- 1Federal College of Animal Health and Production Technology, National Veterinary Research Institute (NVRI) Vom, Vom, Plateau State, Nigeria
- 2Biotechnology Division, National Veterinary Research Institute (NVRI), PMB 01 Vom, Vom, Plateau State, Nigeria
- 3Fleming Laboratory, Diagnostic Services Department, National Veterinary Research Institute (NVRI), PMB 01 Vom, Vom, Plateau State, Nigeria
- 4Department of Medical Microbiology, University of Jos, Jos, Nigeria
Background: Environmental coliform bacteria are frequently the cause of subclinical mastitis (SCM), a serious health issue in the dairy industry. Extended-spectrum β-lactamase (ESBL)-producing coliforms in livestock are a serious public health concern, particularly in environments where people and animals coexist. With an emphasis on their zoonotic and One Health implications, this study sought to evaluate the incidence of SCM and the occurrence of ESBL-producing coliforms in ruminants in Plateau State, Nigeria.
Methods: The California Mastitis Test (CMT) was used to screen 287 milk samples that were taken from cows, ewes, and does. Standard microbiological methods were used to identify the bacterial isolates from CMT-positive samples. The presence of resistance genes (blaTEM and blaCTX-M) was ascertained by PCR, and ESBL production was confirmed phenotypically. Phylogenetic analysis showed genetic diversity and possible horizontal gene transfer among isolates.
Results: Out of 287 milk samples, 79 (27.5%) had subclinical mastitis through the CMT, with a higher prevalence recorded in does 18(22.8%) while ewes and cows recorded 23(29.1%), and 38(48.1%) respectively. Of the 79 CMT-positive samples, the following isolates were identified: Citrobacter freundii (6.3%), Klebsiella pneumoniae (21.6%), K. oxytoca (2.5%), K. aerogenes (6.3%), and E. coli, being the most prevalent in cows (71%). Through PCR, 46 isolates expressed two important ESBL genes, blaTEM and blaCTX-M.
Conclusion: A possible zoonotic reservoir for antibiotic resistance in Nigeria is highlighted by the increased frequency of ESBL-producing coliforms in ruminants with SCM. These results highlight the necessity of implementing integrated One Health initiatives, such as public education, surveillance, and antimicrobial stewardship, in order to reduce the risk of resistant pathogen transmission from animals to people.
Introduction
Mastitis, an inflammation of the mammary gland that is particularly common in sub-Saharan Africa, including Nigeria, is one of the most economically important conditions affecting the intrinsic quality of ruminant dairy products globally (Halasa et al., 2007; Abebe et al., 2022). Based on the clinical condition of the mammary gland, mastitis is classified into two groups: clinical and subclinical mastitis. Clinical mastitis presents with sudden redness and a painful udder that secretes low-quality milk with higher somatic cell counts (Cobirka et al., 2020). In contrast, subclinical mastitis is characterized by the absence of visible signs in the udder and does not visibly reveal the quality of poor milk (Ruegg, 2017).
Due to their opportunistic tendencies and ubiquitous nature in the environment, coliform bacteria, particularly Escherichia coli (E.coli), Klebsiella spp., and Enterobacter spp., are becoming increasingly implicated in both clinical and subclinical cases as causal agents (Bachaya et al., 2021). The rise of coliforms that produce extended-spectrum beta-lactamase (ESBL) in mastitic milk is a growing concern (Klibi et al., 2019). Particularly when this is connected with toxigenic pathogens such as Staphylococcus aureus, which produces enterotoxins in milk-based products, leading to toxic shock syndrome, food poisoning, and cramps (Galal et al., 2006). These coliforms are resistant to a variety of beta-lactam antibiotics, which limits the available treatment choices and poses serious health hazards to the general public (Iroha et al., 2023).
Because ESBL-producing coliforms can spread through direct contact with infected animals, ingestion of raw or inadequately pasteurized milk, and environmental pollution, their zoonotic potential is especially concerning (Tansawai et al., 2022). Antimicrobial-resistant bacteria can proliferate and spread throughout animal and human populations in Nigeria, mainly through the food chain and through direct contact with farmers in the smallholder and informal dairy systems. This is exacerbated due to inadequate veterinary supervision, poor biosecurity, excessive antibiotic usage, and unsanitary practices (Odetokun et al., 2020).
Mastitis in Nigerian livestock has been extensively studied in small ruminants, especially goat, with data on subclinical mastitis appearing in cattle in the late 2000s (Shittu et al. 2012). The disease has been described as common in Nigeria due to the farming system and poor hygiene around farms, which promote infection of the udder and subsequently low-quality milk and milk-based products. While several microbes have been implicated in the pathogenesis of mastitis, coliforms have been identified as the most notorious group of pathogens with a higher incidence of cow death and agalactia-related culling when compared to other pathogens (Mbuk et al., 2016). Food animals consume 69,455 tonnes of antimicrobials globally (Ardakani et al., 2024), and a significant portion of these antimicrobials goes into the treatment of mastitis in dairy animals (Theodoridou Oxinou et al., 2025). The excessive use of antibiotics in dairy animals is a major cause of antibiotic use in dairy animals. This is further compounded by the fact that up to 20% of subclinical mastitis (SCM) are unrelated to microbial infection (Mdegela et al., 2009), underscoring the need for caution in the administration of antibiotics.
In the context of ruminant mastitis, this study provides a critical framework for addressing the shared burden of antimicrobial resistance (AMR) caused by ESBL-producing pathogens (WHO, 2022). Understanding the prevalence, drivers, and health risks associated with these resistant coliforms in Nigerian livestock systems is essential for developing integrated strategies to safeguard both public health and animal productivity. This study aims to determine the zoonotic and one health implications of ESBL-Producing Coliforms in ruminants with Subclinical Mastitis in Plateau State, Nigeria.
Materials and methods
Data collection
This study was conducted in Jos North and Jos South Local Government Areas (LGAs) of Plateau State, Nigeria, between September 2019 and March 2020. It employed a cross-sectional study using snowball sampling. Herds containing three different ruminant groups that consented to participate in the study were chosen. The choice of snowball sampling was necessitated by the limited number of herders in these areas and due to the security challenges involved in accessing remote areas. The identified herders recommended more farms in the LGA that also fit the criteria. Because each herd includes a variety of strata, such as meat ruminants, lactating but dry ruminants, and lactating ruminants, the stratified random sampling approach was used to select which ruminants would be included in the study. Out of 349 ruminants, 95 were meat ruminants, 132 were dry female ruminants, while 122 lactating ruminants were sampled.
Sample size determination
The sample size was calculated using the formula for estimating the prevalence of mastitis with a 95% confidence level and a 5% margin of error. Using the formula, N = Z2xP(1-P)/d2,where N is the minimum sample size, Z is the Z-statistic of 95% confidence level (1.96 for two-tailed tests), P is the prevalence of mastitis, and d is the allowable error margin of 5%. In this study, P was taken as 10.3% based on previous studies (Mbuk et al., 2016). By substitution, N was calculated to be 141.49, however, a total of 287 milk samples from ruminants were collected to ensure robustness of the study, account for both effects due to clustering, and the potential for nonresponse. About 213 milk samples were collected from 4 quarters of each of 54 lactating cows, except for one cow that had three blind teats, 68 milk samples collected from 34 does, and 34 ewes, respectively, and 12 fecal samples from pastoralists.
California Mastitis Test
Individual ruminants were properly restrained and given a vet’s clinical inspection. Briefly, visual inspection and palpation were used to examine for clinical mastitis, while the CMT was used to further investigate the ruminants that did not have clinical mastitis but subclinical mastitis Ruminants that did not have clinical mastitis were subjected to further investigation for subclinical mastitis by using the CMT on milk samples from each half of the sampled ruminant. It was carried out by adding equal amounts of CMT reagent and milk from each half on the test paddle and was rocked for 10 seconds. Samples with a CMT score of 0 or T (trace) were considered negative, while those with CMT scores of 1 (mild clumping), 2 (moderate clumping), or 3 (heavy clumping) were considered positive for subclinical mastitis, according to (Anueyiagu et al., 2020).
Sample collection
Following teat cleaning with 70% ethanol, aseptic methods were used to collect 10 mL of milk from each afflicted quarter or half of the ruminants’ udder, as well as samples of pastoralists’ feces (Zeryehun and Abera, 2017). Samples were transported in sterile screw-capped containers placed in cool boxes containing ice packs, maintaining a temperature of approximately 4 °C throughout the transport period.
Samples were transported to the Microbiology laboratory of the Federal College of Animal Health and Production Technology, Vom, within 4–6 hours of collection to ensure sample integrity. Human fecal samples of the pastoralists were taken in order to determine if there was any correlation between coliforms that would be isolated from ruminants and the pastoralists.
Bacterial isolation and identification
Milk and fecal samples were first enriched in peptone water according to Geser et al. (2012) and incubated at 37 °C for 24 hours. Using the quadrant streaking technique, a loopful of broth culture was streaked on sterile MacConkey (Oxoid, UK) and Eosin Methylene Blue (EMB) agars (Oxoid, UK) and incubated at 37 °C for 24–48 hours. Lactose-fermenting colonies with characteristic morphology were subjected to Gram staining and a battery of biochemical tests (indole, methyl red, Voges-Proskauer, citrate utilization, oxidase, and catalase) for presumptive identification of coliforms.
The confirmatory screening was done using Oxiod Microbact GNB 24E following the manufacturer’s recommendations on presumptive Gram-stained coliforms. From an 18–24-hour culture, one to three isolated colonies were selected, emulsified in 5.0ml of sterile saline, and vigorously mixed to obtain a homogeneous suspension. The plate containing the substrates was put in the holding tray, and 4 drops (about 100 µl) of the bacterial suspension were added using a sterile Pasteur pipette. Except for well 20, which was used to detect oxidase-positive and other Gram-negative bacilli, the substrates underlined in the wells were covered with sterile mineral oil. Results were read following the manufacturer’s instructions after an 18–24 hour incubation period at 37 °C. The steps of the method were carried out in the order that Balows et al. (1991) recommended.
A prepared suspension of isolates to a turbidity equivalent to 0.5 McFarland standards was placed on Brilliance ESBL Chromogenic Culture Medium (Oxoid, UK) following the report by Ezeanya et al. (2017). At 37 °C between 24 hours and 48 hours, inoculated plates were incubated aerobically; color changes of colonies were observed and interpreted according to Oxoid, UK guidelines. The positive control used was Klebsiella pneumoniae ATCC 700603, while the negative control was E. coli ATCC 25922. Confirmed ESBL-producing coliform isolates were stored in glycerol stock at –20 °C for further analysis.
Molecular detection of ESBL genes
Genomic DNA was extracted from phenotypically confirmed ESBL-producing isolates using the boiling lysis method. PCR was conducted to amplify common ESBL-encoding genes, including blaTEM, and blaCTX-M, using gene-specific primers: F-TCCGCTCATGAGACAATAACC, R-TTGGTCTGACAGTTACCAATGC (Kiratisin et al., 2008); and F-CGCTTTGCGATGTGCAG, R-ACCGCGATATCGTTGGT (Villegas et al., 2008), respectively. PCR products were visualized by gel electrophoresis on 1.5% agarose gels stained with ethidium bromide. Molecular weights were compared against a 931 bp, 550 bp and 868 bp for blaTEM, blaSHV, and blaCTX-M DNA ladders. Positive and negative control strains were included in each run.
Phylogeny
Phylogenetic relationships were inferred using the maximum likelihood method based on the Jukes-Cantor model. The corresponding taxa’s percentage of clustered trees is displayed next to the branches. The initial tree(s) for the heuristic search were constructed using the Neighbor-Joining method based on a matrix of pairwise distance calculated with the Maximum Composite Likelihood approach. With branch lengths expressed as the number of substitutions per site, the tree is drawn to scale. The analysis involved 36 nucleotide sequences. Evolutionary analyses were conducted in MEGA v.6.06 (Tamura et al., 2013) with bootstrap replicate values set at 1,000.
Ethical considerations
Ethical approval was obtained from the Animal Ethics Committee of NVRI, Vom with reference number NVRI/AEC/02/64/19. Ethical approval for the collection of fecal samples from pastoralists was obtained from the Health Research Ethics Committee of Plateau Specialist Hospital, Jos, Plateau State, under reference number NHREC/09/23/2010b.
Statistical analysis
Data were entered and analyzed using Microsoft Excel and R Commander version 2.10-0. Descriptive statistics such as frequencies and percentages were computed for relevant variables. Inferential statistics were applied to determine associations between variables. Chi-square tests were used to compare proportions of pathogen detection across the groups, and a p-value < 0.05 was considered statistically significant. Results were presented using tables and graphs for clarity and ease of interpretation.
Results
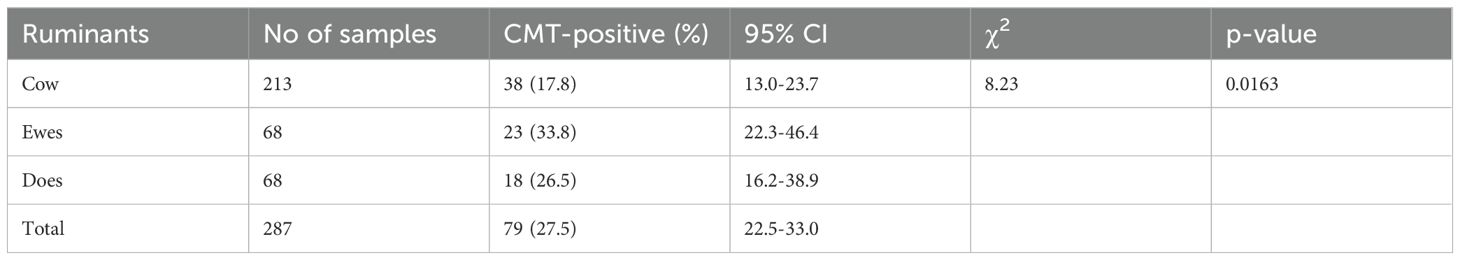
In Plateau State, 287 milk samples were taken from 54 cows, 34 ewes, and 34 does. Of them, the CMT revealed that 79 (27.5%) had subclinical mastitis. Among the ruminant species with SCM, the prevalence rates were 18 (22.8%) in does, 23 (29.1%) in ewes, and 38 (48.1%) in cows. The prevalence of SCM varied significantly among the ruminant groups, according to statistical analysis (χ² = 8.23, p = 0.0163), suggesting that species type affected the chance of SCM occurrence (Table 1).
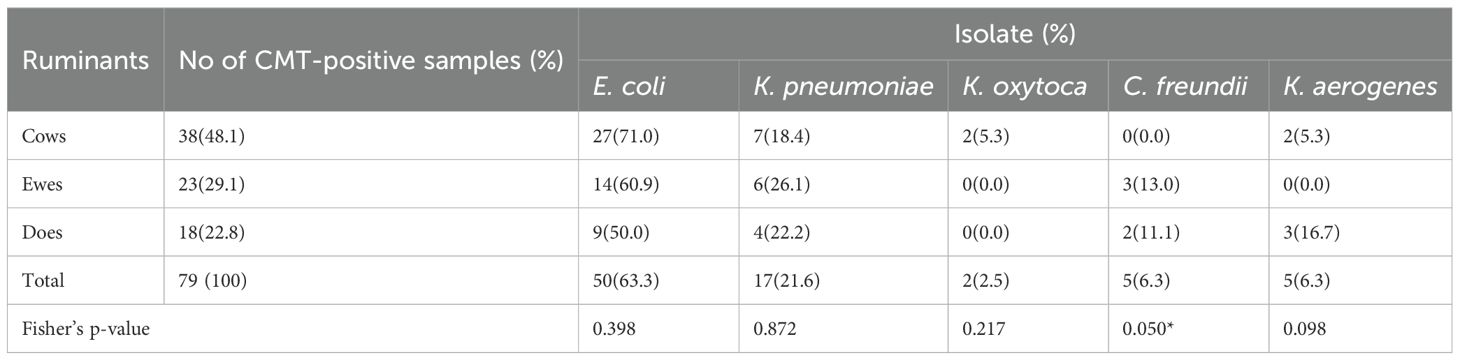
E. coli was the most commonly isolated coliform among the 79 CMT-positive samples, appearing in 50 (63.3%) of them. Citrobacter freundii (6.3%), K. pneumoniae (21.6%), K. oxytoca (2.5%), and K. aerogenes (6.3%) came next. E. coli was most prevalent in cows (71%), followed by ewes (60.9%) and does (50%), according to the distribution of these germs among the ruminant species. Notwithstanding these variations, the Fisher’s exact tests for the bacteria showed only C. freundii had a marginal statistically significant difference in the prevalence of coliform across the ruminant groups (Table 2). E. coli was isolated from 4 out of the 12 fecal samples of pastoralists examined.
It was determined that 42 (53.2%) of the 79 CMT-positive samples were coliform isolates that produced ESBLs. The most common isolate that produced ESBLs was E. coli (50%), which was followed by K. pneumoniae (21.4%), K. aerogenes (16.7%), C. freundii (9.5%), and K. oxytoca (2.4%). These isolates were found in all three ruminant species, with the ESBL-positive pool consisting of cows (16), ewes (18), and does (8) (Table 3).
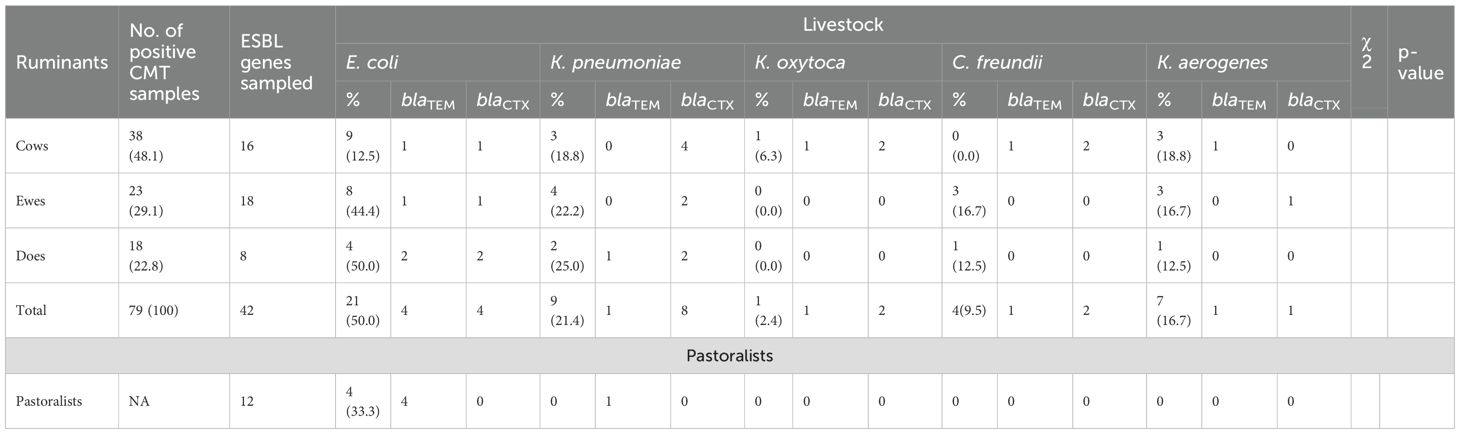
Table 3. Occurrence of ESBL-producing coliforms in ruminants and pastoralist and their respective genes in ruminants with SCM.
Two important ESBL genes, blaTEM and blaCTX-M, were found in 46 coliform isolates by PCR analysis. Among all coliform species and ruminant sources, the blaTEM gene was more common. K. pneumoniae and K. aerogenes from cows and does reveal dual carriage of both genes in several isolates, while E. coli isolates from pastoralists (4 samples) demonstrated 100% carriage of the blaTEM gene with no detection of blaCTX-M (Table 3). Using the Maximum Likelihood Method, phylogenetic analysis further verified the blaTEM (Figure 1) and blaCTX-M genes’ (Figure 2) genetic relatedness across species.
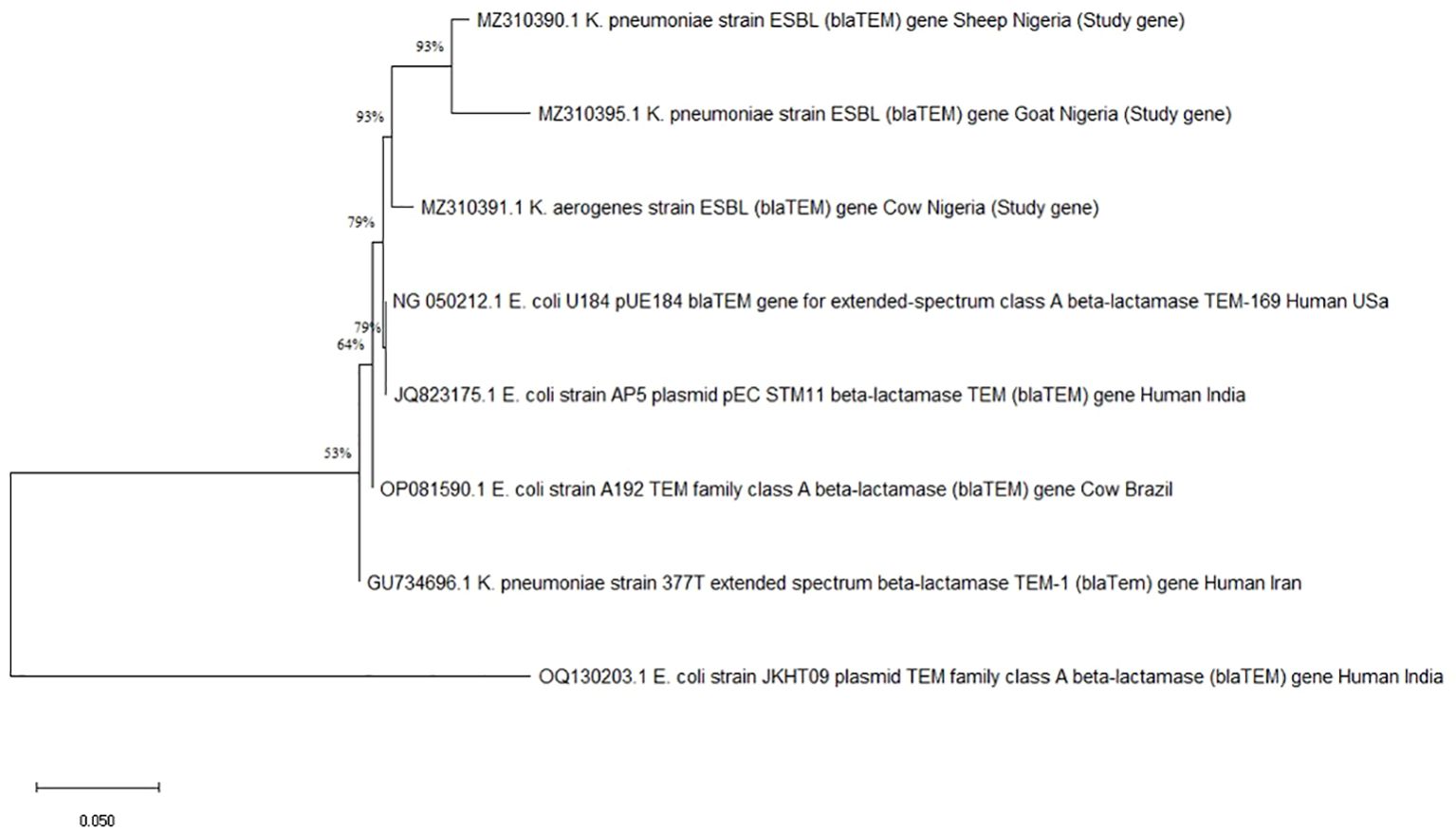
Figure 1. Phylogenetic tree showing genetic relationships based on the blaTEM gene. Each branch represents a gene, identified by names and origins, with percentage values indicating bootstrap support for the nodes.

Figure 2. Phylogenetic tree showing the genetic relationship of various beta-lactamase genes. Branches indicate strains: K. pneumoniae from goat, E. coli from cows, and K. oxytoca and K. michiganensis from humans, with bootstrap values of fortyseven and eighty-four percent.
Discussion
In this study, we identified ESBL-producing microorganisms implicated in SCM among ruminants, some of which are known to possess zoonotic potential. While direct transmission from animals to humans was not assessed, our findings contribute to the growing body of evidence suggesting that individuals in close contact with livestock may be at increased risk of exposure to antimicrobial-resistant pathogens and resistance genes (Agusi et al.., 2024). This underscores the importance of continued surveillance within a One Health framework (Aworh et al., 2019; Johnson et al., 2007).
This study showed that subclinical mastitis in ruminants was more prevalent than clinical mastitis. This is attributed to the subtle nature of SCM, making it less problematic for farmers as it scarcely affects the physical quality of milk; hence, farmers focus more on clinical mastitis since subclinical mastitis is difficult to detect. As a result, untreated subclinical mastitis festers and has the potential to spread from animal to animal. Cows, Ewes, and Does have a prevalence of 38%, 23%, and 18%, respectively. These figures corroborate earlier findings by Gebrewahid et al. (2012), who reported a prevalence of 18.03% for Does but a higher prevalence of 28.14% for Ewes. This may be connected to Nigeria’s prevalent poor environmental hygiene and farm management practices, which tend to increase the risk of infectious agents spreading among herds in feedlots and during transhumance, a common practice among local pastoralists. In this study, 79 ruminant milk samples with SCM contained 50 coliform isolates identified as E. coli, K. pneumoniae, K. oxytoca, C. freundii, and K. aerogenes. This is in close agreement with Garedew et al. (2012), who identified the primary mastitogens as E. coli, Klebsiella spp., Enterobacter spp., Citrobacter, Serratia, and Proteus. Organic materials, such as bedding and manure, typically contain large amounts of coliform bacteria (environment). Therefore, from an epidemiologic perspective, environmental factors accounted for the majority of pathogen infections in this study. When coliform bacteria come into contact with teat ends, they enter the udder through the teat sphincter. Coliform bacteria either proliferate quickly or go dormant after entering the mammary gland.
E. coli had the highest prevalence rate of 63.3% among all the ruminants sampled, followed by K. pneumoniae with a prevalence of 21.6%, and K. aerogenes with a prevalence of 6.3%. This might be because E. coli is a common commensal organism in the gastrointestinal tract of ruminants and is more readily shed in feces, making it more likely to be isolated during sampling, especially in environments with suboptimal hygiene and management practices. In a cross-sectional study comparable to this one, 54 distinct bacterial species were found in Gondar, Ethiopia. However, the frequently isolated Gram-negative bacterial pathogens were E. coli (29.6%), Pseudomonas aeruginosa (18.5%), and K. pneumoniae (16.7%) (Garedew et al., 2012).
ESBL-producers strains identified in this study carried either blaCTX-M or blaTEM. The overall prevalence rate of blaCTX-M and blaTEM in ruminant mastitis was 36.96% and 26.08%, respectively. Ali et al. (2016) reported that blaCTX-M was the predominant ESBL gene detected from bovine mastitis, with a higher prevalence of 77.78% isolates. However, Yu et al. (2019) had a contrary result where blaTEM was the most frequently detected resistance gene with 83.1% prevalence in bovine mastitis, followed by blaCTX-M with 66.3% prevalence.
The resistance gene, blaTEM, has increasingly been identified in many different sources, including humans, animals, and the environment. During the last decade, it has virtually displaced the other ESBLs within Enterobacteriaceae (Canton et al., 2012). Among 12 stool samples collected from pastoralists, only four E. coli were isolated, and all were blaTEM. This is significant since it reflects AMR in human isolates, and this may reflect likely transmission of resistant strains or resistance genes from humans to animals or vice versa, particularly in environments with close animal-human contact. Despite the small sample size, this raises concerns about the likely reservoir of resistance genes among the population in contact with livestock.
According to phylogenetic analysis, some of the blaCTX-M and blaTEM gene sequences from the ruminants in this study shared ancestry with human genes from various geographical locations. Due to the high level of phylodiversity found in ruminants, clones with different genetic backgrounds may be responsible for the transmission of coliforms carrying the blaCTX-M and blaTEM genes (Jena et al., 2017). Surprisingly, none of the samples screened in this study revealed the presence of the blaSHV gene. This trend was found in Yang et al., 2018 where blaSHV was not detected, while blaCTX-M and blaTEM had 97.26% and 71.23% of isolates in cows with mastitis. The absence of blaSHV genes in this study may be due to the difference in type and volume of consumption of antibiotics and the difference in time in which the isolates were collected (Al-Agamy et al., 2009). Also, it has become evident that once a blaCTX-M type enters an area, it becomes prevalent, replacing blaTEM and blaSHV as the dominating ESBL (Canton et al., 2012).
This study highlights the One Health implications of ESBL-producing coliforms in Nigerian ruminants with SCM. The veterinary, medical, and environmental sectors must work together to address this issue in order to stop the spread of resistance and protect animal and human populations.
Conclusion
This study emphasizes the zoonotic threat posed by (ESBL)-producing coliform bacteria in Nigeria as well as the public health importance of SCM in ruminants. The hidden but significant danger of AMR transmission from animals to people is highlighted by the high prevalence of ESBL-producing E. coli and Klebsiella species, especially those carrying the blaTEM and blaCTX-M genes, in otherwise healthy nursing animals.
There is a significant risk of zoonotic spillover of these resistant infections due to the informal selling and consumption of raw milk, as well as the frequent close human-animal contacts found in Nigeria’s pastoral and smallholder dairy systems. According to the findings, a One Health strategy that encourages better farm hygiene, prudent antibiotic usage, integrated surveillance, and livestock handler education should be put into place.
In the end, combating ESBL-producing organisms in animal health systems is crucial for maintaining the efficacy of vital antibiotics for future generations as well as for protecting animal productivity and reducing the growing threat of AMR to human health.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The animal studies were approved by Animal Ethics Committee, National Veterinary Research Institute, Vom. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
KA: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. EA: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. DK: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. GA: Conceptualization, Supervision, Validation, Visualization, Writing – review & editing. EI: Conceptualization, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors would like to acknowledge and appreciate the support of the head of the department of Biotechnology Centre, National Veterinary Research Institute (NVRI) Vom Dr Mosun Ogendegbe, and the entire staff for providing technical support during the the laboratory analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abebe R., Tufa T. B., and Tsegaye Y. (2022). Bovine mastitis and its association with antimicrobial-resistant pathogens in dairy cows: A systematic review. Veterinary Med. Int. 2022, 1–9. doi: 10.1155/2022/1824932
Agusi E. R., Kabantiyok D., Mkpuma N., Atai R. B., Okongwu-Ejike C., Bakare E. L., et al. (2024). Prevalence of multidrug-resistant Escherichia coli isolates and virulence gene expression in poultry farms in Jos, Nigeria. Front. Microbiol. 15. doi: 10.3389/FMICB.2024.1298582/BIBTEX
Al-Agamy M. H. M., Shibl A. M., and Tawfik A. F. (2009). Prevalence and molecular characterization of extended-spectrum β-lactamase-producing Klebsiella pneumoniae in Riyadh, Saudi Arabia. Annu. Saudi Med. Rep. 29, 253–257. doi: 10.4103/0256-4947.55306
Ali T., Rahman S., Zhang L., Shahid M., Zhang S., Liu G., et al. (2016). ESBL-producing Escherichia coliform cows suffering mastitis in China contain clinical class 1 integrons with CTX-M linked to ISCR 1. Front. Microbiol. 7, 1931. doi: 10.3389/fmicb.2016.01931
Anueyiagu K. N., Audu S. K., Joshua B. D., Pelumi O. E., and Haji S. A. (2020). Prevalence and antibiogram of coliform bacteria, occurrence of fungi in subclinical mastitis in small ruminants in Plateau State, Nigeria. J. Vet. Med. Anim. Health 5 (3), 83–911. doi: 10.31248/JASVM2020.206
Ardakani Z., Aragrande M., and Canali M. (2024). Global antimicrobial use in livestock farming: an estimate for cattle, chickens, and pigs. Animal 18, 101060. doi: 10.1016/J.ANIMAL.2023.101060
Aworh M. K., Kwaga J., Okolocha E., Mba N., and Thakur S. (2019). Prevalence and risk factors for multi-drug resistant Escherichia coli among poultry workers in the Federal Capital Territory, Abuja, Nigeria. PloS One 14, e0225379. doi: 10.1371/JOURNAL.PONE.0225379
Bachaya H. A., Abbas R. Z., Khan A., Raza M. A., and Hassan M. M. (2021). Etiology and antibiotic susceptibility profile of bacterial isolates from bovine clinical mastitis in Southern Punjab, Pakistan. Veterinary World 14, 74–79. doi: 10.14202/vetworld.2021.74-79
Balows A., Hausier W. J., Hermann K. L., Isengeng J. D., and Shadomy J. (eds.) (1991). Manual of Clinical Microbiology, 5th Edition, (Washington D. C.: American Society of Microbiology). .
Canton R., Gonzalez-Alba J. M., and Galan J. C. (2012). CTX-M-enzymes: origin and diffusion. Front. Microbiol. 3, 110. doi: 10.3389/fmicb.2012.00110
Ezeanya C. C., Agbakoba N. R., Ejike C. E., and Okwelogu S. I. (2017). Evaluation of a Chromogenic Medium for the Detection of ESBL with Comparison to Double Disk Synergy Test. Br. J. Med. Med. Res. 21(12), 1–11.
Cobirka M., Tancin V., and Slama P. (2020). Epidemiology and classification of mastitis. Anim. 2020 10, 2212. doi: 10.3390/ANI10122212
Galal K., Hameed A., Sender G., and Korwin-Kossakowska A. (2006). Public health hazard due to mastitis in dairy cows. Anim. Sci. Papers Rep. 25, 73–85. Available online at: https://www.igbzpan.pl/uploaded/FSiBundleContentBlockBundleEntityTranslatableBlockTranslatableFilesElement/filePath/290/strona73-86.pdf (Accessed July 12, 2025).
Garedew L., Berhanu A., Mengesha D., and Tsegay G. (2012). Identification of gram-negative bacteria from critical control points of raw and pasteurized Cow milk consumed at Gondar town and its suburbs, Ethiopia. Biomed. Cent. Public Health 12, 950. doi: 10.1186/1471-2458-12-950
Gebrewahid T. T., Abera B. H., and Menghistu H. T. (2012). Prevalence and etiology of subclinical mastitis in small ruminants of tigray regional state, North Ethiopia. Veterinary World 5, 103–109. doi: 10.5455/vetworld.2012.103-109
Geser N., Stephan R., and Hachler H. (2012). Occurrence and characteristics of extended-spectrum β-lactamase (ESBL) producing Enterobacteriaceae in food producing animals, minced meat and raw milk. BMC Vet. Res. 8, 21. Available online at: http://www.biomedcentral.com/1746-6148/8/21 (Accessed February 11, 2017).
Halasa T., Huijps K., Østerås O., and Hogeveen H. (2007). Economic effects of bovine mastitis and mastitis management: A review. Veterinary Q. 29, 18–31. doi: 10.1080/01652176.2007.9695224
Iroha I. R., Ugwu M. C., and Moses I. B. (2023). Prevalence and molecular detection of ESBL genes in coliforms isolated from dairy cattle in Nigeria. J. Global Antimicrobial Resistance 32, 100–107. doi: 10.1016/j.jgar.2023.04.010
Jena J., Debata N. K., Sahoo R. K., Gaur M., and Subudhi E. (2017). Genetic diversity study of various β-lactamase-producing multidrug-resistant Escherichia coli isolates from a tertiary care hospital using ERIC-PCR. Indian J. Med. Res. 146, S23–S29. doi: 10.4103/ijmr.IJMR_575_16
Johnson J. R., Sannes M. R., Croy C., Johnston B., Clabots C., Kuskowski M. A., et al. (2007). Antimicrobial drug–resistant Escherichia coli from humans and poultry products, Minnesota and Wisconsin 2002–2004. Emerging Infect. Dis. 13, 838. doi: 10.3201/EID1306.061576
Kiratisin P., Apisarnthanarak A., Laesripa C., and Saifon P.. (2008). Molecular Characterization and Epidemiology of Extended-Spectrum-Lactamase-Producing Escherichia coli and Klebsiella pneumoniae Isolates Causing Health Care-Associated Infection in Thailand, Where the CTX-M Family Is Endemic. Antimicrobial Agents and Chemother. 52, 2818–2824. doi: 10.1128/aac.00171-08
Klibi A., Jouini A., El-Andolsi R. B., Kmiha S., Hamda C. B., Ghedira K., et al. (2019). Epidemiology of β-lactamase-producing staphylococci and gram negative bacteria as cause of clinical bovine mastitis in Tunisia. Biomed. Res. Int. 9. doi: 10.1155/2019/2165316
Mbuk E. U., Kwaga J. K. P., Bale J. O. O., Boro L. A., and Umoh J. U. (2016). Coliform organisms associated with milk of cows with mastitis and their sensitivity to commonly available antibiotics in Kaduna State, Nigeria. J. Vet. Med. Anim. Health, 8 (12), 228–236.
Mdegela R. H., Ryoba R., Karimuribo E. D., Phiri J., Løken T., Reksen O., et al. (2009). Prevalence of clinical and subclinical mastitis and quality of milk on smallholder dairy farms in Tanzania. J. South Afr. Veterinary Assoc. 80 (3), 163–168. doi: 10.10520/EJC99827
Odetokun I. A., Akpabio U., Adetunji V. O., Fasina F. O., and Antia R. E. (2020). Risk of zoonotic transmission of antimicrobial-resistant bacteria among pastoralists in Nigeria: A One Health perspective. Trop. Anim. Health Production 52, 1045–1055. doi: 10.1007/s11250-019-02132-6
Ruegg P. L. (2017). A 100-Year Review: Mastitis detection, management, and prevention. J. Dairy Sci. 100, 10381–10397. doi: 10.3168/JDS.2017-13023
Shittu A., Abdullahi J., Jibril A., Mohammed A. A., and Fasina F. O. (2012). Sub-clinical mastitis and associated risk factors on lactating cows in the Savannah Region of Nigeria. BMC Vet. Res. 8 (1), 134. doi: 10.1186/1746-6148-8-134
Tamura K., Stecher G., Daniel P. D., Alan F. A., and Kumar S. (2013). MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol., 30, 2725–2759.
Tansawai U., Pumipuntu N., and Santajit S. (2022). Antimicrobial resistance and zoonotic transmission of ESBL-producing E. coli from animals to humans: A growing global concern. Antibiotics 11, 945. doi: 10.3390/antibiotics11070945
Theodoridou Oxinou D., Lamnisos D., Filippou C., Spernovasilis N., and Tsioutis C. (2025). Antimicrobial use and antimicrobial resistance in food-producing animals: cross-sectional study on knowledge, attitudes, and practices among veterinarians and operators of establishments in the republic of Cyprus. Antibiotics 14, 251. doi: 10.3390/antibiotics14030251
Villegas M. V., Kattan J. N., Quinteros M. G., and Casellas J. M. (2008). Prevalence of extended-spectrum beta-lactamases in South America. Clin. Microbiol. Infect. 14 (1), 154. doi: 10.1111/J.1469-0691.2007.01869.X
World Health Organisation (WHO) (2022).Global antimicrobial resistance and use surveillance system (GLASS) report 2022. Available online at: https://www.who.int/publications/i/item/9789240062702 (Accessed August 1, 2025).
Yang F., Zhang S.-d., Shang X., Wang X., Wang L., Yan Z., et al. (2018). Prevalence and characteristics of extended spectrum β-lactamaseproducing Escherichia coli from bovine mastitis cases in China. J. Integr. Agric. 17, 1246–1251. doi: 10.1016/S2095-3119(17)61830-6
Yu Z. N., Wang J., Ho H., Wang Y. T., Huang S. N., and Han R. W. (2019). Prevalence, Antimicrobial-Resistance Phenotypes and Genotypes of Escherichia coli Isolated from Raw Milk Samples from Mastitis Cases in Four Regions of China. J. Global Antimicrobial Resistance. Available online at: https://www.sciencedirect.com/science/article/pii/S2213716519303303 (Accessed February 05, 2025).
Keywords: subclinical mastitis, ESBL-producing coliforms, antimicrobial resistance, one health, zoonosis, ruminants, Nigeria
Citation: Anueyiagu KN, Agusi ER, Kabantiyok D, Ayanbimpe GM and Ikeh EI (2025) Zoonotic potential of ESBL-producing coliforms in pastorally managed ruminants with subclinical mastitis in Plateau State, Nigeria. Front. Antibiot. 4:1632264. doi: 10.3389/frabi.2025.1632264
Received: 21 May 2025; Accepted: 18 August 2025;
Published: 15 September 2025.
Edited by:
Mabel Kamweli Aworh, North Carolina State University, United StatesReviewed by:
Shamsudeen Fagbo, Saudi Center for Disease Control and Prevention (CDC), Saudi ArabiaIgbaver Ieren, Government of Saskatchewan, Canada
Dharamdeo Singh, University of Guelph, Canada
Copyright © 2025 Anueyiagu, Agusi, Kabantiyok, Ayanbimpe and Ikeh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ebere Roseann Agusi, cm9zZWViZXJlOEBnbWFpbC5jb20=; Keneth Nnamdi Anueyiagu, YW51ZXlpYWd1bm5hbWRpQHlhaG9vLmNvbQ==
 Kenneth Nnamdi Anueyiagu1
Kenneth Nnamdi Anueyiagu1 Ebere Roseann Agusi
Ebere Roseann Agusi Dennis Kabantiyok
Dennis Kabantiyok