- 1ICAR-Indian Grassland and Fodder Research Institute, Jhansi, Uttar Pradesh, India
- 2ICAR-Central Agroforestry Research Institute, Jhansi, Uttar Pradesh, India
- 3ICAR-Research Complex for North Eastern Hill Region, Lembucherra, Tripura, India
- 4ICAR-Research Complex for North Eastern Hill Region, Umiam, Meghalaya, India
- 5ICAR-Central Coastal Agricultural Research Institute, Old Goa, Goa, India
- 6ICAR-Indian Institute of Soil Science, Bhopal, Madhya Pradesh, India
- 7ICAR-Indian Institute of Soil and Water Conservation, Research Centre, Chandigarh, India
Understanding the link between tree root architecture and organic carbon dynamics is critical for enhancing carbon sequestration in semi-arid regions. This study, conducted from 2017 to 2019 in central India, evaluated the root structure and carbon sequestration potential of three tree species: Neolamarckia cadamba (Kadam), Leucaena leucocephala (Subabul), and Melia dubia (Malabar neem). The species exhibited distinct root architectures: Subabul had a symmetric, sparse root system; Kadam had moderately dense roots; and Malabar neem developed a compact and massive root system. The highest root density was recorded in the 0–30 cm topsoil layer near the collar region. Primary roots initially grew vertically (0.15–0.30 m), then extended horizontally, with Malabar neem showing the widest lateral spread (up to 4.4 m). Secondary roots displayed greater angular spread than tertiary and quaternary roots. Lateral root pruning, recommended after the first two years, could enhance resource use efficiency and improve understory crop performance in agroforestry systems. Malabar neem demonstrated significantly higher carbon sequestration potential, storing 25.64 Mg C ha-¹ at three years—2.96 to 3.86 times greater than Subabul (8.62 Mg C ha-¹) and Kadam (6.62 Mg C ha-¹). Annual sequestration rates ranged from 2.20 to 2.87 Mg C ha-¹ yr-¹. Aboveground biomass contributed 80.4–84.3% of total carbon stocks, with belowground biomass contributing 15.7–19.6%. At a planting density of 500 trees ha-¹, Malabar neem achieved the highest CO2-equivalent sequestration (94.09 Mg CO2e ha-¹). These findings highlight Malabar neem-based agroforestry as a viable strategy for restoring degraded lands while improving carbon storage and climate resilience in semi-arid ecosystems.
Introduction
Agroforestry is a holistic and multifunctional land use system that has been widely acknowledged for enhancing both livelihood security and environmental sustainability, particularly in developing countries such as India (Jemal et al., 2018; Kmoch et al., 2018; Ansari et al., 2022; Jinger et al., 2023). Its perennial component play a vital role in combating climate change by absorbing atmospheric carbon dioxide, regulating temperatures, conserving soil and water, and supporting biodiversity. They act as natural carbon sinks, reduce the effects of extreme weather, and provide renewable resources, making them essential for both climate mitigation and adaptation. By integrating trees with crops and/or livestock, agroforestry systems not only capture atmospheric CO2 but also improve soil health, reduce erosion, and increase farm productivity—making them powerful tools for climate change mitigation and sustainable land management. Few recent studies have highlighted the carbon sequestration potential of the agroforestry systems in both soil and biomass (Bettles et al., 2021; Siarudin et al., 2021; Singh et al., 2024). As India has taken a pioneering role in promoting agroforestry by becoming the first country to introduce a National Agroforestry Policy in 2014. This policy has since been integrated into several national initiatives, including the National Bamboo Mission, National Biofuel Policy, Green India Mission, National Horticulture Mission, and the National Mission on Sustainable Agriculture. As of 2023, approximately 28.42 million hectares of land in India are under agroforestry (Ram et al., 2023). Moreover, land suitability assessments reveal that 32.8%, 40.0%, and 11.7% of the country’s total land area are classified as highly, moderately, and marginally suitable, respectively, for the expansion of agroforestry (Ahmad et al., 2019). Evidently, the strategic promotion of agroforestry could play a crucial role in achieving India’s target of 33% forest cover (Dhyani et al., 2016; Sahoo and Wani, 2019; Dhyani et al., 2021).
To ensure the success of agroforestry systems, it is essential to evaluate the structural and developmental characteristics of tree root systems, as they play a vital role in enhancing and optimizing overall system productivity (Arunkumar and Chauhan, 2018). Root architecture studies are crucial for selecting tree species that can effectively survive, establish, and grow under challenging agroclimatic and edaphic conditions, as they influences a plant’s ability to access water and nutrients, thereby enhancing resilience and sustainability in forestry and agroforestry systems (Ma et al., 2024). In agroforestry, understanding root architecture helps reduce competition with crops, improve soil health, and enhance carbon sequestration, contributing to sustainable and climate-resilient land use. Minimizing competition between trees and associated agricultural components such as seasonal crops, semi-perennial fruit trees, or fodder is critical for developing compatible agroforestry models. In this regard, root system manipulation becomes a key intervention to reduce competition for belowground resources such as water and nutrients. Despite of having a crucial role of root architecture in the growth and productivity of a system, no enough studies have been conducted on the topic so far therefore a systematic studies on structure of tree roots urgently required to unveil important insights into belowground carbon sequestration and other ecosystem services. Understanding root architecture dynamics can aid in the selection of suitable tree species for designing productive and resilient agroforestry systems. Such knowledge allows for the targeted exploitation and manipulation of root traits to improve both tree and crop yields, thereby contributing to optimized land use (Herder et al., 2010; Kaushal et al., 2019).
The commercial success of fast-growing tree species such as Populus and Eucalyptus in the states of Haryana and Punjab has sparked growing interest among farmers, primarily due to their short rotation periods and early economic returns (Chavan and Dhillon, 2019). These species provide a sustainable alternative to low-carbon land use systems, offering high yields of renewable woody biomass along with multiple ecosystem benefits (Bredemeier et al., 2015; Jinger et al., 2024). Tree species such as Neolamarckia cadamba (kadam), Leucaena leucocephala (subabul), and Melia dubia (malabar neem) have demonstrated strong potential for commercial agroforestry in semi-arid regions (Chaturvedi et al., 2016; Handa et al., 2019; Jinger et al., 2024). These species not only meet the increasing demand for timber, pulpwood, fuelwood, and fodder, but also contribute to environmental restoration by conserving soil moisture, reducing erosion, restoring degraded lands, and sequestering significant amounts of atmospheric carbon dioxide—thereby improving overall farm income (Hombegowda et al., 2020; Ahlawat et al., 2024; Jinger et al., 2025). In addition, these multipurpose tree species can be effectively integrated with a wide range of food crops, vegetables, medicinal plants, fruit trees, and grasses, enhancing system diversity and resilience (Karmini and Karyati, 2017; Karthikeyan et al., 2018; Thakur et al., 2019). Planting such fast-growing tree species might not necessarily support carbon tunnel vision, as long as broader ecological and social impacts are also considered (Santiago and Doughty, 2018). Competition in agroforestry can lead to both positive and negative ecological consequences. While competition for resources like light, water, and nutrients may reduce the productivity of certain components, it can also drive niche differentiation and improve overall system efficiency. Properly managed competition can enhance biodiversity, promote soil health, and support ecosystem resilience. However, if unmanaged, it may lead to resource depletion and reduced yields of understory crops.
Interestingly, the semi-arid regions, which account for approximately 35–40% of India’s total geographical area, are typically characterized by low and erratic rainfall, high temperatures, and sparse vegetation cover (Kumar et al., 2021) where, soils are often shallow, low in organic carbon, and nutrient-deficient, making them highly susceptible to erosion and degradation (Jinger et al., 2022). Subsequently, degraded and wastelands are prevalent in these areas, where farmers largely rely on rainfed agriculture (Ghosh et al., 2021). Improving soil health and enhancing carbon sequestration in these vulnerable ecosystems is, therefore, an urgent priority. Against this backdrop, we hypothesize that the three fast-growing, multipurpose tree species—kadam, subabul, and malabar neem could serve as viable alternatives for restoring degraded lands by improving soil health and carbon sequestration potential. To test this hypothesis, the present study was conducted with the following objectives: (a) to assess the root system architecture and carbon sequestration potential of these species, and (b) to identify the most promising species for establishing climate-resilient agroforestry systems in the semi-arid tropics of India.
Materials and methods
Study site
The study was conducted in an area of 1.4 ha at research farm of ICAR-Central Agroforestry Research Institute (CAFRI), Jhansi, Uttar Pradesh, India during three consecutive years (2016–17 to 2018-19). The study site is geographically located between 25°30’25°32’ N latitude and 78°32’78°34’ E longitudes, and it is situated 272 m above mean sea level (Figure 1). The average annual rainfall of the region is around 867 mm with large temporal and spatial variability while the mean maximum temperature ranges from 23.5°C in January to 47.4°C in June, and mean minimum temperature ranges from 4.1°C in December to 27.2°C in June (Dev et al., 2020). The soil of the study site was a mixture of red and black soils with poor fertility. The initial (before study) soil pH, organic carbon (%), available N, P and K (kg ha-1) was 7.76, 0.48, 185.5, 8.46 and 172.3, respectively.
Figure 1. Map of India with Bundelkhand region of Uttar Pradesh (Green) and study locations (Light Yellow).
Experimental design and tree/crop management
With three replications, the experiment was conducted using a randomized block design (RBD) in 2016. The seedlings of kadam, malabar neem and subabul were planted at a spacing of 5.0 m (row to row) and 4.0 m (plant to plant) during August, 2016. Kadam and subabul plants were raised from seeds of local plus trees, whereas of malabar neem plants were raised from stem cuttings from local plus trees in the nursery and transplanted to the field after six months. The Vigna mungo (kharif) and Triticum aestivum (rabi) cropping system were intercropped with recommended practices during the study that ran from 2017 to 2019. For proper establishment of tree seedlings, approximately 50 liters of water were given every 15 days during the first four months. Three lifesaving irrigations were given annually during the summer season after establishment. In the wheat crop, five irrigations were given during the rabi season. To reduce crown competition, three species of trees were pruned by 50% every year (before sowing kharif and rabi crops).
Parameters studied
Root architecture parameters
In the winter season, when trees undergo dormancy, data on root architecture of three tree species was collected for three consecutive years (2017-2019). Considering a single plant as one replication, every year, three healthy, uniform trees of each species were uprooted and studied for root architecture, totaling nine trees per species. Selected trees of each species were manually excavated (Figure 2) and the root morphological structures of trees were exposed using hydraulic sluicing. Hydraulic sluicing was carried out downwards from the tree stump to ensure to expose sufficient depth of root system (Czernin and Phillips, 2005; Phillips et al., 2014; Marden et al., 2018). Root system parameters were measured by following procedures given by Czernin and Phillips (2005) and Marden et al. (2018). Before uprooting, aboveground growth parameters, such as tree height, collar diameter, diameter at breast height (DBH), and crown width, were measured using a Ravi Altimeter and a digital Vernier calliper. The uprooted root system was re-aligned into original positions and all the qualitative and quantitative parameters were recorded. The qualitative parameters measured were root symmetry, root distribution pattern, and general root morphology as per the (Czernin and Phillips, 2005; Phillips et al., 2014; Marden et al., 2018). Quantitative parameters included root system spread and depth, length, number, diameter, angle, and biomass of roots from different orders, as well as root:shoot ratio, root spread, and crown spread as per the (Czernin and Phillips, 2005; Phillips et al., 2014). The main root in Malabar neem was not distinct, so the most prominent central root was chosen as the main/tap root for recording various parameters in root architecture study. Secondary roots were designated for roots that originated from the main root/primary root (tap root), regardless of their size. Those that came from secondary and tertiary roots were labeled as tertiary and quaternary roots, respectively. Non-woody and white roots that come from woody secondary, tertiary, and quaternary roots with a diameter of less than 1 mm were deemed fine roots (Czernin and Phillips, 2005; Phillips et al., 2014). A digital Vernier caliper, measuring tape, and scale were used to measure quantitative parameters such as horizontal spread of roots, root system depth, mean root length, and diameter. Further, the angle at which a secondary root emerges from a primary root was recorded to determine the branching angle or root fork angle (Czernin and Phillips, 2005). The root system was compared by calculating root and shoot biomass, crown, and root spread among the three tree species. For above- and below-ground dry biomass estimates, the samples were oven dried at 70 ± 2°C till constant weight (Peng and Dang, 2003). Values of individual parameters were averaged to obtain the mean values.
Carbon stock parameters
The carbon stock (aboveground and belowground) was estimated by using the trees that were uprooted for root system architecture studies. Aboveground portions were divided into stem, leaves, and branches. The belowground portions were divided into tap/primary root, secondary, tertiary, quaternary roots, and fine roots. The fresh weight of all the tree components was taken separately in the field to avoid any variation in weight. Three samples with known fresh weights of all the tree components (species wise) were kept in an oven for drying at 70 ± 2°C till constant weight and then their oven dry weight was recorded. Carbon stock in the various parts of trees (dry biomass × 0.5) as well as their potential to mitigate carbon-dioxide (carbon stock × 3.67) was calculated (IPCC, 2006). All the tree parts were added to calculate the total carbon storage in biomass, which was then used to calculate carbon storage and carbon dioxide mitigation for each species on a per hectare basis.
Statistical analysis
Statistical analysis was carried out using ANOVA and t-test to determine the effects of year and species on above- and below-ground parameters as per Gomez and Gomez (1984). Data were analyzed using one factor analysis using IBM SPSS (v 25.0). Quantitative data pertaining to quaternary root properties were analyzed using a t-test (Gomez and Gomez, 1984). The mean values were compared at a 5% level of significance (p <0.05).
Results and discussion
Tree growth parameters
Most tree growth parameters exhibited a significant difference (p <0.05) in all the species. At three years of age, the tree species had a height range of 5.04 to 9.92 m, while the crown spread ranged from 3.88 to 4.51 m. Malabar neem showed the best growth performance, followed by subabul and kadam (Figure 3). The diameter at breast height (DBH) followed a similar trend. Kadam and Malabar neem had larger collar diameters than subabul. The studied species were growing at a faster rate than common tree species in the region, such as Azadirachta indica, Tectona grandis, Albizia procera, Dalbergia sissoo, Hardwickia binata, and others (Dagar and Tomar, 2002; Loushambam et al., 2017). This indicates their adaptability to the semi-arid environment of central India.
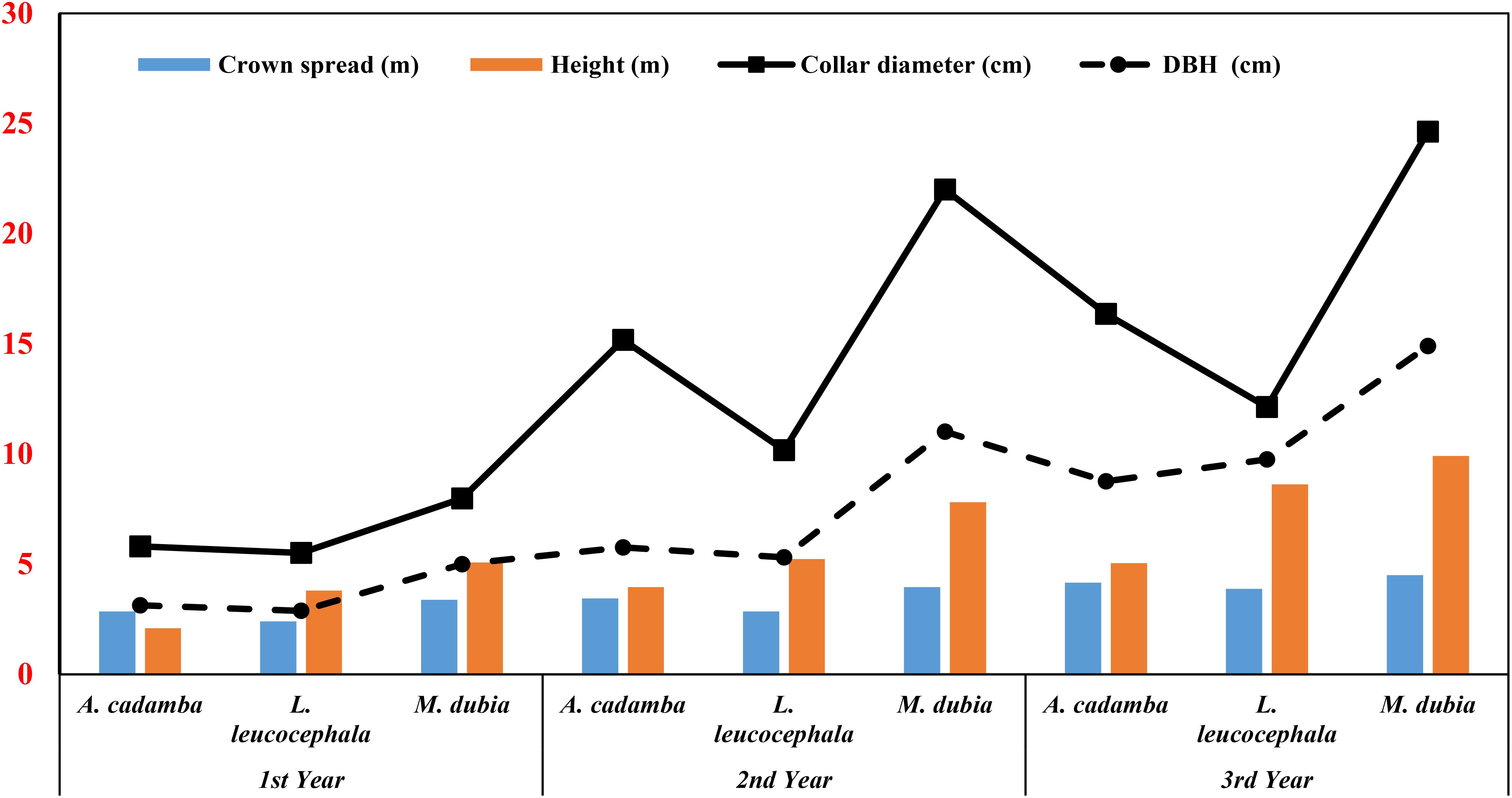
Figure 3. Increased pattern (yearly) in crown spread (m), height (m), collar diameter (cm) and DBH in three tree species.
Qualitative parameters of root system
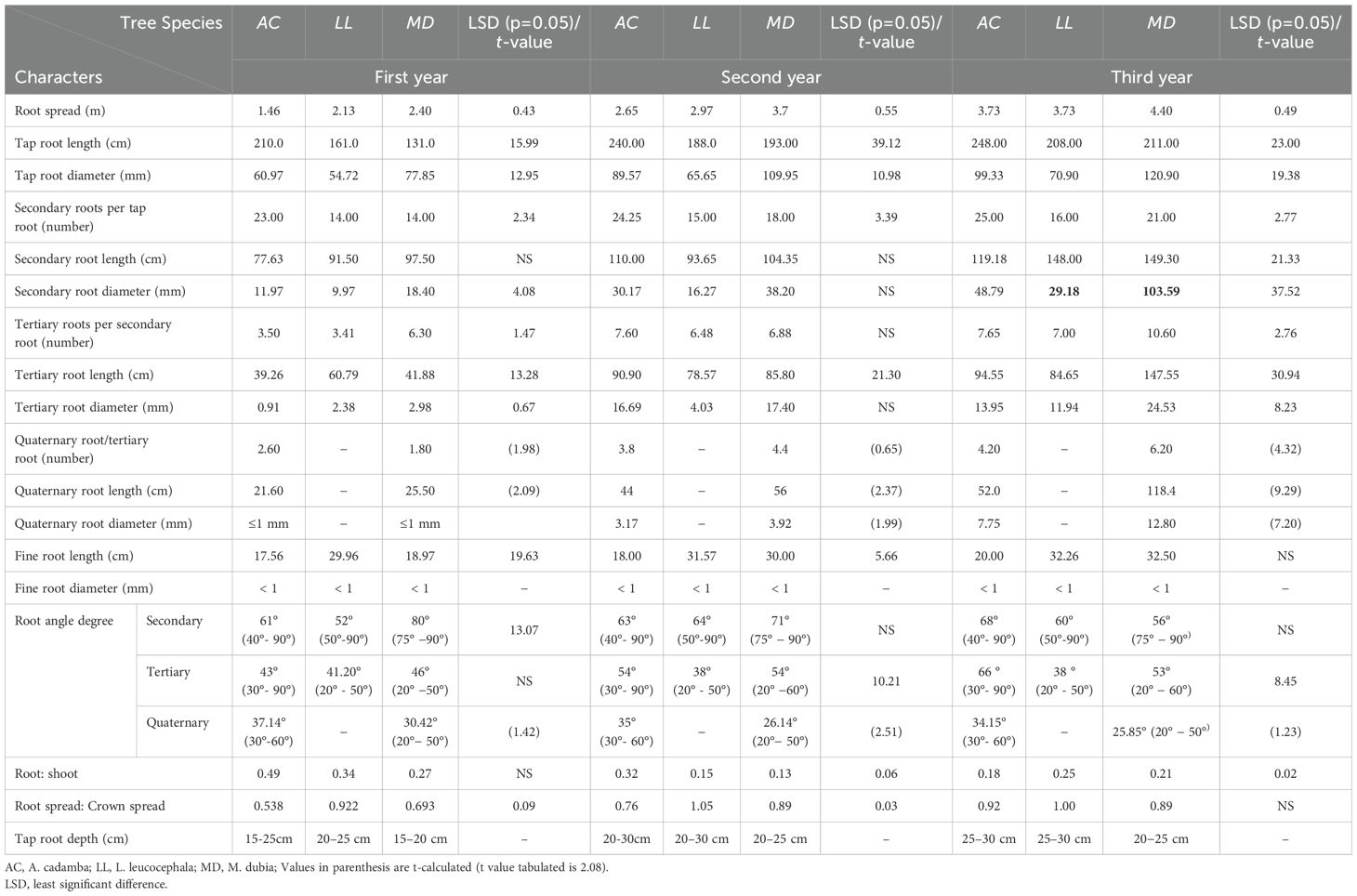
All three tree species had well-developed root systems with maximum root density found in the topsoil (0.30 m) and close to the collar region (Table 1). The root system was almost symmetric in terms of an even distribution of biomass around all axes and an almost equal root spread in the NS and EW directions in these tree species. Due to hard seed coat–induced dormancy and low germination rates, M. dubia is commercially propagated through stem cuttings, which offer higher success and uniformity (Jayanandan, 2016). The roots of subabul were found to be less dense or diffuse, while those of kadam were found to be medium dense, and the roots of malabar neem were both compact and massive. This difference in rooting pattern might be also attributed to the difference in origin of root development and hormonal regulation during early growth stages (Albrecht et al., 2017). Subabul and kadam showed a well-developed, easily recognizable primary (tap) root system. The main root of malabar neem was not distinct, since it was raised from cuttings. Instead, the root with the most prominent central fibrous structure that became a main one was labelled as the primary (tap) root. All three species showed sturdy primary (tap) roots, with the maximum diameter at the collar region and tapering towards the end. However, the primary root grew partially vertically in the soil. Their development was limited to vertical growth until 0.3 m, then horizontal growth until 0.50-2.0 m, and then downwards because of positive geotropism. The secondary, tertiary, and quaternary root systems were present in kadam and malabar neem, while there was no quaternary root system present in subabul. Secondary roots in all these tree species grew laterally towards the surface and then displayed positive geotropism, which was also visible from wider angles of secondary roots (Table 1). These secondary roots produced the maximum number of fine roots. The root color of kadam and subabul was paler yellow to yellowish brown, while malabar neem was reddish brown with black streaks on its surface. The root surface of subabul had a distinct feature: small round dots that looked like superficial growth and were white. The tree species that were evaluated all showed rapid growth and a moderately deep vertical taproot growth in the soil. These tree species confirmed that they had root systems that were almost symmetrical to ensure anchorage, structural support, and stability (Coutts et al., 1999). The root system’s symmetric growth indicates that they are competing for below-ground resources (water, nutrients, etc.), which makes it suitable for inclusion in agroforestry systems (Rewald and Leuschner, 2009). Nonetheless, previous researchers have already documented a shallow root system in malabar neem (Mulatya et al., 2002; Arunkumar and Chauhan, 2018). Contrary to our observations under semi-arid climate, it has been reported that the root system of subabul has a slightly deeper tap root system with maximum root density at the topsoil under humid sub-tropical climate of North-Western Himalaya (Dhyani et al., 1990).
The spread of secondary roots near the collar and topsoil, followed by vertical growth through positive geotropism, enhances tree stability by providing additional anchorage and structural support. Similar root distribution patterns have been observed in species like Acacia auriculiformis, Azadirachta indica, and Bombax ceiba (Das and Chaturvedi, 2008). The concentration of roots in the topsoil indicates potential competition with seasonal crops, which also have shallow, fibrous roots. Therefore, selecting compatible intercrops in agroforestry systems requires careful planning, along with practices like lateral root wrenching and pruning to improve resource use and reduce competition (Chaturvedi and Das, 2002; Das and Chaturvedi, 2008). Additionally, the centralization of root systems near the stem enhances wind resistance and structural stability (Stokes, 2013).
The less dense or diffused roots systems in subabul and medium dense root systems in kadam lend themselves to integration with agricultural crops in an agroforestry system. The massive and compact root system of malabar neem indicates its suitability for wasteland restoration. This tree species’ extensive root network in the surface soil may improve soil fertility and aggregation while also preventing erosion and topsoil loss (Das and Chaturvedi, 2008; Verma et al., 2014; Borden et al., 2016). For establishing malabar neem-based agroforestry and silvipastural systems, spacing between tree rows needs to be kept wider to avoid shading effects and competition for underground resources on associated crops (Das and Chaturvedi, 2008; Verma et al., 2014).
Quantitative root system parameters
In the 3rd year of the study (2019) when trees attained 3-year, kadam (248 cm) had a longer primary (tap) root, followed by malabar neem (211 cm) and subabul (208 cm) (Table 1). The primary (tap) roots of all three tree species grew vertically only within the first 0.30m of soil depth, then spread horizontally across a distance of 0.50 to 20m from their source, and finally exhibited positive geotropism. At the age of 3, malabar neem (4.40 m) displayed the highest horizontal root spread, while subabul (3.73m) and kadam (3.73 m) had a similar horizontal spread. In the case of kadam and malabar neem, root spread was less than canopy spread, but it was equal in the case of subabul (Table 1). The number of secondary, tertiary and quaternary roots varied among all tree species. Secondary root number varied from 16.0 (subabul) to 25.0 (kadam); tertiary root from 7.0 (subabul) to 10.6 (malabar neem); and quaternary roots from 0.0 in subabul to in 6.2 in malabar neem. Quaternary root numbers were only observed in malabar neem and kadam. Tree species had a difference in their secondary root length (119.2 to 149.3 cm) and diameter (29.18 to 103.6 cm), with malabar neem being the longest and widest with subabul and kadam following them (Table 1). Tertiary roots also varied in length (84.7-147.5 cm) and diameter (11.9-24.5 cm), following similar trends with malabar neem producing the longest and widest roots, followed by kadam and subabul. The longest and widest quaternary roots were also produced by malabar neem, followed by kadam. Quaternary roots were not present in subabul until three years old. Fine roots (<1.0 mm) in all three tree species had a range of lengths between 20 cm and 32 cm and mostly originated from secondary roots. They were evenly distributed throughout the root zone area. The increment of root diameter was greater than that of root length with age in all three tree species. In the studied species, an 18.1-61.1% increase in length and a 30-63% increase in diameter was observed in three-year-old tap roots over one-year-old tap roots. The diameter of secondary roots exhibited multifold increase i.e. 11.97-48.79 mm, 9.97 to 29.18 mm and 18.40 to 103.59 mm, for kadam, subabul and malabar neem, respectively.(Table 1). The corresponding increase in mean root length was 39-252%, while the diameter increased by multiple times (4 to 16). It is axiomatic that the species with deep main roots and more spreading lateral roots take up nutrient and water more efficiently from deeper layers and over a wider area and provide firm anchorage to the tree in soil, thereby making the tree wind firm. Das and Chaturvedi (2008) that Azadirachta indica and Wendlandia exserta with deep main roots and Moderate lateral root length, can be more efficiently grown in dry conditions. Sinacore et al. (2017) recorded maximum and mean horizontal rooting distance for six species (Anacardium excelsum, Cedrela odorata, Dalbergia retusa, Pachira quinata, Tabebuia rosea, Terminalia amazonia) scaled to basal diameter. They found that T. amazonia had mean root distances significantly greater than A. excelsum, C. odorata, and P. quinata. They also compared maximum and mean rooting depth by basal diameter among species and found no significant differences among species for either maximum or mean depth of roots. Compared to subabul and kadam, malabar neem has a larger root spread, which makes it more stable and less vulnerable to wind blow (Coutts et al., 1999; Eamus et al., 2002; Barton and Montagu, 2006). The rapid growth and maximum stem diameter are the reason for the large and thick primary, secondary, tertiary, and quaternary roots in malabar neem (Eamus et al., 2002; Barton and Montagu, 2006). Malabar neem compact and dense root system may have encouraged an increase in the uptake of nutrients and water. Due to this, it has more diverse types (e.g. Primary/secondary/tertiary, etc.) of roots and a greater potential for producing aboveground biomass. We recorded a concentrated and uniform distribution of fine roots in the root zone, regardless of the tree species. Yanai et al., 2006 and Macinnis-Ng et al., 2010 also reported that tree fine roots spread uniformly in the root zone. We observed variable root spread corresponding to canopy or crown spread in the three tree species. This indicates that these species are still growing and accumulating aboveground biomass (Das and Chaturvedi, 2008).
Root angles
The three species had varying angles for secondary, tertiary, and quaternary roots. The three-year study revealed a significant (P>0.05) and relatively higher secondary root angle in all three species compared to tertiary and quaternary roots (Table 1). This could have resulted in a greater spread of secondary roots than tertiary and quaternary roots. When compared, the angle of secondary roots in the topsoil was more inclined than those in the deep subsoil. Secondary and tertiary roots showed angles that ranged from 20° to 90°, and there were significant differences among the three species. Quaternary roots also showed wide variation in angles that ranged from 20° to 60°, and were observed only in two tree species, namely malabar neem and kadam. Subabul tree species lacked any quaternary roots (Table 1). Root angles vary due to the tendency of primary, secondary, and further branches of roots to show positive geotropism, diageotropism, and ageotropism, respectively (Fitter, 1987). In the present study, the secondary roots were almost grown horizontally, compared to tertiary and quaternary roots. The topsoil had roots with greater angles than the subsoil. More horizontal spread of roots was indicated by wide root angles, while narrow angles were associated with more vertical growth. Similar features have been reported in many forest tree species, such as Acacia nilotica, Acacia lenticularis, Cassia fistula, Dalbergia sissoo, Eucalyptus tereticornis, etc., grown in India (Toky and Bisht, 1992; Chaturvedi and Das, 2002). The wider root angles and greater root spread in malabar neem compared to other two tree species indicate that roots were spreading laterally instead of vertically. Narrow root angles of trees are desirable characteristics for an agroforestry system. However, the combination of wider and narrow root angles is crucial in binding soil particles, reducing soil erosion, and enhancing water conservation (Verma et al., 2014; Borden et al., 2016). When agricultural crops need to be integrated as intercrops with the studied tree species, root pruning will be necessary due to wider root angles in the topsoil.
Root:shoot ratio
The root:shoot (RS) ratio is crucial for understanding plant growth strategies, resource allocation, and environmental adaptability, particularly under stress conditions. Studies on species like Eucalyptus and Azadirachta indica show how R:S ratios help assess growth and stress tolerance (Gupta and Gupta, 2015; Panda and Bandyopadhyay, 2012). Low RS ratio (Table 1) was recorded in all the three tree species. The RS ratios of tree species varied significantly (P>0.05) between two and three years of age and subabul had the highest ratio, followed by malabar neem and kadam. Earlier, our reported RS ratio was comparable to the range (0.19–0.22) reported by Chaturvedi and Das (2002) for multipurpose agroforestry tree species such as Acacia lenticularis, Acacia procera, Dalbergia sissoo, and Sesbania grandiflora. However, our reported values were less than other MPTs (0.33-0.59), such as Acacia nilotica, Pithecellobium dulce, Senna fistula and Syzygium cumini (Chaturvedi and Das, 2002). In the early growth phase (first and second years), we saw a low RS ratio, which is an indication of momentum in the development stage. This signifies the accumulation of more biomass above ground due to the extensive development of the tree canopy (Chaturvedi and Das, 2002).
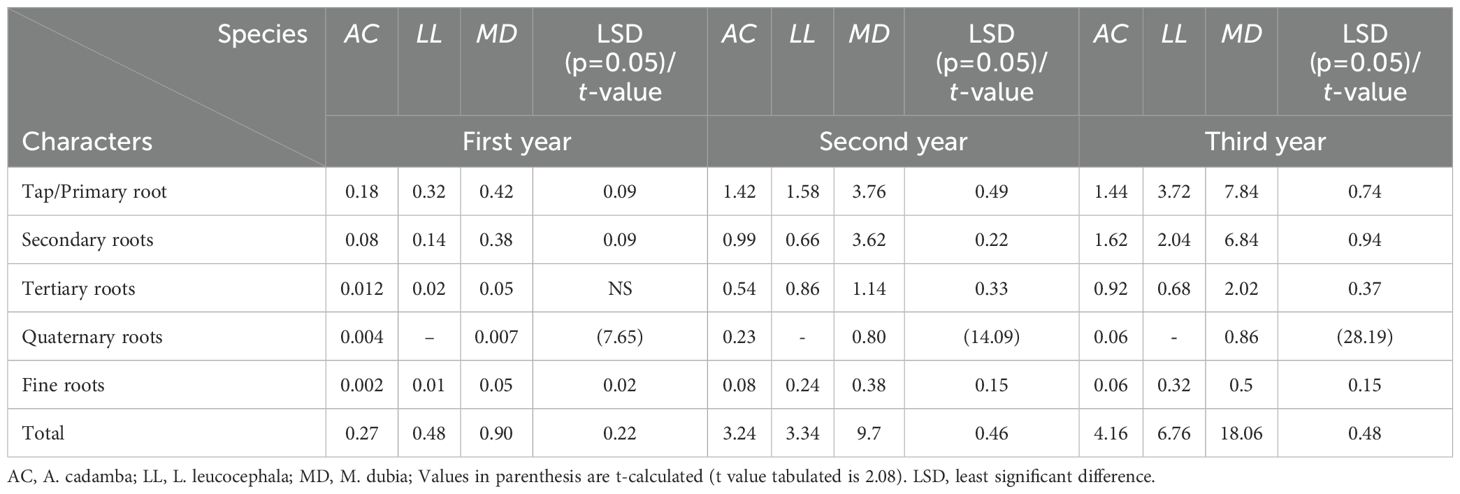
Root biomass
The root biomass varied significantly (P>0.05) between 0.27 and 18.06 kg per tree during their growth periods (first to third years). Malabar neem produced the maximum followed by subabul and the least by kadam (Table 2). Maximum biomass was allocated to primary/tap roots rather than higher order roots in all species during all three years of growth. An exception was observed in kadam at 3-years-old: secondary roots had a higher biomass than primary/tap roots (Table 2). The order of biomass allocation for three tree species at three years old was as:
Kadam: Secondary root > Primary root > Tertiary root > Quaternary root > Fine root.
Subabul: Primary root > Secondary root > Tertiary root > Fine root.
Malabar neem: Primary root > Secondary root > Quaternary root > Tertiary root > Fine root.
The overall root biomass varied significantly in all the species like 0.27 to 4.16 kg tree-1 in A. cadamba; 0.48 to 6.76 kg tree-1 in L. leucocephala and 0.90 to 18.06 kg tree-1 in M. dubia from first to third year. Numerous researchers have observed a variation in biomass accumulation among tree species and types of roots (Das and Chaturvedi, 2008; Chauhan et al., 2009; 2019; Sinacore et al., 2017). In the present study, greater accumulation of primary (tap) root biomass was observed in kadam and malabar neem, followed by the secondary roots, tertiary and quaternary roots. Similar pattern of biomass accumulation was observed in Acacia auriculiformis, Anacardium excelsum, Azadirachta indica, Pachira quinata and Wendlandia exserta (Das and Chaturvedi, 2008; Sinacore et al., 2017). The accumulation of primary (tap) root biomass was more significant in kadam and malabar neem, while secondary, tertiary, and quaternary roots were the next. Comparable biomass accumulation patterns were reported by Das and Chaturvedi (2008) and Sinacore et al. (2017) in Acacia auriculiformis, Anacardium excelsum, Azadirachta indica, Pachira quinata and Wendlandia exserta. Stokes (2013) reported similar findings, with secondary roots accumulating more biomass than primary roots. Secondary roots grow horizontally to a certain distance before showing positive geotropism, providing stability to the tree anchorage. Fast-growing tree species have huge aboveground biomass, which makes the tertiary and quaternary roots act as a guy rope to stabilize the tree (Stokes, 2013).
Biomass carbon stock and its partitioning
The biomass carbon stock varied among the tree species during three years of study (Table 3). Compared to the other two species, malabar neem accumulated the significantly (P>0.05) higher biomass carbon during the entire three-year growth period. At the age of 3 years, malabar neem accumulated approximately 3 to 3.9 times more biomass carbon than subabul and kadam (Table 3; Figure 4). Malabar neem’s above ground biomass carbon stock increased by 23.73 times over the course of one to three years. When the total biomass carbon stock accumulation was compared from the 1st year to the 3rd year, the maximum average increase in the biomass carbon stock was observed in the case of malabar neem (0.37 to 8.55 Mg C ha-¹ yr-¹), followed by subabul (0.177 to 2.87 Mg C ha-¹ yr-¹) and kadam (0.085 to 2.20 Mg C ha-¹ yr-¹). Biomass carbon partitioning (Table 3; Figure 4) showed that the aboveground part contributed 80.4 to 84.3%, while the below-ground part had only 15.7 to 19.6% contribution in all three species (Figure 5). The order of distribution of biomass carbon stocks among various parts in three tree species was identified as:
Kadam: Stem > Branches > Leaves >Secondary roots > Primary roots > Tertiary roots > Quaternary roots > Fine roots.
Subabul: Stem > Branches> Leaves >Primary roots > Secondary roots > Tertiary roots > Fine roots.
Malabar neem: Stem > Branches > Leaves >Primary roots > Secondary roots > Quaternary roots >Tertiary roots > Fine roots.
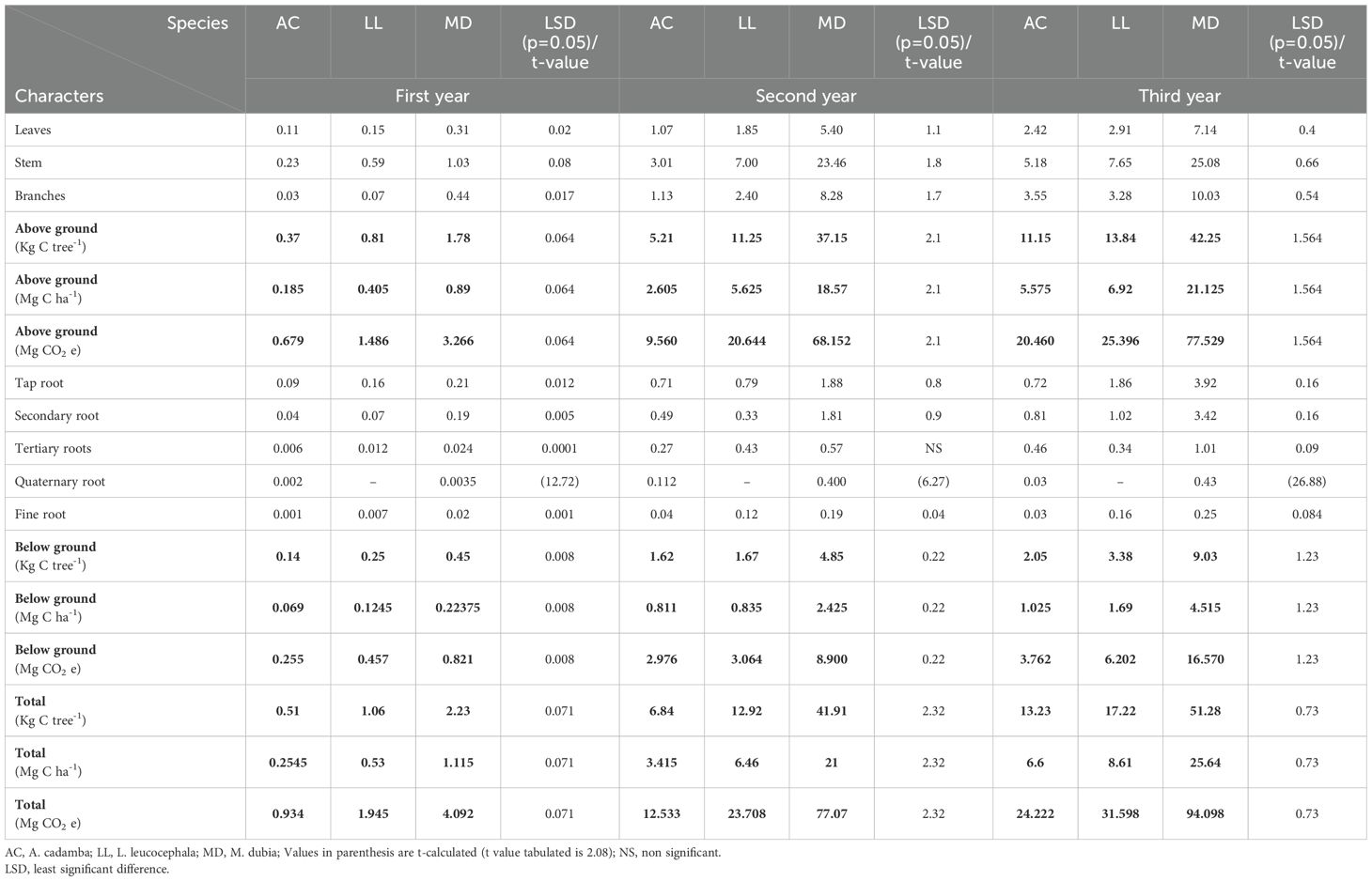
Table 3. Biomass carbon stock (kg C tree-1, Mg C ha-1 & Mg CO2 e) partitioning among various tree parts in three species.
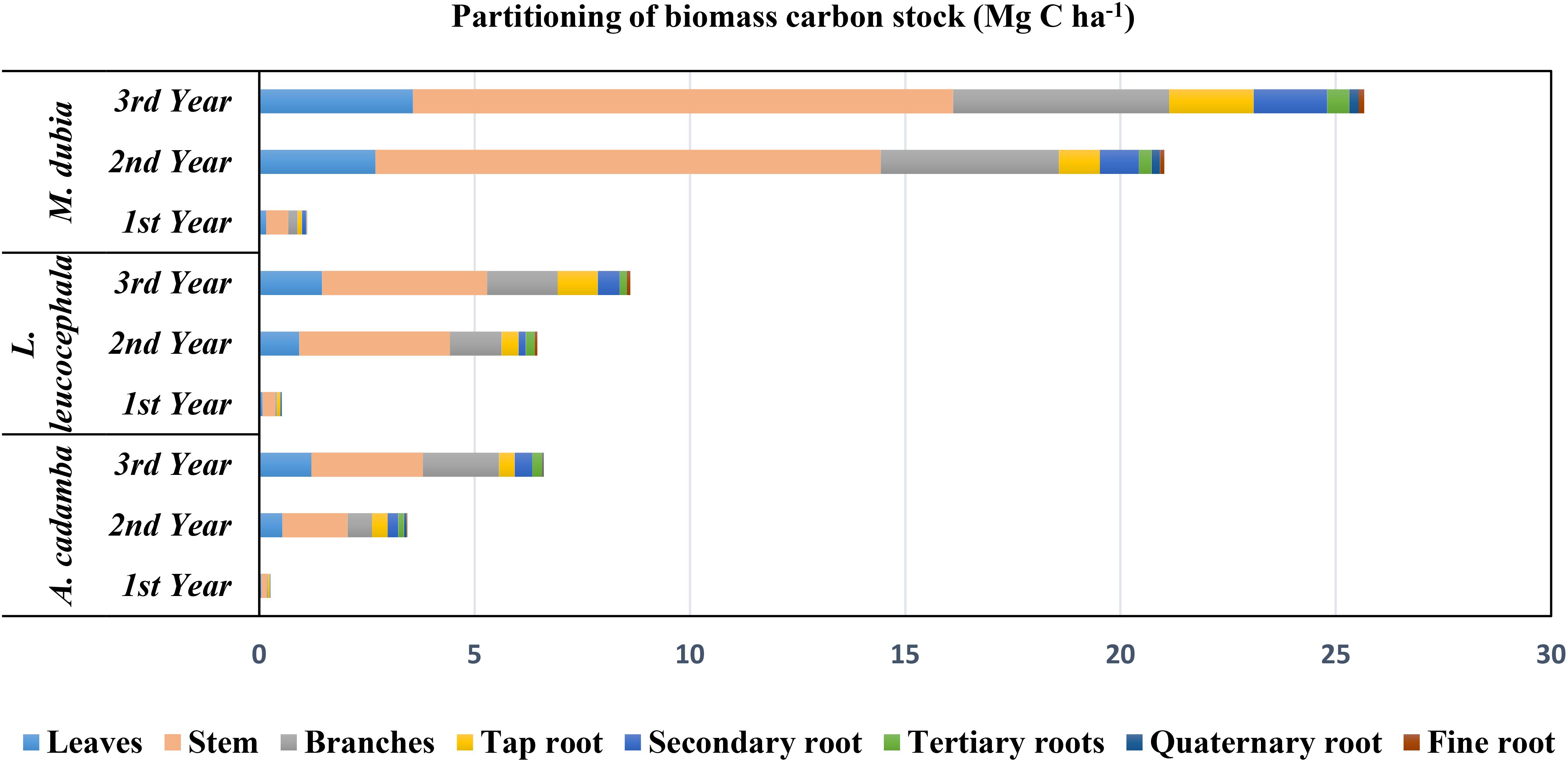
Figure 4. Partitioning of biomass carbon stock (Mg C ha-1) among various plant parts in three fast growing tree species.
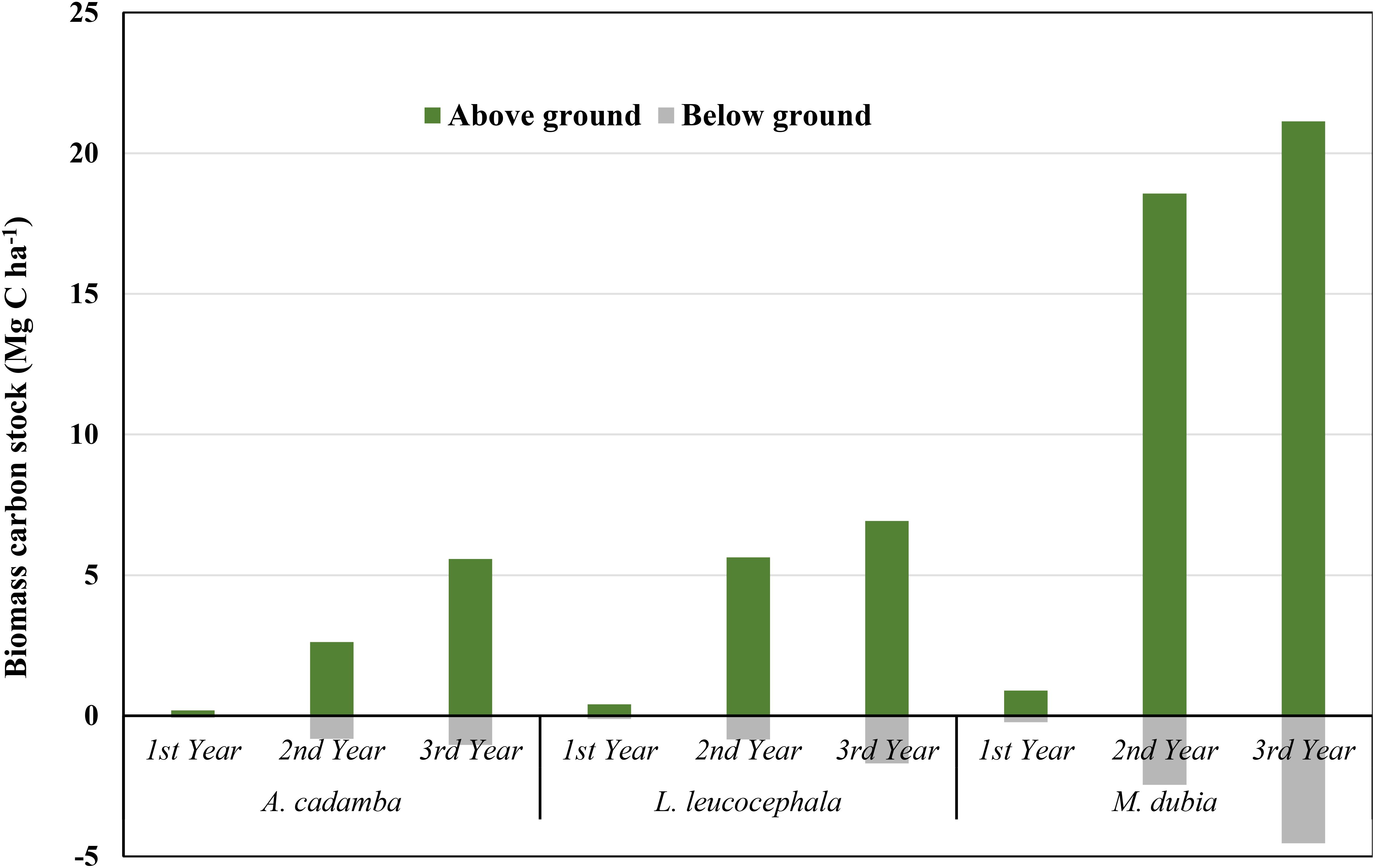
Figure 5. Above and below ground biomass carbon stock (Mg C ha-1) during the study periods (2017-2019).
At a density of 500 trees per hectare, three species had carbon sequestration potential ranging from 2.20 to 8.55 Mg C ha-1 yr-1. As they get older, it is expected that the carbon sequestration potential of these studied species will increase further. Kaul et al. (2010) recorded carbon sequestration potential of short-duration poplar species at 8 Mg C ha-1 yr-1, which is comparable to malabar neem. Our reported values were also comparable to the reported ranges of 3.44 to 9.54 Mg C ha-1 yr-1 for species like Terminalia arjuna, Acacia catechu, Dalbergia sissoo and Melia azedarach (Chauhan et al., 2019).
Carbon sequestration potential of studied species was higher than the other tree species of the region like Acacia catechu, Eucalyptus tereticornis, Dalbergia sissoo, Azadirachta indica, Hardwickia binata, Albizia lebbeck etc (Chauhan et al., 2019; Kumar et al., 2021). According to our findings, these rapidly growing tree species, particularly malabar neem, possess a significant capacity to mitigate climate change by storing a significant amount of carbon in their biomass. The contribution of the root to the shoot varies depending on the tree species, its root system, and ecological conditions. Eslamdoust and Sohrabi (2018) reported that in Populus deltoides, an industrially important fast-growing tree, more than 80% biomass and carbon stock is in above ground part of the tree. Further, compared to our reported ranges of 15 to 19%, Jha (2017) reported a 25 to 40% contribution of roots to total carbon stock in hybrid poplar in sub-humid climate of Mediterranean France. Of all the parts, the stem had the most carbon stored followed by branches, leaves, tap root, secondary root, tertiary root, quaternary root, and fine roots. The variation in order of carbon content observed during the study in various parts of the tree species has also been found in P. crassifolia and P. sylvestris (Shangguan et al., 2025). Similar pattern was also observed in Eucalyptus urophylla and E. grandis plantations, with the stem accounting for the maximum carbon storage (Du et al., 2015).
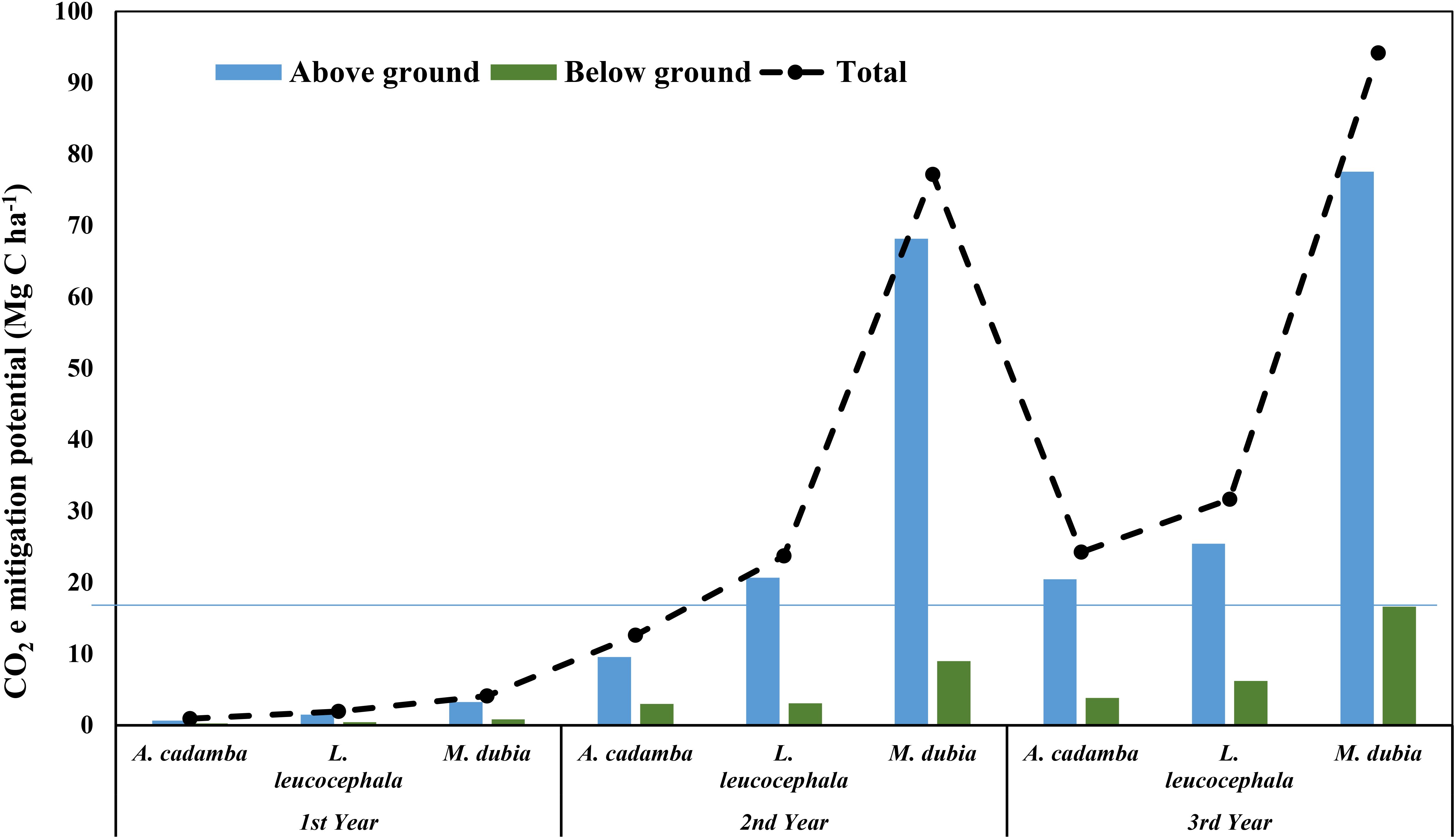
Carbon dioxide equivalent (CO2e) mitigation potential
Under the stand density of 500 trees ha-1, malabar neem sequestered a maximum of 94.09 Mg C ha-1 CO2e, followed by subabul (31.64 Mg C ha-1 CO2e) and kadam (24.29 Mg C ha-1 CO2e) upon reaching three years of age in semi-arid conditions (Figure 6). Implementation of carbon finance mechanism as a projection to this CO2 e sequestration can fetch extra monetary gain of USD 314.53 (≈23158.8 Indian rupees) in malabar neem, 105.68 (≈7787.56 Indian rupees) in subabul and 81.03 (≈5971.10 Indian rupees) in kadam on per hectare basis at the age of three years (market value of one CO2e = 3.34 USD (Asia), Forest Trends’ Ecosystem Marketplace report, 2021). The current prices of carbon offsets are low and vary across different continents from 2.96 USD in Europe to 32.93 USD in Oceania (Forest Trends’ Ecosystem Marketplace, 2021). But a rise in carbon credit prices to USD 20–50 t-1 CO2e is expected by 2030, as more investment is required in projects that sequester high amounts of atmospheric carbon-dioxide to mitigate climate change in the long-run (ScienceDaily, 2021). Thus, these species can ensure additional monetary gains to people on a sustainable basis.
Conclusion
This study demonstrates that tree root architecture plays a crucial role in determining carbon sequestration potential under semi-arid conditions. Among the species evaluated, M. dubia emerged as the most promising candidate due to its compact, dense root system and superior lateral spread, which not only facilitates higher carbon accumulation but also improves soil structure and moisture retention. The study also emphasizes the importance of targeted root management—particularly lateral root pruning after the initial two years—to optimize tree-crop interactions in agroforestry systems. Although root architecture studies typically rely on destructive methods such as root excavation—which are labor-intensive, time-consuming, and costly—the insights they provide are invaluable for developing climate-resilient, resource-efficient agroforestry models tailored to degraded and water-limited landscapes. From a scientific and futuristic perspective, integrating high-performing species like M. dubia into land-use strategies aligns with global efforts to combat climate change through nature-based solutions. Further research should explore long-term carbon dynamics, root-soil-microbe interactions, and genomic traits linked to root development and carbon storage efficiency. Additionally, deploying remote sensing and AI-based root phenotyping tools could enhance large-scale assessment and management. Policy frameworks must now pivot toward incentivizing such species in carbon farming, land restoration, and bioeconomy initiatives, positioning agroforestry as a cornerstone of sustainable development and carbon neutrality in semi-arid and marginal environments.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
KG: Conceptualization, Investigation, Writing – original draft. NK: Investigation, Methodology, Writing – original draft. AR: Formal analysis, Methodology, Writing – original draft. ID: Data curation, Formal analysis, Writing – original draft. BC: Writing – review & editing. NS: Data curation, Supervision, Writing – review & editing. AH: Supervision, Visualization, Writing – original draft. AY: Data curation, Writing – review & editing. HA: Writing – review & editing. AU: Resources, Visualization, Writing – review & editing. DK: Resources, Software, Writing – review & editing. AA: Methodology, Validation, Writing – original draft. DJ: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
Authors are highly thankful to Indian Council for Agricultural Research (ICAR) and Director ICAR-Central Agroforestry Research Institute, Jhansi, India for the research support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahlawat K. S., Kumari A., Bishnoi D. K., Chaudhary K., Sirohi C., Satpal, et al. (2024). Performance of sorghum and barley intercropped with Kadam based agroforestry system in semi-arid ecosystem of Haryana. Forage Res. 49, 461–466.
Ahmad F., Uddin M. M., and Goparaju L. (2019). Agroforestry suitability mapping of India: geospatial approach based on FAO guidelines. Agrofor. Syst. 93, 1319–1336. doi: 10.1007/s10457-018-0233-7
Albrecht U., Bordas M., Lamb B., Meyering B., and Bowman K. D. (2017). Influence of propagation method on root architecture and other traits of young citrus rootstock plants. HortScience horts 52, 1569–1576. doi: 10.21273/HORTSCI12320-17
Ansari M. A., Choudhury B., Mandal S., Jat S. L., and Meitei C. B. (2022). Converting primary forests to cultivated lands: Long-term effects on the vertical distribution of soil carbon and biological activity in the foothills of Eastern Himalaya. J. Environ. Manage. 301, 113886. doi: 10.1016/j.jenvman.2021.113886
Arunkumar A. N. and Chauhan S. (2018). Assessment of root plate structure in wind-thrown trees of Melia dubia. J. Trop. For. Environ. 8, 50–54. doi: 10.31357/jtfe.v8i1.3482
Barton C. V. M. and Montagu K. D. (2006). Effect of spacing and water availability on root:shoot ratio in Eucalyptus camaldensis. Ecol. Manage. 221, 52–62. doi: 10.1016/j.foreco.2005.09.007
Bettles J. D. S., Battisti D. S., Cook-Patton S. C., Kroeger Dev I., Ram A., Ahlawat S. P., et al. (2021). Agroforestry and non-state actors: a review. For. Policy Econ. 130, 102538. doi: 10.1016/j.forpol.2021.102538
Borden K. A., Thomas S. C., and Isaac M. E. (2016). Interspecific variation of tree root architecture in a temperate agroforestry system characterized using ground-penetrating radar. Plant Soil 410, 323–334. doi: 10.1007/s11104-016-3015-x
Bredemeier M., Busch G., Hartmann L., Richter F., and Lamersdorf N. P. (2015). Fast growing plantations for wood production - Integration of ecological effects and economic perspectives. Front. Bioeng. Biotechnol. 3. doi: 10.3389/fbioe.2015.00072
Chaturvedi O. P. and Das D. K. (2002). Studies on rooting patterns of 5-year-old important agroforestry tree species in North Bihar, India. For. Trees Livelihoods 12, 329–339. doi: 10.1080/14728028.2002.9752436
Chaturvedi O. P., Sikka A. K., Handa A. K., and Bajpai C. K. (2016). Agroforestry technologies for different agro-climatic zones of the country (Jhansi, Uttar Pradesh, India: All India Coordinated Research Project on Agroforestry, ICAR-Central Agroforestry Research Institute).
Chauhan S. K., Gupta N., Ritu N., Yadav S., and Chauhan R. (2009). Biomass and carbon allocation in different parts of agroforestry tree species. Indian For. 135, 981–993.
Chauhan S. K., Singh S., Sharma S., Sharma R., and Saralch H. S. (2019). Tree biomass and carbon sequestration in four short rotation tree plantations. Range Manage. Agrofor. 40, 77–82.
Chavan S. B. and Dhillon R. S. (2019). Doubling farmers’ income through Populus deltoides-based agroforestry systems in northwestern India: An economic analysis. Curr. Sci. 117, 219–226. doi: 10.18520/cs/v117/i2/219-226
Coutts M. P., Nielsen C. C. N., and Nicoll B. C. (1999). The development of symmetry, rigidity and anchorage in the structural root system of conifers. Plant Soil 217, 1–15. doi: 10.1023/A:1004578032481
Czernin A. and Phillips C. (2005). Below-ground morphology of Cordyline australis (New Zealand cabbage tree) and its suitability for river bank stabilisation. N. Z. J. Bot. 43, 851–864. doi: 10.1080/0028825X.2005.9512995
Dagar J. C. and Tomar O. S. (2002). “Utilization of salt affected soils and poor quality waters for sustainable biosaline agriculture in arid and semiarid regions of India,” in Proceedings of 12th ISCO Conference, Beijing, Ministry of Water Resources, People's Republic of China, Vol. 12. 340–347.
Das D. K. and Chaturvedi O. P. (2008). Root biomass and distribution of five agroforestry tree species. Agrofor. Syst. 74, 223–230. doi: 10.1007/s10457-008-9159-9
Dev I., Ram A., Ahlawat S. P., Palsaniya D. R., Singh R., Dhyani S. K., et al. (2020). Bamboo-based agroforestry system (Dendrocalamus strictus + sesame–chickpea) for enhancing productivity in semi-arid tropics of central India. Agrofor. Syst. 94, 1725–1739. doi: 10.1007/s10457-020-00492-8
Dhyani S., Murthy I. K., Kadaverugu R., Dasgupta R., Kumar M., and Gadpayle K. A. (2021). Agroforestry to achieve global climate adaptation and mitigation targets: Are South Asian countries sufficiently prepared Forests 12, 303. doi: 10.3390/f12030303
Dhyani S. K., Narain P., and Singh R. K. (1990). Studies on root distribution of five multipurpose tree species in Doon Valley, India. Agrofor. Syst. 12, 149–161. doi: 10.1007/BF00123470
Dhyani S. K., Ram A., and Dev I. (2016). Potential of agroforestry systems in carbon sequestration in India. Indian J. Agric. Sci. 86, 1103–1112. doi: 10.56093/ijas.v86i9.61348
Du H., Zeng F., Peng W., Wang K., Zhang H., Liu L., et al. (2015). Carbon storage in a Eucalyptus plantation chronosequence in Southern China. Forests 6, 1763–1778. doi: 10.3390/f6061763
Eamus D., Chen X., Kelley G., and Hutley L. B. (2002). Root biomass and root fractal analyses of an open Eucalyptus forest in a savanna of north Australia. Aust. J. Bot. 50, 31–41. doi: 10.1071/BT01054
Eslamdoust J. and Sohrabi H. (2018). Carbon storage in biomass, litter, and soil of different native and introduced fast-growing tree plantations in the South Caspian Sea. J. For. Res. 29, 449–457. doi: 10.1007/s11676-017-0469-5
Fitter A. H. (1987). An architectural approach to the comparative ecology of plant root systems. New Phytol. 106, 61–77. doi: 10.1111/j.1469-8137.1987.tb04683.x
Ghosh A., Kumar R. V., Manna M. C., Singh A. K., Parihar C. M., Kumar S., et al. (2021). Eco-restoration of degraded lands through trees and grasses improves soil carbon sequestration and biological activity in tropical climates. Ecol. Eng. 162, 106176. doi: 10.1016/j.ecoleng.2021.106176
Gomez K. A. and Gomez A. A. (1984). Statistical procedures for agricultural research. 2nd Edn (New York: John Wiley).
Gupta S. and Gupta A. (2015). Effect of water stress on growth and root:shoot ratio in Eucalyptus and Acacia species. J. Environ. Biol. 36, 345–352.
Handa A. K., Dev I., Rizvi R. H., Kumar N., Ram A., Kumar D., et al. (2019). Successful agroforestry models for different agro-ecological regions in India (Jhansi, India: CAFRI and New Delhi: ICRAF).
Herder G. D., Van Isterdael G., Beeckman T., and De Smet I. (2010). The roots of a new green revolution. Trends Plant Sci. 15, 600–607. doi: 10.1016/j.tplants.2010.08.009
Hombegowda H. C., Adhikary P. P., Jakhar P., Madhu M., and Barman D. (2020). Hedge row intercropping impact on run-off, soil erosion, carbon sequestration and millet yield. Nutr. Cycl. Agroecosyst 116, 103–116. doi: 10.1007/s10705-019-10031-2
IPCC (2006). IPCC Guidelines for National Greenhouse Inventories – A primer Prepared by the National Greenhouse Gas Inventories Programme. Eggleston H.S., Miwa K., Srivastava N., and Tanabe K. (eds). IGES, Japan.
Jayanandan V. K. (2016). Scientific techniques for Melia dubia-based agroforestry systems: An emerging indigenous tree species for wood-based industries in India (KrishiKosh). Available at: https://krishikosh.egranth.ac.in/items/64c5a73e-c950-49e0-b83a-7d5f1305251c.
Jemal O., Callo-Concha D., and Van Noordwijk M. (2018). Local agroforestry practices for food and nutrition security of smallholder farm households in southwestern Ethiopia. Sustainability 10, 2722. doi: 10.3390/su10082722
Jha K. K. (2017). Root structure and belowground biomass of hybrid poplar in forestry and agroforestry systems in Mediterranean France. Not. Sci. Biol. 9, 422–432. doi: 10.15835/nsb9310155
Jinger D., Kakade V., Bhatnagar P. R., Paramesh V., Dinesh D., Singh G., et al. (2024). Enhancing productivity and sustainability of ravine lands through horti-silviculture and soil moisture conservation: a pathway to land degradation neutrality. J. Environ. Manage. 364, 121425. doi: 10.1016/j.jenvman.2024.121425
Jinger D., Kakade V., Kaushal R., Bhatnagar P. R., Ghosh A., Mahawer S. K., et al. (2025). Nature-based solutions for enhancing soil health and CO2 sequestration in degraded ravine lands through silvo-aromatic system and soil moisture conservation techniques. J. Environ. Manage. 380, 124904. doi: 10.1016/j.jenvman.2025.124904
Jinger D., Kaushal R., Kumar R., Paramesh V., Verma A., Shukla M., et al. (2023). Degraded land rehabilitation through agroforestry in India: achievements, current understanding, and future prospectives. Front. Ecol. Evol. 11. doi: 10.3389/fevo.2023.1088796
Jinger D., Kumar R., Kakade V., Dinesh D., Singh G., Pande V. C., et al. (2022). Agroforestry for controlling soil erosion and enhancing system productivity in ravine lands of Western India under climate change scenario. Environ. Monit. Assess. 194, 267. doi: 10.1007/s10661-022-09910-z
Karmini S. and Karyati S. (2017). Economic analysis of groundnut (Arachis hypogaea) and soybean (Glycine max) as intercropping plants in two agroforestry systems. Biodiversitas 18, 483–493. doi: 10.13057/biodiv/d180206
Karthikeyan K., Sudhagar R. J., Radhakrishnan S., and Fernandaz C. C. (2018). Compatibility studies of fodder crops with Melia dubia Cav. Trends Biosci. 11, 3119–3123.
Kaul M., Mohren G. M. J., and Dadhwal V. K. (2010). Carbon storage and sequestration potential of selected tree species in India. Mitig. Adapt. Strateg. Glob. Change 15, 489–510. doi: 10.1007/s11027-010-9230-5
Kaushal R., Jayaprakash J., Mandal D., Kumar A., Alam N. M., Tomar J. M. S., et al. (2019). Canopy management practices in mulberry: impact on fine and coarse roots. Agrofor. Syst. 93, 545–556. doi: 10.1007/s10457-017-0148-8
Kmoch L., Pagella T., Palm M., and Sinclair F. (2018). Using local agroecological knowledge in climate change adaptation: A study of tree-based options in Northern Morocco. Sustainability 10, 3719. doi: 10.3390/su10103719
Kumar P., Mishra A. K., Chaudhari S. K., Sharma D. K., Rai A. K., Singh K., et al. (2021). Carbon sequestration and soil carbon build-up under Eucalyptus plantation in semi-arid regions of north-west India. J. Sustain. For. 40, 319–331. doi: 10.1080/10549811.2020.1749856
Loushambam R. S., Raju N., Aido S., and Shankar T. (2017). Bamboo in North East India. Indian J. Hill Farm. 30, 181–185.
Ma Y., Yang N., Wang S., Huo C., Yu L., and Gu J. (2024). Climatic and edaphic controls of root-tip production and mortality in five temperate tree species. J. Forestry Res. 35, 145. doi: 10.1007/s11676-024-01797-5
Macinnis-Ng C. M. O., Fuentes S., O’Grady A. P., Palmer R., Taylor D., Whitley R. J., et al. (2010). Root biomass distribution and soil properties of an open woodland on a duplex soil. Plant Soil 327, 377–388. doi: 10.1007/s11104-009-0061-7
Marden M., Lambie S., and Phillips C. (2018). Biomass and root attributes of eight of New Zealand’s most common indigenous evergreen conifer and broadleaved forest species during the first 5 years of establishment. N. Z. J. For. Sci. 48, 1–26. doi: 10.1186/s40490-018-0115-9
Mulatya J. M., Wilson J., Ong C. K., Deans J. D., and Sprent J. I. (2002). Root architecture of provenances, seedlings and cuttings of Melia volkensii: implications for crop yield in dryland agroforestry. Agrofor. Syst. 56, 65–72. doi: 10.1023/A:1021165830511
Panda S. K. and Bandyopadhyay A. (2012). Root:shoot ratio and its implications in species adaptation to semi-arid environments: A study on Malabar neem and Subabul. J. Forestry Res. 23, 569–576.
Peng Y. Y. and Dang Q. L. (2003). Effects of soil temperature on biomass production and allocation in seedlings of four boreal tree species. For. Ecol. Manage. 180, 1–9. doi: 10.1016/S0378-1127(02)00486-3
Phillips C. J., Marden M., and Suzanne L. M. (2014). Observations of root growth of young poplar and willow planting types. N. Z. J. For. Sci. 44, 15. doi: 10.1186/s40490-014-0015-6
Ram A., Dev I., Kumar S., Arunachalam A., and Kumar A. (2023). “Agroforestry for cleaner production,” in Frontiers in agronomy for sustainable agriculture. Eds. Babu S., Das A., Rathore S. S., Singh R., and Shivadhar (Indian Society of Agronomy, New Delhi, New Delhi), 91–116.
Rewald B. and Leuschner C. (2009). Belowground competition in a broad-leaved temperate mixed forest: pattern analysis and experiments in a four-species stand. Eur. J. For. Res. 128, 387–398. doi: 10.1007/s10342-009-0276-4
Sahoo G. and Wani A. M. (2019). Multifunctional agroforestry systems in India for livelihoods. Ann. Hortic. 12, 139–149. doi: 10.5958/0976-4623.2019.00022.7
Santiago L. S. and Doughty C. E. (2018). The role of fast-growing tree species in carbon sequestration and biodiversity conservation. Front. Ecol. Environ. 16, 444–451. doi: 10.1002/fee.2010
ScienceDaily (2021). Ten-fold increase in carbon offset cost predicted. Available online at: https://www.sciencedaily.com/releases/2021/06/210604122439.htm (Accessed October 15, 2023).
Shangguan Y., Zhao H., Zhang Z., Xu E., Lv D., Wang Y., et al. (2025). Carbon storage and sequestration of five planting patterns of Picea crassifolia plantations in Qilian mountains. Front. Earth Sci. 13. doi: 10.3389/feart.2025.1560899
Siarudin M., Rahman S. A., Artati Y., Indrajaya Y., Narulita S., Ardha M. J., et al. (2021). Carbon sequestration potential of agroforestry systems in degraded landscapes in West Java, Indonesia. Forests 12, 1–13. doi: 10.3390/f12060714
Sinacore K., Hall J. S., Potvin C., Royo A. A., Ducey M. J., and Ashton M. S. (2017). Unearthing the hidden world of roots: root biomass and architecture differ among species within the same guild. PloS One 12, e0185934. doi: 10.1371/journal.pone.0185934
Singh A., Choudhury B. U., Balusamy A., and Sahoo U. K. (2024). Restoring the inventory of biomass and soil carbon in abandoned croplands: An agroforestry system approach in India's eastern Himalayas. Agric. Ecosyst. Environ. 362, 108843. doi: 10.1016/j.agee.2023.108843
Stokes A. (2013). The supporting roots of trees and woody plants: form, function and physiology (Dordrecht, The Netherlands: Springer).
Thakur N. S., Mohanty S., Gunaga R. P., and Gajbhiye N. A. (2019). Melia dubia Cav. spatial geometries influence the growth, yield and essential oil principles content of Cymbopogon flexuosus (Nees Ex Steud.) W. Watson. Agrofor. Syst. 8, 1–11.
Toky O. P. and Bisht R. P. (1992). Observations on the rooting patterns of some agroforestry trees in an arid region of north-western India. Agrofor. Syst. 18, 245–263. doi: 10.1007/BF00123320
Verma K. S., Kohli S., Kaushal R., and Chaturvedi O. P. (2014). Root structure, distribution and biomass in five multipurpose tree species of Western Himalayas. J. Mt. Sci. 11, 519–525. doi: 10.1007/S11629-013-2479-X
Keywords: agroforestry, biomass, carbon stock, crown spread, fine roots, root architecture
Citation: Gautam K, Kumar N, Ram A, Dev I, Choudhury BU, Singh NR, Handa AK, Yadav A, Anuragi H, Uthappa AR, Kumar D, Arunachalam A and Jinger D (2025) Root architecture and carbon sequestration potential of fast-growing agroforestry tree species in semi-arid Central India. Front. Agron. 7:1597122. doi: 10.3389/fagro.2025.1597122
Received: 20 March 2025; Accepted: 15 May 2025;
Published: 04 June 2025.
Edited by:
Nasim Ahmad Yasin, University of the Punjab, PakistanReviewed by:
Muhammad Muneeb Hashmi, University of the Punjab, PakistanC. N. Hari Prasath, Tamil Nadu Agricultural University, India
Tarun Verma, Dr. Yashwant Singh Parmar University of Horticulture and Forestry, India
Nyatwere Mganga, University of Dar es Salaam, Tanzania
Copyright © 2025 Gautam, Kumar, Ram, Dev, Choudhury, Singh, Handa, Yadav, Anuragi, Uthappa, Kumar, Arunachalam and Jinger. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Naresh Kumar, bmFyZXNoYmhhcmR3YWo1MEBnbWFpbC5jb20=; Asha Ram, YXNodXNpcnZpODRAZ21haWwuY29t
 Kamini Gautam
Kamini Gautam Naresh Kumar
Naresh Kumar Asha Ram
Asha Ram Inder Dev2
Inder Dev2 Burhan U. Choudhury
Burhan U. Choudhury Nongmaithem Raju Singh
Nongmaithem Raju Singh Ashok Yadav
Ashok Yadav Hirdayesh Anuragi
Hirdayesh Anuragi A. R. Uthappa
A. R. Uthappa Dhiraj Kumar
Dhiraj Kumar Ayyanadar Arunachalam
Ayyanadar Arunachalam Dinesh Jinger
Dinesh Jinger