- 1Department of Crop Sciences, Tshwane University of Technology, Pretoria, South Africa
- 2Savanna Agricultural Research Institute, CSIR, Tamale, Ghana
- 3Department of Chemistry, Faculty of Science, Tshwane University of Technology, Pretoria, South Africa
African soils are inherently low in mineral nutrients. Incorporating N2-fixing legumes into cropping systems can improve soil fertility and increase crop yields. This study assessed N2 fixation, carbon assimilation, grain mineral accumulation and water-use efficiency of 30 groundnut genotypes grown in the field at the Mpumalanga Province, South Africa, using the 15N and 13C natural abundance techniques. The results revealed marked differences in symbiotic performance between and among the groundnut genotypes, with IS-07273 and ICGV13910 exhibiting greater symbiotic dependency on N2 fixation for their N nutrition and higher amounts of N-fixed. The two high N2-fixing symbioses (IS-07273 and ICGV13910) also accumulated significantly high levels of K, Na, Zn, Cu, Mn, and B in their grain. As a result, there were strong correlations between amounts of N-fixed and K, Na, B, Cu, Zn and Mn for genotype ICGV13910. Genotype IS-07273 also showed significant correlations between N-fixed and S, N concentration (%N) and P, %N and K, as well as nodule number and Ca. As to be expected, genotypes with the highest shoot %N accumulated the most protein in their grain. Out of the 30 groundnut genotypes tested in the field, YENYAWOSO, ICGV13848, ICGV13851, ICGV15033 and ICGV131065 showed greater shoot δ13C values, and hence higher water-use efficiency. The high N2 fixation in genotypes ICGV13910 and IS-07273 correlated positively with macro- and micro-nutrient concentrations in their grain, indicating their potential for use in breeding programmes to enhance nutritional security in groundnut.
Introduction
Groundnut (Arachis hypogaea L.) is an important source of protein (25%–28%), oil (48%–50%), and vitamins for human consumption and cash income for farmers (Nyambok, 2011; Janila et al., 2013). Groundnut is also a source of symbiotic nitrogen for plant growth and for boosting crop production in farmers’ fields because of its ability to form effective symbioses with soil rhizobial bacteria (Vieira et al., 2010). Groundnut is nodulated by Bradyrhizobium bacteria (Zhang et al., 1999; Chen et al., 2003; Yang et al., 2005), Rhizobium giardini, and Rhizobium tropici (Taurian et al., 2006; Ibañez et al., 2008; Ibanez et al., 2009). The groundnut plant has been shown to meet much of its N requirement from symbiotic N2 fixation and to contribute to the N economy of cropping systems (Mokgehle et al., 2014).
Groundnut can contribute a considerable amount of nitrogen to cropping systems in Africa. In fact, many studies (Dakora and Keya, 1997; Bado et al., 2006; Nyemba and Dakora, 2010) have found that significant levels of nitrogen have been contributed by groundnut in African cropping systems. In South Africa, groundnut is reported to fix between 58 and 188 kg N.ha-1 (Mokgehle et al., 2014), thus improving soil N fertility in resource-poor farmers’ fields and increasing the yields of intercrops and succeeding cereal crops (Kumawat et al., 2022).
N2-fixing rhizobia in root nodules can convert nitrogen (N2) in the air to NH3, which is then incorporated into amino acids and protein, and stored in leaves and seeds. This explains why the edible leaves and seeds of legumes (or pulses) are rich in protein. Soybean and cowpea grain can contain up to 40% seed protein (Oso and Ashafa, 2021; Zarkadas et al., 2007; Dakora and Belane, 2019), common bean 20%–25% (Broughton et al., 2003; de Paiva Gouvêa et al., 2024), Bambara groundnut 20.6% (Hlanga et al., 2021; Mazahib et al., 2013), Kersting’s bean 21.3% (Ikujenlola et al., 2022; Ayenan and Ezin, 2016), pigeon pea 27%–29% (Obala et al., 2020; Saxena and Singh, 1987) mungbean 21%–31% (Yi-Shen et al., 2018), chickpea 21.8%–25.8% (Xu et al., 2016), and groundnut 20%–30% (Toomer, 2018; Syed et al., 2021). Furthermore, cowpea can contain 34.9% protein in edible leaves and up to 40% in some genotypes (Dakora and Belane, 2019).
In addition to protein, the edible leaves and grain of legumes contain significant levels of nutritionally important minerals that are required for human nutrition and health, especially to combat trace element deficiency and promote brain development. The leaves and seeds of cowpea respectively contain 142–626 mg.kg−1 Fe, 49–104 mg.kg−1 Zn, 196–394 mg.kg−1 Mn, 8.6–19.7 mg.kg−1 Cu, and 42–55 mg.kg−1 B (Belane and Dakora, 2011). Groundnut grain also contained 22.6 mg.kg−1 Fe, 33.1 mg.kg−1 Zn, 6.7 mg.kg−1 Mn, and 7.5 mg.kg−1 Cu (Toomer, 2018), while chickpea seed is reported to have 500.0 mg.kg−1 Fe, 405.0 mg.kg−1 Zn, 480.0 mg.kg−1 Mn, and 85.0 mg.kg−1 Cu (Xu et al., 2016).
African soils are inherently low in nutrients and require fertilization for high crop yields. However, the high cost of chemical fertilizers and their damaging effect on the environment have prohibited their use. There is therefore a need to develop sustainable, green, and affordable technologies to increase crop yields and nutritional quality of grains for smallholder farmers in Africa. Furthermore, with climate change, there is a need to develop drought-tolerant groundnut genotypes that can produce economic yields even under low rainfall conditions. Carbon isotope discrimination during photosynthesis in C3 plants is known to correlate with water-use efficiency (WUE) and can therefore be used as a measure of plant WUE (Farquhar et al., 1989; Oteng-Frimpong and Dakora, 2018; Muhaba and Dakora, 2020; Mohammed et al., 2022; Pampa et al., 2023). Plants experiencing water limitation tend to show high WUE, measured as greater shoot δ13C values. However, with adequate soil moisture, plants exhibit lower shoot δ13C values due to increased discrimination against 13CO2 (Cernusak et al., 2013). Carbon isotope discrimination has therefore been successfully employed to assess plant water relations in legumes and other C3 species (Belane et al., 2014; Mokgehle et al., 2014; Mapope and Dakora, 2016). The aim of this study was to evaluate groundnut genotypes for increased plant growth, high symbiotic performance, greater WUE, and increased grain mineral accumulation under field conditions using the 15N and 13C natural abundance techniques.
Materials and methods
Site description, experimental design, and treatments
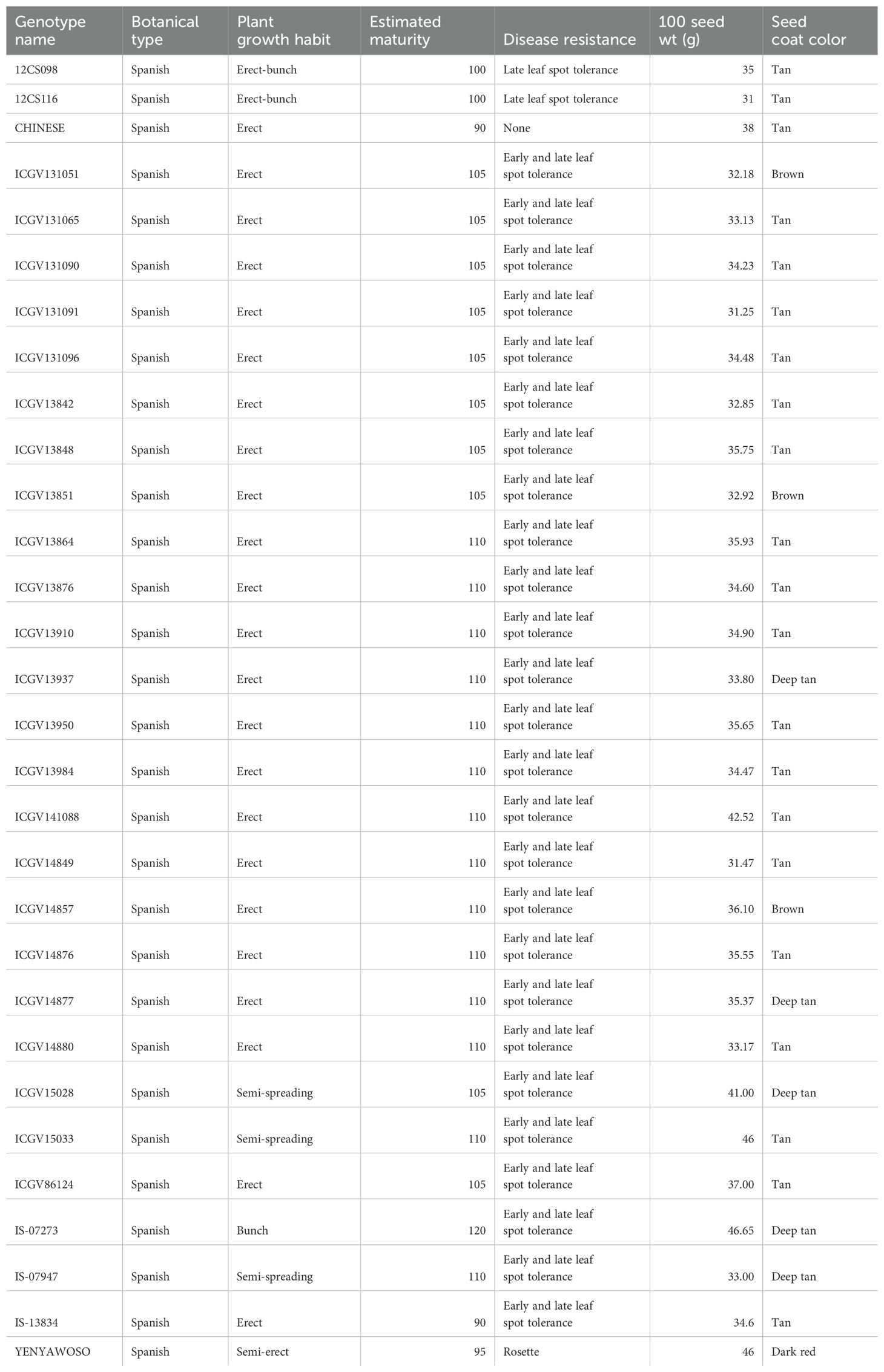
The experiment was conducted during the rainy season of 2019 at Blesbokfontein farm, Bronkhorspruit, Mpumalanga, South Africa. The area is characterized by warmer temperatures and low rainfall with a unimodal rainy season that starts in October each year and ends in April the following year. The map reference coordinates were 25°19’0” S, 31°6’0” E, and the elevation was 199 m above sea level. The optimal rainfall ranged from 500 mm to 900 mm, with a temperature range of 17.5°C to 26°C per annum. Before planting, the soil at Blesbokfontein farm was classified as sandy loam. The experiment was laid out in a randomized complete block design with three replicates in a total of 90 plots. Seeds were sown on the flat with 80 cm between the rows and 10 cm within each row. Each plot size was 6 m2 (3 m × 2 m). Each plot contained three rows with 30 plants per row. Soil samples were collected at a depth of 15–20cm and processed for chemical analysis prior to planting. The characteristics of the 30 groundnut genotypes use in the study are shown in Table 1.
Plant sampling and processing
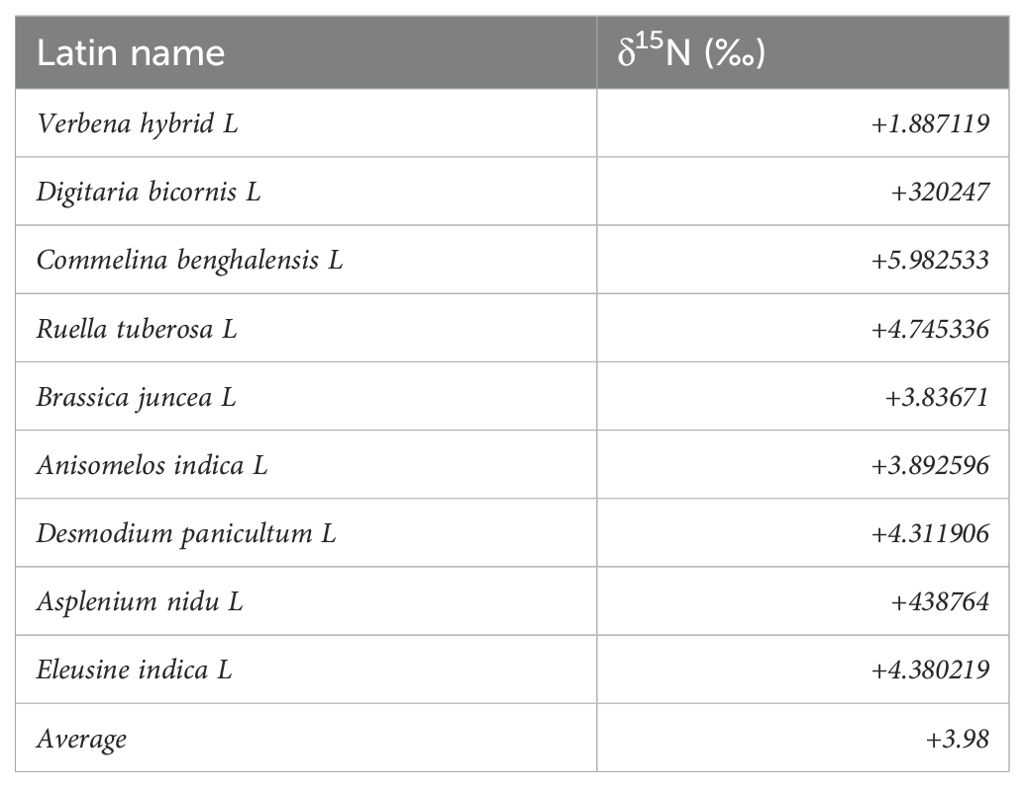
Plant sampling for dry matter yield and the 15N isotopic analysis were done at the flowering to early pod-filling stage. Three plants were randomly sampled from the central rows of each plot. Plant samples were carefully separated into shoots, roots, and nodules, and the nodule number was recorded. The shoots and nodules were oven-dried at 60°C to a constant weight (48 h). The dried shoots were ground to a fine powder (0.85 mm sieve) and stored in vials prior to 15N and 13C analysis. Nine non-leguminous weed species (namely, Verbena hybrid L., Digitaria bicomis L., Commelina benghalensis L., Ruella tuberosa L., Brassica juncea L., Anisomelos indica L., Desmodium panicultum L., Asplenium nidu L., and Eleusine indica L.) growing within the experimental plots were sampled as reference plants to determine the soil N uptake by the groundnut plants (Table 2).
Measurement of shoot N2 fixation and C accumulation
15N/14N isotopic analysis
The analyses of 15N/14N isotopic ratios were performed at the Stable Light Isotope Laboratory, University of Cape Town, Rondebosch, South Africa. For this, 2.0 mg of finely ground groundnut shoots and 2.5 mg of the reference plant samples were weighed into tin aluminum capsules and loaded onto a Thermo 2000 Elemental Analyzer coupled with a Thermo Conflo IV Plus stable light isotope mass spectrometer (Thermo Corporation, Bremen Germany). Samples were combusted in an evacuated quartz tube and analyzed to determine the ratio of 15N/14N and the N concentration (%N) in the plant material. An internal standard of Nasturtium spp. was included after every five runs of the plant samples to correct for machine errors during the isotopic fractionation. The results were normalized against in-house reference material and reported relative to an international standard (N in air). The 15N/14N ratio was used to calculate the isotopic composition (δN), as in Mariotti et al. (1981):
Where 15N/14Nsample is the abundance ratio of 15N and 14N in the sample and 15N/14Natm is the abundance ratio of 15N and 14N in the atmosphere.
Shoot N content
The N content of the shoots was calculated as the product of shoot %N and shoot dry matter, using the method of Pausch et al. (1996), where %N was obtained directly from the mass spectrometer: Ncontent = %Nshoot x dry mass shoot.
The B-value
The 15N natural abundance technique was used for N fixation analyses. It is based on the principle that an effectively nodulated legume growing on a medium free from combined N (mineral N or organic N) is expected to be completely reliant upon symbiotic N2 fixation for its growth, hence, the isotopic composition of the legume would be expected to be similar to that of atmospheric N2. A legume’s δ15N value, when grown in soil containing mineral N, is expected to resemble that of the soil mineral N (Unkovich et al., 2008). Different non-legume plant species were collected from the experimental plots and used for the δ15N analyses and a B-value of -2.70 ‰ (Nyemba & Dakora, 2010) was used for the calculations.
Percent N derived from atmospheric fixation
The proportion of N derived from the atmospheric N2 fixation (%Ndfa) was estimated as in Shearer and Kohl (1986):
Where δ15Nref is the 15N natural abundance of the reference plant, δ15Nleg is the 15N natural abundance of the legume, and the B-value is the 15N natural abundance of groundnut plants deriving all their N nutrition from N2 fixation.
Amount of N-fixed
The amount of fixed N (N-fixed) by the groundnut plant was calculated as in Unkovich et al. (2008): N-fixed = %Ndfa x legume biomass N
Where legume biomass N is the N content of the groundnut shoots.
Soil N uptake
The soil N uptake by the groundnut plant was calculated as in Unkovich et al. (2008):
Soil N uptake = (100 - %Ndfa) x legume biomass N
13C/12C isotopic analysis
Sub-samples of the ground shoots were analyzed at the Stable Light Isotope Laboratory, University of Cape Town, Rondebosch, South Africa as described above for N. The results were normalized and reported relative to Pee Dee Belemnite (PDB). The C concentration (%C) was also concurrently determined following combustion of the sample. The ratio of 13C/12C in each shoot sample was used to calculate δ13C (Mohale et al., 2014):
Where (13C/12C) sample is the isotopic ratio of the sample and (13C/12C) standard is the isotopic ratio of PDB (Craig, 1957).
C content
The C content of each genotype was calculated as the product of %C and the shoot dry mass (biomass) of each plant.
Measurement of nutrient accumulation in groundnut grain
To measure the mineral elements P, K, Ca, Mg, Cu, Zn, Mn, Fe, and B in groundnut grain, 1 g of ground sample was ashed in a porcelain crucible at 500°C overnight. This was followed by dissolving the ash in 5 ml 6 M HCl (analytical grade) and placing it in an oven at 50°C for 30 min, after which 35 ml de-ionized water was added. The mixture was filtered through Whatman No. 1 filter paper. The mineral element concentration in the plant extracts was determined from three replicate samples using inductively coupled plasma mass spectrometry (ICP-MS) (Ataro et al., 2008).
Data analysis
The data were subjected to analysis of variance (ANOVA) to compare the means of the treatments using the STATISTICA program (version 10) and GenStat 11th edition. Where there were differences, Duncan’s multiple range test was used to separate the means at p ≤ 0.05. Correlation analysis was performed to ascertain if any relationship existed between symbiotic parameters (e.g. nodule number, N-fixed, and %N) and mineral nutrient concentrations in grain/shoots.
Results
Soil analysis
The physico-chemical properties of soil samples collected before planting were measured. The soil contained 0.070% N, 39 mg P.kg-1, 196 mg K.kg-1, 117 mg Ca.kg-1, 60 mg Mg.kg-1, 91.35 mg Fe.kg-1, 1.20 mg Cu.kg-1, 34.18 mg Mn.kg-1, 5 mg Na.kg-1, and 3.18 mg Zn.kg-1.
Shoot δ15N of the reference plants
Nine non-legume reference plant species were sampled and analyzed to calculate the %Ndfa of the groundnut genotypes. The δ15N values of the reference plants ranged from +1.89‰ to +5.98‰ with a combined mean of +3.98‰ (Table 3).
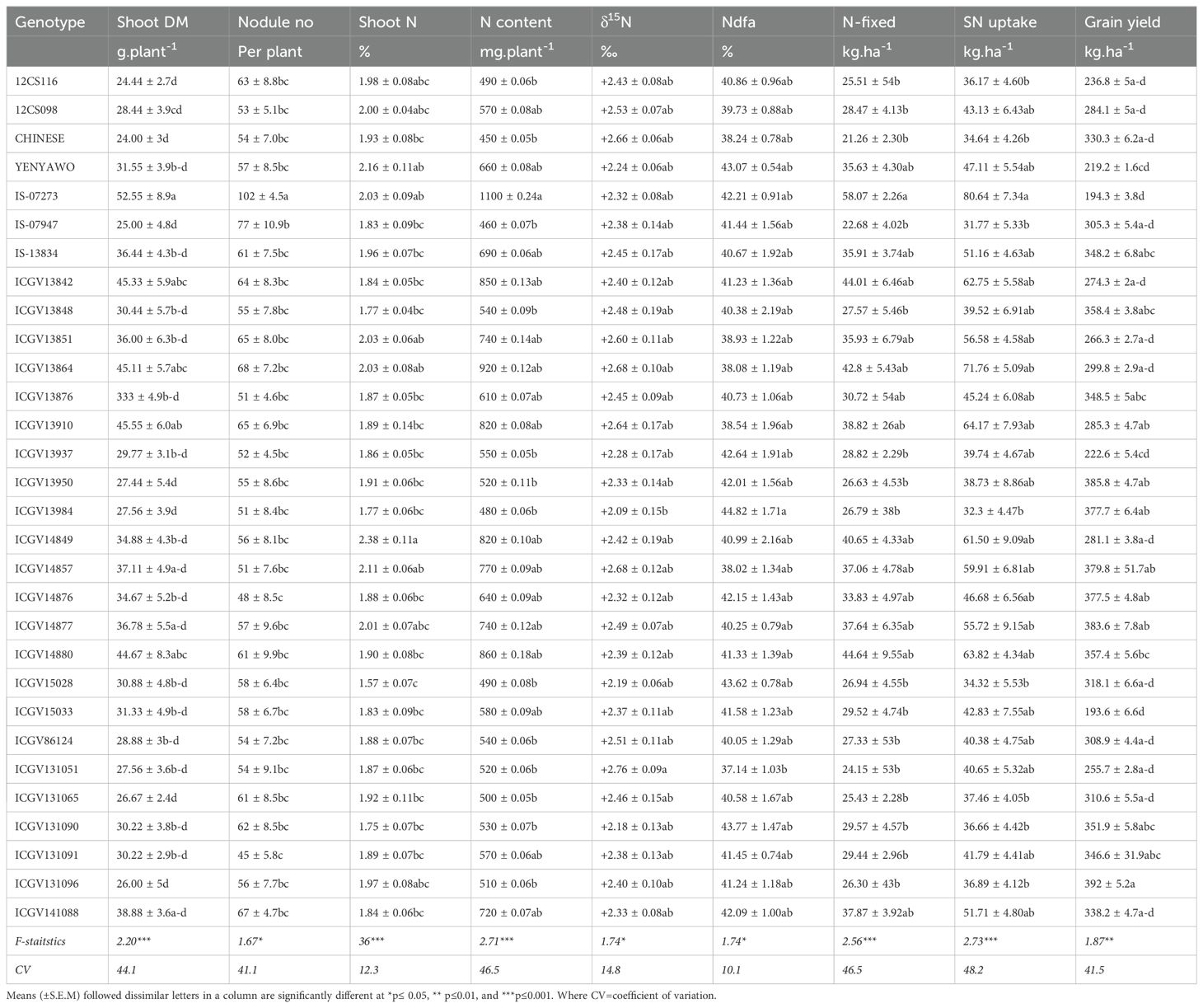
Table 3. Plant growth, symbiotic performance, and grain yield of groundnut genotypes planted in the field at Blesbokfontein, South Africa, in 2019. Values with dissimilar letters in a column are significantly different at P ≤ 0.05.
Plant growth, nodulation, and grain yield
A one-way ANOVA revealed significant differences in plant growth and root nodulation between and among the 30 groundnut genotypes (Table 2). Genotype IS-07273 produced higher nodule numbers and high dry matter. The shoot DM ranged from 24 g.plant-1 in genotypes 12CS116 and CHINESE to 53 g.plant-1 in genotype IS-07273. Nineteen of the 30 groundnut genotypes produced significantly more dry matter compared to 12CS116 and CHINESE, which recorded the lowest biomass together with genotype IS-07273. The other genotypes with greater nodulation included IS-07947, ICGV13864, ICGV13910, ICGV13851, ICGV13842, and 12CS116. Furthermore, 28 of the 30 groundnut genotypes produced high nodule numbers (Table 2). Due to poor rainfall, grain yield was generally low among the 30 groundnut genotypes, with ICGV131096, ICGV13950, ICGV14877, ICGV14857, ICGV14876, ICGV13848, and ICGV13876 producing relatively higher yields (Table 2). Genotypes ICGV15033, IS-07273, and YENYAWOSO recorded the lowest grain yields (Table 2).
N concentration and N content
The N concentration and N content differed between and among the 30 groundnut genotypes, with ICGV14849 showing the highest N concentration (2.3%), followed by genotypes YENYAWOSO, ICGV14857, ICGV13851, ICGV13864, IS-07273,12CS098, and ICGV14877. In contrast, genotypes ICGV15028 and ICGV131090 exhibited the lowest N concentrations (1.57% and 1.75%, respectively; Table 2). Shoot N content was highest in genotype IS-07273 (which recorded the highest dry matter of 1,100 mg.plant-1), followed by genotypes ICGV13864, ICGV14880, and ICGV13842, and lowest in genotypes CHINESE and IS-07947 (Table 2). The shoot N concentration ranged from 1.75% to 2.3%, and shoot N content from 450 to 1,100 mg.plant-1 (Table 2).
δ15N and %Ndfa values of groundnut plants
The δ15N values of groundnut genotypes ranged from +2.09‰ in genotype ICGV13984 to +2.76‰ in genotype ICGV131051 (Table 2). The shoot δ15N of all the groundnut genotypes were statistically similar except for genotype ICGV131051. The genotypes with much lower δ15N values (i.e. ICGV13984, ICGV131090, ICGV15028, and YENYAWOSO) derived the most N from atmospheric N2 fixation, while those with the highest δ15N values (i.e. genotypes CHINESE, ICGV14857, and 12CS098) recorded the lowest %Ndfa (Table 2). Of the 30 groundnut genotypes tested, 23 derived 40% or more of their N nutrition from symbiotic fixation, but none obtained up to 50% of its N nutrition from symbiosis.
Amount of fixed N
The amount of N-fixed by groundnut ranged from 21 kg.ha-1 for genotype CHINESE to 58 kg.ha-1 for IS-07273 (the highest fixer), which was followed by genotypes ICGV14880, ICGV13842, ICGV13864, and ICGV14849 with 44.64, 44.01, 42.8, and 40.65 kg N-fixed.ha-1, respectively (Table 2). The genotypes that produced the most symbiotic N recorded the largest biomass, while genotypes CHINESE, 12CS116, ICGV131096, ICGV131065, and ICGV131051, which contributed the least N, produced the lowest biomass (Table 2). Moreover, 14 of the 30 groundnut genotypes (namely, YENYAWOSO, IS-07273, IS-13834, ICGV13842, ICGV13851, ICGV13864, ICGV13876, ICGV13910, ICGV14849, ICGV14857, ICGV14876, ICGV14877, ICGV14880, and ICGV141088) contributed over 30 kg N.ha-1 from symbiotic N2 fixation (Table 2).
Soil N uptake
The 30 groundnut genotypes differed markedly in their levels of soil N uptake. A comparison of the source of N utilized by the groundnut genotypes showed that 11 of the 30 genotypes (namely, IS-07273, IS-13834, ICGV13842, ICGV13851, ICGV13864, ICG13910, ICGV14849, ICG14857, ICGV14877, ICGV14880, and ICGV141088) obtained more N from the soil than from symbiotic fixation for their N nutrition (Table 2).
Carbon concentration and C content
Genotypes ICGV14849, ICGV131051, ICGV86124, ICGV13937, ICGV13864, and 12CS098 showed the highest shoot C concentration, with genotypes ICGV15033, ICGV131090, and ICGV131065 having the lowest. Genotypes IS-07273, ICGV13864, ICGV14880, and ICGV13910 recorded the highest C content per plant due to their greater shoot biomass (Table 4).
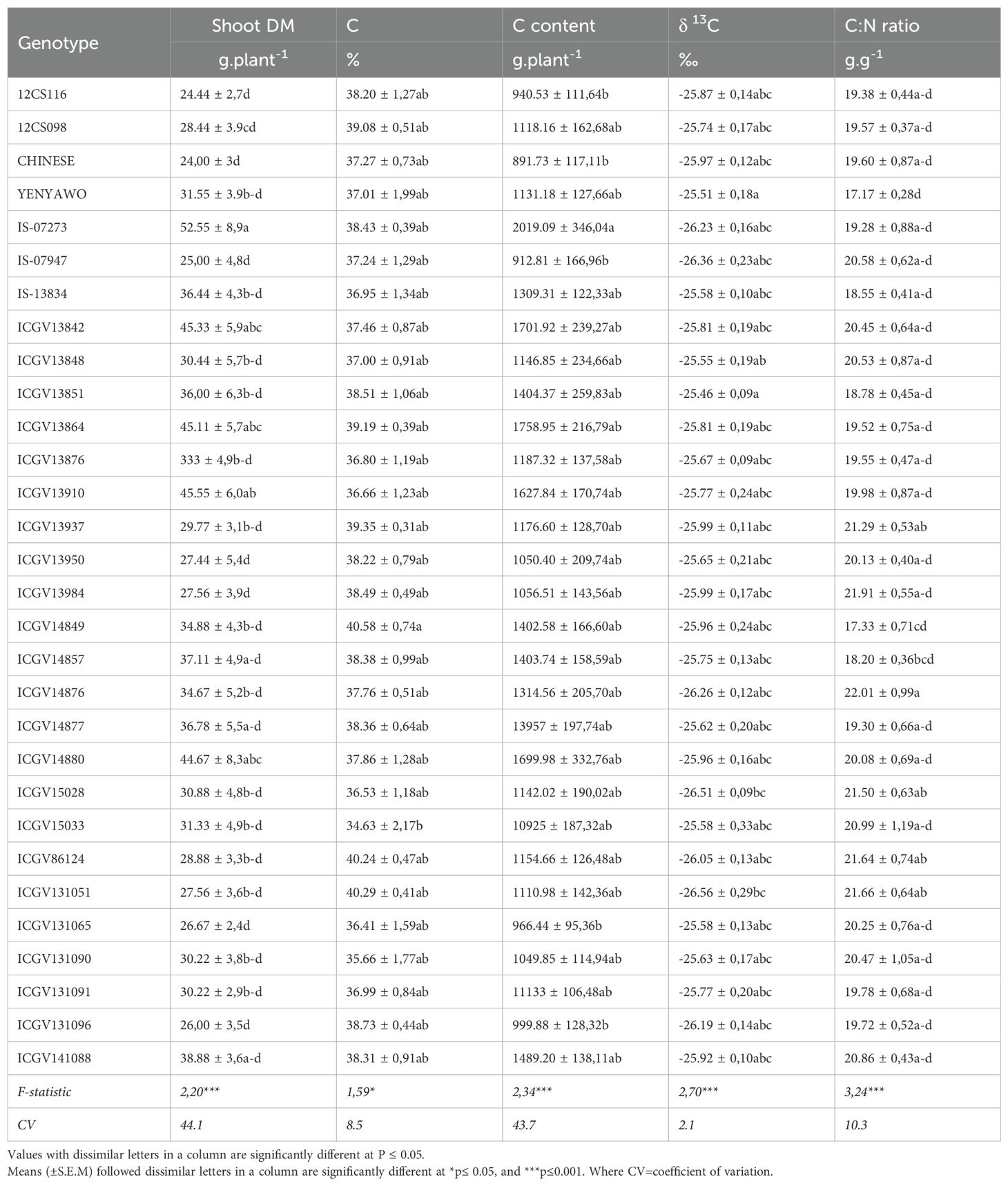
Table 4. Comparison of dry matter yield, %C, C content, C/N ratio, δ13C, and grain yield of groundnut genotypes planted in the field at Blesbokfontein, South Africa, in 2019.
Shoot C:N ratio and δ13C
The C/N ratio of groundnut shoots was highest in genotypes ICGV14876, ICGV131051, ICGV86124, ICGV15033, ICGV15028, ICGV13950, ICGV13937, ICGV13848, ICGV13842, and IS-07947, and lowest in YENYAWOSO, IS-13834, ICGV14849, and ICGV13851 (Table 4). Shoot δ13C values were much greater in genotypes ICGV13851, YENYAWOSO, and ICGV13950, followed by ICGV131090 and ICGV131091 (-25.58 to -25.63‰). Furthermore, 24 of the 30 groundnut genotypes recorded similar shoot δ13C values, which ranged from -25.99‰ to -25.46‰.
Protein content and macronutrient and micronutrient concentrations in groundnut grain
The levels of P, K, Ca, N, Mg, and S in the grain differed significantly among and between the groundnut genotypes (Table 5), with 10 of the 30 groundnut genotypes (namely, IS-07273, IS-07947, 12CS116, ICGV13842, ICGV14876, ICGV14877, ICGV86124, ICGV131051, ICGV131090, and ICGV131096) recording more than 0.50% P. Genotypes ICGV1315, ICGV13910, IS-07273, and ICGV15028 accumulated more than 1% K in groundnut grain. Genotypes IS-07273, ICGV13910, and ICGV 86124 also showed a high concentration of Mg in their grain (Table 5). The protein content in groundnut grain ranged from 20.64% in genotype ICGV131090 to 29.82% in cultivar ICGV13937 (Table 5). The N concentration in groundnut grain was greater in cultivars ICGV13937, 12CS098, ICGV14857, ICGV131091, ICGV13842, and CHINESE, and lowest in ICGV131090 (Table 4). The Ca and S concentrations were higher in cultivars ICGV1315 and ICGV131091 (Table 5). The highest concentration of Na in groundnut grain was in genotype 12CS098, followed by ICGV13910, ICGV13987, and ICGV14877, and the lowest in genotypes ICGV14880, ICGV15028, ICGV1315, and IS-07273 (Table 5). The trace elements in groundnut grain differed significantly between and among the 30 groundnut genotypes (Table 5). The Fe concentration in groundnut grain was highest in genotype 12CS116, followed by IS-13834, 12CS098, CHINESE, ICGV1315, and IS-07273 and lowest in ICGV131051, ICGV131065, ICGV131096, and ICGV141088 (Table 5). The concentration of Cu was also highest in genotypes IS-07273, ICGV13910, ICGV86124, ICGV15028, and IS-07947 and lowest in ICGV13851, ICGV1315, and ICGV13987 (Table 5). Seed Zn concentration of the groundnut grain was significantly higher in cultivar YENYAWOSO, followed by ICGV13842 and 12CS116, and lowest in ICGV1315, ICGV13987, and ICGV14849 (Table 5). The Mn concentration in groundnut grain was highest in genotype ICGV14876, followed by ICGV13848, CHINESE, and ICGV13876, and lowest in ICGV1315, ICGV15033, and ICGV15028 (Table 5). The concentration of B was relatively higher in cultivars IS-07273, IS-07947, and ICGV15028 (Table 5).
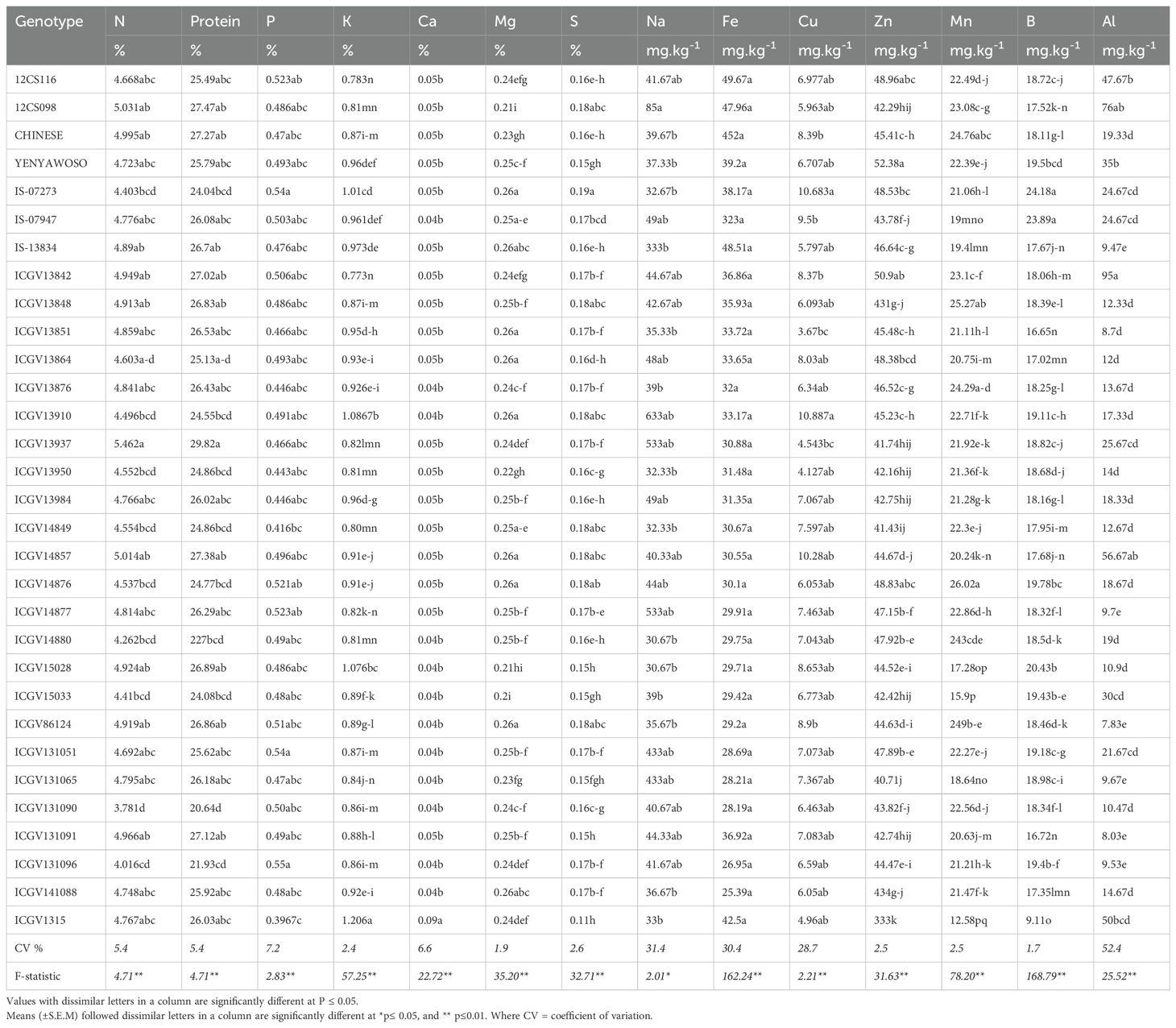
Table 5. Mineral accumulation in the grain of field-grown groundnut genotypes planted at Bronkhorspruit, South Africa, in 2019.
Discussion
Low soil N fertility is a major factor affecting agricultural yields in sub-Saharan Africa. The selection and incorporation of symbiotically superior grain legumes into cropping systems can improve soil N fertility and increase crop yields. In this study, the 15N and 13C natural abundance technique was used to evaluate N contribution and plant water relations of 30 groundnut genotypes grown at Bleskbokfontein farm, near Bronkhorspruit in Mpumalanga, South Africa. The results revealed differences in plant growth, symbiotic performance, grain mineral accumulation, and shoot δ13C (Mohammed et al., 2021, 2022; Ngwenya et al., 2024; Samago and Dakora, 2024). There were also differences in root nodulation among the groundnut genotypes. For example, genotype IS-07947 produced greater shoot biomass from increased nodulation, which resulted in higher N concentration and N content and a greater amount of N-fixed (Table 2). In contrast, genotypes 12CS116 and CHINESE performed poorly in terms of plant growth, N content, and amount of N-fixed, indicating genotypic differences in plant growth and symbiotic performance among the 30 groundnut genotypes. However, these differences could also be attributed to erratic rainfall and intermittent drought (Table 2). Furthermore, 22 of the 30 groundnut cultivars exhibited greater biomass accumulation. As a result, the N level was higher in 26 of the 30 genotypes (Table 2). Possibly due to the poor rainfall, all 30 genotypes (except for ICGV13984) showed markedly higher shoot δ15N values, and hence lower %Ndfa values. Genotype IS-07273 was an exception as it obtained the most N from symbiosis and still took up higher amounts of soil N (Table 2). Moreover, 17 of the 30 groundnut genotypes produced a grain yield of around 300 kg ha-1 (Table 2), which was quite low due to poor rainfall. The low grain yield and impaired symbiotic performance found in this study contrast with the findings by Mokgehle et al. (2014) where rainfall was optimal.
The shoot concentration of C in plants is directly linked to the photosynthetic activity in leaves. In this study, the C concentration of groundnut shoots ranged from 34.6% in genotype ICGV15033 to 40.6% in ICGV14849 (Table 4), levels similar to those reported by Sprent et al. (1996) for nodulated legumes. Although genotypes ICGV86124, ICGV14849, ICGV131065, ICGV13937, and 12CS098 exhibited the highest C concentrations, they did not produce the highest C content due to very low dry matter (Table 4). The groundnut genotypes that produced the most C content also recorded the highest biomass (Table 4), which was consistent with previous reports (Oteng-Frimpong and Dakora, 2019; Taiz and Zeiger, 2002).
The C/N ratio of N2-fixing species tends to be less than 24 g.g-1 while non-legumes have C/N ratios greater than 24 g.g-1 (Hobbie Macko & Shugart, 1998). In this study, the C/N ratios ranged from 17.2 g.g-1 in genotype YENYAWOSO to 22.0 g.g-1 in genotype ICGV14876, which are values much lower than 24 g.g-1 (Table 4). Two groundnut genotypes (namely, ICGV131090 and ICG13984) showed an increase in C/N ratios as the δ15N levels decreased, indicating a relationship between C and N in symbiotic species (Table 4).
Shoot δ 13C is often considered an integrated measure of water-use efficiency in C3 species, which include legumes such as groundnut (Yoneyama et al., 1998). The δ13C of C3 plant species has therefore been used as an indicator of WUE (Farquhar et al., 1989). Here, the higher the δ13C (i.e., less negative or lower discrimination), the greater the WUE, whilst the lower the 13C (i.e., more positive or high discrimination), the lower the WUE (Muhaba and Dakora, 2020). In this regard, genotypes YENYAWOSO, ICGV13848, ICGV13851, ICGV15033, and ICGV131065 showed less 13C discrimination (i.e., less negative δ13C value) and therefore had much greater WUE (Table 4).
The 30 field-grown groundnut genotypes in this study were evaluated for grain protein content. The results revealed marked differences in the levels of protein in groundnut grain. Seed protein levels ranged from 20.6% for genotype ICGV131090 to 29.8% in genotype ICGV13937, with five other genotypes recording approximately 27% grain protein, levels consistent with previous reports for protein in groundnut (Namrata et al., 2016; Dhakar, 2015; Janila et al., 2016; Toomer, 2018). As expected, the genotypes with the highest shoot %N accumulated the most protein in their grain, which is an indication of a direct link between shoot %N and protein level in groundnut grain (Dakora and Belane, 2019). The seeds of N2-fixing legumes, such as groundnut, are rich in N due to the species’ ability to reduce N2 into NH3 and subsequently into nitrogenous solutes for plant use (Belane et al., 2014).
In this investigation, there were significant differences in macro- and micronutrient (P, K, Ca, Mg, S, Fe, Cu, Zn, Mn, and B) uptake and accumulation in the grain. For example, Fe concentration was highest in genotypes 12CS116 (49.6 mg.kg-1) and IS-07273 (48.5 mg.kg-1), followed by 12CS098 (47.9 mg.kg-1), CHINESE (45 mg.kg-1), ICGV1315 (42 mg.kg-1), YENYAWOSO (39.2 mg.kg-1), IS-07273 (38.2 mg.kg-1), and ICGV141088 (25.4 mg.kg-1). However, the grain concentrations of the macronutrients P, K, Ca, Mg, and S were low when compared to the micronutrients (Table 5). Grain P concentration was highest in nine of the 30 groundnut genotypes. However, all 30 groundnut genotypes showed high levels of Zn in edible groundnut seed. However, Mn concentration was highest in genotype ICGV14876 (26.0 mg.kg-1) and lowest in ICGV15033 (15.9 mg.kg-1). While genotype 12CS098 recorded relatively higher Na concentration in the grain (85 mg.kg-1), genotypes ICGV14880 (30.7 mg.kg-1) and ICGV15028 (30.7 mg.kg-1) showed the lowest Na. Genotype IS-07273 was consistent in recording higher accumulation of P (0.54%), Mg (0.26%), S (0.19%), Cu (10.68%), and B (24.18 mg.kg-1) in its grain (Gunununu and Dakora, 2024; Table 6). The greater uptake of P, Mg, S, Cu, and B and their accumulation in the grain would no doubt enhance the nutritional quality of groundnut seeds relative to the other genotypes, with implications for human nutrition/health. Furthermore, genotypes IS-07273, ICGV13842, ICGV13864, ICGV13910, ICGV14880, and ICGV13876, which recorded higher mineral concentrations in the grain, also fixed the most N in shoots. This is evidenced by the strong correlation found between the amount of N-fixed and K, Na, B, Cu, Zn, and Mn in genotype ICGV13910 and the positive correlation obtained between N-fixed and S, nodule number and Ca, %N and P, and %N vs. K in genotype IS-07273 (Table 6). An earlier report by Belane et al. (2014) found a strong relationship between N2-fixing efficiency and mineral accumulation in nodulated cowpea by showing that rhizobia with higher symbiotic efficiency induced greater mineral accumulation in shoots, while strains with low N2-fixing ability elicited reduced mineral accumulation. In this study, the high-fixing groundnut genotype ICGV13910 also accumulated significantly increased levels of K, Na, Zn, Cu, Mn, and B in the grain, in the same manner that the high-fixing genotype IS-07273 also showed greater concentrations of Ca, P, K, and S in grain (Table 5). As a result, K, Na, Cu, Zn, Mn, and B were strongly correlated with the amount of N-fixed in genotype ICGV13910, while Ca, P, K, and S were also positively correlated with symbiotic parameters (nodule number, N-fixed and %N) in genotype IS-07273 (Table 6). These results are therefore consistent with the report by Belane et al. (2014), which indicates that this relationship between N2 fixing and mineral accumulation is not unique to cowpea. Taken together, genotype ICGV13910 and IS-07273 were high in N2-fixation, which also correlated well with macro- and micronutrients in their grain, and therefore have huge potential for inclusion in breeding programs. While the mechanisms underlying the increase in mineral accumulation with high N2 fixation remain unknown, there are many ways that symbiotic rhizobia can increase mineral supply to their host plants. For example, rhizobia are capable of solubilizing bound-P for use by their host plants and producing siderophores for increased Fe availability to plants (Dakora et al., 2024). Rhizobial bacteria can also synthesize and release plant hormones, including indole acetic acid (IAA), abscisic acid (ABA), lumichrome, riboflavin, cytokinins, and gibberellins that promote root proliferation and root hair formation for greater nutrient uptake (Dakora, 2015).
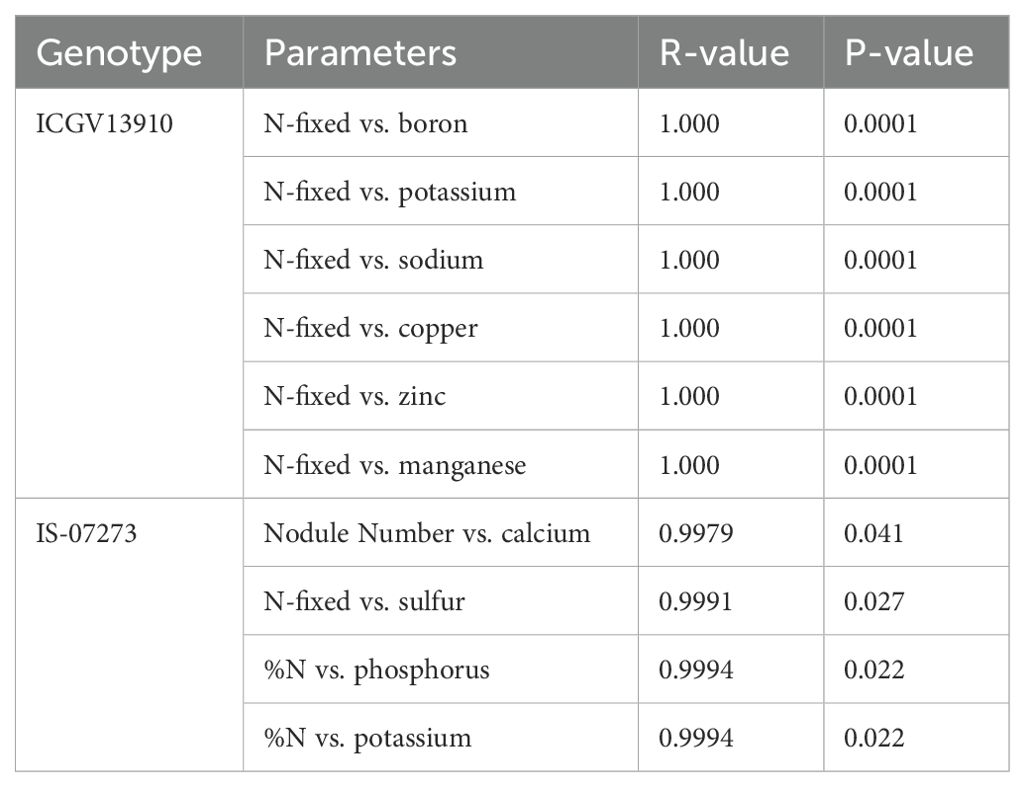
Table 6. Correlation analysis of symbiotic parameters (N-fixed,nodule number, and %N) and grain mineral accumulation in two groundnut genotypes.
Conclusion
In conclusion, we found marked differences in symbiotic performance between and among 30 groundnut genotypes, with IS-07273 and ICGV13910 exhibiting greater symbiotic dependency on N2 fixation for their N nutrition, and higher amounts of N-fixed. These two high N2-fixing symbioses (IS-07273 and ICGV13910) also accumulated significantly higher levels of K, Na, Zn, Cu, Mn, and B in their grain. As a result, there were strong positive correlations between the amount of N-fixed and K, Na, B, Cu, Zn, and Mn for genotype ICGV13910. Genotype IS-07273 also showed significant correlations between N-fixed and S, %N and P, %N and K, and nodule number and Ca. The genotypes with the highest shoot %N accumulated the most protein in their grain. Of the 30 groundnut cultivars tested in the field, YENYAWOSO, ICGV13848, ICGV13851, ICGV15033, and ICGV131065 showed greater shoot δ13C values, and hence higher water-use efficiency. The high N2 fixation genotypes ICGV13910 and IS-07273 correlated positively with macronutrient and micronutrient concentrations in the grain, indicating their potential for use in breeding programs to enhance nutritional security in groundnut.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
TN: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. RF: Resources, Supervision, Visualization, Writing – review & editing. FD: Conceptualization, Funding acquisition, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors appreciate support from Dr Richard Oteng-Frimpong of the CSIR-Savanna Agricultural Research Institute at Nyankpala, Ghana for the supply of groundnut genotypes. We are also grateful to the Masombuka Farms for the provision of site for the experimental trial. Many thanks to the South African Research Chair in Agrochemurgy and Plant Symbioses, the National Research Foundation, and the Tshwane University of Technology (TUT) for financial support to FD’s research, and for a competitive TUT Doctoral Bursary to NTY.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ataro A., Mccrindle R. I., Botha B. M., Mccrindle C. M. E., and Ndibewu P. P. (2008). Quantification of trace elements in raw cow’s milk by inductively coupled plasma mass spectrometry (ICP-MS). Food Chem. 111, 243–248. doi: 10.1016/j.foodchem.2008.03.056
Ayenan M. A. T. and Ezin V. A. (2016). Potential of Kersting’s groundnut [Macrotyloma geocarpum (Harms) Maréchal & Baudet] and prospects for its promotion. Agric. Food Secur. 5, 1–9. doi: 10.1186/s40066-016-0058-4
Bado B. V., Bationo A., and Cescas M. P. (2006). Assessment of cowpea and groundnut contributions to soil fertility and succeeding sorghum yields in the Guinean savannah zone of Burkina Faso (West Africa). Biol. Fertility Soils 43, 171–176. doi: 10.1007/s00374-006-0076-7
Belane A. K. and Dakora F. D. (2011). Photosynthesis, symbiotic N and C accumulation in leaves of 30 nodulated cowpea genotypes grown in the field at Wa in the Guinea savanna of Ghana. Field Crops Res. 124, 279–287. doi: 10.1016/j.fcr.2011.07.014
Belane A. K., Pule-Meulenberg F., Makhubedu T. I., and Dakora F. D. (2014). Nitrogen fixation and symbiosis-induced accumulation of mineral nutrients by cowpea (Vigna unguiculata L. Walp.). Crop Pasture Sci. 65, 250–258. doi: 10.1071/CP13283
Broughton W. J., Hernández G., Blair M., Beebe S., Gepts P., and Vanderleyden J. (2003). Beans (Phaseolus spp.)–model food legumes. Plant Soil 252, 55–128. doi: 10.1023/A:1024146710611
Cernusak L. A., Ubierna N., Winter K., Holtum J. A., Marshall J. D., and Farquhar G. D. (2013). Environmental and physiological determinants of carbon isotope discrimination in terrestrial plants. New Phytol. 200, 950–965. doi: 10.1111/nph.2013.200.issue-4
Chen F., D’Auria J. C., Tholl D., Ross J. R., Gershenzon J., Noel J. P., et al. (2003). An Arabidopsis thaliana gene for methylsalicylate biosynthesis, identified by a biochemical genomics approach, has a role in defense. Plant J. 36, 577–588. doi: 10.1046/j.1365-313X.2003.01902.x
Craig H. (1957). Isotopic standards for carbon and oxygen and correction factors for mass-spectrometric analysis of carbon dioxide. Geochimica cosmochimica Acta 12, 133–149. doi: 10.1016/0016-7037(57)90024-8
Dakora F. D. (2015). Lumichrome: a bacterial signal molecule influencing plant growth. Biol. Nitrogen Fixation, 389–396. doi: 10.1002/9781119053095.ch38
Dakora F. D. and Belane A. K. (2019). Evaluation of protein and micronutrient levels in edible cowpea (Vigna Unguiculata L. Walp.) leaves and seeds. Front. Sustain. Food Syst. 3, 70. doi: 10.3389/fsufs.2019.00070
Dakora F. D., Li H., and Zhao J. (2024). Exploring the impacts of elevated CO2 on food security: nutrient assimilation, plant growth, and crop quality. Engineering. 44, 234–244. doi: 10.1016/j.eng.2024.12.018
Dakora F. D. and Keya S. O. (1997). Contribution of legume nitrogen fixation to sustainable agriculture in Sub-Saharan Africa. Soil Biol. Biochem. 29, 809–817. doi: 10.1016/S0038-0717(96)00225-8
de Paiva Gouvêa L., Caldeira R. F., de Lima Azevedo T., Antoniassi R., Galdeano M. C., Felberg I., et al. (2024). Nutritional properties of common bean protein concentrate compared to commercial legume ingredients for the plant-based market. Curr. Res. Food Sci. 9, 100937. doi: 10.1016/j.crfs.2024.100937
Dhakar T. R. (2015). Character Association and Genetic Divergence in Groundnut (Arachis hypogaea L.) Doctoral dissertation (Udaipur: MPUAT).
Farquhar G. D., Ehleringer J. R., and Hubick K. T. (1989). Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Biol. 40, 503–537. doi: 10.1146/annurev.pp.40.060189.002443
Gunununu R. P. and Dakora F. D. (2024). Variation in grain mineral concentrations of 63 common bean genotypes planted at malkerns, Eswatini. Africa. Legume Sci. 6, e70007. doi: 10.1002/leg3.70007
Hlanga N. C., Modi A. T., and Mathew I. (2021). Evaluating nutritional content among Bambara groundnut lines. J. Food Composition Anal. 102, 104053. doi: 10.1016/j.jfca.2021.104053
Ibañez F., Taurian T., Angelini J., Tonelli M. L., and Fabra A. (2008). Rhizobia phylogenetically related to common bean symbionts Rhizobium giardinii and Rhizobium tropici isolated from peanut nodules in Central Argentina. Soil Biol. Biochem. 40, 537–539. doi: 10.1016/j.soilbio.2007.08.017
Ibañez F., Angelini J., Taurian T., Tonelli M. L., and Fabra A. (2009). Endophytic occupation of peanut root nodules by opportunistic Gammaproteobacteria. Syst. Appl. Microbiol. 321, 49–55. doi: 10.1016/j.syapm.2008.10.001
Ikujenlola A. V., Osungbade O. R., and Gbadamosi S. O. (2022). Bioactive and chemical properties of Kersting’s groundnut proteins. Food Hydrocolloids Health 2, 100043. doi: 10.1016/j.fhfh.2021.100043
Janila P., Nigam S. N., Pandey M. K., Nagesh P., and Varshney R. K. (2013). Groundnut improvement: use of genetic and genomic tools. Front. Plant Sci. 4, 23. doi: 10.3389/fpls.2013.00023
Janila P., Variath M. T., Pandey M. K., Desmae H., Motagi B. N., Okori P., et al. (2016). Genomic tools in groundnut breeding program: status and perspectives. Front. Plant Sci. 7, 289. doi: 10.3389/fpls.2016.00289
Kumawat A., Bamboriya S. D., Meena R. S., Yadav D., Kumar A., Kumar S., et al. (2022). “Legume-based inter-cropping to achieve the crop, soil, and environmental health security,” in Advances in legumes for sustainable intensification (Academic Press), 307–328.
Mapope N. and Dakora F. D. (2016). N2 fixation, carbon accumulation, and plant water relations in soybean (Glycine max L. Merrill) varieties sampled from farmers’ fields in South Africa, measured using 15N and 13C natural abundance. Agriculture Ecosystems Environ. 221, 174–186. doi: 10.1016/j.agee.2016.01.023
Mariotti A., Germon J. C., Hubert P., Kaiser P., Letolle R., Tardieux A., et al. (1981). Experimental determination of nitrogen kinetic isotope fractionation: some principles; illustration for the denitrification and nitrification processes. Plant Soil 62, 413–430. doi: 10.1007/BF02374138
Mazahib A. M., Nuha M. O., Salawa I. S., and Babiker E. E. (2013). Some nutritional attributes of bambara groundnut as influenced by domestic processing. Int. Food Res. J. 20, 1165. Available online at: http://www.ifrj.upm.edu.my
Mohale K. C., Belane A. K., and Dakora F. D. (2014). Symbiotic N nutrition, C assimilation, and plant water use efficiency in Bambara groundnut (Vigna subterranea L. Verdc) grown in farmers’ fields in South Africa, measured using 15N and 13C natural abundance. Biol. Fertil. Soils 50, 307–319. doi: 10.1007/s00374-013-0841-3
Mohammed M., Mbah G. C., Sowley E. N., and Dakora F. D. (2021). Bradyrhizobium inoculation of field-523 grown kersting’s groundnut [Macrotyloma geocarpum (Harms) marechal & Baudet] increased grain yield and N2 fixation, measured using the ureide, and 15N natural abundance techniques. Front. 525 Sustain. Food Syst. 5, 672247. doi: 10.3389/fsufs.2021.672247
Mohammed M., Mbah G. C., Sowley E. N., and Dakora F. D. (2022). Cowpea genotypic variations in N2 fixation, Water Use Efficiency (δ13C), and grain yield in response to bradyrhizobium inoculation in the field, 520 measured using xylem N solutes, 15N, and 13C natural abundance. Front. Agron. 4, 764070. doi: 10.3389/fagro.2022.764070
Mokgehle S. N., Dakora F. D., and Mathews C. (2014). Variation in N2 fixation and N contribution by 25 groundnut (Arachis hypogaea L.) varieties grown in different agro-ecologies, measured using 15N natural abundance. Agriculture ecosystems Environ. 195, 161–172. doi: 10.1016/j.agee.2014.05.014
Muhaba S. K. and Dakora F. D. (2020). Symbiotic performance, shoot biomass and water-use efficiency of 527 three groundnut (Arachis hypogaea L.) genotypes in response to phosphorus supply under field conditions in Ethiopia. Front. Agric. Sci. Eng. 7, 455–466. doi: 10.15302/J-FASE-2020354
Namrata M., Sharma H., Ranwah B. R., and Bisen P. (2016). Variability assessment and path coefficient analysis in groundnut (Arachis hypogaea L.) genotypes in sub-humid southern plains of Rajasthan. Trends Biosci. 9, 642–646.
Ngwenya Z. D., Mohammed M., and Dakora F. D. (2024). Monocropping and Intercropping of Maize with Six Food Legumes at Malkerns in Eswatini: Their Effects on Plant Growth, Grain Yield and N2 Fixation, Measured using the 15N Natural Abundance and Ureide Techniques. Symbiosis 92, 257–269. doi: 10.1007/s13199-024-00971-x
Nyambok E. O. (2011). Chemical contaminants in Chinese aquaculture imports, US import security, and exposure assessment amongst vulnerable sub-populations (Doctoral dissertation). Kansas, United States: Kansas State University.
Nyemba R. C. and Dakora F. D. (2010). Evaluating N2 fixation by food grain legumes in farmers’ fields in three agro-ecological zones of Zambia, using 15N natural abundance. Biol. Fertil. Soils 46, 461–470. doi: 10.1007/s00374-010-0451-2
Obala J., Saxena R. K., Singh V. K., Kale S. M., Garg V., Kumar C. S., et al. (2020). Seed protein content and its relationships with agronomic traits in pigeonpea is controlled by both main and epistatic effects QTLs. Sci. Rep. 10, 214. doi: 10.1038/s41598-019-56903-z
Oso A. A. and Ashafa A. O. (2021). “Nutritional composition of grain and seed proteins,” in Grain and Seed Proteins Functionality (Intech Open).
Oteng-Frimpong R. and Dakora F. D. (2018). Selecting elite groundnut (Arachis hypogaea L) genotypes for symbiotic N nutrition, water-use efficiency and pod yield at three field sites, using 15 N and 13 C natural abundance. Symbiosis 75, 229–243. doi: 10.1007/s13199-017-0524-1
Oteng-Frimpong R. and Dakora F. D. (2019). Multienvironment testing for trait stability and G× E interaction on N2 Fixation, plant development, and water-use efficiency of 21 elite groundnut (Arachis hypogaea L.) genotypes in the Guinea Savanna. Front. Plant Sci. 10, 446640. doi: 10.3389/fpls.2019.01070
Pampa J. M., Ngwenya Z. D., Mpai T., and Dakora F. D. (2023). An assessment of symbiotic N nutrition in 545 species of the genus Aspalathus endemic to the Cape Floristic Region of South Africa. South Afr. J. Bot. 158, 312–318. doi: 10.1016/j.sajb.2023.05.023
Pausch R. C., Mulchi C. L., Lee E. H., and Meisinger J. J. (1996). Use of 13C and 15N isotopes to investigate O3 effects on C and N metabolism in soybeans. Part II. Nitrogen uptake, fixation, and partitioning. Agriculture ecosystems Environ. 60, 61–69. doi: 10.1016/S0167-8809(96)01062-6
Samago T. Y. and Dakora F. D. (2024). Combined use of Rhizobium inoculation and low phosphorus 553 application increased plant growth, root nodulation and grain yield of common bean (Phaseolus vulgaris) in Ethiopia. Front. Agric. Sci. Eng. 12(1). doi: 10.15302/J-FASE-2024556
Saxena K. B., Faris D. G., Singh U., and Kumar R. V. (1987). Relationship between seed size and protein content in newly developed high protein lines of pigeonpea. Plant Foods Hum. Nutr. 36, 335–340.
Shearer G. and Kohl D. H. (1986). N2-fixation in field settings: estimations based on natural 15N abundance. Funct. Plant Biol. 13, 699–756. doi: 10.1071/PP9860699
Sprent J. I., Geoghegan I. E., Whitty P. W., and James E. K. (1996). Natural abundance of 15 N and 13 C in nodulated legumes and other plants in the cerrado and neighbouring regions of Brazil. Oecologia 105, 440–446. doi: 10.1007/BF00330006
Syed F., Arif S., Ahmed I., and Khalid N. (2021). Groundnut (peanut) (Arachis hypogaea). Oilseeds: Health attributes Food Appl., 93–122. doi: 10.1007/978-981-15-4194-0_4
Taiz L. and Zeiger E. (2002). Photosynthesis: physiological and ecological considerations. Plant Physiol. 9, 172–174. doi: 10.1086/394162
Taurian T., Ibañez F., Fabra A., and Aguilar O. M. (2006). Genetic diversity of rhizobia nodulating Arachis hypogaea L. in central Argentinean soils. Plant Soil 282, 41–52. doi: 10.1007/s11104-005-5314-5
Toomer O. T. (2018). Nutritional chemistry of the peanut (Arachis hypogaea). Crit. Rev. Food Sci. Nutr. 58, 3042–3053. doi: 10.1080/10408398.2017.1339015
Unkovich M., Herridge D.A.V.I.D., Peoples M., Cadisch G., Boddey B., Giller K., et al. (2008). Measuring plant-associated nitrogen fixation in agricultural systems (Australian Centre for International Agricultural Research (ACIAR).
Vieira R. F., C Mendes I., Reis-Junior F. B., and Hungria M. (2010). Symbiotic nitrogen fixation in tropical food grain legumes: current status (Springer Vienna), 427–472.
Xu Y., Cartier A., Obielodan M., Jordan K., Hairston T., Shannon A., et al. (2016). Nutritional and anti-nutritional composition, and in vitro protein digestibility of Kabuli chickpea (Cicer arietinum L.) as affected by differential processing methods. J. Food Measurement Characterization 10, 625–633. doi: 10.1007/s11694-016-9346-8
Yang J. K., Xie F. L., Zou J., Zhou Q., and Zhou J. C. (2005). Polyphasic characteristics of bradyrhizobia isolated from nodules of peanut (Arachis hypogaea) in China. Soil Biol. Biochem. 37, 141–153. doi: 10.1016/j.soilbio.2004.06.016
Yi-Shen Z., Shuai S., and FitzGerald R. (2018). Mung bean proteins and peptides: Nutritional, functional and bioactive properties. Food Nutr. Res. 62, 10–29219. doi: 10.29219/fnr.v62.1290
Yoneyama T., Fujihara S., and Yagi K. (1998). Natural abundance of 15N in amino acids and polyamines from leguminous nodules: unique 15N enrichment in homospermidine. J. Exp. Bot. 49, 521–526. doi: 10.1093/jxb/49.320.521
Zarkadas C. G., Gagnon C., Gleddie S., Khanizadeh S., Cober E. R., and Guillemette R. J. (2007). Assessment of the protein quality of fourteen soybean [Glycine max (L.) Merr.] cultivars using amino acid analysis and two-dimensional electrophoresis. Food Res. Int. 40, 129–146. doi: 10.1016/j.foodres.2006.08.006
Keywords: N2-fixation, grain yield, soil N uptake, seed protein content, grain mineral content
Citation: Ngmenzuma TY, Oteng-Frimpong R and Dakora FD (2025) N2 fixation, grain mineral accumulation, and water-use efficiency in 30 field-grown groundnut (Arachis hypogaea L.) genotypes in Mpumalanga, South Africa, measured using 15N and 13C natural abundance techniques. Front. Agron. 7:1483741. doi: 10.3389/fagro.2025.1483741
Received: 20 August 2024; Accepted: 02 May 2025;
Published: 13 June 2025.
Edited by:
Naser A. Anjum, Aligarh Muslim University, IndiaReviewed by:
John M. Maingi, Kenyatta University, KenyaVirender Sardana, Punjab Agricultural University, India
Copyright © 2025 Ngmenzuma, Oteng-Frimpong and Dakora. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Felix Dapare Dakora, RGFrb3JhRkRAdHV0LmFjLnph
 Titus Y. Ngmenzuma
Titus Y. Ngmenzuma Richard Oteng-Frimpong
Richard Oteng-Frimpong Felix Dapare Dakora
Felix Dapare Dakora