- Department of Orthopaedics, Yongchuan Hospita of Traditional Chinese Medicine, Chongqing Medical Uninersity, Chongqing, China
According to World Health Organization (WHO), osteoporosis is a systematic bone disability marked by reduced bone mass and microarchitectural degeneration of osseous cells, which leads to increased bones feebleness and fractures vulnerability. It is a polygenetic, physiological bone deformity that frequently leads to osteoporotic fractures and raises the risk of fractures in minimal trauma. Additionally, the molecular changes that cause osteoporosis are linked to decreased fracture repair and delayed bone regeneration. Bones have the ability to regenerate as part of the healing mechanism after an accident or trauma, including musculoskeletal growth and ongoing remodeling throughout adulthood. The principal treatment approaches for bone loss illnesses, such as osteoporosis, are hormone replacement therapy (HRT) and bisphosphonates. In this review, we searched literature regarding the Traditional Chinese medicines (TCM) in osteoporosis and bone regeneration. The literature results are summarized in this review for osteoporosis and bone regeneration. Traditional Chinese medicines (TCM) have grown in popularity as a result of its success in curing ailments while causing minimal adverse effects. Natural Chinese medicine has already been utilized to cure various types of orthopedic illnesses, notably osteoporosis, bone fractures and rheumatism with great success. TCM is a discipline of conventional remedy that encompasses herbal medication, massage (tui na), acupuncture, food, and exercise (qigong) therapy. It is based on more than 2,500 years of Chinese healthcare profession. This article serves as a comprehensive review summarizing the osteoporosis, bone regeneration and the traditional Chinese medicines used since ancient times for the management of osteoporosis and bone regeneration.
Introduction
According to WHO, by 2022, the number of persons living with osteoporosis in the America is projected to boost from around 10 million to over 14 million (Bartl and Frisch, 2004; Burge et al., 2007). Due to modern civilizations’ constantly expanding life expectancy, the global implications of osteoporosis and delayed bone repair are massive. As a result, the clinical need to reverse bone loss, promote bone development, and enhance osteogenesis is rising, and it is a critical problem for medical professionals (Russow et al., 2019). Hormonal and metabolic abnormalities are the most common etiologies of osteoporosis. Nevertheless, osteoimmunology has shown that the immune cells and immunological parameters perform key regulating roles in the progression of osteoporosis. The immune system’s aberrant stimulation disrupts the equilibrium between osteoclasts and osteoblasts, creating instability in bone remodeling and osteoporosis (Guerrini and Takayanagi, 2014; Limmer and Wirtz, 2017). Over 50 years of age, about one-third of women and one-fifth of men will develop at least one bone fracture due to osteoporosis (Sözen et al., 2017).
For the prophylaxis and therapy of osteoporosis, the Federal Drug Administration (FDA) of United States has authorized a number of medications that function by preventing bone resorption or reduce the incidence of fractures (anabolic agents). Bisphosphonates, competitive oestrogen receptor modulators, and the monoclonal antibody denosumab are examples of drugs that prevent bone degradation but do not induce bone regeneration (Russow et al., 2019). Abaloparatide and Teriparatide are the currently US Food and Drug Administration (FDA) approved anabolic agents for the treatment of osteoporosis in the United States approved in 2017 and 2002 respectively (Miller et al., 2016; Haas and LeBoff, 2018). The principal treatment approaches for bone loss illnesses, such as osteoporosis, are hormone replacement therapy (HRT) and bisphosphonates. Continuous HRT is associated to high risk of mammary cancer and endometrial, as well as coronary artery problems and other cardiac disorders whereas bisphosphonates are responsible for the osteonecrosis of the jaws and bones (Dinger et al., 2016; He et al., 2017; Spivakovsky, 2017). The clinical usage of HRT and bisphosphonates is limited due to these adverse effects. As a result, new treatment strategies are needed to produce osteoporosis treatments that are somewhat liable to cause fewer adverse reactions (Lungu et al., 2018). Cathepsin-K is a cysteine protease that is found in significant amounts in osteoclasts. Even though there are various additional cathepsins with large anatomical concentrations, any antagonists for clinical usage must have a higher selectivity if they are to be safe. Balicatib preceded through phase 2 clinical studies, however due to cutaneous and respiratory adverse effects, onward research was halted (Reid, 2008). Odanacatib concluded effectively phase 2 studies with no apparent potential risks. It decreases osteoclastogenesis indicators by around 70% and induces variations in spine bone concentrations that are similar in many aspects with active bisphosphonates if administered as a fortnightly oral route (Bone et al., 2007).
Traditional Chinese medicines (TCM) have grown in popularity as a result of its success in curing ailments while causing minimal adverse affects. Natural Chinese medicine has already been utilized to cure various types of orthopaedic illnesses, notably osteoporosis, bone fractures and rheumatism with great success (Mukwaya et al., 2014; Suvarna et al., 2018). The widespread use of TCM for musculoskeletal disorders has been existed for many years, both in rural as well as in urban regions. Topical herbal formulations are especially beneficial in cases of joint injuries, inflammations, but potentially in fractured bones (Jiang and Ye, 2005). According to latest research findings, these Chinese medicinal approaches for the management of osteoporosis emerge to have equally anticatabolic and anabolic impacts by boosting osteogenesis and minimizing extremely unbalanced bone turnover, resulting in improved bone mineral density as well as minimal bone microstructural degradation (Wang et al., 2017a; He et al., 2017).
This review focuses on and acknowledges the scientific proof for the possible usage of traditional Chinese medicine in the management of osteoporosis and bone regeneration.
Osteoporosis and Bone Regeneration
Osteoporosis is a skeletal disorder that is characterized by poor bone microarchitecture/mineralization, reduced bones’ mineral density (BMD), and/or diminished bone growth. This asymptomatic illness typically goes untreated till it emerges as minimal fractures of the spine, wrist, pelvis, proximal humerus, and/or hips, which commonly necessitates hospitalizations (Cosman et al., 2014; Jeremiah et al., 2015). There are two types of osteoporosis: primary and secondary. Primary osteoporosis: 1) postmenopausal affects mostly the vertebral column trabeculae and is common in women aged 45 to 65. It is caused by the oestrogen insufficiency and impairment of ovarian function. 2) senile in women over the age of 70–75, which is caused by a reduction in vitamin D and calcium consumption, decreased vitamin D generation and metabolism in the body, diminished intestinal absorption, and the ageing process: 3) Idiopathic (juvenile) fractures—a rare type that affects people of reproductive age (below 40). Disorders or medicines that damage bone cells cause secondary osteoporosis. Continuous utilization of selective serotonin receptor inhibitors, glucocorticoids, androgen deprivation therapy, proton pump inhibitors, thiazolidinedione, calcineurin inhibitors, heparin, and various chemotherapies, such as methotrexate, might cause osteoporosis (Ivanova et al., 2015).
Primary osteoporosis is frequently linked to advanced age and a lack of sex hormones. The ongoing degradation of the trabeculae in bone causes age-related osteoporosis. Furthermore, the decrease in oestrogen levels in older women leads to greater osteoporosis. Sex-hormone–binding globulin downregulates the male sex hormones as they mature, which might also result in bone loss over time (Jeremiah et al., 2015; Society, 2021). Multiple concomitant conditions and/or drugs can lead to secondary osteoporosis. The imbalance of vitamin D, calcium, and sex hormones is frequently linked in disorders associated with osteoporosis. Men on androgen-deprivation therapy (ADT) for prostate cancer, for example, have a higher likelihood of osteoporosis (Shahinian et al., 2005; Schnatz et al., 2011). Excessive glucocorticoid synthesis in Cushing’s syndrome has now been reported to hasten osteoporosis (Kawamata et al., 2008). Furthermore, several inflammatory disorders, including rheumatoid arthritis, may necessitate long-term glucocorticoid medication, which has now been linked to secondary osteoporosis. Glucocorticoids, in particular, are thought to be even more prevalent drugs associated to drug-induced osteoporosis (Buckley et al., 2017). Reduced blood supply in radiotherapy and chemotherapeutic therapies focused resistant cancerous cells via modifying the bones microenvironment as well as inhibiting them from establishing a quiescent state. Such results indicate that limiting radiation- and chemotherapy-induced pericyte growth via blood supply manipulation may augment existing anticancer therapeutic strategies for managing or preventing bone metastases (Singh et al., 2019).
Bones have the ability to regenerate as part of the healing mechanism after an accident or trauma, including musculoskeletal growth and ongoing remodeling throughout adulthood. In order to maximize osseous fixing and re-establish skeletal function, bone rehabilitation is composed of a very well sequence of biochemical pathways of bone initiation and conduction, comprising a set of cell types and extracellular and intracellular molecular-signaling pathways, with a distinguishable spatial and temporal sequence (Einhorn, 1998; Cho et al., 2002; Bates et al., 2018). The much more prevalent form of osteogenesis in the clinical context is fracture repair, which mimics the typical embryonic osteogenic cascade, comprising of endochondral and intramembranous bone formation. Almost all of the orthopaedic traumas cure without the development of scar tissue, and bone regenerates like its pre-existing qualities completely recovered, with the freshly formed bones finally unidentifiable from the nearby healthy bone (Ferguson et al., 1999; Dimitriou et al., 2011). Conversely, there are examples where bone regrowth is hindered during bone remodeling, about 13% of tibia fractures being linked with fracture non-union or disorder union. Furthermore, there are other situations in oral and maxillofacial and orthopaedic surgical procedure where substantial quantities of bone healing are needed, including bony restoration of large bone defects caused by tumour resection, trauma, bone deformities, and infection, or situations wherein the regenerative procedure is impaired, like osteoporosis and a vascular necrosis (Audigé et al., 2005).
Presently there are a variety of medical procedures in the surgeon’s therapeutic strategies for all of the mentioned scenarios wherein the natural process of bone healing is either damaged or simply ineffective, that could be employed by itself or in conjunction for the augmentation or management of these complicated clinical situations, which can frequently be resistive to therapy, constituting a medical and economical challenge. Distraction osteogenesis and bones transfer are two common techniques used in clinical practice to accelerate or enhance bone healing. A variety of bone-grafting techniques, including allografts, autologous bone grafts, growth factors, and bone-graft replacements are also used (Aronson, 1997; Giannoudis et al., 2005; Giannoudis and Einhorn, 2009).
Traditional Chinese Medicines in Osteoporosis and Bone Regeneration
Since ancient times, Traditional Chinese medicines (TCM) were used in hospitals and private clinics to cure skeletal disorders, and it is aneconomical substitute to commercialized pharmaceutical medicines. TCM are used in more than 130 countries across the globe. It is assumed that the application of TCM on damage tissues effect directly on the traumatized organ and trigger improvements and bone healing. Traditional Chinese Medicine’s extensive use for musculoskeletal disorders has endured the test of time in both rural and urban settings. In joint sprains, inflammatory disorders, and even fractured bones, topical applications of natural remedies are useful (Hsiao, 2007; Mukwaya et al., 2014). TCM has already been utilized for the management of musculoskeletal disorders, particularly bone fractures, osteoporosis, and rheumatism with great success (Mukwaya et al., 2014). TCM evolved from mythological therapy into a herbal medicine system. The impacts of several Chinese medicines were examined and vetted over many years of clinical practice. TCM differs greatly from modern medicine in terms of practice and theory (Wong and Rabie, 2006). TCM was added in the 11th edition of the International Statistical Classification of Diseases and Related Health Problems by the World Health Organization (WHO) in 2018 (Organization, 2004).
TCM is a discipline of conventional remedy that encompasses herbal medication, massage (tui na), acupuncture, food, and exercise (qigong) therapy. All these approaches are equally practiced, in this review, however we will be more focusing on herbal medication component of the Traditional Chinese Medicine. It is based on more than 2,500 years of Chinese healthcare profession (Sheng et al., 2012). The zàng-fu theory is among the fundamental concepts of TCM. The terminology zàng focuses on five key components, which include the liver, heart, lung, kidney and spleen, which include the stomach, gallbladder, small intestine, urine bladder, large intestine, and Sānjiaō. The kidneys are some of them, and it is thought to be associated to skeletons. In contrast to the Modern health conception of kidneys, the TCM idea of kidneys seems more like a method of characterizing a group of interconnected components than a physiological organ. The kidney’s primary duties are to build bones, control human growth and development, and generate marrow to fill the brain. Numerous kidney-nourishing herbal medications can restore bones, as per the Chinese medicine kidney theory, and are thus prescribed to cure bone-related disorders including osteoporosis. One of these medicines’ mode of action might increase osteoblastogenesis (Liu, 2018; Matuk, 2006). According to published research findings, such traditional Chinese medicines for the therapy of osteoporosis seem to have both anabolic and anticatabolic impacts besides boosting osteogenesis and minimising unbalance osteoclast activity, resulting in enhanced bone mineral density and biomechanical properties, as well as diminished bone microstructural degeneration (Figure 1) (He et al., 2017; Suvarna et al., 2018).
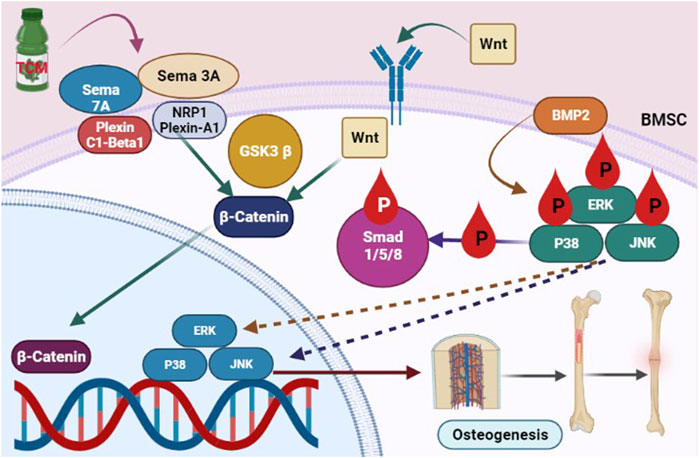
FIGURE 1. Natural Chinese medicine’s therapeutic promise in the treatment of osteoporosis. When faced with the obstacles of oestrogen or androgen shortage, excessive hormone medicines, and weightlessness, bone quality will be severely harmed. Although various natural Chinese medications may be good choice for improving skeleton growth and preventing bone loss.
Bone regeneration is a sophisticated biochemical procedure that starts with localized hemorrhage and inflammation, and then proceeds to the production of cartilage, soft extracellular matrix tissue, and fresh bone via the complex actions of mesenchymal progenitor cells. TCM emphasizes on the total influence of the patient’s body on the bone lesions in healing musculoskeletal disorders, rather than just the local therapy as is usual in western treatment. Therefore, TCM uses both interior therapies in conjunction to external therapy. TCM considers 3 phases when it comes to bone deformities: 1) during first phase, there is harm to the blood vessels due to stagnation of “qi” and blood, and also obstructed meninges, which together leads to pain; 2) in the second phase, the inflammation eventually subsides and the pain is significantly reduced, but the bruises remain, and connective tissues are not repaired; 3) in the third phase, the overall bone damage has now been healed, but remedy to pacify and improve bone regeneration, and improve the internal environment is yet required (Tang et al., 2021; Zhang et al., 2021). Icariin (ICA) being a traditional Chinese medicine which was found to repair the vascular system and successfully modulate intracellular senescence in bones. Furthermore, it is unknown if ICA can impact endothelial cells’ angiogenic capacity through changing apoptosis (Li et al., 2022). Traditional Chinese medicines having bone regeneration and antiosteoporosis activity are summarized in Table 1.
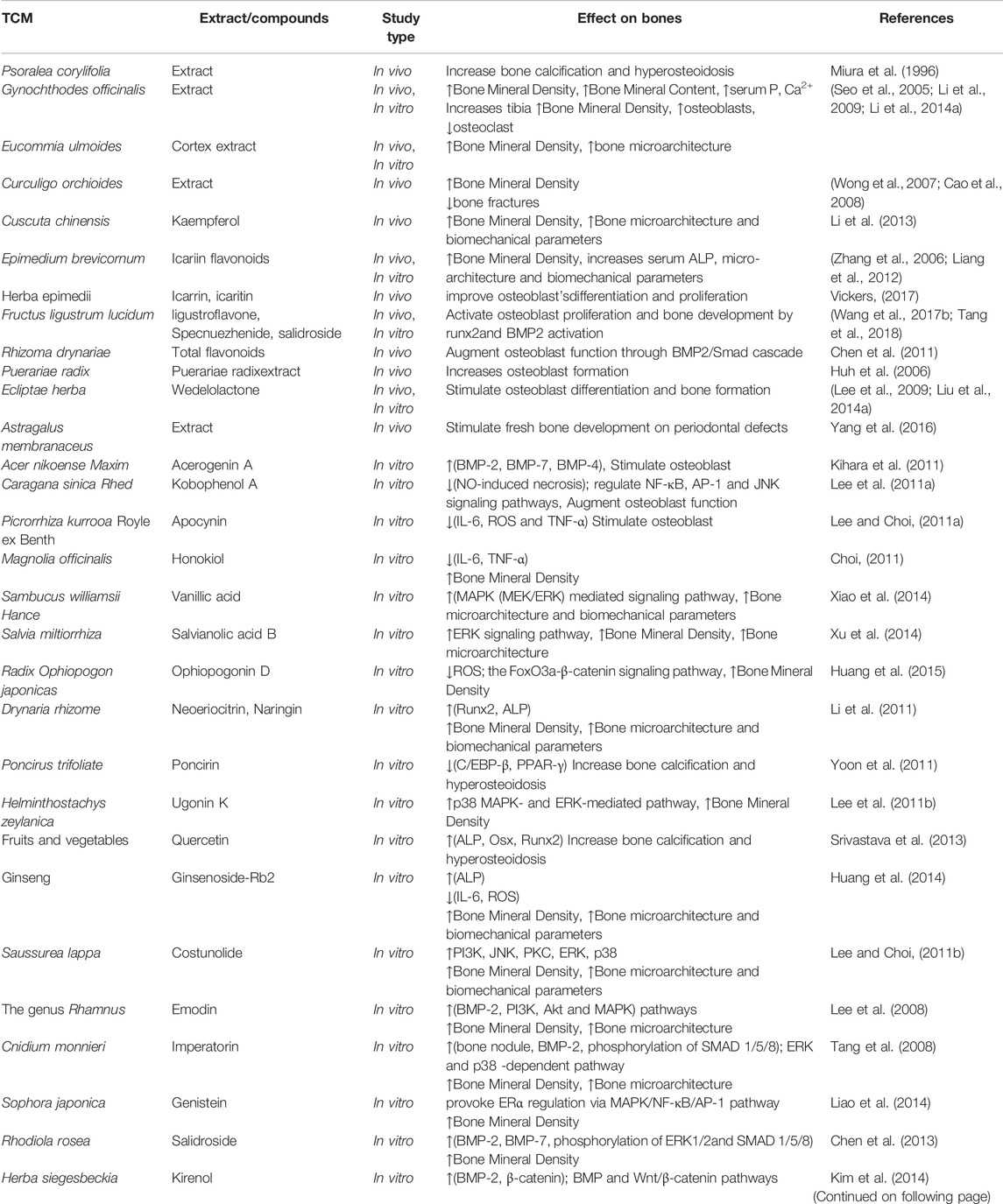
TABLE 1. Summary and mechanism of Traditional Chinese medicines (TCM) used for bone regeneration and antiosteoporosis.
Herba Epimedii
The dried aerial component of Epimedium species like Epimedium sagittatum (Yin Yang Huo) is known as Herba Epimedii (HE). HE is among the best commonly recommended herbs in Chinese osteoporosis formulations (Qin et al., 2005). The plant is harvested, stripped of stiff stems and unwanted particles, and shade or Sun drying is done in the autumn and summer. Sichuan, Shaanxi, Guangxi, Liaoning, and Hubei states yield the majority of it. Flavonoids, phenolic glycosides, ligans, penethylol glycosides, ionones and sesquiterpenes have all been extracted from Epimedium species. In an aging or ovariecto-mized (OVX) rat model, HE as a solo herb or even as a herb combination could prevent bone resorption (Zhu, 1998; Wong and Rabie, 2006). According to Gao, in vitro production of the chick embryo femur was stimulated by Epimedium in injectable formulation (Gao, 1985). HE extract has been shown in clinical trials to inhibit bone resorption and enhance E2 and osteocalcin concentrations. In OVX rats, the total flavonoid fraction of HEP started to improve BMD, increased E2 levels, and lowered systemic IL-6 levels (Jiang et al., 2002). Icariin, a flavonoid biomarker in HE, is thought to be the main bioactive element responsible for its bone-regeneration properties (Wong and Zhang, 2013). In an experiment conducted, OVX C57BL/6 test mice were injected icariin at a dose of 0.3 mg/g day for 6 weeks to determine the in vivo bones protecting properties of the compound. Icariin was reported to prevent the degradation of bone density and integrity in the femur bone (Mok et al., 2010).
Bone and bone marrow vascular are essential for supply of nutrients, oxygen, and secrete angiocrine factors essential for maintenance, survival, and self-renewal of progenitor and stem cells. Vasculature produces nurturing slots for blood-forming stem cells and bones in the skeletal system. Hematopoiesis is regulated by intact blood vessels and also aid in the formation of bones during repair, development, and re-generation. Malfunctioning of vasculature induces bone diseases, skeletal aging, and blood disorders (Chen et al., 2020). Physical activity can improve blood supply and vascularization to an organ, however, the curative/preventive potentials of physical activity on bone repair due to contribution of vascularization due to such activities have not been investigated specifically (Wazzani et al., 2021). The two major components Icaritin and Icariin of Epimedium sagittatum (Yin Yang Huo) also known as Herba Epimedii (HE) have been shown to have prominent effects in vascularization and bone formation. Icaritin reduced the incidence of steroid-associated osteonecrosis and inhibited intravascular thrombosis and extravascular lipid-deposition upon oral administration to rabbits (Zhang et al., 2009a). Similarly, an icariin/tricalcium phosphate porous scaffold enhanced new vascular and bone formation in a rabbit model of femoral head osteonecrosis after 12 weeks of treatment (Xie et al., 2015).
By methodologies of cell culture in vitro and detection of 3H-TdR integrating DNA of bone marrow cells, Liu et al. investigated the impact of Epimedium sagittatum polysaccharides on DNA replication of bone marrow cells of “yang deficiency” animal model produced by hydroxyurea. The findings demonstrated that following 100 μg dose of Epimedium sagittatum polysaccharides were applied, cell multiplication improved by 72% and DNA replication enhanced by 68 percent (Liu et al., 1991). Yu et al. investigated the effects of the Chinese herb Guizhou epimedium on osteoporosis and osteoclastic bone resorption. In order to evaluate the activity of the Chinese herb in vitro, osteoclasts were extracted and cultivated. The in vivo activity of this herb had also been studied in rats that had osteoporosis caused by ovariectomy, and the findings were correlated to the impact of estradiol in the similar group of rats. The epimedium was found to decrease osteoclastic degradation of bones in an in vitro research. Apparently the estradiol and epimedium proved to boost up mineral content and accelerate bone regeneration in the in vivo study (Yu et al., 1999).
Fortune’s Drynaria Rhizome (Rhizoma Drynariae)
The dried rhizome of the perennial Drynaria fortunei is known as Gu Sui Bu. It has been assumed to be an effective bones healer in traditional Chinese medicine. All year long, the rhizome can be assembled and processed. Guangdong, Zhejiang, Sichuan Hubei, and Guangxi provinces yield the Gu Sui Bu (Xu et al., 1991). Drynaria fortunei is a perennial herb that is often used to treat musculoskeletal diseases. Drynaria fortunei also possesses anti-osteoporosis properties. Drynaria fortunei’s anti-osteoporosis activity was also supported by the finding in rats that it inhibited ovariectomy-induced bone resorption thus maintained the fine particle surfaces of the trabeculae (Anderson et al., 2002). In an in vitro model, Drynaria fortunei extract was also demonstrated to have a powerful blocking action on cathepsin-K induced decomposition of collagen via reducing cathepsin-K activities. Furthermore, naringin is a flavonoid with estrogenic action that is found naturally in Drynaria fortune as bioactive component. Naringin when administered orally, increases BMP-2 expression, inhibits retinoic acid induced osteoporosis in rodents, enhances the growth and osteoblast development of bone mesenchymal stem cells, and induces osteogenesis through stimulating oestrogen receptor phosphorylation in osteocytes (Jeong et al., 2005; Wei et al., 2007; Dai et al., 2009).
Lin and his colleagues used an in vitro bone cell culture to look into the biochemical impact of ten various traditional Chinese medicines. The research model focused on the rat osteoblast-osteoclast co-culture technique. Different testing compounds were introduced when the cells had grown to 80% confluences. A colorimetric technique was used to assess the mitochondrial actions of osteocytes, following exposure to several Chinese medicine treatments. To assess bone cell activity, biochemical indicators like lactate dehydrogenase (LDH), protein content, acid phosphatase (ACP) titer, and alkaline phosphatase (ALP) were analysed. Just 4 out of 10 Chinese herbs had possible good results on bone cell culture when cultured for 24 h, and only Drynaria fortunei showed ubiquitous significant positive impact on bone cell metabolism. Drynaria fortunei’s main positive activity on bone cells is thought to be due to the triggering of apoptosis in the osteoclast cell (Lin et al., 2002).
Curculigo orchioides Gaertn
Curculigo plants have been reported in over 20 different species around the globe. They are indigenous to South America, Africa, Asia and Oceania’s tropical and subtropical climates. Curculigo orchioides is a kind of curculigo. In Chinese medicine, Curculigo orchioides Gaertn (COG, “Xian Mao”), it was applied topically for the management of knee and spine joints arthritis, leg fatigue and diarrhoea. COG seems to have antitumor effects and antioxidant, and could be employed as an antiosteoporotic herbal agent, according to latest research (Ramchandani et al., 2014; Vickers, 2017; Hejazi et al., 2018; Cui et al., 2019). COG has been studied in vivo for its antiosteoporotic activities in TCM for the management of postmenopausal women with osteoporosis. COG reduced trabecular bone degradation in ovariectomized rats’ tibias by reducing osteoclast activity and boosting serum calcium, phosphorus and OPG levels while having no effect on body or uterus size. After COG dosing, serum concentrations of bones degradation associated makers like corticosterone, TRAcP, DPD/Cr, and ACTH were reduced (Cao et al., 2008). COG might even stimulate bone regeneration after healing process, according to Wong et al. COG’s principal biologically active component, curculigoside (CCG; phenolic glycoside), is reported to have antiosteoclastic and osteogenic properties (Wong et al., 2007; Liu et al., 2014b).
Curculigoside upregulated the osteogenic action in human amniotic fluid derived stem cells in a dose-dependent way, according to a latest in vitro analysis, which revealed that CCG up-regulated osteogenic action in a dose-dependent way, such as upregulation of Collagen I and osteopontin, increased the ALP action, and calcium accumulation. The observation that Cyclin-D1 and β-catenin are both upregulated at the same time suggests that such actions are regulated through the β-catenin/Wnt signaling cascade (Liu et al., 2014b). By modulating osteogenic growth, differentiation, and proinflammatory cytokine concentrations, CCG has indeed been demonstrated to prevent rat calvarial osteoblasts from dexamethasone (DXM)-induced toxicity. The consequences of DXM on the concentrations of osteoblast development biomarkers such as OPG, ALP and β-catenin were reverted, suggesting that CCG could be a good candidate for the management of postmenopausal osteoporosis (Zhu et al., 2015). Phytochemicals extracted from COG have been demonstrated to exhibit antiosteoporotic effects in vitro and in rat calvarial investigations, such as the encouragement of osteoblast differentiation and proliferation, enhanced bone forming activities, and suppression of osteoclastogenesis (Jiao et al., 2009; Wang et al., 2017c). In summary, C. orchioides’ induction of osteogenesis may be a good impact resulting in reduction of osteoporosis, and the medicine’s suppression of ROS is a valuable quality. Furthermore, several COG-derived compounds have been shown to inhibit osteoclastic demineralization. More exploration is needed to establish the therapeutic constituents of COG, molecular pathways, and signalling routes as well as the biochemical basis of osteoporosis.
Fructus Ligustri Lucidi
Fructus Ligustri Lucidi (FL) is most often used to purify the kidneys and augment the bones as a singular herb. In ovariectomized rats which were fed whether with a regular 0.6% Ca dietor a reduced 0.1% Ca diet, orally, FL extract enhanced bone density and bone’s mechanical integrity at the diaphysis of femurs, tibias and lumbar vertebrae. Additionally, FL modulates calcium equilibrium in older women by modifying the parathormone’s vitamin-D axis and increasing absorption of calcium in vivo, implying that FL might be an appropriate therapy for restoring calcium and vitamin-D equilibrium (Haung and Yang, 2003; Zhang et al., 2008a; Zhang et al., 2008b). Even though oleanolic acid, the significant bioactive compound in FL, had enhanced the osteoblastic delineation of osseous mesenchymal stem cells and osteoprotective activity in ovariectomy-induced osteoporotic rats in vitro, more research is needed to determine whether this chemical contributes to the regulatory oversight of FL on calcium equllibrium (Haung and Yang, 2003).
Er-Xian Decoction
Er-Xian decoction (EX) is a multi-herb composition consisting of six different herbs: Cortex phellodendri, Radix morindae officinalis, H. epimedii, Rhizome curculiginis, Radix angelicae sinensis and Rhizome anemarrhenae. It has proven effective in reducing osteoporosis, perimenopausal syndrome, and ageing problems in older patients. It is therapeutically useful in alleviating menopausal symptoms by boosting the level of circulating estradiol. EX alleviated the menopausal symptoms by increasing antioxidant and endocrine activity, through the stimulation of catalase (CAT) and aromatase detoxification channels (Liu et al., 2005; Sze et al., 2009). EX has been proven to have anti-osteoporotic actions comparable to estrogens and has attributed enormously to the prophylaxis or therapy of bone loss caused by ovariectomy in rats. It has beneficial benefits on bone while having just minimal impact on the uterus, demonstrating that EX is acceptable to be used for bone health management (Nian et al., 2006).
Eucommia Ulmoides Oliv
Eucommia Ulmoides Oliv (EO) is the extract of leaf and cortex that has reported to inhibit osteolysis and bones density depletion, as well as stimulate osteogenesis. Ha and his co-researchers investigated the phytochemicals in a portion of extract of Eucommiae cortex and found that these constituents play active role in every sequence of the pathways for initiating osteoblast to expedite osteogenesis and impeding osteoclast action to block osteolysis by evaluating on osteoporosis the clinical efficiency of these crude extract, like methanol, ethyl acetate, chloroform, aqueous, and butanol fractions. The oral administration of EO over a 4-month time frame in adult OVX rats was found to prohibit the degradation of trabecular micro-architecture and loss of estrogen deficiency-induced osseous micronutrients, thus sustaining physiological fluency of the bone. Moreover, EO juice was reported to activate the secretion of GH, controlling bone growth, development and bone formation, implying that EO could be a promising candidate for the treatment of osteoporosis (Ha et al., 2003; Zhang et al., 2009b).
Fructus Psoraleae (Bu Gu Zhi)
The dehydrated mature fruits of the perennial herb Psoralea corylifolia L. are known as Bu Gu Zhi. It belongs to family Leguminosae. The plant is harvested and processed in the autumn. After that, the fruit is scraped clean and free of extraneous debris. Anhui, Shanxi, Henan, and Sichuan states yield the majority of the plant. Healthy rats were given the acetone seed extract (the n-hexane ethyl acetate elution) of P. corylifolia, which resulted in a considerable increase in bones mineralization and serum inorganic phosphorus. Bone regeneration and serum levels of phosphorus were elevated in rachitic rats (already nourished with vitamin D free diet) when n-hexane elution were orally administered. In rachitic rats, administration of n-hexane elution showed a significant reduction in osteoid quantity and an enhancement in hyperosteoides. These findings indicate that n-hexane elution could be effective treatments for osteomalacia, osteoporosis, broken bone, and other skeletal disorders (Ma et al., 1996; Miura et al., 1996). Psoralea corylifolia L. fruit preparations stimulated osteoblastic development in an in vitro grown UMR106 cell line, according to Wang et al. By using activity-guided fractionation, the flavonoids bavachin and corylin have been recovered and defined as bioactive components. These findings indicate that Psoralea corylifolia L. fruit preparations, as well as bavachin and corylin, may induce bone production or have anti-osteoporosis effect (Wang et al., 2001).
Epimedium davidii Franch (Icariin, and Icaritin)
Epimedium davidii Franch (EDF) was used to cure osteoporosis for decades, attributed to its ability to “develop” bones. Icariin is the major bioactive flavonoid glucoside extracted from EDFand it is linked to the herb’s pharmacological activities. Nevertheless, in current years, its bone-strengthening properties have gotten a lot of publicity. Icariin boosts antiosteoporotic action by promoting osteogenic development and calcification, decreasing adipogenesis, blocking osteoclast formation, and causing osteoclast death, all of which reduce osteoclast activity and bone resorption (Li et al., 2014b; Xu et al., 2016). Icariin may boost Nitric oxide (NO) production by increasing osteoblast development and matrix calcification. Subsequent research revealed that icariin therapy increased the expression of SMAD4, BMP-2, OPG, and Cbfa1/Runx2 genes while decreasing the expression of RANKL. This action might lead to its effects on osteoblast multiplication and development, which results in osteogenesis (Li et al., 2014b). Liang’s findings also showed that icariin might enhance osteogenesis in hFOB 1.19 cells via activating the BMP-2/Smad4 signaling cascade (Figure 2) (Liang et al., 2012). Icaritin, an icariin endogenous product, has a comparable performance to icariin. In vitro and in an OVX rat, oral administration of icaritin suppressed osteoclastogenesis by downregulating TRAF6 and inhibiting the NF-κB, MAPK/AP-1, and reactive oxygen species signalling cascades in a synchronized approach to diminish NFATc1 expression and activities (Tan et al., 2017). According to Wu and Sheng, icaritin increased the protein content of osteocalcin BMPs, and Runx2 in human adipose tissue-derived stem cells and derived mesenchymal stem cells while impeding adipogenesis in marrow mesenchymal stem cells by suppressing peroxisome proliferator-activated receptor gamma and glycogen synthase kinase-3 (Sheng et al., 2013).
TCM enhanced the Sema7A and sema3A-mediated signaling cascade, which is stimulated downstream of β-catenin nuclear translocation, and boosted the stimulation of the Smad1/5/8 and BMP2 pathway, leading to an increase in osteoblastogenesis.
Eclipta prostrata (Mo-Han-Lian)
The aerial components of Eclipta prostrataL. (Asteraceae), often termed as “Mo-Han-Lian,” contain antiosteoporotic potential. Ecliptae herba produces the chemical wedelolactone. Though this Ecliptae herba ethyl acetate extract and wedelolactone had no effect on BMSC growth, the extract and wedelolactone did improve BMSC development into osteoblasts. The extract was given orally as well as applied topically on the fractured site. When BMSCs are incubated with wedeloloactone, the production of ALP, a diagnostic enzyme for mature osteoblasts, increases in a dose-dependent way. In addition, wedelolactone caused an elevation in bones calcification. Wedelolactone suppressed GSK3 function and increased GSK3 phosphorylation at the molecular scale, which therefore boosted nuclear translocation of β-catenin. The expression of genes associated to osteoblastogenesis, such as osteocalcin, osteorix, and runx2, was elevated. Wedelolactone treatment to ovariectomized rats reduced ovariectomy induced bone resorption by increasing osteoblast activation and stimulating osteogenesis (Zhang et al., 2008c; Zhang et al., 2013; Liu et al., 2016).
Salvia miltiorrhiza
Researchers investigated the anti-osteoporotic potential of 99 distinct Chinese herbal remedies in 108 randomized studies involving 10,655 Chinese subjects. Salvia miltiorrhiza was present in 16 of the investigations. TCM therapy was contrasted to Western medication (like vitamin D2, caltrate, alendronate, and calcitonine) in 61 studies, including 23 natural treatment investigations showing a considerable benefit in boosting bone mineral density (BMD). In 48 researches the impact of combining Western medicines with Chinese herbal medicine, 26 found that the combined treatment had a greater effect on BMD versus Conventional medicine solo. The subsequent studies had equivalent results or were significantly lesser effective. Standard medicines such as denosumab, bisphosphonates, and selective oestrogen receptor stimulators have been often or not included in the controlled research utilizing Western medicine. Calcitriol caltrate, calcitonin, and vitamin D were the most often utilized Western medicines in the normal control in investigations including Salvia miltiorrhiza, whereas a bisphosphonate (alendronate) was given once only (Liu et al., 2014c).
Network Pharmacology Approach for Active Ingredient Identification
Traditional Chinese medicine usually contain many components and the main issue of application of such combinations is that they contain the effective therapeutic components that have been identified or not yet and involve various unknown elements, some of which may have underlying toxicities. Therefore, it is extremely important to extract only these therapeutic components and avoid risks posed by the administration of raw herbal medicine. Therefore, there is a need to propose potent approaches to identify those therapeutic elements and extract them. In recent years, computer technology along with endless innovations and development in systems biology, a network pharmacology (NP) approach has been adopted which has shown applicational value in many fields, including drug target identification, discovery of active ingredients, investigation of mechanism of action, and safety evaluations (Ye et al., 2014). It was proposed for the first time in 2007 by Hopkins and is now believed as a promising approach which combine systems medicine with information science (Hopkins, 2007). It give an efficient approach for evaluation of the synergy of components of TCM and their mechanisms (Zhang et al., 2020) and has been widely used for the overall molecular mechanism of TCM preparations or multi-drug combinations (Ye et al., 2016). Bioinformatics tools are used to obtain differentially expressed genes (DEGs) as disease targets (e.g., Osteoporosis), and then effective components and pharmacological mechanism of TCM in the treatment of the disease are analyzed by network pharmacology. Various studies have reported this approach for the investigation of mechanism of TCM in treatment of Osteoporosis. For example, Li et al. used this approach for investigation of the mechanism of Xianlinggubao capsule in the treatment of osteoporosis (Li et al., 2021). They found that the active components (luteolin, quercetin, apigenin and ursolic acid) of Xianlinggubao capsule could be responsible for the mineralization of MC3T3-E1 cells. Similarly, Yang et al., used Network pharmacology approach combined with molecular docking to investigate the Potential Mechanism of Jintiange Capsule for the treatment of Osteoporosis and found that calcium phosphate (the main active ingredient), may interact with CALR and CALM1 targets and regulate multiple signaling pathways to treat osteoporosis (Yang et al., 2021). Gan et al. used NP approach for investigation of the mechanism of Rhizoma drynariae (TCM) against Osteoporosis and found 16 active ingredients that directly or indirectly target multiple signaling pathways and affect the differentiation and proliferation of multiple types of cells (Gan et al., 2019). There are many other examples where this approach has been used for identification of major components responsible for the treatment of osteophorosis and their possible mechanism has been provided. Yet, it is extremely important to extract only these therapeutic components and avoid risks posed by the administration of raw herbal medicine.
Future Prospects
Accidental injuries and malignancies create bone abnormalities, which are widespread in clinical practice. High costs and long treatment cycles, uncontrolled curative impact, and problems such as infection, nonunion and malunion, of bones define existing bones defect therapies. These concerns not only have a negative impact on patients’ physiological and emotional health, but they also pose a difficulty for orthopaedic surgeons. The natural Chinese medications discussed in this article are both traditional and bone-specific. Therapeutic skills, as we all knew, are quite significant in Chinese medicine. As per the rich practises and expertise in the clinics, as well as Chinese medicine concepts, Chinese medications were grouped into several groups with specific functions. Many of them have been traditional and bone-specific medications used to manage bone injuries and bone—related illnesses because they improved bone production. In traditional Chinese medicine, the majority of them have the impacts and activities of tonifying the “Yang,” which improves bone formation and metabolism. In Chinese medicine, “yang-tonifying” medications are common and traditional type of natural remedies used to prevent osteoporosis (Ju et al., 2014; Li et al., 2015). Several clinical trials have demonstrated the antiosteoporosis actions of well substances, such as Epimedium derived phytoestrogen flavonoids, which were employed in a clinical study to cure and suppress osteoporosis and bone resorption in older women (Zhang et al., 2007; Zhu et al., 2012; Wang et al., 2013; Shi et al., 2017; Liang et al., 2020). TCM formulations offer less adverse effects, have become less expensive, and may be used for a prolonged time. TCM formulations not only can restore bones microarchitecture, enhance bone mass, and promote bone physiological qualities, but could also lessen or eradicate spinal debilitation, backache, as well as other complaints, according to a large body of clinical settings and animal experimentation (Zhu et al., 2012).
The modes of action of natural Chinese medicines that are efficacious in curing osteoporosis has not been thoroughly examined, emphasising the urgency for more research. To fully evaluate substances for pharmaceutical use, more studies are needed to identify and describe active antiosteoporotic molecules from traditional and bone-specific medications, as well as their safety, effectiveness, and probable interactions with other treatments. To establish their promising implications for the therapy of osteoporosis, as an efficient, potential substitute to principal treatment interventions, or in conjunction with existing main treatment modalities, research works to establish the special and focused molecular and cellular mechanisms of Traditional Chinese medicine substances are required.
Conclusion
Countless trials have been performed to examine the pathophysiology of osteoporosis. Immunological modulation of osteoporosis is a new scientific focus that presents a novel method of osteoporosis etiology. The therapeutic potential of Traditional Chinese herbs in the management of osteoporosis has been demonstrated. Further investigation is necessary, but a growing number of researchers have shown that these herbs have an essential part in the rehabilitation of osteoporosis by modulating the immune system. Traditional Chinese medicine’s mode of action is notable for being multiroute and multitargeted, rather than a singular mechanism. Traditional Chinese medicine has therapeutic value for the management of osteoporosis, according to current in vitro and in vivo findings. To fully understand the therapeutic potential of Chinese medicines, more investigation is required to confirm their safety, potency, and accuracy. Further high excellence clinical trials using these traditional remedies are required to offer additional evidence for the candidate’s antiosteoporotic usage to be efficacious and non-toxic.
Author Contributions
ZP: Manuscript writing, RX: Reviewing and finalizing the manuscript, QY: Data collectioin.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ACP, acid phosphatase; ACTH, Adrenocorticotropic hormone; BMD, bones mineral density; CCG, curculigoside; COG, Curculigo orchioides Gaertn; DXM, dexamethasone; EO, eucommia ulmoides olive; ERK, extracellular signal-regulated kinase; EX, Er-Xian decoction; FDA, Federal Drug Administration; FL, Fructus ligustri lucidi; GSK3-β, Glycogen Synthase Kinase 3-β; HE, Herba Epimedii; HRT, hormone replacement therapy; JNK, c-Jun N-terminal kinases; OPG, Orthopantomogram; OVX, ovariecto-mized; ROS, reactive oxygen species; TCM, Traditional Chinese medicines.
References
Anderson, G., Sun, J., and Theriault, B. (2002). The Effect of Gu-Sui-Bu (Drynaria Fortunei) on Bone Cell Activity: A Preliminary Study. J. bone mineral Res. 17, S454.
Aronson, J. (1997). Current Concepts Review - Limb-Lengthening, Skeletal Reconstruction, and Bone Transport with the Ilizarov Method. J. Bone & Jt. Surg. 79 (8), 1243–1258. doi:10.2106/00004623-199708000-00019
Audigé, L., Griffin, D., Bhandari, M., Kellam, J., and Rüedi, T. P. (2005). Path Analysis of Factors for Delayed Healing and Nonunion in 416 Operatively Treated Tibial Shaft Fractures. Clin. Orthop. Relat. Research® 438, 221–232. doi:10.1097/01.blo.0000163836.66906.74
Bartl, R., and Frisch, B. (2004). “Definition of Osteoporosis,” in Osteoporosis (Springer), 24–32. doi:10.1007/978-3-662-09163-0_3
Bates, P., Yeo, A., and Ramachandran, M. (2018). Bone Injury, Healing and Grafting. Basic Orthop. Sci. 1, 205–222. doi:10.1201/9781315117294-14
Bone, H., Mcclung, M. R., Ince, A., and Verbruggen, N. (2007). A Randomized Double-Blind, Placebo-Controlled Study of a Cathepsin K Inhibitor in the Treatment of Postmenopausal Women with Low BMD: One Year Results. J. Bone Min. Res. 22 (Suppl. 1), S37. doi:10.1016/j.bone.2007.12.136
Buckley, L., Guyatt, G., Fink, H. A., Cannon, M., Grossman, J., Hansen, K. E., et al. (2017). 2017 American College of Rheumatology Guideline for the Prevention and Treatment of Glucocorticoid-Induced Osteoporosis. Arthritis & Rheumatology 69 (8), 1521–1537. doi:10.1002/art.40137
Burge, R., Dawson-Hughes, B., Solomon, D. H., Wong, J. B., King, A., and Tosteson, A. (2007). Incidence and Economic Burden of Osteoporosis-Related Fractures in the United States, 2005-2025. J. Bone Min. Res. 22 (3), 465–475. doi:10.1359/jbmr.061113
Cao, D. P., Zheng, Y. N., Qin, L. P., Han, T., Zhang, H., Rahman, K., et al. (2008). Curculigo Orchioides, a Traditional Chinese Medicinal Plant, Prevents Bone Loss in Ovariectomized Rats. Maturitas 59 (4), 373–380. doi:10.1016/j.maturitas.2008.03.010
Chen, J.-J., Zhang, N.-F., Mao, G.-X., He, X.-B., Zhan, Y.-C., Deng, H.-B., et al. (2013). Salidroside Stimulates Osteoblast Differentiation through BMP Signaling Pathway. Food Chem. Toxicol. 62, 499–505. doi:10.1016/j.fct.2013.09.019
Chen, J., Hendriks, M., Chatzis, A., Ramasamy, S. K., and Kusumbe, A. P. (2020). Bone Vasculature and Bone Marrow Vascular Niches in Health and Disease. J. Bone Min. Res. 35 (11), 2103–2120. doi:10.1002/jbmr.4171
Chen, L.-l., Lei, L.-h., Ding, P.-h., Tang, Q., and Wu, Y.-m. (2011). Osteogenic Effect of Drynariae Rhizoma Extracts and Naringin on MC3T3-E1 Cells and an Induced Rat Alveolar Bone Resorption Model. Archives Oral Biol. 56 (12), 1655–1662. doi:10.1016/j.archoralbio.2011.06.008
Cho, T.-J., Gerstenfeld, L. C., and Einhorn, T. A. (2002). Differential Temporal Expression of Members of the Transforming Growth Factor β Superfamily during Murine Fracture Healing. J. Bone Min. Res. 17 (3), 513–520. doi:10.1359/jbmr.2002.17.3.513
Choi, E. M. (2011). Honokiol Isolated from Magnolia Officinalis Stimulates Osteoblast Function and Inhibits the Release of Bone-Resorbing Mediators. Int. Immunopharmacol. 11 (10), 1541–1545. doi:10.1016/j.intimp.2011.05.011
Cosman, F., de Beur, S. J., LeBoff, M. S., Lewiecki, E. M., Tanner, B., Randall, S., et al. (2014). Clinician’s guide to prevention and treatment of osteoporosis Osteop. Int. 25 (10), 2359–2381. doi:10.1007/s00198-014-2794-2
Cui, X., Shang, S., Lv, X., Zhao, J., Qi, Y., and Liu, Z. (2019). Perspectives of Small Molecule Inhibitors of Activin Receptor-like K-inase in A-nti-tumor T-reatment and S-tem C-ell D-ifferentiation (Review). Mol. Med. Rep. 19 (6), 5053–5062. doi:10.3892/mmr.2019.10209
Dai, K.-R., Zhang, P., Yan, S. G., Yan, W. Q., Zhang, C., Chen, D. Q., et al. (2009). Effects of Naringin on the Proliferation and Osteogenic Differentiation of Human Bone Mesenchymal Stem Cell. Eur. J. Pharmacol. 607 (1-3), 1–5. doi:10.1016/j.ejphar.2009.01.035
Dimitriou, R., Jones, E., McGonagle, D., and Giannoudis, P. V. (2011). Bone Regeneration: Current Concepts and Future Directions. BMC Med. 9 (1), 66–10. doi:10.1186/1741-7015-9-66
Dinger, J., Bardenheuer, K., and Heinemann, K. (2016). Drospirenone Plus Estradiol and the Risk of Serious Cardiovascular Events in Postmenopausal Women. Climacteric 19 (4), 349–356. doi:10.1080/13697137.2016.1183624
Einhorn, T. A. (1998). The Cell and Molecular Biology of Fracture Healing. Clin. Orthop. Relat. Res. 355S, S7–S21. doi:10.1097/00003086-199810001-00003
Ferguson, C., Alpern, E., Miclau, T., and Helms, J. A. (1999). Does Adult Fracture Repair Recapitulate Embryonic Skeletal Formation? Mech. Dev. 87 (1-2), 57–66. doi:10.1016/s0925-4773(99)00142-2
Gan, D., Xu, X., Chen, D., Feng, P., and Xu, Z. (2019). Network Pharmacology-Based Pharmacological Mechanism of the Chinese Medicine Rhizoma Drynariae against Osteoporosis. Med. Sci. Monit. 25, 5700–5716. doi:10.12659/msm.915170
Gao, Z. F. (1985). Stimulant Effect of Epimedium in an Injection Form on the Growth of the Chick Embryo Femur In Vitro. Zhong Xi Yi Jie He Za Zhi 5 (3), 172133–133.
Giannoudis, P. V., Dinopoulos, H., and Tsiridis, E. (2005). Bone Substitutes: an Update. Injury 36 (3), S20–S27. doi:10.1016/j.injury.2005.07.029
Giannoudis, P. V., and Einhorn, T. A. (2009). Bone Morphogenetic Proteins in Musculoskeletal Medicine. Injury 40, S1–S3. doi:10.1016/s0020-1383(09)00642-1
Guerrini, M. M., and Takayanagi, H. (2014). The Immune System, Bone and RANKL. Archives Biochem. Biophysics 561, 118–123. doi:10.1016/j.abb.2014.06.003
Ha, H., Ho, J., Shin, S., Kim, H., Koo, S., Kim, I.-H., et al. (2003). Effects of Eucommiae Cortex on Osteoblast-like Cell Proliferation and Osteoclast Inhibition. Arch. Pharm. Res. 26 (11), 929–936. doi:10.1007/bf02980202
Haas, A. V., and LeBoff, M. S. (2018). Osteoanabolic Agents for Osteoporosis. J. Endocr. Soc. 2 (8), 922–932. doi:10.1210/js.2018-00118
Haung, W., and Yang, Y.-F. (2003). Pharmacological and Clinical Research Progression of Privet Berry and its Active Ingredient. Mod. J. Integr. Chin. Traditional West. Med. 12 (7), 772–775.
He, J. B., Chen, M. H., and Lin, D. K. (2017). New Insights into the Tonifying Kidney-Yin Herbs and Formulas for the Treatment of Osteoporosis. Arch. Osteoporos. 12 (1), 14–13. doi:10.1007/s11657-016-0301-4
Hejazi, I. I., Khanam, R., Mehdi, S. H., Bhat, A. R., Rizvi, M. M. A., Thakur, S. C., et al. (2018). Antioxidative and Anti-proliferative Potential of Curculigo Orchioides Gaertn in Oxidative Stress Induced Cytotoxicity: In Vitro, Ex Vivo and In Silico Studies. Food Chem. Toxicol. 115, 244–259. doi:10.1016/j.fct.2018.03.013
Hopkins, A. L. (2007). Network Pharmacology. Nat. Biotechnol. 25 (10), 1110–1111. doi:10.1038/nbt1007-1110
Hsiao, J. I.-H. (2007). Patent Protection for Chinese Herbal Medicine Product Invention in Taiwan. J. World Intellect. Prop. 10 (1), 1–21. doi:10.1111/j.1422-2213.2007.00311.x
Huang, Q., Gao, B., Jie, Q., Wei, B.-Y., Fan, J., Zhang, H.-Y., et al. (2014). Ginsenoside-Rb2 Displays Anti-osteoporosis Effects through Reducing Oxidative Damage and Bone-Resorbing Cytokines during Osteogenesis. Bone 66, 306–314. doi:10.1016/j.bone.2014.06.010
Huang, Q., Gao, B., Wang, L., Zhang, H.-Y., Li, X.-J., Shi, J., et al. (2015). Ophiopogonin D: a New Herbal Agent against Osteoporosis. Bone 74, 18–28. doi:10.1016/j.bone.2015.01.002
Huh, J.-E., Yang, H.-R., Park, D.-S., Choi, D.-Y., Baek, Y.-H., Cho, E.-M., et al. (2006). Puerariae Radix Promotes Differentiation and Mineralization in Human Osteoblast-like SaOS-2 Cells. J. Ethnopharmacol. 104 (3), 345–350. doi:10.1016/j.jep.2005.09.041
Ivanova, S., Vasileva, L., Ivanova, S., Peikova, L., and Obreshkova, D. (2015). Osteoporosis: Therapeutic Options. Folia Med. Plovdiv. 57 (3/4), 181–190. doi:10.1515/folmed-2015-0037
Jeong, J.-C., Lee, J.-W., Yoon, C.-H., Lee, Y.-C., Chung, K.-H., Kim, M.-G., et al. (2005). Stimulative Effects of Drynariae Rhizoma Extracts on the Proliferation and Differentiation of Osteoblastic MC3T3-E1 Cells. J. Ethnopharmacol. 96 (3), 489–495. doi:10.1016/j.jep.2004.09.038
Jeremiah, M. P., Unwin, B. K., Greenawald, M. H., and Casiano, V. E. (2015). Diagnosis and Management of Osteoporosis. Am. Fam. Physician 92 (4), 261–268.
Jiang, S., and Ye, G. (2005). Authentication Of “Bone Penetrating Herbs”. Lishizhen Med. Materia Medica Res. 16, 1014–1015.
Jiang, Y. N., Mo, H. Y., and Chen, J. M. (2002). Effects of Epimedium Total Flavonoids Phytosomes on Preventing and Treating Bone-Loss of Ovariectomized Rats. Zhongguo Zhong Yao Za Zhi 27 (3), 221–224.
Jiao, L., Cao, D.-P., Qin, L.-P., Han, T., Zhang, Q.-Y., Zhu, Z., et al. (2009). Antiosteoporotic Activity of Phenolic Compounds from Curculigo Orchioides. Phytomedicine 16 (9), 874–881. doi:10.1016/j.phymed.2009.01.005
Ju, D., Liu, M., Zhao, H., and Wang, J. (2014). Mechanisms Of “Kidney Governing Bones” Theory in Traditional Chinese Medicine. Front. Med. 8 (3), 389–393. doi:10.1007/s11684-014-0362-y
Kawamata, A., Iihara, M., Okamoto, T., and Obara, T. (2008). Bone Mineral Density before and after Surgical Cure of Cushing's Syndrome Due to Adrenocortical Adenoma: Prospective Study. World J. Surg. 32 (5), 890–896. doi:10.1007/s00268-007-9394-7
Kihara, T., Ichikawa, S., Yonezawa, T., Lee, J.-W., Akihisa, T., Woo, J. T., et al. (2011). Acerogenin A, a Natural Compound Isolated from Acer Nikoense Maxim, Stimulates Osteoblast Differentiation through Bone Morphogenetic Protein Action. Biochem. Biophysical Res. Commun. 406 (2), 211–217. doi:10.1016/j.bbrc.2011.02.017
Kim, M.-B., Song, Y., and Hwang, J.-K. (2014). Kirenol Stimulates Osteoblast Differentiation through Activation of the BMP and Wnt/β-Catenin Signaling Pathways in MC3T3-E1 Cells. Fitoterapia 98, 59–65. doi:10.1016/j.fitote.2014.07.013
Lee, C.-H., Huang, Y.-L., Liao, J.-F., and Chiou, W.-F. (2011). Ugonin K Promotes Osteoblastic Differentiation and Mineralization by Activation of P38 MAPK- and ERK-Mediated Expression of Runx2 and Osterix. Eur. J. Pharmacol. 668 (3), 383–389. doi:10.1016/j.ejphar.2011.06.059
Lee, M. K., Ha, N. R., Yang, H., Sung, S. H., and Kim, Y. C. (2009). Stimulatory Constituents ofEclipta Prostrataon Mouse Osteoblast Differentiation. Phytother. Res. 23 (1), 129–131. doi:10.1002/ptr.2560
Lee, S.-R., Kwak, J.-H., Park, D.-S., and Pyo, S. (2011). Protective Effect of Kobophenol A on Nitric Oxide-Induced Cell Apoptosis in Human Osteoblast-like MG-63 Cells: Involvement of JNK, NF-Κb and AP-1 Pathways. Int. Immunopharmacol. 11 (9), 1251–1259. doi:10.1016/j.intimp.2011.04.004
Lee, S.-U., Shin, H. K., Min, Y. K., and Kim, S. H. (2008). Emodin Accelerates Osteoblast Differentiation through Phosphatidylinositol 3-kinase Activation and Bone Morphogenetic Protein-2 Gene Expression. Int. Immunopharmacol. 8 (5), 741–747. doi:10.1016/j.intimp.2008.01.027
Lee, Y. S., and Choi, E. M. (2011). Apocynin Stimulates Osteoblast Differentiation and Inhibits Bone-Resorbing Mediators in MC3T3-E1 Cells. Cell. Immunol. 270 (2), 224–229. doi:10.1016/j.cellimm.2011.05.011
Lee, Y. S., and Choi, E. M. (2011). Costunolide Stimulates the Function of Osteoblastic MC3T3-E1 Cells. Int. Immunopharmacol. 11 (6), 712–718. doi:10.1016/j.intimp.2011.01.018
Li, F., Yang, X., Yang, Y., Guo, C., Zhang, C., Yang, Z., et al. (2013). Antiosteoporotic Activity of Echinacoside in Ovariectomized Rats. Phytomedicine 20 (6), 549–557. doi:10.1016/j.phymed.2013.01.001
Li, G.-W., Xu, Z., Chang, S.-X., Nian, H., Wang, X.-Y., and Qin, L.-D. (2014). Icariin Prevents Ovariectomy-Induced Bone Loss and Lowers Marrow Adipogenesis. Menopause 21 (9), 1007–1016. doi:10.1097/gme.0000000000000201
Li, L., Zeng, Z., and Cai, G. (2011). Comparison of Neoeriocitrin and Naringin on Proliferation and Osteogenic Differentiation in MC3T3-E1. Phytomedicine 18 (11), 985–989. doi:10.1016/j.phymed.2011.03.002
Li, N., Qin, L.-P., Han, T., Wu, Y.-B., Zhang, Q.-Y., and Zhang, H. (2009). Inhibitory Effects of Morinda Officinalis Extract on Bone Loss in Ovariectomized Rats. Molecules 14 (6), 2049–2061. doi:10.3390/molecules14062049
Li, X., Wen, Y., Sheng, L., Guo, R., Zhang, Y., and Shao, L. (2022). Icariin Activates Autophagy to Trigger TGFβ1 Upregulation and Promote Angiogenesis in EA.Hy926 Human Vascular Endothelial Cells. Bioengineered 13 (1), 164–177. doi:10.1080/21655979.2021.2011637
Li, Y., Lü, S. S., Tang, G. Y., Hou, M., Tang, Q., Zhang, X. N., et al. (2014). Effect of Morinda Officinalis Capsule on Osteoporosis in Ovariectomized Rats. Chin. J. Nat. Med. 12 (3), 204–212. doi:10.1016/S1875-5364(14)60034-0
Li, Y., Li, R., Zeng, Z., Li, S., Liao, S., Ma, W., et al. (2021). Network Pharmacology Combined with Bioinformatics to Investigate the Mechanism of Xianlinggubao Capsule in the Treatment of Osteoporosis. Phytomedicine Plus 1 (3), 100049. doi:10.1016/j.phyplu.2021.100049
Li, Y., Liang, W., Li, X., Gao, B., Gan, H., Yin, L., et al. (2015). Effect of Serum from Postmenopausal Women with Osteoporosis Exhibiting the Kidney-Yang Deficiency Pattern on Bone Formation in an hFOB 1.19 Human Osteoblastic Cell Line. Exp. Ther. Med. 10 (3), 1089–1095. doi:10.3892/etm.2015.2616
Liang, J., Wang, F., Huang, J., Xu, Y., and Chen, G. (2020). The Efficacy and Safety of Traditional Chinese Medicine Tonifying-Shen (Kidney) Principle for Primary Osteoporosis: a Systematic Review and Meta-Analysis of Randomized Controlled Trials. Evidence-Based Complementary Altern. Med. 2020, 5687421. doi:10.1155/2020/5687421
Liang, W., Lin, M., Li, X., Li, C., Gao, B., Gan, H., et al. (2012). Icariin Promotes Bone Formation via the BMP-2/Smad4 Signal Transduction Pathway in the hFOB 1.19 Human Osteoblastic Cell Line. Int. J. Mol. Med. 30 (4), 889–895. doi:10.3892/ijmm.2012.1079
Liao, M.-H., Tai, Y.-T., Cherng, Y.-G., Liu, S.-H., Chang, Y.-A., Lin, P.-I., et al. (2014). Genistein Induces Oestrogen Receptor-α Gene Expression in Osteoblasts through the Activation of Mitogen-Activated Protein kinases/NF-κB/activator Protein-1 and Promotes Cell Mineralisation. Br. J. Nutr. 111 (1), 55–63. doi:10.1017/s0007114513002043
Limmer, A., and Wirtz, D. (2017). Osteoimmunology: Influence of the Immune System on Bone Regeneration and Consumption. Z Orthop. Unf. 155 (03), 273–280. doi:10.1055/s-0043-100100
Lin, C.-Y., Sun, J.-S., Sheu, S.-Y., Lin, F.-H., Wang, Y.-J., and Chen, L.-T. (2002). The Effect of Chinese Medicine on Bone Cell Activities. Am. J. Chin. Med. 30 (02n03), 271–285. doi:10.1142/s0192415x02000351
Liu, F., Ding, G., and Li, J. (1991). [Effects of Epimedium Sagittatum Maxim. Polysaccharides on DNA Synthesis of Bone Marrow Cells of "yang Deficiency" Animal Model Caused by Hydroxyurea]. Zhongguo Zhong Yao Za Zhi 16 (10), 620.
Liu, M., Li, Y., and Yang, S.-T. (2014). Curculigoside Improves Osteogenesis of Human Amniotic Fluid-Derived Stem Cells. Stem cells Dev. 23 (2), 146–154. doi:10.1089/scd.2013.0261
Liu, Q., Shi, J. R., Yang, Y., Fang, Z. Q., Liang, S. H., and Guo, R. X. (2005). Effects on Secretory Function of Rat Gonad by Erxian Decoction and its Disassembled Prescriptions. Zhongguo Zhong Yao Za Zhi 30 (13), 1023–1026.
Liu, Y., Liu, J. P., and Xia, Y. (2014). Chinese Herbal Medicines for Treating Osteoporosis. Cochrane Database Syst. Rev. 1 (3), CD005467. doi:10.1002/14651858.CD005467.pub2
Liu, Y.-Q., Zhan, L.-B., Liu, T., Cheng, M.-C., Liu, X.-Y., and Xiao, H.-B. (2014). Inhibitory Effect of Ecliptae Herba Extract and its Component Wedelolactone on Pre-osteoclastic Proliferation and Differentiation. J. Ethnopharmacol. 157, 206–211. doi:10.1016/j.jep.2014.09.033
Liu, Y. Q., Hong, Z. L., Zhan, L. B., Chu, H. Y., Zhang, X. Z., and Li, G. H. (2016). Wedelolactone Enhances Osteoblastogenesis by Regulating Wnt/β-Catenin Signaling Pathway but Suppresses Osteoclastogenesis by NF-κB/c-fos/NFATc1 Pathway. Sci. Rep. 6 (1), 32260–32272. doi:10.1038/srep32260
Liu, Y. (2018). “Traditional Chinese Medicine Therapy for Targeting Osteoblastogenesis,” in Osteogenesis and Bone Regeneration (London: IntechOpen).
Lungu, A. E., Lazar, M. A., Tonea, A., Rotaru, H., Roman, R. C., and Badea, M. E. (2018). Observational Study of the Bisphosphonate-Related Osteonecrosis of Jaws. Med. Pharm. Rep. 91 (2), 209–215. doi:10.15386/cjmed-838
Ma, K.-C., Zhu, T.-Y., and Wang, F.-X. (1996). Stimulative Effects of Gusuibu (Drynaria Baronii) Injection on Chick Embryo Bone Primordium Calcification In Vitro. Am. J. Chin. Med. 24 (01), 77–82. doi:10.1142/s0192415x96000104
Matuk, C. (2006). Seeing the Body: The Divergence of Ancient Chinese and Western Medical Illustration. J. Biocommunication 32 (1), 1–8.
Miller, P. D., Hattersley, G., Riis, B. J., Williams, G. C., Lau, E., Russo, L. A., et al. (2016). Effect of Abaloparatide vs Placebo on New Vertebral Fractures in Postmenopausal Women with Osteoporosis. Jama 316 (7), 722–733. doi:10.1001/jama.2016.11136
Miura, H., Nishida, H., and linuma, M. (1996). Effect of Crude Fractions ofPsoralea corylifoliaSeed Extract on Bone Calcification. Planta Med. 62 (02), 150–153. doi:10.1055/s-2006-957839
Mok, S.-K., Chen, W.-F., Lai, W.-P., Leung, P.-C., Wang, X.-L., Yao, X.-S., et al. (2010). Icariin Protects against Bone Loss Induced by Oestrogen Deficiency and Activates Oestrogen Receptor-dependent Osteoblastic Functions in UMR 106 Cells. Br. J. Pharmacol. 159 (4), 939–949. doi:10.1111/j.1476-5381.2009.00593.x
Mukwaya, E., Xu, F., Wong, M.-S., and Zhang, Y. (2014). Chinese Herbal Medicine for Bone Health. Pharm. Biol. 52 (9), 1223–1228. doi:10.3109/13880209.2014.884606
Nian, H., Qin, L.-P., Zhang, Q.-Y., Zheng, H.-C., Yu, Y., and Huang, B.-K. (2006). Antiosteoporotic Activity of Er-Xian Decoction, a Traditional Chinese Herbal Formula, in Ovariectomized Rats. J. Ethnopharmacol. 108 (1), 96–102. doi:10.1016/j.jep.2006.04.020
Organization W. H. (2004). International Statistical Classification of Diseases and Related Health Problems: Alphabetical Index. Geneva: World Health Organization. 3.
Qin, L., Zhang, G., Hung, W. Y., Shi, Y., Leung, K., Yeung, H. Y., et al. (2005). Phytoestrogen-rich Herb Formula "XLGB" Prevents OVX-Induced Deterioration of Musculoskeletal Tissues at the Hip in Old Rats. J. Bone Min. Metab. 23 Suppl (1), 55–61. doi:10.1007/BF03026324
Ramchandani, D., Ganeshpurkar, A., Bansal, D., Karchuli, M. S., and Dubey, N. (2014). Protective Effect of Curculigo Orchioides Extract on Cyclophosphamide-Induced Neurotoxicity in Murine Model. Toxicol. Int. 21 (3), 232–235. doi:10.4103/0971-6580.155323
Reid, I. R. (2008). “Anti-resorptive Therapies for Osteoporosis,” in Seminars in Cell & Developmental Biology (Elsevier). doi:10.1016/j.semcdb.2008.08.002
Russow, G., Rau, D., Heyland, M., Wulsten, D., Kösters, C., Schmölz, W., et al. (2019). Anabolic Therapies in Osteoporosis and Bone Regeneration. Int. J. Mol. Sci. 20 (1), 83. doi:10.3390/ijms20010083
Schnatz, P. F., Marakovits, K. A., DuBois, M., and O'Sullivan, D. M. (2011). Osteoporosis Screening and Treatment Guidelines. Menopause 18 (10), 1072–1078. doi:10.1097/gme.0b013e318215101a
Seo, B. I., Ku, S. K., Cha, E. M., Park, J. H., Kim, J. D., Choi, H. Y., et al. (2005). Effect of Mornidae Radix Extracts on Experimental Osteoporosis in Sciatic Neurectomized Mice. Phytother. Res. 19 (3), 231–238. doi:10.1002/ptr.1683
Shahinian, V. B., Kuo, Y.-F., Freeman, J. L., and Goodwin, J. S. (2005). Risk of Fracture after Androgen Deprivation for Prostate Cancer. N. Engl. J. Med. 352 (2), 154–164. doi:10.1056/nejmoa041943
Sheng, H., Rui, X.-f., Sheng, C.-J., Li, W.-J., Cheng, X.-Y., Jhummon, N. P., et al. (2013). A Novel Semisynthetic Molecule Icaritin Stimulates Osteogenic Differentiation and Inhibits Adipogenesis of Mesenchymal Stem Cells. Int. J. Med. Sci. 10 (6), 782–789. doi:10.7150/ijms.6084
Sheng, X., Zhang, H., and Weng, Q. (2012). China's Bear Farms Prompt Public Outcry. Nature 484 (7395), 455. doi:10.1038/484455c
Shi, Z.-Y., Zhang, X. G., Li, C. W., Liu, K., Liang, B. C., Shi, X. L., et al. (2017). Effect of Traditional Chinese Medicine Product, QiangGuYin, on Bone Mineral Density and Bone Turnover in Chinese Postmenopausal Osteoporosis. Evidence-Based Complementary Altern. Med. 2017, 6062707. doi:10.1155/2017/6062707
Singh, A., Veeriah, V., Xi, P., Labella, R., Chen, J., Romeo, S. G., et al. (2019). Angiocrine Signals Regulate Quiescence and Therapy Resistance in Bone Metastasis. JCI Insight 4 (13). doi:10.1172/jci.insight.125679
Society, N. A. M. (2021). Management of Osteoporosis in Postmenopausal Women: The 2021 Position Statement of the North American Menopause Society. New York, NY): Menopause, 973–997. 28.9
Sözen, T., Özışık, L., and Başaran, N. Ç. (2017). An Overview and Management of Osteoporosis. Eur. J. Rheumatology 4 (1), 46. doi:10.5152/eurjrheum.2016.048
Spivakovsky, S. (2017). Treatment for Bisphosphonate-Related Osteonecrosis of the Jaw. Evid. Based Dent. 18 (2), 56. doi:10.1038/sj.ebd.6401243
Srivastava, S., Bankar, R., and Roy, P. (2013). Assessment of the Role of Flavonoids for Inducing Osteoblast Differentiation in Isolated Mouse Bone Marrow Derived Mesenchymal Stem Cells. Phytomedicine 20 (8-9), 683–690. doi:10.1016/j.phymed.2013.03.001
Suvarna, V., Sarkar, M., Chaubey, P., Khan, T., Sherje, A., Patel, K., et al. (2018). Bone Health and Natural Products- an Insight. Front. Pharmacol. 9, 981. doi:10.3389/fphar.2018.00981
Sze, S. C. W., Tong, Y., Zhang, Y. B., Zhang, Z. J., Lau, A. S. L., Wong, H. K., et al. (2009). A Novel Mechanism: Erxian Decoction, a Chinese Medicine Formula, for Relieving Menopausal Syndrome. J. Ethnopharmacol. 123 (1), 27–33. doi:10.1016/j.jep.2009.02.034
Tan, E. M., Li, L., Indran, I. R., Chew, N., and Yong, E.-L. (2017). TRAF6 Mediates Suppression of Osteoclastogenesis and Prevention of Ovariectomy-Induced Bone Loss by a Novel Prenylflavonoid. J. Bone Min. Res. 32 (4), 846–860. doi:10.1002/jbmr.3031
Tang, C. H., Yang, R. S., Chien, M. Y., Chen, C. C., and Fu, W. M. (2008). Enhancement of Bone Morphogenetic Protein-2 Expression and Bone Formation by Coumarin Derivatives via P38 and ERK-dependent Pathway in Osteoblasts. Eur. J. Pharmacol. 579 (1-3), 40–49. doi:10.1016/j.ejphar.2007.10.013
Tang, H., Hosein, A., and Mattioli-Belmonte, M. (2021). Traditional Chinese Medicine and Orthopedic Biomaterials: Host of Opportunities from Herbal Extracts. Mater. Sci. Eng. C 120, 111760. doi:10.1016/j.msec.2020.111760
Tang, Y. Q., Li, C., Sun, X. J., Liu, Y., Wang, X. T., Guo, Y. B., et al. (2018). Fructus Ligustri Lucidi Modulates Estrogen Receptor Expression with No Uterotrophic Effect in Ovariectomized Rats. BMC Complement. Altern. Med. 18 (1), 118–8. doi:10.1186/s12906-018-2171-3
Vickers, N. J. (2017). Animal Communication: When I'm Calling You, Will You Answer Too? Curr. Biol. 27 (14), R713–R715. doi:10.1016/j.cub.2017.05.064
Wang, D., Li, F., and Jiang, Z. (2001). Osteoblastic Proliferation Stimulating Activity ofPsoralea corylifoliaExtracts and Two of its Flavonoids. Planta Med. 67 (08), 748–749. doi:10.1055/s-2001-18343
Wang, L., Ma, R., Guo, Y., Sun, J., Liu, H., Zhu, R., et al. (2017). Antioxidant Effect of Fructus Ligustri Lucidi Aqueous Extract in Ovariectomized Rats Is Mediated through Nox4-ROS-NF-Κb Pathway. Front. Pharmacol. 8, 266. doi:10.3389/fphar.2017.00266
Wang, T., Liu, Q., Tjhioe, W., Zhao, J., Lu, A., Zhang, G., et al. (2017). Therapeutic Potential and Outlook of Alternative Medicine for Osteoporosis. Curr. Drug Targets 18 (9), 1051–1068. doi:10.2174/1389450118666170321105425
Wang, X., Zhang, M., Zhang, D., Wang, S., and Yan, C. (2017). An O-Acetyl-Glucomannan from the Rhizomes of Curculigo Orchioides: Structural Characterization and Anti-osteoporosis Activity In Vitro. Carbohydr. Polym. 174, 48–56. doi:10.1016/j.carbpol.2017.06.051
Wang, Z.-Q., Li, J. L., Sun, Y. L., Yao, M., Gao, J., Yang, Z., et al. (2013). Chinese Herbal Medicine for Osteoporosis: a Systematic Review of Randomized Controlled Trails. Evidence-Based Complementary Altern. Med. 2013, 356260. doi:10.1155/2013/356260
Wazzani, R., Pallu, S., Bourzac, C., Ahmaïdi, S., Portier, H., and Jaffré, C. (2021). Physical Activity and Bone Vascularization: a Way to Explore in Bone Repair Context? Life 11 (8), 783. doi:10.3390/life11080783
Wei, M., Yang, Z., Li, P., Zhang, Y., and Sse, W. C. (2007). Anti-osteoporosis Activity of Naringin in the Retinoic Acid-Induced Osteoporosis Model. Am. J. Chin. Med. 35 (04), 663–667. doi:10.1142/s0192415x07005156
Wong, M.-S., and Zhang, Y. (2013). “Flavonoids of Herba Epimedii and Bone Metabolism in Experimental Ovarian Deficiency,” in Nutrition and Diet in Menopause (Springer), 427–439. doi:10.1007/978-1-62703-373-2_32
Wong, R. W., Rabie, B., Bendeus, M., and Hägg, U. (2007). The Effects of Rhizoma Curculiginis and Rhizoma Drynariae Extracts on Bones. Chin. Med. 2 (1), 13–17. doi:10.1186/1749-8546-2-13
Wong, R. W. K., and Rabie, A. B. M. (2006). Traditional Chinese Medicines and Bone Formation-A Review. J. Oral Maxillofac. Surg. 64 (5), 828–837. doi:10.1016/j.joms.2006.01.017
Xiao, H.-H., Gao, Q.-G., Zhang, Y., Wong, K.-C., Dai, Y., Yao, X.-S., et al. (2014). Vanillic Acid Exerts Oestrogen-like Activities in Osteoblast-like UMR 106 Cells through MAP Kinase (MEK/ERK)-mediated ER Signaling Pathway. J. steroid Biochem. Mol. Biol. 144, 382–391. doi:10.1016/j.jsbmb.2014.08.002
Xie, X., Pei, F., Wang, H., Tan, Z., Yang, Z., and Kang, P. (2015). Icariin: A Promising Osteoinductive Compound for Repairing Bone Defect and Osteonecrosis. J. Biomater. Appl. 30 (3), 290–299. doi:10.1177/0885328215581551
Xu, D., Xu, L., Zhou, C., Lee, W. Y. W., Wu, T., Cui, L., et al. (2014). Salvianolic Acid B Promotes Osteogenesis of Human Mesenchymal Stem Cells through Activating ERK Signaling Pathway. Int. J. Biochem. Cell Biol. 51, 1–9. doi:10.1016/j.biocel.2014.03.005
Xu, J.-h., Yao, M., Ye, J., Wang, G.-d., Wang, J., Cui, X.-j., et al. (2016). Bone Mass Improved Effect of Icariin for Postmenopausal Osteoporosis in Ovariectomy-Induced Rats: a Meta-Analysis and Systematic Review. Menopause 23 (10), 1152–1157. doi:10.1097/gme.0000000000000673
Xu, X., Lei, X., and Cao, Y. (1991). The English-Chinese Encyclopedia of Practical Traditional Chinese Medicine: Orthopedics and Traumatology. Beijing: Higher Education Press, 469–470.
Yang, F., Yan, G., Li, Y., Han, Z., Zhang, L., Chen, S., et al. (2016). Astragalus Polysaccharide Attenuated Iron Overload-Induced Dysfunction of Mesenchymal Stem Cells via Suppressing Mitochondrial ROS. Cell Physiol. Biochem. 39 (4), 1369–1379. doi:10.1159/000447841
Yang, Z., Yuan, Z. Z., and Ma, X. L. (2021). Network Pharmacology-Based Strategy and Molecular Docking to Explore the Potential Mechanism of Jintiange Capsule for Treating Osteoporosis. Evid. Based Complement. Altern. Med. 2021, 5338182. doi:10.1155/2021/5338182
Ye, H., Liu, Q., and Wei, J. (2014). Construction of Drug Network Based on Side Effects and its Application for Drug Repositioning. PloS one 9 (2), e87864. doi:10.1371/journal.pone.0087864
Ye, H., Wei, J., Tang, K., Feuers, R., and Hong, H. (2016). Drug Repositioning through Network Pharmacology. Ctmc 16 (30), 3646–3656. doi:10.2174/1568026616666160530181328
Yoon, H. Y., Yun, S. I., Kim, B. Y., Jin, Q., Woo, E. R., Jeong, S. Y., et al. (2011). Poncirin Promotes Osteoblast Differentiation but Inhibits Adipocyte Differentiation in Mesenchymal Stem Cells. Eur. J. Pharmacol. 664 (1-3), 54–59. doi:10.1016/j.ejphar.2011.04.047
Yu, S., Chen, K., Li, S., and Zhang, K. (1999). In Vitro and In Vivo Studies of the Effect of a Chinese Herb Medicine on Osteoclastic Bone Resorption. Chin. J. Dent. Res. 2 (1), 7–11.
Yuan, H., Xiao, L., Min, W., Yuan, W., Lu, S., and Huang, G. (2018). Bu-Shen-Tong-Luo Decoction Prevents Bone Loss via Inhibition of Bone Resorption and Enhancement of Angiogenesis in Ovariectomy-Induced Osteoporosis of Rats. J. Ethnopharmacol. 220, 228–238. doi:10.1016/j.jep.2018.01.007
Zhang, G., Qin, L., Hung, W. Y., Shi, Y. Y., Leung, P. C., Yeung, H. Y., et al. (2006). Flavonoids Derived from Herbal Epimedium Brevicornum Maxim Prevent OVX-Induced Osteoporosis in Rats Independent of its Enhancement in Intestinal Calcium Absorption. Bone 38 (6), 818–825. doi:10.1016/j.bone.2005.11.019
Zhang, G., Qin, L., Sheng, H., Wang, X.-L., Wang, Y.-X., Yeung, D. K.-W., et al. (2009). A Novel Semisynthesized Small Molecule Icaritin Reduces Incidence of Steroid-Associated Osteonecrosis with Inhibition of Both Thrombosis and Lipid-Deposition in a Dose-dependent Manner. Bone 44 (2), 345–356. doi:10.1016/j.bone.2008.10.035
Zhang, G., Qin, L., and Shi, Y. (2007). Epimedium-Derived Phytoestrogen Flavonoids Exert Beneficial Effect on Preventing Bone Loss in Late Postmenopausal Women: A 24-Month Randomized, Double-Blind and Placebo-Controlled Trial. J. Bone Min. Res. 22 (7), 1072–1079. doi:10.1359/jbmr.070405
Zhang, H., Xing, W.-W., Li, Y.-S., Zhu, Z., Wu, J.-Z., Zhang, Q.-Y., et al. (2008). Effects of a Traditional Chinese Herbal Preparation on Osteoblasts and Osteoclasts. Maturitas 61 (4), 334–339. doi:10.1016/j.maturitas.2008.09.023
Zhang, R., Liu, Z. G., Li, C., Hu, S. J., Liu, L., Wang, J. P., et al. (2009). Du-Zhong (Eucommia Ulmoides Oliv.) Cortex Extract Prevent OVX-Induced Osteoporosis in Rats. Bone 45 (3), 553–559. doi:10.1016/j.bone.2008.08.127
Zhang, X., Robles, H., Magee, K. L., Lorenz, M. R., Wang, Z., Harris, C. A., et al. (2021). A Bone-specific Adipogenesis Pathway in Fat-free Mice Defines Key Origins and Adaptations of Bone Marrow Adipocytes with Age and Disease. Elife 10, e66275. doi:10.7554/eLife.66275
Zhang, X., Shen, T., Zhou, X., Tang, X., Gao, R., Xu, L., et al. (2020). Network Pharmacology Based Virtual Screening of Active Constituents of Prunella Vulgaris L. And the Molecular Mechanism against Breast Cancer. Sci. Rep. 10 (1), 15730–15742. doi:10.1038/s41598-020-72797-8
Zhang, Y., Dong, X.-L., Leung, P.-C., Che, C.-T., and Wong, M.-S. (2008). Fructus Ligustri Lucidi Extract Improves Calcium Balance and Modulates the Calciotropic Hormone Level and Vitamin D-dependent Gene Expression in Aged Ovariectomized Rats. Menopause 15 (3), 558–565. doi:10.1097/gme.0b013e31814fad27
Zhang, Y., Leung, P.-C., Che, C.-T., Chow, H.-K., Wu, C.-F., and Wong, M.-S. (2008). Improvement of Bone Properties and Enhancement of Mineralization by Ethanol Extract of Fructus Ligustri Lucidi. Br. J. Nutr. 99 (3), 494–502. doi:10.1017/s0007114507801589
Zhang, Z.-G., Bai, D., Liu, M.-J., Li, Y., Pan, J.-H., Liu, H., et al. (2013). Therapeutic Effect of Aqueous Extract from Ecliptae Herba on Bone Metabolism of Ovariectomized Rats. Menopause 20 (2), 232–240. doi:10.1097/gme.0b013e318265e7dd
Zhu, F. B., Wang, J. Y., Zhang, Y. L., Quan, R. F., Yue, Z. S., Zeng, L. R., et al. (2015). Curculigoside Regulates Proliferation, Differentiation, and Pro-inflammatory Cytokines Levels in Dexamethasone-Induced Rat Calvarial Osteoblasts. Int. J. Clin. Exp. Med. 8 (8), 12337–12346.
Zhu, H. M., Qin, L., Garnero, P., Genant, H. K., Zhang, G., Dai, K., et al. (2012). The First Multicenter and Randomized Clinical Trial of Herbal Fufang for Treatment of Postmenopausal Osteoporosis. Osteoporos. Int. 23 (4), 1317–1327. doi:10.1007/s00198-011-1577-2
Keywords: osteoporosis, bone regeneration, traditional Chinese medicines, decoction, adrenocorticotropic hormone (ACTH)
Citation: Peng Z, Xu R and You Q (2022) Role of Traditional Chinese Medicine in Bone Regeneration and Osteoporosis. Front. Bioeng. Biotechnol. 10:911326. doi: 10.3389/fbioe.2022.911326
Received: 02 April 2022; Accepted: 12 May 2022;
Published: 31 May 2022.
Edited by:
Antonella Motta, University of Trento, ItalyReviewed by:
Anjali P. Kusumbe, University of Oxford, United KingdomFrancesco Grassi, Rizzoli Orthopedic Institute (IRCCS), Italy
Copyright © 2022 Peng, Xu and You. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ronghua Xu, WHVyb25naHVhZGUxMjNAMTYzLmNvbQ==
 Zhicai Peng
Zhicai Peng Ronghua Xu
Ronghua Xu