- Department of Grain Science, Kansas State University, Manhattan, KS, United States
Introduction: Microbially fermented plant protein (FPP) has been demonstrated to have high protein digestibility and palatability for terrestrial animals although no work has previously been published describing this for pets. The objectives of this study were to evaluate the nutritional value of FPP, its performance in extrusion processing to produce pet food, and graded inclusion levels on diet utilization in dogs.
Methods: Four experimental diets were produced on a single-screw extruder with processing data and samples collected at 15-min intervals. The control diet without FPP contained 15% soybean meal (SBM); soybean meal was replaced by FPP at 5%, 10%, and 15% to create three diets with graded levels of FPP (5FPP, 10FPP, and 15FPP). The experimental diets were fed to 12 adult dogs in a 4 × 4 replicated Latin square design. Dogs were given these diets for 9 days followed by a 5-day total fecal collection. Fresh fecal samples were collected for hindgut fermentation evaluation. Apparent total tract digestibility was calculated by total fecal collection and titanium dioxide marker methods. Data were analyzed using a generalized linear mixed model with diet as a fixed effect and dog and period as random effects. Least-square means were analyzed with a single degree of freedom contrasts at significance level of a = 0.05.
Results: The preconditioner steam injection rate showed a linear decrease (P < 0.05) as FPP increased in dog diets. The sectional expansion index (SEI) was greater (P < 0.05) in kibbles with 5FPP and 15FPP compared to SBM. Food intake and dog fecal scores were not impacted by FPP inclusion. Dogs fed 15FPP had greater (P < 0.05) crude protein digestibility than those fed SBM, with no significant effects on fecal pH, ammonia, or short-chain fatty acid production. For palatability, dogs preferred SBM over 5FPP and 10FPP but did not show a difference between SBM and 15FPP.
Discussion: Overall, including up to 15% FPP in extruded dog diets promoted kibble expansion without negatively affecting animal acceptability, fecal quality, nutrient digestibility, or hindgut fermentation.
Introduction
According to the survey of the American Pet Products Association, there were 98 million U.S. households that owned at least one cat or dog, and the pet food and treat industry sales in the U.S. were projected to reach 66.9 billion US dollars in 20251. The pet food industry is a sophisticated consumer-oriented industry providing nutrition to companion animals, which may be fed a given diet chronically for the life of the animal. Ingredients that bring extra nutrition and health benefits are much better accepted by pet owners than those which are simply an economic choice by the manufacturer (Schleicher et al., 2019). Animal-based proteins are generally considered to provide a better balance of essential amino acids for dogs and cats compared to plant-based proteins commonly used in pet foods2. However, the impacts of animal agriculture and animal slaughtering for consumption on climate change and animal welfare have become a public concern (Koneswaran and Nierenberg, 2008); thus, plant proteins have the potential to play a greater role in the future pet food industry as a more sustainable option.
Defatted soybean meal is a by-product of the soybean oil extraction process, primarily used as a protein supplement in feed for poultry, swine, and cattle (Stein et al., 2008). Soybean meal is also a common complementary plant protein source in pet food because of its high protein content and metabolizable energy (Stein et al., 2008). The amino acid profile and bioavailability of limiting amino acids (LAA) are critical for the protein quality of a feed ingredient. Traditional soybean meal has a low methionine content, which is an essential amino acid for dogs and cats (Berry et al., 1962; Zarkadas et al., 2007). Furthermore, antinutritional factors (ANFs) such as trypsin inhibitors, flatulence- and diarrhea-causing oligosaccharides (OSs), and phytic acid also limit the utilization of soybean meal in pet food (Yamka et al., 2006; Kim et al., 2023).
Enzyme-coated soybean-based foods have been compared to poultry-based diets in dogs and showed that the former led to increased gut fermentation activity and improved the SBM protein value through amino acid absorption but not as effectively as a poultry-based diet (Tortola et al., 2013). Fermentation is a natural process in which microorganisms produce bioactive enzymes to metabolize substrates and convert them into different chemicals. Fermented or bioprocessed soybean meal has shown benefits in the nutrition of calves, piglets, broilers, and dogs (Mathivanan et al., 2006; Yang et al., 2007; Kim et al., 2012; Beloshapka et al., 2016). Protéger®, a fermented plant protein (FPP), is an enzyme enhanced fungal fermentation product (Gibbons and Brown, 2016) that has been reported to have high protein digestibility, palatability, and capability to promote greater fecal quality in weaned pigs and calves (Senevirathne et al., 2017; Sinn et al., 2017). However, fermented soybean meal products vary in physical–chemical properties and nutrient composition depending on the production protocols; this may impact extrusion processing and diet utilization when fed to animals. A fermented maize–soybean blend was found to decrease sectional expansion at high moisture content (35% compared to 20%) (Ojokoh et al., 2015). Furthermore, fermented rice–black gram flour improved expansion at 10% feed moisture content (Rani et al., 2018). Our hypothesis was that inclusion of FPP in dog diets would not negatively influence food production (extrusion at low feed moisture content), fecal quality, nutrient utilization, or palatability. Therefore, the objective of this study was to evaluate the extrusion performance of FPP and the effects of graded levels of FPP in extruded food on fecal quality, nutrient digestibility, markers of hindgut fermentation, and palatability in dogs.
Materials and methods
Diet formulation and production
The FPP evaluated was a commercial product derived from soybean meal through a proprietary fermentation process (Protéger®, Prairie AquaTech, Brookings, SD, USA). Four experimental dog diets were formulated to have similar nutritional composition and to be consistent with a premium dog food with high-protein and moderate levels of fat (>25% CP and >10% CF). Soybean meal was used exclusively as the plant-based protein source in the control diet (SBM) and was replaced by FPP at 5%, 10%, and 15% (5FPP, 10FPP, and 15FPP, respectively). All diets included titanium dioxide (TiO2; 0.4%) as an indigestible marker for the determination of apparent total tract digestibility (ATTD) of dietary nutrients. As predicted by the formulation software, the concentrations of minerals (calcium, phosphorus, potassium, magnesium, sodium, sulfur, manganese, copper, iron, and zinc) were similar among diets and met the AAFCO nutrient profile recommendations for maintenance of adult dogs (Table 1).

Table 1. Dog diet formulations with nutrient compositions of the experimental diets with increasing levels of FPP (SBM, 0%; 5FPP, 5%; 10FPP, 10%; and 15FPP, 15%).
The four dog foods were produced using a single screw extruder (model X115, Wenger Manufacturing, Sabetha, KS, USA). The preconditioner (model HIP 150, Wenger Manufacturing, Sabetha, KS, USA) was operated at a constant speed (20% mix intensity) and welded paddles. The extruder had a defined profile and barrel temperatures based on a typical commercial pet food configuration. At the end of the extruder barrel, there were two inserts with three holes (6.7 mm diameter) per insert. Fixed input parameters were kept constant throughout each individual food production and included dry blends feed rate (739.5 ± 2.5 kg/h), preconditioner water (132 ± 2.0 kg/h), extruder screw speed (350 rpm, except for 5FPP which was 325 rpm), extruder barrel temperature (70°C and 80°C for zones 1 and 2 for all diets; 95°C, 95°C, and 60°C for zones 3–5 for SBM and 5FPP; and 90°C, 80°C, and 60°C for zones 3–5 for 10FPP and 15FPP), and extruder knife speed (1,300 ± 50 rpm).
During the extrusion processing, preconditioner and extruder parameters were collected from sensor readouts every 15 min to estimate the effects of different inclusion levels of FPP on extrusion. Output variables were those parameters resulting from the input variables and included extruder die temperature, extruder motor load, and specific mechanical energy (SME). Measurements were collected every 15 min during production of at least 2,000 lb per experimental diet for statistical analysis as treatment replicates.
Wet extrudates were conveyed pneumatically through an air hood system and deposited onto an oscillating belt spreader that spread the kibbles evenly across the dryer bed. The kibbles were dried on a belt passing two zones of a three-zone dryer (Airflow II, Wenger Manufacturing, Sabetha, KS, USA) to achieve a moisture content of kibbles below 10%. The dryer zones had varied temperatures and retention times, such as 19 min in zone 1, 10 min in zone 2, and 29 minutes in zone 3. Dried kibbles were coated with chicken fat and dry digest (Manx, AFB International, St. Charles, MO) in a drum mixer. Coated kibbles were stored at room temperature in poly-lined paper bags until fed.
SME was calculated using the following formula:
where τ is the extruder motor load, τ0 is the extruder no load % torque, N is the extruder screw speed (rpm), Nr is the rated extruder screw speed, Pr is the rated extruder motor power, and m is the dry feed rate (kg/s).
Kibble diameter and thickness (length) of 10 randomly selected kibbles from each collection point of each diet production off the extruder and off the dryer were measured with a digital caliper. The sectional expansion index (SEI) was determined by comparing the squared diameter of the dried extruded kibbles by the squared die diameter of the extruder:
where D is the extrudate diameter and d is the extruder die diameter.
Kibble bulk density was measured manually off the extruder in each data collection point during each treatment processing using a 1-L cup and leveling the kibbles with a metal ruler and weighing on a digital scale with 1.0-g sensitivity.
Feeding trial
The dog digestibility trial was conducted at the Kansas State University Large Animal Research Center (LARC) under the Institutional Animal Care and Use Committee (IACUC) protocol #4348. Dogs were housed in USDA-approved kennels (Large Animal Research Center at Kansas State University; Manhattan, KS, USA). Twelve healthy adult beagles (six neutered male and six spayed female) of similar age (1.0 ± 0.3 year) and similar body weights (8.4 ± 2.5 kg) were individually housed in pens (1.83 m × 1.20 m) equipped with an acrylic-mesh floor to allow for the separation of urine and feces. The dogs were housed on a 12-h light cycle with lights off from 19:00 to 07:00. During the study, dogs were allowed daily group playing time under supervision either indoors with toys or outdoors in a dedicated area with many types of enrichments (e.g., slides, sandpit, and water pond). They were also pair-housed during adaptation days to minimize stress. The dogs were randomly assigned to square and sequentially fed experimental diets. Initial dietary intake on day 0 was determined by calculating daily metabolizable energy (ME) requirements for laboratory kennel dogs [130 × BWkg0.75; National Research Council (2006)].
The ME (kcal/100 g) of experimental dog diets was calculated by the formulation software using the modified Atwater prediction factors in pet food [(8.5 × CF% + (3.5 × CP% + (3.5 × NFE%], where the NFE% was calculated as [100 − (CP% + moisture% + crude fiber% + CF% + ash%)] (National Research Council, 2006). The daily food allowance was calculated using the daily ME requirement divided by the predicted ME of the diets. Dogs were fed twice daily (8:00 a.m. and 4:00 p.m.) in equal rations to meet daily food allowance. The body weights of dogs were measured weekly in the mornings before feeding, and their food allowance was adjusted by 5% or 10% for the following week to maintain their BW. Water was provided ad libitum.
The feeding trial used a replicated Latin square design with no carryover effect (Kim and Stein, 2009), where each animal served as its own control. Each of the four periods was composed of 9 days for adaptation followed by 5 days of collection. During the collection period, all feces and orts were collected at least twice daily around feeding time. Every fecal sample was weighed and scored on a 5-point scale, where 1 = liquid diarrhea; 2 = very soft consistency, unformed feces; 3 = soft feces that retain shape; 4 = well-formed firm feces that do not leave residue when picked up; and 5 = very hard, dry feces. Fecal samples were stored at −20°C until the end of the trial and then thawed at room temperature, pooled by dog, weighed, and dried in a forced air oven at 55°C for up to 48 h until the moisture level was below 10%. The partially dried fecal samples were also weighed, and the values were used when calculating the dry matter (DM) of the fecal samples. Diet samples and partially dried fecal samples were ground through a 1-mm screen by a laboratory fixed blade impact mill (Retsch, type ZM200, Haan, Germany) and stored in mason jars at room temperature for further chemical analysis.
In addition, one fresh fecal sample per dog per period (within 15 min of defecation) was also collected and pH recorded in triplicate with a calibrated glass-electrode pH probe (FC240B, Hanna Instruments, Smithfield, RI) immediately after collection. Fresh fecal samples were stored at −80°C until further analysis.
Chemical analysis
The FPP ingredient, ground experimental diet samples (after coating), and fecal samples were analyzed for DM, organic matter (OM), and ash according to the methods in AOAC 934.01 and 942.05. Crude protein was determined (AOAC 990.03) using a nitrogen analyzer (FP928, LECO Corporation, Saint Joseph, MI). Crude fat was determined by acid hydrolysis followed by hexane extraction (ISO 11085:2008) using semi-automated equipment (Hydrotec 8000 and ST 255 Soxtec, Foss, Denmark). Gross energy was measured as the total heat of sample combustion by calorimetry (Parr 6200 Calorimeter, Parr Instrument Company, Moline, IL). The titanium dioxide content in diet and fecal samples was analyzed with a colorimetric method (Myers et al., 2004). The soluble dietary fiber (SDF), insoluble dietary fiber (IDF), and total dietary fiber (TDF) contents of the diet samples were measured (AOAC 991.43) by an ANKOM Dietary Fiber Analyzer (ANKOM Technology, Macedon, NY, USA).
Additional nutrient analysis was conducted for the FPP ingredient including long-chain fatty acids (LCFAs), minerals, and amino acids at the University of Missouri Agricultural Experiment Station Chemical Laboratories (Columbia, MO). The LCFAs were analyzed (AOAC 996.06) by detection of fatty acid methyl esters (FAMEs) via gas chromatography. Elements were analyzed [AOAC 985.01(A, B, D)] with inductively coupled plasma-optical emission spectroscopy. All amino acids, except methionine, cysteine, and tryptophan, were digested with 6 N of HCl for 24 h at 110°C. The amino acids were then separated by ion-exchange chromatography, and the concentration was determined with a Beckman 6300 amino acid analyzer (Beckman, Palo Alto, CA). Methionine and cysteine were first oxidized by performic acid to methionine sulfone and cysteic acid, respectively, prior to acid hydrolysis. Tryptophan was hydrolyzed in 3 M of mercaptoethanesulfonic acid before analysis. Available lysine was determined (AOAC 975.44) and lysine availability (%) was calculated as the ratio of available lysine to total lysine.
Ammonia concentration in the fresh fecal samples was analyzed through the colorimetric method described by Chaney and Marbach (1962). The fresh fecal samples were thawed and diluted with deionized water and 0.1 N of HCl and homogenized. The homogenized samples were centrifuged at 3,000×g for 20 min to separate the suspended solids. One milliliter of the supernatant of the centrifuged samples was collected and kept frozen at −20°C for at least 24 h to complete deproteinization. The acidified samples were thawed, centrifuged at 20,000×g for 15 min, and plated.
Fecal short-chain fatty acids (SCFAs) including branched-chain fatty acid (BCFA) contents were analyzed on a gas chromatography (GC; Agilent 7890, Agilent Technologies, Santa Clara, CA). The fresh fecal samples were thawed and diluted with deionized water and homogenized, followed by centrifugation at 3,000×g for 20 min to separate the suspended solids. One milliliter of the supernatant of the centrifuged samples was collected and acidified with 0.25 mL of 25% m-phosphoric acid before deproteinization at −20°C for at least 24 h. The GC was equipped with a flame ionization detector (FID) and a capillary column (DB-WAX, Agilent 127-7012, 10 m × 0.1 mm × 0.1 µm, Agilent Technologies, Santa Clara, CA). Hydrogen was used as a carrier gas with a flow rate of 35 mL/min, and the split ratio was 100:1 with an injection volume of 0.2 µL. Nitrogen was used as the makeup gas with a flow rate of 25 mL/min. The detector and injector temperatures were set at 300°C, and the initial oven temperature was set to 40°C with a ramp rate of 20°C/min to 180°C for a total run time of 8 min. The peak area of the chromatograms was determined using an integrative software (OpenLab CDS, Agilent Technologies, Santa Clara, CA). The concentrations of SCFAs (acetate, propionate, and butyrate) and BCFAs (isobutyrate, isovalerate, and valerate) in the samples were quantified by comparing the sample peak area to three standards with known concentrations of each volatile free acid and correcting for the fecal dry matter content.
Digestibility calculation
Two methods were utilized to estimate apparent total tract nutrient digestibility. The total fecal collection (TFC) method requires the collection of all feces excreted by the experimental animals. The marker method uses TiO2 as an indigestible dietary marker. In the current study, the ATTD of DM, OM, CP, CF, and GE was calculated according to the TFC and marker methods by the following equations:
1. TFC method:
2. Marker method:
In-vitro protein digestibility
The in-vitro pepsin–pancreatin protein digestibility was determined in duplicates as 1 g of the FPP sample was weighed into 50-mL centrifuge tubes, followed by the addition of 15 mL of 0.1 N HCL–pepsin (porcine; Merck Millipore 516360-2.5GM) solution to each tube and placed in a shaking water bath for 6 h at 37°C. After pepsin incubation, the tubes were removed and 7.5 mL of 0.5 N NaOH was added to neutralize the sample and stop pepsin hydrolysis. Next, porcine pancreatin (4 mg; Sigma Aldrich P1750-100G) was added to 7.5 mL of phosphate buffer (pH 8), followed by 1 mL of sodium azide (for microbial control). The tubes were then placed in the shaking water bath for 18 additional hours at 37°C.
After 18 h of incubation, 1 mL of 10% TCA was added to each tube to help with protein precipitation. Vials were then centrifuged at 3,700×g for 20 min. The supernatant was discarded by filtration (Whatman 541 filter paper). Then, the samples were washed with distilled water, centrifuged, and filtered again three times. The final sediments were oven-dried overnight at 105°C and then analyzed for nitrogen utilizing the Dumas combustion method (AOAC 990.03). In-vitro protein digestibility was calculated using the following equations:
Protein-corrected amino acid scores (PDCAAS) were calculated using the Food and Agricultural Organization suggested equation (Food and Agriculture Organization, 1991):
Where the reference amino acid pattern is dog or cat maintenance recommended values by the National Research Council (2006). The LAA refers to limiting amino acid, which has the lowest AAS. Values for PDCAAS greater than 1.00 were treated as 1.00.
Palatability trials
Palatability trials for dog diets were conducted at a commercial research kennel (Summit Ridge Farms; Susquehanna, PA, USA). The kennel is registered with the USDA No. 23-R-0126 under the Animal Welfare Act. All dogs had access to outdoor “Puppy Parks” with play structures for ample exercise. Dedicated staff and the designated Animal Enrichment Team ensured the personal attention and constant socialization that enhanced the dogs’ quality of life. Each FPP containing dog diets (5FPP, 10FPP, and 15FPP) was compared to the control diet (SBM) where each comparison was determined in beagle dogs with a two-bowl forced-choice test: 30 male and female beagles identified by ear tattoo and cage number were presented the test diets on an individual basis. Two stainless steel bowls, each containing approximately 400 g of diet, were offered once daily for 2 days. Bowl placement was reversed daily and both bowls were presented for 30 min. If one diet was completely consumed prior to the end of the 30 min, both bowls were removed. Food consumption and first-choice preference were recorded for each dog. Preference was determined based on the dogs’ first choice (first bite of the food) and total food consumption (Aldrich and Koppel, 2015). Data from consumption were represented as the following ratio:
Statistical analysis
Least square means of extrusion data and digestibility trial data were estimated by one-way ANOVA using the GLIMMIX procedure in SAS (version 9.4, SAS Institute INC, Cary, NC, USA). Pairwise treatment comparisons were conducted using a Tukey’s post-hoc test. Contrasts comparing control (SBM) vs. treatments (5FPP, 10FPP, and 15FPP) and linear, quadratic, and cubic relationships among all diets were considered significant at P <0.05. For each diet production, sampling was conducted at evenly spaced intervals, which were considered replicates. For digestibility trial analysis, dog and period were considered random effects in the model for analysis of data. In the palatability experiments, the intake ratio was analyzed using a one-way ANOVA for total consumption ratio analysis followed by a paired t-test for intake ratio analysis within each single day. The first-choice preference was analyzed using the χ2 test.
Results
Chemical analysis of FPP and diets
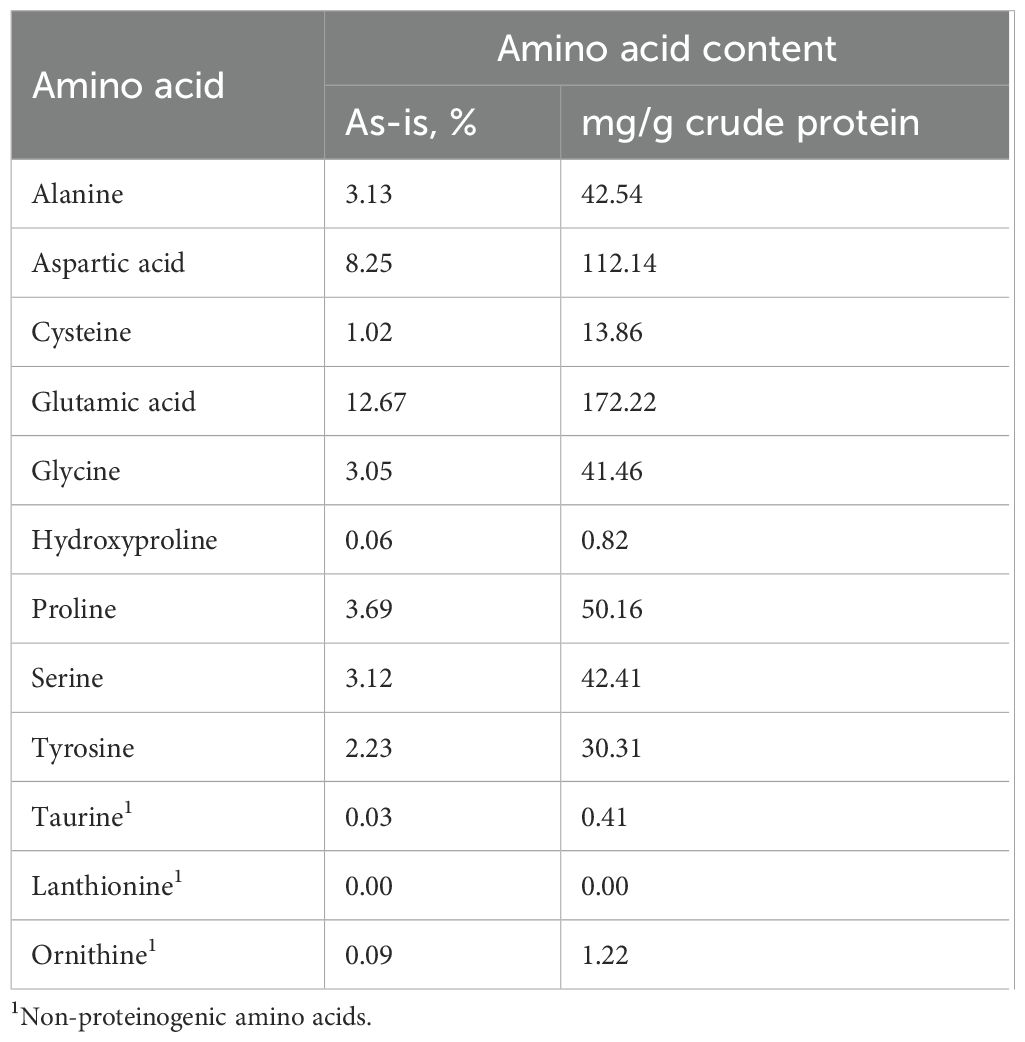
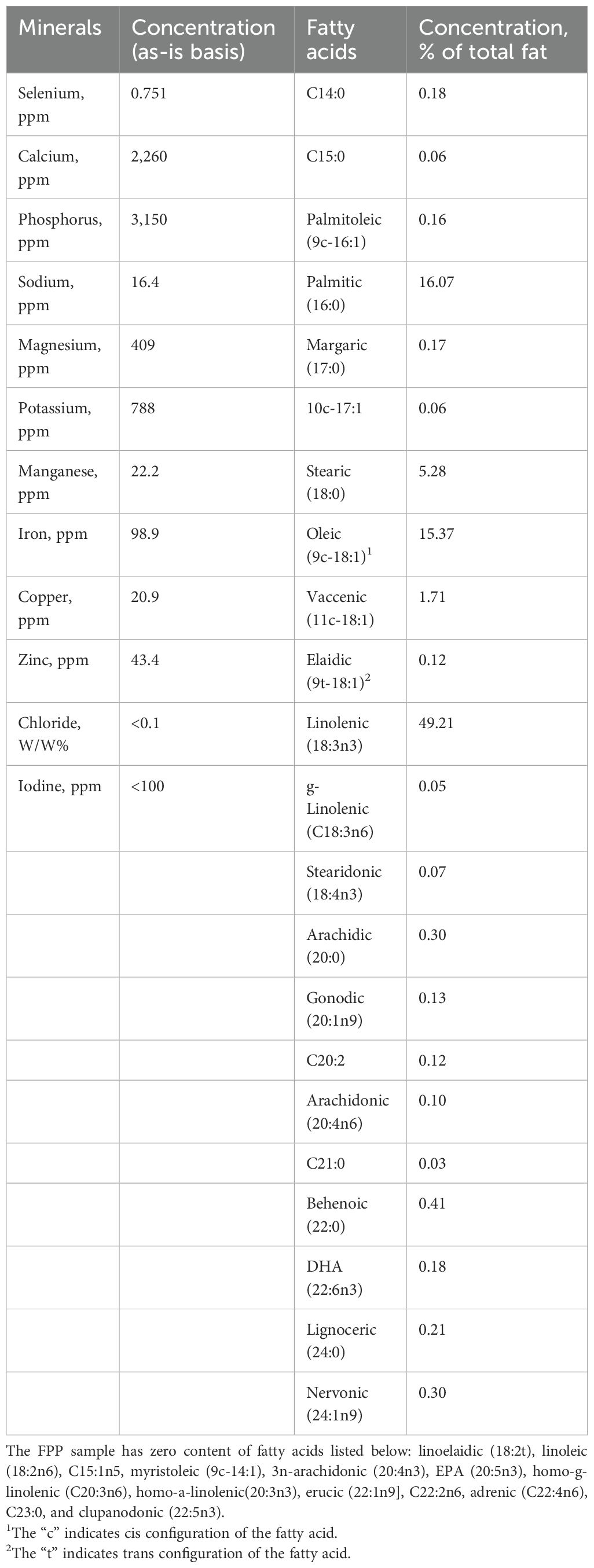
DM, crude protein, and crude fat content of the FPP samples were 95.5%, 77.03% (DM basis), and 0.66% (DM basis), respectively. The in-vitro protein digestibility of FPP was 98.46% ± 0.12%. The essential amino acid analysis and PDCAAS (0.41) of FPP showed that methionine was the limiting amino acid for dogs (Table 2). Total non-essential amino acids comprised 52.63% of total amino acids in FPP with aspartic acid (112.1 mg/g CP) and glutamic acid (172.2 mg/g CP) being the most abundant (Table 3). Phosphorus (3,150 ppm, as-is basis) and calcium (2,260 ppm, as-is basis) had the highest concentrations in FPP among the 12 minerals analyzed (Table 4). Long-chain fatty acids comprised over 90% of the total fatty acids in CF in FPP (Table 4). Among all the long-chain fatty acids, over 54.5% was α-linolenic acid (18:3n3), almost 0.2% was docosahexaenoic acid (DHA, 22:6n3), and no eicosapentaenoic acid (EPA, 20:5n3) was detected. All diets were similar in DM, CP, CF, and GE content (DM basis). The IDF content increased numerically as FPP increased, while SDF was the highest in SBM and 15FPP.
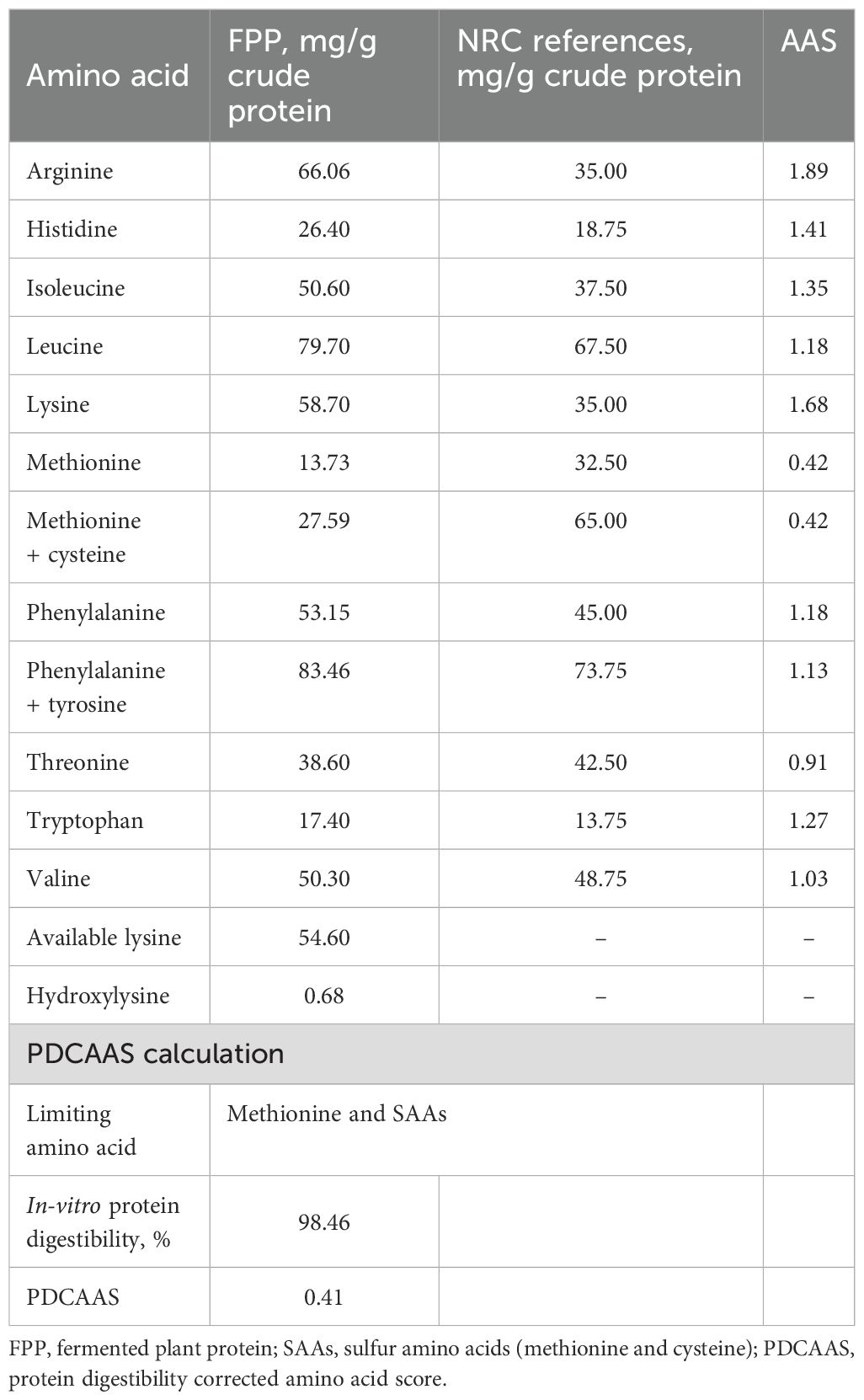
Table 2. Essential amino acid (for dogs) composition in FPP and their amino acid scores (AAS) based on the National Research Council-stated minimal requirements for adult dogs.
Extrusion process
A linear decrease (P < 0.05) of preconditioner steam was observed as FPP inclusion increased (Table 5). A cubic relationship (P < 0.05) between extruder shaft speed and FPP inclusion was observed while this parameter was not different among the diets. Neither did the SME differ among the diets, but a cubic relationship (P < 0.05) between SME and FPP inclusion was observed. The 15FPP required 59.67 kg/h extruder water injection, which was greater (P < 0.001) than that of all the other dog diets (22.00 kg/h for both SBM and 5FPP, 21.83 kg/h for 10FPP). Extrudates of 5FPP had lower (P < 0.05) bulk density (374.0 kg/m3) than that of the SBM (408.6 kg/m3). A cubic relationship (P < 0.05) between bulk density and FPP inclusion was observed. Extrudates of 5FPP and 15FPP had a similar diameter (11.89 and 11.59 mm, respectively) but were greater (P < 0.001) than that of the other two diets (11.08 mm for SBM and 11.28 mm for 10FPP). Such influence was consistently reflected on the SEI that also showed a quadratic relationship (P < 0.001) with FPP inclusion.
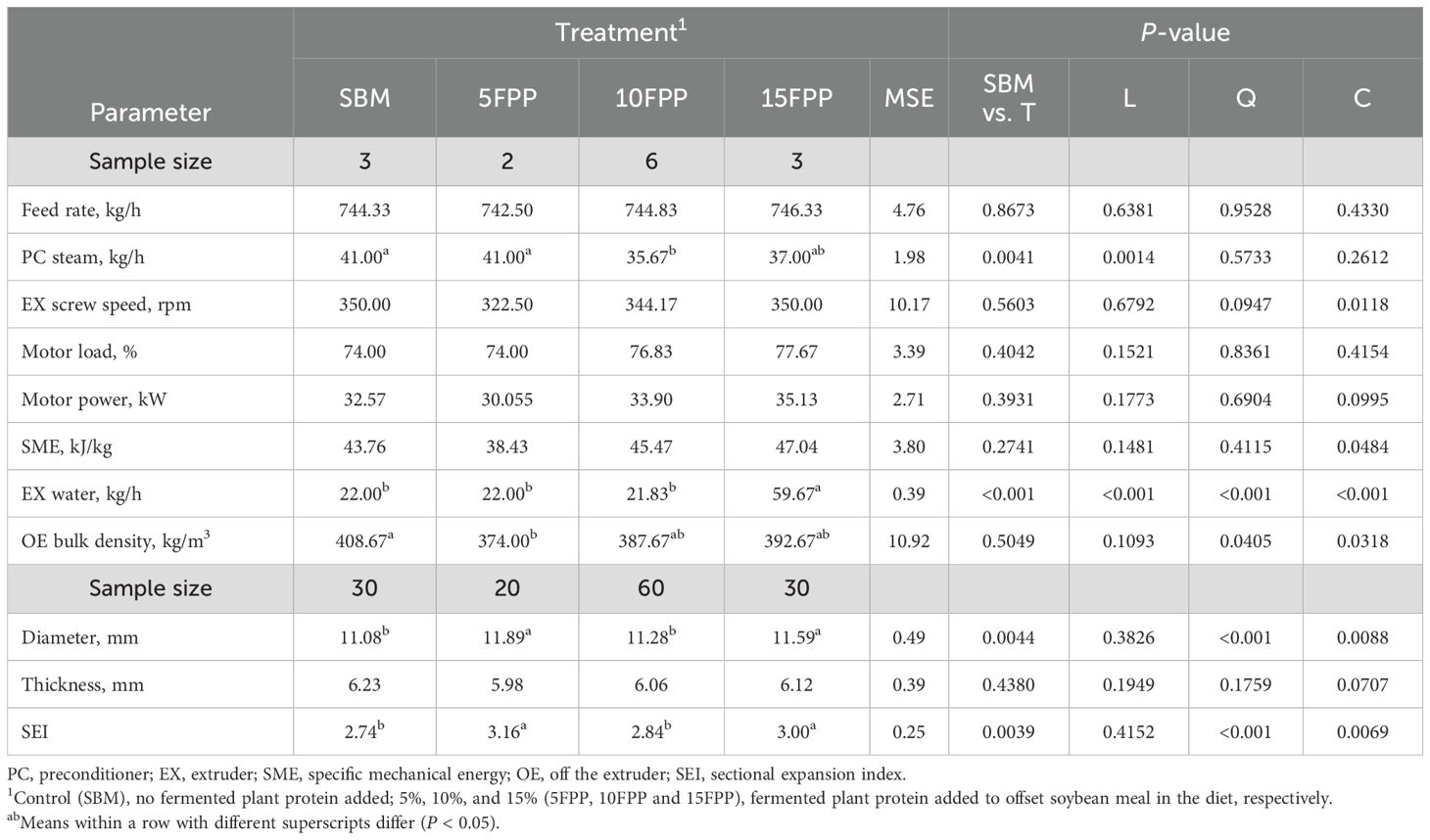
Table 5. Extrusion processing data and post-extrusion kibble measurements for the experimental dog diets.
Food intake and fecal characteristics
Food intake was similar among the diets and no refusal to food was observed during this feeding trial. Fecal wet output in dogs fed with 15FPP (113.4 g/day) was lower than that of dogs fed with SBM (128.8 g/day), and a linear relationship (P < 0.05) was observed between fecal wet output and FPP inclusion (Table 6). Fecal dry matter output also showed a linear relationship (P < 0.05) with FPP inclusion although the inclusion level from 0% to 15% did not lead to a statistical difference in fecal dry matter output (averagely 36.8 g/day) among the dogs irrespective of diets. Food intake, fecal moisture, defecation frequency, and fecal score all remained similar among the dogs fed with different experimental diets.
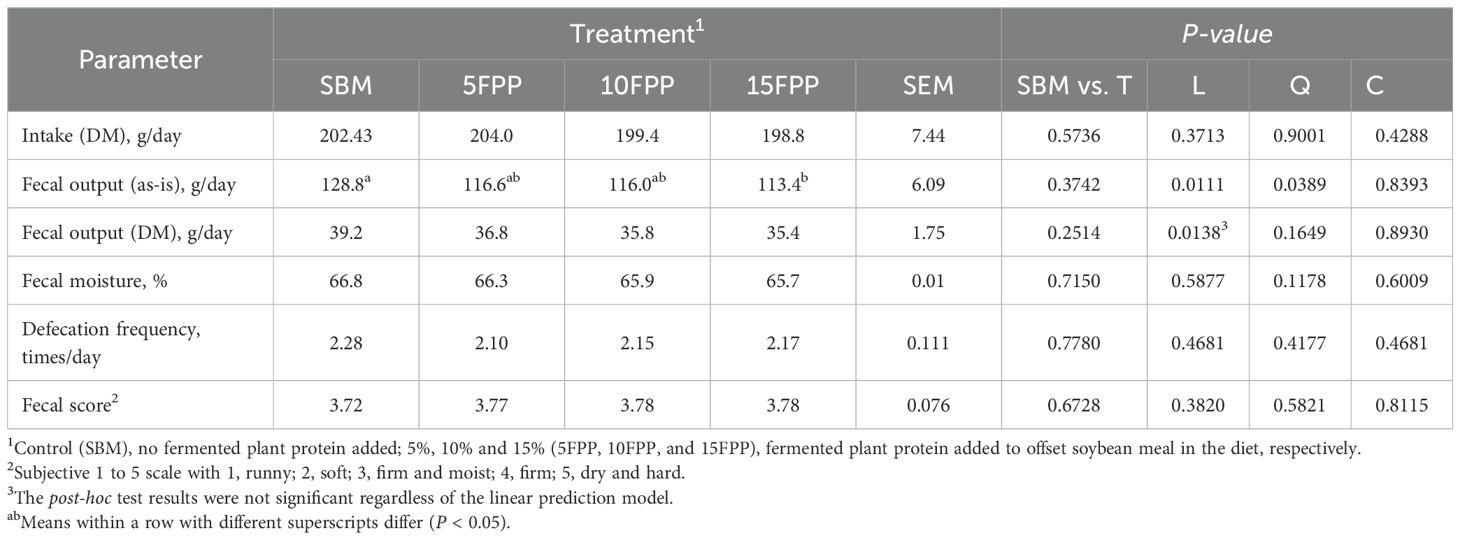
Table 6. Least square means and contrasts (SBM vs. FPP5–15 [T]; linear [L]; quadratic [Q]; cubic [C]) for food intake, fecal output, fecal score, and defecation frequency of dogs fed diets containing increasing levels of FPP.
Apparent total tract digestibility
With the total TFC method, the ATTD of dry matter increased linearly (P < 0.05) as FPP inclusion increased in dog diets, indicating that dogs fed with 15FPP (82.12%) had greater dry matter ATTD than dogs fed with SBM (80.61%) (Table 7). The same pattern applied to ATTD of crude protein (P < 0.05). There was a quadratic relationship in ATTD of organic matter (P < 0.05), crude protein (P < 0.05), and crude fat (P < 0.001) among the diets with different FPP inclusion levels. The ATTD of crude fat was the greatest (P < 0.05) in dogs fed with 15FPP (98.10%) and the lowest in dogs fed with 10FPP and SBM (96.91% and 96.49%, respectively), while that of dogs fed with 5FPP (97.55%) was intermediate.
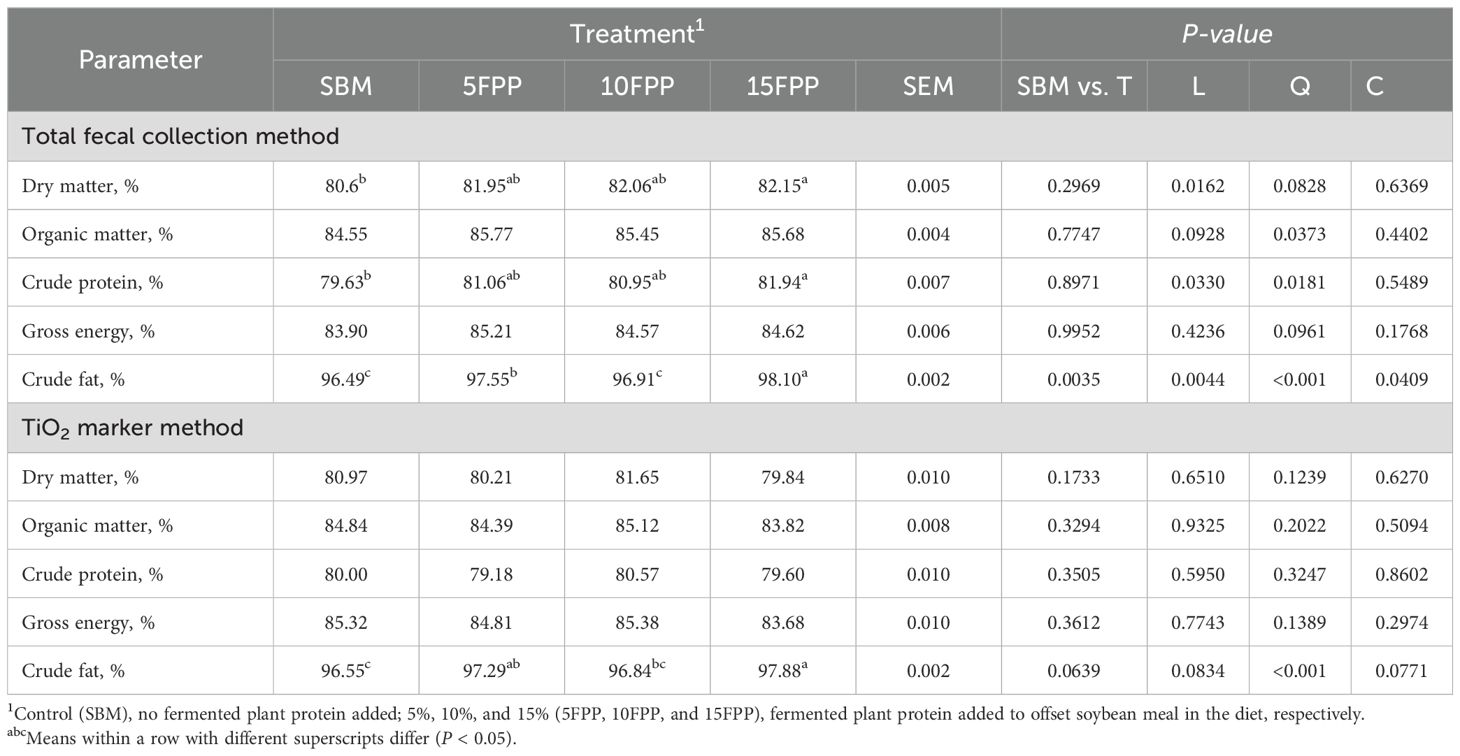
Table 7. Least square means and contrasts (SBM vs. FPP5–15 [T]; linear [L]; quadratic [Q]; cubic [C]) for nutrient ATTD calculated using the total fecal collection method (TFC) and marker method by dogs fed diets with increasing levels (0%, 5%, 10%, and 15%) of FPP.
In comparison, the ATTD calculated with the titanium dioxide marker method only revealed a cubic relationship P < 0.001) between crude fat digestibility and FPP inclusion level, indicating that the ATTD of crude fat in dogs fed with 15FPP (97.88%) was the greatest compared to dogs fed with SBM (96.55%).
Hindgut fermentation
The pH of fresh fecal samples all fell into the range of 5.0–6.0 and did not differ among the dogs fed with different diets (Table 8). The ammonia concentration in fresh fecal samples was numerically the greatest in dogs fed with the 5FPP (103.2 μmol/g DM feces), while that from dogs fed with other three diets was similar to each other (at an average of 98.6 μmol/g DM feces). None of the fatty acid concentrations in fresh feces differed among the dogs fed with different diets. The propionate concentration in fecal samples showed a tendency of quadratic relationship (P = 0.0804) with FPP inclusion.
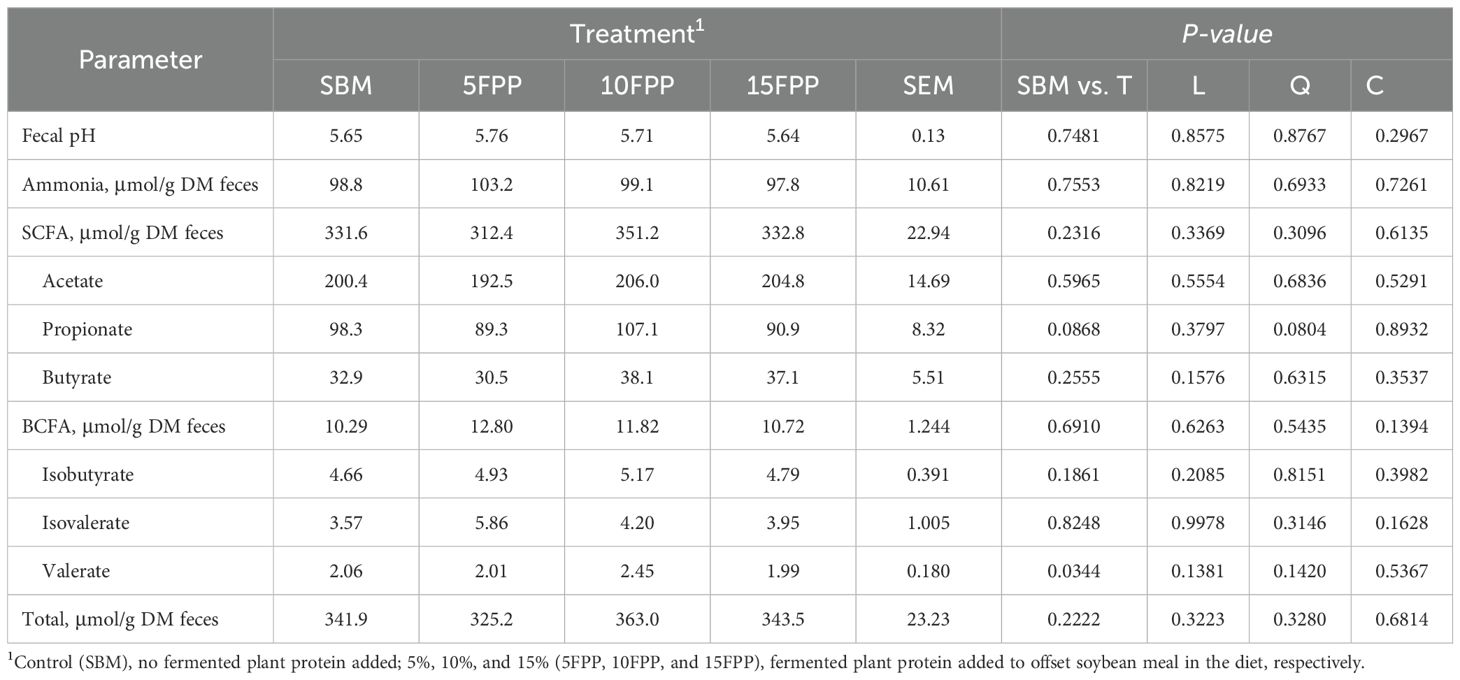
Table 8. Least square means and contrasts (SBM vs. FPP10–30 [T]; linear [L]; quadratic [Q]; cubic [C]) for short-chain fatty acid (SCFA), branched-chain fatty acid (BCFA), and total fatty acid (SCFA + BCFA) production from the fresh fecal sample collected from the dogs fed diets with increasing levels (0%, 5%, 10%, and 15%) of FPP expressed in μmol/g of feces in dry matter basis.
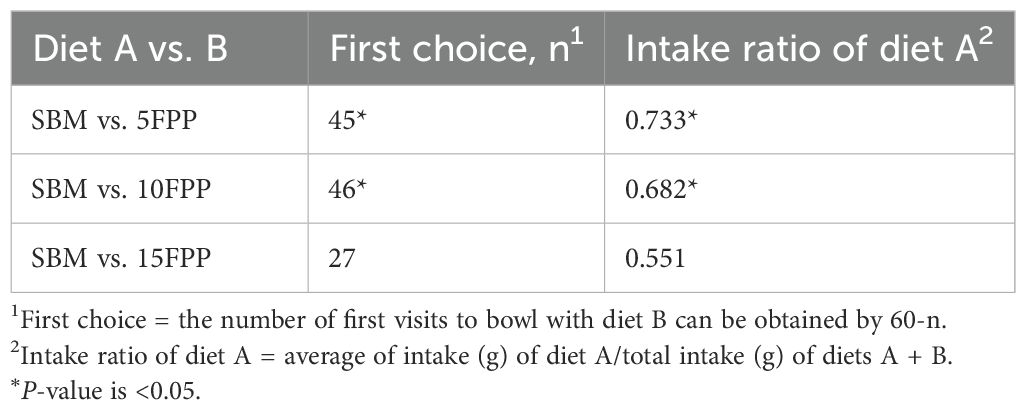
Palatability
When comparing SBM to 5FPP or 10FPP, dogs preferred (P < 0.05) SBM as indicated by both first choice and intake ratio (Table 9). The SBM was chosen firstly over 5FPP for 45 out of 60 times and over 10FPP for 46 out of 60 times by 30 dogs in 2 days, respectively. However, such preference for SBM to FPP-containing diet did not last to 15FPP where two diets had similar palatability.
Discussion
Chemical analysis of FPP and diets
The crude protein content of FPP (77.03% on DM basis) was higher than that of soybean meal (44%–50%) and intermediate between that of soybean protein concentrate (over 65%) and soybean protein isolate (over 90%; Murphy, 2008). This could be a result of fungal protein accumulation and decrease of carbohydrates during the production of FPP (Mukherjee et al., 2015). A cross-sectional study evaluated amino acid compositions of 14 soybean cultivars in Canada, and the average methionine and cysteine contents were 20.92 (16.14–22.22) and 20.93 (18.49–23.96) mg/g total protein (Zarkadas et al., 2007). Another study involving soybean meal produced in the USA reported a methionine and (methionine + cysteine) content of 14.2 and 29.2 mg/g CP, respectively (Reilly et al., 2021). Grieshop et al. (2003) reported that the mean methionine content in soybean meals from 10 U.S. processing plants was 13.47 (10.93–16.33) mg/g CP. In comparison, methionine (13.73 mg/g CP) and (methionine + cysteine) content (27.59 mg/g CP) in FPP did not provide advantages over regular soybean meal. Surprisingly, the in-vitro protein digestibility of FPP exceeded 98%, while Ou et al. (2004) found that the in-vitro protein digestibility of soybean protein isolate powder was approximately 86.4%. Another study found that the in-vitro protein digestibility of soybean protein increased from 83% to 93% (Amadou et al., 2010). Note that in the study of Amadou, only trypsin was used to determine protein digestibility, while pepsin and pancreatin were used in our study; however, the effect of fermentation on increasing in-vitro protein digestibility was consistent. Despite the ultra-high in-vitro protein digestibility, the PDCAAS score of FPP was relatively low (0.41) with methionine or SAAs being the limiting amino acids just like in traditional soybean protein (Zarkadas et al., 2007; Reilly et al., 2021). The mineral analysis provided valuable information when incorporating FPP into certain formulas. Considering the low crude fat content of FPP, it would not be considered as a major lipid source in the diet or for essential fatty acids.
The similar moisture content among dietary treatments was expected as drying conditions were controlled during processing. The 15FPP had slightly higher CP content compared to SBM although the much lower chicken meal content in the former was expected as the CP content in FPP (77.03% DM basis) was much higher than soybean meal (53.9% DM basis) used in this study. Even though chicken meal was replaced with brewers’ rice as FPP increased, it was still challenging to perfectly balance CP content while keeping other macronutrients similar among the diets. The increase in IDF content when soybean meal was replaced by FPP was also observed in a study evaluating FPP in calf feed (Senevirathne et al., 2017) and in a study evaluating enzyme-treated soybean meal in extruded dog diet (Beloshapka et al., 2016). The decrease in SDF in 5FPP and 10FPP was expected as FPP was low in OSs that are considered soluble fiber (Gibbons and Brown, 2016). However, the greater amount of SDF in 15FPP was surprising. The extrusion may have contributed to the conversion of IDF into SDF with greater SME and potentially greater in-barrel moisture content compared to the other diets, as such impacts have been reviewed before (Robin et al., 2012).
Extrusion process
The differences in dry blend feed rate, preconditioner water, and extruder knife speed were minimal and did not appear to be of practical importance as it relates to equipment operation. The decreased preconditioner steam measured for 10FPP compared to SBM and 5FPP was due to the extruder operator and not the dietary matrix. There is scant research on the influence of microbially enhanced soybean protein on pet food extrusion. Despite the fact that not only fiber but also protein was the major variant in diet formulas in the current study, some of the concepts from studies of fiber sources on extruded pet food may be as valuable to the interpretation of results from this study as those of protein composition.
Usually, increased insoluble fiber and decreased soluble fiber led to less sectional expansion (Robin et al., 2012). This seemed to be true in this study when switching production from SBM to 5FPP with all input parameters unchanged. To achieve an extrudate bulk density close to the SBM samples, extruder shaft speed was intentionally decreased in 5FPP. This subsequent increased expansion in 5FPP could be explained by extended retention time for cooking due to decreased shaft speed. The decreased motor power and SME regardless of increased barrel fill indicated that the melt 5FPP had good flowability. The SME is usually positively related to expansion, while other factors such as fiber content, fiber particle size, retention time, and nutrient composition also play a role (Riaz, 2000; Robin et al., 2012). It has been reported that insoluble fibers provide additional friction in the extruder barrel (Donadelli et al., 2021) and dietary fiber water solubility can be significantly increased when increasing SME during extrusion (Redgwell et al., 2011). It seems that the 15FPP had the greatest SME due to abundant insoluble fiber content. With the increase of SME, fiber solubility increased and competed with starch for water as both insoluble and soluble fiber are considered to compete with starch for water (Donadelli et al., 2021). Therefore, the greater extruder water requirement for 15FPP to achieve the target extrudate bulk density was probably due to its high dietary fiber and starch contents compared to the other diets. The increased fiber solubility in 15FPP may also have contributed to viscosity of the melt, thus greater kibble expansion.
It was reported that even though the soluble fiber mixes with the melt material in the extruder, it does not always provide structural forming like gelatinized starch, which leads to less expansion (Parada et al., 2011). This may be the case for the SBM diet as it had the greatest soluble fiber content among all the diets and had the least expanded kibbles. Fiber seemed to have acted differently in SBM and 15FPP. This may be the result of the different chemical–physical properties between fibers in soybean meal and in FPP, which remained to be investigated. Also, the 15FPP had more fiber from brewer’s rice. Fat also has negative impacts in creating mechanical energy in extrusion since it acts like a lubricant between the shaft and melt material (Riaz, 2000). The SBM had the greatest chicken meal content which was the only variation in fat source in the extrusion raw mix. This may have caused higher fat content in the melt and also led to less expansion of SBM extrudates.
In summary, we did not observe a pattern of changes in extrusion conditions and kibble expansion as FPP increased in the diets. With the extrusion input parameters slightly adjusted, it did not seem to be challenging to replace soybean meal with FPP at up to 15% of the formula. Additionally, replacing soybean meal with FPP benefited kibble expansion.
Food intake and fecal characteristics
Since the diets had similar gross energy content (4.7–5.0 kcal/g), the food DM intake was expected to be close among the treatments. Dog fecal contamination is a major social conflict with dog ownership and poses zoonotic risks (Mori et al., 2023). Diets that promote decreased fecal output and defecation are appreciated by dog owners and would aid cleanup. The linear decrease in wet fecal output as FPP increased in the diet provided such advantages. Though fecal dry matter output, fecal moisture content, and fecal score were not different among the treatments, we did see a consistent numerical change to the desired direction of these parameters as FPP increased in the diets. Another study on piglets observed reduced diarrhea when regular soybean meal was replaced by FPP (Sinn et al., 2017). It would be valuable to further investigate if FPP could provide benefit in dogs with diarrhea-causing gastric-intestinal conditions. Yamka et al. (2006) reported a decrease in wet fecal output in dogs fed with a diet of 30% low-OS low-phytate soybean meal compared to that of dogs fed with a diet of 30% regular soybean meal. As the OSs were nearly removed from the FPP used in the study of Sinn et al. (2017), both our work and Yamka’s supported that the removal of soybean OSs contributed to decreased dog fecal wet output compared to regular soybean meal. Beloshapka et al. (2016) found that when gradually replacing poultry by-product meal with bioprocessed soybean meal in extrude dog diets, wet fecal output did not change until replacing the level reached over 24%. Yamka et al. (2006) also observed that diets with 30% low-OS low-phytate soybean meal or 30% regular soybean meal both increased dog wet fecal output compared to the diet with 22% poultry meal. In the current study, chicken meal inclusion also decreased as FPP increased in the diets. The wet fecal output did not differ among the three FPP-containing diets, which agreed with the results from the study of Beloshapka et al. (2016). It would be interesting to evaluate fecal output in future studies when replacing chicken meal with FPP at over 30% to determine if FPP is comparable to chicken meal in regard to fecal output characteristics.
Apparent total tract digestibility
Many studies have discussed the influences on nutrient digestibility when soybean products were included in dog diets, among which soybean meal was the most studied one (Zuo et al., 1996; Bednar et al., 2000; Yamka et al., 2003; Carciofi et al., 2009; Félix et al., 2012; Tortola et al., 2013; Menniti et al., 2014; Maria et al., 2017; Vanelli et al., 2021). The soybean meal inclusion level in previous studies ranged from 9% to 47% with variable results on alternations to nutrient digestibility. The ATTDs ranged from 73% to 87% for dry matter, over 80% for protein, approximately 90% for crude fat, and close to 85% for gross energy. For ileal protein digestibility, Yamka et al. (2003) found that inclusion of soybean meal decreased dry matter and crude protein digestibility. Other studies found that soybean meal increased ileal protein digestibility in dogs compared to animal protein ingredients (Zuo et al., 1996; Bednar et al., 2000; Clapper et al., 2001). The variation in the results and the minimal impact on differences in nutrient digestibility between soybean meal and common animal ingredients does not seem adequate to support statements that soybean meal provides inadequate values compared to animal proteins. To promote the utilization of soybean meal products in pet food, the enzyme-assisted fermentation technique has been explored with the idea that it might promote improved nutrient bioavailability in soybeans. Numerous studies have demonstrated that microbial fermentation can increase protein content, decrease protein molecular weight, reduce antinutritional factors, and bring health benefits to monogastric animals (Amadou et al., 2010; Hoa and Hung, 2013; Mukherjee et al., 2015; Yuan et al., 2017; Nkhata et al., 2018; Mikawa et al., 2021). There are also studies in which fermented soybean meal or enzyme-treated soybean meal was fed to dogs and pigs (Yamka et al., 2006; Cervantes-Pahm and Stein, 2010; Beloshapka et al., 2016). To our knowledge, this is the first study evaluating the FPP, an enzyme-assisted fungal fermentation product from soybean meal, as an extruded dog food ingredient.
In this study, dogs were healthy and fed to maintain body weight throughout the study by adjusting food allowance based on weekly weight checks. Stable body weight ensures consistent metabolic state, body composition, and energy requirements to minimize confounding factors on nutrient digestibility (Rosenbaum et al., 2000). The linear increase in ATTD of dry matter calculated by the TFC method was consistent with the linear decrease in wet fecal output and dry fecal output, while fecal moisture content remained constant. In the study of Yamka et al. (2006), low-OS low-phytate soybean meal also increased the ATTD of dry matter in dogs compared to regular soybean meal. The FPP used in this study was reported to have 0% raffinose and 0.02% stachyose (Sinn et al., 2017), which may be the reason of increased ATTD of DM. Zuo et al. (1996) and Yamka et al. (2003, 2006) both indicated that diets containing stachyose less than 27–30 g/kg and raffinose less than 2 g/kg did not decrease nutrient digestibility in dogs. Such observations could explain why the differences in ATTD of DM, CP, and CF between FPP-containing diets and SBM diet did not happen until FPP inclusion increased to 15%. Only when the soybean meal was replaced by FPP at an adequately high level (15% in this study) could the negative influence on nutrient digestibility from OSs be removed.
The study of Beloshapka et al. (2016) evaluated a similar product (HP-300) which is only enzyme treated (low ANFs) but not fermented soybean meal. They found that the ATTD of DM, OM, CF, and energy did not differ until the poultry meal was replaced by HP-300 as high as 48% in dog diets, while the ATTD of CP did not differ among the treatments at all. In our study, the FPP was derived from soybean meal through a combination of enzyme hydrolysis and fungal fermentation. It is intended to have greater protein/AA digestibility than an enzyme-hydrolyzed protein. The SBM had the highest level of soybean meal and chicken meal but no FPP, while the 15FPP had the highest level of FPP and chicken meal but no soybean meal. The improved CP digestibility in 15FPP could be a combined effect of replacing regular soybean meal protein and chicken meal protein by the FPP protein. Additionally, Zuo et al. (1996) also found that both soybean meal and low-OS soybean meal increased ileal digestibility and ATTD of CP in dogs compared to poultry meal. A reasonable next step would be to compare FPP and poultry meal at different replacement levels in dog diets. A favorable combination ratio of FPP and other animal protein products (such as poultry meal, deboned chicken, and frozen beef) is also worth investigating.
Hindgut fermentation
Mammals are not able to efficiently digest soybean OSs due to the lack of α-galactosidases in their intestinal mucosa to break down α-1,6-glycosidic bonds (Medic et al., 2014). Undigested OSs pass to the large intestine, where they become substrates for microbial fermentation, producing SCFAs (mostly acetate, propionate, and butyrate) and gases such as hydrogen, carbon dioxide, and methane (Sunvold et al., 1995). Malabsorbed dietary proteins/peptides can also be fermented in the colon to produce BCFAs, gases, ammonia, amines, and phenolic and indolic compounds (Macfarlane et al., 1986). Proper colonic fermentation benefits the host by providing nutrients (SCFAs) and competitively excluding potential pathogenic bacterial in the gut by competition for oxygen, nutrients, and mucosal adhesion sites and creating an unfavorable environment for non-commensal species by lowering colonic pH, etc (Suchodolski, 2011). Though colonic fermentation of dietary fiber is generally beneficial, the fast gas production from excessive soluble OSs (i.e., part of the soluble dietary fiber) in soybean meal is believed to cause flatulence and altered gut mobility (Suarez et al., 1999; Yamka et al., 2003, 2006). Research also reported that a diet low in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols considerably relieved irritated bowl syndrome in human patients (Staudacher et al., 2012).
Soybean meal dietary fiber consists of both non-starch polysaccharides (NSPs) and OSs where the OSs only contribute to approximately 20% of it (Middelbos and Fahey, 2008; Choct et al., 2010; Opazo et al., 2012). The similar SCFA concentrations observed in this study were possibly due to similar NSP content among the diets that overweighed the difference in OSs. In the study of Beloshapka et al. (2016), SCFA content did not differ until greater than 24% of the diet contained HP-300 when compared to poultry by-product meal. Considering the conditions in which the FPP inclusion level was less than 15% in our study and dietary fiber profile in FPP was closer to soybean meal than to poultry by-product meal, it probably would require much higher inclusion level of FPP and soybean meal to induce changes in SCFA content if any.
The similar ammonia, individual BCFA, and total BCFA contents observed in this study were unexpected as the FPP was postulated to decrease colonic fermentation of proteins/peptides. The results indicate that while the ATTD of CP was statistically different among the diets, the difference was too small to have practical significance. The study of Nery et al. (2012) on dog diets indicated that highly digestible plant proteins are superior to moderately digestible poultry proteins in regard to reduced concentrations of protein-based fermentation products in feces together with improved fecal quality in dogs. Based on our results, the FPP has the potential to be a cost-efficient protein source in pet food compared to moderately digestible animal protein sources. Further research to compare FPP with various animal ingredients would be justified.
Palatability
The two-bowl forced-choice method is commonly used in pet food palatability evaluation. The trial consists of two parts of results: first choice and intake ratio—the first choice reflects the olfactory characteristics and the intake ratio is considered to better reflect an overall preference (Aldrich and Koppel, 2015).
In this study, both first choice and intake ratio indicated that dogs preferred SBM to either 5FPP or 10FPP, but such preference did not exist between SBM and 15FPP. That means that as the FPP inclusion level increased, some contributing factors outweighed negative factors in regard to palatability. Félix et al. (2012) suggested that low molecular sugars contribute to the palatability of dog diets. This might also be true in our study as the oligosaccharides were decomposed into smaller sugar molecules by added enzymes and/or fungal glycosidases during the production of FPP. It was reported that dogs preferred diets with no added fiber to the diet with fiber from sugarcane or wheat bran (Koppel et al., 2015). However, the TDF contents in their treatment diets were approximately twice the amount in the control diet from this study. In our study, it seemed to be contradictory as the diets with higher TDF content were preferred to diets with lower TDF. This gives rise to two indications: a) diet palatability in this study was determined by other factor(s) instead of minor differences in TDF content; or b) source, solubility, and other characteristics of dietary fiber are more influential on palatability than merely the amount (Koppel et al., 2015). In summary, the results in this study indicated that a 15% inclusion level of FPP was better than lower levels in regard to preference by dogs.
Conclusion
The results of this study indicated that even though FPP has an imbalanced amino acid profile, it is an acceptable plant protein source in extruded dog foods. Compared to traditional soybean meal, the FPP seems to be able to decrease fecal output and improve total tract protein digestibility at the 15% inclusion level while maintaining practical extrusion processes and comparable palatability. The potential influences of FPP on colonic fermentation may require more specific diet formulations and/or more dogs to be observed. It is possible that FPP may be preferable to regular soybean meal and even some animal protein sources in regard to fecal quality, digestion, and colonic fermentation, which requires further research to verify.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by the Kansas State University under the Institutional Animal Care and Use Committee. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
YC: Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. CA: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was funded by Prairie AquaTech (Brookings, SD; grant #BH0739). The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Acknowledgments
We wish to express our gratitude to Prairie AquaTech for supporting this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
- ^ https://americanpetproducts.org/industry-trends-and-stats
- ^ https://www.gapfa.org/files/download/GAPFA_Factsheet_The_Science_of_Pet_Food-Role_of_Protein.pdf
References
Aldrich G. C. and Koppel K. (2015). Pet Food Palatability Evaluation: A Review of Standard Assay Techniques and Interpretation of Results with a Primary Focus on Limitations. Anim. (Basel) 5, 43–55. doi: 10.3390/ani5010043
Amadou I., Amza T., Foh M., and Le M. (2010). Influence of Lactobacillus plantarum Lp6 fermentation on the functional properties of soybean protein meal. Emir. J. Food Agric. 22, 456. doi: 10.9755/ejfa.v22i6.4663
Bednar G. E., Murray S. M., Patil A. R., Flickinger E. A., Merchen N. R., and Fahey G. C. (2000). Selected animal and plant protein sources affect nutrient digestibility and fecal characteristics of ileally cannulated dogs. Archiv für Tierernaehrung 53, 127–140. doi: 10.1080/17450390009381942
Beloshapka A. N., De Godoy M. R. C., Detweiler K. B., Newcomb M., Ellegård K. H., Fahey G. C., et al. (2016). Apparent total tract macronutrient digestibility, fecal characteristics, and fecal fermentative end-product concentrations of healthy adult dogs fed bioprocessed soy protein1. J. Anim. Sci. 94, 3826–3834. doi: 10.2527/jas.2016-0449
Berry T. H., Becker D. E., Rasmussen O. G., Jensen A. H., and Norton H. W. (1962). The Limiting Amino Acids in Soybean Protein. J. Anim. Sci. 21, 558–561. doi: 10.2527/jas1962.213558x
Carciofi A. C., de-Oliveira L. D., Valério A. G., Borges L. L., De Carvalho F. M., Brunetto M. A., et al. (2009). Comparison of micronized whole soybeans to common protein sources in dry dog and cat diets. Anim. Feed Sci. Technol. 151, 251–260. doi: 10.1016/j.anifeedsci.2009.01.002
Cervantes-Pahm S. K. and Stein H. H. (2010). Ileal digestibility of amino acids in conventional, fermented, and enzyme-treated soybean meal and in soy protein isolate, fish meal, and casein fed to weanling pigs1. J. Anim. Sci. 88, 2674–2683. doi: 10.2527/jas.2009-2677
Chaney A. L. and Marbach E. P. (1962). Modified Reagents for Determination of Urea and Ammonia. Clin. Chem. 8, 130–132. doi: 10.1093/clinchem/8.2.130
Choct M., Dersjant-Li Y., Mcleish J., and Peisker M. (2010). Soy Oligosaccharides and Soluble Non-starch Polysaccharides: A Review of Digestion, Nutritive and Anti-nutritive Effects in Pigs and Poultry. Asian-Australasian J. Anim. Sci. 23, 1386–1398. doi: 10.5713/ajas.2010.90222
Clapper G. M., Grieshop C. M., Merchen N. R., Russett J. C., Brent J. L., and Fahey G. C. (2001). Ileal and total tract nutrient digestibilities and fecal characteristics of dogs as affected by soybean protein inclusion in dry, extruded diets. J. Anim. Sci. 79, 1523. doi: 10.2527/2001.7961523x
Donadelli R. A., Dogan H., and Aldrich G. (2021). The effects of fiber source on extrusion parameters and kibble structure of dry dog foods. Anim. Feed Sci. Technol. 274, 114884. doi: 10.1016/j.anifeedsci.2021.114884
FAO (1991). Protein quality evaluation: report of the Joint FAO/WHO Expert Consultation, FAO food and nutrition paper (Rome: Food and Agriculture Organization of the United Nations).
Félix A. P., Carvalho M. P., Alarça L. G., Brito C. B. M., Oliveira S. G., and Maiorka A. (2012). Effects of the inclusion of carbohydrases and different soybean meals in the diet on palatability, digestibility and faecal characteristics in dogs. Anim. Feed Sci. Technol. 174, 182–189. doi: 10.1016/j.anifeedsci.2012.03.013
Gibbons W. R. and Brown M. L. (2016). US 9,370,200B2. Washington, DC: U.S. Patent and Trademark Office.
Grieshop C. M., Kadzere C. T., Clapper G. M., Flickinger E. A., Bauer L. L., Frazier R. L., et al. (2003). Chemical and nutritional characteristics of united states soybeans and soybean meals. J. Agric. Food Chem. 51, 7684–7691. doi: 10.1021/jf034690c
Hoa B. T. and Hung P. V. (2013). Optimization of nutritional composition and fermentation conditions for cellulase and pectinase production by Aspergillus oryzae using response surface methodology. Int. Food Res. J. 20, 3269–3274.
Kim B. G. and Stein H. H.. (2009). Chemical and Nutritional Characteristics of United States Soybeans and Soybean Meals. J. Agric. Food Chem. 51, 7684–7691. doi: 10.1021/jf034690c
Kim H. S., Titgemeyer E. C., Curles E., Olsen L. M., and Aldrich C. G. (2023). Evaluation of Soybean Ingredients in Pet Foods Applications: Systematic Review. Animals 14, 16. doi: 10.3390/ani14010016
Kim M. H., Yun C. H., Lee C. H., and Ha J. K. (2012). The effects of fermented soybean meal on immunophysiological and stress-related parameters in Holstein calves after weaning. J. Dairy Sci. 95, 5203–5212. doi: 10.3168/jds.2012-5317
Koneswaran G. and Nierenberg D. (2008). Global farm animal production and global warming: impacting and mitigating climate change. Environ. Health Perspect. 116, 578–582. doi: 10.1289/ehp.11034
Koppel K., Monti M., Gibson M., Alavi S., Donfrancesco B. D., and Carciofi A. C. (2015). The effects of fiber inclusion on pet food sensory characteristics and palatability. Animals 5, 110–125. doi: 10.3390/ani5010110
Macfarlane G. T., Cummings J. H., and Allison C. (1986). Protein Degradation by Human Intestinal Bacteria. Microbiology 132, 1647–1656. doi: 10.1099/00221287-132-6-1647
Maria A. P. J., Ayane L., Putarov T. C., Loureiro B. A., Neto B. P., Casagrande M. F., et al. (2017). The effect of age and carbohydrate and protein sources on digestibility, fecal microbiota, fermentation products, fecal IgA, and immunological blood parameters in dogs1,2. J. Anim. Sci. 95, 2452–2466. doi: 10.2527/jas.2016.1302
Mathivanan R., Selvaraj P., and Nanjappan K. (2006). Feeding of fermented soybean meal on broiler performance. International Journal of Poultry Science 5, 868–872. doi: 10.3923/ijps.2006.868.872
Medic J., Atkinson C., and Hurburgh C. R. (2014). Current Knowledge in Soybean Composition. J. Am. Oil Chem. Soc. 91, 363–384. doi: 10.1007/s11746-013-2407-9
Menniti M. F., Davenport G. M., Shoveller A. K., Cant J. P., and Osborne V. R. (2014). Effect of graded inclusion of dietary soybean meal on nutrient digestibility, health, and metabolic indices of adult dogs1. J. Anim. Sci. 92, 2094–2104. doi: 10.2527/jas.2013-7226
Middelbos I. S. and Fahey G. C. (2008). “Soybean Carbohydrates,” in Soybeans (Philadelphia, PA: Elsevier), 269–296. doi: 10.1016/B978-1-893997-64-6.50012-3
Mikawa S., Matsuda A., Kamemori Y., Asanuma S., and Kitagawa H. (2021). Enhancement of natural killer cell activity by oral administration of a fermented soybean product in dogs. Open Vet. J. 11, 394–400. doi: 10.5455/OVJ.2021.v11.i3.10
Mori K., Rock M., McCormack G., Liccioli S., Giunchi D., Marceau D., et al. (2023). Fecal contamination of urban parks by domestic dogs and tragedy of the commons. Sci. Rep. 13, 3462. doi: 10.1038/s41598-023-30225-7
Mukherjee R., Chakraborty R., and Dutta A. (2015). Role of Fermentation in Improving Nutritional Quality of Soybean Meal — A Review. Asian Australas. J. Anim. Sci. 29, 1523–1529. doi: 10.5713/ajas.15.0627
Murphy P. A. (2008). “Soybean Proteins,” in Soybeans. Eds. Johnson L. A., White P. J., and Galloway R. (Philadelphia, PA: Elsevier), 229–267. doi: 10.1016/B978-1-893997-64-6.50011-1
Myers W. D., Ludden P. A., Nayigihugu V., and Hess B. W. (2004). Technical Note: A procedure for the preparation and quantitative analysis of samples for titanium dioxide1. J. Anim. Sci. 82, 179–183. doi: 10.2527/2004.821179x
National Research Council (2006). Nutrient Requirements of Dogs and Cats., 2nd Edn. Washington (DC): National Academies Press.
Nery J., Goudez R., Biourge V., Tournier C., Leray V., Martin L., et al. (2012). Influence of dietary protein content and source on colonic fermentative activity in dogs differing in body size and digestive tolerance1. J. Anim. Sci. 90, 2570–2580. doi: 10.2527/jas.2011-4112
Nkhata S. G., Ayua E., Kamau E. H., and Shingiro J. (2018). Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Sci. Nutr. 6, 2446–2458. doi: 10.1002/fsn3.846
Ojokoh A. O., Yimin W., and Eromosele O. S. (2015). Effect of some extrusion variables on fermented maize-soybean blend. J Food Sci Technol 52, 5763–5771. doi: 10.1007/s13197-014-1689-8
Opazo R., Ortúzar F., Navarrete P., Espejo R., and Romero J. (2012). Reduction of Soybean Meal Non-Starch Polysaccharides and α-Galactosides by Solid-State Fermentation Using Cellulolytic Bacteria Obtained from Different Environments. PLoS One 7, e44783. doi: 10.1371/journal.pone.0044783
Ou S., Kwok K. C., and Kang Y. (2004). Changes in in vitro digestibility and available lysine of soy protein isolate after formation of film. Journal of Food Engineering 64, 301–305. doi: 10.1016/j.jfoodeng.2003.10.013
Parada J., Aguilera J. M., and Brennan C. (2011). Effect of guar gum content on some physical and nutritional properties of extruded products. J. Food Eng. 103, 324–332. doi: 10.1016/j.jfoodeng.2010.11.001
Rani P., Kumar A., Purohit S. R., and Rao P. S. (2018). Impact of fermentation and extrusion processing on physicochemical, sensory and bioactive properties of rice-black gram mixed flour. LWT 89, 155–163. doi: 10.1016/j.lwt.2017.10.050
Redgwell R. J., Curti D., Robin F., Donato L., and Pineau N. (2011). Extrusion-Induced Changes to the Chemical Profile and Viscosity Generating Properties of Citrus Fiber. J. Agric. Food Chem. 59, 8272–8279. doi: 10.1021/jf201845b
Reilly L. M., von Schaumburg P. C., Hoke J. M., Davenport G. M., Utterback P. L., Parsons C. M., et al. (2021). Use of the precision-fed cecectomized rooster assay to determine standardized amino acid digestibility, true metabolizable energy content, and digestible indispensable amino acid scores of plant-based protein by-products used in canine and feline diets. Trans. Anim. Sci. 5, txab025. doi: 10.1093/tas/txab025
Robin F., Schuchmann H. P., and Palzer S. (2012). Dietary fiber in extruded cereals: Limitations and opportunities. Trends Food Sci. Technol. 28, 23–32. doi: 10.1016/j.tifs.2012.06.008
Rosenbaum M., Hirsch J., Murphy E., and Leibel R. L. (2000). Effects of changes in body weight on carbohydrate metabolism, catecholamine excretion, and thyroid function. Am. J. Clin. Nutr. 71, 1421–1432. doi: 10.1093/ajcn/71.6.1421
Schleicher M., Cash S. B., and Freeman L. M. (2019). Determinants of pet food purchasing decisions. Can. Vet. J. 60, 644–650.
Senevirathne N. D., Anderson J. L., Gibbons W. R., and Clapper J. A. (2017). Growth performance of calves fed microbially enhanced soy protein in pelleted starters. J. Dairy Sci. 100, 199–212. doi: 10.3168/jds.2016-11221
Sinn S. M., Gibbons W. R., Brown M. L., DeRouchey J. M., and Levesque C. L. (2017). Evaluation of microbially enhanced soybean meal as an alternative to fishmeal in weaned pig diets. Animal 11, 784–793. doi: 10.1017/S1751731116002020
Staudacher H. M., Lomer M. C. E., Anderson J. L., Barrett J. S., Muir J. G., Irving P. M., et al. (2012). Fermentable Carbohydrate Restriction Reduces Luminal Bifidobacteria and Gastrointestinal Symptoms in Patients with Irritable Bowel Syndrome. J. Nutr. 142, 1510–1518. doi: 10.3945/jn.112.159285
Stein H. H., Berger L. L., Drackley J. K., Fahey G. C., Hernot D. C., and Parsons C. M. (2008). Nutritional Properties and Feeding Values of Soybeans and Their Coproducts. Eds. Johnson L. A., White P. J., and Galloway R. (Philadelphia, PA: Soybeans. AOCS Press), 613–660. doi: 10.1016/B978-1-893997-64-6.50021-4
Suarez F. L., Springfield J., Furne J. K., Lohrmann T. T., Kerr P. S., and Levitt M. D. (1999). Gas production in humans ingesting a soybean flour derived from beans naturally low in oligosaccharides. Am. J. Clin. Nutr. 69, 135–139. doi: 10.1093/ajcn/69.1.135
Suchodolski J. S. (2011). Companion Animal Symposium: Microbes and gastrointestinal health of dogs and cats1. J. Anim. Sci. 89, 1520–1530. doi: 10.2527/jas.2010-3377
Sunvold G. D., Hussein H. S., Fahey G. C. Jr., Merchen N. R., and Reinhart G. A. (1995). In vitro fermentation of cellulose, beet pulp, citrus pulp, and citrus pectin using fecal inoculum from cats, dogs, horses, humans, and pigs and ruminal fluid from cattle. J. Anim. Sci. 73, 3639–3648. doi: 10.2527/1995.73123639x
Tortola L., Souza N. G., Zaine L., Gomes M. O. S., Matheus L. F. O., Vasconcellos R. S., et al. (2013). Enzyme effects on extruded diets for dogs with soybean meal as a substitute for poultry by-product meal. J. Anim. Physiol. Anim. Nutr. 97, 39–50. doi: 10.1111/jpn.12009
Vanelli K., FonsecadeOliveira A., Sotomaior C. S., Weber S. H., and Costa L. (2021). Soybean meal and poultry offal meal effects on digestibility of adult dogs diets: Systematic review. PLoS One 16, e0249321. doi: 10.1371/journal.pone.0249321
Yamka R. M., Harmon D. L., Schoenherr W. D., Khoo C., Gross K. L., Davidson S. J., et al. (2006). In vivo measurement of flatulence and nutrient digestibility in dogs fed poultry by-product meal, conventional soybean meal, and low-oligosaccharide low-phytate soybean meal. doi: 10.2460/ajvr.67.1.88
Yamka R. M., Jamikorn U., True A. D., and Harmon D. L. (2003). Evaluation of soyabean meal as a protein source in canine foods. Anim. Feed Sci. Technol. 109, 121–132. doi: 10.1016/S0377-8401(03)00203-7
Yang Y. X., Kim Y. G., Lohakare J. D., Yun J. H., Lee J. K., Kwon M. S., et al. (2007). Comparative Efficacy of Different Soy Protein Sources on Growth Performance, Nutrient Digestibility and Intestinal Morphology in Weaned Pigs. Asian-Australasian J. Anim. Sci. 20, 775–783. doi: 10.5713/ajas.2007.775
Yuan L., Chang J., Yin Q., Lu M., Di Y., Wang P., et al. (2017). Fermented soybean meal improves the growth performance, nutrient digestibility, and microbial flora in piglets. Anim. Nutr. 3, 19–24. doi: 10.1016/j.aninu.2016.11.003
Zarkadas C. G., Gagnon C., Gleddie S., Khanizadeh S., Cober E. R., and Guillemette R. J. D. (2007). Assessment of the protein quality of fourteen soybean [Glycine max (L.) Merr.] cultivars using amino acid analysis and two-dimensional electrophoresis. Food Res. Int. 40, 129–146. doi: 10.1016/j.foodres.2006.08.006
Keywords: plant protein, dogs, digestibility, extrusion, fecal quality, hindgut fermentation, palatability
Citation: Chen Y and Aldrich CG (2025) The impact of graded levels of fermented plant protein (Proteger®) in extruded foods on fecal quality, nutrient digestibility, and colonic fermentation in beagle dogs. Front. Anim. Sci. 6:1571097. doi: 10.3389/fanim.2025.1571097
Received: 04 February 2025; Accepted: 06 May 2025;
Published: 02 June 2025.
Edited by:
Bianca Castiglioni, National Research Council (CNR), ItalyReviewed by:
Fabiola Espinosa-Gómez, Popular Autonomous University of the State of Puebla, MexicoClaire Timlin, Four Rivers Kennel, LLC, United States
Copyright © 2025 Chen and Aldrich. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Charles Gregory Aldrich, YWxkcmljaDRAa3N1LmVkdQ==
 Youhan Chen
Youhan Chen Charles Gregory Aldrich
Charles Gregory Aldrich