- 1Department of Agricultural Machinery, University of Applied Sciences, Neubrandenburg, Germany
- 2Institute of Animal Science, University of Bonn, Bonn, Germany
- 3Department of Agricultural Process Engineering, Faculty of Agricultural and Environmental Sciences, University of Rostock, Rostock, Germany
- 4Psychophysiology Working Group, Research Institute for Farm Animal Biology (FBN), Dummerstorf, Germany
The introduction of precision dairy farming has led to increasing automation of core tasks in dairy farming. However, the impact of these technologies on animal welfare remains the subject of ongoing debate. A previous study using the Welfare Quality® (WQ) Assessment protocol to examine the impact of dairy farm automation on cattle welfare found an effect on animals’ behavior. While the WQ protocol is widely used to evaluate dairy cattle welfare, it is often criticized for subjectivity. Thus, more objective indicators are demanded. Concurrently, hair cortisol concentration (HCC) has emerged as a promising objective indicator of long-term stress in animals, offering a potential indirect welfare indicator. Thus, the present study aimed to investigate the relationship between farm automation levels and dairy cattle welfare, using HCC as a biomarker of animal stress. Furthermore, associations between HCC and WQ indicators are examined. Therefore, German farms (n = 32) were categorized into three automation levels based on a newly developed classification system. On each farm, welfare assessment was performed using the WQ protocol, and hair samples were collected from 15 cows to determine HCCs. Median HCC values were compared across automation levels using the non-parametric Kruskal–Wallis test. Associations between HCC and WQ indicators were examined using multiple linear regression analysis. A trend of lower HCC levels with increasing automation was observed. Even so, the differences were not statistically significant, likely due to substantial variabilities in housing, management, and settings of automatic systems such as individual milking intervals or frequencies of feeding, bedding, and so forth. among farms. Significant correlations were found between median HCC per farm and the WQ protocol indicators “percentage of moderately lame cows,” “cows with at least one hairless patch and no lesion,” “tendency to be apathetic,” and “absence of injuries.” However, these indicators are not recommended as standalone measures of welfare. Nevertheless, consistent with the existing literature, our findings support lameness and integument alterations as key indicators of poor welfare in dairy cattle, which was also reflected in elevated HCC levels. As the number of highly automated farms is expected to increase in upcoming years, future studies with larger sample sizes are recommended.
1 Introduction
Dairy farming in recent years has been characterized by two major developments: growing public concern about animal welfare (Clark et al., 2016; Wolf et al., 2016) as well as increasing automation of the sector (Weary and von Keyserlingk, 2023). Here, application of “smart” or “precision” farming technologies is viewed ambivalently. On the one hand, warnings are issued about risks, including animals experiencing stress from the adaptation to new precision livestock systems (Tuyttens et al., 2022), farmers’ dependency on technologies (Kleen and Guatteo, 2023), and difficulty of data interpretation (Bianchi et al., 2022). On the other hand, precision livestock farming (PLF) offers a wide range of potential welfare advantages, including earlier disease detection, objective and consistent measurements of animal-related information, and predicting risks for animal welfare (Bianchi et al., 2022; Kleen and Guatteo, 2023).
The impact of precision livestock technologies is manifold and therefore poorly explored (Kleen and Guatteo, 2023). Accordingly, there is a consensus in the research field of dairy cattle farming that the impact of PLF technologies on dairy cattle welfare requires further research (Schillings et al., 2021; Veissier et al., 2019). Measuring dairy cattle welfare is challenging given that there is no universal and standardized measurement method for doing so. Instead, a variety of different evaluation systems have been developed, all containing different variations of welfare indicators (Leliveld and Provolo, 2020; Krueger et al., 2020). The most recognized evaluation system in dairy cattle research is the Welfare Quality® (WQ) Assessment protocol, which includes measures that were tested for validity, reliability, and feasibility (Knierim et al., 2021). Still, there are critics of the protocol, including the lack of validation by objective measurements (Krueger et al., 2020), the weighting of measures (de Vries et al., 2013), and inconsistency of scorings by trained users (de Graaf et al., 2017).
As welfare assessment using protocols involves a certain degree of subjectivity and animal welfare cannot be measured directly, alternative indicators considered to be associated with animal welfare are therefore necessary. Thus, biomarkers designed to measure stress levels or emotional responses of cattle have been investigated as indicators of animal welfare. de Almeida et al. (2019) defined biomarkers as biological molecules used to understand a physiological process or diagnose an abnormal process or a disease. These molecules are contained in various biological matrices, such as blood, saliva, milk, or hair, which makes it possible to objectively measure whether an animal is in a normal or abnormal state depending on their concentration (Hirsch and Watkins, 2020; Ataallahi et al., 2022). Multiple biomarkers have been investigated for different physiological and emotional states in dairy cattle research. Considering animal welfare, stress is an important aspect (Grelet et al., 2022), describing a condition in which animals are confronted with sudden and threatening stimuli leading to activation of the hypothalamic-pituitary-adrenal (HPA) axis (Moberg, 1985; Ghassemi Nejad et al., 2022). Stimuli causing stress can be physiological (e.g., pain and disease), nutritional (e.g., hunger, thirst, metabolic disorders), management-related (e.g., inappropriate human–cattle relationships), or environmental (e.g., poor housing comfort) (Jurkovich et al., 2024).
These different kinds of stressors can lead to chronic or acute stress reactions, depending on their duration and intensity (Ataallahi et al., 2022). Acute stress responses are characterized by a short-time experience of stressors leading to a rapid and complete recovery of physiological balance and finally to full adaptation. During this, the HPA axis is activated and hormones are secreted within seconds or minutes, leading to changes in the physiological or metabolic state of cattle [e.g., increased heart and respiratory rate, higher blood pressure, increased energy mobilization, or reduced appetite; Trevisi and Bertoni (2009)]. Contrary to acute stress, chronic stress appears when cattle experience multiple stressors or repeated acute stress responses, leading to the autonomic nervous system becoming incapable of activating normal physiological and behavioral adaptations (Moberg, 1985; Burnard et al., 2016). Overstimulation of coping responses caused by chronic stress results in direct effects, such as increased body temperature, low energy, and anxious behavior, or indirect effects, such as changes at the functional levels of the endocrine system, immune system, and metabolic system. Such changes are, in turn, responsible for pre-pathological or pathological consequences that negatively affect animal health and welfare (Moberg, 1985; Trevisi and Bertoni, 2009; Romero, 2004).
To detect stress in dairy cattle, biomarkers can reveal whether an animal is in physiological comfort or not. Stress biomarkers can be subdivided into physiological, endocrine, and biochemical biomarkers. They can also include immune indices and performance and health indices (Moberg, 1985; Trevisi and Bertoni, 2009). According to Kelly et al. (1997), a good marker of chronic stress should lead to subtle and long-term changes in physiological function (e.g., endocrine, metabolic, and immune systems), even if the individual appears to have accepted its living conditions. Furthermore, a reliable stress marker should be strongly correlated with the specific pathophysiological aspect of stress, be easy to sample, be stable and durable during storage and evaluation periods, and make use of assays with adequate specificity and sensitivity (Ghassemi Nejad et al., 2022; Ataallahi et al., 2022; Dhama et al., 2019). These criteria are well fulfilled by cortisol. Therefore, it is widely used in animal research (Heimbürge et al., 2019). Cortisol is a glucocorticoid hormone released in response to the activation of the HPA axis, which is a central neuroendocrine system involved in the physiological stress response of organisms (Dallmann et al., 1987). Upon exposure to a stressor, neurons of the paraventricular nuclei of the hypothalamus are stimulated to secrete corticotropin-releasing hormone (CRH). Corticotropin-releasing hormone stimulates the G-protein–coupled CRH-receptor-1 in the endocrine cells of the anterior pituitary, which induces the release of the adrenocorticotropic hormone (ACTH) into the blood. Subsequently, ACTH stimulates cortisol secretion via the adrenal cortex (Burdett, 2019; Meyer and Novak, 2017; Jacobson, 2005). Cortisol is accordingly an indicator for increased HPA axis activity and therefore considered an indicator of acute or chronic stress (Comin et al., 2013; Burnett et al., 2015; Ataallahi et al., 2022).
Cortisol concentrations have been measured in various biomatrices such as plasma (Breuer et al., 2003; Almoosavi et al., 2020), saliva (Lürzel et al., 2015), urine (Higashiyama et al., 2007), milk (Hemsworth et al., 1989), feces (Pesenhofer et al., 2006; Palme et al., 2000; Ebinghaus et al., 2020) and hair (Ghassemi Nejad et al., 2022; Koenneker et al., 2023; Braun et al., 2019).
Apart from hair, the biological matrices noted above all exhibit some limitations that restrict their utility as biomarkers of long-term stress. For example, cortisol levels in blood undergo diurnal fluctuations (Hucklebridge et al., 2005). Moreover, liquid matrices such as serum and saliva primarily reflect HPA axis activity shortly after its activation; cortisol levels in urine and feces represent HPA axis activity from a few hours to days prior to the measurement (Ghassemi Nejad et al., 2022). On the contrary, hair cortisol provides a retrospective assessment of HPA axis activity over extended periods ranging from weeks to months, given that hair of dairy cattle grows approximately one centimeter per month (Ataallahi et al., 2022; Wennig, 2000).
The incorporation of hair cortisol into the hair shaft is not fully understood yet, though the drug incorporation into human hair has been extensively researched. Possible mechanisms of cortisol incorporation into hair are accordingly based on findings of drug research. According to Pragst and Balikova (2006), four possible methods of incorporation exist: active or passive diffusion into the hair follicle during hair growth, diffusion from body secretions such as sweat and sebum or by uptake from the deep skin structure during formation of the hair shaft, and external contamination after the formation of the hair shaft (Ghassemi Nejad et al., 2022; Heimbürge, 2021). The last method was also confirmed by Otten et al. (2022) in an in-vitro experiment showing that contamination of hair with saliva, urine, and feces can cause cortisol to be incorporated into the hair. It is therefore recommended that researchers obtain hair samples for cortisol analysis from clean regions of an animal’s body. In addition to external contamination of the hair, there are other factors that influence hair cortisol concentration (HCC) values and should be considered before sampling. Significant differences between HCC values of different cattle breeds have been found by Braun et al. (2017), comparing HCC levels of Brown Swiss, Swiss Fleckvieh, and water buffalo cows. Furthermore, multiparous cows were found to exhibit slightly higher HCCs than primiparous cows (Burnett et al., 2014, 2015). The color of hair samples affects HCC values, as white hair seems to be less stable against photodegradation (Otten et al., 2023). Heimbürge et al. (2020b) also found an effect of seasonality on HCC. Regarding the effects of the lactation stage on HCC levels, there is currently no consensus. Some studies have reported peak HCC between 60 and 200 days postpartum (Otten et al., 2023; Endo et al., 2017); others have noted elevated levels around calving and a decline after 120 days (Braun et al., 2017; Hayashi et al., 2021; Burnett et al., 2014, 2015). However, it is widely accepted that the transition period (i.e., 3 weeks before calving to three weeks after calving) significantly affects metabolism and involves multiple stressors (Grummer, 1995; Mezzetti et al., 2021), which could result in elevated HCC levels.
As hair grows slowly and cortisol is incorporated into the hair consistently, analysis of HCC can reflect HPA axis activity after prolonged stress situations or in response to frequently repeated stressors. It is therefore considered a suitable indicator for chronic stress and animal welfare and health under exposure to environmental stressors (Ataallahi et al., 2022).
Thus, HCC has been used for these purposes in various studies. For example, Nejad et al. (2021) found that increased access to pasture decreased hair cortisol levels significantly. An Indian study found that high HCC levels were related to poor housing conditions and health problems (Sharma et al., 2019). Grelet et al. (2022) were able to demonstrate significantly higher levels of HCC in cows housed under conditions known to cause stress, compared with cows housed under normal conditions. On the other hand, Fischer-Tenhagen et al. (2018) found no differences between the HCC of lame cows compared with non-lame cows. Ninomiya et al. (2024) studied correlations between human–animal relationships and HCC but found no significant effects. A comparison of hair cortisol of two groups of veal calves, with one group reared under enhanced welfare standards and one group reared under conventional standards, found no significant differences between both groups (Braun et al., 2019).
The housing condition of dairy cattle in farms is rapidly changing due to the automation technology of the farm operations. This shift raises the question of whether this could also affect chronic stress and/or well-being of dairy cattle. Veissier et al. (2019) claimed that animal well-being could be improved via PLF improvements, as PLF makes use of behavioral signals associated with health status, social relations, and human–animal relationships, for instance. On the other hand, some scientists have noted that PLF might induce stress since animals have to adapt to new technologies. Furthermore, researchers have raised concern about housing environments being designed to adhere to new technologies rather than animal behavior; such environments could possibly limit animals’ ability to perform species-specific behavior (Tuyttens et al., 2022). However, there has only been one study so far concerning the effect of increased automation on dairy cattle welfare; impact of three automation levels on dairy cattle welfare at 32 German dairy farms was investigated using the WQ protocol. The authors found that highly automated farms scored significantly higher for appropriate behavior and positive emotional state of cattle; those farms also exhibited improved human–animal interactions as well as a decreased percentage of cows with dirty lower legs and severe lameness (Lavrijsen-Kromwijk et al., 2024). Building on these results, it is assumed that cows living on highly automated farms might exhibit lower levels of HCC than animals living on non-automated farms.
It is also of interest to determine whether there are correlations between HCCs of dairy cows and animal welfare assessment scores. van Eerdenburg et al. (2021b) previously investigated correlations between welfare assessments of nine different protocols, including the WQ protocol, and HCC levels in cows, finding no significant correlations. Vesel and Pavič (2019) used pooled hair samples collected from eight dairy farms but also failed to find a correlation between the outcome of the WQ protocol and HCC levels of cows. However, it is recommended that similar research be conducted with larger sample sizes using standardized protocols for hair sampling, processing, and analysis (Vesel et al., 2020).
The aim of this study is to investigate differences between median HCC levels of cows living on farms with different automation levels using standardized procedures. It is hypothesized that increasing automation levels of dairy farms are negatively correlated with HCC levels of cows living there. On the other hand, this study aims to examine correlations between welfare assessment with the WQ protocol and median HCC levels, hypothesizing to find negative correlations between improved welfare measures and criteria and median HCC levels.
2 Materials and methods
2.1 Farms and animals
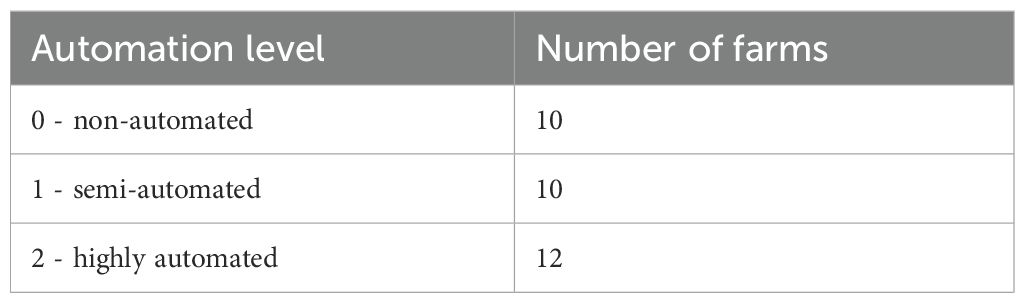
The study was conducted from October 2023 to April 2024 on 32 German dairy farms located in both Northern and Central Germany. Only farms keeping Holstein and Red Holstein cows in conventional cubicle housing systems without access to pasture were included in the study. Trial farms were grouped into three different automation levels according to the classification system of Lavrijsen-Kromwijk et al. (2024) (Table 1). Given that farms can use different automation techniques in various combinations, the classification is based on a scoring system across the four functional areas of milking, feeding, dung removal, and bedding. Each area is scored from 0 to 2 points, with 0 points for application of the least automated technology and 2 points for the highest level of available technology. For milking and feeding, 1 point is applied to farms using intermediate stages of automation. Points are summed across all four areas to yield automation scores ranging from 0 for the least automated farms to 8 for the most automated farms. Finally, automation scores are classified into automation levels. Farms with an automation score from 0 to 2 are classified as non-automated, farms with an automation score from 3 to 5 are classified as semi-automated, and farms with an automation score ranging from 6 to 8 points are classified as highly automated (Lavrijsen-Kromwijk et al., 2024).
2.2 Welfare assessment and hair sampling, storage and analysis
The study design was approved by the State Office for Agriculture, Food Safety, and Fisheries, Mecklenburg–Western Pomerania, Germany (approval number 7221.3-17033_23).
Welfare assessments were conducted on all farms using the WQ Protocol (Welfare Quality® 2009) by one single person. The welfare assessment procedures, the selection of sample sizes, and the requirements for the assessor are documented in the study of Lavrijsen-Kromwijk et al. (2024). Hair samples were collected from 15 cows on each farm. Sample size was determined according to the calculations of Grelet et al. (2022). As previous investigations found various factors influencing HCC, it is recommended to use standardized sampling protocols in terms of age, body region, hair color, and season and to avoid external contaminations of hair samples (Heimbürge et al., 2019, 2020). Therefore, hair samples were only taken from multiparous Holstein–Friesian (HF) and Red Holstein cows. Only colored hair samples (black and brown) were taken, and the period of hair sampling was restricted to the winter half-year, from the end of October to the beginning of April. Farms of each automation level were visited in random order during the study period. Hair samples were taken as clean as possible from the hip; this region is considered to be less exposed to fecal contamination than the tail switch. Although application of the shave–reshave method is recommended for analysis of HCC, this method could not be used due to long distances between trial farms, therefore making this method too time consuming and cost-intensive (Otten et al., 2022). To avoid potential stress factors related to the transition period of dairy cattle, cows with fewer than 140 days in milk (DIM) were excluded from hair sampling. Given that cow hair grows about 0.6–1.0 cm per month and is fully renewed every 3 months, it is assumed that any calving-related effects are no longer detectable in the hair after 140 days postpartum (Schwertl et al., 2003; Comin et al., 2012).
Hair sampling was conducted by one single person. This person was given a list of cows from 140 to 400 DIM by the herdsmen at each trial farm. Animals on the list that satisfied the selection criteria were randomly selected in the barn. Hair samples were collected from the cleanest possible place on the flank of the cow as described by van Eerdenburg et al. (2021b). A 10 cm × 10 cm on the animal’s coat was shaved using an electric clipper (Aesculap Durato GT434-RS, Suhl, Germany). Hair was clipped 0.2 mm close to the skin by usage of a size 50 blade (Oster Cryogen-X Nr. 50, McMinnville, TN, USA). Each hair sample was placed in a small plastic bag labeled with the cow’s ID number and then put in a dry paper envelope for each farm. All envelopes were stored together in a dark box at room temperature until processing. In total, 480 hair samples were collected. Lactation and DIM were documented for all cows included in the study.
Extraction and analysis of hair cortisol was performed as described in Heimbürge et al. (2020b). Briefly, the hair samples were washed twice with isopropanol, dried at room temperature, snap-frozen in liquid nitrogen, and pulverized using a ball mill (MM 400, Retsch GmbH, Haan, Germany). For cortisol extraction, 1 ml of methanol (HPLC grade) was added to approximately 50 mg of pulverized hair and incubated at room temperature for 18h–24h with slow shaking. The samples were then centrifuged for 2 min at 12,000 rpm in a microcentrifuge, and 0.6 ml of the supernatants were finally dried using a vacuum centrifuge (SpeedVac Concentrator, Thermo Fisher Scientific Inc., Waltham, MA, USA) and stored at −20°C. The dried extracts were reconstituted in 0.4 ml phosphate buffer and analyzed for hair cortisol by ELISA (Demeditec Diagnostics GmbH, Kiel, Germany) according to the manufacturer’s instructions. The cross-reactivities with other steroids were as follows: testosterone, <0.1%; corticosterone, 6.2%; cortisone, 0.8%; 11-deoxycorticosterone, 2.6%; 11-deoxycortisol, 50%; dexamethasone, <0.1%; estriol, <0.1%; estrone, <0.1%; prednisolone, 100%; prednisone, 0.9%; progesterone, <0.1%; 17-hydroxyprogesterone, 1.3%; danazole, <0.1%; pregnenolone, <0.1%; estradiol, <0.1%; and androstenedione, <0.1%. The sensitivity of the assay was 0.8 pg/mg. The intra- and inter-assay coefficients of variation were 4.2% and 7.2%.
2.3 Statistical analysis
Statistical analyses were performed using SPSS 29 (IBM, Armonk, NY, USA). For each farm, median HCC was used as a representative value. Median HCCs were tested for normality with the Shapiro–Wilk test. Levene’s test was used to test the homogeneity of variance.
2.3.1 Analysis of correlations between automation level and HCC of farms
The Shapiro–Wilk test revealed that the data were not normally distributed. Therefore, the non-parametric Kruskal–Wallis test was used to compare median cortisol levels across the three automation levels. Statistical significance was determined at a threshold of p ≤ 0.05. In addition, correlations between single automation systems and median HCC levels were investigated using a non-parametric test. The Mann–Whitney U test was applied for dung removal and bedding systems, and the Kruskal–Wallis test for milking and feeding systems. Furthermore, several covariates were included in the statistical calculations. Since none of these covariates significantly impacted the results, they were removed from the statistical model.
2.3.2 Analysis of correlations between HCC and Welfare Quality® assessment
Given that data were not normally distributed, Spearman rank correlations between median HCCs per farm and indicators of the WQ Assessment protocol were calculated. Measures and criteria exhibiting significant p-values (p ≤ 0.05) and correlation coefficients with values greater than or equal to 0.3 were considered to have significant effects on HCC and were therefore included in regression analysis (Akoglu, 2018). Preselection of variables was performed to reduce the risk of overfitting and to improve interpretability of the model (Heinze et al., 2018). The selected criteria were also checked for homoscedasticity using the Breusch-Pagan test. A multiple linear regression was performed for the following WQ measures: “duration of lying down moments,” “percentage of cows with dirty lower legs,” “percentage of cows with dirty udder,” “percentage of cows with dirty flanks and upper legs,” “percentage of not lame cows,” “percentage of moderately lame cows,” “percentage of severely lame cows,” “percentage of cows with no lesion,” “percentage of cows with at least one hairless patch and no lesion,” “percentage of cows with at least one lesion,” “tendency to be friendly,” “tendency to be positively occupied,” “tendency to be inquisitive,” and “tendency to be apathetic.” The following WQ criteria were included in multiple regression analysis: “absence of prolonged thirst,” “absence of injuries,” and “positive emotional state.” A stepwise regression approach was employed to select variables based on their statistical contribution to the model. Thus, “percentage of moderately lame cows,” “percentage of cows with at least one hairless patch and no lesion,” and “tendency to be apathetic” were included in the model for WQ measures, as described by the following equation:
The total adjusted r2 was 67.8% and residuals were normally distributed. For WQ criteria, only “absence of injuries” was included in the following model:
The total adjusted r2 was 57.1% and residuals were normally distributed. For both WQ measures and criteria, multicollinearity was tested using variance inflation factor (VIF) and tolerance values. The VIF had to be lower than 10 and the tolerance had to be below 0.1.
3 Results
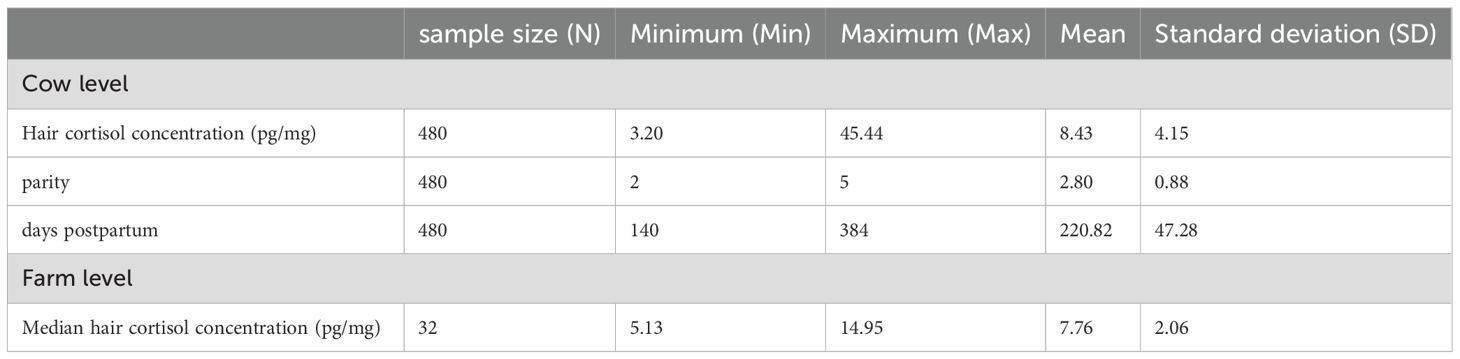
Median HCC was 8.43 pg/mg (SD = 4.15 pg/mg). Descriptive statistics for HCC values based on cow and farm level are listed in Table 2. Data were not normally distributed.
3.1 Correlations between automation level and HCC of farms
The Kruskal–Wallis test revealed no significant correlation between farm automation level and median HCC (p = 0.733). However, there was a tendency toward lower HCCs with increasing automation level (Figure 1). The median HCC of all trial farms was 7.76 ± 2.06 pg/mg. For non-automated farms, median HCC was 8.30 ± 2.62 pg/mg. For semi-automated farms, median HCC was 7.63 ± 2.14 pg/mg, and for highly automated farms, median HCC was 7.43 ± 1.50 pg/mg.
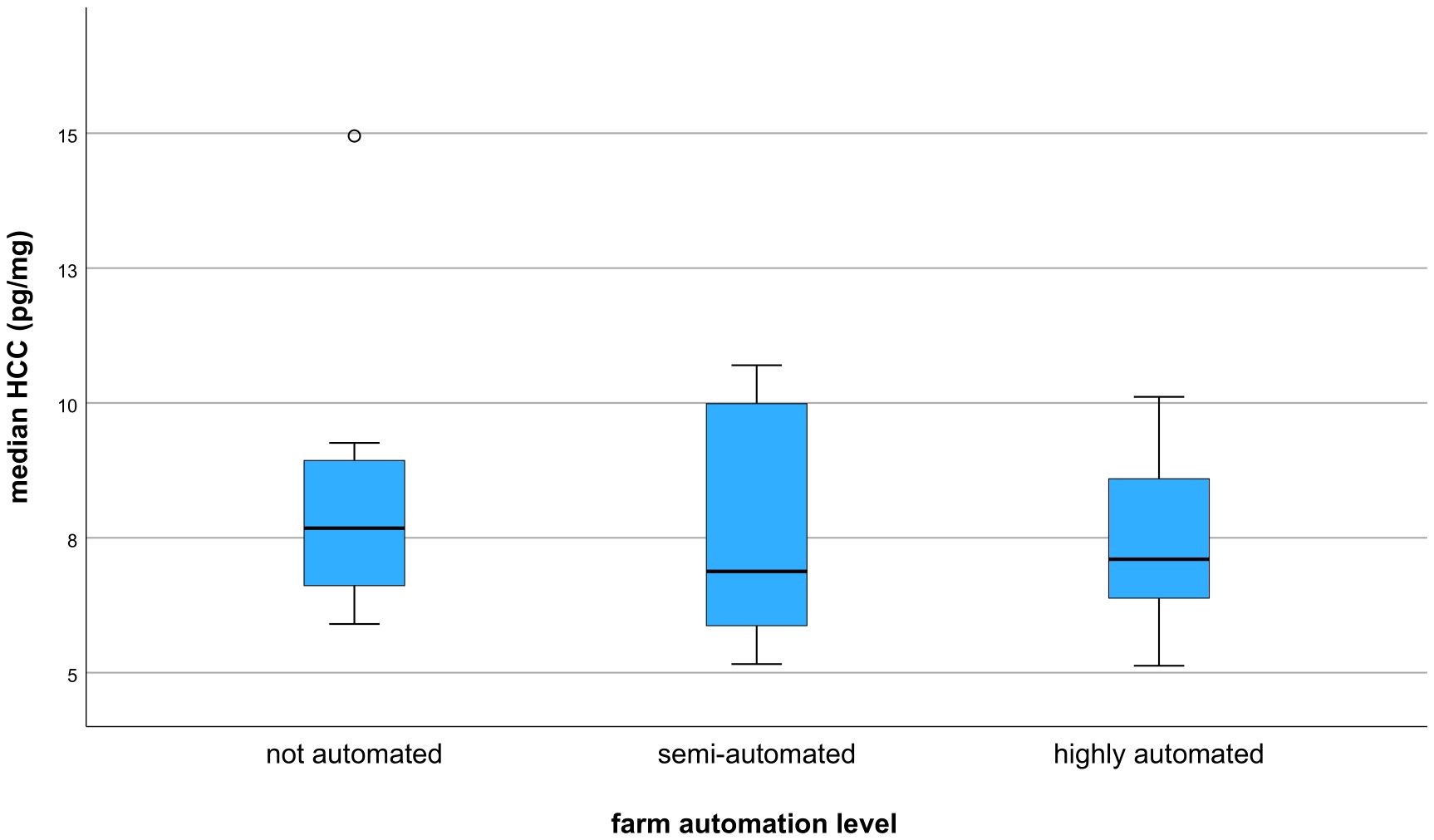
Figure 1. Boxplot showing the Kruskal–Wallis test between median HCC (pg/mg) and farm automation level.
3.2 Correlations between HCC and Welfare Quality® assessment
Table 3 lists the WQ measures and criteria that exhibited statistically significant associations (p ≤ 0.05) with median HCC, as indicated by Spearman’s rank correlation coefficients of 0.3 or larger. Subsequently, these variables were included in multiple linear regression analysis.
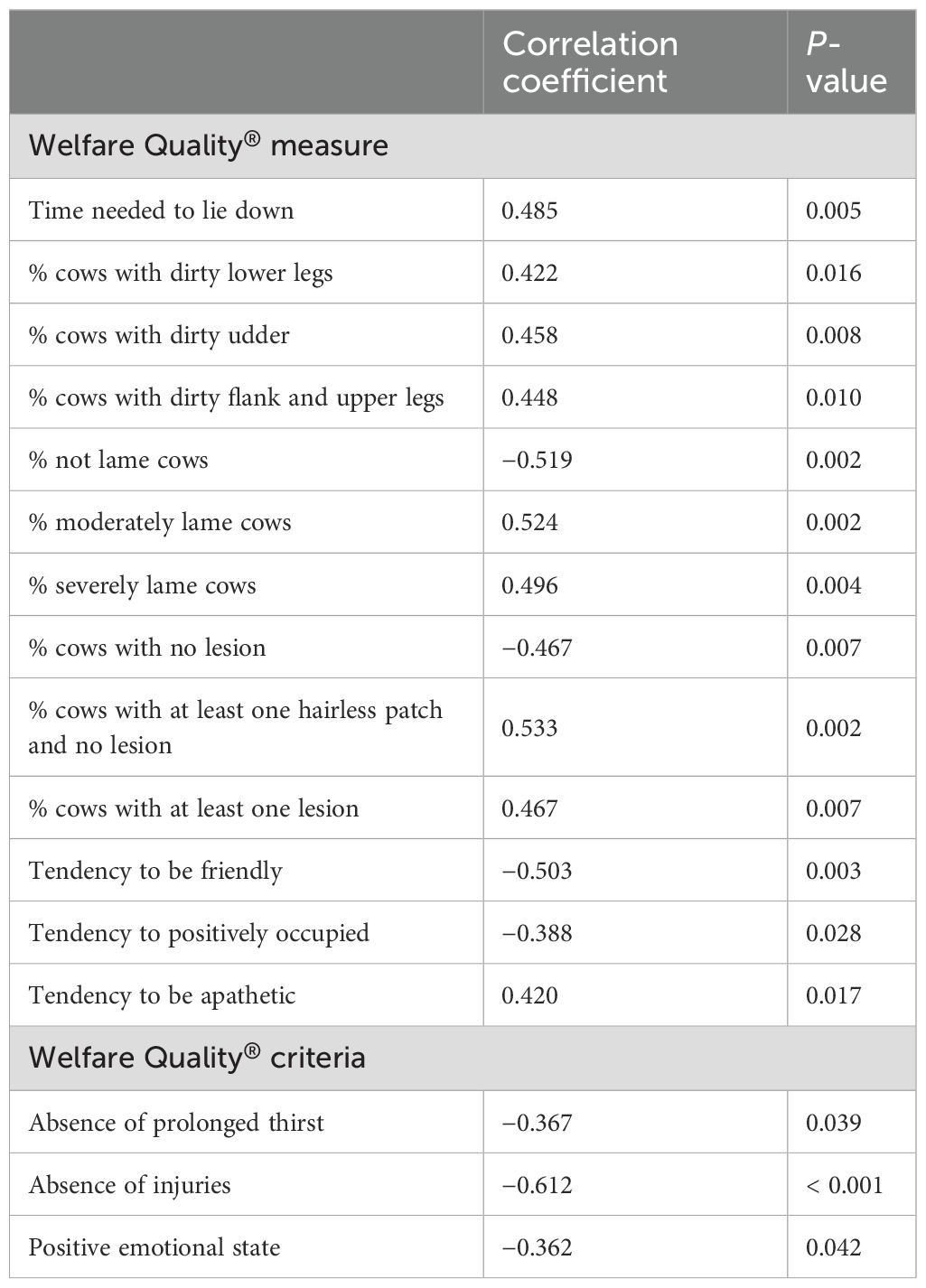
Table 3. Spearman’s rank correlation coefficients for median HCC (pg/mg) with Welfare Quality® measures and criteria on a per-farm basis.
Regression analysis was used with the stepwise procedure. For multiple regression analysis of WQ measures and median HCC per farm, “percentage of moderately lame cows,” “tendency to be apathetic,” and “percentage of cows with at least one hairless patch and no lesion” were included in the model. For WQ criteria, only “absence of injuries” was included in the model (Table 4).
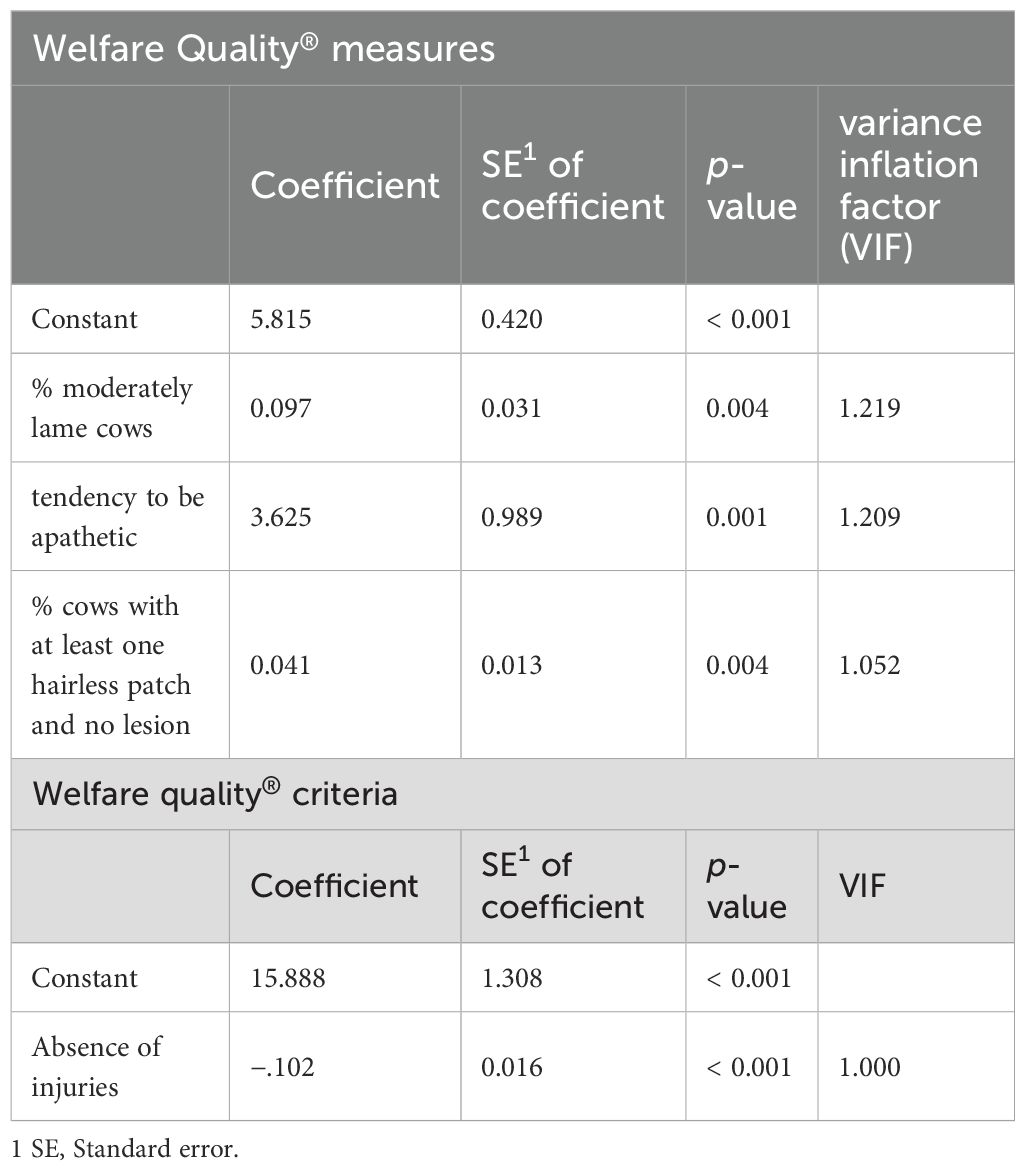
Table 4. Multiple linear regression analysis of Welfare Quality® measures and criteria significantly correlated (p ≤ 0.05) with HCC (pg/mg).
4 Discussion
Hair cortisol concentrations of 480 dairy cows on 32 dairy farms characterized by three different automation levels were measured. It was found that HCC ranged from 3.20 pg/mg to 45.44 pg/mg. Median HCC across all animals was 8.43 pg/mg, which is similar to the values measured in previous studies that also took hair from caudo-dorsal body sites. For example, Otten et al. (2023) found mean HCC in black hair from postpartum dairy cows of 8.09 ± 0.28 pg/mg. Heimbürge et al. (2020a) found a mean HCC of 9.8 ± 0.5 pg/mg for dairy cattle in an experiment prior to the administration of ACTH. A study investigating the effect of seasonality on HCC noted a mean value of 8.5 ± 0.3 pg/mg for black hair. A significant difference between HCC levels in the summer and winter seasons was found for dairy cattle; mean HCC in winter was 8.6 ± 0.3 pg/mg, and mean HCC in summer was 6.2 ± 0.3 pg/mg (Heimbürge et al., 2020b). A Dutch study including white hair samples of 548 dairy cows found a mean HCC of 20.50 pg/mg [range: 3.93–127.42 pg/mg; van Eerdenburg et al. (2021b)]. In our study, median HCC level per farm (n = 32) was 7.76 pg/mg (range: 5.13 - 14.95 pg/mg), whereas mean HCC in the Dutch study was 20.41 ± 1.92 pg/mg (n = 58). Mean HCC in the Dutch study was higher than the median in our study since the Dutch study examined a total of 60 farms, with 20 farms each classified as weak, sufficient, or good in terms of animal welfare based on a veterinarian-defined classification system. Farms in our study were predominantly classified as “Enhanced” (n = 29) according to the WQ Assessment protocol; only a few were rated “Acceptable” (n = 3). Therefore, welfare levels of farms in our study were generally higher, and HCC was accordingly lower. At this point, further studies comparing HCC values at the farm level are not available.
Furthermore, our study did not find any significant correlation between median HCC and automation level of dairy farms. However, we did note that median HCC levels were slightly lower for semi- and highly automated farms compared with non-automated farms; this finding indicates that cows living in farms with higher levels of automation might experience lower levels of stress. Given that farms of different automation levels can include various combinations of automation systems, it would be interesting to determine whether correlations exist among milking, feeding, dung removal, and bedding systems and median HCC levels. However, no significant correlations were found between any of the single automation technologies mentioned above and HCC levels, either. Studies investigating effects of milking, feeding, dung removal, or bedding systems on HCC are not yet available. Nevertheless, there have been studies comparing conventional and automatic systems with other welfare-related indicators. For instance, studies have compared conventional and automatic milking systems concerning milk cortisol, restlessness behavior, heart rate, and plasma adrenaline and noradrenaline. Studies have reported contradictory results but concluded that both conventional and automatic milking systems are equally acceptable and do not affect the welfare of dairy cattle (Gygax et al., 2006, 2008; Wenzel et al., 2003; Hopster et al., 2002).
For feeding systems, investigations have focused on the effects of different feeding frequencies on feeding behavior, rumination, lying behavior, and agonistic behavior of cows (Grothmann et al., 2014; Mattachini et al., 2019). Feeding frequencies in our study varied significantly among farms, with conventional feeding systems feeding 1–3 times per day and automatic feeding systems operating 5–13 times per day; automatic feeders provided feed up to ten times more frequently per day. Results of feeding-related studies have also been contradictory. It was found that automatic feeding can positively affect cow welfare, as cow traffic at the feed bunk and at the milking robot becomes more uniform, and cows experience decreased waiting times and fewer disturbances there (Oberschätzl-Kopp et al., 2016; DeVries et al., 2005). Gaworski and Kic (2024) noted that automatic feeding is beneficial concerning animal welfare, as such feeding devices produce less noise and emissions compared with conventional feeders since automatic feeders are electrically powered.
Comparing farms with automatic and manual dung removal systems, we did not find significant differences in HCC levels, either. Although farms with manual dung removal systems only cleaned cows’ walkways up to three times per day and farms with automatic manure scrapers cleaned walkways anywhere from 6 to 24 times per day, this difference did not affect HCC of dairy cows.
Studies of the effects of automatic dung removers and cattle welfare have investigated the prevalence of hoof disorders and lameness; more frequent dung removal decreases the likelihood of both conditions (Doerfler et al., 2017; King et al., 2016). Although lameness is considered to cause pain and negatively affect animal welfare, a study comparing lame and non-lame cows concerning HCCs found no significant difference between the groups (Fischer-Tenhagen et al., 2018). Several studies investigating the reaction of cows toward the operation of automatic scrapers all concluded that cows experience mild stress when being confronted with scrapers. However, the animals are able to quickly adapt to them (Buck et al., 2013; Doerfler et al., 2016; Stülpner et al., 2014; Leinweber et al., 2019).
There is a paucity of studies of the effects of automatic bedding systems on dairy cattle welfare. However, our data have shown that farms with automatic bedding systems provide fresh bedding material into cows’ cubicles at least once a day; farms that manually spread bedding in cows’ cubicles do so on average five times per month. Cows prefer dry cubicles with more bedding, so it is assumed that cows spend more time lying in farms with automatic bedding systems. This might in turn positively affect animal welfare (Fregonesi et al., 2007). Even so, HCC in our study did not differ between animals kept in farms of each bedding system.
Overall, investigating the impact of automation level on HCC and dairy cattle welfare remains challenging given that every farm is unique. For instance, all visited farms had their own individual frequencies of milking, feeding, bedding, and dung removal; there was a high level of variance in these parameters. Moreover, different suppliers are available on the market for automatic milking, feeding, dung removal, and bedding. Consequently, robots differ in their technical construction and functionality. Therefore, their operation can be experienced differently by the animals. Physiological and behavioral reactions of dairy cattle to technology from different manufacturers remain unexplored.
Additionally, the success of the implementation of precision dairy farming (PDF) technologies also depends on the technology skills of the farmer (Tuyttens et al., 2022). Various settings can be programmed for each PDF device, determining how well the technology is working in each individual herd.
But more than the management of automation technologies can affect animal welfare and the amount of stress dairy cattle experience, including general management routines, for example, appropriate handling of animals, controlling of suitable feeding rations, and appropriate health management to prevent cows from experiencing pain and disease (Jurkovich et al., 2024; Abeni and Bertoni, 2009). Wide variations in the implementation of these factors persist across farms. Therefore, fully standardizing commercial farms to investigate the impact of automation levels presents a significant challenge. That situation is exacerbated by the fact that only a few farms in Northern and Central Germany are already highly automated.
Studies exploring the impact of automation on dairy cattle welfare are generally lacking. Only one other previous study by our team investigated this subject using the WQ Assessment protocol. It was found that higher automation levels positively affected human–animal relationships and the positive emotional state of animals (Lavrijsen-Kromwijk et al., 2024). This may be due to a shift in the nature of contact with the animals. As farmers intervene less in cows’ daily routines, they are given more freedom to express their natural behaviors, perceiving farmers’ actions as less disruptive (Wildridge et al., 2020; Jacobs and Siegford, 2012). Furthermore, highly automated farms exhibited significantly lower scores for the prevalence of severe lameness and cows with dirty lower legs (Lavrijsen-Kromwijk et al., 2024). However, these effects, which are assumed to be correlated with lower stress levels in dairy cattle, did not lead to a significant decrease in HCC levels on farms with higher automation levels. However, we did note a tendency for lower HCC values with increasing automation levels in the descriptive statistics.
The goal of the present study was to determine whether WQ measures and criteria might correlate with median HCC levels of trial farms. Regression analysis of WQ measures revealed that “percentage of moderately lame cows” (b = 0.097; p = 0.004), “percentage of cows with at least one hairless patch and no lesion” (b = 0.041; p = 0.004), and “tendency to be apathetic” (b = 3.625; p = 0.001) had a positive impact on HCC and explained a significant proportion of the variance of the model, R2 = 0.678, F(3, 28) = 19.648; p < 0.001.
The higher percentage of “moderately lame cows” that was correlated with higher HCCs is unlike the findings of Fischer-Tenhagen et al. (2018). Those authors could not find an effect of lameness on levels of HCC extracted from hair of the tail switch. Even so, various studies have noted lameness causing pain and distress in dairy cattle and therefore negatively affecting animal welfare (Weigele et al., 2018; Sadiq et al., 2020, 2017). The positive relationship between “percentage of cows with at least one hairless patch and no lesion” and median HCCs is consistent with the findings of Sharma et al. (2019). This team found associations between high HCC levels and carpal joint injuries and body lesions; injuries and lesions are painful for cattle and can lead to inflammation. Consequently, animals experience stress, possibly leading to higher levels of hair cortisol (Sharma et al., 2019; Burnett et al., 2014).
Studies investigating correlations between HCC and the emotional state of cattle are currently lacking. However, a study investigating the welfare of Pakistani monkeys found a positive correlation between the monkeys’ fear score and HCCs. That finding could support the positive correlation between apathetic behaviors and HCC levels noted in our study (Akbar and Evans, 2023). Studies of dogs have also revealed that chronic stress causing sustained high cortisol levels is related to fearful behavior, aggression, and anxiety (Mârza et al., 2024). Correlations between cortisol concentrations and human–animal relationships have been explored in studies involving cows and dogs: Ninomiya et al. (2024) did not find significant correlations between cows’ reactions to humans and HCC levels; Ebinghaus et al. (2020) found increased human–animal contact to be associated with a decrease in fecal cortisol concentrations. Roth et al. (2016) found a negative correlation between positive human interactions and hair cortisol levels in dogs. Additional studies investigating the link between human–animal relationships and cortisol levels are necessary.
Our regression analysis revealed that “absence of injuries” was the only criterion negatively correlated with median HCC levels (b = −0.102; p < 0.001). This term explained a portion of the variance: R2 = 0.571, F(1, 30) = 39.966, p < 0.001. As “absence of injuries” is calculated based on the prevalence of lameness and integument alterations, it seems plausible that this criterion also helps to explain the HCC of the cows on the farms that we visited. This finding raises the question of whether it is sufficient to only assess measures for “absence of injuries” instead of all of the measures included in the WQ protocol when it comes to evaluating cattle welfare. Since execution of the WQ protocol is very time consuming, several studies have investigated whether it is possible to reduce the number of measures and still achieve a reliable welfare assessment. Heath et al. (2014) aimed to identify potential “iceberg indicators” potentially predicting overall classification. They found that “absence of prolonged thirst” predicted 88% classification of farms correctly. However, it was concluded that the prediction of overall welfare scores by “absence of prolonged thirst” was due to the strong weighting of the criterion in the WQ overall score. Based on the results of our study, this is viewed critically. As regression analysis only included “absence of injuries” in the model, higher weighting of this criterion would be recommended for WQ overall classification instead of “absence of prolonged thirst.” de Vries et al. (2013) also criticized strong weighting of a limited number of measures, including “absence of prolonged thirst” on WQ overall scores and underrating of measures of the WQ principle “Good health,” including prevalence of lameness and integument alterations; WQ principles are four overarching categories concerning feeding, housing, health, and behavior of cattle. Principle scores are derived from the WQ measures and criteria that represent quantifiable welfare indicators. The Danish Cattle Federation (DCF) developed an assessment protocol containing 10 simplified measures rather than 30 measures, including lameness and integument alterations. Comparison of the WQ Assessment protocol and the extended protocol designed by the DCF revealed significant correlations across all four WQ principles and overall scores (Andreasen et al., 2014). Tuyttens et al. (2021) similarly developed a simplified welfare monitoring protocol. Therefore, trained users of the WQ Assessment protocol have ranked all WQ measures and selected six animal-based measures considered to have the largest impact on cattle welfare. These measures, also including lameness and integument alterations, were combined with severity scores to rate how strongly each measure was assumed to affect cattle welfare. The severity score was highest for severe lameness (92) and lowest for hairless patches (18-34) and wounds/swellings (40-58). A high rating of lameness aligns well with the findings of our study; a low rating of integument alterations contradicts the results of our study. We found that regression analysis revealed correlations with HCC levels merely for “percentage of moderately lame cows,” “percentage of cows with at least one hairless patch and no lesion,” and “tendency to be apathetic,” which is part of the Qualitative Behavior Assessment (QBA), a scientific approach for evaluating the emotional state of animals via behavioral observation. The fact that the regression model of our study included “tendency to be apathetic” as a part of the QBA contradicts Dutch studies conducted by van Eerdenburg et al. (2018, 2021a). Those authors modified the WQ protocol since they found the protocol to not be discriminating enough. Farmers considered the results of the QBA to give no important contribution to the welfare evaluation of their animals. Thus, QBA was omitted from the newly developed “Welfare Monitor” protocol. Results of that study revealed increased reliability of the modified protocol.
On the other hand, Andreasen et al. (2012) investigated whether performing QBA alone could be an alternative welfare assessment method for dairy cattle farms. Results of the study revealed that correlations between QBA and WQ measures were low. That finding supports farmers’ opinions that QBA makes only a superficial contribution to welfare assessments. This, in turn, is inconsistent with the regression model of our study that exhibited a correlation between “tendency to be apathetic” as part of the QBA and median HCCs. Ebinghaus et al. (2022) found no association between herd health indicators and QBA but found a positive relationship between QBA and enhanced housing conditions and more intensive animal care. Moreover, omitting QBA from welfare evaluations is viewed critically as more and more studies are calling for positive welfare indicators, rather than focusing on negative welfare indicators (Keeling et al., 2021; Papageorgiou and Simitzis, 2022). For instance, Mattiello et al. (2019) argued that the absence of negative welfare indicators such as lameness, disease, and lesions does not automatically indicate a high level of animal welfare. Consequently, positive welfare indicators assessing the emotional state of animals, such as the QBA, are being demanded. Two reviews evaluating possible positive welfare indicators came to the conclusion that the QBA appears to be one of the most promising indicators (Napolitano et al., 2009; Papageorgiou and Simitzis, 2022).
Overall, the hypothesis that it could be sufficient to only evaluate “absence of injuries,” including indicators of lameness and integument alterations, for animal welfare assessment cannot be confirmed by other studies that have developed new protocols incorporating fewer indicators. Although lameness and integument alterations are also included in the modified protocols, they are still supplemented by other indicators and therefore considered not applicable as stand-alone indicators for welfare assessment. While our regression analysis revealed a correlation between HCC levels and the three aforementioned welfare measures, this finding is inconsistent with other studies. Those investigations developed shorter and simplified welfare protocols that included more measures due to their strong links with overall welfare scores and principles. One would expect that measures of the simplified protocols would show correlations with HCC levels. Nonetheless, this assumption was not borne out.
This situation in turn leads to the assumption that HCC is not suitable as an alternative animal welfare assessment method. A study by van Eerdenburg et al. (2021b) comparing HCC and the results of nine different welfare assessment protocols assumed that HCC levels would negatively correlate with the outcome of the protocols and therefore possibly be a valid dairy cattle welfare measure. However, the findings of the study revealed weak negative correlations with HCC for only five parameters out of all nine welfare protocols, including the modified WQ protocol parameter “housing” and the “Welfare Monitor” parameters “health” and “milk yield.” These results can be interpreted in one of two ways. On the one hand, those results might indicate that HCC levels are not suitable for assessing dairy cattle welfare. On the other hand, those results revealed that welfare assessment protocols were only weakly correlated with one another, which in turn raises concerns as to whether the protocols are reliable for assessing dairy cattle welfare. Unlike the design of our study, the Dutch study took samples from only 10 cows per farm, and the cows were not standardized in terms of DIM. Moreover, half of the collected samples were analyzed, 1 year later than the first half; higher cortisol concentrations were recorded for the samples that were analyzed immediately. Furthermore, white hair samples were analyzed although other studies have demonstrated that white hair samples are more vulnerable to photodegradation (Otten et al., 2023). In addition, there were slight differences in the sample preparation and the analysis of cortisol in hair samples compared with our study. Vesel and Pavič (2019) compared WQ scores of eight Slovenian farms with HCC levels. Those authors failed to find negative correlations between welfare scores and HCC. In that study, HCC levels were measured from pooled samples that included anywhere from 17 to 33 randomly selected cows of each herd. Thus, their sampling protocol also reveals a lack of standardization. Consequently, future research with larger sample sizes and standardized protocols for sampling, processing, and analysis of hair cortisol samples was recommended (Vesel et al., 2020). In our study, we used standardized methods for sampling and analysis of HCC levels. However, interpreting correlations between measures of the WQ protocol is still challenging since the validity and reliability of the WQ Assessment remain controversial issues (Sandøe et al., 2019; de Vries et al., 2013; Knierim and Winckler, 2009).
5 Conclusion
This study is the first to investigate the effect of dairy farm automation on HCCs in cattle. It was hypothesized that higher levels of automation may negatively impact long-term animal stress. Results of the study revealed that the median HCC of farms declined with increased automation level. However, the trend did not attain statistical significance. High variability among farms regarding housing, management, and automation techniques with individual settings likely masked an underlying trend. Our study is limited by its small number of trial farms, since only a few farms in Northern and Central Germany are currently highly automated. Moreover, correlations between WQ measures and criteria and HCC levels of trial farms were examined. Multiple linear regression revealed associations between HCC levels and “percentage of moderately lame cows,” “percentage of cows with at least one hairless patch and no lesion,” “tendency to be apathetic,” and “absence of injuries.”
This underlines existing consensus about lameness and integument alterations as being some of the most severe welfare issues in dairy cattle. Nevertheless, these indicators are not recommended as stand-alone measures for animal welfare assessment. Overall, linking the WQ protocol to HCC levels remains difficult; previous studies also lacked consensus among different welfare protocols. While HCC levels are a promising objective welfare indicator, they cannot yet be validated due to the absence of reliable reference standards. This situation highlights a gap in research about objective validation tools for both animal welfare protocols and HCC levels.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Study design was submitted to Landesamt für Landwirtschaft, Lebensmittelsicherheit und Fischerei, Mecklenburg-Vorpommern, Germany, who confirmed that the study is not classified an animal experiment within the meaning of Section 7 (2) of the German Animal Protection law. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
LL-K: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Writing – original draft, Writing – review & editing. UM: Formal analysis, Writing – review & editing. SD: Conceptualization, Funding acquisition, Resources, Writing – review & editing. UG: Methodology, Resources, Writing – review & editing. SR: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study is part of the project “Gewinnung und Entwicklung von professoralem Personal– neue Qualifizierungswege an der Hochschule Neubrandenburg” (ProfQua NB; 03FHP121), which is part of the “Bund-Länder-Programm FH Personal”. The research was also funded by internal research funding from the University of Applied Sciences Neubrandenburg (“Hochschulinterne Forschungsförderung” and “Förderung des wissenschaftlichen Nachwuchses”) and the “Professorinnenprogramm III,” which is funded by the Federal Ministry of Education and Research. We acknowledge support for the article processing charge from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation, 491232355) and the Open Access Publication Fund of the Hochschule Neubrandenburg (Neubrandenburg University of Applied Sciences).
Acknowledgments
The authors would like to thank the managers and the staff of the trial farms for their great support during this investigation. The authors are also grateful to Dr. Winfried Otten for valuable advice and support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abeni F. and Bertoni G. (2009). Main causes of poor welfare in intensively reared dairy cows. Ital. J. Anim. Sci. 8, 45–66. doi: 10.4081/ijas.2009.s1.45
Akbar M. and Evans N. P. (2023). Elevated hair cortisol and decreased hair testosterone indicates chronic disruption of the HPA/HPG axis and is reflective of poor welfare in Rhesus Macaques used as performing (dancing) monkeys in Pakistan. Appl. Anim. Behav. Sci. 269, 106111. doi: 10.1016/j.applanim.2023.106111
Akoglu H. (2018). User’s guide to correlation coefficients. Turk. J. Emerg. Med. 18, 91–93. doi: 10.1016/j.tjem.2018.08.001
Almoosavi S. S., Ghoorchi T., Naserian A. A., Ramezanpor S., and Ghaffari M. (2020). Long-term impacts of late-gestation maternal heat stress on growth performance, blood hormones and metabolites of newborn calves independent of maternal reduced feed intake. Domest. Anim. Endocrinol. 72, 106433. doi: 10.1016/j.domaniend.2019.106433
Andreasen S., Sandøe P., and Forkman B. (2014). Can animal-based welfare assessment be simplified? A comparison of the Welfare Quality® protocol for dairy cattle and the simpler and less time-consuming protocol developed by the Danish Cattle Federation. Anim. Welf. 23, 81–94. doi: 10.7120/09627286.23.1.081
Andreasen S., Wemelsfelder F., Sandøe P., and Forkman B. (2012). The correlation of Qualitative Behavior Assessments with Welfare Quality® protocol outcomes in on-farm welfare assessment of dairy cattle. Appl. Anim. Behav. Sci. 143, 9–17. doi: 10.1016/j.applanim.2012.11.013
Ataallahi M., Nejad J. G., and Park K.-H. (2022). Selection of appropriate biomatrices for studies of chronic stress in animals: A review. J. Anim. Sci. Technol. 64, 621. doi: 10.5187/jast.2022.e38
Bianchi M., Bava L., Sandrucci A., Tangorra F., Tamburini A., Gislon G., et al. (2022). Diffusion of precision livestock farming technologies in dairy cattle farms. Animal 16, 100650. doi: 10.1016/j.animal.2022.100650
Braun U., Michel N., Baumgartner M. R., Hässig M., and Binz T. M. (2017). Cortisol concentration of regrown hair and hair from a previously unshorn area in dairy cows. Res. Vet. Sci. 114, 412–415. doi: 10.1016/j.rvsc.2017.07.005
Braun U., Wiest A., Lutz T., Riond B., Stirn M., Hilbe M., et al. (2019). Hair cortisol concentration in veal calves reared under two different welfare production labels. Res. Vet. Sci. 123, 286–292. doi: 10.1016/j.rvsc.2019.01.027
Breuer K., Hemsworth P. H., and Coleman G. J. (2003). The effect of positive or negative handling on the behavioural and physiological responses of nonlactating heifers. Appl. Anim. Behav. Sci. 84, 3–22. doi: 10.1016/S0168-1591(03)00146-1
Buck M., Friedli K., Steiner B., Gygax L., Wechsler B., and Steiner A. (2013). Influence of manure scrapers on dairy cows in cubicle housing systems. Livest. Sci. 158, 129–137. doi: 10.1016/j.livsci.2013.10.011
Burdett S. (2019). Exploring the relationships between behavioural responses to stress and performance, carcass composition, energy expenditure and macronutrient oxidation in restrict fed female Yorkshire pigs. University of Guelph, Guelph, Canada.
Burnard C., Ralph C., Hynd P., Edwards J. H., and Tilbrook A. (2016). Hair cortisol and its potential value as a physiological measure of stress response in human and non-human animals. Anim. Prod. Sci. 57, 401–414. doi: 10.1071/AN15622
Burnett T. A., Madureira A. M., Silper B. F., Nadalin A., Tahmasbi A., Veira D. M., et al. (2014). Factors affecting hair cortisol concentrations in lactating dairy cows. J. Dairy. Sci. 97, 7685–7690. doi: 10.3168/jds.2014-8444
Burnett T. A., Madureira A. M., Silper B. F., Tahmasbi A., Nadalin A., Veira D. M., et al. (2015). Relationship of concentrations of cortisol in hair with health, biomarkers in blood, and reproductive status in dairy cows. J. Dairy. Sci. 98, 4414–4426. doi: 10.3168/jds.2014-8871
Clark B., Stewart G. B., Panzone L. A., Kyriazakis I., and Frewer L. J. (2016). A systematic review of public attitudes, perceptions and behaviours towards production diseases associated with farm animal welfare. J. Agric. Environ. Ethics. 29, 455–478. doi: 10.1007/s10806-016-9615-x
Comin A., Peric T., Corazzin M., Veronesi M., Meloni T., Zufferli V., et al. (2013). Hair cortisol as a marker of hypothalamic-pituitary-adrenal axis activation in Friesian dairy cows clinically or physiologically compromised. Livest. Sci. 152, 36–41. doi: 10.1016/j.livsci.2012.11.021
Comin A., Peric T., Montillo M., Faustini M., Zufferli V., Cappa A., et al. (2012). Hair cortisol levels to monitor hypothalamic-pituitary-adrenal axis activity in healthy dairy cows. J. Anim. Vet. Adv. 11, 3623–3626. doi: 10.3923/javaa.2012.3623.3626
Dallmann M. F., Akana S. F., Cascio C. S., Darlington D. N., Jacobson L., and Levin N. (1987). “Regulation of ACTH secretion: variations on a theme of B,” in Proc. Laurentian Hormone Conf (Elsevier) Cambridge, Massachusetts, United States :Academic Press, 113–173. doi: 10.1016/B978-0-12-571143-2.50010-1
de Almeida A. M., Zachut M., Hernández-Castellano L. E., Šperanda M., Gabai G., and Mobasheri A. (2019). Biomarkers of fitness and welfare in dairy animals: healthy living. J. Dairy Res. 86, 379–387. doi: 10.1017/S0022029919000803
de Graaf S., Ampe B., Winckler C., Radeski M., Mounier L., Kirchner M., et al. (2017). Trained-user opinion about Welfare Quality measures and integrated scoring of dairy cattle welfare. J. Dairy. Sci. 100, 6376–6388. doi: 10.3168/jds.2016-12255
de Vries M., Bokkers E., Van Schaik G., Botreau R., Engel B., Dijkstra T., et al. (2013). Evaluating results of the Welfare Quality multi-criteria evaluation model for classification of dairy cattle welfare at the herd level. J. Dairy. Sci. 96, 6264–6273. doi: 10.3168/jds.2012-6129
DeVries T., Von Keyserlingk M., and Beauchemin K. (2005). Frequency of feed delivery affects the behavior of lactating dairy cows. J. Dairy. Sci. 88, 3553–3562. doi: 10.3168/jds.S0022-0302(05)73040-X
Dhama K., Latheef S. K., Dadar M., Samad H. A., Munjal A., Khandia R., et al. (2019). Biomarkers in stress related diseases/disorders: diagnostic, prognostic, and therapeutic values. Front. Mol. Biosci. 691. doi: 10.3389/fmolb.2019.00091
Doerfler R. L., Lehermeier C., Kliem H., Möstl E., and Bernhardt H. (2016). Physiological and behavioral responses of dairy cattle to the introduction of robot scrapers. Front. Vet. Sci. 3. doi: 10.3389/fvets.2016.00106
Doerfler R. L., Martin R., and Bernhardt H. (2017). Implications of robotic walkway cleaning for hoof disorders in dairy cattle. Int. J. Eng. Res. Appl. 7, 98–104. doi: 10.9790/9622-07010498104
Ebinghaus A., Knierim U., Simantke C., Palme R., and Ivemeyer S. (2020). Fecal cortisol metabolites in dairy cows: a cross-sectional exploration of associations with animal, stockperson, and farm characteristics. Animals 10, 1787. doi: 10.3390/ani10101787
Ebinghaus A., Matull K., Knierim U., and Ivemeyer S. (2022). Associations between dairy herds’ Qualitative behavior and aspects of herd health, stockperson and farm factors—A cross-sectional exploration. Animals 12, 182. doi: 10.3390/ani12020182
Endo N., Kuroki R., and Tanaka T. (2017). Comparison of productive and reproductive performance and hair cortisol levels between Brown Swiss cross-bred and Holstein cows housed in the same barn. Anim. Sci. J. 88, 1506–1512. doi: 10.1111/asj.12828
Fischer-Tenhagen C., Ladwig-Wiegard M., Heuwieser W., and Thöne-Reineke C. (2018). Is hair cortisol a potential indicator for stress caused by chronic lameness in dairy cows? J. Dairy. Sci. 101, 5439–5443. doi: 10.3168/jds.2017-13967
Fregonesi J., Veira D., Von Keyserlingk M., and Weary D. (2007). Effects of bedding quality on lying behavior of dairy cows. J. Dairy. Sci. 90, 5468–5472. doi: 10.3168/jds.2007-0494
Gaworski M. and Kic P. (2024). Assessment of production technologies on dairy farms in terms of animal welfare. Appl. Sci. 14, 6086. doi: 10.3390/app14146086
Ghassemi Nejad J., Ghaffari M. H., Ataallahi M., Jo J.-H., and Lee H.-G. (2022). Stress concepts and applications in various matrices with a focus on hair cortisol and analytical methods. Animals 12, 3096. doi: 10.3390/ani12223096
Grelet C., Dries V. V., Leblois J., Wavreille J., Mirabito L., Soyeurt H., et al. (2022). Identification of chronic stress biomarkers in dairy cows. Animal 16, 100502. doi: 10.1016/j.animal.2022.100502
Grothmann A., Moser L., Nydegger F., Steiner A., and Zähner M. (2014). Influence of different feeding frequencies on the rumination and lying behaviour of dairy cows. Proc. Int. Conf. Agric. Eng., 6–10.
Grummer R. R. (1995). Impact of changes in organic nutrient metabolism on feeding the transition dairy cow. J. Anim. Sci. 73, 2820–2833. doi: 10.2527/1995.7392820x
Gygax L., Neuffer I., Kaufmann C., Hauser R., and Wechsler B. (2006). Milk cortisol concentration in automatic milking systems compared with auto-tandem milking parlors. J. Dairy. Sci. 89, 3447–3454. doi: 10.3168/jds.S0022-0302(06)72382-7
Gygax L., Neuffer I., Kaufmann C., Hauser R., and Wechsler B. (2008). Restlessness behaviour, heart rate and heart-rate variability of dairy cows milked in two types of automatic milking systems and auto-tandem milking parlours. Appl. Anim. Behav. Sci. 109, 167–179. doi: 10.1016/j.applanim.2007.03.010
Hayashi H., Arai C., Ikeuchi Y., Yamanaka M., and Hirayama T. (2021). Effect of growth and parturition on hair cortisol in Holstein cattle. Anim. Sci. J., 92, e13518. doi: 10.1111/asj.13518
Heath C., Browne W., Mullan S., and Main D. (2014). Navigating the iceberg: reducing the number of parameters within the Welfare Quality® assessment protocol for dairy cows. Animal 8, 1978–1986. doi: 10.1017/S1751731114002018
Heimbürge S. (2021). Hair cortisol concentration in cattle and pigs: Investigation of influencing factors and the potential as an indicator of long-term stress. Leipzig University, Leipzig, Germany.
Heimbürge S., Kanitz E., and Otten W. (2019). The use of hair cortisol for the assessment of stress in animals. Gen. Comp. Endocrinol. 270, 10–17. doi: 10.1016/j.ygcen.2018.09.016
Heimbürge S., Kanitz E., Tuchscherer A., and Otten W. (2020a). Is it getting in the hair?–Cortisol concentrations in native, regrown and segmented hairs of cattle and pigs after repeated ACTH administrations. Gen. Comp. Endocrinol. 295, 113534. doi: 10.1016/j.ygcen.2020.113534
Heimbürge S., Kanitz E., Tuchscherer A., and Otten W. (2020b). Within a hair’s breadth–Factors influencing hair cortisol levels in pigs and cattle. Gen. Comp. Endocrinol. 288, 113359. doi: 10.1016/j.ygcen.2019.113359
Heinze G., Wallisch C., and Dunkler D. (2018). Variable selection–a review and recommendations for the practicing statistician. Biom. J. 60, 431–449. doi: 10.1002/bimj.201700067
Hemsworth P., Barnett J., Tilbrook A., and Hansen C. (1989). The effects of handling by humans at calving and during milking on the behaviour and milk cortisol concentrations of primiparous dairy cows. Appl. Anim. Behav. Sci. 22, 313–326. doi: 10.1016/0168-1591(89)90026-9
Higashiyama Y., Nashiki M., Narita H., and Kawasaki M. (2007). A brief report on effects of transfer from outdoor grazing to indoor tethering and back on urinary cortisol and behaviour in dairy cattle. Appl. Anim. Behav. Sci. 102, 119–123. doi: 10.1016/j.applanim.2006.03.007
Hirsch M. S. and Watkins J. (2020). A comprehensive review of biomarker use in the gynecologic tract including differential diagnoses and diagnostic pitfalls. Adv. Anat. Pathol. 27, 164–192. doi: 10.1097/PAP.0000000000000238
Hopster H., Bruckmaier R., van der Werf J., Korte S., Macuhova J., Korte-Bouws G., et al. (2002). Stress responses during milking; comparing conventional and automatic milking in primiparous dairy cows. J. Dairy. Sci. 85, 3206–3216. doi: 10.3168/jds.S0022-0302(02)74409-3
Hucklebridge F. S., Hussain T., Evans P., and Clow A. (2005). The diurnal patterns of the adrenal steroids cortisol and dehydroepiandrosterone (DHEA) in relation to awakening. Psychoneuroendocrinology 30, 51–57. doi: 10.1016/j.psyneuen.2004.04.007
Jacobs J. and Siegford J. (2012). Invited review: The impact of automatic milking systems on dairy cow management, behavior, health, and welfare. J. Dairy. Sci. 95, 2227–2247. doi: 10.3168/jds.2011-4943
Jacobson L. (2005). Hypothalamic–pituitary–adrenocortical axis regulation. Endocrinol. Metab. Clin. 34, 271–292. doi: 10.1016/j.ecl.2005.01.003
Jurkovich V., Hejel P., and Kovács L. (2024). A review of the effects of stress on dairy cattle behaviour. Animals 14, 2038. doi: 10.3390/ani14142038
Keeling L. J., Winckler C., Hintze S., and Forkman B. (2021). Towards a positive welfare protocol for cattle: a critical review of indicators and suggestion of how we might proceed. Front. Anim. Sci. 2. doi: 10.3389/fanim.2021.753080
Kelly S., Hertzman C., and Daniels M. (1997). Searching for the biological pathways between stress and health. Annu. Rev. Public Health 18, 437–462. doi: 10.1146/annurev.publhealth.18.1.437
King M., Pajor E., Leblanc S., and Devries T. (2016). Associations of herd-level housing, management, and lameness prevalence with productivity and cow behavior in herds with automated milking systems. J. Dairy. Sci. 99, 9069–9079. doi: 10.3168/jds.2016-11329
Kleen J. L. and Guatteo R. (2023). Precision livestock farming: What does it contain and what are the perspectives? Animals 13, 779. doi: 10.3390/ani13050779
Knierim U. and Winckler C. (2009). On-farm welfare assessment in cattle: validity, reliability and feasibility issues and future perspectives with special regard to the Welfare Quality® approach. Anim. Welf. 18, 451–458. doi: 10.1017/S0962728600000865
Knierim U., Winckler C., Mounier L., and Veissier I. (2021). “Developing effective welfare measures for cattle,” in Understanding the behaviour and improving the welfare of dairy cattle (Burleigh Dodds Science Publishing, Cambridge, UK).
Koenneker K., Schulze M., Pieper L., Jung M., Schmicke M., and Beyer F. (2023). Comparative assessment of the stress response of cattle to common dairy management practices. Animals 13, 2115. doi: 10.3390/ani13132115
Krueger A., Cruickshank J., Trevisi E., and Bionaz M. (2020). Systems for evaluation of welfare on dairy farms. J. Dairy Res. 87, 13–19. doi: 10.1017/S0022029920000461
Lavrijsen-Kromwijk L., Demba S., Müller U., and Rose S. (2024). Impact of automation level of dairy farms in Northern and central Germany on dairy cattle welfare. Animals 14, 3699. doi: 10.3390/ani14243699
Leinweber T., Zähner M., and Schrade S. (2019). Evaluation of a dung-removal robot for use in dairy housing from an ethological and process-engineering point of view. Landtechnik 74, 55–68. doi: 10.15150/lt.2019.3204
Leliveld L. M. C. and Provolo G. (2020). A review of welfare indicators of indoor-housed dairy cow as a basis for integrated automatic welfare assessment systems. Animals 10, 1430. doi: 10.3390/ani10081430
Lürzel S., Windschnurer I., Futschik A., Palme R., and Waiblinger S. (2015). Effects of gentle interactions on the relationship with humans and on stress-related parameters in group-housed calves. Anim. Welf. 24, 475–484. doi: 10.7120/09627286.24.4.475
Mârza S. M., Munteanu C., Papuc I., Radu L., Diana P., and Purdoiu R. C. (2024). Behavioral, physiological, and pathological approaches of cortisol in dogs. Animals 14, 3536. doi: 10.3390/ani14233536
Mattachini G., Pompe J., Finzi A., Tullo E., Riva E., and Provolo G. (2019). Effects of feeding frequency on the lying behavior of dairy cows in a loose housing with automatic feeding and milking system. Animals 9, 121. doi: 10.3390/ani9040121
Mattiello S., Battini M., De Rosa G., Napolitano F., and Dwyer C. (2019). How can we assess positive welfare in ruminants? Animals 9, 758. doi: 10.3390/ani9100758
Meyer J. S. and Novak M. (2017). “HPA axis,” in The international encyclopedia of primatology (Wiley Blackwell, Chichester, UK). doi: 10.1002/9781119179313
Mezzetti M., Cattaneo L., Passamonti M. M., Lopreiato V., Minuti A., and Trevisi E. (2021). The transition period updated: A review of the new insights into the adaptation of dairy cows to the new lactation. Dairy 2, 617–636. doi: 10.3390/dairy2040048
Moberg G. P. (1985). “Biological response to stress: key to assessment of animal well-being?,” in Animal Stress (Springer, New York, US). doi: 10.1007/978-1-4614-7544-6_3
Napolitano F., Knierim U., Grass F., and De Rosa G. (2009). Positive indicators of cattle welfare and their applicability to on-farm protocols. Ital. J. Anim. Sci. 8, 355–365. doi: 10.4081/ijas.2009.s1.355
Nejad J. G., Lee B.-H., Kim J.-Y., Chemere B., Sung K.-I., and Lee H.-G. (2021). Effect of alpine grazing on plasma and hair cortisol, serotonin, and DHEA in dairy cows and its welfare impact. Domest. Anim. Endocrinol. 75, 106581. doi: 10.1016/j.domaniend.2020.106581
Ninomiya S., Nishi A., Nakamura R., and Shibata M. (2024). Positive correlation of social rank and hair cortisol concentration in group-housed pregnant cows. Animals 15, 13. doi: 10.3390/ani15010013
Oberschätzl-Kopp R., Haidn B., Peis R., Reiter K., and Bernhardt H. (2016). Studies on dairy cow behaviour with automatic feeding in a herd milked by an AMS. Landtechnik 7, 55–65. doi: 10.15150/lt.2016.3122
Otten W., Heimbürge S., Tuchscherer A., and Kanitz E. (2022). The age of hair matters–the incorporation of cortisol by external contamination is enhanced in distal hair segments of pigs and cattle. Animal 16, 100495. doi: 10.1016/j.animal.2022.100495
Otten W., Heimbürge S., Tuchscherer A., and Kanitz E. (2023). Hair cortisol concentration in postpartum dairy cows and its association with parameters of milk production. Domest. Anim. Endocrinol. 84, 106792. doi: 10.1016/j.domaniend.2023.106792
Palme R., Robia C., Baumgartner W., and Möstl E. (2000). Transport stress in caftle as reflected by an increase in faecal cortisol metabolite concentrations. Vet. Rec. 146, 108. doi: 10.1136/vr.146.4.108
Papageorgiou M. and Simitzis P. E. (2022). Positive welfare indicators in dairy animals. Dairy 3, 814–841. doi: 10.3390/dairy3040056
Pesenhofer G., Palme R., Pesenhofer R., and Kofler J. (2006). Comparison of two methods of fixation during functional claw trimming-walk-in crush versus tilt table-in dairy cows using faecal cortisol metabolite concentrations and daily milk yield as parameters. Vet. Med. Austria 93, 288–294.
Pragst F. and Balikova M. A. (2006). State of the art in hair analysis for detection of drug and alcohol abuse. Clin. Chim. Acta 370, 17–49. doi: 10.1016/j.cca.2006.02.019
Romero L. M. (2004). Physiological stress in ecology: lessons from biomedical research. Trends Ecol. Evol. 19, 249–255. doi: 10.1016/j.tree.2004.03.008
Roth L. S., Faresjö Å., Theodorsson E., and Jensen P. (2016). Hair cortisol varies with season and lifestyle and relates to human interactions in German shepherd dogs. Sci. Rep. 6, 19631. doi: 10.1038/srep19631
Sadiq M. B., Ramanoon S. Z., Mansor R., Syed-Hussain S. S., and Shaik Mossadeq W. M. (2020). Claw trimming as a lameness management practice and the association with welfare and production in dairy cows. Animals 10, 1515. doi: 10.3390/ani10091515
Sadiq M. B., Ramanoon S. Z., Shaik Mossadeq W. M., Mansor R., and Syed-Hussain S. S. (2017). Association between lameness and indicators of dairy cow welfare based on locomotion scoring, body and hock condition, leg hygiene and lying behavior. animals 7, 79. doi: 10.3390/ani7110079
Sandøe P., Corr S. A., Lund T. B., and Forkman B. (2019). Aggregating animal welfare indicators: can it be done in a transparent and ethically robust way? Anim. Welf. 28, 67–76. doi: 10.7120/09627286.28.1.067
Schillings J., Bennett R., and Rose D. C. (2021). Exploring the potential of precision livestock farming technologies to help address farm animal welfare. Front. Anim. Sci. 2. doi: 10.3389/fanim.2021.639678
Schwertl M., Auerswald K., and Schnyder H. (2003). Reconstruction of the isotopic history of animal diets by hair segmental analysis. Rapid. Commun. Mass. Spectrom. 17, 1312–1318. doi: 10.1002/rcm.1042
Sharma A., Umapathy G., Kumar V., and Phillips C. J. (2019). Hair cortisol in sheltered cows and its association with other welfare indicators. Animals 9, 248. doi: 10.3390/ani9050248
Stülpner A., Adeili S., Haidn B., Dörfler R., and Bernhardt H. (2014). Reactions of dairy cows during the operation of a robotic slat cleaner. Landtechnik 69, 5.
Trevisi E. and Bertoni G. (2009). Some physiological and biochemical methods for acute and chronic stress evaluationin dairy cows. Ital. J. Anim. Sci. 8, 265–286. doi: 10.4081/ijas.2009.s1.265
Tuyttens F. A., De Graaf S., Andreasen S. N., De Boyer Des Roches A., Van Eerdenburg F. J., Haskell M. J., et al. (2021). Using expert elicitation to abridge the Welfare Quality® protocol for monitoring the most adverse dairy cattle welfare impairments. Front. Vet. Sci. 8. doi: 10.3389/fvets.2021.634470
Tuyttens F. A., Molento C. F., and Benaissa S. (2022). Twelve threats of precision livestock farming (PLF) for animal welfare. Front. Vet. Sci. 9. doi: 10.3389/fvets.2022.889623
Van Eerdenburg F. J., Di Giacinto A. M., Hulsen J., Snel B., and Stegeman J. A. (2021a). A new, practical animal welfare assessment for dairy farmers. Animals 11, 881. doi: 10.3390/ani11030881
van Eerdenburg F. J., Hof T., Doeve B., Ravesloot L., Zeinstra E. C., Nordquist R. E., et al. (2021b). The relation between hair-cortisol concentration and various welfare assessments of Dutch dairy farms. Animals 11, 821. doi: 10.3390/ani11030821
van Eerdenburg F. J., Hulsen J., Snel B., Van Den Broek J., and Stegeman J. (2018). “Proposal for three modifications for the Welfare Quality© protocol for dairy cattle,” in Bienestar animal en la práctica, en producciones lecheras, desde la perspectiva europea. Ed. Van Eerdenburg F. J. C. M. Utrecht, The Netherlands: Utrecht University, 46–54.
Veissier I., Kling-Eveillard F., Mialon M. M., Silberberg M., De Boyer Des Roches A., Terlouw C., et al. (2019). Precision livestock farming and animal welfare: is the numerical revolution in agriculture able to take into account animals’ and farmers’ needs? Inrae Productions Animales 32, 281–290. doi: 10.20870/productions-animales.2019.32.2.2478
Vesel U. and Pavič T. (2019). Welfare assessment of dairy cows in large Slovenian farms using Welfare Quality® assessment protocol and hair cortisol measurement. Clinic for reproduction and large animals. University of Ljubljana, Ljubljana, Slovenia.
Vesel U., Pavič T., Ježek J., Snoj T., and Starič J. (2020). Welfare assessment in dairy cows using hair cortisol as a part of monitoring protocols. J. Dairy Res. 87, 72–78. doi: 10.1017/S0022029920000588
Weary D. M. and von Keyserlingk M. A. (2023). Using animal welfare to frame discussion on dairy farm technology. Animal 17, 100836. doi: 10.1016/j.animal.2023.100836
Weigele H., Gygax L., Steiner A., Wechsler B., and Burla J.-B. (2018). Moderate lameness leads to marked behavioral changes in dairy cows. J. Dairy. Sci. 101, 2370–2382.. doi: 10.3168/jds.2017-13120
Welfare Quality® (2009). Assessment Protocol for Cattle (Lelystad, Netherlands: Welfare Quality® Consortium).
Wennig R. (2000). Potential problems with the interpretation of hair analysis results. Forensic Sci. In. 107, 5–12. doi: 10.1016/S0379-0738(99)00146-2
Wenzel C., Schönreiter-Fischer S., and Unshelm J. (2003). Studies on step–kick behavior and stress of cows during milking in an automatic milking system. Livest. Prod. Sci. 83, 237–246. doi: 10.1016/S0301-6226(03)00109-X
Wildridge A., Thomson P., Garcia S., Jongman E., and Kerrisk K. (2020). Transitioning from conventional to automatic milking: Effects on the human-animal relationship. J. Dairy. Sci. 103, 1608–1619. doi: 10.3168/jds.2019-16658
Keywords: precision dairy farming, animal welfare, stress reaction, biomarker, lameness, injuries, qualitative behavior assessment
Citation: Lavrijsen-Kromwijk L, Müller U, Demba S, Gimsa U and Rose S (2025) Hair cortisol concentration of dairy cattle is unrelated to automation level of dairy farms—however, it can reflect Welfare Quality® measures. Front. Anim. Sci. 6:1688775. doi: 10.3389/fanim.2025.1688775
Received: 19 August 2025; Accepted: 24 September 2025;
Published: 22 October 2025.
Edited by:
Jeremy N. Marchant, Organic Plus Trust Inc., United StatesReviewed by:
Arvind Sharma, The University of Queensland, AustraliaMuchamad Luthfi, National Research and Innovation Agency (BRIN), Indonesia
Copyright © 2025 Lavrijsen-Kromwijk, Müller, Demba, Gimsa and Rose. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lianne Lavrijsen-Kromwijk, bGF2cmlqc2VuQGhzLW5iLmRl
 Lianne Lavrijsen-Kromwijk
Lianne Lavrijsen-Kromwijk Ute Müller
Ute Müller Susanne Demba3
Susanne Demba3 Ulrike Gimsa
Ulrike Gimsa