- 1Department of Research and Clinical Trial, Kilimanjaro Clinical Research Institute, Moshi, Tanzania
- 2Boyd Orr Centre for Population and Ecosystem Health, School of Biodiversity, One Health and Veterinary Medicine, College of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow, United Kingdom
- 3Paediatric Department, Kilimanjaro Christian Medical Centre, Moshi, Tanzania
- 4Paediatric and Child Health Department, KCMC University, Moshi, Tanzania
- 5Sydney School of Veterinary Science, Faculty of Science, University of Sydney, Camden, NSW, Australia
Introduction: Infections are a major driver of broad-spectrum antibiotic use. This wide use of antibiotics contributes to the emergence of antimicrobial resistance globally that poses a threat to human and animal health. Infections continue to be a major cause of death among pregnant women and neonates. Therefore, this study aimed to assess the burden of extended-spectrum beta-lactamase (ESBL)-producing E. coli and K. pneumoniae carriage among neonates and their surroundings admitted to a referral hospital in Northeast Tanzania.
Methodology: The burden of ESBL-producing E. coli and K. pneumoniae in a neonatal ward was assessed by screening neonates’ rectums, maternal and healthcare workers’ hands, and neonatal cots. Isolates were cultured, identified, and tested for antimicrobial resistance, while generalized linear models identified risk factors for carriage.
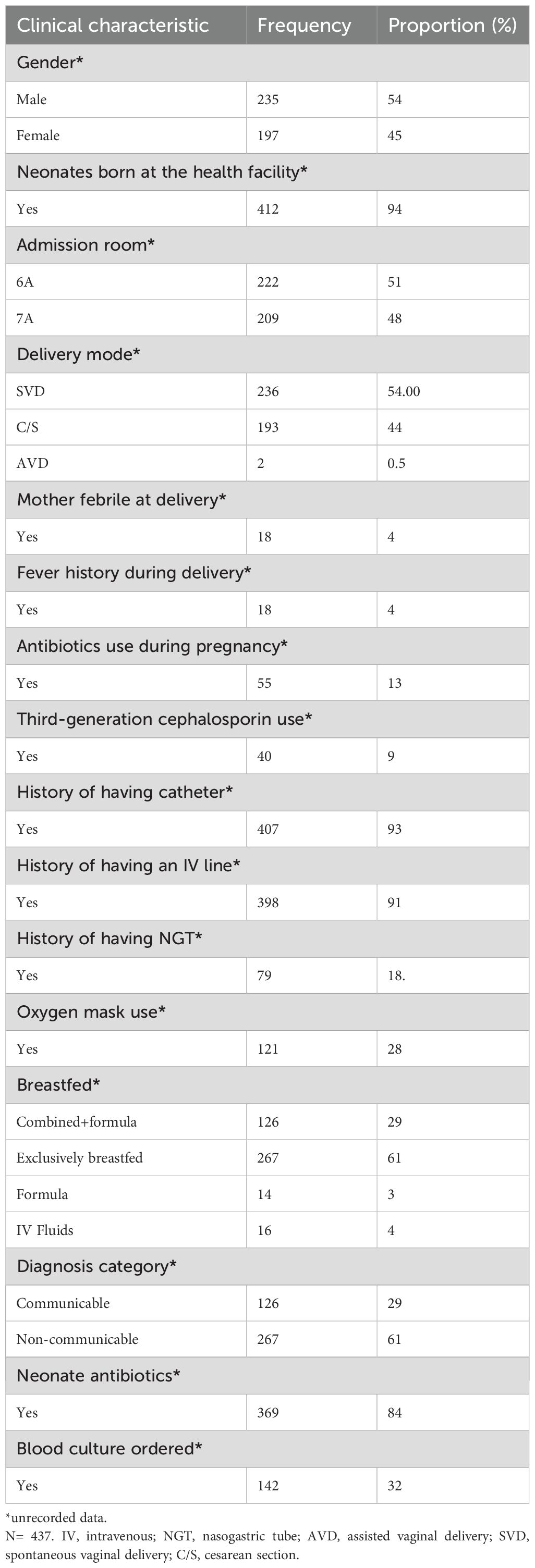
Results: A total of 437 neonates were screened for ESBL-producing E. coli and K. pneumoniae, with 235 (54%) being male. In addition, 77 maternal hand swabs, 118 neonatal cots, and 45 healthcare workers’ hand swabs were collected. ESBL-producing K. pneumoniae was isolated from 198 neonates (45%), and E. coli from 96 (23%). Additionally, 5% of maternal hands and 22% of neonatal cots were contaminated with these resistant bacteria. Overall ampicillin resistance was frequent in ESBL-producing E. coli and ESBL K. pneumoniae neonatal colonization (n=261,100%), as was resistance to trimethoprim-sulfamethoxazole (n = 233,89%), gentamicin (n = 169, 66%), and tetracycline (n = 140,54%). Only three (1%) of the ESBL-producing E. coli and ESBL K. pneumoniae isolates were resistant to meropenem. Risk factors significantly associated with carriage of either ESBL-producing E. coli or K. pneumoniae were being born in an admission room [odds ratio (OR)=1.95, confidence interval (CI)=1.31-3.13, p=0.006] and delivery mode, with vaginal delivery associated with a reduced risk of carriage (OR=0.57, CI=0.35–0.92, p=0.023).
Conclusion: The study reveals a high burden of ESBL-producing K. pneumoniae and E. coli in neonates and their environment, with frequent resistance to ampicillin and gentamicin. Hospital admission and cesarean delivery increase the risk of carriage, while vaginal delivery lowers it. Active screening upon admission and advanced diagnostic methods can help reduce transmission and guide effective antimicrobial treatment.
Introduction
Antimicrobial resistance (AMR) occurs when bacteria, viruses, fungi, and parasites no longer respond to treatment, making infections harder to treat and also increasing the risk of transmission, severe illness, and death (World Health Organization, 2015). The global prevalence of extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae (EPE) is increasing (Raphael et al., 2021). In East Africa, EPE prevalence ranges from 6% to 17% and 38% to 83% in community and hospital settings, respectively. In Tanzania, different studies have reported the prevalence of fecal carriage of EPE to be 50% among children admitted to a tertiary hospital and 12% among healthy community children (Tellevik et al., 2016). ESBL-producing Escherichia coli and Klebsiella pneumoniae are major multidrug-resistant bacteria that are known to cause serious hospital and community-acquired infections worldwide (Teklu et al., 2019; Husna et al., 2023). Infections such as sepsis, urinary tract infections, and diarrhea are major drivers of broad-spectrum antibiotic use. These contribute to the emerging global threat of antimicrobial resistance and decrease the effectiveness of first-line antimicrobial therapies.
Global efforts to tackle the threat of AMR have been discussed at the highest level, including the World Health Assemblies in 2014, 2015, and 2019, and the United Nations General Assembly in 2016 (World Health Organization, 2015). In 2015, the General Assembly of the World Health Organization (WHO) adopted a global action plan on AMR in recognition of the immediate threat AMR poses to human and animal health, productivity, and prosperity. The WHO urged its Member States to develop National Action Plans (NAPs) for AMR. In 2017, Tanzania adopted its NAP for 2017–2022, recognizing the need for action (Ministry of Health, n.d.). However, despite the existence of regulation and training programs, adherence to the guidelines does not always take place accordingly, and therefore antimicrobial resistance is accelerating. In Tanzania, antimicrobial resistance is a major problem and there are high levels of inappropriate use of antimicrobials in the human and animal sectors (Sangeda et al., 2021). There is, however, no process for systematically collecting data on the prevalence of antibiotic resistance in common pathogens. One of the objectives of NAPs is to strengthen the knowledge and evidence through surveillance and research, which are important for infection prevention and control measures, reducing the risk of transmission and acquisition of bacteria (Ministry of Health, n.d.). Therefore, this study aimed to address gaps identified in Tanzania’s National Action Plan to Combat Antimicrobial Resistance, including the lack of national surveillance for pathogens such as E. coli and K. pneumoniae and the absence of a systematic data collection approach for effective infection control, by assessing the burden, risk factors, and antimicrobial susceptibility patterns associated with the carriage of ESBL-producing E. coli and K. pneumoniae among neonates, and in their surroundings, at a referral hospital in north-eastern Tanzania.
Methods
Study design and area
This was a cross-sectional study conducted at the neonatal ward at a referral hospital, Kilimanjaro Christian Medical Center, in the Kilimanjaro region of Tanzania. The neonatal ward has a total of 45 neonatal cots, 3 incubators, and 18 beds. The majority of the neonates are born at the facility while others are referred from other facilities or born at home. The majority of caretakers/mothers practice subsistence farming, livestock keeping, and small businesses.
Sample size
The sample size for active surveillance (measuring within-hospital transmission of E. coli in neonatal wards) was calculated based on the expected prevalence of ESBL-producing coliforms (25% of rectal swabs), a colonization period of approximately 1 week, and a Poisson distribution for the size of patient clusters colonized by ESBL-producing coliforms. To gather sufficient data on the actual cluster size, which reflects the risk of colonization with ESBL-producing coliforms in the neonatal ward, we aimed to collect samples from 330 neonates.
Sample collection
Samples were collected between 2019 and 2021 across different seasons from the neonates at the neonatal ward after admission and consent was obtained. The exact start date was August to November 2019 for the first round, and the second round was from August to November 2021.
A sterile swab was gently inserted into the neonate’s anal sphincter. The sample was then stored in a sterile container with Amies transport medium and labeled with the patient’s identification number and date of collection. The specimen was then transported to the laboratory and processed on the same day. Swabs of maternal hands, healthcare workers’ hands, and neonatal cots were also collected and transported to the laboratory for microbiological processing.
Laboratory procedures
Neonatal rectal, hand, and bed swabs were inoculated onto MacConkey agar supplemented with cefotaxime 2 μg/ml for screening of ESBL-producing organisms. E. coli and K. pneumoniae were phenotypically identified based on lactose fermentation on MacConkey agar, urease negativity, Simmons citrate negativity, sulfur indole motility test results (i.e., no production of hydrogen sulfide gas, indole positivity, and motility), and the fermentation on triple sugar iron test. AST was done on Mueller Hinton agar using the Clinical Laboratory Standard Institute (CLSI) guideline.
ESBL and antibiotic susceptibility testing
Cefotaxime-resistant E. coli and K. pneumoniae isolates were screened for ESBL production with the combined diffusion method using ceftazidime (30 µg), cefotaxime (30 µg), and amoxicillin/clavulanate (30 µg discs) (Oxoid Ltd) as the inhibitory substances. The test was considered positive for ESBL when there was a synergy between any two antibiotics with amoxicillin/clavulanate. Susceptibility to 11 antibiotics was tested. Specifically, the disks used were ampicillin (10 μg), trimethoprim-sulfamethoxazole (1.25/23.75 μg), amoxicillin-clavulanic acid (20/10 μg), ciprofloxacin (5 μg), gentamicin (10 μg), piperacillin-tazobactam (100/10 μg), ceftriaxone (30 μg), ceftazidime (30 μg), and meropenem (10 μg). Confirmation of ESBL carriage was done using the combined disk diffusion method. The results of the susceptibility test were interpreted as sensitive, intermediate, and resistant based on the CLSI guideline (Weinstein and Clinical and Laboratory Standards Institute (CLSI), 2018). The control strains used were E. coli ATCC 25922, and K. pneumoniae ATCC 8868.
Statistics
Data from the questionnaires were entered using RedCap (https://project-redcap.org/), downloaded to MS Excel, and analyzed using the analysis tool pack software. Descriptive statistics were used to summarize the data, with frequencies and percentages reported for categorical variables, and measures of central tendency and their dispersion for numerical data. The Chi-square test was used to conduct an initial univariable analysis of the association between potential risk factors and ESBL-producing E. coli and K. pneumoniae carriage.
We also conducted a multivariable analysis using generalized linear models (GLMs) in R (R Core Team, 2023) with a binomial family. The outcome was a binary variable indicating the presence of either ESBL-producing E. coli or K. pneumoniae. Our initial model contained all samples for which there was complete data on the following variables: time period (i.e., pre- or post-June 2020), infant gender, whether the infant was born in a healthcare facility, admission room, delivery mode, maternal fever at delivery, maternal fever during pregnancy, maternal use of antibiotics, maternal use of third generation cephalosporins, infant nasogastric tube, infant intravenous line, infant oxygen mask, feeding method, and whether a blood culture was taken. As fewer than 4% of the infants included in the analysis had a catheter, this variable was excluded. The diagnostic category (communicable or non-communicable illness) was excluded due to collinearity with the feeding mode. Backward model selection using the Akaike information criterion (AIC) was used to identify the best-fitting model. The final model residuals were examined using the Dharma package (Hartig, 2022). A p-value < 0.05 indicated statistical significance.
Inclusion and exclusion criteria
We included healthcare professionals in the neonatal wards, neonates, and the caretakers of the babies who were enrolled in the study. In consultation with the neonatal ward management, all neonates present on the neonatal ward and meeting the inclusion criteria were included if parental consent was obtained. Students rotating in the neonatal care unit, visiting workers, short-term volunteers, neonates who were severely ill, preterm babies, and participants who withdrew informed consent were excluded from the study.
Ethical clearance
Ethical permission for the study was granted by the Kilimanjaro Christian Medical University College (KCMUCo) research ethical review committee in Moshi and the Medical Research Coordinating Committee of the National Institute for Medical Research in Tanzania (NIMR-MRCC).
Results
Clinical and demographic characteristics of the study participant
A total of 437 neonates were screened for ESBL-producing E. coli and K. pneumoniae in their rectums. Of them, 235/437 (54%) were male. Most study participants were born in health facilities (94%, n=412) and 6% (n=25) were born at home. Of the 437 neonates with recorded data, 54.00% (236/437) were born through spontaneous vaginal delivery (SVD), 44% (193/437) were born through cesarean section (C/S), and 0.5% (2/437) were born through assisted vaginal delivery (AVD). The median gestational age and birth weight of the study participants was 38 weeks and 2.87 kg, respectively. Overall, 84% (n=369) of the neonates were on antibiotics. Some (4%, n=18) mothers were febrile during delivery with 4% (n=18) having a history of fever during delivery. Some (13%, n=55) of the mothers received antibiotics during their pregnancy, with 9% (n=40) taking third-generation cephalosporins (Table 1).
Overall prevalence of ESBL-producing E. coli and K. pneumoniae species from neonatal feces, maternal hands, and neonatal cots
A total of 201 (46%) K. pneumoniae and 96 (22%) E. coli isolates were from the neonates. Out of the 297 K. pneumoniae and E. coli isolates, 294 were ESBL positive, of which, 198 (67%) were ESBL-producing K. pneumoniae and 96 (32%) were ESBL-producing E. coli. A total of 50 (11%) of the neonates had both ESBL-producing K. pneumoniae and E. coli species isolated in one sample. One (1%) K. pneumoniae isolate and one (1%) E. coli isolate were isolated from maternal hands. Furthermore, 23 (21%) K. pneumoniae and 4 (4%) E. coli isolates were from neonatal cots, while healthcare workers were not contaminated with E. coli or K. pneumoniae species. K. pneumoniae was the most isolated organism, followed by E. coli.
Potential risk factors associated with fecal carriage of ESBL-producing E. coli and K. pneumoniae in neonates
Descriptive statistics
High rates of ESBL-producing E. coli and K. pneumoniae carriage were found in the neonates born at the health facility (n=282, 65%) compared to those born at home (n=25, 6%). There was a slight difference in the number of those with ESBL fecal carriage between males (n=164, 38%) and females (n=131, 30%) and in ESBL fecal carriage among neonates with a history of having an intravenous (IV) line (n= 274, 63%) compared to those without an IV line. Carriage was also greater among neonates who were exclusively breastfed compared to those who also received formula (n= 174, 40%). There was a slight difference in the number of ESBL carriages among neonates with communicable (n= 100, 23%) and non-communicable diseases (n= 174, 40%). ESBL fecal carriage was high among neonates who received antibiotics during their hospital stay (n=256, 59%) (Table 2).
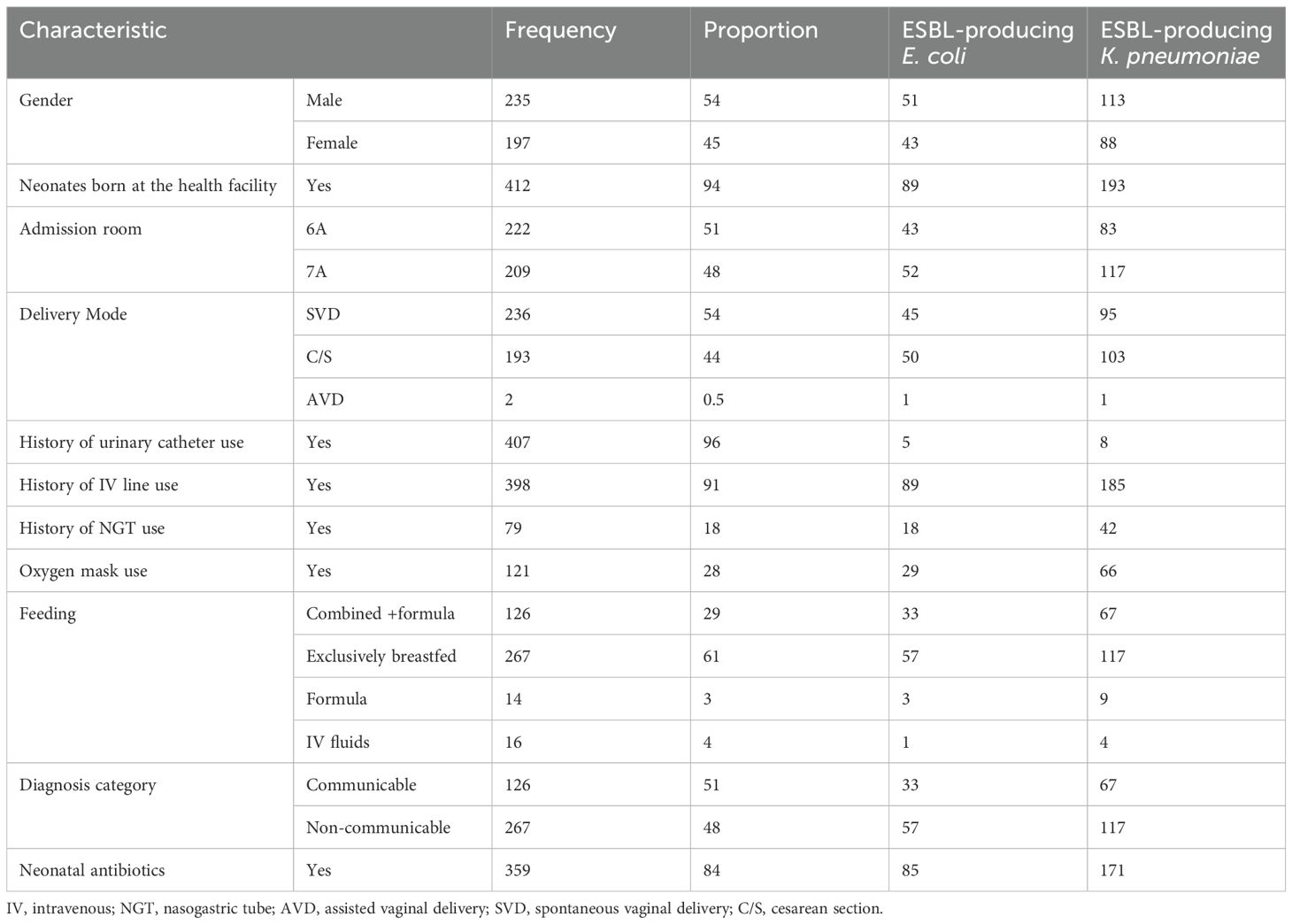
Table 2. Potential risk factors associated with neonatal fecal ESBL-producing E. coli and K. pneumoniae carriage.
Risk factor analysis using a generalized linear model
After selecting entries with complete information (i.e., excluding entries with missing entries or “unknown”) the analysis was performed on 300 infants. The best-fitting model for the presence of ESBL-producing E. coli or K. pneumoniae (Table 3) showed significant associations between the presence of the organisms and the admission room and delivery mode. Associated with a significant increase in the risk of ESBL-producing E. coli or K. pneumoniae were samples from infants admitted to rooms 7 or 7A [odds ratio (OR): 1.95, confidence interval (CI): 1.21 – 3.13, p = 0.006]. Samples from infants born by vaginal delivery had a reduced risk of ESBL-producing E. coli or K. pneumoniae carriage (odds ratio: 0.57, CI: 0.35 – 0.92, p=0.023).
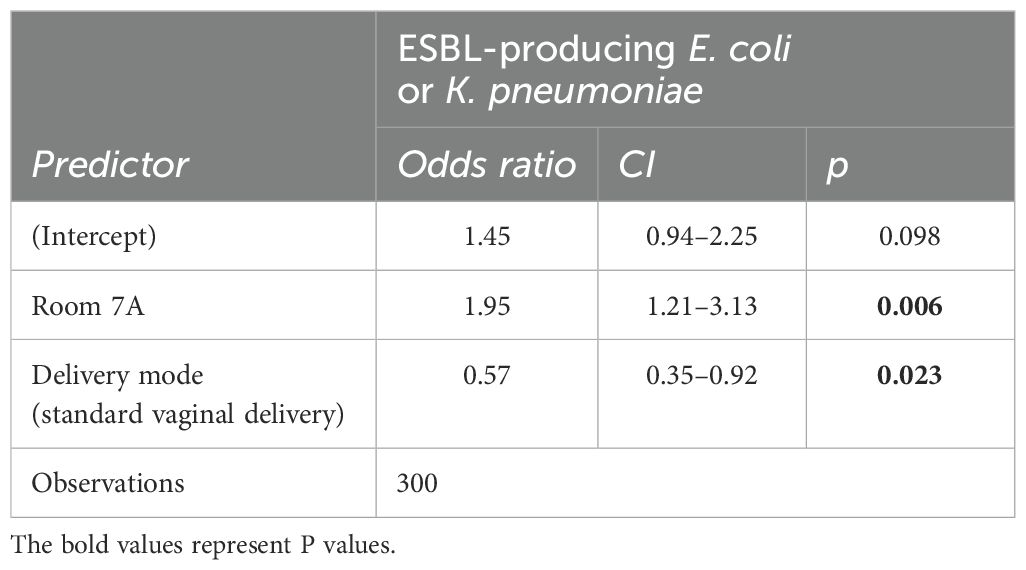
Table 3. Final model showing the risk factors associated with neonatal fecal ESBL-producing E. coli or K. pneumoniae carriage in 300 neonates.
Antimicrobial resistance pattern for ESBL E. coli and K. pneumoniae
Overall, ESBL-producing E. coli and ESBL-producing K. pneumoniae were frequently resistant to ampicillin (n=261, 100%), trimethoprim-sulfamethoxazole (n = 233, 89%), gentamicin (n = 169, 66%), and tetracycline (n = 140, 54%). Only three (1%) of the ESBL-producing E. coli and ESBL K. pneumoniae isolates were resistant to meropenem.
Multi-drug resistance in ESBL-producing E. coli and K. pneumoniae
Overall, 82% (253/310) of the ESBL-producing E. coli and K. pneumoniae isolates were multidrug-resistant (MDR, resistance to ≥3 antibiotics). Among the total isolates for each species, MDR E. coli comprised 75% (62/83) and MDR K. pneumonia 86% (191/222). Only one ESBL-producing K. pneumoniae isolate was resistant to all antibiotics including meropenem.
Discussion
ESBL-producing E. coli and K. pneumoniae are major multidrug-resistant bacteria that are known to cause serious hospital and community-acquired infections worldwide. E. coli and Klebsiella infections are usually treated with common antibiotics, such as penicillin and cephalosporin. However, when these bacteria produce ESBLs, they become resistant to these antibiotics and this significantly limits treatment options, contributing to the progression of infection and in some cases death (Kumburu et al., 2019). In the current study, we assessed the burden, risk factors, and antimicrobial susceptibility patterns associated with the carriage of ESBL-producing E. coli and K. pneumoniae among neonates, and in their surroundings, at a referral hospital in north-eastern Tanzania.
In our study, we found a high burden of ESBL-producing K. pneumoniae and E. coli among neonates and the environment, while resistance to ampicillin and gentamicin was also frequent. This is similar to other findings in other studies in Africa (Herindrainy et al., 2011; Isendahl et al., 2012; Farra et al., 2016; Karanika et al., 2016; Tellevik et al., 2016; Sanneh et al., 2018; Letara et al., 2021).
In our study, the overall prevalence of ESBL-producing K. pneumoniae and E. coli fecal carriage among neonates was 67%. The overall prevalence of ESBL-producing K. pneumoniae and E. coli fecal carriage among neonates was 67% and 32%, respectively. This prevalence is higher compared to the observations from other studies (Tellevik et al., 2016; Letara et al., 2021).
This difference could be due to differences in study participants and risk factors associated with carriage. This finding aligns with the results of other studies conducted in the Mwanza region of Tanzania, where a notably high prevalence of ESBL-producing E. coli and K. pneumoniae was observed among street children. These studies have highlighted the issue of antimicrobial resistance in this vulnerable population, emphasizing the increased risk of infections caused by these drug-resistant pathogens (Moremi et al., 2017).
In total, 5% of maternal hands and 22% of neonatal cots were found to be contaminated with ESBL-producing E. coli and K. pneumoniae. This is consistent with a study conducted in the Mwanza region, where maternal hands and neonatal cots were also found to be contaminated with multidrug-resistant Gram-negative bacteria (Silago et al., 2020).
This could be due to less emphasis on the importance of hand washing and frequent decontamination of neonatal cots, which may result in the acquisition of ESBLs; therefore, these surfaces may serve as reservoirs for transmission, highlighting the importance of regular disinfection and hand hygiene within the neonatal ward. Healthcare workers were not contaminated with either E. coli or K. pneumoniae. All healthcare workers involved in the study were observed to have carried portable hand sanitizers during their daily routine and the results suggest a proper adherence to hand decontamination. ESBL-producing K. pneumoniae (67%) was predominantly isolated from the neonates followed by ESBL-producing E. coli (32%), in contrast with studies that reported E. coli to be predominant (Kumburu et al., 2019). This calls for more research in this area.
In this study, we assessed the risk factors associated with fecal carriage of ESBL-producing E. coli and K. pneumoniae in neonates. High rates of ESBL-producing E. coli and K. pneumoniae carriage were found in those born at the health facility (n=282, 65%) compared to those born at home (n=25, 6%). There was a slightly significant difference in the number of those with ESBL fecal carriage between males (n=164,38%) and females (n=131, 30%). ESBL fecal carriage was slightly higher among neonates with a history of having an IV line (n= 274, 63%) and neonates who were exclusively breastfed (n= 174, 40%). There was no significant difference in ESBL carriage among neonates with communicable (n= 100, 23%) and non-communicable diseases (n= 174, 40%). ESBL fecal carriage was high among neonates who were on antibiotics during their hospital stay (n=256, 59%) (Table 2). The results suggest that the admission room is a significant risk factor for the carriage of ESBL-producing E. coli or K. pneumoniae in neonates, with an increased likelihood of carriage observed in neonates admitted to the hospital (OR = 1.95, p = 0.006). Additionally, delivery mode appears to influence the risk, with vaginal delivery being associated with a reduced risk of carriage (OR = 0.57, p = 0.023), indicating that neonates born via vaginal delivery may have a lower likelihood of acquiring these resistant pathogens compared to those delivered by other methods and, therefore, optimizing hospital room hygiene and reconsidering delivery practices, especially in the case of delivering neonates, are recommended. In this study, ESBL-producing E. coli and K. pneumonia isolates were frequently resistant to ampicillin (n=261, 100%), trimethoprim-sulfamethoxazole (n = 233, 89%), gentamicin (n = 169, 66%), and tetracycline (n = 140, 54%). This is in line with other studies from Tanzania, which reported frequent resistance to tetracycline (100%), trimethoprim-sulfamethoxazole (97%), ciprofloxacin (69%), and gentamicin (44%) (Moremi et al., 2017). In Madagascar, frequent resistance was reported to trimethoprim-sulfamethoxazole (91%), gentamicin (76%), and ciprofloxacin (50%) (Andriatahina et al., 2010). In Spain, resistance to nalidixic acid (65%), ciprofloxacin (32%), levofloxacin (32%), and trimethoprim-sulfamethoxazole (41%) were frequent in ESBL-producing K. pneumoniae (Fernández-Reyes et al., 2014). These findings are consistent with another study conducted in the Mwanza region of Tanzania, where resistance to ampicillin and gentamicin was also reported (Marando et al., 2018). This similarity could be due to the presence of resistance genes. Our study also observed high frequencies of multidrug resistance in ESBL-producing E. coli and K. pneumoniae. Overall, 82% (253/310) of the ESBL-producing E. coli and K. pneumoniae isolates were MDR, defined as resistance to ≥3 antibiotics. Among the total isolates for each species, MDR E. coli comprised 75% (62/83) and MDR K. pneumonia 86% (191/222). Only one ESBL-producing K. pneumoniae isolate was resistant to all antibiotics, including meropenem. This highlights the need for antimicrobial stewardship in neonatal wards to limit the use of broad-spectrum antibiotics. It will be of interest to study the molecular characterization of ESBL-producing E. coli and K. pneumoniae to determine the role of resistance genes and their mechanisms in accelerating drug resistance. Meanwhile, to ensure the effectiveness of infection control measures and prevent the transmission of resistant bacteria, active screening on admission to a specific ward could effectively limit and prevent the spread of resistant bacteria. In addition, antimicrobial stewardship should promote the appropriate use of antimicrobials (including antibiotics), which would improve patient outcomes, reduce antimicrobial resistance, and decrease the spread of infections caused by multidrug-resistant organisms. Our study has several limitations including its cross-sectional design, which allows for the collection of data at a single point in time and is therefore unable to examine changes over time. There is a need for more in-depth research in the future. Furthermore, the study was conducted at only one site which may not represent the practices, infection rates, or population demographics of other healthcare settings, and the single-center design limits the external validity.
Conclusion
Our findings indicated a high burden of ESBL-producing bacteria colonizing neonates, maternal hands, and neonatal cots. In addition, resistance to first-line antibiotics, ampicillin and gentamicin, was also frequent. To ensure the effectiveness of infection control measures and prevent the transmission of resistant bacteria, active screening on admission to a specific ward could limit and prevent the spread of resistant bacteria, especially by implementing routine screening for ESBL-producing bacteria upon neonatal admission, strengthening hand hygiene protocols, and frequently disinfecting neonatal cots and surfaces. The establishment of microbiological methods to detect resistant bacteria could help guide empiric antimicrobial chemotherapy.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical permission for the study was granted by the Kilimanjaro Christian Medical University College (KCMUCo) research ethical review committee in Moshi and the Medical Research Coordinating Committee of the National Institute for Medical Research in Tanzania (NIMR-MRCC). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
HM: Data curation, Formal Analysis, Investigation, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing. DK: Data curation, Formal Analysis, Investigation, Methodology, Supervision, Writing – review & editing. FM: Investigation, Methodology, Supervision, Writing – review & editing. KO: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Writing – review & editing. LM: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Software, Supervision, Validation, Writing – review & editing. BM: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – review & editing, Resources. RZ: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing, Data curation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The project was funded by the Antimicrobial Resistance Cross-Council Initiative through a grant from the Medical Research Council, a Council of UK Research and Innovation, and the National Institute for Health Research, UK (award reference MR/S004815/1).
Acknowledgments
The authors are grateful to the participants in the Kilimanjaro region for their dedicated cooperation and to the technical staff from KCMC/KCRI-Moshi, namely, Victor V. Mosha, Anthon G. Mwingwa, Ignas Mushi, Rose Uisso, Judith Njau, Asia Hemed, Loreen Laizer, and Anna Sechu, for their assistance in the field and the laboratory.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Andriatahina T., Randrianirina F., Hariniana E. R., Talarmin A., Raobijaona H., Buisson Y., et al. (2010). High prevalence of fecal carriage of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in a pediatric unit in Madagascar. BMC Infect. Dis. 10, 204. doi: 10.1186/1471-2334-10-204
Farra A., Frank T., Tondeur L., Bata P., Gody J., and Onambale M. (2016). High rate of faecal carriage of extended-spectrum beta-lactamase-producing Enterobacteriaceae in healthy children in Bangui, Central African Republic. Clin. Microbiol. Infect. 22, 891.e1–891.e4. doi: 10.1016/j.cmi.2016.07.001
Fernández-Reyes M., Vicente D., Gomariz M., Matee TG, Legido-Quigley H, Mboera LEG, et al. (2014). High rate of fecal carriage of extended-spectrum-β-lactamase-producing Escherichia coli in healthy children in Gipuzkoa, northern Spain. Antimicrob. Agents Chemother. 58, 1822–1824. doi: 10.1016/j.eimc.2017.05.005
Hartig F. (2022). Residual diagnostics for hierarchical (multi-level/mixed) regression models. R package version 0.4.6. Available from https://CRAN.R-project.org/package=DHARMa
Herindrainy P., Randrianirina F., Ratovoson R., Hariniana E. R., Buisson Y., Genel N., et al. (2011). Rectal carriage of extended-spectrum beta-lactamase-producing gram-negative bacilli in community settings in Madagascar. PloS One 6, e22738. doi: 10.1371/journal.pone.0022738
https://project-redcap.org/. Available online at: https://project-redcap.org/ (Accessed October 28, 2022).
Husna A., Rahman M., Badruzzaman A. T. M., Sikder M. H., Islam M. R., Rahman M., et al. (2023). Extended-spectrum β-lactamases (ESBL): challenges and opportunities. Biomedicine 11, 2937. doi: 10.3390/biomedicines11112937
Isendahl J., Turlej-Rogacka A., Manjuba C., Rodrigues A., Giske C. G., and Nauclér P. (2012). Fecal carriage of ESBL-producing E coli and K pneumoniae in children in Guinea-Bissau: a hospital-based cross-sectional study. PloS One 7, e51981. doi: 10.1371/journal.pone.0051981
Karanika S., Karantanos T., Arvanitis M., Grigoras C., and Mylonakis E. (2016). Fecal colonization with extended-spectrum beta-lactamase-producing enterobacteriaceae and risk factors among healthy individuals: a systematic review and metaanalysis. Clin. Infect. Dis. 63, 310–318. doi: 10.1093/cid/ciw283
Kumburu H. H., Sonda T., Zwetselaar M.v., Leekitcharoenphon P., Lukjancenko O., Mmbaga B. T., et al. (2019). Using WGS to identify antibiotic resistance genes and predict antimicrobial resistance phenotypes in MDR Acinetobacter baumannii in Tanzania. J. Antimicrob. Chemother. 74, 1484–1493. doi: 10.1093/jac/dkz055
Letara N., Ngocho J. S., Karami N., Msuya S. E., Nyombi B., Kassam N. A., et al. (2021). Prevalence and patient related factors associated with Extended-Spectrum Beta-Lactamase producing Escherichia coli and Klebsiella pneumoniae carriage and infection among pediatric patients in Tanzania. Sci. Rep. 11, 22759. doi: 10.1038/s41598-021-02186-2
Marando R., Senii J., Miramboi M. M., Falgenhaueri L., Moremii N., Mushii M. F., et al. (2018). Predictors of the extended-spectrum-beta lactamases producing Enterobacteriaceae neonatal sepsis at a tertiary hospital, Tanzania. Int. J. Med. Microbiol. IJMM 308, 803–811. doi: 10.1016/j.ijmm.2018.06.012
Ministry of Health. (n.d.). Community development, gender, elderly and children (MOHCDGEC). The national action plan on antimicrobial resistance 2017-2022. Available online at: https://afro.who.int/publications/national-action-plan-antimicrobial-resistance-2017-2022 (Accessed October 28, 2022).
Moremi N., Claus H., Vogel U., and Mshana S. E. (2017). Faecal carriage of CTX-M extended-spectrum beta-lactamase-producing Enterobacteriaceae among street children dwelling in Mwanza city, Tanzania. PloS One 12, e0184592. doi: 10.1371/journal.pone.0184592
R Core Team. (2023). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available from https://www.R-project.org/
Raphael E., Glymour M. M., and Chambers H. F. (2021). Trends in prevalence of extended-spectrum beta-lactamase-producing Escherichia coli isolated from patients with community- and healthcare-associated bacteriuria: results from 2014 to 2020 in an urban safety-net healthcare system. Antimicrob. Resist. Infect. Control 10, 118. doi: 10.1186/s13756-021-00983-y
Sangeda R. Z., Saburi H. A., Masatu F. C., Aiko B. G., Mboya E. A., Mkumbwa S., et al. (2021). National antibiotics utilization trends for human use in Tanzania from 2010 to 2016 inferred from Tanzania medicines and medical devices authority importation data. Antibiot. (Basel) 10, 1249. doi: 10.3390/antibiotics10101249
Sanneh B., Kebbeh A., Jallow H. S., Camara Y., Witson Mwamakamba F., Ceesay I. F., et al. (2018). Prevalence and risk factors for faecal carriage of extended spectrum beta-lactamase producing Enterobacteriaceae among food handlers in lower basic schools in West Coast Region of the Gambia. PloS One 13, e0200894. doi: 10.1371/journal.pone.0200894
Silago V., Mboera LEG, Mshana SE, Seni J., Matthews L., Oravcová K., et al. (2020). Bacteremia in critical care units at Bugando Medical Centre, Mwanza, Tanzania: The role of colonization and contaminated cots and mothers’ hands in cross-transmission of multidrug resistant Gram-negative bacteria. Antimicrob. Resist. Infect. Control 9, 58. doi: 10.1186/s13756-020-00721-w
Teklu D. S., Negeri A. A., Legese M. H., Bedada T. L., Woldemariam H. K., and Tullu K. D. (2019). Extended-spectrum beta-lactamase production and multi-drug resistance among Enterobacteriaceae isolated in Addis Ababa, Ethiopia. Antimicrob. Resist. Infect. Control 8, 39. doi: 10.1186/s13756-019-0488-4
Tellevik M. G., Blomberg B., Kommedal Ø, Maselle S. Y., Langeland N., and Moyo S. J. (2016). High prevalence of faecal carriage of ESBL-producing enterobacteriaceae among children in Dar es Salaam, Tanzania. PloS One 11, e0168024. doi: 10.1371/journal.pone.0168024
Weinstein M. P. and Clinical and Laboratory Standards Institute (CLSI) (2018). Performance standards for antimicrobial susceptibility testing. 28th (950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087 USA: Clinical and Laboratory Standards Institute).
World Health Organization (2015). Global action plan on antimicrobial resistance. Available online at: https://www.who.int/publications/i/item/9789241509763 (Accessed October 28, 2022).
Keywords: burden, risk factor, antimicrobial susceptibility, extended-spectrum beta-lactamase (ESBL), neonates
Citation: Mshana HJ, Kovacs D, Muro F, Zadoks R, Oravcova K, Matthews L and Mmbaga BT (2025) The burden, risk factors, and antimicrobial susceptibility pattern associated with extended-spectrum beta-lactamase-producing E. coli and K. pneumoniae carriage among neonates and their surroundings at a referral hospital in the Moshi municipality. Front. Antibiot. 4:1556842. doi: 10.3389/frabi.2025.1556842
Received: 07 January 2025; Accepted: 07 April 2025;
Published: 21 May 2025.
Edited by:
Salome N. Seiffert, Zentrum für Labormedizin (ZLM), SwitzerlandReviewed by:
Ronni Mol Joji, Arabian Gulf University, BahrainMojtaba Akbari, Isfahan University of Medical Sciences, Iran
Copyright © 2025 Mshana, Kovacs, Muro, Zadoks, Oravcova, Matthews and Mmbaga. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Happyness J. Mshana, amVyZW1pYWhhcHBpbmVzc0B5YWhvby5jb20=
 Happyness J. Mshana
Happyness J. Mshana Dorottya Kovacs2
Dorottya Kovacs2 Ruth Zadoks
Ruth Zadoks Katarina Oravcova
Katarina Oravcova Louise Matthews
Louise Matthews Blandina T. Mmbaga
Blandina T. Mmbaga