- 1Allergy & Immunology Service, Walter Reed National Military Medical Center, Bethesda, MD, United States
- 2Department of Pediatrics, Uniformed Services University, Bethesda, MD, United States
- 3Henry M. Jackson Foundation for the Advancement of Military Medicine, Bethesda, MD, United States
- 4Center for Military Precision Health, Uniformed Services University, Bethesda, MD, United States
- 5ARUP Laboratories and Huntsman Cancer Institute, Department of Pathology, University of Utah, Salt Lake, UT, United States
- 6Department of Medicine, Uniformed Services University, Bethesda, MD, United States
Tryptase genotyping has an expanding role in the screening, diagnosis, and management of patients with systemic mastocytosis (SM). Reference ranges for basal serum tryptase (BST) based on increased TPSAB1 gene copy number can guide whether a patient's BST value is normal according to their specific tryptase genotype. Patients with an elevated BST based upon their tryptase genotype should be offered a bone marrow biopsy with sample evaluation by a hematopathologist. Tryptase genotyping is required when assessing patients for the WHO minor criterion, BST > 20 ng/ml, especially in those with monoclonal mast cell activation syndrome, bone marrow mastocytosis (BMM), and indolent systemic mastocytosis (ISM) when the major criterion is not met. Additionally, in patients with non-advanced SM, tryptase genotyping helps determine whether a patient with hereditary-alpha tryptasemia (HαT) has BMM with a BST < 125 ng/ml or fulfills the B-finding of BST > 200 ng/ml through application of a correction factor. Understanding a patient's BST level based upon their tryptase genotype also is helpful in guiding when to pursue a repeat bone marrow biopsy in patients with SM treated with a tyrosine kinase inhibitor (TKI). However, TKIs have variable KIT D816V as well as wild type KIT inhibition. Given this variable KIT inhibition, ongoing and future clinical trials with selective TKIs should report whether patients with SM and HαT experience normalization or persistent elevation of BST values as this is essential in understanding the expected treatment response and when to assess for pathological remission in the bone marrow.
Introduction
Mast cells (MCs) are a group of tissue-resident white blood cells characterized by metachromatic granules that contain mediators released during the early- and late-phase allergic responses. Tryptases are serine proteases that were first identified in MC granules in 1981 and they are the most abundant protein mediator in MC secretory granules (1–3). In humans, MCs can be identified through granular cytoplasmic expression of tryptase by immunohistochemistry and MC subpopulations in various tissues can be further delineated based upon the granular cytoplasmic co-expression of both tryptase and a second serine protease known as chymase (4). Tryptase and other MC mediators, including chymase, histamine, heparin, prostaglandins, leukotrienes, cytokines, and acid hydrolases, are released from secretory granules during degranulation and collectively contribute to allergic disease. MC mediators may affect the expression and stability of one another. For instance, exogenous histamine induces a concentration-dependent increase in tryptase (5). Conversely, exogenous tryptase can also induce a concentration-dependent increase in histamine (6). Heparin combined with an acidic pH serves to stabilize tetrameric tryptase via four histidine residues found in tryptase (7–10). An individual role for tryptase in human disease has not yet been established (11). By contrast, histamine has been demonstrated to cause cardiovascular instability when infused directly into human subjects and histamine receptor antagonists are widely used in the treatment of allergic disease (12). There is no clear role for tryptase or other MC mediators in human health (13).
Elevations in basal serum tryptase (BST) values occur at increased frequency in patients with the myeloid neoplasm systemic mastocytosis (SM) (11, 12). SM is characterized by the abnormal accumulation of neoplastic MCs in one or more organ systems. BST values ≥20 ng/ml are incorporated into the 2022 World Health Organization (WHO) and International Consensus Classification (ICC) diagnostic criteria for SM (13, 14). Additionally, BST values are used as a disease burden biomarker corresponding to the quantity of neoplastic mast cells in patients with SM. BST >200 ng/ml is a marker of high neoplastic MC burden. Serial monitoring of BST values is an important aspect of SM disease management in patients treated with tyrosine kinase inhibitors (15). It has recently been determined that the most common etiology for elevated BST in the general population is due to copy number variation at the tryptase locus gene, TPSAB1, rather than SM and this has led to refinements in SM screening (11, 16). Further, BST reference ranges specific to an individual's tryptase genotype, based on copy number variation at TPSAB1, have been reported (17). Thus, tryptase genotyping is becoming an important clinical tool in the management of patients with SM. Here, we explore the specific use of tryptase genotyping for SM screening, application of SM diagnostic criteria and subtype determination, and guiding SM management in patients treated with tyrosine kinase inhibitors.
Tryptase locus
The tryptase locus found at chromosome 16p13.3 is a 1.64 megabase region containing tryptase genes encoding soluble and membrane-bound serine proteases (Figure 1A). Tryptase genes are part of an ancient gene family that arose in non-mammalian vertebrates (18). From telomere to centromere, these genes include TPSG1, TPSB2, and TPSAB1 (19, 20). TPSB2 and TPSAB1 are >90% similar in sequence while TPSG1 is only 47% similar to TPSB2 (20, 21). Additional related genes of reduced function or pseudogenes are distal to TPSAB1 towards the centromere including TPSD1 and PRSS22 (22, 23). The tryptase locus genes were first cloned and described in humans in 1999 (20, 21, 24). The TPSG1 gene encodes γ tryptase isoforms. γ tryptases have a hydrophobic tail that results in them being membrane-bound (21, 24). Genomes of non-mammalian vertebrates encode homologs of TPSG1, but not TPSB2 or TPSAB1, suggesting that membrane-bound tryptase isoforms evolved first (18). The TPSB2 and TPSAB1 genes likely arose in mammals. They encode soluble tryptases that localize to MC granules as tetramers and are also constitutively secreted as pro-tryptase monomers (25). The TPSB2 gene encodes β tryptase isoforms. The three TPSB2 alleles described in humans includes the βI minor allele, βII major allele, and βIII major allele. Approximately 20% of the population may have a frameshifted TPSB2 βIII null allele (βIIIFS) (26). The TPSAB1 gene encodes either the α major allele, βI major allele, or βII minor allele. β tryptase homotetramers are functional serine proteases while homotetrameric α tryptases are likely non-functional based on crystal structure and biochemical analysis, due to amino acid substitutions at residues −3 and 215. The Arg-3Gln substitution in the N-terminal pro-peptide of α tryptase evolved recently and leads to faulty zymogen activation. The Gly215Asp substitution in the catalytic primary specificity pocket leads to reduced substrate binding and flawed catalytic activity (27–29). αβ heterotetramers occur in proportion to the number of α alleles present. The protease activity of heterotetramers has been incompletely characterized, although one study showed that heterotetramers in vitro can activate protease activated receptor-2 and cleave EGF-like module—containing mucin-like hormone receptor-like 2 (30). The mutation causing the Gly215Asp substitution in α tryptase arose in Old World monkeys after they split from New World monkeys. This mutation also arose before the split of α and β alleles at TPSAB1 (18). Site directed mutagenesis to swap Gly for Asp at residue 215 was sufficient for α tryptase to gain βII enzymatic activity (29).
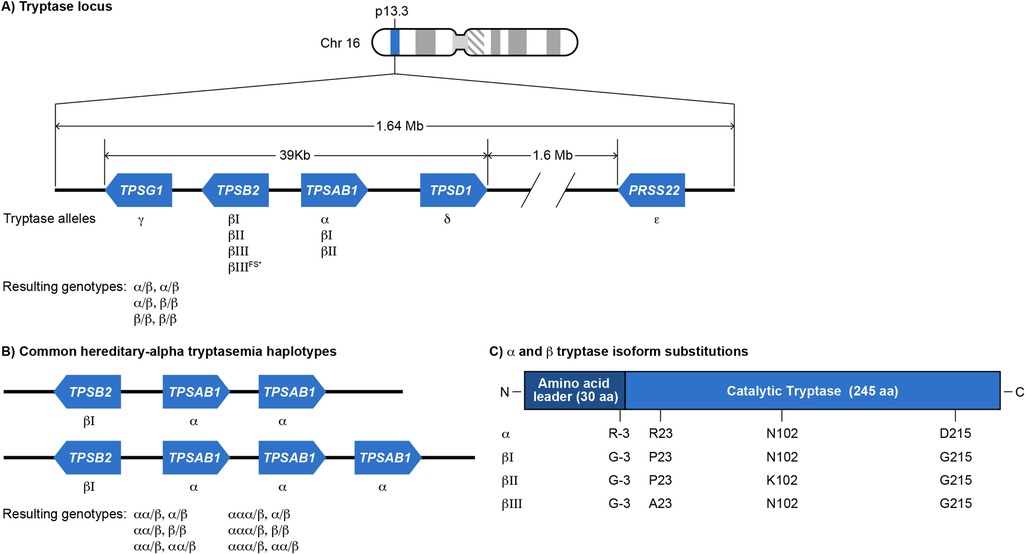
Figure 1. (A) Schematic of tryptase gene cluster and its wild type genotypes. Tryptase locus on chromosome 16p13.3 comprises a homologous cluster of genes: TPSAB1, TPSB2, TPSD1 and TPSG1. TPSAB1 and TPSD1 are located on the positive strand of DNA, while TPSB2 and TPSG1 are on the negative strand. (23) An additional human tryptase (ε) encoded by PRSS22 also exists on 16p just outside of this cluster on the negative strand. TPSAB1 and TPSB2 encode soluble tryptases (α and β). The resulting normal TPSAB1/TPSB2 genotypes are shown (specific β alleles are not indicated). (B) Hereditary-α tryptasemia (HαT) alleles and genotypes. The HαT genetic trait is defined as one or more tandem increased copies of the TPSAB1 α allele. (97) Tandem TPSAB1 α allele duplications and triplications are the most commonly reported copy number variants that underly HαT, though even higher tandem copy numbers can occur. (16) The resulting most common HαT TPSAB1/TPSB2 genotypes are shown (specific β alleles are not indicated). (C) Each α− and β-tryptase allele encodes a 275-amino acid peptide with a 30-amino acid-leader sequence and a mature catalytic portion of 245 amino acids. Each of these isoforms is highly similar, being at least 97% identical. (98) The key amino acids differentiating each isoform are Arg/Gly at −3, Pro/Arg 23, Asn/Lys at 102 and Asp/Gly at 215. (26, 27). *bIIIFS-tryptase p.M123Dfs*14 is inactive.
Hereditary-alpha tryptasemia (HαT) genetic trait
Tandem increased copy number of the α allele of TPSAB1 on one or more chromosomes is known as the HαT genetic trait and was first identified in 2016 (Figure 1B) (16). The tandem α alleles in HαT have an expanded promoter repetitive element that is linked to increased α tryptase expression (17). It is not clear how many founder events for the HαT genetic trait may have occurred during human evolution, in which human populations these founder events occurred, and whether the trait occurred due to genetic drift or positive selection. Two studies have reported on the prevalence of HαT in a general population sample. In 2016, when HαT was first reported, the authors assessed a sample of 98 individuals and found 8 (8.2%) that had both elevated BST values and HαT. A subsequent study in the UK of 423 individuals found that 22 (5%) had HαT (16, 31). BST references ranges based upon the number of tandem TPSAB1 α alleles were reported in 2021 (Table 1) (32). Modeled BST reference range values were subsequently reported in 2023 and showed that each additional tandem TPSAB1 α allele contributes around 10 ng/ml to BST on average (17). The genes at the tryptase locus, particularly TPSB2 and TPSAB1, are under strong linkage disequilibrium and are inherited as haplotypes (26). There are two major tryptase locus haplotypes of TPSB2-TPSAB1 for individuals who do not have HαT that occur at a frequency of >15% and they are βII-α and βIII-βI. There are additional minor haplotypes that occur at a frequency of <15% (26). The major haplotype for TPSB2-TPSAB1 for individuals who have HαT appears to be βI-αDUP (17).
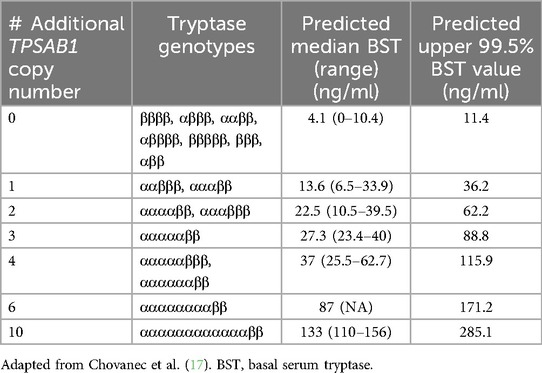
Table 1. Predicted basal serum tryptase median, range, and upper 99% interval according to TPSAB1 α allele copy number.
Assays for both tryptase genotyping and serum tryptase
Comparison of an individual's tryptase genotype to their BST value is necessary to determine whether their BST is elevated. Tryptase genotyping refers to clinical assays designed to report a summation of the α and β tryptase allele copy numbers encoded at the TPSAB1 and TPSB2 genes. A digital droplet polymerase chain reaction (ddPCR) assay using primer and probe sets for α- and β-tryptase to quantify α- and β-tryptase allele copy number was developed in 2016 (16). This method is now available in a few select commercial laboratories in the United States. The α and β allele copy number reported in ddPCR assays typically allows one to determine tryptase haplotypes for both chromosomes in the individual tested. However, in some cases, α and β copy number may correspond to multiple possible haplotypes instead of only one haplotype. Additionally, the β tryptase allele copy number reported does not distinguish between βI, βII, βIII, or βIIIFS alleles.
Since 2016, three additional clinical tryptase genotyping assays have been reported. Two studies have reported a multiplex ddPCR assay, which allows for quantification of α- and β-tryptase copy counts in a single reaction. Compared to the original ddPCR assay, the multiplex ddPCR may have a lower cost and runtime, and a strong correlation with BST and overall accuracy (33, 34). A second reported method is the target amplicon next-generation sequencing (NGS) assay, which utilizes machine learning models to identify polymorphisms at TPSAB1 and TPSB2 genes (35). In this study, this NGS assay accurately estimated 96% of both α and βIIIFS tryptase alleles, and 94% of extra α alleles on TPSAB1 (35). Neither the multiplex nor NGS methods are commercially available.
Several serum tryptase assays have been developed over time and a few are described here. The first assays utilized a low sensitivity mouse monoclonal G5 anti-tryptase antibody which can detect linear epitopes on denatured tryptase. Initially, a sandwich enzyme-linked immunosorbent assay (ELISA) utilizing the G5 capture antibody with a goat polyclonal anti-tryptase detector antibody was developed (36). Then, a sandwich radioimmunoassay was developed utilizing the G5 capture antibody with a mouse monoclonal G4 detector antibody (37). The assays developed next utilize the more sensitive mouse monoclonal B12 antibody. The B12 antibody detects epitopes of tetrameric and denatured linear tryptase. Initially, an ELISA was developed that utilized the B12 capture antibody with a biotin-G4 detector antibody (38). Clinical serum tryptase testing now utilizes quantitative fluorescent-based immunoassays (e.g., ImmunoCAP) using the B12 capture antibody to measure total tryptase, reported as one value corresponding to the sum of both mature tetrameric tryptase and monomeric α- and β-protryptases.
Systemic mastocytosis
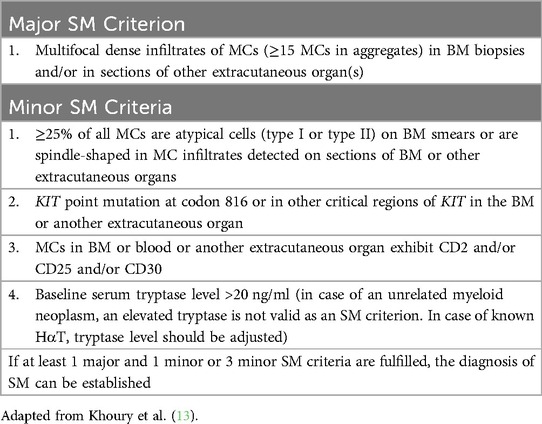
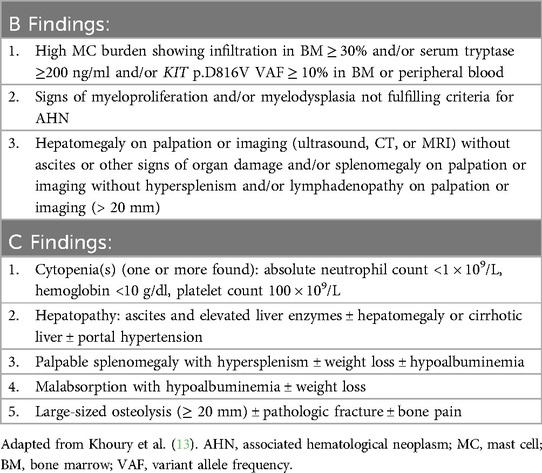
There are three types of mastocytosis: cutaneous mastocytosis (CM), systemic mastocytosis (SM), and MC sarcoma. The three subtypes of CM include maculopapular CM (MPCM), diffuse CM, and mastocytoma (39). MPCM can be polymorphic or monomorphic. SM subtypes include bone marrow mastocytosis (BMM), indolent SM (ISM), smoldering SM (SSM), aggressive SM (ASM), SM with an associated hematologic neoplasm (SM-AHN), and MC leukemia (MCL). BMM, ISM, and SSM are non-advanced (non-AdvSM) and ASM, SM-AHN, and MCL are advanced subtypes (AdvSM). Well-differentiated SM is a morphologic pattern occurring in any SM subtype and is characterized by enlarged round and well-granulated MCs (13). A diagnosis of SM is established when at least 1 major and 1 minor or 3 minor criteria are met, as detailed in the 5th edition of the WHO diagnostic criteria (Table 2) (13). Monoclonal mast cell activation syndrome (MMAS) occurs when the WHO major criterion is not met and only two minor criteria are met. SM subtypes are determined based on the presence of B (disease “burden”) and C (need for “cytoreductive” treatment) findings (Table 3) (13). Patients with BMM have no B or C findings, the BST should be <125 ng/ml, and they have no skin involvement. ISM patients can have only 1 B finding, SSM patients must have 2 or more B findings, and ASM patients have one or more C findings. MCL requires ≥20% neoplastic mast cells in the BM aspirate (13). Greater than 90% of patients with SM have the activating mutation KIT c.2447 C > T p.D816V (40).
SM is rare with a prevalence of 0.9–1.7 per 10,000 individuals, of which ISM is the most common subtype (41–44). ISM with skin lesions and BMM together represent approximately 82%–91% of all SM patients (42, 45, 46). Other less prevalent subtypes include SSM occurring in 4.3%–7.1%, SM-AHN in 3.1–13.5%, ASM in 5.1%–9.7%, and MCL in 1-7%–4.8% of all SM patients (41, 42, 45–49). Each SM subtype has different prognostic implications. Patients with SSM have a higher risk of progression to AdvSM at 9.4%–15% compared to ISM and BMM at 4.9% and 1.7%, respectively, over a median follow-up period of 2.0–4.3 years (48, 50, 51). A large cohort of patients with a median follow-up time of 62 months demonstrated a 10-year progression-free survival rate of 100% in BMM, 98.1% in ISM with skin lesions, 87.4% in SM-AHN, 62.5% in SSM, and 55.6% in ASM (41). Although patients with ISM generally have a near-normal life expectancy, the median survival in advanced forms is significantly less with 3–5 years for SM-AHN, 3–4 years for ASM, and 0.5–1.6 years for MCL (50, 52–54).
Heterogeneity of SM clinical presentations and access to high complexity testing likely contributes to diagnostic delays with a median time to diagnosis of 7 years across all subtypes, with delays being highest in patients with non-AdvSM (55). Patients may experience frequent and debilitating cutaneous, gastrointestinal, musculoskeletal, and neurocognitive symptoms that impact quality of life (56–59). Around half of SM patients report a history of one or more anaphylaxis episodes and the grade of anaphylaxis is usually severe. Anaphylaxis in SM patients can be triggered by Hymenoptera envenomation, foods, drugs, or be idiopathic (53, 60–62). Osteoporosis and fragility fractures may occur in up to 30% and 50% of SM patients, respectively. Osteoporosis and fractures are more prevalent in ISM than AdvSM, with the latter showing more osteosclerosis (46, 63–66). Skin lesions are also reported in around half of SM patients (46, 51, 67). Additionally, nearly half of patients with non-AdvSM may present with BST values <20 ng/ml. SM patients with low BST values may experience delays in obtaining diagnostic bone marrow (BM) biopsies due to a perception that their BST values are too low to be consistent with SM. This is most often true of patients with ISM and BMM, and less commonly with SM-AHN (67–70). Access to high-complexity testing, including mast cell flow cytometry, high-sensitivity quantitative KIT p.D816V assays, and tryptase genotyping is not uniform across institutions. Overcoming these barriers is important as there are now disease-modifying selective TKIs that improve the morbidity and prognosis of SM.
Tryptase genotyping in SM screening
Screening for SM has historically been challenging due to the disease's variable clinical presentation. Advances in the last 15 years have led to an optimized screening strategy (Figure 2). The first milestone occurred in 2010 with the development of a clinical and laboratory scoring system known as the Red Española de Mastocitosis (REMA) score (71). Eighty-three patients with a history of anaphylaxis, but no mastocytosis-in-skin, were assessed for variables that might predict the presence of SM. A multivariate analysis demonstrated that male sex, BST values >25 ng/ml, as well as clinical manifestations during anaphylaxis of syncope or presyncope, and the absence of urticaria and angioedema were linked to SM. Syncope or presyncope during an episode of anaphylaxis were the most tightly linked clinical findings. The specificity of the REMA score is 74% (67, 72). The REMA score sensitivity is challenging to determine since approximately half of patients with SM may have no history of anaphylaxis. Among SM patients who experience at least one episode of anaphylaxis but without skin involvement, the sensitivity of the REMA score is 87% (72). Among all SM patients, regardless of history of anaphylaxis or skin involvement, the sensitivity of the REMA score is 34% (67).
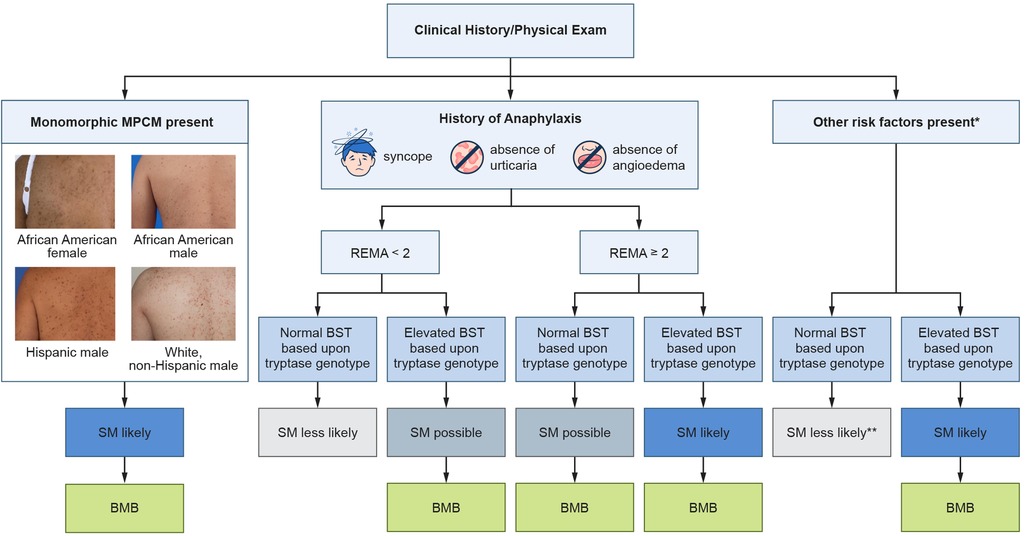
Figure 2. Screening algorithm for systemic mastocytosis. Clinicians should assess for a history of anaphylaxis, perform a skin exam, and assess for other SM risk factors. Tryptase genotyping is especially important in patients with anaphylaxis as well as patients with other SM risk factors. Additionally, some patients with monomorphic MPCM may be lacking a confirmatory skin biopsy and an elevated BST based upon an individual's tryptase genotype could obviate the need to perform a skin biopsy. *Other risk factors include flushing, unexplained osteoporosis, pathologic fracture, splenomegaly, and blood count abnormalities. Additional less specific risk factors include headache, diarrhea, fatigue, difficulty concentrating, and poor memory. Adapted with permission from “Screening approach to clonal mast cell disease in patients with Hymenoptera venom allergy (HVA)” by Nathan A. Boggs, Ilaria Tanasi, Karin Hartmann, Roberta Zanotti, and David Gonzalez-de-Olano, licensed under CC BY-NC-ND.
The second major change to the overall SM screening approach was proposed in 2014. An analysis of 59 patients with adult-onset MPCM demonstrated that 97% of these patients had SM, indicating that adult-onset MPCM is specific for systemic disease (73). It is not clear whether any of these adult patients might have had polymorphic MPCM since polymorphic MPCM is nearly always found in children and polymorphic and monomorphic terminology was not in use at the time of the study. Notably, around half of SM patients may have skin involvement and most of these patients are thought to have adult-onset MPCM. A subsequent study in 2024 demonstrated that monomorphic MPCM is tightly linked to SM with a specificity of 97% (67).
Tryptase genotyping became clinically available around 2020 in the United States. The first study to assess the benefit of tryptase genotyping in SM screening was published in 2022 and assessed a group of 58 patients. SM and other myeloid neoplasms were found to be enriched in patients with elevated BST values who did not have HαT. Specifically, in patients with a BST value ≥11.5 ng/ml, 63% had HαT, 20% had myeloid neoplasms, and 12% had CKD. These results suggested that myeloid neoplasms are much more likely to be present in those with elevated BST, when HαT is absent (11). A second study to assess the benefit of tryptase genotyping in patients with elevated BST who do not have CKD was published in 2023. A group of 409 patients with elevated BST were screened for HαT and myeloid neoplasms. Of these 409 with BST ≥11.5 ng/ml, 74% had HαT and 29% had SM or another myeloid neoplasm (17). Finally, a third study assessed screening testing accuracy among a variety of screening tests including an elevated BST based upon tryptase genotype, BST ≥11.5 ng/ml, BST ≥20 ng/ml, and REMA combined with an elevated BST based upon tryptase genotype. This study demonstrated that the single most accurate screening test for SM, based on Youden's index which is a value based on the sum of the sensitivity and specificity, was elevated BST based upon tryptase genotype with a sensitivity of 84% and specificity of 90%. Further, it was found that the REMA score combined with elevated BST based upon tryptase genotype had a substantially improved specificity over the REMA score alone (70). An elevated BST based upon an individual's tryptase genotype may also be helpful when there is uncertainty in the diagnosis of anaphylaxis due to absence of a trigger (e.g., Hymenoptera venom) or when anaphylaxis occurs with mild symptoms such as lightheadedness rather than syncope.
SM is linked to other clinical manifestations that, on their own, may be less specific for SM, including osteoporosis, large osteosclerotic bone lesions, flushing, chronic diarrhea, fatigue, frequent headaches, and more. In 2024, one study assessed the combination of unexplained osteoporosis in patients with either elevated BST or symptoms of MC activation (74). The authors noted that common causes of osteoporosis had been excluded though it was not explicitly stated which causes those were. Nonetheless, the authors assessed 139 patients and showed that SM is more common in patients with osteoporosis and BST ≥11.5 ng/ml, if they also had BST >19 ng/ml, vertebral fractures, and were <54 years old. They developed several scoring systems designed to predict who might have SM in those with unexplained osteoporosis and elevated BST. The scoring test has a sensitivity of 71% and specificity of 67%. When BST >19 ng/ml was removed and replaced with an elevated BST based upon genotype, the scoring system had an improved sensitivity of 87% and specificity of 76%.
Some centers may not yet have access to tryptase genotyping. Measurement of MC mediators or metabolites, including leukotriene E4 (LTE4), N-methylhistamine (NMH), and 11 β-prostaglandin F2 α (BPG), in urine samples represent another means to assess the pretest probability of SM (75–77). The most specific urinary mediator was found to be NMH with a specificity of 88% (78). It is not clear how sensitive NMH and other urinary mediators are in SM screening in patients with a low disease burden as prior studies excluded patients with low BST values or did not describe the SM disease burden of their cohort. The relative accuracy of urinary mediators in predicting SM has not yet been directly compared to BST combined with tryptase genotyping, monomorphic MPCM, or the REMA score.
Importance of tryptase genotyping in SM diagnostic testing and subtyping
The diagnosis of SM relies on determining whether the WHO major and minor criteria are met by an experienced hematopathologist. Notably, the minor criterion of BST ≥20 ng/ml was first introduced into the WHO SM diagnostic criteria in 2001, based on the idea that most patients with SM have BST values >20 ng/ml (79). Data supporting a specific diagnostic BST cutoff of >20 ng/ml, rather than other elevated BST values, is limited. One study showed that as many as 50% of SM patients may have BST values <20 ng/ml (67). The 5th edition of the WHO classification published in 2022 recommended adjustment of BST in case of HαT, although a specific manner of adjustment was not provided (13). The 2022 International Consensus Classification (ICC) of myeloid neoplasms and acute leukemias did not include adjustment of BST in the case of HαT (14). Neither the WHO nor the ICC included adjustment of BST in the case of HαT for a BST >200 ng/ml, which is a B-finding, or for a BST >125 ng/ml in patients with non-AdvSM and no skin lesions. Predicted median and upper 99.5% BST values for incremental tandem TPSAB1 α allele replications has been reported (17). An online calculator that adjusts BST using a correction factor based on TPSAB1 α allele replication number also has been published (https://bst-calculater.niaid.nih.gov) (17). Finally, a recent proposal has recommended harmonization of diagnostic criteria across organizations and to adjust for HαT by dividing the BST by 1 plus the extra copies of the TPSAB1 α allele in order to determine whether SM criteria are met and whether the B-finding of BST >200 ng/ml is present. The importance of tryptase genotyping to determine whether an SM subtype is BMM in a patient with non-AdvSM, no B or C findings, and without skin lesions when the BST is >125 ng/ml was not specifically addressed (80).
There are several factors to consider when an SM diagnosis or subtype depends specifically on BST values. First, greater than 95% of patients with SM have disease driven by the KIT p.D816V mutation. MC spindling and expression of CD25 are found in nearly all cases where KIT p.D816V is detected in either peripheral blood or BM, even when the VAF approaches the limit of detection using clinically validated high-sensitivity PCR clinical assays (81, 82). Thus, BST values are not typically required to establish an SM diagnosis unless suboptimal diagnostic testing is employed. Second, it would be advantageous for allergy, hematology, and pathology teams to consistently employ tryptase genotyping in every suspected case of SM. This may be challenging in the short term as some centers currently do not have access to tryptase genotyping. It is worth noting that SM patients with relatively high BST values and a higher rate of HαT occurrence may be preferentially referred for BM biopsies compared to SM patients with lower BST values and this supports the need to employ tryptase genotyping in all SM diagnostic evaluations (67). Third, it is likely best to avoid the use of BST ≥20 ng/ml as a minor criterion, BST > 200 ng/ml as a B-finding, and BST >125 ng/ml for BMM/ISM subtype determination in the absence of tryptase genotyping. The impact of not having tryptase genotyping available when BST is >200 ng/ml (B-finding of increased MC burden) is partially mitigated by the fact that the same B finding can be met in other ways (i.e., high KIT p.D816 V VAF ≥ 10% and/or MCs ≥ 30% in the BM biopsy). Also, the BST >20 ng/ml minor criterion seems to be less important in most cases of AdvSM and SSM as the major criterion is typically met. Lastly, the original use of BST ≥20 ng/ml, as opposed to other elevated BST values, appears to be based on limited data. Future discussion should consider what role BST values should play in SM diagnostic criteria. The value of using BST as a minor criterion may be highest in individuals with atypical KIT mutations when the major SM criterion is not met, when all other BM diagnostic testing is adequate, and the case has been reviewed by an experienced hematopathologist.
BST monitoring in SM patients treated with tyrosine kinase inhibitors
Tryptase genotyping is important in the management of SM patients treated with TKIs. There have been several recent advances in the use of TKI treatments for SM. Three TKIs have been FDA approved for AdvSM including midostaurin, imatinib, and avapritinib. Tryptase genotypes of patients with AdvSM enrolled in these TKI trials were not assessed (83–87). Most patients with SM have a non-advanced subtype (BMM, ISM, or SSM). Low dose avapritinib at 25 mg daily was recently FDA approved for patients with ISM and is the first FDA approved treatment for patients with this SM subtype (88). Preliminary data from the PIONEER study shows a similar percentage of reduction in MC burden (i.e., serum tryptase and KIT p.D816V VAF) in patients with and without HαT treated with a low dose of the selective KIT p.D816V inhibitor avapritinib (89). There are several ongoing clinical trials including with avapritinib (NCT06327685, NCT03731260), bezuclastinib (NCT04996875, NCT05186753), elenestinib (NCT05609942, NCT04910685), and masitinib (NCT04333108).
Serial BST values, in addition to KIT p.D816V VAFs, measured in SM patients after a period of TKI treatment, may help guide when to repeat a BM biopsy to determine whether the SM neoplasm is in pathological remission. BST values are an indirect marker of BM MC burden. It is important to note that BST values in patients with SM likely represent a summation of the tryptase secreted by both neoplastic as well as wild-type MCs, and basophils to a lesser degree. BST values in SM patients, excluding those with SM-AHN where the AHN may also contribute to elevated BST values, who do not have HαT would be expected to fully normalize on a disease-modifying TKI therapy. In contrast, based on our experience, BST values in SM patients with HαT (excluding those with SM-AHN) assessed after a period of treatment with a disease-modifying TKI may not normalize BST values. Persistent BST elevations in patients with SM and unknown HαT status on TKI therapy are at significant risk. First, they risk effective therapy being discontinued under a false assumption that the TKI therapy is not effectively reducing the BST to “normal” values. Second, they risk unnecessary TKI dose escalations which are not indicated. Thus, tryptase genotyping is recommended for all patients with SM undergoing TKI treatment.
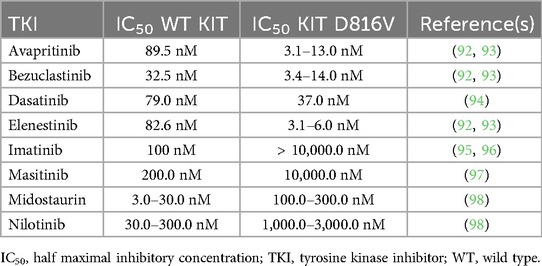
The half maximal inhibitory concentrations (IC50) of TKIs for wild type KIT and KIT D816V assessed in patients with SM have been previously reported and representative values are shown for a variety of TKIs in Table 4 (90–96). It is not clear what IC50 level and what dose of each specific TKI might lead to BST normalization in SM patients with HαT. Our experience has been that avapritinib 25 mg daily does not lead to normalization of BST values (BST <11.5 ng/ml) in patients with SM and HαT while it is too early to tell for other TKIs. Future studies should aim to determine whether each specific KIT inhibitor might lead to normalization of BST values in SM patients with and without HαT. Knowing whether to expect BST normalization or not would be helpful to guide when to perform an interval BM biopsy in these patients to assess for remission.
Conclusions
Tryptase and tryptase genotyping assessments are essential in the screening, diagnosis, and management of SM. Patients with an elevated BST based upon their tryptase genotype should be offered a BM biopsy at a center with a hematopathologist expert to evaluate for SM. Tryptase genotyping as it relates to the WHO minor criterion BST >20 ng/ml, may be most important in the diagnosis of MMAS, BMM, and ISM, when there is minimal involvement of MCs in the BM such that the major criterion is not met. Tryptase genotyping is also needed to determine whether a patient with non-AdvSM has BMM if their BST is <125 ng/ml and whether the B-finding of BST >200 ng/ml is present. In patients with SM treated with a TKI, understanding a patient's BST value in relation to their tryptase genotype may help guide the decision on when to repeat a BM biopsy to assess for remission. Finally, ongoing clinical trials with selective TKIs should report on whether patients with SM and HαT have normalization or persistent elevation of BST values due to variable inhibition of wild type KIT and KIT D816V, as the potential BST nadir impacts the decision on when to pursue a BM biopsy.
Author contributions
JCM: Investigation, Resources, Writing – review & editing, Writing – original draft, Conceptualization. BJS: Writing – review & editing, Writing – original draft. JV: Visualization, Writing – review & editing. TIG: Investigation, Writing – review & editing. NAB: Resources, Writing – review & editing, Investigation, Conceptualization, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We thank Sofia C. Echelmeyer of the Uniformed Services University for her assistance with figure illustrations. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, Uniformed Services University, or Department of Defense; nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Conflict of interest
Author JCM has received consulting fees from Cogent Biosciences. Author NAB has received consultng fees from Blueprint Medicines Corporation. Author TIG receives salary support from ARUP Laboratories. TIG has received consulting fees from Beckman Coulter, Blueprint Medicines Corporation, Cogent Biosciences, and Incyte.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ASM, aggressive systemic mastocytosis; BM, bone marrow; BMM, bone marrow mastocytosis; BST, basal serum tryptase; CM, cutaneous mastocytosis; DCM, diffuse cutaneous mastocytosis; HαT, hereditary-alpha tryptasemia; ICC, International Consensus Classification; ISM, indolent systemic mastocytosis; MC, mast cell; MCL, mast cell leukemia; MPCM, maculopapular cutaneous mastocytosis; SSM, smoldering systemic mastocytosis; SM, systemic mastocytosis; SM-AHN, systemic mastocytosis with an associated hematologic neoplasm; VAF, variant allele frequency; WHO, World Health Organization.
References
1. Schwartz LB, Lewis RA, Austen KF. Tryptase from human pulmonary mast cells. Purification and characterization. J Biol Chem. (1981) 256:11939–43. doi: 10.1016/S0021-9258(19)68496-6
2. Schwartz LB, Lewis RA, Seldin D, Austen KF. Acid hydrolases and tryptase from secretory granules of dispersed human lung mast cells. J Immunol. (1981) 126:1290–4. doi: 10.4049/jimmunol.126.4.1290
3. Glenner GG, Cohen LA. Histochemical demonstration of a species-specific trypsin-like enzyme in mast cells. Nature. (1960) 185:846–7. doi: 10.1038/185846a0
4. Irani AA, Schechter NM, Craig SS, DeBlois G, Schwartz LB. Two types of human mast cells that have distinct neutral protease compositions. Proc Natl Acad Sci USA. (1986) 83:4464–8. doi: 10.1073/pnas.83.12.4464
5. He S-H, Xie H. Modulation of tryptase secretion from human colon mast cells by histamine. World J Gastroenterol. (2004) 10:323–6. doi: 10.3748/wjg.v10.i3.323
6. He S, Gaça MD, Walls AF. A role for tryptase in the activation of human mast cells: modulation of histamine release by tryptase and inhibitors of tryptase. J Pharmacol Exp Ther. (1998) 286:289–97. doi: 10.1016/S0022-3565(24)37586-X
7. Hallgren J, Lindahl S, Pejler G. Structural requirements and mechanism for heparin-dependent activation and tetramerization of human betaI- and betaII-tryptase. J Mol Biol. (2005) 345:129–39. doi: 10.1016/j.jmb.2004.10.029
8. Hallgren J, Karlson U, Poorafshar M, Hellman L, Pejler G. Mechanism for activation of mouse mast cell tryptase: dependence on heparin and acidic pH for formation of active tetramers of mouse mast cell protease 6. Biochemistry. (2000) 39:13068–77. doi: 10.1021/bi000973b
9. Hallgren J, Bäckström S, Estrada S, Thuveson M, Pejler G. Histidines are critical for heparin-dependent activation of mast cell tryptase. J Immunol. (2004) 173:1868–75. doi: 10.4049/jimmunol.173.3.1868
10. Sakai K, Ren S, Schwartz LB. A novel heparin-dependent processing pathway for human tryptase. Autocatalysis followed by activation with dipeptidyl peptidase I. J Clin Invest. (1996) 97:988–95. doi: 10.1172/JCI118523
11. Waters AM, Park HJ, Weskamp AL, Mateja A, Kachur ME, Lyons JJ, et al. Elevated basal serum tryptase: disease distribution and variability in a regional health system. J Allergy Clin Immunol Pract. (2022) 10:2424–2435.e5. doi: 10.1016/j.jaip.2021.12.031
12. Schwartz LB, Sakai K, Bradford TR, Ren S, Zweiman B, Worobec AS, et al. The alpha form of human tryptase is the predominant type present in blood at baseline in normal subjects and is elevated in those with systemic mastocytosis. J Clin Invest. (1995) 96:2702–10. doi: 10.1172/JCI118337
13. Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, et al. The 5th edition of the world health organization classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia. (2022) 36:1703–19. doi: 10.1038/s41375-022-01613-1
14. Arber DA, Orazi A, Hasserjian RP, Borowitz MJ, Calvo KR, Kvasnicka H-M, et al. International consensus classification of myeloid neoplasms and acute leukemias: integrating morphologic, clinical, and genomic data. Blood. (2022) 140:1200–28. doi: 10.1182/blood.2022015850
15. Gotlib J, Gerds AT, Abdelmessieh P, Ali H, Castells M, Dunbar A, et al. NCCN Guidelines® insights: systemic mastocytosis, version 3.2024. J Natl Compr Canc Netw. (2024) 22:8. doi: 10.6004/jnccn.2024.0030
16. Lyons JJ, Yu X, Hughes JD, Le QT, Jamil A, Bai Y, et al. Elevated basal serum tryptase identifies a multisystem disorder associated with increased TPSAB1 copy number. Nat Genet. (2016) 48:1564–9. doi: 10.1038/ng.3696
17. Chovanec J, Tunc I, Hughes J, Halstead J, Mateja A, Liu Y, et al. Genetically defined individual reference ranges for tryptase limit unnecessary procedures and unmask myeloid neoplasms. Blood Adv. (2023) 7:1796–810. doi: 10.1182/bloodadvances.2022007936
18. Trivedi NN, Tong Q, Raman K, Bhagwandin VJ, Caughey GH. Mast cell alpha and beta tryptases changed rapidly during primate speciation and evolved from gamma-like transmembrane peptidases in ancestral vertebrates. J Immunol. (2007) 179:6072–9. doi: 10.4049/jimmunol.179.9.6072
19. Vanderslice P, Ballinger SM, Tam EK, Goldstein SM, Craik CS, Caughey GH. Human mast cell tryptase: multiple cDNAs and genes reveal a multigene serine protease family. Proc Natl Acad Sci USA. (1990) 87:3811–5. doi: 10.1073/pnas.87.10.3811
20. Pallaoro M, Fejzo MS, Shayesteh L, Blount JL, Caughey GH. Characterization of genes encoding known and novel human mast cell tryptases on chromosome 16p13.3. J Biol Chem. (1999) 274:3355–62. doi: 10.1074/jbc.274.6.3355
21. Caughey GH, Raymond WW, Blount JL, Hau LW, Pallaoro M, Wolters PJ, et al. Characterization of human gamma-tryptases, novel members of the chromosome 16p mast cell tryptase and prostasin gene families. J Immunol. (2000) 164:6566–75. doi: 10.4049/jimmunol.164.12.6566
22. Trivedi NN, Raymond WW, Caughey GH. Chimerism, point mutation, and truncation dramatically transformed mast cell delta-tryptases during primate evolution. J Allergy Clin Immunol. (2008) 121:1262–8. doi: 10.1016/j.jaci.2008.01.019
23. Wong GW, Yasuda S, Madhusudhan MS, Li L, Yang Y, Krilis SA, et al. Human tryptase epsilon (PRSS22), a new member of the chromosome 16p13.3 family of human serine proteases expressed in airway epithelial cells. J Biol Chem. (2001) 276:49169–82. doi: 10.1074/jbc.M108677200
24. Wong GW, Tang Y, Feyfant E, Sali A, Li L, Li Y, et al. Identification of a new member of the tryptase family of mouse and human mast cell proteases which possesses a novel COOH-terminal hydrophobic extension. J Biol Chem. (1999) 274:30784–93. doi: 10.1074/jbc.274.43.30784
25. Schwartz LB, Min H-K, Ren S, Xia H-Z, Hu J, Zhao W, et al. Tryptase precursors are preferentially and spontaneously released, whereas mature tryptase is retained by HMC-1 cells, mono-Mac-6 cells, and human skin-derived mast cells. J Immunol. (2003) 170:5667–73. doi: 10.4049/jimmunol.170.11.5667
26. Trivedi NN, Tamraz B, Chu C, Kwok P-Y, Caughey GH. Human subjects are protected from mast cell tryptase deficiency despite frequent inheritance of loss-of-function mutations. J Allergy Clin Immunol. (2009) 124:1099–105.e1. doi: 10.1016/j.jaci.2009.07.026
27. Marquardt U, Zettl F, Huber R, Bode W, Sommerhoff C. The crystal structure of human alpha1-tryptase reveals a blocked substrate-binding region. J Mol Biol. (2002) 321:491–502. doi: 10.1016/s0022-2836(02)00625-3
28. Selwood T, Wang Z-M, McCaslin DR, Schechter NM. Diverse stability and catalytic properties of human tryptase alpha and beta isoforms are mediated by residue differences at the S1 pocket. Biochemistry. (2002) 41:3329–40. doi: 10.1021/bi015662v
29. Huang C, Li L, Krilis SA, Chanasyk K, Tang Y, Li Z, et al. Human tryptases alpha and beta/II are functionally distinct due, in part, to a single amino acid difference in one of the surface loops that forms the substrate-binding cleft. J Biol Chem. (1999) 274:19670–6. doi: 10.1074/jbc.274.28.19670
30. Le QT, Lyons JJ, Naranjo AN, Olivera A, Lazarus RA, Metcalfe DD, et al. Impact of naturally forming human α/β-tryptase heterotetramers in the pathogenesis of hereditary α-tryptasemia. J Exp Med. (2019) 216:2348–61. doi: 10.1084/jem.20190701
31. Robey RC, Wilcock A, Bonin H, Beaman G, Myers B, Grattan C, et al. Hereditary alpha-tryptasemia: UK prevalence and variability in disease expression. J Allergy Clin Immunol Pract. (2020) 8:3549–56. doi: 10.1016/j.jaip.2020.05.057
32. Glover SC, Carter MC, Korošec P, Bonadonna P, Schwartz LB, Milner JD, et al. Clinical relevance of inherited genetic differences in human tryptases: hereditary alpha-tryptasemia and beyond. Ann Allergy Asthma Immunol. (2021) 127:638–47. doi: 10.1016/j.anai.2021.08.009
33. Svetina M, Šelb J, Lyons JJ, Korošec P, Rijavec M. Clinically accessible amplitude-based multiplex ddPCR assay for tryptase genotyping. Sci Rep. (2024) 14:2416. doi: 10.1038/s41598-024-52983-8
34. Alheraky A, Wierenga ATJ, Simpelaar A, Hesp LB, Minovic I, Bagheri N, et al. Hereditary alpha tryptasemia: validation of a single-well Multiplex digital droplet PCR assay in a cohort of symptomatic patients. Clin Chem. (2024) 70:425–33. doi: 10.1093/clinchem/hvad206
35. Li O, Hackney JA, Choy DF, Chang D, Nersesian R, Staton TL, et al. A targeted amplicon next-generation sequencing assay for tryptase genotyping to support personalized therapy in mast cell-related disorders. PLoS One. (2024) 19:e0291947. doi: 10.1371/journal.pone.0291947
36. Wenzel S, Irani AM, Sanders JM, Bradford TR, Schwartz LB. Immunoassay of tryptase from human mast cells. J Immunol Methods. (1986) 86:139–42. doi: 10.1016/0022-1759(86)90277-2
37. Enander I, Matsson P, Nystrand J, Andersson AS, Eklund E, Bradford TR, et al. A new radioimmunoassay for human mast cell tryptase using monoclonal antibodies. J Immunol Methods. (1991) 138:39–46. doi: 10.1016/0022-1759(91)90062-k
38. Schwartz LB, Bradford TR, Rouse C, Irani AM, Rasp G, Van der Zwan JK, et al. Development of a new, more sensitive immunoassay for human tryptase: use in systemic anaphylaxis. J Clin Immunol. (1994) 14:190–204. doi: 10.1007/BF01533368
39. Hartmann K, Escribano L, Grattan C, Brockow K, Carter MC, Alvarez-Twose I, et al. Cutaneous manifestations in patients with mastocytosis: consensus report of the European competence network on mastocytosis; the American academy of allergy, asthma & immunology; and the European academy of allergology and clinical immunology. J Allergy Clin Immunol. (2016) 137:35–45. doi: 10.1016/j.jaci.2015.08.034
40. Arock M, Sotlar K, Akin C, Broesby-Olsen S, Hoermann G, Escribano L, et al. KIT Mutation analysis in mast cell neoplasms: recommendations of the European competence network on mastocytosis. Leukemia. (2015) 29:1223–32. doi: 10.1038/leu.2015.24
41. Zanotti R, Bonifacio M, Isolan C, Tanasi I, Crosera L, Olivieri F, et al. A multidisciplinary diagnostic approach reveals a higher prevalence of indolent systemic mastocytosis: 15-Years’ experience of the GISM network. Cancers (Basel). (2021) 13:1–13. doi: 10.3390/cancers13246380
42. Cohen SS, Skovbo S, Vestergaard H, Kristensen T, Møller M, Bindslev-Jensen C, et al. Epidemiology of systemic mastocytosis in Denmark. Br J Haematol. (2014) 166:521–8. doi: 10.1111/bjh.12916
43. Schuler CF, Volertas S, Khokhar D, Yuce H, Chen L, Baser O, et al. Prevalence of mastocytosis and hymenoptera venom allergy in the United States. J Allergy Clin Immunol. (2021) 148:1316–23. doi: 10.1016/j.jaci.2021.04.013
44. Bergström A, Hägglund H, Berglund A, Nilsson G, Lambe M. Epidemiology of mastocytosis: a population-based study (Sweden). Acta Oncol. (2024) 63:44–50. doi: 10.2340/1651-226X.2024.31406
45. Hermans MAW, Rietveld MJA, van Laar JAM, Dalm VASH, Verburg M, Pasmans SGMA, et al. Systemic mastocytosis: a cohort study on clinical characteristics of 136 patients in a large tertiary centre. Eur J Intern Med. (2016) 30:25–30. doi: 10.1016/j.ejim.2016.01.005
46. Ungerstedt J, Ljung C, Klimkowska M, Gülen T. Clinical outcomes of adults with systemic mastocytosis: a 15-year multidisciplinary experience. Cancers (Basel). (2022) 14:1–15. doi: 10.3390/cancers14163942
47. Tiryaki TO, Özkan SG, Erdem S, Aday AD, Hindilerden İY, Gelincik A, et al. Comprehensive mastocytosis data analysis from a single center. BMC Cancer. (2023) 23:82. doi: 10.1186/s12885-022-10498-3
48. Sperr WR, Kundi M, Alvarez-Twose I, van Anrooij B, Oude Elberink JNG, Gorska A, et al. International prognostic scoring system for mastocytosis (IPSM): a retrospective cohort study. Lancet Haematol. (2019) 6:e638–49. doi: 10.1016/S2352-3026(19)30166-8
49. Sánchez-Muñoz L, Alvarez-Twose I, García-Montero AC, Teodosio C, Jara-Acevedo M, Pedreira CE, et al. Evaluation of the WHO criteria for the classification of patients with mastocytosis. Mod Pathol. (2011) 24:1157–68. doi: 10.1038/modpathol.2011.84
50. Trizuljak J, Sperr WR, Nekvindová L, Elberink HO, Gleixner KV, Gorska A, et al. Clinical features and survival of patients with indolent systemic mastocytosis defined by the updated WHO classification. Allergy. (2020) 75:1927–38. doi: 10.1111/all.14248
51. Pieri L, Bonadonna P, Elena C, Papayannidis C, Grifoni FI, Rondoni M, et al. Clinical presentation and management practice of systemic mastocytosis. A survey on 460 Italian patients. Am J Hematol. (2016) 91:692–9. doi: 10.1002/ajh.24382
52. Damaj G, Joris M, Chandesris O, Hanssens K, Soucie E, Canioni D, et al. ASXL1 but not TET2 mutations adversely impact overall survival of patients suffering systemic mastocytosis with associated clonal hematologic non-mast-cell diseases. PLoS One. (2014) 9:e85362. doi: 10.1371/journal.pone.0085362
53. Lim K-H, Tefferi A, Lasho TL, Finke C, Patnaik M, Butterfield JH, et al. Systemic mastocytosis in 342 consecutive adults: survival studies and prognostic factors. Blood. (2009) 113:5727–36. doi: 10.1182/blood-2009-02-205237
54. Kennedy VE, Perkins C, Reiter A, Jawhar M, Lübke J, Kluin-Nelemans HC, et al. Mast cell leukemia: clinical and molecular features and survival outcomes of patients in the ECNM registry. Blood Adv. (2022) 7:1713–24. doi: 10.1182/bloodadvances.2022008292
55. Jennings SV, Slee VM, Zack RM, Verstovsek S, George TI, Shi H, et al. Patient perceptions in mast cell disorders. Immunol Allergy Clin North Am. (2018) 38:505–25. doi: 10.1016/j.iac.2018.04.006
56. Jennings S, Russell N, Jennings B, Slee V, Sterling L, Castells M, et al. The mastocytosis society survey on mast cell disorders: patient experiences and perceptions. J Allergy Clin Immunol Pract. (2014) 2:70–6. doi: 10.1016/j.jaip.2013.09.004
57. Russell N, Jennings S, Jennings B, Slee V, Sterling L, Castells M, et al. The mastocytosis society survey on mast cell disorders: part 2-patient clinical experiences and beyond. J Allergy Clin Immunol Pract. (2019) 7:1157–1165.e6. doi: 10.1016/j.jaip.2018.07.032
58. van Anrooij B, Kluin-Nelemans JC, Safy M, Flokstra-de Blok BMJ, Oude Elberink JNG. Patient-reported disease-specific quality-of-life and symptom severity in systemic mastocytosis. Allergy. (2016) 71:1585–93. doi: 10.1111/all.12920
59. Mesa RA, Sullivan EM, Dubinski D, Carroll B, Slee VM, Jennings SV, et al. Perceptions of patient disease burden and management approaches in systemic mastocytosis: results of the TouchStone healthcare provider survey. Cancer. (2022) 128:3700–8. doi: 10.1002/cncr.34421
60. Brockow K, Jofer C, Behrendt H, Ring J. Anaphylaxis in patients with mastocytosis: a study on history, clinical features and risk factors in 120 patients. Allergy. (2008) 63:226–32. doi: 10.1111/j.1398-9995.2007.01569.x
61. Gülen T, Hägglund H, Dahlén B, Nilsson G. High prevalence of anaphylaxis in patients with systemic mastocytosis - a single-centre experience. Clin Exp Allergy. (2014) 44:121–9. doi: 10.1111/cea.12225
62. González de Olano D, de la Hoz Caballer B, Núñez López R, Sánchez Muñoz L, Cuevas Agustín M, Diéguez MC, et al. Prevalence of allergy and anaphylactic symptoms in 210 adult and pediatric patients with mastocytosis in Spain: a study of the Spanish network on mastocytosis (REMA). Clin Exp Allergy. (2007) 37:1547–55. doi: 10.1111/j.1365-2222.2007.02804.x
63. van der Veer E, van der Goot W, de Monchy JGR, Kluin-Nelemans HC, van Doormaal JJ. High prevalence of fractures and osteoporosis in patients with indolent systemic mastocytosis. Allergy. (2012) 67:431–8. doi: 10.1111/j.1398-9995.2011.02780.x
64. Rossini M, Zanotti R, Bonadonna P, Artuso A, Caruso B, Schena D, et al. Bone mineral density, bone turnover markers and fractures in patients with indolent systemic mastocytosis. Bone. (2011) 49:880–5. doi: 10.1016/j.bone.2011.07.004
65. Makovoz A, Wang J, Oshegbo G, Park YH, Lyons JJ, Eisch AR, et al. Assessment of osteoporosis and fracture risk in mastocytosis within a north American cohort. J Allergy Clin Immunol Pract. (2021) 9:4459–4467.e10. doi: 10.1016/j.jaip.2021.08.001
66. Degboé Y, Eischen M, Nigon D, Apoil P-A, Mailhol C, Tournier E, et al. Prevalence and risk factors for fragility fracture in systemic mastocytosis. Bone. (2017) 105:219–25. doi: 10.1016/j.bone.2017.09.005
67. McMurray JC, Pacheco CS, Schornack BJ, Sun X, Brunader JA, Scott AE, et al. Standardized indolent systemic mastocytosis evaluations across a health care system: implications for screening accuracy. Blood. (2024) 144:408–19. doi: 10.1182/blood.2023023347
68. Boggs NA, Sun X, Lyons JJ, McMurray JC, Rose DM, Pryor EM, et al. Challenges in applying diagnostic criteria for systemic mastocytosis. Blood Adv. (2023) 7:3150–4. doi: 10.1182/bloodadvances.2023009826
69. Šelb J, Rijavec M, Eržen R, Zidarn M, Kopač P, Škerget M, et al. Routine KIT p.D816V screening identifies clonal mast cell disease in patients with hymenoptera allergy regularly missed using baseline tryptase levels alone. J Allergy Clin Immunol. (2021) 148:621–626.e7. doi: 10.1016/j.jaci.2021.02.043
70. Jendoubi F, Shourick J, Negretto M, Laurent C, Apoil PA, Evrard S, et al. Cutaneous mastocytosis in adults with a serum tryptase level < 20ng mL-1 : why we should investigate further. Br J Dermatol. (2021) 185:453–5. doi: 10.1111/bjd.20098
71. Alvarez-Twose I, González de Olano D, Sánchez-Muñoz L, Matito A, Esteban-López MI, Vega A, et al. Clinical, biological, and molecular characteristics of clonal mast cell disorders presenting with systemic mast cell activation symptoms. J Allergy Clin Immunol. (2010) 125:1269–1278.e2. doi: 10.1016/j.jaci.2010.02.019
72. Alvarez-Twose I, González-de-Olano D, Sánchez-Muñoz L, Matito A, Jara-Acevedo M, Teodosio C, et al. Validation of the REMA score for predicting mast cell clonality and systemic mastocytosis in patients with systemic mast cell activation symptoms. Int Arch Allergy Immunol. (2012) 157:275–80. doi: 10.1159/000329856
73. Berezowska S, Flaig MJ, Ruëff F, Walz C, Haferlach T, Krokowski M, et al. Adult-onset mastocytosis in the skin is highly suggestive of systemic mastocytosis. Mod Pathol. (2014) 27:19–29. doi: 10.1038/modpathol.2013.117
74. Tanasi I, Crosera L, Taus F, Orsolini G, Adami G, Olivieri F, et al. Underlying systemic mastocytosis in patients with unexplained osteoporosis: score proposal. Bone. (2024) 186:117141. doi: 10.1016/j.bone.2024.117141
75. Keyzer JJ, de Monchy JG, van Doormaal JJ, van Voorst Vader PC. Improved diagnosis of mastocytosis by measurement of urinary histamine metabolites. N Engl J Med. (1983) 309:1603–5. doi: 10.1056/NEJM198312293092603
76. Morrow JD, Guzzo C, Lazarus G, Oates JA, Roberts LJ. Improved diagnosis of mastocytosis by measurement of the major urinary metabolite of prostaglandin D2. J Invest Dermatol. (1995) 104:937–40. doi: 10.1111/1523-1747.ep12606209
77. Divekar R, Butterfield J. Urinary 11β-PGF2α and N-methyl histamine correlate with bone marrow biopsy findings in mast cell disorders. Allergy. (2015) 70:1230–8. doi: 10.1111/all.12668
78. Lueke AJ, Meeusen JW, Donato LJ, Gray AV, Butterfield JH, Saenger AK. Analytical and clinical validation of an LC-MS/MS method for urine leukotriene E4: a marker of systemic mastocytosis. Clin Biochem. (2016) 49:979–82. doi: 10.1016/j.clinbiochem.2016.02.007
79. Valent P, Horny HP, Escribano L, Longley BJ, Li CY, Schwartz LB, et al. Diagnostic criteria and classification of mastocytosis: a consensus proposal. Leuk Res. (2001) 25:603–25. doi: 10.1016/s0145-2126(01)00038-8
80. Valent P, Hartmann K, Hoermann G, Reiter A, Alvarez-Twose I, Brockow K, et al. Harmonization of diagnostic criteria in mastocytosis for use in clinical practice: WHO vs ICC vs AIM/ECNM. J Allergy Clin Immunol Pract. (2024) 12:3250–60. doi: 10.1016/j.jaip.2024.08.044
81. Sotlar K, Horny H-P, Simonitsch I, Krokowski M, Aichberger KJ, Mayerhofer M, et al. CD25 Indicates the neoplastic phenotype of mast cells: a novel immunohistochemical marker for the diagnosis of systemic mastocytosis (SM) in routinely processed bone marrow biopsy specimens. Am J Surg Pathol. (2004) 28:1319–25. doi: 10.1097/01.pas.0000138181.89743.7b
82. Shean RC, Hellwig S, Saadalla A, George TI, Rets AV. High-sensitivity KIT D816V variation analysis by droplet digital polymerase chain reaction: the reference laboratory perspective. Am J Clin Pathol. (2025):1–5. doi: 10.1093/ajcp/aqaf008
83. Gotlib J, Kluin-Nelemans HC, George TI, Akin C, Sotlar K, Hermine O, et al. Efficacy and safety of midostaurin in advanced systemic mastocytosis. N Engl J Med. (2016) 374:2530–41. doi: 10.1056/NEJMoa1513098
84. DeAngelo DJ, George TI, Linder A, Langford C, Perkins C, Ma J, et al. Efficacy and safety of midostaurin in patients with advanced systemic mastocytosis: 10-year median follow-up of a phase II trial. Leukemia. (2018) 32:470–8. doi: 10.1038/leu.2017.234
85. Gotlib J, Reiter A, Radia DH, Deininger MW, George TI, Panse J, et al. Efficacy and safety of avapritinib in advanced systemic mastocytosis: interim analysis of the phase 2 PATHFINDER trial. Nat Med. (2021) 27:2192–9. doi: 10.1038/s41591-021-01539-8
86. DeAngelo DJ, Radia DH, George TI, Robinson WA, Quiery AT, Drummond MW, et al. Safety and efficacy of avapritinib in advanced systemic mastocytosis: the phase 1 EXPLORER trial. Nat Med. (2021) 27:2183–91. doi: 10.1038/s41591-021-01538-9
87. Alvarez-Twose I, Matito A, Morgado JM, Sánchez-Muñoz L, Jara-Acevedo M, García-Montero A, et al. Imatinib in systemic mastocytosis: a phase IV clinical trial in patients lacking exon 17 KIT mutations and review of the literature. Oncotarget. (2017) 8:68950–63. doi: 10.18632/oncotarget.10711
88. Gotlib J, Castells M, Elberink HO, Siebenhaar F, Hartmann K, Broesby-Olsen S, et al. Avapritinib versus placebo in indolent systemic mastocytosis. NEJM evid. (2023) 2:1–15. doi: 10.1056/EVIDoa2200339
89. Castells M, Akin C, Maurer M, Hartmann K, Broesby-Olsen S, George TI, et al. Analysis of hereditary alpha tryptasemia and AssociationWith baseline characteristics in patients with IndolentSystemic mastocytosis enrolled on the PIONEER study. American Initiative in Mast Cell Diseases (AIM) Conference; Boston, MA; May 18–19, 2024. (2024). p. 1–10.
90. Guarnieri AL. Preclinical data identifies bezuclastinib as a differentiated KIT inhibitor with unique selectivity to KIT D816V and minimal evidence of brain penetration. Mol Cancer Ther. (2021) 20(12_Supplement):1–10. doi: 10.1158/1535-7163.TARG-21-P257
91. Tashi T, Hermine O, Castells M, Guilarte M, Sabato V, Maurer M, et al. Elenestinib, an investigational, next generation KIT D816V inhibitor, reduces mast cell burden, improves symptoms, and has a favorable safety profile in patients with indolent systemic mastocytosis: analysis of the harbor trial. Blood. (2023) 142:76–76. doi: 10.1182/blood-2023-188904
92. Shah NP, Lee FY, Luo R, Jiang Y, Donker M, Akin C. Dasatinib (BMS-354825) inhibits KITD816V, an imatinib-resistant activating mutation that triggers neoplastic growth in most patients with systemic mastocytosis. Blood. (2006) 108:286–91. doi: 10.1182/blood-2005-10-3969
93. Heinrich MC, Griffith DJ, Druker BJ, Wait CL, Ott KA, Zigler AJ. Inhibition of c-kit receptor tyrosine kinase activity by STI 571, a selective tyrosine kinase inhibitor. Blood. (2000) 96:925–32. doi: 10.1182/blood.V96.3.925
94. Growney JD, Clark JJ, Adelsperger J, Stone R, Fabbro D, Griffin JD, et al. Activation mutations of human c-KIT resistant to imatinib mesylate are sensitive to the tyrosine kinase inhibitor PKC412. Blood. (2005) 106:721–4. doi: 10.1182/blood-2004-12-4617
95. Dubreuil P, Letard S, Ciufolini M, Gros L, Humbert M, Castéran N, et al. Masitinib (AB1010), a potent and selective tyrosine kinase inhibitor targeting KIT. PLoS One. (2009) 4:e7258. doi: 10.1371/journal.pone.0007258
96. Gleixner KV, Mayerhofer M, Aichberger KJ, Derdak S, Sonneck K, Böhm A, et al. PKC412 inhibits in vitro growth of neoplastic human mast cells expressing the D816V-mutated variant of KIT: comparison with AMN107, imatinib, and cladribine (2CdA) and evaluation of cooperative drug effects. Blood. (2006) 107:752–9. doi: 10.1182/blood-2005-07-3022
97. Lyons JJ, Yi T. Mast cell tryptases in allergic inflammation and immediate hypersensitivity. Curr Opin Immunol. (2021) 72:94–106. doi: 10.1016/j.coi.2021.04.001
Keywords: systemic mastocytosis (SM), tryptase genotyping, basal serum tryptase (BST), hereditary alpha tryptasemia, tyrosine kinase inhibitors (TKIs)
Citation: McMurray JC, Schornack BJ, Villar J, George TI and Boggs NA (2025) Utility of tryptase genotyping in the screening, diagnosis, and management of systemic mastocytosis. Front. Allergy 6:1599358. doi: 10.3389/falgy.2025.1599358
Received: 24 March 2025; Accepted: 5 May 2025;
Published: 27 May 2025.
Edited by:
Peter Kopac, University Clinic of Pulmonary and Allergic Diseases Golnik, SloveniaReviewed by:
Carmen Fava, University of Turin, ItalyMatevž Škerget, University Medical Centre Ljubljana, Slovenia
Copyright: © 2025 McMurray, Schornack, Villar, George and Boggs. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jeremy C. McMurray, amVyZW15bWNtdXJyYXkubWRAZ21haWwuY29t
 Jeremy C. McMurray
Jeremy C. McMurray Brandon J. Schornack
Brandon J. Schornack Joaquin Villar
Joaquin Villar Tracy I. George
Tracy I. George Nathan A. Boggs
Nathan A. Boggs