- The Allergy Immunology Unit, Department of Pediatrics, Advanced Pediatrics Centre, Post Graduate Institute of Medical Education and Research, Chandigarh, India
Primary atopic disorders (PADs) are monogenic conditions associated with severe, early-onset atopic diseases. Clinically, they often overlap with polygenic allergic conditions, making specialized laboratory testing necessary to distinguish them from polygenic atopy. Multisystem involvement, such as growth failure, recurrent infections, and autoimmunity, points towards PADs warranting further investigations. PADs associated with immune dysregulation can be broadly categorized into four mechanistic groups: those affecting the regulation of cell cytoskeleton dynamics, T-cell receptor (TCR) signaling and repertoire diversity, and function of regulatory T cell (Treg), and cytokine signaling. In this review, we have examined the defects in cytokine signaling pathways associated with PADs. Key cytokine signaling pathways implicated in PADs include the STAT3, JAK1/STAT5b, and TGF-β pathways. Pathogenic variants in these pathways result in complex clinical phenotypes but share a common theme of Th2 polarization and severe atopic manifestations. Early and accurate differentiation between polygenic atopy and PADs is crucial, as it allows for timely, targeted immunological or genetic interventions that may significantly improve patient outcomes.
1 Introduction
Inborn errors of immunity (IEIs) are heritable disorders with a heterogenous presentation due to variants in genes impairing the activity of the immune system (1). Clinically, they may manifest as heightened susceptibility to infections, autoinflammation, autoimmunity, atopy, and malignancies (2). A subset of these IEIs features a distinct atopic phenotype marked by chronic Th2 skewing, aberrant mast cell degranulation, eosinophilic inflammation, and elevated IgE levels (3). In 2018, Lyons and Milner introduced the term primary atopic disorders (PADs) to categorize monogenic conditions associated with early-onset atopic symptoms driven by immune dysregulation (4). However, it is now recognized that PADs are not a strict subcategory of IEIs. Only a subset of PADs are immune in origin and may be classified as IEI. Many PADs result from non-immune mechanisms involving structural or barrier defects. These PADs primarily disrupt epithelial integrity, leading to heightened allergen penetration and subsequent atopic responses. Prototypic disorders of these PADs are attributed to variants in genes encoding epidermal barrier proteins like FLG (filaggrin), protease inhibitors such as SPINK5, and intercellular adhesion molecules CDSN, DSG1, and DSP (5–7).
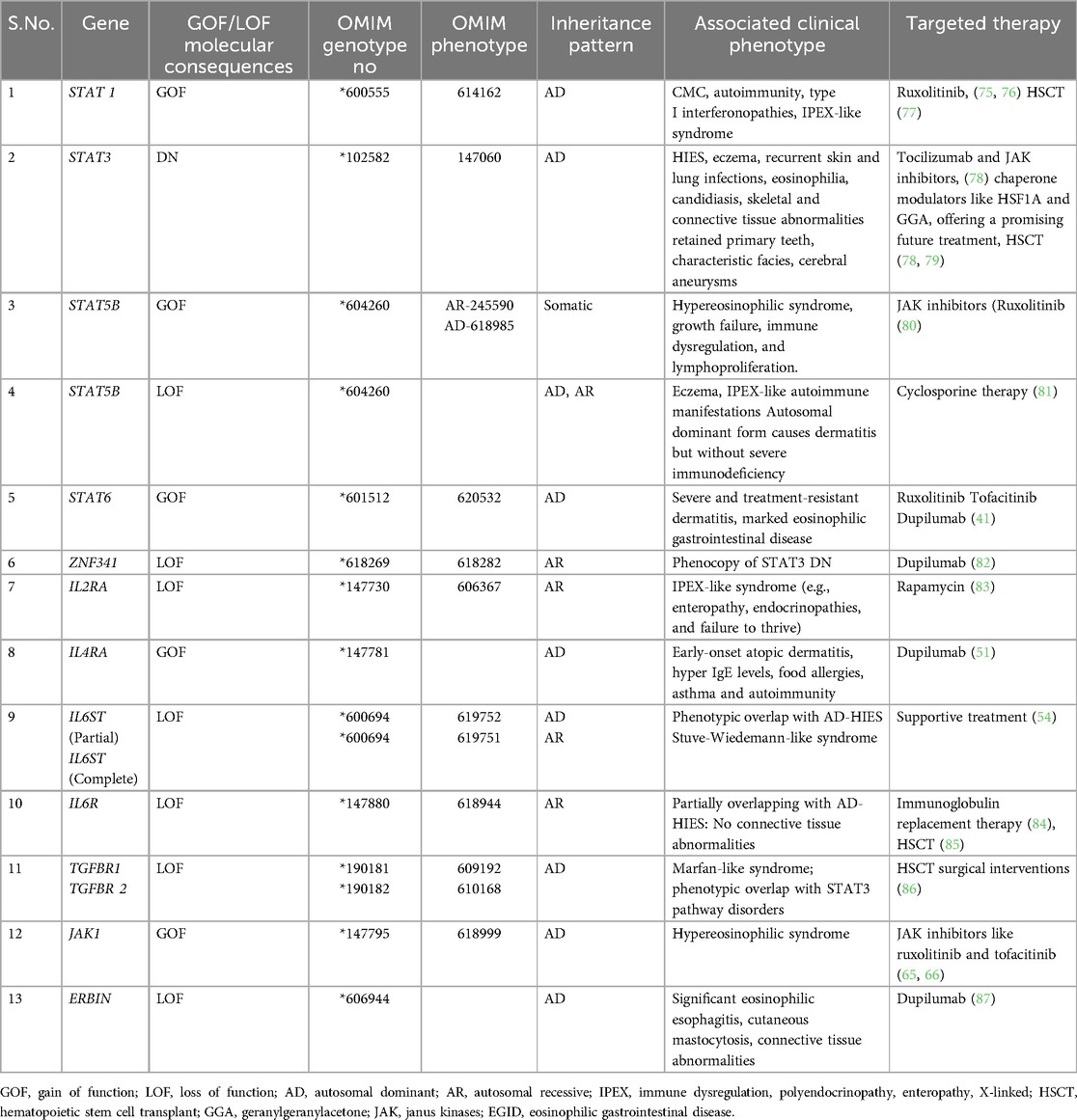
IEIs with atopic manifestations exhibit overlapping clinical and immunological phenotypes and have been grouped into key syndromic categories. These are classified based on clinical presentation and immunological profiling as Hyper-IgE syndromes, immune dysregulation poly-endocrinopathy enteropathy X-linked (IPEX) and IPEX-like conditions, Omenn syndrome, Wiskott–Aldrich syndrome, CBM-opathies, and other atopy predominant IEIs (8). Although clinical features of PADs often overlap with polygenic allergic conditions, they are distinguished by early onset and severe manifestations with complex comorbidities such as growth failure, recurrent infections, and autoimmunity, to name a few (9) (Figure 1).
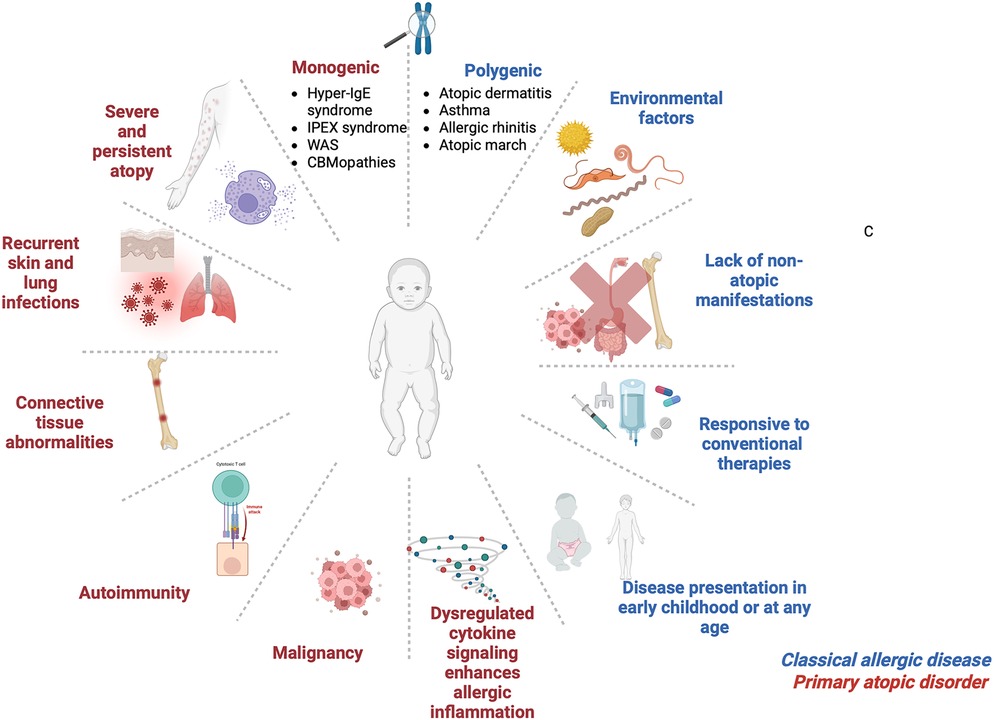
Figure 1. Clinical characteristics for classical allergic disease and primary atopic disorder. IPEX, Immune dysregulation, polyendocrinopathy, enteropathy, X-linked; WAS, Wiskott–Aldrich syndrome; CBMopathies, CBM complex—CARD11, BCL10 and MALT1. Figure was created using BioRender.
Atopy is a genetic tendency to mount exaggerated IgE-mediated immune responses to environmental allergens. These patients often present with a constellation of clinical symptoms, such as atopic dermatitis, food allergy, allergic rhinitis, and asthma - collectively referred to as the ‘atopic march’ (10–13). In PADs, these manifestations are often accompanied by immune dysregulation due to Th2 polarization and overproduction of IL-4, IL-5, and IL-13. These Th2 effector cytokines drive downstream signaling cascades that recruit eosinophils, mast cells, and other effector cells (11). While allergic phenotypes are common in the general population, those seen in IEIs are frequently more severe and are rooted in well-defined genetic defects (9).
Diagnosing PADs begins with a thorough clinical evaluation, including physical examination, family history, and immunological investigations (14) (Figure 2). Family medical history is important as PADs typically exhibit distinct inheritance patterns, although de-novo variants may arise spontaneously as well. Hallmark laboratory findings include eosinophilia, elevated serum IgE, abnormal immunoglobulin profiles, and T-cell subsets, which are typically assessed via flow cytometry (14). Genetic testing plays a pivotal role in confirming the diagnosis by identifying causal variants (15).
Figure 2. Diagnostic algorithm in patients with suspected primary atopic disorder. Figure was created using BioRender.
Key immune pathways involved include those regulating the cellular cytoskeleton along with immune synapse formation, T-cell receptor (TCR) signaling and repertoire diversity, T regulatory cell (Treg) function, and innate immune cell effector mechanisms (16). Among the molecular mechanisms implicated in PADs, signaling pathways of cytokines have a cardinal role. Disruption in cytokine signaling can severely impair host immune response and tolerance, predisposing individuals to infections, inflammation, and allergic diseases (17). Specifically, alterations in STAT3, JAK1/STAT5, and TGF-β pathways through molecular consequences, such as dominant-negative (DN), loss or gain-of-function (LOF, GOF) mutations, are closely associated with Th2 polarization and atopic manifestations (14).
In this review, we focus on PADs arising from genetic defects that disrupt cytokine signaling networks. We examine mutations affecting transcription factors such as STAT1, STAT3, STAT5B, STAT6, ZNF341, cell surface receptors including IL2RA, IL4RA, IL6R, IL6ST, TGFBR1/2, and intracellular signaling mediators such as JAK1 and ERBB2IP (18). Understanding these pathways not only enhances our insight into the pathogenesis of PADs but also provides a foundation for developing precision medicine. The key primary atopic disorders resulting from cytokine signaling defects have been summarised in Table 1.
2 Role of transcription factors in altered cytokine signaling
2.1 STAT1 GOF
Autosomal dominant- GOF variants in STAT1 are among the commonest monogenic defects linked to chronic mucocutaneous candidiasis (CMC), with over 400 reported cases (19, 20). These mutations prevent dephosphorylation of STAT1, leading to its constitutive nuclear localization and enhanced type I/II interferon signaling (21). Consequently, Th17 differentiation is impaired, which directly impacts antifungal defenses, and enhanced interferon signaling drives autoimmunity (e.g., hypothyroidism, cytopenias) that resemble IPEX-like phenotypes (22). A novel N-terminal mutation (c.194A>C; p.D65A) has even been tied to eosinophilic esophagitis, underscoring the link between STAT1 hyperactivity and atopic inflammation (21, 23).
2.2 STAT3 DN
First described clinically as Job's syndrome in 1966, autosomal -dominant DN mutations in STAT3 are the molecular basis of Hyper -IgE syndrome (HIES) (24–26). Patients exhibit severe eczema, elevated IgE (>1,000 IU/ml), eosinophilia, and recurrent staphylococcal skin and pulmonary infections (27, 28). Dampened IL-6 and IL-10 signaling through STAT3 reduces Th17 cell numbers and IL-17 production, heightening susceptibility to Staphylococcus and Candida infections (27, 29). Diagnostic criteria combine IgE quantification, Th17 enumeration, a clinical scoring system (>30 points), and genetic confirmation of a STAT3 DN variant (30).
2.3 STAT5B GOF
Somatic GOF variants in the SH2 or transactivation domains of STAT5B enhance STAT5 signaling, driving clonal T-cell expansion with a Th2 bias (31). Thus, STAT5B remains constitutively active instead of responding appropriately to growth hormone signals that regulate IGF-1–dependent growth but also skew T-cell differentiation towards a Th2 phenotype. Clinically, affected individuals present with treatment-refractory atopic dermatitis, persistent urticaria, elevated numbers of eosinophilia, alopecia, and angioedema (32).
2.4 STAT5B LOF
STAT5B loss-of-function leads to atopic dermatitis with dwarfism, hyper IgE, autoimmunity and lymphocytic interstitial pneumonitis (33, 34). STAT5B acts as a key mediator in growth hormone signaling, and its deficiency results in growth hormone insensitivity and growth failure (35). Additionally, STAT5B LOF impairs IL-2-dependent signaling, leading to recurrent viral infections associated with reduced function and even decreased numbers of T regs (34). Atopic symptoms such as eczema are prevalent and affected individuals may develop conditions resembling IPEX like syndrome (33). In addition to LOF variants, the STAT5B deficiency can also result from the autosomal dominant form of STAT5B, causing stunted growth and eczema, but it does not lead to severe immunodeficiency (36).
2.5 STAT6 GOF
STAT6 is the major transcription factor activated by IL-4 and IL-13. Upon activation, STAT6 dimerizes and translocates to the nucleus. STAT6 promotes differentiation of naive CD4+ T cells to Th2 cells along with class-switch from IgM to IgE on B-cells (37). This process is initiated on binding of IL-4 and IL-13 to the IL-4 receptor complex, triggering the phosphorylation of tyrosine residues on the IL-4 receptor alpha (IL-4Rα) subunit (38). This is mediated via Janus kinases (JAK) (39). Src homology 2 (SH2) domains recruit STAT6, which bind to the phosphorylated tyrosine residues on IL-4Rα (39). In STAT6 GOF, hyperphosphorylation of STAT6 intensifies IL-4 and IL-13 signaling. A STAT6 heterozygous misense variant (c.1129G>A; p.Glu377Lys) linked to atopy was initially identified by Suratannon et al. (40). Another missense variant in exon 22 (c.1114G>A; p.E372K) was identified in a patient with early-onset eczema, food allergies, eosinophilia, and eosinophilic esophagitis (41). Functional studies demonstrated heightened IL-4/IL-13 responsiveness, reversed by JAK inhibition (ruxolitinib) or IL-4Rα blockade, which normalized IgE levels and tissue eosinophilia (41). To date, STAT6-GOF mutations have been reported in 21 persons (42).
2.6 ZNF341 LOF
ZNF341 is a zinc-finger transcription factor that upregulates both STAT1 and STAT3 expression (43). Autosomal-recessive LOF mutations in ZNF341 phenocopy STAT3-HIES, causing elevated IgE, eosinophilia, eczema, and recurrent bacterial and fungal infections (43, 44). Till now, 20 patients with autosomal recessive LOF ZNF341 have been reported (44). Unlike STAT3 DN, connective tissue defects tend to be milder, but the underlying mechanism, diminished STAT3 transcription remains the same.
3 Cell surface receptor defects in altered cytokine signaling
3.1 IL-2Rα (CD25) LOF
The α-chain of the high-affinity IL-2 receptor is encoded by IL2RA and is essential for regulatory T-cell development and peripheral tolerance (45). Biallelic loss-of-function mutations in IL2RA produce an IPEX-like syndrome characterized by severe atopic dermatitis, eosinophilia, elevated IgE, autoimmunity, and chronic infections (22, 46). Defective IL-2 signaling impairs Treg homeostasis, which results in unchecked Th2 and Th17 responses that drive both allergic and autoimmune pathology (22).
3.2 IL-4RA GOF
Gain-of-function (GOF) variants in IL4RA, particularly the R576 allele, are strongly associated with increased susceptibility to atopic diseases (47). The Q576R variant in IL-4RA disrupts the formation of the STAT3–ERBIN–SMAD2/3 complex (48). Impaired STAT3 and ERBIN function intensifies Th2 polarization by reducing TGF-β signaling and increasing IL-4RA expression on lymphocytes (49). These changes culminate in a clinical phenotype characterised by early-onset atopic dermatitis, elevated serum IgE, asthma, food allergies, and, in some cases, autoimmune features (50). Importantly, dupilumab, an IL-4Rα antagonist, has demonstrated clinical efficacy in treating patients with variants in IL-4RA (51).
3.3 IL6ST LOF
IL6ST encodes GP130, the shared signal-transducing subunit for all IL-6 family cytokines. The main cytokines of IL-6 family include IL-6, IL-11, IL-27, IL-35, IL-39, and oncostatin M (52, 53). Recessive LOF variants abolish JAK/STAT3 activation, manifesting as an autosomal-recessive Hyper-IgE syndrome with eczema, high IgE, eosinophilia, and recurrent bacterial infections (54). Recently, dominant-negative IL6ST mutations (c.2261C>A, p.Ser754Ter) have been linked to autosomal-dominant HIES phenotypes (54, 55), and secondary glycosylation defects (e.g., in PGM3 deficiency) can similarly impair GP130 surface expression and STAT3 phosphorylation (56).
3.4 IL-6R LOF
One of the key functions of IL-6 signaling is to differentiate activated Th cells into IL-17 and IL-22 secreting Th17 and Th22 cells (53).). On the other hand, IL-6 suppresses the differentiation of CD4+ T regulatory cells, which regulate inflammation. Individuals deficient in IL-6R develop atopic dermatitis, eosinophilia, recurring pulmonary infections, skin abscesses due to Staphylococcus sp., high IgE levels, but no skeletal abnormalities (57, 58).
3.5 TGF-β receptor (TGFBR1/2) deficiency in Loeys–Dietz syndrome
Heterozygous mutations in TGFBR1 or TGFBR2 cause Loeys–Dietz syndrome (59). This is an autosomal-dominant connective-tissue disorder marked by arterial aneurysms, craniofacial abnormalities, and severe atopic features in the form of asthma, food allergy, and eosinophilic gastrointestinal disease (60). The TGFBR1/2 complex recognises TGF-β, and variants in the receptor may lead to dysregulated TGF-β signaling, which may enhance SMAD2/3 phosphorylation (61). This results in conversion of a tolerogenic pathway to a pro-allergic one by producing dysfunctional Tregs and by enhancing transcription of IL-9 and other pro-allergic mediators (62).
4 Key defects in intracellular signaling components leading to altered cytokine signaling defects
4.1 JAK1 GOF
Germline JAK1 GOF mutations lead to constitutive activation of the Janus kinase 1 protein, leading to immune dysregulation due to unchecked STAT phosphorylation (63). Mechanistically, JAK1 hyperactivity skews CD4+ T-cell differentiation toward a Th2 phenotype while suppressing Th1 responses (63). This results in amplification of allergic inflammation (63). These variants lead to novel monogenic immune dysregulation syndrome, termed JAACD—JAK1-associated Autoimmunity, Atopy, Colitis, and Dermatitis. Affected individuals exhibit a syndromic phenotype characterized by early-onset atopic disease, autoimmune features, severe dermatitis, and inflammatory bowel manifestations such as colitis (64). Del Bel et al. described the first germline GOF mutation in humans in JAK1, resulting in an alanine to aspartate substitution at position 634 (65). Three more variants were identified in JAK1, namely, S703I, H596D, and C787F, from patients with a similar clinical phenotype (66–68). Recently, Horesh et al. described 59 patients with JAACD spectrum harbouring four JAK1-GOF variants (p.E139K, p.R506C, p.S700N, and p.V985I). These patients share a common phenotype of severe atopy with autoimmunity and immune dysregulation (64).
4.2 ERBB2IP (ERBIN) LOF
ERBB2IP encodes ERBIN, a scaffold protein that links activated STAT3 to SMAD2/3 complexes, sequestering them in the cytoplasm and thereby restricting TGF-β signaling (49, 69). Impaired STAT3 signaling can decrease ERBIN levels resulting in disrupted regulation of TGF-β and consequent increase in Tregs. Autosomal-recessive LOF variants in ERBB2IP disrupt this regulatory axis, leading to excessive SMAD2/3 nuclear translocation, enhanced Treg proliferation, and paradoxical Th2 polarization (49). Clinically, ERBIN-deficient patients exhibit anomalies reminiscent of STAT3-HIES, despite a distinct molecular etiology. Patients may present with severe atopic dermatitis, eosinophilic gastrointestinal diseases, elevated IgE, and connective-tissue disorders. Emerging biologic therapy, such as IL-4Rα blockade with dupilumab has shown promise in reducing Th2-driven inflammation in this disorder (70).
5 Future perspectives
Next-generation sequencing (NGS) is revolutionizing the diagnosis of primary atopic disorders (PADs) by enabling rapid identification of disease-causing variants. This technology is especially valuable in patients with complex or treatment-refractory presentations, where it can resolve long-standing diagnostic challenges. However, as the use of NGS expands, a growing number of variants of uncertain significance (VUS) or cases lacking identifiable pathogenic variants are being reported. These findings often complicate clinical decision-making and may necessitate extensive additional testing.
Large-scale analyses of genetic testing across hereditary diseases have underscored the growing burden of variants of uncertain significance (VUS). In a study involving over 1.6 million individuals, 41% had at least one VUS, and nearly one-third received only VUS results (71). Despite efforts, only 7% of unique VUSs were reclassified as pathogenic or likely pathogenic, often taking over two years (71). These findings underscore the broader systemic challenge posed by VUSs across rare disease diagnostics and highlight the need for structured interpretive frameworks that could also benefit the PAD diagnostic landscape.
Looking forward, decision-making around functional validation of variants must be guided by integrated criteria, including in silico prediction tools, family segregation analysis, phenotypic concordance, and population frequency data (72). While functional assays remain the gold standard for confirming pathogenicity, they are resource-intensive and often inaccessible in routine clinical settings.
Future diagnostic frameworks must prioritise variants with the highest clinical relevance. Innovative tools such as multiplexed assays of variant effect (MAVEs) offer a promising, high-throughput approach to functional validation (73). Concurrently, emerging efforts to harmonise the interpretation of single-nucleotide variants (SNVs) and copy-number variants (CNVs) are streamlining variant classification in rare diseases (74).
To fully realise the promise of precision medicine in PADs, future efforts should focus on building standardised, scalable, and integrative diagnostic pipelines. Such systems will be essential for accelerating diagnosis, guiding targeted therapies, and ultimately improving clinical outcomes for patients.
6 Conclusion
PADs represent a critical intersection between monogenic immune dysregulation and severe allergic inflammation. Cytokine signaling defects involving JAK-STAT and TGF-β pathways can result in profound allergic phenotypes often misdiagnosed as common atopy. Timely diagnosis of the pathogenetic defect using advanced next-generation sequencing is essential to deliver targeted, immune-based therapies. As our understanding of PADs continues to expand, personalized approach will eventually be the standard of care for affected individuals.
Author contributions
VT: Writing – review & editing, Writing – original draft. RP: Writing – original draft, Conceptualization, Writing – review & editing. AS: Writing – review & editing, Writing – original draft. SS: Writing – review & editing, Writing – original draft. AM: Writing – review & editing, Writing – original draft. TG: Writing – original draft, Writing – review & editing. MS: Writing – original draft, Writing – review & editing. GV: Writing – review & editing, Writing – original draft. PV: Writing – review & editing, Writing – original draft. SS: Writing – review & editing, Resources, Writing – original draft, Conceptualization. MD: Supervision, Writing – review & editing, Writing – original draft, Visualization. AR: Supervision, Writing – review & editing, Writing – original draft, Conceptualization, Resources.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Amit Rawat received research grant from the Indian Council of Medical Research, New Delhi, India vide Grant ID: 33/29-1/2019-TF/Rare/BMS. However, the funding agency had no role in preparation of the manuscript or its final approval.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Notarangelo LD, Bacchetta R, Casanova JL, Su HC. Human inborn errors of immunity: an expanding universe. Sci Immunol. (2020) 5(49):eabb1662. doi: 10.1126/sciimmunol.abb1662
2. Tangye SG, Al-Herz W, Bousfiha A, Chatila T, Cunningham-Rundles C, Etzioni A, et al. Human inborn errors of immunity: 2019 update on the classification from the international union of immunological societies expert committee. J Clin Immunol. (2020) 40(1):24–64. doi: 10.1007/s10875-019-00737-x
3. Amaya-Uribe L, Rojas M, Azizi G, Anaya J-M, Gershwin ME. Primary immunodeficiency and autoimmunity: a comprehensive review. J Autoimmun. (2019) 99:52–72. doi: 10.1016/j.jaut.2019.01.011
4. Lyons JJ, Milner JD. Primary atopic disorders. J Exp Med. (2018) 215(4):1009–22. doi: 10.1084/jem.20172306
5. Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. (2006) 38(4):441–6. doi: 10.1038/ng1767
6. Bitoun E, Micheloni A, Lamant L, Bonnart C, Tartaglia-Polcini A, Cobbold C, et al. LEKTI proteolytic processing in human primary keratinocytes, tissue distribution and defective expression in netherton syndrome. Hum Mol Genet. (2003) 12(19):2417–30. doi: 10.1093/hmg/ddg247
7. Gaddameedhi S, Selby CP, Kemp MG, Ye R, Sancar A. The circadian clock controls sunburn apoptosis and erythema in mouse skin. J Invest Dermatol. (2015) 135(4):1119–27. doi: 10.1038/jid.2014.508
8. Castagnoli R, Lougaris V, Giardino G, Volpi S, Leonardi L, La Torre F, et al. Inborn errors of immunity with atopic phenotypes: a practical guide for allergists. World Allergy Organ J. (2021) 14(2):100513. doi: 10.1016/j.waojou.2021.100513
9. Vaseghi-Shanjani M, Smith KL, Sara RJ, Modi BP, Branch A, Sharma M, et al. Inborn errors of immunity manifesting as atopic disorders. J Allergy Clin Immunol. (2021) 148(5):1130–9. doi: 10.1016/j.jaci.2021.08.008
10. Zhu J, Paul WE. CD4T cells: fates, functions, and faults. Blood. (2008) 112(5):1557–69. doi: 10.1182/blood-2008-05-078154
11. Nelson RW, Geha RS, McDonald DR. Inborn errors of the immune system associated with atopy. Front Immunol. (2022) 13:860821. doi: 10.3389/fimmu.2022.860821
12. Umetsu DT, DeKruyff RH. Th1 and Th2 CD4+ cells in the pathogenesis of allergic diseases. Proc Soc Exp Biol Med. (1997) 215(1):11–20. doi: 10.3181/00379727-215-44109
13. Zheng T, Yu J, Oh MH, Zhu Z. The atopic march: progression from atopic dermatitis to allergic rhinitis and asthma. Allergy Asthma Immunol Res. (2011) 3(2):67–73. doi: 10.4168/aair.2011.3.2.67
14. Vaseghi-Shanjani M, Samra S, Yousefi P, Biggs CM, Turvey SE. Primary atopic disorders: inborn errors of immunity causing severe allergic disease. Curr Opin Immunol. (2025) 94:102538. doi: 10.1016/j.coi.2025.102538
15. Erman B, Aba U, Ipsir C, Pehlivan D, Aytekin C, Cildir G, et al. Genetic evaluation of the patients with clinically diagnosed inborn errors of immunity by whole exome sequencing: results from a specialized research center for immunodeficiency in Türkiye. J Clin Immunol. (2024) 44(7):157. doi: 10.1007/s10875-024-01759-w
16. Sogkas G, Atschekzei F, Adriawan IR, Dubrowinskaja N, Witte T, Schmidt RE. Cellular and molecular mechanisms breaking immune tolerance in inborn errors of immunity. Cell Mol Immunol. (2021) 18(5):1122–40. doi: 10.1038/s41423-020-00626-z
17. Barnes PJ. Pathophysiology of allergic inflammation. Immunol Rev. (2011) 242(1):31–50. doi: 10.1111/j.1600-065X.2011.01020.x
18. Milner JD. Primary atopic disorders. Annu Rev Immunol. (2020) 38(1):785–808. doi: 10.1146/annurev-immunol-042718-041553
19. Zhang W, Chen X, Gao G, Xing S, Zhou L, Tang X, et al. Clinical relevance of gain- and loss-of-function germline mutations in STAT1: a systematic review. Front Immunol. (2021) 12:654406. doi: 10.3389/fimmu.2021.654406
20. Toubiana J, Okada S, Hiller J, Oleastro M, Lagos Gomez M, Aldave Becerra JC, et al. Heterozygous STAT1 gain-of-function mutations underlie an unexpectedly broad clinical phenotype. Blood. (2016) 127(25):3154–64. doi: 10.1182/blood-2015-11-679902
21. Okada S, Asano T, Moriya K, Boisson-Dupuis S, Kobayashi M, Casanova JL, et al. Human STAT1 gain-of-function heterozygous mutations: chronic mucocutaneous candidiasis and type I interferonopathy. J Clin Immunol. (2020) 40(8):1065–81. doi: 10.1007/s10875-020-00847-x
22. Verbsky JW, Chatila TA. Immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) and IPEX-related disorders: an evolving web of heritable autoimmune diseases. Curr Opin Pediatr. (2013) 25(6):708–14. doi: 10.1097/MOP.0000000000000029
23. Scott O, Sharfe N, Dadi H, Vong L, Garkaby J, Abrego Fuentes L, et al. Case report: eosinophilic esophagitis in a patient with a novel STAT1 gain-of-function pathogenic variant. Front Immunol. (2022) 13:801832. doi: 10.3389/fimmu.2022.801832
24. Davis SD, Schaller J, Wedgwood RJ. Job’s syndrome. Recurrent, “cold”, staphylococcal abscesses. Lancet. (1966) 1(7445):1013–5. doi: 10.1016/S0140-6736(66)90119-X
25. Holland SM, DeLeo FR, Elloumi HZ, Hsu AP, Uzel G, Brodsky N, et al. STAT3 mutations in the hyper-IgE syndrome. N Engl J Med. (2007) 357(16):1608–19. doi: 10.1056/NEJMoa073687
26. Minegishi Y, Saito M, Tsuchiya S, Tsuge I, Takada H, Hara T, et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature. (2007) 448(7157):1058–62. doi: 10.1038/nature06096
27. Grimbacher B, Holland SM, Gallin JI, Greenberg F, Hill SC, Malech HL, et al. Hyper-IgE syndrome with recurrent infections–an autosomal dominant multisystem disorder. N Engl J Med. (1999) 340(9):692–702. doi: 10.1056/NEJM199903043400904
28. Tsilifis C, Freeman AF, Gennery AR. STAT3 hyper-IgE syndrome-an update and unanswered questions. J Clin Immunol. (2021) 41(5):864–80. doi: 10.1007/s10875-021-01051-1
29. Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis. (2004) 190(3):624–31. doi: 10.1086/422329
30. Woellner C, Gertz EM, Schäffer AA, Lagos M, Perro M, Glocker E-O, et al. Mutations in STAT3 and diagnostic guidelines for hyper-IgE syndrome. J Allergy Clin Immunol. (2010) 125(2):424–32.e8. doi: 10.1016/j.jaci.2009.10.059
31. Rajala HL, Eldfors S, Kuusanmäki H, van Adrichem AJ, Olson T, Lagström S, et al. Discovery of somatic STAT5b mutations in large granular lymphocytic leukemia. Blood. (2013) 121(22):4541–50. doi: 10.1182/blood-2012-12-474577
32. Ma CA, Xi L, Cauff B, DeZure A, Freeman AF, Hambleton S, et al. Somatic STAT5b gain-of-function mutations in early onset nonclonal eosinophilia, urticaria, dermatitis, and diarrhea. Blood. (2017) 129(5):650–3. doi: 10.1182/blood-2016-09-737817
33. Kanai T, Jenks J, Nadeau KC. The STAT5b pathway defect and autoimmunity. Front Immunol. (2012) 3:234. doi: 10.3389/fimmu.2012.00234
34. Smith MR, Satter LRF, Vargas-Hernández A. STAT5b: a master regulator of key biological pathways. Front Immunol. (2022) 13:1025373. doi: 10.3389/fimmu.2022.1025373
35. Hwa V. STAT5B deficiency: impacts on human growth and immunity. Growth Horm IGF Res. (2016) 28:16–20. doi: 10.1016/j.ghir.2015.12.006
36. Klammt J, Neumann D, Gevers EF, Andrew SF, Schwartz ID, Rockstroh D, et al. Dominant-negative STAT5B mutations cause growth hormone insensitivity with short stature and mild immune dysregulation. Nat Commun. (2018) 9(1):2105. doi: 10.1038/s41467-018-04521-0
37. Luo Y, Alexander M, Gadina M, O’Shea JJ, Meylan F, Schwartz DM. JAK-STAT signaling in human disease: from genetic syndromes to clinical inhibition. J Allergy Clin Immunol. (2021) 148(4):911–25. doi: 10.1016/j.jaci.2021.08.004
38. Goenka S, Kaplan MH. Transcriptional regulation by STAT6. Immunol Res. (2011) 50(1):87–96. doi: 10.1007/s12026-011-8205-2
39. Walford HH, Doherty TA. STAT6 and lung inflammation. Jakstat. (2013) 2(4):e25301. doi: 10.4161/jkst.25301
40. Suratannon N, Ittiwut C, Dik WA, Ittiwut R, Meesilpavikkai K, Israsena N, et al. A germline STAT6 gain-of-function variant is associated with early-onset allergies. J Allergy Clin Immunol. (2023) 151(2):565–71.e9. doi: 10.1016/j.jaci.2022.09.028
41. Sharma M, Leung D, Momenilandi M, Jones LCW, Pacillo L, James AE, et al. Human germline heterozygous gain-of-function STAT6 variants cause severe allergic disease. J Exp Med. (2023) 220(5):e20221755. doi: 10.1084/jem.20221755
42. Bariş S, Chatila T. Identification of a novel primary atopic disorder due to STAT6 gain-of-function mutations. Turk J Immunol. (2024) 12(Suppl 1):53–9. doi: 10.4274/tji.galenos.2023.39200
43. Béziat V, Li J, Lin JX, Ma CS, Li P, Bousfiha A, et al. A recessive form of hyper-IgE syndrome by disruption of ZNF341-dependent STAT3 transcription and activity. Sci Immunol. (2018) 3(24):eaat4956. doi: 10.1126/sciimmunol.aat4956
44. Béziat V, Fieschi C, Momenilandi M, Migaud M, Belaid B, Djidjik R, et al. Inherited human ZNF341 deficiency. Curr Opin Immunol. (2023) 82:102326. doi: 10.1016/j.coi.2023.102326
45. Kim HP, Kelly J, Leonard WJ. The basis for IL-2-induced IL-2 receptor alpha chain gene regulation: importance of two widely separated IL-2 response elements. Immunity. (2001) 15(1):159–72. doi: 10.1016/S1074-7613(01)00167-4
46. Hoffjan S, Beygo J, Akkad DA, Parwez Q, Petrasch-Parwez E, Epplen JT. Analysis of variation in the IL7RA and IL2RA genes in atopic dermatitis. J Dermatol Sci. (2009) 55(2):138–40. doi: 10.1016/j.jdermsci.2009.05.001
47. Hershey GK, Friedrich MF, Esswein LA, Thomas ML, Chatila TA. The association of atopy with a gain-of-function mutation in the alpha subunit of the interleukin-4 receptor. N Engl J Med. (1997) 337(24):1720–5. doi: 10.1056/NEJM199712113372403
48. Tachdjian R, Mathias C, Al Khatib S, Bryce PJ, Kim HS, Blaeser F, et al. Pathogenicity of a disease-associated human IL-4 receptor allele in experimental asthma. J Exp Med. (2009) 206(10):2191–204. doi: 10.1084/jem.20091480
49. Lyons JJ, Liu Y, Ma CA, Yu X, O'Connell MP, Lawrence MG, et al. ERBIN deficiency links STAT3 and TGF-β pathway defects with atopy in humans. J Exp Med. (2017) 214(3):669–80. doi: 10.1084/jem.20161435
50. Banzon TM, Kelly MS, Bartnikas LM, Sheehan WJ, Cunningham A, Harb H, et al. Atopic dermatitis mediates the association between an IL4RA variant and food allergy in school-aged children. J Allergy Clin Immunol Pract. (2022) 10(8):2117–24.e4. doi: 10.1016/j.jaip.2022.04.042
51. Thibodeaux Q, Smith MP, Ly K, Beck K, Liao W, Bhutani T. A review of dupilumab in the treatment of atopic diseases. Hum Vaccin Immunother. (2019) 15(9):2129–39. doi: 10.1080/21645515.2019.1582403
52. Chen YH, Spencer S, Laurence A, Thaventhiran JE, Uhlig HH. Inborn errors of IL-6 family cytokine responses. Curr Opin Immunol. (2021) 72:135–45. doi: 10.1016/j.coi.2021.04.007
53. Murakami M, Kamimura D, Hirano T. Pleiotropy and specificity: insights from the interleukin 6 family of cytokines. Immunity. (2019) 50(4):812–31. doi: 10.1016/j.immuni.2019.03.027
54. Schwerd T, Twigg SRF, Aschenbrenner D, Manrique S, Miller KA, Taylor IB, et al. A biallelic mutation in IL6ST encoding the GP130 co-receptor causes immunodeficiency and craniosynostosis. J Exp Med. (2017) 214(9):2547–62. doi: 10.1084/jem.20161810
55. Basu S, Goel S, Rawat A, Vignesh P, Saikia B. An Indian family with autosomal dominant hyper-IgE syndrome due to IL6ST defect. J Clin Immunol. (2024) 44(4):90. doi: 10.1007/s10875-024-01695-9
56. Ben-Ali M, Ben-Khemis L, Mekki N, Yaakoubi R, Ouni R, Benabdessalem C, et al. Defective glycosylation leads to defective gp130-dependent STAT3 signaling in PGM3-deficient patients. J Allergy Clin Immunol. (2019) 143(4):1638–40.e2. doi: 10.1016/j.jaci.2018.12.987
57. Spencer S, Köstel Bal S, Egner W, Lango Allen H, Raza SI, Ma CA, et al. Loss of the interleukin-6 receptor causes immunodeficiency, atopy, and abnormal inflammatory responses. J Exp Med. (2019) 216(9):1986–98. doi: 10.1084/jem.20190344
58. Nahum A, Sharfe N, Broides A, Dadi H, Naghdi Z, Mandola AB, et al. Defining the biological responses of IL-6 by the study of a novel IL-6 receptor chain immunodeficiency. J Allergy Clin Immunol. (2020) 145(3):1011–5.e6. doi: 10.1016/j.jaci.2019.11.015
59. Loeys BL, Chen J, Neptune ER, Judge DP, Podowski M, Holm T, et al. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet. (2005) 37(3):275–81. doi: 10.1038/ng1511
60. Niehues T, von Hardenberg S, Velleuer E. Rapid identification of primary atopic disorders (PAD) by a clinical landmark-guided, upfront use of genomic sequencing. Allergol Select. (2024) 8:304–23. doi: 10.5414/ALX02520E
61. Abonia JP, Wen T, Stucke EM, Grotjan T, Griffith MS, Kemme KA, et al. High prevalence of eosinophilic esophagitis in patients with inherited connective tissue disorders. J Allergy Clin Immunol. (2013) 132(2):378–86. doi: 10.1016/j.jaci.2013.02.030
62. Li MO, Flavell RA. TGF-beta: a master of all T cell trades. Cell. (2008) 134(3):392–404. doi: 10.1016/j.cell.2008.07.025
63. Biggs CM, Cordeiro-Santanach A, Prykhozhij SV, Deveau AP, Lin Y, Del Bel KL, et al. Human JAK1 gain of function causes dysregulated myelopoeisis and severe allergic inflammation. JCI Insight. (2022) 7(24):e150849. doi: 10.1172/jci.insight.150849
64. Horesh ME, Martin-Fernandez M, Gruber C, Buta S, Le Voyer T, Puzenat E, et al. Individuals with JAK1 variants are affected by syndromic features encompassing autoimmunity, atopy, colitis, and dermatitis. J Exp Med. (2024) 221(6):e20232387. doi: 10.1084/jem.20232387
65. Del Bel KL, Ragotte RJ, Saferali A, Lee S, Vercauteren SM, Mostafavi SA, et al. JAK1 gain-of-function causes an autosomal dominant immune dysregulatory and hypereosinophilic syndrome. J Allergy Clin Immunol. (2017) 139(6):2016–20.e5. doi: 10.1016/j.jaci.2016.12.957
66. Gruber CN, Calis JJA, Buta S, Evrony G, Martin JC, Uhl SA, et al. Complex autoinflammatory syndrome unveils fundamental principles of JAK1 kinase transcriptional and biochemical function. Immunity. (2020) 53(3):672–84.e11. doi: 10.1016/j.immuni.2020.07.006
67. Takeichi T, Lee JYW, Okuno Y, Miyasaka Y, Murase Y, Yoshikawa T, et al. Autoinflammatory keratinization disease with hepatitis and autism reveals roles for JAK1 kinase hyperactivity in autoinflammation. Front Immunol. (2021) 12:737747. doi: 10.3389/fimmu.2021.737747
68. Fayand A, Hentgen V, Posseme C, Lacout C, Picard C, Moguelet P, et al. Successful treatment of JAK1-associated inflammatory disease. J Allergy Clin Immunol. (2023) 152(4):972–83. doi: 10.1016/j.jaci.2023.06.004
69. Dai F, Chang C, Lin X, Dai P, Mei L, Feng XH. Erbin inhibits transforming growth factor beta signaling through a novel smad-interacting domain. Mol Cell Biol. (2007) 27(17):6183–94. doi: 10.1128/MCB.00132-07
70. Dell’Edera A. Use of dupilumab in HyperIgE syndrome with ERBIN-deficiency: case report. Clin Immunol. (2023) 250:109430. doi: 10.1016/j.clim.2023.109430
71. Chen E, Facio FM, Aradhya KW, Rojahn S, Hatchell KE, Aguilar S, et al. Rates and classification of variants of uncertain significance in hereditary disease genetic testing. JAMA Netw Open. (2023) 6(10):e2339571. doi: 10.1001/jamanetworkopen.2023.39571
72. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American college of medical genetics and genomics and the association for molecular pathology. Genet Med. (2015) 17(5):405–24. doi: 10.1038/gim.2015.30
73. Fowler DM, Adams DJ, Gloyn AL, Hahn WC, Marks DS, Muffley LA, et al. An atlas of variant effects to understand the genome at nucleotide resolution. Genome Biol. (2023) 24(1):147. doi: 10.1186/s13059-023-02986-x
74. Durkie M, Cassidy E-J, Berry I, Owens M, Turnbull C, Scott RH, et al. ACGS Best Practice Guidelines for Variant Classification in Rare Disease 2024. Exeter: Royal Devon University Healthcare NHS Foundation Trust (2024). p. 5.
75. Forbes LR, Vogel TP, Cooper MA, Castro-Wagner J, Schussler E, Weinacht KG, et al. Jakinibs for the treatment of immune dysregulation in patients with gain-of-function signal transducer and activator of transcription 1 (STAT1) or STAT3 mutations. J Allergy Clin Immunol. (2018) 142(5):1665–9. doi: 10.1016/j.jaci.2018.07.020
76. Higgins E, Al Shehri T, McAleer MA, Conlon N, Feighery C, Lilic D, et al. Use of ruxolitinib to successfully treat chronic mucocutaneous candidiasis caused by gain-of-function signal transducer and activator of transcription 1 (STAT1) mutation. J Allergy Clin Immunol. (2015) 135(2):551–3. doi: 10.1016/j.jaci.2014.12.1867
77. Kiykim A, Charbonnier LM, Akcay A, Karakoc-Aydiner E, Ozen A, Ozturk G, et al. Hematopoietic stem cell transplantation in patients with heterozygous STAT1 gain-of-function mutation. J Clin Immunol. (2019) 39(1):37–44. doi: 10.1007/s10875-018-0575-y
78. Faletti L, Ehl S, Heeg M. Germline STAT3 gain-of-function mutations in primary immunodeficiency: impact on the cellular and clinical phenotype. Biomed J. (2021) 44(4):412–21. doi: 10.1016/j.bj.2021.03.003
79. Ciullini Mannurita S, Goda R, Schiavo E, Coniglio ML, Azzali A, Fotzi I, et al. Case report: signal transducer and activator of transcription 3 gain-of-function and spectrin deficiency: a life-threatening case of severe hemolytic anemia. Front Immunol. (2020) 11:620046. doi: 10.3389/fimmu.2020.620046
80. Eisenberg R, Gans MD, Leahy TR, Gothe F, Perry C, Raffeld M, et al. JAK inhibition in early-onset somatic, nonclonal STAT5B gain-of-function disease. J Allergy Clin Immunol Pract. (2021) 9(2):1008–10.e2. doi: 10.1016/j.jaip.2020.11.050
81. Acres MJ, Gothe F, Grainger A, Skelton AJ, Swan DJ, Willet JDP, et al. Signal transducer and activator of transcription 5B deficiency due to a novel missense mutation in the coiled-coil domain. J Allergy Clin Immunol. (2019) 143(1):413–6.e4. doi: 10.1016/j.jaci.2018.08.032
82. Joshi TP, Anvari S, Gupta MR, Davis CM, Hajjar J. Case report: dupilumab successfully controls severe eczema in a child with elevated IgE levels and recurrent skin infections. Front Pediatr. (2021) 9:646997. doi: 10.3389/fped.2021.646997
83. Lai N, Liu L, Lin L, Cui C, Wang Y, Min Q, et al. Effective and safe treatment of a novel IL2RA deficiency with rapamycin. J Allergy Clin Immunol Pract. (2020) 8(3):1132–5.e4. doi: 10.1016/j.jaip.2019.09.027
84. Orange JS, Hossny EM, Weiler CR, Ballow M, Berger M, Bonilla FA, et al. Use of intravenous immunoglobulin in human disease: a review of evidence by members of the primary immunodeficiency committee of the American academy of allergy, asthma and immunology. J Allergy Clin Immunol. (2006) 117(4 Suppl):S525–53. doi: 10.1016/j.jaci.2006.01.015
85. Tie R, Li H, Cai S, Liang Z, Shan W, Wang B, et al. Interleukin-6 signaling regulates hematopoietic stem cell emergence. Exp Mol Med. (2019) 51(10):1–12. doi: 10.1038/s12276-019-0320-5
86. MacCarrick G, Black JH III, Bowdin S, El-Hamamsy I, Frischmeyer-Guerrerio PA, Guerrerio AL, et al. Loeys–Dietz syndrome: a primer for diagnosis and management. Genet Med. (2014) 16(8):576–87. doi: 10.1038/gim.2014.11
87. Droghini HR, Abonia JP, Collins MH, Milner JD, Lyons JJ, Freeman AF, et al. Targeted IL-4Rα blockade ameliorates refractory allergic eosinophilic inflammation in a patient with dysregulated TGF-β signaling due to ERBIN deficiency. J Allergy Clin Immunol Pract. (2022) 10(7):1903–6. doi: 10.1016/j.jaip.2022.01.012
Keywords: primary atopic disorders, monogenic allergic diseases, primary immunodeficiencies, inborn errors of immunity, allergy, atopy
Citation: Thakur V, Pilania RK, Sharma A, Sharma S, Mario AT, Goyal T, Sharma M, Vaitheeswaran GC, Vignesh P, Singh S, Dhaliwal M and Rawat A (2025) Cytokine signaling defects in primary atopic diseases—an updated review. Front. Allergy 6:1617714. doi: 10.3389/falgy.2025.1617714
Received: 24 April 2025; Accepted: 6 June 2025;
Published: 1 July 2025.
Edited by:
Mahnaz Jamee, Research Institute for Children’s Health (RICH), IranReviewed by:
Mehul Sharma, Boston University, United StatesCopyright: © 2025 Thakur, Pilania, Sharma, Sharma, Mario, Goyal, Sharma, Vaitheeswaran, Vignesh, Singh, Dhaliwal and Rawat. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Manpreet Dhaliwal, bWFucHJlZXQzMjZAZ21haWwuY29t; Amit Rawat, cmF3YXRhbWl0QHlhaG9vLmNvbQ==
†These authors share first authorship
‡These authors share senior authorship
 Vaishali Thakur
Vaishali Thakur Rakesh Kumar Pilania
Rakesh Kumar Pilania Arunima Sharma
Arunima Sharma Saniya Sharma
Saniya Sharma Taru Goyal
Taru Goyal Madhubala Sharma
Madhubala Sharma Pandiarajan Vignesh
Pandiarajan Vignesh Surjit Singh
Surjit Singh Manpreet Dhaliwal
Manpreet Dhaliwal Amit Rawat
Amit Rawat