- 1Subcenter for Stem Cell Clinical Translation, First Affiliated Hospital of Gannan Medical University, Ganzhou, Jiangxi, China
- 2School of Rehabilitation Medicine Gannan Medical University, Ganzhou, Jiangxi, China
- 3Ganzhou Key Laboratory of Stem Cell and Regenerative Medicine, Ganzhou, Jiangxi, China
- 4College of Nursing, Gannan Medical University, Ganzhou, Jiangxi, China
- 5Rehabilitation Assessment and Treatment Center, The Third Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China
- 6Key Laboratory of Prevention and Treatment of Cardiovascular and Cerebrovascular Diseases, Ministry of Education, Gannan Medical University, Ganzhou, Jiangxi, China
- 7Jiangxi Provincal Key Laboratory of Tissue Engineering, Gannan Medical University, Ganzhou, Jiangxi, China
Background: Liver damage due to long-term viral infection, alcohol consumption, autoimmune decline, and other factors could lead to the gradual development of liver fibrosis. Unfortunately, until now, there has been no effective treatment for liver fibrosis. Mesenchymal stem cells, as a promising new therapy for liver fibrosis, can slow the progression of fibrosis by migrating to the site of liver injury and by altering the microenvironment of the fibrotic area.
Aim: By including all relevant studies to date to comprehensively assess the efficacy of mesenchymal stem cells for the treatment of hepatic fibrosis and to explore considerations for clinical translation and therapeutic mechanisms.
Methods: Data sources included PubMed, Web of Science, Embase, and Cochrane Library, and were constructed until October 2023. Data for each study outcome indicator were extracted for comprehensive analysis.
Results: The overall meta-analysis showed that mesenchymal stem cells significantly improved liver function. Moreover, it inhibited the expression level of transforming growth factor-β [SMD = 4.21, 95% CI (3.02,5.40)], which in turn silenced hepatic stellate cells and significantly reduced the area of liver fibrosis [SMD = 3.61, 95% CI (1.41,5.81)].
Conclusion: Several outcome indicators suggest that mesenchymal stem cells therapy is relatively reliable in the treatment of liver fibrosis. The therapeutic effect is cell dose-dependent over a range of doses, but not more effective at higher doses. Bone-marrow derived mesenchymal stem cells were more effective in treating liver fibrosis than mesenchymal stem cells from other sources.
Systematic Review Registration: Identifier CRD42022354768.
1 Introduction
Liver fibrosis due to viral infection or alcohol intake is a major threat to human health. Persistent hepatic fibrosis is a key factor in many diseases including cirrhosis and hepatocellular carcinoma (Roehlen et al., 2020). Hepatocyte responses to inflammation allow fibrosis to develop through processes such as the formation of pro- and inflammation-suppressive cells and the recruitment of macrophages and monocytes. These inflammatory cells and chemokines accelerate the onset and progression of fibrosis by activating hepatic stellate cells which in turn accelerate fibrosis. However, there is no specific or effective treatment for fibrosis treatment (Hu et al., 2019). An increasing number of studies have shown that mesenchymal stem cells (MSCs) have been considered an attractive application in studies related to the treatment of hepatic fibrosis, with prominent characterizations of self-renewal, multidirectional differentiation, and immunomodulation. There have been demonstrated to have an ameliorative effect on the progression of hepatic fibrosis in animal models (Nomura et al., 2022; Wang et al., 2022) and clinical trials (Liu et al., 2022a).
MSCs, as one type of adult stem cells, can not only differentiate into liver cells but regulate the liver microenvironment (Eom et al., 2015). However, in clinical trials, the use of MSCs for the treatment of hepatic fibrosis has been controversial, as evidenced by the limited homing ability of MSCs after transplantation and their ability to differentiate into myofibroblasts rather than hepatocytes (di Bonzo et al., 2008). Several scholars have conducted meta-analyses of MSC therapy for liver disease, but their study subjects were mainly clinical patients. These clinical randomized controlled trial (RCT) meta-analyses targeted the recovery of overall liver function but did not involve histopathologic findings. In addition, the results of the meta-analysis are difficult to objectively reflect the effectiveness of MSCs in treating hepatic fibrosis because the physical conditions of patients were not consistent at baseline and were easily influenced by external factors (Zhou et al., 2020; Lu et al., 2023). Therefore, we conducted the first meta-analysis of MSCs therapy for hepatic fibrosis using an animal model to explore the effectiveness of MSCs in treating liver fibrosis potential precautions, and therapeutic mechanisms. In addition, this meta-analysis reports for the first time 11 key metrics of hepatic fibrosis including hyaluronic acid, laminin, hydroxyproline, collagen type III, the collagen fiber area, and transforming growth factor-β (TGF-β).
2 Methods
Systematic reviews and meta-analyses were interpreted and evaluated as per the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) and PRISMA 2020. The registration number for this study is CRD42022354768.
2.1 Search strategies
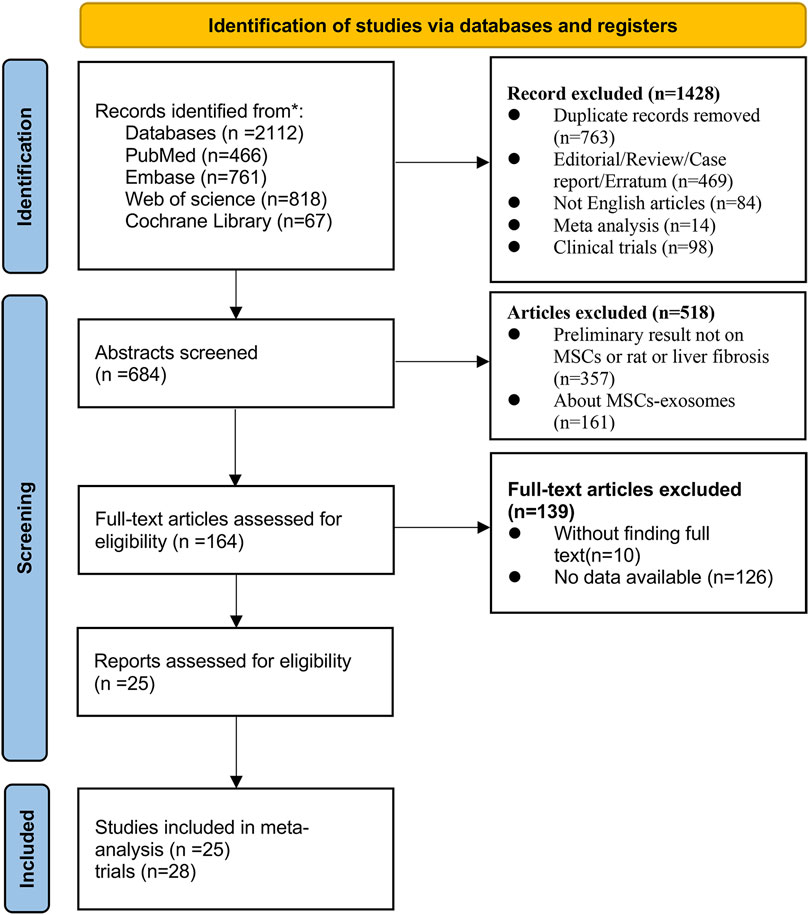
The first author of this article (Xuesong Wang) searched the PubMed, Embase, Web of Science, and Cochrane Library databases for the following terms (“mesenchymal stem cells” OR “ mesenchymal stromal cells” OR “mesenchymal progenitor cells” OR “wharton jelly cells”) AND (“liver fibrosis” OR “liver cirrhosis” OR “hepatic cirrhosis”) (the detailed search terms are presented in Supplementary Table S1) The database was searched from the time it was created until 2 October 2023. A total of 3 screenings were conducted, initially by screening the types of articles to exclude reviews, conferences, non-English literature, etc., followed by selecting animal model experiments that matched the topic through the titles and abstracts, and finally by reading the full text to identify a total of 25 relevant studies (28 trials) for meta-analysis (Figure 1).
2.2 Study selection
2.2.1 Inclusion criteria
A. 1) The language used in this study was English; 2) The study involved a RCT; 3) The article involved the use of rats as an animal model of liver fibrosis.
B. In the experimental group, MSCs were used for the intervention in the rat liver fibrosis model, while in the control group, healthy rats were simply induced into liver fibrosis.
C. The study focused on the effects of MSCs on liver fibrosis and included at least one of the following metrics. 1) Albumin (ALB); 2) Alanine aminotransferase (ALT); 3) Aspartate aminotransferase (AST); 4) Alkaline phosphatase (ALP); 5) Total bilirubin (TBIL); 6) Hyaluronic acid (HA); 7) Laminin (LN); 8) Hydroxyproline (HYP); 9) Collagen fibers; 10) Type III collagen; 11) TGF-β.
2.2.2 Exclusion criteria
A. The subject of the study was human, or an animal model other than rats.
B. The study is not experimental, but rather a review, conference, clinical case, or meta-analysis.
C. The complete article could not be queried or the data were incomplete, where incomplete data were included simply using statistical graphs or qualitative pictures and no quantitative data could be provided.
D. In the experimental group, MSCs were pre-treated with drugs or gene editing. This treatment may affect the results of the experiment.
E. The experiment explicitly involved extracellular vesicle therapy, not MSCs therapy.
F. The animals were not sacrificed immediately after treatment, which could have affected the outcome indicators.
2.3 Required data extraction
First, two researchers discussed the inclusion criteria for the data and extracted the data (Yue Wang and Wenming Lu). The extracted data were then handed over to two other researchers (Jiayang Qu and Yang Zhang), who organized and checked the data. If an article was found to have missing data or incomplete information, the corresponding author (Junsong Ye) then consulted the corresponding author of the raw article by email and ultimately supplemented the data with complete information.
2.4 Quality assessment
The quality of the studies was independently assessed by two researchers (Yue Wang and Yang Zhang) using an assessment tool downloaded from the Cochrane Library. The quality assessment consisted of the following steps: 1. Random sequence generation. 2. Allocation concealment. 3. Blinding of participants and personnel. 4. Blinding of outcome assessment. 5. Incomplete outcome data. 6. Selective reporting. 7. Other bias. After the assessment was performed by another researcher (Wenming Lu) for review, and when there was a disagreement, the three researchers negotiated to eliminate the disagreement and solve the problem.
2.5 Statistical analysis
We analyzed the mean and standard deviation of the data using software (Review Manager 5.4 and StatsSE 14) to determine the weighted mean difference (WMD) and a standardized mean difference (SMD). SMD is selected as a valid indicator when the difference in means is large. The fixed-effects model was applied on the assumption that all studies were in the same population, but animal model studies cannot be based on such a premise; therefore, a random-effects model was used. When heterogeneity was high, we also performed subgroup analyses and sensitivity analyses, which were stratified by injection route, stem cell type, stem cell injection dose, and animal model.
Funnel plots were used to assess publication bias when a single metric was greater than or equal to 10 studies. The funnel plot asymmetry was tested for accuracy by Egger’s test. Finally, the statistical significance was set at p < 0.05.
3 Results
3.1 Study selection
A total of 2,112 articles were identified that matched the topic of mesenchymal stem cell therapy for liver fibrosis. After removing duplicates, conference proceedings, reviews, and other studies, 25 studies (28 trials) met the inclusion criteria after the full texts of the articles were reviewed by the researchers. The complete flow chart is presented in (Figure 1).
3.2 Characteristics of the included studies
This study included 25 prospective studies (28 trials) involving 530 rats. A total of 278 rats with liver fibrosis were treated with MSCs. Of these 25 studies, 12 were conducted in China (Zhao et al., 2005; Chang et al., 2009; Qiao et al., 2011; Wang et al., 2012; Wang et al., 2014a; Wang et al., 2014b; Hong et al., 2014; Ma et al., 2016; Hao et al., 2017; Zhang et al., 2017; Zhang et al., 2018; Zhang et al., 2023), 8 in Egypt (Abdel Aziz et al., 2007; Ahmed et al., 2014; Raafat et al., 2015; Elberry et al., 2016; Mohamed et al., 2016; Ewida et al., 2017; Jang et al., 2018; Khalil et al., 2021), and the other 5 were from the Republic of Korea (Kim et al., 2014), Iran (Mortezaee et al., 2017), India (Aithal et al., 2018), Japan (Fathy et al., 2020), and Italy (Pietrosi et al., 2020) (Supplementary Figure S1). MSCs used to treat liver fibrosis are derived from a variety of tissues, including bone marrow, adipose tissue, the human umbilical cord, and the human amniotic membrane. Additionally, the dose and route of injection of MSCs for the treatment of liver fibrosis have been reported to vary from study to study. We extracted the basic information of each study and put all the information in Supplementary Table S2. The basic information included the authors, the year of publication, the number of rats, the type of rats, the source of MSCs, the dosage and route of MSCs injection, and the metrics corresponding to the therapeutic effect.
3.3 Risk assessment of bias
A total of 20 studies out of 28 trials mentioned the principle of random allocation in the text, therefore, we categorized these 20 studies as having a low risk of bias. However, the other seven studies did not mention whether they were randomized or not. No study has shown that trials were conducted by designation, concealment, or blinding of researchers. Blinding of outcome assessments was reported in only six trials and was determined to be at low risk of detection bias. Absent data, selective reporting of data, or other trial biases were not included in the meta-analysis. A total of 28 trials had reliable and acceptable methodological quality (Supplementary Figure S2).
3.4 Primary outcome
3.4.1 ALB
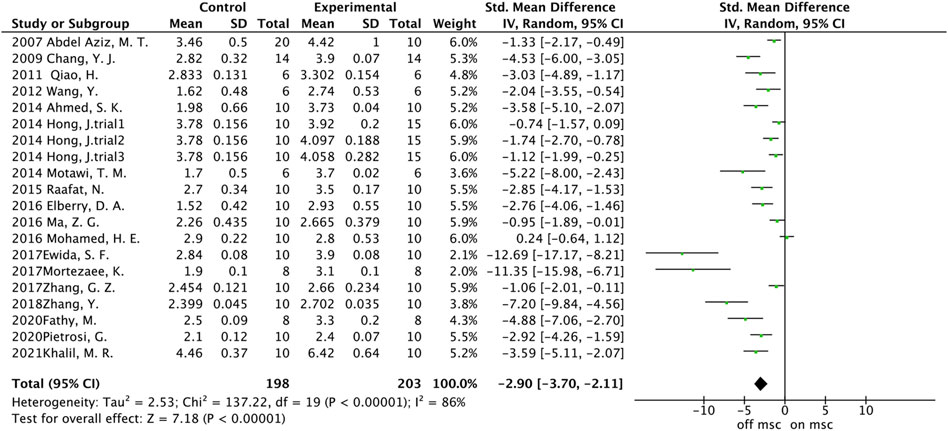
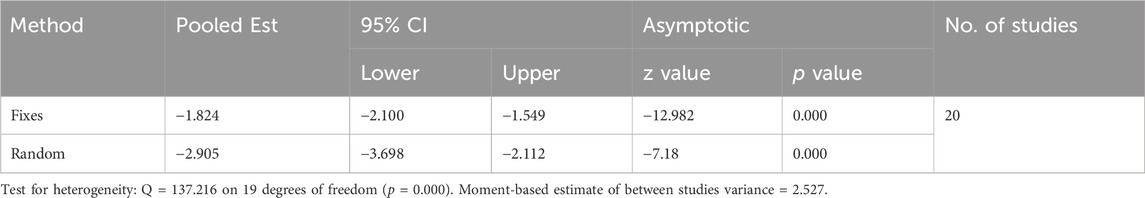
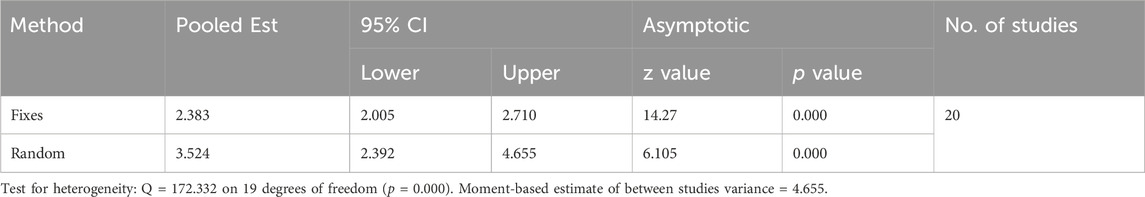
A total of 20 trials from 18 studies reported differences in the serum ALB concentration between the experimental groups and control groups of MSCs for liver fibrosis (Figure 2). Although each trial showed an increase in ALB levels after MSCs treatment for liver fibrosis, there was significant heterogeneity [p < 0.00001, I2 = 86%; SMD = −2.90, 95% CI (−3.70, −2.11), p < 0.00001]. Subgroups were defined based on the route of injection, MSCs source, injection dose, and animal model. However, no source of heterogeneity was found (Figures 3A, B) (Supplementary Figures S3A, B). The effect of publication bias was demonstrated with a funnel plot (Figure 4A). The funnel plot showed the existence of publication bias, which was found to exist by Egger’s test (p = 0.000 < 0.05) (Figure 4B). The stabilization of combined effect sizes was assessed using trim-and-fill methods. A random effects model was applied (Q = 137.216, p = 0.000), which yielded an estimate (Est) of −2.905 and 95% CI (−3.698, −2.112) (Table 1). However, there are no virtual reports on this topic, and the results are robust and reliable (Figure 4C). Moreover, the results of the sensitivity analysis likewise confirmed the stability of the meta-analysis results, despite the presence of publication bias and high heterogeneity (Figure 5A). Therefore, it is plausible that MSCs therapy can reverse the resulting decrease in serum ALB concentration due to liver fibrosis.
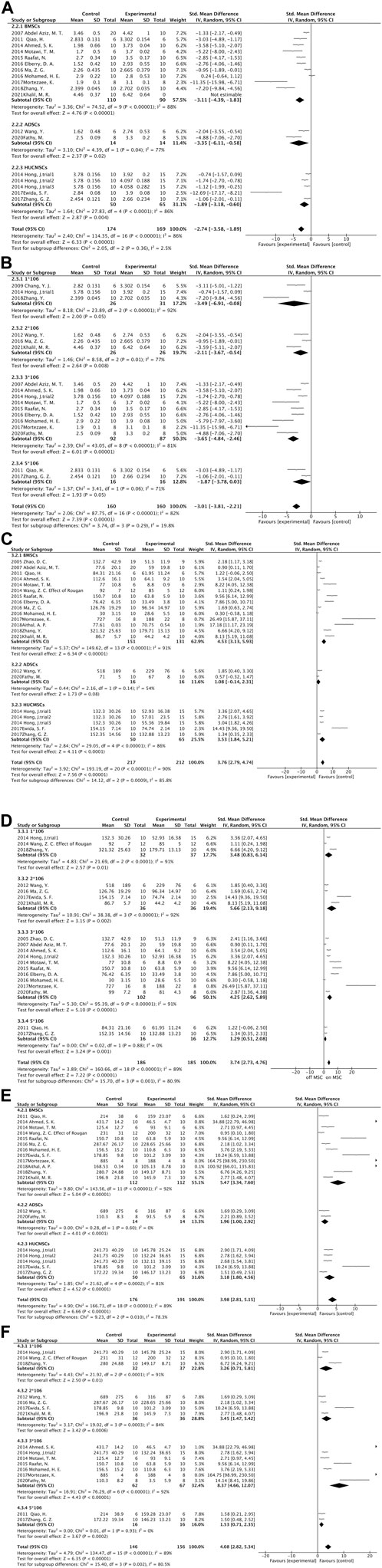
Figure 3. Forest plot: (A) MSCs source subgroup for ALB levels; (B) Injection dose subgroup of ALB levels; (C) MSCs source subgroup for ALT levels; (D) Injection dose subgroup for ALT levels; (E) MSCs source subgroup for AST levels; (F) Injection dose subgroup for AST levels.
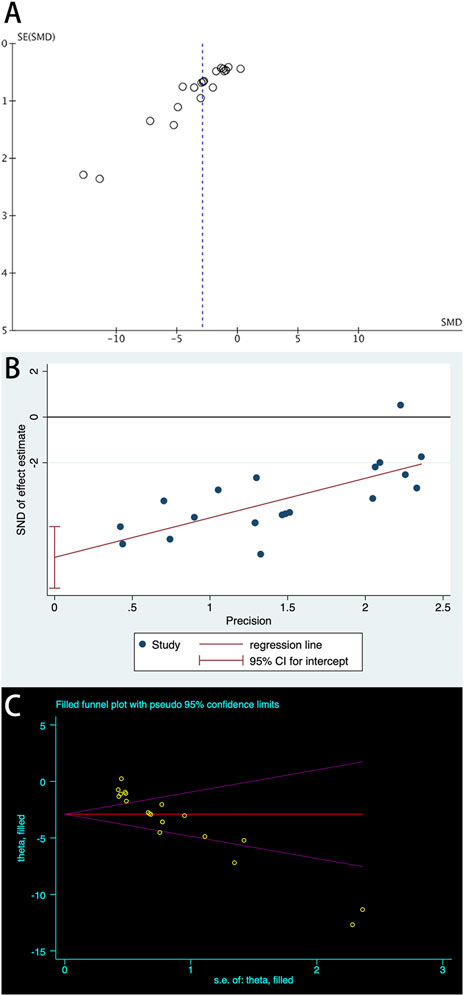
Figure 4. Plots of ALB: (A) Funnel plot with pseudo-95% confidence limits; (B) Egger’s publication bias plot; (C) Filled funnel plot with pseudo-95% confidence limits.
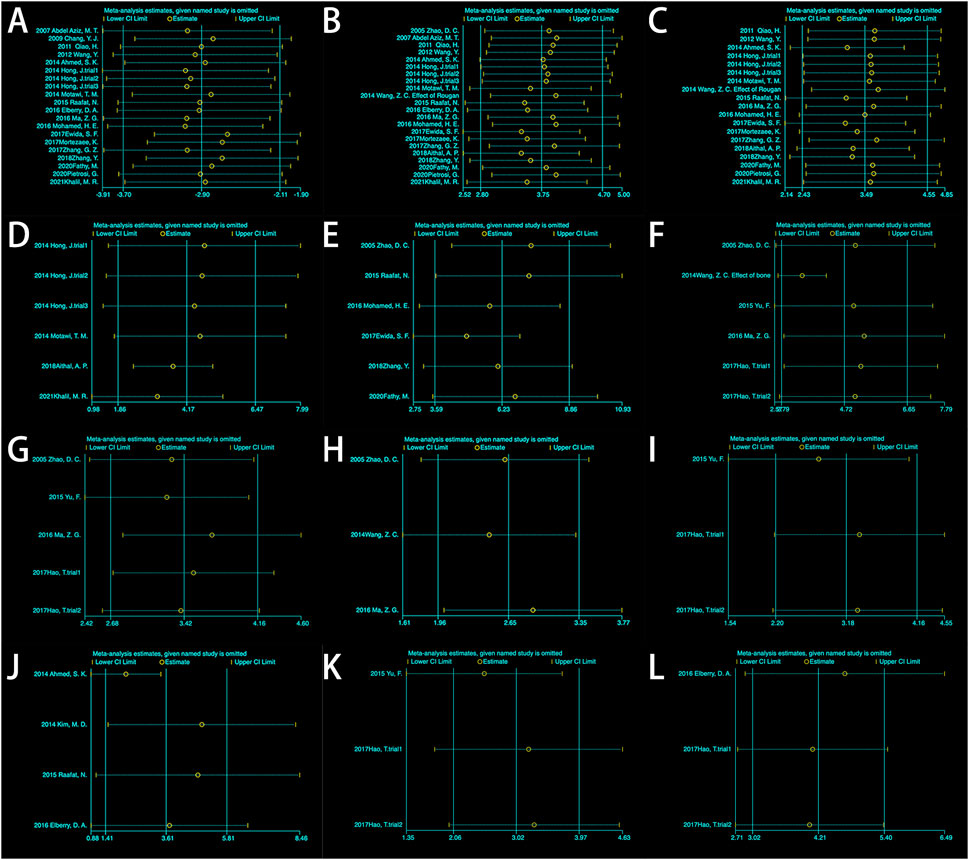
Figure 5. Sensitivity analysis: (A) Albumin (ALB); (B) Alanine aminotransferase (ALT); (C) Aspartate aminotransferase (AST); (D) Alkaline phosphatase (ALP); (E) Total bilirubin (TBIL); (F) Hyaluronic acid (HA); (G) HA (excluding 2014Wang); (H) Laminin (LN); (I) Hydroxyproline (HYP); (J) Area percentage of collagen fibers; (K) Type III collagen. (L) TGF-β.
3.4.2 ALT
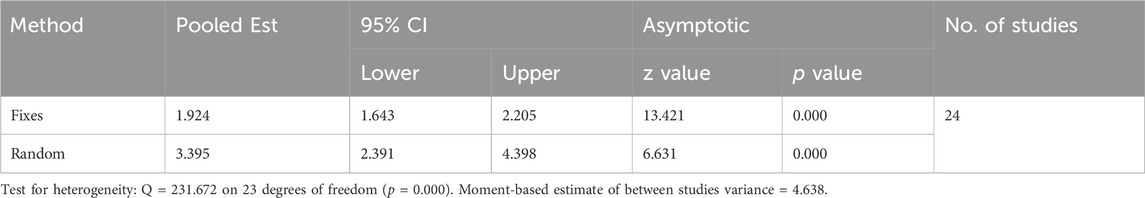
The meta-analysis of the endpoints comprising 22 trials showed a statistically significant difference in the serum ALT between the treatment and control groups [SMD = 3.75, 95% CI (2.80, 4.70), p < 0.00001; heterogeneity test p < 0.00001, I2 = 89%] (Supplementary Figure S4). Subgroups were analyzed by four aspects: cell input route, source of MSCs, administration dose of MSCs, and model of animals. No significant change in heterogeneity was found (Figures 3C, D) (Supplementary Figures S3C, D). Sensitivity analyses were used to verify the stability of the results (Figure 5B), which did not significantly change the combined size effect after excluding the experiment alone. We also used funnel plots to detect publication bias (Supplementary Figure S5A). The funnel plot showed asymmetry and Egger’s test verified publication bias (p = 0.000 < 0.05) (Supplementary Figure S5B). The results were supplemented by the trim-and-fill approach, which uses a random effects model (Q = 198.07, p = 0.000) and yielded an Est of 3.747 and 95% CI (2.797,4.697). Two virtual experiments were performed (Supplementary Figure S5C), and the results were Q = 231.672, p = 0.000 < 0.05, and the combined effect sizes were Est = 3.395 and 95% CI (2.391,4.398) (Table 2). This finding indicates that despite publication bias, this does not affect the trend of the results, in which MSCs injections reduced ALT levels in the blood.
3.4.3 AST
Nineteen experiments reported differences in AST expression levels between the experimental and control groups. The data revealed a statistically significant difference between the two groups [SMD = 3.49, 95% CI (2.43,4.55), p < 0.00001; Heterogeneity testing p < 0.00001, I2 = 88%] (Supplementary Figure S6). Subgroup analyses did not reveal sources of heterogeneity (Figures 3E, F) (Supplementary Figures S3E, F). However, sensitivity analyses showed that heterogeneity did not affect the trend of the results (Figure 5C). Funnel plots were used to evaluate publication bias (Supplementary Figure S7A). The results indicated the presence of publication bias, which was likewise verified by Egger’s test (p = 0.000 < 0.05) (Supplementary Figure S7B). A virtual experiment was merged using the trim-and-fill method (Supplementary Figure S7C). A random effects model was used before virtual experiments were merged [Q = 149.470, p = 0.000, Est = 3.490, 95% CI (2.431,4.548)]. There was no effect on a verdict after combining the virtual experiments [Q = 172.332, p = 0.000, Est = 3.524, 95% CI (2.392,4.655)] (Table 3). These findings illustrate that the level of AST in the MSCs-treated group was lower than that in the control group.
3.4.4 ALP
Six experiments containing 107 rats were involved in the analysis of differences in ALP. All six experiments showed statistically significant differences in the data between the two groups. MSCs treatment significantly reduced the expression level of ALP in rats. The data from the six experiments were then analyzed by meta-analysis using a random effects model [SMD = 4.17, 95% CI (1.86,6.47), p = 0.0004, Heterogeneity: p < 0.00001, I2 = 89%] (Supplementary Figure S8A). Subgroup analyses were performed using the cell injection dose, and heterogeneity was significantly reduced when the cell injection dose was 3*106 [SMD = 2.92, 95% CI (1.92,3.92), p < 0.00001, Heterogeneity: p = 0.87, I2 = 0%] (Supplementary Figure S8B). Sensitivity analyses further tested the effect of heterogeneity on the results and presented that statistical differences between the two groups are relatively reliable (Figure 5D).
3.4.5 TBIL
A total of six studies reported differences in total bilirubin expression levels in the blood of experimental and control rats. The study showed a statistically significant variation between the two groups [SMD = 6.23, 95% CI (3.59,8.86), p < 0.00001, Heterogeneity: p < 0.00001, I2 = 89%] (Supplementary Figure S9A). Subsequent subgroup analyses based on animal model categorization reduced heterogeneity [Wister rats: SMD = 2.65, 95% CI (1.87,3.43), p < 0.00001, Heterogeneity: p = 0.34, I2 = 8%; SD rats: SMD = 5.81,95% CI (3.60,8.02), p < 0.00001, Heterogeneity: p = 0.005, I2 = 42%] (Supplementary Figure S9B). Sensitivity analyses manifested plausible differences in total bilirubin expression levels between the two groups (Figure 5E). It indicates that MSCs effectively reduced the level of total bilirubin in the blood of rats with liver fibrosis.
3.4.6 HA
Five studies containing six experiments evaluated the differences in HA expression between the two groups. A random effects model was used for the meta-analysis. The results showed that intervention of hepatic fibrosis using MSCs significantly reduced the level of HA [p < 0.00001, SMD = 4.72, 95% CI (2.79,6.65)]. There was a large degree of heterogeneity in the endings (p < 0.00001, I2 = 84%) (Supplementary Figure S10A). Selecting the route of injection as a means of subgroup analysis did not reveal a source of heterogeneity (Supplementary Figure S3G). However, when (Wang et al., 2014b) was excluded, it was found that the heterogeneity decreased from 86% to 0% (Supplementary Figure S10B), indicating that the study brought about heterogeneity. Sensitivity analysis using the exclusion-by-exclusion method found no effect on the results (Figure 5F), regardless of whether (Wang et al., 2014b) was excluded or not (Figure 5G), suggesting that the combined results were stable.
3.4.7 LN
Three studies showed the expression levels of LN before and after MSCs treatment. A meta-analysis showed that MSCs significantly decreased the level of LN in liver fibrosis compared with control [SMD = 2.55, 95% CI (1.84,3.25), p < 0.00001, Heterogeneity: p = 0.66, I2 = 0%] (Supplementary Figure S11). The results showed low heterogeneity, while the outcome of the sensitivity analysis indicated that MSCs could reduce the level of LN in liver fibrosis with plausible assertion (Figure 5H).
3.4.8 HYP
A meta-analysis of three trials demonstrated that there was a significant difference between the treatment group and the control group (p < 0.00001) (Supplementary Figure S12). The results from the fixed effect model were SMD = 3.18, 95% CI [2.20,4.16]. The results did not show the presence of heterogeneity (I2 = 0%). Although only three experiments yielded HYP values, the sensitivity analysis showed that the results were statistically stable when the experiments were excluded one by one (Figure 5I). The above results indicate that HYP levels can be reduced by MSC intervention in rats with liver fibrosis, and the results are stable and reliable.
3.4.9 Area percentage of collagen fibers (%)
The area of collagen fibers is one of the most intuitive manifestations of hepatic fibrosis. A total of four studies reported the area of collagen fibers in results between the two groups. Comparative calculations were performed in the area between the treatment group and the control group. The results show that MSCs reduced the area of collagen fibers in the liver [SMD = 3.61, 95% CI (1.41,5.81), p = 0.001] (Supplementary Figure S13). However, when we performed a sensitivity analysis, we found that the results were not stable (Figure 5J). Therefore, caution is needed when extrapolating the effect of MSCs on the area of collagen fibers in hepatic fibrosis.
3.4.10 Type III collagen
There were three experiments in which type III collagen was measured. The analysis was performed using a fixed-effects model, and there was a significant difference between the two groups [SMD = 3.02, 95% CI (2.06,3.97), p < 0.00001] (Supplementary Figure S14). Low heterogeneity was shown between the two groups (p = 0.39, I2 = 0%). To verify the reliability of the results, a sensitivity analysis was performed (Figure 5K), and the results showed that it was trustworthy that MSCs could reduce type III collagen.
3.4.11 TGF-β
Two studies, including three experiments, have reported levels of TGF-ß. Fixed-effects modeling was used for the meta-analysis of TGF-β [SMD = 4.21, 95% CI (3.02,5.40), p < 0.00001] (Supplementary Figure S15). The outcome indicated that there was not a high degree of heterogeneity and that the difference between the two groups was stable as shown by a sensitivity analysis in which the studies were excluded one by one (Figure 5L). TGF-β is a typical effector of liver fibrosis, and MSCs reduce fibrosis by decreasing the level of TGF-β expression.
4 Discussion
Liver fibrosis is a crucial step in the progression of chronic liver disease to cirrhosis and even liver cancer. The pathogenesis of liver fibrosis is complicated, which makes treatment challenging. It develops mainly through the activation of myofibroblasts in the liver, which in turn secrete extracellular matrix proteins. The main source of these myofibroblasts is the resident hepatic stellate cells (Gupta et al., 2022). Hepatic stellate cells are quiescent, lipid-containing, pericyte-like cells. By tracing these resident hepatic stellate cells, 82%–96% of the total myofibroblasts were found to be involved in carbon tetrachloride-induced liver fibrosis (Mederacke et al., 2013). Therefore, it is critical to regulate the activation of hepatic stellate cells. Among the various efforts to alleviate fibrosis, mesenchymal stem cells are considered a promising therapeutic approach. It can treat liver fibrosis in various aspects. 1) Immunomodulatory properties (Xie et al., 2021; Zheng et al., 2022). 2) Differentiate into hepatocytes to fight fibrosis (Bi et al., 2017). 3) Silencing and inhibiting activation of hepatic stellate cells (Zhou et al., 2021). 4) Anti-inflammatory, antioxidant, and anti-apoptotic capabilities (Ye et al., 2019) (Figure 6). TGF-β is a major factor involved in inducing hepatic stellate cell activation (He et al., 2021).
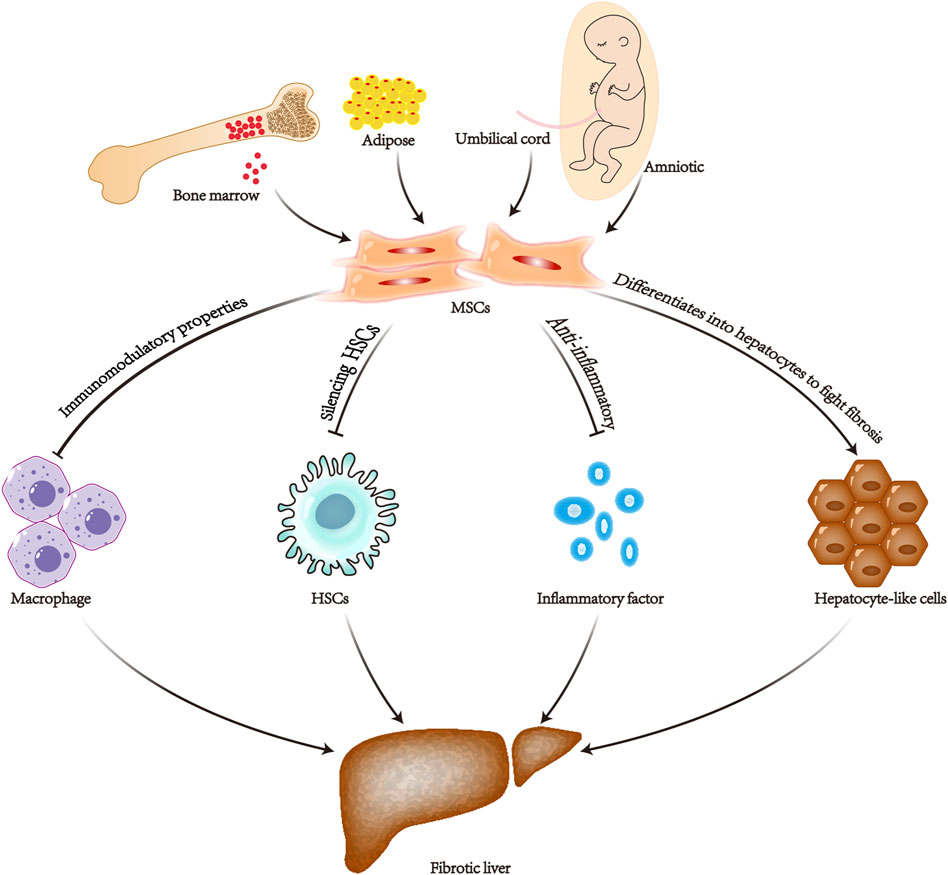
Figure 6. Diagram of the mechanism of mesenchymal stem cell therapy for liver fibrosis. MSCs, mesenchymal stem cells; HSCs, hepatic stellate cells.
The exploration of therapeutic mechanisms has continued in recent years. It has been found that one of the main reasons why MSCs can effectively ameliorate the level of fibrosis is due to it can significantly downregulate the mRNA expression of TGF-β and the TGF-β downstream factor SAMD3 point mRNA in myofibroblasts, as well as increase the mRNA level of SMAD7 (Yin et al., 2020; Liu et al., 2022b; Liu et al., 2022c). It has also been shown that MSCs also significantly inhibit the protein expression of SAMD2 (Kao et al., 2020). High expression of SMAD2 and SMAD3 is a major factor in the activation of the pro-fibrotic factors α-SMA and Collagen I (Peng et al., 2023). SAMD7 is an inhibitor of fibrosis and reduces hyaluronic acid levels (Su et al., 2020; Liu et al., 2022a). Bone marrow mesenchymal transplantation promotes the expression of M2-type macrophages and matrix metalloproteinase 13. Moreover, the inhibition of M1-type macrophages further inhibits the activation of hepatic stellate cells (Luo et al., 2019). Mesenchymal stem cell-based therapies have been shown to have positive effects on liver fibrosis in most animal experiments and clinical trials (Abou Rayia et al., 2023; Terai et al., 2023). However, many reports have suggested the opposite conclusion.
It has been reported that the injection of MSCs through the spleen into mice results in liver fibrosis, and only a few extraneous mesenchymal stem cells migrate to the liver (Popp et al., 2007). There are even studies claiming that bone marrow MSCs derived from adults or children by transplantation into the liver could improve α-SMA and exhibit fibrotic activity. The finding indicates potential harm to the liver parenchyma (Baertschiger et al., 2009). Moreover, some clinical trials have shown no significant improvement in liver function after MSC therapy. For example, several clinical trials have shown no significant changes in the serum ALB concentration, MELD score, or serum aminotransferase level in patients with hepatic fibrosis who underwent autologous bone marrow MSCs transplantation at the 12-month follow-up (Yang et al., 2020a). There is an urgent need to evaluate the therapeutic efficacy of MSCs in treating liver fibrosis comprehensively.
To comprehensively assess the effectiveness of MSCs in treating liver fibrosis, we performed a meta-analysis of 28 experiments using animal models. For the first time, we comprehensively pooled important indicators that can reflect the degree of hepatic fibrosis damage and performed a meta-analysis based on the pooled results. The results showed that MSCs treatment significantly ameliorated the degree of hepatic fibrosis in rats compared with the control group. Treatment resulted in similar to normal levels of ALB, ALT, ALP, AST, and TBIL for routine liver function. Heterogeneity was found to be relatively high during our analysis, and after going through subgroup and sensitivity analyses, no source of heterogeneity was found. Subsequently, we also tested for publication bias in the included studies for the three indicators of ALB, ALT, and AST, and found that publication bias did not affect the stability of the conclusions according to the trim-and-fill approach. It indicates that ALB levels can be elevated and ALT, AST, and TBIL levels can be reduced by MSCs intervention referred to liver fibrosis.
The dose of MSCs administered to patients with end-stage liver disease has usually been based solely on experience (Alfaifi et al., 2018). In 2016, scholar Suk used bone marrow mesenchymal stem cells to compare the therapeutic effects of injecting doses of 5*107 once a month with 5*107 twice a month for alcoholic cirrhosis patients. The results showed that there was no significant difference between the quantitative fibrosis results of the two groups (Suk et al., 2016). It has also been shown that there was a significant improvement in liver fibrosis when MSCs was injected at a dose of 1*107, but not when MSCs were injected at a dose of 2*108 (Amin et al., 2013; Mohamadnejad et al., 2013). Therefore, we were concerned about whether the dose of MSCs injections had an impact on the treatment effect in our meta-analysis. Interestingly in the subgroup analysis of ALB, ALT, and AST according to the MSCs injection dose, it was found that 1*106 to 3*106 as the number of cells increased, the greater the difference between the two groups. However, when the cell dose was increased to 5*106, the therapeutic effect compared to 3*106 was not satisfactory (Figures 3B, D, F). Therefore, we speculate that a limited range of MSCs doses can effectively ameliorate liver fibrosis, beyond which perhaps there is no further improvement in liver fibrosis, and even oversized doses can be harmful. Correspondingly, when the cell injection dose is within this stated range, the therapeutic effect becomes more obvious as the cell dose increases. The range of cell dosage depends on the body weight, variety of diseases, and age distribution. We are concerned about cell dosage in clinical investigations, and we will continue to follow up (Wang et al., 2023). In addition, different sources of MSCs have different therapeutic effects on liver fibrosis; however, there are no established standards for MSCs for treating liver disease, but the most well-studied MSCs in clinical practice originate from the umbilical cord and bone marrow. The umbilical cord can provide a much greater number of MSCs than the bone marrow (Mueller and Glowacki, 2001; Batsali et al., 2013). Moreover, umbilical cord MSCs have increased proliferation and differentiation capacity, lower immunogenicity, and superior allograft capacity (Cho et al., 2008; Hsieh et al., 2010). However, the results of our meta-analysis suggest that bone marrow-derived MSCs may be more effective in treating hepatic fibrosis than umbilical cord-derived or adipose-derived ones (Figures 3A, C, E). Thus, due to the advantages of umbilical cord MSCs, multiple considerations may be needed in the future before they can be used as seed cells for the treatment of liver fibrosis in the clinic.
Hyaluronic acid is synthesized mainly by hepatic stellate cells and is metabolized in the liver, and a decrease in hyaluronic acid levels in combination with TGF-β predicts a decrease in the level of hepatic stellate cell activation (Cheng et al., 2019). The results of the sensitivity analysis of the included studies indicated that MSCs attenuate hepatic fibrosis by reducing hepatic stellate cell activation.
Laminin is a non-collagenous sugar that constitutes the intercellular matrix and is synthesized in the liver mainly by endothelial cells and lipid storage cells, and together with collagen, it constitutes a component of the basement membrane (Kanninen et al., 2016). In addition, hydroxyproline, a non-essential amino acid, is one of the main components of collagen tissue and is a unique amino acid in collagen (Shi et al., 2016; Zhang et al., 2022). In clinical practice, serum laminin and hydroxyproline are mostly used as an indicator of liver disease and mainly reflect the degree of liver fibrosis activity. The results of this meta-analysis showed that the cell therapy significantly reduced the levels of laminin and hydroxyproline. The low heterogeneity of the results indicated the reliability of our analysis.
Type III collagen is generally an important index in liver biopsy and can reflect the status of collagen synthesis in the liver and is important for the diagnosis of liver fibrosis. The collagen concentration and collagen fiber area are both relatively intuitive indicators of the degree of fibrosis. It is difficult to obtain data in clinical trials and clinical meta-analyses. Our results showed that MSCs treatment effectively reduced the level of type III collagen and the area of collagen fibers in the liver. However, when sensitivity analyses were performed on experiments incorporating the fibrotic area, we found that the heterogeneity was high, and the cutoff results were unstable. Due to the insufficient sample size of the experiments, we could not specifically analyze the source of heterogeneity.
There are several limitations of the current meta-analysis that need to be mentioned. First, studies have shown that the route of cell input, the source of cells, the dose of cell input, and the animal model all affect the effectiveness of the treatment. However, due to the insufficient sample size, it is difficult to fix the other variables to further analyze which experimental protocol optimizes the therapeutic efficacy of MSCs. This also makes the heterogeneity of some indicators relatively high. Second, it would be interesting to know how safe MSCs are, especially when the dosage is different, and whether they cause adverse reactions in animals. However, during our analysis, we found that some of the studies had inconsistent numbers of models in the control and experimental groups, and the authors did not explain this inconsistency or provide the mortality rates of the animals (Abdel Aziz et al., 2007; Hong et al., 2014). Finally, we did not explain the route of injection because of the differences between animals and humans. The most common method of administration in clinical practice is peripheral intravenous injection. However, other methods of administration, such as portal vein, hepatic artery, and intrasplenic injection, need to be considered (Yang et al., 2020b). Peripheral intravenous injection has the obvious advantage of convenience; however, in the treatment of miniature pigs suffering from acute liver failure (Cao et al., 2012; Li et al., 2012), portal venous injection was found to restore liver function, whereas a similar effect was not observed for peripheral venous injection. One study compared the effectiveness of portal vein and intrasplenic injections for the treatment of liver failure and showed that portal vein injections were more effective than intrasplenic injections (Amer et al., 2011). However, given the invasive nature of portal venous injection, the choice of which route of injection should be used as a standard in the future needs to be explored in a large number of clinical randomized controlled trials.
In conclusion, our meta-analysis aggregated as comprehensively as possible the indicators concerning liver fibrosis to explore the effectiveness of MSCs in treating liver fibrosis potential precautions, and therapeutic mechanisms. The results suggest that MSCs are effective in treating liver fibrosis. Within a certain cell dose range, the higher the injection volume, the more favorable the improvement of fibrosis, but if the cell dose is blindly increased, the result will be counterproductive. Although umbilical cord MSCs are less immunogenic, bone marrow MSCs are superior in terms of therapeutic efficacy. Therefore, before MSCs can be introduced into the clinic on a large scale for the treatment of liver diseases, clinical trials are needed to determine the relevant therapeutic standards. For example, the gold standard for injection should be fixed in terms of the injection dose, injection route, and source of cells. In recent years, research related to the treatment of liver diseases by MSCs has been developing rapidly, for example, many researchers have combined gene technology and drugs to pretreat MSCs to further improve their therapeutic efficacy. There has also been much research on inducing MSCs to undergo hepatic differentiation through 2D or 3D culture in vitro to harvest hepatocytes for the successful treatment of liver diseases; moreover, exosomes from MSCs have also been proven to have therapeutic effects on liver diseases. However, all of the above studies may require further development before reaching the stage where they can be implemented in clinical settings.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
XW: Writing–original draft, Writing–review and editing. YW: Data curation, Investigation, Writing–review and editing. WL: Data curation, Formal Analysis, Writing–review and editing. JQ: Data curation, Resources, Writing–review and editing. YZ: Formal Analysis, Project administration, Writing–review and editing. JY: Funding acquisition, Supervision, Visualization, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The National Natural Science Foundation of China (Grant No. 32060232), the Jiangxi Provincial Natural Science Foundation (Grant No. 20212BAB206075), the First Affiliated Hospital of Gannan Medical University Doctor Start-up Fund (QD076), the Stem Cell Clinical Research Bi-Filing Project of First Affiliated Hospital of Gannan Medical University (SC-BiFR-001), and the Science and Technology Project of Ganzhou (202101034530) were used.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2024.1424253/full#supplementary-material
SUPPLEMENTARY FIGURE S1 | Publication country of the included studies.
SUPPLEMENTARY FIGURE S2 | Risk of bias graph and summary.
SUPPLEMENTARY FIGURE S3 | Forest plot: (A) Route of injection subgroup of ALB levels; (B) Animal model subgroup of ALB levels; (C) Route of the injection subgroup for ALT levels; (D) Animal model subgroup for ALT levels; (E) Route of injection subgroup for AST levels; (F) Animal model subgroup for AST levels; (G) Injection dose subgroup for HA levels.
SUPPLEMENTARY FIGURE S4 | The forest plot of ALT.
SUPPLEMENTARY FIGURE S5 | Plots of ALT: (A) Funnel plot with pseudo-95% confidence limits; (B) Egger’s publication bias plot; (C) Filled funnel plot with pseudo-95% confidence limits.
SUPPLEMENTARY FIGURE S6 | The forest plot of AST.
SUPPLEMENTARY FIGURE S7 | Plots of AST: (A) Funnel plot with pseudo-95% confidence limits; (B) Egger’s publication bias plot; (C) Filled funnel plot with pseudo-95% confidence limits.
SUPPLEMENTARY FIGURE S8 | The forest plots of ALP. (A) ALP levels; (B) The cell injection dose subgroup of ALP levels.
SUPPLEMENTARY FIGURE S9 | The forest plots of TBIL. (A) ALP levels; (B) Animal model subgroup of TBIL levels.
SUPPLEMENTARY FIGURE S10 | The forest plots of HA. (A) HA levels; (B) HA levels when 2014 Wang was excluded.
SUPPLEMENTARY FIGURE S11 | The forest plot of LN.
SUPPLEMENTARY FIGURE S12 | The forest plot of HYP.
SUPPLEMENTARY FIGURE S13 | The forest plot of the area percentage of collagen fibers.
SUPPLEMENTARY FIGURE S14 | The forest plot of type III collagen.
SUPPLEMENTARY FIGURE S15 | The forest plot of TGF-β.
Abbreviations
MSCs, Mesenchymal stem cells; BMSCs, Bone marrow MSCs; HUCMSCs, Human umbilical cord MSCs; ALB, Albumin; ALT, Alanine aminotransferase; ALP, Alkaline phosphatase; HA, Hyaluronic acid; HYP, Hydroxyproline; CCl4, Carbon tetrachloride; DMN, Dimethylnitrosamine; HSCs, Hepatic stellate cells; ADSCs, Adipose MSCs; HAMSCs, Human amniotic MSCs; AST, Aspartate aminotransferase; TBIL, Total bilirubin; LN, Laminin; TGF-β, Transforming growth factor-β; DEN, Diethylnitrosamine; TAA, Thioacetamide.
References
Abdel Aziz, M. T., Atta, H. M., Mahfouz, S., Fouad, H. H., Roshdy, N. K., Ahmed, H. H., et al. (2007). Therapeutic potential of bone marrow-derived mesenchymal stem cells on experimental liver fibrosis. Clin. Biochem. 40, 893–899. doi:10.1016/j.clinbiochem.2007.04.017
Abou Rayia, D. M., Ashour, D. S., Abo Safia, H. S., Abdel Ghafar, M. T., Amer, R. S., and Saad, A. E. (2023). Human umbilical cord blood mesenchymal stem cells as a potential therapy for schistosomal hepatic fibrosis: an experimental study. Pathog. Glob. Health 117, 190–202. doi:10.1080/20477724.2022.2064795
Ahmed, S. K., Mohammed, S. A., Khalaf, G., and Fikry, H. (2014). Role of bone marrow mesenchymal stem cells in the treatment of CCL4 induced liver fibrosis in albino rats: a histological and immunohistochemical study. Int. J. Stem Cells 7, 87–97. doi:10.15283/ijsc.2014.7.2.87
Aithal, A. P., Bairy, L. K., Seetharam, R. N., and Kumar, N. (2018). Haemostatic potential of human bone marrow-derived mesenchymal stromal cells in Wistar rats with carbon tetrachloride induced liver cirrhosis. Stem Cell Investig. 5, 21. doi:10.21037/sci.2018.07.04
Alfaifi, M., Eom, Y. W., Newsome, P. N., and Baik, S. K. (2018). Mesenchymal stromal cell therapy for liver diseases. J. Hepatol. 68, 1272–1285. doi:10.1016/j.jhep.2018.01.030
Amer, M. E., El-Sayed, S. Z., El-Kheir, W. A., Gabr, H., Gomaa, A. A., El-Noomani, N., et al. (2011). Clinical and laboratory evaluation of patients with end-stage liver cell failure injected with bone marrow-derived hepatocyte-like cells. Eur. J. Gastroenterol. Hepatol. 23, 936–941. doi:10.1097/meg.0b013e3283488b00
Amin, M. A., Sabry, D., Rashed, L. A., Aref, W. M., El-Ghobary, M. A., Farhan, M. S., et al. (2013). Short-term evaluation of autologous transplantation of bone marrow-derived mesenchymal stem cells in patients with cirrhosis: Egyptian study. Clin. Transplant. 27, 607–612. doi:10.1111/ctr.12179
Baertschiger, R. M., Serre-Beinier, V., Morel, P., Bosco, D., Peyrou, M., Clément, S., et al. (2009). Fibrogenic potential of human multipotent mesenchymal stromal cells in injured liver. PLoS One 4, e6657. doi:10.1371/journal.pone.0006657
Batsali, A. K., Kastrinaki, M. C., Papadaki, H. A., and Pontikoglou, C. (2013). Mesenchymal stem cells derived from Wharton’s Jelly of the umbilical cord: biological properties and emerging clinical applications. Curr. Stem Cell Res. Ther. 8, 144–155. doi:10.2174/1574888x11308020005
Bi, H., Ming, L., Cheng, R., Luo, H., Zhang, Y., and Jin, Y. (2017). Liver extracellular matrix promotes BM-MSCs hepatic differentiation and reversal of liver fibrosis through activation of integrin pathway. J. Tissue Eng. Regen. Med. 11, 2685–2698. doi:10.1002/term.2161
Cao, H., Yang, J., Yu, J., Pan, Q., Li, J., Zhou, P., et al. (2012). Therapeutic potential of transplanted placental mesenchymal stem cells in treating Chinese miniature pigs with acute liver failure. BMC Med. 10, 56. doi:10.1186/1741-7015-10-56
Chang, Y. J., Liu, J. W., Lin, P. C., Sun, L. Y., Peng, C. W., Luo, G. H., et al. (2009). Mesenchymal stem cells facilitate recovery from chemically induced liver damage and decrease liver fibrosis. Life Sci. 85, 517–525. doi:10.1016/j.lfs.2009.08.003
Cheng, Q., Li, C., Yang, C. F., Zhong, Y. J., Wu, D., Shi, L., et al. (2019). Methyl ferulic acid attenuates liver fibrosis and hepatic stellate cell activation through the TGF-β1/Smad and NOX4/ROS pathways. Chem. Biol. Interact. 299, 131–139. doi:10.1016/j.cbi.2018.12.006
Cho, P. S., Messina, D. J., Hirsh, E. L., Chi, N., Goldman, S. N., Lo, D. P., et al. (2008). Immunogenicity of umbilical cord tissue derived cells. Blood 111, 430–438. doi:10.1182/blood-2007-03-078774
Di Bonzo, L. V., Ferrero, I., Cravanzola, C., Mareschi, K., Rustichell, D., Novo, E., et al. (2008). Human mesenchymal stem cells as a two-edged sword in hepatic regenerative medicine: engraftment and hepatocyte differentiation versus profibrogenic potential. Gut 57, 223–231. doi:10.1136/gut.2006.111617
Elberry, D. A., Amin, S. N., Esmail, R. S., Rashed, L. A., and Gamal, M. M. (2016). Effect of undifferentiated versus hepatogenic partially differentiated mesenchymal stem cells on hepatic and cognitive functions in liver cirrhosis. Excli J. 15, 652–670. doi:10.17179/excli2016-645
Eom, Y. W., Shim, K. Y., and Baik, S. K. (2015). Mesenchymal stem cell therapy for liver fibrosis. Korean J. Intern Med. 30, 580–589. doi:10.3904/kjim.2015.30.5.580
Ewida, S. F., Abdou, A. G., El-Rasol Elhosary, A. A., and El-Ghane Metawe, S. A. (2017). Hepatocyte-like versus mesenchymal stem cells in CCl4-induced liver fibrosis. Appl. Immunohistochem. Mol. Morphol. 25, 736–745. doi:10.1097/pai.0000000000000373
Fathy, M., Okabe, M., E, M. O., Saad Eldien, H. M., and Yoshida, T. (2020). Preconditioning of adipose-derived mesenchymal stem-like cells with eugenol potentiates their migration and proliferation in vitro and therapeutic abilities in rat hepatic fibrosis. Molecules 25, 2020. doi:10.3390/molecules25092020
Gupta, S., Pinky, VISHAL, Sharma, H., Soni, N., Rao, E. P., Dalela, M., et al. (2022). Comparative evaluation of anti-fibrotic effect of tissue specific mesenchymal stem cells derived extracellular vesicles for the amelioration of CCl4 induced chronic liver injury. Stem Cell Rev. Rep. 18, 1097–1112. doi:10.1007/s12015-021-10313-9
Hao, T., Chen, J., Zhi, S., Zhang, Q., Chen, G., and Yu, F. (2017). Comparison of bone marrow-vs. adipose tissue-derived mesenchymal stem cells for attenuating liver fibrosis. Exp. Ther. Med. 14, 5956–5964. doi:10.3892/etm.2017.5333
He, Y., Guo, X., Lan, T., Xia, J., Wang, J., Li, B., et al. (2021). Human umbilical cord-derived mesenchymal stem cells improve the function of liver in rats with acute-on-chronic liver failure via downregulating Notch and Stat1/Stat3 signaling. Stem Cell Res. Ther. 12, 396. doi:10.1186/s13287-021-02468-6
Hong, J., Jin, H., Han, J., Hu, H., Liu, J., Li, L., et al. (2014). Infusion of human umbilical cord-derived mesenchymal stem cells effectively relieves liver cirrhosis in DEN-induced rats. Mol. Med. Rep. 9, 1103–1111. doi:10.3892/mmr.2014.1927
Hsieh, J. Y., Fu, Y. S., Chang, S. J., Tsuang, Y. H., and Wang, H. W. (2010). Functional module analysis reveals differential osteogenic and stemness potentials in human mesenchymal stem cells from bone marrow and Wharton’s jelly of umbilical cord. Stem Cells Dev. 19, 1895–1910. doi:10.1089/scd.2009.0485
Hu, C., Zhao, L., Duan, J., and Li, L. (2019). Strategies to improve the efficiency of mesenchymal stem cell transplantation for reversal of liver fibrosis. J. Cell Mol. Med. 23, 1657–1670. doi:10.1111/jcmm.14115
Jang, Y. O., Kim, S. H., Cho, M. Y., Kim, K. S., Park, K. S., Cha, S. K., et al. (2018). Synergistic effects of simvastatin and bone marrow-derived mesenchymal stem cells on hepatic fibrosis. Biochem. Biophysical Res. Commun. 497, 264–271. doi:10.1016/j.bbrc.2018.02.067
Kanninen, L. K., Harjumäki, R., Peltoniemi, P., Bogacheva, M. S., Salmi, T., Porola, P., et al. (2016). Laminin-511 and laminin-521-based matrices for efficient hepatic specification of human pluripotent stem cells. Biomaterials 103, 86–100. doi:10.1016/j.biomaterials.2016.06.054
Kao, Y. H., Lin, Y. C., Lee, P. H., Lin, C. W., Chen, P. H., Tai, T. S., et al. (2020). Infusion of human mesenchymal stem cells improves regenerative niche in thioacetamide-injured mouse liver. Tissue Eng. Regen. Med. 17, 671–682. doi:10.1007/s13770-020-00274-4
Khalil, M. R., El-Demerdash, R. S., Elminshawy, H. H., Mehanna, E. T., Mesbah, N. M., and Abo-Elmatty, D. M. (2021). Therapeutic effect of bone marrow mesenchymal stem cells in a rat model of carbon tetrachloride induced liver fibrosis. Biomed. J. 44, 598–610. doi:10.1016/j.bj.2020.04.011
Kim, M. D., Kim, S. S., Cha, H. Y., Jang, S. H., Chang, D. Y., Kim, W., et al. (2014). Therapeutic effect of hepatocyte growth factor-secreting mesenchymal stem cells in a rat model of liver fibrosis. Exp. Mol. Med. 46, e110. doi:10.1038/emm.2014.49
Li, J., Zhang, L., Xin, J., Jiang, L., Li, J., Zhang, T., et al. (2012). Immediate intraportal transplantation of human bone marrow mesenchymal stem cells prevents death from fulminant hepatic failure in pigs. Hepatology 56, 1044–1052. doi:10.1002/hep.25722
Liu, P. Y., Mao, Y. C., Xie, Y., Wei, J. Y., and Yao, J. (2022a). Stem cells for treatment of liver fibrosis/cirrhosis: clinical progress and therapeutic potential. Stem Cell Res. Ther. 13, 356. doi:10.1186/s13287-022-03041-5
Liu, Q., Lv, C., Huang, Q., Zhao, L., Sun, X., Ning, D., et al. (2022b). ECM1 modified HF-MSCs targeting HSC attenuate liver cirrhosis by inhibiting the TGF-β/Smad signaling pathway. Cell Death Discov. 8, 51. doi:10.1038/s41420-022-00846-4
Liu, Q., Lv, C., Jiang, Y., Luo, K., Gao, Y., Liu, J., et al. (2022c). From hair to liver: emerging application of hair follicle mesenchymal stem cell transplantation reverses liver cirrhosis by blocking the TGF-β/Smad signaling pathway to inhibit pathological HSC activation. PeerJ 10, e12872. doi:10.7717/peerj.12872
Luo, X. Y., Meng, X. J., Cao, D. C., Wang, W., Zhou, K., Li, L., et al. (2019). Transplantation of bone marrow mesenchymal stromal cells attenuates liver fibrosis in mice by regulating macrophage subtypes. Stem Cell Res. Ther. 10, 16. doi:10.1186/s13287-018-1122-8
Lu, W., Qu, J., Yan, L., Tang, X., Wang, X., Ye, A., et al. (2023). Efficacy and safety of mesenchymal stem cell therapy in liver cirrhosis: a systematic review and meta-analysis. Stem Cell Res. Ther. 14, 301. doi:10.1186/s13287-023-03518-x
Ma, Z. G., Lv, X. D., Zhan, L. L., Chen, L., Zou, Q. Y., Xiang, J. Q., et al. (2016). Human urokinase-type plasminogen activator gene-modified bone marrow-derived mesenchymal stem cells attenuate liver fibrosis in rats by down-regulating the Wnt signaling pathway. World J. Gastroenterol. 22, 2092–2103. doi:10.3748/wjg.v22.i6.2092
Mederacke, I., Hsu, C. C., Troeger, J. S., Huebener, P., Mu, X., Dapito, D. H., et al. (2013). Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat. Commun. 4, 2823. doi:10.1038/ncomms3823
Mohamadnejad, M., Alimoghaddam, K., Bagheri, M., Ashrafi, M., Abdollahzadeh, L., Akhlaghpoor, S., et al. (2013). Randomized placebo-controlled trial of mesenchymal stem cell transplantation in decompensated cirrhosis. Liver Int. 33, 1490–1496. doi:10.1111/liv.12228
Mohamed, H. E., Elswefy, S. E., Rashed, L. A., Younis, N. N., Shaheen, M. A., and Ghanim, A. M. (2016). Bone marrow-derived mesenchymal stem cells effectively regenerate fibrotic liver in bile duct ligation rat model. Exp. Biol. Med. (Maywood) 241, 581–591. doi:10.1177/1535370215627219
Mortezaee, K., Khanlarkhani, N., Sabbaghziarani, F., Nekoonam, S., Majidpoor, J., Hosseini, A., et al. (2017). Preconditioning with melatonin improves therapeutic outcomes of bone marrow-derived mesenchymal stem cells in targeting liver fibrosis induced by CCl4. Cell Tissue Res. 369, 303–312. doi:10.1007/s00441-017-2604-1
Mueller, S. M., and Glowacki, J. (2001). Age-related decline in the osteogenic potential of human bone marrow cells cultured in three-dimensional collagen sponges. J. Cell Biochem. 82, 583–590. doi:10.1002/jcb.1174
Nomura, M., George, J., Hashizume, C., Saito, T., Ueda, Y., Ishigaki, Y., et al. (2022). Surgical implantation of human adipose derived stem cells attenuates experimentally induced hepatic fibrosis in rats. Mol. Med. 28, 143. doi:10.1186/s10020-022-00566-6
Peng, X., Yang, H., Tao, L., Xiao, J., Zeng, Y., Shen, Y., et al. (2023). Fluorofenidone alleviates liver fibrosis by inhibiting hepatic stellate cell autophagy via the TGF-β1/Smad pathway: implications for liver cancer. PeerJ 11, e16060. doi:10.7717/peerj.16060
Pietrosi, G., Fernandez-Iglesias, A., Pampalone, M., Ortega-Ribera, M., Lozano, J. J., Garcia-Caldero, H., et al. (2020). Human amniotic stem cells improve hepatic microvascular dysfunction and portal hypertension in cirrhotic rats. Liver Int. 40, 2500–2514. doi:10.1111/liv.14610
Popp, F. C., Slowik, P., Eggenhofer, E., Renner, P., Lang, S. A., Stoeltzing, O., et al. (2007). No contribution of multipotent mesenchymal stromal cells to liver regeneration in a rat model of prolonged hepatic injury. Stem Cells 25, 639–645. doi:10.1634/stemcells.2006-0515
Qiao, H., Tong, Y., Han, H., Xu, W., Ren, Z., Ouyang, J., et al. (2011). A novel therapeutic regimen for hepatic fibrosis using the combination of mesenchymal stem cells and baicalin. Pharmazie 66, 37–43. doi:10.1691/ph.2011.9840
Raafat, N., Abdel Aal, S. M., Abdo, F. K., and El Ghonaimy, N. M. (2015). Mesenchymal stem cells: in vivo therapeutic application ameliorates carbon tetrachloride induced liver fibrosis in rats. Int. J. Biochem. Cell Biol. 68, 109–118. doi:10.1016/j.biocel.2015.09.003
Roehlen, N., Crouchet, E., and Baumert, T. F. (2020). Liver fibrosis: mechanistic concepts and therapeutic perspectives. Cells 9, 875. doi:10.3390/cells9040875
Shi, H., Shi, A., Dong, L., Lu, X., Wang, Y., Zhao, J., et al. (2016). Chlorogenic acid protects against liver fibrosis in vivo and in vitro through inhibition of oxidative stress. Clin. Nutr. 35, 1366–1373. doi:10.1016/j.clnu.2016.03.002
Su, D. N., Wu, S. P., and Xu, S. Z. (2020). Mesenchymal stem cell-based Smad7 gene therapy for experimental liver cirrhosis. Stem Cell Res. Ther. 11, 395. doi:10.1186/s13287-020-01911-4
Suk, K. T., Yoon, J. H., Kim, M. Y., Kim, C. W., Kim, J. K., Park, H., et al. (2016). Transplantation with autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: phase 2 trial. Hepatology 64, 2185–2197. doi:10.1002/hep.28693
Terai, S., Tsuchiya, A., Watanabe, Y., and Abe, H. (2023). Mesenchymal stem/stromal cells: MSC and exosome therapy for liver cirrhosis. Cytotherapy 25, S95. doi:10.1016/s1465-3249(23)00302-x
Wang, Z. C., Yang, S., Huang, J. J., Chen, S. L., Li, Q. Q., and Li, Y. (2014a). Effect of bone marrow mesenchymal stem cells on the Smad expression of hepatic fibrosis rats. Asian Pac J. Trop. Med. 7, 321–324. doi:10.1016/s1995-7645(14)60048-1
Wang, Z. C., Yang, S., Huang, J. J., Chen, S. L., Li, Q. Q., and Li, Y. (2014b). Effect of Rougan Huaqian granules combined with human mesenchymal stem cell transplantation on liver fibrosis in cirrhosis rats. Asian Pac J. Trop. Med. 7, 576–581. doi:10.1016/s1995-7645(14)60097-3
Wang, P., Cui, Y., Wang, J., Liu, D., Tian, Y., Liu, K., et al. (2022). Mesenchymal stem cells protect against acetaminophen hepatotoxicity by secreting regenerative cytokine hepatocyte growth factor. Stem Cell Res. Ther. 13, 94. doi:10.1186/s13287-022-02754-x
Wang, Y., Lian, F., Li, J., Fan, W., Xu, H., Yang, X., et al. (2012). Adipose derived mesenchymal stem cells transplantation via portal vein improves microcirculation and ameliorates liver fibrosis induced by CCl4 in rats. J. Transl. Med. 10, 133. doi:10.1186/1479-5876-10-133
Wang, Z., Li, T., Zhang, Z., Yuan, M., Shi, M., Wang, F. S., et al. (2023). Human umbilical cord-derived mesenchymal stem cells for the treatment of decompensated cirrhosis (MSC-DLC-1): a dose-escalation, phase I trial protocol. BMJ Open 13, e078362. doi:10.1136/bmjopen-2023-078362
Xie, Y., Liu, S., Wang, L., Yang, H., Tai, C., Ling, L., et al. (2021). Individual heterogeneity screened umbilical cord-derived mesenchymal stromal cells with high Treg promotion demonstrate improved recovery of mouse liver fibrosis. Stem Cell Res. Ther. 12, 359. doi:10.1186/s13287-021-02430-6
Yang, X., Meng, Y., Han, Z. P., Ye, F., Wei, L. X., and Zong, C. (2020a). Mesenchymal stem cell therapy for liver disease: full of chances and challenges. Cell Biosci. 10, 123. doi:10.1186/s13578-020-00480-6
Yang, X., Meng, Y., Han, Z., Ye, F., Wei, L., and Zong, C. (2020b). Mesenchymal stem cell therapy for liver disease: full of chances and challenges. Cell Biosci. 10, 123. doi:10.1186/s13578-020-00480-6
Ye, Z., Lu, W., Liang, L., Tang, M., Wang, Y., Li, Z., et al. (2019). Mesenchymal stem cells overexpressing hepatocyte nuclear factor-4 alpha alleviate liver injury by modulating anti-inflammatory functions in mice. Stem Cell Res. Ther. 10, 149. doi:10.1186/s13287-019-1260-7
Yin, F., Wang, W. Y., Mao, L. C., Cai, Q. Q., and Jiang, W. H. (2020). Effect of human umbilical cord mesenchymal stem cells transfected with HGF on TGF-β1/smad signaling pathway in carbon tetrachloride-induced liver fibrosis rats. Stem Cells Dev. 29, 1395–1406. doi:10.1089/scd.2020.0060
Zhang, G. Z., Sun, H. C., Zheng, L. B., Guo, J. B., and Zhang, X. L. (2017). In vivo hepatic differentiation potential of human umbilical cord-derived mesenchymal stem cells: therapeutic effect on liver fibrosis/cirrhosis. World J. Gastroenterol. 23, 8152–8168. doi:10.3748/wjg.v23.i46.8152
Zhang, L., Zhang, H., Gu, J., Xu, W., Yuan, N., Sun, J., et al. (2022). Glabridin inhibits liver fibrosis and hepatic stellate cells activation through suppression of inflammation and oxidative stress by activating PPARγ in carbon tetrachloride-treated mice. Int. Immunopharmacol. 113, 109433. doi:10.1016/j.intimp.2022.109433
Zhang, Y., Li, R., Rong, W., Han, M., Cui, C., Feng, Z., et al. (2018). Therapeutic effect of hepatocyte growth factor-overexpressing bone marrow-derived mesenchymal stem cells on CCl(4)-induced hepatocirrhosis. Cell Death Dis. 9, 1186. doi:10.1038/s41419-018-1239-9
Zhang, Z., Shang, J., Yang, Q., Dai, Z., Liang, Y., Lai, C., et al. (2023). Exosomes derived from human adipose mesenchymal stem cells ameliorate hepatic fibrosis by inhibiting PI3K/Akt/mTOR pathway and remodeling choline metabolism. J. Nanobiotechnology 21, 29. doi:10.1186/s12951-023-01788-4
Zhao, D. C., Lei, J. X., Chen, R., Yu, W. H., Zhang, X. M., Li, S. N., et al. (2005). Bone marrow-derived mesenchymal stem cells protect against experimental liver fibrosis in rats. World J. Gastroenterology 11, 3431–3440. doi:10.3748/wjg.v11.i22.3431
Zheng, X. H., Zhou, X., Ma, G., Yu, J. H., Zhang, M., Yang, C. M., et al. (2022). Endogenous Follistatin-like 1 guarantees the immunomodulatory properties of mesenchymal stem cells during liver fibrotic therapy. Stem Cell Res. Ther. 13, 403. doi:10.1186/s13287-022-03042-4
Zhou, G. P., Jiang, Y. Z., Sun, L. Y., and Zhu, Z. J. (2020). Therapeutic effect and safety of stem cell therapy for chronic liver disease: a systematic review and meta-analysis of randomized controlled trials. Stem Cell Res. Ther. 11, 419. doi:10.1186/s13287-020-01935-w
Zhou, Q., Gu, T., Zhang, Y., Li, H., Zhuansun, X., Xu, S., et al. (2021). Human umbilical cord mesenchymal stem cells ameliorate hepatic stellate cell activation and liver fibrosis by upregulating MicroRNA-455-3p through suppression of p21-activated kinase-2. Biomed. Res. Int. 2021, 1–13. doi:10.1155/2021/6685605
Keywords: mesenchymal stem cell, stem cell therapy, liver fibrosis, cirrhosis, meta-analysis
Citation: Wang X, Wang Y, Lu W, Qu J, Zhang Y and Ye J (2024) Effectiveness and mechanisms of mesenchymal stem cell therapy in preclinical animal models of hepatic fibrosis: a systematic review and meta-analysis. Front. Bioeng. Biotechnol. 12:1424253. doi: 10.3389/fbioe.2024.1424253
Received: 27 April 2024; Accepted: 26 June 2024;
Published: 22 July 2024.
Edited by:
Chunying Li, Georgia State University, United StatesReviewed by:
Xiaomeng Shi, Emory University, United StatesRajeev Nema, Manipal University Jaipur, India
Copyright © 2024 Wang, Wang, Lu, Qu, Zhang and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junsong Ye, eWpzMTIxMUAxNjMuY29t
 Xuesong Wang
Xuesong Wang Yue Wang4
Yue Wang4 Jiayang Qu
Jiayang Qu Junsong Ye
Junsong Ye