- 1Psychiatry Department, University of California San Diego, La Jolla, CA, United States
- 2Pharmacology and Toxicology Department, Medical College of Wisconsin, Milwaukee, WI, United States
Introduction: The endocannabinoid (eCB) system is a regulatory mechanism that helps to maintain homeostasis in the brain. Cannabis use and circulating eCB disruptions have been linked with altered memory; however, this work has largely been done in animal models with minimal investigation into these relationships by sex. We aim to investigate how circulating eCB concentrations in cannabis using young adults are associated verbal memory. We hypothesize that greater amounts of self-reported cannabis use and lower eCB circulating concentrations will be associated with worse learning and memory. Sex as a potential moderator was explored.
Method: Eighty-seven participants between the ages of 18–20 (63% female) completed measures on past 30-day cannabis use, verbal learning and memory (Rey Auditory Verbal Learning Task; RAVLT), and a blood draw. Serum sample analysis from blood draws assessed concentrations of the eCBs 2-arachidonoylglycerol (2-AG) and N-arachidonoylethanolamine (AEA). Linear regressions examining cannabis group status based on past 30 day cannabis use [no use, light use (<8 use days), and heavy cannabis use (≥8 use days)], eCB concentrations, and their interaction on RAVLT learning and memory scores. Three-way sex by cannabis status by eCB concentrations interactions were also explored.
Results: Heavy cannabis use was associated with worse verbal memory performance. Significant interactions between 2-AG and heavy cannabis use were observed, revealing that individuals with elevated 2-AG concentrations and heavy cannabis use showed better performance on verbal learning and memory tasks. In contrast, heavy cannabis use with higher AEA concentrations performed worse on verbal learning tasks. There was a significant three-way interaction with sex, cannabis use group, and 2-AG concentrations on verbal learning where men with heavy cannabis use and high 2-AG concentrations had better verbal learning compared to men with lower 2-AG concentrations.
Discussion: Distinct patterns emerged between 2-AG and AEA concentrations and memory performance among individuals with heavy cannabis. Higher 2-AG concentrations and lower AEA concentrations correlated with improved memory performance in the heavy cannabis use group, suggesting that circulating eCB profiles may serve as a biomarker for cannabis-related cognitive deficits. More work is needed to disentangle the complex relationships between circulating eCB concentrations, cannabis use, and neurocognitive functioning.
Introduction
Endocannabinoids (eCBs) are endogenous signaling molecules that regulate human psychophysiology through activation of cannabinoid type-1 and type-2 receptors [CB1 and CB2 (1);]. The two best well-characterized eCBs, 2-arachidonoylglycerol (2-AG) and N-arachidonoylethanolamine (AEA), are synthesized “on demand” and serve as high-efficacy agonists of CB1 receptors (2, 3). CB1 receptors are present at high density in many brain areas, including the basal ganglia, amygdala, and the hippocampus (4). The binding of eCBs to CB1 receptors in these brain regions are thought to modulate both cognitive and emotional processes (5). Memory consolidation and maintenance in particular are thought to be heavily regulated by eCB signaling (6). Thus, eCBs are crucial in the role of retaining and remembering new information.
Cannabis use has been closely associated with adverse cognitive effects (7), particularly in adolescents and young adults [AYA (8);]. This is concerning given that in 2023 18% of 12th graders have reported past month cannabis use and 29% have reported past year cannabis use (9). Poorer verbal memory performance in particular has been linked with AYA cannabis use, as cannabis using AYAs often show worse verbal learning and immediate and delayed memory performance than their non-using counterparts (10–12). Cognitive alterations observed with cannabis use are believed to result from the principal psychotropic compound of cannabis, delta-9-tetrahydrocannabinol (THC), which binds to CB1 receptors (13, 14). Chronic cannabis exposure has been demonstrated to lead to CB1 receptor downregulation and reductions in eCB concentrations (15, 16). Given that the hippocampus, a brain region crucial for learning and memory, is densely populated with CB1 receptors, changes in receptor density and eCB concentrations contribute to downstream cannabis-related memory changes (5, 17).
Preclinical research suggests that continuous activation of CB1 receptors with repeated exogenous cannabis exposure may lead to a disruption of the eCB system and interfere with the homeostasis of hippocampal neuronal signaling, and therefore, impact memory performance (18). Indeed, preclinical models examining hippocampal neurons show that THC at high rates antagonized endogenous 2-AG signaling (19). The continuous activation of CB1 receptors may have particularly pronounced effects during adolescence and young adulthood, a critical period of neurodevelopment in which eCB signaling is highly influential. The eCB system is essential for regulating neuronal cell proliferation, migration, differentiation, and survival (20). Disrupting this signaling during such a sensitive period may help explain the distinct brain and cognitive effects observed with AYA cannabis use compared to adults (21, 22). However, while the effects of the eCB system and cannabis on memory performance are well documented in pre-clinical models, human studies are limited (11). Thus, additional clinical studies focusing on cannabis use and eCB signaling in AYA are crucial to bridge the gap between preclinical research findings and their relevance to emerging young adults.
Further complicating these relationships is the role of biological sex. Sex differences are observed in the eCB system and in the effects of cannabis exposure on the brain and cognition (23, 24). Past preclinical studies have shown that females demonstrate greater downregulation of CB1 receptors compared to males with repeated THC exposure (25). However, males show greater CB1 receptor density compared to their female counter parts (26). Additionally, sex specific effects of the eCB system play a role in modulating both neural and cognitive development and have been characterized in preclinical adolescent models (27) as well as several human studies suggesting preclinical findings may generalize to clinical samples (28); however, these studies are limited and more sex-specific investigations into cannabis use and eCBs on cognition are imperative to better understand their relationships in humans.
Here we aim to investigate the relationship between AYA cannabis use and circulating eCB concentrations on verbal learning and memory performance. We hypothesize that AYAs who have engaged in both light cannabis use and heavy cannabis use in the past 30 days will perform worse on measures of verbal learning and memory compared to individuals with no-use in the past 30 days (9, 11). Further, lower circulating concentrations of both 2-AG and AEA in AYAs will be associated with worse verbal learning and memory performance (29). Finally, we hypothesize that there will be an interaction between light and heavy cannabis use group status and eCB concentrations where heavy cannabis use and lower eCB concentrations, both 2-AG and AEA, will be related to worse verbal learning and memory (18, 30). In addition to the above aims, we will conduct preliminary analyses exploring the role of sex assigned at birth on cannabis use and eCB concentrations on memory performance.
Method
Participants
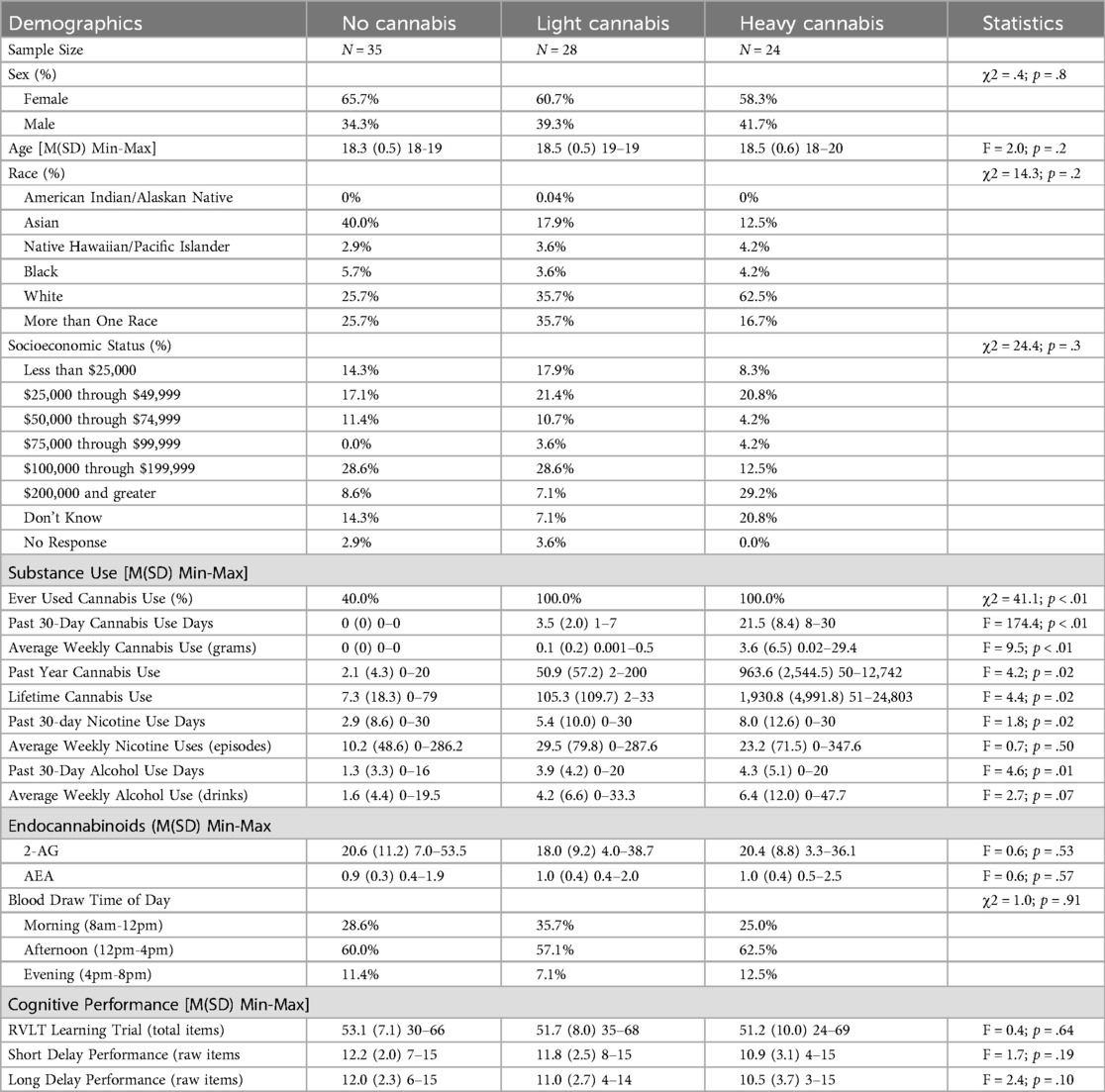
Eighty-nine participants between the ages of 18–20 years old were selected as a subsample from a larger parent study investigating the effects of cannabis and nicotine co-use. Participants included individuals with and without cannabis use and who provided a blood sample to quantify circulating eCB concentrations. Cannabis use groups included light cannabis use [n = 28 (F = 17)], defined as less than 8 cannabis use episodes in the past 30 days (≤2x/week on average), and heavy cannabis use [n = 24 (F = 14)], defined as greater than 8 cannabis use episodes in the past 30 days (>2x/week, on average). Control participants [n = 34 (F = 23)] endorsed no cannabis use in the past 30 days.
Inclusionary criteria for the parent project were for enrollment purposes only and required meeting criteria for one of four groups based on cannabis and nicotine patterns in the past six months. Groups included: (1) single substance cannabis and (2) single substance nicotine and tobacco product use with a pattern >48 use episodes in the past 6 months, or >2 episodes/week for the past 6 months, on average of cannabis or nicotine and tobacco products, and no use of the other substance within the past 6 months at enrollment; (3) a co-use group which included individuals using both cannabis and nicotine and tobacco products in the past 6 months and defined as consuming both substances weekly, or ≥2 episodes/week for the past 6 months of both cannabis and nicotine and tobacco products; and (4) a control group, including individuals reporting ≤1 use episode of either cannabis or nicotine and tobacco product use in the past 6 months at enrollment.
Exclusionary criteria included greater than 100 alcohol use episodes, prenatal alcohol, tobacco, or illicit drug exposure, premature birth (<24 week gestation or birth weight <5 lbs), history of serious medical or neurological problems including major neurological disorder or head trauma with loss of consciousness >2 min, current or past DSM-5 psychiatric disorder (other than cannabis and/or tobacco use disorder, which was not screened for), >10 illicit substance use episodes, history of learning disability or pervasive developmental disorder, non-correctable visual or hearing problems, non-fluency in English, MRI contraindications, pregnant on day of scan, or failure to abstain from alcohol or cannabis use twelve hours prior to their visit (acute nicotine was allowed so that cognitive performance was not impacted by nicotine withdrawal).
Procedures
Participants were recruited from the San Diego County area using digital and physical flyer postings, in-person tabling events, and social media postings. Interested participants were screened via phone calls with trained study staff. Eligible participants were brought into the study site and completed a comprehensive battery of mental health, substance use, neurocognitive assessments, biological specimen collection, and structural and functional neuroimaging acquisitions. Participants were screened for acute intoxication. Those with positive urine toxicology results for any drug were asked to provide a saliva sample for Draeger testing to confirm they were not intoxicated at the time of their visit. All study protocol were approved by the local Institutional Review Board and adhered to the Declaration of Helsinki.
Materials
Substance use
The Timeline Follow Back (TLFB) was used to measure past 30-day cannabis use (31), and other substance use episodes. The TLFB is the gold standard for measuring substance use where a calendar displaying memory cues of significant events in the participants past 30 days is used to help recall substance use patterns. A trained research assistant worked with participants to identify how much cannabis they consumed each day. In this way, the number of cannabis use days were calculated and utilized to define cannabis use groups.
Verbal learning and memory
The Rey Auditory Verbal Learning Test (RAVLT) is a verbal list learning task (32) where participants complete five trials during which they were read a list of words and were asked to repeat as many words as they can remember (verbal learning). After hearing and completing an immediate recall trial with a distractor list, participants were asked to recall the initial list of words they learned (short-term memory) and again after a thirty-minute delay (long-term memory). Raw item level scores (i.e., total words learned during learning trials, and words recalled during short and long-term delay trials) were used as the outcome variable.
Endocannabinoids
A trained phlebotomist performed the venipuncture following standard procedures. The 5 ml blood samples were collected in serum-separated tubes (SST) and immediately inverted to ensure proper mixing with additives. Within 30 min of collection, the samples were centrifuged at 1,300 × g for 10 min to separate the serum, which was then aliquoted into pre-labeled cryovials and stored at −80° C. Blood samples were shipped on dry ice to the Hillard laboratory at the Medical College of Wisconsin in Milwaukee, WI. Serum sample analysis was conducted using liquid chromatography-tandem mass spectrometry (LC-MS/MS) to procure circulating eCB concentrations including 2-AG and AEA [pmol/ml (33);].
The time and date of blood draws were noted. For descriptive purposes, the time blood draws occurred were put into categories of morning (8am-12pm), afternoon (12pm-4pm), and evening (after 4pm).
Analyses
Analysis of variance (ANOVA) and chi-square tests were run to examine differences in eCB concentrations by demographics and by cannabis group; eCB concentrations were grand mean centered. A series of linear regressions were run to examine the effects of cannabis group, eCB concentrations, and their interaction on RAVLT learning and memory scores. eCB analyses were run separately (2-AG and AEA). Sex assigned at birth and time between the blood draw and neurocognitive battery were included as covariates. Given the importance of sex as a potential moderator in cannabis and eCB relationships (25, 27), exploratory analyses were run investigating the three-way interaction of sex by cannabis use group by eCB concentrations interaction term for each verbal memory outcome. Analyses were conducted in R (R 4.2.1) utilizing the stats (version 4.2.1) and psych (version 2.2.5) packages. All statistical decisions were made at p < 0.05.
Outliers
Potential outliers within eCBs (i.e., 2-AG & AEA) and RAVLT performance were removed utilizing difference in Beta values (DFBETAS) at a threshold of |2/. In this way, two participants were removed from analyses for having 2-AG concentrations that exceeded DFBETAS threshold (0.35). One participant in the control group (DFBETA = 2.48) and one participant in the light cannabis use group (DFBETA = −0.81) were removed.
Results
Participants
A total of N = 87 participants were included ranging between the ages of 18–20 (M = 18.45, SD = 0.52). The sample was predominately female (63.2% female) and mostly identified as White (40.2%). Most participants had completed 12 years of education (59.8%) and 24.1% had a household income of $100,000 through $199,000. Participants included 35 controls (F = 23), 28 individuals with light cannabis use (F = 17), and 24 individuals with heavy cannabis use (F = 14). There were no significant differences in demographics by cannabis group (see Table 1). Neither AEA nor 2-AG endocannabinoid concentrations differed by cannabis use group. Concentrations of 2-AG significantly differed by sex [F(1,85) = 9.08, p < 0.01], with male participants recording higher 2-AG concentrations (M = 23.62; SD = 9.39) than female participants (M = 17.32; SD = 9.51), but no other demographic variables. Concentrations of AEA did not significantly differ by any demographic variables.
Verbal learning & memory
Cannabis
Heavy cannabis use group status was related with lower short delay verbal memory [F(3,83) = 2.18, b = −1.29, SE = 0.65, p = 0.05, r2 = 0.08] and long delay verbal memory [F(3,83) = 1.68, b = −1.60, SE = 0.76, p = 0.04, r2 = 0.06]. There were no significant differences based on light cannabis use group status on RAVLT performance.
Sex
There were no significant differences on RAVLT performance based on sex.
2-AG endocannabinoid concentrations
There was not a significant effect of 2-AG. There was a significant interaction between the heavy cannabis use group and 2-AG concentrations where individuals in the heavy cannabis use group had a positive relationships between 2-AG concentrations and verbal learning [F(7,79) = 1.68, b = 0.47, SE = 0.23, p = 0.04, r2 = 0.05; see Table 2], short delay recall [F(7,79) = 2.48, b = 0.21, SE = 0.07, p < 0.01, r2 = 0.11; see Figure 1], and long-delay recall [F(7,79) = 1.50, b = 0.17, SE = 0.08, p = 0.04, r2 = 0.04].
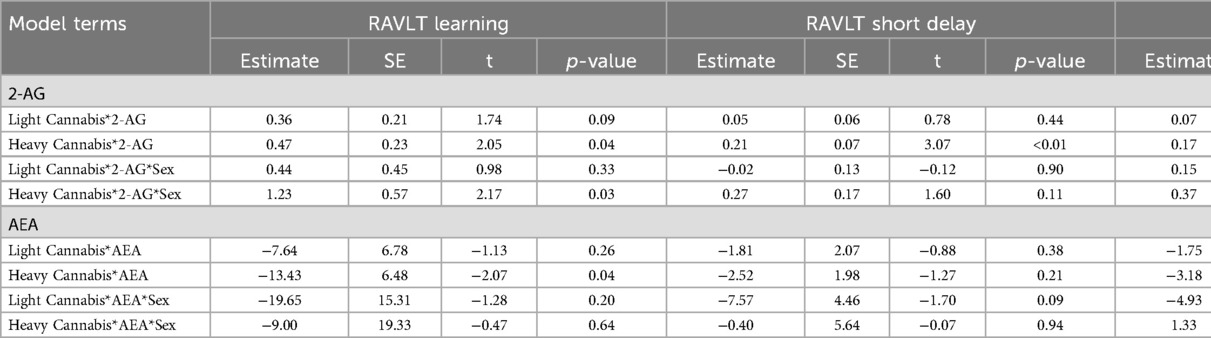
Table 2. Interaction effects between Cannabis Use, eCB concentrations, and Sex effects on verbal learning and memory.
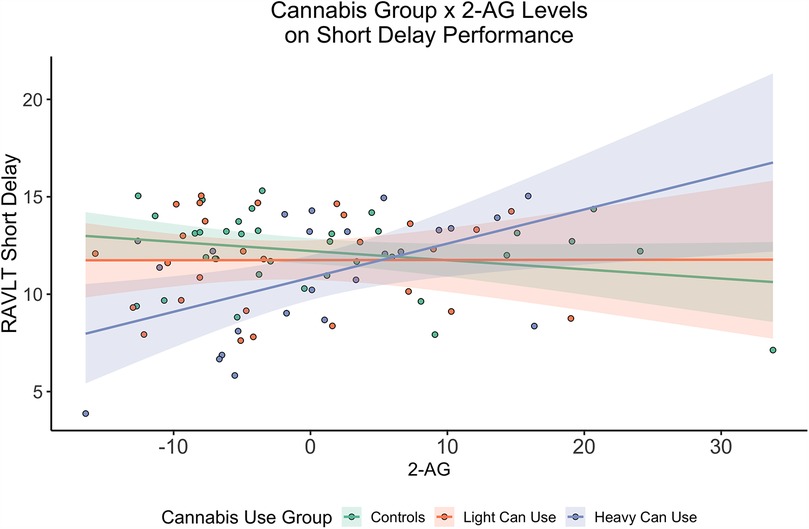
Figure 1. Cannabis group by 2-AG concentrations on short delay verbal memory performance. Interaction showing 2-AG concentrations (x-axis) by cannabis use group on RAVLT short delay performance (y-axis). There was a significant interaction with the heavy cannabis use group and 2-AG concentrations on short delay performance (p < 0.01). 2-AG values were mean centered to allow for direct interpretation of results. Shading from mean regression depicts Standard Error.
AEA endocannabinoid concentrations
Higher AEA eCB concentrations were associated with better verbal learning performance [F(7,79) = 1.62, b = 10.51, SE = 5.09, p = 0.04, r2 = 0.13]. There was a significant interaction between AEA concentrations and cannabis group status, indicating that for individuals with heavy cannabis use there was a negative relationship between AEA concentrations and verbal learning scores [F(7,79) = 1.62, b = −13.43, SE = 6.48, p = 0.04, r2 = 0.13].
Preliminary Sex interactions
2-AG endocannabinoid concentrations
There were sex specific findings between 2-AG concentrations and sex assigned at birth. Males with lower 2-AG concentrations showed worse verbal learning [F(12,74)= 1.63, b = −0.53, SE = 0.26, p = 0.04, r2 = 0.21] and long delay memory [F(12,74) = 1.42, b = −0.20, SE = 0.09, p = 0.03, r2 = 0.19] compared to females with higher 2-AG concentrations who showed better performance. There was a significant three-way interaction between heavy cannabis group status, 2-AG concentrations, and sex assigned at birth on verbal learning [F(12,74)= 1.63, b = 1.23, SE = 0.57, p = 0.03, r2 = 0.21]. Men in the heavy cannabis use group with higher concentrations of 2-AG demonstrated better RAVLT learning performance compared to men with lower 2-AG concentrations in the heavy cannabis use group (see Figure 2).
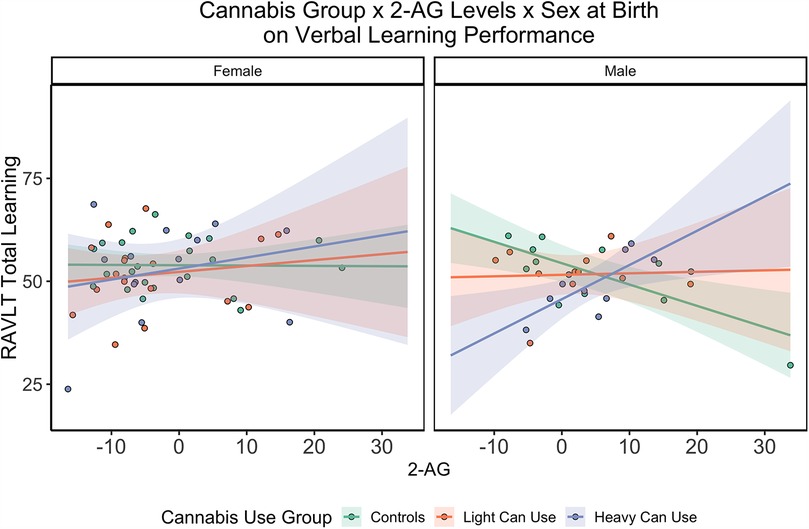
Figure 2. Three-Way interaction of Cannabis group by Sex and 2-AG concentrations on verbal learning performance. Three-way interaction between cannabis use group, sex, and 2-AG concentrations (x-axis) on verbal learning performance (y-axis). There was a significant interaction within male participants between heavy cannabis use 2-AG concentrations on verbal learning performance (p = 0.03). Sex assigned at birth is separated across both boxes. Shading from mean regression depicts Standard Error.
AEA endocannabinoid concentrations
There were no significant sex effects between AEA concentrations and cannabis use groups on cognition.
Discussion
Our study aimed to characterize the relationships among serum eCB concentrations on verbal learning and memory in AYAs who use cannabis regularly. As hypothesized, heavy cannabis use was related to worse short and long-term verbal memory. While serum 2-AG concentrations were not directly related to learning and memory, they did moderate the relationship between cannabis use and memory, such that for individuals in the heavy cannabis use group only, higher circulating 2-AG concentrations were associated with better verbal learning. In contrast, serum AEA concentrations had a direct positive relationship with verbal learning, as higher AEA concentrations were associated with improved learning and memory. However, with heavy cannabis use, elevated AEA concentrations were linked to poorer learning and memory performance, indicating a significant moderating effect of AEA on the relationship between group status and learning outcomes. Additionally, exploratory analyses revealed sex-specific differences: in men within the heavy cannabis use group, lower 2-AG concentrations were associated with reduced verbal learning performance.
Consistent with the broader literature demonstrating greater deleterious effects with higher frequency of use (34), we found that heavy (≥2 weekly uses) but not light cannabis use (≤2 weekly uses) was related to memory deficits. With chronic cannabis use, research suggests that the brain adapts and downregulates CB1 receptors, and potentially eCB concentrations (35). Our findings expand into human studies preclinical work demonstrating that eCBs may moderate the relationships between cannabis use and verbal learning performance.
The eCB concentrations were determined in serum isolated from venous blood. It is likely that circulating eCBs arise from different sources, including blood cells, peripheral, metabolic organs and skeletal muscle (36). It is unclear how these concentrations relate to eCB concentrations and eCB mediated signaling in the brain. It is not likely that they are influenced significantly by brain overflow as synaptic eCBs are tightly regulated by their degradative enzymes (37). On the other hand, considerable data, including the findings in this report, indicate that serum eCB concentrations are associated with a variety of measures of CNS function and psychopathology (38). However, the mechanistic basis for these relationships is not known.
In our study, we found that verbal memory performances improved specifically for individuals with heavy cannabis use as a function of increasing 2-AG concentrations. Interestingly, in a sample of individuals diagnosed with Alzheimer's disease, increased 2-AG were associated with improved memory performance (39), suggesting similar 2-AG patterns on cognition in varying populations. Conversely, lower eCB concentrations may represent a disruption from heavy cannabis use or, perhaps, premorbid susceptibilities that result in exogenous cannabis use. If so, eCB concentrations may provide an opportunity to identify individuals who are at greater cognitive risk with cannabis use. Indeed, studies in cannabis using adults have shown that more frequent cannabis use was associated with lower baseline 2-AG (40). However, we also found the inverse relationship in AEA and heavy cannabis use, with lower AEA associated with worse performance, further complicating results. There are many cannabis-related factors (THC potency, modality of use, age of onset etc.) that may contribute to varying impacts of cannabis use on the eCB system; thus, differentiating eCB concentrations may help clarify the mechanisms underlying diverse effects of regular cannabis use (34). Longitudinal data on these relationships are needed to fully disentangle these complex effects.
Notably, only AEA concentrations showed a main effect on verbal learning performance, suggesting it may play a unique role in cognitive function. In contrast, the lack of a main effect of 2-AG on verbal learning and memory may highlight potential differences in the relevance of circulating eCBs on neurocognitive functioning. This may explain the variability of findings in relation to eCBs and cognition in human studies (36). Higher AEA has been correlated with improved cognitive performance in a healthy sample of women (41). Conversely, in the same sample, higher 2-AG was negatively correlated with cognitive performance (41). Our findings of greater AEA concentrations on memory performance also aligns with the broader literature demonstrating associations with AEA tone and memory consolidation, particularly under stress (42). As preclinical work has demonstrated that greater AEA concentrations have been shown to counteract the deleterious effects of stress in learning performance (43), our findings are an important step in replicating these findings in humans. Considering the vast changes that occur in the brain during adolescent and young adult development, including eCB system development (20), it is possible that higher AEA concentrations in human AYAs may be an advantageous biological response in verbal learning. However, due to the paucity of human research on eCBs, cannabis use, and memory, further work in clinical samples are needed to better understand these relationships.
As clinical studies on sex differences related to cannabis use and eCB concentrations on cognition are limited (27), we sought to build on the current literature. We found a three-way interaction by sex where heavy male cannabis users with lower 2-AG concentrations showed poorer verbal learning compared to male heavy cannabis users with higher 2-AG concentrations. Previous work has highlighted sex differences in the eCB system in molecular signaling pathways, with these differences leading to downstream behavioral changes (44). Sex specific changes have also been noted in eCB modulation during neurodevelopment including fluctuations in CB1 receptor density, receptor coupling, and eCB metabolism and distribution (27). These exploratory findings expand this literature, but more work is needed to decipher the clinical utility of these varying behavioral responses to eCB dysregulation by sex and cannabis use history.
There are several limitations of note for the current study. Blood sampling occurred on the same day of testing in 74% of the sample. While we controlled for the time interval between blood draws and verbal memory testing in our analyses, this could influence the observed associations. Future investigations should aim to examine eCB concentrations collected in closer temporal proximity to cognitive testing to better characterize their associations. Additionally, while circulating eCBs denote peripheral system eCB synthesis, they are not a direct marker of central nervous system eCB functioning (36). Research utilizing PET scans in conjunction with circulating eCBs may help to identify potential mechanisms. Further, while sex analyses were only preliminary, there was a smaller sample of men compared to women by cannabis use group (see Table 1). Well-powered studies are needed to explore potential moderators of sex differences (e.g., pubertal hormones, etc.) and will help to replicate our findings from our modestly powered study. Indeed, while our predictors explain only a small proportion of variance in the outcome, several associations were statistically significant and theoretically meaningful. Finally, our analyses are cross-sectional; and therefore, causality cannot be disentangled. Large scale longitudinal studies that include adequate representation of both males and females will be critical to disentangling the complex interplay between cannabis use, eCB concentrations, and cognitive performance during adolescence and young adulthood.
Our study adds to the limited literature of the role of eCBs in cannabis using AYAs. Specifically, we demonstrated that eCBs moderate the impact of heavy cannabis use on verbal learning and memory in young adults, with higher 2-AG but lower AEA concentrations associated with better verbal learning and memory performance among individuals using cannabis at least twice weekly. These modulatory effects may represent a premorbid susceptibility to exogenous cannabis or a mechanistic response to heavy cannabis exposure within some individuals. We also conducted exploratory analyses on the effects of sex, which demonstrated that men compared to women showed greater susceptibility to these effects. Longitudinal studies in developing adolescents and young adults who use cannabis are imperative to help disentangle these complex effects.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: Datasets will be uploaded to the National Addiction & HIV Data Archive Program upon study completion in line with the grants Data management and Sharing Plan. Requests to access these datasets should be directed to Joanna Jacobus:amphY29idXNAaGVhbHRoLnVjc2QuZWR1.
Ethics statement
The studies involving humans were approved by the Institutional Review Board at UC San Diego. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AW: Conceptualization, Formal analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing. RB: Data curation, Methodology, Writing – original draft, Writing – review & editing. GA: Data curation, Methodology, Writing – original draft, Writing – review & editing. CH: Conceptualization, Data curation, Writing – review & editing. JH: Visualization, Writing – review & editing. NW: Conceptualization, Writing – review & editing. KC: Writing – review & editing. MH: Writing – review & editing. JJ: Conceptualization, Data curation, Funding acquisition, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study received funding from the National Institute on Drug Abuse (NIDA), grant R01 DA054106. Salary support while writing the manuscript was provided by NIDA grants U01 DA041089, R01 DA054106, R01 DA054980, and T32 DA031098 (PI: Moore to Happer), and the National Institute on Alcohol Abuse and Alcoholism (NIAAA) grant T32 AA013525 (PI: Riley/Spadoni to Wallace).
Conflict of interest
CH is a member of the Scientific Advisory Board and has equity in Formulate Bioscience, Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Battista N, Di Tommaso M, Bari M, Maccarrone M. The endocannabinoid system: an overview. Front Behav Neurosci. (2012) 6:9. doi: 10.3389/fnbeh.2012.00009
2. Duncan RS, Riordan SM, Gernon MC, Koulen P. Cannabinoids and endocannabinoids as therapeutics for nervous system disorders: preclinical models and clinical studies. Neural Regen Res. (2024) 19(4):788. doi: 10.4103/1673-5374.382220
3. Lu H-C, Mackie K. Review of the endocannabinoid system. Biol Psych Cog Neurosci Neuroimaging. (2021) 6(6):607–15. doi: 10.1016/j.bpsc.2020.07.016
4. Mechoulam R, Parker LA. The endocannabinoid system and the brain. Annu Rev Psychol. (2013) 64:21–47. doi: 10.1146/annurev-psych-113011-143739
5. Zanettini C, Panlilio LV, Aliczki M, Goldberg SR, Haller J, Yasar S. Effects of endocannabinoid system modulation on cognitive and emotional behavior. Front Behav Neurosci. (2011) 5. doi: 10.3389/fnbeh.2011.00057
6. Drumond A, Madeira N, Fonseca R. Endocannabinoid signaling and memory dynamics: a synaptic perspective. Neurobiol Learn Mem. (2017) 138:62–77. doi: 10.1016/j.nlm.2016.07.031
7. Bourque J, Potvin S. Cannabis and cognitive functioning: from acute to residual effects, from randomized controlled trials to prospective designs. Front Psychiatry. (2021) 12. doi: 10.3389/fpsyt.2021.596601
8. Dellazizzo L, Potvin S, Giguère S, Dumais A. Evidence on the acute and residual neurocognitive effects of cannabis use in adolescents and adults: a systematic meta-review of meta-analyses. Addiction. (2022) 117(7):1857–70. doi: 10.1111/add.15764
9. Miech RA, Johnston LD, Patrick ME, O'Malley PM, Bachman JG. Monitoring the Future National Survey Results on Drug Use, 1975-2023: Overview and Detailed Results for Secondary School Students. University of Michigan, Ann Arbor, MI: Institute for Social Research (2024).
10. Lorenzetti V, Hoch E, Hall W. Adolescent cannabis use, cognition, brain health and educational outcomes: a review of the evidence. Eur Neuropsychopharmacol. (2020) 36:169–80. doi: 10.1016/j.euroneuro.2020.03.012
11. Prini P, Zamberletti E, Manenti C, Gabaglio M, Parolaro D, Rubino T. Neurobiological mechanisms underlying cannabis-induced memory impairment. Eur Neuropsychopharmacol. (2020) 36:181–90. doi: 10.1016/j.euroneuro.2020.02.002
12. Solowij N, Battisti R. The chronic effects of Cannabis on memory in humans: a review. Curr Drug Abuse Rev. (2008) 1(1):81–98. doi: 10.2174/1874473710801010081
13. McPartland JM, Guy GW, Marzo VD. Care and feeding of the endocannabinoid system: a systematic review of potential clinical interventions that upregulate the endocannabinoid system. PLoS One. (2014) 9(3):e89566. doi: 10.1371/journal.pone.0089566
14. Stella N. THC And CBD: similarities and differences between siblings. Neuron. (2023) 111(3):302–27. doi: 10.1016/j.neuron.2022.12.022
15. Fischer AS, Tapert SF, Louie DL, Schatzberg AF, Singh MK. Cannabis and the developing adolescent brain. Curr Treat Options Psychiatry. (2020) 7(2):144–61. doi: 10.1007/s40501-020-00202-2
16. Hirvonen J, Goodwin RS, Li C-T, Terry GE, Zoghbi SS, Morse C, et al. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol Psychiatry. (2011) 17(6):642. doi: 10.1038/mp.2011.82
17. Robledo-Menendez A, Vella M, Grandes P, Soria-Gomez E. Cannabinoid control of hippocampal functions: the where matters. FEBS J. (2022) 289(8):2162–75. doi: 10.1111/febs.15907
18. Kilaru A, Chapman KD. The endocannabinoid system. Essays Biochem. (2020) 64(3):485–99. doi: 10.1042/EBC20190086
19. Roloff AM, Thayer SA. Modulation of excitatory synaptic transmission by Δ9-tetrahydrocannabinol switches from agonist to antagonist depending on firing rate. Mol Pharmacol. (2009) 75(4):892–900. doi: 10.1124/mol.108.051482
20. Gomes TM, Dias da Silva D, Carmo H, Carvalho F, Silva JP. Epigenetics and the endocannabinoid system signaling: an intricate interplay modulating neurodevelopment. Pharmacol Res. (2020) 162:105237. doi: 10.1016/j.phrs.2020.105237
21. Dhein S. Different effects of Cannabis abuse on adolescent and adult brain. Pharmacology. (2020) 105(11–12):609–17. doi: 10.1159/000509377
22. Scheyer AF, Laviolette SR, Pelissier A-L, Manzoni OJJ. Cannabis in adolescence: lasting cognitive alterations and underlying mechanisms. Cannabis and Cannabinoid Research. (2023) 8(1):12–23. doi: 10.1089/can.2022.0183
23. Calakos KC, Bhatt S, Foster DW, Cosgrove KP. Mechanisms underlying sex differences in Cannabis use. Curr Addict Rep. (2017) 4(4):439–53. doi: 10.1007/s40429-017-0174-7
24. Matheson J, Bourgault Z, Le Foll B. Sex differences in the neuropsychiatric effects and pharmacokinetics of cannabidiol: a scoping review. Biomolecules. (2022) 12(10):1462. Article 10. doi: 10.3390/biom12101462
25. Farquhar CE, Breivogel CS, Gamage TF, Gay EA, Thomas BF, Craft RM, et al. Sex, THC, and hormones: effects on density and sensitivity of CB1 cannabinoid receptors in rats. Drug Alcohol Depend. (2019) 194:20–7. doi: 10.1016/j.drugalcdep.2018.09.018
26. Paola Castelli M, Fadda P, Casu A, Sabrina Spano M, Casti A, Fratta W, et al. Male and female rats differ in brain cannabinoid CB1 receptor density and function and in behavioural traits predisposing to drug addiction: effect of ovarian hormones. Curr Pharm Des. (2014) 20(13):2100–13. doi: 10.2174/13816128113199990430
27. Simone JJ, Green MR, McCormick CM. Endocannabinoid system contributions to sex-specific adolescent neurodevelopment. Progress in Neuro-Psychopharmacology and Biological Psychiatry. (2022) 113:110438. doi: 10.1016/j.pnpbp.2021.110438
28. Francis AM, Bissonnette JN, MacNeil SE, Crocker CE, Tibbo PG, Fisher DJ. Interaction of sex and cannabis in adult in vivo brain imaging studies: a systematic review. Brain and Neuroscience Advances. (2022) 6:23982128211073431. doi: 10.1177/23982128211073431
29. Lemtiri-Chlieh F, Levine ES. 2-AG And anandamide enhance hippocampal long-term potentiation via suppression of inhibition. Front Cell Neurosci. (2022) 16:1023541. doi: 10.3389/fncel.2022.1023541
30. Bliss T. Long-Term potentiation. In: Pfaff DW, Volkow ND, Rubenstein JL, editors. Neuroscience in the 21st Century: From Basic to Clinical. Cham: Springer International Publishing (2022). p. 3053–75. doi: 10.1007/978-3-030-88832-9_143
31. Sobell LC, Sobell MB. Timeline follow-back. In: Litten RZ, Allen JP, editors. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Totowa: Humana Press (1992). p. 41–72. doi: 10.1007/978-1-4612-0357-5_3
32. Bean J. Rey auditory verbal learning test, rey AVLT. In: Kreutzer JS, DeLuca J, Caplan B, editors. Encyclopedia of Clinical Neuropsychology. New York: Springer (2011). p. 2174–5. doi: 10.1007/978-0-387-79948-3_1153
33. Spagnolo PA, Ramchandani VA, Schwandt ML, Kwako LE, George DT, Mayo LM, et al. FAAH Gene variation moderates stress response and symptom severity in patients with posttraumatic stress disorder and comorbid alcohol dependence. Alcoholism: Clinical and Experimental Research. (2016) 40(11):2426–34. doi: 10.1111/acer.13210
34. Scott JC, Slomiak ST, Jones JD, Rosen AFG, Moore TM, Gur RC. Association of Cannabis with cognitive functioning in adolescents and young adults: a systematic review and meta-analysis. JAMA Psychiatry. (2018) 75(6):585–95. doi: 10.1001/jamapsychiatry.2018.0335
35. Connor JP, Stjepanović D, Le Foll B, Hoch E, Budney AJ, Hall WD. Cannabis use and cannabis use disorder. Nat Rev Dis Primers. (2021) 7(1):1–24. doi: 10.1038/s41572-021-00247-4
36. Hillard CJ. Circulating endocannabinoids: from whence do they Come and where are they going? Neuropsychopharmacology. (2018) 43(1):155–72. doi: 10.1038/npp.2017.130
37. Hillard CJ. The endocannabinoid signaling system in the CNS: a primer. Int Rev Neurobiol. (2015) 125:1–47. doi: 10.1016/bs.irn.2015.10.001
38. Gowatch LC, Evanski JM, Ely SL, Zundel CG, Bhogal A, Carpenter C, et al. Endocannabinoids and stress-related neurospsychiatric disorders: a systematic review and meta-analysis of basal concentrations and response to acute psychosocial stress. Cannabis and Cannabinoid Research. (2024) 9(5):1217–34. doi: 10.1089/can.2023.0246
39. Altamura C, Ventriglia M, Martini MG, Montesano D, Errante Y, Piscitelli F, et al. Elevation of plasma 2-arachidonoylglycerol levels in Alzheimer’s disease patients as a potential protective mechanism against neurodegenerative decline. Journal of Alzheimer’s Disease: JAD. (2015) 46(2):497–506. doi: 10.3233/JAD-142349
40. Kearney-Ramos T, Herrmann ES, Belluomo I, Matias I, Vallée M, Monlezun S, et al. The relationship between circulating endogenous cannabinoids and the effects of smoked Cannabis. Cannabis and Cannabinoid Research. (2023) 8(6):1069–78. doi: 10.1089/can.2021.0185
41. Fagundo AB, de la Torre R, Jiménez-Murcia S, Agüera Z, Pastor A, Casanueva FF, et al. Modulation of the endocannabinoids N-arachidonoylethanolamine (AEA) and 2-arachidonoylglycerol (2-AG) on executive functions in humans. PLoS One. (2013) 8(6):e66387. doi: 10.1371/journal.pone.0066387
42. Morena M, Santori A, Campolongo P. Circadian regulation of memory under stress: endocannabinoids matter. Neurosci Biobehav Rev. (2022) 138:104712. doi: 10.1016/j.neubiorev.2022.104712
43. Santori A, Morena M, Hill MN, Campolongo P. Hippocampal 2-arachidonoyl glycerol signaling regulates time-of-day- and stress-dependent effects on rat short-term memory. Int J Mol Sci. (2020) 21(19):7316. doi: 10.3390/ijms21197316
Keywords: cannabis, adolescent young adults, endocannabinoids, verbal memory and learning, sex differences
Citation: Wallace AL, Baca R, Andrade G, Hillard CJ, Happer JP, Wade NE, Courtney KE, Hernandez Mejia M and Jacobus J (2025) Young adult cannabis use and circulating endocannabinoid concentrations on cognitive performance. Front. Adolesc. Med. 3:1538448. doi: 10.3389/fradm.2025.1538448
Received: 2 December 2024; Accepted: 20 May 2025;
Published: 17 June 2025.
Edited by:
Mónica Méndez-Díaz, National Autonomous University of Mexico, MexicoReviewed by:
Rodrigo Erick Escartín-Pérez, National Autonomous University of Mexico, MexicoBarkha Yadav-Samudrala, University of North Carolina at Chapel Hill, United States
Copyright: © 2025 Wallace, Baca, Andrade, Hillard, Happer, Wade, Courtney, Hernandez Mejia and Jacobus. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joanna Jacobus, amphY29idXNAaGVhbHRoLnVjc2QuZWR1
 Alexander L. Wallace
Alexander L. Wallace Rachel Baca1
Rachel Baca1 Cecilia J. Hillard
Cecilia J. Hillard Joseph P. Happer
Joseph P. Happer Natasha E. Wade
Natasha E. Wade Joanna Jacobus
Joanna Jacobus