- 1Department of Precision and Regenerative Medicine and Jonian Area, Section of Veterinary Science and Animal Production, University of Bari Aldo Moro, Bari, Italy
- 2Department of Veterinary Medicine, University of Bari Aldo Moro, Bari, Italy
- 3Department of Pharmacy-Pharmaceutical Sciences, University of Bari Aldo Moro, Bari, Italy
- 4Institute of Science of Food Production (ISPA), Research National Council (CNR), Bari, Italy
- 5Italian Rabbit Breeders Association (ANCI-AIA) , Foggia, Italy
Marrubium vulgare L., commonly known as horehound in Europe, belongs to the Lamiaceae family, one of the most prominent medicinal plant families in the Mediterranean region. Traditionally used in herbal medicine, horehound contains a broad spectrum of bioactive compounds, supporting its potential use as a natural feed additive in animal nutrition. This study evaluated the effects of dietary horehound powder (HP) on growth performance, serum antioxidant status, digestive enzyme activity, intestinal microbial populations, and gut and liver histomorphology in growing rabbits. Eighty weaned male Bianca Italiana rabbits (42 days old) were randomly assigned to two dietary groups for a 6-week feeding trial: a control group fed a basal diet and a treatment group receiving the same diet supplemented with 0.15% HP (1.5 g/kg). At slaughter (84 days of age), samples of meat, blood, liver, and intestinal tissue were collected for analysis. Dietary HP significantly improved final body weight, feed conversion ratio, and carcass yield (P < 0.01), with no notable differences in proximate meat composition. However, meat from HP-fed rabbits showed increased total polyunsaturated fatty acids (PUFAs) (P < 0.05) and n-6 fatty acids (P < 0.01). Serum lipid profiles remained unaffected, while serum antioxidant parameters—total antioxidant capacity (TAC), malondialdehyde (MDA), and superoxide dismutase (SOD)—were significantly enhanced in the HP group. Additionally, HP supplementation increased the activity of duodenal α-amylase, maltase, lipase, and trypsin (P < 0.05). Although caecal morphology did not differ significantly between groups, rabbits receiving HP exhibited higher Lactobacillus spp. counts and reduced Escherichia coli populations. Duodenal histomorphometry showed significant improvements in villus height, crypt depth, and the villus height to crypt depth ratio (P < 0.01). No histological alterations were observed in the liver of rabbits. In conclusion, dietary inclusion of horehound powder positively influenced growth performance, oxidative status, digestive enzyme activity, and intestinal health in rabbits, supporting its use as a functional feed additive in sustainable rabbit production.
1 Introduction
From ancient times, plant species have been used in folk medicine as a source of valuable biocompounds and applied as practical remedies for a variety of ailments, including metabolic, gastrointestinal, and inflammatory disorders (Popoola et al., 2013; Caceres et al., 2022). In the Mediterranean area, traditional plant knowledge (TPK) on wild or cultivated botanicals represents a significant part of local culture (Menale et al., 2016). Preserving popular traditions is crucial for the recovery of both autochthonous plant varieties and animal breeds, and thus for maintaining ethnobiodiversity, especially in rural areas or small villages where traditional agri-food products (TAP) represent the main economic revenue. In view of these aspects, TPK may play a key role in the development of rural production systems. Indeed, the use of natural resources according to local traditions and availability offers the opportunity to enhance the yield and quality of animal products (Colonna et al., 2023).
Current research on animal health and welfare management emphasized the need for natural nutritional approaches that may positively influence livestock health status and performance indices. In this context, plant extracts or phytogenic feed additives may represent a viable alternative as natural growth promoters, thereby reducing reliance on antibiotics and synthetic additives (Falcão-e-Cunha et al., 2007; Seidavi et al., 2021). In addition, natural feed additives in livestock nutrition align with consumer preferences for sustainable and “clean label” animal products (Kehlbacher et al., 2012; Placha et al., 2022; Pugliese et al., 2024). However, traditional knowledge of herbal remedy practices was mainly passed down orally, resulting in a lack of scientific evidence (Viegi et al., 2003). Therefore, ethnobotanical and ethnopharmacological surveys on herb species and medicinal plants, supported by in vivo studies, may represent an important tool to deepen the understanding of the biological properties of these products and to discover and develop new compounds that could be employed in livestock feeding strategies.
Marrubium vulgare L. belongs to the Lamiacea family, one of the most useful plant families in the Mediterranean area (Amessis-Ouchemoukh et al., 2014; Rezgui et al., 2021). It is a robust perennial herb growing in marginal areas, with dense cottony stems and white flowers. It is commonly known as “horehound” in Europe and as “Marrubia” or “Om rubia” in Tunisia. Marrubium species are indigenous to Europe, the Mediterranean area, and Asia, among other regions. They also grow naturally in North and South America and are extensively distributed in areas where sheep are raised, especially around bedding and watering locations (Lodhi et al., 2017). Available literature reports that M. vulgare is widely used in traditional medicine worldwide (Boulila et al., 2015; El-Gharbaoui et al., 2017), where it serves as a remedy for various conditions such as gastrointestinal and respiratory disorders, intestinal infections, inflammatory processes, and bile secretion disorders (Lodhi et al., 2017; Acmovic et al., 2020). For instance, African ethnopharmacology and ethnoveterinary surveys list horehound botanical preparations for the treatment of gastrointestinal pain, inflammation, and diarrhoea in farm animals, as well as for improving livestock production (Stark et al., 2013; Rahman et al., 2023). Moreover, M. vulgare is included among the plants used as antimicrobials in traditional folk medicine in Algerian animal husbandry (Kahlouche-Riachi et al., 2012; Saidi et al., 2019). In Italy, both M. vulgare and M. incanum are considered medicinal plants employed in ethnomedicine within the Lucanian Arbëreshë communities of southern Italy, a belief supported by a local refrain stating, “the horehound destroys every disease” (Pieroni et al., 2002). Administration of M. vulgare leaves is traditionally reported as a phyto-remedy in ethnoveterinary medicine to treat common gastrointestinal disorders in rabbits and sheep caused by the fermentation of fresh grass or hay (Manganelli et al., 2001; Viegi et al., 2003). Leaves of M. vulgare are also often administered to cattle and horses when their stomachs are swollen from consuming fodder that is too fresh and wet (Manganelli et al., 2001). Local farmers have used plant-based formulations as a remedy for diarrhea or as a vermifuge or anthelmintic. Furthermore, an ethnoveterinary study on the antimicrobial activity of M. vulgare methanolic extract found it effective against resistant bacterial strains of Staphylococci and Enterobacteria isolated from bovine mastitis (Saidi et al., 2019). A recent phytochemical and pharmacological overview (Acmovic et al., 2020) reported that, in veterinary medicine, M. vulgare preparations may be employed as a chicken lice repellent and as an anthelmintic against the eggs and larvae of bovine strongyle parasites (Moussouni et al., 2018). Consequently, due to its traditional use in ethnomedicine, M. vulgare is included in a number of monographs and pharmacopoeias, such as those of the European Medicines Agency (EMA), European Scientific Cooperative on Phytotherapy (ESCOP), British Herbal Pharmacopoeia (BHP), American Botanical Council, German Commission, and European Pharmacopoeia Commission (Acmovic et al., 2020). For example, the Committee on Herbal Medicinal Products (HMPC) of the EMA reports that M. vulgare medicinal preparations are used in modern phytotherapy as expectorants for cough and for the symptomatic treatment of mild dyspeptic disorders, such as bloating, flatulence, or temporary loss of appetite (EMA, 2013).
Plant extracts and botanical preparations represent a natural source of bioactives with promising health-promoting properties. Earlier phytochemical assays of M. vulgare extracts identified a variety of biocompounds, among which marrubiin stands as the main chemical component. This labdane diterpene is considered the chemotaxonomic marker of the Marrubium genus and exhibits remarkable pharmacological properties with high safety margins in several animal models (Masoodi et al., 2015; Yousefi et al., 2016; Ahvazi et al., 2018). The biological activity of M. vulgare is the result of its chemical complexity, and its preparations have demonstrated hepatoprotective, choleretic, and cholagogue effects (Akther et al., 2013; Elberry et al., 2010). Moreover, horehound, being a rich source of polyphenolic compounds—such as phenolic acids, phenylpropanoid (cinnamic) acids, esters, and flavonoids—is an effective antioxidant in stress-related disorders (Lodhi et al., 2017; Acmovic et al., 2020). The redox properties of these compounds enable them to act as reducing agents, preventing or delaying oxidative processes happening within animal tissues (Tarnawski et al., 2006; Boulila et al., 2015; Mbah et al., 2019).
The ethnoveterinary use, the abundance of biocompounds with proven pharmacological properties, and the increasing interest in natural additives suggest that M. vulgare may be used in animal nutrition to improve animal health, sustain growth, and maintain a balanced gut environment (Tufarelli et al., 2017; Schlemper et al., 2021). Notably, the antioxidant compounds present in M. vulgare may positively influence gut health and microbiota, hinder pathogenic bacterial growth, and prevent tissue oxidation, thereby increasing growth performance and product quality. This highlights the potential for its inclusion as a multifunctional feed supplement, including in rabbit diets.
Regrettably, despite the rising number of studies published in the scientific literature, the biological activity of M. vulgare has mainly been investigated in vitro, and the current state of knowledge on in vivo trials remains limited.
For the abovementioned reasons, the intent of this study was to explore the potential of dietary supplementation with horehound powder (HP) in growing rabbits by evaluating productive traits, serum antioxidant status, digestive enzyme activity, intestinal bacterial counts, and gut health. Moreover, given the hepatoprotective effects of M. vulgare, its influence on rabbit liver architectural features was also assessed.
2 Materials and methods
2.1 Animals, management and diet
The study was carried out at the rabbit facility of the Genetic Center affiliated with the Italian Rabbit Breeders Association (ANCI-AIA), located in Volturara Appula, Foggia, Italy. All procedures involving animals complied with European Directive 2010/63/EU on the protection of animals used for scientific purposes, which replaced Directive 86/609/EEC, and adhered to the ethical standards established by the Department of Emergency and Organ Transplantation (DiMePRe-J) of the University of Bari Aldo Moro (Approval code: 09/2022).
A total of 80 male Italian White (Bianca Italiana) growing rabbits, aged 42 days and with an average body weight of 1,097 ± 24.1 g (mean ± SEM), were randomly allocated to two experimental groups (n = 40 per group) based on dietary treatment. The feeding trial continued until the rabbits reached 84 days of age. The control group received a standard diet with no additives, whereas the experimental group was fed the same basal diet supplemented with 0.15% horehound powder (HP), equivalent to 1.5 g/kg of feed.
Each rabbit was housed individually in a galvanized wire cage (35 × 40 × 50 cm; width × height × length), part of an open-housing system (Italian battery) raised 90 cm above the concrete floor. Animals had ad libitum access to fresh drinking water supplied through an automated nipple drinker, and feed was provided manually once a day in the morning. Throughout the study, environmental and hygienic conditions were kept uniform for all animals. Microclimate conditions were maintained by a temperature and humidity monitoring system, ensuring compliance with rabbit welfare parameters.
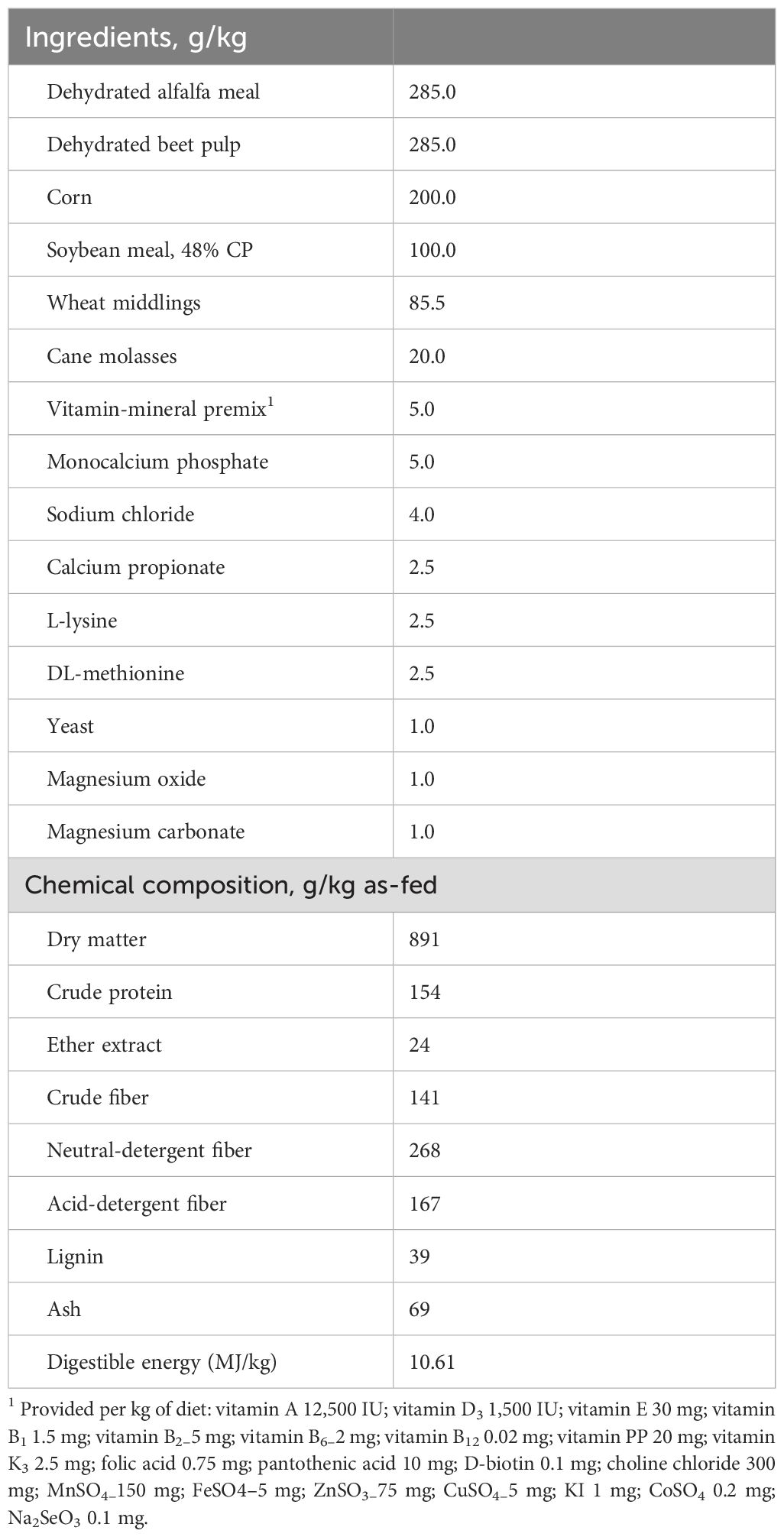
No pharmaceutical treatments were administered via feed or water, and the health status of each rabbit was monitored daily through individual clinical observation. The composition and proximate analysis of the basal diet are presented in Table 1.
2.2 Horehound collection and analysis
Horehound aerial parts were collected during the spring season in the Taranto province of southern Italy at the early flowering stage. The material was then air-dried at room temperature and ground into a fine powder to be included in the pellet. Horehound powder (HP) was characterized for volatile organic compounds (VOCs), total phenolic compounds (TPC), total antioxidant capacity (TAC), 2,2-diphenyl-1-picrylhydrazyl (DPPH) activity, polyphenol, and fatty acid composition, as presented in Table 2.
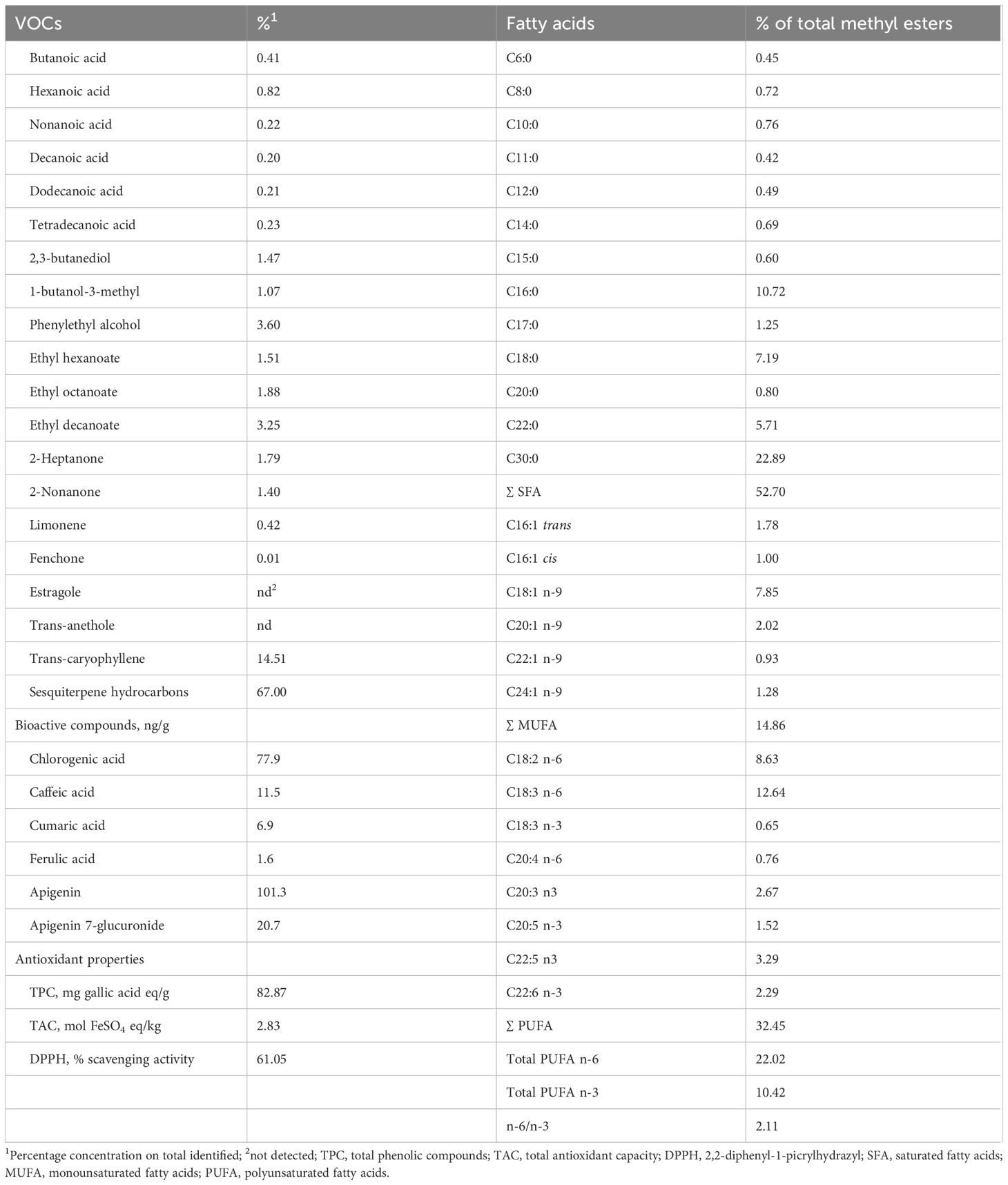
Table 2. Volatile organic compounds (VOCs), bioactive compounds, antioxidant properties, and fatty acid composition of horehound (Marrubium vulgare).
2.3 Horehound volatile organic compounds and phenolic composition
VOCs were extracted from HP samples using solid-phase microextraction (SPME), and the GC–MS analysis was carried out with a gas chromatograph (Perkin Elmer, Waltham, MA, USA) coupled with a mass spectrometer (SQ8S; Perkin Elmer, USA), following the procedures of Iommelli et al. (2025). Compounds were considered correctly identified when their spectra showed a library match factor >85. VOCs were expressed as percentages of the total volatile compounds detected.
The solid–liquid extraction procedure was performed using an orbital shaker (PSU-10i, BIOSAN, Riga, Latvia) and a multispeed refrigerated centrifuge (ALC PK 121R; ALC International, Cologno Monzese, Italy). HPLC-DAD analyses were performed by using an Agilent 1260 Infinity liquid chromatograph (Agilent Technologies Inc., Wilmington, DE, USA) equipped with a binary pump (G1312B), an autosampler (G1367E) with a 100 μL loop, a spectrophotometric diode array detector (DAD; G4212B), and a software for Microsoft Windows 7 (OpenLAB, CSB, ChemStation Edition). The separation was achieved using a Zorbax SB-C18 column, 5 μm (4.6x150 mm) (Agilent Technologies Inc.).
Two aqueous ethanol solutions were prepared as extracting solvents: ethanol/water 50% (wt/wt) and ethanol/water 70% (wt/wt). Extraction was performed for 1 h at room temperature under shaking (230 rpm) in closed jars, as similarly reported by Chamorro et al. (2012). In the following step, the solid was excluded from the extract and the subsequent centrifugation (4500 rpm at 25°C for 10 min) was performed before storing the samples in Eppendorf tubes (1.5 ml) at -80°C for further analyses. All experiments were carried out in duplicate.
High-performance liquid chromatography (HPLC) was performed to detect and quantify the extracted phenolic compounds. Extracts were diluted with water (1 mL of water per 1 mL of extract), centrifuged (14,400 rpm at 30°C for 2 min), and filtered through 0.45 μm regenerated cellulose filters. The method and parameters were based on Sergio et al. (2020) with minor modifications. A 20 μL aliquot of each sample was injected into the column at 25°C. The flow rate was set to 0.7 mL/min, and the mobile phase consisted of a gradient of H2O with CH3COOH 5% and methanol (MeOH). The DAD monitored five wavelengths: 260, 280, 325, 360, and 560 nm.
Standard solutions of phenolic compounds—including gallic acid, catechin, syringic acid, rutin, quercetin, kaempferol 3-galactoside, apigenin, apigenin 7-glucuronide, resveratrol, ferulic acid, chlorogenic acid, caffeic acid, and coumaric acid—were prepared using methanol as the solvent.
2.4 Horehound antioxidant properties and fatty acid composition
The TPC in samples was determined using the Folin–Ciocalteu colorimetric method described by Taga et al. (1984). The concentration was calculated using gallic acid as the standard (Sigma-Aldrich), and the results were expressed as mg gallic acid equivalents (GAE)/g.
The TAC of the samples was assessed using the ferric ion reducing antioxidant power assay, following the method reported by Benzie and Strain (1997). The standard curve was constructed using iron sulfate heptahydrate (FeSO4·7H2O) and data were expressed as mol FeSO4 equivalents per kg of HP.The antioxidant activity was determined using the DPPH photometric assay with a UV–Vis spectrophotometer (Infinite M1000 Pro multiplate reader; Tecan, Cernusco S. N., Italy), according to the procedure of Carocci et al. (2022). A volume of 100 μL of diluted extract (1:50, 1:150, 1:450, 1:1350, 1:4050, 1:12150, 1:36450) (v/v) with H2O:CH3OH (50:50) was mixed with 100 μL of 200 μM DPPH solution in methanol, obtained from a freshly prepared 10 μM solution. The mixtures were incubated in dark conditions for 30 min at room temperature. Absorbance was measured using the UV–Vis spectrophotometer set at 640 nm, as at the conventional wavelength (520 nm), the absorption could be affected by the color of the extracts at the highest concentrations. Results were expressed in mg GAE/mL.
The HP was analyzed for FA composition. Briefly, the lipid fraction of HP was extracted from 10 g of sample according to Gray et al. (1967). The lipid extract was methylated by adding 1 mL of hexane and 0.05 mL of 2 N methanolic KOH. Separation of the methyl esters in forage samples was performed according to Di Trana et al (2015). FAMEs were identified with reference to the retention times of the FA standard mixture (Supelco 37 Component FAME Mix; Supelco, Bellefonte, PA, USA).
The content of FA was quantified using internal standards (Supelco, Bellefonte, PA, USA) added during the methylation step. Briefly, 100 mg of lipid extract was mixed with 50 μL of 2 N methanolic KOH and 1 mL of hexane containing the internal standards (20 mg/mL), according to Giorgio et al. (2019). FAMEs were fractionated over a CP-SIL 88 column (100 × 0.25 mm i.d., film thickness 0.20 μm, fused silica; Varian, Palo Alto, CA, USA) in a Shimadzu (Model 2GC17-A) gas chromatograph equipped with a Hewlett-Packard HP 6890 gas system and flame ionization detection. Helium was used as the carrier gas at a constant flow rate of 1.7 mL/min. The oven temperature was programmed as follows: 175°C, held for 4 min; 175–250°C at 3°C/min; and then maintained at 250°C for 20 min. The injector port and detector temperatures were 250°C. Samples (1 μL) were injected with an autosampler. Output signals were identified and quantified based on the retention times and peak areas of known calibration standards. Composition was expressed as percentages of the total FA. All determinations were carried out in triplicate.
2.5 Growth, carcass traits, and meat quality
From 6 to 12 weeks of age, individual body weights were recorded weekly, while daily feed intake was monitored to calculate the feed conversion ratio (FCR). Animal health was observed daily, with particular attention to clinical signs of illness and mortality. Mortality data were incorporated into the FCR calculation.
At the end of the fattening period (84 days of age), 10 rabbits were randomly selected from each group in the afternoon for slaughter evaluation. The following morning, the selected animals were transported in small groups to a nearby slaughter facility. Slaughtering procedures, including carcass assessment, were conducted within 2 h post-arrival and adhered to the guidelines of the World Rabbit Science Association (WRSA), as outlined by Blasco and Ouhayoun (1996).
Prior to slaughter, rabbits were weighed and then subjected to electrical stunning. Following exsanguination, the skin, gastrointestinal tract, and distal limbs were removed. The hot carcasses—retaining the head, thoracic organs, liver, kidneys, and perirenal and scapular fat—were weighed and subsequently chilled at 4°C for 24 h in a ventilated cold room. After chilling, the carcasses were reweighed, and slaughter yield was determined.
Meat samples from the Longissimus lumborum muscle were collected and stored at −80°C for lipid and fatty acid analyses. Each sample was analyzed in triplicate. Dry matter was determined by oven drying (AOAC 934.01), ash content by muffle furnace incineration (AOAC 942.05), and crude protein using the Kjeldahl method (AOAC 954.01). Total lipids were extracted using the protocol of Folch et al. (1957), and fatty acid profiles were assessed following the method described by Tufarelli et al. (2023). Fatty acid composition was expressed as a percentage of total fatty acid methyl esters (FAMEs).
2.6 Blood serum lipids and redox status analysis
Ten blood samples from each treatment group were taken from the rabbits’ marginal ear veins at the end of the trial (84 days of age), 1 h before the regular feeding time. After the samples were gathered into sterile tubes and centrifuged, the serum was separated and stored at −20°C for further analysis.
The obtained serum samples were subjected to measurements of the following parameters: total cholesterol (mg/dL), low-density lipoprotein (LDL; mg/dL), high-density lipoprotein (HDL; mg/dL), and triglycerides (mg/dL). Using commercial kits (Randox Laboratories, Ltd., Crumlin, UK), serum antioxidant-related biomarkers—including total antioxidant capacity (TAC), malondialdehyde (MDA), and superoxide dismutase (SOD)—were evaluated. All samples were analyzed in triplicate according to the specifications of the kits.
2.7 Digestive enzyme activity, caecal characteristics, and microbiota
At slaughter, the duodenum of each rabbit was gently squeezed from one end to the other to obtain a uniform sample. Ice-cold PBS (pH 7.0) was used to dilute each sample 10-fold after weighing. Each sample was homogenized using a portable glass homogenizer and then centrifuged at 5,000 g for 20 min at 4°C. After centrifugation, the supernatants were carefully separated and stored at 4°C until further analysis.
Following the extraction process, the duodenal digestive enzymes (α-amylase, lipase, maltase, and trypsin) were evaluated within 24 h using the methods described by Badawy et al. (2025). Enzyme activity units were calculated and expressed per mg of protein in the tissue supernatant to minimize errors during sample preparation, as described by Wang et al. (2021).
Volatile fatty acids (VFAs) were assessed in the caecal contents of slaughtered rabbits using gas chromatography, as described by Abu Hafsa et al. (2017), and ammonia was analyzed using an automated distillation unit. The pH of caecal contents was determined using a portable pH meter.
The caecal content was then collected into sterile plastic tubes and frozen at −80°C for microbiota analysis. Bacterial counts were conducted on the same caecal material to determine the main bacterial species present. Total bacterial count was assessed using Plate Count agar; Escherichia coli was enumerated using chromogenic coliform agar; and Lactobacillus spp. were assessed using De Man–Rogosa–Sharpe agar, according to the method of Maturin and Peeler (1998). Bacterial colonies were counted using an optical colony counter.
2.8 Histomorphometric examination
On day 84, all slaughtered rabbits were subjected to histomorphometric evaluation. Samples of duodenum and liver were collected. Duodenal segments approximately 2 cm in length were excised 3 cm distal to the pylorus. These segments were immediately flushed with 0.9% saline to remove the contents and then fixed in fresh 10% buffered formalin, dehydrated, cleared, and embedded in paraffin. Sections were trimmed to a thickness of 5 μm –7 μm, mounted on glass slides, and stained with hematoxylin and eosin (H&E; Merck, Darmstadt, Germany) for histomorphometric detection and with Azan–Mallory (AM) trichrome stain (Merck, Darmstadt, Germany) to evaluate extracellular matrix and collagen fiber distribution.
For histomorphometric evaluations, 20 villi containing a lamina propria were randomly selected per slide. The morphometric indices evaluated included villus height (VH), measured from the tip of the villus to the crypt; crypt depth (CD), measured from the base of the villus to the submucosa; and the ratio of villus height to crypt depth (VH: CD), calculated as described by Laudadio et al. (2012). Apparent villus surface area was calculated using the following formula: [(villus width at one-third + villus width at two-thirds of the height of the villus) × 2−1 × villus height], according to Tufarelli et al. (2023). All morphological measurements (VH and CD) were evaluated at 10× and 25× magnification using an image analysis system (X-Series, Alexasoft).
For histological examination, liver specimens collected from slaughtered rabbits were washed in normal saline and fixed in 10% formalin. Routine histological methods, including paraffin embedding, were applied. Liver serial sections (5 μm thick) were mounted on glass slides and stained using the H&E method (Merck, Darmstadt, Germany) for morphological evaluation and periodic acid–Schiff (PAS) staining to detect glycogen deposition in hepatocytes, which appears as scattered red to magenta particles in the cytoplasm. Stained sections were examined at different magnifications (2.5×, 4×, and 10×), and images were captured using a digital camera (DFC 420, Leica, Cambridge, UK) connected to a light microscope (DIAPLAN, Leitz, Wetzlar, Germany).
2.9 Statistical analysis
Data were analyzed using a one-way ANOVA design with the GLM procedure in SAS (version 9.2; SAS, 2008). Means were separated and compared using Tukey’s honestly significant difference (HSD) test. Microbial count data were logarithmically transformed and analyzed using a normal probability plot at a 95% confidence interval. Results were reported as least squares means and pooled standard error of the mean (SEM). Statistical significance was considered at P ≤ 0.05.
3 Results
3.1 Growth, carcass traits, and meat quality
The effects of horehound powder (HP) inclusion in the meat-rabbit diet on growth performance, carcass traits, and meat characteristics are shown in Table 3. Dietary supplementation had a significant effect on final body weight (FBW) and average daily gain (ADG), which were both higher in HP rabbits (P<0.05). No significant differences were found between groups in feed intake (FI) (P > 0.05); however, feed conversion ratio (FCR), as well as carcass traits (slaughter weight, hot carcass weight, and carcass yield), were improved by HP (P<0.001 and P<0.05, respectively). The meat nutritional composition did not differ significantly between the tested groups.
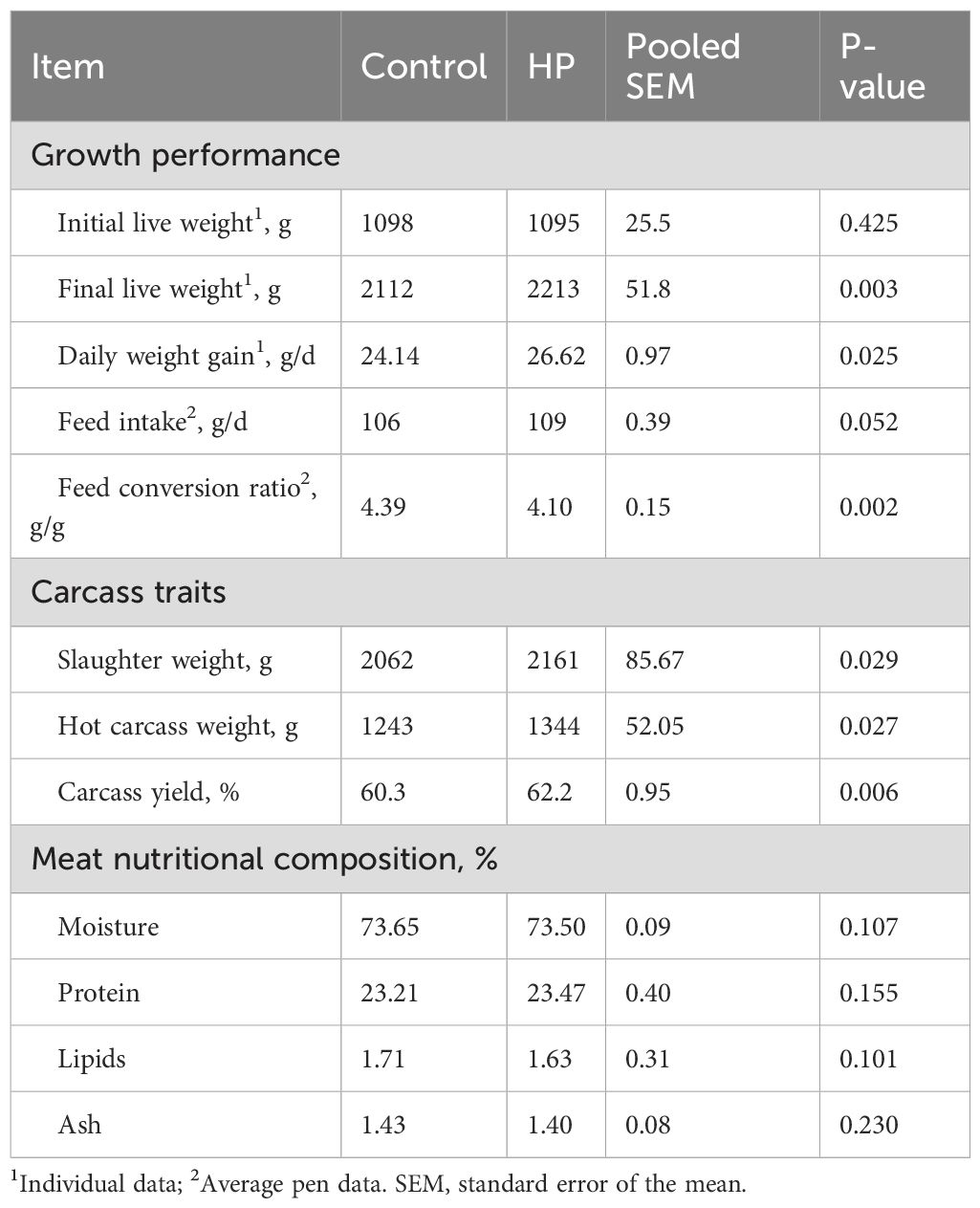
Table 3. Effect of horehound powder (HP) on growth performance, carcass yield, and meat nutritional composition of rabbits.
3.2 Meat fatty acid profile
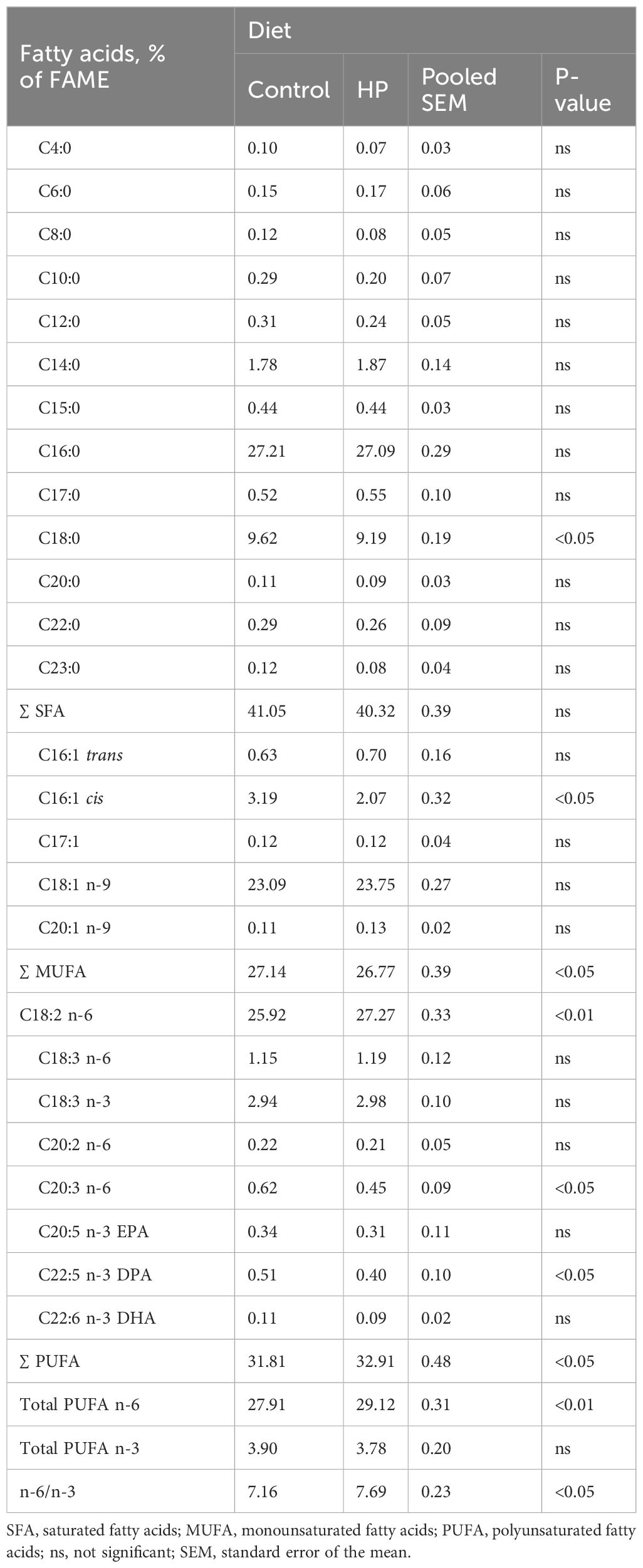
The effects of HP on fatty acid (FA) composition of the L. lumborum muscle are shown in Table 4. No significant differences between groups were found for total saturated fatty acids (SFA), except for C18:0 (stearic acid), which was significantly reduced in the HP group (P<0.05). Results for L. lumborum monounsaturated fatty acid (MUFA) content showed a significant decrease in C16:1 cis (palmitoleic acid) and in total MUFA in rabbits fed the HP diet.
Conversely, dietary HP inclusion led to a significant increase in total polyunsaturated fatty acids (PUFA), with a significant rise in n-6 linoleic acid (C18:2 n-6, LA), and a significant reduction in C20:3 n-6 (dihomo-γ-linolenic acid, DGLA) and C22:5 n-3 (docosapentaenoic acid, DPA). Consequently, total PUFA n-6 and n6/n3 ratio were significantly augmented (P<0.01) in the HP group.
3.3 Blood serum lipids and antioxidant capacity
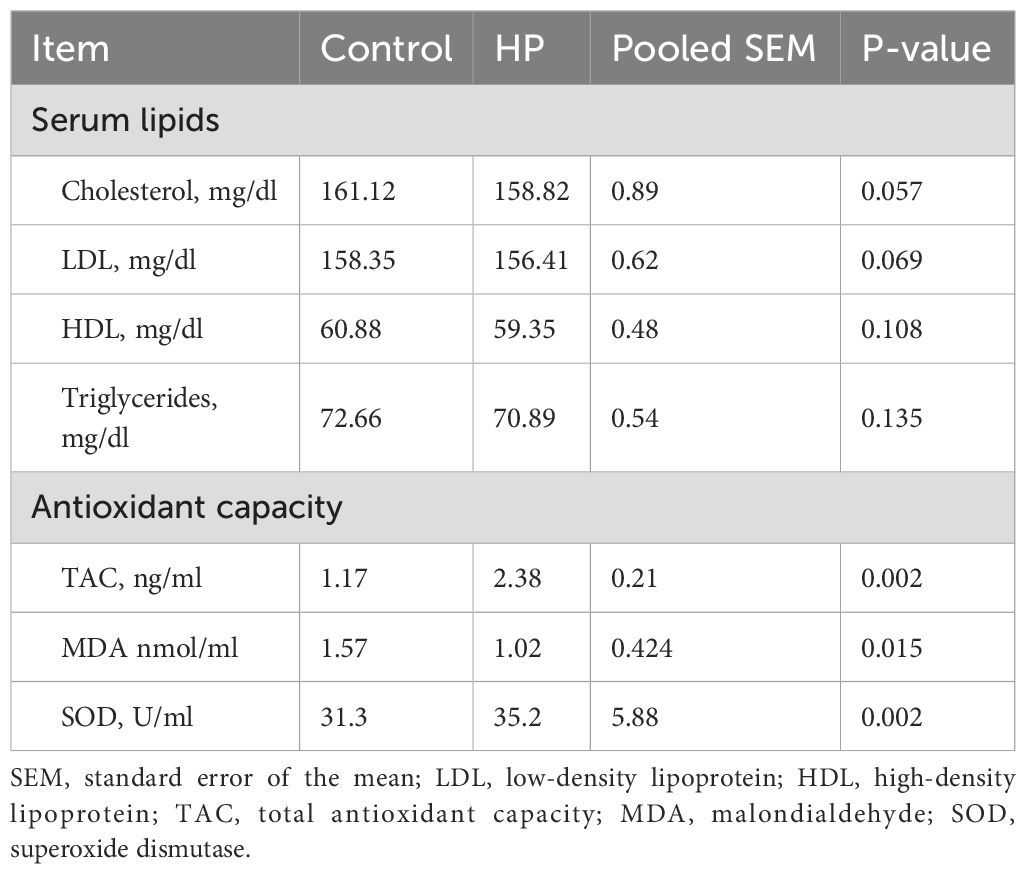
The effect of HP on blood serum lipids and redox status in rabbits is shown in Table 5. Serum cholesterol, LDL, HDL, and triglycerides (TG) were not affected by the dietary treatment.
Regarding oxidative status, the findings indicated that TAC and SOD levels increased by 50.8% and 11.1%, respectively (P<0.01), while the MDA level decreased by 53.9% (P=0.015) in the HP-fed group.
3.4 Digestive enzyme activity, caecal characteristics and microbiota
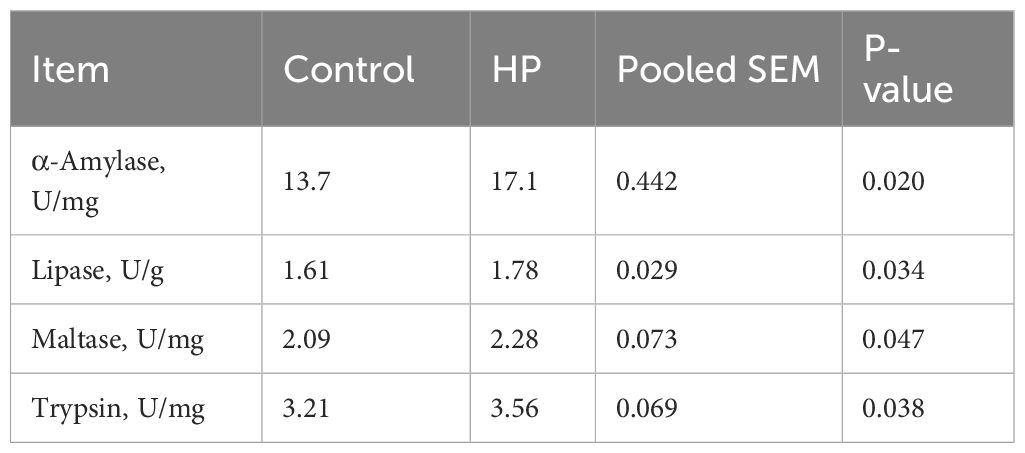
The impact of HP on intestinal digestive enzyme activity is shown in Table 6. Compared to the control group, HP supplementation increased the activities of α-amylase, lipase, maltase, and trypsin by 19.9%, 9.5%, 8.3%, and 9.8%, respectively (P<0.05).
The caecal environmental characteristics and microflora population in rabbits fed the HP-supplemented diet are reported in Table 7. The values of caecal pH, ammonia-N, total VFAs, and the propionate-to-butyrate ratio did not differ significantly between groups.
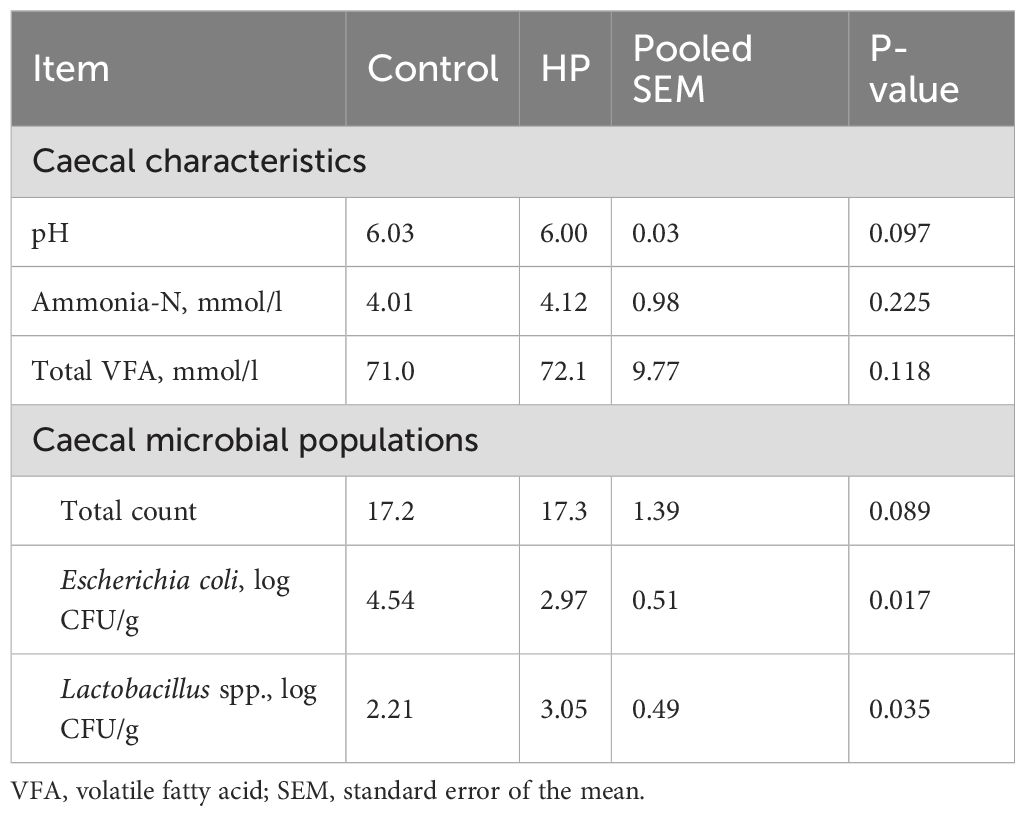
Table 7. Effect of horehound powder (HP) on caecal characteristics and microflora population of rabbits.
However, caecal microbiota analysis showed that dietary treatment with HP reduced E. coli populations by 52.8% (P=0.017), while Lactobacillus spp. populations increased by 27.5% (P=0.035).
3.5 Histomorphometric examination
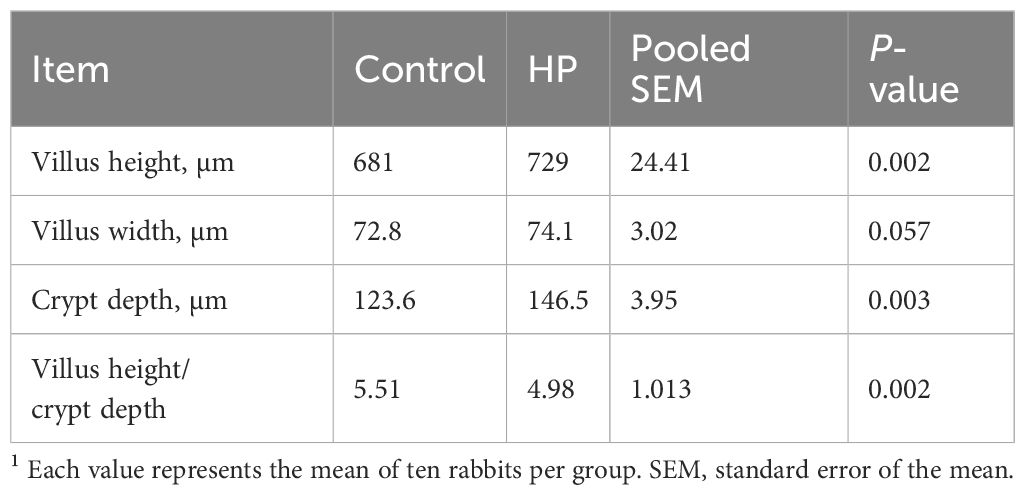
Histological examination of the duodenal mucosa and liver sections of rabbits fed the HP diet displayed morphological features similar to those of the control group (Figures 1–3). The intestinal evaluation included morphometric values of villus height (VH), villus width (VW), crypt depth (CD), and the ratio of villus height to crypt depth (VH/CD). The duodenal mucosa consisted of regular villi lined by simple columnar epithelium and basal crypts attached to a regular muscularis mucosa layer. Basal crypts, or crypts of Lieberkühn, were constituted by invaginations of the epithelium that surrounded the villi. The microscopic evaluation revealed globet cells (GCs) regularly scattered among enterocytes, which produced a protective mucous layer that lined the epithelium surface (Figures 1A, B). The Azan–Mallory trichrome stain allowed for the evaluation of intestinal structural architecture, showing a balanced arrangement of extracellular matrix and collagen fibers stained in blue in both groups (Figures 1C, D).
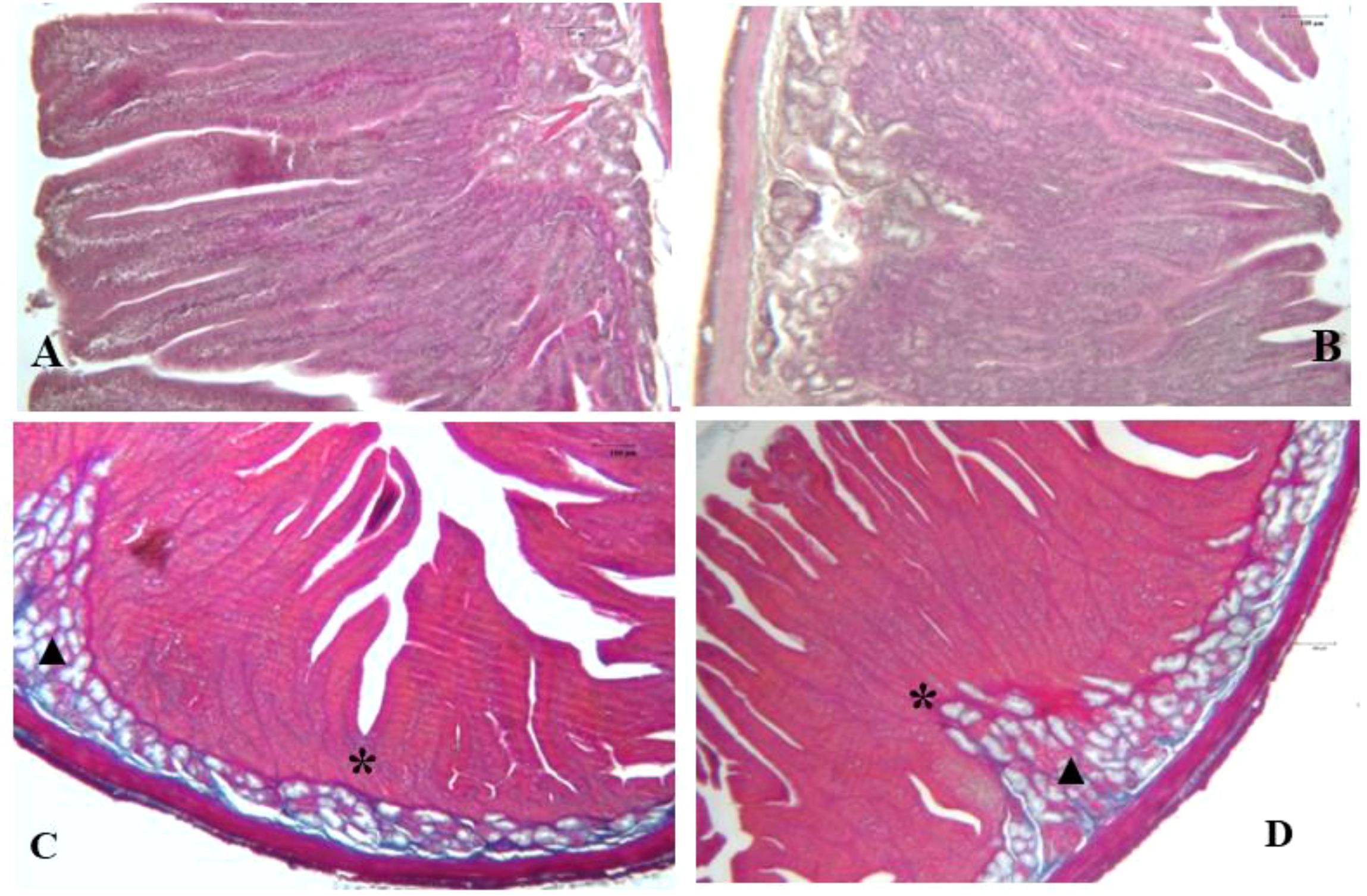
Figure 1. Photomicrographs of rabbit duodenal sections from the control diet group (A) and HP diet group (B), stained with H&E (40× magnification), and from the control group and HP group (D), stained with Azan-Mallory (25× magnification). In panels (A, B), the duodenal mucosa shows regular villi lined by simple columnar epithelium, crypts of Lieberkühn (*), and Brunner’s glands (▲), anchored on a regular muscularis mucosae layer. Azan-Mallory stain evidenced a balanced arrangement of the intestinal scaffold in both (C, D).
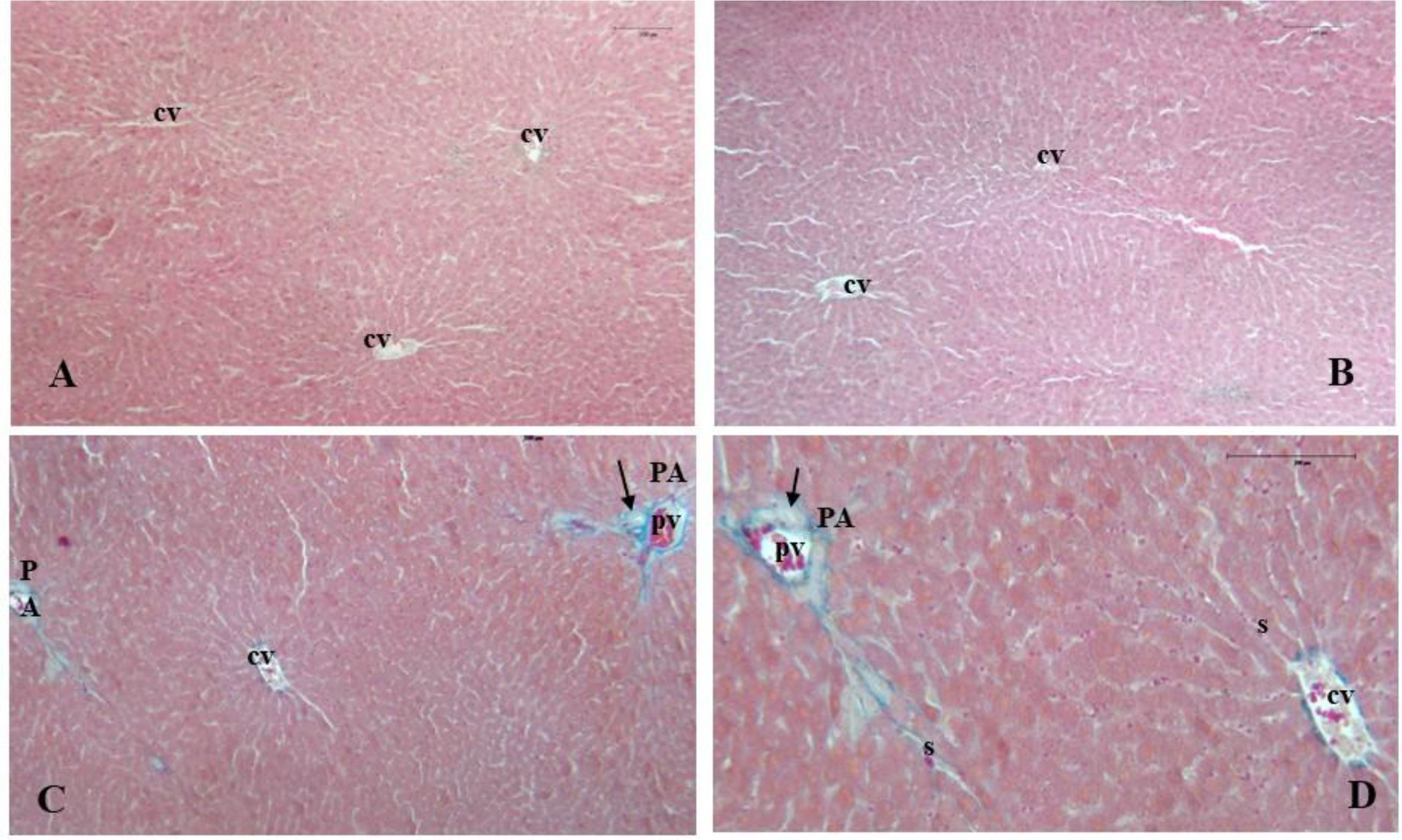
Figure 2. Photomicrographs of rabbit liver sections from the control group and HP group (B, D). (A) (H&E, 25×) and (B) (H&E, 25×) show the regular arrangement of liver lobules and hepatocytes disposed in plates radiating from the central vein (cv), along with blood sinusoids. (C) (Azan-Mallory, 25×) and (D) (Azan-Mallory, 40×) show the characteristic pattern of the portal area (PA), with dense connective tissue surrounding the portal triad containing a branch of the portal vein (pv), the hepatic artery, and the bile duct (arrow).
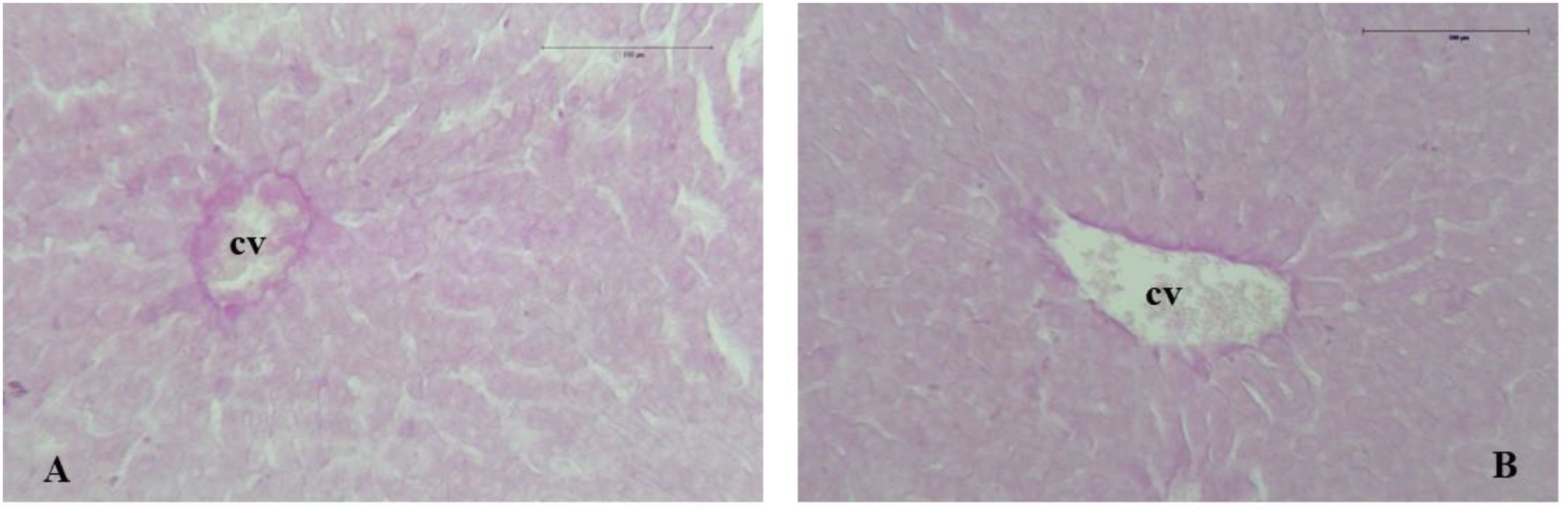
Figure 3. Photomicrographs of rabbit liver sections from the control group (A) and HP group (B) stained with PAS (100×). A portion of the hepatic lobule with centrilobular vein (cv) shows a weak positive reaction to PAS reagents and no signs of intrahepatic glycogen or triglyceride deposits.
The effect of HP dietary supplementation on the histomorphometric measurements of the rabbit duodenum is shown in Table 8 and Figure 1. VH and CD were significantly increased (P<0.05) in rabbits fed HP, while villus width was similar between groups. Histomorphometric analysis also revealed that HP induced a significant reduction in the VH/CD ratio in the experimental group compared to the control (P<0.05).
Morphological analysis of liver tissues showed well-organized structures in all groups, with no signs of alteration (Figures 2 and 3). The structural features of liver sections stained with H&E consisted of regularly shaped hexagonal lobules, typically surrounded by minimal or absent connective membranes and featuring central veins located at the center or randomly dispersed (Figures 2A, B).
Light microscopic examination disclosed a harmonious distribution of polyhedral hepatocytes characterized by clear cytoplasm and rounded nuclei. These were arranged in plates or cords radiating from the central vein toward the periphery of the hepatic lobule. Hepatic sinusoids, lined with endothelial cells, were also observed between hepatocytes. No signs of intralobular cord distortion, intracellular degeneration, or dilation of central veins or blood sinusoids were found.
Photomicrographs of liver samples stained with Azan–Mallory detected normal deposition of dense connective tissue, stained blue, in the portal triad region (Figures 2C, D). In rabbits, as described in the literature (Al-Hamdany, 2019), the portal area lies at the corners of hepatic lobules and contains branches of the hepatic artery, portal vein, and bile duct, as evidenced in Figures 2C, D).
The PAS method revealed glycogen content in the cytoplasm of hepatocytes, with intrahepatic glycogen staining dark pink. PAS staining confirmed the presence of glycogen deposition, whether or not combined with triglyceride accumulation, such as in glycogen storage diseases or metabolic or stress-related syndromes. In PAS-stained sections, liver samples from both rabbit groups displayed a weak positive reaction, indicating no significant hepatic glycogen accumulation (Figures 3A, B). No signs of hepatocyte degeneration, vacuolization, or intrahepatic lipid accumulation were found in the collected samples.
4 Discussion
This study aimed to explore the modes of action and effects of a phytobiotic dietary supplement based on M. vulgare powder in rabbit production and health. As mentioned above, in vivo studies on the biological activity of M. vulgare extracts in animals are still scarce; therefore, data discussion in this trial was mainly supported by the extensive literature on phytochemicals used in rabbit nutrition, with a focus on the bioactive compounds of M. vulgare.
In recent years, a number of chemical studies have demonstrated that M. vulgare extracts are complex mixtures composed of a wide range of compounds that may exert multiple biological functions in the animal body. To our knowledge, no evaluation of the phytochemical profile of M. vulgare herbal extract grown in Italy has been reported until now. Data obtained in this study were consistent with previous investigations conducted on the same herbal species in different regions of the world and showed that M. vulgare powder contains significant amounts of phenolic and bioactive compounds, as reported in Table 2.
Recently, Aitbaba et al. (2024), in a comprehensive study exploring the phytochemical composition of an aqueous extract of M. vulgare leaves, revealed a significant presence of bioactive compounds, with considerable amounts of total polyphenols (232.6 ± 4.22 mg GAE/g), flavonoids (89.2 ± 2.07 mg QE/g), and condensed tannins (26.94 ± 1.02 mg CE/g). Wojdyło et al. (2007) showed that in a Polish M. vulgare extract the total phenolic content was 3.86 ± 0.05 mg GAE/g and that the major phenolic compounds identified were caffeic acid, p-coumaric acid, and ferulic acid. Furthermore, Rezgui et al. (2020) reported that in a Tunisian M. vulgare extract, the main phenolic compounds were sinapic acid, quercetin, ferulic acid, p-coumaric acid, caffeic acid, apigenin, and luteolin.
The determination of total phenols and flavonoids in an M. vulgare leaf extract conducted by Salaj et al. (2018) found 59.87 ± 7.31 mg GAE/g of total phenols and 14.47 ± 0.54 mg QE/g of total flavonoids (apigenin, luteolin, and their 7-O-glucosides), confirming high levels of these secondary plant metabolites. The HPLC analysis of a Saudi M. vulgare methanolic extract (Al-Zaban et al., 2021) revealed higher phenol content (26.8 ± 0.01 mg/GAE g) than flavonoid content (0.61 ± 0.05 mg EC/mL), and identified ferulic acid (28.9 mg/g), luteolin-7-O-d-glucoside (26.35 mg/g), and premarrubiin (28.28 mg/g) as the dominant compounds.
Similarly, the M. vulgare phytochemical profiling in this study evidenced the presence of chlorogenic acid, caffeic acid, coumaric acid, ferulic acid, apigenin, and apigenin 7-glucuronide, with a prevalence of apigenin (101.3 ng/g). In addition, total phenolic content (TPC) was 82.87 mg/GAE g—a notable amount compared to other data found in the literature (Wojdyło et al., 2007; Salaj et al., 2018; Al-Zaban et al., 2021; Mulondo et al., 2023).
Several reports conducted on various plant species have detected the free radical scavenging ability and protection from oxidative stress exerted by these secondary plant metabolites (Sengul et al., 2009; Kozlowska and Szostak-Wegierek, 2014; Kabach et al., 2019; Al-Zaban et al., 2021). Gulcin et al. (2003) also suggested that polyphenolic compounds seem to play a role in the stabilization of lipid oxidation, coupled with their antioxidant activity. Notably, multiple assessments have displayed a significant linear correlation between TPC values and the antioxidant capacity of plants, implying that extracts with the highest polyphenol contents exert higher antioxidant activity (Verzelloni et al., 2007; Al-Zaban et al., 2021; Rezgui et al., 2020).
For instance, in a study on methanolic extracts of four Lamiaceae spp. (Ajuga iva, Marrubium vulgare, Mentha pulegium, and Teucrium polium), Khaled-Khodja et al. (2014) found significant positive correlations between antioxidant activities and both TPC and total flavonoid (TF) contents. In particular, the M. vulgare extract had a TPC of 20.07 ± 0.44 mg GAE/g and a TF content of 7.03 ± 0.28 mg QE/g, while the antioxidant activity measured by the DPPH assay was IC50 0.522 ± 0.001 mg/mL. Earlier, Okur et al. (2019) obtained an IC50 of 2.08 mg/mL and a TPC of 48.97 mg GAE/g for a methanolic extract of M. vulgare. Another study, comparing the free radical scavenging activity of methanolic and aqueous extracts of M. vulgare, reported IC50 values of 2.49 mg/mL and 3.09 mg/mL, respectively, with corresponding total polyphenolic values of 60.40± 6.63 mg GAE/g and 32.71± 3.82 mg GAE/g (Kabach et al., 2019).In the present study, the M. vulgare extract exhibited good activity in the DPPH assay with an IC50 equal to 1.31 mg/mL and a TPC of 82.87 mg/GAE g (Table 2). As is known, the lower the IC50 value, the higher the antioxidant capacity of the plant extract. Thus, it may be concluded that the aerial parts of the M. vulgare tested in our study demonstrated notable antioxidant capacity.
Lastly, in this trial, the quantitative analysis of the fatty acid composition of horehound powder (HP) revealed a high amount of ursolic acid (UA), accounting for 22.9% of total methyl esters. Literature reports that UA is widely present in medicinal plants, and in recent years it has gained considerable attention for its pharmacological properties, such as antioxidant and anti-inflammatory effects (Peng et al., 2021). In addition, UA has demonstrated both direct and indirect antibacterial activity (Podder et al., 2015; Grace et al., 2016). Many studies on experimental animal models reported that UA may play a role in the modulation of gut microbiota structure and intestinal homeostasis (Zhang et al., 2019), restoring mucosal integrity after antibiotic damage or in enteritis and colitis affections (Chun et al., 2014; Liu et al., 2016; Seow and Lau, 2017; Peng et al., 2021).
Considering the large amounts of the above-mentioned biocompounds found in our plant samples, it can be supposed that dietary supplementation with HP may hold potential for enhancing rabbit nutrition and health, contributing to the stimulation of metabolic processes, growth, production, and overall welfare of the animals (Schlemper et al., 2021; da Silva et al., 2024). Moreover, several studies have reported the growth-promoting effects of using phytobiotics as additives in rabbit diets (Dalle Zotte et al., 2020; Abdel-Wareth et al., 2022; Menchetti et al., 2020; Pugliese et al., 2024).
The results of the present study showed that dietary HP supplementation was effective in improving in vivo performance (FBW, ADG, and FCR) in male rabbits; however, no effects were observed on feed intake or carcass traits (Table 3). According to the literature, the presented data may be related to the influence of M. vulgare bioactive compounds on animal productive performance (Yang et al., 2009). The growth-promoting effect of M. vulgare has previously been investigated in experimental models such as rodents. Scientific studies have found that dietary inclusion of M. vulgare at different doses induced a slight increase in body weight and had a positive influence on liver function in treated rats (Haouari et al., 1991; Ahmed et al., 2010; Elberry et al., 2010; El-Hallous et al., 2018). Chabane et al. (2020), in a toxicity study on a methanol extract of M. vulgare (MEMV), detected that at the dose of 800 mg/kg of body weight, MEMV significantly affected weight gain and the weight of various organs (liver, intestine, colon, kidney, spleen, heart, and lung) without any adverse effects in treated rats. In a survey on weaned piglets, da Silva et al. (2024) indicated that M. vulgare infusion up to 10% (corresponding to 0.1 mg/kg BW) promoted weight gain with no significant alterations in liver and kidney functions or serum biochemical values. Similarly, in broilers, Molinetti et al. (2017), comparing the effects of a diet supplemented with M. vulgare infusion (IMV) at concentrations of 2%, 4%, and 6% versus a diet with an antibiotic growth promoter (tylosin 1%), found a significant improvement in FCR and increased weight gain in subjects that received dietary IMV.
Interestingly, Schlemper et al. (2021) examined the productive performance and meat sensory characteristics of broilers after administration of an infusion of M. vulgare and L. sibiricus water extracts. The authors found that groups receiving plant infusions showed improved final body weight (FBW) and FCR, without any alteration in meat color or sensory qualities. The same authors concluded that the influence of M. vulgare on productive parameters was probably due to multiple modes of action linked to the antioxidant, anti-inflammatory, gastroprotective, and antimicrobial properties of phytobiotic compounds, mainly phenols (Batiha et al., 2020; Mahfuz et al., 2021). In particular, these compounds may operate through a synergistic mechanism that simultaneously acts on feed utilization, the stimulation of digestive secretions, and on gut microenvironment and morphology—ultimately optimizing nutrient utilization and growth indices (Paula de Oliveira et al., 2011; Zarai et al., 2011; Ghedadba et al., 2016).
The literature reports that bioactive compounds from herbs may enhance digestion and absorption by influencing the motility of the stomach and intestine (Dhama et al., 2015; Mendel et al., 2017; Ademolue et al., 2024). Gastrointestinal (GI) motility plays a pivotal role in animal digestive physiology, especially in rabbits. GI transit is governed by both the enteric nervous system and hormonal networks (Fukui et al., 2018). Moreover, since contractions of intestinal smooth muscles are Ca2+ dependent, it is hypothesized that the effects produced by plant extracts might also be due to interference with Ca2+ influx or Ca2+ release from intracellular stores (Brading, 1981; Zarga et al., 1995; Schlemper et al., 1996).
In vitro studies have found that hydroalcoholic (50% ethanol) extracts of roots or aerial parts of M. vulgare exert significant antispasmodic activity on several smooth muscles, including those of guinea pig ileum and rat duodenum (Schlemper et al., 1996). Analysis of the gathered data revealed that the spasmolytic effect of M. vulgare extracts may be mediated by inhibition of neurotransmitters or via calcium channel blockade, and that these effects may be attributed to the presence of steroids and terpenes, particularly marrubiin (Lodhi et al., 2017). For instance, tests conducted on isolated rabbit jejunum found that marrubiin exhibits strong spasmolytic and Ca2+ antagonistic activity (Hussain et al., 2011). Additionally, there is some evidence that GI contractility may be regulated by plant secondary metabolites, such as flavonoid compounds, which might exert a Ca2+ channel-blocking activity on gut smooth muscles (Capasso et al., 1991; Schlemper et al., 1996). Thus, it is reasonable to assume that the effects on rabbit weight gain observed in this trial may be attributed, among other factors, to a regulatory influence of the plant extract on GI motility and intestinal transit, thereby improving nutrient absorption (Schlemper et al., 2021).
Notably, many studies have reported that one of the core modes of action of bioactive compounds in certain plant extracts in improving digestion rates and growth consists in enhancing the production of digestive secretions such as saliva (Rodriguez Villanueva and Martin Esteban, 2016; Büyükkartal et al., 2016), digestive enzymes (Arram et al., 2018; Zaikina et al., 2022), bile, and mucus (Ferdous et al., 2019; Elwan et al., 2020; Alagawany et al., 2021; Abdel-Wareth et al., 2022; Reda et al., 2025). The activity of intestinal digestive enzymes is a reliable indicator of digestive and metabolic processes (Wang et al., 2024). In the GI environment, the actions of digestive enzymes such as trypsin, lipase, and amylase on dietary particles increase the availability of essential nutrients for absorption, thereby optimizing nutrient utilization in the digestive tract (Hashemi and Davoodi, 2011; Yang et al., 2017; Bedford and Apajalahti, 2022; Pugliese et al., 2024). For instance, pancreatic enzyme activity (i.e., amylase, lipase, and protease) may be linked to body and intestinal weight gain due to its significant role in the breakdown of macromolecules at the small intestinal level (Sklan and Noy, 2000; Choct, 2009). In the same manner, the stimulation of α-amylase activity improves the hydrolysis of glycosidic bonds present in starch, while enhanced protease activity promotes protein digestion and assimilation (Abdel-Wareth et al., 2018; Gourich et al., 2023).
In monogastric species, Windisch et al. (2008) reported that the use of phytogenics as dietary supplements can boost the production of endogenous digestive enzymes, such as trypsin and amylase, thereby fostering nutrient utilization. Existing literature reports a positive association between the promotion of growth indices and the stimulation of digestive enzymes (Wenk, 2003; Hashemi and Davoodi, 2011). Alagawany et al. (2021) stated that medicinal plants may exert a marked stimulation of many digestive enzymes in the small intestine and pancreas, and that the increased activity of endogenous enzymes may be related to improved nutrient digestibility and increased productive performance.
Notably, some natural digestive enhancers can promote both the synthesis and activity of enzymes and increase the production of bile acids (Abd-El-Hady, 2014). In particular, some herb extracts may exert choleretic and/or cholagogue effects by promoting the synthesis and/or secretion of bile acids that facilitate nutrient solubilization through strong emulsifier activity on liberated lipids (Choct, 2009; Dhama et al., 2015).
Traditionally, M. vulgare juice and infusion have been used to promote gastric and bile secretion due to the presence of bitter constituents, particularly marrubinic acid (Rodriguez et al., 2016; Lodhi et al., 2017; Acmovic et al., 2020). Indeed, the sesquiterpenes marrubiin and premarrubiin have shown the ability to stimulate the secretion of saliva and gastric juice (Büyükkartal et al., 2016). Moreover, M. vulgare is also used as a choleretic and cholagogue agent (Capasso, 2003; Al-Snafi et al., 2021). In particular, marrubiinic acid, which arises from the breakdown of marrubiin in the intestine, has demonstrated a strong choleretic effect (Sahpaz et al., 2002; Lodhi et al., 2017). Thus, the bioactives found in this herb may offer multiple advantages for the digestive system, simultaneously promoting digestive and metabolic processes (Placha et al., 2022).
In the current study, dietary HP inclusion exerted a favorable influence on digestive enzyme secretion, as shown by the significant increases in α-amylase, lipase, maltase, and trypsin levels in treated rabbits (Table 6). To the best of our knowledge, no previous research has explored the influence of dietary HP on digestive enzyme stimulation in livestock species. This improvement could be associated with some of the identified chemical components in the HP extract. Although the mode of action is still not clearly established, it may be supposed that the active ingredients in HP—such as phenols, flavonoids, and tannins—could trigger digestive enzyme activity under certain conditions. These effects may be linked to their influence on the functional units of gut epithelial architecture, namely villus enterocytes (Shehata et al., 2022), and their modulatory effects on gut microbiota balance, which in turn regulate metabolism and improve local epithelial proliferation (Feng et al., 2021; Shehata et al., 2022; El-Sabrout et al., 2023).
In this regard, Peng et al. (2021) stated that ursolic acid—found in significant amounts in our herb—may be effective in supporting gut homeostasis and increasing digestive enzyme secretion by strengthening the intestinal barrier function and influencing gut microbial ecology. Notably, the antioxidant activity of phenolic compounds in M. vulgare may protect digestive tract cell membranes from oxidative damage, thereby improving the gut environment and enzyme capacity (Mutwedu et al., 2022). Furthermore, the choleretic and cholagogue effects of HP phytochemicals may have influenced lipid metabolism and feed utilization by increasing the availability of bile acids. The improvements in both feed efficiency and enzymatic activity found in this trial support the hypothesis that HP bioactive molecules favorably modulate digestive activity and gut function, resulting in a regulatory effect on rabbit growth performance.
It is noteworthy that the meat FA profile may be influenced by feeding treatments aimed at maximizing the content of FAs that provide nutritional benefits (Saini and Keum, 2018; Jachimowicz et al., 2022). In particular, rabbit meat is a valuable source of polyunsaturated fatty acids (PUFA), and it has been found that manipulating the rabbit diet is a viable strategy to optimize its meat lipid profile (Dalle Zotte, 2002; Dalle Zotte and Szendro, 2011; Elazab et al., 2022). For instance, it has been reported that rabbits fed a diet enriched with n-3 or n-6 precursors (C18:3 n-3, α-linolenic acid, ALA; C18:2 n-6, linoleic acid, LA) exhibit meat with higher levels of long-chain PUFA (Dal Bosco et al., 2004; Li et al., 2012; Zubiri-Gaitán et al., 2022).
In this study, the effect of dietary HP on the FA composition of L. lumborum muscle in the examined rabbits is presented in Table 4. Although no significant differences were found for total SFA, a significant decrease in C18:0 (stearic acid) was observed in the HP-fed group. Additionally, differences were found in total MUFA and C16:1 cis (palmitoleic acid), which were significantly higher in the control group. Conversely, muscles of HP-fed rabbits exhibited a significant increase in C18:2 n-6 (linoleic acid, LA), total PUFA, and PUFA n-6, along with a significant reduction in C20:3 n-6 (dihomo-γ-linolenic acid, DGLA) and C22:5 n-3 (docosapentaenoic acid, DPA), resulting in increased n-6 PUFA content and a higher n-6/n-3 ratio.
Dietary recommendations for human health suggest restricting SFA intake and increasing PUFA content in animal-derived products to reduce the risk of cardiovascular diseases (Palazzo et al., 2020; Adli et al., 2024). As reported by Adli et al. (2024), the use of plant extracts contributes to the production of healthier rabbit meat, increasing PUFA levels at the expense of SFA. Winiarska-Mieczan et al. (2024) demonstrated in poultry that a ΣPUFA/ΣSFA ratio higher than 0.45 is a quality requirement for meat, as lower values are associated with hypercholesterolemic effects in humans. In the present research, although the HP-enriched diet did not significantly reduce total SFA, the ΣPUFA/ΣSFA ratio was above this value (0.81). This finding aligns with Peiretti et al. (2011), who reported that consumption of meat enriched with MUFA and PUFA reduces the atherogenic index and the risk of cardiovascular and metabolic disorders.
On the other hand, as mentioned above, rabbit meat is naturally rich in PUFA, which makes the intramuscular fat more susceptible to oxidative processes occurring both in vivo and postmortem. This trait negatively impacts the nutritional value, physical properties, and stability of rabbit meat (Dalle Zotte, 2002; Emami et al., 2020; Prates, 2025). To counter these effects, previous studies have reported that dietary supplementation with plant extracts may be an effective strategy for introducing natural antioxidants into phospholipid membranes, thereby modulating tissue oxidative stability (Botsoglou et al., 2004; Vizzarri et al., 2017; Untea et al., 2022; Bešlo et al., 2023) and stimulating the endogenous antioxidant systems of animal tissues (Lee et al., 2017; Amer et al., 2022).
In this feeding trial, the HP group showed a significant increase in n-6 LA, ΣPUFA, and total n-6 PUFA, along with a lower MUFA content. These findings suggest that the UFA content observed in the experimental group may be related to the antioxidant activity of HP bioactives and their capacity to directly inhibit lipid peroxidation or indirectly enhance endogenous antioxidant enzyme activity. Notably, in vitro assays on M. vulgare revealed an antioxidant activity twice as high as that of the synthetic antioxidant butylated hydroxytoluene (BHT) (Abadi and Hassani, 2013; Acmovic et al., 2020). It was assumed that the antioxidant activity of HP polyphenols halted the lipid peroxidation cascade in L. lumborum muscle lipids, particularly targeting the more oxidation-sensitive PUFAs (Rezgui et al., 2021; Bešlo et al., 2023).
Gulcin et al (2003) stated that polyphenols play a crucial role in stabilizing lipid oxidation due to their antioxidant properties. Palazzo et al. (2015) also reported increased oxidative stability in the Longissimus thoracis and lumborum muscles of rabbits fed plant polyphenols. Furthermore, numerous studies have highlighted a linear correlation between the phenolic content of plants and their antioxidant capacity (Rezgui et al., 2020; Al-Zaban et al., 2021). Therefore, our findings may be explained by the hypothesis that HP polyphenols protect oxidation-sensitive PUFA from peroxidation, rather than the more stable MUFA or SFA (Hashemipour et al., 2013). Similarly, the increased level of LA found in treated rabbits can be attributed to the presence of antioxidants, which protect this FA from oxidation. Remarkably, Rezgui et al. (2021), exploring the antioxidant activity of M. vulgare for potential use as a human dietary supplement, demonstrated its capacity to inhibit the oxidation of LA through the β-carotene bleaching assay.
Rabbits receiving HP showed both greater concentrations of PUFA and elevated serum antioxidant enzyme activities (Table 5). Similar results were reported by Hashemipour et al. (2013), who found that in broilers, a diet supplemented with phenols (thymol and carvacrol) improved endogenous oxidative status and delayed lipid oxidation in thigh muscles, resulting in enhanced tissue levels of UFA. Thus, the active components in the HP diet may affect muscle lipid metabolism by strengthening the endogenous antioxidant defense system, which aids in hindering lipid oxidation and improves the nutritional value, sensory attributes, and shelf life of meat (Elghalid et al., 2020; Prates, 2025).
The blood profile is a relevant marker of biological balance and the proper functioning of rabbits’ metabolic processes (Ogbuewu et al., 2017; Levchehko et al., 2024). This feeding trial investigated the effect of HP on the serum lipid profile and antioxidant capacity in rabbits (Table 5). In agreement with da Silva et al (2024), our study found that serum cholesterol, LDL, HDL, and TG were not affected by dietary treatment, while oxidative capacity was significantly improved by HP supplementation. Indeed, the HP group exhibited enhanced antioxidant enzyme activity, with a significant increase in serum TAC and SOD and a significant reduction in MDA.
According to Elghalid et al. (2020), herbal mixtures rich in phenolic compounds can elicit antioxidant effects by scavenging reactive oxygen species (ROS) and by upregulating endogenous antioxidant activity. Polyphenolic compounds, such as phenols and flavonoids, can protect cells by modulating the primary intracellular antioxidant pool (e.g., SOD, CAT, and GSH-px), increasing the capacity of the antioxidant defense system (Asadi-Samani et al., 2015). Superoxide dismutase (SOD), together with TAC, serves as an indicator of plasma capacity to neutralize free radicals generated by oxidation, thereby preventing or slowing free radical chain reactions in tissues (Habashy et al., 2019; Abdelnour et al., 2020; Sheiha et al., 2020). The greatest levels of SOD and TAC, and the lowest MDA values in rabbits fed HP, suggest that it acts as an effective antioxidant capable of alleviating oxidative stress in plasma.
Moreover, in this study, the improvement of antioxidant biomarkers and the associated decline in MDA levels suggest that HP supplementation may serve as both an exogenous antioxidant and a protective agent against tissue damage. Literature reports that M. vulgare can improve tissue antioxidant status by enhancing TAC and enzymatic defenses against oxidative stress, while reducing MDA concentrations in animal tissues, indicating decreased lipid peroxidation in membranes (Acmovic et al., 2020; Al-Snafi et al., 2021). For example, M. vulgare was found to upregulate GSH, SOD, and CAT enzyme levels and overall TAC, along with a significant decrease in MDA in liver tissues exposed to hepatotoxic agents (Elberry et al., 2010; Akther et al., 2013; Ibrahim et al., 2014; Ettaya et al., 2016; El-Hallous et al., 2018). Investigating the hepatoprotective activity of a methanol extract of M. vulgare, Akther et al (2013) linked the increased activity of primary intracellular antioxidant enzymes with its role in preventing excessive ROS accumulation in the liver. According to Ettaya et al. (2016), the protective effect of M. vulgare on liver tissue may be attributed to the presence of phenolic acids and flavonoids with proven antioxidant properties.
Liver and gut functional states play a vital role in digestion, metabolism, and nutrient storage in animals (Carabano et al., 1998; Flees et al., 2017). In rabbits, the liver is the primary site of lipogenesis, playing a pivotal role in lipid metabolism, transport, and fat deposition (Gondret et al., 1997; Zubiri-Gaitán et al., 2022). The study of liver and intestinal morphological features provides insight into their structural and functional states (Levchehko et al., 2024). Thus, to support the biochemical findings, this trial also investigated the influence of HP on rabbit gut and liver histological traits.
To sustain animal production, gut and liver tissues bear a substantial workload in handling nutrient metabolism and lipogenesis. These tissues are among the most susceptible to stress-related disorders, such as redox imbalance, oxidative toxicity, or lipid peroxidation. In the liver, hepatocyte degeneration or vacuolization, intracellular glycogen or triglyceride storage, as well as changes in sinusoidal arrangement, are among the initial signs of liver injury and thus indicate metabolic disruption. For instance, it has been reported that oxidative stress reactions induced by heat stress in rabbits impair liver lipid metabolism, promote hepatic lipid aggregation, and alter the availability of precursors for gluconeogenesis (Naji et al., 2017; Abdelnour et al., 2019). Similarly, Lu et al. (2019) detected abnormal hepatic lipid accumulation in broilers exposed to heat stress.
At the cellular level, oxidative metabolism imbalance leads to damage within the cellular system due to excessive free radical production, which binds to the cellular membrane and induces cytologic degeneration (Naji et al., 2017; Wu et al., 2019). These radicals interfere with lipid metabolism, decreasing lipid transport out of the hepatocytes and thereby promoting fat accumulation or hepatic steatosis. Additionally, degradation of reactive radicals produces reactive aldehydes, which increase membrane permeability, induce cellular vacuolization and hydropic degeneration, and may ultimately lead to cell death (Naji et al., 2017).
In the current study, liver histological analysis in treated groups revealed compact parenchyma with a normal arrangement of hepatic cells forming the typical liver functional unit—hepatic lobules—characterized by a normal central vein and hepatocytes with well-preserved cytoplasm and nuclei (Figures 2A, B). Light microscopic examination of H&E-stained samples did not reveal signs of intralobular cord distortion, intracellular degenerative changes such as hepatocyte ballooning, nor dilated or congested central veins or sinusoids.
Furthermore, the histochemical evaluation of PAS-stained sections did not reveal abnormal glycogen deposition or intracellular lipid droplets (Figures 3A, B). Azan-Mallory trichrome staining showed no abnormal connective tissue deposition surrounding hepatic lobules or portal triads (Figures 2C, D).
These liver histological findings appear to support the serum enzyme assay results. Although serum biochemical markers of liver function (e.g., ALT, ALP, AST) were not directly measured, the significant increase in serum TAC and SOD and the reduction in MDA may indicate a positive influence of M. vulgare on tissue antioxidant status.
Consistent with this, available literature reports that HP can counteract oxidative toxicity in liver tissue via its antioxidant and free radical scavenging properties. These effects are attributed to polyphenolic compounds and ursolic acid, which act by inhibiting microsomal enzymes and lipid peroxidation (Asadi-Samani et al., 2015).
Intestinal disorders are a common issue in intensive rabbit farming, especially at weaning, leading to a significant decline in animal performance and welfare (Palazzo et al., 2020). Oxidative stress is known to interfere with intestinal cell function and structure, impair digestive enzyme activity, and shift caecal microbial balance (Xu et al., 2014; Zhu et al., 2012). It is widely reported that the bioactive compounds in plant extracts have demonstrated the ability to modulate gut microbiota populations. Moreover, as described in various studies, phytogenic compounds with antioxidant properties may exert beneficial effects on gut morphology by protecting the intestinal mucosal layer, thereby impacting villus height (VH), crypt depth (CD), and the total intestinal absorptive surface (Kamboh and Zhu, 2014; Darmawan et al., 2022).
Hence, intestinal architecture evaluation may provide a reliable indicator of gut health status and offer further insight into the potential benefits of horehound. To date, no previous studies have assessed the influence of M. vulgare on gut morphology and the intestinal microenvironment. In this study, duodenal histological features were comparable between rabbit groups. The intestinal mucosa exhibited a well-organized architecture, consisting of villi and basal crypts, both covered by regular enterocytes and dispersed mucus-secreting goblet cells. Morphometric evaluation revealed that HP significantly increased VH and CD in the duodenum, resulting in an improved VH/CD ratio.
The VH, CD, and VH/CD ratio are indirect markers of the functional capacity of the enteric mucosa (Martínez et al., 2022; Tufarelli et al., 2025). Taller villi increase the intestinal absorptive surface, while deeper crypts promote rapid villus renewal—both supporting nutrient digestion and absorption (Patra et al., 2019). Additionally, increased VH is associated with enhanced brush border enzyme activity and improved nutrient transport (Murugesan et al., 2014, Murugesan et al., 2015). It is possible to argue that HP biocompounds exerted a positive influence on gut architecture enhancing the small intestine absorptive efficiency, thus explaining the increased growth performance and digestive enzyme content in rabbits.
Improvement of the gut environment in HP-treated rabbits was also confirmed by caecal microenvironment evaluation. Although no significant differences were observed in caecal characteristics between groups, microbiota counts revealed that dietary HP supplementation significantly increased Lactobacillus spp. and significantly reduced E. coli populations. The caecal microbial population plays a pivotal role in the regulation of animal growth and health. It is important to consider that some phytogenic feed additives beneficially affect gut microflora, either directly or indirectly (Hashemi and Davoodi, 2011). For example, polyphenols may exhibit prebiotic properties, promoting the development of beneficial gut microorganisms (e.g., Bacillus spp. and Lactobacillus spp.) at the expense of certain pathogenic bacteria, thus positively modulating gut microbiome activity (Hashemi and Davoodi, 2011; Qiao et al., 2014; Serra et al., 2018; Scott et al., 2022; Bešlo et al., 2023; Simitzis, 2017).This favorable influence on caecal microbiota balance may, in turn, promote intestinal morphology by supporting villus cell regeneration and enhancing absorptive capacity and enzymatic activity (Elghalid et al., 2020). It is worth noting that, in this study, the chemical profile of HP revealed the presence of the triterpenoid ursolic acid (UA), which has been reported to exert antioxidant and anti-inflammatory properties (Mancha-Ramirez and Slaga, 2016; Seow and Lau, 2017; Wan et al., 2019). In particular, Peng et al. (2021) investigated the effects of UA on intestinal health and gut flora in mice, finding that dietary supplementation improved intestinal morphology and mucosal barrier integrity, regulated the expression of nutrient transport carriers, and positively influenced microbiota structure. Finally, the same authors argued that UA had positive effects on growth performance through its positive impact on gut health and resident microbial flora. Taking into account these findings, our results highlight that HP had a beneficial effect on multiple aspects of intestinal digestive efficiency and cecal environment.
5 Conclusions
From the results of the present study, it can be stated that horehound dietary inclusion in rabbits exerted a positive influence on growth performance, gut health, and oxidative metabolism, as confirmed by serum antioxidant capacity. In addition, the balancing effect on redox status resulted in reduced lipid oxidation susceptibility in the muscle tissue of rabbits fed the herb. Feeding rabbits with horehound also had a remarkable effect on duodenal digestive enzyme activity, histomorphometry, and caecal microenvironment.
The effect of horehound on oxidative balance suggests that our data on liver health status could be enhanced by specific assays, such as antioxidant markers and enzymes. Further studies are needed to gain insight into the mechanisms of action and the specific role of bioactives in M. vulgare through in vivo studies. Moreover, additional in vivo research may delve into the long-term influence of horehound supplementation on rabbit productive traits and welfare at different physiological stages, as well as determine the appropriate inclusion rate to achieve consistent results.
These findings contribute to the growing body of evidence supporting the consideration of horehound as a valuable source of phytochemicals, capable of introducing natural antioxidants into the diet and offering a promising way to optimize sustainable rabbit production and welfare.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Animals were handled and cared in compliance with the EU legislation on animal welfare regulations (Directive 2010/63/EU, which updates and replaces the 1986 Directive 86/609/EEC) and following the research policies of the DiMePRe-J of the University of Bari Aldo Moro, Italy (Approval code 09/2022). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
CL: Formal Analysis, Writing – original draft, Investigation. GP: Writing – original draft, Formal Analysis, Investigation. LeP: Formal Analysis, Investigation, Writing – original draft. FG: Conceptualization, Funding acquisition, Writing – original draft. EC: Formal Analysis, Methodology, Writing – original draft. VR: Writing – original draft, Formal Analysis. LG: Formal Analysis, Writing – original draft. MS: Investigation, Writing – original draft. VL: Conceptualization, Writing – review & editing. LuP: Methodology, Writing – original draft, Formal Analysis. VT: Conceptualization, Funding acquisition, Writing – original draft, Investigation, Data curation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. Part of this study was carried out within the Agritech National Research Center and received funding from the European Union Next-GenerationEU (PIANO NAZIONALE DI RIPRESA E RESILIENZA (PNRR) – MISSIONE 4 COMPONENTE 2, INVESTIMENTO 1.4 – D.D. 1032 17/06/2022, CN00000022). This manuscript reflects only the authors’ views and opinions, neither the European Union nor the European Commission can be considered responsible for them.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abadi A. and Hassani A. (2013). Essential oil composition and antioxidant activity of Marrubium vulgare L. growing wild in Eastern Algeria. Int. Lett. Chemistry Phys. Astronomy 9, 17–24. doi: 10.56431/v-02o4j8
Abd-El-Hady A. M. (2014). Performance, physiological parameters and slaughter characteristics in growing rabbits as affected by a herbal feed additives (DIGESTAROM). J. Int. Sci. Publications: Agric. Food 2, 353–365.
Abdelnour S. A., Abd El-Hack M. E., Khafaga A. F., Arif M., Taha A. E., and Noreldin A. E. (2019). Stress biomarkers and proteomics alteration to thermal stress in ruminants: A review. J. thermal Biol. 79, 120–134. doi: 10.1016/j.jtherbio.2018.12.013
Abdelnour S. A., El-Saadony M. T., Saghir S. A. M., Abd El-Hack M. E., Al-Shargi O. Y. A., Al-Gabri N., et al. (2020). Mitigating negative impacts of heat stress in growing rabbits via dietary prodigiosin supplementation. Livestock Sci. 240, 104220. doi: 10.1016/j.livsci.2020.104220
Abdel-Wareth A. A., Kehraus S., and Südekum K. H. (2022). Evaluation of oregano leaves and plant bioactive lipid compounds as feed additives for growing rabbits: Effects on performance, nutrient digestibility, serum metabolic profile and carcass traits. Anim. Feed Sci. Technol. 284, 115208. doi: 10.1016/j.anifeedsci.2022.115208
Abdel-Wareth A. A., Taha E. M., Südekum K. H., and Lohakare J. (2018). Thyme oil inclusion levels in a rabbit ration: Evaluation of productive performance, carcass criteria and meat quality under hot environmental conditions. Anim. Nutr. 4, 410–416. doi: 10.1016/j.aninu.2018.02.004
Abu Hafsa S. H., Ibrahim S. A., and Hassan A. A. (2017). Carob pods (Ceratonia siliqua L.) improve growth performance, antioxidant status and caecal characteristics in growing rabbits. J. Anim. Physiol. Anim. Nutr; 101, 1307–1315. doi: 10.1111/jpn.12651
Acmovic M., Jeremić K., Salaj N., Gavarić N., Kiprovski B., Sikora V., et al. (2020). Marrubium vulgare L.: A phytochemical and pharmacological overview. Molecules 25, 2898. doi: 10.1080/13510002.1997.11747115
Ademolue R. O., Sanwo K. A., Sobayo R. A., Ayo-Ajasa O. Y., and Okanlawon E. O. (2024). Effect of turmeric, garlic, ginger powder and their blends on growth performance and carcass yield of rabbits. Adan J. Of Agric. 5. doi: 10.36108/adanja
Adli D. N., Sugiharto S., Irawan A., Tribudi Y. A., Wibowo S., Azmi A. F. M., et al. (2024). The effects of herbal plant extract on the growth performance, blood parameters, nutrient digestibility and carcase quality of rabbits: A meta-analysis. Heliyon 10. doi: 10.1016/j.heliyon.2024.e25724
Ahmed B., Masoodi M. H., Siddique A. H., and Khan S. (2010). A new monoterpene acid from Marrubium vulgare with potential antihepatotoxic activity. Natural Product Res. 24, 1671–1680. doi: 10.1080/14786410802280976
Ahvazi M., Balali G. R., Jamzad Z., and Saeidi H. (2018). A taxonomical, morphological and pharmacological review of Marrubium vulgare L., an old medicinal plant in Iran. J. Medicinal Plants 2018 7–24.
Aitbaba A., Kabdy H., Baslam A., Azraida H., Aboufatima R., El Yazouli L, et al. (2024). Chemical investigation and antinociceptive activity evaluation of Marrubium vulgare L. aqueous extract. Chem. Biodiversity 21, e202400228. doi: 10.1002/cbdv.202400228
Akther N., Shawl A. S., Sultana S., Chandan B. K., and Akhter M. (2013). Hepatoprotective activity of Marrubium vulgare against paracetamol induced toxicity. J. Pharm. Res. 7, 565–570. doi: 10.1016/j.jopr.2013.06.023
Alagawany M., El-Saadony M. T., Elnesr S. S., Farahat M., Attia G., Madkour M., et al. (2021). Use of lemongrass essential oil as a feed additive in quail’s nutrition: its effect on growth, carcass, blood biochemistry, antioxidant and immunological indices, digestive enzymes and intestinal microbiota. Poultry Sci. 100, 101172. doi: 10.1016/j.psj.2021.101172
Al-Hamdany M. Z. (2019). Comparative anatomical, histological, and histochemical study of liver in human and domestic rabbit. Iraqi J. Veterinary Sci. 33, 437–446. doi: 10.33899/ijvs.2019.163193
Al-Snafi A. E., Al-Saedy H. A., Talab T. A., Majid W. J., Batiha G. E. S., and Abofazi J. S. (2021). The bioactive ingredients and therapeutic effects of Marrubium vulgare-A review. Int. J. Biol. Pharm. Sci. Arch. 1, 9–21. doi: 10.30574/ijbpsa.2021.1.2.0301
Al-Zaban M., Naghmouchi S., and AlHarbi N. K. (2021). HPLC-analysis, biological activities and characterization of action mode of Saudi Marrubium vulgare against foodborne diseases bacteria. Molecules 26, 5112. doi: 10.3390/molecules26175112
Amer S. A., Abdel-Wareth A. A., Gouda A., Saleh G. K., Nassar A. H., Sherief W. R., et al. (2022). Impact of dietary lavender essential oil on the growth and fatty acid profile of breast muscles, antioxidant activity, and inflammatory responses in broiler chickens. Antioxidants 11, 1798. doi: 10.3390/antiox11091798
Amessis-Ouchemoukh N., Abu-Reidah I. M., Quirantes-Piné R., Madani K., and Segura-Carretero A. (2014). Phytochemical profiling, in vitro evaluation of total phenolic contents and antioxidant properties of Marrubium vulgare (horehound) leaves of plants growing in Algeria. Ind. Crops Products 61, 120–129. doi: 10.1016/j.indcrop.2014.06.049
Arram H. M., Radwan A. A., El-Sayed A. I., Elhodairy F. A., Farid O. A., and Amer M. M. (2018). Ann. Agric. Sci. (Moshtohor) 56, 987–996. Available online at: http://aasj.bu.edu.eg/index.php (Accessed March 5, 2024).
Asadi-Samani M., Kafash-Farkhad N., Azimi N., Fasihi A., Alinia-Ahandani E., and Rafieian-Kopaei M. (2015). Medicinal plants with hepatoprotective activity in Iranian folk medicine. Asian Pacific J. Trop. Biomedicine 5, 146–157. doi: 10.1016/S2221-1691(15)30159-3
Badawy M., Attia A. I., Reda F., Sherasiya A., Swelum A. A., and El-Mekkawy M. M. (2025). Effects of dietary supplementation with Laurus nobilis extract on growth performance, carcass features, blood lipid profile, immunity, antioxidative status, digestive enzymes, and gut microbial load in growing New Zealand white rabbits. Trop. Anim. Health Production 57, 1–12. doi: 10.1007/s11250-025-04425-4
Batiha G. E. S., Magdy Beshbishy A., Wasef L., Elewa Y. H., Abd El-Hack M. E., Taha A. E., et al. (2020). Uncaria tomentosa (Willd. ex Schult.) DC.: A review on chemical constituents and biological activities. Appl. Sci. 10, 2668. doi: 10.3390/app10082668
Bedford M. R. and Apajalahti J. H. (2022). The role of feed enzymes in maintaining poultry intestinal health. J. Sci. Food Agric. 102, 1759–1770. doi: 10.1002/jsfa.11670
Benzie I. F. F. and Strain J. J. (1997). Simultaneous automated measurement of total ‘antioxidant’(reducing) capacity and ascorbic acid concentration. Redox Rep. 3, 233–238. doi: 10.1080/13510002.1997.11747115
Bešlo D., Golubić N., Rastija V., Agić D., Karnaš M., Šubarić D., et al. (2023). Antioxidant activity, metabolism, and bioavailability of polyphenols in the diet of animals. Antioxidants 12, 1141. doi: 10.3390/antiox12061141
Blasco A. and Ouhayoun J. (1996). Harmonization of criteria and terminology in rabbit meat research. Revised proposal. World Rabbit Sci. 4, 93–99. doi: 10.4995/wrs.1996.278
Botsoglou N. A., Florou-Paneri P., Christaki E., Giannenas I., and Spais A. B. (2004). Performance of rabbits and oxidative stability of muscle tissues as affected by dietary supplementation with oregano essential oil. Arch. Anim. Nutr. 58, 209–218. doi: 10.1080/00039420410001701404
Boulila A., Sanaa A., Salem I. B., Rokbeni N., M’rabet Y., Hosni K., et al. (2015). Antioxidant properties and phenolic variation in wild populations of Marrubium vulgare L.(Lamiaceae). Ind. Crops products 76, 616–622. doi: 10.1016/j.indcrop.2015.07.069
Brading A. F. (1981). How do drugs initiate contraction in smooth muscles? Trends Pharmacol. Sci. 2, 261–265. doi: 10.1016/0165-6147(81)90334-5
Büyükkartal H. N., Çölgeçen H., and Akgül G. (2016). Comparative leaf, stem and root anatomies of taxa Marrubium bourgaei and Marrubium heterodon (Lamiaceae). Aust. J. Crop Sci. 10, 1516–1522. doi: 10.21475/ajcs.2016.10.11.PNE44
Caceres F., Vallès J., Garnatje T., Parada M., and Gras A. (2022). Gastrointestinal, metabolic, and nutritional disorders: A plant-based ethnoveterinary meta-analysis in the Catalan linguistic areaFront. Veterinary Sci. 9, 908491. doi: 10.3389/fvets.2022.908491
Capasso F. (2003). Phytotherapy: a quick reference to herbal medicine (New York: Springer Science & Business Media).
Capasso A., Pinto A., Mascolo N., Autore G., and Capasso F. (1991). Reduction of agonist-induced contractions of Guinea-pig isolated ileum by flavonoids. Phytotherapy Res. 5, 85–87. doi: 10.1002/ptr.2650050210
Carabano R., Piquer J., Menoyo D., and Badiola I. (1998) Nutrition of the rabbit. Eds. de Blas C. and Wiseman J. Wallington, UK.
Carocci A., Barbarossa A., Leuci R., Carrieri A., Brunetti L., Laghezza A., et al. (2022). Novel phenothiazine/donepezil-like hybrids endowed with antioxidant activity for a multi-target approach to the therapy of Alzheimer’s disease. Antioxidants 11, 1631. doi: 10.3390/antiox11091631
Chabane M. A., Touil A. T., Khelladi B., Meddah B., and Mokhtar M. (2020). In Vivo Toxicological and Microbiological Activity of Marrubium vulgare L. @ on Candida albicans Isolated from Nosocomial Infections. Pharm. Sci. 26, 239–251. doi: 10.34172/PS.2020.35
Chamorro S., Goñi I., Viveros A., Hervert-Hernández D., and Brenes A. (2012). Changes in polyphenolic content and antioxidant activity after thermal treatments of grape seed extract and grape pomace. Eur. Food Res. Technol. 234, 147–155. doi: 10.1007/s00217-011-1621-7
Choct M. (2009). Managing gut health through nutrition. Br. poultry Sci. 50, 9–15. doi: 10.1080/00071660802538632
Chun J., Lee C., Hwang S. W., Im J. P., and Kim J. S. (2014). Ursolic acid inhibits nuclear factor-κB signaling in intestinal epithelial cells and macrophages, and attenuates experimental colitis in mice. Life Sci. 110, 23–34. doi: 10.1016/j.lfs.2014.06.018
Colonna M. A., Karatosidi D., Cosentino C., Freschi P., Carbonara C., Giannico F., et al. (2023). Dietary supplementation with oregano and linseed in autochthonous “Facciuta lucana” Goats: effects on meat quality traits in suckling kids. Animals 13, 3050. doi: 10.3390/ani13193050
Dal Bosco A., Castellini C., Bianchi L., and Mugnai C. (2004). Effect of dietary α-linolenic acid and vitamin E on the fatty acid composition, storage stability and sensory traits of rabbit meat. Meat Sci. 66, 407–413. doi: 10.1016/S0309-1740(03)00127-X
Dalle Zotte A. (2002). Perception of rabbit meat quality and major factors influencing the rabbit carcass and meat quality. Livestock production Sci. 75, 11–32. doi: 10.1016/S0301-6226(01)00308-6
Dalle Zotte A., Celia C., Cullere M., Szendro Z., Kovács M., Gerencsér Z., et al. (2020). Effect of an in-vivo and/or in-meat application of a liquorice (Glycyrrhiza glabra L.) extract on fattening rabbits live performance, carcass traits and meat quality. Anim. Feed Sci. Technol. 260, 114333. doi: 10.1016/j.anifeedsci.2019.114333
Dalle Zotte A. and Szendro Z. (2011). The role of rabbit meat as functional food. Meat Sci. 88, 319–331. doi: 10.1016/j.meatsci.2011.02.017
Darmawan A., Hermana W., Suci D. M., Mutia R., Jayanegara A., and Ozturk E. (2022). Dietary phytogenic extracts favorably influence productivity, egg quality, blood constituents, antioxidant and immunological parameters of laying hens: a meta-analysis. Animals 12, 2278. doi: 10.3390/ani12172278
da Silva A. C., Da Cunha G. S., Tizziani T., Schlemper V., and Sandjo L. P. (2024). Phytogenic effects of Marrubium vulgare on the growth performance of weaned piglets: biochemical parameters and liquid chromatographic–electrospray ionization–mass spectrometric profile of plant and animal serum. J. Sci. Food Agric. 104, 9758–9771. doi: 10.1002/jsfa.13799
Dhama K., Latheef S. K., Mani S., Samad H. A., Karthik K., Tiwari R., et al. (2015). Multiple beneficial applications and modes of action of herbs in poultry health and production-A review. Int. J. Pharmacol. 11, 152–176. doi: 10.3923/ijp.2015.152.176
Di Trana A., Bonanno A., Cecchini S., Giorgio D., Di Grigoli A., and Claps S. (2015). Effects of Sulla forage (Sulla coronarium L.) on the oxidative status and milk polyphenol content in goats. J. Dairy Sci. 98, 37–46. doi: 10.3168/jds.2014-8414
Elazab M. A., Khalifah A. M., Elokil A. A., Elkomy A. E., Rabie M. M., Mansour A. T., et al. (2022). Effect of dietary rosemary and ginger essential oils on the growth performance, feed utilization, meat nutritive value, blood biochemicals, and redox status of growing NZW rabbits. Animals 12, 375. doi: 10.3390/ani12030375
Elberry A. A., Harraz F. M., Ghareib S. A., Nagy A. A., Gabr S. A., Suliaman M. I., et al. (2010). Antihepatotoxic effect of Marrubium vulgare and Withania somnifera extracts on carbon tetrachloride-induced hepatotoxicity in rats. J. basic Clin. Pharm. 1, 247.
Elghalid O. A., Kholif A. E., El-Ashry G. M., Matloup O. H., Olafadehan O. A., El-Raffa A. M., et al. (2020). Oral supplementation of the diet of growing rabbits with a newly developed mixture of herbal plants and spices enriched with special extracts and essential oils affects their productive performance and immune status. Livestock Sci. 238, 104082. doi: 10.1016/j.livsci.2020.104082
El-Gharbaoui A., Benítez G., González-Tejero M. R., Molero-Mesa J., and Merzouki A. (2017). Comparison of Lamiaceae medicinal uses in eastern Morocco and eastern Andalusia and in Ibn al-Baytar’s Compendium of Simple Medicaments (13th century CE). J. ethnopharmacology 202, 208–224. doi: 10.1016/j.jep.2017.03.014
El-Hallous E. I., Alsanie W. F., Ismail I. A., and Dessoky E. S. (2018). Utilization of Marrubium vulgare extract as a therapeutic to hepatic damage induced by Carbon Tetrachloride in rats. Int. J. Pharm. Res. Allied Sci. 7, 168–178.
El-Sabrout K., Khalifah A., and Ciani F. (2023). Current applications and trends in rabbit nutraceuticals. Agriculture 13, 1424. doi: 10.3390/agriculture13071424
Elwan H., Abdelhakeam M., El-Shafei S., El-Rahman A. A., Ismail Z., Zanouny A., et al. (2020). Efficacy of dietary supplementation with Capsicum Annum L on performance, hematology, blood biochemistry and hepatic antioxidant status of growing rabbits. Animals 10, 2045. doi: 10.3390/ani10112045
EMA—European Medicines Agency and Committee on Herbal Medicinal Products (HMPC) (2013). Community Herbal Monograph on Marrubium vulgare L., herba; (London, UK: HMPC).
Emami N. K., Jung U., Voy B., and Dridi S. (2020). Radical response: effects of heat stress-induced oxidative stress on lipid metabolism in the avian liver. Antioxidants 10, 35. doi: 10.3390/antiox10010035
Ettaya A., Dhibi S., Samout N., Elfeki A., and Hfaiedh N. (2016). Hepatoprotective activity of white horehound (Marrubium vulgare) extract against cyclophosphamide toxicity in male rats. Can. J. Physiol. Pharmacol. 94, 441–447. doi: 10.1139/cjpp-2015-0405
Falcão-e-Cunha L., Castro-Solla L., Maertens L., Marounek M., Pinheiro V., Freire J., et al. (2007). Alternatives to antibiotic growth promoters in rabbit feeding: a review. World Rabbit Sci. 15. doi: 10.4995/wrs.2007.597
Feng J., Lu M., Wang J., Zhang H., Qiu K., Qi G., et al. (2021). Dietary oregano essential oil supplementation improves intestinal functions and alters gut microbiota in late-phase laying hens. J. Anim. Sci. Biotechnol. 12, 1–15. doi: 10.1186/s40104-021-00600-3
Ferdous M. F., Arefin M. S., Rahman M. M., Ripon M. M. R., Rashid M. H., Sultana M. R., et al. (2019). Beneficial effects of probiotic and phytobiotic as growth promoter alternative to antibiotic for safe broiler production. J. advanced veterinary Anim. Res. 6, 409. doi: 10.5455/javar.2019.f361
Flees J., Rajaei-Sharifabadi H., Greene E., Beer L., Hargis B. M., Ellestad L., et al. (2017). Effect of Morinda citrifolia (noni)-enriched diet on hepatic heat shock protein and lipid metabolism-related genes in heat stressed broiler chickens. Front. Physiol. 8, 919. doi: 10.3389/fphys.2017.00919
Folch J., Lees M., and Stanley G. S. (1957). A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226, 497–409. doi: 10.1016/S0021-9258(18)64849-5
Fukui H., Xu X., and Miwa H. (2018). Role of gut microbiota-gut hormone axis in the pathophysiology of functional gastrointestinal disorders. J. Neurogastroenterol. Motil. 24, 367. doi: 10.5056/jnm18071
Ghedadba N., Hambaba L., Bousselsela H., Hachemi M., Drid A., Abd-Essmad A., et al. (2016). Evaluation of in vitro antioxidant and in vivo anti-inflammatory potential of white horehound (Marrubium vulgare L.) leaves. Int. J. Pharm. Sci. Rev. Res. 41, 252–259.
Giorgio D., Di Trana A., Di Napoli M. A., Sepe L., Cecchini S., Rossi R., et al. (2019). Comparison of cheeses from goats fed 7 forages based on a new health index. J. Dairy Sci. 102, 6790–6801. doi: 10.3168/jds.2018-15857
Gondret F., Mourot J., and Bonneau M. (1997). Developmental changes in lipogenic enzymes in muscle compared to liver and extramuscular adipose tissues in the rabbit (Oryctolagus cuniculus). Comp. Biochem. Physiol. Part B: Biochem. Mol. Biol. 117, 259–265. doi: 10.1016/S0305-0491(97)00049-7
Gourich A. A., Touijer H., Drioiche A., Asbabou A., Remok F., Saidi S., et al. (2023). Insight into biological activities of chemically characterized extract from Marrubium vulgare L. in vitro, in vivo and in silico approaches. Front. Chem. 11, 1238346. doi: 10.3389/fchem.2023.1238346
Grace D., Khan M. S., Friesen K., and Ata A. (2016). Antimicrobial compounds from Drypetes staudtii. Chem. Biodiversity 13, 913–917. doi: 10.1002/cbdv.201500298
Gray I. K., Rumsby M. G., and Hawke J. C. (1967). The variations in linolenic acid and galactolipid levels in Graminaceae species with age of tissue and light environment. Phytochemistry 6, 107–113. doi: 10.1016/0031-9422(67)85014-3
Gulcin İ., Oktay M., Kıreçcı E., and Küfrevlu Ö.İ. (2003). Screening of antioxidant and antimicrobial activities of anise (Pimpinella anisum L.) seed extracts. Food Chem. 83, 371–382. doi: 10.1016/S0308-8146(03)00098-0
Habashy W. S., Milfort M. C., Rekaya R., and Aggrey S. E. (2019). Cellular antioxidant enzyme activity and biomarkers for oxidative stress are affected by heat stress. Int. J. biometeorology 63, 1569–1584. doi: 10.1007/s00484-019-01769-z
Haouari M., Sfaxi A., Nagati K., and Kallal Z. L. (1991). Marrube blanc: Marrubium vulgare L.(Lamiaceae) plant hypoglycemiante. Rev. Med. Pharm. Afr 5, 11–16.
Hashemi S. R. and Davoodi H. (2011). Herbal plants and their derivatives as growth and health promoters in animal nutrition. Veterinary Res. Commun. 35, 169–180. doi: 10.1007/s11259-010-9458-2
Hashemipour H., Kermanshahi H., Golian A., and Veldkamp T. J. P. S. (2013). Effect of thymol and carvacrol feed supplementation on performance, antioxidant enzyme activities, fatty acid composition, digestive enzyme activities, and immune response in broiler chickens. Poultry Sci. 92, 2059–2069. doi: 10.3382/ps.2012-02685
Hussain J., Ullah R., Khan A. U., Mabood F., Shah M. R., Al-Harrasi A., et al. (2011). Antispasmodic and Ca++ antagonist potential of marrubiin, a labdane type diterpene from Phlomis bracteosa. J. Pharm. Res. 4, 178.
Ibrahim F. M., Ibrahim A. Y., and Omer E. A. (2014). Potential Effect of Marrubium vulgare L. extracts on CCL4 model induced hepatotoxicity in albino mice. World J. Pharm. Sci. 2 (12), 1664–1670.
Iommelli P., Musco N., Lombardi P., Spina A. A., Morittu V. M., Sarubbi F, et al. (2025). Dietary fennel (Foeniculum vulgare Mill) seeds supplementation affects yield, fatty acid composition and flavour profile of milk and cheese in grazing goats. Trop. Anim. Health Production 57, 1–17. doi: 10.1007/s11250-025-04456-x
Jachimowicz K., Winiarska-Mieczan A., and Tomaszewska E. (2022). The impact of herbal additives for poultry feed on the fatty acid profile of meat. Animals 12, 1054. doi: 10.3390/ani12091054
Kabach I. M. A. D., Mrid R. B. E. N., Bouchmaa N. A. J. A. T., Bouargalne Y. O. U. S. S. E. F., and Nhiri A. (2019). Phytochemical screening, antioxidant and cytotoxic activities of M. vulgare. Int. J. Pharm. Res. 11, 338–345.
Kahlouche-Riachi F., Mansour-Djaalab H., Serakta-Delmi M., Djerrou Z., Hamimed S., Moulahoum T., et al. (2012). Evaluation of antibacterial activity of some wild plants of Algeria. Int. J. Med. Aromat Plant 2, 275–280.
Kamboh A. A. and Zhu W. Y. (2014). Individual and combined effects of genistein and hesperidin on immunity and intestinal morphometry in lipopolysacharide-challenged broiler chickens. Poultry Sci. 93, 2175–2183. doi: 10.3382/ps.2014-03971
Kehlbacher A., Bennett R., and Balcombe K. (2012). Measuring the consumer benefits of improving farm animal welfare to inform welfare labelling. Food Policy 37, 627–633. doi: 10.1016/j.foodpol.2012.07.002
Khaled-Khodja N., Boulekbache-Makhlouf L., and Madani K. (2014). Phytochemical screening of antioxidant and antibacterial activities of methanolic extracts of some Lamiaceae. Ind. Crops products 61, 41–48. doi: 10.1016/j.indcrop.2014.06.037
Kozlowska A. and Szostak-Wegierek D. (2014). Flavonoids-food sources and health benefits. Roczniki Państwowego Zakładu Higieny 65.
Laudadio V., Passantino L., Perillo A., Lopresti G., Passantino A., Khan R. U., et al. (2012). Productive performance and histological features of intestinal mucosa of broiler chickens fed different dietary protein levels. Poultry Sci. 91, 265–270. doi: 10.3382/ps.2011-01675
Lee M. T., Lin W. C., Yu B., and Lee T. T. (2017). Antioxidant capacity of phytochemicals and their potential effects on oxidative status in animals—A review. Asian Australas. J. Anim. Sci. 30, 299. doi: 10.5713/ajas.16.0438
Levchehko I. V., Belchenko A. S., Ostahenko V. I., Baidak L. I., and Prykhodko M. F. (2024). Effectiveness of using phyto-additive “VATAGANIMAL” in feeding young rabbits. Archivos zootecnia 73, 149–158.
Li R. G., Wang X. P., Wang C. Y., Ma M. W., and Li F. C. (2012). Growth performance, meat quality and fatty acid metabolism response of growing meat rabbits to dietary linoleic acid. Asian-Australasian J. Anim. Sci. 25, 1169. doi: 10.5713/ajas.2012.12085
Liu B., Piao X., Guo L., Liu S., Chai F., and Gao L. (2016). Ursolic acid protects against ulcerative colitis via anti-inflammatory and antioxidant effects in mice. Mol. Med. Rep. 13, 4779–4785. doi: 10.3892/mmr.2016.5094
Lodhi S., Vadnere G. P., Sharma V. K., and Usman M. R. (2017). Marrubium vulgare L.: A review on phytochemical and pharmacological aspects. J. Intercult. Ethnopharmacol 6, 429–452. doi: 10.5455/jice.20170713060840
Lu Z., He X., Ma B., Zhang L., Li J., Jiang Y., et al. (2019). Dietary taurine supplementation decreases fat synthesis by suppressing the liver X receptor α pathway and alleviates lipid accumulation in the liver of chronic heat-stressed broilers. J. Sci. Food Agric. 99, 5631–5637. doi: 10.1002/jsfa.9817
Mahfuz S., Shang Q., and Piao X. (2021). Phenolic compounds as natural feed additives in poultry and swine diets: A review. J. Anim. Sci. Biotechnol. 12, 1–18. doi: 10.1186/s40104-021-00565-3
Mancha-Ramirez A. M. and Slaga T. J. (2016). Ursolic acid and chronic disease: an overview of UA’s effects on prevention and treatment of obesity and cancer. Anti-inflammatory Nutraceuticals Chronic Dis. 928, 75–96. doi: 10.1007/978-3-319-41334-1_4
Manganelli R. U., Camangi F., and Tomei P. E. (2001). Curing animals with plants: traditional usage in Tuscany (Italy). J. Ethnopharmacology 78, 171–191. doi: 10.1016/S0378-8741(01)00341-5
Martínez Y., Iser M., Valdivié M., Rosales M., Albarrán E., and Sánchez D. (2022). Dietary supplementation with Agave tequilana (weber var. Blue) stem powder improves the performance and intestinal integrity of broiler rabbits. Animals 12, 1117. doi: 10.3390/ani12091117
Masoodi M., Ali Z., Liang S., Yin H., Wang W., and Khan I. A. (2015). Labdane diterpenoids from Marrubium vulgare. Phytochem. Lett. 13, 275–279. doi: 10.1016/j.phytol.2015.07.005
Maturin L. and Peeler J. (1998). “Aerobic plate count; Chapter 3,” in Food and drug administration bacteriological analytical manual, 8th Edn (AOAC International, Baltimore, MD).
Mbah C. J., Orabueze I., and Okorie N. H. (2019). Antioxidants properties of natural and synthetic chemical compounds: Therapeutic effects on biological system. Acta Sci. Pharm. Sci. 3, 28–42. doi: 10.31080/ASPS.2019.03.0273
Menale B., De Castro O., Cascone C., and Muoio R. (2016). Ethnobotanical investigation on medicinal plants in the Vesuvio National Park (Campania, southern Italy). J. ethnopharmacology 192, 320–349. doi: 10.1016/j.jep.2016.07.049
Menchetti L., Brecchia G., Branciari R., Barbato O., Fioretti B., Co dini M., et al. (2020). The effect of Goji berries (Lycium barbarum) dietary supplementation on rabbit meat quality. Meat Sci. 161, 108018. doi: 10.1016/j.meatsci.2019.108018
Mendel M., Chłopecka M., Dziekan N., and Karlik W. (2017). Phytogenic feed additives as potential gut contractility modifiers—A review. Anim. feed Sci. Technol. 230, 30–46. doi: 10.1016/j.anifeedsci.2017.05.008
Molinetti W. T., Prando D. G., Schran R., Pedroso A. C., and Schlemper V. (2017). “Utilização da Marrubium vulgare em frangos de corte como promotor de cresciment,” in Anais da VII Jornada de Iniciação Científica e Tecnológica - VII JIC(Portuguese).
Moussouni L., Benhanifia M., and Ayad A. (2018). In-vitro anthelmintic effects of aqueous and ethanolic extracts of Marrubium vulgare leaves against bovine digestive strongyles. Türkiye Parazitolojii Dergisi 42, 262. doi: 10.5152/tpd.2018.5972
Mulondo S., Afaf L., Hayet C., and Muzamiru K. (2023). Assessment of Marrubium vulgare hydro-alcoholic extract’s biological activities. Algerian J. Biosci. 4, 001–008. doi: 10.57056/ajb.v4i1.102
Murugesan G. R., Romero L. F., and Persia M. E. (2014). Effects of protease, phytase and a Bacillus sp. direct-fed microbial on nutrient and energy digestibility, ileal brush border digestive enzyme activity and cecal short-chain fatty acid concentration in broiler chickens. PloS One 9, e101888. doi: 10.1371/journal.pone.0101888
Murugesan G. R., Syed B., Haldar S., and Pender C. (2015). Phytogenic feed additives as an alternative to antibiotic growth promoters in broiler chickens. Front. veterinary Sci. 2, 21. doi: 10.3389/fvets.2015.00021
Mutwedu V. B., Nyongesa A. W., Kitaa J. M., Ayagirwe R. B., Baharanyi C., and Mbaria J. M. (2022). Effects of Moringa oleifera aqueous seed extracts on reproductive traits of heat-stressed New Zealand White female rabbits. Front. Vet. Sci. 9, 883976. doi: 10.3389/fvets.2022.883976
Naji K. M., Al-Shaibani E. S., Alhadi F. A., Al-Soudi S. A. A., and D’souza M. R. (2017). Hepatoprotective and antioxidant effects of single clove garlic against CCl 4-induced hepatic damage in rabbits. BMC complementary Altern. Med. 17, 1–12. doi: 10.1186/s12906-017-1916-8
Ogbuewu I. P., Emenalom O. O., and Okoli I. C. (2017). Alternative feedstuffs and their effects on blood chemistry and haematology of rabbits and chickens: a review. Comp. Clin. Pathol. 26, 277–286. doi: 10.1007/s00580-015-2210-0
Okur M., Karakaş N., Karadag A., Yilmaz R., and Demirci F. (2019). In vitro cytotoxicity evaluation of Marrubium vulgare L. methanol extract. J. Res. Pharm. 23. doi: 10.12991/jrp.2019.180
Palazzo M., Vizzarri F., Arvay J., D’Alessandro A. G., Martemucci G., Casamassima D., et al. (2020). Dietary effect of dried bay leaves (Laurus nobilis) meal on selected productive performances and on quality meat traits in growing rabbits. Livestock Sci. 242, 104301. doi: 10.1016/j.livsci.2020.104301
Palazzo M., Vizzarri F., Nardoia M., Ratti S., Pastorelli G., and Casamassima D. (2015). Dietary Lippia citriodora extract in rabbit feeding: effects on quality of carcass and meat. Arch. Anim. Breed. 58, 355–364. doi: 10.5194/aab-58-355-2015
Patra A. K., Amasheh S., and Aschenbach J. R. (2019). Modulation of gastrointestinal barrier and nutrient transport function in farm animals by natural plant bioactive compounds–a comprehensive review. Crit. Rev. Food Sci. Nutr. 59, 3237–3266. doi: 10.1080/10408398.2018.1486284
Paula de Oliveira A., Santin J. R., Lemos M., Klein Júnior L. C., Couto A. G., Meyre da Silva Bittencourt ,. C., et al. (2011). Gastroprotective activity of methanol extract and marrubiin obtained from leaves of Marrubium vulgare L.(Lamiaceae). J. Pharm. Pharmacol. 63, 1230–1237. doi: 10.1111/j.2042-7158.2011.01321.x
Peiretti P. G., Masoero G., and Meineri G. (2011). Effects of replacing palm oil with maize oil and Curcuma longa supplementation on the performance, carcass characteristics, meat quality and fatty acid profile of the perirenal fat and muscle of growing rabbits. Animal 5, 795–801. doi: 10.1017/S175173111000234X
Peng F., Zhang H., He X., and Song Z. (2021). Effects of ursolic acid on intestinal health and gut bacteria antibiotic resistance in mice. Front. Physiol. 12, 650190. doi: 10.3389/fphys.2021.650190
Pieroni A., Quave C., Nebel S., and Heinrich M. (2002). Ethnopharmacy of the ethnic Albanians (Arbëreshë) of northern Basilicata, Italy. Fitoterapia 73, 217–241. doi: 10.1016/S0367-326X(02)00063-1
Placha I., Gai F., and Pogány Simonová M. (2022). Natural feed additives in animal nutrition—Their potential as functional feed. Front. Veterinary Sci. 9, 1062724. doi: 10.3389/978-2-8325-0843-5
Podder B., Jang W. S., Nam K. W., Lee B. E., and Song H. Y. (2015). Ursolic acid activates intracellular killing effect of macrophages during Mycobacterium tuberculosis infection. J. Microbiol. Biotechnol. 25, 738–744. doi: 10.4014/jmb.1407.07020
Popoola O. K., Elbagory A. M., Ameer F., and Hussein A. A. (2013). Marrubiin. Molecules 18, 9049–9060. doi: 10.3390/molecules18089049
Prates J. A. (2025). Nutritional value and health implications of meat from monogastric animals exposed to heat stress. Nutrients 17, 1390. doi: 10.3390/nu17081390
Pugliese G., Losacco C., Laudadio V., Schiavitto M., and Tufarelli V. (2024). Plant extracts and essential oils in rabbit diet: A practical green-way for sustainable and resilient production systems. J. Hellenic Veterinary Med. Soc. 75, 7935–7942. doi: 10.12681/jhvms.3608
Qiao Y., Sun J., Xia S., Tang X., Shi Y., and Le G. (2014). Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity. Food Funct. 5, 1241–1124. doi: 10.1039/c3fo60630a
Rahman I. U., Ijaz F., and Bussmann R. W. (2023). Ethnoveterinary practices in livestock: Animal production, healthcare, and livelihood development. Front. Veterinary Sci. 9, 1086311. doi: 10.3389/fvets.2022.1086311
Reda F. M., Alagawany M., Mahmoud H. K., Al-Marakby K. M., Ismail T. A., and Elnesr S. (2025). The use of moringa leaves extract in rabbit diets: its effect on performance, lipid profile, kidney and liver function, immunity, antioxidant, digestive enzymes, and cecal microbiota. Ann. Anim. Sci. 25, 259–269. doi: 10.2478/aoas-2024-0097
Rezgui M., Basma M., Neng N., Nogueira J. M., Bettaieb Ben-Kaab L., and MaChado Araújo M. E. (2021). Evaluation of marrubium vulgare growing wild in Tunisia for its potential as a dietary supplement. Foods 10, 2864. doi: 10.3390/foods10112864
Rezgui M., Majdoub N., Mabrouk B., Baldisserotto A., Bino A., Kaab L. B., et al. (2020). Antioxidant and antifungal activities of marrubiin, extracts and essential oil from Marrubium vulgare L. against pathogenic dermatophyte strains. J. Mycologie Médicale 30, 100927. doi: 10.1016/j.mycmed.2020.100927
Rodriguez Villanueva J. and Martin Esteban J. (2016). An insight into a blockbuster phytomedicine; Marrubium vulgare L. herb. More of a myth than a reality? Phytotherapy Res. 30, 1551–1558. doi: 10.1002/ptr.5661
Sahpaz S., Garbacki N., Tits M., and Bailleul F. (2002). Isolation and pharmacological activity of phenylpropanoid esters from Marrubium vulgare. J. ethnopharmacology 79, 389–392. doi: 10.1016/S0378-8741(01)00415-9
Saidi R., Mimoune N., Baazizi R., Benaissa M. H., Khelef D., and Kaidi R. (2019). A study of ethno-veterinary medicinal plants and in vitro antimicrobial activities against bovine mastitis isolated bacterial pathogens in Algeria. Bull. UASVM Vet. Med. 76, 154–161.
Saini R. K. and Keum Y. S. (2018). Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 203, 255–267. doi: 10.1016/j.lfs.2018.04.049
Salaj N., Barjaktarović J., Kladar N., and Gavarić N. (2018). Biomedical potential of horehound extract (Marrubium vulgare, Lamiaceae). Medicinski pregled 71, 21–26. doi: 10.2298/MPNS1802021S
Schlemper V., Molinetti W. T., Prando D. G., Weber J., and Schlemper S. R. D. M. (2021). Phytobiotic effect of Marrubium vulgare and Leonurus sibiricus on productive performance of griller-type broilers. Ciec. Rural 51, e20200855. doi: 10.1590/0103-8478cr20200855
Schlemper V., Ribas A., Nicolau M., and Cechinel Filho V. (1996). Antispasmodic effects of hydroalcoholic extract of Marrubium vulgare on isolated tissues. Phytomedicine 3, 211–216. doi: 10.1016/S0944-7113(96)80038-9
Scott M. B., Styring A. K., and McCullagh J. S. (2022). Polyphenols: bioavailability, microbiome interactions and cellular effects on health in humans and animals. Pathogens 11, 770. doi: 10.3390/pathogens11070770
Seidavi A., Tavakoli M., Slozhenkina M., Gorlov I., Hashem N. M., Asroosh F., et al. (2021). The use of some plant-de rived products as effective alternatives to antibiotic growth promoters in organic poultry production: A review. Environ. Sci. pollut. Res. 28, 47856–47868. doi: 10.1007/s11356-021-15460-7
Sengul M., Yildiz H., Gungor N., Cetin B., Eser Z., and Ercisli S. (2009). Total phenolic content, antioxidant and antimicrobial activities of some medicinal plants. Pakistan J. Pharm. Sci. 22.
Seow C. L. and Lau A. J. (2017). Differential activation of pregnane X receptor by carnosic acid, carnosol, ursolic acid, and rosmarinic acid. Pharmacol. Res. 120, 23–33. doi: 10.1016/j.phrs.2017.03.007
Sergio L., Boari F., Pieralice M., Linsalata V., Cantore V., and Di Venere D. (2020). Bioactive phenolics and antioxidant capacity of some wild edible greens as affected by different cooking treatments. Foods 9, 1320. doi: 10.3390/foods9091320
Serra D., Almeida L. M., and Dinis T. C. (2018). Dietary polyphenols: A novel strategy to modulate microbiota-gut-brain axis. Trends Food Sci. Technol. 78, 224–233. doi: 10.1016/j.tifs.2018.06.007
Shehata A. A., Yalçın S., Latorre J. D., Basiouni S., Attia Y. A., Abd El-Wahab A., et al. (2022). Probiotics, prebiotics, and phytogenic substances for optimizing gut health in poultry. Microorganisms 10, 395. doi: 10.3390/microorganisms10020395
Sheiha A. M., Abdelnour S. A., Abd El-Hack M. E., Khafaga A. F., Metwally K. A., Ajarem J. S., et al. (2020). Effects of dietary biological or chemical-synthesized nano-selenium supplementation on growing rabbits exposed to thermal stress. Animals 10, 430. doi: 10.3390/ani10030430
Simitzis P. E. (2017). Enrichment of animal diets with essential oils—a great perspective on improving animal performance and quality characteristics of the derived products. Medicines 4, 35. doi: 10.3390/medicines4020035
Sklan D. and Noy Y. (2000). Hydrolysis and absorption in the small intestines of posthatch chicks. Poultry Sci. 79, 1306–1310. doi: 10.1093/ps/79.9.1306
Stark T. D., Mtui D. J., and Balemba O. B. (2013). Ethnopharmacological survey of plants used in the traditional treatment of gastrointestinal pain, inflammation and diarrhea in Africa: future perspectives for integration into modern medicine. Animals 3, 158–227. doi: 10.3390/ani3010158
Taga M., Miller E., and Pratt D. (1984). Chia seeds as a source of natural lipid antioxidants. J. Am. Oil Chem. Soc. 6, 928–931. doi: 10.1007/BF02542169
Tarnawski M., Depta K., Grejciun D., and Szelepin B. (2006). HPLC determination of phenolic acids and antioxidant activity in concentrated peat extract—a natural immunomodulator. J. Pharm. Biomed. Anal. 41, 182–188. doi: 10.1016/j.jpba.2005.11.012
Tufarelli V., Casalino E., D’Alessandro A. G., and Laudadio V. (2017). Dietary phenolic compounds: biochemistry, metabolism and significance in animal and human health. Curr. Drug Metab. 18, 905–913. doi: 10.2174/1389200218666170925124004
Tufarelli V., Losacco C., Pugliese G., Tateo A., Schiavitto M., Iarussi F., et al. (2025). Effects of a multi-strain probiotic on productive traits, antioxidant defence, caecal microbiota and short-chain fatty acid profile, and intestinal histomorphology in rabbits. Anim. Bioscience 38, 1247. doi: 10.5713/ab.24.0716
Tufarelli V., Losacco C., Tedone L., Passantino L., Tarricone S., Laudadio V., et al. (2023). Hemp seed (Cannabis sativa L.) cake as sustainable dietary additive in slow-growing broilers: effects on performance, meat quality, oxidative stability and gut health. Veterinary Q. 43, 1–12. doi: 10.1080/01652176.2023.2260448
Untea A. E., Turcu R. P., Saracila M., Vlaicu P. A., Panaite T. D., and Oancea A. G. (2022). Broiler meat fatty acids composition, lipid metabolism, and oxidative stability parameters as affected by cranberry leaves and walnut meal supplemented diets. Sci. Rep. 12, 21618. doi: 10.1038/s41598-022-25866-z
Verzelloni E., Tagliazucchi D., and Conte A. (2007). Relationship between the antioxidant properties and the phenolic and flavonoid content in traditional balsamic vinegar. Food Chem. 105, 564–571. doi: 10.1016/j.foodchem.2007.04.014
Viegi L., Pieroni A., Guarrera P. M., and Vangelisti R. (2003). A review of plants used in folk veterinary medicine in Italy as basis for a databank. J. Ethnopharmacology 89, 221–244. doi: 10.1016/j.jep.2003.08.003
Vizzarri F., Palazzo M., D’Alessandro A. G., and Casamassima D. (2017). Productive performance and meat quality traits in growing rabbit following the dietary supplementation of Lippia citriodora, Raphanus sativus and Solanum lycopersicum extracts. Livestock Sci. 200, 53–59. doi: 10.1016/j.livsci.2017.04.007
Wan S. Z., Liu C., Huang C. K., Luo F. Y., and Zhu X. (2019). Ursolic acid improves intestinal damage and bacterial dysbiosis in liver fibrosis mice. Front. Pharmacol. 10, 1321. doi: 10.3389/fphar.2019.01321
Wang X., Jiang D., An X., Li S., Qi Y., Yang Y., et al. (2024). Effects of wheat germ diet on intestinal antioxidant capacity, immunological function and gut microbiota of Sichuan white geese. Front. Microbiol. 15, 1435454. doi: 10.3389/fmicb.2024.1435454
Wang J., Luo Y., Li P., Zhang F., and Liu N. (2021). Effect of Salvia miltiorrhiza aerial parts on growth performance, nutrient digestibility, and digestive enzymes in rabbits. Anim. Bioscience 34, 1981. doi: 10.5713/ab.21.0070
Wenk C. (2003). Herbs and botanicals as feed additives in monogastric animals. Asian-Australasian J. Anim. Sci. 16 (2), 282–289. doi: 10.5713/ajas.2003.282
Windisch W., Schedle K., Plitzner C., and Kroismayr A. (2008). Use of phytogenic products as feed additives for swine and poultry. J. Anim. Sci. 86, 140–148. doi: 10.2527/jas.2007-0459
Winiarska-Mieczan A., Kwiecień M., Purwin C., Jachimowicz-Rogowska K., Borsuk-Stanulewicz M., Pogorzelska-Przybyłek P., et al. (2024). Fatty acid profile and dietary value of thigh meat of broiler chickens receiving mineral or organic forms of zn. Animals 14, 1156. doi: 10.3390/ani14081156
Wojdyło A., Oszmiański J., and Czemerys R. (2007). Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 105, 940–949. doi: 10.1016/j.foodchem.2007.04.038
Wu Z. L., Yang X., Chen S. Y., Deng F. L., Jia X. B., Hu S. Q., et al. (2019). Liver transcriptome changes of hyla rabbit in response to chronic heat stress. Animals 9, 1141. doi: 10.3390/ani9121141
Xu C. C., Yang S. F., Zhu L. H., Cai X., Sheng Y. S., Zhu S. W., et al. (2014). Regulation of N-acetyl cysteine on gut redox status and major microbiota in weaned piglets. J. Anim. Sci. 92, 1504–1511. doi: 10.2527/jas.2013-6755
Yang Y., Iji P. A., and Choct M. (2009). Dietary modulation of gut microflora in broiler chickens: a review of the role of six kinds of alternatives to in-feed antibiotics. World’s Poultry Sci. J. 65, 97–114. doi: 10.1017/S0043933909000087
Yang J., Yang L., Wang Y., Zhai S., Wang S., Yang Z., et al. (2017). Effects of dietary protein and energy levels on digestive enzyme activities and electrolyte composition in the small intestinal fluid of geese. Anim. Sci. J. 88, 294–299. doi: 10.1111/asj.12557
Yousefi K., Hamedeyazdan S., Torbati M., and Fathiazad F. (2016). Chromatographic fingerprint analysis of marrubiin in Marrubium vulgare L. via HPTLC technique. Advanced Pharm. Bull. 6, 131. doi: 10.15171/apb.2016.019
Zaikina A. S., Buryakov N. P., Buryakova M. A., Zagarin A. Y., Razhev A. A., and Aleshin D. E. (2022). Impact of supplementing phytobiotics as a substitute for antibiotics in broiler chicken feed on growth performance, nutrient digestibility, and biochemical parameters. Veterinary Sci. 9, 672. doi: 10.3390/vetsci9120672
Zarai Z., Kadri A., Ben Chobba I., Ben Mansour R., Bekir A., Mejdoub H., et al. (2011). The in-vitro evaluation of antibacterial, antifungal and cytotoxic properties of Marrubium vulgare L. essential oil grown in Tunisia. Lipids Health Dis. 10, 1–8. doi: 10.1186/1476-511X-10-161
Zarga M. A., Qauasmeh R., Sabri S., Munsoor M., and Abdalla S. (1995). Chemical constituents of Artemisia arborescens and the effect of the aqueous extract on rat isolated smooth muscle. Planta Med. 61, 242–245. doi: 10.1055/s-2006-958064
Zhang W., Gan D., Jian J., Huang C., Luo F., Wan S., et al. (2019). Protective effect of ursolic acid on the intestinal mucosal barrier in a rat model of liver fibrosis. Front. Physiol. 10, 956. doi: 10.3389/fphys.2019.00956
Zhu L. H., Zhao K. L., Chen X. L., and Xu J. X. (2012). Impact of weaning and an antioxidant blend on intestinal barrier function and antioxidant status in pigs. J. Anim. Sci. 90, 2581–2589. doi: 10.2527/jas.2011-4444
Keywords: rabbit, feed additive, horehound, heath, production
Citation: Losacco C, Pugliese G, Passantino L, Giannico F, Ceci E, Roselli V, Gambacorta L, Schiavitto M, Laudadio V, Piemontese L and Tufarelli V (2025) Horehound (Marrubium vulgare L.) as natural dietary feed additive in rabbit: effects on productive traits, antioxidant status, caecal environment, and gut morphology. Front. Anim. Sci. 6:1658188. doi: 10.3389/fanim.2025.1658188
Received: 02 July 2025; Accepted: 30 July 2025;
Published: 28 August 2025.
Edited by:
Petru Alexandru Vlaicu, National Research Development Institute for Animal Biology and Nutrition, RomaniaReviewed by:
Yuwen Dong, University of Pennsylvania, United StatesEva Voslarova, University of Veterinary and Pharmaceutical Sciences Brno, Czechia
Gabriela Cornescu, National Research Development Institute for Animal Biology and Nutrition, Romania
Copyright © 2025 Losacco, Pugliese, Passantino, Giannico, Ceci, Roselli, Gambacorta, Schiavitto, Laudadio, Piemontese and Tufarelli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesco Giannico, ZnJhbmNlc2NvLmdpYW5uaWNvQHVuaWJhLml0; Vincenzo Tufarelli, dmluY2Vuem8udHVmYXJlbGxpQHVuaWJhLml0
 Caterina Losacco1
Caterina Losacco1 Gianluca Pugliese
Gianluca Pugliese Francesco Giannico
Francesco Giannico Vincenzo Roselli
Vincenzo Roselli Vito Laudadio
Vito Laudadio Luca Piemontese
Luca Piemontese Vincenzo Tufarelli
Vincenzo Tufarelli