- 1Laboratorio de Biotecnología, Instituto de Nutrición y Tecnología de los Alimentos (INTA) - Universidad de Chile, Santiago, Chile
- 2Facultad de Medicina Veterinaria y Agronomía, Universidad de Las Américas, Santiago, Chile
Introduction: The broiler chicken industry has grown rapidly, suggesting that this sector plays a key role in ensuring global food security. However, to meet future needs, how chickens are raised must be improved, as probiotics are promising feed additives.
Methods: We conducted a systematic review of 338 articles retrieved from four scientific databases to evaluate the effectiveness of different probiotic formulations in broiler chickens. The analysis focused on body weight gain (BWG) and feed conversion ratio (FCR).
Results: The most common probiotics were Bacillus, Lactobacillus, and a mixture of different genera types (Probiotic Mix). The results showed that these probiotic formulations had a significant positive effect on both BWG and FCR. The combined effect sizes for BWG were as follows: Lactobacillus (1.08); Probiotic Mix (0.96); and Bacillus (0.87). The effect sizes for FCR were as follows: Probiotic Mix (-1.32) Lactobacillus, (-1.22); and Bacillus (-1.04). Except for BWG in Bacillus category, there was considerable variation in the results. Researchers have also looked at factors such as probiotic dose (CFU/kg) and the number of strains in the Probiotic Mix, but these did not have a significant influence on heterogeneity. When converted combined effect sizes to metric units (g or kg), Lactobacillus showed the best results, with a 221.69 (g) increase in BWG and 0.17 (kg) decrease in FCR.
Conclusion: This study demonstrates that probiotic supplementation, particularly Lactobacillus, improves growth performance and feed efficiency in broiler chickens. These findings support the inclusion of probiotics in poultry farming as a strategy to enhance production efficiency and contribute to future global food security.
1 Introduction
It is estimated that the global human population will reach approximately 8.7 billion people by 2033 (OECD/FAO, 2024), leading to provide by animal-based sources (meat, eggs, and milk) (Drewnowski and Hooker, 2025), and the demand for this type of protein is expected to increase by 12% by 2033 (OECD/FAO, 2024), especially in high-income and upper-middle-income countries (Godber and Wall, 2014).
Animal meat production has grown by 55% over the past two decades, with chicken meat showing the highest growth rate compared to pork or beef, reaching 34% of the total meat production in 2022 (123 million tons) (FAO, 2024). Based on this growth observed in recent decades, it is evident (unless major changes occur) that the poultry industry—especially broiler chicken production—should be one of the key sectors to support future food security in terms of animal-based protein (Mottet and Tempio, 2017; Govoni et al., 2021).
However, poultry production is not free from negative externalities, including environmental degradation and public health risks (MacMahon et al., 2008; Mottet and Tempio, 2017; Kheiralipour et al., 2024). One of the primary concerns in poultry nutrition is the heavy reliance on oats or corn as energy sources and soybeans as the principal protein provider in feed formulation (Govoni et al., 2021). These crops are also used in human nutrition, meaning that the expansion of poultry production places additional pressure on food markets by affecting the availability and pricing of these feedstuffs (Mengesha, 2012; Govoni et al., 2021), a particularly critical issue in low-income countries (Mengesha, 2012). Thus, improvements in broiler productivity, including faster growth and better feed efficiency, will positively influence future food supplies (Kheiralipour et al., 2024).
A wide range of additives, including growth promoters (e.g., zinc bacitracin), exogenous enzymes, organic acids, probiotics, and prebiotics, have been used in poultry farming with different success levels to improve productive performance (Castanon, 2007; Munir and Maqsood, 2017; Abd El-Ghany, 2024; Salahi and Abd El-Ghany, 2024). Moreover, the use of growth promoters—has been increasingly questioned due to their contribution to antibiotic resistance and the potential for residue accumulation in meat products. Consequently, this type of additive has been banned in the European Union since 2006 (Castanon, 2007).
Probiotics have emerged as a promising strategy to support the health and sustainable growth of the global poultry industry (Idowu et al., 2025). Probiotics are defined as “live microorganisms which, when administered in adequate amounts, confer a health benefit on the host” (FAO/WHO, 2001). They play a beneficial role in the gastrointestinal tract by promoting the stability and protection of the intestinal ecosystem, enhancing the functionality of microbial communities, and stimulating the immune response, among other effects (Markowiak and Śliżewska, 2018; Wieërs et al., 2020; Kogut, 2022; Nourizadeh et al., 2022; Idowu et al., 2025). Furthermore, probiotics promote host health through various mechanisms, including strengthening the intestinal barrier by acting on the epithelium and mucosal lining, producing antimicrobial substances, competing with pathogenic bacteria, and regulating luminal acidity (Barko et al., 2018; Hou et al., 2020). Many of these mechanisms are directly related to protection against pathogenic microorganisms (Halder et al., 2024; Idowu et al., 2025). Additionally, the administration of probiotics has been shown to improve growth performance and feed conversion in broiler chickens (Al-Khalaifa et al., 2019; Abd El-Hack et al., 2020; Yaqoob et al., 2022; Halder et al., 2024).
Research on probiotic additives has encompassed a wide range of formulations and strategies. It is essential to recognize that the traditional concept of probiotics, which involves the administration of viable exogenous bacteria, is formally designated by the Food and Drug Administration (FDA) as “direct-fed microbials” (DFM) (Zoumpopoulou et al., 2018). An important aspect of probiotics is their regulatory requirements. For instance, if a probiotic is marketed to cure, mitigate, treat, or prevent a disease, the FDA requires the product to submit an Investigational New Drug Application (IND). However, if the product is considered a dietary supplement regulated by the FDA’s Center for Food Safety and Applied Nutrition, it does not require FDA approval (Venugopalan et al., 2010). Nonetheless, viable microorganism can also be administered through fermented feed or via the consumption of fermented dairy products (Makled et al., 2019; Bishehkolaei et al., 2021; Abeddargahi et al., 2022; Abdel-Raheem et al., 2023; Wang et al., 2023).
Probiotic additives can be derived from microorganisms across various taxonomic groups, including bacteria, yeasts, and molds (Yaqoob et al., 2022). These additives may be formulated as either single-strain (mono-strain) or multi-strain combinations, encompassing different species, genera, kingdoms, or domains (mono-genus or multi-genus mixtures) (Timmerman et al., 2004). Furthermore, these formulations can be integrated with non-nutritional additives such as enzymes, phages, plant extracts, and notably, prebiotics (Dev et al., 2020; Shaufi et al., 2023; Such et al., 2023; El-kahal Hassanien et al., 2024; Marchal et al., 2024; Golshahi et al., 2025). Prebiotics are defined as substrates selectively utilized by the microbiota, thereby conferring health benefits (Gibson et al., 2017). A symbiotic refers to an additive that combines both a prebiotic and a probiotic (Markowiak and Śliżewska, 2018). Several other terminologies are employed to describe additives closely related to probiotics, which have been comprehensively reviewed by Salahi and El-Ghany (2024).
Given the wide diversity of probiotic formulations and microbiota-modulating additives, the development of a meta-analysis in the field of probiotics must involve classifying and organizing these studies to form groups with relatively uniform formulations, ultimately enabling a comparison of their effects.
The objective of this study was to conduct a systematic review aimed at selecting articles that assessed the effects of probiotics as DFM supplements, synbiotics, or probiotics in combination with other non-nutritional additives on the productive performance of broiler chickens. The selected studies were subsequently categorized based on the types of probiotic formulations evaluated and also the routes of administration.
A bibliometric analysis allowed us to identify the probiotic formulations that have been most frequently evaluated over the past two decades. This process enabled the selection of multiple formulations or the most representative groups, ensuring a robust number of studies for meta-analysis. Consequently, our study provides a basis for comparing different formulations in terms of their impact on productive factors, such as body weight gain (BWG) and feed conversion ratio (FCR). This unique strategy represents a distinct methodological contribution that sets it apart from previous systematic reviews on probiotics in broilers.
2 Material and methods
2.1 Search strategy
The systematic review associated with this research examined the extant scientific literature concerning studies on the utilization of probiotics and their effects on the productive performance of chickens, particularly those bred for meat production. This investigation adhered to the PRISMA 2020 guidelines for conducting systematic reviews and meta-analyses (Page et al., 2021). A thorough electronic search was executed using the databases Scopus (Elsevier), EBSCO, PubMed (NCBI), and Web of Science (Clarivate), employing the following search equation:
“Probiotic* AND “Growth performance” AND (Poultry OR Domesticated birds OR Aviculture) AND (Chick* OR Hen OR Rooster OR Cockerel OR Pullet OR Broiler*)”
The search was updated as of January 2025, with no limitations imposed on the initial date.
2.2 Study eligibility criteria
2.2.1 Type of birds and housing
Only studies or experimental groups involving chickens (Gallus gallus) of broiler genetic lines or dual-purpose breeds were included. The birds had to be in good health and were not exposed to any pathogenic challenge, either before or during the study. Additionally, the animals were kept under calm conditions and free from stress-inducing factors.
2.2.2 Type of intervention
Studies were selected based on the criterion that at least one experimental group received a daily administration of a probiotic, symbiotic, or probiotic combined with a non-nutritional additive. There were no restrictions on the age at which probiotic formulations were initiated. However, only studies involving postnatal individuals were included, thereby excluding those in which probiotics were administered during the embryonic stages.
2.2.3 Types of comparators
The control groups were maintained under identical environmental and nutritional conditions to the experimental groups, with the sole distinction being the administration of the probiotic or symbiotic formulation. Furthermore, the control group did not receive antibiotics or any probiotic formulation.
2.2.4 Types of studies
Studies employing a completely randomized design or factorial arrangement were included. For factorial designs, only studies in which both the control and experimental groups strictly conformed to the principles and criteria of this systematic review were selected.
2.2.5 Types of outcomes
Studies selected for inclusion were required to report BWG) and/or FCR for both control and experimental groups. Alternatively, studies were considered if they provided sufficient data within their results to enable the calculation of at least one of these performance indicators.
2.2.6 Types of probiotics
All studies were included irrespective of the probiotic formulation employed, encompassing single-strain preparations, mono-genus or multi-genus mixtures, and formulations with non-nutritional additives, particularly synbiotic. All genera and species of microorganisms, including bacteria, yeasts, and molds, were accepted based on their taxonomy. However, the probiotic formulation was administered as direct-fed microbes (DFM), indicating that the organisms had to be in a viable form. Consequently, studies utilizing probiotics in the form of fermented feed (except fermented dairy products) or containing inactivated microorganisms such as postbiotic, were excluded. An additional criterion is that the probiotic microorganisms used must not have been genetically modified.
2.3 Data extraction
Two independent reviewers extracted data using a standardized form. A third reviewer (PS) fully checked all records against the original article to ensure their accuracy and completeness.
2.4 Probiotics strategies classification
The diverse array of strategies employed in probiotic research poses a challenge in establishing standardized groupings for meta-analyses, particularly for determining robust and comparable combined effects. Consequently, this study necessitated a classification stage of probiotic strategies at the experimental group level, with a primary focus on two aspects: the type of probiotic formulation and route of administration. In this context, we conducted three separate meta-analyses based on the collected data, each corresponding to one of the three most frequently utilized formulation strategies, all administered through the most commonly employed route.
2.5 Statistical methods used in the meta-analysis
For each of the selected studies, the mean values for BWG and FCR, along with their standard deviations, were recorded for each experimental group, whether control, treated with probiotics, or symbiotic. In instances in which only the standard error (SE) was reported in the published data, the standard deviation (SD) was calculated using the following equation:
n = number of replicates
In certain cases, specifically concerning BWG, where this index was calculated as the difference between final and initial weights, the standard deviation was estimated by propagating the error from the variance (Krüger, 2017), using the following equation:
n = number of replicates
Meta-analyses were performed using the metafor package in R (Viechtbauer, 2010). Before this, the effect size and its standard errors were calculated using the escalc command, employing the standardized mean difference (“SMD”) method (Hedges, 1981). The meta-analyses utilized a random-effects model via the Restricted Maximum Likelihood (REML) method (Tanriver-Ayder et al., 2021). The aggregated effect size was expressed as the standardized mean difference (SMD) with a 95% confidence interval. Heterogeneity among studies was assessed using the parameters tau² and I², and significance was evaluated using Cochran’s Q test (Higgins et al., 2003). Additionally, for meta-analyses showing significant heterogeneity, two moderator variables were investigated: the dose of probiotic administration, measured as colony-forming units per kilogram of diet (CFU/kg), and, in probiotic mixture formulations, the number of strains included in the formulation. This analysis was performed by meta-regression (Viechtbauer, 2010).
Publication bias was analyzed using the methodology proposed by Rosenthal (1979), commonly known as the fail-safe N procedure.
Considering that effect size measures (Cohen’s d or Hedges’ g) represent differences in means expressed in units of standard deviation (Borenstein et al., 2009), it is possible to estimate an approximate real productive impact in metric units (grams or kilos) for both evaluated indices, BWG and FCR. This estimation was performed by multiplying the combined effect size by the pooled standard deviation (SD pooled combined) (Guyatt et al., 2019), using the following formulas:
k = number of studies
, = Sample size (replicates) of each control (C) and experimental group (T).
= Standard deviation of each study for the control (C) and experimental group (T).
The combined SDpooled formula was proposed to appropriately weight variability by group size and degrees of freedom, thereby deriving a normalized SD for the calculation of metric units.
3 Results
3.1 Identification and screening of full-text articles
Utilizing the proposed key equations, 1,895 records were retrieved from searches conducted across four selected databases: EBSCO, PubMed, Scopus, and Web of Science. The number of articles retrieved from each database was as follows: EBSCO, 357; PUBMED, 380; Scopus, 184; Web of Science (WoS), 974. After the deduplication process, 1,235 unique records were obtained, spanning from December 1997 to January 2025.
From the 1,235 records obtained, a screening process was implemented utilizing the title and abstract of each article. This step excluded review articles, studies that did not involve the administration of a probiotic formulation, and those that solely reported in vivo analyses, among other criteria. The filtering process resulted in the selection of 491 full-text articles for further examination. Subsequently, a second evaluation was conducted on these articles by analyzing their complete content. The exclusion criteria applied in this stage are shown in Figure 1. Following three screening phases, 338 full-text articles met the criteria and were included in this systematic review (see Figure 1).
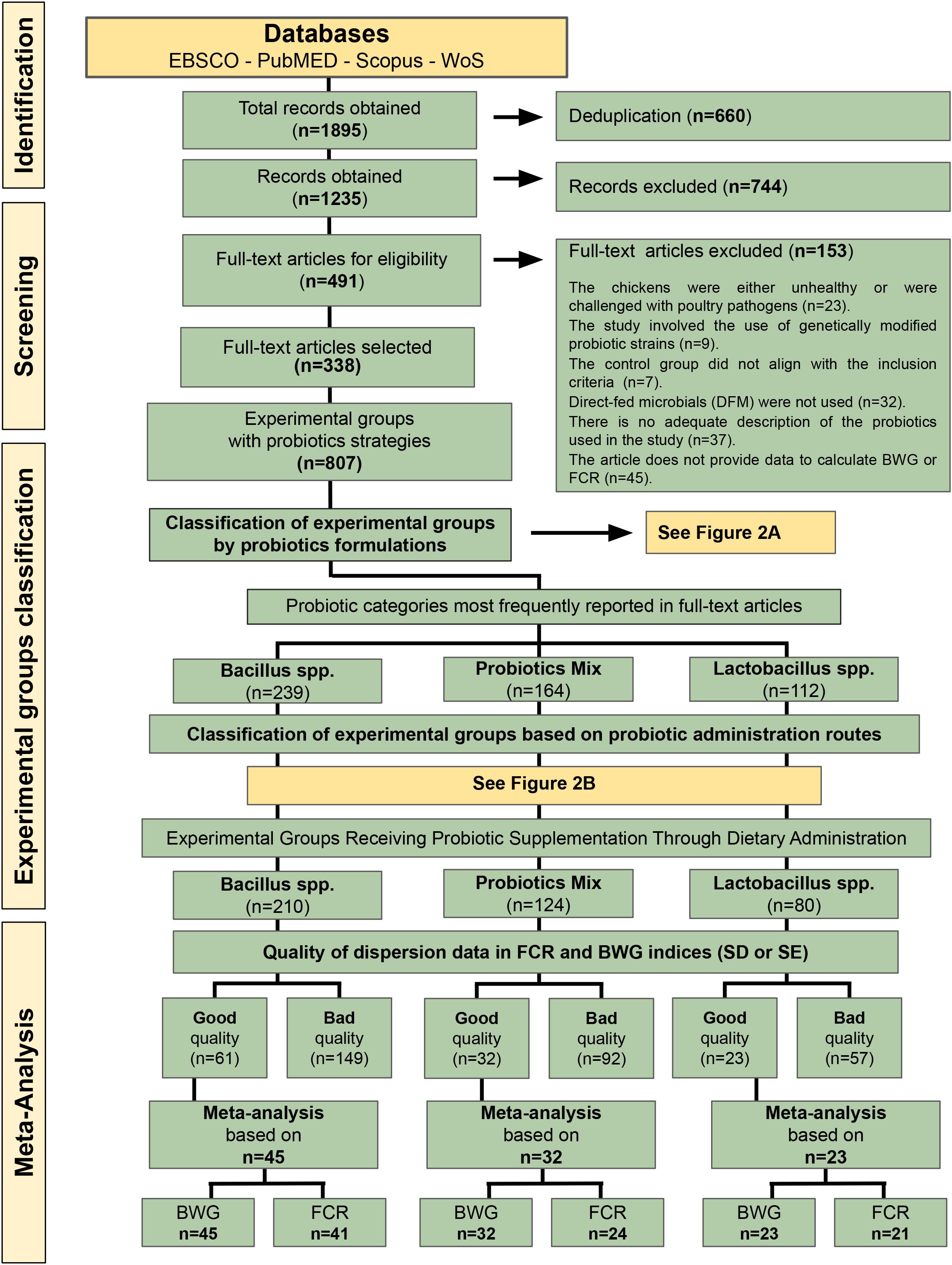
Figure 1. Flowchart illustrating the identification and screening process of the systematic review analyzing studies on the effects of various probiotic formulations on broiler productivity performance. The figure also outlines the classification of the experimental groups and the selection of full-text articles included in the meta-analysis. BWG, Body Weight Gain; FCR, Feed Conversion Ratio.
3.2 Experimental groups classification
To ensure consistency in the categorization of probiotic strategies and their routes of administration, a systematic classification process was implemented. It is important to recognize that several studies have encompassed multiple probiotic formulation strategies and/or administration routes. Consequently, classification was applied at the level of each experimental group within the selected studies.
The analysis of experimental groups involving probiotic administration identified a total of 807 groups (Figure 1). Probiotic strategies were classified along two dimensions: type of formulation and route of administration.
Probiotic formulations were categorized according to the following criteria: for experimental groups using single-strain probiotics or mono-genus mixtures, classification was based on the genus of the microorganism (e.g., Bacillus spp., Lactobacillus spp., or Saccharomyces spp.). In contrast, multi-genus mixtures were classified under the term Probiotics Mix.
When probiotics were administered in combination with a non-nutritional additive, the classification included the genus of the microorganism followed by the term plus (e.g., Bacillus spp. Plus or Lactobacillus spp. plus). For multi-genus mixtures combined with such additives, the classification term used was Probiotics Mix plus (Figure 2A).

Figure 2. Distribution of probiotic research strategies. (A) Pie chart illustrating the proportions of different probiotic formulation strategies identified among the 807 experimental groups recorded from 338 selected studies. (B) Stacked bar chart showing the distribution of probiotic administration routes across the same experimental groups.
The classification results of the probiotic formulation strategy are shown in Figure 2A. Among the 807 experimental groups analyzed, the classification categories with the highest proportions were Bacillus spp. (29.6%, n = 239); Probiotic Mix, 16.6% (n = 164); and Lactobacillus spp., 14.4% (n = 112) (Figure 2A). These three classification categories were selected for the development of separate meta-analyses to assess their combined effects on BWG and FCR.
In Figure 2A, a classification group labeled “Others<3%” can be observed, accounting for 16.6% of the total. This category represents the aggregate of all classification groups, with individual proportions below 3%. A detailed breakdown of this category is presented in Supplementary Figure 1.
Subsequently, the experimental groups were classified based on the route of probiotic administration. Among the 807 experimental groups analyzed, four different administration routes were used: diet, water, gavage, and nasal spray. The diet administration route was the most commonly used method overall, representing 87.9% of the experimental groups in the Bacillus spp. category, 79.5% in the Probiotics Mix category, and 69.0% in the Lactobacillus spp. category. Given the importance of this administration route for the three probiotic formulation groups, it was the only route selected to normalize the experimental groups (Figure 2B), allowing for a larger number of experimental groups to be included in the subsequent phases. Based on this criterion, 210 experimental groups were selected for the Bacillus spp. group, 124 for the Probiotics Mix group, and 80 for the Lactobacillus spp. group (Figure 1).
After selecting classification terms and administration routes for the meta-analyses, it was crucial to assess the quality of the dispersion data related to BWG and/or FCR indices in each selected article (Figure 1). High-quality data were identified by the presence of tabulated values of standard deviation (SD) or standard error of the mean (SEM) for each experimental group. Unfortunately, only a small fraction of the studies offered high-quality dispersion data: 29.0% (n = 61) of the experimental groups in the Bacillus spp. category, 25.8% (n = 32) in the Probiotics Mix category, and 28.7% (n = 23) in the Lactobacillus spp. category (Figure 1). Approximately 50% of the articles presented their dispersion data as pooled SEM, while another 24% reported results only through graphs or did not provide any dispersion values in their tables.
In the development of the meta-analyses, the total number of selected experimental groups was utilized for the Probiotics Mix and Lactobacillus spp. classification categories, comprising 32 and 23 groups, respectively (Figure 1). However, given that 61 experimental groups were identified for Bacillus spp., it was appropriate to conduct a more homogeneous analysis by selecting groups based on the most frequently occurring species. Based on this criterion, 45 experimental groups associated with Bacillus subtilis, Bacillus coagulans, Bacillus licheniformis, and Bacillus amyloliquefaciens were selected (Figure 1).
Supplementary Figures 2A-C further enriches the results from the classification of probiotic formulations by depicting the proportions of primary species or mixtures (either mono-genus or multi-genus) as subgroups within each specified classification category (Bacillus spp., Probiotics Mix, and Lactobacillus spp.). In the Bacillus spp. classification category (Supplementary Figure 2A), the subgroups with the largest proportions, listed in descending order, were B. subtilis (54.4%), B. licheniformis + B. subtilis (10.5%), B. licheniformis (6.7%), and B. coagulans (6.3%). Within the Probiotics Mix classification category (Supplementary Figure 2B), the subgroups with the largest proportions, in descending order, were: a mixture of A. oryzae, B. bifidum, C. pintolopesii, E. faecium, L. acidophilus, L. delbrueckii, L. plantarum, L. rhamnosus, and S. salivarius (Protexin™) at 17.4%; a mixture of B. lactis, L. casei, and L. acidophilus at 3.9%; a mixture of B. subtilis, C. butyricum, and L. acidophilus at 3.9%; and a mixture of B. subtilis and P. acidilactici at 3.2%. For the Lactobacillus spp. classification category (Supplementary Figure 2C), the subgroups with the largest proportions, in descending order, were L. plantarum (23.3%), L. acidophilus (10.3%), L. salivarius (6%), and L. reuteri (5.2%).
3.3 Meta-analysis of BWG and FCR for three probiotic formulation categories
The findings of the analysis, conducted using a random-effects model with heterogeneity estimated via restricted maximum likelihood (REML), are presented in Table 1. Detailed results are shown in the corresponding forest plots: for the Bacillus spp. group, BWG and FCR are illustrated in Figures 3 and 4, respectively; for the Probiotics Mix group, Figures 5 and 6 display BWG and FCR; and for the Lactobacillus spp. group, BWG and FCR are presented in Figures 7 and 8.
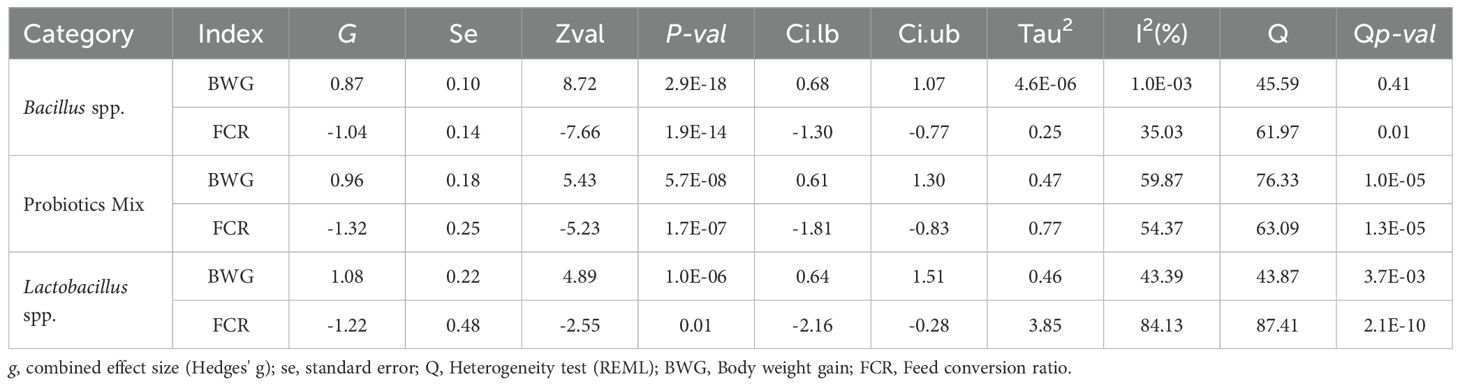
Table 1. Meta-analysis results for body weight gain (BWG) and feed conversion ratio (FCR) across three classification categories: Bacillus spp., Probiotic Mix, and Lactobacillus spp.
![Forest plot showing the standardized mean difference (SMD) with 95% confidence intervals for various studies on Bacillus and body weight gain (BWG). Each study is listed along the y-axis with SMD values plotted horizontally. The overall effect size is depicted as a diamond at the bottom, with an SMD of 0.87 [0.68, 1.07]. Heterogeneity is noted with \(I^2 = 0\%.](https://www.frontiersin.org/files/Articles/1679614/fanim-06-1679614-HTML/image_m/fanim-06-1679614-g003.jpg)
Figure 3. Forest plot of standardized mean differences (SMD) and combined effect size (Hedges' g) for body weight gain (BWG) in broilers following dietary administration of Bacillus spp. SMDs represent differences compared with their respective control groups. Q, heterogeneity test (REML), CI, confidence interval, n.s., not significant, n(articles) = 22, and n(experimental groups) = 45. References cited: Sikandar et al., (2017); Dong et al., (2020); Gong et al., (2018); Li et al., (2014) ; Wang and Gu, (2010) ; Bai et al., (2017) ; Li et al., (2019) ; Zhu et al., (2017); Saleh et al., (2020); Park et al., (2020); Al-Seraih et al., (2022); Qin et al., (2024); Ma et al., (2018) ; Zhou et al., (2010) ; Mazanko et al., (2022) ; Hairui et al., (2024); Popov et al., (2024); Liu et al., (2023) ; Molnár et al., (2011); (Li et al., 2016); Aristides et al., (2012) ; Manafi, (2015).

Figure 4. Forest plot of standardized mean differences (SMD) and combined effect size (Hedges' g) for feed conversion ratio (FCR) in broilers following dietary administration of Bacillus spp. SMDs represent differences compared with their respective control groups. CFU/kg: total number of colony-forming units of the probiotic formulation per kilogram of diet. Q, heterogeneity test (REML), CI, confidence interval (95%), ** signifies p < 0.01, n(articles) = 19, and n(experimental groups) = 41. References cited: Al-Seraih et al., (2022); Aristides et al., (2012); Ma et al., (2018); Mazanko et al., (2022); Bai et al., (2017); Li et al., (2019); Sikandar et al., (2017); Zhu et al., (2017); Zhou et al., (2010); Gong et al., (2018); Molnár et al., (2011); Wang and Gu, (2010); Manafi, (2015); Qin et al., (2024); Park et al., (2020); Li et al., (2016); Saleh et al., (2020); Hairui et al., (2024); Liu et al., (2023).
![Forest plot titled “Probiotics Mix - BWG” displaying standardized mean differences (SMD) with 95% confidence intervals for various studies on probiotics. Each study is listed with CFU per kilogram and strains used. The plot shows SMD values ranging from negative to positive, with a summary effect size of 0.96 [0.61, 1.30]. The plot also notes a random-effects model with heterogeneity statistics: \(I^2 = 59.9\%\), \(\tau^2 = 0.47\), and \(Q(31) = 76.3\).](https://www.frontiersin.org/files/Articles/1679614/fanim-06-1679614-HTML/image_m/fanim-06-1679614-g005.jpg)
Figure 5. Forest plot of standardized mean differences (SMD) and combined effect size (Hedges' g) for body weight gain (BWG) in broilers following dietary administration of Probiotic Mix formulations. SMDs represent differences compared with their respective control groups. CFU/kg: denotes the total colony-forming units per kilogram of diet, Strains: number of different microbial strains in the probiotic mixture, Q, heterogeneity test (REML), CI, confidence interval (95%), *** signifies p ≈ 0, n(articles) = 19, and n(experimental groups) = 32. References cited: Saleh et al., (2013); LI, (2023); Khan et al., (2011); Hossain et al., (2024); Buahom et al., (2018); Salih and Mirza, (2023); Alizadeh et al., (2023); Sobczak et al., (2018); Abdel-Latif et al., (2018); Agustono et al., (2022); Yu et al., (2020); Shaufi et al., (2023); Aluwong et al., (2013); Gholami-Ahangaran et al., (2021); Houshmand et al., (2011); Derakhshan et al., (2023); Alaedini-Shourmasti et al., (2024); Aziz and Al-Hawezy, (2021) ; Naghibi et al., (2023).

Figure 6. Forest plot of standardized mean differences (SMD) and combined effect size (Hedges' g) for feed conversion ratio (FCR) in broilers following dietary administration of Probiotics Mix formulation. SMDs represent differences compared with their respective control groups. CFU/kg: total colony-forming units per kilogram of diet, Strains: indicates the number of different microbial strains in the probiotic mixture, Q, heterogeneity test (REML), CI, confidence interval (95%), *** = p ≈ 0, n(articles) = 15, and n(experimental groups)=24. References cited: Naghibi et al., (2023); Alaedini-Shourmasti et al., (2024); Aziz and Al-Hawezy, (2021); Hossain et al., (2024); Houshmand et al., (2011); Salih and Mirza, (2023); Agustono et al., (2022); Derakhshan et al., (2023); Abdel-Latif et al., (2018); LI, (2023); Gholami-Ahangaran et al., (2021); Khan et al., (2011); Shaufi et al., (2023); Alizadeh et al., (2023); Saleh et al., (2013).
![Forest plot showing the standardized mean difference (SMD) of various studies on Lactobacillus and body weight gain (BWG) in chickens. Each study is represented with a square (effect size) and horizontal line (confidence interval). The vertical line at zero indicates no effect. The summary effect size is shown by a diamond at the bottom, with a SMD of 1.08 [0.64, 1.51]. Heterogeneity is indicated as I² = 43.4%.](https://www.frontiersin.org/files/Articles/1679614/fanim-06-1679614-HTML/image_m/fanim-06-1679614-g007.jpg)
Figure 7. Forest plot of standardized mean differences (SMD) and combined effect size (Hedges' g) for body weight gain (BWG) in broilers following dietary administration of Lactobacillus spp.. SMDs represent differences compared with their respective control groups. CFU/kg: total number of colony-forming units of the probiotic formulation per kilogram of diet, Q, heterogeneity test (REML), CI, confidence interval (95%), *** = p ≈ 0, n(articles) = 15, and n(experimental groups) = 23. References cited: Geeta et al., (2021); Chen et al., (2017); Tsega et al., (2024); Chen et al., (2018); Shokryazdan et al., (2017); Li et al., (2014); Saleh et al., (2020); Trabelsi et al., (2016); Buahom et al., (2018); Chai et al., (2023); Al-Surrayai et al., (2022); Fesseha et al., (2021); Leal et al., (2023); Altaher et al., (2015); Gyawali et al., (2022).

Figure 8. Forest plot of standardized mean differences (SMD) and combined effect size (Hedges' g) for feed conversion ratio (FCR) in broilers following dietary administration of Lactobacillus spp.. SMDs represent differences compared with their respective control groups. CFU/kg: total number of colony-forming units of the probiotic formulation per kilogram of diet, Q, heterogeneity test (REML), CI, confidence interval (95%), *** = p ≈ 0, n(articles) = 13, and n(experimental groups) = 21. References cited: Tsega et al., (2024); Fesseha et al., (2021); Al-Surrayai et al., (2022); Leal et al., (2023); Gyawali et al., (2022); Chai et al., (2023); Chen et al., (2017); Saleh et al., (2020); Shokryazdan et al., (2017); Altaher et al., (2015); Li et al., (2014); Geeta et al., (2021); Chen et al., (2018).
Administration of all three probiotic formulation categories to broiler chickens resulted in significant and statistically robust combined effects on both BWG and FCR indices (Table 1). Regarding the BWG index, the combined effects for each probiotic formulation group, ranked in descending order, were as follows: Lactobacillus spp. (g = 1.08; 95% CI [0.64, 1.51]; p-value< 0.0001), Probiotic Mix (g = 0.96; 95% CI [0.61, 1.30]; p-value< 0.0001), and Bacillus spp. (g = 0.87; 95% CI [0.68, 1.07]; p-value< 0.0001). In terms of the FCR index, the observed combined effects, ranked in ascending order, were: Probiotic Mix (g = -1.32; 95% CI [-1.81, -0.83]; p-value< 0.0001), Lactobacillus spp. (g = -1.22; 95% CI [-2.16, -0.28]; p-value< 0.01), and Bacillus spp. (g = -1.04; 95% CI [-1.30, -0.77]; p-value< 0.0001).
Substantial heterogeneity was observed in both evaluated indices for the Probiotic Mix and Lactobacillus spp. categories. Conversely, in the Bacillus spp. category, significant heterogeneity was detected only in the FCR index (Table 1). The BWG index for the Bacillus spp. group demonstrated low Tau² and I² values, and the heterogeneity test was not significant (p > 0.05) (Table 1).
To facilitate the interpretation of the results, the combined effect sizes were converted into metric units (g or kg), results presented in Table 2. Dietary supplementation with Lactobacillus spp. strains resulted in an approximate increase of 221.69 g in the body weight gain of broilers. This was followed by an increase of 197.05 g for those receiving Probiotic Mix formulations and 152.04 g for those treated with Bacillus spp., compared with their respective control groups.
In terms of the FCR, dietary supplementation with Bacillus spp. strains resulted in a reduction of approximately 0.10 metric units (g). Supplementation with Probiotic Mix formulations led to a decrease of 0.14 metric units (g), whereas treatments based on Lactobacillus spp. exhibited the most significant reduction, with a decrease of 0.17 metric units (g) compared with the respective control groups.
3.4 Analysis of moderator variables
Except for the BWG index within the Bacillus spp. group, all heterogeneity analyses conducted using Cochran’s Q test yielded significant results. Consequently, moderator variable analyses were performed for the FCR index in the Bacillus spp. group as well as for both indices in the probiotic mix and Lactobacillus spp. groups. In the Probiotics Mix group, two moderators were identified: the probiotic dose as a colony-forming unit per kilogram (CFU/kg), and the number of microbial strains included in the probiotic mixture proposed formulation (Figures 5 and 6). For the Bacillus spp. and Lactobacillus spp. categories, only the probiotic dose (CFU/kg) was assessed as a moderator (Figures 4, 7, and 8). Meta-regression tests for moderators did not yield significant results for any category or index evaluated. Specifically, for the Probiotics Mix category, the results were BWG-QM(2) = 0.58, p = 0.75, and FCR-QM(2) = 3.50, p = 0.18; for the Bacillus spp. category, FCR-QM(1) = 0.10, p = 0.75; and for the Lactobacillus spp. category, BWG-QM(1) = 0.06, p = 0.80, and FCR-QM(1) = 0.03, p = 0.87.
3.5 Assessment of publication bias
Sensitivity analysis using Rosenthal’s Fail-Safe N method indicated the following: for the Bacillus spp. category, 763 and 505 unpublished null studies were required to nullify the statistical significance of the combined effect observed (p< 0.05) for BWG and FCR, respectively. For the Probiotics Mix category, 155 and 120 unpublished null studies would be needed to overturn the statistical significance of the combined effect for BWG and FCR, respectively. Finally, for the Lactobacillus spp. category, 100 and 13 unpublished null studies were necessary to negate the statistical significance of BWG and FCR, respectively. Overall, these results suggest that the findings of the meta-analysis were robust against publication bias.
4 Discussion
A notable aspect of this systematic review is the marked increase, commencing in 2017, in the number of studies examining the use of probiotic formulations to enhance productive performance in broiler chickens. Since then, over 50 articles have been published annually, culminating in a peak of 151 publications in 2024, nearly five times the 32 studies reported in 2012. Research on the use of probiotics in poultry production has attracted increasing interest in recent years, as described in bibliometric additive–poultry analysis (Wickramasuriya et al., 2024).
The meta-analysis was conducted within a framework of experimental groups that were homogeneous in terms of probiotic formulation strategies and administration routes. The results of all meta-analyses indicated that the administration of probiotic formulations (Bacillus spp., Probiotic Mix, and Lactobacillus spp.) had significant effects (p< 0.05) on productive performance, as measured by BWG and FCR. Furthermore, the combined effect sizes observed were classified as large based on Cohen’s effect size interpretation (Hedges, 2024).
An essential component of this meta-analysis was the capacity to translate the combined effect sizes into metric units, thereby offering more intuitive value for interpreting the results. For a better interpretation, it is necessary previously to note that 75% of the studies selected for the meta-analysis assessed a growth period of 31 to 50 days. Consequently, many of these studies reported an average final weight close to 1.8–2.5 kg (Al-Dawood and Al-Atiyat, 2022). The observed weight gain values in this meta-analysis must be evaluated in the context of the final body weights achieved. Based on these final weights, the estimated percentage increase BWG, relative to the control groups (those without probiotic administration), was between 6.0% and 8.4% for the Bacillus spp. group, between 7.8% and 11.0% for the Probiotic Mix group, and between 8.8% and 12.3% for the Lactobacillus spp. group.
Regarding the feed conversion ratio (FCR), the Probiotic Mix group demonstrated the highest combined effect size of -1.32. However, upon conversion to metric units, the Lactobacillus spp. category exhibited a greater pooled standard deviation (SDpooled) value of 0.14. This corresponded to an FCR reduction of -0.17, indicating a more substantial improvement compared to the Probiotic Mix group, which showed a decrease of -0.14. Practically, this implies that administering strains or mixtures of Lactobacillus spp. could reduce the feed required to achieve a 1 kg increase in body weight by 170 g relative to control groups without probiotics. This is a highly relevant analysis, as it enables producers to improve the economic assessment of probiotic administration by allowing them to contrast the productive gains achieved through its use with the costs associated with administering it in poultry flocks.
The primary probiotic formulation strategy assessed for enhancing the productive performance of broiler chickens, as identified through a systematic review and classification process, involved the utilization of strains or mixtures from the Bacillus genus. Bacillus is classified under the phylum Bacillota (formerly Firmicutes) (Pallen, 2023), which exhibits the highest prevalence across various intestinal segments in chickens, including the small and large intestine (Mohd Shaufi et al., 2015; Rychlik, 2020). Although Bacillus can be detected within the chicken gut microbiota (Barbosa Teresa et al., 2005; Mazanko et al., 2022), it is not considered a dominant genus, and is instead regarded as an allochthonous member (Cartman Stephen et al., 2008; Tellez et al., 2012). One of the primary reasons for the extensive use of this genus as a probiotic is its classification as an exogenous spore-forming bacterium (Tellez et al., 2012). This attribute confers significant resistance to low pH, bile salts, and other adverse conditions encountered within the gastric environment (Setlow, 2006; Cartman Stephen et al., 2008), thereby enhancing their viability as probiotic additives and ensuring their survival throughout the gastrointestinal tract.
Conversely, the genus Lactobacillus, which also belongs to the phylum Bacillota, is a representative genus within chicken microbiota, particularly in the small intestine, where it constitutes up to 3% of the microbial population (Mohd Shaufi et al., 2015; Rychlik, 2020). A potential reason for the relatively lower interest in utilizing this genus as a probiotic compared to Bacillus may be attributed to the fact that certain Lactobacillus strains are more fastidious regarding cultivation and handling, thereby presenting greater challenges for their development into a viable commercial probiotic additive (Hammes and Hertel, 2006).
Although the Lactobacillus genus has received less research attention compared to Bacillus, our meta-analysis revealed that this probiotic genus has yielded more substantial combined effects on both BWG and FCR. Nonetheless, comparative studies involving different formulations of these genera have not reported statistically significant differences in performance outcomes (Al-Khalaifa et al., 2019; Saleh et al., 2023).
The mechanisms through which probiotics may enhance productive performance include the production of enzymes such as xylanase, amylase, protease, and phytase (Flores et al., 2016; Sharma et al., 2020); an increase in villus height and crypt depth in the intestinal epithelium (Bogucka et al., 2019; Wieërs et al., 2020; Younas et al., 2025), which expands the surface area for nutrient absorption; and the promotion of tight junction protein gene expression in the gut epithelia (Gadde et al., 2017), which could provide a protective effect that supports epithelial integrity and enhances nutrient uptake efficiency. The productive variations observed between different probiotic formulations could be associated with the differential modulation of these mechanisms; however, this is an area that requires further research.
Regarding the variation observed across the different studies for each probiotic formulation, measured as heterogeneity, it was possible to establish that the only analysis that did not yield significant results was the BWG index for the Bacillus spp. group. This outcome suggests that across the various studies administering probiotic strains of the Bacillus genus, the overall effects were not influenced by any moderating variables. This indicates a high level of consistency in the results across studies, which is a favorable attribute.
For probiotic formulations showing significant heterogeneity, potential moderators were examined, such as probiotic dose across all analyses and, in the case of the Probiotic Mix group, the number of strains included in the formulation. However, none of the meta-regression models reached statistical significance, indicating that these moderators did not explain a substantial portion of the heterogeneity observed in BWG and FCR outcomes.
Previous studies investigating probiotic dose influence on probiotic effects, but often report results that are difficult to interpret, either showing diminished effects at higher doses or no significant differences between dose levels, which commonly range from 107 to 1010 CFU/kg (Jin et al., 1998; Mountzouris et al., 2010; Aluwong et al., 2013; Al-Seraih et al., 2022). These findings are consistent with the present meta-regression results.
The heterogeneity observed across studies in this meta-analysis can be attributed to a primary factor: interspecies differences within the same genus concerning probiotic mechanisms. Variations in the enzymatic repertoire have been well documented among different Lactobacillus species (Maske et al., 2021). This variability likely extends to other mechanisms, including immune response modulation, bacteriocin production, and other factors influencing host health. Such differences can even be identified at the strain level, as the activity of probiotics is unique to their specific strains. Consequently, the effects observed with one strain cannot be extrapolated to other strains within the same genus or species (Marteau, 2011).
Research on multi-genus probiotic mixtures has been predominantly influenced by commercial formulations, some of which incorporate up to nine distinct microorganisms, including bacteria, yeasts, and molds. It is reasonable to assume that multi-genus formulations might yield superior outcomes compared to single-strain or single-genus formulations (Tong et al., 2023). Although multi-genus probiotic mixtures exhibited the strongest combined effect on the feed conversion ratio (FCR), formulations containing Lactobacillus demonstrated better results for both performance indices when expressed in metric units. These findings challenge the assumption that a greater number of strains leads to better outcomes, which is also consistent with the analysis of the moderating variables.
Finally, based on the findings of this study, we consider it important to highlight the growing normalization of editorial policies that favor the presentation of data variability as pooled standard errors. In our review, we found that approximately 50% of the articles employed this method to report variability in their tables. However, this practice complicates the direct use of such data in meta-analyses, particularly when calculating standardized mean differences (SMDs). This limitation is particularly concerning for probiotic strategies, for which research output is relatively scarce. This trend underscores the need for journal editorial boards to reconsider the acceptance of this reporting format. Alternatively, the authors could address this issue by presenting the standard deviations (SD) or group-specific standard errors of the mean (SEMs) in tabular form, either within the manuscript or as Supplementary Material.
5 Conclusions
Among the diverse range of probiotic formulations evaluated for their effects on the productive performance of broiler chickens, the most frequently studied are those based on Bacillus, multi-genus mixtures, and Lactobacillus. All of these probiotic formulations have demonstrated benefits by significantly increasing body weight gain (BWG) and reducing feed conversion ratio (FCR). Notably, although Lactobacillus was investigated less extensively than the other groups, it exhibited the most pronounced effects when the combined effect sizes were converted into standard metric units. The meta-analysis indicated that probiotics significantly enhanced the productive performance of broilers, which has important implications for food security. Notably, formulations containing Lactobacillus produced stronger effects than multi-genus formulations with a wide diversity of microorganisms, which remain the most commonly used in the industry, a practice that should be reconsidered.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
RO: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. CS: Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. AV: Conceptualization, Data curation, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The author(s) declare that financial support for the publication of this article was provided by Grant ENLACE (Código ENL 17/24) from the University of Chile.
Conflict of interest
The authors declare that this review was completed in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fanim.2025.1679614/full#supplementary-material
Supplementary Figure 1 | The pie chart provides a detailed breakdown of the “Other <3%” category from Figure 2A, illustrating probiotic formulation strategies with individual proportions below 3%.
Supplementary Figure 2 | Pie charts illustrating the proportions of subgroups (individual species or species/genus combinations) within each selected classification group: (A) Bacillus spp., (B) Probiotics Mix, and (C) Lactobacillus spp. The data encompassed all experimental groups derived from the prior selection process, including all probiotic formulations and routes of administration.
References
Abd El-Ghany W. A. (2024). Applications of organic acids in poultry production: an updated and comprehensive review. Agriculture 14 (10), 1-19. doi: 10.3390/agriculture14101756
Abd El-Hack M. E., El-Saadony M. T., Shafi M. E., Qattan S. Y. A., Batiha G. E., Khafaga A. F., et al. (2020). Probiotics in poultry feed: A comprehensive review. J. Anim. Physiol. Anim. Nutr. 104, 1835–1850. doi: 10.1111/jpn.13454
Abdel-Latif M. A., Abd El-Hack M. E., Swelum A. A., Saadeldin I. M., Elbestawy A. R., Shewita R. S., et al. (2018). Single and Combined Effects of Clostridium butyricum and Saccharomyces cerevisiae on Growth Indices, Intestinal Health, and Immunity of Broilers. Animals 8 (10), 1-13. doi: 10.3390/ani8100184
Abdel-Raheem S. M., Mohammed E. S., Mahmoud R. E., El Gamal M. F., Nada H. S., El-Ghareeb W. R., et al. (2023). Double-fermented soybean meal totally replaces soybean meal in broiler rations with favorable impact on performance, digestibility, amino acids transporters and meat nutritional value. Animals 13 (6), 1-19. doi: 10.3390/ani13061030
Abeddargahi F., Hassan D. K., Farjad R., Mohammad R.-A., Zeynab T., Hassan S. R., et al. (2022). The effect of probiotic and fermented soybean meal based on Bacillus subtilis spore on growth performance, gut morphology, immune response and dry matter digestibility in broiler chickens. Ital. J. Anim. Sci 21, 1642–1650. doi: 10.1080/1828051X.2022.2148577
Agustono B., Lokapirnasari W. P., Yunita M. N., Kinanti R. N., Cesa A. E., and Windria S. (2022). Efficacy of dietary supplementary probiotics as substitutes for antibiotic growth promoters during the starter period on growth performances, carcass traits, and immune organs of male layer chicken. Veterinary World 15, 324–330. doi: 10.14202/vetworld.2022.324-330
Alaedini-Shourmasti A., Aliakbarpour H.-r., and Shokrzaseh M. (2024). Oxidative status, immune response and growth performance of broiler chickens treated with different multi-strain probiotics. J. Cent. Eur. Agric. 25, 325–332. doi: 10.5513/JCEA01/25.2.4212
Al-Dawood A. and Al-Atiyat R. (2022). A comparative study on growth parameters of three broiler chicken strains from Jordan. Braz. J. Poultry Sci 24 (2), 1-8. doi: 10.1590/1806-9061-2021-1534
Alizadeh M. R., Aliakbarpour H.-R., and Hashemi Karouei S. M. (2023). Effect of dietary supplementation of Iranian multi-strain probiotic or P. acidilactici of camel milk isolate on broilers performance, blood parameters, intestinal histology, and microbiota. Ital. J. Anim. Sci 22, 660–665. doi: 10.1080/1828051X.2023.2234937
Al-Khalaifa H., Al-Nasser A., Al-Surayee T., Al-Kandari S., Al-Enzi N., Al-Sharrah T., et al. (2019). Effect of dietary probiotics and prebiotics on the performance of broiler chickens. Poultry Sci 98, 4465–4479. doi: 10.3382/ps/pez282
Al-Seraih A. A., Alsereah B. A., Alwaely W. A., and Al-Hejaj M. Y. (2022). Effect of bacillus subtilis as a probiotic on the productive and physiological performance of broilers. Arch. Razi Institute 77, 1647–1653. doi: 10.22092/ari.2022.357803.2100
Al-Surrayai T., Al-Khalaifah H., Al-Mansour H., Kishk M., Al-Mutairi A., Sultan H., et al. (2022). Evaluation of the lactic acid bacteria based formulated probiotic product for poultry. Front. Anim. Sci 3. doi: 10.3389/fanim.2022.1026958
Altaher Y. W., Faseleh jahromi M., Ebrahim R., Idrus Z., and Liang J. (2015). Lactobacillus pentosus ita23 and L. Acidipiscis ita44 enhance feed conversion efficiency and beneficial gut microbiota in broiler chickens. Rev. Bras. Ciec. Avícola 17, 159–164. doi: 10.1590/1516-635x1702159-164
Aluwong T., Hassan F., Dzenda T., Kawu M., and Ayo J. (2013). Effect of different levels of supplemental yeast on body weight, thyroid hormone metabolism and lipid profile of broiler chickens. J. Veterinary Med. Sci 75, 291–298. doi: 10.1292/jvms.12-0368
Aristides L. G. A., Paiao F. G., Murate L. S., Oba A., and Shimokomaki M. (2012). The effects of biotic additives on growth performance and meat qualities in broiler chickens. Int. J. Poultry Sci 11, 599–604. doi: 10.3923/ijps.2012.599.604
Aziz H. I. and Al-Hawezy D. J. (2021). Effect of probiotics, prebiotics and symbiotic on growth performance of broiler under differents stock density. Iraqi J. Agric. Sci. 52, 1126–1138. doi: 10.36103/ijas.v52i5.1451
Bai K., Huang Q., Zhang J., He J., Zhang L., and Wang T. (2017). Supplemental effects of probiotic Bacillus subtilis fmbJ on growth performance, antioxidant capacity, and meat quality of broiler chickens. Poultry Sci 96, 74–82. doi: 10.3382/ps/pew246
Barbosa Teresa M., Serra Cláudia R., La Ragione Roberto M., Woodward Martin J., and Henriques Adriano O. (2005). Screening for bacillus isolates in the broiler gastrointestinal tract. Appl. Environ. Microbiol. 71, 968–978. doi: 10.1128/AEM.71.2.968-978.2005
Barko P. C., McMichael M. A., Swanson K. S., and Williams D. A. (2018). The gastrointestinal microbiome: A review. J. Veterinary Internal Med. 32, 9–25. doi: 10.1111/jvim.14875
Bishehkolaei R., Rezaeipour V., Abdullahpour R., Jarrahi M., Ghasemi H., Daryaei S., et al. (2021). Milk kefir as a natural probiotic, individually or in combination with organic acids in broiler chickens: influence on the immune-related gene expression, intestinal morphology, microbiota activity, and serum biochemistry. Poultry Sci J. 9, 97–105. doi: 10.22069/psj.2021.18783.1665
Bogucka J., Ribeiro D. M., Bogusławska-Tryk M., Dankowiakowska A., da Costa R. P. R., and Bednarczyk M. (2019). Microstructure of the small intestine in broiler chickens fed a diet with probiotic or synbiotic supplementation. J. Anim. Physiol. Anim. Nutr. 103, 1785–1791. doi: 10.1111/jpn.13182
Borenstein M., Hedges L. V., Higgins J. P. T., and Rothstein H. R. (2009). “Effect sizes based on means,” in Introduction to meta-analysis (Chichester, West Sussed, United Kingdom: Wiley & Sons, Ltd), 21–32.
Buahom J., Siripornadulsil S., and Siripornadulsil W. (2018). Feeding with single strains versus mixed cultures of lactic acid bacteria and bacillus subtilis KKU213 affects the bacterial community and growth performance of broiler chickens. Arabian J. Sci Eng. 43, 3417–3427. doi: 10.1007/s13369-017-3045-6
Cartman Stephen T., La Ragione Roberto M., and Woodward Martin J. (2008). Bacillus subtilis spores germinate in the chicken gastrointestinal tract. Appl. Environ. Microbiol. 74, 5254–5258. doi: 10.1128/AEM.00580-08
Castanon J. I. R. (2007). History of the use of antibiotic as growth promoters in European poultry feeds. Poultry Sci 86, 2466–2471. doi: 10.3382/ps.2007-00249
Chai C., Guo Y., Mohamed T., Bumbie G. Z., Wang Y., Zeng X., et al. (2023). Dietary lactobacillus reuteri SL001 improves growth performance, health-related parameters, intestinal morphology and microbiota of broiler chickens. Animals 13 (10), 1-16. doi: 10.3390/ani13101690
Chen F., Gao S. S., Zhu L. Q., Qin S. Y., and Qiu H. L. (2018). Effects of dietary Lactobacillus rhamnosus CF supplementation on growth, meat quality, and microenvironment in specific pathogen-free chickens. Poultry Sci 97, 118–123. doi: 10.3382/ps/pex261
Chen F., Zhu L., and Qiu H. (2017). Isolation and probiotic potential of lactobacillus salivarius and pediococcus pentosaceus in specific pathogen free chickens. Rev. Bras. Ciec. Avícola 19, 325–332. doi: 10.1590/1806-9061-2016-0413
Derakhshan M., Ghasemian S. O., and Gholami-Ahangaran M. (2023). The effects of probiotic and phytase on growth performance, biochemical parameters and antioxidant capacity in broiler chickens. Veterinary Med. Sci 9, 860–866. doi: 10.1002/vms3.1075
Dev K., Mir N. A., Biswas A., Kannoujia J., Begum J., Kant R., et al. (2020). Dietary synbiotic supplementation improves the growth performance, body antioxidant pool, serum biochemistry, meat quality, and lipid oxidative stability in broiler chickens. Anim. Nutr. 6, 325–332. doi: 10.1016/j.aninu.2020.03.002
Dong Y., Li R., Liu Y., Ma L., Zha J., Qiao X., et al. (2020). Benefit of dietary supplementation with bacillus subtilis BYS2 on growth performance, immune response, and disease resistance of broilers. Probiotics Antimicrobial Proteins 12, 1385–1397. doi: 10.1007/s12602-020-09643-w
Drewnowski A. and Hooker K. (2025). The protein transition: what determines the animal-to-plant (A:P) protein ratios in global diets. Front. Nutr. 12. doi: 10.3389/fnut.2025.1518793
El-kahal Hassanien A., El-Kaiaty A. M., Sobhy H. M., El Moghazy G. M., Gomaa A. H., Abdel A’al M. H., et al. (2024). Evaluation of using probiotics, prebiotics and symbiotic as growth promoters in broilers and their effects on some growth performance-related genes. J. Hellenic Veterinary Med. Soc. 75, 7621–7628. doi: 10.12681/jhvms.35116
FAO (2024). World Food and Agriculture – Statistical Yearbook 2024 (Rome: Food and Agriculture Organization of the United Nations).
FAO/WHO (2001). Probiotics in food. Health and nutritional properties and guidelines for evaluation. Report of a joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria (Cordoba, Argentina, Rome: FAO).
Fesseha H., Demlie T., Mathewos M., and Eshetu E. (2021). Effect of lactobacillus species probiotics on growth performance of dual-purpose chicken. Veterinary Med.: Res. Rep. 12, 75–83. doi: 10.2147/VMRR.S300881
Flores C., Williams M., Pieniazek J., Dersjant-Li Y., Awati A., and Lee J. T. (2016). Direct-fed microbial and its combination with xylanase, amylase, and protease enzymes in comparison with AGPs on broiler growth performance and foot-pad lesion development. J. Appl. Poultry Res. 25, 328–337. doi: 10.3382/japr/pfw016
Gadde U., Oh S. T., Lee Y. S., Davis E., Zimmerman N., Rehberger T., et al. (2017). The effects of direct-fed microbial supplementation, as an alternative to antibiotics, on growth performance, intestinal immune status, and epithelial barrier gene expression in broiler chickens. Probiotics Antimicrobial Proteins 9, 397–405. doi: 10.1007/s12602-017-9275-9
Geeta S., Yadav A., Pradhan S., Rajoria R., Kumar A. M., Gopi M., et al. (2021). Probiotic attributes of lactobacillus fermentum NKN51 isolated from yak cottage cheese and the impact of its feeding on growth, immunity, caecal microbiology and jejunal histology in the starter phase of broiler birds. Indian J. Anim. Res. 55, 451–456. doi: 10.18805/ijar.B-3970
Gholami-Ahangaran M., Haj-Salehi M., Ahmadi-Dastgerdi A., and Zokaei M. (2021). The advantages and synergistic effects of Gunnera (Gundelia tournefortii L.) extract and protexin in chicken production. Veterinary Med. Sci 7, 2374–2380. doi: 10.1002/vms3.624
Gibson G. R., Hutkins R., Sanders M. E., Prescott S. L., Reimer R. A., Salminen S. J., et al. (2017). Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nature Reviews Gastroenterology & Hepatology 14 (8), 491–502. doi: 10.1038/nrgastro.2017.75
Godber O. F. and Wall R. (2014). Livestock and food security: vulnerability to population growth and climate change. Global Change Biol. 20, 3092–3102. doi: 10.1111/gcb.12589
Golshahi A., Shams Shargh M., Dastar B., and Rahmatnejad E. (2025). The effect of thymus vulgaris extract and probiotic on growth performance, blood parameters, intestinal morphology, and litter quality of broiler chickens fed low-protein diets. Poultry Sci 104, 104554. doi: 10.1016/j.psj.2024.104554
Gong L., Wang B., Mei X., Xu H., Qin Y., Li W., et al. (2018). Effects of three probiotic Bacillus on growth performance, digestive enzyme activities, antioxidative capacity, serum immunity, and biochemical parameters in broilers. Anim. Sci J. 89, 1561–1571. doi: 10.1111/asj.13089
Govoni C., Chiarelli D. D., Luciano A., Ottoboni M., Perpelek S. N., Pinotti L., et al. (2021). Global assessment of natural resources for chicken production. Adv. Water Resour. 154, 103987. doi: 10.1016/j.advwatres.2021.103987
Guyatt G. H., Oxman A. D., Vist G. E., Kunz R., Falck-Ytter Y., Alonso-Coello P., et al. (2019). “Chapter 15: interpreting results,” in Cochrane handbook for systematic reviews of interventions, 2nd ed. Eds. Higgins J. P. T., Thomas J., Chandler J., Cumpston M., Li T., Page M. J., and Welch V. A. (John Wiley & Sons, Chichester (UK), 469–500.
Gyawali I., Zeng Y., Zhou J., Li J., Wu T., Shu G., et al. (2022). Effect of novel Lactobacillus paracaesi microcapsule on growth performance, gut health and microbiome community of broiler chickens. Poultry Sci 101, 101912. doi: 10.1016/j.psj.2022.101912
Hairui Y., Rahman A., Sadique M. A., Batool T., Imtiaz B., Zaman M. A., et al. (2024). Impact of bacillus subtilis probiotic on growth performance, bone health, intestinal morphology, and cecal microbiota in cobb broiler chicks. Pakistan Veterinary J. 44, 1243–1248. doi: 10.29261/pakvetj/2024.254
Halder N., Sunder J., De A. K., Bhattacharya D., and Joardar S. N. (2024). Probiotics in poultry: a comprehensive review. J. Basic Appl. Zool. 85, 23. doi: 10.1186/s41936-024-00379-5
Hammes W. P. and Hertel C. (2006). “The Genera Lactobacillus and Carnobacterium,” in The Prokaryotes. Eds. Dworkin M., Falkow S., Rosenberg E., Schleifer K.-H., and Stackebrandt E. (Springer, New York), 320–403.
Hedges L. V. (1981). Distribution theory for glass’s estimator of effect size and related estimators. J. Educ. Stat 6, 107–128. doi: 10.2307/1164588
Hedges L. V. (2024). Interpretation of the standardized mean difference effect size when distributions are not normal or homoscedastic. Educ. psychol. Measurement 85, 245–257. doi: 10.1177/00131644241278928
Higgins J. P. T., Thompson S. G., Deeks J. J., and Altman D. G. (2003). Measuring inconsistency in meta-analyses. BMJ 327, 557. doi: 10.1136/bmj.327.7414.557
Hossain M. T., Sardar D., Afsana S., Datta M., and Habib M. A. (2024). Comparative analysis between multi-strain probiotics and antibiotic as starter feed supplement of poultry on growth performance, serum metabolites and meat quality. Veterinary Anim. Sci 24, 100346. doi: 10.1016/j.vas.2024.100346
Hou Q., Feiyan Z., Wenjun L., Ruirui L., Thwe K. W. W., Jia H., et al. (2020). Probiotic-directed modulation of gut microbiota is basal microbiome dependent. Gut Microbes 12, 1736974. doi: 10.1080/19490976.2020.1736974
Houshmand M., Azhar K., Zulkifli I., Bejo M. H., and Kamyab A. (2011). Effects of nonantibiotic feed additives on performance, nutrient retention, gut pH, and intestinal morphology of broilers fed different levels of energy. J. Appl. Poultry Res. 20, 121–128. doi: 10.3382/japr.2010-00171
Idowu P. A., Mpofu T. J., Magoro A. M., Modiba M. C., Nephawe K. A., and Mtileni B. (2025). Impact of probiotics on chicken gut microbiota, immunity, behavior, and productive performance—a systematic review. Front. Anim. Sci 6. doi: 10.3389/fanim.2025.1562527
Jin L. Z., Ho Y. W., Abdullah N., and Jalaludin S. (1998). Growth performance, intestinal microbial populations, and serum cholesterol of broilers fed diets containing Lactobacillus cultures. Poultry Sci 77, 1259–1265. doi: 10.1093/ps/77.9.1259
Khan S. H., Hasan S., Sardar R., and Dil S. (2011). Effect of dietary supplementation of probiotic on the performance of F1 crossbred (Rhode Island red male × Fayoumi female) cockerels. J. Anim. Physiol. Anim. Nutr. 95, 523–532. doi: 10.1111/j.1439-0396.2010.01079.x
Kheiralipour K., Shahin R., Mahmoud K., Mohammad N., and Paliwal J. (2024). The environmental impacts of commercial poultry production systems using life cycle assessment: a review. World’s Poultry Sci J. 80, 33–54. doi: 10.1080/00439339.2023.2250326
Kogut M. H. (2022). Role of diet-microbiota interactions in precision nutrition of the chicken: facts, gaps, and new concepts. Poultry Sci 101, 101673. doi: 10.1016/j.psj.2021.101673
Krüger D. (2017). Introduction to Error Calculation and Propagation (London: Division of Imaging Sciences & Biomedical Engineering, King’s College).
Leal K., Truong L., Maga E., and King A. (2023). Lactobacillus (L. plantarum & L. rhamnosus) and Saccharomyces (S. cerevisiae): effects on performance, biochemical parameters, ammonium ion in manure, and digestibility of broiler chickens. Poultry Sci 102, 102525. doi: 10.1016/j.psj.2023.102525
Li W.-j. (2023). Effect of probiotics, acidifiers and enzymes on growth performance, meat quality, serum biochemical indexes and antioxidant capacity of Dagu chickens. Feed Res. 46, 36–41. doi: 10.13557/j.cnki.issn1002-2813.2023.05.008
Li C.-l., Wang J., Zhang H.-j., Wu S.-g., Hui Q.-r., Yang C.-b., et al. (2019). Intestinal morphologic and microbiota responses to dietary bacillus spp. in a broiler chicken model. Front. Physiol. 9. doi: 10.3389/fphys.2018.01968
Li Y., Xu Q., Huang Z., Lv L., Liu X., Yin C., et al. (2016). Effect of Bacillus subtilis CGMCC 1.1086 on the growth performance and intestinal microbiota of broilers. J. Appl. Microbiol. 120, 195–204. doi: 10.1111/jam.12972
Li Y.-B., Xu Q.-Q., Yang C.-J., Yang X., Lv L., Yin C.-h., et al. (2014). Effects of probiotics on the growth performance and intestinal micro flora of broiler chickens. Pakistan J. Pharm. Sci. 27, 713–717.
Liu S., Xiao G., Wang Q., Zhang Q., Tian J., Li W., et al. (2023). Effects of dietary bacillus subtilis HC6 on growth performance, antioxidant capacity, immunity, and intestinal health in broilers. Animals 13 (18), 1-19. doi: 10.3390/ani13182915
Ma Y., Wang W., Zhang H., Wang J., Zhang W., Gao J., et al. (2018). Supplemental Bacillus subtilis DSM 32315 manipulates intestinal structure and microbial composition in broiler chickens. Sci. Rep. 8, 15358. doi: 10.1038/s41598-018-33762-8
MacMahon K. L., Delaney L. J., Kullman G., Gibbins J. D., Decker J., and Kiefer M. J. (2008). Protecting poultry workers from exposure to avian influenza viruses. Public Health Rep. 123, 316–322. doi: 10.1177/003335490812300311
Makled M. N., Abouelezz K. F. M., Gad-Elkareem A. E. G., and Sayed A. M. (2019). Comparative influence of dietary probiotic, yoghurt, and sodium butyrate on growth performance, intestinal microbiota, blood hematology, and immune response of meat-type chickens. Trop. Anim. Health Production 51, 2333–2342. doi: 10.1007/s11250-019-01945-8
Manafi M. (2015). Comparison study of a natural non-antibiotic growth promoter and a commercial probiotic on growth performance, immune response and biochemical parameters of broiler chicks. J. Poultry Sci 52 (4), 274-281. doi: 10.2141/jpsa.0150027
Marchal L., Bello A., Archer G., Sobotik E. B., and Dersjant-Li Y. (2024). Total replacement of soybean meal with alternative plant-based ingredients and a combination of feed additives in broiler diets from 1 day of age during the whole growing period. Poultry Sci 103, 103854. doi: 10.1016/j.psj.2024.103854
Markowiak P. and Śliżewska K. (2018). The role of probiotics, prebiotics and synbiotics in animal nutrition. Gut Pathog. 10, 21. doi: 10.1186/s13099-018-0250-0
Marteau P. (2011). Evidence of probiotic strain specificity makes extrapolation of results impossible from a strain to another, even from the same species. J. Clin. Gastroenterol 45, S1–S5.
Maske B. L., de Melo Pereira G. V., da S. Vale A., de Carvalho Neto D. P., Karp S. G., Viesser J. A., et al. (2021). A review on enzyme-producing lactobacilli associated with the human digestive process: From metabolism to application. Enzyme Microbial Technol. 149, 109836. doi: 10.1016/j.enzmictec.2021.109836
Mazanko M. S., Popov I. V., Prazdnova E. V., Refeld A. G., Bren A. B., Zelenkova G. A., et al. (2022). Beneficial effects of spore-forming bacillus probiotic bacteria isolated from poultry microbiota on broilers’ Health, growth performance, and immune system. Front. Veterinary Sci 9. doi: 10.3389/fvets.2022.877360
Mengesha M. (2012). The issue of feed-food competition and chicken production for the demands of foods of animal origin. Asian J. Poultry Sci 6, 31–43. doi: 10.3923/ajpsaj.2012.31.43
Mohd Shaufi M. A., Sieo C. C., Chong C. W., Gan H. M., and Ho Y. W. (2015). Deciphering chicken gut microbial dynamics based on high-throughput 16S rRNA metagenomics analyses. Gut Pathog. 7, 4. doi: 10.1186/s13099-015-0051-7
Molnár A. K., Podmaniczky B., Kürti P., Tenk I., Glávits R., Virág G. Y., et al. (2011). Effect of different concentrations of Bacillus subtilis on growth performance, carcase quality, gut microflora and immune response of broiler chickens. Br. Poultry Sci 52 (6), 658–665. doi: 10.1080/00071668.2011.636029
Mottet A. and Tempio G. (2017). Global poultry production: current state and future outlook and challenges. World’s Poultry Sci J. 73, 245–256. doi: 10.1017/S0043933917000071
Mountzouris K. C., Tsitrsikos P., Palamidi I., Arvaniti A., Mohnl M., Schatzmayr G., et al. (2010). Effects of probiotic inclusion levels in broiler nutrition on growth performance, nutrient digestibility, plasma immunoglobulins, and cecal microflora composition1. Poultry Sci 89, 58–67. doi: 10.3382/ps.2009-00308
Munir K. and Maqsood S. (2017). A review on role of exogenous enzyme supplementation in poultry production. Emirates J. Food Agric. 25, 66–80. doi: 10.9755/ejfa.v25i1.9138
Naghibi F., Aliakbarpour H. R., and Rezaeipour V. (2023). Effects of different sources of probiotics on performance, carcass, intestinal morphology and bone characteristics in broiler chickens. Iranian J. Appl. Anim. Sci 13, 535–543. Available online at: http://sanad.iau.ir/fa/Article/1023942.
Nourizadeh R., Sepehri B., Abbasi A., Sayyed R. Z., and Khalili L. (2022). “Impact of Probiotics in Modulation of Gut Microbiome,” in Microbiome-Gut-Brain Axis: Implications on Health. Eds. Sayyed R. Z. and Khan M. (Springer Nature Singapore, Singapore), 401–409.
Page M. J., McKenzie J. E., Bossuyt P. M., Boutron I., Hoffmann T. C., Mulrow C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, n71. doi: 10.1136/bmj.n71
Pallen M. J. (2023). Request for an Opinion on the standing and retention of Firmicutes as a phylum name. Int. J. Systematic Evolutionary Microbiol. 73 (7), 1-2. doi: 10.1099/ijsem.0.005933
Park I., Zimmerman N. P., Smith A. H., Rehberger T. G., Lillehoj E. P., and Lillehoj H. S. (2020). Dietary supplementation with bacillus subtilis direct-fed microbials alters chicken intestinal metabolite levels. Front. Veterinary Sci 7. doi: 10.3389/fvets.2020.00123
Popov I. V., Skripkin V. S., Mazanko M. S., Epimakhova E. E., Prazdnova E. V., Dilekova O. V., et al. (2024). Effects of spore-forming Bacillus probiotics on growth performance, intestinal morphology, and immune system of broilers housed on deep litter. J. Appl. Poultry Res. 33, 100396. doi: 10.1016/j.japr.2023.100396
Qin S., Xiao X., Dai Z., Zhao G., Cui Z., Wu Y., et al. (2024). Effects of Bacillus licheniformis on growth performance, immune and antioxidant functions, and intestinal microbiota of broilers. Poultry Sci 103, 103210. doi: 10.1016/j.psj.2023.103210
Rosenthal R. (1979). The “file drawer problem” and tolerance for null results. psychol. Bull. 86, 638–641. doi: 10.1037/0033-2909.86.3.638
Rychlik I. (2020). Composition and function of chicken gut microbiota. Animals 10 (1), 1-20. doi: 10.3390/ani10010103
Salahi A. and Abd El-Ghany W. A. (2024). Beyond probiotics, uses of their next-generation for poultry and humans: A review. J. Anim. Physiol. Anim. Nutr. 108, 1336–1347. doi: 10.1111/jpn.13972
Saleh A. A., Hayashi K., and Ohtsuka A. (2013). Synergistic effect of feeding aspergillus awamori and saccharomyces cerevisiae on growth performance in broiler chickens; promotion of protein metabolism and modification of fatty acid profile in the muscle. J. Poultry Sci 50, 242–250. doi: 10.2141/jpsa.0120153
Saleh H., Jangjou O., Mirakzehi M. T., Agah M. J., and Bostani A. (2023). The effects of various feed forms and dietary supplements (Probiotic and antibiotic) on performance, immune system, cecal microbiota, and intestinal morphology in broiler chickens. Poultry Sci J. 11, 59–71. doi: 10.22069/psj.2022.20187.1818
Saleh A. A., Paray B. A., and Dawood M. A. O. (2020). Olive cake meal and bacillus licheniformis impacted the growth performance, muscle fatty acid content, and health status of broiler chickens. Animals 10 (4), 1-15. doi: 10.3390/ani10040695
Salih Y. G. and Mirza R. A. (2023). The efficacy of using olive cake as a by-product and probiotic supplementation on growth performance and blood characteristics of broiler chickens. Passer J. Basic Appl. Sci. 5, 213–217. doi: 10.24271/psr.2023.388533.1273
Setlow P. (2006). Spores of Bacillus subtilis: their resistance to and killing by radiation, heat and chemicals. J. Appl. Microbiol. 101, 514–525. doi: 10.1111/j.1365-2672.2005.02736.x
Sharma N., Angural S., Rana M., Puri N., Kondepudi K. K., and Gupta N. (2020). Phytase producing lactic acid bacteria: Cell factories for enhancing micronutrient bioavailability of phytate rich foods. Trends Food Sci Technol. 96, 1–12. doi: 10.1016/j.tifs.2019.12.001
Shaufi M. A., Sieo C. C., Chong C. W., Geok Hun T., Omar A. R., Han Ming G., et al. (2023). Effects of phage cocktail, probiotics, and their combination on growth performance and gut microbiota of broiler chickens. Animals 13 (8), 1-16. doi: 10.3390/ani13081328
Shokryazdan P., Faseleh Jahromi M., Liang J. B., Ramasamy K., Sieo C. C., and Ho Y. W. (2017). Effects of a Lactobacillus salivarius mixture on performance, intestinal health and serum lipids of broiler chickens. PloS One 12, e0175959. doi: 10.1371/journal.pone.0175959
Sikandar A., Zaneb H., Younus M., Masood S., Aslam A., Shah M., et al. (2017). Growth performance, immune status and organ morphometry in broilers fed Bacillus subtilis-supplemented diet. South Afr. J. Anim. Sci 47, 378–388. doi: 10.4314/sajas.v47i3.14
Sobczak J., Stangierski J., Marek P., and Kijowski J. (2018). Effect of probiotic administration on productivity and quality of broiler chicken meat. Anim. Nutr. Feed Technol. 18, 79. doi: 10.5958/0974-181X.2018.00007.0
Such N., Mezőlaki Á., Rawash M. A., Tewelde K. G., Pál L., Wágner L., et al. (2023). Diet composition and using probiotics or symbiotics can modify the urinary and faecal nitrogen ratio of broiler chicken’s excreta and also the dynamics of in vitro ammonia emission. Animals 13 (3), 1-12. doi: 10.3390/ani13030332
Tanriver-Ayder E., Faes C., van de Casteele T., McCann S. K., and Macleod M. R. (2021). Comparison of commonly used methods in random effects meta-analysis: application to preclinical data in drug discovery research. BMJ Open Sci 5, e100074. doi: 10.1136/bmjos-2020-100074
Tellez G., Pixley C., Wolfenden R. E., Layton S. L., and Hargis B. M. (2012). Probiotics/direct fed microbials for Salmonella control in poultry. Food Res. Int. 45, 628–633. doi: 10.1016/j.foodres.2011.03.047
Timmerman H. M., Koning C. J. M., Mulder L., Rombouts F. M., and Beynen A. C. (2004). Monostrain, multistrain and multispecies probiotics—A comparison of functionality and efficacy. Int. J. Food Microbiol. 96, 219–233. doi: 10.1016/j.ijfoodmicro.2004.05.012
Tong D.-q., Lu Z.-j., Zeng N., Wang X.-q., Yan H.-c., and Gao C.-q. (2023). Dietary supplementation with probiotics increases growth performance, improves the intestinal mucosal barrier and activates the Wnt/β-catenin pathway activity in chicks. J. Sci Food Agric. 103, 4649–4659. doi: 10.1002/jsfa.12562
Trabelsi I., Ktari N., Ben Slima S., Bouchaala K., and Ben Salah R. (2016). Effects of supplementation with L. plantarum TN8 encapsulated in alginate-chitosan in broiler chickens. Int. J. Biol. Macromolecules 89, 677–681. doi: 10.1016/j.ijbiomac.2016.05.044
Tsega K. T., Maina K. J., Berhane T. N., and Mekuria S. A. (2024). Effects of Lactobacillus probiotics supplemented with concentrate feed on growth performance, carcass characteristics, and caecal microflora of RIR chickens. Cogent Food Agric. 10, 2311959. doi: 10.1080/23311932.2024.2311959
Venugopalan V., Shriner K. A., and Wong-Beringer A. (2010). Regulatory oversight and safety of probiotic use. Emerging Infect. Dis. J. 16, 1661. doi: 10.3201/eid1611.100574
Viechtbauer W. (2010). Conducting meta-analyses in R with the metafor package. J. Stat. Software 36, 1–48. doi: 10.18637/jss.v036.i03
Wang Y. and Gu Q. (2010). Effect of probiotic on growth performance and digestive enzyme activity of Arbor Acres broilers. Res. Veterinary Sci 89, 163–167. doi: 10.1016/j.rvsc.2010.03.009
Wang J., Yao L., Su J., Fan R., Zheng J., and Han Y. (2023). Effects of Lactobacillus plantarum and its fermentation products on growth performance, immune function, intestinal pH, and cecal microorganisms of Lingnan yellow chicken. Poultry Sci 102, 102610. doi: 10.1016/j.psj.2023.102610
Wickramasuriya S. S., Ault J., Ritchie S., Gay C. G., and Lillehoj H. S. (2024). Alternatives to antibiotic growth promoters for poultry: a bibliometric analysis of the research journals. Poultry Sci 103, 103987. doi: 10.1016/j.psj.2024.103987
Wieërs G., Belkhir L., Enaud R., Leclercq S., Philippart de Foy J.-M., Dequenne I., et al. (2020). How probiotics affect the microbiota. Front. Cell. Infection Microbiol. 9. doi: 10.3389/fcimb.2019.00454
Yaqoob M. U., Wang G., and Wang M. (2022). An updated review on probiotics as an alternative of antibiotics in poultry — A review. Anim. Biosci. 35, 1109–1120. doi: 10.5713/ab.21.0485
Younas S., Bukhari D. A., Bibi Z., Ullah A., and Rehman A. (2025). Impact of multistrain probiotics on growth performance, immune response, and gut morphometry in broiler chicken Gallus gallus domesticus. Poultry Sci 104, 105026. doi: 10.1016/j.psj.2025.105026
Yu W., Hao X., Zhiyue W., Haiming Y., and Lei X. (2020). Evaluation of the effect of bacillus subtilis and pediococcus acidilactici mix on serum biochemistry, growth promotation of body and visceral organs in lohmann brown chicks. Rev. Bras. Ciec. Avícola 22 (3), 1-7. doi: 10.1590/1806-9061-2020-1274
Zhou X., Wang Y., Gu Q., and Li W. (2010). Effect of dietary probiotic, Bacillus coagulans, on growth performance, chemical composition, and meat quality of Guangxi Yellow chicken. Poultry Sci 89, 588–593. doi: 10.3382/ps.2009-00319
Zhu P., Xu X., Qi Y., Shi S., and Wang H. (2017). Effects of Bacillus subtilis on growth performance and intestinal digestive function of yellow broilers. Eur. Poultry Sci 81, 1–8. doi: 10.1399/eps.2017.176
Zoumpopoulou G., Kazou M., Alexandraki V., Angelopoulou A., Papadimitriou K., Pot B., et al. (2018). “Probiotics and Prebiotics: An Overview on Recent Trends,” in Probiotics and Prebiotics in Animal Health and Food Safety. Eds. Di Gioia D. and Biavati B. (Springer International Publishing, Cham), 1–34.
Keywords: broilers, probiotics, meta-analysis, body weight gain, feed conversion ratio, Lactobacillus, Bacillus
Citation: Opazo R, Salinas C and Villasante A (2025) Formulation strategies of probiotics in broilers: systematic review and meta-analysis of their effects on production performance. Front. Anim. Sci. 6:1679614. doi: 10.3389/fanim.2025.1679614
Received: 04 August 2025; Accepted: 04 September 2025;
Published: 29 September 2025.
Edited by:
Teodora Popova, Institute of Animal Sciences, BulgariaReviewed by:
Monika Madheswaran, ICAR-Directorate of Poultry Research (DPR), IndiaArtun Yibar, Bursa Uludag Universitesi, Türkiye
Alaa Al-Seraih, University of Basrah, Iraq
Copyright © 2025 Opazo, Salinas and Villasante. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rafael Opazo, cm9wYXpvQGludGEudWNoaWxlLmNs
 Rafael Opazo
Rafael Opazo Catalina Salinas
Catalina Salinas Alejandro Villasante1,2
Alejandro Villasante1,2