- 1Instutute of Animal Science - Kostinbrod, Agricultural Academy, Kostinbrod, Bulgaria
- 2Department of Meat and Fish Technology, University of Food Technologies, Plovdiv, Bulgaria
- 3Bulgarian Academy of Sciences, Sofia, Bulgaria
- 4Institute of Cryobiology and Food Technologies, Agricultural Academy, Sofia, Bulgaria
The study aimed to investigate the effect of the inclusion of low-fat Tenebrio molitor meal (LFTM) as partial replacement of soybean in the diet of broilers on the physical properties, chemical composition and fatty acid profile of the meat. The trial included a total of 120 male one-day-old Ross 308 broilers allocated to 5 groups. After 14 days of adaptation, the birds were fed a basal diet (C) and diets including LFTM in amounts of 2.5% (T2.5), 5% (T5), 7.5% (T7.5), and 10% (T10). At 35 days of age, 8 birds per group were sacrificed and subjected to analysis of the meat quality. The higher dietary levels of LFTM were associated with decrease in pH24 (linear, P<0.0001), but increased L* (linear, P=0.0004), drip loss (linear, P=0.0001) and cooking loss (linear, P=0.0058) in the breast. Furthermore, increasing the LFTM concentrations led to decline in protein (linear, P<0.0001), but increase in fat (linear, P<0.0001) and moisture (linear, P=0.0006) of the breast meat. The inclusion of the LFTM in the diet affected the fatty acid composition in breast and thigh meat in a dose dependent manner. Increasing the dietary levels of LFTM increased the saturated (SFA) fatty acids in the breast (linear, P=0.0012) and thigh (linear, P<0.0001) as well as the content of monounsaturated (MUFA) fatty acids in both cuts (linear, P<0.0001). This was associated with decrease in the polyunsaturated (PUFA) fatty acids in the meat of the birds fed higher levels of LFTM (linear, P<0.0001), thus producing higher atherogenic (AI) (linear, P<0.0001) and thrombogenic indices (TI) (linear, P<0.0001). The results of this study suggest that LFTM might be included in amount up to 5% in the diet of broilers without adverse effects on meat quality.
1 Introduction
Effective genetic selection has enhanced the growth performance, feed efficiency, and meat yield in broilers, thus helping to achieve broiler production at mass level (Choi et al., 2023; Mir et al., 2017). Since the intensive growth of broilers is often associated with abnormalities in meat (Petracci et al., 2019; Baldi et al., 2020; Wang et al., 2023; Wu et al., 2024), the emphasis is now on striving to improve its quality. Meat quality is a broad concept. It includes physical traits, chemical composition and sensory attributes of meat, all of which are important and affect the consumers. While the chemical composition is crucial for the nutritive and healthy value of meat, physical characteristics are closely related to the sensory ones, the latter having effect on the acceptability of the consumers and their willingness to purchase meat. Some of the most important quality attributes of poultry meat are the appearance, texture and functionality (Fletcher, 2002) and they are related mainly to the pH, color and water holding capacity. According to Swatland (1989) the research on the physical aspects of meat quality is important since they are of commercial significance, objectively measurable through rapid methods, and can explain the full effect of the biochemical processes in meat.
Nutrition may have significant impact on the body composition in broilers and the quality of their meat (Mir et al., 2017), as the protein and energy contents being the key factors (Choi et al., 2023). They affect the yield of the main carcass parts, particularly breast (Berri et al., 2008), and have influence on the pH, water holding capacity and color (Zhao et al., 2012; Jlali et al., 2012). The latter have strong impact on the consumers’ acceptability. Furthermore, nutrition has considerable influence on the dietary and healthy value of the poultry meat, particularly the content of protein, intramuscular fat and the fatty acid profile. According to Bordoni and Danesi (2017) the protein content in poultry meat is within the range of 19.4 -22.5 g/100 g, while lipids vary between 2.6-3.7g/100 g. Poultry provides high quality protein and its lipids contain high amounts of polyunsaturated fatty acids (Soriano-Santos, 2010). In recent years there is increasing interest in alternative protein sources that may successfully replace the soybean and fishmeal in the animal and poultry diets. Insects offer a promising and sustainable solution for addressing the challenges associated with conventional protein sources while providing comparable nutritional value and a reduced environmental footprint (Veldkamp et al., 2022; Van Huis, 2022). In addition to the high-quality protein, the edible insects provide a wide range of beneficial biologically active compounds, such as fatty acids, antimicrobial peptides and chitin (Solecka and Drazbo, 2024) that have positive immunomodulatory effect, and could be used in animal and poultry diets without adverse effect on the production (Malematja et al., 2023). As stated by Grau et al. (2017) Tenebrio molitor (TM) is economically among the most important species used for large-scale conversion of plant biomass into protein. The mealworms can also breakdown low-nutrient byproducts of common crops such as maize, wheat, millet and peanuts and quickly recycle them into high-quality food (Heidari-Parsa et al., 2018). Meals derived from TM larvae have been extensively studied as a protein source in poultry diets, with focus mainly on the performance and carcass composition, showing different effect depending on the levels of the meal. Bovera et al. (2016) did not observe any significant effect on the growth performance and carcass in broilers associated with the intake of TM larvae meal, however the groups consumed the insects displayed improved feed conversion. Biasato et al. (2018) suggested that increasing the level of TM up to 15% in the diet may improve the body weight and feed intake but negatively affect the feed efficiency and gut morphology. Nieto et al. (2022) reported lower body weight and weight gain in slow-growing chickens fed TM meal as full replacement of the soybean meal. This was later confirmed by Petkov et al. (2024) showing that broilers receiving LFTM responded with linear decrease of the body weight and weight gain with increasing the insect meal up to 10%. So far, only few studies examined also the effect of the mealworm meals on the meat quality parameters in poultry. The results differed depending on the dose of the meals but no negative effect on the meat quality parameters were reported in broilers with full (Bovera et al., 2016) or partial (Elahi et al., 2020; Šťastník et al., 2021; Shaviklo et al., 2021) replacement of soybean meal, up to 25% replacement of fish meal (Parlar and Ustundag, 2024) and free-range hybrid chickens (Dabbou et al., 2020). No negative effect was reported in the quality of meat in native chickens (Selaledi et al., 2021) fed up to 15% mealworm meal. Others reported improvement in the meat quality of broilers fed 1% TM meal (Kim et al., 2014) and quails (Zadeh et al., 2019). Full fat TM meals were used for the purposes of these trials. Among the insect-derived meals, the defatted ones are of major interest since they are richer in crude protein and have lower ether extract. In addition, the lower fat content in these meals makes them more stable during storage due to reduced risk of lipid oxidation (Renna et al., 2023) and they are easier to include in feed formulations than the full-fat ones (Gasco et al., 2023). The highly and partially defatted insect meals examined in regard to the meat quality traits in poultry are mainly derived from Black soldier fly larvae (Schiavone et al., 2019; Popova et al., 2020; Gross-Bošković et al., 2024) and silkworm pupae (Zsedely et al., 2023; Dalle Zotte et al., 2024). To date there is no research of the effect of defatted or low-fat Tenebrio molitor meal on the quality of broilers meat. Hence, the aim of this study was to investigate the meat quality attributes in broilers fed diets partially replaced with different doses of low-fat Tenebrio molitor meal.
2 Material and methods
2.1 Experimental design and diets
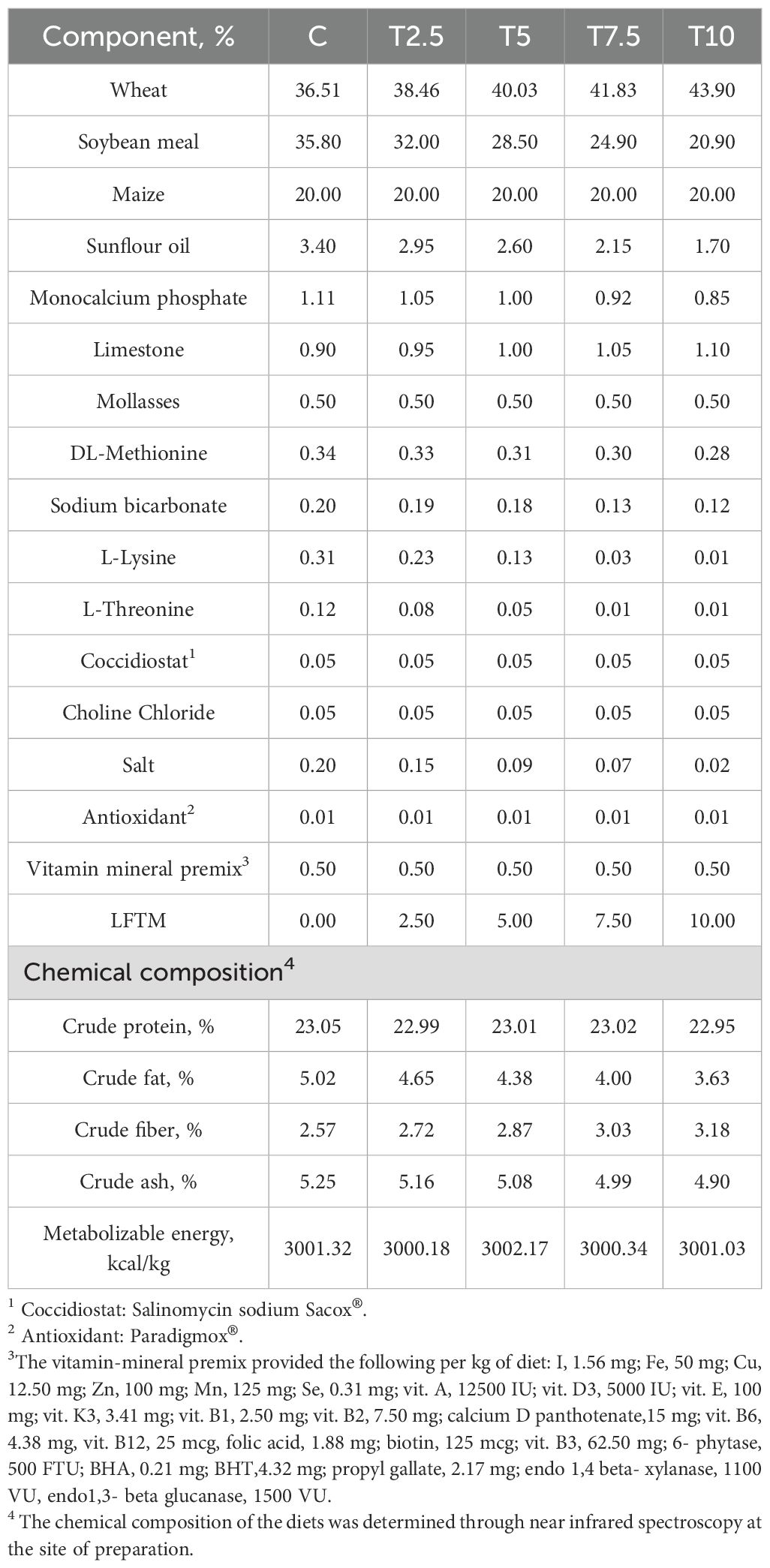
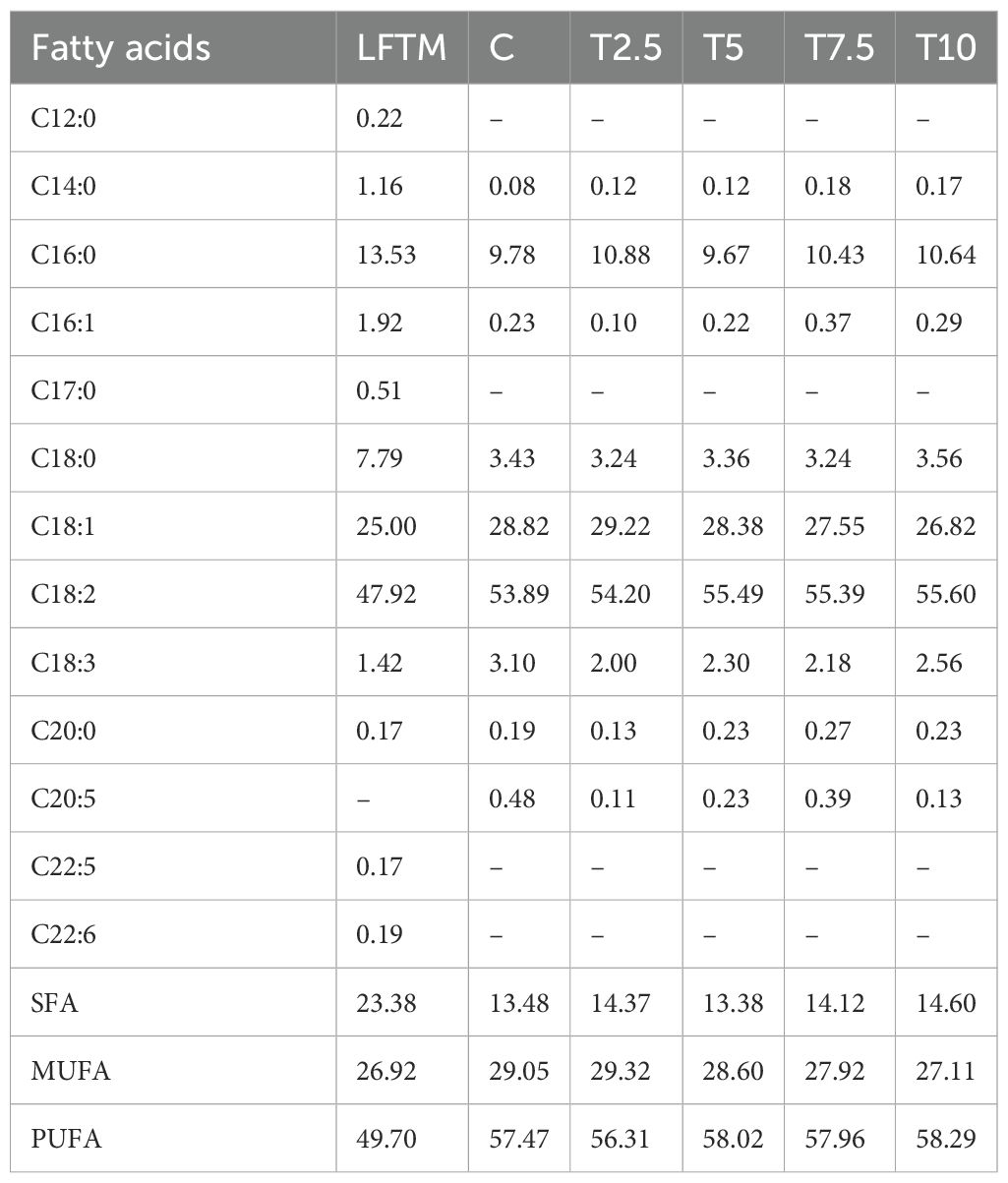
The trial was carried out in the experimental poultry farm of the Institute of Animal Science-Kostinbrod, Bulgaria. The experimental design is provided in full details elsewhere (Petkov et al., 2024). Briefly, 120 male Ross 308 broilers (BW 41.19 ± 0.55 g) were randomly allocated to 5 groups (each including 4 replicate cages with 6 birds per cage). The birds were kept in controlled microclimate in appropriately designed premises with artificial fluorescent light and forced ventilation. Heating was done through local brooders on each cage. The low-fat Tenebrio molitor meal was supplied by VBF Pro Ltd., Petrich, Bulgaria. Its chemical composition was analyzed in the Laboratory for Feed Analysis at the Institute of Animal Science-Kostinbrod and summarized as follows: crude protein: 62.8%, moisture: 11.74%, crude fat: 4.02%, crude fiber: 9.46%; crude ash: 7.47%; nitrogen free extracts: 4.51%; Ca: 0.082%; P:1.536%. The feeding of the broilers was in two phases, as during the first two weeks of the trial (1d-14d), all the groups were fed the same starter diet adjusted to the recommendations for Ross broilers. For the grower period, four diets were formulated containing respectively 2.5% (T2.5), 5% (T5), 7.5(T7.5) and 10% (T10) LFTM. The control (C) group was fed the basal diet. The composition of the diets as previously reported by Petkov et al. (2024) is presented in Table 1. The formulations were designed to be isocaloric and isonitrogenous to meet the nutritional requirements for broilers (Ross Broiler Management Handbook, 2018). They were prepared and their nutritional composition analyzed by Viand JSC. The fatty acid composition of the diets and the insect meal was analyzed in the Laboratory of Lipid Analysis in the Institute of Animal Science-Kostinbrod and presented in Table 2. During the trial period, the birds received feed and water ad libitum, and were individually weighed each week to calculate their weight gain.
2.2 Slaughtering and sampling
At the end of the trial (35 d) two birds per replicate cage were selected from each group (n=8), based on the average live weight (Petkov et al., 2024). The broilers were stunned, decapitated and bled. The carcasses were then plucked, eviscerated and their feet were removed. Hot carcass weight was recorded and dressing percentage was calculated (Petkov et al., 2024). The carcasses were then stored at 4°C for 24 h and weighed again. Further, the abdominal fat was removed from the carcasses, and they were separated into breast, thigh, back and wings. The neck was also separated from the carcass. Тhe carcass parts were weighed and calculated as percentage of the cold carcass. Further, the skin was removed, the breast and thigh muscles were separated from the bones and subjected to analysis.
2.3 Measurement of pH and color
Muscle pH and color were measured 24 h post mortem at the time of deboning of the breast and thigh cuts. The pH24 measurements were taken using a portable pH meter equipped with a glass electrode HI 83141 (Hanna Instruments Ltd., Woonsocket, Rhode Island, USA). Calibration prior to use at pH 4.0 and 7.0 was performed. The measurements were taken directly in the breast (m. Pectoralis major) and thigh (m. Iliotibialis laterialis) at three different points. The color of the breast and thigh muscles was measured by Chroma meter CR-410 (Konica Minolta Inc., Osaka, Japan) using CIE values expressed as lightness (L*), redness (a*), and yellowness (b*). A 50 mm diameter measurement area, illuminant D65, and 2° standard observer were used. The instrument was calibrated using a standard white plate. The color measurements were done on the medial surface at the bone side of the breast and thigh muscle. Three measurements of each cut were taken and averaged.
2.4 Drip loss
Drip loss was measured as described by Honikel (1998) with slight modifications. After deboning portions of approximately 50 g and 30 g were excised respectively from the fresh m. Pectoralis major and the upper part of m. Iliotibialis lateralis. The samples were immediately placed in plastic bags, hung from a hook and stored for 24 h at 4°C. The samples were taken out of the bags immediately before measurement, gently blotted dry and weighed on electronic balance (KERN EG220-3NM, Kern & Sohn GmbH, Balingen, Germany). Drip loss was expressed as percentage of the initial weight, following the equation:
where W1 is the initial weight of the sample (g), W2 is the final weight of the sample (g).
2.5 Cooking loss
Cooking loss was measured as described by Echegaray et al. (2022). The breast and thigh meat samples, taken as described above, were weighed, put into plastic bags, sealed and placed in a multiple water bath (W3, VEB MLW Prüfgeräte-Werk Medingen/Sitz Freital, Germany) set at 80°C. The meat samples were cooked until the temperature in the core reached 70°C measured by digital thermometer, (SIKA, TT 7110, Dr. Siebert & Kunh GmbH &Co KG, Kaufungen, Germany). Then the bags were removed and the meat was left to cool at room temperature for approximately 30 min. The meat pieces were dried to eliminate any water left on the surface and then weighed. The cooking loss was expressed as a percentage using the following equation:
Where W1 is the initial weight of the sample (g), W2 is the weight after cooking (g).
2.6 Proximate analysis
The content of moisture, protein, fat, and ash in the breast and thigh meat was assessed according to the methods of AOAC (2004). Each sample was analyzed in triplicate.
2.7 Fatty acid composition of the meat
The fatty acid composition of the LFTM, feeds and meat was determined according to the method of Bligh and Dyer (1959) with slight modifications described by Vargas-Ramella et al. (2020). Lipids were extracted from 10 g of the muscle/feed sample and homogenized using a HG-15D homogenizer (Witeg Labortechnik GmbH, Wertheim, Germany) with 10 mL of chloroform and 20 mL of methanol for 30 s. Following this, 10 mL of chloroform and 10 mL of NaCl (1% in distilled water) were added to the mixture and homogenized for 30 s. The samples were centrifuged (4000 rpm for 10 min) and finally the chloroform layer was evaporated. The fatty acids were trans esterified as described by Domínguez et al. (2015) with some modifications: 20 mg of extracted fat dissolved in 1 mL of toluene was mixed with 2 mL of a sodium methoxide (0.5 N) solution, vortexed for 10 s, and allowed to stand for 15 min at room temperature. Then, 4 mL of a H2SO4 solution (10% of H2SO4 in methanol) was added, vortexed for 10 s, and left for 5 min before adding 2 mL of saturated sodium bicarbonate solution. Fatty acid methyl esters were extracted as 1 mL of hexane was added to the samples, vortexed for 10 s, and the organic phase was transferred to an appropriate GC vial. Separation and quantification of FAMEs were carried out using a gas chromatograph (CSi 200 series, Cambridge Scientific Instruments Ltd., Ely, UK) equipped with a capillary column (DM-2330:30 m × 0.25 mm × 0.20µm) and hydrogen as a carrier gas. The oven temperature was first set to 160°C for 0.2 min, then raised to 220°C at a rate of 5°C/min and then held for 5 min. The temperatures of the detector and injector were 230°C. Methyl esters were identified through comparison of the retention times of the standards. Fatty acids are presented as percentages of the total amount of the methyl esters (FAME) identified. The amount of each fatty acid was used to calculate the atherogenic (AI) and thrombogenic (TI) indices (Ulbricht and Southgate, 1991):
The Δ 9-desaturase index, as an indirect index of stearoyl-CoA desaturase (SCD) activity, was calculated using the following formulae (Zhang et al., 2007):
The indices of Δ5 (D5D) and Δ6 desaturase (D6D) activity were calculates as ratio between product and precursor (Haug et al., 2014): D5D=C20:4n-6/C20:3n-6;
The elongase index was calculated as 100×[(C18:0 + C18:1 n-9)/(C16:0 + C16:1 + C18:0 + C18:1 n-9)] (Green et al., 2010).
2.8 Statistical evaluation
Statistical processing was done using JMP pro 17 software package. Shapiro-Wilk test was applied to check the normality of the distribution of the data. Тhe physical parameters, proximate composition and the fatty acid profile were analyzed through one-way ANOVA, followed by post-hoc comparisons (Tukey HSD, p<0.05) to assess the significance of the differences. Polynomial contrasts were used to assess the linear and quadratic effects of the levels of LFTM in the diet of the birds on the meat quality parameters. Data was presented as Mean and Standard error of the mean (SEM).
3 Results
3.1 Physical attributes
The effect of the incorporation of the LFTM in the diet on pH24 and color was observed only in the breast (Table 3). The pH24 values in this cut decreased linearly (P<0.0001) with increasing the levels of the insect meal. The L* parameter showed linear (P=0.0004) response to the inclusion of the LFTM. The darkest color of the breast meat was observed in the birds from the control group. The inclusion of the insect meal and the increase of its content was associated with increase in the L* values. The redness of the meat showed the opposite changes, responding with linear decrease (P=0.0481) to the higher concentration of the insect meal in the diet. The highest a* values were measured in the breast of the control group, and the lowest in T7.5.
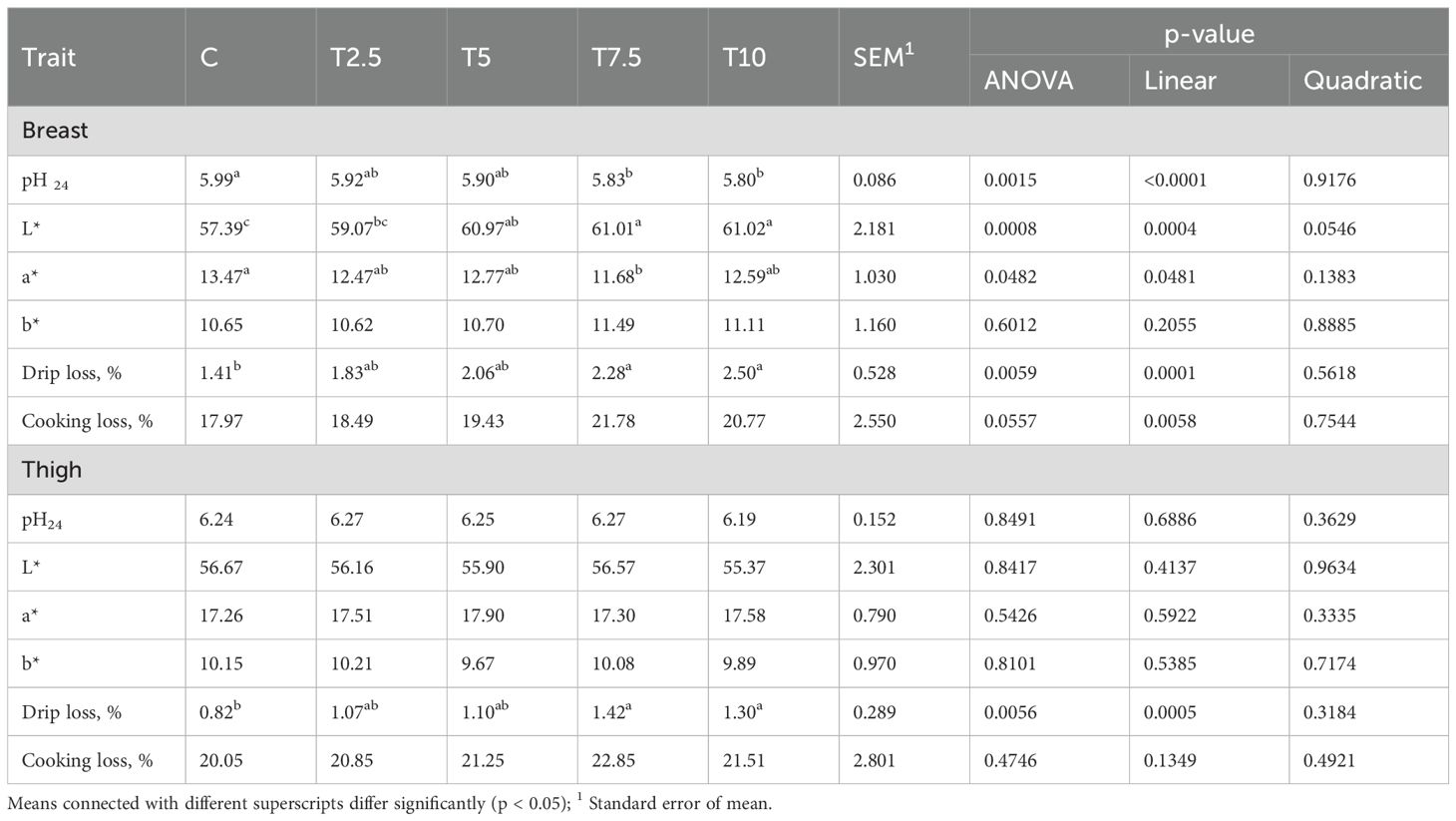
Table 3. Effect of LFTM in the diet on the physical parameters of breast and thigh meat in broilers.
In the thigh meat, pH24 values showed no significant difference between groups (Table 3). Similarly, no effect of the incorporation of LFTM in the diet on the color parameters of the thigh meat was observed.
The drip losses were affected by the inclusion of the insect meal in the diet of broilers in both breast and thigh meat. In the breast there was significant linear response of this trait and its values increased with increasing the contents of the LFTM. The lowest drip loss (1.41%) was measured in the control group, while the breast of T7.5 and T10 exhibited the highest values of this trait (2.28% and 2.50% respectively). The same linear effect was observed in the drip loss of the thigh meat (P=0.0005).
The cooking losses in the breast increased linearly in response to the higher doses of LFTM (P=0.0058) and followed the pattern of the drip losses. Although a slight increase of the cooking loss was detected in the thigh meat of the groups fed higher doses of the insect meal, the effect was not significant.
3.2 Proximate composition
The effect of LFTM in the diet of the broilers on the proximate composition was different for the breast and the thigh meat. There was significant linear response of the protein and ash content of the breast meat towards the levels of LFTM (P<0.0001) (Table 4). The values of both components decreased with increasing of the meal content in the diet with highest values in the control group and lowest in T7.5 and T10. The intramuscular fat content (IMF) of the breast showed the opposite trend and increased linearly (P<0.0001) in the groups receiving higher doses of the low-fat meal. Similarly, the moisture content of the breast showed significant linear response (P=0.0006), increasing significantly in T7.5 and T10.
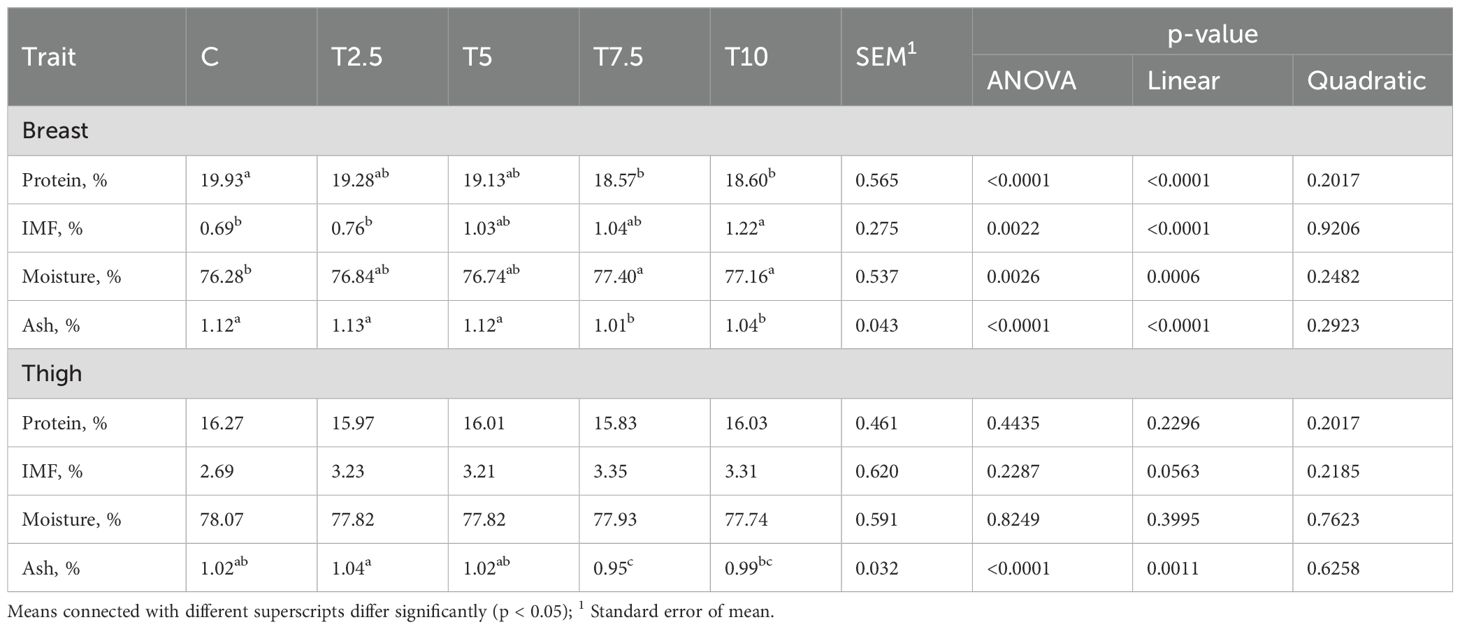
Table 4. Effect of LFTM in the diet on the proximate composition of breast and thigh meat in broilers.
In thighs, only the ash content of the meat was influenced by the insect meal in the diet of the broilers. Similar to the breast meat, in the meat of the thighs we observed linear response of the ash content (P=0.0011) that showed decrease with increasing of the insect meal levels (Table 4).
3.3 Fatty acid composition
The inclusion of the LFTM in the diet affected the fatty acids profile of the breast meat according to its percentage in the diet (Table 5).
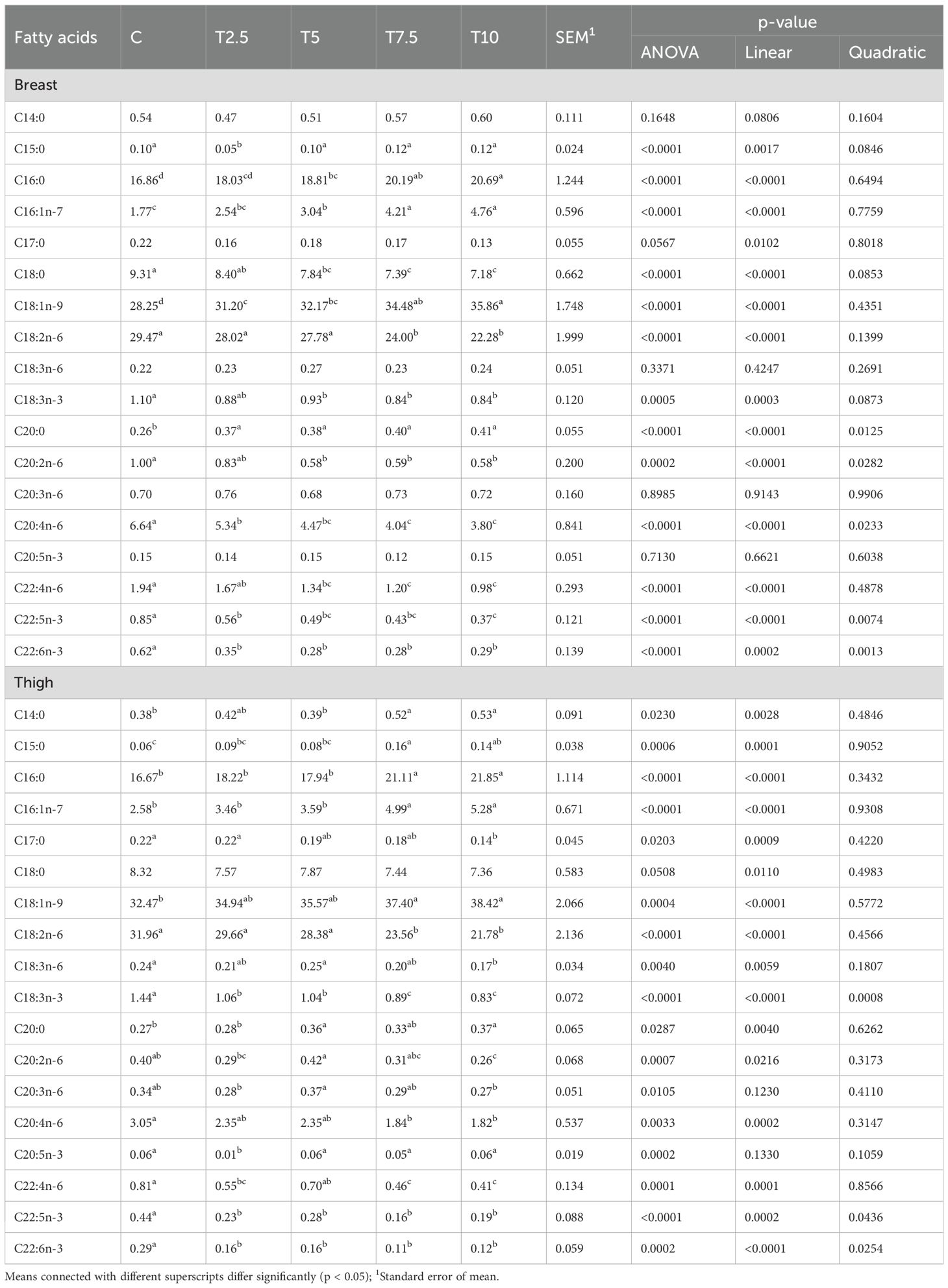
Table 5. Effect of LFTM in the diet on the fatty acid composition (%FAME) of breast and thigh meat in broilers.
While C14:0 in the breast remained unaffected, linear increase was observed in the percentage of C15:0 and C16:0 (P<0.0001). C20:0 increased linearly (P<0.0001) and quadratically (P=0.0125) with the higher levels of LFTM in the diet. On the other hand, significant linear decrease was found in the percentage of C17:0 (P=0.0102) and C18:0 (P<0.0001). The monounsaturated C16:1n-7 and C18:1n-9 in the beast meat showed significant linear increase with the higher content of LFTM in the diet (P<0.0001). The higher doses of LFTM in the diet were associated with linear decrease in C18:2n-6 (P<0.0001), C18:3n-3 (P=0.0003), C20:2n-6 (P<0.0001), C20:4n-6 (P<0.0001), C22:4n-6 (P<0.0001), C22:5n-3 (P<0.0001) and C22:6n-3 (P=0.0002). Quadratic response to the incorporation of LFTM in the diet was observed in C20:2n-6 (P=0.0282), C20:4n-6 (P=0.0233), C22:5n-3 (P=0.0074) and C 22:6n-3 (P=0.0013).
Similar to the breast meat, in thighs the effect of the incorporation of LFTM in the diet of the broiler was associated with linear increase in the percentage of C15:0 (P=0.0001), C16:0 (P<0.0001), C20:0 (P=0.0040) and also C14:0 (P=0.0028). Linear decrease was found in the percentage of C17:0 (P=0.0009) and C18:0 (P=0.0110). The contents of C16:1n-7 and C18:1n-9 were linearly increased (P<0.0001) with increase of LFTM showing maximum values in T7.5 and T10. The increase in the amount of LFTM in the diet lead to significant linear decline in the n-6 fatty acids including C18:2n-6 (P<0.0001), C18:3n-6 (P=0.0059), C20:2n-6 (P=0.0216), C20:4n-6 (P=0.0002) and C22:4n-6 (P=0.0001). Linear and quadratic response was observed in C18:3n-3 (P<0.0001 linear, P=0.0008 quadratic); C22:5n-3 (P=0.0002 linear, P=0.0436 quadratic) and C22:6n-3 (P<0.0001 linear, P=0,0254 quadratic), however all these fatty acids showed reduced content with the increase of LFTM in the diet.
Despite the different pattern of changes in the individual saturated fatty acids the total amount of SFA increased linearly in the breast (P=0.0012) and thigh meat (P<0.0001) following the increase of the levels of LFTM (Table 6). The total amount of MUFA showed the same linear response as the SFA in both meat cuts (P<0.0001) and both displayed considerably higher values in the T7.5 and T10 in comparison to the control, T2.5 and T5. The total amount of PUFA linearly decreased with the increase of TLFM in the diet (P<0.0001) and the minimal values of this indicator were found in the breast and thigh meat of T7.5 and T10.
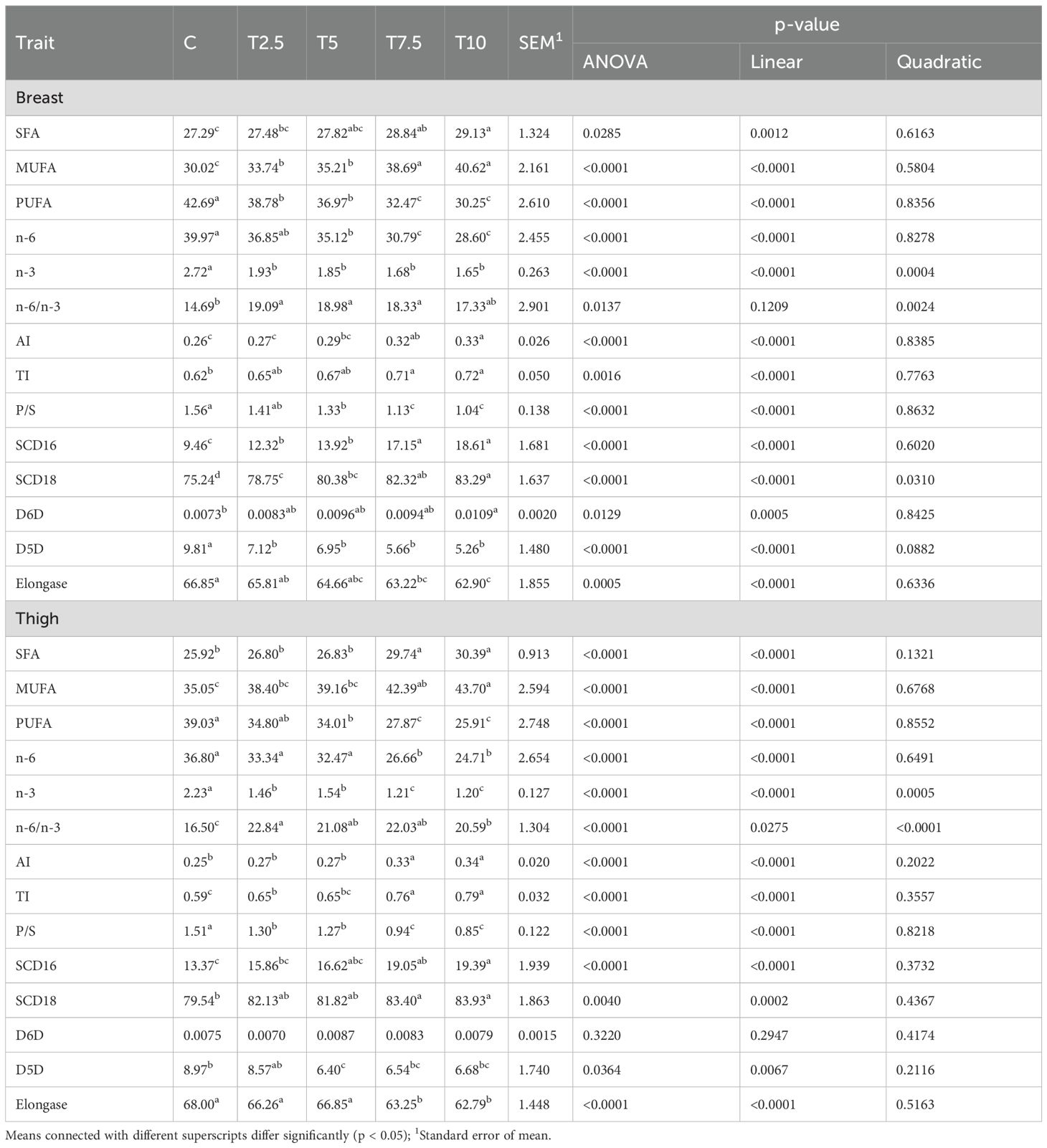
Table 6. Effect of LFTM in the diet on the total amounts of fatty acids and lipid nutritional indices of breast and thigh meat in broilers.
The incorporation of the LFTM in the diet of the broilers affected significantly SCD 16 (P<0.0001 linear) and SCD 18 (P<0.0001 linear, P= 0.0310 quadratic) in the breast, as both indices increased with the growing contents of the LFTM. The same pattern was observed in the thigh meat, where the desaturase indices increased linearly and showed considerably higher values in T7.5 and T10. On the other hand, the elongase index linearly decreased in both breast and thighs (P<0.0001) with increasing the dietary LFTM. The activity of D6D was affected by the LFTM only in breast showing linear increase in the experimental groups (P<0.0001). The D5D index decreased in all the groups receiving LFTM in both breast (P<0.0001) and thigh (P=0.0067).
With regard to the lipid nutritional indices of the meat in this study, we observed linear increase in AI and TI and decrease in P/S (P<0.0001). The n-6/n-3 ratio showed quadratic response to the incorporation of LFTM (P=0.0024) in the breast, however its values were lowest in the control group. In the thighs, this ratio responded both linearly (P=0.0275) and quadratically (P<0.0001), however it remained the lowest in the birds that did not receive LFTM.
4 Discussion
The results of this study showed that substantial effect of LFTM on the pH24, color and water holding capacity, depending on the dose might be expected in the breast meat rather than in thighs in broilers. pH is an important parameter closely related to the meat quality attributes and its shelf life (Mir et al., 2017) as well as the possibilities for further processing. The values of pH24 in the breast that were measured varied within 5.99-5.80 and fell in the “normal” range according to Zhang and Barbut (2005). On the other hand, the values of pH24 in the thigh meat varied within 6.18-6.27. Such values are due to the greater activity of these muscles before slaughter associated with lower glycogen content and hence lower production lactic acid (Kokoszyński et al., 2016). The values of pH24 in the breast decreased significantly with increasing the LFTM levels in the diet. In thighs, although not significant, the lowest pH24 was measured in T10. Similarly, Shaviklo et al. (2021) observed decrease in the pH of breast meat in broilers fed 1, 2 and 3% mealworm meal. Also, Selaledi et al. (2021) reported decreased pH in the meat of Boschveld indigenous chickens as a result of incorporation of 15% TM larvae. In contrast, Bovera et al. (2016) reported higher pH of the breast muscle in broilers when fed TM larvae in comparison to soybean meal. A possible reason for the linear decrease in the breast pH24 with the higher levels of LFTM that was observed here might be the significant linear decline in the breast weight that was reported in previous work (Petkov et al., 2024). The weight of the breast decreased with 26.58% for the group consuming 7.5 and 10% LFTM. Lower weight of the breast muscle is associated with higher glycolytic potential and hence lower pH24 (Le Bihan-Duval et al., 2008; Kaya et al., 2024). The lower pH in the meat of the groups fed LFTM resulted in paler color of the breast as indicated by the increase in L* and decrease in a* values. According to Anadon (2002), the lower pH of meat (≤ 6.0) is associated with higher extent of protein denaturation and hence the increased scattering and opaqueness of the meat. The effects of the insect meals on the meat color reported in the literature are contradictory. Selaledi et al. (2021) and Vasilopoulos et al. (2023) reported higher L* in broilers fed TM larvae, however, the latter also observed significant increase in the redness and yellowness. In contrast, Bovera et al. (2016); Elahi et al. (2020) and Dabbou et al. (2020) did not find any significant effect of TM meal on the color of broilers meat, while Parlar and Ustundag (2024) reported quadratic decrease in L* of the breast in broilers fed diet containing 25, 50, 75 and 100% TM as replacement of fish meal. With regard to meals derived from other insects, in a previous study (Popova et al., 2020) significant increase in the lightness of both breast and thigh meat in broilers fed partially defatted and full fat Hermetia illucens meal was found, while Schiavone et al. (2019) observed increased redness of the breast in broilers fed 5 to 15% defatted Hermetia illucens meal. On the other hand, no effect on the meat color was reported in slow growing chickens fed Acheta domesticus larval meal (Nieto et al., 2024). The higher lightness of the breast in the birds consumed LFTM also corresponded to the higher content of IMF observed in the experimental groups. Such relationship in broiler meat was demonstrated by Kokoszyński et al. (2025) who reported increased L* in breast meat with higher IMF content. The values of L* in this study fall within the normal range (56<L*<62) according to Lee et al. (2022). Thus the changes in the lightness of the meat due to the incorporation of the low-fat insect meal might not be of concern to the consumers and not adversely affect consumers’ perception.
Drip loss is an important parameter closely related to the water holding capacity of meat that affect both its technological qualities and sensory attributes. Drip and cooking losses in the breast meat increased in linear fashion with increasing the levels of LFTM in the diet of broilers corresponding to the changes in pH and color. Linear increase in the drip loss in the birds consuming higher levels of LFTM was also observed in thighs, indicating decrease in the water holding capacity of the meat. Linearly reduced water holding capacity due to inclusion of TM in the diet was observed in quails by Secci et al. (2022). The results of our study, however, were not consistent with the results of Parlar and Ustundag (2024), who reported better water holding capacity and reduced cooking loss in broilers with increasing the amounts of TML, and Zadeh et al. (2019) who demonstrated improved WHC in quails. On the other hand, Bovera et al. (2016) and Dabbou et al. (2020) did not find any effect of TM on the water retention capacity of meat in broilers. The values of drip loss in this study are within the range reported by Kralik et al. (2014) for normal broiler meat. The results indicate that the incorporation of LFTM does not have a negative effect on the water retention of the broiler meat in terms of cooking and drip loss. This is especially important for the latter, since excessive drip losses may be associated with significant economic loss for the industry.
With regard to the chemical composition of the meat, the incorporation of the insect meal in the diet of the broilers had more pronounced effect in the breast when compared to the thigh meat. In the breast meat, we observed lower protein content in all the groups consuming LFTM that decreased linearly with increasing levels of the insect meal. No such effect of the LFTM was found in the thigh meat. On the other hand, the groups fed LFTM showed increased IMF content and moisture in the breast. This corresponded to the lower protein content and is indicator of dystrophic processes due to impaired protein utilization in the diets containing LFTM in amounts higher than 5%. Similarly, Parlar and Ustundag (2024) reported linear decrease of the meat protein content in the birds with increasing levels of TM meal in the diet. However, the authors demonstrated also decrease in the fat content which is not consistent with our findings. Contrary to our results, Vasilopoulos et al. (2023) observed stronger effect of the TM on the chemical composition of the thighs in broilers, and reported increased protein but decreased fat content of the meat. The results here corresponded to the changes in the growth performance and carcass parameters that were reported recently for the same experimental birds (Petkov et al., 2024). In this work, we found linear decrease in the weight of the broilers as well as the breast yield, while no such changes were registered for the thighs. This indicated a possible negative effect of the insect meal, especially in higher doses, on the protein utilization and breast muscle deposition that might be associated with the lower protein content in the meat. The ash content decreased linearly in both breast and thigh meat, especially in T7.5 and T10. Shaviklo et al. (2021) reported different pattern of change in the ash content depending on the dose and also on the age of slaughter. In their experiment involving broilers fed 24 days with mealworm meal they found lower ash in the groups receiving insect meal. However, in another experiment the ash of the experimental groups increased after shifting to normal feeding. The decline in the ash content observed in this study suggests that high doses of the low-fat insect meal might induce impaired absorption of the dietary minerals in the broilers.
The fatty acid composition of meat is an important characteristic associated with its dietetic and healthy quality. In this study, the incorporation of LFTM in the diet of the broilers affected considerably the fatty acid profile of the breast and thigh meat, however it did not reflect entirely the fatty acid profile of the diets. Both individual and total saturated fatty acids in the breast and thigh responded in a similar way in to the increase of the dietary levels of LFTM in the diet. With exception to the C17:0 and C18:0, that decreased considerably in the groups fed higher levels of LFTM, the other saturated fatty acids increased with the higher amount of LFTM. Biasato et al. (2025) when comparing the effect of different doses of partially defatted meals derived of Hermetia illucens and Tenebrio molitor as well as mix between both in the diet of poultry found that C14:0 remained unaffected while C16:0 increased in the in the breast of the group fed 10% TM compared to 5%. The authors also reported increased content of total SFA in the group receiving higher amount of TM which is in agreement with the results of this study. Loponte et al. (2018) reported significant increase in C12:0 and C14:0 in the breast of broilers receiving TML meal compared to soybean meal. In line with the results of the present experiment, Dabbou et al. (2020) reported increased content of C14:0 in the thighs of free range chickens fed diet containing TM meal. The authors however found significant decrease in C16:0 and SFA in the breast that contradicts to our results. Since many studies report the hypercholesterolemic effect of C14:0 and C16:0, the increase of their content in the groups fed higher levels of LFTM indicates certain negative effect on the healthy value of the meat, especially in the groups fed 7.5 and 10%. On the other hand, the total content of MUFA increased linearly in both breast and thigh with increasing the levels of LFTM in the diet of the birds. This increase was determined by the amounts of C16:1n-7 and C18:1n-9 that raised in the groups receiving higher percentage of the insect meal. The same effect was observed by Biasato et al. (2025). The increase of the MUFA in the meat of the broilers fed higher amounts of LFTM corresponds to the increase in the SCD16 and SC18 indices and also to the decreased amounts of PUFA. This can be associated with higher activity of Stearoyl-CoA desaturase, in conditions of low levels of PUFA. According to Choi et al. (2001), SCD expression can be considerably suppressed by C18:2n-6, C18:3n-3 and C20:4n-6. Furthermore, as it has been shown by Lefevr et al. (2001), that in chickens SCD is up-regulated by low-fat high-carbohydrate diets, and is down-regulated by the addition of dietary PUFA.
The most abundant PUFA in the chicken meat is C18:2n-6. In this study its linear decrease in the meat despite its increasing levels in the broilers’ diets was observed. The same was found in regard to the C18:3n-3. Both fatty acids are essential and derived exclusively from the diet. These fatty acids are also precursors for the synthesis of long chain n-6 and n-3 PUFA and their lower amounts corresponded with the decreased percentages of the other PUFA as well as the total amount of the PUFA. Similarly, decrease in the levels of C18:2n-6, C18:3n-3 and total PUFA in the meat of broilers fed 10% TM in comparison to the group receiving 5% were reported (Biasato et al., 2025). The opposite was observed by Dabbou et al. (2020), who found increased content of C18:3n-3 and total amount of n-3 fatty acids in the breast of free-range chickens fed diet containing TM meal. No effect of full fat TM meal in comparison to soybean meal was found in the breast meat of broilers (Loponte et al., 2018). The higher D6D corresponded to the lower percentage of its substrate C18:2n-6 in the breast of the groups consuming LFTM, while no such changes were observed in the thighs. On the other hand, the activity of D5D showed marked decrease in both breast and thighs in the chicken that received the insect meal. The higher values of D5D and D6D mean better conversion of the precursors and synthesis of long chain fatty acids. The lower percentage of the individual PUFA and particularly C20:4n-6 in our research found in the groups receiving LFTM corresponded to the increased content of C18:1. Høstmark and Haug (2014) demonstrated a strong inverse relationship between the percentage of C18:1 and C20:4n-6 in chicken breast. According to these authors, C18:1 acts as an inhibitor for the Δ-5 and Δ-6 desaturase and/or 5-elongase systems, while at the same time C20:4n-6 might inhibit the SCD. The decreasing percentage of the PUFA in the meat of the birds receiving the insect meat can also be associated with their higher IMF content. Such relationship was observed by Sirri et al. (2010), in different chicken genotypes. Low relative content in PUFA in animals with higher IMF is due to the higher percentage of triacylglycerols and low of phospholipids, as the latter present high content of PUFA (Bartoň et al., 2008).
The changes of the fatty acids due to the inclusion of the LFTM in the diet affected the lipid nutritional indices. Both AI and TI increased with increasing the percentage of LFTM. The values of AI varied between 0.26-0.33 for the breast and 0.25-0.34 for the high meat. The TI index had values within the range of 0.62-0.72 for the breast and 0.59-0.79 for the thighs. The values of both indices were lower than those reported in a study of Dal Bosco et al. (2024), for breast from slow and fast-growing broilers and within the range reported by Ciobanu et al. (2019). This indicated that despite the adverse effect of the increasing level of LFTM on the hypercholesterolemic C14:0 and C16:0, they do not impair considerably the healthy quality of the chicken meat in this study.
The ratio between n-6 and n-3 PUFA ranged within 14.69-19.09 for the breast and 16.50-22.84 for the thigh meat. In contrast to the AI and TI, its values are high regardless of the incorporation of LFTM and considerably exceed the recommended value of 4 (Simopoulos, 2002). These values can be attributed to the high levels of C18:2n-6 that is abundant in the broilers meat and fall within the range reported in other studies (Untea et al., 2022). The values of the n-6/n-3 ratio found in this study are indicator for a certain imbalance of the fatty acid profile of meat of the broilers and suggest the need of further studies in order to adapt feeding strategies that will help achieve a more favorable profile in regard to the polyunsaturated fatty acids.
5 Conclusions
The study provides new data and insight on the possible use of low-fat Tenebrio molitor meal in the diet of broilers concerning the quality attributes of meat. With regard to the physical properties and chemical composition, the results indicated that the effect of the incorporation of the LFTM was stronger in breast meat and depended on the dose. Although it could not be considered as adverse, it was associated with lower pH24, paler meat, increased drip and cooking loss, lower protein and higher fat of the meat that changed linearly as the levels of the insect meal increased. In regard to the fatty acid profile, the increase of the doses of LFTM produced higher amount of SFA and MUFA, while lowered PUFA which was associated with higher atherogenic and thrombogenic indices, as well as n-6/n-3 ratio. The results suggest that amounts up to 5% LFTM might be appropriate for broilers diets. However, further research will be necessary to formulate feeding strategies for incorporation of the LFTM that will not negatively affect the qualities of broiler meat.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by Bulgarian Animal Ethics Committee, prot. No.18/02.07.2020. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
TP: Conceptualization, Formal analysis, Investigation, Methodology, Writing – original draft. EP: Conceptualization, Investigation, Resources, Writing – review & editing. DV-V: Conceptualization, Investigation, Resources, Project administration, Funding Acquisition, Writing – review & editing. NK: Investigation, Data curation, Formal analysis, Writing – review & editing. DB: Conceptualization, Investigation, Resources, Project administration, Writing – review & editing. SD: Conceptualization, Methodology, Investigation, Writing – review & editing. KD: Investigation, Formal analysis, Data curation, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was funded by Bulgarian National Science Fund, Ministry of Education and Science in Bulgaria (Grant number KP-06-N76/7, 5 December 2023).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Anadon H. L. S. (2002). Biological, nutritional, and processing factors affecting breast meat quality of broilers (Blacksburg, VA: Virginia Polytechnic Institute and State University).
AOAC (2004). Official Methods of Analysis. 18th ed (Arlington, VA, USA: Association of Official Analytical Chemists).
Ross Broiler Management Handbook. (2018) Available online at: https://aviagen.com/assets/Tech_Center/Ross_Broiler/Ross-BroilerHandbook2018-EN.pdf (Accessed August 20, 2024).
Baldi G., Soglia F., and Petracci M. (2020). Current status of poultry meat abnormalities. Meat Muscle Biol. 4, 1–7. doi: 10.22175/mmb.9503
Bartoň L., Marounek M., Kudrna V., Bures D., and Zahradkova R. (2008). Growth, carcass traits, chemical composition and fatty acid profile in beef from Charolais and Simmental bulls fed different types of dietary lipids. J. Sci. Food Agric. 88, 2622–2630. doi: 10.1002/jsfa.3381
Berri C., Besnard J., and Relandeau C. (2008). Increasing dietary lysine increases final pH and decreases drip loss of broiler breast meat. Poult. Sci. 87, 480–484. doi: 10.3382/ps.2007-00226
Biasato I., Gariglio M., Bongiorno V., Fiorilla E., Cappone E. E., Oddon S. B., et al. (2025). Can a mixture of Hermetia illucens and Tenebrio molitor meals be feasible to feed broiler chickens? A focus on bird productive performance, nutrient digestibility, and meat quality. Poultry Sci. 104, 105150. doi: 10.1016/j.psj.2025.105150
Biasato I., Gasco L., De Marco M., Renna M., Rotolo L., Dabbou S., et al. (2018). Yellow mealworm larvae (Tenebrio molitor) inclusion in diets for male broiler chickens: effects on growth performance, gut morphology, and histological findings. Poultry Sci. 97, 540–548. doi: 10.3382/ps/pex308
Bligh E. G. and Dyer W. Y. (1959). A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917. doi: 10.1139/y59-099
Bordoni A. and Danesi F. (2017). “Chapter 11 - Poultry meat nutritive value and human health,” in Woodhead Publishing Series in Food Science, Technology and Nutrition, Poultry Quality Evaluation. Eds. Petracci M. and Berri C. (Woodhead Publishing), 279–290.
Bovera F., Loponte R., Marono S., Piccolo G., Parisi G., Iaconisi V., et al. (2016). Use of Tenebrio molitor larvae meal as protein source in broiler diet: Effect on growth performance, nutrient digestibility, and carcass and meat traits. J. Anim. Sci. 94, 639–647. doi: 10.2527/jas.2015-9201
Choi J., Kong B., Bowker B. C., Zhuang H., and Kim W. K. (2023). Nutritional strategies to improve meat quality and composition in the challenging conditions of broiler production: a review. Animals 13, 1386. doi: 10.3390/ani13081386
Choi Y. J., Prak Y. H., Michael W. P., and Ntambi J. M. (2001). Regulation of stearoyl-CoA desaturase activity by the trans-10, cis-12 isomer of conjugated linoleic acid in HepG2 cells. Biochem. Biophys. Res. Commun. 284, 689–693. doi: 10.1006/bbrc.2001.5036
Ciobanu M. M., Boisteanu P. C., Simeanu D., Postolache A. N., Lazar R., and Vintu C. R. (2019). Study on the profile of fatty acids of broiler chicken raised and slaughtered in industrial system. Rev. Chim. 70, 4089–4094. doi: 10.37358/Rev.Chim.1949
Dabbou S., Gasco L., Lussiana C., Brugiapaglia A., Biasato I., Renna M., et al. (2020). Yellow mealworm (Tenebrio molitor L.) larvae inclusion in diets for free-range chickens: effects on meat quality and fatty acid pro-file. Renew. Agr. Food Syst. 35, 571–578. doi: 10.1017/S1742170519000206
Dal Bosco A., Cavallo M., Menchetti L., Angelucci E., Cartoni Mancinelli A., Vaudo G., et al. (2024). The healthy fatty index allows for deeper insights into the lipid composition of foods of animal origin when compared with the atherogenic and thrombogenicity indexes. Foods 13, 1568. doi: 10.3390/foods13101568
Dalle Zotte A., Singh Y., Zsedely E., Contiero B., Palumbo B., and Cullere M. (2024). Dietary inclusion of defatted silkworm (Bombyx mori L.) pupa meal in broiler chickens: phase feeding effects on nutritional and sensory meat quality. Poult. Sci. 103, 103812. doi: 10.1016/j.psj.2024.103812
Domínguez R., Crecente S., Borrajo P., Agregan R., and Lorenzo J. M. (2015). Effect of slaughter age on foal carcass traits and meat quality. Animal 9, 1713–1720. doi: 10.1017/S1751731115000671
Echegaray N., Rosmini M., Pateiro M., Domìnguez R., Munekata P. E. S., Lorenzo J. M., et al. (2022). “Texture analysis,” in Methods to assess the quality of meat products. Eds. Lorenzo J. M., Domìnguez R., Pateiro M., and Munekata P. E. S. (Humana, New York, NY, USA), 29–40.
Elahi U., Wang J., Ma Y.-B., Wu S.-G., Wu J., Qi G.-H., et al. (2020). Evaluation of yellow mealworm meal as a protein feedstuff in the diet of broiler chicks. Animals 10, 224. doi: 10.3390/ani10020224
Fletcher D. L. (2002). Poultry meat quality. World’s Poult. Sci. J. 58, 131–145. doi: 10.1079/WPS20020013
Gasco L., Renna M., Bellezza Oddon S., Rezaei Far A., Naser El Deen S., and Veldkamp T. (2023). Insect meals in a circular economy and applications in monogastric diets. Anim. Front. 13, 81–90. doi: 10.1093/af/vfad016
Grau T., Vilcinskas A., and Joop G. (2017). Sustainable farming of the mealworm Tenebrio molitor for the production of food and feed. Z. für Naturforschung C 72, 337–349. doi: 10.1515/znc-2017-003
Green C. D., Ozguden-Akkoc C. G., Wang Y., Jump D. B., and Olson L. K. (2010). Role of fatty acid elongases in determination of de novo synthesized monounsaturated fatty acid species. J. Lipid Res. 51, 1871–1877. doi: 10.1194/jlr.M004747
Gross-Bošković A., Medić H., Pleadin J., Janječić Z., Šperanda M., Rebekić A., et al. (2024). Effect of Black Soldier Fly (Hermetia illucens) defatted flour on technological properties and quality of chicken meat. J. Cent. Eur. Agric. 25, 55–69. doi: 10.5513/JCEA01/25.1.4115
Haug A., Nyquist N. F., Thomassen M., Høstmark A. T., and Østbyeet T.-K. (2014). N-3 fatty acid intake altered fat content and fatty acid distribution in chicken breast muscle, but did not influence mRNA expression of lipid-related enzymes. Lipids Health Dis. 13, 92. doi: 10.1186/1476-511X-13-92
Heidari-Parsa S., Imani S., Fathipour Y., Kheiri F., and Chamani M. (2018). Determination of yellow mealworm (Tenebrio molitor) nutritional value as an animal and human food supplementation. Arthropods 7, 94–102.
Honikel K. O. (1998). Reference methods for the assessment of physical characteristics of meat. Meat Sci. 49, 447–457. doi: 10.1016/S0309-1740(98)00034-5
Høstmark A. T. and Haug A. (2014). The inverse association between relative abundancies of oleic and arachidonic acid is relate of alfa-linolenic acid. Lipids Health Dis. 13, 76. doi: 10.1186/1476-511X-13-76
Jlali M., Gigaud V., Métayer-Coustard S., Sellier N., Tesseraud S., Le Bihan-Duval E., et al. (2012). Modulation of glycogen and breast meat processing ability by nutrition in chickens: effect of crude protein level in 2 chicken genotypes. J. Anim. Sci. 90, 447–455. doi: 10.2527/jas.2011-4405
Kaya M., Karaarslan S., Oral Toplu H. D., Dereli Fidan E., Türkyilmaz K. M., and Nazligül A. (2024). Growth performance, carcass, and meat quality traits in broiler chickens reared on plastic-grid flooring, wood shavings, and zeolite-supplemented wood shavings. Trop. Anim. Health Prod. 56, 66. doi: 10.1007/s11250-024-03915-1
Kim S. G., Kim J. E., Oh H. K., Kang S. J., Koo H. Y., Kim H. J., et al. (2014). Feed supplementation of Yellow Mealworms (Tenebrio molitor L.). improves blood characteristics and meat quality in broiler. J. Agric. Sci. Technol. 49, 9–18. doi: 10.29335/tals.2014.49.9
Kokoszyński D., Bernacki Z., Stęczny K., Saleh M., Wasilewski P. D., Kotowicz M., et al. (2016). Comparison of carcass composition, physicochemical and sensory traits of meat from spent broiler breeders with broilers. Europ. Poult. Sci. 80, 1–11. doi: 10.1399/eps.2016.131
Kokoszyński D., Włodarczyk K., Żochowska-Kujawska J., Kotowicz M., Wegner M., Stęczny K., et al. (2025). Effect of intramuscular fat level on carcass composition, physicochemical characteristics, texture, and microstructure of breast muscle of broiler chickens. Poult. Sci. 104, 104772. doi: 10.1016/j.psj.2025.104772
Kralik G., Djurkin I., Kralik Z., Skrtic Z., and Radisic Z. (2014). Quality indicators of broiler breast meat in relation to colour. Anim. Sci. Paper Rep. 32, 173–178.
Le Bihan-Duval E., Debut M., Berri C. M., Sellier N., Santé-Lhoutellier V., Jégo Y., et al. (2008). Chicken meat quality: genetic variability and relationship with growth and muscle characteristics. BMC Genet. 9, 53. doi: 10.1186/1471-2156-9-53
Lee S.-K., Chon J.-W., Yun Y.-K., Lee J.-C., Jo C., Song K.-Y., et al. (2022). Properties of broiler breast meat with pale colour and a new approach for evaluating meat freshness in poultry processing plants. Poult. Sci. 101, 101627. doi: 10.1016/j.psj.2021.101627
Lefevr P., Tripon E., Plumelet C., Douaire M., and Diot C. (2001). Effects of polyunsaturated fatty acids and clofibrate on chicken Stearoyl-CoA Desaturase 1 gene expression. Biochem. Biophys. Res. Commun. 280, 25–31. doi: 10.1006/bbrc.2000.4070
Loponte R., Bovera F., Piccolo G., Gasco L., Secci G., Iaconisi V., et al. (2018). Fatty acid profile of lipids and caeca volatile fatty acid production of broilers fed a full fat meal from Tenebrio molitor larvae. It. J. Anim. Sci. 18, 168–173. doi: 10.1080/1828051X.2018.1502053
Malematja E., Manyelo T. G., Sebola N. A., and Mabelebele M. (2023). The role of insects in promoting the health and gut status of poultry. Comp. Clin. Path. 32, 501–513. doi: 10.1007/s00580-023-03447-4
Mir N. A., Rafiq A., Kumar F., Singh V., and Shukla V. (2017). Determinants of broiler chicken meat quality and factors affecting them: a review. J. Food Sci. Technol. 54, 2997–3009. doi: 10.1007/s13197-017-2789-z
Nieto J., Plaza J., Hernández-Jiménez M., Revilla I., and Palacios C. (2024). Substitution of soybean meal for yellow mealworm meal in the diet of slow-growing chickens provides comparable carcass traits and meat quality. Br. Poult. Sci. 65, 730–739. doi: 10.1080/00071668.2024.2369671
Nieto J., Plaza J., Lara J., Abecia J.-A., Revilla I., and Palacios C. (2022). Performance of slow-growing chickens fed with Tenebrio molitor larval meal as a full replacement for soybean meal. Vet. Sci. 9, 131. doi: 10.3390/vetsci9030131
Parlar M. and Ustundag A. O. (2024). Effects of adding mealworm (Tenebrio molitor L.) as a replacement for fish meal to broiler chicken diet on performance, carcass parameters, meat quality and nutrient digestibility. Trop. Anim. Health Prod. 56, 386. doi: 10.1007/s11250-024-04230-5
Petkov E., Popova T., Dimov K., Vlahova-Vangelova D., Balev D., Kolev N., et al. (2024). Low-fat Tenebrio molitor meal as a component in the broiler diet: growth performance and carcass composition. Insects 15, 979. doi: 10.3390/insects15120979
Petracci M., Soglia F., Madruga M., Carvalho L., Ida Е., and Estévez М. (2019). Wooden-breast, white striping, and spaghetti meat: causes, consequences and consumer perception of emerging broiler meat abnormalities. Compr. Rev. Food Sci. Food Saf. 18, 565–583. doi: 10.1111/1541-4337.12431
Popova T. L., Petkov E., and Ignatova M. (2020). Effect of Black Soldier Fly (Hermetia illucens) meals on the meat quality in broilers. Agric. Food Sci. 29, 177–188. doi: 10.23986/afsci.88098
Renna M., Rastello L., Veldkamp T., Toral P. G., Gonzalez-Ronquillo M., Jimenez L. E. R., et al. (2023). Are insects a solution for feeding ruminants? Legislation, scientific evidence, and future challenges. Anim. Front. 13, 102–111. doi: 10.1093/af/vfad026
Schiavone A., Dabbou S., Petracci M., Zampiga M., Sirri F., Biasato I., et al. (2019). Black soldier fly defatted meal as a dietary protein source for broiler chickens: effects on carcass traits, breast meat quality and safety. Animal 13, 2397–2405. doi: 10.1017/S1751731119000685
Secci G., Dabbou S., Lira de Medeiros A. C., Addeo N. F., Atallah E., Parisi G., et al. (2022). Low dietary inclusion levels of Tenebrio molitor larva meal slightly modify growth performance, carcass and meat traits of Japanese quail (Coturnix japonica). J. Sci. Food Agric. 102, 6578–6585. doi: 10.1002/jsfa.12023
Selaledi L., Baloyi J., Mbajiorgu C., Sebola A. N., Kock H. D., and Mabelebele M. (2021). Meat quality parameters of Boschveld indigenous chickens as influenced by dietary yellow mealworm meal. Foods 10, 3094. doi: 10.3390/foods10123094
Shaviklo A. R., Alizadeh-Ghamsari A. H., and Hosseini S. A. (2021). Sensory attributes and meat quality of broiler chickens fed with mealworm (Tenebrio molitor). J. Food Sci. Technol. 58, 4587–4597. doi: 10.1007/s13197-020-04946-w
Simopoulos A. P. (2002). The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 56, 365–379. doi: 10.1016/S0753-3322(02)00253-6
Sirri F., Castellini C., Roncarati A., Franchini A., and Meluzzi A. (2010). Effect of feeding and genotype on the lipid profile of organic chicken meat. Eur. J. Lipid Sci. Technol. 112, 994–1002. doi: 10.1002/ejlt.200900204
Solecka K. and Drazbo A. (2024). The use of insects in poultry nutrition – a review. Anim. Sci. Genet. 20, 23–36. doi: 10.5604/01.3001.0054.8389
Soriano-Santos J. (2010). “Chemical composition and nutritional content of raw poultry meat,” in Handbook of Poultry Science and Technology, Volume 1: Primary Processing. Eds. Guerrero-Legarreta I. and Hui Y. H. (John Wiley & Sons, Inc), 467–489.
Šťastník O., Novotný J., Roztočilová A., Jůzl M., Piechowiczová M., Kouřil P., et al. (2021). The effect of yellow mealworm larvae meal supplementation in broiler diets on meat quality. Acta Vet. Brno 90, 349–356. doi: 10.2754/avb202190030349
Swatland H. J. (1989). Objective measurements of physical aspects of meat quality. Reciprocal Meat Conf. Proc. 42, 65–74.
Ulbricht T. L. and Southgate D. A. T. (1991). Coronary heart disease: Seven dietary factors. Lancet 338, 985–992. doi: 10.1016/0140-6736(91)91846-M
Untea A. E., Turcu R. P., Saracila M., Vlaicu P. A., Panaite T. D., and Oancea A. G. (2022). Broiler meat fatty acids composition, lipid metabolism, and oxidative stability parameters as affected by cranberry leaves and walnut meal supplemented diets. Sci. Rep. 12, 21618. doi: 10.1038/s41598-022-25866-z
Van Huis A. (2022). Edible insects: challenges and prospects. Entomol. Res. 52, 161–177. doi: 10.1111/1748-5967.12582
Vargas-Ramella M., Pateiro M., Barba F. J., Franco D., Campagnol P. C. B., Munekata P. E. S., et al. (2020). Microencapsulation of healthier oils to enhance the physicochemical and nutritional properties of deer páté. LWT Food Sci. Technol. 125, 109223. doi: 10.1016/j.lwt.2020.109223
Vasilopoulos S., Giannenas I., Savvidou S., Bonos E., Rumbos C. I., Papadopoulos E., et al. (2023). Growth performance, welfare traits and meat characteristics of broilers fed diets partly replaced with whole Tenebrio molitor larvae. Anim. Nutr. 13, 90–100. doi: 10.1016/j.aninu.2022.12.002
Veldkamp T., Meijer N., Alleweldt F., Deruytter D., Van Campenhout L., Gasco L., et al. (2022). Overcoming technical and market barriers to enable sustainable large-scale production and consumption of insect proteins in Europe: A SUSINCHAIN Perspective. Insects 13, 281. doi: 10.3390/insects13030281
Wang C., Che S., Susta L., and Barbut S. (2023). Textural and physical properties of breast fillets with myopathies (wooden breast, white striping, spaghetti meat) in Canadian fast-growing broiler chickens. Poult. Sci. 2, 102309. doi: 10.1016/j.psj.2022.102309
Wu T., Liu P., Wu J., Jiang Y., Zhou N., Zhang Y., et al. (2024). Broiler spaghetti meat abnormalities: muscle characteristics and metabolomic profiles. Animals 14, 1236. doi: 10.3390/ani14081236
Zadeh Z. S., Kheiri F., and Faghani M. (2019). Use of yellow mealworm (Tenebrio molitor) as a protein source on growth performance, carcass traits, meat quality and intestinal morphology of Japanese quails (Coturnix japonica). Vet. Anim. Sci. 8, 100066. doi: 10.1016/j.vas.2019.100066
Zhang L. and Barbut S. (2005). Rheological characteristics of fresh and frozen PSE, normal and DFD chicken breast meat. Br. Poult. Sci. 46, 687–693. doi: 10.1080/00071660500391516
Zhang S., Knight T. J., Stalder K. J., Goodwin R. N., Lonergan S. M., and Beitz D. C. (2007). Effects of breed, sex, and halothane genotype on fatty acid composition of pork longissimus muscle. J. Anim. Sci. 85, 583–591. doi: 10.2527/jas.2006-239
Zhao J. P., Zhao G. P., Jiang R. R., Zheng M. Q., Chen J. L., Liu R. R., et al. (2012). Effects of diet-induced differences in growth rate on metabolic, histological, and meat-quality properties of 2 muscles in male chickens of 2 distinct broiler breeds. Poult. Sci. 91, 237–247. doi: 10.3382/ps.2011-01667
Keywords: low-fat Tenebrio molitor meal, broilers, meat quality, fatty acids, lipid nutritional indices
Citation: Popova T, Petkov E, Vlahova-Vangelova D, Kolev N, Balev D, Dragoev S and Dimov K (2025) Meat quality and fatty acid profile in broilers as affected by low-fat Tenebrio molitor meal in the diet. Front. Anim. Sci. 6:1629411. doi: 10.3389/fanim.2025.1629411
Received: 15 May 2025; Accepted: 31 July 2025;
Published: 22 August 2025.
Edited by:
Samiru Sudharaka Wickramasuriya, Agricultural Research Service (USDA), United StatesReviewed by:
Arda Yıldırım, Gaziosmanpaşa University, TürkiyeQianqian Zhang, Dankook University, Republic of Korea
Anca Gheorghe, Research Station of Sericulture Baneasa Bucharest, Romania
Copyright © 2025 Popova, Petkov, Vlahova-Vangelova, Kolev, Balev, Dragoev and Dimov. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Teodora Popova, dF9wb3BvdmFAaWFzLmJn
 Teodora Popova
Teodora Popova Evgeni Petkov1
Evgeni Petkov1