- 1Department of Psychological and Brain Sciences, University of California Santa Barbara, Santa Barbara, CA, United States
- 2Department of Molecular, Cellular and Developmental Biology, University of California Santa Barbara, Santa Barbara, CA, United States
- 3Neuroscience Research Institute, University of California Santa Barbara, Santa Barbara, CA, United States
Introduction: Early-onset binge-drinking and biological sex are critical risk factors for the development of cognitive decline and neurodegeneration associated with Alzheimer’s disease and related dementias (ADRDs). Recently, we demonstrated that a prior history of binge-drinking during adolescence induces what appears to be latent (>6 months post-drinking) changes in the expression of glutamate receptors and neuropathology markers within brain regions governing working and spatial memory, many of which precede the manifestation of overt cognitive anomalies.
Methods: To determine whether alcohol-induced changes in protein expression manifest within the hippocampus and prefrontal cortex at earlier times post-drinking, we conducted immunoblotting on tissue from mice with a subchronic history of binge-drinking (14 days of 2-h access to 10, 20 and 40% ethanol) during either adolescence or adulthood.
Results: We previously reported that this binge-drinking regimen produces mild, age- and sex-selective, changes in working memory and spatial recall when behavior was assayed starting a 1 or 30 days withdrawal. Here, we provide evidence a subchronic binge-drinking history is sufficient to alter the expression of certain glutamate receptors and ADRD-related proteins during the first few months following drinking cessation. Further, these alcohol-induced protein changes are regionally specific and sex-selective.
Discussion: The present results add to our growing understanding of the long-term consequences of adolescent-onset binge-drinking of potential relevance to understanding individual variability in the cognitive consequences of heavy drinking.
Introduction
A history of excessive alcohol-drinking is one of the most common risk factors for the development of dementia and cognitive decline (Wiegmann et al., 2020; Nunes et al., 2019; Schwarzinger et al., 2018) and there exists a high rate of co-occurrence between alcohol use disorder (AUD) and dementia, particularly Alzheimer’s Disease and related dementia (ADRD) (Hersi et al., 2017; Hoffman et al., 2019). The age of drinking-onset and amount of alcohol consumed during adolescence are major predictors of both AUD and early-onset ADRDs (Huang et al., 2018; Ledesma et al., 2021), with evidence from both rodent and human studies demonstrating that excessive alcohol intake during adolescence perturbs the development of the mesocorticolimbic system, which is presumed to contribute to cognitive anomalies in later life (Cservenka and Brumback, 2017; Novier et al., 2015). Consistent with this, we (Chavez et al., 2024; Jimenez Chavez et al., 2023; Szumlinski et al., 2019) and others (e.g., Van Hees et al., 2021) demonstrated recently that both subchronic (14-day) and more chronic (30-day) histories of binge-drinking during adolescence are sufficient to induce certain cognitive impairments in both the short (<2 months) and longer (≥ 6 months) term in C57BL/6J (B6J) mice. Further, the longer term (i.e., ≥ 6 months) cognitive effects of a prior adolescent binge-drinking history appear to be more apparent in female mice, while those observed in earlier withdrawal (≤ 30–40 days) appear to be less sex-dependent. Such findings align with evidence from rodent studies of forced “binge-like” alcohol exposure during adolescence (e.g., Pascual et al., 2007), as well as studies of humans indicating that women tend to exhibit higher vulnerability to alcohol-induced dementia in later life than men (Agabio et al., 2017; Ferretti et al., 2018; Hebert et al., 2013).
The onset of psychiatric symptoms indicative of, and comorbid with, an AUD has been linked to compensatory changes related to an overactive glutamate system that can persist during alcohol abstinence (e.g., Bergink et al., 2004; Hoffman and Tabakoff, 1994; Kamal et al., 2020; Koob, 2021), notably within key brain regions gating executive function, learning, and memory such as the prefrontal cortex (PFC) (Crews and Vetreno, 2014; Kalluri et al., 1998; Medina et al., 2008), entorhinal cortex (Ibáñez et al., 1995; Crews et al., 2000), and hippocampus (De Bellis et al., 2000; Kalluri et al., 1998; Nagel et al., 2005). Consistent with this, glutamate-dependent excitotoxicity has long been implicated in neurodegenerative diseases, as well as the capacity of alcohol to accelerate disease progression (e.g., Heymann et al., 2016; Kamal et al., 2020; Mira et al., 2019; Peng et al., 2020). Sex differences have been reported in the effects of chronic alcohol consumption on the expression of NMDA receptor subunits within the cortex and hippocampus (Chavez et al., 2024; Devaud and Morrow, 1999). Despite this, and the facts that women are approximately twice as likely to develop ADRD than men (Ferretti et al., 2018; Hebert et al., 2013) and the gender gap in binge-alcohol consumption is nearly closed (White, 2020), little preclinical research work has examined for sex differences in the effects of repeated alcohol exposure during adolescence on the expression of glutamate-related proteins and their relationship to indices of neuropathology within PFC and hippocampus. These regions are of particular interest as human studies indicate a more pronounced volumetric reduction within the PFC of women versus men with adolescent-onset heavy drinking (Medina et al., 2008), while hippocampal volumes appear to be similarly impacted by adolescent drinking in both sexes (De Bellis et al., 2000; Nagel et al., 2005).
Recently, we showed that female mice with a prior adolescent binge-drinking history exhibit more anomalies in the expression of glutamate receptor-related proteins, indices of kinase activity and markers of neuropathology within PFC, amygdala and entorhinal cortex when assayed following very prolonged periods of alcohol abstinence (6–12 months) (Chavez et al., 2024). These immunoblotting data indicate that a prior history of adolescent-onset binge-drinking produces latent or long-lasting (6–12 month) changes in brain biochemistry to which females may be more susceptible. Whether or not the same might be true with respect to sex differences in binge drinking-induced changes in brain biochemistry earlier during alcohol withdrawal remains to be determined. The present study sought to address this question by examining for sex by age interactions in the expression of glutamate receptor-related proteins, as well as protein indices of ADRD-related neuropathology within the hippocampus and PFC of the B6J mice from our prior behavioral study examining for sex differences in the effect of withdrawal from subchronic (14 days) binge-drinking during either adolescence or adulthood on negative affect and spatial cognition (Jimenez Chavez et al., 2023). Specifically, we immunoblotted for the obligatory GluN1 subunit of the NMDA receptor to index total NMDA receptor expression, as well as the alcohol-sensitive GluN2B NMDA receptor subunit (e.g., Wills et al., 2017). Both of these NMDA receptor subunits are up-regulated within hippocampus and PFC by alcohol exposure (e.g., Devaud and Morrow, 1999; Littleton et al., 2001; Mira et al., 2019; Nagy, 2004). Moreover, these subunits are up-regulated in adult rodents with a prior history of adolescent alcohol exposure (Carpenter-Hyland and Chandler, 2007; Swartzwelder et al., 2016; but see also Chavez et al., 2024). As the mGlu1 and mGlu5 subtypes of metabotropic glutamate receptors (mGluRs) are also upregulated in both adult mice with a history of adult-onset (Cozzoli et al., 2009, 2012, 2014; Szumlinski et al., 2023) or adolescent-onset binge-drinking (Chavez et al., 2024; Lee et al., 2016; Lee et al., 2017b), we examined their expression along with that of their highly alcohol-sensitive Homer scaffolding proteins (Campbell et al., 2019; Chavez et al., 2024; Cozzoli et al., 2009, 2012, 2014; Lee et al., 2016; Szumlinski et al., 2023). As in our recent reports (Campbell et al., 2019; Chavez et al., 2024), we also examined for p(Tyr204)-ERK1/2 expression to index cellular activity. Finally, we immunoblotted for certain proteins that currently serve as strong and reliable biomarkers of AD in human brain [e.g., amyloid precursor protein (APP), amyloid-β peptides (Aβ), hyper-phosphorylated tau proteins and beta secretase (BACE); Banning et al., 2021; Cheignon et al., 2018; Dodart et al., 2002; Hamley, 2012; Hersi et al., 2017; Perl, 2010]. These proteins accumulate in brain during normal aging in both humans and rodents (Arriagada et al., 1992; Haroutunian et al., 1998; O'Brien et al., 2009; Troncoso et al., 1998) and can be induced by prior alcohol experience in laboratory rodents (Hoffman et al., 2019; Liu et al., 2022; Salling et al., 2016; Szumlinski et al., 2023).
Based on studies of sex differences in alcohol-induced protein expression (Jimenez Chavez et al., 2022; Chavez et al., 2024), we hypothesized that females would exhibit more alcohol-related changes in protein expression than males, particularly in later withdrawal, when females exhibit more cognitive impairment than males (Jimenez Chavez et al., 2023).
Materials and methods
Subjects
The male and female C57BL/6J (B6J) mice employed in the present study were the same as those described previously in Jimenez Chavez et al. (2023). In brief, adolescent and early adult mice were shipped from The Jackson Laboratory (Sacramento, CA, United States) at ages PND21 and PND 49, respectively. Mice were then housed under a reverse light cycle (lights off: 1,100 h) in same-sex and -age groups of 4 for 1 week prior to the commencement of study. Prior to tissue collection for the present study, subjects underwent a 14-day 2 h/day Drinking-in-the-Dark (DID) procedure, followed by a 1-day test battery for negative affect consisting of a light–dark box, a forced swim and a marble-burying assay, a 7-day Morris water maze and a 14-day radial arm maze procedure (see Figure 1). Procedural details of these behavioral assays are provided in Jimenez Chavez et al. (2023). All experimental procedures aligned with The Guide for the Care and Use of National Research Council (2011) and all protocols were approved by the Institutional Animal Care and Use Committee of the University of California, Santa Barbara.
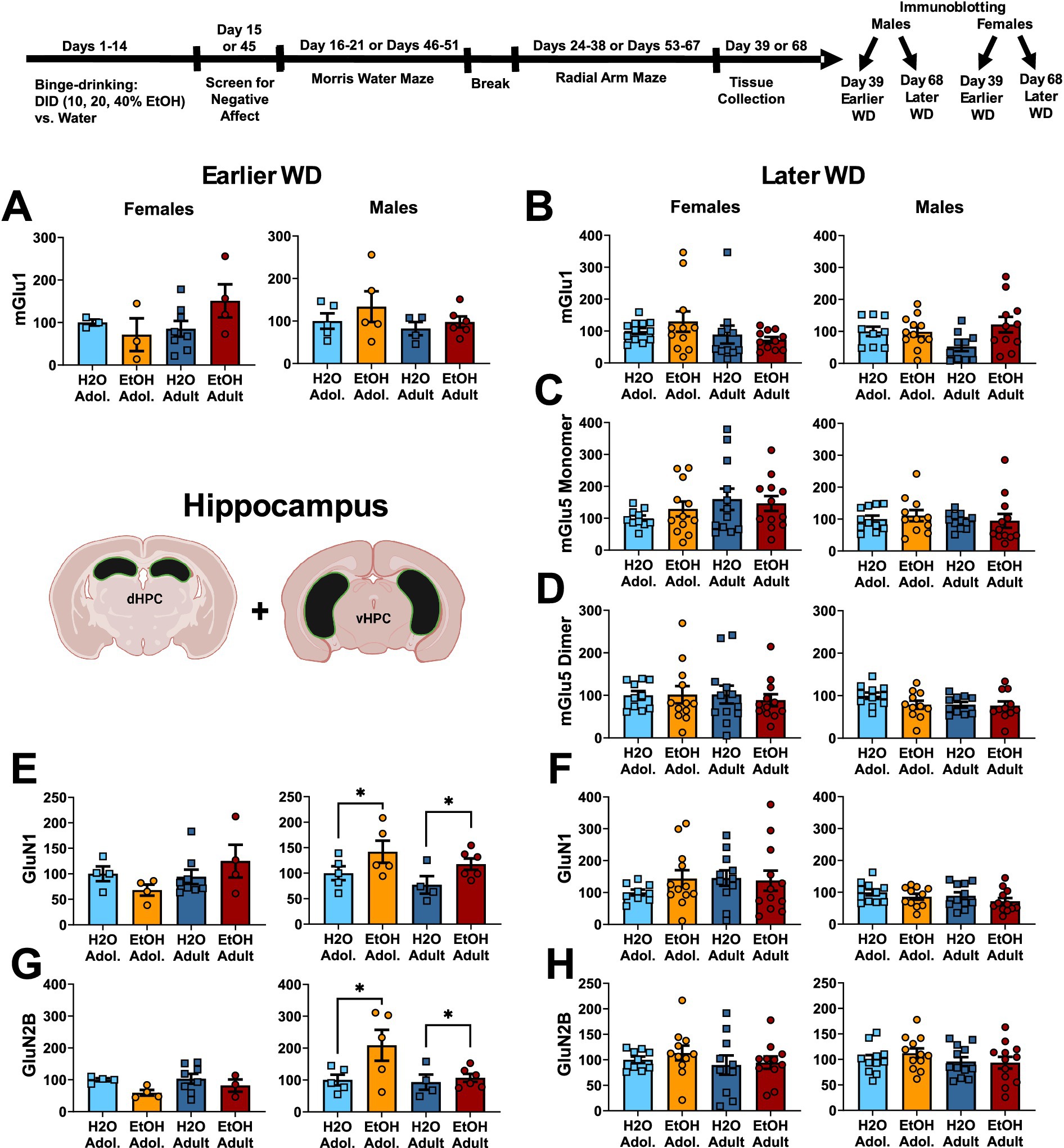
Figure 1. Summary of the changes in mGlu1 (A,B), the monomer (C) and dimer form of mGlu5 (D), GluN1 (E,F) and GluN2B (G,H) within the hippocampus of mice with a 2-week history of binge-drinking (EtOH) during either adolescence (Adol.) or adulthood (Adult) observed during early (left) or later alcohol withdrawal (right). H2O = water-drinking controls. Immunoblotting for mGlu5 was not conducted on hippocampus tissue from mice tested in early withdrawal. A cartoon illustrating the sites of tissue dissection is also presented. The data represent the means ± SEMs of the number of individual mice indicated. *p < 0.05 H2O vs. age-matched EtOH group (alcohol effect).
Immunoblotting
One day following the end of radial arm maze procedures (e.g., 25 or 54 days following the last drinking session for mice tested at the earlier versus later withdrawal time-point; see Figure 1), mice were decapitated, brains were extracted, cooled on ice, and then sectioned in 1 mm-thick coronal slices. Using blunt forceps, the entire PFC was dissected out and then both the ventral and dorsal hippocampus removed and hippocampal subregions combined into a single sample as illustrated in Figures 1–3 for hippocampus and Figures 2–4 for PFC.
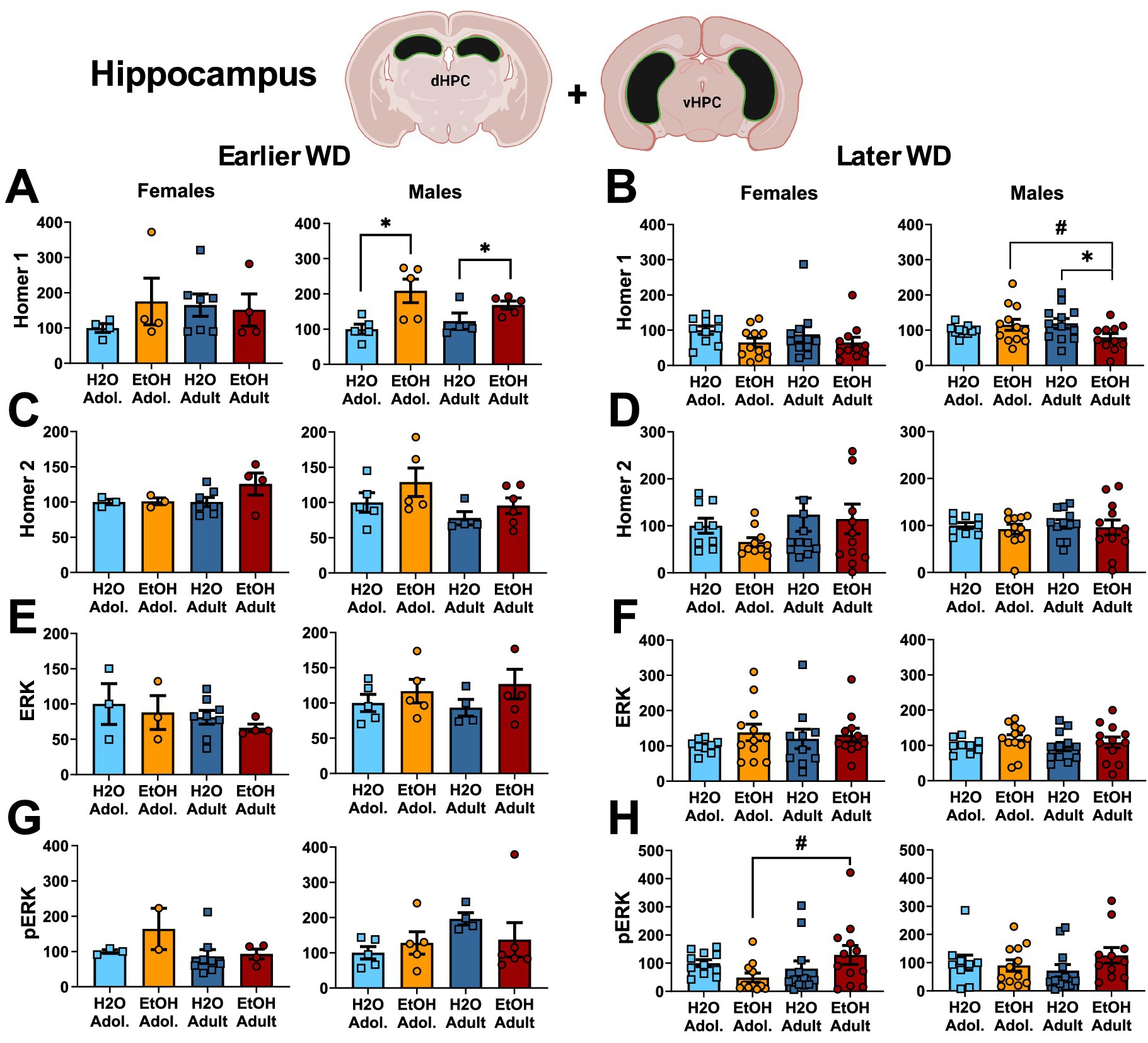
Figure 2. Summary of the changes in Homer1 (A,B), Homer2 (C,D), ERK (E,F) and p(Tyr204)-ERK (G,H) within the hippocampus of mice with a 2-week history of binge-drinking (EtOH) during either adolescence (Adol.) or adulthood (Adult) observed during early (left) or later alcohol withdrawal (right). H2O = water-drinking controls. A cartoon illustrating the sites of tissue dissection is also presented. The data represent the means ± SEMs of the number of individual mice indicated. *p < 0.05 H2O vs. age-matched EtOH group (alcohol effect); #p < 0.05 Adol. vs. Adult (age effect).
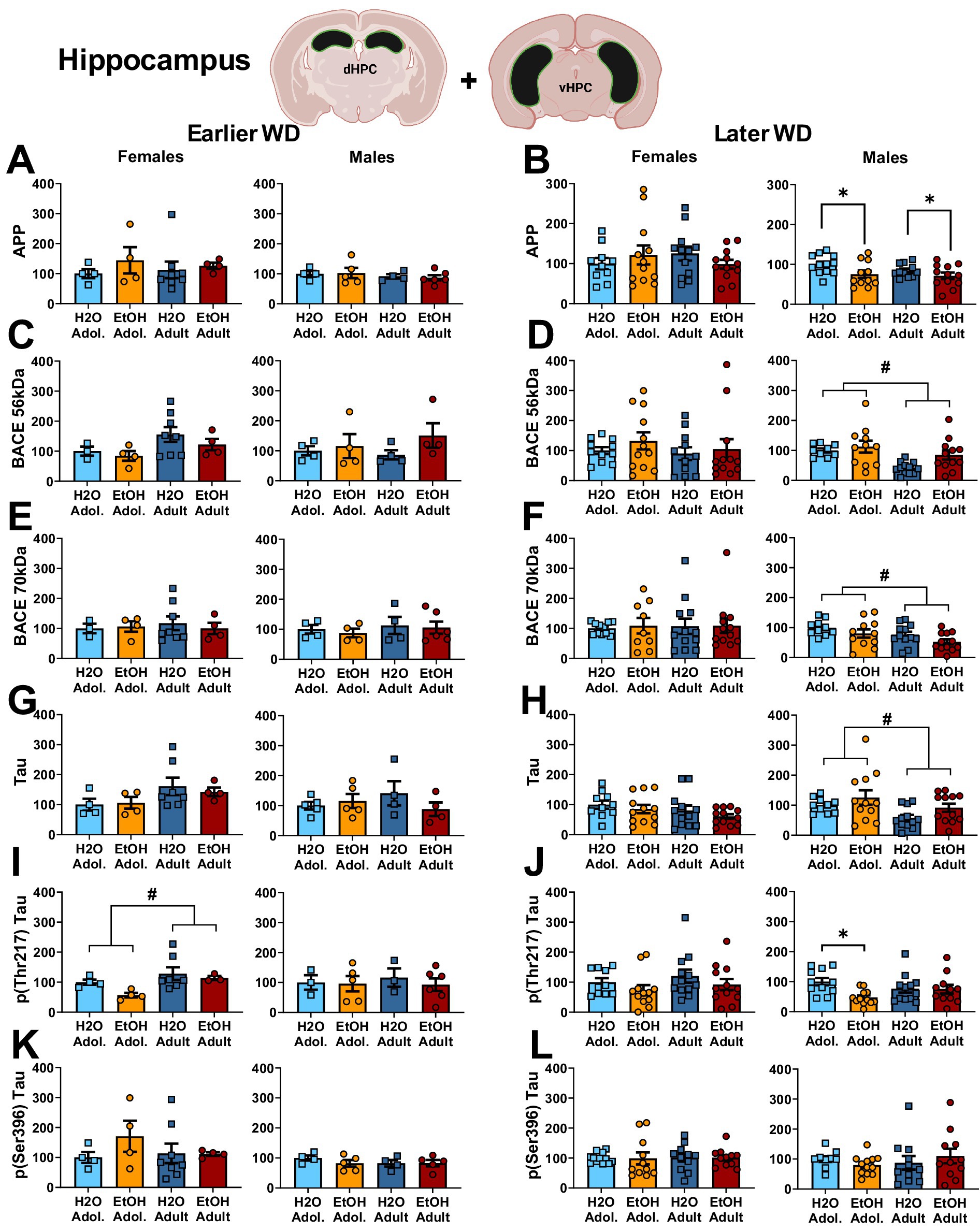
Figure 3. Summary of the changes in APP (A,B), the 56 kDa (C,D) and the 70 kDa isoforms of BACE (E,F), Tau (G,H), p(Thr217)-Tau (I,J) and p(Ser396)-Tau (K,L) within the hippocampus of mice with a 2-week history of binge-drinking (EtOH) during either adolescence (Adol.) or adulthood (Adult) observed during early (left) or later alcohol withdrawal (right). H2O = water-drinking controls. A cartoon illustrating the sites of tissue dissection is also presented. The data represent the means ± SEMs of the number of individual mice indicated. *p < 0.05 H2O vs. age-matched EtOH group (alcohol effect); #p < 0.05 Adol. vs. Adult (age effect).
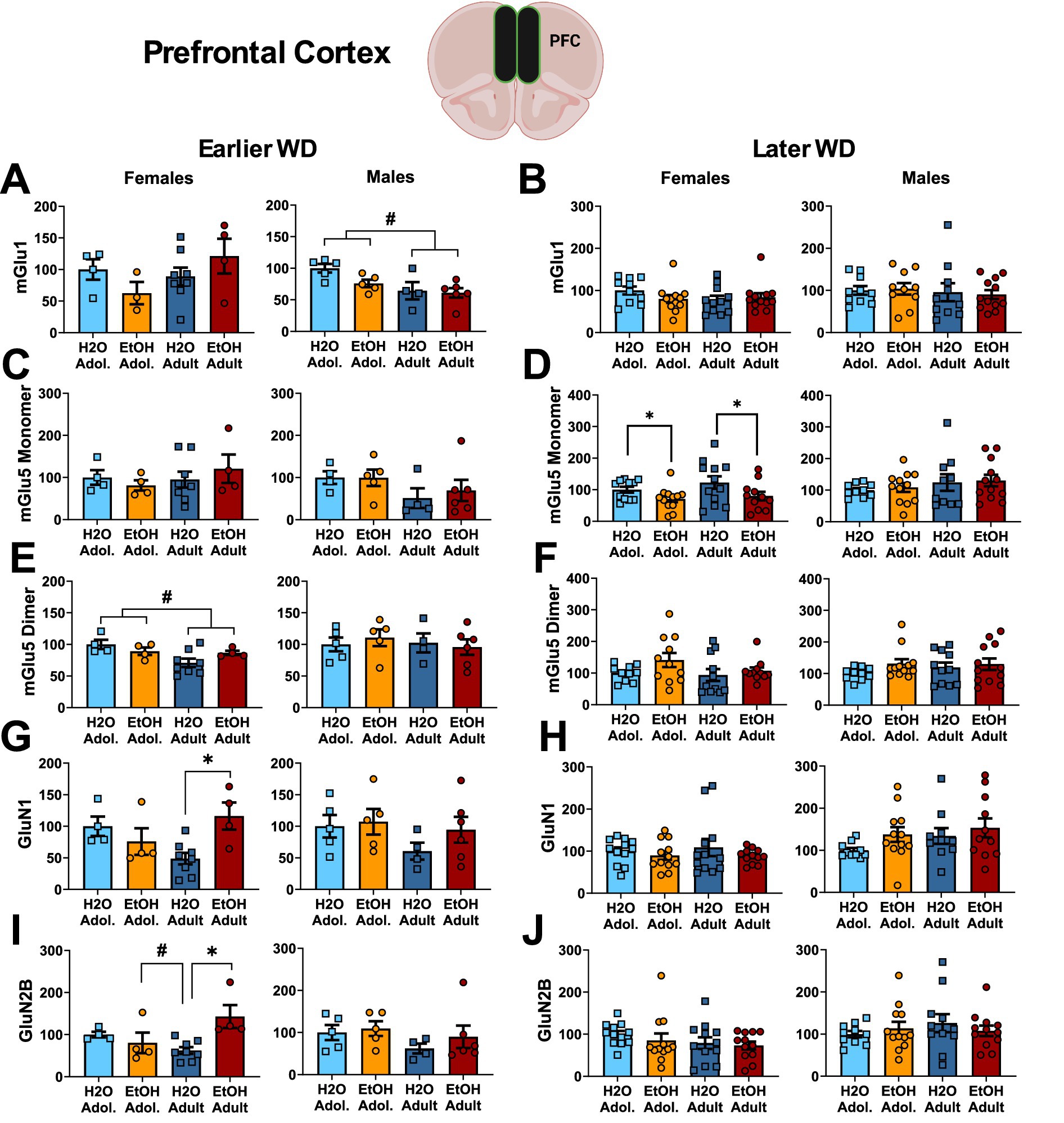
Figure 4. Summary of the changes in mGlu1 (A,B), the monomer (C,D) and dimer form of mGlu5 (E,F), GluN1 (G,H) and GluN2B (I,J) within the prefrontal cortex of mice with a 2-week history of binge-drinking (EtOH) during either adolescence (Adol.) or adulthood (Adult) observed during early (left) or later alcohol withdrawal (right). H2O = water-drinking controls. A cartoon illustrating the sites of tissue dissection is also presented. The data represent the means ± SEMs of the number of individual mice indicated. *p < 0.05 H2O vs. age-matched EtOH group (alcohol effect).
Due to the large number of experimental groups, immunoblotting was conducted separately on the tissue from male and female mice, with separate experiments examining for protein expression at the different withdrawal time-points, akin to recent large-scale projects employing immunoblotting by our group (e.g., Chavez et al., 2024; Denning et al., 2024; Szumlinski et al., 2023). Gel electrophoresis was conducted on 16 gels total for each brain region, with each gel containing the tissue from 2 to 3 same-sex mice from each of our four conditions: adolescent water-drinking (H2O-Adol.), adolescent alcohol-drinking (EtOH-Adol.), adult water-drinking (H2O-Adult) and adult alcohol-drinking (EtOH-Adult). For each sex, 4 gels compared tissue from mice euthanized following testing in earlier withdrawal and 4 gels compared tissue from mice euthanized following testing in later withdrawal. The tissue homogenization and immunoblotting procedures employed in the present study were very similar to those detailed in our recent reports (e.g., Chavez et al., 2024; Denning et al., 2024; Huerta Sanchez et al., 2023; Szumlinski et al., 2023). We employed the same antibodies as those in Chavez et al. (2024), including the rabbit primary antibodies: mGlu5 (metabotropic glutamate receptor 5; 1:1,000 dilution; Millipore; AB5675), GluN1 (NMDA receptor subunit 1; 1:500 dilution; Cell Signaling Technology; 5704S), Homer2a/b (1:500 dilution; Synaptic Systems; 160,203), p-(Tyr204)ERK1/2 (1:750 dilution; R&D systems; AF1018), APP (1:1,000 dilution; Millipore-Sigma; 07–667), amyloid beta (1:500 dilution; Abcam, ab180956), p (Ser396)-tau (1:750 dilution; Abcam; ab109390) and p (Thr217)-tau (1:500 dilution; Invitrogen, 44–744) and the mouse primary antibodies: mGlu1 (metabotropic glutamate receptor 1; 1:500 dilution; BD Biosciences; 610,965), GluN2B (NMDA subunit 2B; 1:500 dilution; Invitrogen; MA1-2014), Homer1b/c (1:1,000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA; sc-25271), ERK1/2 (1:1,000 dilution; Invitrogen, MA5-15605), tau (1:750 dilution; Invitrogen, AHB0042) and BACE (1:500 dilution; Millipore Sigma; MAB5308). Note that as reported in our earlier studies (Chavez et al., 2024; Huerta Sanchez et al., 2023), our selected mGlu1 antibody does not reliably detect the dimer form of the receptor and thus, only the monomer form of mGlu1 is reported herein. To control for protein loading and transfer, we immunoblotted for calnexin expression using either a rabbit or mouse primary anti-calnexin antibody (for rabbit, 1:1000 dilution; Enzo Life Sciences; ADI-SPA-860; for mouse, 1:500 dilution; Invitrogen, MA5-31501).
Membranes were then washed with phosphate-buffered saline with tween, incubated in either a goat anti-rabbit IRDye 800CW secondary antibody (1:10,000 dilution; Li-Cor; 925–3,221) or a goat anti-mouse IRDye 680RD secondary antibody (1:10,000 dilution; Li-Cor; 925–68,070), and imaged on an Odyssey Infrared Imaging System (Li-Cor Biosciences, Lincoln, NE, USA). Raw values for each band were measured, and first normalized to their corresponding calnexin signal and then to the average value of the H2O-Adol. water controls for that particular gel (see more details below). It should be noted that several technical difficulties, including accidental tissue warming, were encountered when immunoblotting the tissue collected at the earlier withdrawal time-point and there was insufficient tissue to repeat immunoblotting procedures. As such, the sample sizes for all the proteins from the earlier withdrawal time-point are a small fraction of the original n = 12/sex/group originally collected and we were unsuccessful immunoblotting for mGlu5 expression in the hippocampus during early withdrawal. For transparency, the available data were plotted and analyzed but it is recognized that the statistical power of these analyses is low, likely precluding detection of group differences and subject factor interactions.
Statistical analysis
As immunoblotting was conducted separately for male and female mice at the two different withdrawal time-points, the data for male and females were analyzed separately for protein expression determined at either the earlier or later withdrawal time-point (i.e., 25 or 54 days following the last binge-drinking session; see time-line in Figure 1) using Age x Drinking History ANOVAs. To increase statistical power to identify lower-level age differences in protein expression, alpha was set at 0.05 for all analyses and post-hoc LSD comparisons were performed. For all analyses where sphericity was violated, a Greenhouse–Geisser correction was used. Outliers were identified and excluded from the analyses using the ± 1.5 × IQR rule, however, in instances where too many outliers were identified, we adopted the ± 3 × IQR rule to ensure that only the most extreme outliers were removed. IBM SPSS Statistics software (version 27.0 for Macintosh) was used for all statistical tests, and GraphPad Prism software (version 9.3.1 for Macintosh) was used to create all graphs.
Results
Table 1 summarizes the means ± SEMS of the average alcohol intake and the blood ethanol levels of the mice employed in this study. The results pertaining to the effects of binge-drinking on affective and cognitive behavioral measures are detailed in Jimenez Chavez et al. (2023).
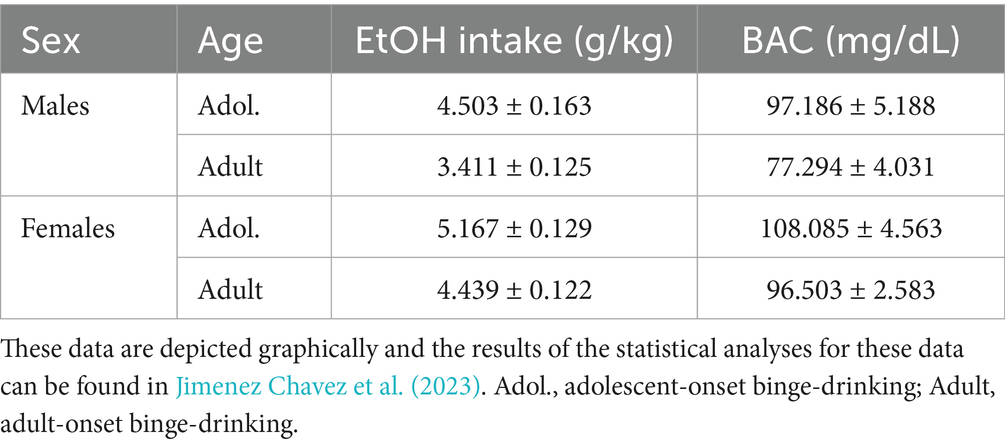
Table 1. Summary of the average alcohol intake (g/kg) and blood alcohol levels (BACs; mg/dL) of the mice employed for immunoblotting in the present study.
Immunoblotting of hippocampal tissue
Glutamate receptor-related proteins
The results of the statistical analyses for glutamate receptor-related protein expression within the hippocampus are provided in Table 2 and representative immunoblots are provided in Supplementary Figure 1. When assayed on WD25, it appeared that mGlu1 monomer expression was higher in female mice with a history of adult-onset binge-drinking, compared to the other female groups. However, no significant group differences were detected for either female or male mice at the earlier time-point (Figure 1A) nor did we detect group differences in hippocampal mGlu1 monomer expression in mice assayed on WD54 (Figure 1B). Unfortunately, we could not immunoblot for the monomer and dimer form of mGlu5 on the hippocampal tissue collected from the earlier withdrawal time-point; thus, no data is available. However, we failed to detect any changes in the expression of the mGlu5 monomer (Figure 1C) or dimer (Figure 1D) within the hippocampus of mice on WD54. While no group differences were detected for hippocampal GluN1 expression in female mice at either withdrawal time-point (Figures 1E,F), GluN1 expression was higher in alcohol- versus water-drinking males when assayed on WD25 (Figure 1E). However, this alcohol effect was not apparent 1 month later (Figure 1F). Similarly, no group differences were detected for hippocampal GluN2B expression in female mice at either withdrawal time-point (Figure 1G, H). In contrast, GluN2B expression was higher in alcohol- versus water-drinking males in earlier (Figure 1G), but not later in withdrawal (Figure 1H).
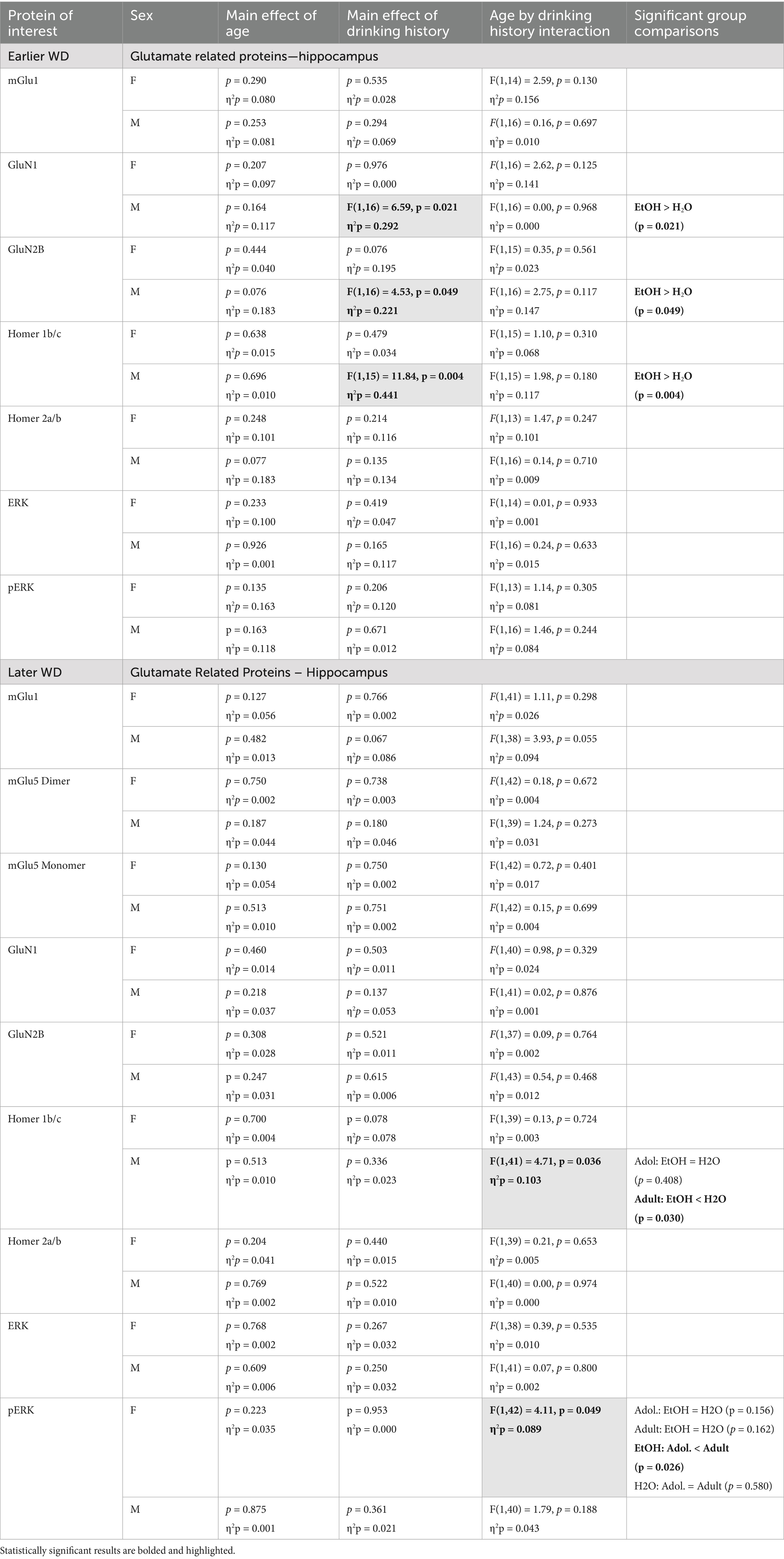
Table 2. Summary of the statistical results from the study of glutamate-related protein expression and ERK activation within the hippocampus of male (M) and female (F) mice euthanized following behavioral testing in earlier (top) or later withdrawal (respectively, 25 and 54 days following the last binge-drinking session).
Homer proteins and ERK activation
The results of the statistical analyses for Homer protein expression and ERK phosphorylation within the hippocampus are also provided in Table 2 and representative immunoblots are provided in Supplementary Figure 1. As illustrated in Figure 2, females exhibited no group differences in either Homer1b/c (Figures 2A,B) or Homer2a/b (Figures 2C,D) within hippocampus. In contrast, binge-drinking history increased Homer1b/c expression within the hippocampus of male mice on WD25 (Figure 2A) and a significant interaction was detected for this Homer isoform on WD54 (Figure 2B). This interaction reflected lower Homer1b/c expression in alcohol- versus water-experienced adults, with no alcohol-water difference detected in adolescent males (see Table 2). No changes in Homer2a/b were detected within the hippocampus of male mice during either withdrawal time-point (Figures 2C,D). Neither male nor female mice exhibited any change in hippocampal levels of ERK (Figures 2E,F) and males also did not differ in p(Tyr204)-ERK expression at either withdrawal time-point (Figures 2G,H). However, a significant interaction for this phospho-kinase was detected within the hippocampus of female mice on WD54 (Figures 2G,H). Post-hoc analyses indicated that this interaction did not reflect alcohol-water differences in either age group but rather reflected lower p(Tyr204)-ERK expression in alcohol-experienced adolescents versus adults, with no age difference noted in water-drinking controls (see Table 2).
Neuropathology markers
The results of the statistical analyses for the neuropathology markers examined within the hippocampus are provided in Table 3 and representative immunoblots are provided in Supplementary Figure 2. Neither male nor female mice exhibited changes in the hippocampal expression of APP on WD25 (Figure 3A), while binge-drinking males exhibited higher APP expression than their water controls ion WD54, irrespective of age of drinking-onset (Figure 3B). Females also did not exhibit any group differences in the expression of the 56 kDa (Figure C, D) or the 70 kDa (Figure E, F) BACE isoform at either withdrawal time-point. Males exhibited no group differences in the expression of either BACE isoform on WD25 (Figures 3C,E); however, the expression of both isoforms was higher in adolescent versus adult males and higher in alcohol- versus water-experienced males tested at the later withdrawal time-point (Figures 3D,F).
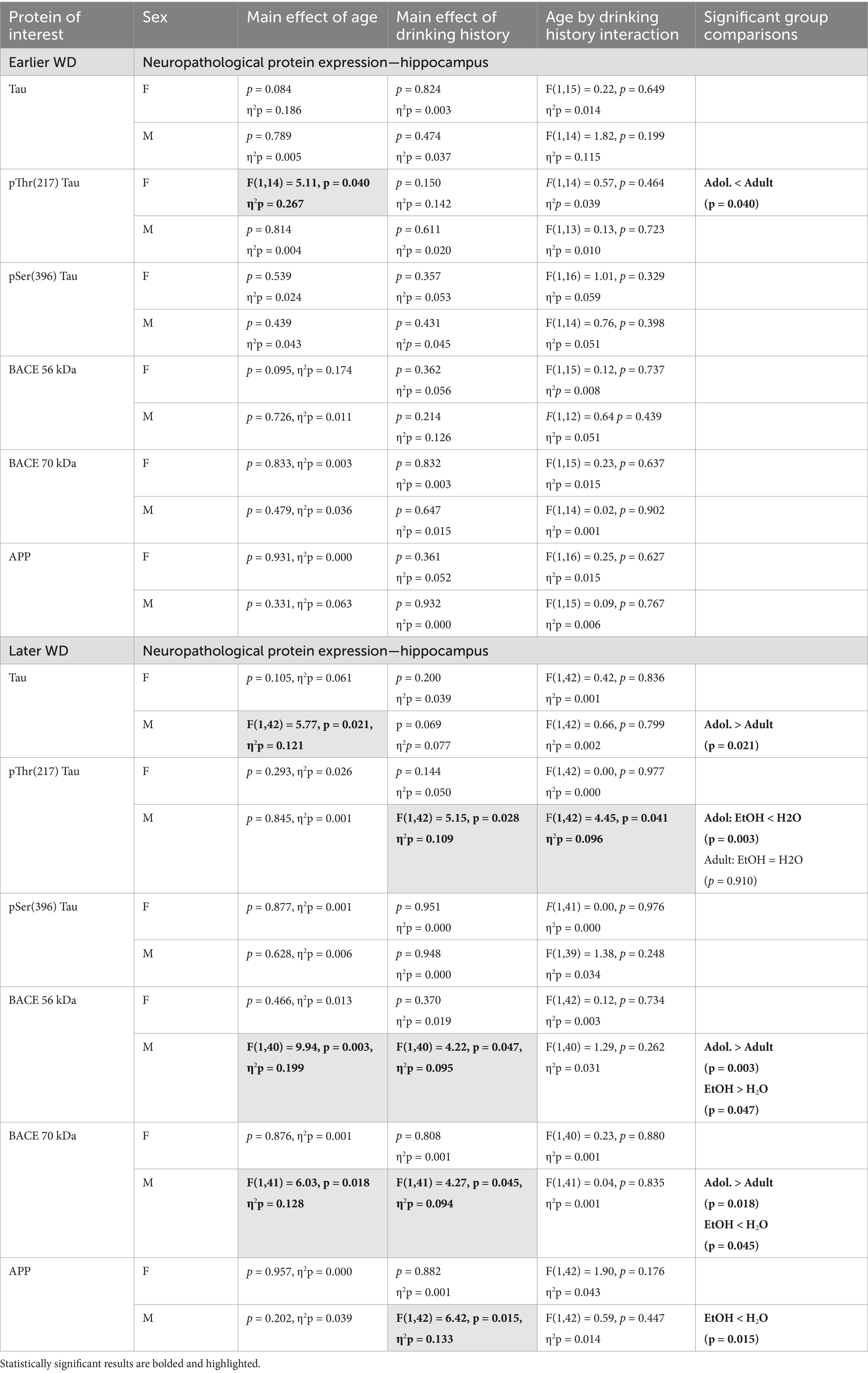
Table 3. Summary of the statistical results from the study of neuropathology-related proteins within the hippocampus of male (M) and female (F) mice euthanized following behavioral testing in earlier (top) or later (bottom) withdrawal (respectively, 25 and 54 days following the last binge-drinking session).
Females also did not differ in terms of hippocampal levels of Tau at either withdrawal time-point (Figures 3G,H) nor were group differences detected in females for the expression of p(Ser396)-Tau (Figures 3K,L). Although p(Thr217)-Tau expression was higher in adult versus adolescent females on WD25 (Figure 3I), no group differences were noted later on WD54 (Figure 3J). In males, we detected no group differences in total Tau (Figures 3G,H), p(Ser217)-Tau (Figures 3I,J) or p(Thr396)-Tau (Figures 3K,L) at either time-point.
Immunoblotting of prefrontal cortex tissue
Glutamate receptor-related proteins
The results of the statistical analyses for glutamate receptor-related protein expression within the PFC are provided in Table 4 and representative immunoblots are provided in Supplementary Figure 3. Female mice exhibited no group differences in the expression of the mGlu1 monomer within the PFC at either withdrawal time-point (Figures 4A,B). Although adolescent males exhibited higher mGlu1 monomer expression than male adults on WD25 (Figure 4A), no group differences were noted in later withdrawal (Figure 4B). mGlu5 monomer levels were unchanged in either female or male PFC on WD25 (Figure 4C), while binge-drinking females exhibited lower mGlu5 monomer levels on WD54, compared to their water-drinking controls (Figure 4D). Adult females also exhibited higher mGlu5 dimer expression within the PFC than adolescent females on WD25 (Figures 4E,F), but no differences in mGlu5 dimer expression were detected in the PFC of males. In females, Drinking History X Age interactions were detected for PFC expression of both the GluN1 (Figure 4G) and GluN2B (Figure 4I) subunit of the NMDA receptor on WD25. In both cases, these interactions reflected higher NMDA subunit expression in adult-onset binge-drinking females versus their water controls (see Table 4). These alcohol effects were not apparent in female mice tested later in withdrawal (Figures 4H,J) nor were group differences in the expression of either NMDA subunit apparent within the PFC of male mice (Figures 4G–J).
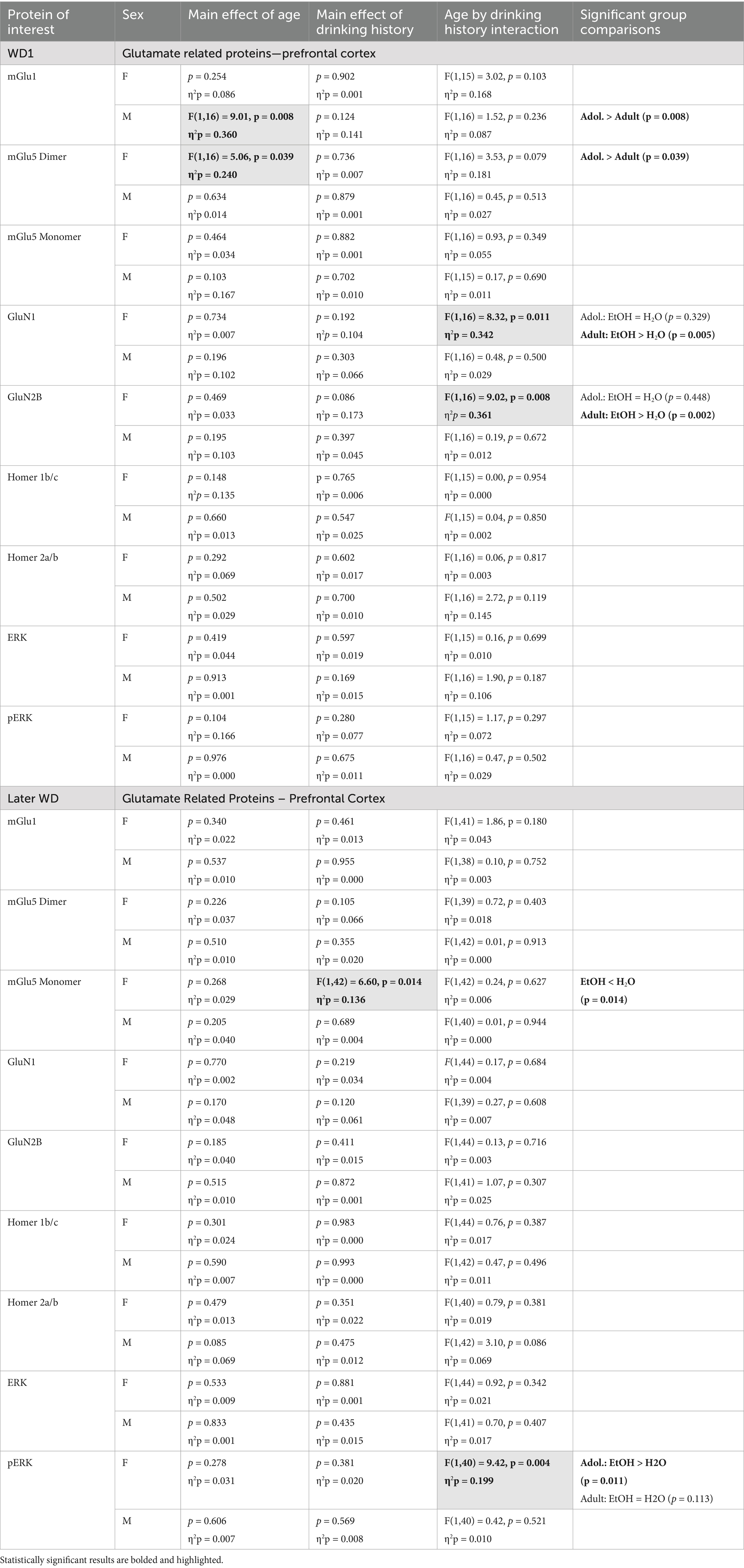
Table 4. Summary of the statistical results from the study of glutamate-related protein expression and ERK activation within the prefrontal cortex of male (M) and female (F) mice euthanized following behavioral testing in earlier (top) or later (bottom) withdrawal (respectively, 25 and 54 days following the last binge-drinking session).
Homer proteins and ERK activation
The results of the statistical analyses for Homer protein expression and ERK phosphorylation within the PFC are also provided in Table 4 and representative immunoblots are provided in Supplementary Figure 3. We detected no group differences in the PFC expression of either Homer1b/c (Figures 5A,B) or Homer2a/b (Figures 5C,D). We also did not detect any group differences in total ERK expression within PFC (Figures 5E,F). While mice of neither sex exhibited changes in p(Tyr204)-ERK expression within the PFC on WD25 (Figure 5G), a significant Drinking History X Age interaction was detected in female mice later in withdrawal (Figure 5H), that reflected higher p(Tyr204)-ERK expression in adolescent-onset binge-drinking mice, compared to their adult counterparts, with no age difference noted for water-drinking controls (see Table 4).
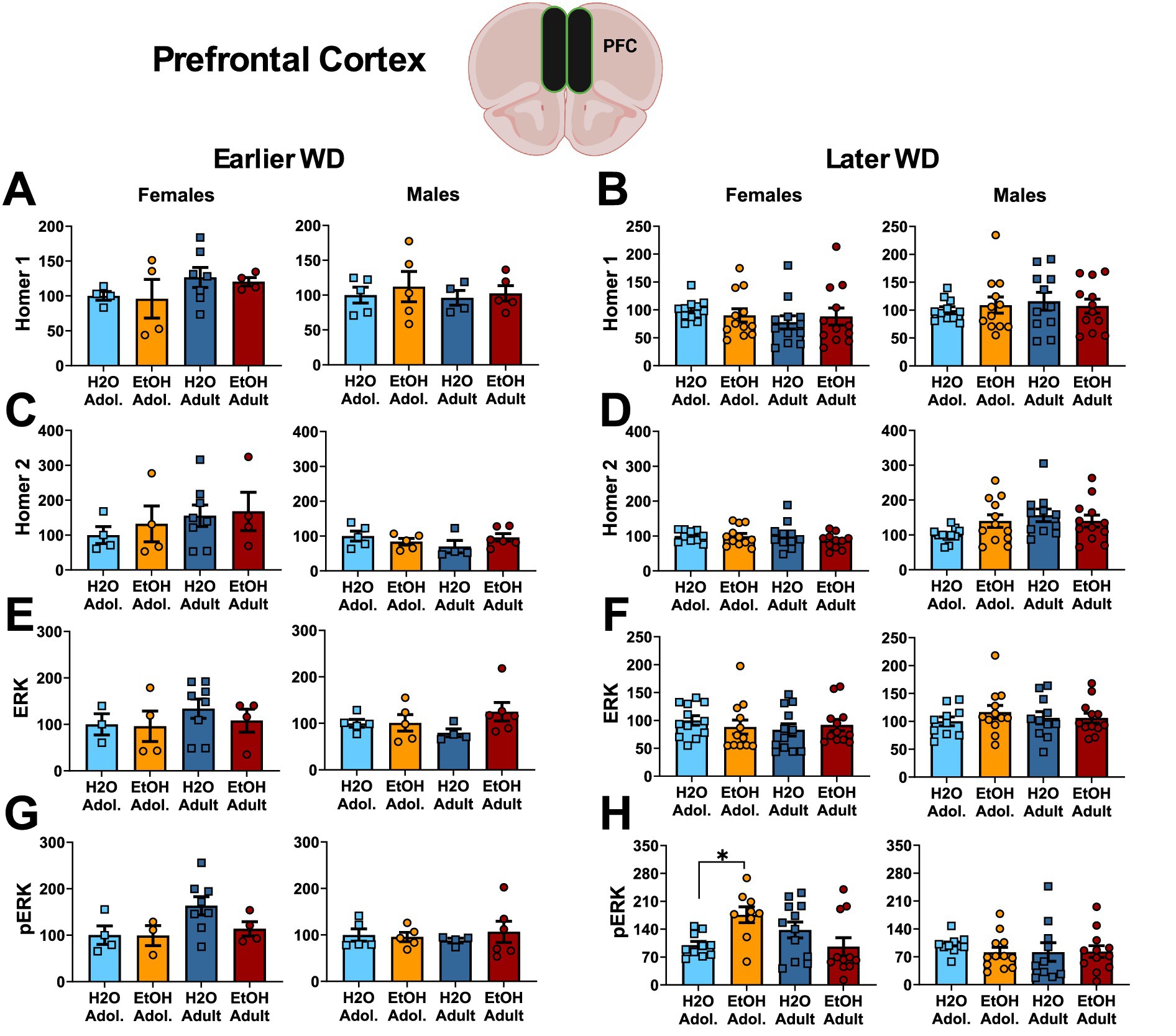
Figure 5. Summary of the changes in Homer1 (A,B), Homer2 (C,D), ERK (E,F) and p(Tyr204)-ERK (G,H) within the prefrontal cortex of mice with a 2-week history of binge-drinking (EtOH) during either adolescence (Adol.) or adulthood (Adult) observed during early (left) or later alcohol withdrawal (right). H2O = water-drinking controls. A cartoon illustrating the sites of tissue dissection is also presented. The data represent the means ± SEMs of the number of individual mice indicated. *p < 0.05 H2O vs. age-matched EtOH group (alcohol effect).
Neuropathology markers. The results of the statistical analyses for the neuropathology markers examined within the hippocampus are provided in Table 5 and representative immunoblots are provided in Supplementary Figure 4. We detected no group differences in the PFC expression of APP (Figures 6A,B) or the 56 kDa BACE isoform within PFC (Figures 6C,D). While no group differences in the 70 kDa BACE isoform were detected on WD25 (Figure 6E), a Drinking History X Age interaction was detected in female mice later in withdrawal (Figure 6F). As presented in Table 5, this interaction did not reflect alcohol-water differences in either age group, but rather higher BACE 70 kDa expression in adolescent- versus adult-onset water-drinking controls, with no age difference detected in alcohol-drinking females.
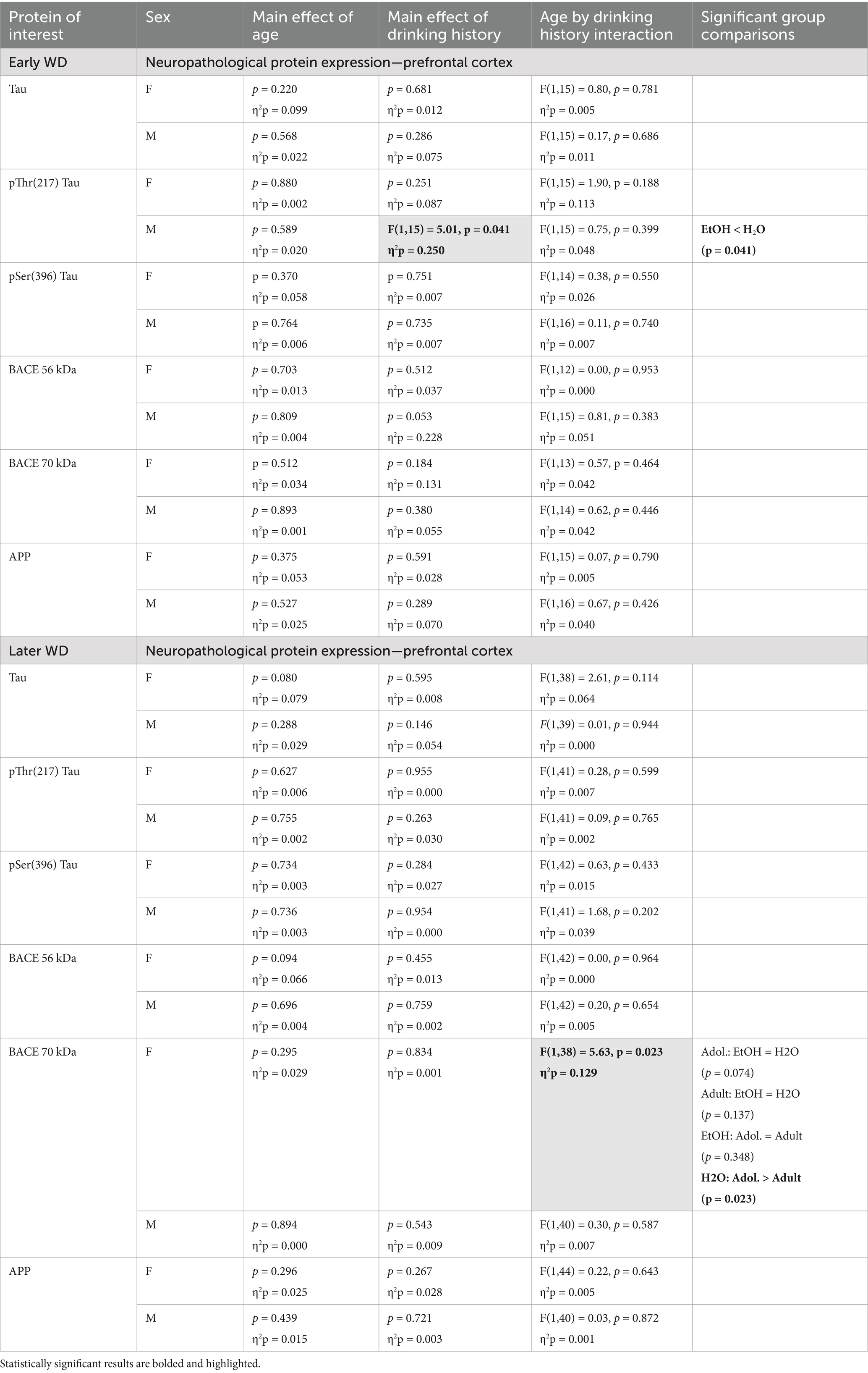
Table 5. Summary of the statistical results from the study of neuropathology-related proteins within the prefrontal cortex of male (M) and female (F) mice euthanized following behavioral testing in earlier (top) or later (bottom) withdrawal (respectively, 25 and 54 days following the last binge-drinking session).
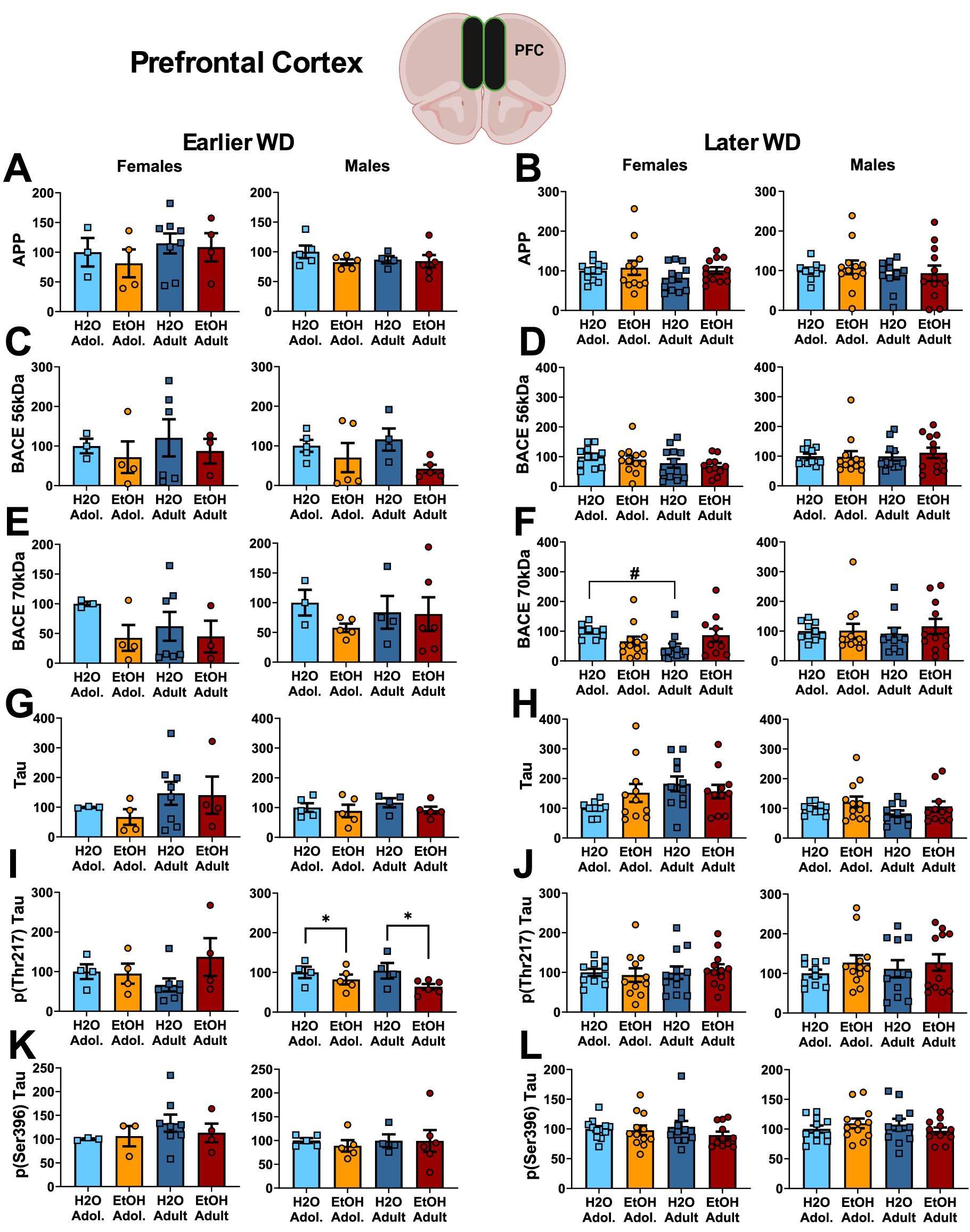
Figure 6. Summary of the changes in APP (A,B), the 56 kDa (C,D) and the 70 kDa isoforms of BACE (E,F), Tau (G,H), p(Thr217)-Tau (I,J) and p(Ser396)-Tau (K,L) within the prefrontal cortex of mice with a 2-week history of binge-drinking (EtOH) during either adolescence (Adol.) or adulthood (Adult) observed during early (left) or later alcohol withdrawal (right). H2O = water-drinking controls. A cartoon illustrating the sites of tissue dissection is also presented. The data represent the means ± SEMs of the number of individual mice indicated. *p < 0.05 H2O vs. age-matched EtOH group (alcohol effect); #p < 0.05 Adol. vs. Adult (age effect).
No group differences were detected in the PFC expression of Tau (Figures 6G,H) or p(Ser396)-Tau (Figures 6K,L). Further, no group differences were detected for the PFC expression of p(Thr217)-Tau in female mice (Figures 6I,J). However, binge-drinking males exhibited higher p(Thr217)-Tau levels on WD25 only (Figures 6I,J).
Discussion
Summary of results
Consistent with the mild behavioral effects observed in our prior study (Jimenez Chavez et al., 2023), a subchronic (2-week) history of binge-drinking during either adolescence or adulthood elicited relatively few changes in the expression of glutamate receptors, neuropathology markers or ERK activation within the hippocampus and PFC when assayed at approximately 1 or 2 months following the last binge-drinking session. Although the loss of tissue samples and the resulting low sample sizes for the mice tested at the WD25 time-point likely contributed to the negative outcomes, we nevertheless detected an alcohol-dependent increase in the expression of the NMDA receptor subunits GluN1 and GluN2b, as well as their scaffolding protein, Homer1b/c, within the hippocampus of both adolescent- and adult-onset alcohol-drinking male mice approximately 1 month into withdrawal (WD25). Interestingly, when examined a month later (WD54), hippocampal Homer1b/c expression was significantly lower in adult-onset alcohol-drinking males, relative to their water-drinking controls, but the changes in NMDA subunit expression were no longer apparent. Finally, although age-related differences in BACE isoforms and Tau were detected in the hippocampus of male mice on WD54, the only alcohol-related effect was lower p(Thr217)-Tau expression in adolescent-onset binge-drinking males. In contrast to males, females exhibited no detectable alcohol-related protein changes within hippocampus at either withdrawal time-point examined herein.
In PFC, male mice exhibited no alcohol-related changes in glutamate receptor or Homer protein expression at either withdrawal time-point, nor did prior alcohol history affect ERK activation. The only alcohol-related effect observed within the PFC of male mice was an age-independent reduction in p(Thr217)-Tau expression on WD25 that was no longer apparent a month later. In contrast to males, a few alcohol-related changes were detected within the PFC of female mice that varied with the age of binge-drinking onset. These included increased GluN1 and GluN2b expression on WD25 in adult-onset binge-drinking females (with comparable trends noted also for the monomer forms of mGlu1 and mGlu5 monomer) and increased p(Tyr204)-ERK expression on WD54 in adolescent-onset binge-drinking females on WD54. No alcohol-related changes in neuropathology-related markers were apparent in the PFC of female mice. Taken together, the present results align with our prior work examining how subject factors, such as sex and age of binge-drinking onset (Chavez et al., 2024; Jimenez Chavez et al., 2023) interact in a complex manner with the duration of alcohol withdrawal to influence protein expression within PFC and hippocampus, even during the first few months post-drinking.
Sex differences in alcohol-related changes in glutamate receptors at 1–2 months into withdrawal
This study is the first in our laboratory to compare subchronic (2-week) histories of adolescent- versus adult-onset binge-drinking on hippocampal and PFC protein expression in male versus female mice at any time-point following cessation of binge-drinking. While considerable evidence indicates that a history of adolescent alcohol exposure can induce long-lasting perturbations in indices of glutamate signaling within hippocampus (e.g., Beaudet et al., 2016; Contreras et al., 2019; Nwachukwu et al., 2022) and PFC (Galaj et al., 2020; Hardy et al., 1999; Salling et al., 2016; but see Raeder et al., 2008), the majority of these studies were conducted in male rodents only. However, a prior study of rats consuming a 6% alcohol liquid diet over a 14-day period demonstrated some clear sex differences in the effects of alcohol on NMDA receptor subunit expression within hippocampus and PFC (Devaud and Morrow, 1999). Consistent, in part, with evidence for some sex-selective effects of alcohol (Devaud and Morrow, 1999), alcohol binge-related changes in glutamate-related protein expression were detected on WD25 in the hippocampus of male mice only (Table 2). Given that the female rodents consumed more alcohol and achieved higher BACs than males in the current study (Table 1; Jimenez Chavez et al., 2022) and that by Devaud and Morrow (1999), the male-selectivity of the alcohol effects in hippocampus cannot be readily explained by sex differences in the amount of alcohol consumed or alcohol metabolism. It is possible that our low sample size for the WD25 time-point may have reduced our chances of detecting alcohol-water differences in female hippocampus. However, some female-selective changes in NMDA receptor subunits were detected in the PFC on WD25 (Table 4), despite the low sample size. Further, only females exhibited increased mGlu5 expression on WD54, when the sample size was sufficiently powered to detect alcohol effects in mice of both sexes. Yet, no alcohol-related changes in glutamate receptor proteins were detected in the PFC of males at either withdrawal time-point (Table 4). Such a double-dissociation in findings argues more in favor of genuine sex by brain region interactions in the alcohol sensitivity of glutamate receptor-related protein expression, rather than issues related to statistical power as accounting for our results, at least when protein expression is examined at 1–2 months into withdrawal.
Alternatively, a 2-week period of binge-drinking may simply be insufficient to alter glutamate receptor expression in the hippocampus of females or in the PFC of males. Indeed, comparable binge alcohol-related increases in the expression of both AMPA and NMDA receptor subunits, as well as Group 1 mGluRs, were observed in both mature adult and aged male and female subjects at 25 days withdrawal from a month-long multi-bottle DID binge-drinking procedure (Szumlinski et al., 2023). Moreover, patch-clamp electrophysiology studies indicate higher and lower intrinsic excitability of PFC neurons, respectively, in adult- and adolescent-onset alcohol-drinking male rats when assessed at 21 days withdrawal following a chronic, intermittent, alcohol procedure (Galaj et al., 2020). This being said, the duration of withdrawal is also a key modifier of alcohol’s effects on glutamate transmission within PFC as a female-selective reduction in NMDA and AMPA receptor cell surface expression and spontaneous excitatory postsynaptic potentials was detected within the prelimbic cortex of the PFC when assayed immediately following the last drinking session of a 4-week DID protocol when alcohol was still on board (Crowley et al., 2019), whereas increased intrinsic excitability of pyramidal neurons is observed in both male and female mice at 24 h withdrawal (Dao et al., 2021) and this increased excitability persists in both sexes for at least 6 months into withdrawal (Smith et al., 2025).
However, in contrast to the PFC, only males exhibited age- and alcohol-related changes in glutamate receptor expression within hippocampus in our prior study of older mice (Szumlinski et al., 2023). As we have yet to examine for sex differences in hippocampal protein expression during acute (1 day) alcohol withdrawal, we do not yet know if our results to date (Szumlinski et al., 2023; Table 2) reflect an insensitivity of the female hippocampus to alcohol drinking-induced changes in glutamate-related protein expression or sex differences in the persistence of any alcohol-induced changes that might occur earlier than 25 days into withdrawal. Arguing in favor of the latter possibility, both male and female alcohol-dependent adult rats exhibit increased hippocampal expression of GluN1 in acute alcohol withdrawal (Devaud and Morrow, 1999). To the best of our knowledge, only one other study has examined for sex differences in the effects of more protracted withdrawal (11 days) from a binge-like drinking paradigm (schedule-induced drinking) on hippocampal NMDA subunit mRNA expression and observed no alcohol-induced changes in mRNA in either sex at this time-point, despite a male-selective alcohol-induced impairment in spatial memory, as well as synaptic plasticity within hippocampus (Sanz-Martos et al., 2023). Given these disparate results, the sex differences in glutamate receptor-related protein expression observed herein at 1–2 months into alcohol withdrawal, and clinical evidence that women are more vulnerable to alcohol-induced cognitive impairment than men (e.g., Bahorik et al., 2021; Mumenthaler et al., 1999; Squeglia et al., 2009, 2011a, 2011b), it will be important in future work to systemically examine how the age of binge-drinking onset interacts with sex to impact both pre- and postsynaptic aspects of glutamate transmission within the PFC and hippocampus earlier during alcohol withdrawal in light of their important roles in executive and cognitive processing, respectively.
Alcohol-related changes in neuropathology markers during protracted withdrawal in male and female mice
Despite detecting several age-related differences in the expression of proteins associated with ADRD-related neuropathology in the present study, particularly within hippocampus, we detected only a few alcohol-related changes in protein expression. Perhaps this result should not be surprising given the relatively young age of the mice at both binge-drinking onset and tissue collection (< 4 months of age). Supporting age at the time of study as a modifying factor in both alcohol-related and baseline neuropathology marker expression, p(Thr217)-Tau expression was lower within the PFC of binge-drinking males in early withdrawal, irrespective of their age of drinking-onset and age-related decreases in APP, the 56 and 70 kDa BACE isoforms and Tau were apparent within the hippocampus of male mice during later withdrawal, irrespective of prior alcohol history. Further, female water-drinking mice exhibited an age-related reduction in 70 kDa BACE expression within the PFC. These findings contrast with the results from our study of older male mice in which: (1) the expression of our neuropathology markers tended to be higher within hippocampus of 18- versus 6-month-old mice, with fewer age-related changed detected for PFC and (2) prior alcohol-drinking history either increased or did not affect the expression of our neuropathology markers within hippocampus and PFC (Szumlinski et al., 2023). As these latter data for alcohol-experienced older mice align with the limited literature on age- and alcohol-related changes in ADRD-related genes/proteins in wild-type mice (Hoffman et al., 2019; Liu et al., 2022; Salling et al., 2016; Wisniewski et al., 1973), the present results argue that the age at tissue collection may be important for not only detecting, but also the direction of, both baseline and alcohol-related changes in, ADRD-related marker expression.
Supporting this idea, adolescent-onset binge-drinking elicited alcohol-related increases in p(Thr217)-Tau expression in entorhinal cortex and PFC, BACE (70 kDa) in entorhinal cortex, APP in entorhinal cortex, PFC and amygdala, and Aβ within amygdala during very protracted withdrawal, while the expression of what is considered to be one of the earliest markers of ADRD, (Ser396)-Tau (Janelidze et al., 2020), is reduced in all three brain regions during very protracted withdrawal (Chavez et al., 2024). In fact, the only age-related difference in ADRD marker expression observed in the present study that is consistent with the extant literature was p(Thr217)-Tau within the hippocampus of females tested in early withdrawal. As p(Thr217)-Tau is considered a highly specific biomarker of AD in both preclinical and advanced stages of AD in humans (e.g., Fukumoto et al., 2002; Johnson and Stoothoff, 2004), it is tempting to speculate that female hippocampus may telescope through normal aging-related neuropathology. However, this age-related difference in p(Thr217)-Tau expression was no longer apparent in females at the later withdrawal time-point, suggesting that the early withdrawal effect may be a spurious result related to our low sample sizes. Given the ample evidence that heavy alcohol drinking increases dementia vulnerability (Huang et al., 2018; Mumenthaler et al., 1999; Sabia et al., 2018; Weyerer et al., 2011; Xu et al., 2017) and reduces the age of dementia-onset in both humans (Kilian et al., 2023; Koch et al., 2019; Zarezadeh et al., 2024) and laboratory rodents (Crews and Vetreno, 2014; Hoffman et al., 2019; Jimenez Chavez et al., 2020; Ledesma et al., 2021), it is clear that more work is required to gain a more thorough understanding of the biomolecular impact of developmental exposure to alcohol, not only during critical periods of neuroplasticity (e.g., adolescence), but also in later life, in order to better predict behavioral outcomes and the efficacy of therapeutic interventions.
Conclusion
Herein we show that a 2-week history of binge-drinking by male and female, adult and adolescent, B6 mice is sufficient to elicit some sex- and region-selective changes in glutamate receptor-related and ADRD-related protein expression within the hippocampus and PFC that vary as a function of the duration of alcohol withdrawal and may contribute to mild cognitive impairment reported following adolescent- and adult-onset binge-drinking.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Institutional Animal Care and Use Committee of the University of California Santa Barbara. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
LJ: Supervision, Visualization, Funding acquisition, Methodology, Formal analysis, Writing – review & editing, Investigation, Data curation, Writing – original draft. LM: Supervision, Methodology, Investigation, Data curation, Writing – review & editing. CD: Data curation, Investigation, Writing – review & editing, Methodology, Supervision. EL: Writing – review & editing, Investigation, Methodology. DN: Data curation, Writing – review & editing, Investigation. GS: Investigation, Writing – review & editing, Data curation, Methodology. KS: Funding acquisition, Project administration, Formal analysis, Supervision, Conceptualization, Writing – original draft, Investigation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by NIH grant AA024044 (KKS; National Institute on Alcohol Abuse and Alcoholism). CLJC was supported by a National Science Foundation Graduate Research Fellowship Program award 2139319.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnbeh.2025.1619889/full#supplementary-material
References
Agabio, R., Pisanu, C., Luigi Gessa, G., and Franconi, F. (2017). Sex differences in alcohol use disorder. Curr. Med. Chem. 24, 2661–2670. doi: 10.2174/0929867323666161202092908
Arriagada, P. V., Marzloff, K., and Hyman, B. T. (1992). Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer's disease. Neurology 42, 1681–1688. doi: 10.1212/wnl.42.9.1681
Bahorik, A., Bobrow, K., Hoang, T., and Yaffe, K. (2021). Increased risk of dementia in older female US veterans with alcohol use disorder. Addiction 116, 2049–2055. doi: 10.1111/add.15416
Banning, L. C. P., Ramakers, I. H. G. B., Rosenberg, P. B., Lyketsos, C. G., and Leoutsakos, J. S. (2021). Alzheimer's disease biomarkers as predictors of trajectories of depression and apathy in cognitively normal individuals, mild cognitive impairment, and Alzheimer's disease dementia. Int. J. Geriatr. Psychiatry 36, 224–234. doi: 10.1002/gps.5418
Beaudet, G., Valable, S., Bourgine, J., Lelong-Boulouard, V., Lanfumey, L., Freret, T., et al. (2016). Long-lasting effects of chronic intermittent alcohol exposure in adolescent mice on object recognition and hippocampal neuronal activity. Alcohol. Clin. Exp. Res. 40, 2591–2603. doi: 10.1111/acer.13256
Bergink, V., Van Megen, H. J., and Westenberg, H. G. (2004). Glutamate and anxiety. Eur. Neuropsychopharmacol. 14, 175–183. doi: 10.1016/S0924-977X(03)00100-7
Campbell, R. R., Domingo, R. D., Williams, A. R., Wroten, M. G., McGregor, H. A., Waltermire, R. S., et al. (2019). Increased alcohol-drinking induced by manipulations of mGlu5 phosphorylation within the bed nucleus of the Stria terminalis. J. Neurosci. 39, 2745–2761. doi: 10.1523/JNEUROSCI.1909-18.2018
Carpenter-Hyland, E. P., and Chandler, L. J. (2007). Adaptive plasticity of NMDA receptors and dendritic spines: implications for enhanced vulnerability of the adolescent brain to alcohol addiction. Pharmacol. Biochem. Behav. 86, 200–208. doi: 10.1016/j.pbb.2007.01.016
Chavez, C. L. J., Scheldrup, G. P., Madory, L. E., Denning, C. J. E., Lee, E. C., Nguyen, D. T., et al. (2024). Biochemical changes precede affective and cognitive anomalies in aging adult C57BL/6J mice with a prior history of adolescent alcohol binge-drinking. Addict. Biol. 29:e70006. doi: 10.1111/adb.70006
Cheignon, C., Tomas, M., Bonnefont-Rousselot, D., Faller, P., Hureau, C., and Collin, F. (2018). Oxidative stress and the amyloid beta peptide in Alzheimer's disease. Redox Biol. 14, 450–464. doi: 10.1016/j.redox.2017.10.014
Contreras, A., Morales, L., and Del Olmo, N. (2019). The intermittent administration of ethanol during the juvenile period produces changes in the expression of hippocampal genes and proteins and deterioration of spatial memory. Behav. Brain Res. 372:112033. doi: 10.1016/j.bbr.2019.112033
Cozzoli, D. K., Courson, J., Caruana, A. L., Miller, B. W., Greentree, D. I., Thompson, A. B., et al. (2012). Nucleus accumbens mGluR5-associated signaling regulates binge alcohol drinking under drinking-in-the-dark procedures. Alcohol. Clin. Exp. Res. 36, 1623–1633. doi: 10.1111/j.1530-0277.2012.01776.x
Cozzoli, D. K., Courson, J., Wroten, M. G., Greentree, D. I., Lum, E. N., Campbell, R. R., et al. (2014). Binge alcohol drinking by mice requires intact group 1 metabotropic glutamate receptor signaling within the central nucleus of the amygdala. Neuropsychopharmacology 39, 435–444. doi: 10.1038/npp.2013.214
Cozzoli, D. K., Goulding, S. P., Zhang, P. W., Xiao, B., Hu, J. H., Ary, A. W., et al. (2009). Binge drinking upregulates accumbens mGluR5-Homer2-PI3K signaling: functional implications for alcoholism. J. Neurosci. 29, 8655–8668. doi: 10.1523/JNEUROSCI.5900-08.2009
Crews, F. T., Braun, C. J., Hoplight, B., Switzer, R. C., and Knapp, D. J. (2000). Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin Exp Res. 24:1712–1723. doi: 10.1097/00000374-200011000-00014
Crews, F. T., and Vetreno, R. P. (2014). Neuroimmune basis of alcoholic brain damage. Int. Rev. Neurobiol. 118, 315–357. doi: 10.1016/B978-0-12-801284-0.00010-5
Crowley, N. A., Magee, S. N., Feng, M., Jefferson, S. J., Morris, C. J., Dao, N. C., et al. (2019). Ketamine normalizes binge drinking-induced defects in glutamatergic synaptic transmission and ethanol drinking behavior in female but not male mice. Neuropharmacol 149, 35–44. doi: 10.1016/j.neuropharm.2019.02.003
Cservenka, A., and Brumback, T. (2017). The burden of binge and heavy drinking on the brain: effects on adolescent and young adult neural structure and function. Front. Psychol. 8:1111. doi: 10.3389/fpsyg.2017.01111
Dao, N. C., Brockway, D. F., Suresh Nair, M., Sicher, A. R., and Crowley, N. A. (2021). Somatostatin neurons control an alcohol binge drinking prelimbic microcircuit in mice. Neuropsychopharmacology 46, 1906–1917. doi: 10.1038/s41386-021-01050-1
De Bellis, M. D., Clark, D. B., Beers, S. R., Soloff, P. H., Boring, A. M., Hall, J., et al. (2000). Hippocampal volume in adolescent-onset alcohol use disorders. Am. J. Psychiatry 157, 737–744. doi: 10.1176/appi.ajp.157.5.737
Denning, C. J. E., Madory, L. E., Herbert, J. N., Cabrera, R. A., and Szumlinski, K. K. (2024). Neuropharmacological evidence implicating drug-induced glutamate receptor dysfunction in affective and cognitive sequelae of subchronic methamphetamine self-administration in mice. Int. J. Mol. Sci. 25:1928. doi: 10.3390/ijms25031928
Devaud, L. L., and Morrow, A. L. (1999). Gender-selective effects of ethanol dependence on NMDA receptor subunit expression in cerebral cortex, hippocampus and hypothalamus. Eur. J. Pharmacol. 369, 331–334. doi: 10.1016/s0014-2999(99)00103-x
Dodart, J. C., Mathis, C., Bales, K. R., and Paul, S. M. (2002). Does my mouse have Alzheimer's disease? Genes Brain Behav. 1, 142–155. doi: 10.1034/j.1601-183x.2002.10302.x
Ferretti, M. T., Iulita, M. F., Cavedo, E., Chiesa, P. A., Schumacher Dimech, A., Santuccione Chadha, A., et al. (2018). Sex differences in Alzheimer disease—the gateway to precision medicine. Nat. Rev. Neurol. 14, 457–469. doi: 10.1038/s41582-018-0032-9
Fukumoto, H., Cheung, B. S., Hyman, B. T., and Irizarry, M. C. (2002). Beta-secretase protein and activity are increased in the neocortex in Alzheimer disease. Arch. Neurol. 59, 1381–1389. doi: 10.1001/archneur.59.9.1381
Galaj, E., Guo, C., Huang, D., Ranaldi, R., and Ma, Y. Y. (2020). Contrasting effects of adolescent and early-adult ethanol exposure on prelimbic cortical pyramidal neurons. Drug Alcohol Depend. 216:108309. doi: 10.1016/j.drugalcdep.2020.108309
Hamley, I. W. (2012). The amyloid beta peptide: a chemist's perspective. Role in Alzheimer's and fibrillization. Chem. Rev. 112, 5147–5192. doi: 10.1021/cr3000994
Hardy, P. A., Chen, W., and Wilce, P. A. (1999). Chronic ethanol exposure and withdrawal influence NMDA receptor subunit and splice variant mRNA expression in the rat cerebral cortex. Brain Res. 819, 33–39. doi: 10.1016/s0006-8993(98)01340-7
Haroutunian, V., Perl, D. P., Purohit, D. P., Marin, D., Khan, K., Lantz, M., et al. (1998). Regional distribution of neuritic plaques in the nondemented elderly and subjects with very mild Alzheimer disease. Arch. Neurol. 55, 1185–1191. doi: 10.1001/archneur.55.9.1185
Hebert, L. E., Weuve, J., Scherr, P. A., and Evans, D. A. (2013). Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 80, 1778–1783. doi: 10.1212/WNL.0b013e31828726f5
Hersi, M., Irvine, B., Gupta, P., Gomes, J., Birkett, N., and Krewski, D. (2017). Risk factors associated with the onset and progression of Alzheimer’s disease: a systematic review of the evidence. Neurotoxicology 61, 143–187. doi: 10.1016/j.neuro.2017.03.006
Heymann, D., Stern, Y., Cosentino, S., Tatarina-Nulman, O., Dorrejo, N., and Gu, Y. (2016). The association between alcohol use and the progression of Alzheimer’s disease. Curr. Alzheimer Res. 13, 1356–1362. doi: 10.2174/1567205013666160603005035
Hoffman, J. L., Faccidomo, S., Kim, M., Taylor, S. M., Agoglia, A. E., May, A. M., et al. (2019). Alcohol drinking exacerbates neural and behavioral pathology in the 3xTg-AD mouse model of Alzheimer's disease. Int. Rev. Neurobiol. 148, 169–230. doi: 10.1016/bs.irn.2019.10.017
Hoffman, P. L., and Tabakoff, B. (1994). The role of the NMDA receptor in ethanol withdrawal. EXS 71, 61–70. doi: 10.1007/978-3-0348-7330-7_7
Huang, D., Yu, M., Yang, S., Lou, D., Zhou, W., Zheng, L., et al. (2018). Ethanol alters APP processing and aggravates Alzheimer-associated phenotypes. Mol. Neurobiol. 55, 5006–5018. doi: 10.1007/s12035-017-0703-3
Huerta Sanchez, L. L., Sankaran, M., Li, T. L., Doan, H., Chiu, A., Shulman, E., et al. (2023). Profiling prefrontal cortex protein expression in rats exhibiting an incubation of cocaine craving following short-access self-administration procedures. Front. Psych. 13:1031585. doi: 10.3389/fpsyt.2022.1031585
Ibáñez, J., Herrero, M. T., Insausti, R., Belzunegui, T., Tuñón, T., García-Bragado, F., et al. (1995). Chronic alcoholism decreases neuronal nuclear size in the human entorhinal cortex. Neurosci. Lett. 183, 71–74. doi: 10.1016/0304-3940(94)11117-2
Janelidze, S., Stomrud, E., Smith, R., Palmqvist, S., Mattsson, N., Airey, D. C., et al. (2020). Cerebrospinal fluid p-tau217 per-forms better than p-tau181 as a biomarker of Alzheimer’s disease. Nat. Commun. 11:1683. doi: 10.1038/s41467-020-15436-0
Jimenez Chavez, C. L., Coelho, M. A., Brewin, L. W., Swauncy, I., Tran, T., Albanese, T., et al. (2020). Incubation of negative affect during protracted alcohol withdrawal is age-, but not sex-selective. Brain Sci. 10:405. doi: 10.3390/brainsci10060405
Jimenez Chavez, C. L., Van Doren, E., Matalon, J., Ogele, N., Kharwa, A., Madory, L., et al. (2022). Alcohol-drinking under limited-access procedures during mature adulthood accelerates the onset of cognitive impairment in mice. Front. Behav. Neurosci. 16:732375. doi: 10.3389/fnbeh.2022.732375
Jimenez Chavez, C. L., Van Doren, E., Scheldrup, G., Rivera, E., Torres-Gonzalez, J., Herbert, J. N., et al. (2023). A subchronic history of binge-drinking elicits mild, age- and sex-selective, affective, and cognitive anomalies in C57BL/6J mice. Front. Behav. Neurosci. 17:1192076. doi: 10.3389/fnbeh.2023.1192076
Johnson, G. V., and Stoothoff, W. H. (2004). Tau phosphorylation in neuronal cell function and dysfunction. J. Cell Sci. 117, 5721–5729. doi: 10.1242/jcs.01558
Kalluri, H. S., Mehta, A. K., and Ticku, M. K. (1998). Up-regulation of NMDA receptor subunits in rat brain following chronic ethanol treatment. Brain Res. Mol. Brain Res. 58, 221–224. doi: 10.1016/s0169-328x(98)00112-0
Kamal, H., Tan, G. C., Ibrahim, S. F., Shaikh, M. F., Mohamed, I. N., Mohamed, R. M. P., et al. (2020). Alcohol use disorder, neurodegeneration, Alzheimer's and Parkinson's disease: interplay between oxidative stress, Neuroimmune response and excitotoxicity. Front. Cell. Neurosci. 14:282. doi: 10.3389/fncel.2020.00282
Kilian, C., Klinger, S., Rehm, J., and Manthey, J. (2023). Alcohol use, dementia risk, and sex: a systematic review and assessment of alcohol-attributable dementia cases in Europe. BMC Geriatr. 23:246. doi: 10.1186/s12877-023-03972-5
Koch, M., Fitzpatrick, A. L., Rapp, S. R., Nahin, R. L., Williamson, J. D., Lopez, O. L., et al. (2019). Alcohol consumption and risk of dementia and cognitive decline among older adults with or without mild cognitive impairment. JAMA Netw. Open 2:e1910319. doi: 10.1001/jamanetworkopen.2019.10319
Koob, G. F. (2021). Drug addiction: Hyperkatifeia/negative reinforcement as a framework for medications development. Pharmacol. Rev. 73, 163–201. doi: 10.1124/pharmrev.120.000083
Ledesma, J. C., Rodríguez-Arias, M., Gavito, A. L., Sánchez-Pérez, A. M., Viña, J., Medina Vera, D., et al. (2021). Adolescent binge-ethanol accelerates cognitive impairment and β-amyloid production and dysregulates endocannabinoid signaling in the hippocampus of APP/PSE mice. Addict. Biol. 26:e12883. doi: 10.1111/adb.12883
Lee, K. M., Coehlo, M. A., Solton, N. R., and Szumlinski, K. K. (2017). Negative affect and excessive alcohol intake incubate during protracted withdrawal from binge-drinking in adolescent, but not adult, mice. Front. Psychol. 8:1128. doi: 10.3389/fpsyg.2017.01128
Lee, K. M., Coelho, M. A., McGregor, H. A., Solton, N. R., Cohen, M., and Szumlinski, K. K. (2016). Adolescent mice are resilient to alcohol withdrawal-induced anxiety and changes in indices of glutamate function within the nucleus accumbens. Front. Cell. Neurosci. 10:265. doi: 10.3389/fncel.2016.00265
Littleton, J. M., Lovinger, D., Liljequist, S., Ticku, R., Matsumoto, I., and Barron, S. (2001). Role of polyamines and NMDA receptors in ethanol dependence and withdrawal. Alcohol. Clin. Exp. Res. 25, 132S–136S. doi: 10.1097/00000374-200105051-00023
Liu, M., Guo, S., Huang, D., Hu, D., Wu, Y., Zhou, W., et al. (2022). Chronic alcohol exposure alters gene expression and neurodegeneration pathways in the brain of adult mice. J. Alzheimers Dis. 86, 315–331. doi: 10.3233/JAD-215508
Medina, K. L., McQueeny, T., Nagel, B. J., Hanson, K. L., Schweinsburg, A. D., and Tapert, S. F. (2008). Prefrontal cortex volumes in adolescents with alcohol use disorders: unique gender effects. Alcohol. Clin. Exp. Res. 32, 386–394. doi: 10.1111/j.1530-0277.2007.00602.x
Mira, R. G., Tapia-Rojas, C., Pérez, M. J., Jara, C., Vergara, E. H., Quintanilla, R. A., et al. (2019). Alcohol impairs hippocampal function: From NMDA receptor synaptic transmission to mitochondrial function. Drug and alcohol dependence. 205:107628. doi: 10.1016/j.drugalcdep.2019.107628
Mumenthaler, M. S., Taylor, J. L., O'Hara, R., and Yesavage, J. A. (1999). Gender differences in moderate drinking effects. Alcohol Res. Health 23, 55–64.
Nagel, B. J., Schweinsburg, A. D., Phan, V., and Tapert, S. F. (2005). Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Res. Neuroimaging 139, 181–190. doi: 10.1016/j.pscychresns.2005.05.008
Nagy, J. (2004). The NR2B subtype of NMDA receptor: a potential target for the treatment of alcohol dependence. Curr. Drug Targets CNS Neurol. Disord. 3, 169–179. doi: 10.2174/1568007043337409
National Research Council. (2011). Guide for the Care and Use of Laboratory Animals: Eighth Edition. Washington, DC: The National Academies Press.
Novier, A., Diaz-Granados, J. L., and Matthews, D. B. (2015). Alcohol use across the lifespan: an analysis of adolescent and aged rodents and humans. Pharmacol. Biochem. Behav. 133, 65–82. doi: 10.1016/j.pbb.2015.03.015
Nunes, P. T., Kipp, B. T., Reitz, N. L., and Savage, L. M. (2019). Aging with alcohol-related brain damage: critical brain circuits associated with cognitive dysfunction. Int. Rev. Neurobiol. 148, 101–168. doi: 10.1016/bs.irn.2019.09.002
Nwachukwu, K. N., King, D. M., Healey, K. L., Swartzwelder, H. S., and Marshall, S. A. (2022). Sex-specific effects of adolescent intermittent ethanol exposure-induced dysregulation of hippocampal glial cells in adulthood. Alcohol 100, 31–39. doi: 10.1016/j.alcohol.2022.02.002
O'Brien, R. J., Resnick, S. M., Zonderman, A. B., Ferrucci, L., Crain, B. J., Pletnikova, O., et al. (2009). Neuropathologic studies of the Baltimore longitudinal study of aging (BLSA). J. Alzheimers Dis. 18, 665–675. doi: 10.3233/JAD-2009-1179
Pascual, M., Blanco, A. M., Cauli, O., Miñarro, J., and Guerri, C. (2007). Intermittent ethanol exposure induces inflammatory brain damage and causes long-term behavioural alterations in adolescent rats. Eur. J. Neurosci. 25, 541–550. doi: 10.1111/j.1460-9568.2006.05298.x
Peng, B., Yang, Q. B., Joshi, R., Liu, Y., Akbar, M., Song, B. J., et al. (2020). Role of alcohol drinking in Alzheimer's disease, Parkinson's disease, and amyotrophic lateral sclerosis. Int. J. Mol. Sci. 21:2316. doi: 10.3390/ijms21072316
Perl, D. P. (2010). Neuropathology of Alzheimer's disease. Mt. Sinai J. Med. 77, 32–42. doi: 10.1002/msj.20157
Raeder, H., Holter, S. M., Hartmann, A. M., Spanagel, R., Moller, H. J., and Rujescu, D. (2008). Expression of N-methyl-d-aspartate (NMDA) receptor subunits and splice variants in an animal model of long-term voluntary alcohol self-administration. Drug Alcohol Depend. 96, 16–21. doi: 10.1016/j.drugalcdep.2007.12.013
Sabia, S., Fayosse, A., Dumurgier, J., Dugravot, A., Akbaraly, T., Britton, A., et al. (2018). Alcohol consumption and risk of dementia: 23 year fol-low-up of Whitehall II cohort study. BMJ 362:k2927. doi: 10.1136/bmj.k2927
Salling, M. C., Faccidomo, S. P., Li, C., Psilos, K., Galunas, C., Spanos, M., et al. (2016). Moderate alcohol drinking and the amygdala proteome: identification and validation of calcium/calmodulin dependent kinase II and AMPA receptor activity as novel molecular mechanisms of the positive reinforcing effects of alcohol. Biol. Psychiatry 79, 430–4342. doi: 10.1016/j.biopsych.2014.10.020
Sanz-Martos, A. B., Fuentes-Verdugo, E., Merino, B., Morales, L., Pérez, V., Capellán, R., et al. (2023). Schedule-induced alcohol intake during adolescence sex dependently impairs hippocampal synaptic plasticity and spatial memory. Behav. Brain Res. 452:114576. doi: 10.1016/j.bbr.2023.114576
Schwarzinger, M., Pollock, B. G., Hasan, O. S., Dufouil, C., Rehm, J., Baillot, S., et al. (2018). Contribution of alcohol use disorders to the burden of dementia in France 2008–13: a nationwide retrospective cohort study. Lancet Public Health 3, e124–e132. doi: 10.1016/S2468-2667(18)30022-7
Smith, G. C., Griffith, K. R., Sicher, A. R., Brockway, D. F., Proctor, E. A., and Crowley, N. A. (2025). Alcohol consumption confers lasting impacts on prefrontal cortical neuron intrinsic excitability and spontaneous neurotransmitter signaling in the aging brain in mice. Neurobiol. Aging 145, 42–54. doi: 10.1016/j.neurobiolaging.2024.09.014
Squeglia, L. M., Schweinsburg, A. D., Pulido, C., and Tapert, S. F. (2011a). Adolescent binge drinking linked to abnormal spatial working memory brain activation: differential gender effects. Alcohol. Clin. Exp. Res. 35, 1831–1841. doi: 10.1111/j.1530-0277.2011.01527.x
Squeglia, L. M., Sorg, S. F., Schweinsburg, A. D., Wetherill, R. R., Pulido, C., and Tapert, S. F. (2011b). Binge drinking differentially affects adolescent male and female brain morphometry. Psychopharmacology 220, 529–539. doi: 10.1007/s00213-011-2500-4
Squeglia, L. M., Spadoni, A. D., Infante, M. A., Myers, M. G., and Tapert, S. F. (2009). Initiating moderate to heavy alcohol use predicts changes in neuropsychological functioning for adolescent girls and boys. Psychol. Addict. Behav. 23, 715–722. doi: 10.1037/a0016516
Swartzwelder, H. S., Risher, M. L., Miller, K. M., Colbran, R. J., Winder, D. G., and Wills, T. A. (2016). Changes in the adult GluN2B associated proteome following adolescent intermittent ethanol exposure. PLoS One 11:e0155951. doi: 10.1371/journal.pone.0155951
Szumlinski, K. K., Coelho, M. A., Lee, K. M., Tran, T., Sern, K. R., Bernal, A., et al. (2019). DID it or DIDn't it? Exploration of a failure to replicate binge-like alcohol-drinking inC57BL/6J mice. Pharmacol. Biochem. Behav. 178, 3–18. doi: 10.1016/j.pbb.2018.12.002
Szumlinski, K. K., Herbert, J. N., Espinoza, B. M., Madory, L. E., and Scudder, S. L. (2023). Alcohol-drinking during later life by C57BL/6J mice induces sex- and age-dependent changes in hippocampal and prefrontal cortex expression of glutamate receptors and neuropathology markers. Addict. Neurosci. 7:100099. doi: 10.1016/j.addicn.2023.100099
Troncoso, J. C., Cataldo, A. M., Nixon, R. A., Barnett, J. L., Lee, M. K., Checler, F., et al. (1998). Neuropathology of preclinical and clinical late-onset Alzheimer's disease. Ann. Neurol. 43, 673–676. doi: 10.1002/ana.410430519
Van Hees, L., Didone, V., Charlet-Briart, M., Van Ingelgom, T., Alexandre, A., Quertemont, E., et al. (2021). Voluntary alcohol binge-drinking in adolescent C57Bl6 mice induces delayed appearance of behavioural defects in both males and females. Addict. Biol. 27:e13102. doi: 10.1111/adb.13102
Weyerer, S., Schaufele, M., Wiese, B., Maier, W., Tebarth, F., van den Bussche, H., et al. (2011). German AgeCoDe study g. current alcohol consumption and its relationship to incident dementia: results from a 3-year follow-up study among primary care attenders aged 75 years and older. Age Ageing 40, 456–463. doi: 10.1093/ageing/afr007
White, A. M. (2020). Gender differences in the epidemiology of alcohol use and related harms in the United States. Alcohol Res. 40:01. doi: 10.35946/arcr.v40.2.01
Wiegmann, C., Mick, I., Brandl, E. J., Heinz, A., and Gutwinski, S. (2020). Alcohol and dementia–what is the link? A systematic review. Neuropsychiatr. Dis. Treat. 16, 87–99. doi: 10.2147/NDT.S198772
Wills, T. A., Baucum, A. J. 2nd, Holleran, K. M., Chen, Y., Pasek, J. G., Delpire, E., et al. (2017). Chronic intermittent alcohol disrupts the GluN2B-associated proteome and specifically regulates group I mGlu receptor-dependent long-term depression. Addict. Biol. 22, 275–290. doi: 10.1111/adb.12319
Wisniewski, H. M., Ghetti, B., and Terry, R. D. (1973). Neuritic (senile) plaques and filamentous changes in aged rhesus monkeys. J. Neuropathol. Exp. Neurol. 32, 566–584. doi: 10.1097/00005072-197310000-00007
Xu, W., Wang, H., Wan, Y., Tan, C., Li, J., Tan, L., et al. (2017). Alcohol consumption and de-mentia risk: a dose response meta-analysis of prospective studies. Eur. J. Epidemiol. 32, 31–42. doi: 10.1007/s10654-017-0225-3
Zarezadeh, M., Mahmoudinezhad, M., Faghfouri, A. H., Mohammadzadeh Honarvar, N., Regestein, Q. R., Papatheodorou, S. I., et al. (2024). Alcohol consumption in relation to cognitive dysfunction and dementia: a systematic review and dose-response meta-analysis of comparative longitudinal studies. Ageing Res. Rev. 100:102419. doi: 10.1016/j.arr.2024.102419
Keywords: adolescence, group 1 metabotropic glutamate receptors, tau, prefrontal cortex, hippocampus, sex differences
Citation: Jimenez Chavez CL, Madory LE, Denning CJE, Lee EC, Nguyen DT, Scheldrup GP and Szumlinski KK (2025) Examination of age- and sex-related changes in protein expression within the hippocampus and prefrontal cortex during withdrawal from a subchronic history of binge-drinking in C57BL/6J mice. Front. Behav. Neurosci. 19:1619889. doi: 10.3389/fnbeh.2025.1619889
Edited by:
Laura B. Tucker, Uniformed Services University of the Health Sciences, United StatesReviewed by:
Liana Asatryan, University of Southern California, United StatesXavier Maddern, University of Melbourne, Australia
Copyright © 2025 Jimenez Chavez, Madory, Denning, Lee, Nguyen, Scheldrup and Szumlinski. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Karen K. Szumlinski, c3p1bWxpbnNraUB1Y3NiLmVkdQ==
 C. Leonardo Jimenez Chavez
C. Leonardo Jimenez Chavez Lauren E. Madory1
Lauren E. Madory1 Chris J. E. Denning
Chris J. E. Denning Gavin P. Scheldrup
Gavin P. Scheldrup Karen K. Szumlinski
Karen K. Szumlinski