125(6):1127–33. doi: 10.1542/peds.2009-0238
30. Morean ME, Krishnan-Sarin S, O’Malley SS. Assessing nicotine dependence in adolescent e-cigarette users: the 4-item patient-reported outcomes measurement information system (PROMIS) nicotine dependence item bank for electronic cigarettes. Drug Alcohol Depend. (2018) 188:60–3. doi: 10.1016/j.drugalcdep.2018.03.029
31. Rubinstein ML, Thompson PJ, Benowitz NL, Shiffman S, Moscicki A-B. Cotinine levels in relation to smoking behavior and addiction in young adolescent smokers. Nicotine Tob Res. (2007) 9(1):129–35. doi: 10.1080/14622200601078517
32. Carpenter MJ, Baker NL, Gray KM, Upadhyaya HP. Assessment of nicotine dependence among adolescent and young adult smokers: a comparison of measures. Addict Behav. (2010) 35(11):977–82. doi: 10.1016/j.addbeh.2010.06.013
33. Chaffee BW, Halpern-Felsher B, Jacob P III, Helen GS. Biomarkers of nicotine exposure correlate with the hooked on nicotine checklist among adolescents in California, United States. Addict Behav. (2022) 128:107235. doi: 10.1016/j.addbeh.2022.107235
34. Zhan W, Dierker LC, Rose JS, Selya A, Mermelstein RJ. The natural course of nicotine dependence symptoms among adolescent smokers. Nicotine Tob Res. (2012) 14(12):1445–52. doi: 10.1093/ntr/nts031
35. Leslie FM. Unique, long-term effects of nicotine on adolescent brain. Pharmacol Biochem Behav. (2020) 197:173010. doi: 10.1016/j.pbb.2020.173010
36. Hu M-C, Davies M, Kandel DB. Epidemiology and correlates of daily smoking and nicotine dependence among young adults in the United States. Am J Public Health. (2006) 96(2):299–308. doi: 10.2105/AJPH.2004.057232
37. Behrendt S, Wittchen H-U, Höfler M, Lieb R, Beesdo K. Transitions from first substance use to substance use disorders in adolescence: is early onset associated with a rapid escalation? Drug Alcohol Depend. (2009) 99(1–3):68–78. doi: 10.1016/j.drugalcdep.2008.06.014
38. Buchmann AF, Blomeyer D, Jennen-Steinmetz C, Schmidt MH, Esser G, Banaschewski T, et al. Early smoking onset may promise initial pleasurable sensations and later addiction. Addict Biol. (2013) 18(6):947–54. doi: 10.1111/j.1369-1600.2011.00377.x
39. Lanza ST, Vasilenko SA. New methods shed light on age of onset as a risk factor for nicotine dependence. Addict Behav. (2015) 50:161–4. doi: 10.1016/j.addbeh.2015.06.024
40. Sharapova S, Reyes-Guzman C, Singh T, Phillips E, Marynak KL, Agaku I. Age of tobacco use initiation and association with current use and nicotine dependence among US middle and high school students, 2014–2016. Tob Control. (2020) 29(1):49–54. doi: 10.1136/tobaccocontrol-2018-054593
41. Yuan ML, Cross SJ, Loughlin SE, Leslie FM. Nicotine and the adolescent brain. J Physiol Lond. (2015) 593(16):3397–412. doi: 10.1113/Jp270492
42. Zeid D, Kutlu MG, Gould TJ. Differential effects of nicotine exposure on the hippocampus across lifespan. Curr Neuropharmacol. (2018) 16(4):388–402. doi: 10.2174/1570159x15666170714092436
43. Mahajan SD, Homish GG, Quisenberry A. Multifactorial etiology of adolescent nicotine addiction: a review of the neurobiology of nicotine addiction and its implications for smoking cessation pharmacotherapy. Front Public Health. (2021) 9:664748. doi: 10.3389/fpubh.2021.664748
44. Jordan CJ, Andersen SL. Sensitive periods of substance abuse: early risk for the transition to dependence. Dev Cogn Neurosci. (2017) 25:29–44. doi: 10.1016/j.dcn.2016.10.004
45. Chaarani B, Kan KJ, Mackey S, Spechler PA, Potter A, Orr C, et al. Low smoking exposure, the adolescent brain, and the modulating role of CHRNA5 polymorphisms. Biol Psychiatry Cogn Neurosci Neuroimaging. (2019) 4(7):672–9. doi: 10.1016/j.bpsc.2019.02.006
46. Kangiser MM, Thomas AM, Kaiver CM, Lisdahl KM. Nicotine effects on white matter microstructure in young adults. Arch Clin Neuropsychol. (2020) 35(1):10–21. doi: 10.1093/arclin/acy101
47. Mejia MH, Wade NE, Baca R, Diaz VG, Jacobus J. The influence of cannabis and nicotine co-use on neuromaturation: a systematic review of adolescent and young adult studies. Biol Psychiatry. (2021) 89(2):162–71. doi: 10.1016/j.biopsych.2020.09.021
48. Courtney KE, Sorg S, Baca R, Doran N, Jacobson A, Liu TT, et al. The effects of nicotine and cannabis co-use during late adolescence on white matter fiber tract microstructure. J Stud Alcohol Drugs. (2022) 83(2):287–95. doi: 10.15288/jsad.2022.83.287
49. Happer JP, Courtney KE, Baca RE, Andrade G, Thompson C, Shen Q, et al. Nicotine use during late adolescence and young adulthood is associated with changes in hippocampal volume and memory performance. Front Neurosci. (2024) 18:1436951. doi: 10.3389/fnins.2024.1436951
50. Hernandez Mejia M, Courtney KE, Wade NE, Wallace A, Baca RE, Shen Q, et al. The combined effects of nicotine and Cannabis on cortical thickness estimates in adolescents and emerging adults. Brain Sci. (2024) 14(3):195. doi: 10.3390/brainsci14030195
51. Wallace AL, Courtney KE, Wade NE, Hatz LE, Baca R, Jacobson A, et al. Neurite orientation dispersion and density imaging (NODDI) of brain microstructure in adolescent Cannabis and nicotine use. Behavioral Sciences. (2024) 14(3):231. doi: 10.3390/bs14030231
52. Subramaniyan M, Dani JA. Dopaminergic and cholinergic learning mechanisms in nicotine addiction. Addiction Reviews. (2015) 1349(1):46–63. doi: 10.1111/nyas.12871
53. Courtney KE, Baca R, Doran N, Jacobson A, Liu TT, Jacobus J. The effects of nicotine and cannabis co-use during adolescence and young adulthood on white matter cerebral blood flow estimates. Psychopharmacology (Berl). (2020) 237(12):3615–24. doi: 10.1007/s00213-020-05640-7
54. Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the customary drinking and drug use record (CDDR): a measure of adolescent alcohol and drug involvement. J Stud Alcohol. (1998) 59(4):427–38. doi: 10.15288/jsa.1998.59.427
55. Jacobus J, Taylor CT, Gray KM, Meredith LR, Porter AM, Li I, et al. A multi-site proof-of-concept investigation of computerized approach-avoidance training in adolescent cannabis users. Drug Alcohol Depend. (2018) 187:195–204. doi: 10.1016/j.drugalcdep.2018.03.007
56. Karoly HC, Schacht JP, Jacobus J, Meredith LR, Taylor CT, Tapert SF, et al. Preliminary evidence that computerized approach avoidance training is not associated with changes in fMRI cannabis cue reactivity in non-treatment-seeking adolescent cannabis users. Drug Alcohol Depend. (2019a) 200:145–52. doi: 10.1016/j.drugalcdep.2019.04.007
57. Karoly HC, Schacht JP, Meredith LR, Jacobus J, Tapert SF, Gray KM, et al. Investigating a novel fMRI cannabis cue reactivity task in youth. Addict Behav. (2019b) 89:20–8. doi: 10.1016/j.addbeh.2018.09.015
58. Wheeler KC, Fletcher KE, Wellman RJ, Difranza JR. Screening adolescents for nicotine dependence: the hooked on nicotine checklist. J Adolesc Health. (2004) 35(3):225–30. doi: 10.1016/S1054-139X(03)00531-7
59. Brandon TH, Baker TB. The smoking consequences questionnaire: the subjective expected utility of smoking in college students. Psychol Assessment. (1991) 3(3):484. doi: 10.1037/1040-3590.3.3.484
60. Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. Br J Addict. (1991) 86(11):1467–76. doi: 10.1111/j.1360-0443.1991.tb01732.x
61. Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. (2001) 3(1):7–16. doi: 10.1080/14622200020032051
62. Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. (2002) 33(3):341–55. doi: 10.1016/s0896-6273(02)00569-x
63. Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. (2004) 14(1):11–22. doi: 10.1093/cercor/bhg087
64. Gould TJ, Davis JA. Associative learning, the hippocampus, and nicotine addiction. Curr Drug Abuse Rev. (2008) 1(1):9–19. doi: 10.2174/1874473710801010009
65. Volkow ND, Michaelides M, Baler R. The neuroscience of drug reward and addiction. Physiol Rev. (2019) 99(4):2115–40. doi: 10.1152/physrev.00014.2018
66. Froeliger B, Kozink RV, Rose JE, Behm FM, Salley AN, McClernon FJ. Hippocampal and striatal gray matter volume are associated with a smoking cessation treatment outcome: results of an exploratory voxel-based morphometric analysis. Psychopharmacology. (2010) 210:577–83. doi: 10.1007/s00213-010-1862-3
67. McClernon FJ, Conklin CA, Kozink RV, Adcock RA, Sweitzer MM, Addicott MA, et al. Hippocampal and insular response to smoking-related environments: neuroimaging evidence for drug-context effects in nicotine dependence. Neuropsychopharmacology. (2016) 41(3):877–85. doi: 10.1038/npp.2015.214
68. Huntley ED, Marusak HA, Berman SE, Zundel CG, Hatfield JR, Keating DP, et al. Adolescent substance use and functional connectivity between the ventral striatum and hippocampus. Behav Brain Res. (2020) 390:112678. doi: 10.1016/j.bbr.2020.112678
69. Johnson A, van der Meer MA, Redish AD. Integrating hippocampus and striatum in decision-making. Curr Opin Neurobiol. (2007) 17(6):692–7. doi: 10.1016/j.conb.2008.01.003
70. Lipton DM, Gonzales BJ, Citri A. Dorsal striatal circuits for habits, compulsions and addictions. Front Syst Neurosci. (2019) 13:28. doi: 10.3389/fnsys.2019.00028
71. Janes AC, Park MTM, Farmer S, Chakravarty MM. Striatal morphology is associated with tobacco cigarette craving. Neuropsychopharmacology. (2015) 40(2):406–11. doi: 10.1038/npp.2014.185
72. Das D, Cherbuin N, Anstey KJ, Sachdev PS, Easteal S. Lifetime cigarette smoking is associated with striatal volume measures. Addict Biol. (2012) 17(4):817–25. doi: 10.1111/j.1369-1600.2010.00301.x
73. Boer OD, El Marroun H, Franken IH. Brain morphology predictors of alcohol, tobacco, and cannabis use in adolescence: a systematic review. Brain Res. (2022) 1795:148020. doi: 10.1016/j.brainres.2022.148020
74. Xiang S, Jia T, Xie C, Cheng W, Chaarani B, Banaschewski T, et al. Association between vmPFC gray matter volume and smoking initiation in adolescents. Nat Commun. (2023) 14(1):4684. doi: 10.1038/s41467-023-40079-2
75. Cheetham A, Allen NB, Whittle S, Simmons J, Yücel M, Lubman DI. Amygdala volume mediates the relationship between externalizing symptoms and daily smoking in adolescence: a prospective study. Psychiatry Research: Neuroimaging. (2018) 276:46–52. doi: 10.1016/j.pscychresns.2018.03.007
76. Wade NE, Bagot KS, Cota CI, Fotros A, Squeglia LM, Meredith LR, et al. Orbitofrontal cortex volume prospectively predicts cannabis and other substance use onset in adolescents. J Psychopharmacol. (2019) 33(9):1124–31. doi: 10.1177/0269881119855971
77. Jacobus J, Castro N, Squeglia LM, Meloy M, Brumback T, Huestis MA, et al. Adolescent cortical thickness pre-and post marijuana and alcohol initiation. Neurotoxicol Teratol. (2016) 57:20–9. doi: 10.1016/j.ntt.2016.09.005
78. Volkow ND, Koob GF, Croyle RT, Bianchi DW, Gordon JA, Koroshetz WJ, et al. The conception of the ABCD study: from substance use to a broad NIH collaboration. Dev Cogn Neurosci. (2018) 32:4–7. doi: 10.1016/j.dcn.2017.10.002
79. Miller AP, Baranger DA, Paul SE, Garavan H, Mackey S, Tapert SF, et al. Neuroanatomical variability and substance use initiation in late childhood and early adolescence. JAMA Netw Open. (2024) 7(12):e2452027. doi: 10.1001/jamanetworkopen.2024.52027
80. Squeglia LM, Jacobus J, Tapert SF. The effect of alcohol use on human adolescent brain structures and systems. Handb Clin Neurol. (2014) 125:501–10. doi: 10.1016/B978-0-444-62619-6.00028-8
Keywords: hippocampus, age of onset, nicotine, vaping, adolescent, emerging adult
Citation: Happer JP, Courtney KE, Baca RE, Andrade G, Shen Q, Liu TT and Jacobus J (2025) Age of onset of nicotine use and severity of nicotine addiction symptoms are associated with hippocampal volume in late adolescents and emerging adults. Front. Adolesc. Med. 3:1532450. doi: 10.3389/fradm.2025.1532450
Received: 22 November 2024; Accepted: 26 May 2025;
Published: 10 June 2025.
Edited by:
Alessandra Maria Passarotti, University of Illinois Chicago, United StatesReviewed by:
Tim Durazzo, Stanford University, United StatesFrances Leslie, University of California, Irvine, United States
Copyright: © 2025 Happer, Courtney, Baca, Andrade, Shen, Liu and Jacobus. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joanna Jacobus, amphY29idXNAaGVhbHRoLnVjc2QuZWR1
 Joseph P. Happer
Joseph P. Happer Kelly E. Courtney
Kelly E. Courtney Rachel E. Baca1
Rachel E. Baca1 Qian Shen
Qian Shen Thomas T. Liu
Thomas T. Liu Joanna Jacobus
Joanna Jacobus