- Department of Animal Science, Iowa State University, Ames, IA, United States
This study examined the role of Zn status on muscle glucose and other metabolites. Angus steers (144; 525 ± 30 kg) with varying plasma Zn concentrations and implant status were used for this secondary experiment. Steers were assigned to implant (IMP) treatments: no implant (NO) or Component TE-200 (TE-200; Elanco, Greenfield, IN) on d 0. Zinc sulfate was supplemented at 0 (analyzed 54 mg Zn/kg DM), 30, or 100 mg Zn/kg DM starting d -60. Steers were fed in two blocks via GrowSafe bunks, and steer was the experimental unit. Jugular blood and longissimus thoracis biopsies were collected d 40 post-implant. Plasma Zn was quantified via ICP-OES and stratified into quintiles by concentration and IMP treatment. Samples (n = 48; 12 low and 12 high from each IMP group) were identified and designated to plasma Zn treatments (PLZN): low (LO, 1.1 mg Zn/L) or high (HI, 1.6 mg Zn/L). Corresponding muscle samples were analyzed via gas chromatography-mass spectrometry for non-targeted metabolomics. Data were analyzed using ProcMixed of SAS with fixed effects of PLZN, IMP, BLOCK, and PLZN×IMP. No interactions were noted. β-alanine, 3-hydroxybutyric acid, and glycine were greater in HI than LO (P ≤ 0.05), while 3-hydroxybutyric acid, 2,3,4-trihydroxybutyric acid, and glycine were greater in TE200 than NO (P ≤ 0.03). Lactic and malic acids tended to be greater in TE200 than NO (P ≤ 0.10). Although both Zn groups were adequate, greater plasma Zn altered metabolites indicative of enhanced energy metabolism, potentially explaining benefits of Zn supplementation to feedlot cattle.
1 Introduction
Zinc is an essential trace mineral crucial to whole-body growth. It is a cofactor to over 300 enzymes, a component of many transcription factors, and is implicated in nearly every signaling pathway in higher organisms (Beyersmann and Haase, 2001; Cousins et al., 2006). The current requirement for Zn is 30 mg Zn/kg dry matter (DM) and has remained unchanged for four decades (NASEM, 2016). However, consulting nutritionists often supplement at concentrations as high as 300% of the requirement (Samuelson et al., 2016). This may be to accommodate the 44% increase in average daily gain (ADG) of beef cattle between 1977 and 2007 (Capper, 2011). While the required Zn concentration prevents deficiency, modern feedlot cattle may need greater Zn to support growth.
In implanted cattle, we have observed improved growth when supplementing up to 150 mg Zn/kg DM (Messersmith et al., 2022; Messersmith and Hansen, 2024) but the mechanisms are not entirely understood. Several of our studies have noted decreased plasma Zn concentration in implanted cattle, which is often overcome by Zn supplementation, suggesting circulating Zn may be important in the Zn-induced growth response (Messersmith and Hansen, 2021; Messersmith et al., 2022; Smerchek et al., 2024; Messersmith and Hansen, 2024). In the prior study, growth performance was not influenced by supplemental Zn, potentially driven by high plasma Zn concentrations across dietary treatments and limited growth potential noted in the steers. However, Zn impacted circulating glucose and insulin, corresponding with increased d 20 skeletal muscle mRNA abundance of GLUT4 in implanted steers, implicating altered muscle energy demand (Smerchek et al., 2024).
In this study, like others who have examined extreme populations (Russell et al., 2016; Carlson et al., 2017), we analyzed selected highs and lows in plasma Zn within implanted and non-implanted steers to examine the muscle metabolome. We hypothesized that implanted steers and steers with greater plasma Zn concentration would have greater concentrations of muscle metabolites related to growth and energy metabolism.
2 Methods
All procedures and protocols were approved by the Iowa State University Institutional Animal Care and Use Committee (IACUC-20-127).
2.1 Animals and experimental design
This study utilized samples from a subset of a larger study (Smerchek et al., 2024). Briefly, 144 single-source Angus-cross steers (525 ± 30 kg) were used in a 2 × 3 randomized design with steers blocked by body weight (BW) to one of two blocks to accommodate sampling logistics and assigned to one of two implant treatments: no implant or Component TE-200 (200 mg trenbolone acetate + 20 mg estradiol; Elanco Animal Health, Greenfield, IN) on d 0. Zinc was supplemented as ZnSO4 at 0 mg Zn/kg DM (analyzed 53 mg Zn/kg DM), 30 mg Zn/kg DM (CON + 30 mg Zn/kg DM), or 100 mg Zn/kg DM (CON + 100 mg Zn/kg DM), starting 60 d prior to implant. Steers were stratified by BW into pens (n = 6 steers/pen) equipped with GrowSafe bunks (GrowSafe Systems Ltd., Airdrie, AB, Canada) to determine individual feed disappearance and steer was the experimental unit. Steers were fed a dry-rolled corn-based diet ad libitum delivered daily at 0800 h (45% dry-rolled corn, 20% Sweet Bran, 10% DDGS, 15% corn silage, and 5% basal premix on a dry matter basis), and Zn treatments were delivered via premix utilizing dry distillers grains plus solubles as a carrier at 5% diet DM.
2.2 Sample collection and analysis
Blood and longissimus thoracis (LT) muscle samples for this experiment were collected 40 days after terminal implant (Smerchek et al., 2024), approximately aligned with peak hormone payout from uncoated implant pellets (Parr et al., 2014).
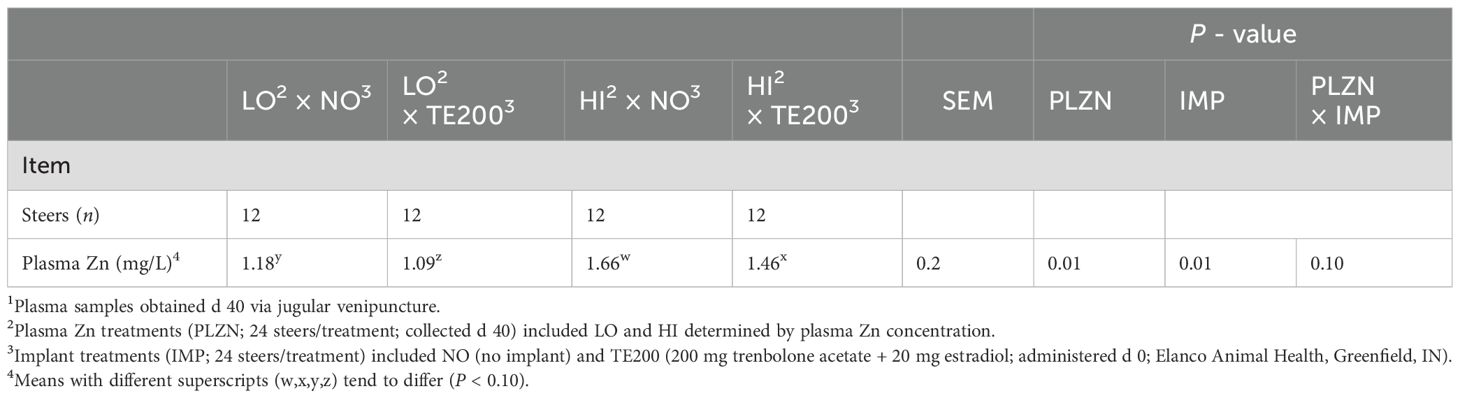
Trace mineral concentration of plasma samples was measured using inductively coupled plasma optical emission microscopy (Optima 7000 DV, Perkin Elmer, Waltham, MA; Pogge et al., 2012). Standards were used to verify instrument accuracy (UTAK Laboratories INC., Valencia, CA). Plasma Zn samples (n = 144) were stratified into quintiles by plasma Zn concentration to identify the 12 highest (HI) and 12 lowest (LO) samples within non implanted (NO) or implanted (TE-200) groups (n = 48 total; Table 1). Samples were selected from both the early and late BW blocks.
For these 48 steers, snap-frozen LT samples from the same day were pulverized and 100 mg of sample was weighed while frozen prior to metabolomics analysis. Muscle metabolites were extracted, dried, and analyzed in accordance with methods adapted from Heiderscheit and Hansen (2022). Metabolites were identified by the W.M. Keck Metabolomics Research Laboratory at Iowa State University (Ames, IA) using an Agilent Technologies Model 6890 GC coupled to Model 5975 controlled by the Agilent ChemStation software. The reference library was based on metabolites observed in Heiderscheit and Hansen (2022) which utilized the 2017 mass spectral library from the National Institutes of Standards and Technology. Metabolites that could not be assigned to a metabolite reference were excluded from analysis.
2.3 Statistical analysis
Data were analyzed as a complete randomized design using the MIXED procedure of SAS 9.4 (SAS Inst. Inc., Cary, NC) with fixed effects of plasma zinc grouping (PLZN), implant status (IMP), PLZN × IMP, and block. Metabolites with >30% of values missing were removed from the dataset. Data were logarithmically transformed to achieve normality and presented means were back-transformed. Outliers were characterized as greater than three standard deviations from the treatment mean and were excluded from analysis. No interactions were observed for any muscle metabolite and thus main effects are presented (n = 12 per PLZN × IMP treatment).
3 Results
Plasma Zn concentrations are displayed in Table 1. There was a tendency for a PLZN×IMP interaction (P = 0.09) where, by design, HI was greater than LO, but implanted steers had lesser plasma Zn in HI and LO. In longissimus thoracis, there was a PLZN effect for beta-alanine, 3-Hydroxybutyric acid (BHB), and glycine, where HI was greater than LO (P ≤ 0.05; Figure 1). An IMP effect was noted for BHB, 2,3,4-trihydroxybutyric acid, and glycine in which TE200 was greater than NO (P = 0.03; Figure 2). Additionally, lactic acid and malic acid tended to be greater in TE200 than NO (P ≤ 0.10; Figure 2). Several other metabolites related to amino acid and energy metabolism were identified but not affected by treatment (P > 0.10; Table 2).
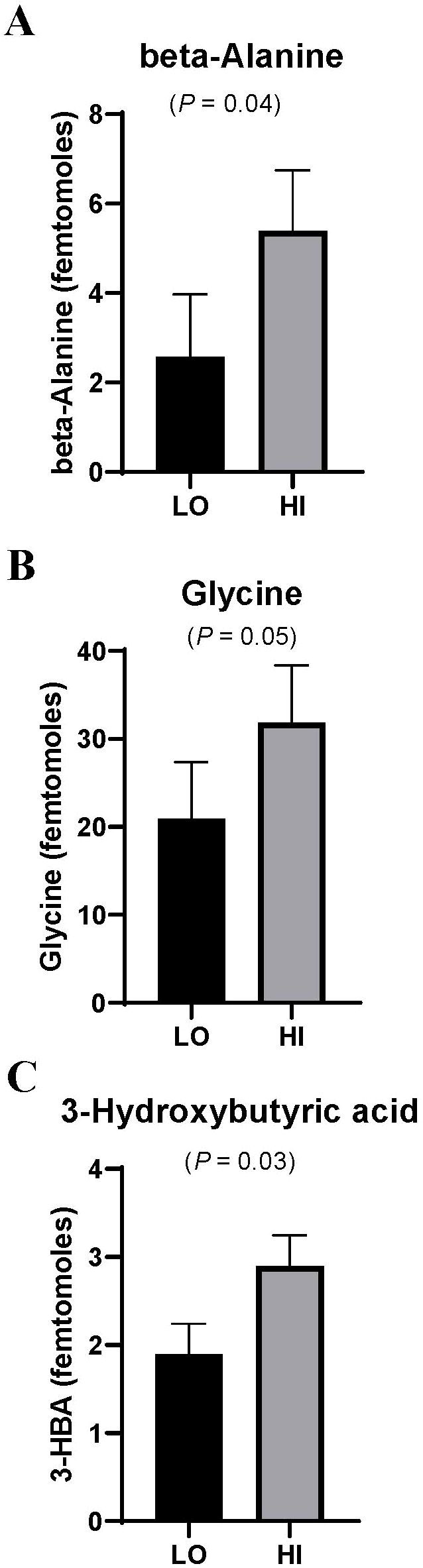
Figure 1. (A-C) Effects of LO or HI plasma Zn (PLZN) on muscle metabolites of beef steers (P ≤ 0.05). Steers were selected for PLZN treatment groups from the lowest and highest quintile of plasma Zn concentration (PLZN; LO = 1.1 mg Zn/L; HI = 1.6 mg Zn/L; 24 steers/treatment).
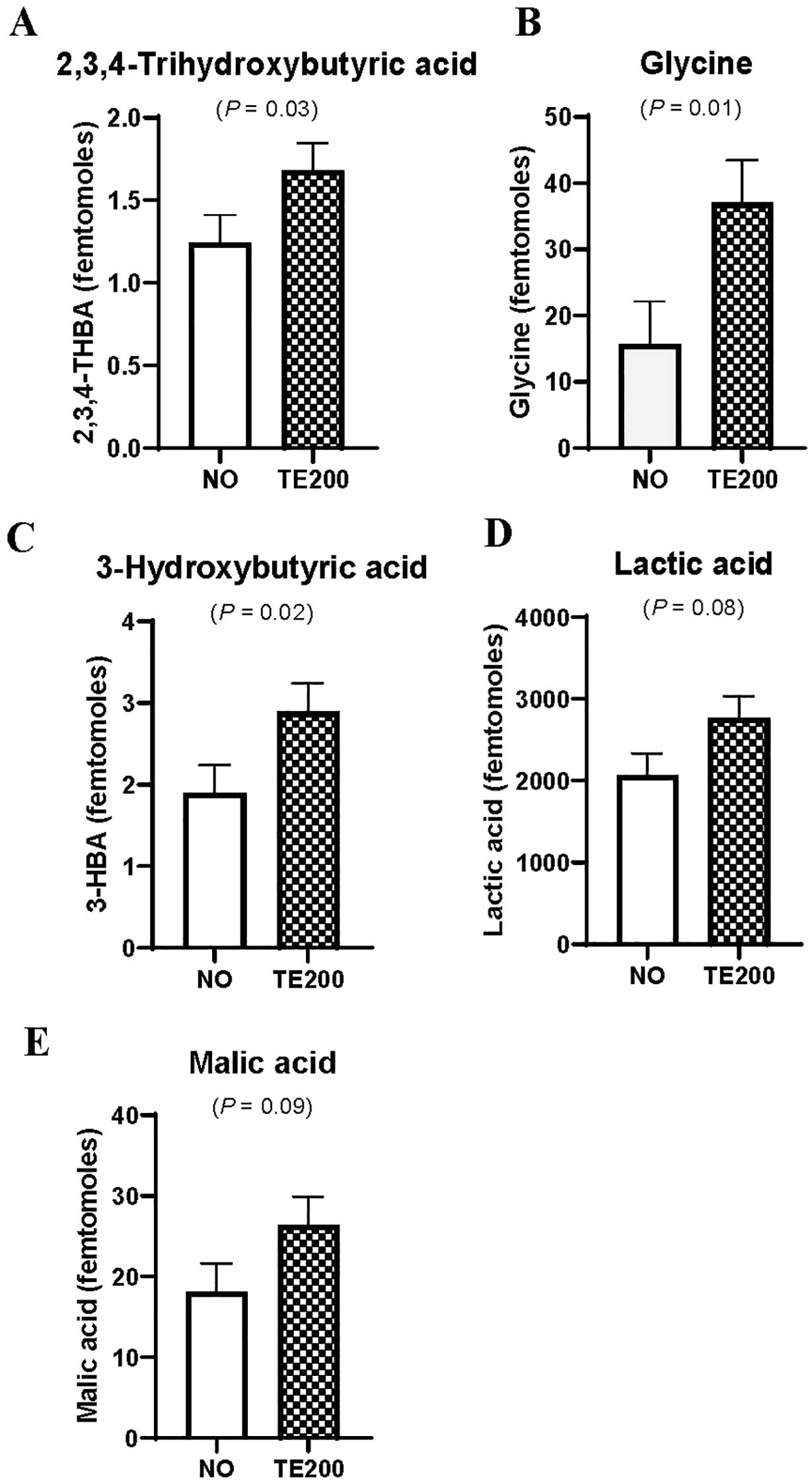
Figure 2. (A–E) Effects of NO or TE200 on muscle metabolites of beef steers collected 40 d after implant (P ≤ 0.10). Steers received a steroidal implant treatment (IMP) on d 0: 1) no implant; NO; or 2) high potency combination implant; TE200 (200 mg trenbolone acetate + 20 mg estradiol; administered d 0; Elanco Animal Health, Greenfield, IN; 24 steers/treatment).
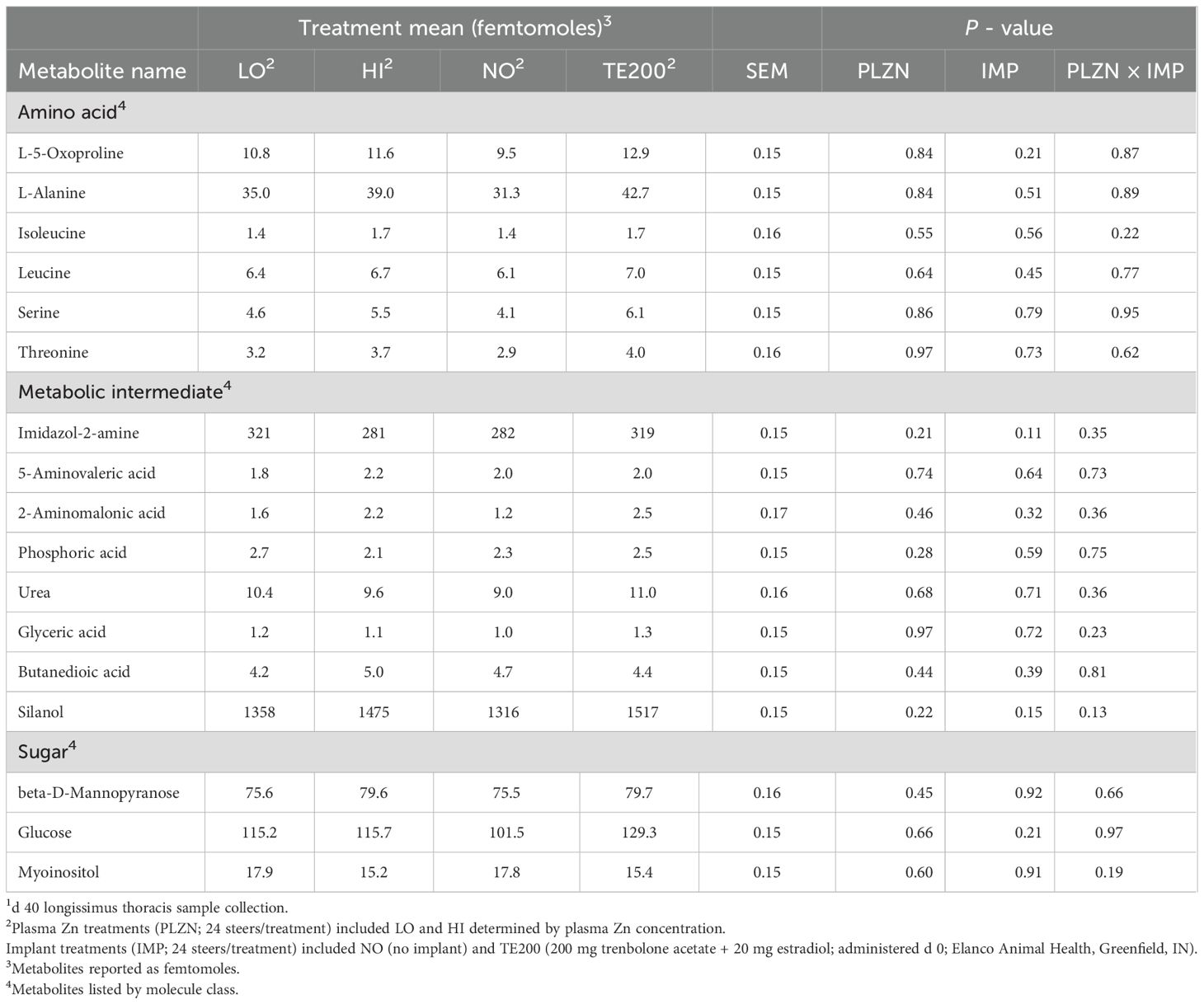
Table 2. Longissimus thoracis metabolites detected that were not different reported as concentrations1.
4 Discussion
Trace minerals support many biological pathways that profoundly impact functions related to cattle growth (Suttle, 2010). This study investigated the effects of plasma Zn concentration on muscle metabolites. As supplementing Zn increased plasma Zn concentration and hot carcass weight of steers (Messersmith and Hansen, 2021; Messersmith et al., 2022) and post-mortem rate of tenderness and LT metabolites related to energy metabolism (Schulte et al., 2023) we hypothesized steers with greater plasma Zn concentration would have more favorable energetic metabolites to support growth.
β-alanine is the rate-limiting metabolite of carnosine synthesis (Artioli et al., 2010), and was increased in HI PLZN. Carnosine helps buffer cellular H+ ions, making it an important antioxidant and transition metal chelator. It is also present at higher concentrations in Type II muscle fibers (Dunnett and Harris, 1997). β-alanine is used to improve stamina in athletes (Artioli et al., 2010). Cônsolo et al. (2020) found Nellore steers with greater genetic potential for growth had greater muscle carnosine compared to low-growth potential counterparts. Similarly, cattle with more tender steaks had greater β-alanine and β-alanine was negatively correlated with Warner-Bratzler Shear Force (WBSF; r = -0.45) (Antonelo et al., 2020), aligning with Schulte et al. (2023) who observed improved post-mortem LT tenderness in Zn-supplemented steers. Heiderscheit and Hansen (2022) examined the effects of three concentrations of Zn supplementation (0, 70, and 120 mg Zn/kg DM) on LT metabolites before and after an 18 h transit event. Pre-transit, steers supplemented 70 mg Zn/kg DM had lesser L-alanine than steers supplemented 120 mg Zn/kg DM. Porcine satellite cells treated with carnosine had greater proliferation and Akt/mTOR activity (Liu et al., 2022; Kalbe et al., 2023), potentially offering a mechanism by which Zn improves the response to steroidal implants in previous live-animal studies (Messersmith and Hansen, 2021).
We observed greater glycine concentration in the LT of both HI PLZN and TE200 steers. Glycine is acquired from dietary sources or de novo synthesis, resulting mainly from serine and its precursors (Alves et al., 2019). Glycine is readily catabolized, donating nitrogen to the greater pool for transamination (Matthews et al., 1981) and is integral to collagen and the extracellular matrix (Parry, 1988). Steroidal implants increase circulating insulin-like growth factor-1, promoting Type II collagen synthesis (Fortier et al., 1999; Preston, 1999). Genther-Schroeder et al. (2018) found no effects of increasing dietary Zn on meat collagen content when all steers were fed ractopamine hydrochloride (Genther-Schroeder et al., 2018). However, Schulte et al. (2023) found that ractopamine-fed steers had greater glycine concentrations in post-mortem LT than in control. While steroidal implants and β-agonists have different mechanisms of action, growth induced by these technologies may influence glycine metabolism. Matrix metalloproteinase 9 is implicated in the biological response to steroidal implants (Kamanga-Sollo et al., 2014; Thornton et al., 2015) and is involved in extracellular matrix remodeling (Koulicoff et al., 2023). In postmortem LT, steers supplemented 60 mg Zn/kg DM as ZnSO4 + 60 mg Zn/kg DM as Zn-AA had increased matrix metalloproteinase 9 activity compared to unsupplemented steers, contributing to altered extracellular matrix degradation (Koulicoff et al., 2023). In contemporaries to steers from the present study, increasing supplemental Zn increased mRNA abundance of matrix metalloproteinase 2 in muscle 20 days post implant (Smerchek et al., 2024). Further, Zn treatment of HTR-8/SVneo cells has been shown to influence expression of STAT3 and matrix metalloproteinase 2/9 (Zong et al., 2017), known to impact satellite cell proliferation (Thornton et al., 2015). Increased glycine in both HI PLZN and TE200 groups may be related to extracellular matrix remodeling associated with increased protein synthesis and satellite cell fusion to muscle fibers, both key modes of implant-induced growth (Johnson et al., 1998; Reichhardt et al., 2021) and influenced by Zn (Ninh et al., 1998; Tang and Shay, 2001; Haase and Maret, 2003; Plum et al., 2014).
Both HI PLZN and TE200 steers had greater concentrations of BHB in LT. Similarly, 2,3,4-trihydroxybutyric acid was greater in TE200 and is an oxidized derivative of BHB. Butyric acid is preferentially absorbed by the rumen epithelium for its energy requirements. In cattle fed energy-dense diets, epithelial monocarboxylate transporters provide the animal with ketone bodies produced by intraepithelial breakdown of volatile fatty acids (Gäbel et al., 2002). Additionally, β-Hydroxy-β-methylbutyrate (HMB), a metabolite of leucine metabolism, leads to increased BHB levels. Rats injected with HMB showed higher BHB concentrations in plasma and gastrocnemius (Ikeda et al., 2021). In aged rats with limb disuse for 14 days, HMB treatment led to a higher proportion of paired box 7 and myogenic differentiation-1 positive stem cells in the plantaris compared to controls (Alway et al., 2013). In the live-animal study (Smerchek et al., 2024), myogenic regulatory factor 5 expression increased with increasing ZnSO4, but not due to implant status. While not assessed in the present study, it is intriguing to consider differences in LT BHB may reflect satellite cell differentiation differences.
Prior to transit, Zn-supplemented steers had greater BHB in LT relative to control, and BHB decreased from pre-transit to post-transit to support energy demands (Heiderscheit and Hansen, 2022). Schulte et al. (2023) found that post-mortem LT of steers fed greater Zn concentrations or fed ractopamine hydrochloride had greater BHB concentrations than their respective control treatments. In support of these findings, we found that steers with greater plasma Zn concentration had greater BHB concentrations, indicating HI PLZN improved energy availability to muscle. Steroidal implants impact both satellite cell fusion into myofibers and fiber type-specific differences in energy metabolism, and differences to ketones in the present study suggest differential energy metabolism and satellite cell activity. Fiber type was not assessed in the present study.
Both lactic acid and malic acid tended to be greater in TE200 steers. In the forward reaction, Zn-dependent lactate dehydrogenase converts pyruvate to lactate while oxidizing nicotinamide adenine dinucleotide (Price, 1962). Similarly, malic acid is an energy metabolism intermediate of the TCA cycle, contributing to energy production through NADPH generation. Differential lactic and malic acid levels suggest altered energy metabolism in implanted cattle, potentially due to a shift towards glycolytic metabolism. Increased malic acid may result from decreased conversion to oxaloacetate via malate dehydrogenase, indicating a bottleneck or regulatory change in the TCA cycle in which malate is spared for fatty acid synthesis. A study examining the effects of Nellore cattle fed to achieve high and low growth rates in feedlot and pasture systems noted decreased malonate, a key compound in fatty acid synthesis, in low ADG feedlot steers compared to high ADG pastured steers, indicating greater energy demand to support de novo fatty acid synthesis in the feedlot steers (Gómez et al., 2022). Relatedly, feedlot steers with high ADG had greater circulating BHB (Gómez et al., 2022), which is rapidly sequestered from blood in high performance cattle to support lipid metabolism (Imaz et al., 2022), matching our findings. Ractopamine-fed steers supplemented 120 mg Zn/kg DM compared to non-ractopamine-fed steers with equal Zn supplementation had lesser abundance of malate dehydrogenase in post-mortem LT (Schulte et al., 2023).
5 Conclusions
Plasma Zn concentration influenced several metabolites related to satellite cell proliferation and energy metabolism that may explain why Zn supplementation enhances cattle growth. However, unlike previous studies, energy metabolites affected by PLZN or IMP indicate modification of TCA cycle intermediates. While there are limitations with the use of metabolites for inferences of muscle metabolism, data obtained from this study, specifically metabolites affecting intracellular pH and satellite cell proliferation, highlight areas affected by Zn status to be validated in further research to substantiate these exploratory findings.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Iowa State University Institutional Animal Care and Use Committee (IACUC-20-127). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
BO: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. DS: Conceptualization, Investigation, Methodology, Writing – review & editing. SH: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was partially funded by a competitive grant from the USDA-NIFA, grant number 2022-08296.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fanim.2025.1640542/full#supplementary-material
References
Alves A., Bassot A., Bulteau A.-L., Pirola L., and Morio B. (2019). Glycine metabolism and its alterations in obesity and metabolic diseases. Nutrients. 11, 1356. doi: 10.3390/nu11061356
Alway S. E., Pereira S. L., Edens N. K., Hao Y., and Bennett B. T. (2013). β-Hydroxy-β-methylbutyrate (HMB) enhances the proliferation of satellite cells in fast muscles of aged rats during recovery from disuse atrophy. Exp Gerontol. 48 (9), 973–984. doi: 10.1016/j.exger.2013.06.005
Antonelo D., Gómez J. F. M., Cônsolo N. R. B., Beline M., Colnago L. A., Schilling W., et al. (2020). Metabolites and metabolic pathways correlated with beef tenderness. Meat Muscle Biol. 4. doi: 10.22175/mmb.10854
Artioli G. G., Gualano B., Smith A., Stout J., and Lancha A. H. (2010). Role of β-alanine supplementation on muscle carnosine and exercise performance. Med. Sci. Sports Exerc 42, 1162–1173. doi: 10.1249/MSS.0b013e3181c74e38
Beyersmann D. and Haase H. (2001). Functions of zinc in signaling, proliferation and differentiation of mammalian cells. BioMetals. 14, 331–341. doi: 10.1023/A:1012905406548
Capper J. L. (2011). The environmental impact of beef production in the United States: 1977 compared with 2007. J. Anim. Sci. 89, 4249–4261. doi: 10.2527/jas.2010-3784
Carlson K. B., Prusa K. J., Fedler C. A., Steadham E. M., Huff-Lonergan E., and Lonergan S. M. (2017). Proteomic features linked to tenderness of aged pork loins. J. Anim. Sci. 95, 2533–2546. doi: 10.2527/jas2016.1122
Cônsolo N. R. B., da Silva J., Buarque V. L. M., Higuera-Padilla A., Barbosa L. C. G. S., Zawadzki A., et al. (2020). Selection for growth and precocity alters muscle metabolism in nellore cattle. Metabolites 10, 58. doi: 10.3390/metabo10020058
Cousins R. J., Liuzzi J. P., and Lichten L. A. (2006). Mammalian zinc transport, trafficking, and signals. J. Biol. Chem. 281, 24085–24089. doi: 10.1074/jbc.R600011200
Dunnett M. and Harris R. C. (1997). High-performance liquid chromatographic determination of imidazole dipeptides, histidine, 1-methylhistidine and 3-methylhistidine in equine and camel muscle and individual muscle fibres. J. Chromatogr B BioMed. Sci. Appl. 688, 47–55. doi: 10.1016/S0378-4347(97)88054-1
Fortier L. A., Lust G., Mohammed H. O., and Nixon A. J. (1999). Coordinate upregulation of cartilage matrix synthesis in fibrin cultures supplemented with exogenous insulin-like growth factor-I. J. Orthopaedic Res. 17, 467–474. doi: 10.1002/jor.1100170403
Gäbel G., Aschenbach J. R., and Müller F. (2002). Transfer of energy substrates across the ruminal epithelium: implications and limitations. Anim. Health Res. Rev. 3, 15–30. doi: 10.1079/AHRR200237
Genther-Schroeder O, N.., Branine M. E., and Hansen S. L. (2018). Effects of increasing supplemental dietary Zn concentration on growth performance and carcass characteristics in finishing steers fed ractopamine hydrochloride. J. Anim. Sci. 96 (5), 1903–1913. doi: 10.1093/jas/sky094
Gómez J. F. M., Cônsolo N. R. B., Antonelo D. S., Beline M., Gagaoua M., Higuera-Padilla A., et al. (2022). Impact of cattle feeding strategy on the beef metabolome. Metabolites. 12, 640. doi: 10.3390/metabo12070640
Haase H. and Maret W. (2003). Intracellular zinc fluctuations modulate protein tyrosine phosphatase activity in insulin/insulin-like growth factor-1 signaling. Exp. Cell Res. 291, 289–298. doi: 10.1016/S0014-4827(03)00406-3
Heiderscheit K. J. and Hansen S. L. (2022). Effect of increasing zinc supplementation on post-transit performance, behavior, blood and muscle metabolites, and gene expression in growing beef feedlot steers. J. Anim. Sci. 100. doi: 10.1093/jas/skac246
Ikeda K., Takahashi M., Aburaya S., Harada D., Ikeda M., Kitagawa Y., et al. (2021). Produced β-hydroxybutyrate after β-hydroxy-β-methylbutyrate (HMB) administration may contribute HMB function in mice. Biochem. Biophys. Rep. 27, 101097. doi: 10.1016/j.bbrep.2021.101097
Imaz J. A., García S., and González L. A. (2022). The metabolomics profile of growth rate in grazing beef cattle. Sci. Rep. 12, 2554. doi: 10.1038/s41598-022-06592-y
Johnson B. J., Halstead N., White M. E., Hathaway M. R., DiCostanzo A., and Dayton W. R. (1998). Activation state of muscle satellite cells isolated from steers implanted with a combined trenbolone acetate and estradiol implant. J. Anim. Sci. 76, 2779. doi: 10.2527/1998.76112779x
Kalbe C., Metzger K., Gariépy C., and Palin M.-F. (2023). Effect of muscle fibre types and carnosine levels on the expression of carnosine-related genes in pig skeletal muscle. Histochem Cell Biol. 160, 63–77. doi: 10.1007/s00418-023-02193-6
Kamanga-Sollo E., Thornton K. J., White M. E., and Dayton W. R. (2014). Role of G protein-coupled estrogen receptor-1, matrix metalloproteinases 2 and 9, and heparin binding epidermal growth factor-like growth factor in estradiol-17β-stimulated bovine satellite cell proliferation. Domest Anim. Endocrinol. 49, 20–26. doi: 10.1016/j.domaniend.2014.04.004
Koulicoff L. A., Heilman T., Vitanza L., Welter A., Jeneske H., O’Quinn T. G., et al. (2023). Matrix metalloproteinase- 9 may contribute to collagen structure modification during postmortem aging of beef. Meat Sci. 205, 109321. doi: 10.1016/j.meatsci.2023.109321
Liu Y., Shen W., Liu T., Mosenthin R., Bao Y., Chen P., et al. (2022). Improved satellite cell proliferation induced by L-carnosine benefits muscle growth of pigs in part through activation of the akt/mTOR/S6K signaling pathway. Agriculture. 12, 988. doi: 10.3390/agriculture12070988
Matthews D. E., Conway J. M., Young V. R., and Bier D. M. (1981). Glycine nitrogen metabolism in man. Metabolism. 30, 886–893. doi: 10.1016/0026-0495(81)90067-6
Messersmith E. M. and Hansen S. L. (2021). 162 increasing concentrations of supplemental zinc influence performance, carcass characteristics, and trace mineral status of non-implanted and implanted steers. J. Anim. Sci. 99, 123–123. doi: 10.1093/jas/skab054.204
Messersmith E. M. and Hansen S. L. (2024). Effects of increasing supplemental zinc to non-implanted and implanted finishing steers. J. Anim. Sci. 102. doi: 10.1093/jas/skae365
Messersmith E. M., Smerchek D. T., and Hansen S. L. (2022). Effects of increasing supplemental zinc in beef feedlot steers administered a steroidal implant and beta agonist. Transl. Anim. Sci. 6. doi: 10.1093/tas/txac029
NASEM. (2016). Nutrient Requirements of Beef Cattle. 8th Revised Edition (Washington, D.C: National Academies Press).
Ninh N. X., Maiter D., Lause P., Chrzanowska B., Underwood L. E., Ketelslegers J. M., et al. (1998). Continuous administration of growth hormone does not prevent the decrease of IGF-I gene expression in zinc-deprived rats despite normalization of liver GH binding. Growth Hormone IGF Res. 8, 465–472. doi: 10.1016/S1096-6374(98)80299-2
Parr S. L., Brown T. R., Ribeiro F. R. B., Chung K. Y., Hutcheson J. P., Blackwell B. R., et al. (2014). Biological responses of beef steers to steroidal implants and zilpaterol hydrochloride1. J. Anim. Sci. 92, 3348–3363. doi: 10.2527/jas.2013-7221
Parry D. A. D.. (1988). The molecular fibrillar structure of collagen and its relationship to the mechanical properties of connective tissue. Biophys. Chem. 29.1-2, 195–209. doi: 10.2527/jas.2013-7221
Plum L. M., Brieger A., Engelhardt G., Hebel S., Nessel A., Arlt M., et al. (2014). PTEN-inhibition by zinc ions augments interleukin-2-mediated Akt phosphorylation. Metallomics. 6, 1277. doi: 10.1039/c3mt00197k
Pogge D. J., Richter E. L., Drewnoski M. E., and Hansen S. L. (2012). Mineral concentrations of plasma and liver after injection with a trace mineral complex differ among Angus and Simmental cattle. J. Anim. Sci. 90, 2692–2698. doi: 10.2527/jas.2011-4482
Preston R. L. (1999). Hormone containing growth promoting implants in farmed livestock. doi: 10.1016/S0169-409X(99)00012-5
Price C. (1962). A zinc-dependent lactate dehydrogenase in Euglena gracilis. Biochem. J. 82, 61–66. doi: 10.1042/bj0820061
Reichhardt C. C., Messersmith E. M., Brady T. J., Motsinger L. A., Briggs R. K., Bowman B. R., et al. (2021). Anabolic implants varying in hormone type and concentration influence performance, feeding behavior, carcass characteristics, plasma trace mineral concentrations, and liver trace mineral concentrations of angus sired steers. Animals. 11, 1964. doi: 10.3390/ani11071964
Russell J. R., Sexten W. J., Kerley M. S., and Hansen S. L. (2016). Relationship between antioxidant capacity, oxidative stress, and feed efficiency in beef steers. J. Anim. Sci. 94, 2942–2953. doi: 10.2527/jas.2016-0271
Samuelson K. L., Hubbert M. E., Galyean M. L., and Löest C. A. (2016). Nutritional recommendations of feedlot consulting nutritionists: The 2015 New Mexico State and Texas Tech University survey1. J. Anim. Sci. 94, 2648–2663. doi: 10.2527/jas.2016-0282
Schulte M. D., Hochmuth K. G., Steadham E. M., Lonergan S. M., Hansen S. L., and Huff-Lonergan E. J. (2023). Early postmortem muscle proteome and metabolome of beef longissimus thoracis muscle classified by pH at 6 hours postmortem. J. Proteomics. 271, 104756. doi: 10.1016/j.jprot.2022.104756
Smerchek D. T., Rients E. L., McLaughlin A. M., Thornton K. J., and Hansen S. L. (2024). Influence of steroidal implants and zinc sulfate supplementation on growth performance, trace mineral status, circulating metabolites, and transcriptional changes in skeletal muscle of feedlot steers. J. Anim. Sci. 102, skae154. doi: 10.1093/jas/skae154
Suttle N. F. (2010). Mineral Nutrition of Livestock. 4th ed (Wallingford, Oxfordshire, UK: CAB International).
Tang X. and Shay N. F. (2001). Zinc has an insulin-like effect on glucose transport mediated by phosphoinositol-3-kinase and akt in 3T3-L1 fibroblasts and adipocytes. J. Nutr. 131, 1414–1420. doi: 10.1093/jn/131.5.1414
Thornton K. J., Kamange-Sollo E., White M. E., and Dayton W. R. (2015). Role of G protein–coupled receptors (GPCR), matrix metalloproteinases 2 and 9 (MMP2 and MMP9), heparin-binding epidermal growth factor–like growth factor (hbEGF), epidermal growth factor receptor (EGFR), erbB2, and insulin-like growth factor 1 receptor (IGF-1R) in trenbolone acetate–stimulated bovine satellite cell proliferation1. J. Anim. Sci. 93, 4291–4301. doi: 10.2527/jas.2015-9191
Keywords: anabolic implant, feedlot cattle, muscle, metabolite, zinc
Citation: Ortner BM, Smerchek DT and Hansen SL (2025) Differences in longissimus thoracis metabolites in feedlot steers with differing plasma Zn concentration and implant status. Front. Anim. Sci. 6:1640542. doi: 10.3389/fanim.2025.1640542
Received: 03 June 2025; Accepted: 24 July 2025;
Published: 20 August 2025.
Edited by:
Michael D. Flythe, United States Department of Agriculture, United StatesReviewed by:
Ahmed Ismaeel, University of Kentucky, United StatesBenjamin Burke, University of Kentucky, United States
Copyright © 2025 Ortner, Smerchek and Hansen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stephanie L. Hansen, c2xoYW5zZW5AaWFzdGF0ZS5lZHU=
 Brock M. Ortner
Brock M. Ortner Dathan T. Smerchek
Dathan T. Smerchek Stephanie L. Hansen
Stephanie L. Hansen