- Department of Allergy and Clinical Immunology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea
Introduction: Airway epithelial cells function as the first physical barrier against pathogens and are key regulators of immune responses by producing a wide array of cytokines involved in both innate and adaptive immunity.
Methods: This review summarizes recent advances in our understanding of epithelial-derived cytokines in severe asthma (SA) pathogenesis and highlights promising therapeutic strategies.
Results: Epithelial-derived cytokines can be functionally classified into the following four main groups: alarmins [interleukin [IL]-25, IL-33, thymic stromal lymphopoietin [TSLP]], proinflammatory cytokines (IL-1, IL-6, tumor necrosis factor-α), chemokines (CCL2, CCL5), and antiviral cytokines [interferon (IFN)-α, IFN-β, IFN-λ]. Alarmins are rapidly released in response to epithelial injury and play a pivotal role in initiating immune responses by activating dendritic cells, type 2 innate lymphoid cells, and eosinophils. Proinflammatory cytokines intensify inflammation by promoting immune cell activation and cytokine cascades, while chemokines guide immune cells to sites of injury. Antiviral cytokines enhance epithelial defenses by inducing the expression of antiviral genes. In SA, epithelial-derived cytokines play a central role in initiating and sustaining type 2 (T2) inflammation by activating the IL-4, IL-5, and IL-13 axis, leading to increased eosinophils, elevated serum IgE, and heightened airway hyperresponsiveness. These cytokines are also implicated in non-T2 inflammation, particularly in refractory asthma phenotypes.
Discussion: Growing insights into epithelial cytokines and their complex signaling networks with the airway microenvironment have opened new avenues for developing targeted and personalized treatment in SA.
Introduction
Airway epithelial cells form the first line of defense against inhaled pathogens and environmental toxins, serving both physical and immunological barrier functions (1). These cells develop, maintain, and repair the respiratory tract by producing mucus, regulating inflammation, and facilitating tissue remodeling (2). Beyond their structural role, epithelial cells are actively involved in innate immunity (3). Through pattern recognition receptors, including Toll-like receptors, NOD-like receptors, and RIG-I-like receptors, airway epithelial cells detect microbial components and initiate intracellular signaling cascades (4). In response, they secrete a variety of antimicrobial peptides, such as defensins, cathelicidins, and lysozymes, which directly contribute to the elimination of pathogens (5). Epithelial cells also release cytokines and chemokines that direct immune cell recruitment and activation (3). Following injury, airway epithelial cells rapidly proliferate and differentiate to restore barrier integrity and prevent secondary infections (6).
Asthma is a chronic inflammatory disease of the airways characterized by variable respiratory symptoms, including wheezing, shortness of breath, chest tightness, cough, and expiratory airflow limitation (7). Severe asthma (SA), affecting approximately 5%–10% of patients with asthma, is a complex clinical condition characterized by persistent symptoms that remain uncontrolled despite adhering to guideline-recommended therapy (8). SA encompasses multiple inflammatory phenotypes, including type 2 (T2) inflammation with elevated IL-4, IL-5, and IL-13 driving eosinophilia, IgE production, and airway hyperresponsiveness (9), as well as non-T2 phenotypes such as neutrophilic airway inflammation, obesity-associated asthma, and paucigranulocytic asthma (10). Recent studies have highlighted the importance of airway epithelial cell-derived cytokines in SA (6, 11). These cytokines activate both innate and adaptive immune responses, amplifying inflammation and perpetuating chronic disease. In this review, we systematically summarize the latest findings on the role of epithelial-derived cytokines in SA, focusing on their immunological function and potential as therapeutic targets.
Airway epithelium as a physical barrier
The airway epithelial barrier comprises epithelial cells interconnected by adhesion proteins, such as zonula occludens-1, occludin, and claudins, which form tight junctions that establish the first line of defense against airborne environmental threats, including house dust mites, pollen, and pollutants (12, 13). This barrier comprises three key cell types: ciliated cells, which coordinate mucociliary clearance by propelling mucus to remove inhaled particles and pathogens; basal cells, located at the base of the epithelium, which function as progenitor cells to regenerate damaged or senescent epithelial cells; and secretory cells (such as goblet cells), which produce mucins to form the protective mucus layer, and in some cases, cytokines (11, 14) (Figure 1).
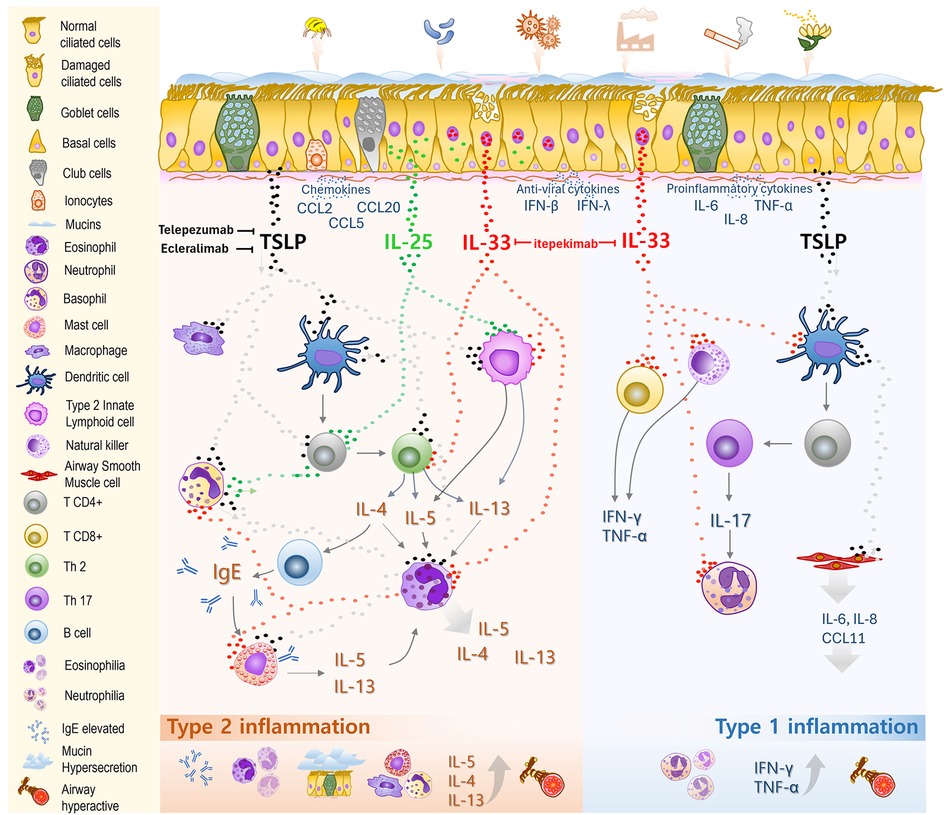
Figure 1. Roles of epithelial-derived cytokines in the pathogenesis of severe asthma. The airway epithelial barrier comprises ciliated and basal cells, along with secretory cells such as goblet cells, club cells, and ionocytes, all covered by a mucus layer propelled by ciliary motion. This forms the first line of defense against inhaled allergens, pollutants, and pathogens. Upon exposure, these agents trigger the release of various cytokines from epithelial cells. Epithelial-derived alarmins, thymic stromal lymphopoietin (TSLP), interleukin (IL)-25, and IL-33, play key roles as initiators and amplifiers of inflammation. TSLP drives dendritic cells (DCs) to induce CD4+ T cell differentiation into Th2 cells, enhances IgG and IgE production, activates ILC2s and basophils, and stimulates the proliferation and differentiation of mast cells and macrophages toward a type 2 inflammatory response. TSLP indirectly promotes Th17 differentiation, leading to IL-17 production and neutrophilic inflammation, driving a type 1 inflammatory response. It also stimulates airway smooth muscle cells to release proinflammatory cytokines. IL-33, stored in the nucleus and released upon cellular or tissue damage, acts on both type 2 (Th2 cells, ILC2s, eosinophils) and type 1 (CD8+ T cells, neutrophils) immune cells, thereby bridging and amplifying both inflammatory pathways. IL-25, normally stored in the cytoplasm of epithelial cells, primarily targets T2 immune cells such as ILC2s, Th2 cells, and basophils, boosting T2 inflammatory responses. Together, these cytokine pathways drive airway inflammation, mucus hypersecretion, bronchoconstriction, and airway remodeling in severe asthma. Targeting epithelial-derived cytokines has become a promising therapeutic approach, with tezepelumab (anti-TSLP) already approved for clinical use.
In asthma, the airway epithelial barrier can be disrupted by the breakdown of tight junctions, leading to increased permeability to pathogens and environmental allergens (15, 16). This disruption is observed across all asthma phenotypes. In severe T2-high asthma, epithelial barrier dysfunction is associated with FcεRI–IgE cross-linking, which induces activation of Src family kinases, alarmin expression, loss of junctional proteins, and increased epithelial permeability (17).
Mucus hypersecretion is common in asthma, particularly SA. The mucus obstructs the airways and leads to uncontrolled airway inflammation and recurrent exacerbations (18). In SA, overactivation of the IL-4/IL-13 pathway drives excessive mucus production (19). Additionally, eosinophils release eosinophil peroxidase (EPO), which promotes the formation of dense and highly viscous mucins, further impairing airflow (20). Goblet cell hyperplasia, combined with a reduction in the number and function of ciliated epithelial cells, worsens mucus retention (21).
Basal cells serve as the primary progenitors of the airway epithelium, playing a crucial role in tissue regeneration. They maintain epithelial homeostasis by proliferating and differentiating to replenish specialized cells, such as goblet and ciliated cells after injury (11, 21). However, in SA, airway epithelial cells, including basal cells, exhibit markedly increased proliferation compared to those in mild asthma or healthy individuals, potentially contributing to airway remodeling (22). Moreover, structural damage to the epithelium may trigger the release of growth factors such as TGF-β, which impairs the ability of basal cells to regenerate and perpetuates epithelial dysfunction (23).
Club cells (also known as Clara cells) are non-ciliated epithelial cells located in the bronchioles. They secrete anti-inflammatory proteins (club cell secretory protein/CC10), secrete protective proteins and surfactant components, detoxify inhaled substances via cytochrome P450 enzymes, and support epithelial regeneration after injury (24). Ionocytes are rare, specialized epithelial cells characterized by high expression of cystic fibrosis transmembrane conductance regulator, a chloride channel essential for ion transport and regulation of airway surface liquid (ASL). This function maintains mucosal hydration and effectively supports mucociliary clearance (25, 26).
Beyond structural cells, innate immune cells, including dendritic cells (DCs) and mast cells, are scattered within or adjacent to the airway epithelium. Although they do not directly contribute to the physical barrier, they are critical in recognizing environmental stimuli and activating other innate immune cells as well as adaptive immune responses, thereby protecting the airways from harmful agents.
Immune functions of airway epithelial cells
In addition to forming a physical barrier, airway epithelial cells also play a central role in orchestrating immune responses by releasing a wide range of cytokines. Epithelial-derived cytokines are functionally grouped into four categories: alarmins, proinflammatory cytokines, chemokines, and antiviral cytokines. Alarmins play a pivotal role in airway immune responses in patients with severe asthma and will be discussed in detail in the following sections.
Following injury or IL-13 stimulation, epithelial cells—particularly basal cells and goblet cells—upregulate the secretion of proinflammatory cytokines such as IL-6, IL-8, and TNF-α via NF-κB and AP-1 signaling pathways (27–29), thereby recruiting inflammatory cells, amplifying inflammatory responses, and promoting remodeling through the release of growth factors (30, 31).
Chemokines are small (∼15 kDa) signaling molecules secreted by epithelial cells that guide the migration of immune cells to sites of inflammation (32). According to their structural motifs, chemokines are classified into two main families (CC and CXC) and two subgroups (C and CX3C) (33). CC chemokines primarily attract monocytes, lymphocytes, dendritic cells, eosinophils, and basophils, but not neutrophils (34). Viral or bacterial infections induce the release of epithelial chemokines, including CCL2, CCL5, and CCL20 (35), while house dust mite allergens trigger thymus- and activation-regulated chemokine, enhancing Th2 cell recruitment (36). In asthma, CCL2 is often overexpressed, and blocking CCL2 or its receptor alleviates symptoms (37). CC chemokines such as CCL11, CCL24, CCL26, and CCL5 recruit eosinophils to allergic sites and activate them via CCR3 signaling, promoting inflammation and the release of intracellular granules (38–40). Meanwhile, CXC chemokines predominantly recruit neutrophils and other inflammatory cells (34). The association of CXCL5 with its receptor CXCR2 has been linked to eosinophilia during asthma exacerbations (41). Additionally, the CXCL10/CXCR3 axis is involved in the recruitment of mast cells to inflamed regions, contributing to the contraction of airway smooth muscle (ASM) cells in asthma (42).
The airway epithelium produces type I interferons, particularly IFN-β, in response to viral infections. IFN-β enhances antiviral defenses by inducing interferon-stimulated genes and recruiting NK, CD4+, and CD8+ T cells (43). IFN-β deficiency is associated with asthma severity, and clinical trials with inhaled IFN-β have shown reduced asthma exacerbations caused by viruses (44). The mechanisms underlying IFN-β deficiency in asthma and the therapeutic role of epithelial cytokines require further investigation (45). Due to the functional diversity and complex interactions, non-alarmin epithelial-derived cytokines present considerable difficulties in pinpointing precise therapeutic targets. The understanding of the role of non-alarmin cytokines in SA remains limited. To date, no large-scale studies have demonstrated significant success in developing targeted treatments in this field.
Epithelial alarmins: initiators and boosters of inflammation
The key alarmins include TSLP, IL-25, and IL-33. Their release may occur concurrently or selectively, depending on the nature of the environmental insult.
TSLP: the spark of inflammation
TSLP, a member of the IL-2 cytokine family, was first isolated from thymic stromal cell cultures and initially linked to B cell development (46). Structurally composed of four α-helical bundles, TSLP is encoded by the TSLP gene on chromosome 5q22. It signals through a heterodimeric receptor comprising the TSLP receptor chain (TSLPR) and the IL-7 receptor alpha chain (IL-7Rα) (47). In humans, TSLP exists in two isoforms: the short form (sfTSLP) and the long form (lfTSLP), each with distinct functions (48). sfTSLP is constitutively expressed in healthy barrier tissues such as the epithelium of the lungs, gut, and skin, contributing to immune homeostasis and displaying potential anti-inflammatory and antimicrobial functions by suppressing pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6 (49). In contrast, lfTSLP is induced by inflammatory stimuli, including allergens, infections, and cytokines, and strongly promotes type 2 (T2) inflammatory responses (50). Recent studies have demonstrated that lfTSLP disrupts airway epithelial barrier function, whereas sfTSLP can counteract these detrimental effects (51). Additionally, lfTSLP has been shown to induce autophagy in ASM cells, thereby promoting airway inflammation and remodeling in both in vitro and in vivo asthma models, while sfTSLP exerts inhibitory effects on these processes (52).
In the lungs, TSLP is primarily secreted by epithelial cells but is also produced by fibroblasts, DCs, basophils, and mast cells (53). TSLP secretion is triggered by various factors, including PAR-2-activating proteases (e.g., airborne fungi like Alternaria) (54), dsRNA from viruses such as rhinovirus or RSV (55), and uric acid from house dust mites (56). There are several mechanisms of TSLP-mediated immune activation. TSLP acts on DCs, promoting their ability to stimulate naive CD4+ T cells to differentiate into Th2 cells, which produce T2 cytokines such as IL-4, IL-5, and IL-13 (57). TSLP may also directly influence CD4+ T cells to differentiate into T follicular helper cells, enhancing the production of IgG and IgE (58). TSLP inhibits regulatory T cells, removing negative regulation and allowing unchecked T2 inflammation (59, 60). TSLP and IL-33 jointly activate group 2 innate lymphoid cells, leading to IL-5 and IL-13 production (61). TSLP also promotes basophil production independently of IL-3 (62, 63), enhances the expression of CD203c, supports eotaxin-mediated migration, and forms an IL-3 autocrine loop, contributing to allergic airway inflammation (64).
When stimulated by TNF-α, the expression of TSLPR and TSLPR mRNA increases, activating eosinophils and the release of eosinophil-derived neurotoxin (65). In bronchial epithelial cells, TSLP delays apoptosis, enhances fibronectin adhesion, and induces secretion of IL-6, IL-8, CXCL1, and CXCL2 (66). TSLP also promotes the proliferation and differentiation of mast cells from bone marrow by activating signal transducer and activator of transcription 6 (STAT6) and upregulating mouse double minute 2 homolog (MDM2), intensifying allergic inflammation (67). TSLP drives the differentiation of macrophages into an alternatively activated T2 inflammatory phenotype (68).
TSLP also contributes to non-type 2 (non-T2) inflammatory processes, including non-allergic asthma and non-eosinophilic asthma. In the context of viral infections or non-allergic triggers, TSLP-stimulated DCs promote the differentiation of naive CD4+ T cells into Th2 cells and promote Th17 differentiation, particularly in the presence of IL-1β, IL-6, and IL-23 (69). These Th17 cells secrete IL-17, which contributes to the recruitment of neutrophils and induces neutrophilic inflammation, a hallmark of non-T2 asthma (10). TSLP also activates ASM cells, enhancing secretion of IL-6, CXCL8 (IL-8), and CCL11 (eotaxin-1) (70).
Clinically, elevated TSLP levels in bronchoalveolar lavage fluid and airway tissues correlate with disease severity and airway obstruction in children with asthma (71). TSLP gene expression is increased in the airway epithelium and mucosa of adult patients with SA (72), and high plasma TSLP levels correlate with persistent asthma exacerbations (73). In SA, TSLP has been implicated in corticosteroid resistance, through regulation of STAT5 phosphorylation and Bcl-xL expression in natural helper cells (74). Beyond T2 inflammation, TSLP promotes airway remodeling, by inducing bronchial smooth muscle cell migration and proliferation, contributing to bronchial wall thickening and remodeling in chronic asthma (75). In mouse models of chronic asthma, TSLP inhibition improved airway remodeling features, such as peribronchial collagen deposition and goblet cell hyperplasia (76).
IL-25: amplifier of T2-inflammation
IL-25, also known as IL-17E, is a member of the IL-17 cytokine family encoded on chromosome 14 (77). IL-25 is produced by airway epithelial cells (78), intestines, and colon, and is also produced by Th2 cells, alveolar macrophages (79), eosinophils, basophils (80), and mast cells (81). IL-25 signals through a heterodimeric receptor composed of IL-17RA and IL-17RB (also known as IL-17Rh1 or EVI27), both of which need to be present to activate downstream signaling pathways (82). Under normal conditions, IL-25 is continuously produced and stored in the cytoplasm of resting normal human bronchial epithelial cells and secreted upon exposure to allergen-derived proteases (83).
IL-25 mRNA expression increases upon exposure to airborne allergens (78, 84). IL-25 directly promotes the differentiation of CD4+ T cells into Th2 cells, via the production of cytokines such as IL-4, IL-5, and IL-13, which are central to T2 inflammation. This process depends on the initial autocrine production of IL-4 by T cells and the presence of the signaling protein STAT6 (78). IL-25 enhances Th2 cytokine production, recruits eosinophils and CD4+ T cells to the airways, and induces goblet cell hyperplasia, which are hallmarks of allergic airway inflammation (78, 85). IL-25 also upregulates eotaxin and arginase-1, two key factors in recruiting eosinophils into lung tissue, thereby amplifying the T2 inflammatory response (86).
IL-25 is a potent activator of group 2 innate lymphoid cells (ILC2), which are immune cells lacking specific antigen receptors but capable of producing type 2 inflammatory cytokines such as IL-5, IL-9, and IL-13 (87). Activated ILC2 promotes mucus secretion, bronchoconstriction (88), and the recruitment of eosinophils and mast cells, which enhances the inflammatory response (89, 90). This cytokine loop supports IgE production, further fueling allergic type 2 inflammation (91). The initiation and amplification of type 2 inflammation via ILC2 also involves other alarmins such as TSLP and IL-33. Furthermore, IL-25 directly enhances antigen uptake in eosinophils and activates Th2 cells in allergic inflammation (92). IL-25 promotes eosinophil activation and migration (93), while reducing apoptosis (94), thereby amplifying type 2 inflammation. Unlike TSLP and IL-33, IL-25 is currently not associated with non-T2 inflammation (10, 95), reinforcing its role as a T2-specific amplifier.
In asthma, IL-25 is elevated (96), particularly during rhinovirus-triggered exacerbations (97). High IL-25 levels, even in the absence of TSLP or IL-33, correlate with greater T2 inflammation and increased responsiveness to inhaled corticosteroids (96). IL-25 expression in the sputum of asthma patients is associated with asthma severity (98). Evidence suggests that IL-25 contributes to airway remodeling in SA by inducing a fibrotic phenotype shift in airway epithelial cells and circulating fibroblasts (99), promoting fibroblast proliferation, extracellular matrix deposition (100), and a fibrotic epithelial phenotype.
IL-33: tissue damage alarm
IL-33 is a member of the IL-1 cytokine family, acting as a vital alarmin that helps maintain tissue balance and repair while participating in both type 1 and type 2 immune responses (101). IL-33 is encoded on chromosome 9p24.1 (102). IL-33 is predominantly expressed in the nucleus of epithelial, endothelial, and mesenchymal cells, including basal cells of the airway epithelium (103). IL-33 is stored in the nucleus and can be released in response to proteases derived from allergens or pathogens (104–106), during cellular stress (107), or in the course of apoptosis (108, 109).
IL-33 is synthesized and stored in the nucleus as a full-length form (IL-33-FL), which has modest biological activity. Upon cellular stress or necrosis, IL-33-FL is released into the extracellular space. Its biological fate then depends on the proteases involved: (i) proteolytic processing by allergen-derived or endogenous proteases (e.g., elastase, cathepsin G, chymase, or tryptase) generates mature IL-33 forms, which are up to 60-fold more potent and bind with high affinity to its receptor ST2, promoting a rapid type 2 inflammatory response (110); (ii) conversely, cleavage by apoptotic caspases produces inactive IL-33 fragments, thereby preventing inappropriate immune activation (108).
ST2 is also known as IL-1 receptor-like 1 (IL1RL1) (111) and exists in two forms: the membrane-anchored form (ST2l) and the soluble form (sST2) (112). ST2l mediates IL-33 signaling, while sST2 acts as a “decoy” receptor binding to IL-33 in the extracellular fluid to prevent it from interacting with ST2l, thereby regulating or inhibiting IL-33 signaling (113). Upon IL-33 binding, ST2l associates with a co-receptor, IL-1 receptor accessory protein (IL-1RAcP). The formation of the IL-33/ST2l/IL-1RAcP complex activates intracellular signaling pathways such as nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and mitogen-activated protein kinase, leading to T2 cytokine production (114).
IL-33 impacts the immune system and tissue repair through various mechanisms. In type 2 inflammation, IL-33 activates Th2 cells via the transcription factor GATA3, promoting the production of IL-5 and IL-13 (115, 116). The IL-33/ST2 signaling axis activates ILC2, increasing the production of IL-5 and IL-13 (117), and collaborates with TSLP to phosphorylate STAT5, amplifying type 2 inflammation (118). For eosinophils, IL-33 stimulates degranulation, enhances superoxide production similar to IL-5, prolongs cell survival, and increases adhesion capacity (119, 120). In basophils, IL-33 upregulates the surface expression of the CD11b antigen, enhances adhesion, promotes migration to inflammatory sites (121), and increases IgE-dependent degranulation (122), reinforcing its role in T2 inflammation. IL-33 also stimulates mast cell differentiation, releasing cytokines (IL-5, IL-6, IL-10, and IL-13) and chemokines (CCL1, CXCL8), contributing to allergic inflammation (123).
Respiratory infections, such as viral (e.g., rhinovirus) or bacterial (e.g., influenza) infections, are key factors that trigger and aggravate exacerbations in patients with non-T2 SA (124). IL-33 drives non-T2 inflammation to clear these pathogens, potentially worsening airway inflammation. IL-33 cooperates with IL-12 and TCR signaling to increase IFN-γ production in CD8+ T cells, promoting viral clearance and CD8+ T cell differentiation (125, 126). It also stimulates DCs to produce IL-6 and upregulate surface molecules, thereby driving type 1 inflammation via TNF-α and IFN-γ (127), and contributes to neutrophil activation in patients with uncontrolled SA (128).
In ASM cells, IL-33 aids wound healing through IL-13 from mast cells (129). IL-33 also increases collagen and fibronectin production in fibroblasts, contributing to airway remodeling in asthma (130, 131). Since IL-33 is released upon cellular or tissue damage, initiating inflammatory and tissue repair responses, it is considered a “tissue damage alarmin.”
Clinically, IL-33 and ST2 levels are elevated in the serum and sputum of asthma patients and correlate with disease severity (132, 133). IL-33 expression is markedly elevated in ASM cells from endobronchial biopsy samples of patients with SA (134). Unlike TSLP, IL-33 plays a key role in driving virus-induced exacerbations in asthma patients (135).
Alarmin-targeted therapies for SA
Recent advances in SA treatment have coincided with improved phenotypic classification, particularly the recognition of T2 inflammation (9). The development of monoclonal antibodies targeting T2 pathways, such as anti-IL5 or anti-IL4/IL13, has substantially alleviated the burden of SA (136). However, a subset of patients, especially those lacking T2 inflammatory biomarkers, do not respond to these therapies (10). In this context, targeting TSLP, a cytokine that initiates inflammatory responses upon allergen exposure, may provide therapeutic benefits for various SA phenotypes, including those that do not respond to T2-targeted biologic therapies.
Tezepelumab, a fully humanized IgG2λ monoclonal antibody targeting TSLP, is currently the only successful anti-TSLP therapy (137–139). Approved for commercial use since 2021, it is indicated for the treatment of uncontrolled SA in patients aged 12 and older and administered via subcutaneous injection (140). Tezepelumab significantly reduces asthma exacerbation rates according to two major clinical trials. In the PATHWAY trial (phase 2), patients receiving tezepelumab experienced a 62%–71% reduction in annual exacerbation rates compared to placebo, alongside improvements in lung function, with efficacy independent of baseline eosinophil counts (138). In the NAVIGATOR trial (phase 3), tezepelumab reduced exacerbation rates by 56% compared to placebo, including in patients with elevated blood eosinophils, while also improving lung function, asthma control, quality of life, and symptom severity (139). To further reduce systemic side effects and improve safety, inhaled anti-TSLP products, such as ecleralimab, are being developed. Ecleralimab (CSJ117), an inhaled anti-TSLP antibody fragment, binds to soluble TSLP and prevents TSLP receptor activation (141). Phase IIa studies indicate that ecleralimab can reduce bronchoconstriction and airway inflammation caused by allergens in patients with mild allergic asthma (142).
Monoclonal antibodies targeting IL-25 (anti-IL-25) (143) or its receptor IL-17RB (144, 145) remain in preclinical development or early research stages. Studies in mice indicate that IL-25 inhibition can prevent airway hyperresponsiveness, reduce the production of T2 inflammation-related cytokines, decrease eosinophil infiltration, limit goblet cell hyperplasia, and lower serum IgE levels (146). Anti-IL-25 agents, originally developed for treating respiratory viral infections, may also help manage severe asthma exacerbations associated with viral infections (143). Despite promising findings, no anti-IL-25 agents have advanced to phase II clinical trials.
The IL-33/ST2 axis is under investigation as a therapeutic target for patients with SA, particularly those who have not responded to previous biologic therapies. Several monoclonal antibodies in trials include GSK3772847, REGN3500 (itepekimab), and ANB020 (etokimab). A phase IIa trial of GSK3772847 in patients with moderate to SA and allergic fungal airway disease showed no significant efficacy, possibly due to a small sample size (147). Itepekimab, an anti-IL-33 monoclonal antibody, reduced exacerbation rates and improved lung function compared to placebo in patients with moderate to SA but did not provide superior benefits compared to dupilumab (148). A phase 2b trial of etokimab in patients with severe eosinophilic asthma has been completed, but official results have not yet been published (149). Overall, drugs targeting the IL-33/IL1RL1 axis hold potential as alternatives to current type 2 monoclonal antibodies, but the efficacy of combining these therapies requires further investigation.
Conclusion
Airway epithelial cells play a central role in asthma by secreting proinflammatory cytokines, chemokines, antiviral cytokines, and alarmins, driving diverse inflammatory responses, including T2 and non-T2 inflammation. Understanding their mechanisms of action and interaction networks offers opportunities for targeted therapies in SA, particularly for non-T2 phenotypes. Among current treatments, tezepelumab (a commercialized anti-TSLP agent) has demonstrated efficacy in reducing exacerbation rates and improving lung function, while therapies targeting IL-25 and IL-33 show promise in preclinical studies. However, clinical data on IL-25 and IL-33 remain limited, and large-scale studies on non-alarmin epithelial cytokines are lacking. Future research should focus on cytokine-specific pathways, evaluating combination therapies, and developing inhaled biologics to optimize efficacy and minimize systemic side effects. Large-scale clinical trials targeting non-T2 phenotypes and epithelial-driven airway remodeling pathways are essential to advance personalized asthma treatments.
Author contributions
DDP: Conceptualization, Data curation, Writing – original draft, Writing – review & editing, Formal analysis, Investigation, Methodology. TBK: Conceptualization, Writing – review & editing, Funding acquisition, Methodology.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: RS-2024-00403700).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Davis JD, Wypych TP. Correction: cellular and functional heterogeneity of the airway epithelium. Mucosal Immunol. (2022) 15(3):528. doi: 10.1038/s41385-022-00500-3
2. Ganesan S, Comstock AT, Sajjan US. Barrier function of airway tract epithelium. Tissue Barriers. (2013) 1(4):e24997. doi: 10.4161/tisb.24997
3. Whitsett JA, Alenghat T. Respiratory epithelial cells orchestrate pulmonary innate immunity. Nat Immunol. (2015) 16(1):27–35. doi: 10.1038/ni.3045
4. Opitz B, van Laak V, Eitel J, Suttorp N. Innate immune recognition in infectious and noninfectious diseases of the lung. Am J Respir Crit Care Med. (2010) 181(12):1294–309. doi: 10.1164/rccm.200909-1427SO
5. Hiemstra PS, Amatngalim GD, van der Does AM, Taube C. Antimicrobial peptides and innate lung defenses: role in infectious and noninfectious lung diseases and therapeutic applications. Chest. (2016) 149(2):545–51. doi: 10.1378/chest.15-1353
6. Noureddine N, Chalubinski M, Wawrzyniak P. The role of defective epithelial barriers in allergic lung disease and asthma development. J Asthma Allergy. (2022) 15:487–504. doi: 10.2147/JAA.S324080
7. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention (2022). Available online at: https://ginasthma.org/wp-content/uploads/2022/07/GINA-Main-Report-2022-FINAL-22-07-01-WMS.pdf (Accessed June 2025)
8. Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. (2014) 43(2):343–73. doi: 10.1183/09031936.00202013
9. Maison N, Omony J, Illi S, Thiele D, Skevaki C, Dittrich AM, et al. T2-high asthma phenotypes across lifespan. European Respiratory Journal. (2022) 60(3):2102288.35210326
10. Sze E, Bhalla A, Nair P. Mechanisms and therapeutic strategies for non-T2 asthma. Allergy. (2020) 75(2):311–25. doi: 10.1111/all.13985
11. Dorscheid D, Gauvreau GM, Georas SN, Hiemstra PS, Varricchi G, Lambrecht BN, et al. Airway epithelial cells as drivers of severe asthma pathogenesis. Mucosal Immunol. (2025) 18(3):524–36. doi: 10.1016/j.mucimm.2025.03.003
12. Georas SN, Rezaee F. Epithelial barrier function: at the front line of asthma immunology and allergic airway inflammation. J Allergy Clin Immunol. (2014) 134(3):509–20. doi: 10.1016/j.jaci.2014.05.049
13. Xiao C, Puddicombe SM, Field S, Haywood J, Broughton-Head V, Puxeddu I, et al. Defective epithelial barrier function in asthma. J Allergy Clin Immunol. (2011) 128(3):549–56e1-12. doi: 10.1016/j.jaci.2011.05.038
14. Davis JD, Wypych TP. Cellular and functional heterogeneity of the airway epithelium. Mucosal Immunol. (2021) 14(5):978–90. doi: 10.1038/s41385-020-00370-7
15. Wan H, Winton HL, Soeller C, Tovey ER, Gruenert DC, Thompson PJ, et al. Der p 1 facilitates transepithelial allergen delivery by disruption of tight junctions. J Clin Invest. (1999) 104(1):123–33. doi: 10.1172/JCI5844
16. Bouley J, Groeme R, Le Mignon M, Jain K, Chabre H, Bordas-Le Floch V, et al. Identification of the cysteine protease amb a 11 as a novel major allergen from short ragweed. J Allergy Clin Immunol. (2015) 136(4):1055–64. doi: 10.1016/j.jaci.2015.03.001
17. Weng CM, Lee MJ, Chao W, Lin YR, Chou CJ, Chen MC, et al. Airway epithelium IgE-FcepsilonRI cross-link induces epithelial barrier disruption in severe T2-high asthma. Mucosal Immunol. (2023) 16(5):685–98. doi: 10.1016/j.mucimm.2023.07.003
18. Garrido C V, Mukherjee M, Svenningsen S, Nair P. Eosinophil-mucus interplay in severe asthma: implications for treatment with biologicals. Allergol Int. (2024) 73(3):351–61. doi: 10.1016/j.alit.2024.03.001
19. Whittaker L, Niu N, Temann UA, Stoddard A, Flavell RA, Ray A, et al. Interleukin-13 mediates a fundamental pathway for airway epithelial mucus induced by CD4T cells and interleukin-9. Am J Respir Cell Mol Biol. (2002) 27(5):593–602. doi: 10.1165/rcmb.4838
20. Dunican EM, Elicker BM, Gierada DS, Nagle SK, Schiebler ML, Newell JD, et al. Mucus plugs in patients with asthma linked to eosinophilia and airflow obstruction. J Clin Invest. (2018) 128(3):997–1009. doi: 10.1172/JCI95693
21. Raby KL, Michaeloudes C, Tonkin J, Chung KF, Bhavsar PK. Mechanisms of airway epithelial injury and abnormal repair in asthma and COPD. Front Immunol. (2023) 14:1201658. doi: 10.3389/fimmu.2023.1201658
22. Cohen L, E X, Tarsi J, Ramkumar T, Horiuchi TK, Cochran R, et al. Epithelial cell proliferation contributes to airway remodeling in severe asthma. Am J Respir Crit Care Med. (2007) 176(2):138–45. doi: 10.1164/rccm.200607-1062OC
23. Makinde T, Murphy RF, Agrawal DK. The regulatory role of TGF-beta in airway remodeling in asthma. Immunol Cell Biol. (2007) 85(5):348–56. doi: 10.1038/sj.icb.7100044
24. Reynolds SD, Malkinson AM. Clara cell: progenitor for the bronchiolar epithelium. Int J Biochem Cell Biol. (2010) 42(1):1–4. doi: 10.1016/j.biocel.2009.09.002
25. Sato Y, Kim D, Turner MJ, Luo Y, Zaidi SSZ, Thomas DY, et al. Ionocyte-specific regulation of cystic fibrosis transmembrane conductance regulator. Am J Respir Cell Mol Biol. (2023) 69(3):281–94. doi: 10.1165/rcmb.2022-0241OC
26. Shah VS, Chivukula RR, Lin B, Waghray A, Rajagopal J. Cystic fibrosis and the cells of the airway epithelium: what are ionocytes and what do they do? Annu Rev Pathol. (2022) 17:23–46. doi: 10.1146/annurev-pathol-042420-094031
27. Liu X, Yin S, Chen Y, Wu Y, Zheng W, Dong H, et al. LPS-induced proinflammatory cytokine expression in human airway epithelial cells and macrophages via NF-κB, STAT3 or AP-1 activation. Mol Med Rep. (2018) 17(4):5484–91. doi: 10.3892/mmr.2018.8542
28. Xie B, Laxman B, Hashemifar S, Stern R, Gilliam TC, Maltsev N, et al. Chemokine expression in the early response to injury in human airway epithelial cells. PLoS One. (2018) 13(3):e0193334. doi: 10.1371/journal.pone.0193334
29. Tanabe T, Rubin BK. Airway goblet cells secrete pro-inflammatory cytokines, chemokines, and growth factors. Chest. (2016) 149(3):714–20. doi: 10.1378/chest.15-0947
30. Chavda VP, Bezbaruah R, Ahmed N, Alom S, Bhattacharjee B, Nalla LV, et al. Proinflammatory cytokines in chronic respiratory diseases and their management. Cells. (2025) 14(6):400. doi: 10.3390/cells14060400
31. Zhang JM, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin. (2007) 45(2):27–37. doi: 10.1097/AIA.0b013e318034194e
32. Liu C, Zhang X, Xiang Y, Qu X, Liu H, Liu C, et al. Role of epithelial chemokines in the pathogenesis of airway inflammation in asthma (review). Mol Med Rep. (2018) 17(5):6935–41. doi: 10.3892/mmr.2018.8739
33. Castan L, Magnan A, Bouchaud G. Chemokine receptors in allergic diseases. Allergy. (2017) 72(5):682–90. doi: 10.1111/all.13089
34. Balestrieri ML, Balestrieri A, Mancini FP, Napoli C. Understanding the immunoangiostatic CXC chemokine network. Cardiovasc Res. (2008) 78(2):250–6. doi: 10.1093/cvr/cvn029
35. Schneider D, Hong JY, Bowman ER, Chung Y, Nagarkar DR, McHenry CL, et al. Macrophage/epithelial cell CCL2 contributes to rhinovirus-induced hyperresponsiveness and inflammation in a mouse model of allergic airways disease. Am J Physiol Lung Cell Mol Physiol. (2013) 304(3):L162–9. doi: 10.1152/ajplung.00182.2012
36. Heijink IH, Marcel Kies P, van Oosterhout AJ, Postma DS, Kauffman HF, Vellenga E. Der p, IL-4, and TGF-beta cooperatively induce EGFR-dependent TARC expression in airway epithelium. Am J Respir Cell Mol Biol. (2007) 36(3):351–9. doi: 10.1165/rcmb.2006-0160OC
37. Mellado M, de Ana A M, Gómez L, Martínez C, Rodríguez-Frade JM. Chemokine receptor 2 blockade prevents asthma in a cynomolgus monkey model. J Pharmacol Exp Ther. (2008) 324(2):769–75. doi: 10.1124/jpet.107.128538
38. Kitayama J, Mackay CR, Ponath PD, Springer TA. The C-C chemokine receptor CCR3 participates in stimulation of eosinophil arrest on inflammatory endothelium in shear flow. J Clin Invest. (1998) 101(9):2017–24. doi: 10.1172/JCI2688
39. Alam R, Stafford S, Forsythe P, Harrison R, Faubion D, Lett-Brown MA, et al. RANTES is a chemotactic and activating factor for human eosinophils. J Immunol. (1993) 150(8 Pt 1):3442–8. doi: 10.4049/jimmunol.150.8.3442
40. Fujisawa T, Kato Y, Nagase H, Atsuta J, Terada A, Iguchi K, et al. Chemokines induce eosinophil degranulation through CCR-3. J Allergy Clin Immunol. (2000) 106(3):507–13. doi: 10.1067/mai.2000.108311
41. Qiu Y, Zhu J, Bandi V, Guntupalli KK, Jeffery PK. Bronchial mucosal inflammation and upregulation of CXC chemoattractants and receptors in severe exacerbations of asthma. Thorax. (2007) 62(6):475–82. doi: 10.1136/thx.2006.066670
42. Bradding P, Walls AF, Holgate ST. The role of the mast cell in the pathophysiology of asthma. J Allergy Clin Immunol. (2006) 117(6):1277–84. doi: 10.1016/j.jaci.2006.02.039
43. Fensterl V, Sen GC. Interferons and viral infections. Biofactors. (2009) 35(1):14–20. doi: 10.1002/biof.6
44. Djukanovic R, Harrison T, Johnston SL, Gabbay F, Wark P, Thomson NC, et al. The effect of inhaled IFN-β on worsening of asthma symptoms caused by viral infections. A randomized trial. Am J Respir Crit Care Med. (2014) 190(2):145–54. doi: 10.1164/rccm.201312-2235OC
45. Ritchie AI, Jackson DJ, Edwards MR, Johnston SL. Airway epithelial orchestration of innate immune function in response to virus infection. A focus on asthma. Ann Am Thorac Soc. (2016) 13(Suppl 1):S55–63. doi: 10.1513/AnnalsATS.201507-421MG
46. Reche PA, Soumelis V, Gorman DM, Clifford T, Liu M, Travis M, et al. Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J Immunol. (2001) 167(1):336–43. doi: 10.4049/jimmunol.167.1.336
47. He R, Geha RS. Thymic stromal lymphopoietin. Ann N Y Acad Sci. (2010) 1183:13–24. doi: 10.1111/j.1749-6632.2009.05128.x
48. Fornasa G, Tsilingiri K, Caprioli F, Botti F, Mapelli M, Meller S, et al. Dichotomy of short and long thymic stromal lymphopoietin isoforms in inflammatory disorders of the bowel and skin. J Allergy Clin Immunol. (2015) 136(2):413–22. doi: 10.1016/j.jaci.2015.04.011
49. Bjerkan L, Schreurs O, Engen SA, Jahnsen FL, Baekkevold ES, Blix IJ, et al. The short form of TSLP is constitutively translated in human keratinocytes and has characteristics of an antimicrobial peptide. Mucosal Immunol. (2015) 8(1):49–56. doi: 10.1038/mi.2014.41
50. Harada M, Hirota T, Jodo AI, Doi S, Kameda M, Fujita K, et al. Functional analysis of the thymic stromal lymphopoietin variants in human bronchial epithelial cells. Am J Respir Cell Mol Biol. (2009) 40(3):368–74. doi: 10.1165/rcmb.2008-0041OC
51. Dong H, Hu Y, Liu L, Zou M, Huang C, Luo L, et al. Distinct roles of short and long thymic stromal lymphopoietin isoforms in house dust mite-induced asthmatic airway epithelial barrier disruption. Sci Rep. (2016) 6:39559. doi: 10.1038/srep39559
52. Zhao J, Zhang J, Tang S, Wang J, Liu T, Zeng R, et al. The different functions of short and long thymic stromal lymphopoietin isoforms in autophagy-mediated asthmatic airway inflammation and remodeling. Immunobiology. (2021) 226(5):152124. doi: 10.1016/j.imbio.2021.152124
53. West EE, Kashyap M, Leonard WJ. TSLP: a key regulator of asthma pathogenesis. Drug Discov Today Dis Mech. (2012) 9(3–4):e83–8. doi: 10.1016/j.ddmec.2012.09.003
54. Kouzaki H, O'Grady SM, Lawrence CB, Kita H. Proteases induce production of thymic stromal lymphopoietin by airway epithelial cells through protease-activated receptor-2. J Immunol. (2009) 183(2):1427–34. doi: 10.4049/jimmunol.0900904
55. Lee HC, Headley MB, Loo YM, Berlin A, Gale M Jr, Debley JS, et al. Thymic stromal lymphopoietin is induced by respiratory syncytial virus-infected airway epithelial cells and promotes a type 2 response to infection. J Allergy Clin Immunol. (2012) 130(5):1187–1196.e5. doi: 10.1016/j.jaci.2012.07.031
56. Hara K, Iijima K, Elias MK, Seno S, Tojima I, Kobayashi T, et al. Airway uric acid is a sensor of inhaled protease allergens and initiates type 2 immune responses in respiratory mucosa. J Immunol. (2014) 192(9):4032–42. doi: 10.4049/jimmunol.1400110
57. Ito T, Wang Y-H, Duramad O, Hori T, Delespesse GJ, Watanabe N, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. (2005) 202(9):1213–23. doi: 10.1084/jem.20051135
58. Pattarini L, Trichot C, Bogiatzi S, Grandclaudon M, Meller S, Keuylian Z, et al. TSLP-activated dendritic cells induce human T follicular helper cell differentiation through OX40-ligand. J Exp Med. (2017) 214(5):1529–46. doi: 10.1084/jem.20150402
59. Lee JY, Lim YM, Park MJ, Min SY, Cho ML, Sung YC, et al. Murine thymic stromal lymphopoietin promotes the differentiation of regulatory T cells from thymic CD4(+)CD8(-)CD25(-) naive cells in a dendritic cell-independent manner. Immunol Cell Biol. (2008) 86(2):206–13. doi: 10.1038/sj.icb.7100127
60. Mazzucchelli R, Hixon JA, Spolski R, Chen X, Li WQ, Hall VL, et al. Development of regulatory T cells requires IL-7Ralpha stimulation by IL-7 or TSLP. Blood. (2008) 112(8):3283–92. doi: 10.1182/blood-2008-02-137414
61. Han M, Rajput C, Hong JY, Lei J, Hinde JL, Wu Q, et al. The innate cytokines IL-25, IL-33, and TSLP cooperate in the induction of type 2 innate lymphoid cell expansion and mucous metaplasia in rhinovirus-infected immature mice. J Immunol. (2017) 199(4):1308–18. doi: 10.4049/jimmunol.1700216
62. Siracusa MC, Saenz SA, Hill DA, Kim BS, Headley MB, Doering TA, et al. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature. (2011) 477(7363):229–33. doi: 10.1038/nature10329
63. Salter BM, Oliveria JP, Nusca G, Smith SG, Watson RM, Comeau M, et al. Thymic stromal lymphopoietin activation of basophils in patients with allergic asthma is IL-3 dependent. J Allergy Clin Immunol. (2015) 136(6):1636–44. doi: 10.1016/j.jaci.2015.03.039
64. Li X, Li Z, Tang M, Zhang K, Yang T, Zhong W, et al. Thymic stromal lymphopoietin-activated basophil promotes lung inflammation in mouse atopic march model. Front Immunol. (2025) 16:1573130. doi: 10.3389/fimmu.2025.1573130
65. Cook EB, Stahl JL, Schwantes EA, Fox KE, Mathur SK. IL-3 and TNFα increase thymic stromal lymphopoietin receptor (TSLPR) expression on eosinophils and enhance TSLP-stimulated degranulation. Clin Mol Allergy. (2012) 10(1):8. doi: 10.1186/1476-7961-10-8
66. Wong CK, Hu S, Cheung PF, Lam CW. Thymic stromal lymphopoietin induces chemotactic and prosurvival effects in eosinophils: implications in allergic inflammation. Am J Respir Cell Mol Biol. (2010) 43(3):305–15. doi: 10.1165/rcmb.2009-0168OC
67. Han NR, Oh HA, Nam SY, Moon PD, Kim DW, Kim HM, et al. TSLP induces mast cell development and aggravates allergic reactions through the activation of MDM2 and STAT6. J Invest Dermatol. (2014) 134(10):2521–30. doi: 10.1038/jid.2014.198
68. Han H, Headley MB, Xu W, Comeau MR, Zhou B, Ziegler SF. Thymic stromal lymphopoietin amplifies the differentiation of alternatively activated macrophages. J Immunol. (2013) 190(3):904–12. doi: 10.4049/jimmunol.1201808
69. Tanaka J, Watanabe N, Kido M, Saga K, Akamatsu T, Nishio A, et al. Human TSLP and TLR3 ligands promote differentiation of Th17 cells with a central memory phenotype under Th2-polarizing conditions. Clin Exp Allergy. (2009) 39(1):89–100. doi: 10.1111/j.1365-2222.2008.03151.x
70. Shan L, Redhu NS, Saleh A, Halayko AJ, Chakir J, Gounni AS. Thymic stromal lymphopoietin receptor-mediated IL-6 and CC/CXC chemokines expression in human airway smooth muscle cells: role of MAPKs (ERK1/2, p38, and JNK) and STAT3 pathways. J Immunol. (2010) 184(12):7134–43. doi: 10.4049/jimmunol.0902515
71. Chorvinsky E, Nino G, Salka K, Gaviria S, Gutierrez MJ, Pillai DK. TSLP bronchoalveolar lavage levels at baseline are linked to clinical disease severity and reduced lung function in children with asthma. Front Pediatr. (2022) 10:971073. doi: 10.3389/fped.2022.971073
72. Shikotra A, Choy DF, Ohri CM, Doran E, Butler C, Hargadon B, et al. Increased expression of immunoreactive thymic stromal lymphopoietin in patients with severe asthma. J Allergy Clin Immunol. (2012) 129(1):104–11.e1–9. doi: 10.1016/j.jaci.2011.08.031
73. Ibrahim B, Achour D, Zerimech F, de Nadai P, Siroux V, Tsicopoulos A, et al. Plasma thymic stromal lymphopoietin (TSLP) in adults with non-severe asthma: the EGEA study. Thorax. (2023) 78(2):207–10. doi: 10.1136/thorax-2022-219192
74. Kabata H, Moro K, Fukunaga K, Suzuki Y, Miyata J, Masaki K, et al. Thymic stromal lymphopoietin induces corticosteroid resistance in natural helper cells during airway inflammation. Nat Commun. (2013) 4:2675. doi: 10.1038/ncomms3675
75. Redhu NS, Shan L, Movassagh H, Gounni AS. Thymic stromal lymphopoietin induces migration in human airway smooth muscle cells. Sci Rep. (2013) 3:2301. doi: 10.1038/srep02301
76. Chen ZG, Zhang TT, Li HT, Chen FH, Zou XL, Ji JZ, et al. Neutralization of TSLP inhibits airway remodeling in a murine model of allergic asthma induced by chronic exposure to house dust mite. PLoS One. (2013) 8(1):e51268. doi: 10.1371/journal.pone.0051268
77. Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. (2001) 15(6):985–95. doi: 10.1016/S1074-7613(01)00243-6
78. Angkasekwinai P, Park H, Wang YH, Wang YH, Chang SH, Corry DB, et al. Interleukin 25 promotes the initiation of proallergic type 2 responses. J Exp Med. (2007) 204(7):1509–17. doi: 10.1084/jem.20061675
79. Kang CM, Jang AS, Ahn MH, Shin JA, Kim JH, Choi YS, et al. Interleukin-25 and interleukin-13 production by alveolar macrophages in response to particles. Am J Respir Cell Mol Biol. (2005) 33(3):290–6. doi: 10.1165/rcmb.2005-0003OC
80. Wang YH, Angkasekwinai P, Lu N, Voo KS, Arima K, Hanabuchi S, et al. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J Exp Med. (2007) 204(8):1837–47. doi: 10.1084/jem.20070406
81. Ikeda K, Nakajima H, Suzuki K, Kagami S, Hirose K, Suto A, et al. Mast cells produce interleukin-25 upon fc epsilon RI-mediated activation. Blood. (2003) 101(9):3594–6. doi: 10.1182/blood-2002-09-2817
82. Yuan Q, Peng N, Xiao F, Shi X, Zhu B, Rui K, et al. New insights into the function of interleukin-25 in disease pathogenesis. Biomark Res. (2023) 11(1):36. doi: 10.1186/s40364-023-00474-9
83. Kouzaki H, Tojima I, Kita H, Shimizu T. Transcription of interleukin-25 and extracellular release of the protein is regulated by allergen proteases in airway epithelial cells. Am J Respir Cell Mol Biol. (2013) 49(5):741–50. doi: 10.1165/rcmb.2012-0304OC
84. Hurst SD, Muchamuel T, Gorman DM, Gilbert JM, Clifford T, Kwan S, et al. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J Immunol. (2002) 169(1):443–53. doi: 10.4049/jimmunol.169.1.443
85. Tamachi T, Maezawa Y, Ikeda K, Kagami S, Hatano M, Seto Y, et al. IL-25 enhances allergic airway inflammation by amplifying a TH2 cell-dependent pathway in mice. J Allergy Clin Immunol. (2006) 118(3):606–14. doi: 10.1016/j.jaci.2006.04.051
86. Sharkhuu T, Matthaei KI, Forbes E, Mahalingam S, Hogan SP, Hansbro PM, et al. Mechanism of interleukin-25 (IL-17E)-induced pulmonary inflammation and airways hyper-reactivity. Clin Exp Allergy. (2006) 36(12):1575–83. doi: 10.1111/j.1365-2222.2006.02595.x
87. Huang Y, Guo L, Qiu J, Chen X, Hu-Li J, Siebenlist U, et al. IL-25-responsive, lineage-negative KLRG1(hi) cells are multipotential ‘inflammatory’ type 2 innate lymphoid cells. Nat Immunol. (2015) 16(2):161–9. doi: 10.1038/ni.3078
88. Kuperman DA, Huang X, Koth LL, Chang GH, Dolganov GM, Zhu Z, et al. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat Med. (2002) 8(8):885–9. doi: 10.1038/nm734
89. Takatsu K, Nakajima H. IL-5 and eosinophilia. Curr Opin Immunol. (2008) 20(3):288–94. doi: 10.1016/j.coi.2008.04.001
90. Pajulas A, Fu Y, Cheung CCL, Chu M, Cannon A, Alakhras N, et al. Interleukin-9 promotes mast cell progenitor proliferation and CCR2-dependent mast cell migration in allergic airway inflammation. Mucosal Immunol. (2023) 16(4):432–45. doi: 10.1016/j.mucimm.2023.05.002
91. Licona-Limón P, Kim LK, Palm NW, Flavell RA. TH2, allergy and group 2 innate lymphoid cells. Nat Immunol. (2013) 14(6):536–42. doi: 10.1038/ni.2617
92. Peng B, Sun L, Zhang M, Yan H, Shi G, Xia Z, et al. Role of IL-25 on eosinophils in the initiation of Th2 responses in allergic asthma. Front Immunol. (2022) 13:842500. doi: 10.3389/fimmu.2022.842500
93. Salter BM, Oliveria JP, Nusca G, Smith SG, Tworek D, Mitchell PD, et al. IL-25 and IL-33 induce type 2 inflammation in basophils from subjects with allergic asthma. Respir Res. (2016) 17:5. doi: 10.1186/s12931-016-0321-z
94. Wang H, Mobini R, Fang Y, Barrenäs F, Zhang H, Xiang Z, et al. Allergen challenge of peripheral blood mononuclear cells from patients with seasonal allergic rhinitis increases IL-17RB, which regulates basophil apoptosis and degranulation. Clin Exp Allergy. (2010) 40(8):1194–202. doi: 10.1111/j.1365-2222.2010.03542.x
95. Niessen NM, Fricker M, McDonald VM, Gibson PG. T2-low: what do we know?: past, present, and future of biologic therapies in noneosinophilic asthma. Ann Allergy Asthma Immunol. (2022) 129(2):150–9. doi: 10.1016/j.anai.2022.04.020
96. Cheng D, Xue Z, Yi L, Shi H, Zhang K, Huo X, et al. Epithelial interleukin-25 is a key mediator in Th2-high, corticosteroid-responsive asthma. Am J Respir Crit Care Med. (2014) 190(6):639–48. doi: 10.1164/rccm.201403-0505OC
97. Beale J, Jayaraman A, Jackson DJ, Macintyre JDR, Edwards MR, Walton RP, et al. Rhinovirus-induced IL-25 in asthma exacerbation drives type 2 immunity and allergic pulmonary inflammation. Sci Transl Med. (2014) 6(256):256ra134. doi: 10.1126/scitranslmed.3009124
98. Paplinska-Goryca M, Grabczak EM, Dabrowska M, Hermanowicz-Salamon J, Proboszcz M, Nejman-Gryz P, et al. Sputum interleukin-25 correlates with asthma severity: a preliminary study. Postepy Dermatol Alergol. (2018) 35(5):462–9. doi: 10.5114/ada.2017.71428
99. Yao X, Chen Q, Wang X, Liu X, Zhang L. IL-25 induces airway remodeling in asthma by orchestrating the phenotypic changes of epithelial cell and fibrocyte. Respir Res. (2023) 24(1):212. doi: 10.1186/s12931-023-02509-z
100. Yao X, Wang W, Li Y, Lv Z, Guo R, Corrigan CJ, et al. Characteristics of IL-25 and allergen-induced airway fibrosis in a murine model of asthma. Respirology. (2015) 20(5):730–8. doi: 10.1111/resp.12546
101. Cayrol C, Girard JP. Interleukin-33 (IL-33): a critical review of its biology and the mechanisms involved in its release as a potent extracellular cytokine. Cytokine. (2022) 156:155891. doi: 10.1016/j.cyto.2022.155891
102. Liew FY, Girard JP, Turnquist HR. Interleukin-33 in health and disease. Nat Rev Immunol. (2016) 16(11):676–89. doi: 10.1038/nri.2016.95
103. Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin'? PLoS One. (2008) 3(10):e3331. doi: 10.1371/journal.pone.0003331
104. Cayrol C, Duval A, Schmitt P, Roga S, Camus M, Stella A, et al. Environmental allergens induce allergic inflammation through proteolytic maturation of IL-33. Nat Immunol. (2018) 19(4):375–85. doi: 10.1038/s41590-018-0067-5
105. Clark JT, Christian DA, Gullicksrud JA, Perry JA, Park J, Jacquet M, et al. IL-33 promotes innate lymphoid cell-dependent IFN-γ production required for innate immunity to Toxoplasma gondii. Elife. (2021) 10:e65614. doi: 10.7554/eLife.65614
106. Nikonova A, Shilovskiy I, Galitskaya M, Sokolova A, Sundukova M, Dmitrieva-Posocco O, et al. Respiratory syncytial virus upregulates IL-33 expression in mouse model of virus-induced inflammation exacerbation in OVA-sensitized mice and in asthmatic subjects. Cytokine. (2021) 138:155349. doi: 10.1016/j.cyto.2020.155349
107. Uchida M, Anderson EL, Squillace DL, Patil N, Maniak PJ, Iijima K, et al. Oxidative stress serves as a key checkpoint for IL-33 release by airway epithelium. Allergy. (2017) 72(10):1521–31. doi: 10.1111/all.13158
108. Lüthi AU, Cullen SP, McNeela EA, Duriez PJ, Afonina IS, Sheridan C, et al. Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity. (2009) 31(1):84–98. doi: 10.1016/j.immuni.2009.05.007
109. Zhao W, Hu Z. The enigmatic processing and secretion of interleukin-33. Cell Mol Immunol. (2010) 7(4):260–2. doi: 10.1038/cmi.2010.3
110. Scott IC, Majithiya JB, Sanden C, Thornton P, Sanders PN, Moore T, et al. Interleukin-33 is activated by allergen- and necrosis-associated proteolytic activities to regulate its alarmin activity during epithelial damage. Sci Rep. (2018) 8(1):3363. doi: 10.1038/s41598-018-21589-2
111. Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. (2005) 23(5):479–90. doi: 10.1016/j.immuni.2005.09.015
112. De la Fuente M, MacDonald TT, Hermoso MA. The IL-33/ST2 axis: role in health and disease. Cytokine Growth Factor Rev. (2015) 26(6):615–23. doi: 10.1016/j.cytogfr.2015.07.017
113. Hayakawa H, Hayakawa M, Kume A, Tominaga S. Soluble ST2 blocks interleukin-33 signaling in allergic airway inflammation. J Biol Chem. (2007) 282(36):26369–80. doi: 10.1074/jbc.M704916200
114. Lu J, Kang J, Zhang C, Zhang X. The role of IL-33/ST2l signals in the immune cells. Immunol Lett. (2015) 164(1):11–7. doi: 10.1016/j.imlet.2015.01.008
115. KleinJan A, Klein Wolterink RG, Levani Y, de Bruijn MJ, Hoogsteden HC, van Nimwegen M, et al. Enforced expression of Gata3 in T cells and group 2 innate lymphoid cells increases susceptibility to allergic airway inflammation in mice. J Immunol. (2014) 192(4):1385–94. doi: 10.4049/jimmunol.1301888
116. Griesenauer B, Paczesny S. The ST2/IL-33 axis in immune cells during inflammatory diseases. Front Immunol. (2017) 8:475. doi: 10.3389/fimmu.2017.00475
117. Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. (2010) 464(7293):1367–70. doi: 10.1038/nature08900
118. Toki S, Goleniewska K, Zhang J, Zhou W, Newcomb DC, Zhou B, et al. TSLP and IL-33 reciprocally promote each other’s lung protein expression and ILC2 receptor expression to enhance innate type-2 airway inflammation. Allergy. (2020) 75(7):1606–17. doi: 10.1111/all.14196
119. Cherry WB, Yoon J, Bartemes KR, Iijima K, Kita H. A novel IL-1 family cytokine, IL-33, potently activates human eosinophils. J Allergy Clin Immunol. (2008) 121(6):1484–90. doi: 10.1016/j.jaci.2008.04.005
120. Suzukawa M, Koketsu R, Iikura M, Nakae S, Matsumoto K, Nagase H, et al. Interleukin-33 enhances adhesion, CD11b expression and survival in human eosinophils. Lab Invest. (2008) 88(11):1245–53. doi: 10.1038/labinvest.2008.82
121. Bochner BS, McKelvey AA, Sterbinsky SA, Hildreth JE, Derse CP, Klunk DA, et al. IL-3 augments adhesiveness for endothelium and CD11b expression in human basophils but not neutrophils. J Immunol. (1990) 145(6):1832–7. doi: 10.4049/jimmunol.145.6.1832
122. Suzukawa M, Iikura M, Koketsu R, Nagase H, Tamura C, Komiya A, et al. An IL-1 cytokine member, IL-33, induces human basophil activation via its ST2 receptor. J Immunol. (2008) 181(9):5981–9. doi: 10.4049/jimmunol.181.9.5981
123. Allakhverdi Z, Smith DE, Comeau MR, Delespesse G. Cutting edge: the ST2 ligand IL-33 potently activates and drives maturation of human mast cells. J Immunol. (2007) 179(4):2051–4. doi: 10.4049/jimmunol.179.4.2051
124. Liu T, Woodruff PG, Zhou X. Advances in non-type 2 severe asthma: from molecular insights to novel treatment strategies. Eur Respir J. (2024) 64(2):2300826. doi: 10.1183/13993003.00826-2023
125. Bonilla WV, Fröhlich A, Senn K, Kallert S, Fernandez M, Johnson S, et al. The alarmin interleukin-33 drives protective antiviral CD8+ T cell responses. Science. (2012) 335(6071):984–9. doi: 10.1126/science.1215418
126. Yang Q, Li G, Zhu Y, Liu L, Chen E, Turnquist H, et al. IL-33 synergizes with TCR and IL-12 signaling to promote the effector function of CD8+ T cells. Eur J Immunol. (2011) 41(11):3351–60. doi: 10.1002/eji.201141629
127. Matta BM, Lott JM, Mathews LR, Liu Q, Rosborough BR, Blazar BR, et al. IL-33 is an unconventional alarmin that stimulates IL-2 secretion by dendritic cells to selectively expand IL-33R/ST2+ regulatory T cells. J Immunol. (2014) 193(8):4010–20. doi: 10.4049/jimmunol.1400481
128. Quoc QL, Cao TBT, Jang JH, Shin YS, Choi Y, Park HS. ST2-mediated neutrophilic airway inflammation: a therapeutic target for patients with uncontrolled asthma. Allergy Asthma Immunol Res. (2024) 16(1):22–41. doi: 10.4168/aair.2024.16.1.22
129. Kaur D, Gomez E, Doe C, Berair R, Woodman L, Saunders R, et al. IL-33 drives airway hyper-responsiveness through IL-13-mediated mast cell: airway smooth muscle crosstalk. Allergy. (2015) 70(5):556–67. doi: 10.1111/all.12593
130. Guo Z, Wu J, Zhao J, Liu F, Chen Y, Bi L, et al. IL-33/ST2 promotes airway remodeling in asthma by activating the expression of fibronectin 1 and type 1 collagen in human lung fibroblasts. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. (2014) 30(9):975–9.25200162
131. An G, Zhang X, Wang W, Huang Q, Li Y, Shan S, et al. The effects of interleukin-33 on airways collagen deposition and matrix metalloproteinase expression in a murine surrogate of asthma. Immunology. (2018) 154(4):637–50. doi: 10.1111/imm.12911
132. Hamzaoui A, Berraies A, Kaabachi W, Haifa M, Ammar J, Kamel H. Induced sputum levels of IL-33 and soluble ST2 in young asthmatic children. J Asthma. (2013) 50(8):803–9. doi: 10.3109/02770903.2013.816317
133. Li R, Yang G, Yang R, Peng X, Li J. Interleukin-33 and receptor ST2 as indicators in patients with asthma: a meta-analysis. Int J Clin Exp Med. (2015) 8(9):14935–43.26628975
134. Préfontaine D, Lajoie-Kadoch S, Foley S, Audusseau S, Olivenstein R, Halayko AJ, et al. Increased expression of IL-33 in severe asthma: evidence of expression by airway smooth muscle cells. J Immunol. (2009) 183(8):5094–103. doi: 10.4049/jimmunol.0802387
135. Ravanetti L, Dijkhuis A, Dekker T, Sabogal Pineros YS, Ravi A, Dierdorp BS, et al. IL-33 drives influenza-induced asthma exacerbations by halting innate and adaptive antiviral immunity. J Allergy Clin Immunol. (2019) 143(4):1355–1370.e16. doi: 10.1016/j.jaci.2018.08.051
136. Busse WW, Viswanathan R. What has been learned by cytokine targeting of asthma? J Allergy Clin Immunol. (2022) 150(2):235–49. doi: 10.1016/j.jaci.2022.06.010
137. Gauvreau GM, O'Byrne PM, Boulet LP, Wang Y, Cockcroft D, Bigler J, et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med. (2014) 370(22):2102–10. doi: 10.1056/NEJMoa1402895
138. Corren J, Parnes JR, Wang L, Mo M, Roseti SL, Griffiths JM, et al. Tezepelumab in adults with uncontrolled asthma. N Engl J Med. (2017) 377(10):936–46. doi: 10.1056/NEJMoa1704064
139. Menzies-Gow A, Corren J, Bourdin A, Chupp G, Israel E, Wechsler ME, et al. Tezepelumab in adults and adolescents with severe, uncontrolled asthma. N Engl J Med. (2021) 384(19):1800–9. doi: 10.1056/NEJMoa2034975
140. Hoy SM. Tezepelumab: first approval. Drugs. (2022) 82(4):461–8. doi: 10.1007/s40265-022-01679-2
141. O'Byrne PM, Panettieri RA Jr, Taube C, Brindicci C, Fleming M, Altman P. Development of an inhaled anti-TSLP therapy for asthma. Pulm Pharmacol Ther. (2023) 78:102184. doi: 10.1016/j.pupt.2022.102184
142. Gauvreau GM, Hohlfeld JM, FitzGerald JM, Boulet LP, Cockcroft DW, Davis BE, et al. Inhaled anti-TSLP antibody fragment, ecleralimab, blocks responses to allergen in mild asthma. Eur Respir J. (2023) 61(3):2201193. doi: 10.1183/13993003.01193-2022
143. Williams TC, Loo SL, Nichol KS, Reid AT, Veerati PC, Esneau C, et al. IL-25 blockade augments antiviral immunity during respiratory virus infection. Commun Biol. (2022) 5(1):415. doi: 10.1038/s42003-022-03367-z
144. Xu G, Paglialunga S, Qian X, Ding R, Webster K, van Haarst A, et al. Evaluation of the safety, tolerability, pharmacokinetics and pharmacodynamics of SM17 in healthy volunteers: results from pre-clinical models and a first-in-human, randomized, double blinded clinical trial. Front Immunol. (2024) 15:1495540. doi: 10.3389/fimmu.2024.1495540
145. Lam LH, Li W, Wu WC, Chow KC, Au WYD, Xu G, et al. SM17, a new IL-17RB-targeting antibody, ameliorates disease progression in a mouse model of atopic dermatitis. Allergy. (2024) 79(6):1625–8. doi: 10.1111/all.16120
146. Ballantyne SJ, Barlow JL, Jolin HE, Nath P, Williams AS, Chung KF, et al. Blocking IL-25 prevents airway hyperresponsiveness in allergic asthma. J Allergy Clin Immunol. (2007) 120(6):1324–31. doi: 10.1016/j.jaci.2007.07.051
147. Akinseye C, Crim C, Newlands A, Fairman D. Efficacy and safety of GSK3772847 in participants with moderate-to-severe asthma with allergic fungal airway disease: a phase IIa randomized, multicenter, double-blind, sponsor-open, comparative trial. PLoS One. (2023) 18(2):e0281205. doi: 10.1371/journal.pone.0281205
148. Wechsler ME, Ruddy MK, Pavord ID, Israel E, Rabe KF, Ford LB, et al. Efficacy and safety of itepekimab in patients with moderate-to-severe asthma. N Engl J Med. (2021) 385(18):1656–68. doi: 10.1056/NEJMoa2024257
149. NCT03469934. Proof of Concept Study to Investigate Etokimab (ANB020) Activity in Adult Participants with Severe Eosinophilic Asthma. Available online at: https://clinicaltrials.gov/study/NCT03469934
Keywords: severe asthma, epithelial-derived cytokines, alarmin, type 2 inflammation, non-T2 asthma
Citation: Pham DD and Kim T-B (2025) Epithelial-derived cytokines in the pathogenesis of severe asthma. Front. Allergy 6:1681147. doi: 10.3389/falgy.2025.1681147
Received: 7 August 2025; Accepted: 29 September 2025;
Published: 15 October 2025.
Edited by:
Hector Ortega, Prana Therapies, United StatesReviewed by:
Josalyn L. Cho, The University of Iowa, United StatesSarita Thawanaphong, McMaster University, Canada
Copyright: © 2025 Pham and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tae-Bum Kim, dGJraW1AYW1jLnNlb3VsLmty
 Duong Duc Pham
Duong Duc Pham Tae-Bum Kim
Tae-Bum Kim