- 1Department of Endocrinology and Metabolism, Affiliated Hospital of Zunyi Medical University, Zunyi, China
- 2Department of Nuclear Medicine, Affiliated Hospital of Zunyi Medical University, Zunyi, China
Introduction: As the prevalence of overweight/obesity has increased, the prevalence of Polycystic ovary syndrome (PCOS) in adolescents has also increased significantly. The main features of PCOS in adolescents include menstrual irregularities and hyperandrogenism, manifested as hirsutism, acne, and/or elevated testosterone levels. Currently, the main treatment of PCOS is symptomatic supportive therapy. Lifestyle intervention remains the first-line therapeutic approach for adolescents with PCOS. We present a case study of Time-restricted feeding (TRF) intervention in 18-year-old monozygotic twins, both diagnosed with PCOS and insulin resistance (IR).
Methods: The twins underwent an 8-hour TRF intervention for 24 weeks (eating window between 8:00 a.m. and 4:00 p.m., with only water permitted during the remaining time; daily caloric intake was restricted to 1,200–1,500 kcal). Changes in waist circumference, body weight, body mass index (BMI), lipids, alanine aminotransferase (ALT), aspartate aminotransferase (AST), aspartate aminotransferase (GGT), fasting glucose (FBG), fasting insulin (FINS), glucose metabolism (glucose disposal rate, GDR), luteinizing hormone (LH), follicle stimulating hormone (FSH), LH/FSH, total testosterone (TT), sex hormone-binding globulin (SHBG), free androgen index (FAI), anti-Müllerian hormone (AMH), menstrual cycle, Ferryman—gallwey (F-G) index and Self-Rating Depression Scale (SDS) were evaluated.
Results: Significant changes in waist circumference, body weight, BMI, FINS, FBG, ALT, AST, GGT, HDL-L, SHBG, TES, FAI, AMH, LH/FSH were found after the TRF intervention. Additionally, there was significant improvement in IR based on the hyperinsulinemic-euglycemic clamp test. Furthermore, TRF improves menstrual irregularity and hirsutism symptoms, and may also alleviate potentially depression.
Conclusion: This is the first case report of an 8-hour TRF intervention in monozygotic twins with PCOS and IR, with effective control of genetic and environmental confounders. TRF improves IR and clinical symptoms in adolescents with PCOS, and these benefits are more pronounced with higher adherence to the intervention.
Introduction
Polycystic ovary syndrome (PCOS) is one of the most common reproductive endocrine and metabolic disorders in women of reproductive age. The increasing prevalence of overweight and obesity has significantly increased PCOS prevalence in adolescents. PCOS is primarily characterized by insulin resistance (IR), hyperandrogenemia (HA), polycystic ovary morphology (PCOM), and ovulatory dysfunction. The main clinical features of adolescent with PCOS include menstrual irregularity and hyperandrogenism (hirsutism, acne, and/or high testosterone). IR and HA are the key pathophysiological features of PCOS and the main drivers of its clinical manifestations (1–4). PCOS is the most common cause of anovulatory infertility in women and increases the risk of metabolic syndrome, type 2 diabetes mellitus (T2DM), cardiovascular disease, gynecologic cancers, psychiatric disorders (depression and anxiety), and pregnancy-related complications, including gestational diabetes mellitus, pregnancy-induced hypertension (PIH), and preeclampsia (5–10). The treatment of PCOS is crucial for improving preconception health, reducing pregnancy-related and multisystem complications. Improving IR and implementing weight management constitute essential components of PCOS treatment. The treatment of PCOS primarily involves a combination of lifestyle interventions and pharmacological therapies. Lifestyle interventions should be implemented throughout the treatment of PCOS, whether combined with pharmacological therapy or not. Lifestyle interventions, including dietary control, physical activity and behavioral modifications, are the first-line therapy in international evidence-based guidelines for PCOS, among which dietary intervention is the fundamental cornerstone (11). Various dietary patterns have been studied in patients with PCOS, but no consensus on the optimal pattern has been reached. Time-restricted feeding (TRF) involves an eating window of 4–10 h per day, with fasting for the rest of the day, and may or may not include calorie restriction. A clinical study reported the endocrine and metabolic effects of 8 h TRF in patients with PCOS, with a 5-week intervention period, showing that TRF reduces weight and improves metabolism (12).
Current clinical research on TRF in adolescents with PCOS remains limited. We report the effects of 8-hour TRF on IR and metabolism in monozygotic twins with PCOS, and describe the changes and differences biochemical and metabolic markers between twin sisters following a 24-week TRF intervention.
Case report
Clinical findings
A pair of 18-year-old monozygotic twin sisters presented with “menstrual disorders for 8 years”. Years of menstrual disorders led them to consult an endocrinologist at our hospital on July 7, 2022. They had not received any treatment during these 8 years. Their mother had no history of PCOS, and their father had no history of baldness. Sister A had her menarche at age 10, which was mainly characterized by menstrual irregularities (menstrual cycle of 30–60 days), along with marked hirsutism and acne. Sister B experienced menarche at age 10, similarly presenting with menstrual irregularities (menstrual cycle of 30–180 days) and recurrent acne, though with less severe hirsutism compared to sister A.
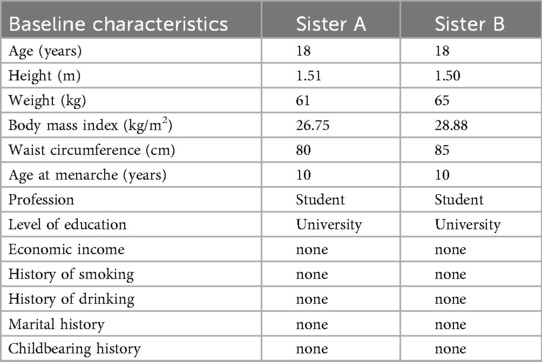
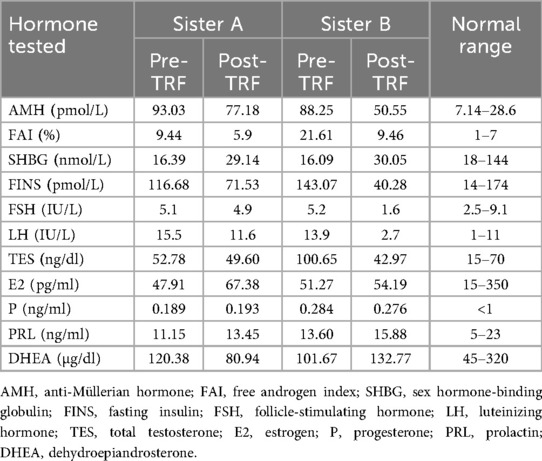
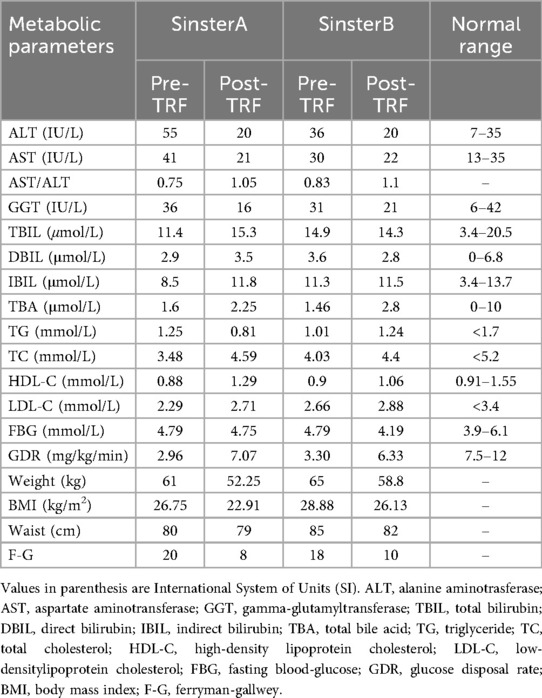
Sister A: height was 1.51 m, weight was 61 kg, body mass index (BMI) was 26.75 kg/m2. Gynecological ultrasound revealed a cystic mass in the left adnexal region. The hormone results: free androgen index (FAI):9.44%; total testosterone (TES): 52.78 ng/dl; sex hormone-binding globulin (SHBG): 16.39 nmol/L; estradiol (E2): 47.91 pg/ml; follicle-stimulating hormone (FSH): 5.1 IU/L; luteinizing hormone (LH):15.5 IU/L; progesterone (P): 0.189 ng/ml; prolactin (PRL): 11.15 ng/ml; dehydroepiandrosterone (DHEA): 120.38 µg/dl; fasting insulin (FINS): 116.68 pmol/L; anti-Müllerian hormone (AMH): 93.03 pmol/L. The euglycemic-hyperinsulinemic clamp test was employed to assess IR, and the measured glucose disposal rate (GDR) was 2.96 mg/kg/min, confirming significant IR. alanine aminotransferase (ALT): 55 U/L; aspartate aminotransferase (AST): 41 U/L; triglyceride (TG): 1.25 mmol/L, total cholesterol (TC): 3.48 mmol/L; high-density lipoprotein cholesterol (HDL-C): 0.88 mmol/L; low-density lipoprotein cholesterol (LDL-C): 2.29 mmol/L; fasting blood-glucose (FBG): 4.79 mmol/L. Ferriman–Gallwey (F-G) score was 20 points, and she had no obvious signs of acanthosis nigricans.
Sister B: height was 1.50 m, weight was 65 kg, BMI: 28.88 kg/m2. Gynecologic ultrasound showed no significant abnormalities in the sonograms of the uterus and bilateral adnexa. Hormone and results of euglycemic-hyperinsulinemic clamp test were as follows: FAI: 21.61 nmol/L; TES: 100.65 ng/dl; SHBG: 16.09 nmol/L; E2: 51.27 pg/ml; FSH: 5.2 IU/L; LH: 13.9 IU/L; P: 0.284 ng/ml; PRL: 13.60 ng/ml; DHEA: 101.67 µg/dl; FINS: 20.6 pmol/L; AMH: 88.25 pmol/L; GDR: 3.30 mg/kg/min. ALT: 36 U/L; AST: 30 U/L; TG: 1.01 mmol/L; TC: 4.03 mmol/L; HDL-C: 0.9 mmol/L; LDL-C: 2.66 mmol/L; FBG: 4.79 mmol/L. F-G score was 18 points. The cervical region and axillae presented with marked acanthosis nigricans.
The twins both exhibited typical PCOS symptoms, including clinical and biochemical hyperandrogenism and menstrual irregularities. After excluding other causes of HA and menstrual disorders (e.g., congenital adrenocortical hyperplasia, androgen-secreting tumors, and Cushing's syndrome), both were diagnosed with PCOS based on the 2008 Rotterdam criteria. Table 1 presents the basic information and characteristics of the twins. The F-G index indicated significant hirsutism. Both twins were found to have significant IR, as confirmed by the hyperinsulinemic-euglycemic clamp testing. IR is one of the main pathophysiological features of PCOS. Given that monozygotic twins share nearly identical genetic and environmental backgrounds, we focused on investigating their lifestyle habits (diet, sleep and exercise) and found that neither had healthy habits. Regarding diet, rice was their staple food, and they frequently consumed high-fat, high-calorie, and high-carbohydrate foods, Additionally, their dietary habits were usually irregular. Regarding sleep, they often stayed up late (going to sleep after 24:00), averaging 8–10 h of sleep per night. We further assessed sleep quality using the Pittsburgh Sleep Quality Index (PSQI), which revealed no significant abnormalities. They had irregular schedules and rarely exercised. To comprehensively assess their quality of life, symptom severity and potential depression, the twins completed the Polycystic Ovary Syndrome Questionnaire (PCOSQ) and the Self-Rating Depression Scale (SDS). The PCOSQ results revealed that they occasionally felt frustrated by irregular menstruation and hirsutism. They were highly concerned about obesity, and felt that it was difficult to lose weight and maintain an ideal weight, which led to frustration with weight loss. Despite their strong desire to lose weight, neither had made any efforts to change their lifestyle habits, resulting in prolonged psychological distress and recurring anxiety. Neither SDS result indicated clinical depression, but the scores were close to the diagnostic threshold. The PSQI results showed that both had acceptable sleep quality.
Therapeutic interventions and follow-up
A 24-week, 8 h TRF intervention was administered to the twins with PCOS and IR. They ate between 8:00 a.m. and 4:00 p.m., with only water allowed during the remaining hours. Daily caloric intake was restricted to 1,200–1,500 kcal and maintained their usual level of physical activity. Both were provided with identical dietary guidelines and closely monitored daily (all food consumed, except water, was required to be photographed and recorded). The aim was to observe changes in metabolic and biochemical indicators and track menstrual patterns during the intervention.
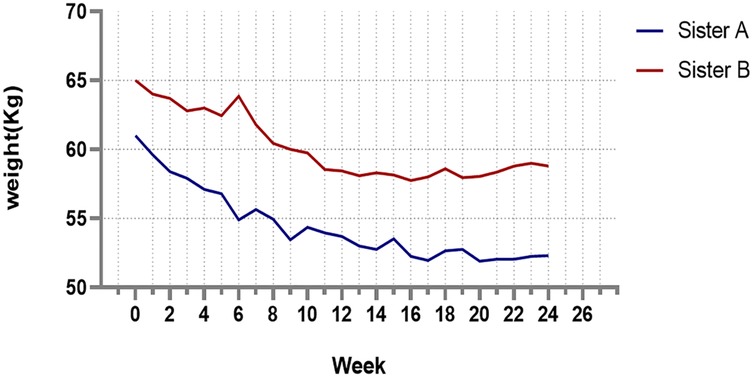
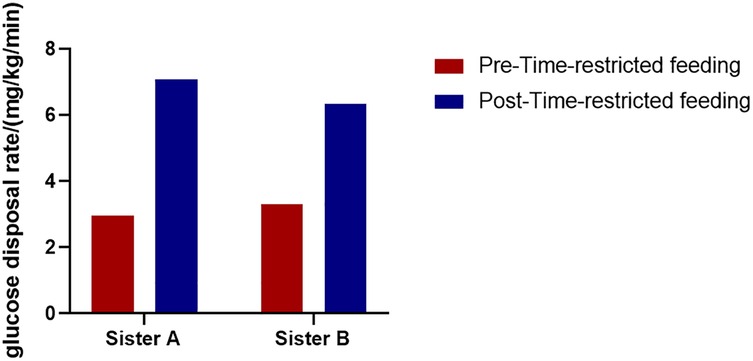
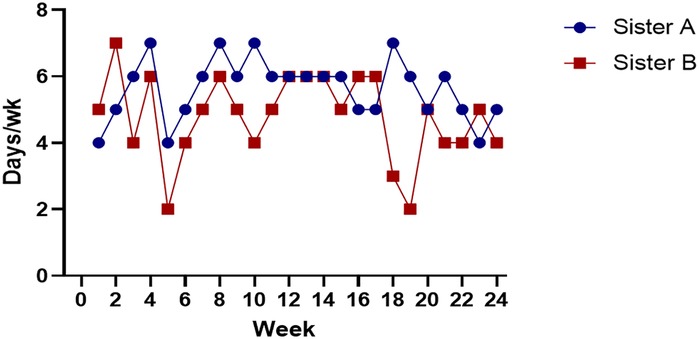
After 24 weeks of the TRF intervention, the twins returned to hospital for a follow-up examination. Figure 1 shows that both sisters experienced significant weight loss, with the most pronounced reduction occurring in the first 8 weeks, followed by minimal change over the subsequent 16 weeks. Both achieved a body weight loss of more than 5%: Sister A lost 13.60%, and Sister B lost 9.23%. The hyperinsulinemic-euglycemic clamp test showed significant improvements in IR, with the following GDR: Sister A, 7.07 mg/kg/min; Sister B, 6.33 mg/kg/min (Figure 2). Levels of FAI, TES and LH/FSH improved significantly, while SHBG increased significantly. Although FBG and INS levels were already within the normal range, post-intervention values were lower than baseline. Additionally, F-G scores indicated that hirsutism symptoms improved after the TRF intervention. We observed a decline in AMH levels compared to those before the intervention (Table 2), and their menstruation records during the intervention showed improvement in menstrual regularity. Levels of ALT, GGT, and AST decreased after the TRF intervention. However, its effects on the blood lipid profile were inconsistent: HDL-C and TC levels increased, whereas LDL-C exhibited only a marginal elevation that remained within the normal physiological range (Table 3). Following TRF intervention, SDS values decreased. Neither twin experienced sleep disorders, no significant changes in sleep quality were observed. The differences in outcomes between the twins may be attributed to differential compliance with the 24-week TRF intervention. Sister A adhered to the regimen for an average of 6 days per week, Sister B followed it for 5 days per week (Figure 3). We recorded the reasons for occasional non-compliance with fasting, most instances were due to social events (such as dinners with family or friends) or busyness with studies, while other reasons included forgetting the time, hunger, or food cravings. Neither twin experienced significant adverse effects during the intervention. Three months later, during our follow-up with the twins, they said that they had attempted to maintain the same fasting habits as during the intervention, albeit with less strict adherence. No significant changes were observed in the twins' body weight, and their menstrual cycles remained relatively regular.
Discussion
In this report, the twins presented with typical clinical manifestations of adolescents with PCOS, including menstrual irregularities, hirsutism, and acne. Hyperinsulinemic-euglycemic clamp test revealed severe insulin resistance in both twins. While the etiology of PCOS remains uncertain, genetic and environmental factors are thought to play a major role (1). In this case, we controlled these factors and focused on the twins' lifestyle behaviors. We observed that long-term unhealthy lifestyle behaviors (poor diet, disorganized routine, and chronic physical inactivity) may induce or exacerbate PCOS symptoms. These findings are consistent with previous epidemiological observations in women with PCOS (13). Unhealthy lifestyle habits contribute to weight gain, which in turn exacerbates clinical symptoms and psychological issues, thereby creating a vicious cycle.
Currently, TRF is one of the most popular dietary patterns for weight loss and a form of intermittent fasting. An increasing number of clinical studies are exploring the effects of TRF on obesity and metabolic disorders, including type 2 diabetes, metabolic syndrome and PCOS, most research has found that TRF can reduce body weight and improve metabolic disorders, however, the majority of studies have focused on short-term interventions (4–12 weeks) (14–16). In this case, the twins underwent a 24-week TRF intervention, a longer duration than most existing studies. Significant improvements were observed in weight, BMI, waist circumference, TES, SHBG, FAI, FINS, FBG, LH/FSH, ALT, AST, and GGT, consistent with previous findings (8, 14). However, most studies assess insulin resistance using the homeostatic model assessment-insulin resistance (HOMA-IR). Notably, twins’ insulin resistance improved significantly as measured by the hyperinsulinemic-euglycemic clamp assay (the gold standard for diagnosing IR). Therefore, this case report found that TRF significantly improves insulin sensitivity. Furthermore, current studies have found that AMH levels are significantly elevated in adolescent with PCOS. Measuring AMH during adolescence is not only meaningful for the diagnosis of PCOS but also as a significant determinant in later life (17, 18). We found that the twin's AMH levels decreased after TRF, which may reflect an improvement in PCOS-related ovulatory dysfunction. Women with PCOS have an increased risk of depression (8), although neither twin in this case was diagnosed with depression, weight loss and symptom improvement significantly reduced the frequency of anxiety after TRF intervention. The primary difference between the twins was intervention adherence, Sister A had higher adherence, which led to more pronounced effects. Those findings indicate that higher adherence is associated with more pronounced benefits.
At present, the mechanism by which TRF improves insulin resistance and hyperandrogenism remains unclear. Current studies have shown that the improvement of metabolic disorders by TRF may be associated with enhanced circadian rhythms, increased autophagy expression, reduced inflammatory responses, and improved gut microbiota (19–21), more basic and clinical studies are required to further confirm the underlying mechanisms.
Conclusion
This is the first case report of monozygotic twins with PCOS and IR who underwent a 24-week TRF intervention, effectively controlling for genetic and environmental confounders. The 8 h TRF intervention significantly reduced waist circumference, BMI, FBG, FINS, ALT, AST and GGT. It also significantly improved IR and HA, regulated menstruation, and promoted ovulation. Long-term TRF adherence may contribute to body weight control, with higher adherence levels associated with greater benefits. TRF may be a suitable pattern for adolescents with PCOS to improve clinical symptoms and restore ovulation. Further large-scale randomized controlled trials are required to elucidate the effects of TRF in adolescents with PCOS.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Affiliated Hospital of Zun Yi Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
DL: Data curation, Formal analysis, Supervision, Writing – original draft. ZT: Investigation, Writing – original draft. JF: Formal analysis, Investigation, Writing – review & editing. YL: Writing – review & editing, Formal analysis, Investigation. QH: Supervision, Writing – review & editing. XL: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. (2018) 14(5):270–84. doi: 10.1038/nrendo.2018.24
2. Ibáñez L, de Zegher F. Adolescent PCOS: a postpubertal central obesity syndrome. Trends Mol Med. (2023) 29(5):354–63. doi: 10.1016/j.molmed.2023.02.006
3. Hart R, Doherty DA, Norman RJ, Franks S, Dickinson JE, Hickey M, et al. Serum antimullerian hormone (AMH) levels are elevated in adolescent girls with polycystic ovaries and the polycystic ovarian syndrome (PCOS). Fertil Steril. (2010) 94(3):1118–21. doi: 10.1016/j.fertnstert.2009.11.002
4. Barber TM, Franks S. Obesity and polycystic ovary syndrome. Clin Endocrinol. (2021) 95(4):531–41. doi: 10.1111/cen.14421
5. Lim SS, Kakoly NS, Tan JWJ, Fitzgerald G, Bahri Khomami M, Joham AE, et al. Metabolic syndrome in polycystic ovary syndrome: a systematic review, meta-analysis and meta-regression. Obes Rev. (2019) 20(2):339–52. doi: 10.1111/obr.12762
6. Osibogun O, Ogunmoroti O, Michos ED. Polycystic ovary syndrome and cardiometabolic risk: opportunities for cardiovascular disease prevention. Trends Cardiovasc Med. (2020) 30(7):399–404. doi: 10.1016/j.tcm.2019.08.010
7. Alur-Gupta S, Boland MR, Barnhart KT, Sammel MD, Dokras A. Postpartum complications increased in women with polycystic ovary syndrome. Am J Obstet Gynecol. (2021) 224(3):280.e1–e13. doi: 10.1016/j.ajog.2020.08.048
8. Dybciak P, Raczkiewicz D, Humeniuk E, Powrózek T, Gujski M, Małecka-Massalska T, et al. Depression in polycystic ovary syndrome: a systematic review and meta-analysis. J Clin Med. (2023) 12(20):6446. doi: 10.3390/jcm12206446
9. Barry JA, Azizia MM, Hardiman PJ. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. (2014) 20(5):748–58. doi: 10.1093/humupd/dmu012
10. Teede H, Deeks A, Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. (2010) 8:41. doi: 10.1186/1741-7015-8-41
11. Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. (2018) 110(3):364–79. doi: 10.1016/j.fertnstert.2018.05.004
12. Li C, Xing C, Zhang J, Zhao H, Shi W, He B. Eight-hour time-restricted feeding improves endocrine and metabolic profiles in women with anovulatory polycystic ovary syndrome. J Transl Med. (2021) 19(1):148. doi: 10.1186/s12967-021-02817-2
13. Kazemi M, Kim JY, Wan C, Xiong JD, Michalak J, Xavier IB, et al. Comparison of dietary and physical activity behaviors in women with and without polycystic ovary syndrome: a systematic review and meta-analysis of 39 471 women. Hum Reprod Update. (2022) 28(6):910–55. doi: 10.1093/humupd/dmac023
14. Feyzioglu BS, Güven CM, Avul Z. Eight-hour time-restricted feeding: a strong candidate diet protocol for first-line therapy in polycystic ovary syndrome. Nutrients. (2023) 15(10):2260. doi: 10.3390/nu15102260
15. Pellegrini M, Cioffi I, Evangelista A, Ponzo V, Goitre I, Ciccone G, et al. Effects of time-restricted feeding on body weight and metabolism. A systematic review and meta-analysis. Rev Endocr Metab Disord. (2020) 21(1):17–33. doi: 10.1007/s11154-019-09524-w
16. Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. (2018) 27(6):1212–21.e3. doi: 10.1016/j.cmet.2018.04.010
17. Rudnicka E, Kunicki M, Calik-Ksepka A, Suchta K, Duszewska A, Smolarczyk K, et al. Anti-Müllerian hormone in pathogenesis, diagnostic and treatment of PCOS. Int J Mol Sci. (2021) 22(22):12507. doi: 10.3390/ijms222212507
18. Tsukui Y, Kitahara Y, Hasegawa Y, Kobayashi M, Osuka S, Iwase A. Anti-Müllerian hormone levels in the diagnosis of adolescent polycystic ovarian syndrome: a systematic review and meta-analysis. Endocr J. (2022) 69(8):897–906. doi: 10.1507/endocrj.EJ22-0081
19. Petersen MC, Gallop MR, Flores Ramos S, Zarrinpar A, Broussard JL, Chondronikola M, et al. Complex physiology and clinical implications of time-restricted eating. Physiol Rev. (2022) 102(4):1991–2034. doi: 10.1152/physrev.00006.2022
20. Jamshed H, Beyl RA, Della Manna DL, Yang ES, Ravussin E, Peterson CM. Early time-restricted feeding improves 24-hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans. Nutrients. (2019) 11(6):1234. doi: 10.3390/nu11061234
Keywords: polycystic ovary syndrome (PCOS), time-restricted feeding (TRF), insulin resistance (IR), hyperandrogenemia, twins
Citation: Luo D, Tan Z, Feng J, Li Y, Huang Q and Liao X (2025) Case Report: Time-restricted feeding improves metabolism in twins with polycystic ovary syndrome and insulin resistance. Front. Adolesc. Med. 3:1557504. doi: 10.3389/fradm.2025.1557504
Received: 8 January 2025; Accepted: 29 August 2025;
Published: 22 September 2025.
Edited by:
Anurag Bajpai, Regency Hospital, IndiaReviewed by:
Swapan Banerjee, Poornima University, IndiaRoya Rozati, Mahavir Dental Hospital and Research Centre, India
Copyright: © 2025 Luo, Tan, Feng, Li, Huang and Liao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Liao, bGlhb3hpbjg2MThAMTYzLmNvbQ==
 Dingyan Luo
Dingyan Luo Zhouying Tan1
Zhouying Tan1 Xin Liao
Xin Liao