- 1 Neuroscience and Behavioral Disorders Program, Duke-NUS Graduate Medical School, Singapore, Singapore
- 2 Center for Cognitive Neuroscience, Duke University, Durham, NC, USA
Even a single night of total sleep deprivation (SD) can have dramatic effects on economic decision making. Here we tested the novel hypothesis that SD influences economic decisions by altering the valuation process. Using functional magnetic resonance imaging we identified value signals related to the anticipation and the experience of monetary and social rewards (attractive female faces). We then derived decision value signals that were predictive of each participant’s willingness to exchange money for brief views of attractive faces in an independent market task. Strikingly, SD altered decision value signals in ventromedial prefrontal cortex (VMPFC) in proportion to the corresponding change in economic preferences. These changes in preference were independent of the effects of SD on attention and vigilance. Our results provide novel evidence that signals in VMPFC track the current state of the individual, and thus reflect not static but constructed preferences.
Introduction
A single night of total sleep deprivation (SD) can result in a host of neurocognitive consequences (Goel et al., 2009). Most comprehensively studied have been impairments in vigilance and attention (Lim and Dinges, 2010). Considerably less is known about how SD influences affective processes (Walker, 2009), for example, those engaged during decision making (Harrison and Horne, 1999; Killgore et al., 2006; McKenna et al., 2007; Venkatraman et al., 2007). Studies to date have evaluated risky decisions using the Iowa Gambling Task (Killgore et al., 2006), risky and ambiguous decision making tasks (McKenna et al., 2007; Venkatraman et al., 2007, 2011), and the Balloon Analog Risk Task (Killgore et al., 2011). Increased propensity to take risks has been observed in some of these studies and an intriguing possibility is that SD affects decision making by influencing the very values that underlie our decisions. Investigating this potential mechanism would benefit from measuring the neural correlates of reward valuation without involving decision making.
Recent studies suggest that decision preferences reflect the subjective valuation of different goods that have been converted into a standard signal, or “common neural currency” (Montague and Berns, 2002; Izuma et al., 2008; Kim et al., 2010; Rademacher et al., 2010; Smith et al., 2010). This common neural currency enables individuals to make decisions about nominally incommensurable rewards, as when sacrificing a physical or monetary good to obtain a desirable social interaction (Smith et al., 2010; Lin et al., 2011). Such common valuation signals have been demonstrated in ventromedial prefrontal cortex (VMPFC), through correlative techniques such as functional magnetic resonance imaging (fMRI) and single-unit recording (Padoa-Schioppa and Assad, 2006, 2008; Chib et al., 2009; Padoa-Schioppa, 2009; Kim et al., 2010; Smith et al., 2010). One limitation of the extant research is that nearly all studies manipulate value by changing the stimuli about which individuals make decisions (e.g., comparing high- and low-valued items). Yet, in the real-world, our valuation of an outcome depends both on its intrinsic features and on our current state. SD provides an ideal milieu for testing state-dependent changes in valuation: it allows for fully within-participant testing, it has no effects on the actual value of economic goods (unlike presenting food items under states of satiation and hunger), and it represents a common and ecologically relevant state that nearly all individuals experience at some time in their lives.
We hypothesized that SD can alter valuation and perturb the neural common currency for value as reflected by VMPFC activation. This hypothesis stems from the observation that VMPFC activation was altered during risky decision making when sleep deprived (Venkatraman et al., 2011). In turn, these changes in decision value signals would be expected to be commensurate with shifts in valuation of different reward types – demonstrating that VMPFC plays an important role in coding a common currency signal. This second prediction stems from observing that SD also affects activation of regions involved in emotional processing (Yoo et al., 2007; Gujar et al., 2011). We used an incentive delay task in which participants anticipated and then received rewards that had either monetary value or social value [e.g., pictures of faces of varying attractiveness (Aharon et al., 2001; Figure 1A)]. Following the scanner session, we measured each subject’s willingness to sacrifice money to view attractive faces (Smith et al., 2010; Figure 1B). This procedure was motivated by evidence that signals reflecting subjective value can be elicited by incentive-compatible stimuli even in the absence of overt decision making (Lebreton et al., 2009; Smith et al., 2010; Tusche et al., 2010). The measurement of these brain signals provides a neural marker for subjective value independent of the effects of SD on decision making (Venkatraman et al., 2007). We anticipated that changes in an individual’s relative willingness to trade social and monetary rewards following SD would be correlated with a corresponding alteration of neural signals corresponding with their valuation. Such a result would provide evidence that SD affects more than attentional and cognitive inputs to a decision – it shapes the very mechanisms of valuation that underlie economic preferences.
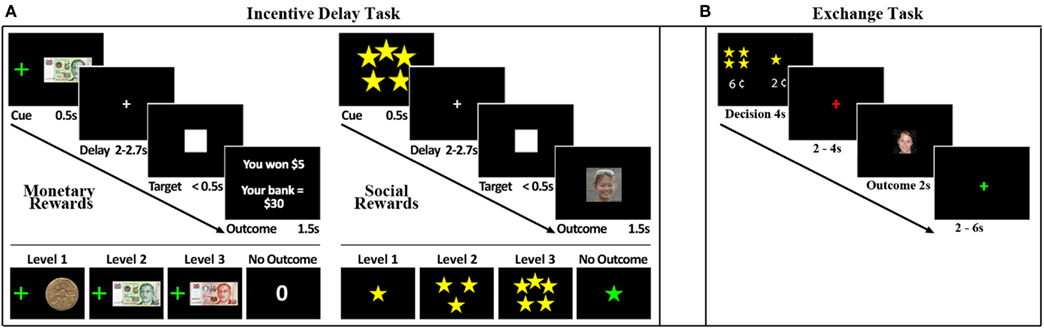
Figure 1. Experimental task. (A) Trial structure of the Incentive Delay Task. Male, heterosexual young adults viewed a cue (0.5 s) predictive of reward type and magnitude. Monetary rewards (left panel) ranged from $1 to $10. A monetary reward control predicted $0. Social rewards (right panel) ranged from 1-star (unattractive) to 5-stars (very attractive), based on ratings from an independent group of participants. A social reward control condition predicted the picture of a scrambled face. After a variable delay (2–2.7 s) a visual target (white square) appeared (<0.5 s). Participants were instructed to respond as quickly as possible to the visual target. After the response, an outcome screen (1.5 s) revealed their reward: money or picture of a face if the response was fast enough, or no money or the picture of a scrambled face if the response was too slow. Reaction time thresholds were defined for each subject and each cue independently in a practice run that preceded the task, such that on average participants succeeded in 60% of the trials. (B) Trial structure for the economic exchange task. Trials began with a decision phase (lasting 4 s) in which the participant was forced to spend a small amount of money to view a face. Participants could choose to spend more money to view a more attractive face or less money to view a less attractive face. Following the decision phase, there was an anticipation phase that lasted either 2 or 4 s. A single face, randomly selected from the chosen attractiveness category, was then displayed for 2 s. Trials were separated by a variable interval of 2–6 s.
Another issue we examine here is whether SD-related changes in valuation correlate with alterations in vigilance (Venkatraman et al., 2011). If SD can result in decoupling of some cognitive and affective processes, it would caution us against expecting that countermeasures effective for one behavioral domain would also benefit other domains (Killgore et al., 2009).
Materials and Methods
Participants
Twenty-two healthy adult males (mean age = 22.7 years, SD = 3.2 years), self-reported as heterosexual, participated in the study. All participants provided informed consent, in compliance with the requirements of the National University of Singapore Institutional Review Board. Participants were selected from a pool of university students who responded to a web-based questionnaire. They had to be right-handed, be between 18 and 30 years of age, not be on any long-term medication, and have no history of any psychiatric or neurologic disorders. They also had to have habitually good sleeping habits (sleeping no less than 6.5 h each night for the past 1 month) and have no symptoms associated with sleep disorders. Participants’ sleep habits were monitored throughout the 2-week duration of the study with an Actiwatch (Philips Respironics, USA). Only participants who maintained a regular sleep schedule (>6.5 h of sleep/night; sleep time no later than 1:00 AM; wake time no later than 9:00 AM) for the week prior to each fMRI scanning session were included in the study. Four participants were removed from the analysis: one for excessive motion during the scan, and three for failure to perform the task appropriately during the SD session. All participants indicated that they did not smoke, consume any medications, stimulants, caffeine, or alcohol for at least 24 h prior to scanning.
Study Procedures
Participants visited the laboratory three times. During the first visit, they were briefed on the study protocol. At the end of this session, they were each given a wrist actigraph, which they were instructed to wear at all times until the conclusion of the experiment. They were also issued sleep diaries on which they recorded the onset and offset of all sleep bouts. The second and third visits (one rested wakefulness, RW session and one SD session) involved the actual in-scanner fMRI experiments. The order of the two sessions was counterbalanced across all the participants and the sessions were separated by 1 week to minimize residual effects of sleep loss in participants whose SD session preceded the RW session.
The first scanning session took place approximately 1 week after the initial visit. For the RW session, scanning commenced at about 8:00 AM. For the SD session, scanning took place at about 6:00 AM, but participants were monitored in the lab from 7:00 PM onward the night before. They remained awake overnight under the constant supervision of a research assistant and were allowed to engage in non-strenuous activities such as reading and watching videos. Participants completed the psychomotor vigilance task (Dinges et al., 1997) and rated their subjective sleepiness using the Karolinska Sleepiness Scale (Akerstedt and Gillberg, 1990) during the first 10 min of every hour from 7:00 PM until 6:00 AM.
Behavioral Tasks
Monetary and social incentive delay task
Participants were scanned while performing an adaptation of the monetary incentive delay task (Knutson et al., 2001; Clithero et al., 2011; Figure 1A). Participants responded as fast as possible to a white square (target). Each target was preceded (between 2 and 2.7 s) by a visual cue (0.5 s) that signaled either a monetary or social reward (presented for 1.5 s) that could be obtained if the response to the upcoming target was fast enough (hit trials). Reaction time threshold was dynamically titrated using information about response times on previous similar trials to adjust the threshold such that on average 60% of the responses resulted in receipt of the reward (Knutson et al., 2001). Eight different visual cues predicted the eight possible rewards: three monetary rewards ($1, $5, and $10), three social rewards (1-star, 3-star, 5-star), and two control cues, one monetary ($0) and one social (0-star, where a scrambled picture of a face was presented). These cues were semi-randomly intermixed in the course of a run. A higher number of stars signaled that a picture of a more attractive female face would be seen.
To establish the attractiveness categories, an independent group of 30 male heterosexual volunteers viewed 5000 pictures of female faces and rated them on a five-point scale. All images were obtained from publicly accessible local dating websites, and the ethnicity proportions matched those of Singapore’s population. We used headshots and did not remove the hair. The average rating of each face was then used to re-bin the face ratings into six categories. The least attractive faces were then discarded as they might elicit aversive responses. Faces belonging to the other five categories were classified from 1-star to 5-star. Faces belonging to the 1, 3, and 5-star categories were used as social rewards. The control cue was constructed by scrambling the pixels of a random picture.
Each scanning session consisted of one in-scanner practice run followed by six runs. Each run consisted of 56 trials: seven semi-randomly intermixed repetitions of each of the eight possible rewards. Intertrial intervals had a uniform distribution between 1.8 and 4.5 s. At the end of the last session subjects received the amount of money earned in two randomly selected runs of the 12 they performed.
Participants viewed stimuli through a set of MR-compatible LCD goggles (Resonance Technology, Los Angeles, USA). Further, they responded to the task using their right hand via a MR-compatible button box. A research assistant monitored participants’ performance throughout the session and noted all lapses and eye closures through use of an eye tracking device. Participants were prompted to attend to the task through an intercom system when they failed to respond to a trial or when epochs of eye closure exceeded 3 s.
Exchange task
After each scanning session participants performed a two-alternative force choice task, in which they had to spend money to view a social reward (female face). On each trial, participants were presented with two options – two different star ratings (1- to 5-stars) each paired with a monetary amount (price tag: 1–12 cents). After making a choice between the two offers participants would view a picture of a female face of the star category they chose (2 s) and pay the monetary amount equal to the number of cents in the price tag. More stars were always paired with a more expensive price tag. Money spent in the exchange task was deducted from each participant’s total compensation.
Face-rating task
Following the exchange task, participants rated the attractiveness of female faces from one to five (one being least attractive and five being most attractive). The faces that were rated all appeared previously in the incentive delay task.
Imaging Procedures
Images were acquired on a 3-T Siemens Tim Trio scanner (Siemens, Erlangen, Germany) fitted with a 12-channel head coil. A high-resolution 3D-MEMPRAGE (Multi-Echo Magnetization-Prepared Rapid-Acquisition Gradient Echo) sequence was obtained so that anatomical images could be normalized into common stereotactic space. Functional images were collected using a gradient echo-planar imaging sequence (TR: 1500 ms; TE: 20 ms; flip angle: 90°; field-of-view: 192 mm × 192 mm; matrix size: 64 × 64). We acquired 34 oblique axial slices (4 mm thick with no inter-slice gap) parallel to the line connecting the anterior and posterior commissures, resulting in whole-brain coverage.
Behavioral Data Analysis
Exchange task
A trial was considered an exchange if the subject chose the more expensive (and more attractive face) out of the two options. In other words, an exchange meant that the subject was willing to spend money to view a more attractive face. For each subject (and each state: RW and SD) we calculated the proportion of exchange trials. We then calculated for each subject the difference between the proportions of exchanges between both states (RW minus SD; Figure 2B).
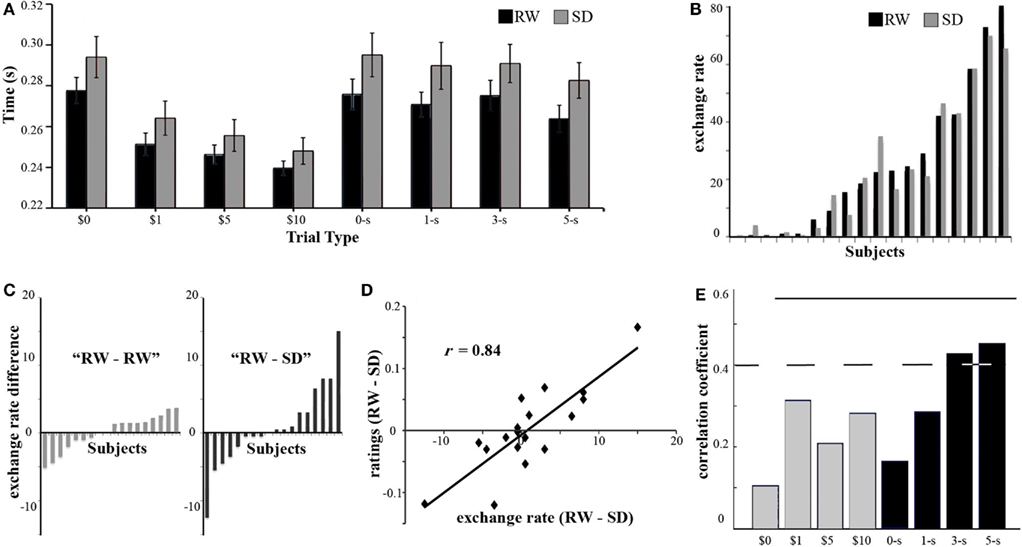
Figure 2. Behavioral results. (A) To quantify the subjective value participants assign to different reward types and magnitudes we measured the reaction time following the different cues. On average, participants responded faster when the cues predicted higher rewards for both monetary and social stimuli. In addition, responses to monetary rewards were significantly faster than those to social rewards. Finally, responses during SD (gray) were slower than responses during RW (black). Error bars denote the SE from the mean. (B) To get a behavioral measure of the relative valuation of monetary with respect to social rewards, participants performed an out-of-scanner exchange task, where they had the option to pay money to view more attractive faces. The percentage of trials in which a subject chose to pay more money to view a more attractive face, or exchange rate, is shown for each subject (sorted by exchange rate in RW) in both RW (black) and SD (gray). (C) Participants showed a range of alterations in exchange rate between RW and SD (right RW–SD, sorted by magnitude of difference). To test whether this difference was related to SD or just test–retest variation, a separate group of participants performed the exchange task in two RW sessions (left). The variance associated with RW–SD was significantly larger than the one associated with RW–RW. (D) Following the exchange task participants performed a face-rating task, where they judged the attractiveness of hundreds of female faces. We observed that the difference in exchange rate (x axis) for each individual subject was strongly correlated (r = 0.84) with the corresponding difference in average attractiveness ratings across states (in the y axis), such that a trend toward more generous attractiveness ratings in one state predicted a higher tendency to exchange money to view a more attractive faces in the same state. This suggests that the change in exchange rate is strongly influenced by a change in social reward valuation. (E) If altered social reward valuation were an important factor determining the alteration of exchange rates, we would expect the alteration of exchange rate to be correlated with changes in reaction times, during the incentive delay task, in response to high social rewards. Reaction times to high social rewards (black, 3- and 5-stars) correlated with each individual’s change in exchange rate, while change in reaction times to low social rewards (0- and 1-star) as well as all monetary rewards (gray) did not correlate with alteration of exchange rate. Dotted line corresponds to significance level p < 0.05 uncorrected, and solid line p < 0.05 Bonferroni corrected.
Face-rating task
Ratings were first converted to z-score, and then we calculated for each subject (and each state: RW and SD) the average difference between their ratings and the ratings of independent group of participants. For each subject, we calculated the difference between the ratings in both states (RW minus SD).
Imaging Data Analysis
The MRI data were analyzed using BrainVoyager QX 1.10.1 (Brain Innovation, Maastricht, The Netherlands) and MATLAB (MathWorks, Natick, USA). Inter-slice timing differences within each functional acquisition were corrected using cubic spline interpolation. Intra-session image alignment to correct for motion was performed using the first image of the first functional run as the reference image. Spatial filtering employed a Gaussian filtering kernel with a 6-mm FWHM for group level activation maps. Linear trend removal and a high-pass filter of 160s were applied to compensate for scanner drifts. Functional volumes were registered to the high-resolution 3D anatomical image. Finally, all images were normalized to Talairach space.
Functional analysis at a voxel-by-voxel-level was performed using a general linear model (GLM) with a total of 24 predictor variables (regressors) in each state (RW, SD). Eight regressors were created for the different cues ($0, $1, $5, $10, 0-star, 1-star, 3-star, 5-stars) of unit amplitude response with 0.5 s duration following the onset of the cue and 16 regressors for the different outcomes (hit or miss) of unit amplitude response with 1.5 s duration following the onset of the outcome. Two additional models were created that included parametric regressors for either anticipation or outcome of rewards, with all other events modeled as nuisance regressors. Parametric regressors included $1, $5, and $10 for monetary rewards and 1-star, 3-star, and 5-star for social rewards. Control rewards – $0 and 0-star, were added as nuisance regressors. Each regressor was convolved with a hemodynamic response function. A voxel-level threshold of at least p < 0.001 (uncorrected) for t-maps was applied. To control for Type I error, remaining voxels were then processed using an iterative cluster size thresholding procedure (Goebel et al., 2006) that considered the spatial smoothness of functional imaging data when generating activation maps based on a corrected cluster threshold (p < 0.05).
To identify brain regions with decision value signals, we performed a whole-brain correlation analysis between exchange rate and the difference in parameter estimates of parametric regressors of social and monetary rewards. This “decision value” signal was computed separately for the cue and outcome-hit periods and also separately for each state. Correlation maps were thresholded (p < 0.05) and cluster corrected (p < 0.05). The conjunction of regions identified in RW and SD were used as ROIs for further analysis. For further details on the rationale for using subjects’ preferences in the exchange task to look at neural measures of decision value (in the absence of choice) see Smith et al. (2010).
We performed a whole-brain correlation analysis between the difference in decision value signal in VMPFC (RW minus SD) and the difference in parameter estimates of parametric regressors during cue or outcome-hit periods for both monetary and social rewards. Correlation maps were masked using the union of parametric response in RW and SD for the specified period (anticipation or receipt phases) and reward type. Correlation maps were thresholded (p < 0.05) and cluster corrected (p < 0.05).
Results
Behavioral Results
The reward value of an item influences the effort expended in acquiring it (Bickel et al., 1992; Aharon et al., 2001). In the Incentive Delay Task (see Materials and Methods), increasing effort is reflected in shorter response times. Three factors were expected to influence response times: behavioral state (SD or RW), reward modality (social or money), and reward magnitude (high, medium, low).
Consistent with prior work, a three-way ANOVA showed a significant interaction between reward type and reward magnitude [F(3,54) = 35.18, p < 0.001; Figure 2A]. A two-way ANOVA of reaction time for monetary rewards showed significant main effects of reward magnitude [F(2,17) = 23.07, p < 0.001] and state [F(1,17) = 6.61), p < 0.05] on response time but no interaction between these factors [F(2,17) = 2, n.s.]. Similarly, for social rewards there were significant main effects of reward magnitude [F(2,17) = 10.89, p < 0.001] and state [F(1,17) = 8.65, p < 0.01] on response times without significant interaction between reward and state [F(2,17) = 0.27, n.s.]. These findings formed the behavioral basis for the identification of brain regions involved in the valuation of monetary and social rewards.
The relative valuation of social and monetary rewards was ascertained by analyzing decisions in the Exchange Task. Following a normal night of sleep (RW), participants ranged from being unwilling to exchange money to view an attractive face, to exchanging in over 90% of the trials (Figure 2B). Although the average exchange rate did not differ across the two sessions – the mean exchange rate being 24.9% in RW and 24.0% in SD [t(17) = 0.62, n.s.] – individual participants showed a range of alterations in willingness to exchange money to view attractive faces, with some participants exchanging more and some exchanging less following SD (Figure 2B).
The heterogeneous alteration in exchange behavior following SD may be the result of random fluctuation in choices rather than a systematic alteration of preferences. To rule out this possibility, we examined the consistency of exchange rates across runs within the same session. Within-session exchange rates were similarly consistent across both states (ICC of 0.939 for RW and 0.936 for SD), arguing against increased randomness in choices following SD. As a control experiment, we recruited an additional independent group of participants to perform the exchange task twice in RW. Consistent with the expectation that SD was the source of altered exchange behavior, we found the variance associated with RW–SD exchanges to be larger than the variance associated with RW–RW exchanges (Levene test, F = 4.58, p < 0.05, Figure 2C). Finally, order of sessions could not explain the shifts in exchange rate [t(17) = −1.56, n.s.].
To ascertain whether SD prompted alteration in exchange behavior by shifting the perceived (social) value of attractive faces, we had subjects rate faces for attractiveness in both states. We found strong correlation between the state-driven alteration in exchange rates between RW and SD and the corresponding difference in attractiveness ratings (r = 0.84, p < 0.001; Figure 2D).
Finally, if participants altered their valuation of social, but not monetary, rewards in a consistent manner during SD, we would expect this to be reflected in the reaction times during the Incentive Delay Task. Indeed, the SD-induced change in exchange rate was significantly correlated with a corresponding change in reaction time for the higher levels of social reward – 5-star and 3-star faces (r = 0.48; p < 0.05) – but not for control cues, 1-star faces, or monetary rewards (r < 0.26; n.s., Figure 2E). Taken together, these behavioral findings suggest that the shift in exchange rates elicited by SD were a consequence of altered valuation of social as opposed to monetary rewards.
Additionally, altered psychomotor vigilance (Dinges et al., 1997), as evidenced by increased RT during SD [RW: 242 ms, SD: 291 ms; t(17) = 6.5, p < 0.001], did not correlate with alteration of exchange behavior (r = −0.04; n.s.). This result concurs with a prior finding that vigilance and the propensity to make gain-maximizing decisions are uncorrelated (Venkatraman et al., 2011).
Imaging Results
Effects of SD on processing of monetary and social rewards
Brain regions carrying decision value signals related to the exchange of monetary for social rewards were identified as those where an individual’s willingness to exchange money to view attractive faces correlated with the corresponding difference in MR signal associated with the receipt of social and monetary rewards. A region in the VMPFC (Figure 3A) was found to carry such decision value signals in both RW and SD (Figure 3B). Of specific interest, SD-driven alteration in exchange rates correlated with SD-driven alteration of decision value signal in the VMPFC (r = 0.58, p < 0.01, Figure 3C).
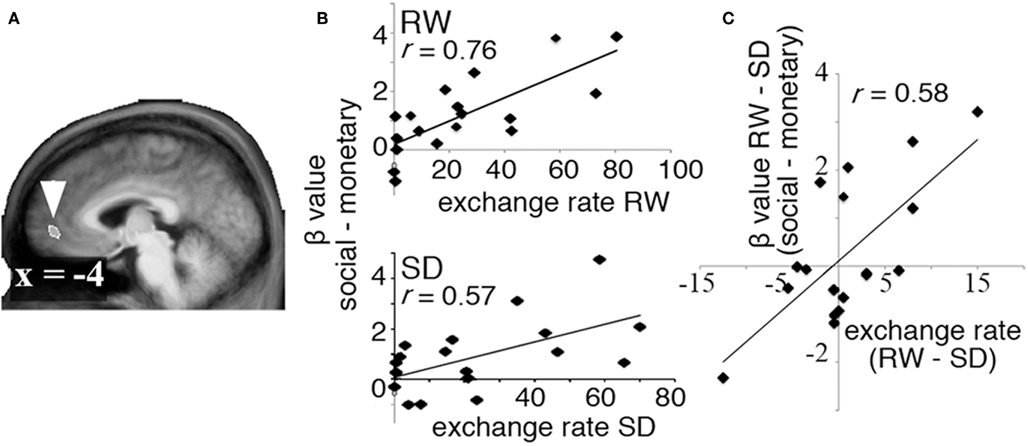
Figure 3. Decision value signals. To identify regions carrying decision value signals in both RW and SD, we computed the correlation between exchange rate in each state and the difference in BOLD signal associated with receipt of social and monetary rewards (the “decision value” signal). (A) A region in VMPFC showed a significant correlation between exchange rate and difference between activation in response to social and monetary rewards in both RW and SD. (B) Correlation between exchange rate and decision value signals in VMPFC in RW (top) and SD (bottom). (C) Change in decision value signals across states (RW–SD, y axis) correlated significantly with change in exchange rate (RW–SD, x axis).
To evaluate signals associated with the processing of the monetary and social reward value, we first identified voxels that showed parametric increase in activation with reward size (Tables 1 and 2). We then determined whether SD-related alteration of decision value signals in VMPFC correlated with signal change associated with the processing of monetary or social rewards. We found significant correlation between SD-related signal shifts (comparison of RW and SD) associated with the receipt of social rewards and the corresponding alteration in decision value signals in VMPFC. Regions showing such correlation were: left amygdala (r = 0.53, p < 0.05; Figure 4A), frontal pole (r = 0.54, p < 0.05; Figure 4B), left putamen (r = 0.49, p < 0.05; Figure 4C), and left anterior insula (r = 0.52, p < 0.05; Figure 4D; Table 3).
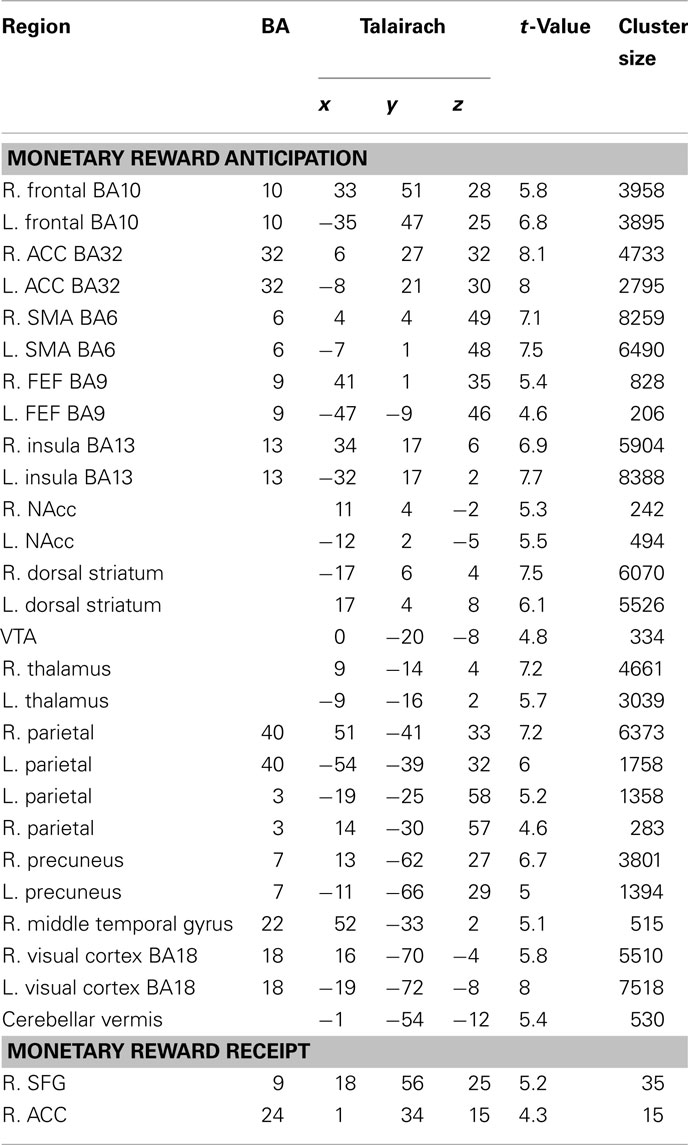
Table 1. Regions exhibiting significant parametric activation in response to anticipation and receipt of monetary rewards (p < 0.001, cluster corrected p < 0.05).
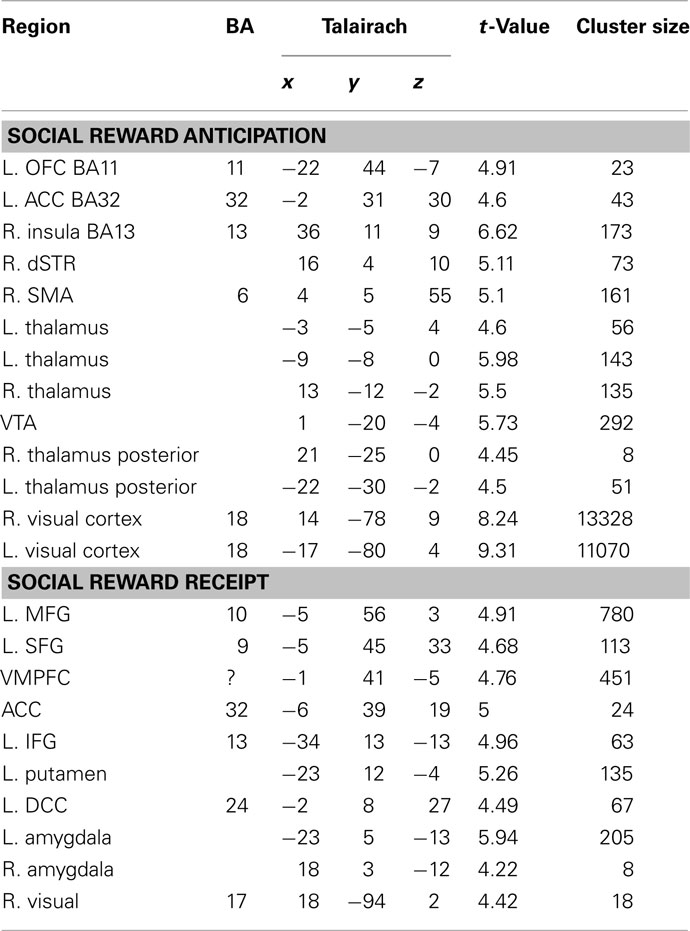
Table 2. Regions exhibiting significant parametric activation in response to anticipation and receipt of social rewards (p < 0.001, cluster corrected p < 0.05).
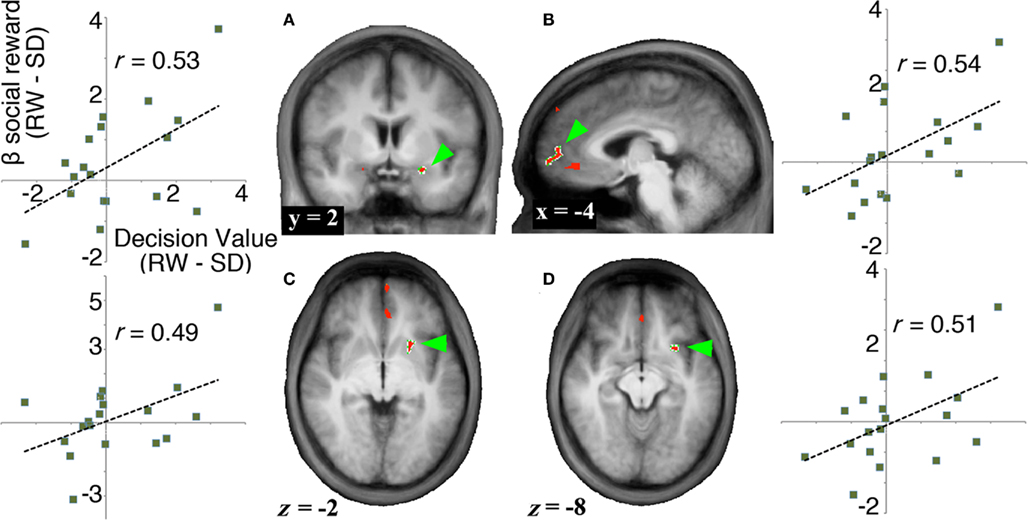
Figure 4. Neural signals associated with the receipt of social rewards. To identify regions related to the SD-induced change in exchange rate, we searched for areas that (1) showed a parametric increase in activation in response to rewards, and (2) showed a significant correlation between change in decision value signals in VMPFC (RW–SD) and change in activation in response to rewards (RW–SD). (A–D) Regions that show both a parametric increase in response to increasing social rewards and a significant correlation between change in decision value signals in VMPFC and change in activation associated with social rewards (no regions were identified for monetary rewards). We show activation maps of the conjunction of regions showing significant parametric increase to social rewards (p < 0.001, cluster corrected to p < 0.05) and regions showing significant correlation with decision value signals in VMPFC across participants (p < 0.05) and plots of each subject’s state-related shift in decision value signals in VMPFC (x axis) versus difference in BOLD signal to social reward (y axis).
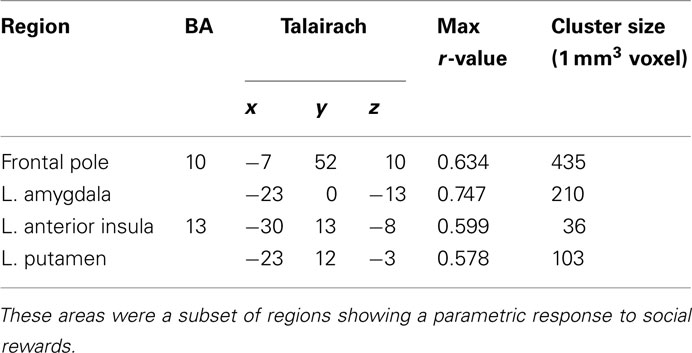
Table 3. Regions exhibiting significant correlation between change in response to social rewards and change in decision value signals in VMPFC.
In contrast, we found no significant correlation between SD-related change in monetary reward signals and the corresponding alteration of decision value signals in VMPFC. There were also no significant associations in the corresponding analyses related to the anticipation of social or monetary rewards.
Discussion
We found that SD alters decision value signals in VMPFC in a manner consistent with state-dependent changes in economic preference. This alteration in decision value signals appears to be driven by neural changes in processes underlying the valuation of the social rewards in this task. Finally, we found that these effects of SD on economic preferences were uncorrelated with its effects on vigilance.
SD Affects Decision Value and Social Reward Signals
Previous studies have suggested that, during decision making, the values of different actions under consideration are converted into signals that can be compared within the VMPFC to select the action with the highest overall benefit (Rangel and Hare, 2010; Smith and Huettel, 2010). The critical evidence in support of this conjecture – previously absent from the literature – would be the manipulation of a participant’s state in a manner that causes idiosyncratic changes in valuation, then leads to concomitant changes in VMPFC activation. Such a result would be consistent with the idea that VMPFC signals are associated with constructed preferences. We found such evidence in the current study: a significant correlation between SD-related shift in behavioral measures of decision value and their corresponding BOLD signals in the VMPFC (Figures 3A,C).
We next tested the hypothesis that the observed change in decision value signals during SD originates from altered valuation of different goods. Previous studies have shown that monetary and social rewards are processed in separate, but overlapping brain regions (Spreckelmeyer et al., 2009; Rademacher et al., 2010; Smith et al., 2010). Changes in decision value signals in VMPFC could thus originate in brain regions involved in assigning value to the different rewards under consideration. Altered inputs from these regions would then be integrated in the VMPFC, resulting in shifts in signals that reflect the observed shifts in neural valuation signals. In support of this line of reasoning, we found that changes in decision value signals in VMPFC correlated with changes in signals elicited by social rewards during the incentive delay task in the amygdala, frontal pole, left putamen, and anterior insula. These structures have been proposed to belong to a network supporting motivation and reward processing (Craig, 2009; Gujar et al., 2011). Of particular interest is the result observed in the amygdala, given its known role in social reward processing as well as its interactions with VMPFC (Price, 2003; Yoo et al., 2007).
One limitation of this study concerns the generalizability of the results. It is possible that other types of reward, such as food or sex, could be differently affected by SD. Furthermore, the observed lack of effect of SD on monetary rewards could be dependent on context as well as reward attributes, such as the magnitude, uncertainty, or delay of the offers.
The Neural Correlates of Monetary and Social Reward Valuation
While we observed activation associated with valuation of monetary and social rewards consistent with prior studies (Aharon et al., 2001; Knutson et al., 2001; O’Doherty et al., 2003; Winston et al., 2007; Rademacher et al., 2010; Smith et al., 2010), we observed ventral striatal activation only during anticipation of monetary rewards, not during anticipation of social rewards. This dissociation might be the product of differences in the rewarding value of the social stimuli used in this study compared to those used in previous studies. Although viewing attractive faces of the opposite sex serves as a social reward (Aharon et al., 2001; O’Doherty et al., 2003; Hayden et al., 2007; Winston et al., 2007; Smith et al., 2010), other types of social interactions may be even more potent. As examples, both receiving social approval signals (Spreckelmeyer et al., 2009; Davey et al., 2010; Rademacher et al., 2010) and donating money to charities (Harbaugh et al., 2007; Carter et al., 2009) have been shown to activate the ventral striatum.
An alternative explanation is that mixing social and monetary rewards within the same run affects the signals associated with social rewards. Social rewards used in this experiment were lower-valued than monetary reward as evidenced by the relatively slower RTs elicited by social rewards. As social and monetary rewards were intermixed in the same runs, the certain anticipation of receiving a (relatively lower-valued) social reward as opposed to monetary reward might have in turn attenuated the value of social cues (Nieuwenhuis et al., 2005; Seymour and McClure, 2008), leading to a different result from that reported when social and monetary rewards appeared in separate runs (Rademacher et al., 2010).
Inter-Individual Differences in the Effects of SD on Different Facets of Behavior
Previous studies have shown the utility of fMRI in augmenting such behavioral assessments of inter-individual variation in vulnerability to SD (Venkatraman et al., 2007). Correlations between behavioral and neural changes under SD have been found in studies involving working memory (Bell-McGinty et al., 2004; Habeck et al., 2004; Lim et al., 2007), visual short-term memory (Chee and Chuah, 2007), episodic memory (Chuah et al., 2009), and selective attention (Chee et al., 2010).
Here, the correlation between state-related alterations in exchange rate and fMRI signal in the amygdala, frontal pole, dorsal striatum, and anterior insula is in agreement with prior work concerning the generation of reward value signals (Baxter and Murray, 2002; O’Doherty et al., 2003; Winston et al., 2007) adding to the neuroanatomical markers that can be used to augment behavioral assessment of state-induced alteration of behavior.
Following SD participants showed individual differences in their willingness to exchange money to view attractive faces. Across-individuals variation in the manner in which SD alters behavior would be of considerable practical relevance (e.g., to determine relative suitability for tasks requiring extreme durations of sustained wakefulness). Previous work has shown that the inter-individual differences in the effects of SD on attention, executive function, and speed of processing are not random, but rather trait-like (Leproult et al., 2003; Van Dongen et al., 2004). Whether the effects of SD on social reward valuation are trait-like remains to be determined, but we did replicate the observation that decision making signals and behavior are altered by SD in a manner that is not correlated with changes in vigilance (Venkatraman et al., 2011). These two independently obtained results warn that a single test in sleep deprived persons is unlikely to predict how SD alters behavior in all domains. As a real-world example, a person who appears to be an ideal operative for all-night missions cannot be assumed to make the same kind of value judgments while sleep deprived as when fully rested.
Conclusion
We have shown that the VMPFC, a region that integrates valuation signals for different rewards into a common scale, demonstrates a shift in activation commensurate with shift in behavior across states. This strengthens the notion that VMPFC is involved in value comparisons during economic decision making independent of state. Additionally, regions involved in social reward valuation, such as the amygdala, alter their responses to social rewards during SD in a manner that is correlated with the shift in decision value signals in VMPFC. This suggests that the changes in VMPFC activation could be the result of state-related alteration of inputs from the amygdala and other regions mediating affective processes.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Annette Chen, Shuwei Koh, and Jack De Havas for collecting data. This work was supported by the Defense Science and Technology Agency, Singapore (POD 00713897), by National Research Foundation STaR Award (0004/2008), by an Incubator Award from the Duke Institute for Brain Sciences (Scott A. Huettel), by NIMH RC1-088680 (Scott A. Huettel), and by NIMH National Research Service Award F31-086248 (David V. Smith).
References
Aharon, I., Etcoff, N., Ariely, D., Chabris, C. F., O’Connor, E., and Breiter, H. C. (2001). Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron 32, 537–551.
Akerstedt, T., and Gillberg, M. (1990). Subjective and objective sleepiness in the active individual. Int. J. Neurosci. 52, 29–37.
Bell-McGinty, S., Habeck, C., Hilton, H. J., Rakitin, B., Scarmeas, N., Zarahn, E., Flynn, J., DeLaPaz, R., Basner, R., and Stern, Y. (2004). Identification and differential vulnerability of a neural network in sleep deprivation. Cereb. Cortex 14, 496–502.
Bickel, W. K., Hughes, J. R., DeGrandpre, R. J., Higgins, S. T., and Rizzuto, P. (1992). Behavioral economics of drug self-administration. IV. The effects of response requirement on the consumption of and interaction between concurrently available coffee and cigarettes. Psychopharmacology (Berl.) 107, 211–216.
Carter, R. M., Macinnes, J. J., Huettel, S. A., and Adcock, R. A. (2009). Activation in the VTA and nucleus accumbens increases in anticipation of both gains and losses. Front. Behav. Neurosci. 3:21. doi: 10.3389/neuro.08.021.2009
Chee, M. W. L., and Chuah, Y. M. L. (2007). Functional neuroimaging and behavioral correlates of capacity decline in visual short-term memory after sleep deprivation. Proc. Natl. Acad. Sci. U.S.A. 104, 9487–9492.
Chee, M. W. L., Tan, J. C., Parimal, S., and Zagorodnov, V. (2010). Sleep deprivation and its effects on object-selective attention. Neuroimage 49, 1903–1910.
Chib, V. S., Rangel, A., Shimojo, S., and O’Doherty, J. P. (2009). Evidence for a common representation of decision values for dissimilar goods in human ventromedial prefrontal cortex. J. Neurosci. 29, 12315–12320.
Chuah, L. Y. M., Chong, D. L., Chen, A. K., Rekshan, W. R. III, Tan, J. C., Zheng, H., and Chee, M. W. (2009). Donepezil improves episodic memory in young individuals vulnerable to the effects of sleep deprivation. Sleep 32, 999–1010.
Clithero, J. A., Reeck, C., Carter, R. M., Smith, D. V., and Huettel, S. A. (2011). Nucleus accumbens mediates relative motivation for rewards in the absence of choice. Front. Hum. Neurosci. 5:87. doi: 10.3389/fnhum.2011.00087
Craig, A. D. (2009). How do you feel – now? The anterior insula and human awareness. Nat. Rev. Neurosci. 10, 59–70.
Davey, C. G., Allen, N. B., Harrison, B. J., Dwyer, D. B., and Yücel, M. (2010). Being liked activates primary reward and midline self-related brain regions. Hum. Brain Mapp. 31, 660–668.
Dinges, D. F., Pack, F., Williams, K., Gillen, K. A., Powell, J. W., Ott, G. E., Aptowicz, C., and Pack, A. I. (1997). Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4-5 hours per night. Sleep 20, 267–277.
Goebel, R., Esposito, F., and Formisano, E. (2006). Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: from single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Hum. Brain Mapp. 27, 392–401.
Goel, N., Rao, H., Durmer, J. S., and Dinges, D. F. (2009). Neurocognitive consequences of sleep deprivation. Semin. Neurol. 29, 320–339.
Gujar, N., Yoo, S. S., Hu, P., and Walker, M. P. (2011). Sleep deprivation amplifies reactivity of brain reward networks, biasing the appraisal of positive emotional experiences. J. Neurosci. 31, 4466–4474.
Habeck, C., Rakitin, B. C., Moeller, J., Scarmeas, N., Zarahn, E., Brown, T., and Stern, Y. (2004). An event-related fMRI study of the neurobehavioral impact of sleep deprivation on performance of a delayed-match-to-sample task. Brain Res. Cogn. Brain Res. 18, 306–321.
Harbaugh, W. T., Mayr, U., and Burghart, D. R. (2007). Neural responses to taxation and voluntary giving reveal motives for charitable donations. Science 316, 1622–1625.
Harrison, Y., and Horne, J. A. (1999). One night of sleep loss impairs innovative thinking and flexible decision making. Organ. Behav. Hum. Decis. Process. 78, 128–145.
Hayden, B. Y., Parikh, P. C., Deaner, R. O., and Platt, M. L. (2007). Economic principles motivating social attention in humans. Proc. Biol. Sci. 274, 1751–1756.
Izuma, K., Saito, D. N., and Sadato, N. (2008). Processing of social and monetary rewards in the human striatum. Neuron 58, 284–294.
Killgore, W. D., Grugle, N. L., Reichardt, R. M., Killgore, D. B., and Balkin, T. J. (2009). Executive functions and the ability to sustain vigilance during sleep loss. Aviat. Space Environ. Med. 80, 81–87.
Killgore, W. D., Kamimori, G. H., and Balkin, T. J. (2011). Caffeine protects against increased risk-taking propensity during severe sleep deprivation. J. Sleep Res. 20, 395–403.
Killgore, W. D. S., Balkin, T. J., and Wesensten, N. J. (2006). Impaired decision making following 49 h of sleep deprivation. J. Sleep Res. 15, 7–13.
Kim, H., Shimojo, S., and O’Doherty, J. P. (2010). Overlapping responses for the expectation of juice and money rewards in human ventromedial prefrontal cortex. Cereb. Cortex 21, 769–776.
Knutson, B., Fong, G. W., Adams, C. M., Varner, J. L., and Hommer, D. (2001). Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport 12, 3683–3687.
Lebreton, M., Jorge, S., Michel, V., Thirion, B., and Pessiglione, M. (2009). An automatic valuation system in the human brain: evidence from functional neuroimaging. Neuron 64, 431–439.
Leproult, R., Colecchia, E. F., Berardi, A. M., Stickgold, R., Kosslyn, S. M., and Van Cauter, E. (2003). Individual differences in subjective and objective alertness during sleep deprivation are stable and unrelated. Am. J. Physiol. Regul. Integr. Comp. Physiol. 284, R280–R290.
Lim, J., Choo, W. C., and Chee, M. W. (2007). Reproducibility of changes in behaviour and fMRI activation associated with sleep deprivation in a working memory task. Sleep 30, 61–70.
Lim, J., and Dinges, D. F. (2010). A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychol. Bull. 136, 375–389.
Lin, A., Adolphs, R., and Rangel, A. (2011). Social and monetary reward learning engage overlapping neural substrates. Soc. Cogn. Affect. Neurosci. doi: 10.1093/scan/nsr006. [Epub ahead of print].
McKenna, B. S., Dickinson, D. L., Orff, H. J., and Drummond, S. P. (2007). The effects of one night of sleep deprivation on known-risk and ambiguous-risk decisions. J. Sleep Res. 16, 245–252.
Montague, P. R., and Berns, G. S. (2002). Neural economics and the biological substrates of valuation. Neuron 36, 265–284.
Nieuwenhuis, S., Heslenfeld, D. J., von Geusau, N. J., Mars, R. B., Holroyd, C. B., and Yeung, N. (2005). Activity in human reward-sensitive brain areas is strongly context dependent. Neuroimage 25, 1302–1309.
O’Doherty, J., Winston, J., Critchley, H., Perrett, D., Burt, D. M., and Dolan, R. J. (2003). Beauty in a smile: the role of medial orbitofrontal cortex in facial attractiveness. Neuropsychologia 41, 147–155.
Padoa-Schioppa, C. (2009). Range-adapting representation of economic value in the orbitofrontal cortex. J. Neurosci. 29, 14004–14014.
Padoa-Schioppa, C., and Assad, J. A. (2006). Neurons in the orbitofrontal cortex encode economic value. Nature 441, 223–226.
Padoa-Schioppa, C., and Assad, J. A. (2008). The representation of economic value in the orbitofrontal cortex is invariant for changes of menu. Nat. Neurosci. 11, 95–102.
Price, J. L. (2003). Comparative aspects of amygdala connectivity. Ann. N. Y. Acad. Sci. 985, 50–58.
Rademacher, L., Krach, S., Kohls, G., Irmak, A., Gründer, G., and Spreckelmeyer, K. N. (2010). Dissociation of neural networks for anticipation and consumption of monetary and social rewards. Neuroimage 49, 3276–3285.
Rangel, A., and Hare, T. (2010). Neural computations associated with goal-directed choice. Curr. Opin. Neurobiol. 20, 262–270.
Seymour, B., and McClure, S. M. (2008). Anchors, scales and the relative coding of value in the brain. Curr. Opin. Neurobiol. 18, 173–178.
Smith, D. V., Hayden, B. Y., Truong, T. K., Song, A. W., Platt, M. L., and Huettel, S. A. (2010). Distinct value signals in anterior and posterior ventromedial prefrontal cortex. J. Neurosci. 30, 2490–2495.
Smith, D. V., and Huettel, S. A. (2010). Decision neuroscience: neuroeconomics. Wiley Interdiscip. Rev. Cogn. Sci. 1, 854–871.
Spreckelmeyer, K. N., Krach, S., Kohls, G., Rademacher, L., Irmak, A., Konrad, K., Kircher, T., and Gründer, G. (2009). Anticipation of monetary and social reward differently activates mesolimbic brain structures in men and women. Soc. Cogn. Affect. Neurosci. 4, 158–165.
Tusche, A., Bode, S., and Haynes, J. D. (2010). Neural responses to unattended products predict later consumer choices. J. Neurosci. 30, 8024–8031.
Van Dongen, H. P. A., Baynard, M. D., Maislin, G., and Dinges, D. F. (2004). Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability. Sleep 27, 423–433.
Venkatraman, V., Chuah, Y. M. L., Huettel, S. A., and Chee, M. W. (2007). Sleep deprivation elevates expectation of gains and attenuates response to losses following risky decisions. Sleep 30, 603–609.
Venkatraman, V., Huettel, S. A., Chuah, L. Y., Payne, J. W., and Chee, M. W. (2011). Sleep deprivation biases the neural mechanisms underlying economic preferences. J. Neurosci. 31, 3712–3718.
Walker, M. P. (2009). The role of sleep in cognition and emotion. Ann. N. Y. Acad. Sci. 1156, 168–197.
Winston, J. S., O’Doherty, J., Kilner, J. M., Perrett, D. I., and Dolan, R. J. (2007). Brain systems for assessing facial attractiveness. Neuropsychologia 45, 195–206.
Keywords: sleep deprivation, decision making, reward, valuation, VMPFC
Citation: Libedinsky C, Smith DV, Teng CS, Namburi P, Chen VW, Huettel SA and Chee MWL (2011) Sleep deprivation alters valuation signals in the ventromedial prefrontal cortex. Front. Behav. Neurosci. 5:70. doi: 10.3389/fnbeh.2011.00070
Received: 05 August 2011; Accepted: 03 October 2011;
Published online: 24 October 2011.
Edited by:
David M. Diamond, University of South Florida, USAReviewed by:
Francesca Cirulli, Istituto Superiore di Sanità, ItalyPhillip R. Zoladz, Ohio Northern University, USA
William Killgore, McLean Hospital, USA
Copyright: © 2011 Libedinsky, Smith, Teng, Namburi, Chen, Huettel and Chee. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: Michael W. L. Chee, Duke-NUS Graduate Medical School, 8 College Road, #06-18, Singapore 169857, Singapore. e-mail:bWljaGFlbC5jaGVlQGR1a2UtbnVzLmVkdS5zZw==



 Praneeth Namburi1
Praneeth Namburi1
