- 1Faculty of Medicine and Medical Sciences, University of Balamand, El Koura, Lebanon
- 2Department of Psychology, Faculty of Arts and Sciences, University of Balamand, El Koura, Lebanon
Homosexuality is an intricate and multifactorial phenomenon affected by the interaction of biological, genetic, neurological and environmental factors. This paper examines the interplay of homosexuality determinants. Biological determinants such as the role of androgen levels, the fraternal birth order effect and maternal immune response contribute to shaping sexual orientation. Additionally, genetic influences are also assessed. These include the potential role of X chromosome, the possible link of fragile X mental retardation neighbor gene (FMR1) to sexual orientation, the function of genetic variants such as COMT an MTHFR, as well as connection with chromosomes 7, 8, 13 and 14. Furthermore, neurologic factors such as the role of the hypothalamus are assessed to highlight their contribution to sexual preference and attraction mediation. Lastly, childhood gender nonconformity and early exposure to traumatic events are among the environmental influences that contribute to the development of homosexuality. By incorporating various perspectives, this paper seeks to present a thorough overview of the multiple factors influencing sexual orientation, while emphasizing the importance of ongoing interdisciplinary research in this area.
1 Introduction
Human Sexual behavior is complex practice that depends on the processing of sexual stimuli that allow individuals to enter the sexual cycle. From an evolutionary perspective, this behavior is fundamental, supporting reproductive interactions that are vital for biological adaptation and species survival (Calabrò et al., 2019). The regulation of sexual behavior involves both subcortical structures, including the hypothalamus, brainstem, and spinal cord, as well as various cortical brain areas that orchestrate together to finely tune this primitive yet complex behavior. Central to this regulation are the dopaminergic and serotonergic systems, which play significant roles in different aspects of sexual response, although adrenergic, cholinergic, and other neuropeptide transmitter systems may also contribute (Calabrò et al., 2019). In humans, sexual behavior should be viewed as a pleasure-seeking impulse that can be appropriately controlled under the influence of cultural factors such as morality and ethics (Calabrò et al., 2019).
Sexual orientation refers to the enduring sexual attraction towards individuals of the opposite sex (heterosexuality), the same sex (homosexuality), both sexes (bisexuality), or a lack of interest in either sex (asexuality) following maturity (Rosario and Schrimshaw, 2014). It is distinct from related concepts like sexual partner preference, gender identity, and sexual behavior (Rosario et al., 2006). Sexual partner preference, which is highly sexually dimorphic, describes an animal’s sexual attraction to a partner of either the same or opposite sex when given a choice. Gender identity is the self-perception of being male, female, both, or neither, while sexual behavior refers to the actual sexual interactions an individual engages in (Bailey et al., 2016; Bailey and Zuk, 2009). Gender identity is an individual’s deeply held internal sense of being female, male, neither, both, or fluid, which may not be outwardly visible and may not align with the sex assigned at birth. It can evolve and change over a person’s life (Ervin et al., 2023). While sexual orientation and gender identity are specific to humans, sexual partner preference and behavior are observed in both humans and other animals.
Sexual orientation, which refers to predisposed sexual behavior, constitutes a fundamental aspect of individual sexual identity (Rahman, 2005a, 2005b). It encompasses enduring patterns of emotional, romantic, and/or sexual attractions to either men, women, or both genders (Roselli, 2018). Research interest in understanding the etiology of male homosexuality has increased significantly over the past decade (Qin et al., 2018).
Homosexuality, defined as the romantic and sexual attraction to individuals of the same gender, has undergone linguistic evolution since its initial introduction in 1868 within the field of sexology (Herek and Garnets, 2007). The term “lesbian,” tracing its roots back to the Greek poet Sappho from the island of Lesbos, similarly underwent linguistic shifts during the 19th century (Duden, 1989). In contrast, the term “gay,” derived from Old French (“gai”) and Germanic languages, has a longer history dating back to the 13th century (Collins, 1995).
Twin studies and segregation analyses have revealed that homosexuality is influenced by a complex interplay of biological, genetic, neurological and environmental factors (Hu et al., 2021; Kendler et al., 2000; Bailey and Pillard, 1991). Genetic examinations rooted in behavioral sciences have provided substantial evidence for the heritability of sexual orientation, estimating genetic influence at approximately 32% (Sanders et al., 2021; Bailey et al., 2016). Multiple biological factors contribute to the occurrence of homosexuality, including prenatal sex hormones, particularly testosterone, which shapes behavioral sex differences across various species (Roselli, 2018). Neural circuitry present during early fetal development is also believed to play a role in the continuum of sexual orientations (Rahman, 2005a, 2005b). Fraternal birth order has been linked to male sexual orientation, possibly due to maternal immunization processes affecting sexual differentiation in fetal life and differing social environments experienced by younger brothers (Rahman, 2005a, 2005b). Additionally, progressive immunization of some mothers to male-specific antigens with each successive male fetus may impact brain sexual differentiation, potentially influencing sexual orientation (Gavrilets and Rice, 2006). Social experiences and sociocultural factors also contribute to the development of homosexuality (Hu et al., 2021; Kendler et al., 2000; Bailey and Pillard, 1991). Psychoanalytic theories further underscore the role of family experiences in shaping same-sex behavior (Qin et al., 2018; Friedman, 1988). Despite all these studies, mechanisms underlying homosexuality remain poorly understood.
In this review, we aim to develop the biological, genetic, neurological and environmental factors that contribute to the development of homosexuality. To our knowledge, this is the first review in the middle east region that discusses this topic.
2 Materials and methods
This review was developed using a narrative approach to synthesize existing literature on the biological, genetic, neurological, and environmental factors influencing homosexuality. A thorough search was performed across three major databases: PubMed, Google Scholar, and ScienceDirect. The search strategy utilized a combination of keywords and MeSH terms, including: homosexuality, sexual orientation, LGBT, biological influences, genetic markers, neurological mechanisms, environmental contributors, prenatal hormones, COMT, MTHFR, fraternal birth order, and gender nonconformity. A total of 63 articles were reviewed in this narrative synthesis. Of these, 17 addressed biological factors, 17 examined genetic influences, 15 explored neurological mechanisms, and 14 investigated environmental determinants. The search focused on studies published in English from 1980 to 2024, encompassing both human and relevant animal research. Inclusion criteria were based on the relevance of the study to at least one of the four domains examined in this review. Priority was given to peer-reviewed empirical studies, genome-wide association studies (GWAS), neuroimaging analyses, twin studies, and systematic reviews. Additional articles were identified by manually reviewing the reference lists of key sources. Although no formal quality assessment tool was applied, studies were selected based on methodological soundness and their contribution to understanding the multifactorial nature of sexual orientation.
3 Influencing factors on homosexuality
3.1 Biological factors
3.1.1 The effect of androgen levels
3.1.1.1 In females
The theory of prenatal androgens suggests that gonadal androgens play a significant role in determining the human sexual orientation. The conducted research explains the different observations in brain structure and behavior between vertebrates (Morris et al., 2004). This theory is supported by preclinical (animals) and clinical studies (Humans).
3.1.1.1.1 Pre-clinical studies
The theory of prenatal androgens received initial backing from experiments involving the manipulation of prenatal sex hormones in animals. In mammals, hormones from the testes, such as androgens or estrogens derived from androgens, influence male differentiation during prenatal or early postnatal stages. These hormones can enhance male-typical behaviors, like mounting, or reduce female-typical behaviors, like lordosis, or both. The significance of masculinization versus defeminization in male development varies by species and depends on whether male or female behaviors are more distinctly different (Baum, 1979). In the absence of testicular hormones, female differentiation occurs, suggesting that ovaries are not essential for developing female behaviors. Experimental evidence shows that females exposed to testosterone or estradiol early in development exhibit male-like behaviors such as increased mounting after testosterone priming and decreased receptivity after estradiol or a combination of estradiol and progesterone priming. Conversely, males that are castrated early develop female-like characteristics, showing reduced mounting and heightened receptivity after appropriate hormone treatments. These effects from early hormonal interventions are permanent and typically occur during a critical developmental period, leading to their classification as “organisational” effects, in contrast to the temporary “activational” effects of the hormones given in adults. Research on the organisational effects of sex hormones on mating behavior has shown that male-typical and female-typical behaviors are distinct dimensions. It is possible for masculinization to occur without defeminization and vice versa (Feder, 1981).
3.1.1.1.2 Clinical studies
In one study, Manning (2002) concluded that elevated levels of prenatal steroid hormones may play a role in the sexual orientation of “butch lesbians.” A “butch lesbian” is a woman who identifies as a lesbian and expresses her gender in a masculine way. This often means she adopts traditionally masculine characteristics in how she looks and behaves. Research shows this is a unique form of female masculinity found within lesbian communities (Rosario et al., 2011). Retrospective indicators of prenatal androgen exposure, such as digit ratios suggest that, on average, lesbians experienced higher levels of prenatal androgens compared to straight women (Breedlove, 2017). Specifically, lesbians that identify as “butch” have more masculine digit ratios compared to those identifying as “femme” suggesting an even higher exposure to prenatal hormones (Brown et al., 2002a). In simple terms, a “femme” is a lesbian woman who expresses her gender in a way that’s traditionally seen as feminine. This often means she enjoys presenting with things like dresses, makeup, and longer hairstyles, which are usually associated with femininity (Maltry and Tucker, 2002). Although digit ratios and long-bone development are frequently used as indicators of prenatal androgen exposure, these measures are indirect and may be influenced by a range of postnatal or ethnic factors. Furthermore, the available evidence is largely correlational and retrospective in nature, which limits the strength of any causal inferences drawn from these associations.
The findings are backed by information about the frequency of play behaviors typically associated with males, decreased contentment with being assigned a female sex at birth, diminished interest in heterosexual activities during childhood and adolescence, and eventual engagement in lesbian relationships among women diagnosed with congenital adrenal hyperplasia (e.g., Money et al., 1984; Hines et al., 2004). Singh et al. (1999) found that self-identified “butch lesbians” recalled exhibiting more childhood behaviors not conforming to traditional gender roles such as playing with boy’s toys instead of girl’s toys. This possibly suggests that they might not see themselves as females but rather as males. They also expressed less desire to have children and had higher waist-to-hip ratios and testosterone levels compared to self-identified “femmes” (Pearcey et al., 1996). Research has identified a link between elevated androgen levels and increased waist-to-hip ratio (WHR) in various groups, including postmenopausal women, women with androgen excess, obese adolescent girls, female-to-male transgender individuals, and women of different sexual orientations. Evidence from female-to-male transgender individuals indicates that testosterone treatment can lead to an increase in abdominal visceral fat without hip fat deposition, resulting in a higher WHR proving that a high WHR is a male characteristic related to a high androgen state (Van Anders and Hampson, 2005). However, the cellular mechanisms by which androgens influence fat distribution and elevate WHR remain poorly understood (Van Anders and Hampson, 2005).
“Proxy markers” for prenatal hormonal exposure have been researched. These are non-invasively explored in the endocrinologically normal population and they consist of somatic features that are understood to be shaped by prenatal exposure to sex hormones. Morris et al. (2004) pointed out that there are three indicators that are believed to indicate the presence of fetal androgens in lesbians that have been more exposed to them in utero than straight women. The indicators are: (1) click-evoked otoacoustic emissions (McFadden and Pasanen, 1998); (2) eye blink reflexes (Rahman et al., 2003a, 2003b, 2003c); and (3) finger length ratios. Therefore, demonstrating differences in these characteristics among adult heterosexuals and homosexuals could offer insight into the early development of sexual preferences influenced by prenatal hormones.
Click-evoked otoacoustic emissions, known as CEOAEs, are echo-like waveforms emitted by cochlea of individuals with normal hearing in response to a brief stimulus. Research indicates that CEOAEs tend to be more pronounced in females compared to males. The CEOAEs of homosexual and bisexual females were intermediate between those of heterosexual females and males. This suggests that the auditory systems and relevant brain structures related to sexual orientation in homosexual and bisexual females may have undergone partial masculinization due to prenatal exposure to elevated androgen levels. Interestingly, no distinction in CEOAEs was noted between homosexual and heterosexual males (McFadden and Pasanen, 1998).
Prepulse inhibition (PPI) denotes a decrease in the startle response triggered by a strong sensory stimulus when preceded by a weaker stimulus, known as the prepulse. It reflects an innate mechanism for regulating sensorimotor functions and exhibits a noticeable gender contrast, with women typically demonstrating lower PPI compared to men. The eyeblink startle responses to auditory stimuli in 59 healthy individuals, encompassing both heterosexual and homosexual men and women revealed that homosexual women displayed a notably more masculinized pattern of PPI compared to heterosexual women, while no notable difference in PPI was observed between homosexual and heterosexual men. These findings provide evidence for within-gender variations in fundamental sensorimotor gating mechanisms and suggest involvement of established neural pathways associated with PPI in human sexual orientation (Rahman et al., 2003a, 2003b, 2003c).
One commonly used “proxy marker” of prenatal hormonal exposure is the second to fourth finger length ratio, known as the 2D:4D ratio (Manning et al., 1998). The presence of reduced ratios in individuals exposed to excess androgens, as seen in conditions like congenital adrenal hyperplasia, strongly suggests that prenatal androgens play a significant role in influencing the 2D:4D ratio (Brown et al., 2002b; Buck et al., 2003). 2D:4D is also linked to variation in the androgen receptor gene (Manning et al., 2003) and the ratio of testosterone to estrogen taken from amniotic fluid during gestation is negatively associated with 2D:4D at 2 years of age (Lutchmaya et al., 2004). Although ultimately correlational, these data strongly indicate that an overabundance of androgens can influence the relative lengths of the second and fourth finger digits. Four separate studies have demonstrated that homosexual women tend to have notably more masculine (lower) 2D:4D ratios compared to heterosexual women, although it seems that this effect is hand-specific (Rahman and Wilson, 2003a, 2003b; Williams et al., 2000; Rahman, 2005a, 2005b; McFadden and Schubel, 2002).
In 1981, Perkins reported that exclusively homosexual women categorized as ‘dominant’ were notably taller, had broader shoulders, and narrower hips compared to those categorized as ‘passive’. Inconsistent findings have emerged regarding self-reported height and weight differences related to sexual orientation (Bogaert and Friesen, 2002; Bogaert et al., 2002). However, it remains uncertain whether these variations are primarily due to prenatal sex steroids or a combination of postnatal factors. One recent study has found that homosexual women have more long-bone growth in the arms, legs and hands compared to heterosexual women (Martin and Nguyen, 2004). During childhood, certain bone characteristics display sexual differences in homosexual individuals, but this distinction diminishes after puberty. These findings imply that homosexual women exhibit partial masculinization in specific anthropometric measures prior to the increase in sex steroid levels during puberty (Martin and Nguyen, 2004).
Similarly, Over-exposure to prenatal androgens leads to homosexuality in women (Ellis and Ames, 1987). Moreover, this classic model of the origins of sexual orientation is supported from the observation of differing sexual orientations in cases of human intersex individuals or those with endocrine disorders like congenital adrenal hyperplasia, which align with the idea of hormonal exposure during prenatal development (Bailey, 2003; Morris et al., 2004). Congenital adrenal hyperplasia (CAH) is a genetic disorder affecting the steroid-producing enzymes in the adrenal cortex, resulting with excess androgens. Depending on the presence of androgens, the fetal brain can develop in a typically male direction suggesting that aspects of sexual development, including sexual orientation, may be influenced by brain structure programming during prenatal development (Daae et al., 2020). Systematic reviews investigated sexual orientation in females with CAH. A review included a sample of 927 assigned females at birth. Twelve studies with control groups indicated that the prevalence of non-heterosexual orientation was greater among assigned females with CAH compared to the control group (Money et al., 1984; Dittmann et al., 1992; Kuhnle et al., 1993; Zucker et al., 1996; Hines et al., 2004; Johannsen et al., 2006; Meyer-Bahlburg et al., 2008; Frisen et al., 2009; Fagerholm et al., 2011; Lesma et al., 2014; Binet et al., 2016; Khorashad et al., 2017).
All research shows that homosexual women are exposed to more prenatal androgens compared to heterosexual women; it was also observed that elevated levels of prenatal estrogen also have been linked to the development of lesbian orientation (Meyer-Bahlburg et al., 1995; Collaer and Hines, 1995).
3.1.1.2 In males
There is increasing evidence suggesting that certain genetic factors contribute to the sexual orientation of some homosexual men. Under-exposure to prenatal androgens leads to homosexuality in men, however, bisexual males are thought to have been exposed to increased prenatal testosterone levels. On average, bisexual men are reported to have a younger age of first sexual experience, tend to be taller than heterosexual men (Bogaert and Friesen, 2002), have a higher sex drive than other men on the average (Comings, 1994) and are more likely to participate in sexual activities with higher levels of risk than control homosexuals or heterosexuals (Doll and Beeker, 1996) with the latter being associated with higher testosterone levels (Zuckerman, 1994). As mentioned above with lesbians, finger-length ratio and higher uterine testosterone levels exposure are seen in bisexual men as well as higher testosterone levels in infancy, childhood, adolescence, and adulthood. Research also found that homosexual men reach puberty earlier than heterosexual men (Bogaert et al., 2002); during a period when males are still predominantly interacting with each other, making it more probable for them to associate their sexual awakening with males rather than females (Storms, 1981; Wyre, 1990). One commonly used “proxy marker” of prenatal hormonal exposure is 2D:4D ratio (Manning et al., 1998). According to this ratio, bisexual males have lower ratios than females (Rahman and Wilson, 2003a, 2003b; Williams et al., 2000; Rahman, 2005a, 2005b; McFadden and Schubel, 2002). One study has found that homosexual men have less long-bone growth in the arms, legs and hands compared to heterosexual men (Martin and Nguyen, 2004). During childhood, certain bone characteristics display sexual differences in homosexual individuals, but this distinction diminishes after puberty. These findings imply that homosexual men exhibit partial feminization prior to the increase in sex steroid levels during puberty (Martin and Nguyen, 2004). Research shows that homosexual men exhibit mosaic traits ranging from sex-typical to sex-atypical and other sex-exaggerated factors. Differences in the timing and amount of androgen exposure, such as below-average or above-average levels, could potentially lead to this variation in heterosexual and homosexual males. For example, Geschwind and Galaburda (1985) proposed that homosexual men might experience elevated androgen levels during early development. On the other hand, homosexual men have a 34% chance of being non-right-handed compared to their heterosexual counterparts while homosexual women have a 91% chance (Lalumiere et al., 2000). Thus, a correlation between homosexuality and being left-handed has emerged. This explanation could be found in developmental instability (DI), which refers to how vulnerable an organism is to environmental and genetic stresses during its development. According to this view, same-sex orientation may result from generalized developmental challenges that push sexual preferences away from the typical pattern of opposite-sex attraction (Lalumiere et al., 2000).
3.1.2 The fraternal birth order
Epidemiological research has consistently indicated that having older brothers raises the likelihood of homosexuality in younger males (Blanchard, 1997; Blanchard and Ellis, 2001) but not in females. This is known as the “fraternal birth order (FBO) effect” (Blanchard and Ellis, 2001). The FBO effect has been observed in various homosexual individuals, including both those from typical community volunteers and those from atypical groups, who have diverse characteristics and preferences in their desired partners (Blanchard and Bogaert, 1996a, 1996b; Blanchard et al., 1998; Ellis and Blanchard, 2001; Robinson and Manning, 2000; Blanchard and Bogaert, 1997; Purcell et al., 2000; Williams et al., 2000). In males, each extra older brother raises the likelihood of homosexuality by around 33% but there no link was observed in older sisters. Nevertheless, this FBO effect explains the sexual orientation of only some gay men (Bogaert and Skorska, 2011; Blanchard and Bogaert, 1996a). By contrast, there is no variation neither in birth order nor in sibling composition between homosexual and heterosexual women (Bogaert, 1997). According to Manning (2002), approximately one in seven homosexual men associate their sexual orientation to the FBO effect (Cantor et al., 2002). The fraternal birth order effect appears to explain only a subset of male homosexuality and has not been observed in females. Additionally, reliance on self-reported familial data introduces potential recall bias. Effect sizes reported in the literature are often modest, and social or environmental variables linked to birth order are frequently not adequately controlled.
3.1.2.1 Antibodies against a Y-linked factor
The primary theory proposing an explanation for the linkage of antibodies against the Y-linked factor suggests that certain mothers produce antibodies against a Y-linked factor which is crucial for the development of the male brain. This response appears to intensify with each subsequent male pregnancy, consequently resulting in changes in the brain structures that influence the sexual orientation of later-born boys. In support of this theory, Bogaert et al. demonstrated that mothers of homosexual sons, particularly those with older brothers, have higher antibody titers to neuroligin 4 (NLGN4Y), which is an extracellular protein involved in synaptic functioning and influencing fetal brain development.
Research has shown that gay men who have older brothers tend to have lower birth weights in comparison to straight men with older brothers (Blanchard and Ellis, 2001; Blanchard et al., 2002). Researchers have suggested that the repeated exposure to male-specific antigens during successive pregnancies may lead to a gradual immune response in some mothers as the maternal immune system identifies male-related antigens as foreign and starts generating antibodies against them (Blanchard, 2004). A potential set of antigens that can play a role here are the Y-linked minor histocompatibility antigens, particularly H-Y. As an illustration, male-specific antibodies might attach to and disable receptors responsible for masculinizing fetal neurons. This action can hinder the development of male-oriented sexual preferences. Neural tissue contains a significant number of H-Y antigens, and the H-Y antigen is exclusively found in male fetuses, so the maternal immune system retains a memory of past male pregnancies and can adjust its response accordingly (Blanchard, 2004; Blanchard and Bogaert, 1996a). Various other potential antigens besides H-Y are being considered, such as unique Y-linked protein groups like protocadherin and neuroligin, both of which have been identified in humans. These cell adhesion proteins are believed to affect communication between cells in the initial development of male-specific brain morphogenesis and could result in male-typical behavioral outcomes (Blanco et al., 2000). Also, the male human brain (including the hypothalamus) has a direct transcription of Y-linked sex determination genes SRY and ZFY (Mayer et al., 1998). While the maternal immune hypothesis offers an intriguing explanation, current findings are largely correlational and supported primarily by animal models. In humans, the evidence remains limited, and individual variability in maternal immune response adds complexity to establishing a consistent and generalizable mechanism.
3.1.2.2 Pre-clinical rodent studies
These findings were supported by preclinical mice in rodents. For instance, a study conducted on mice revealed that maternal immunity to male-derived antigens can impact fetal weight (Gentile et al., 1992; Lu and Dawson, 1986). Likewise, male mice born to mothers who were immunized against H-Y before pregnancy display diminished male-typical consummatory sexual behavior when interacting with receptive females (Singh and Verma, 1987). It is unknown yet whether male-specific antibodies could potentially engage with sexual differentiation processes regulated by sex hormones or operate entirely autonomously from them. Although animal models provide valuable insights into the neurobiological underpinnings of sexual behavior, their applicability to humans is limited. Species differences in brain development and behavior, as well as the artificial nature of experimental manipulations, challenge the direct translation of findings to human sexual orientation.
3.1.2.3 Sibling sex-play
Another possible explanation for the (FBO) effect is that interactions with older brothers during critical stages of sexual development might predispose individuals to a homosexual orientation. However, research conducted by Bogaert (2000) suggests that engaging in sibling sex-play is not a significant factor linking FBO to male sexual orientation (Bogaert, 2000). Instead, it is the frequency of having older brothers, rather than the specific sexual activities, that most accurately predicts the sexual attraction aspect of one’s sexual orientation (Bogaert, 2003). Additionally, the absence of a connection between same-sex play among pairs of gay brothers and later adult homosexual attraction has been noted (Dawood et al., 2000).
3.2 Genetic components
The analysis of the development of physiological and behavioral traits should always examine genetic factors. Many family and twin studies showed evidence of genetic influence on both male and female sexual orientation. Family studies showed a higher occurrence of homosexuality among relatives of homosexual people (Bailey and Pillard, 1995). The likelihood of genetic diversity differs in a significant manner between sex chromosomes and autosomes (Manning, 2002). However, it was found that the 50% similarity in sexual orientation among identical twins is not only due to genetic factors but also to other factors such as non-genetic elements and chance. Additionally, evidence suggests an increased likelihood of maternal transmission of male homosexuality, indicating a possible connection to the X chromosome (Camperio-Ciani et al., 2004; Hamer et al., 1993). However, other research has found no correlation with paternal transmission (Bailey et al., 1999). Females have a complex transmission pattern including both autosomal and sex-linked pathways (Pattatucci and Hamer, 1995). Moderate heritability estimates were shown in twin studies in community and population samples. Genes affecting sexual orientation can easily extend and be found in different forms under many conditions as indicated by the genetic models concerning homosexuality. The idea that alleles linked to homosexuality may provide a selective advantage when they are rare, while becoming disadvantageous when common, is vital for explaining how genetic diversity is maintained. This concept is backed by theories such as frequency-dependent selection and heterozygote advantage. Frequency-dependent selection suggests that the effectiveness of a trait is contingent on its frequency; when an allele is infrequent, it could facilitate enhanced social interactions, foster cooperation, or improve support among relatives, thereby boosting their reproductive success (Sasani and Gardner, 2020; Kirkpatrick and Barton, 1997). Furthermore, the heterozygote advantage implies that individuals with one copy of a certain allele may exhibit traits like enhanced creativity or social skills, which can indirectly benefit the reproductive success of their relatives (Hamer and Copeland, 1998; Rieger et al., 2008a, 2008b). As the frequency of the allele increases, its advantages might wane due to rising competition and evolving social dynamics, which plays a role in maintaining genetic diversity associated with homosexuality in populations (Hamer, 2007). Overall, these evolutionary dynamics ensure that such alleles remain present despite their potential reproductive drawbacks.
3.2.1 The role of X chromosome
Polymorphism is generally less common on the X chromosome compared to autosomes. However, in the case of sexually antagonistic genes—those that exert different effects on males and females—the potential for polymorphism on the X chromosome becomes significantly higher, especially if these genes primarily influence masculine traits. Although this phenomenon is not fully understood, it suggests that if genes linked to homosexuality are located on the X chromosome, it would strongly support the theory of sexual antagonism over that of overdominance (Gavrilets and Rice, 2006). Sexual antagonism and overdominance are two evolutionary mechanisms that help maintain certain alleles in a population. Sexual antagonism occurs when an allele has different effects on males and females, benefiting one sex while disadvantaging the other. Overdominance, by contrast, refers to a heterozygote advantage, where individuals with one copy of an allele have greater fitness than those with two copies of either allele, preserving genetic diversity without regard to sex-specific effects. Since the X chromosome represents a small portion of the genome in most species, the presence of multiple X-linked gene loci associated with homosexuality would emphasize sexual antagonism as a key factor in maintaining genetic variation related to homosexuality.
In the study of the genetics of homosexuality, researchers have mapped male sexual orientation to the Xq28 chromosomal region using microsatellite markers. Hamer et al. (1993) identified genetic markers in this region, although later studies confined this effect to males (Hu et al., 1995). A more recent genome-wide scan, however, did not confirm the Xq28 linkage in new family samples but pointed to other regions, such as 7q36, 8p12, and 10q26, which warrant further investigation (Mustanski et al., 2005). These findings illustrate how polymorphic genes can contribute to genetic diversity and influence traits related to masculinity and femininity, potentially leading to variations in sexual preferences or behaviors.
Despite the progress made, further studies focusing on genetic markers and linkage analysis are needed to better identify the genetic factors associated with sexual behavior, which would deepen our understanding of its development and variability among individuals. The Xq28 region, for instance, includes several genes that may be relevant to sexual orientation. For example, the arginine vasopressin receptor (AVPR) 2 has been linked to social and affiliative behavior (Ebstein et al., 2012), and the cyclic nucleotide-gated channel alpha 2 (CNGA2) plays a critical role in regulating odor-evoked socio-sexual behaviors in mice (Mandiyan et al., 2005).
3.2.2 The COMT and MTHFR genetic variants
Studies have focused extensively on a very important neurotransmitter in sexual behavior which is dopamine (Liu et al., 2008; Hull et al., 2004). Dopamine (DA), also referred to as 3,4-dihydroxytyramine, is a neurotransmitter produced by dopaminergic neurons in the brain. It is synthesized through the action of the enzyme tyrosine hydroxylase, which converts tyrosine into L-DOPA by adding a hydroxyl group. L-DOPA is then decarboxylated to form DA. The vesicular monoamine transporter 2 (VMAT2) transports DA into synaptic vesicles, from which it is released into the synaptic cleft and binds to dopamine receptors (DARs). DA interacts with five receptor subtypes—D1 to D5—all of which are part of the G protein-coupled receptor (GPCR) family (Speranza et al., 2021).
Whether DA exerts an excitatory or inhibitory effect depends on the type of receptor present on the target neuron’s membrane and the cell’s response to changes in cyclic AMP (cAMP) levels. Consequently, the various roles of DA are determined by the receptor subtype, cell type, synaptic characteristics, and interactions with other neurotransmitters (Speranza et al., 2021).
Groundbreaking research in the 1970s first identified dopamine’s role in penile erection and sexual behavior (Melis et al., 2022). This was further confirmed in 1986 when it was discovered that dopamine agonists, known for inducing penile erection by activating dopamine D2 receptors in male rats, produced the same effect when microinjected into the paraventricular nucleus (PVN) of the hypothalamus (Melis et al., 1987). Additionally, dopamine was shown to enhance copulatory behavior when injected into the medial preoptic area (Hull et al., 1986). This provided the first evidence that activation of the incertohypothalamic dopaminergic system, whose neurons originate in the A13 and A14 catecholaminergic cell groups of the hypothalamus (Dahlstroem and Fuxe, 1964), plays a key role in facilitating both penile erection and sexual behavior. More recent findings indicate that other dopaminergic systems, such as the mesolimbic and mesocortical pathways, are also involved in sexual behavior. These systems, with neurons located in the ventral tegmental area and projecting to the nucleus accumbens and medial prefrontal cortex, are critical for motivational and reward processes, including those related to sexual activity (Melis et al., 2022).
When dopaminergic (DAergic) neurons are activated, synaptic vesicles release their contents into the synaptic cleft, allowing dopamine (DA) to interact with postsynaptic DA receptors or presynaptic DA autoreceptors (Werkman et al., 2006; Zhang and Sulzer, 2012). To terminate the signal, extracellular DA must be cleared from the synaptic cleft. This can occur either through reuptake by DAergic neurons for recycling or through uptake by glial cells for degradation (Meiser et al., 2013). In glial cells, DA is rapidly broken down by monoamine oxidase (MAO) and catechol-O-methyltransferase (COMT). COMT gene is located on chromosome 22, specifically at 22q11.21 (Männistö and Kaakkola, 1999). COMT transfers methyl groups from S-adenosylmethionine (SAM) to the hydroxyl groups of catecholic compounds (Männistö et al., 1992; Männistö and Kaakkola, 1999). The 3-O-methylation of DOPAC by COMT produces homovanillic acid (HVA), a primary degradation product of DA. While COMT activity is present in glial cells, it is absent in the dopaminergic nigrostriatal neurons (Myöhänen et al., 2010).
A positive relationship between dopamine levels and male–male courtship has been established in animals’ studies (Cibrian-Llanderal et al., 2012; Chen et al., 2012; Liu et al., 2009; Liu et al., 2008). Several studies support the notion that genes influencing the dopaminergic pathway, like the COMT gene involved in dopamine metabolism and the methylenetetrahydrofolate reductase (MTHFR) gene which might influence COMT function through DNA methylation, make male more susceptible to be homosexual (Männistö and Kaakkola, 1999; de Arruda et al., 2013; Friso et al., 2002).
The COMT enzyme breaks down dopamine and norepinephrine (Männistö and Kaakkola, 1999; Sesack et al., 1998). Polymorphisms in the COMT gene have a direct impact on enzyme activity and estrogen levels leading to changes in biological traits linked to dopamine and estrogen (Camara et al., 2010; Calati et al., 2011). MTHFR is an important enzyme in carbon and folate metabolism and the synthesis of DNA and proteins (Nazki et al., 2014). It is mapped on chromosome 1 at the end of the short arm (1p36.6) (Liew and Gupta, 2015). One study explored specific genetic variabilities in COMT (rs4680, rs4818, and rs6267) and MTHFR (rs1801133) associated with male sexual orientation. Certain specific genetic variations like (MTHFR rs1801133 and COMT rs4818) were found to correlate with male homosexuality especially in homozygous comparisons (T/T vs. C/C at rs1801133; G/G vs. C/C at rs4818,) (Qin et al., 2018). However, in heterozygote comparisons (G/A vs. G/G), the variation in COMT gene at rs4680 indicated a tendency towards reducing the susceptibility to male homosexuality. This underlines the importance of the influence of COMT and MTHFR in male homosexuality susceptibility (Ramagopalan et al., 2010; Mustanski et al., 2005; Melis and Argiolas, 1995; Hull et al., 2004; Cibrian-Llanderal et al., 2012; Chen et al., 2012; Liu et al., 2009).
Genetic polymorphisms in the MTHFR gene might affect COMT methylation and function (Wang et al., 2015). The interaction between the COMT and MTHFR genes may influence male homosexuality through dopamine regulation. However, according to the study by Wang et al. (2015) there was no significant interaction between the two genes affecting this trait.
The interaction between childhood abuse experiences and specific polymorphisms in the COMT and MTHFR genes can influence the development of male homosexuality.
Stress leads to the triggering of excitement by the body’s sympathetic nervous system and raises the secretion of hormones from the pituitary and adrenal glands, such as cortisol (Szyf and Bick, 2013). COMT and MTHFR genes regulate the hypothalamic–pituitary–adrenal axis (Männistö and Kaakkola, 1999; de Arruda et al., 2013; Friso et al., 2002; Sesack et al., 1998). Therefore, by affecting the body’s stress response system, it is suggested that childhood abuse experiences and variations in the COMT and MTHFR genes influence the development of male homosexuality (Andersen et al., 2014; Edwards et al., 2003).
3.2.3 The role of fragile X mental retardation 1 neighbor
Ladd et al. (2007) investigated the role of the fragile X mental retardation 1 neighbor (FMR1NB) in neuronal development and found that it interacts with proteins involved in synaptic function, suggesting its potential role in synaptic plasticity. Using bioinformatics analysis, a study by Hu et al. (2021) explored the potential association of the gene FMR1NB, with male sexual orientation via CRISPR mediated knockout mice. Rs17320865 is the primary single nucleotide polymorphism (SNP) found within the FMR1NB gene. This gene is known to affect brain function and development and has been used to understand dyslexia (Huc-Chabrolle et al., 2013; Liu et al., 2011). FMR1NB was linked previously to sexual preference. However, in this study, knock out mice in which the FMR1NB gene was absent displayed a tendency towards same-sex mounting, making a certain correlation with male sexual orientation (Hu et al., 2021).
3.2.4 The role of ZNF536 gene
Rs7259428 is a single nucleotide polymorphism (SNP) located in the intron of the ZNF536 gene, which belongs to the zinc finger protein family—a group of transcription factors. ZNF536 is predominantly expressed in the brain, particularly in the dorsal root ganglia, cerebral cortex, hippocampus, and hypothalamus, including the suprachiasmatic nucleus (SCN) (Qin et al., 2009). This gene plays a crucial role in various cellular processes, such as gene regulation, transcriptional control, and chromatin remodeling. Notably, studies have shown lower expression of ZNF536 in the SCN region of homosexual individuals compared to heterosexual individuals.
Another study revealed significant genome-wide linkage, particularly on chromosomes 5q31 and 8q24, through the initial Genome-Wide Linkage Study (GWLS) on Cognitive Genes Network (CGN) in males. However, there was no overlap between the strongest linkage peaks for CGN and the most powerful signals identified in previous GWLS studies on male sexual orientation (Sanders et al., 2015). Although CGN and sexual orientation are related, they exhibit distinct and complex genetic patterns.
In a separate study, two loci (5q31 and 10q23) reached genome-wide significance (p < 5 × 10–8) in relation to CGN. Several other regions also showed positive associations (p-values ranging from 10–6 to 10–8). The SNP cluster on 5p13 includes the Solute Carrier Family 1 Member 3 (SLC1A3) gene, while the SNP cluster on 10q23 is associated with the Glutamate Receptor Delta 1 (GRID1) gene. SLC1A3, a glutamate transporter primarily expressed in the brain, is linked to various behavioral traits, including Attention Deficit Hyperactivity Disorder (ADHD), mood disorders, and corticolimbic connectivity during affective regulation (Huang et al., 2019; Medina et al., 2016; Poletti et al., 2018; van Amen-Hellebrekers et al., 2016). Additionally, disruptions in GRID1 have been associated with emotional and social behavioral alterations (Yadav et al., 2012). The complex relationship between GRID1, SLC1A3, and homosexuality suggests that while these genes are not directly responsible for sexual orientation, they contribute to the broader genetic and neurobiological framework influencing cognitive and behavioral traits, which may include sexual orientation. Their impact on brain function and emotional regulation plays a role in shaping complex behaviors and preferences.
3.2.5 Association of homosexuality with chromosomes 7 and 8
In the first genome-wide screening study on male sexual orientation, Mustanski et al. (2005) analyzed 403 microsatellite markers spaced at 10 cM intervals across 146 families with two or more homosexual brothers. They identified a significant association between chromosome 7q36 and homosexuality, though this finding has yet to be confirmed by follow-up studies (Mustanski et al., 2005). Their findings also indicated a possible linkage to the pericentromeric region of chromosome 8 (approximately 60–90 cM, ~8p21–p11) (Mustanski et al., 2005). This locus was further supported by a genome-wide analysis of 409 independent pairs of homosexual brothers (Sanders et al., 2015) and a recent GWAS involving 1,077 homosexual men and 1,231 heterosexual men (Sanders et al., 2017). This region contains several candidate genes involved in the regulation of gonadal hormone levels. For instance, the gene GNRH1, located at 8p21, is secreted by hypothalamic neurons and stimulates the synthesis and release of LH and follicle-stimulating hormone (FSH). This gene plays a crucial role in sex-specific sexual development and behaviors (Desaulniers et al., 2017). Additionally, a gene located at 8p11.23 encodes the steroidogenic acute regulatory protein (STAR), which facilitates pregnenolone synthesis and is involved in the hypothalamic–pituitary regulation of adrenal steroid production (Sugawara et al., 1995). STAR plays a critical role in the development of congenital lipoid adrenal hyperplasia, a potentially life-threatening form of CAH (Fujieda et al., 1997; Nakae et al., 1997; Sahakitrungruang et al., 2010).
3.2.6 Association of homosexuality with chromosomes 13 and 14
The utilization of Genome-Wide Association Screening (GWAS) allowed the identification of multiple single nucleotide polymorphisms (SNPs) on chromosomes 13 and 14 with potential links to male homosexuality. More recently, research analyzing large-scale datasets comprising 477,522 individuals identified five loci significantly associated with same-sex behavior, suggesting that genetic factors account for 8 to 25% of the variation in same-sex sexual behavior (Sanders et al., 2017; Ganna et al., 2019).
One region on chromosome 13, located between the genes SLIT and NTRK-like family member 6 (SLITRK6) and SLITRK5, has been proposed to be associated with male homosexual orientation (Sanders et al., 2017). SLITRK6 and SLITRK5 are primarily expressed in neural tissues (Aruga, 2003). In mice, SLITRK6 knockout leads to deafness and myopia, while in humans, SLITRK6 mutations result in severe myopia and sensorineural deafness (Tekin et al., 2013). Additionally, SLITRK5 deficiency impairs corticostriatal neurotransmission, leading to obsessive-compulsive-like behaviors in mice (Shmelkov et al., 2010). However, further research is needed to determine whether deficiencies in Slitrk5 or Slitrk6 influence sexual behaviors in mice or humans.
Several SNPs on chromosome 14 have also been associated with male homosexual orientation (Ramagopalan et al., 2010; Sanders et al., 2017). Specifically, genetic variation in intron 1 of the thyroid-stimulating hormone receptor (TSHR) gene on chromosome 14 has been suggested to explain the link between familial atypical thyroid function and male homosexuality. TSHR is critical for thyroid cell metabolism (Kleinau et al., 2013). Interestingly, an increased incidence of Graves’ disease, characterized primarily by hyperthyroidism, has been observed in homosexual men (Frisch et al., 2014). Additionally, a retrospective chart review indicated that gestational thyroid dysfunction may be associated with homosexual attraction in men (Sabuncuoglu, 2015). TSHR is expressed in both the thyroid gland and neuron-rich areas of the brain, such as the hippocampus (Crisanti et al., 2001), suggesting its potential role in regulating complex behaviors.
Overall, family and twin studies, along with genetic linkage and association analyses, provide evidence for candidate genetic variants on several chromosomes that may contribute to the development of male sexual orientation. These findings offer valuable clues for subsequent molecular genetic studies, although specific genes controlling sexual orientation have yet to be identified (see Tables 1, 2).
However, it is worth noting that the link between genetics and homosexuality is a complex field. Like all research related to homosexuality in humans, the main limitation is defining the trait. Some studies use a behavioral approach while others rely on the subjects’ definition of sexuality. Therefore, that definition not only differs between two different studies, but also among the subjects of the same research.
There is no single “gay gene,” there are multiple genes of interest involved making genetic testing in itself another limitation as studies are difficult to replicate. For example, genome-wide association studies vary greatly between populations and genes with small effects cannot be detected. Pleiotropy, the phenomenon where one gene locus can affect different phenotypic traits, can also be confounding in genetic research. One gene affecting multiple traits makes causality difficult to determine and lowers the ability to replicate research. The factors mentioned show why genetic testing in homosexuality is such an intricate field. Research fails in replicating previous results (as mentioned in Section 3.2.4 when discussing CGN and sexual orientation) and some studies are yet to be confirmed or reinforced (as mentioned in Section 3.2.5 when discussing chromosome 7q36).
3.3 Neurologic factors
Like various other behaviors, one can understand sexual orientation as a complex interaction involving specific brain processes. These processes involve at least three levels: (1) perception, which involves the feeling of attraction triggered by sensory perception, (2) self-identity, where an individual recognizes this attraction as part of their own identity, and (3) conscious actions directed towards individuals of their desired sex.
The potential biological underpinnings of male homosexuality are likely concentrated in midline regions of the brain. This is especially interesting because these specific brain areas, which include the hypothalamus and thalamus, are known for their involvement in processing sexual arousal (Poeppl et al., 2016; Kim et al., 2006; Motofei and Rowland, 2014). The midline regions of the brain also include the precuneus; it acts as a central hub with robust connections to various cortical regions, including the cuneus and the occipito-temporo parietal junction, which are involved in processing bodily cues. Moreover, the precuneus is linked to the ACC, a region responsible for self-perception also in decision making (Northoff, 2011), as well as subcortical structures that regulate sexual arousal (Poeppl et al., 2016; Kim et al., 2006; Motofei and Rowland, 2014; Cavanna and Trimble, 2006). Consequently, the precuneus may play a pivotal role in shaping choices related to sexual behavior. The findings do not support the idea that the male brain of homosexual individuals is uniformly feminized since there were distinctions in cortical thickness (Cth) and functional connectivity within the precuneus between male and female control groups. These distinctions may hold relevance for understanding sexual behavior.
A 2018 study by Manzouri and Savic employed MRI scans to investigate potential distinctions in brain structure and function among homosexual men (HoM), heterosexual men (HeM), and heterosexual women (HeW). The study was centered on regions of the brain previously identified as having gender-related variations. The primary objective was to determine whether there were any atypical brain distinctions in HoM, and if so, whether these variances were widespread, potentially contributing to behaviors more commonly associated with females in HoM or concentrated in networks related to self-referential sexual arousal.
The analysis encompassed measures such as cortical thickness (Cth), surface area (SA), volumes of subcortical structures, and the functional connectivity observed during resting states in 30 HoM, 35 HeM, and 38 HeW. Findings revealed that HoM exhibited significantly greater thickness in specific brain regions, including the anterior cingulate cortex (ACC), precuneus, and the left occipito-temporal cortex when compared to both control groups. Notably, these differences seemed to be interrelated, as HoM also showed more pronounced associations between these cortical areas.
Furthermore, within the default mode network, responsible for self-referential cognitive processes and inclusive of the ACC and precuneus, HoM exhibited weaker functional connections compared to HeM and HeW. Conversely, functional connectivity between the thalamus and hypothalamus, which are crucial for sexual behavior, was stronger. In addition to these distinctive features, HoM displayed brain characteristics that resembled those typically associated with “female” brain structures, such as similar cortical thickness in the left superior parietal and cuneus cortices to HeW, distinguishing them from HeM (Manzouri and Savic, 2018).
Cth serves as an indicator of neuronal attributes such as size, number, shape, density, and dendritic connections within the columns of the cortex (Rakic, 1988). It can be utilized as a proxy measure for assessing the overall condition of the cerebral cortex. On one hand, it has been observed that the parietal and superior frontal lobe cortex in male controls is thinner compared to that of female controls. This thinning demonstrates a reverse relationship with testosterone levels (Savic and Arver, 2014; Nguyen et al., 2013; Bramen et al., 2012). On the other hand, several studies have shown that the left superior temporal and occipital cortices in male controls are thicker than those in female controls, and this increased thickness is positively associated with testosterone levels (Herting et al., 2015).
Hence, it is reasonable to consider that the thicker parietal and frontal lobe cortex, along with the thinner cuneus cortex observed in homosexual men, may suggest lower androgen levels in specific brain regions. By contrast, homosexual participants demonstrated normal testosterone levels which means that this potential reduction in androgenization could imply a diminished expression or function of androgen receptors (Raznahan et al., 2010).
Neurodevelopmental processes are likely to wire neural circuits differently in individuals with same-sex attractions compared to those with opposite-sex attractions. The initial evidence pointing to neural correlates of sexual partner preference comes from Simon LeVay’s 1991 autopsy study of the third interstitial nucleus of the anterior hypothalamus (INAH-3). LeVay observed that homosexual men had a smaller INAH-3 size in comparison to presumed heterosexual men, resembling the size found in presumed heterosexual women. It’s important to note that the structural distinctions within same-sex groups may not be particularly pronounced.
3.3.1 The role of hypothalamus
A positron emission tomography (PET) study has indicated a more robust hypothalamic response to serotonin in heterosexual men compared to homosexual men (Kinnunen et al., 2004). Furthermore, neuroimaging investigations comparing heterosexual men and women while exposed to their preferred sexual imagery have demonstrated significantly greater hypothalamic activation in heterosexual men (Karama et al., 2002). Taken together with the earlier observations of a smaller INAH-3 in homosexual men, these findings suggest the existence of a distinct functional foundation in the anterior hypothalamus for sexual attraction toward women. Additional support for this concept arises from studies involving lesion models of the preoptic area (POA) within the anterior hypothalamus, which have revealed reduced responses of male animals toward females (Hull et al., 2002).
While discussing the hypothalamus, aromatase and androgens must be mentioned as well since animal models indicate that prenatal androgens may have a role in shaping sexual variations in hypothalamic regions (Morris et al., 2004). For instance, in specific sheep species, some males exclusively prefer the same sex. These males also exhibit reduced aromatase activity and smaller ovine sexually dimorphic nuclei (homolog to the human INAH-3) compared to sheep oriented towards females (Roselli et al., 2004).
Moreover, potential disparities in aromatase activity in human males based on their sexual orientation may help elucidate the complex mix of masculine and feminine traits often observed. For instance, a decline in aromatase activity in homosexual men, as implied by the Roselli study, could lead to reduced availability of aromatized testosterone, particularly estradiol, which is known to contribute to the masculinization of the male mammalian brain (Morris et al., 2004). This could result in less masculinized hypothalamic circuitry, while simultaneously allowing excess non-aromatized testosterone to contribute to the masculinization of other androgen-sensitive traits, such as the 2D:4D ratio, through alternative metabolic pathways (e.g., 5-alpha reductase). Research on hypothalamic structure and function, particularly LeVay’s early work, is constrained by small sample sizes, the use of post-mortem brains, and assumptions regarding the sexual orientation of subjects. Moreover, other confounding factors—such as comorbid medical conditions or the influence of hormone treatment—may contribute to the observed anatomical differences.
3.3.2 The connection between sexual orientation and cognitive processes
Research in neurocognition has provided evidence indicating that differences in brain activity related to sexual orientation might also manifest in higher cortical regions. Multiple independent studies demonstrate that homosexual men tend to perform poorly on fundamental tests of spatial abilities, such as mental rotation and spatial perception, compared to heterosexual men (Rahman and Wilson, 2003a, 2003b). Moreover, homosexual men display heightened spatial location memory, improved recall of spatial landmarks during navigation, and enhanced phonological and semantic fluency—traits typically associated with females—compared to heterosexual men (Rahman et al., 2003a, 2003b, 2003c). These findings suggest that sexual orientation could be linked to variations in brain regions like the parietal, hippocampal-temporal, and prefrontal areas that underlie the mentioned cognitive abilities. Variations in inter-hemispheric pathways might contribute to these cognitive differences but they could not be well replicated (Allen and Gorski, 1992; Wegesin, 1998a, 1998b). A study found a difference in the size of the callosal isthmus between right-handed homosexual and heterosexual men, with homosexual men exhibiting a larger isthmus (Witelson et al., 2008). This finding suggests that right-handed homosexual men may possess brain structures and organization typically seen in left-handers, despite their apparent right-hand preference (Witelson et al., 2008). This anatomical evidence supports the notion that these men might exhibit atypical functional asymmetry. These results align with previous neuropsychological studies, which showed that right-handed homosexual men demonstrate less functional asymmetry compared to their heterosexual counterparts in verbal dichotic tasks (McCormick and Witelson, 1994), divided visual-field tasks (Sanders and Wright, 1997), and neurophysiological EEG measures (Wegesin, 1998a, 1998b).
Further investigations employing various neurophysiological measures provide additional evidence supporting the involvement of parietal and temporal lobes (Howard et al., 1994; Reite et al., 1995; Wegesin, 1998a, 1998b). The parietal lobe seems to be implicated given its role in neural processes related to heterosexual sexual arousal and the visual-configural processing of preferred sexual stimuli (Howard et al., 1994; Waisman et al., 2003). The pre-pulse inhibition of the startle response (a neurobehavioral probe of sexual dimorphism) is notably more masculinized in homosexual women compared to heterosexual women, pointing to the involvement of the pallido-striato-thalamic limbic circuitry (Rahman et al., 2003a, 2003b, 2003c). Cognitive studies indicate that homosexual women tend to exhibit better verbal fluency, suggesting the involvement of the prefrontal cortex, although neurophysiological studies do not consistently reveal differences, except in auditory regions (McFadden and Champlin, 2000). Cognitive and neuroanatomical differences observed between homosexual and heterosexual individuals are intriguing but not yet conclusive. Many studies suffer from small sample sizes and limited replication. Additionally, observed brain differences may be shaped by social experience and plasticity rather than reflecting innate or causal factors (see Table 3).
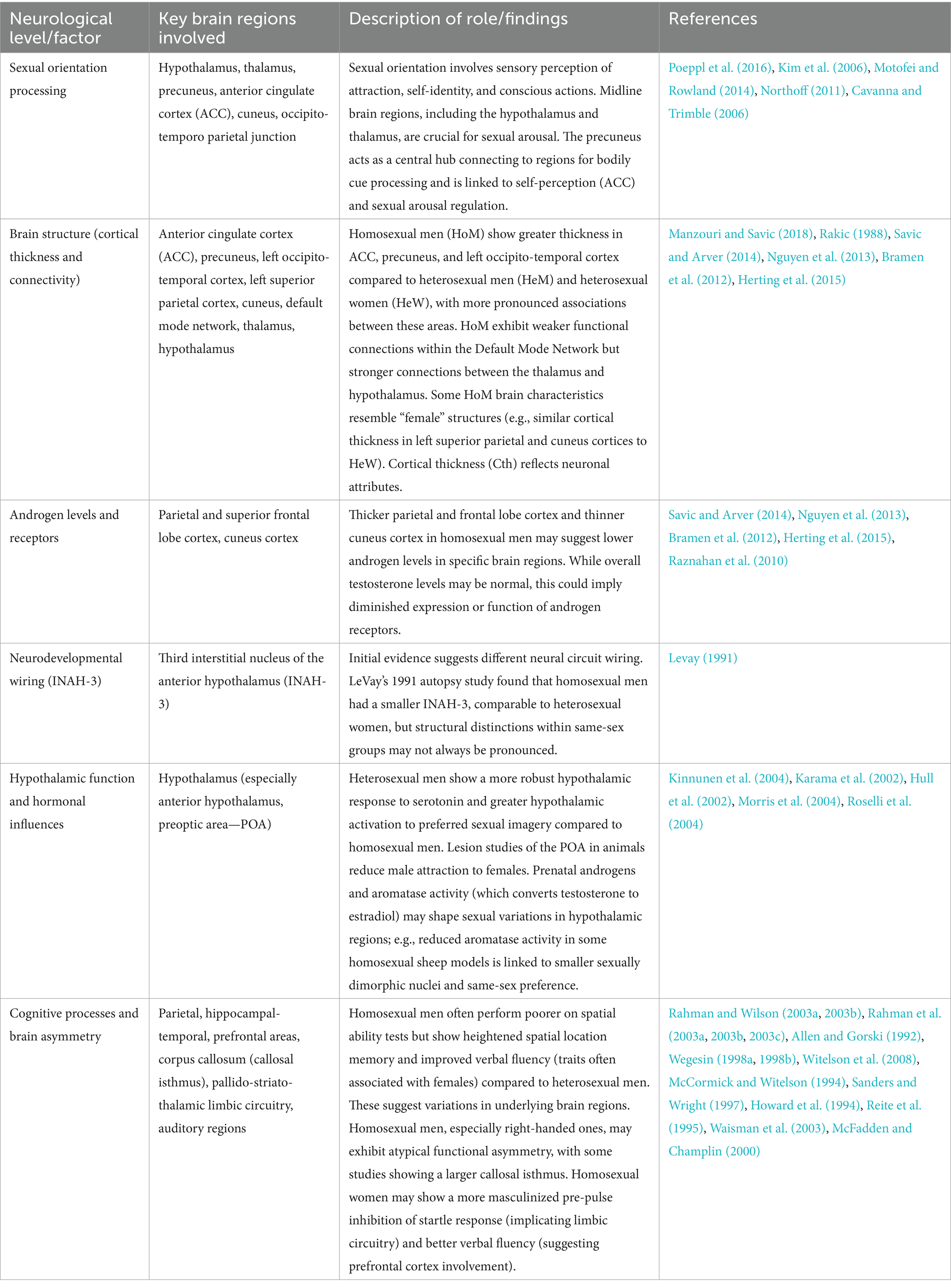
Table 3. Summarizing the neurological factors and brain regions implicated in various aspects of homosexuality, as discussed in the provided text.
3.4 Environmental factors
3.4.1 Childhood gender nonconformity (CGN)
3.4.1.1 Play preferences and gender-typed behaviors
Scientific research has demonstrated a strong link between sexual orientation and various aspects of gender roles. This encompasses childhood behaviors such as play preferences and the formation of gender identity (Bailey and Zucker, 1995), as well as gender-typed behaviors in adulthood, particularly in terms of career choices and leisure activities (Lippa, 2008).
Recent large-scale studies involving population-based prospective research have confirmed the robust association between sexual orientation and Childhood Gender Nonconformity (CGN) (Li et al., 2017; Xu et al., 2019, 2020, 2021). Another study involved assessing behaviors observed in home videos from childhood. This study has found that, consistent with the typical development of gender identity, variations in CGN as assessed by observers become apparent around the age of 3 or 4 (Rieger et al., 2008a, 2008b).
3.4.1.2 Childhood gender nonconformity in males
An alternate method has explored the relationship between homosexual men’s recollections of CGN and their mothers’ memories of their sons and found a strong correlation between the two (r = 0.69) (Bailey et al., 1995). This suggests that retrospective accounts from both individuals and their parents are reliable indicators of past behaviors, supporting the idea that CGN is a noticeable and significant aspect of childhood that may be linked to the later development of sexual orientation. The study also underscored the potential impact of parental perception and societal expectations on how gender-nonconforming behavior is recognized and remembered, contributing to the broader understanding of the factors that influence sexual identity development. The connection between sexual orientation and CGN remains consistent across different cultures (Bailey et al., 2016). A meta-analysis of 41 retrospective studies revealed that individuals with a homosexual orientation recalled significantly more instances of CGN compared to their heterosexual counterparts (Bailey and Zucker, 1995).
In a study involving families, there were moderate family patterns and consistent differences in CGN related to sexual orientation among males (Bailey et al., 2020). It’s noteworthy that twin studies indicate that CGN appears to have a moderate to high heritability among males, with heritability estimates ranging from 29 to 70% (Knafo et al., 2005; van Beijsterveldt et al., 2006; Alanko et al., 2010; Bailey et al., 2000).
Much of the evidence linking childhood gender nonconformity to adult sexual orientation relies on retrospective self- or parent-report, which may be subject to memory and social desirability biases. Furthermore, cultural expectations regarding gender roles may shape both the perception and expression of such behaviors, complicating their interpretation across different contexts.
3.4.2 Early exposure to traumatic events
The development of homosexuality can be influenced by early exposure to traumatic events. These include neglect, sexual, emotional, or physical abuse (Qin et al., 2018). However, the mechanism relating those two variables remains unclear and requires further investigation. Certain studies have shown that some stressful events that occur in life influence both mental and physical health and can impact the development of homosexuality. Male homosexuals declare more incidence of childhood abuse compared to heterosexuals as reported by studies conducted by Wyre (1990), Rind (2001), and Garcia et al. (2002). The abuse episodes have played a role in shaping sexual orientation. Therefore, childhood victimisation is a very sensitive concern since it can have long lasting negative effects on mental and physical health. Childhood victimisation events occur early during a critical period in a child’s brain development and at that time the stress response is extremely sensitive to variables such as abuse, neglect, peer rejection and unstable family environment. It is speculated that all the factors listed previously in combination with the genetic factors like COMT and MTHFR genes collectively contribute to the development of male homosexuality (Qin et al., 2018). While some studies suggest an association between early trauma and later sexual orientation, causality cannot be established. The possibility of recall bias and the stigmatizing nature of such associations call for cautious interpretation. This line of research remains contentious and must be approached with sensitivity and scientific rigor.
A meta-analysis found no statistically significant difference in the overall prevalence of childhood sexual abuse (CSA) among lesbian, gay, and bisexual individuals, with this pattern observed in both men and women. Specifically, CSA prevalence among male sexual minorities was reported at 22.2%, while for female sexual minorities it was 36.2% (Xu and Zheng, 2015). These figures contrast markedly with estimates from heterosexual populations: Pereda et al. (2009b) reported CSA prevalence rates of 7.9% for men and 19.7% for women in a general population sample, and Stoltenborgh et al. (2011) found similar rates of 7.6% for men and 18.0% for women. Taken together, these findings suggest that CSA is more commonly reported among sexual minorities than among heterosexual individuals. However, it is important to emphasize that these data demonstrate an association rather than a definitive causal relationship between CSA and sexual orientation.
Some researchers have proposed that CSA could influence the development of same-sex attraction (Roberts et al., 2013). For example, same-sex sexual abuse during childhood may lead some boys to question or believe they are homosexual (Gartner, 1999), while sexual abuse of girls by male perpetrators may foster aversion toward men and a greater sense of comfort in intimate relationships with women (Marvasti and Dripchak, 2004). These hypotheses, while suggestive, remain the subject of ongoing debate and require further empirical investigation.
Although the meta-analysis sought to estimate the global prevalence of childhood sexual abuse (CSA) among lesbian, gay, and bisexual (LGB) populations, the majority of included studies were conducted in the United States and Canada. Additionally, there was a notable absence of research from African countries, many of which maintain laws criminalizing same-sex behavior, making it challenging to study sexual minorities in these regions. Furthermore, the meta-analysis was restricted to English-language publications, which likely limited the comprehensiveness of the included studies. Hence, the analysis was unable to clarify the temporal sequence between sexual orientation and CSA, meaning that it remains unclear which factor precedes the other (see Table 4).
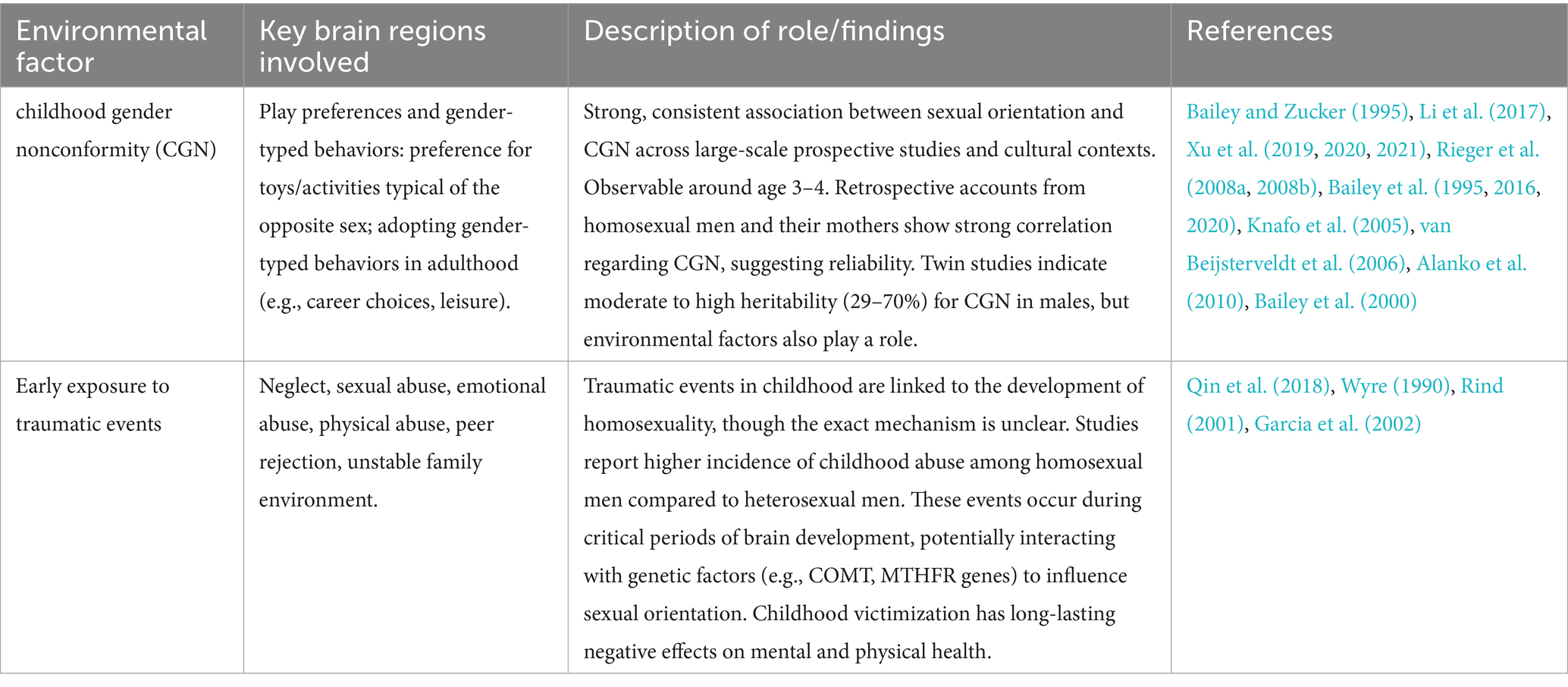
Table 4. Summarizing the environmental factors which have been linked to sexual orientation development.
4 Conclusion
In a narrower context, “sexual orientation” is concerned with an individual’s preference for a sexual partner. Homosexuality is a behavior exhibited among people of the same sex. This behavior is grounded in romantic as well as sexual attraction. The connection between homosexuality and polymorphic genes is supported by two distinct lines of evidence. First, twin studies point to the involvement of genetic factors contributing to homosexuality. Additionally, environmental factors play a role in shaping homosexual characteristics (as seen in Pillard and Bailey, 1998; Bailey et al., 1999; Dawood et al., 2000). Male homosexuality appears to exhibit a heightened inheritance pattern through the maternal lineage (as indicated in Pillard et al., 1981, 1982; Camperio-Ciani et al., 2004). Furthermore, the possibility of polymorphic X-linked genes influencing male homosexuality could also be present. The underlying causes of male and female homosexuality may differ. One basis for is the presence of a well-documented fraternal birth order effect in male homosexuality. In contrast, female homosexuality is independent of ‘sororal’ birth order (Bogaert, 1997).
Childhood abuse has been linked to homosexuality. Additionally, specific genetic variants might contribute to homosexuality. Thus, these factors could potentially have a positive correlation with homosexuality (Qin et al., 2018). These findings could provide valuable clues regarding the genetic foundations of male sexual orientation when considering a wider range of populations were linked to same-sex sexual behavior (Hu et al., 2021).
Understanding the complex interplay of biological, genetic, neurological, and environmental factors in shaping human sexual orientation remains a significant and evolving scientific challenge. Current research suggests that homosexuality cannot be attributed to a single cause but rather arises from a constellation of influences, including but not limited to genetic predispositions, hormonal environments in utero, brain structure differences, and psychosocial contexts. To advance knowledge in this field, interdisciplinary research is crucial. Future studies should integrate genomics, neuroimaging, endocrinology, and longitudinal psychosocial data while ensuring large, diverse, and representative samples. It is also important to study people from different backgrounds and use large, diverse groups in research. Scientists should work together across countries and fields and focus on making research more reliable and respectful of LGBTQ+ individuals. By doing this, we can improve knowledge, reduce prejudice, and help shape more inclusive policies and education.
This narrative review is subject to several inherent limitations. In contrast to systematic reviews, it does not adhere to a standardized, predefined protocol for identifying, selecting, and evaluating the literature, thereby increasing the risk of selection bias. The synthesis presented is shaped by the authors’ interpretations, and the absence of a formal quality appraisal of the included studies constrains the ability to determine the strength and reliability of the evidence. Research on homosexuality is marked by substantial heterogeneity, encompassing variations in study design, definitions of sexual orientation, and the tools used for measurement—factors that complicate both comparability and synthesis of findings. Cultural, political, and legal environments exert a significant influence on research practices and participants’ willingness to disclose personal information, potentially resulting in the underrepresentation of marginalized populations. Moreover, the evidence base is disproportionately drawn from Western, high-income settings, creating both geographical and cultural imbalances. Lastly, given the evolving societal understanding of sexual orientation and identity, some older studies may not align with contemporary conceptual frameworks, thereby limiting their present-day applicability.
5 Future perspective
To truly understand the origins of homosexuality, future research must delve deeper into specific biological, genetic, and neurological mechanisms, moving past broad correlations. This means conducting large-scale genome-wide association studies (GWAS) with diverse groups to pinpoint specific genetic links (Ngun and Vilain, 2014). We also need to explore epigenetic modifications, investigating how prenatal environmental factors might influence brain development and sexual orientation (Rice et al., 2012) Importantly, these studies should include sex-specific analyses, as pathways might differ for males and females (Ngun and Vilain, 2014).
In neurological research, advanced neuroimaging techniques, such as longitudinal fMRI and DTI studies, can examine brain structure and function in young individuals to identify early neural correlates of sexual orientation (American Psychological Association, 2008). Combining this brain data with genetic and hormonal information will offer a more comprehensive view of how these factors shape brain circuitry related to attraction.
For environmental influences, future studies should pinpoint specific prenatal and early postnatal exposures (e.g., in utero hormones, maternal stress) and their dose–response relationships (American Psychological Association, 2008). Understanding gene–environment interactions is also crucial, exploring how genetic predispositions might be expressed differently under various environmental conditions. This will require well-designed prospective cohort studies or controlled animal models.
Ultimately, a complete understanding requires integrative, multi-omics approaches combining genetic, epigenetic, proteomic, and neuroimaging data. It’s also vital to diversify study populations, refine phenotypic measures of sexual orientation, and use longitudinal study designs to track development over time, all while maintaining rigorous ethical standards (American Psychological Association, 2008; Ngun and Vilain, 2014; Rice et al., 2012). By pursuing these avenues, we can gain a more nuanced appreciation of homosexuality as a fundamental aspect of human diversity.
Author contributions
MA: Writing – original draft, Writing – review & editing. FA: Writing – original draft, Writing – review & editing. CB: Writing – original draft, Writing – review & editing. TN: Writing – original draft, Writing – review & editing. NK: Conceptualization, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alanko, K., Santtila, P., Harlaar, N., Witting, K., Varjonen, M., Jern, P., et al. (2010). Common genetic effects of gender atypical behavior in childhood and sexual orientation in adulthood: a study of Finnish twins. Arch. Sex. Behav. 39, 81–92. doi: 10.1007/s10508-008-9457-3
Allen, L. S., and Gorski, R. A. (1992). Sexual orientation and the size of the anterior commissure in the human brain. Proc. Natl. Acad. Sci. USA 89, 7199–7202. doi: 10.1073/pnas.89.15.7199
American Psychological Association (2008). Answers to your questions about sexual orientation and homosexuality. Washington, DC: American Psychological Association.
Andersen, J. P., Hughes, T. L., Zou, C., and Wilsnack, S. C.. (2014). Lifetime victimization and physical health outcomes among lesbian and heterosexual women. PLoS One 9:e101939. doi: 10.1371/journal.pone.0101939
Aruga, J. (2003). Slitrk6 expression profile in the mouse embryo and its relationship to that of Nlrr3. Gene Expr. Patterns 3, 727–733. doi: 10.1016/S1567-133X(03)00141-8
Bailey, J. M., Dunne, M. P., and Martin, N. G. (2000). Genetic and environmental influences on sexual orientation and its correlates in an Australian twin sample. J. Pers. Soc. Psychol. 78, 524–536. doi: 10.1037//0022-3514.78.3.524
Bailey, J. M., Nothnagel, J., and Wolfe, M. (1995). Retrospective measured individual differences in childhood sex-typed behavior among gay men: correspondence between self- and maternal reports. Arch. Sex. Behav. 24, 613–622. doi: 10.1007/BF01542183
Bailey, J. M., and Pillard, R. C. (1991). A genetic study of male sexual orientation. Arch. Gen. Psychiatry 48, 1089–1096. doi: 10.1001/archpsyc.1991.01810360053008
Bailey, J. M., and Pillard, R. C. (1995). Genetics of human sexual orientation. Ann. Rev. Sex. Res. 60, 126–150.
Bailey, J. M., Pillard, R. C., Dawood, K., Miller, M. B., Trivedi, S., Farrer, L. A., et al. (1999). A family history study of male sexual orientation using three independent samples. Behav. Genet. 29, 79–86. doi: 10.1023/a:1021652204405
Bailey, J. M., Rieger, G., Krishnappa, R. S., Kolundzija, A. B., Dawood, K., and Sanders, A. R. (2020). Familiality of gender nonconformity among homosexual men. Arch. Sex. Behav. 49, 2461–2468. doi: 10.1007/s10508-020-01626-w
Bailey, J. M., Vasey, P. L., Diamond, L. M., Breedlove, S. M., Vilain, E., and Epprecht, M. (2016). Sexual orientation, controversy, and science. Psychol. Sci. Public Interest 17, 45–101. doi: 10.1177/1529100616637616
Bailey, J. M., and Zucker, K. J. (1995). Childhood sex-typed behavior and sexual orientation: a conceptual analysis and quantitative review. Dev. Psychol. 31, 43–55. doi: 10.1037/0012-1649.31.1.43
Bailey, N. W., and Zuk, M. (2009). Same-sex sexual behavior and evolution. Trends Ecol. Evol. 24, 439–446. doi: 10.1016/j.tree.2009.03.014
Baum, M. J. (1979). Differentiation of coital behavior in mammals: a comparative analysis. Neurosci. Biobehav. Rev. 3, 265–284. doi: 10.1016/0149-7634(79)90013-7
Binet, A., Lardy, H., Geslin, D., Francois-Fiquet, C., and Poli-Merol, M. L. (2016). Should we question early feminizing genitoplasty for patients with congenital adrenal hyperplasia and XX karyotype? J. Pediatr. Surg. 51, 465–468. doi: 10.1016/j.jpedsurg.2015.10.004
Blanchard, R. (1997). Birth order and sibling sex ratio in homosexual versus heterosexual males and females. Annu. Rev. Sex Res. 8, 27–67. doi: 10.1080/10532528.1997.10559918
Blanchard, R. (2004). Quantitative and theoretical analyses of the relation between older brothers and homosexuality in men. J. Theor. Biol. 230, 173–187. doi: 10.1016/j.jtbi.2004.04.021
Blanchard, R., and Bogaert, A. F. (1996a). Homosexuality in men and the number of older brothers. Am. J. Psychiatry 153, 27–31.
Blanchard, R., and Bogaert, A. F. (1996b). Biodemographic comparisons of homosexual and heterosexual men in the Kinsey interview data. Arch. Sex. Behav. 25, 551–579. doi: 10.1007/BF02437839
Blanchard, R., and Bogaert, A. F. (1997). Additive effects of older brothers and homosexual brothers in the prediction of marriage and cohabitation. Behav. Genet. 27, 45–54. doi: 10.1023/A:1025663325313
Blanchard, R., and Ellis, L. (2001). Birth weight, sexual orientation and the sex of preceding siblings. J. Biosoc. Sci. 33, 451–467. doi: 10.1017/S0021932001004515
Blanchard, R., Zucker, K. J., Cavacas, A., Allin, S., Bradley, S. J., and Schachter, D. C. (2002). Fraternal birth order and birth weight in probably prehomosexual feminine boys. Horm. Behav. 41, 321–327. doi: 10.1006/hbeh.2002.1765
Blanchard, R., Zucker, K. J., Siegelman, M., Dickey, R., and Klassen, P. (1998). The relation of birth order to sexual orientation in men and women. J. Biosoc. Sci. 30, 511–519. doi: 10.1017/S0021932098005112
Blanco, P., Sargent, C. A., Boucher, C. A., Mitchell, M., and Affara, N. A. (2000). Conservation of PCDHX in mammals: expression of human X/Y genes predominantly in brain. Mamm. Genome 11, 906–914. doi: 10.1007/s003350010177
Bogaert, A. F. (1997). Birth order and sexual orientation in women. Behav. Neurosci. 111, 1395–1397. doi: 10.1037/0735-7044.111.6.1395
Bogaert, A. F. (2000). Birth order and sexual orientation in a national probability sample. J. Sex Res. 37, 361–368. doi: 10.1080/00224490009552059
Bogaert, A. F. (2003). Number of older brothers and sexual orientation: new tests and the attraction/behavior distinction in two national probability samples. J. Pers. Soc. Psychol. 84, 644–652. doi: 10.1037/0022-3514.84.3.644
Bogaert, A. F., and Friesen, C. (2002). Sexual orientation and height, weight and age at puberty. Biol. Psychol. 59, 135–145. doi: 10.1016/s0301-0511(01)00131-4
Bogaert, A. F., Friesen, C., and Klentrou, P. (2002). Age of puberty and sexual orientation in a national probability sample. Arch. Sex. Behav. 31, 73–81. doi: 10.1023/a:1014083218773
Bogaert, A. F., and Skorska, M. (2011). Sexual orientation, fraternal birth order, and the maternal immune hypothesis: a review. Front. Neuroendocrinol. 32, 247–254. doi: 10.1016/j.yfrne.2011.02.004
Bramen, J. E., Hranilovich, J. A., Dahl, R. E., Chen, J., Rosso, C., Forbes, E. E., et al. (2012). Sex matters during adolescence: testosterone-related cortical thickness maturation differs between boys and girls. PLoS One 7:e33850. doi: 10.1371/journal.pone.0033850
Breedlove, S. M. (2017). Prenatal influences on human sexual orientation: expectations versus data. Arch. Sex. Behav. 46, 1583–1592. doi: 10.1007/s10508-016-0904-2
Brown, W. M., Finn, C. J., Cooke, B. M., and Breedlove, S. M. (2002a). Differences in finger length ratios between self-identified "butch" and "femme" lesbians. Arch. Sex. Behav. 31, 123–127. doi: 10.1023/A:1014091420590
Brown, W. M., Hines, M., Fane, B., and Breedlove, S. M. (2002b). Masculinised finger length ratios in humans with congenital adrenal hyperplasia. Horm. Behav. 42, 380–386. doi: 10.1006/hbeh.2002.1830
Buck, J. J., Williams, R. M., Hughes, I. A., and Acerini, C. L. (2003). In-utero androgen exposure and 2nd and 4th digit length ratio: comparisons between healthy controls and females with classical congenital adrenal hyperplasia. Hum. Reprod. 18, 976–979. doi: 10.1093/humrep/deg198
Calabrò, R. S., Cacciola, A., Bruschetta, D., Milardi, D., Quattrini, F., Sciarrone, F., et al. (2019). Neuroanatomy and function of human sexual behavior: a neglected or unknown issue? Brain Behav. 9:e01389. doi: 10.1002/brb3.1389
Calati, R., Porcelli, S., Giegling, I., Hartmann, A. M., Möller, H.-J., De Ronchi, D., et al. (2011). Catechol-omethyl-transferase gene modulation on suicidal behavior and personality traits: review, meta-analysis and association study. J. Psychiatr. Res. 45, 309–321. doi: 10.1016/j.jpsychires.2010.07.004
Camara, E., Kramer, U. M., Cunillera, T., Marco-Pallares, J., Cucurell, D., Nager, W., et al. (2010). The effects of COMT (Val108/158Met) and DRD4 (SNP-521) dopamine genotypes on brain activations related to valence and magnitude of rewards. Cereb. Cortex 20, 1985–1996. doi: 10.1093/cercor/bhp263
Camperio-Ciani, A., Corna, F., and Capiluppi, C. (2004). Evidence for maternally inherited factors favouring male homosexuality and promoting female fecundity. Proc. R. Soc. B 271, 2217–2221. doi: 10.1098/rspb.2004.2872
Cantor, J. M., Blanchard, R., Paterson, A. D., and Bogaert, A. F. (2002). How many gay men owe their sexual orientation to fraternal birth order? Arch. Sex. Behav. 31, 63–71. doi: 10.1023/A:1014031201935
Cavanna, A. E., and Trimble, M. R. (2006). The precuneus: a review of its functional anatomy and behavioural correlates. Brain 129, 564–583. doi: 10.1093/brain/awl004
Chen, B., Liu, H., Ren, J., and Guo, A. (2012). Mutation of drosophila dopamine receptor DopR leads to male-male courtship behavior. Biochem. Biophys. Res. Commun. 423, 557–563. doi: 10.1016/j.bbrc.2012.06.003
Cibrian-Llanderal, T., Rosas-Aguilar, V., Triana-Del Rio, R., Perez, C. A., Manzo, J., Garcia, L. I., et al. (2012). Enhanced D2-type receptor activity facilitates the development of conditioned same-sex partner preference in male rats. Pharmacol. Biochem. Behav. 102, 177–183. doi: 10.1016/j.pbb.2012.04.007
Collaer, M. L., and Hines, M. (1995). Human behavioral sex differences: a role for gonadal hormones during early development? Psychol. Bull. 118, 55–107. doi: 10.1037/0033-2909.118.1.55
Comings, D. E. (1994). Role of genetic factors in human sexual behavior based on studies of Tourette syndrome and ADHD probands and their relations. Am. J. Med. Genet. (Neuropsychiatr. Genet.) 54, 227–241.
Crisanti, P., Omri, B., Hughes, E., Meduri, G., Hery, C., Clauser, E., et al. (2001). The expression of thyrotropin receptor in the brain. Endocrinology 142, 812–822. doi: 10.1210/endo.142.2.7943
Daae, E., Feragen, K. B., Waehre, A., Nermoen, I., and Falhammar, H. (2020). Sexual orientation in individuals with congenital adrenal hyperplasia: a systematic review. Front. Behav. Neurosci. 14, 38. doi: 10.3389/fnbeh.2020.00038
Dahlstroem, A., and Fuxe, K. (1964). Evidence for the existence of monoamine neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol. Scand. Suppl. 62, 1–55.
Dawood, K., Pillard, R. C., Horvath, C., Revelle, W., and Bailey, J. M. (2000). Familial aspects of male homosexuality. Arch. Sex. Behav. 29, 155–163. doi: 10.1023/a:1001955721992
de Arruda, I. T., Persuhn, D. C., and de Oliveira, N. F. (2013). The MTHFR C677T polymorphism and global DNA methylation in oral epithelial cells. Genet. Mol. Biol. 36, 490–493. doi: 10.1590/S1415-47572013005000035
Desaulniers, A. T., Cederberg, R. A., Lents, C. A., and White, B. R. (2017). Expression and Role of Gonadotropin-Releasing Hormone 2 and Its Receptor in Mammals. Front. Endocrinol. 8. doi: 10.3389/fendo.2017.00269
Dittmann, R. W., Kappes, M. E., and Kappes, M. H. (1992). Sexual behavior in adolescent and adult females with congenital adrenal hyperplasia. Psychoneuroendocrinology 17, 153–170. doi: 10.1016/0306-4530(92)90054-B
Doll, L. S., and Beeker, C. (1996). Male bisexual behavior and HIV risk in the United States: synthesis of research with implications for behavioral interventions. AIDS Educ. Prev. 8, 205–225.
Duden (1989). The dictionary of origin. Etymology of the German language. Mannheim: Bibliographic Institute & F.A. Brockhaus AG.
Ebstein, R. P., Knafo, A., Mankuta, D., Chew, S. H., and Lai, P. S. (2012). The contributions of oxytocin and vasopressin pathway genes to human behavior. Horm. Behav. 61, 359–379. doi: 10.1016/j.yhbeh.2011.12.014
Edwards, V. J., Holden, G. W., Felitti, V. J., and Anda, R. F. (2003). Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: results from the adverse childhood experiences study. Am. J. Psychiatry 160, 1453–1460. doi: 10.1176/appi.ajp.160.8.1453
Ellis, E., and Ames, M. A. (1987). Neurohormonal functioning and sexual orientation: a theory of homosexuality–heterosexuality. Psychol. Bull. 101, 233–258.
Ellis, L., and Blanchard, R. (2001). Birth order, sibling sex ratio, and maternal miscarriages in homosexual and heterosexual men and women. Pers. Individ. Differ. 30, 543–552. doi: 10.1016/S0191-8869(00)00051-9
Ervin, J., Scovelle, A., Churchill, B., Maheen, H., and King, T. (2023). Gender identity and sexual orientation: a glossary. J. Epidemiol. Community Health 77, 344–348. doi: 10.1136/jech-2022-220009
Fagerholm, R., Santtila, P., Miettinen, P. J., Mattila, A., Rintala, R., and Taskinen, S. (2011). Sexual function and attitudes toward surgery after feminizing genitoplasty. J. Urol. 185, 1900–1904. doi: 10.1016/j.juro.2010.12.099
Feder, H. H. (1981). “Perinatal hormones and their role in the development of sexually dimorphie behaviors” in Neuroendocrinology of reproduction: Physiology and behavior. ed. N. T. Adler (New York: Plenum Press), 127–158.
Friedman, R. C. (1988). Male homosexuality: A contemporary psycho-analytic perspective. New Haven, CT: Yale University Press.
Frisch, M., Nielsen, N. M., and Pedersen, B. V. (2014). Same-sex marriage, autoimmune thyroid gland dysfunction and other autoimmune diseases in Denmark 1989–2008. Eur. J. Epidemiol. 29, 63–71. doi: 10.1007/s10654-013-9869-9
Frisen, L., Nordenstrom, A., Falhammar, H., Filipsson, H., Holmdahl, G., Janson, P. O., et al. (2009). Gender role behavior, sexuality, and psychosocial adaptation in women with congenital adrenal hyperplasia due to CYP21A2 deficiency. J. Clin. Endocrinol. Metab. 94, 3432–3439. doi: 10.1210/jc.2009-0636
Friso, S., Choi, S. W., Girelli, D., Mason, J. B., Dolnikowski, G. G., Bagley, P. J., et al. (2002). A common mutation in the 5, 10-methylenetetrahydrofolate reductase gene affects genomic DNA methylation through an interaction with folate status. Proc. Natl. Acad. Sci. USA 99, 5606–5611. doi: 10.1073/pnas.062066299
Fujieda, K., Tajima, T., Nakae, J., Sageshima, S., Tachibana, K., Suwa, S., et al. (1997). Spontaneous puberty in 46,XX subjects with congenital lipoid adrenal hyperplasia. Ovarian steroidogenesis is spared to some extent despite inactivating mutations in the steroidogenic acute regulatory protein (StAR) gene. J Clin Invest. 99, 1265–1271. doi: 10.1172/jci119284
Ganna, A., Verweij, K. J. H., Nivard, M. G., Maier, R., Wedow, R., Busch, A. S., et al. (2019). Large-scale GWAS reveals insights into the genetic architecture of same-sex sexual behavior. Science 365:aat7693. doi: 10.1126/science.aat7693
Garcia, J., Adams, J., Friedman, L., and East, P. (2002). Links between past abuse, suicide ideation and sexual orientation among San Diego college students. J. Am. Coll. Heal. 51, 9–14. doi: 10.1080/07448480209596322
Gartner, R. B. (1999). Sexual victimization of boys by men: meanings and consequences. J. Gay Lesbian Ment. Health 3, 1–33. doi: 10.1080/19359705.1999.9962210
Gavrilets, S., and Rice, W. R. (2006). Genetic models of homosexuality: generating testable predictions. Proc. Biol. Sci. 273, 3031–3038. doi: 10.1098/rspb.2006.3684
Gentile, T., Malan-Borel, I., Angelucci, J., Miranda, S., and Margni, R. A. (1992). Preferential synthesis of asymmetric antibodies in rats immunized with paternal particulate antigens. J. Reprod. Immunol. 22, 173–183.
Geschwind, N., and Galaburda, A. M. (1985). Cerebral lateralization: biological mechanisms, associations and pathology: I. A hypothesis and program for research. Arch. Neurol. 42, 428–459.
Hamer, D. H. (2007). The genetics of sexual orientation: theories, findings, and implications. Psychol. Sci. Public Interest 8, 181–218. doi: 10.1111/j.1539-6053.2007.00047.x
Hamer, D. H., and Copeland, P. (1998). The genetics of sexual orientation. Annu. Rev. Sex Res. 9, 147–164. doi: 10.1080/10532528.1998.10559719
Hamer, D. H., Hu, S., Magnuson, V. L., Hu, N., and Pattatucci, A. M. (1993). A linkage between DNA markers on the X chromosome and male sexual orientation. Science 261, 321–327. doi: 10.1126/science.8332896
Herek, G. M., and Garnets, L. D. (2007). Sexual orientation and mental health. Annu. Rev. Clin. Psychol. 3, 353–375. doi: 10.1146/annurev.clinpsy.3.022806.091510
Herting, M. M., Gautam, P., Spielberg, J. M., Dahl, R. E., and Sowell, E. R. (2015). A longitudinal study: changes in cortical thickness and surface area during pubertal maturation. PLoS One 10:e0119774. doi: 10.1371/journal.pone.0119774
Hines, M., Brook, C., and Conway, G. S. (2004). Androgens and psychosexual development: core gender identity, sexual orientation and recalled childhood gender role behavior in women and men with congenital adrenal hyperplasia. J. Sex Res. 41, 75–81. doi: 10.1080/00224490409552215
Howard, R. C., Longmore, F. J., Mason, P. A., and Martin, J. L. (1994). Contingent negative variation (CNV) and erotic preference in self-declared homosexuals and in child sex offenders. Biol. Psychol. 38, 169–181. doi: 10.1016/0301-0511(94)90037-X
Huang, X., Zhang, Q., Chen, X., Gu, X., Wang, M., and Wu, J. (2019). A functional variant in SLC1A3 influences ADHD risk by disrupting a hsa-miR-3171 binding site: a two-stage association study. Genes Brain Behav. 18:e12574. doi: 10.1111/gbb.12574
Huc-Chabrolle, M., Charon, C., Guilmatre, A., Vourc’h, P., Tripi, G., Barthez, M. A., et al. (2013). Xq27 FRAXA locus is a strong candidate for dyslexia: evidence from a genome-wide scan in French families. Behav. Genet. 43, 132–140. doi: 10.1007/s10519-012-9575-5
Hull, E., Bitran, D., Pehek, E. A., Warner, R. K., Band, L. C., and Holmes, G. M. (1986). Dopaminergic control of male sex behavior in rats: effects of an intracerebrally-infused agonist. Brain Res. 370, 73–81. doi: 10.1016/0006-8993(86)91106-6
Hull, E.M., Meisel, R.L., and Sachs, B.D. (2002). Male sexual behavior. In D. W. Pfaff, A. P. Arnold, S. E. Fahrbach, A. M. Etgen, R. T. Rubin (Eds.), Hormones, brain and behavior Academic Press (pp. 3–137). doi: 10.1016/B978-012532104-4/50003-2
Hull, E. M., Muschamp, J. W., and Sato, S. (2004). Dopamine and serotonin: influences on male sexual behavior. Physiol. Behav. 83, 291–307. doi: 10.1016/j.physbeh.2004.08.018
Hu, S. H., Li, H. M., Yu, H., Liu, Y., Liu, C. X., Zuo, X. B., et al. (2021). Discovery of new genetic loci for male sexual orientation in Han population. Cell Discov 7:103. doi: 10.1038/s41421-021-00341-7
Hu, S., Pattatucci, A. M. L., Patterson, C., Li, L., Fulker, D. W., Cherny, S. S., et al. (1995). Linkage between sexual orientation and chromosome Xq28 in males but not in females. Nat. Genet. 11, 248–256. doi: 10.1038/ng1195-248
Johannsen, T. H., Ripa, C. P., Mortensen, E. L., and Main, K. M. (2006). Quality of life in 70 women with disorders of sex development. Eur. J. Endocrinol. 155, 877–885. doi: 10.1530/eje.1.02294
Karama, S., Lecours, A. R., Lerous, J. M., Bourgouin, P., Beaudoin, G., Joubert, S., et al. (2002). Areas of brain activation in males and females during viewing of erotic film excerpts. Hum. Brain Mapp. 16, 1–13. doi: 10.1002/hbm.10014
Kendler, K. S., Thornton, L. M., Gilman, S. E., and Kessler, R. C. (2000). Sexual orientation in a U.S. national sample of twin and nontwin sibling pairs. Am. J. Psychiatry 157, 1843–1846. doi: 10.1176/appi.ajp.157.11.1843
Khorashad, B. S., Roshan, G. M., Reid, A. G., Aghili, Z., Hiradfar, M., Afkhamizadeh, M., et al. (2017). Sexual orientation and medical history among Iranian people with complete androgen insensitivity syndrome and congenital adrenal hyperplasia. J. Psychosom. Res. 92, 55–62. doi: 10.1016/j.jpsychores.2016.12.002
Kim, S. W., Sohn, D. W., Cho, Y. H., Yang, W. S., Lee, K. U., Juh, R., et al. (2006). Brain activation by visual erotic stimuli in healthy middle-aged males. Int. J. Impot. Res. 18, 452–457. doi: 10.1038/sj.ijir.3901449
Kinnunen, L. H., Moltz, H., Metz, J., and Cooper, M. (2004). Differential brain activation in exclusively homosexual and heterosexual men produced by the selective serotonin reuptake inhibitor, fluoxetine. Brain Res. 1024, 251–254. doi: 10.1016/j.brainres.2004.07.070
Kirkpatrick, M., and Barton, N. H. (1997). Evolution of a polygenic system under frequency-dependent selection. Genetics 147, 507–519. doi: 10.1093/genetics/147.2.507
Kleinau, G., Neumann, S., Gruters, A., Krude, H., and Biebermann, H. (2013). Novel insights on thyroid-stimulating hormone receptor signal transduction. Endocr. Rev. 34, 691–724. doi: 10.1210/er.2012-1072
Knafo, A., Iervolino, A. C., and Plomin, R. (2005). Masculine girls and feminine boys: genetic and environmental contributions to atypical gender development in early childhood. J. Pers. Soc. Psychol. 88, 400–412. doi: 10.1037/0022-3514.88.2.400
Kuhnle, U., Bullinger, M., Schwarz, H. P., and Knorr, D. (1993). Partnership and sexuality in adult female patients with congenital adrenal hyperplasia. First results of a cross-sectional quality-of-life evaluation. J. Steroid Biochem. Mol. Biol. 45, 123–126. doi: 10.1016/0960-0760(93)90131-F
Ladd, P. D., Smith, L. E., Rabaia, N. A., Moore, J. M., Georges, S. A., Hansen, R. S., et al. (2007). An antisense transcript spanning the CGG repeat region of FMR1 is upregulated in premutation carriers but silenced in full mutation individuals. Hum. Mol. Genet. 16, 3174–3187. doi: 10.1093/hmg/ddm275
Lalumiere, M. L., Blanchard, R., and Zucker, K. J. (2000). Sexual orientation and handedness in men and women: a meta-analysis. Psychol. Bull. 126, 575–592. doi: 10.1037/0033-2909.126.4.575
Lesma, A., Bocciardi, A., Corti, S., Chiumello, G., Rigatti, P., and Montorsi, F. (2014). Sexual function in adult life following Passerini-glazel feminizing genitoplasty in patients with congenital adrenal hyperplasia. J. Urol. 191, 206–211. doi: 10.1016/j.juro.2013.07.097
Levay, S. (1991). A difference in hypothalamic structure between heterosexual and homosexual men. Science (New York, N.Y.), 253, 1034–1037. doi: 10.1126/science.1887219
Liew, S. C., and Gupta, E. D. (2015). Methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism: epidemiology, metabolism and the associated diseases. Eur. J. Med. Genet. 58, 1–10. doi: 10.1016/j.ejmg.2014.10.004
Li, G., Kung, K. T., and Hines, M. (2017). Childhood gender-typed behavior and adolescent sexual orientation: a longitudinal population-based study. Dev. Psychol. 53, 764–777. doi: 10.1037/dev0000281
Lippa, R. A. (2008). Sex differences and sexual orientation differences in personality: findings from the BBC internet survey. Arch. Sex. Behav. 37, 173–187. doi: 10.1007/s10508-007-9267-z
Liu, T., Dartevelle, L., Yuan, C., Wei, H., Wang, Y., Ferveur, J. F., et al. (2008). Increased dopamine level enhances male-male courtship in drosophila. J. Neurosci. 28, 5539–5546. doi: 10.1523/JNEUROSCI.5290-07.2008
Liu, T., Dartevelle, L., Yuan, C., Wei, H., Wang, Y., Ferveur, J. F., et al. (2009). Reduction of dopamine level enhances the attractiveness of male drosophila to other males. PLoS One 4:e4574. doi: 10.1371/journal.pone.0004574
Liu, Y., Jiang, Y., Si, Y., Kim, J. Y., Chen, Z. F., and Rao, Y. (2011). Molecular regulation of sexual preference revealed by genetic studies of 5-HT in the brains of male mice. Nature 472, 95–99. doi: 10.1038/nature09822
Lu, E. Y., and Dawson, W. D. (1986). Paternal antigen and progesterone effects on conceptus size in laboratory mice. Biol. Reprod. 35, 524–530. doi: 10.1095/biolreprod35.3.524
Lutchmaya, S., Baron-Cohen, S., Raggatt, P., Knickmeyer, R., and Manning, J. T. (2004). 2nd to 4th digit ratios, fetal testosterone and estradiol. Early Hum. Dev. 77, 23–28. doi: 10.1016/j.earlhumdev.2003.12.002
Maltry, M., and Tucker, M. (2002). Female Fem(me)ininities: New Articulations in Queer Gender Identities and Subversion. J Lesbian Stud. 6, 89–102. doi: 10.1300/J155v06n02_12
Mandiyan, V. S., Coats, J. K., and Shah, N. M. (2005). Deficits in sexual and aggressive behaviors in Cnga 2 mutant mice. Nat. Neurosci. 8, 1660–1662. doi: 10.1038/nn1589
Manning, J. T. (2002). Digit ratio: A pointer to fertility, behavior and health. London: Rutgers University Press.
Manning, J. T., Bundred, P. E., Newton, D. J., and Flanagan, B. F. (2003). The second to fourth finger length ratio and variation in the androgen receptor gene. Evol. Hum. Behav. 24, 399–405. doi: 10.1016/S1090-5138(03)00052-7
Manning, J. T., Scutt, D., Wilson, J., and Lewis-Jones, D. I. (1998). The ratio of the 2nd to 4th digit length: a predictor of sperm numbers and levels of testosterone, LH and oestrogen. Hum. Reprod. 13, 3000–3004.
Männistö, P. T., and Kaakkola, S. (1999). Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol. Rev. 51, 593–628. doi: 10.1016/S0031-6997(24)01423-6
Männistö, P. T., Ulmanen, I., Lundström, K., Taskinen, J., Tenhunen, J., Tilgmann, C., et al. (1992). Characteristics of catechol O-methyl-transferase (COMT) and properties of selective COMT inhibitors. Prog. Drug Res. 39, 291–350. doi: 10.1007/978-3-0348-7144-0_9
Manzouri, A., and Savic, I. (2018). Multimodal MRI suggests that male homosexuality may be linked to cerebral midline structures. PLoS One 13:e0203189. doi: 10.1371/journal.pone.0203189
Martin, J. T., and Nguyen, D. H. (2004). Anthropometric analysis of homosexuals and heterosexuals: implications for early hormone exposure. Horm. Behav. 45, 31–39. doi: 10.1016/j.yhbeh.2003.07.003
Marvasti, J. A., and Dripchak, V. L. (2004). “The trauma of incest and child sexual abuse: psychobiological perspective” in Psychiatric treatment of victims and survivors of sexual trauma. ed. J. A. Marvasti (Springfield, IL: Charles C Thomas), 3–17.
Mayer, A., Lahr, G., Swaab, D. F., Pilgrim, C., and Reisert, I. (1998). The Y chromosomal genes SRY and ZFY are transcribed in the adult human brain. Neurogenetics 1, 281–288.
McCormick, C. M., and Witelson, S. F. (1994). Functional cerebral asymmetry and sexual orientation in men and women. Behav. Neurosci. 108, 525–531. doi: 10.1037/0735-7044.108.3.525
McFadden, D., and Champlin, C. A. (2000). Comparison of auditory evoked potentials in heterosexual, homosexual and bisexual males and females. J. Assoc. Res. Otolaryngol. 1, 89–99. doi: 10.1007/s101620010008
McFadden, D., and Pasanen, E. G. (1998). Comparison of the auditory systems of heterosexuals and homosexuals: click-evoked otoacoustic emissions. Proc. Natl. Acad. Sci. USA 95, 2709–2713. doi: 10.1073/pnas.95.5.2709
McFadden, D., and Schubel, E. (2002). Relative lengths of fingers and toes in human males and females. Horm. Behav. 42, 492–500. doi: 10.1006/hbeh.2002.1833
Medina, A., Watson, S. J., Bunney, W. Jr., Myers, R. M., Schatzberg, A., Barchas, J., et al. (2016). Evidence for alterations of the glial syncytial function in major depressive disorder. J. Psychiatr. Res. 72, 15–21. doi: 10.1016/j.jpsychires.2015.10.010
Meiser, J., Weindl, D., and Hiller, K. (2013). Complexity of dopamine metabolism. Cell Commun. Signal 11:34. doi: 10.1186/1478-811X-11-34
Melis, M. R., and Argiolas, A. (1995). Dopamine and sexual behavior. Neurosci. Biobehav. Rev. 19, 19–38. doi: 10.1016/0149-7634(94)00020-2
Melis, M. R., Argiolas, A., and Gessa, G. L. (1987). Apomorphine-induced penile erection and yawning: site of action in brain. Brain Res. 415, 98–104. doi: 10.1016/0006-8993(87)90272-1
Melis, M. R., Sanna, F., and Argiolas, A. (2022). Dopamine, erectile function and male sexual behavior from the past to the present: a review. Brain Sci. 12:826. doi: 10.3390/brainsci12070826
Meyer-Bahlburg, H. F., Dolezal, C., Baker, S. W., and New, M. I. (2008). Sexual orientation in women with classical or non-classical congenital adrenal hyperplasia as a function of degree of prenatal androgen excess. Arch. Sex. Behav. 37, 85–99. doi: 10.1007/s10508-007-9265-1
Meyer-Bahlburg, H. F. L., Ehrhardt, A. A., Rosen, L. R., Gruen, R. S., Veridiano, N. P., Vann, F. H., et al. (1995). Prenatal estrogens and the development of homosexual orientation. Dev. Psychol. 31, 12–21. doi: 10.1037/0012-1649.31.1.12
Money, J., Schwartz, M., and Lewis, V. G. (1984). Adult erotosexual status and fetal hormonal masculinization and demasculinization: 46, XX congenital virilizing adrenal hyperplasia and 46, XY androgen-insensitivity syndrome compared. Psychoneuroendocrinology 9, 405–414. doi: 10.1016/0306-4530(84)90048-9
Morris, J. A., Jordan, C. J., and Breedlove, S. M. (2004). Sexual differentiation of the vertebrate nervous systems. Nat. Neurosci. 7, 1034–1039. doi: 10.1038/nn1325
Motofei, I. G., and Rowland, D. L. (2014). The ventral-hypothalamic input route: a common neural network for abstract cognition and sexuality. BJU Int. 113, 296–303. doi: 10.1111/bju.12399
Mustanski, B. S., DuPree, M. G., Nievergelt, C. M., Bocklandt, S., Schork, N. J., and Hamer, D. H. (2005). A genomewide scan of male sexual orientation. Hum. Genet. 116, 272–278. doi: 10.1007/s00439-004-1241-4
Myöhänen, T. T., Schendzielorz, N., and Männistö, P. T. (2010). Distribution of catechol-O-methyltransferase (COMT) proteins and enzymatic activities in wild-type and soluble COMT deficient mice. J. Neurochem. 113, 1632–1643. doi: 10.1111/j.1471-4159.2010.06723.x
Nakae, J., Tajima, T., Sugawara, T., Arakane, F., Hanaki, K., Hotsubo, T., et al. (1997). Analysis of the steroidogenic acute regulatory protein (StAR) gene in Japanese patients with congenital lipoid adrenal hyperplasia. Hum. Mol. Genet. 6, 571–576. doi: 10.1093/hmg/6.4.571
Nazki, F. H., Sameer, A. S., and Ganaie, B. A. (2014). Folate: metabolism, genes, polymorphisms and the associated diseases. Gene 533, 11–20. doi: 10.1016/j.gene.2013.09.063
Ngun, T. C., and Vilain, E. (2014). The biological basis of human sexual orientation: a review. J. Endocrinol. 222, R9–R20. doi: 10.1016/B978-0-12-800222-3.00008-5
Nguyen, T. V., McCracken, J., Ducharme, S., Botteron, K. N., Mahabir, M., Johnson, W., et al. (2013). Testosterone-related cortical maturation across childhood and adolescence. Cereb. Cortex 23, 1424–1432. doi: 10.1093/cercor/bhs125
Northoff, G. (2011). Self and brain: what is self-related processing? Trends Cogn. Sci. 15, 186–187. doi: 10.1016/j.tics.2011.03.001
Pattatucci, A. M. L., and Hamer, D. H. (1995). Development and familiality of sexual orientation in females. Behav. Genet. 25, 407–420. doi: 10.1007/BF02253370
Pearcey, S. M., Docherty, K. J., and Dabbs, J. M.Jr. (1996). Testosterone and sex role identification in lesbian couples. Physiol Behav. 60, 1033–1035. doi: 10.1016/0031-9384(96)00132-1
Pereda, N., Guilera, G., Forns, M., and Gomez-Benito, J. (2009b). The prevalence of child sexual abuse in community and student samples: a meta-analysis. Clin. Psychol. Rev. 29, 328–338. doi: 10.1016/j.cpr.2009.02.007
Pillard, R. C., and Bailey, J. M. (1998). Human sexual orientation has a heritable component. Hum. Biol. 70, 347–365.
Pillard, R. C., Poumadere, J., and Carretta, R. A. (1981). Is homosexuality familial? A review, some data, and a suggestion. Arch. Sex. Behav. 10, 465–475. doi: 10.1007/BF01541437
Pillard, R. C., Poumadere, J., and Carretta, R. A. (1982). A family study of sexual orientation. Arch. Sex. Behav. 11, 511–520. doi: 10.1007/BF01542476
Poeppl, T. B., Langguth, B., Rupprecht, R., Safron, A., Bzdok, D., Laird, A. R., et al. (2016). The neural basis of sex differences in sexual behavior: a quantitative meta-analysis. Front. Neuroendocrinol. 43, 28–43. doi: 10.1016/j.yfrne.2016.10.001
Poletti, S., Riberto, M., Vai, B., Ghiglino, D., Lorenzi, C., Vitali, A., et al. (2018). A glutamate transporter EAAT1 gene variant influences amygdala functional connectivity in bipolar disorder. J. Mol. Neurosci. 65, 536–545. doi: 10.1007/s12031-018-1138-7
Purcell, D. W., Blanchard, R., and Zucker, K. J. (2000). Birth order in a contemporary sample of gay men. Arch. Sex. Behav. 29, 349–356. doi: 10.1023/A:1001966320273
Qin, J. B., Zhao, G. L., Wang, F., Cai, Y. M., Lan, L. N., Yang, L., et al. (2018). Childhood abuse experiences and the COMT and MTHFR genetic variants associated with male sexual orientation in the Han Chinese populations: a case-control study. J. Sex. Med. 15, 29–42. doi: 10.1016/j.jsxm.2017.11.010
Qin, Z., Ren, F., Xu, X., Ren, Y., Li, H., Wang, Y., et al. (2009). ZNF536, a novel zinc finger protein specifically expressed in the brain, negatively regulates neuron differentiation by repressing retinoic acid-induced gene transcription. Mol. Cell. Biol. 29, 3633–3643. doi: 10.1128/MCB.00362-09
Rahman, Q. (2005a). The neurodevelopment of human sexual orientation. Neurosci. Biobehav. Rev. 29, 1057–1066. doi: 10.1016/j.neubiorev.2005.03.002
Rahman, Q. (2005b). Fluctuating asymmetry, 2nd to 4th finger length ratios and human sexual orientation. Psychoneuroendocrinology 30, 382–391. doi: 10.1016/j.psyneuen.2004.10.006
Rahman, Q., Abrahams, S., and Wilson, G. D. (2003a). Sexual orientation related differences in verbal fluency. Neuropsychology 17, 240–246. doi: 10.1037/0894-4105.17.2.240
Rahman, Q., Kumari, V., and Wilson, G. D. (2003b). Sexual orientation related differences in pre-pulse inhibition of the human startle response. Behav. Neurosci. 117, 1096–1102. doi: 10.1037/0735-7044.117.5.1096
Rahman, Q., and Wilson, G. D. (2003a). Large sexual orientation related differences in performance on mental rotation and judgement of line orientation. Neuropsychology 17, 25–31. doi: 10.1037/0894-4105.17.1.25
Rahman, Q., and Wilson, G. D. (2003b). Sexual orientation and the 2nd to 4th finger length ratio: evidence for organising effects of sex hormones or developmental instability? Psychoneuroendocrinology 28, 288–303. doi: 10.1016/s0306-4530(02)00022-7
Rahman, Q., Wilson, G. D., and Abrahams, S. (2003c). Sexual orientation related differences in spatial memory. J. Int. Neuropsychol. Soc. 9, 376–383. doi: 10.1017/S1355617703930037
Rakic, P. (1988). Specification of cerebral cortical areas. Science 241, 170–176. doi: 10.1126/science.3291116
Ramagopalan, S. V., Dyment, D. A., Handunnetthi, L., Rice, G. P., and Ebers, G. C. (2010). A genome-wide scan of male sexual orientation. J. Hum. Genet. 55, 131–132. doi: 10.1038/jhg.2009.135
Raznahan, A., Lee, Y., Stidd, R., Long, R., Greenstein, D., Clasen, L., et al. (2010). Longitudinally mapping the influence of sex and androgen signaling on the dynamics of human cortical maturation in adolescence. Proc. Natl. Acad. Sci. 107, 16988–16993. doi: 10.1073/pnas.1006025107
Reite, M., Sheeder, J., Richardson, D., and Teale, P. (1995). Cerebral laterality in homosexual males: preliminary communication using magnetoencephalography. Arch. Sex. Behav. 24, 585–593. doi: 10.1007/BF01542181
Rice, W. R., Friberg, U., and Gavrilets, S. (2012). Homosexuality as a consequence of epigenetically canalized sexual development. Q. Rev. Biol. 87, 343–368. doi: 10.1086/668167
Rieger, G., Cholewinski, A., and Riemann, B. (2008a). Sexual orientation and genetic factors. Arch. Sex. Behav. 37, 895–903. doi: 10.1007/s10508-008-9290-5
Rieger, G., Linsenmeier, J. A., Gygax, L., and Bailey, J. M. (2008b). Sexual orientation and childhood gender nonconformity: evidence from home videos. Dev. Psychol. 44, 46–58. doi: 10.1037/0012-1649.44.1.46
Rind, B. (2001). Gay and bisexual adolescent boys’ sexual experiences with men: an empirical examination of psychological correlates in a nonclinical sample. Arch. Sex. Behav. 30, 345–368. doi: 10.1023/A:1010210630788
Roberts, A. L., Glymour, M. M., and Koenen, K. C. (2013). Does maltreatment in childhood affect sexual orientation in adulthood? Arch. Sex. Behav. 42, 161–171. doi: 10.1007/s10508-012-0021-9
Robinson, S. J., and Manning, J. T. (2000). The ratio of 2nd to 4th digit length and male homosexuality. Evol. Hum. Behav. 21, 333–345.
Rosario, M., and Schrimshaw, E. W. (2014). “Theories and etiologies of sexual orientation” in APA handbook of sexuality and psychology. eds. D. L. Tolman and L. M. Diamond (Washington, DC: American Psychological Association), 556–559.
Rosario, M., Schrimshaw, E. W., and Hunter, J. (2011) The coming-out process of young lesbian and bisexual women: are there butch/femme differences in sexual identity development? J. Sex Res. 48, 395–408. Available online at: https://pmc.ncbi.nlm.nih.gov/articles/PMC3189348/
Rosario, M., Schrimshaw, E. W., Hunter, J., and Braun, L. (2006). Sexual identity development among gay, lesbian, and bisexual youths: consistency and change over time. J. Sex Res. 43, 46–58. doi: 10.1080/00224490609552298
Roselli, C. E. (2018). Neurobiology of gender identity and sexual orientation. J. Neuroendocrinol. 30:e12562. doi: 10.1111/jne.12562
Roselli, C. E., Larkin, K., Resko, J. A., Stellflug, J. N., and Stormshak, F. (2004). The volume of a sexually dimorphic nucleus in the ovine medial preoptic area/anterior hypothalamus varies with sexual partner preference. Endocrinology 145, 478–483. doi: 10.1210/en.2003-1098
Sabuncuoglu, O. (2015). High rates of same-sex attraction/gender nonconformity in the offspring of mothers with thyroid dysfunction during pregnancy: proposal of prenatal thyroid model. Ment. Illn. 7:5810. doi: 10.4081/mi.2015.5810
Sahakitrungruang, T., Soccio, R. E., Lang-Muritano, M., Walker, J. M., Achermann, J. C., and Miller, W. L. (2010). Clinical, genetic, and functional characterization of four patients carrying partial loss-of-function mutations in the steroidogenic acute regulatory protein (StAR). J Clin Endocrinol Metab. 95, 3352–3359. doi: 10.1210/jc.2010-0437
Sanders, A. R., Beecham, G. W., Guo, S., Badner, J. A., Bocklandt, S., Mustanski, B. S., et al. (2021). Genome-wide linkage study meta-analysis of male sexual orientation. Arch. Sex. Behav. 50, 3371–3375. doi: 10.1007/s10508-021-02035-3
Sanders, A. R., Beecham, G. W., Guo, S., Dawood, K., Rieger, G., Badner, J. A., et al. (2017). Genome-wide association study of male sexual orientation. Sci. Rep. 7:16950. doi: 10.1038/s41598-017-15736-4
Sanders, A. R., Martin, E. R., Beecham, G. W., Guo, S., Dawood, K., Rieger, G., et al. (2015). Genome-wide scan demonstrates significant linkage for male sexual orientation. Psychol. Med. 45, 1379–1388. doi: 10.1017/S0033291714002451
Sanders, G., and Wright, M. (1997). Sexual orientation differences in cerebral asymmetry and in the performance of sexually dimorphic cognitive and motor tasks. Arch. Sex. Behav. 26, 463–480. doi: 10.1023/A:1024551704723
Sasani, H., and Gardner, A. (2020). Frequency-dependent selection and the evolution of sexual orientation. Evol. Biol. 47, 197–207. doi: 10.1007/s11692-020-09504-y
Savic, I., and Arver, S. (2014). Sex differences in cortical thickness and their possible genetic and sex hormonal underpinnings. Cereb. Cortex 24, 3246–3257. doi: 10.1093/cercor/bht180
Sesack, S. R., Hawrylak, V. A., Matus, C., Guido, M. A., and Levey, A. I. (1998). Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J. Neurosci. 18, 2697–2708. doi: 10.1523/JNEUROSCI.18-07-02697.1998
Shmelkov, S. V., Hormigo, A., Jing, D., Proenca, C. C., Bath, K. G., Milde, T., et al. (2010). Slitrk5 deficiency impairs corticostriatal circuitry and leads to obsessive-compulsive-like behaviors in mice. Nat. Med. 16, 598–602. doi: 10.1038/nm.2125
Singh, D., Vidaurri, M., Zambarano, R. J., and Dabbs, J. M. Jr. (1999). Lesbian erotic role identification: Behavioral, morphological, and hormonal correlates. J. Pers. Soc. Psychol. 76, 1035–1049. doi: 10.1037/0022-3514.76.6.1035
Singh, J., and Verma, I. C. (1987). Influence of major histo(in)compatibility complex on reproduction. Am. J. Reprod. Immunol. Microbiol. 15, 150–152. doi: 10.1111/j.1600-0897.1987.tb00007.x
Speranza, L., di Porzio, U., Viggiano, D., de Donato, A., and Volpicelli, F. (2021). Dopamine: the neuromodulator of long-term synaptic plasticity, reward and movement control. Cells 10:735. doi: 10.3390/cells10040735
Stoltenborgh, M., van Ijzendoorn, M. H., Euser, E. M., and Bakermans-Kranenburg, M. J. (2011). A global perspective on child sexual abuse: meta-analysis of prevalence around the world. Child Maltreat. 16, 79–101. doi: 10.1177/1077559511403920
Sugawara, T., Holt, J. A., Driscoll, D., Strauss, J. F.3rd, Lin, D., Miller, W. L., et al. (1995). Human steroidogenic acute regulatory protein: functional activity in COS-1 cells, tissue-specific expression, and mapping of the structural gene to 8p11.2 and a pseudogene to chromosome 13. Proc Natl Acad Sci. 92, 4778–4782. doi: 10.1073/pnas.92.11.4778
Szyf, M., and Bick, J. (2013). DNA methylation: a mechanism for embedding early life experiences in the genome. Child Dev. 84, 49–57. doi: 10.1111/j.1467-8624.2012.01793.x
Tekin, M., Chioza, B. A., Matsumoto, Y., Diaz-Horta, O., Cross, H. E., Duman, D., et al. (2013). SLITRK6 mutations cause myopia and deafness in humans and mice. J. Clin. Invest. 123, 2094–2102. doi: 10.1172/JCI65853
van Amen-Hellebrekers, C. J., Jansen, S., Pfundt, R., Schuurs-Hoeij-makers, J. H., Koolen, D. A., Marcelis, C. L., et al. (2016). Duplications of SLC1A3: associated with ADHD and autism. Eur. J. Med. Genet. 59, 373–376. doi: 10.1016/j.ejmg.2016.06.003
Van Anders, S. M., and Hampson, E. (2005). Waist-to-hip ratio is positively associated with bioavailable testosterone but negatively associated with sexual desire in healthy premenopausal women. Psychosom. Med. 67, 246–250. doi: 10.1097/01.psy.0000151747.22904.d7
van Beijsterveldt, C. E., Hudziak, J. J., and Boomsma, D. I. (2006). Genetic and environmental influences on cross-gender behavior and relation to behavior problems: a study of Dutch twins at ages 7 and 10 years. Arch. Sex. Behav. 35, 647–658. doi: 10.1007/s10508-006-9072-0
Waisman, R., Fenwick, P. B. C., Wilson, G. D., Hewett, T. D., and Lumsden, J. (2003). EEG responses to visual erotic stimuli in men with normal and paraphilic interests. Arch. Sex. Behav. 32, 135–144. doi: 10.1023/a:1022448308791
Wang, L. J., Lee, S. Y., Chen, S. L., Chang, Y.-H., Chen, P. S., Huang, S.-Y., et al. (2015). A potential interaction be-tween COMT and MTHFR genetic variants in Han Chinese patients with bipolar II disorder. Sci. Rep. 5:8813. doi: 10.1038/srep08813
Wegesin, D. J. (1998a). Event related potentials in homosexual and heterosexual men and women: sex dimorphic patterns in verbal asymmetries and mental rotations. Brain Cogn. 36, 73–92. doi: 10.1006/brcg.1997.0964
Wegesin, D. J. (1998b). Relation between language lateralisation and spatial ability in gay and straight men and women. Laterality 3, 227–239.
Werkman, T. R., Glennon, J. C., Wadman, W. J., and McCreary, A. C. (2006). Dopamine receptor pharmacology: interactions with serotonin receptors and significance for the aetiology and treatment of schizophrenia. CNS Neurol. Disord. 5, 3–23. doi: 10.2174/187152706784111614
Williams, T. J., Pepitone, M. E., Christensen, S. E., Cooke, B. M., Huberman, A. D., Breedlove, N. J., et al. (2000). Finger length ratio and sexual orientation. Nature 404, 455–456.
Witelson, S. F., Kigar, D. L., Scamvougeras, A., Kideckel, D. M., Buck, B., Stanchev, P. L., et al. (2008). Corpus callosum anatomy in right-handed homosexual and heterosexual men. Arch. Sex. Behav. 37, 857–863. doi: 10.1007/s10508-007-9276-y
Wyre, R. (1990). “Why do men sexually abuse children?” in Child pornography. ed. T. Tate (London: Methuen), 281–288.
Xu, Y., Norton, S., and Rahman, Q. (2019). Early life conditions and adolescent sexual orientation: a prospective birth cohort study. Dev. Psychol. 55, 1226–1243. doi: 10.1037/dev0000704
Xu, Y., Norton, S., and Rahman, Q. (2020). Childhood maltreatment, gender nonconformity, and adolescent sexual orientation: a prospective birth cohort study. Child Dev. 91, e984–e994. doi: 10.1111/cdev.13317
Xu, Y., Norton, S., and Rahman, Q. (2021). Childhood gender nonconformity and the stability of self-reported sexual orientation from adolescence to young adulthood in a birth cohort. Dev. Psychol. 57, 557–569. doi: 10.1037/dev0001164
Xu, Y., and Zheng, Y. (2015). Prevalence of childhood sexual abuse among lesbian, gay, and bisexual people: a meta-analysis. J. Child Sex. Abus. 24, 315–331. doi: 10.1080/10538712.2015.1006746
Yadav, R., Gupta, S. C., Hillman, B. G., Bhatt, J. M., Stairs, D. J., and Dravid, S. M. (2012). Deletion of glutamate delta-1 receptor in mouse leads to aberrant emotional and social behaviors. PLoS One 7:e32969. doi: 10.1371/journal.pone.0032969
Zhang, H., and Sulzer, D. (2012). Regulation of striatal dopamine release by presynaptic auto- and heteroreceptors. Basal Ganglia. 2, 5–13. doi: 10.1016/j.baga.2011.11.004
Zucker, K. J., Bradley, S. J., Oliver, G., Blake, J., Fleming, S., and Hood, J. (1996). Psychosexual development of women with congenital adrenal hyperplasia. Horm. Behav. 30, 300–318. doi: 10.1006/hbeh.1996.0038
Keywords: homosexuality, LGBT, biological factors, environmental factors, neurologic factors, genetic factors, sexual orientation
Citation: Alagha M, Antoun F, Bacha C, El Nabbout T and El Khoury NB (2025) Biological, genetic, neurological and environmental influences on homosexuality—a narrative review. Front. Behav. Neurosci. 19:1574713. doi: 10.3389/fnbeh.2025.1574713
Edited by:
Walter Adriani, National Institute of Health (ISS), ItalyReviewed by:
Aidas Aglinskas, Boston College, United StatesSebastian Gabini, CONICET Rosario, Argentina
Copyright © 2025 Alagha, Antoun, Bacha, El Nabbout and El Khoury. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Noura B. El Khoury, bm91cmEua2hvdXJ5QGJhbGFtYW5kLmVkdS5sYg==
 Michel Alagha1
Michel Alagha1 Freddy Antoun
Freddy Antoun Noura B. El Khoury
Noura B. El Khoury