- 1Agro-Environmental Protection Institute, Ministry of Agriculture and Rural Affairs, Tianjin, China
- 2Key Laboratory of Rural Toilet and Sewage Treatment Technology, Ministry of Agriculture and Rural Affairs, Tianjin, China
- 3The Fifth Design and Research Institute, Tianjin Municipal Engineering Design and Research Institute Co., Ltd., Tianjin, China
Human urine (HU) is rich in nutrients necessary for plant growth, and recycling HU as fertilizer has multiple positive impacts, such as enhancing agricultural sustainability, reducing wastewater pollution, and decreasing reliance on chemical fertilizers, so it is of great significance to explore the effects of urine agricultural utilization on soil environment. A pot experiment was conducted to evaluate the impacts of varying HU application rates {0 [control (C)], 13, 26, 52, and 104 mL kg−1} on soil physicochemical properties, microbial communities, and enzymatic activities. The findings indicate that HU application enriched soil nutrients and significantly increased soil electrical conductivity, with levels at 104 mL kg−1 reaching an increase of 840% over C. Compared to the C, HU enhanced the activities of soil enzymes such as invertase, urease, and catalase by 7.30%–58.75%, 0.93%–47.77%, and 1.56%–16.62%, respectively, but reduced alkaline phosphatase activity by 6.40%–64.76%. Additionally, increasing HU application was correlated with reductions in both operational taxonomic units and the Shannon–Wiener diversity index. The relative abundance of soil bacteria such as Pseudomonadota and Gemmatimonadota incrementally rose with higher HU input, whereas that of Bacillota declined. Moreover, the composition of the top 20 bacterial genera, including Gaiella (1.49%), Bacillus (1.47%), and Blastococcus (1.02%), was significantly altered by HU application. In conclusion, HU application changes the soil ecological environment and, to some extent, modifies the structure and diversity of soil bacterial communities and enzymatic function. However, the absence of long-term field trials underlines the necessity for comprehensive evaluations of HU’s impact on soil fertility and crop health, and careful attention must be paid to potential environmental safety risks post-HU application.
1 Introduction
In recent years, the application of human urine (HU) as a fertilizer in agriculture has been explored in various countries across Northern Europe and Africa (Pandorf et al., 2019). HU is rich in essential nutrients critical for plant growth, providing an alternative or supplementary source to conventional chemical fertilizers in crop production (Esrey et al., 2001). When stored at temperatures above 20°C for a duration between 2 to 6 months, HU can be utilized directly as a liquid fertilizer (Akpan-Idiok et al., 2012). Pot experiments using HU as fertilizer for cultivating pepper (Capsicum annum L.), ryegrass (Lolium perenne L.), and radish (Raphanus sativus L.) in two different soil textures demonstrated that consumption of these crops poses no health risk to consumers (Migeri et al., 2023). Like chemical fertilizers, over 90% of nitrogen in HU is present in the form of urea or ammonia salts, which are beneficial for comprehensive crop development. Research has demonstrated that applying HU as a liquid fertilizer can significantly increase the yields of cucumber (Cucumis sativus L.), cabbage (Brassica oleracea L. var. capitata L.), and amaranth (Amaranthus tricolor L.) (Adeoluwa and Cofie, 2012). Additionally, (Chrispim and Nolasco, 2012) observed enhancements in leaf count, plant height, root length, and stem fresh weight in celtuce due to HU application. Morgan (2003) also reported on the efficacy of diluted HU (water:HU = 3:1) as a liquid fertilizer in promoting exceptional yields of corn and various vegetables in Zimbabwe. Tang et al. (Tang and Maggi, 2016) used barley (Hordeum vulgare L.) and soybean (Glycine max L.) as experimental crops in the West Wyalong and Moree regions of Australia and found that the absorption of nutrients increased almost linearly with the amount of HU application. It has been found that HU application can affect the shift in root-associated bacterial communities (Van Gerrewey et al., 2021) of lettuce (Lactuca sativa L.) and influence soil community structure (Johansen et al., 2023). Furthermore, previous studies have found that HU can significantly acidify soil by promoting nitrification and volatilization (Raza et al., 2021), and this acidification may alter the soil microbial community (Karimi et al., 2018). According to Rumeau et al (Rumeau et al., 2024), the relative abundance of nitrification and denitrification groups is increased by HU application. Nonetheless, concerns regarding soil salinization from HU usage have emerged, which could potentially result in crop damage, reduced yields, and other negative outcomes (Lienert et al., 2007). Consequently, optimizing the agricultural benefits of HU necessitates more thorough and detailed research into its impacts on the soil’s ecological environment.
Soil enzymatic activity and bacterial community structure serve as crucial biological indicators for assessing soil health and monitoring environmental conditions (Luo et al., 2017; Li et al., 2019). Enzymes and microorganisms are integral to the process of soil biological remediation (Liu et al., 2020). Predominantly produced by microbes, soil enzymes mirror microbial activity and are highly sensitive, providing prompt and accurate reflections of minor changes in soil characteristics such as nutrient content, pH, and salinity (Torres et al., 2015; Yu et al., 2017; Lemanowicz et al., 2020). Consequently, soil enzyme activities are frequently employed as significant indicators of soil properties (Wang et al., 2016). It has been reported that urease facilitates the mineralization of soil organic nitrogen, whereas invertase catalyzes the hydrolysis of sucrose into monosaccharides, supplying energy sources for microbial activities (Wu et al., 2020). Additionally, catalase and phosphatase play crucial roles in the decomposition of exogenous organic compounds and the mineralization of organic matter, respectively (Du et al., 2021). The application of fertilizers during crop cultivation induces physicochemical alterations in soil, impacting soil enzyme activities and microbial community structures (Zhang et al., 2017). However, research on the utilization of HU has primarily focused on its impacts on crop quality and soil physicochemical properties (Kishor et al., 2020; Hilton et al., 2021), with limited exploration into the influence of HU on the interplay between soil enzymatic activities and bacterial communities.
Literature analysis suggests that urine application could potentially alter soil ecosystems, possibly in a dosage-linked manner. Therefore, the objectives of this investigation were to elucidate the impact of varying quantities of HU on soil enzyme activities, the diversity and configuration of soil bacterial communities, as well as the interrelations among these parameters.
2 Materials and methods
2.1 Experimental design
The initial soil sample was collected from the surface layer in Ninghe, Tianjin, China (39°25′N, 117°29′E). This fluvo-aquic soil is representative of the North China region. Its physicochemical properties were characterized as follows: total nitrogen (TN) = 0.91 g kg−1, total phosphorus (TP) = 0.63 g kg−1, available potassium (AK) = 0.34 g kg−1, available phosphorus (AP) = 41.89 mg kg−1, pH = 8.43, and electrical conductivity (EC) = 0.28 mS cm−1. After being transported to the greenhouse at the Agro-Environmental Protection Institute in Tianjin, the soil was mixed and screened to eliminate large stones and other solid impurities. HU was sourced from a urine-diverting toilet in the male restroom at the Institute’s dormitory. The HU was collected, containerized, and stored at 25°C for a duration of 3 months. Subsequent analysis revealed its physicochemical properties as: TN = 3.62 g L−1, TP = 0.26 g L−1, TK = 1.18 g L−1, pH = 9.18, EC = 34 mS cm−1, and chemical oxygen demand (COD) = 12.40 g L−1, sodium ions (Na+) = 1.35 g L−1, chloride ions (Cl−) = 2.23 g L−1.
Pots of uniform size (23 cm in height and 20 cm in diameter) were filled with 5 kg of the homogenized and sieved soil and categorized into five treatment groups: C, T1, T2, T3, and T4. Each group received a different volume of HU: 0, 65, 130, 260, and 520 mL for C, T1, T2, T3, and T4, respectively. The HU application rates were primarily based on its nitrogen content, calculated by referencing the nitrogen fertilizer usage for protected horticultural crops as reported by Zhang et al (Zhang et al., 2018). Treatments were replicated three times. Thorough mixing of the soil and HU was achieved using a mechanical stirrer. To standardize soil moisture across treatments, additional water was added to bring the combined volume of HU and tap water in each pot to 2 L. After a 2-day equilibration period, five pakchoi seeds were sown into each pot. Following germination, thinning was performed to retain only three seedlings per pot. All pots were consistently irrigated to maintain optimal soil moisture levels for plant growth.
2.2 Soil sample collection
After a 40-day growth period, pakchoi plants were harvested (with biomass data presented in Supplementary Figure S1), and the top 0- to 10-cm layer of soil was collected from each pot. Five soil cores from each pot were combined into a single sample, whereas plant debris and other contaminants were removed. These soil samples were then temporarily stored in sterilized, sealable bags within an incubator and promptly transported to the laboratory. Each consolidated sample from the pots was subsequently divided into three sub-samples. The first sub-sample was thoroughly mixed, sifted through a 2-mm sieve, placed in a sterile 10-mL centrifuge tube, and sent to Shanghai Majorbio Bio-Pharm Technology Co., Ltd., for microbial diversity analysis. The second sub-sample was used to assess the soil’s physicochemical properties and enzymatic activities after being ground and sieved. The third sub-sample was utilized to determine the concentrations of soil ammonium nitrogen (NH4+-N) and nitrate nitrogen (NO3−-N).
2.3 Experimental analysis
2.3.1 Measurement of soil and human urine physicochemical characters
Soil pH, EC, SOM, TN, and TP were determined as described by Bao (2008). NO3−-N and NH4+-N were tested using a flow analyzer (AA3, SEAL Analytical, Germany). AP was determined by UV-visible spectrophotometer (TU-1900 double-beam produced by Beijing Pu-analysis General Instrument Co.). AK was determined using a Jena ZEEnit 700P.
The PH and EC of HU were determined, respectively, by a PH meter and a conductivity meter (Hach LC500, USA), and TN and TP were tested using a flow analyzer (AA3, SEAL Analytical, Germany). TK was treated by atomic absorption spectrometry. COD was determined by the acidic potassium permanganate titration method, and Na+ and Cl− were detected by ion chromatography (Donex ICS-6000, China).
2.3.2 Detection of soil enzymatic activity
Soil enzymatic activity was determined using the kit provided by Beijing Solarbio Science & Technology Co., Ltd. (Solarbio, China). Soil enzyme activities were determined using the Solarbio Activity Assay Kit (Spectrophotometer). The air-dried soil after the pot experiment was screened using a 100-mesh sieve, and the activities of soil invertase (INV), urease (URE), catalase (CAT), and alkaline phosphatase (AKP) were determined at wavelengths of 510 nm, 540 nm, 660 nm, and 240 nm, respectively (Akhtar et al., 2018; Han et al., 2024).
2.3.3 DNA extraction and sequencing
High-throughput sequencing analysis of soil samples was conducted at the end of the experiment after crop harvesting. Soil (0.5 g) DNA was extracted using the Fast DNA® Spin Kit for soil (MP Biomedicals, Irvine, CA). DNA concentration and purity were determined using a NanoDrop2000 (Thermo Fisher Scientific, USA). The 338F/806R primers were used to amplify V3-V4 of 16S rRNA gene (Wang et al., 2018).
Amplicons of polymerase chain reaction (PCR) products were purified with the QIAquick Gel Extraction Kit (Qiagen, Germany). The PCRs were pooled and quantified using QuantiFluor™-ST (Promega, USA). Paired-end reads (2 × 300 base pair) were generated by MiSeq (PE300) platform Illumina at Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China). Low-quality sequences were trimmed off using Cutadapt (Martin, 2011), and quality-filtered using the QIIME pipeline (v1.9.1) (Caporaso et al., 2010). UPARSE was employed to divide all sequences into OTUs based on 97% similarity DNA sequences (Wang et al., 2017). OTUs with fewer than two sequences were deleted, and their representative sequences were classified into taxonomic lineages using the Ribosomal Database Project classifier within the SILVA database (v138.1) short-subunit reference database (Quast et al., 2013).
2.4 Statistical analysis
All data were conducted for verify normality and homoscedasticity of variance using SPSS 21.0 Statistics software (IBM Corporation, NY, USA) (Pu et al., 2022). For the data that conformed to the normal distribution and homogeneity of variance test, analysis of variance was conducted again to determine the significant differences among the treatments (Huang et al., 2019). The bar graphs were created using Origin 7.0. The “vegan” package was used for calculating alpha diversity and richness of microorganisms (Oksanen et al., 2017). The statistical method of bacterial diversity index difference was tested by Kruskal–Wallis H. The abundance differences between groups were analyzed using the R (v. 3.3.1) for statistics and mapping. The significance difference between groups was tested by ANCOM difference test (QIIME2) (Nearing et al., 2022). Non-metric multidimensional scaling (NMDS) was carried out using the Bray–Curtis dissimilarity distance to analyze microbial community diversity. Analysis of similarities (ANOSIM) was employed for the significance of separations measured under different treatments (Huang et al., 2019). A model of multivariate analysis of variance was constructed using distance-based redundancy analysis (RDA) (Francioli et al., 2016). ANOSIM, RDA, and Mantel tests were performed using R (v.4.0.3).
3 Results
3.1 Soil physicochemical properties
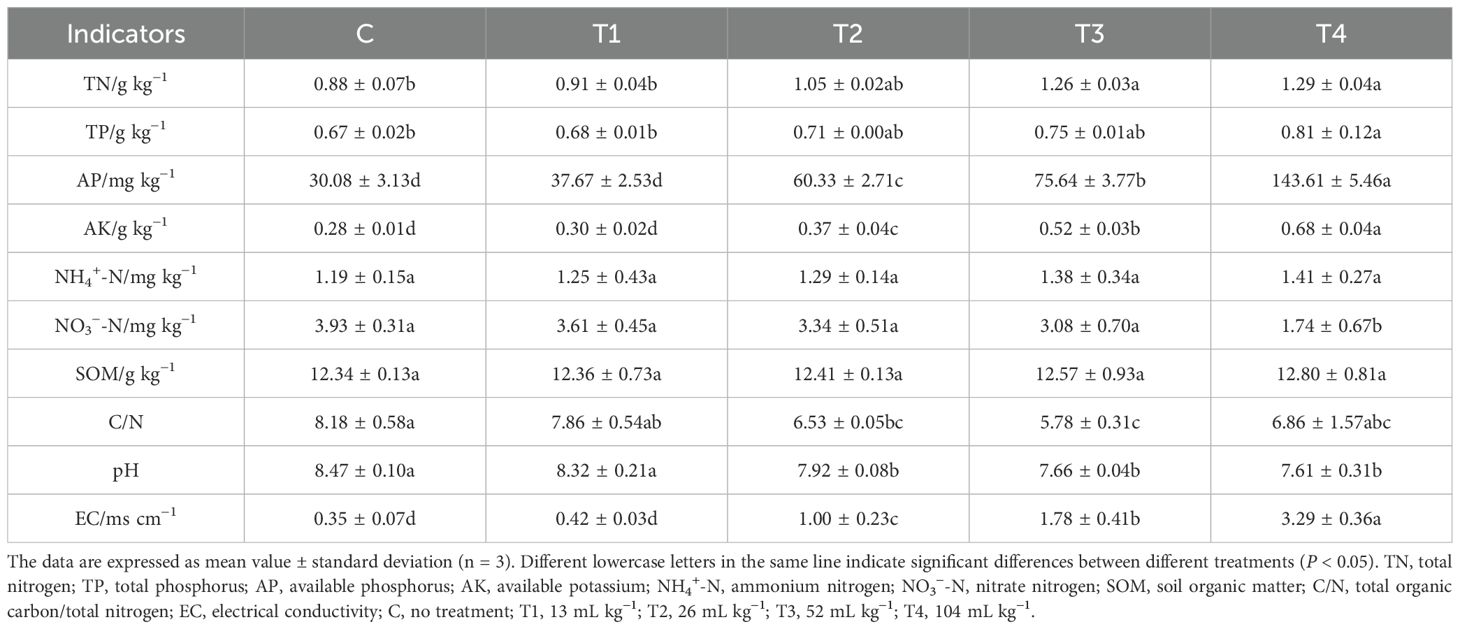
The application of HU to soil induced variations in several parameters such as soil TN, TP, AP, AK, C/N, pH, and EC as shown in Table 1. The content of NH4+-N remained statistically consistent across all treatment groups. TN and TP levels were highest in the T4 treatment, showing increases of 46.59% and 20.90%, respectively, compared to the C. AP and AK also experienced enhancements at various rates with higher volumes of HU, reaching their maximum in T4, which were 377% and 143% higher, respectively, than in C. The C/N ratio decreased under all treatments with T3 exhibiting the most significant reduction, 29.34% lower than C. Similarly, pH values decreased, with T3 showing the most substantial drop from 8.47 in the C to 7.66. EC was greatest in T4, showing an increment of 840%. Moreover, SOM levels slightly increased with greater HU addition.
3.2 Changes in the soil enzymatic activity
As the volume of HU increased, the enzymatic activities of INV, URE, and CAT initially rose and subsequently declined (Figure 1), whereas AKP activity showed a consistent decrease. Increasing the HU volume from 65 mL to 260 mL (T1 to T3) resulted in a linear increase in INV activity, with increments of 7.30% in T1 and peaking at 58.75% in T3, compared to the control (C, 0 mL). However, further increasing the HU volume to 520 mL (T4) led to a reduction in INV activity increment to 39.17% (Figure 1A, Supplementary Figure S2A). Trends in URE activity paralleled those observed for INV activity (Supplementary Figure S2B). Relative to C, URE activities in T1, T2, and T4 increased by 7.10%, 17.66%, and 0.93%, respectively, with T3 exhibiting the highest URE activity at 275.48 μg d−1 g−1, marking a 47.77% increase (Figure 1A). CAT activity reached its maximum in T1 (50.81 μmol d−1 g−1), which was 16.62% higher than C, but diminished in subsequent treatments (T1–T4) reaching its lowest level in T4 at 37.89 μmol d−1 g−1 (Figure 1C). Moreover, AKP activity exhibited a linear decline from C to T4 (Supplementary Figure S2D), with decreases of 6.40%, 9.45%, 34.25%, and 64.76% across T1 to T4, respectively, compared to C (Figure 1D).
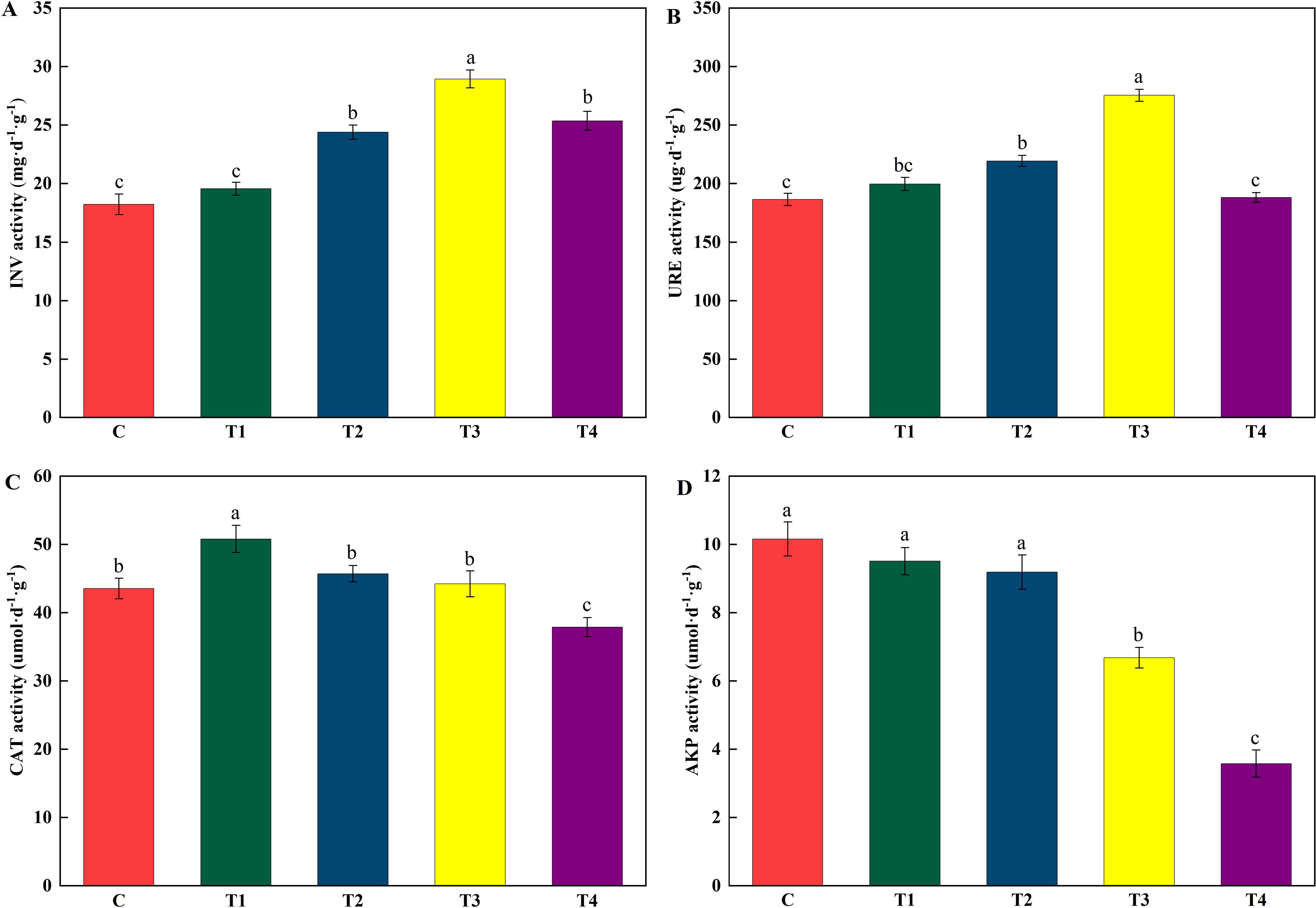
Figure 1. Changes in soil enzyme activity after application of HU. (A) INV activity; (B) URE activity; (C) CAT activity; (D) AKP activity. The Lowercase letters above the columns indicate significant differences between the different HU application treatments at P < 0.05, respectively. Abbreviations: NV = Invertase; URE = Urease; CAT = Catalase; AKP = Alkaline Phosphatase; C = no treatment; T1 = 13 mL∙kg-1; T2 = 26 mL∙kg-1; T3 = 52 mL∙kg-1; T4 = 104 mL∙kg-1.
3.3 Changes in soil bacterial diversity
Venn diagrams are commonly utilized to delineate the shared and unique operational taxonomic units (OTUs) across multiple samples. In our study, subsequent to stringent quality filtering, we amassed 630,747 high-fidelity 16S rRNA gene sequences across 15 samples, with individual samples contributing between 31,858 and 54,312 sequences. Post-chimera removal, non-redundant sequences were clustered into OTUs (excluding singletons) based on a 97% similarity criterion. From this process, a total of 4,356 OTUs were identified. Cluster analysis revealed that the bacterial communities were distributed among 33 phyla, 93 classes, and 827 genera. Additionally, a core set of 2,039 OTUs was present across all samples, whereas individual treatments T1, T2, T3, and T4 contained 3,498, 3,454, 3,219, and 2,902 OTUs, respectively (Figure 2).
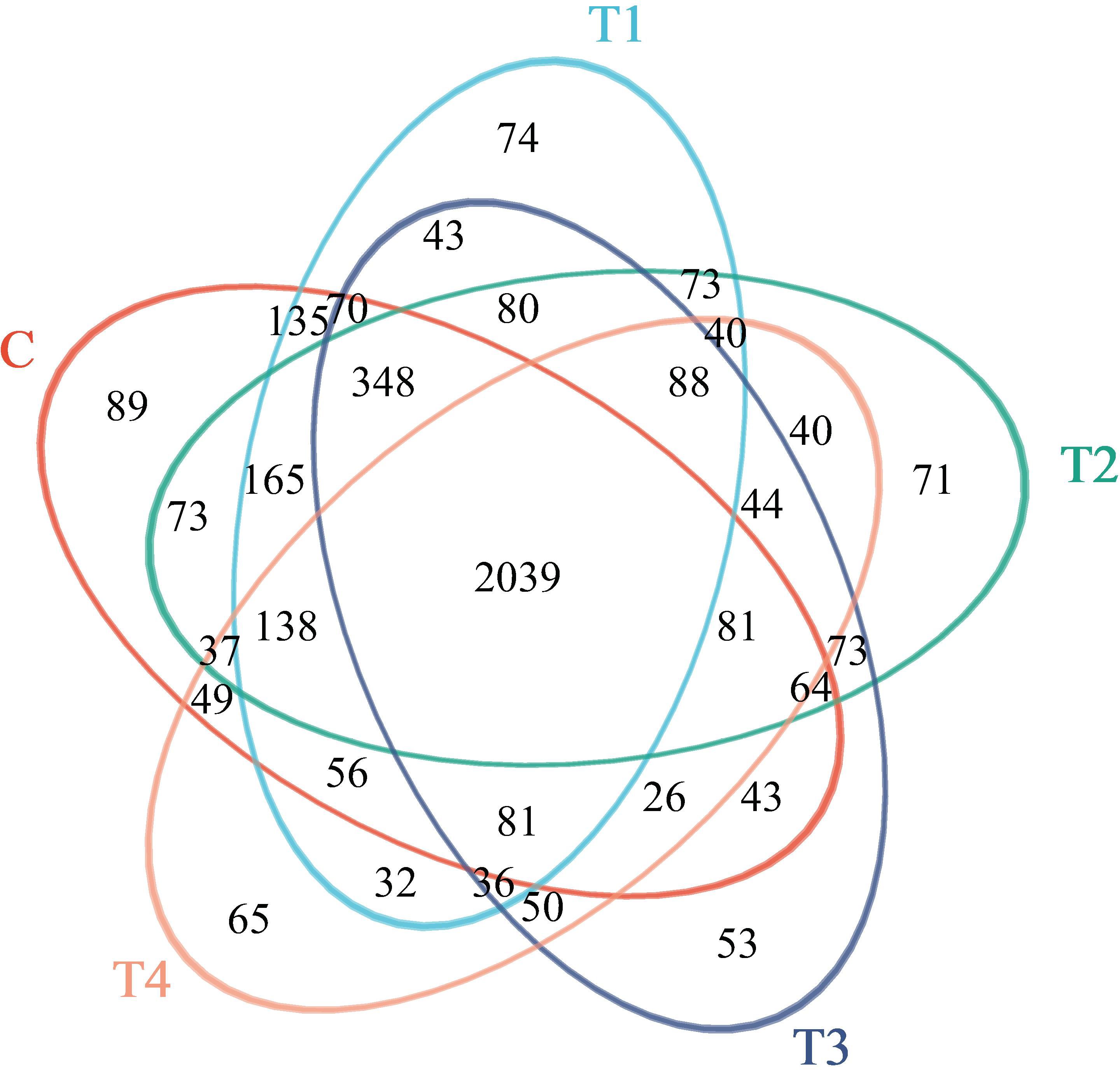
Figure 2. Venn diagram of bacterial communities in four soils with different application of HU.Abbreviations: C = no treatment; T1 = 13 mL∙kg-1; T2 = 26 mL∙kg-1; T3 = 52 mL∙kg-1; T4 = 104 mL∙kg-1.
Figure 3 illustrates that, in comparison to the C, the diversity in other groups decreased variably with increasing HU levels. Both the Shannon–Wiener diversity index and the Evenness index recorded the lowest values in T4, at 6.0815 and 0.7973, respectively. These represent decreases of 8.47% and 5.70% below the C values. The richness index initially increased slightly before declining as the HU addition was augmented. The peak increase in this index was observed in T1, where it reached 3,356.51, marking a 0.74% increase over the C value of 3331.68. A linear regression model was used to explore the linear relationship between the effects of HU on soil quality and bacterial diversity. It is mainly reflected that HU content has a significant positive correlation with SOM (P<0.001) and TN (P<0.05) content, and a significant negative correlation with C/N (P<0.05) and Shannon (P<0.001) (Supplementary Figure S3).
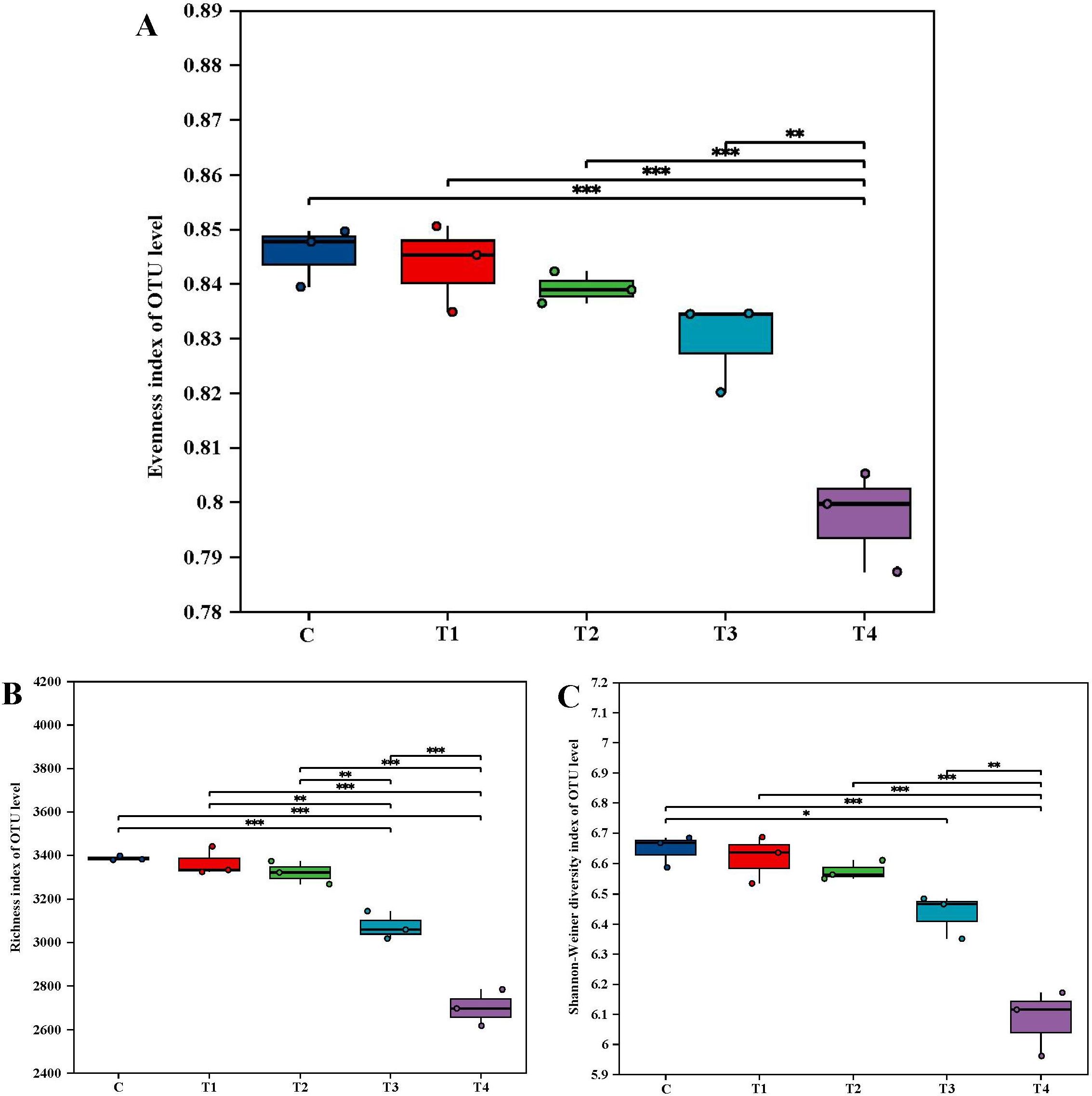
Figure 3. Effect of varying HU dosages on diversity of bacterial community. (A) Evenness index; (B) Richness index; (C) Shannon-Weiner diversity index. The data are expressed as mean value ± standard deviation (n=3). * denotes p < 0.05, ** denotes p < 0.01 and *** denotes p < 0.001. Abbreviations: C = no treatment; T1 = 13 mL∙kg-1; T2 = 26 mL∙kg-1; T3 = 52 mL∙kg-1; T4 = 104 mL∙kg-1.
3.4 Changes in the soil bacterial community structures
The distributions of soil bacterial abundances reveal that, at the phylum level (Figure 4A), the three predominant bacterial phyla were Pseudomonadota (28.79%), Actinomycetota (24.42%), and Chloroflexi (13.79%). These principal groups were accompanied by lesser abundances of Gemmatimonadota (7.21%), Bacteroidota (3.28%), Bacillota (2.98%), and Rokubacteriota (2.20%). In each experimental group, Pseudomonadota abundances were prominently dominant and exhibited an increase concurrent with higher HU additions. Specifically, compared to the control group (C), Pseudomonadota abundances rose by 9.81%, 5.43%, and 23.19% in T1, T2, and T3, respectively, with the most significant surge observed in T4, showing a 40.19% increase over C. Conversely, Actinomycetota displayed a decrease across all treatment groups (T1–T4) correlating with increased HU volumes, with declines of 6.82%, 9.72%, 20.30%, and 20.32% in T1–T4, respectively, relative to C. Similarly, Chloroflexi abundance trended downward, with T4 registering the lowest percentage of 10.50%, which is 30.37% lower than C. Acidobacteriota also showed a decreasing pattern across the treatments, with T4 presenting the minimal proportion of 5.69%, marking a substantial decrease of 64.24% in comparison to the C group. In contrast, with the escalation of HU concentration, both Gemmatimonadota and Bacteroidota phyla demonstrated increments in their richness. Gemmatimonadota richness increased by 15.99%, 27.71%, and 26.64% in T1, T2, and T4, respectively, when compared to C, with T3 experiencing a notable surge in richness (69.80% higher than C). Likewise, Bacteroidota saw increments of 4.05%, 7.21%, and 61.71% in T1, T2, and T3, respectively, with T4 showcasing the most considerable augmentation in richness.
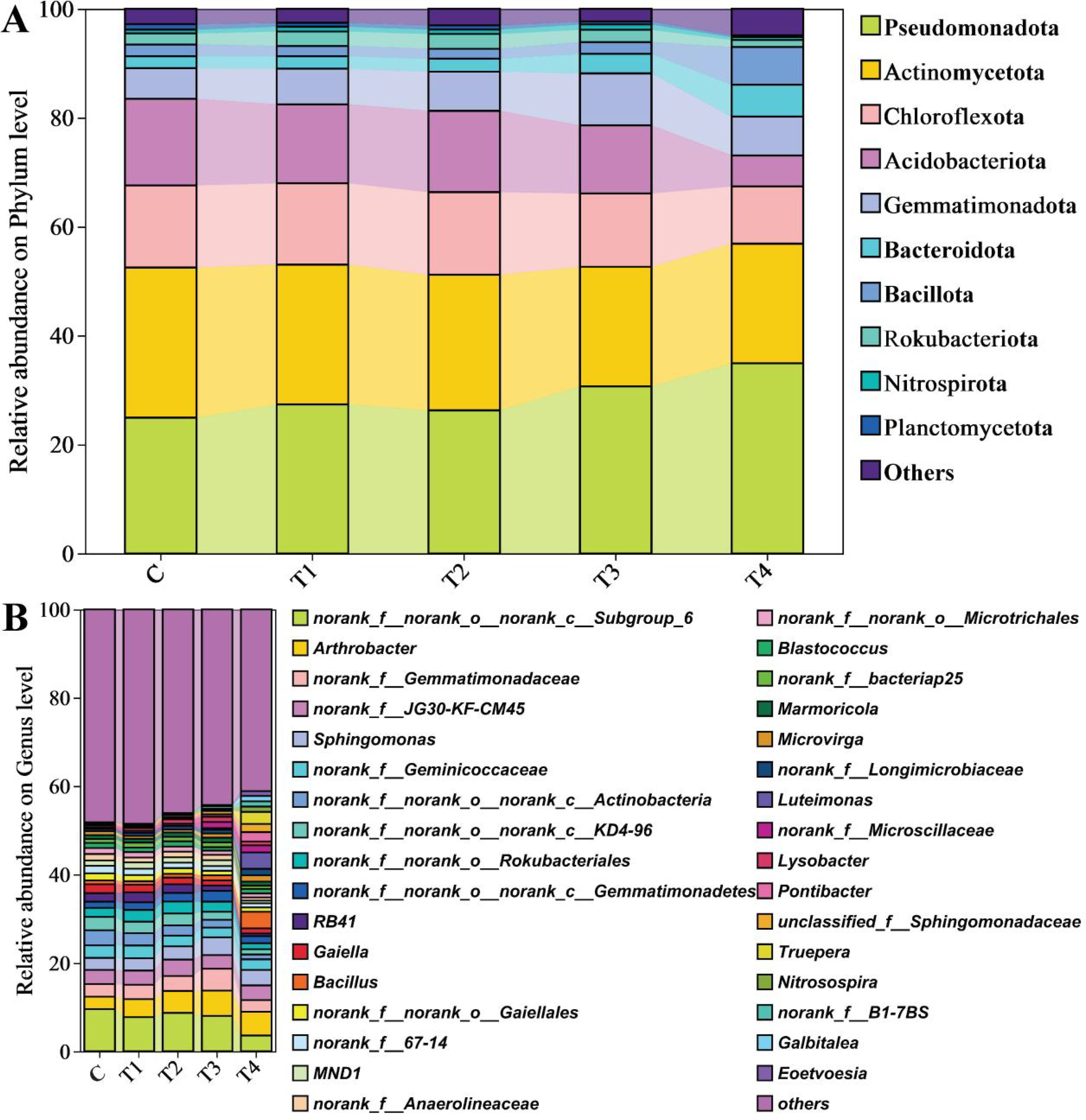
Figure 4. Relative abundances of relative abundance on phylum level (a) and genus level (b) for different application of HU. The phyla and genera accounting for less than 1% of the total composition in each library are represented by Others. Abbreviations: C = no treatment; T1 = 13 mL∙kg-1; T2 = 26 mL∙kg-1; T3 = 52 mL∙kg-1; T4 = 104 mL∙kg-1.
At the genus level, as shown in Figure 4B, predominant bacteria with a relative abundance exceeding 1.0% included unclassified Subgroup_6 members (7.52%), Arthrobacter (4.60%), unclassified JG30-KF-CM45 members (3.41%), unclassified Gemmatimonadaceae members (3.32%), and Sphingomonas (3.20%). Relative to C, the abundance of Sphingomonas increased between 1.10% and 47.25%, reaching a peak of 4.02% in the T3 treatment. Among the top 20 genera, such as Gaiella (1.49%), Bacillus (1.47%), and Blastococcus (1.02%), significant impacts were observed due to HU treatment. With the administration of HU, both Gaiella and Blastococcus showed marked declines. At the 520-mL application rate in T4, the relative abundances of Gaiella and Blastococcus decreased to 1.12% and 0.97%, representing reductions of 42.86% and 15.65% compared to C, respectively. In contrast, Bacillus reached a relative abundance of 3.37% in T4, which is 324% higher than that observed in C. ANCOM difference test analysis shows that g:Eoetvoesia, g:Truepera, g:Luteimonas, and g:norank_f:B1_7BS were significantly different among groups (Figure 5), with W of 823, 821, 800, and 781, respectively (Supplementary Table S5).
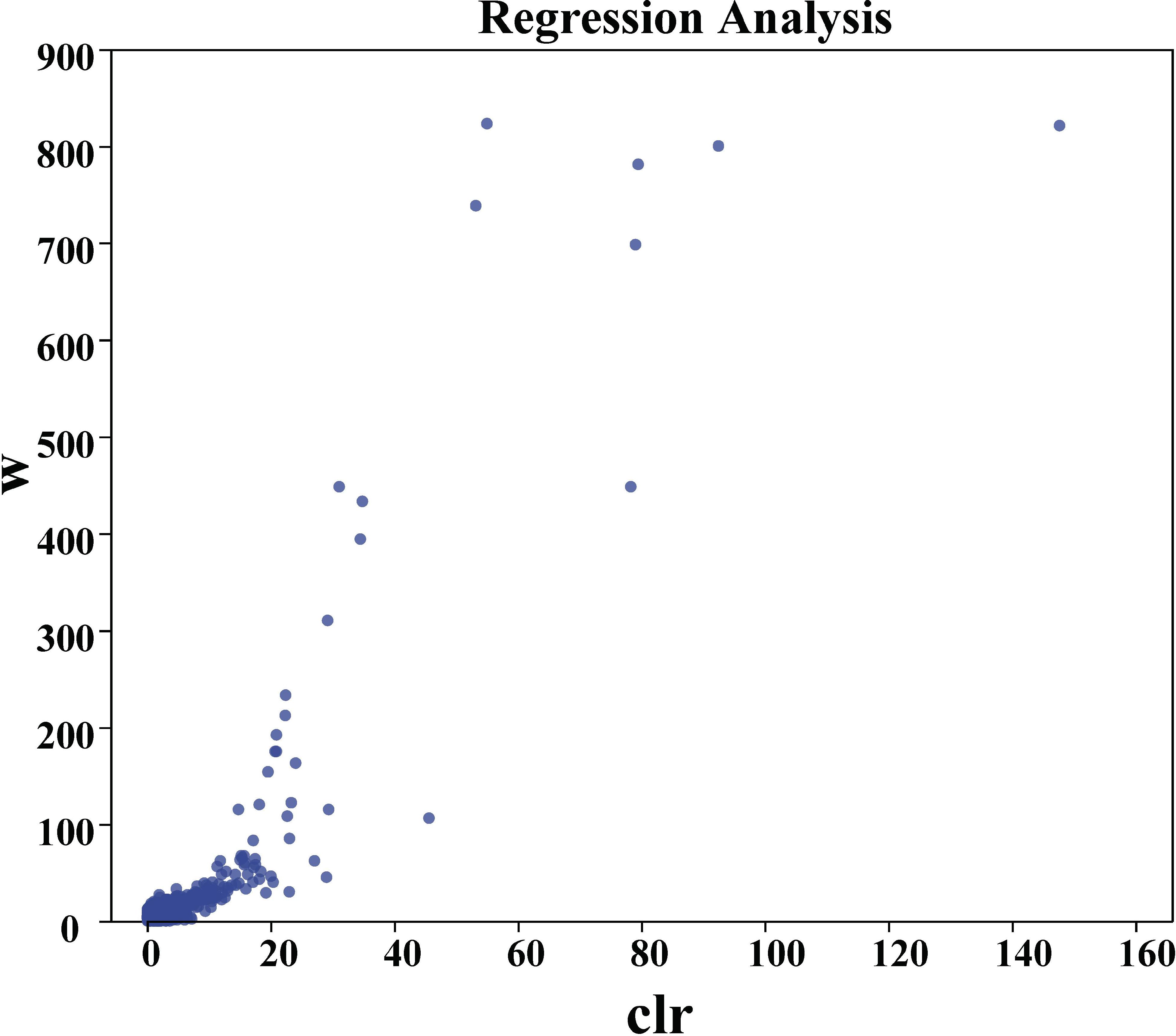
Figure 5. ANCOM Volcano map The points in the figure represent the species, the ordinate represents the W value, and the abscise represents the clr (center log transform) value, which represents the degree of difference in sample abundance between groups. The higher the absolute value of the number, the greater the difference in relative abundance.
3.5 Principal component analysis of the soil bacterial communities
The β-diversity of the soil bacterial communities was assessed using NMDS and ANOSIM. The NMDS analysis, visualized in Figure 6, reveals variations in the composition of the soil bacterial communities across different treatments (C, T1, T2, and T3) in comparison to T4 based on the abundance of OTUs. Notably, significant differences were observed in the structure of the bacterial communities among C, T1, T2, T3, and T4 treatments (R = 0.6533, P = 0.001), as illustrated by the clear separation and independence of samples in the NMDS plot. The distributions observed within the plot—a leftward skew in C, T1, and T2, versus a rightward skew in T4-highlight the substantial impact of HU on the structure of soil bacterial communities.
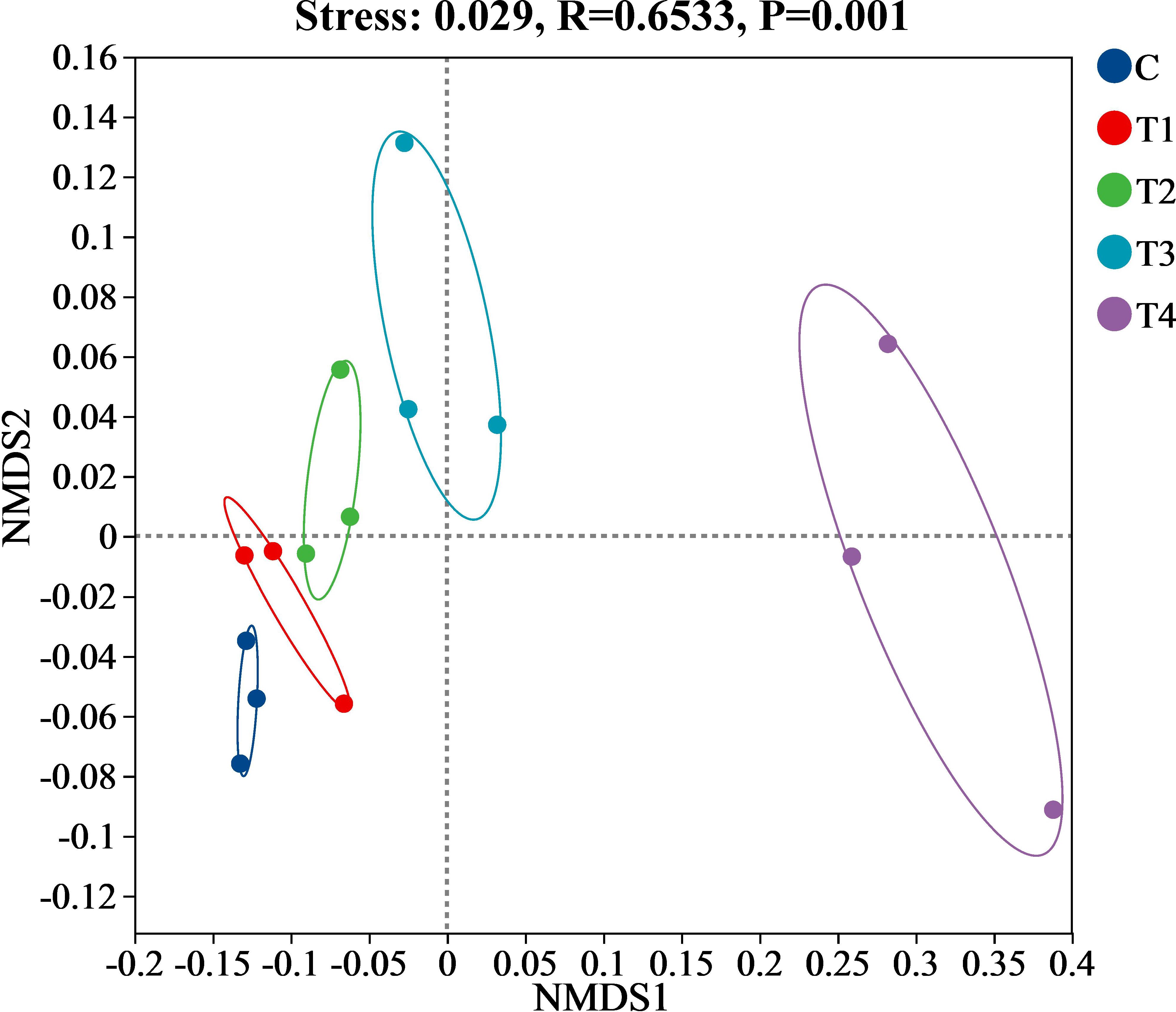
Figure 6. NMDS analysis of soil bacterial communities. Abbreviations: C = no treatment; T1 = 13 mL∙kg-1; T2 = 26 mL∙kg-1; T3 = 52 mL∙kg-1; T4 = 104 mL∙kg-1.
3.6 Correlation between soil enzymatic activity, physicochemical properties, and community structure
Table 2 presents the relationships between the relative abundances of the top 10 most abundant bacterial phyla and the enzymatic activities of four different soil enzymes. Gemmatimonadota were found to be strongly and statistically significantly correlated with INV and URE activities, whereas Pseudomonadota, Bacillota, and Planctomycetota displayed a pronounced positive association with AKP. Notably, Gemmatimonadota and Pseudomonadota exhibited marked negative correlations, in contrast to the positive correlations for Bacillota and Planctomycetota. In addition, Actinomycetota were negatively correlated with INV while showing a substantial positive association with AKP. The Chloroflexi and Acidobacteriota groups were strongly positively correlated with CAT and AKP activities. Conversely, Bacteroidota showed a negative relationship with CAT activity and were strongly inversely correlated with AKP. Lastly, Rokubacteriota had significant strong negative correlations with both CAT and AKP activities.
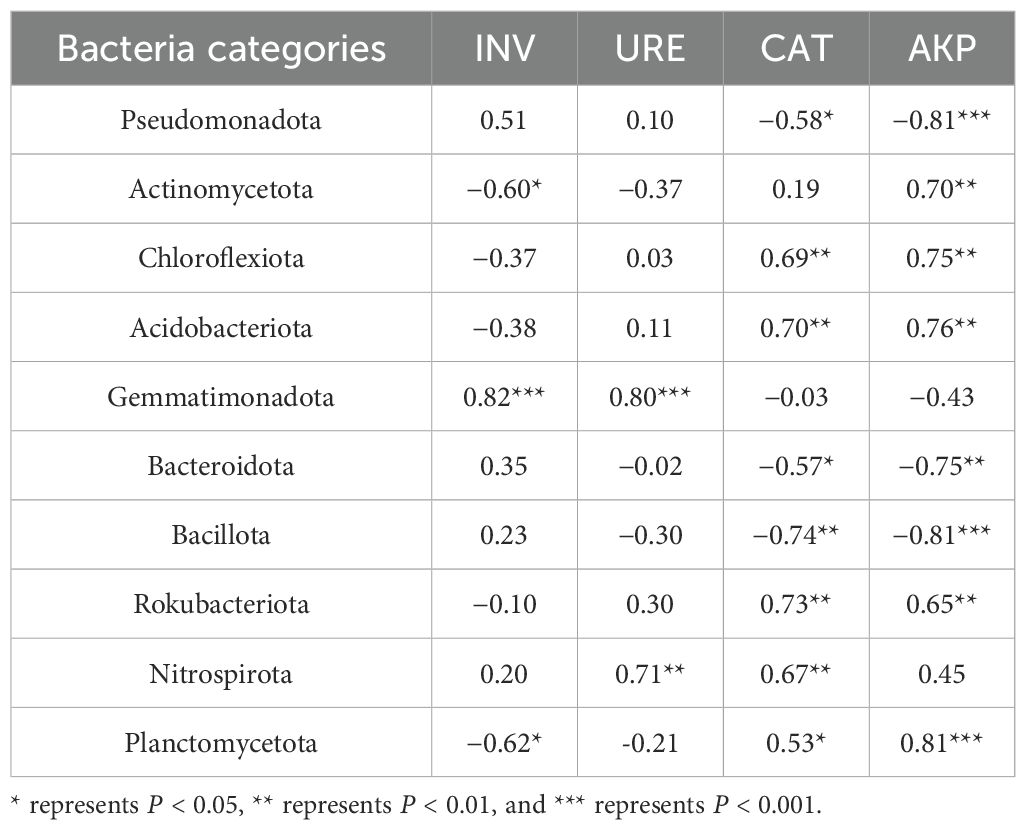
Table 2. Pearson correlation between the top 10 bacteria in phylum level abundance and soil enzyme activity.
RDA of bacterial community structures correlated with soil physicochemical properties, as depicted in Figure 7, revealed that Axis1 and Axis2 explained 63.18% and 8.59% of the variation in soil bacterial communities, respectively. Collectively, both axes accounted for 71.77% of the overall variation. Mantel test outcomes demonstrated that TN (R2 = 0.7524, P = 0.001), NH4+-N (R2 = 0.8027, P = 0.001), and SOM (R2 = 0.8069, P = 0.005) were the primary physicochemical parameters significantly affecting the bacterial community structure.
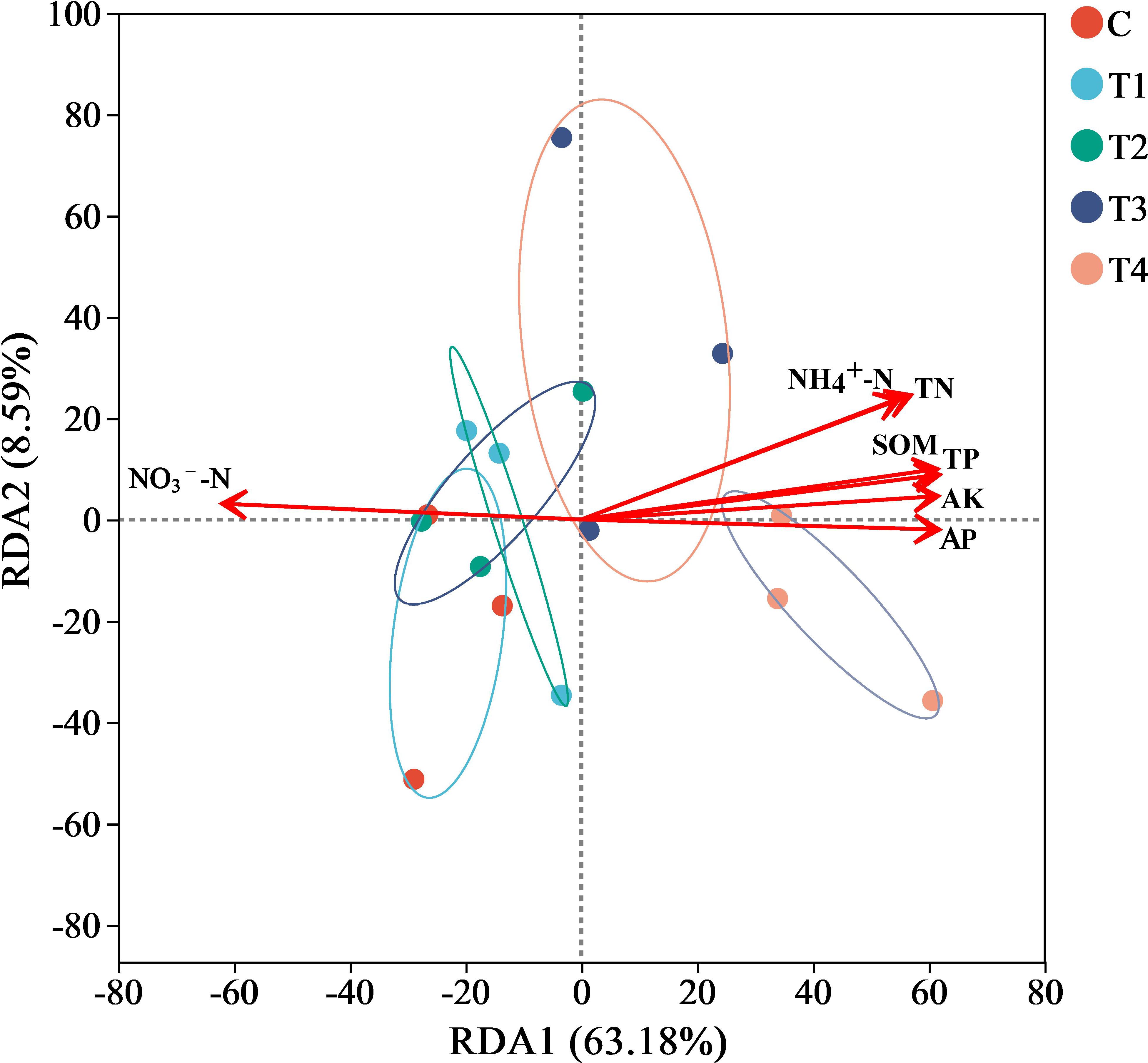
Figure 7. Redundancy analysis (RDA) of soil bacterial as explained by soil physicochemical properties in soil. Abbreviations: TN = Total Nitrogen; TP = Total Phosphorus; AP = Available Phosphorus; AK = Available Potassium; NH4+-N = Ammonium Nitrogen; NO3--N = Nitrate Nitrogen; SOM= Soil Organic Matter; C = no treatment; T1 = 13 mL∙kg-1; T2 = 26 mL∙kg-1; T3 = 52 mL∙kg-1; T4 = 104 mL∙kg-1.
4 Discussion
4.1 Effect of HU on soil physicochemical properties and enzymatic activity
The application of HU led to a decrease in soil pH, which can be attributed to the nitrification of ammonium ions within the soil. This process involves the conversion of ammonium salts into nitrites, releasing two protons and subsequently lowering the soil pH (Schönning, 2001). Despite varying volumes of HU being employed, the soil’s NH4+-N levels remained unchanged. This stability is likely due to the nitrogen within the HU transitioning from urea to ammonia, urine-N would be lost to NH3 evaporating (Rumeau et al., 2023), and a similar phenomenon also exists in the use of liquid ammonium fertilizers (Powlson and Dawson, 2022). Another possible reason is the leaching after nitrification due to high urinary N application; after NH4+-N is converted to NO3− through nitrification, NO3− may be carried with the water to the deeper layers of the soil due to leaching (Ramírez-Sandoval et al., 2022), which is consistent with the results of our study (Supplementary Table S6). Conversely, an increase in soil EC was observed with higher volumes of HU, attributed to the enhanced presence of conductive ions such as chloride ions in the HU. Consequently, as the volume of HU applied escalated, so did the concentration of ions, resulting in an increased soil EC (Kassa et al., 2018). The addition of HU resulted in significant increases in soil TN and TP, indicative of the high nitrogen and phosphorus content of HU. Therefore, extensive application of HU as a fertilizer substantially elevates the soil’s nitrogen and phosphorus levels (Bonzi et al., 2011). SOM also saw a linearly increment with increased HU applications (Supplementary Figure S3A), potentially contributing to the rise in soil TN levels. Additionally, soil AK content experienced increments at various degrees with the escalation of HU volume, a phenomenon likely tied to HU’s high potassium concentration. Thus, the application of greater volumes of HU correspondingly increased the soil potassium level. Moreover, soil NO3−-N content demonstrated a gradual decline with an increase in HU volume, as the lowered soil pH from HU application likely expedited the denitrification rate of NO3−-N (Pradhan et al., 2009).
Soil enzymes are instrumental in the cycling of nitrogen, carbon, and phosphorus in the soil and in activating the plant’s defense mechanisms (Fan et al., 2024; Zhu et al., 2024; Yan et al., 2025). (Tao et al., 2015) noted that the application of organic fertilizers, as opposed to chemical ones, significantly enhanced soil enzyme activities such as INV and URE. HU, as a nutrient-rich organic fertilizer, has been shown to increase the activities of soil INV and URE with increasing application volume (Krause and Rotter, 2018). Consistently, our results demonstrate pronounced enhancements in the activities of INV and URE, alongside significant correlations with changes in AP, AK, SOM, C/N, and EC (Supplementary Table S1). Furthermore, SOM is considered a critical substrate for enzyme synthesis and plays a vital role in stimulating soil enzyme activity (Wei et al., 2015). Such dynamics underscore the close association between soil nutrient status and enzyme activities (Demisie et al., 2014). In the present study, the application of HU corresponded with a linearly reduction in soil C/N ratios (Supplementary Figure S3C), aligning with observations by (Deng et al., 2020) Under certain circumstances, the soil C/N ratio can serve as an indicator of SOM quality (Qin et al., 2023). A significant negative correlation was observed between soil INV and URE activities and the C/N ratio (p < 0.01) (Supplementary Table S1), consistent with findings by Li et al (Li et al., 2017). We noted that the C/N ratio decreased progressively at treatments T1, T2, and T3 but surged dramatically at T4, which was associated with a suppression of soil INV and URE activities (Table 1; Figures 1A, B), this might be that adding too much urine reduces the abundance of Gemmatimonadota, resulting in the inhibition of urease activity (Table 2), which is consistent with the previous research results (Cheng et al., 2020). Additionally, previous studies indicated a positive correlation between Acidobacteriota and AKP activities, and a negative correlation between CAT activities and Bacillota (Ren et al., 2021). Soil AKP activity was found to be inhibited, likely due to increased urine content which subsequently reduced the soil pH. AKP is highly sensitive to soil acidity, and its activity is diminished under low pH conditions. Overall, both the soil physicochemical properties and microbial communities were shown to significantly influence soil enzyme activities.
Moreover, the germination and seedling phases are particularly susceptible during the course of plant development, with salinity serving as a significant environmental constraint on growth, as documented by (Tang et al., 2015) In our study, application rates of HU at 52 mL kg−1 and 104 mL kg−1 (T3 and T4 treatments) inhibited normal germination and growth of pakchoi (Supplementary Figure S1). A contributing factor may be the observed sharp increase in soil electrical conductivity to levels between 1.78 and 3.29 mS cm−1, which represents an elevation of 78% to 229% compared to the T2 treatment. This heightened soil salinity can exert detrimental osmotic pressure, impeding plant development (Acosta-Motos et al., 2017). Furthermore, the inherent sodium and chloride content of HU may also play a role; as application rates rise, the subsequent accumulation of these ions could disrupt nutrient balance across cellular membranes and ultimately prove toxic to the pakchoi plants (Negrao et al., 2017). Consequently, meticulous regulation of HU application rates is imperative in future agricultural practices to circumvent its potential phytotoxic effects and safeguard crop yields.
4.2 Effect of HU on the soil bacterial diversity and community composition
The Shannon–Wiener diversity index (S index) serves as a metric for assessing microbial diversity, where a greater value of the S index signifies enhanced community diversity. In the present study, elevated levels of HU application were correlated with a consistent reduction in the S index during pakchoi cultivation, suggesting that HU application attenuates soil bacterial diversity. This attenuation may be attributable to the increase in soil conductivity and salinity induced by HU, as soil microorganisms are acutely sensitive to shifts in salinity within their habitat. Such increases in salinity can impose osmotic stress upon soil microbes, thereby diminishing microbial diversity (Chen et al., 2022). Concurrently, the Evenness and Richness indices exhibited a generally declining trend as shown in Figure 3, implying that adding HU to the soil during the cultivation of pakchoi reduces bacterial richness. Prior research indicates that soil microbial life is considerably influenced by pH levels (Geisseler and Scow, 2014). In our investigation, the rise in soil EC and the drop in pH associated with greater volumes of HU might be contributing factors to the observed alterations in soil bacterial diversity and community composition (Wu et al., 2020).
Analysis of community structure demonstrated that the abundances of Actinomycetota, Chloroflexi, and Acidobacteriota decreased to varying extents. Given that Acidobacteriota predominantly adopt an oligotrophic lifestyle while HU is nutrient-rich (Ding et al., 2018), it is suggested that HU application could enhance soil fertility and suppress the growth of these bacteria. Conversely, the relative abundances of Pseudomonadota, Bacteroidota, and Gemmatimonadota were observed to increase alongside rising HU volumes. This trend may be attributable to the higher soil fertility requirements of these bacteria, which were met through HU application, thus providing an optimal growth milieu (Guo et al., 2018). Additionally, Chloroflexi have the capability to convert nitrites into nitrates (Daims et al., 2015), whereas Pseudomonadota, Bacteroidota, and Gemmatimonadota possess denitrification capabilities (Ren, 2018). These observations suggest that HU application skewed nitrogen cycling toward reduced nitrification but enhanced denitrification, aligning with the observed declines in NO3−-N within soil physicochemical attributes. Furthermore, genera such as Sphingomonas and Bacillus were noted to elevate the abundance of genes linked to nitrogen metabolism and potentially reduce ammonia nitrogen consumption by influencing nitrogen transport (Sun et al., 2020; Yang et al., 2021). Sphingomonas was also recognized for its capacity to degrade a broad spectrum of harmful compounds (Gatheru et al., 2017). This might be attributable to HU application elevating the soil’s ammonia-nitrogen levels, subsequently increasing Sphingomonas and Bacillus populations, a finding corroborated by (Wang et al., 2022) Moreover, belonging to Actinomycetota, Gaiella was mentioned for its role in soil nitrate assimilation and in facilitating the uptake of amino acids and other nutrients by plants (Leite et al., 2021). These predominant genera are posited as significant ecosystem contributors, notably in soils treated with HU (Roy et al., 2022). Consequently, HU application has a direct impact on soil physicochemical characteristics and nutrient content, inducing environmental shifts that influence soil bacterial communities.
4.3 Relationship between soil enzymatic activity and bacterial communities
Soil enzymes, predominantly synthesized by microorganisms with minor contributions from plants and other soil biota, have been found to play pivotal roles in the nutrient cycling and metabolism within soil ecosystems (Liu et al., 2022). The production of enzymes, which often varies with shifts in microbial community composition, can exert different influences on soil processes. (Baldrian et al., 2010; Qu et al., 2025) reported that soil microorganisms—and, consequently, enzymatic activities—are subject to regulation by a constellation of complex factors that govern microbial community dynamics, with microbial biomass and enzyme activity often showing significant correlation within the upper tens of centimeters of the soil profile (Baldrian et al., 2010). Correlation analyses revealed that the relative abundance of Gemmatimonadota was strongly correlated with INV and URE activities, whereas the relative abundances of Pseudomonadota, Bacillota, and Planctomycetota were closely associated with AKP activity. The former phyla exhibited notably strong negative correlations, whereas the latter displayed significantly positive correlations. These patterns may arise from the enhancement of SOM due to HU amendments, which, in turn, increase bacterial abundance and alter community structure (Jiao et al., 2013). The findings underscore the varied and intricate interconnections between soil enzymatic activities and bacterial communities, underscoring the need for more comprehensive investigation into the specific relationships between distinct bacterial phyla and soil enzymes.
Excessive application of HU for irrigation could potentially lead to soil salinization and eventually promote land erosion (Kassa et al., 2018; Chapman, 1992). Concurrently, risks associated with ammonia volatilization and pathogen contamination persist throughout the urine reuse process (Martin et al., 2022). To address these concerns, it is recommended to either dilute urine or pre-treat HU using appropriate chemical agents such as calcium hydroxide and magnesium hydroxide to convert it into a stable and useful solid form of fertilizer. These chemicals are readily available and cost-effective. Additionally, the introduction of calcium and magnesium can markedly diminish pathogen levels due to the elevated pH values in HU (Kishor et al., 2020). Furthermore, advanced oxidation processes including ozone treatment and electrochemical oxidation have proven effective in sterilization and in decomposing micropollutants such as pharmaceuticals, antibiotics, and hormones in HU (Krishnan et al., 2021).
5 Conclusion
Based on the results of this study, we draw the following conclusions: (1) HU can enhance soil nutrient contents by enriching it with N, P, and K. There is a linear correlation between soil nutrient content and the volume of HU applied. (2) HU increases the enzymatic activities of INV, URE, and CAT, whereas it decreases the activity of AKP. Soil enzymatic activities are closely linked to soil physicochemical properties and the microbial community composition. (3) The application of HU tends to increase the abundance of Pseudomonadota and Gemmatimonadota but decreases the populations of Actinomycetota, Chloroflexi, and Acidobacteriota. In addition, HU increased the abundance of Sphingomonas, but both Gaiella and Blastococcus decreased significantly. HU significantly influences the diversity of soil bacterial communities, which tends to decrease as HU volume increases.
In conclusion, HU serves as a viable alternative to conventional chemical fertilizers by not only enhancing soil nutrient content but also reducing environmental pollution attributable to chemical fertilizers. However, the buffering capacity of soils against urine varies, making application conditions and methods crucial. Unscientific use of urine as fertilizer may lead to soil acidification and salinization. Therefore, based on the current pot experiment, long-term field trials are necessary to comprehensively and accurately evaluate the value and risks of utilizing urine as a fertilizer resource.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
GY: Conceptualization, Data curation, Formal Analysis, Supervision, Validation, Writing – original draft, Writing – review & editing. QW: Conceptualization, Validation, Writing – original draft, Writing – review & editing. XZ: Conceptualization, Investigation, Project administration, Supervision, Writing – review & editing. BY: Formal Analysis, Methodology, Software, Validation, Writing – review & editing. CZ: Formal Analysis, Methodology, Resources, Writing – review & editing. GZ: Conceptualization, Validation, Visualization, Writing – review & editing. XW: Funding acquisition, Investigation, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Special Fund for Basic Scientific Research of Central Public Welfare Institutes (CAAS-ZDRW202306) and Tianjin Agricultural Industry Research and Application ‘Challenge and Appointment’ Project(GBTG202404).
Conflict of interest
Author GZ was employed by the company Tianjin Municipal Engineering Design & Research Institute Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fagro.2025.1610839/full#supplementary-material
References
Acosta-Motos J.R., Ortuño M.F., Bernal-Vicente A., Diaz-Vivancos P., Sanchez-Blanco M.J., and Hernandez J.A. (2017). Plant responses to salt stress, adaptive mechanisms. Agronomy 7, 18. doi: 10.3390/agronomy7010018
Adeoluwa O. O. and Cofie O. (2012). Urine as an alternative fertilizer in agriculture, effects in amaranths (Amaranthus caudatus) production. Renew. Agr. Food Syst. 27, 287–294. doi: 10.1017/S1742170511000512
Akhtar K., Wang W., Ren G., Khan A., Feng Y., and Yang G. (2018). Changes in soil enzymes, soil properties, and maize crop productivity under wheat straw mulching in Guanzhong, China. Soil Tillage Res. 182, 94–102. doi: 10.1016/j.still.2018.05.007
Akpan-Idiok A. U., Udo I. A., and Braide E. I. (2012). The use of human urine as an organic fertilizer in the production of okra (Abelmoschus esculentus) in South Eastern Nigeria. Resour. Conserv. Recycl. 62, 14–20. doi: 10.1016/j.resconrec.2012.02.003
Baldrian P., Merhautová V., Cajthaml T., Petránková M., and Šnajdr J. (2010). Small-scale distribution of extracellular enzymes, fungal, and bacterial biomass in Quercus petraea forest topsoil. Biol. Fertil. Soils 46, 717–726. doi: 10.1007/s00374-010-0478-4
Bao S. (2008). Soil Agricultural Chemistry Analysis Method. 3rd ed. (Beijing, China: China Agriculture Press).
Bonzi M., et al. (2011). A Study of the Agronomic Efficiency of human Stool and urine on Production of Maize and Egg Plant in Burkina Faso (Springer, Dordrecht: Springer Netherlands).
Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K., et al. (2010). QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336. doi: 10.1038/nmeth.f.303
Chapman D. V. (2021). Water quality assessments: A guide to the use of biota, sediments and water in environmental monitoring. Chapman Hall London. 132(5), 1121–1129. doi: 10.1201/9781003062103
Chen H., Ma K., Huang Y., Fu Q., Qiu Y., and Yao Z. (2022). Significant response of microbial community to increased salinity across wetland ecosystems. Geoderma 415, 115778. doi: 10.1016/j.geoderma.2022.115778
Cheng H., Zhang D., Huang B., Song Z., Ren L., Hao B., et al. (2020). Organic fertilizer improves soil fertility and restores the bacterial community after 1, 3-dichloropropene fumigation. Sci. Total Environ. 738, 140345. doi: 10.1016/j.scitotenv.2020.140345
Chrispim M. and Nolasco M. (2012). Human urine as fertilizer, Feasibility study of use in corn and lettuce cultivation in a university campus in Brazil. Study presented at 4th International Dry Toilet Conference. Tampere Finland 8, 22–25.
Daims H., Lebedeva E.V., Pjevac P., Han P., Herbold C., Albertsen M., et al. (2015). Complete nitrification by Nitrospira bacteria. Nat. 528, 504–509. doi: 10.1038/nature16461
Demisie W., Liu Z., and Zhang M. (2014). Effect of biochar on carbon fractions and enzyme activity of red soil. Catena 121, 214–221. doi: 10.1016/j.catena.2014.05.020
Deng X., Ma W., Ren Z., Zhang M., Grieneisen M.L., Chen X., et al. (2020). Spatial and temporal trends of soil total nitrogen and C/N ratio for croplands of East China. Geoderma 361, e114035. doi: 10.1016/j.geoderma.2019.114035
Ding L., Su J., Sun G., Wu J., and Wei W. (2018). Increased microbial functional diversity under long-term organic and integrated fertilization in a paddy soil. Appl. Microbiol. Biotechnol. 102, 1969–1982. doi: 10.1007/s00253-017-8704-8
Du J., Hou F., and Zhou Q. (2021). Response of soil enzyme activity and soil bacterial community to PCB dissipation across different soils. Chemosphere 283, 131229. doi: 10.1016/j.chemosphere.2021.131229
Esrey S. A., Andersson I., and Sawyer R. (2001). Closing the Loop, Ecological sanitation for food security. 18.
Fan Z., Wang J., Lv D., Li S., Miao Y., Hu M., et al. (2024). Effects of cropland-to-orchard conversion on soil multifunctionality, particularly nitrogen cycling in the eastern Loess Plateau. Front. Microbiol. 15, 1471329. doi: 10.3389/fmicb.2024.1471329
Francioli D., Schulz E., Lentendu G., Wubet T., Buscot F., and Reitz T. (2016). Mineral vs. organic amendments, microbial community structure, activity and abundance of agriculturally relevant microbes are driven by long-term fertilization strategies. Front. Microbiol. 7, 1446. doi: 10.3389/fmicb.2016.01446
Waigi M.G. and Sun and Gao K. Y. (2017). Sphingomonads in microbe-assisted phytoremediation: tackling soil pollution. Trends Biotechnol. 35, 883–899. doi: 10.1016/j.tibtech.2017.06.014
Geisseler D. and Scow K. M. (2014). Long-term effects of mineral fertilizers on soil microorganisms-A review. Soil Biol. Biochem. 75, 54–63. doi: 10.1016/j.soilbio.2014.03.023
Guo J., Liu W., Zhu C., Luo G., Kong Y., Ling N., et al. (2018). Bacterial rather than fungal community composition is associated with microbial activities and nutrient-use efficiencies in a paddy soil with short-term organic amendments. Plant Soil 424, 335–349. doi: 10.1007/s11104-017-3547-8
Han H., Song P., Jiang Y., Fan J., Khan A., Liu P., et al. (2024). Biochar immobilized hydrolase degrades PET microplastics and alleviates the disturbance of soil microbial function via modulating nitrogen and phosphorus cycles. J. Hazard. Mater. 474, 134838. doi: 10.1016/j.jhazmat.2024.134838
Hilton S. P., Keoleian G. A., Daigger G. T., Zhou B., and Love N. G. (2021). Life cycle assessment of urine diversion and conversion to fertilizer products at the city scale. Environ. Sci. Technol. 55, 593–603. doi: 10.1021/acs.est.0c04195
Huang F., Liu Z., Mou H., Li J., Zhang P., and Jia Z. (2019). Impact of farmland mulching practices on the soil bacterial community structure in the semiarid area of the loess plateau in China. Eur. J. Soil Biol. 92, 8–15. doi: 10.1016/j.ejsobi.2019.04.001
Jiao K., Qin S., Lyu D., Liu L., and Ma H. (2013). Red clover intercropping of apple orchards improves soil microbial community functional diversity. Acta Agric. Scand. 63, 466–472. doi: 10.1080/09064710.2013.799219
Johansen J.L., Dam M., Kudjordjie E.N., Santos S.S., Palmqvist A., Magid J., et al. (2023). Effects of long-term fertilization with contemporary Danish human urine, composted household waste and sewage sludge on soil nematode abundance and community structure. Sci. Total Environ. 860, 160485. doi: 10.1016/j.scitotenv.2022.160485
Karimi B., Terrat S., Dequiedt S., Saby N. P., Horrigue W., Lelièvre M., et al. (2018). Biogeography of soil bacteria and archaea across France. Sci. Adv. 4, eaat1808. doi: 10.1126/sciadv.aat1808
Kassa K., Ali Y., and Zewdie W. (2018). Human urine as a source of nutrients for maize and its impacts on soil quality at Arba Minch, Ethiopia. J. Water Reuse Desalin. 8, 516–521. doi: 10.2166/wrd.2018.060
Kishor N., Kale S., and Agrawal P. S. (2020). Use of fertilizers derived from urine as a plant growth regulator. Mater. Today: Proc. 32, 504–509. doi: 10.1016/j.matpr.2020.02.761
Krause A. and Rotter V. S. (2018). Recycling improves soil fertility management in smallholdings in Tanzania. Agriculture 8, 31. doi: 10.3390/agriculture8030031
Krishnan R. Y., Manikandan S., Subbaiya R., Biruntha M., Govarthanan M., and Karmegam N. (2021). Removal of emerging micro pollutants originating from pharmevennessuticals and personal care products (PPCPs) in water and wastewater by advanced oxidation processes: A review. Environ. Technol. Innovation 23, 101757. doi: 10.1016/j.eti.2021.101757
Leite H.M.F., Calonego J.C., Rosolem C.A., Mendes L.W., de Moraes L.N., Grotto R.M.T., et al. (2021). Cover crops shape the soil bacterial community in a tropical soil under no-till. Appl. Soil Ecol. 168, 104166. doi: 10.1016/j.apsoil.2021.104166
Lemanowicz J., Haddad S. A., Bartkowiak A., Lamparski R., and Wojewodzki P. (2020). The role of an urban park’s tree stand in shaping the enzymatic activity, glomalin content and physicochemical properties of soil. Sci. Total Environ. 741, 140446. doi: 10.1016/j.scitotenv.2020.140446
Li W.T., Liu M., Jiang C.Y., Wu M., Chen X.F., Ma X.Y., et al. (2017). Changes in soil aggregate-associated enzyme activities and nutrients under long-term chemical fertilizer applications in a phosphorus-limited paddy soil. Soil Use Manage. 33, 25–33. doi: 10.1111/sum.2017.33.issue-1
Li X., Qu C., Bian Y., Gu C., Jiang X., and Song Y. (2019). New insights into the responses of soil microorganisms to polycyclic aromatic hydrocarbon stress by combining enzyme activity and sequencing analysis with metabolomics. Environ. pollut. 255, 113–312. doi: 10.1016/j.envpol.2019.113312
Lienert J., Güdel K., and Escher B. I. (2007). Screening method for ecotoxicological hazard assessment of 42 pharmaceuticals considering human metabolism and excretory routes. Environ. Sci. Technol. 41, 4471–4478. doi: 10.1021/es0627693
Liu B., Xia H., Jiang C., Riaz M., Yang L., Chen Y., et al. (2022). 14 year applications of chemical fertilizers and crop straw effects on soil labile organic carbon fractions, enzyme activities and microbial community in rice-wheat rotation of middle China. Sci. Total Environ. 841, 156608. doi: 10.1016/j.scitotenv.2022.156608
Liu Y., Fan X., Zhang T., He W., and Song F. (2020). Effects of the long-term application of atrazine on soil enzyme activity and bacterial community structure in farmlands in China. Environ. pollut. 262, 114–264. doi: 10.1016/j.envpol.2020.114264
Luo J.Y., Zhang S., Zhu X.Z., Lu L.M., Wang C.Y., Li C.H., et al. (2017). Effects of soil salinity on rhizosphere soil microbes in transgenic Bt cotton fields. J. Integr. Agric. 16, 1624–1633. doi: 10.1016/S2095-3119(16)61456-9
Martin M. (2011). Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17, 10–12. doi: 10.14806/ej.17.1.200
Martin T. M. P., Esculier F., Levavasseur F., Levavasseur F., and Houot S. (2022). Human urine-based fertilizers: A review. Crit. Rev. Environ. Sci. Technol. 52, 890–936. doi: 10.1080/10643389.2020.1838214
Migeri S., Lawal M.A., Hughes J.C., Badza T., Abafe O.A., Martincigh B.S., et al. (2023). Uptake of selected antiretrovirals by pepper (Capsicum annum), radish (Raphanus sativus), and ryegrass (Lolium perenne) grown on two contrasting soils and fertilized with human urine-derived fertilizers. Sci. Total Environ. 892, 164551. doi: 10.1016/j.scitotenv.2023.164551
Morgan P. (2003). Experiments using urine and humus derived from ecological toilets as a source of nutrients for growing crops (Kyoto, Japan: 3rd world water forum), 16–23.
Nearing J.T., Douglas G.M., Hayes M.G., MacDonald J., Desai D.K., Allward N., et al. (2022). Microbiome differential abundance methods produce different results across 38 datasets. Nat. Commun. 13, 342. doi: 10.1038/s41467-022-28034-z
Negrao S., Schmockel S. M., and Tester M. (2017). Evaluating physiological responses of plants to salinity stress. Ann. Bot. 119, 1–11. doi: 10.1093/aob/mcw191
Oksanen J., Simpson J. L., Blanchet Kindt F. G.R., Legendre P., Minchin P. R., et al. (2017). vegan Community Ecology Package (R package version 2.44). Available at: https://CRAN.R-project.org/package=vegan.
Pandorf M., Hochmuth. G., and Boyer T. H. (2019). Human urine as a Fertilizer in the Cultivation of Snap Beans (Phaseolus vulgaris) and Turnips (Brassica rapa). J. Agric. Food Chem. 67, 50–62. doi: 10.1021/acs.jafc.8b06011
Powlson D. S. and Dawson C. J. (2022). Use of ammonium sulphate as a sulphur fertilizer: Implications for ammonia volatilization. Soil Use Manage. 38, 622–634. doi: 10.1111/sum.12733
Pradhan S. K., Holopainen J. K., and Heinonen-Tanski H. (2009). Stored human urine supplemented with wood ash as fertilizer in tomato (Solanum lycopersicum) cultivation and its impacts on fruit yield and quality. J. Agric. Food Chem. 57, 7612–7617. doi: 10.1021/jf9018917
Pu Y., Lang S., Wang A., Zhang S., Li T., Qian H., et al. (2022). Distribution and functional groups of soil aggregate-associated organic carbon along a marsh degradation gradient on the Zoige Plateau, China. Catena 209, 105811. doi: 10.1016/j.catena.2021.105811
Qin W., Zhao X., Yang F., Chen J., Mo Q., Cui S., et al. (2023). Impact of fertilization and grazing on soil N and enzyme activities in a karst pasture ecosystem. Geoderma 437, 116578. doi: 10.1016/j.geoderma.2023.116578
Qu Z., Chen Q., Deng H., Wang Q., Yao S., Chen Q., et al. (2025). Controlled-release phosphate fertilizer improves soil fertility and soybean productivity by regulating soil microbial diversity and composition and increasing enzyme activities. Field Crops Res. 325, 109836. doi: 10.1016/j.fcr.2025.109836
Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., et al. (2013). The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 41, 590–596. doi: 10.1093/nar/gks1219
Ramírez-Sandoval M., Pinochet D., Rivero M. J., and Cardenas L. M. (2022). Effect of cow urine nitrogen rates and moisture conditions on nitrogen mineralization in andisol from Southern Chile. Agronomy 13, 10. doi: 10.3390/agronomy13010010
Raza S., Zamanian K., Ullah S., Kuzyakov Y., Virto I., and Zhou J. (2021). Inorganic carbon losses by soil acidification jeopardize global efforts on carbon sequestration and climate change mitigation. J. Cleaner Prod. 315, 128036. doi: 10.1016/j.jclepro.2021.128036
Ren M. (2018). Microbial community structure in Tarim Basin and its role in carbon and nitrogen cycle (Huazhong Agricultural University).
Ren J., Liu X., Yang W., Yang X., Li W., Xia Q., et al. (2021). Rhizosphere soil properties, microbial community, and enzyme activities: Short-term responses to partial substitution of chemical fertilizer with organic manure. J. Environ. Manage. 299, 113650. doi: 10.1016/j.jenvman.2021.113650
Roy M. A., Sharma S., Braunius K. P., Ajmani A. M., Keyser A. D., Butler C. S., et al. (2022). Lime-treated urine improves sunflower growth without shifting soil bacterial communities. Appl. Soil Ecol. 178, 104575. doi: 10.1016/j.apsoil.2022.104575
Rumeau M., Marsden C., Ait-Mouheb N., Crevoisier D., and Pistocchi C. (2023). Fate of nitrogen and phosphorus from source-separated human urine in a calcareous soil. Environ. Sci. pollut. Res. 30, 65440–65454. doi: 10.1007/s11356-023-26895-5
Rumeau M., Pistocchi C., Ait-Mouheb N., Marsden C., and Brunel B. (2024). Unveiling the impact of human urine fertilization on soil bacterial communities: A path toward sustainable fertilization. Appl. Soil Ecol. 201, 105471. doi: 10.1016/j.apsoil.2024.105471
Schönning C. (2001). “Urine diversion–hygienic risks and microbial guidelines for reuse 1,” in Department of Parasitology, Mycology and Environmental Microbiology (Swedish Institute for Infectious Disease Control).
Sun B., Bai Z., Bao L., Xue L., Zhang S., Wei Y., et al. (2020). Bacillus subtilis biofertilizer mitigating agricultural ammonia emission and shifting soil nitrogen cycling microbiomes. Environ. Int. 144, 105989. doi: 10.1016/j.envint.2020.105989
Tang F. H. M. and Maggi F. (2016). Breakdown, uptake and losses of human urine chemical compounds in barley (Hordeum vulgare) and soybean (Glycine max) agricultural plots: Effectiveness of human urine use in agriculture. Nutr. Cycl. Agroecosyst. 104, 221–245. doi: 10.1007/s10705-016-9768-z
Tang X., Mu X., Shao H., Wang H., and Brestic M. (2015). Global plant-responding mechanisms to salt stress, physiological and molecular levels and implications in biotechnology. Crit. Rev. Biotechnol. 35, 425–437. doi: 10.3109/07388551.2014.889080
Tao R., Liang Y., Wakelin S. A., and Chu G. (2015). Supplementing chemical fertilizer with an organic component increases soil biological function and quality. Appl. Soil Ecol. 96, 42–51. doi: 10.1016/j.apsoil.2015.07.009
Torres I. F., Bastida F., Hernández T., Albaladejo J., and García C. (2015). Enzyme activity, microbial biomass and community structure in a long-term restored soil under semi-arid conditions. Soil Res. 53, 553–560. doi: 10.1071/SR14297
Van Gerrewey T., El-Nakhel C., De Pascale S., De Paepe J., Clauwaert P., Kerckhof F.M., et al. (2021). Root-associated bacterial community shifts in hydroponic lettuce cultured with urine-derived fertilizer. Microorganisms 9, 1326. doi: 10.3390/microorganisms9061326
Wang J., Song Y., Ma T., Raza W., Li J., Howland J.G., et al. (2017). Impacts of inorganic and organic fertilization treatments on bacterial and fungal communities in a paddy soil. Appl. Soil Ecol. 112, 42–50. doi: 10.1016/j.apsoil.2017.01.005
Wang Q., Wang C., Yu W., Turak A., Chen D., Huang Y., et al. (2018). Effects of nitrogen and phosphorus inputs on soil bacterial abundance, diversity and community composition in Chinese fir plantations. Front. Microbiol. 9, 1543. doi: 10.3389/fmicb.2018.01543
Wang B., Sun H., Yang W., Gao M., Zhong X., Zhang L., et al. (2022). Potential utilization of vitamin C industrial effluents in agriculture, Soil fertility and bacterial community composition. Sci. Total Environ. 851, 158253. doi: 10.1016/j.scitotenv.2022.158253
Wang P., Wang Y., and Wu Q. (2016). Effect of soil tillage and planting grass on arbuscular mycorrhizal fungal propagules and soil properties in citrus orchards in southeast China. Soil Tillage Res. 155, 54–61. doi: 10.1016/j.still.2015.07.009
Wei T., Zhang P., Wang K., Ding R., Yang B., Nie J., et al. (2015). Effects of wheat straw incorporation on the availability of soil nutrients and enzyme activities in semiarid areas. PLoS One 10, e0120994. doi: 10.1371/journal.pone.0120994
Wu L., Ma H., Zhao Q., Zhang S., Wei W., and Ding X. (2020). Changes in soil bacterial community and enzyme activity under five years straw returning in paddy soil. Eur. J. Soil Biol. 100, 103215. doi: 10.1016/j.ejsobi.2020.103215
Yan Q., Tian H., Huang Y., Mu X., Tang G., and Ma H. (2025). Recycled wheat straw biochar enhances nutrient-poor soil: Enzymatic kinetics of carbon, nitrogen, and phosphorus cycling. J. Environ. Manage. 380, 124950. doi: 10.1016/j.jenvman.2025.124950
Yang W., Zhang X., Wu L., Rensing C., and Xing S. (2021). Short-term application of magnesium fertilizer affected soil microbial biomass, activity, and community structure. J. Soil Sci. Plant Nutr. 21, 675–689. doi: 10.1007/s42729-020-00392-x
Yu P., Liu S., Han K., Guan S., and Zhou D. (2017). Conversion of cropland to forage land and grassland increases soil labile carbon and enzyme activities in northeastern China. Agric. Ecosyst. Environ. 245, 83–91. doi: 10.1016/j.agee.2017.05.013
Zhang X., et al. (2017). Thirty-one years of rice-rice-green manure rotations shape the rhizosphere microbial community and enrich beneficial bacteria. Soil Biol. Biochem. 104, 208–217. doi: 10.1016/j.soilbio.2016.10.023
Zhang H., et al. (2018). APP implementation and application of simple and rapid recommendation system for vegetable fertiliser application. China Agric. Inf. (in Chinese) 30, 68–75.
Keywords: urine agricultural utilization, soil nutrients, soil ecological environment, bacterial community structure, enzyme function
Citation: Yu G, Wang Q, Zheng X, Yang B, Zhang C, Zhang G and Wei X (2025) Effects of human urine application on soil physicochemical properties, microbial communities, and enzymatic activities. Front. Agron. 7:1610839. doi: 10.3389/fagro.2025.1610839
Received: 13 April 2025; Accepted: 26 May 2025;
Published: 01 July 2025.
Edited by:
Behnam Asgari Lajayer, Dalhousie University, CanadaReviewed by:
Hermes Pérez Hernández, National Institute of Forestry and Agricultural Research (INIFAP), MexicoMuazzez Gurgan, Namik Kemal University, Türkiye
Copyright © 2025 Yu, Wang, Zheng, Yang, Zhang, Zhang and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaocheng Wei, d2VpeGlhb2NoZW5nQGNhYXMuY24=
†These authors have contributed equally to this work
 Guangquan Yu1†
Guangquan Yu1† Xiangqun Zheng
Xiangqun Zheng Xiaocheng Wei
Xiaocheng Wei