- Department of Orthopedics and Traumatology, Affiliated Hospital of Nanjing University of Traditional Chinese Medicine, Nanjing, China
Objective: To compare the accuracy of implant positioning and early functional recovery between direct anterior approach (DAA) and posterolateral approach (PLA) in total hip arthroplasty (THA) guided by an artificial intelligence preoperative planning system (AIHIP).
Methods: The study population consisted of 206 patients who underwent DAA surgery and 81 patients who underwent PLA surgery, all of whom were designed preoperatively using AI-HIP, and postoperatively using artefact-reduced CT reconstruction for prosthesis mounting angle measurements and follow-up such as postoperative outcomes. The main assessments included prosthesis positioning accuracy (compared to the preoperative plan): acetabular anterior inclination (AA), femur anterior inclination (FNA), combined anterior inclination (CA), alignment of femoral stem prosthesis and femur; clinical outcomes: operative time, hospital stay, and time to grounding; functional scores: Harris Hip Score, WOMAC, and VAS Pain Score; and biomarkers: haemoglobin, CRP, and IL-6, among others.
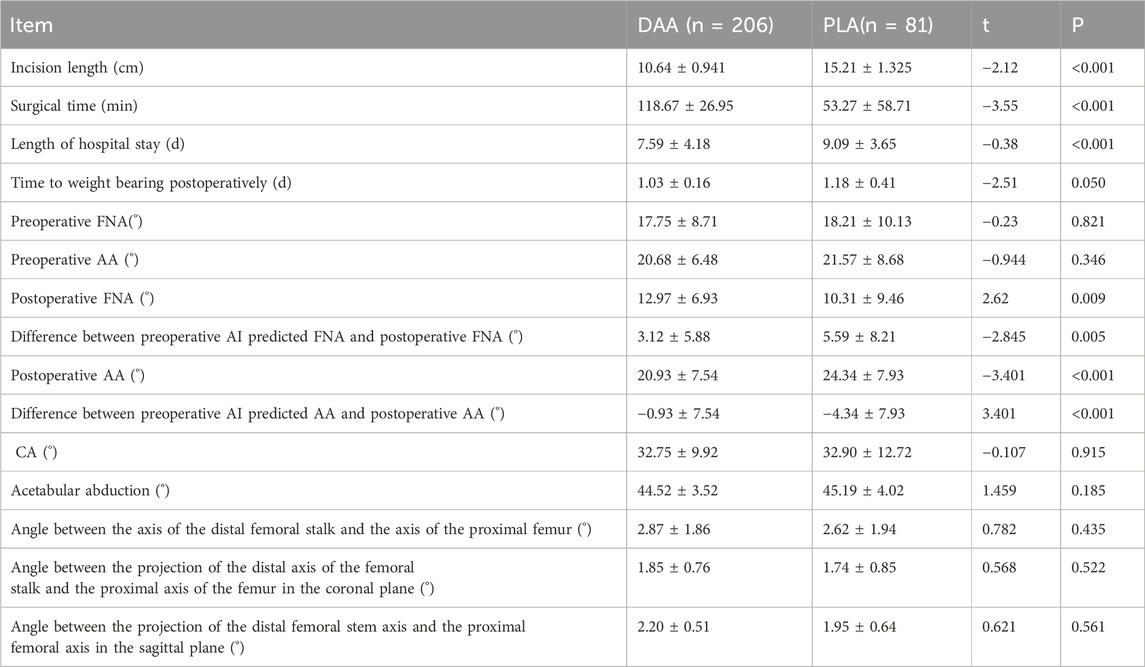
Results: All 287 patients completed ≥6-month follow-up. While preoperative femoral/acetabular anteversion showed no intergroup differences (p > 0.05), the direct anterior approach (DAA) demonstrated superior postoperative acetabular anteversion control (20.93 ± 7.54° vs. 24.34 ± 7.93°, p < 0.001) despite comparable femoral anteversion (12.97 ± 6.93° vs. 14.56 ± 7.21°, p = 0.009). AI-assisted predictions exhibited smaller deviations in DAA for both parameters (FNA: 3.12 ± 5.88° vs. 5.59 ± 8.21°, p = 0.005; AA: 0.93 ± 7.54° vs. −4.34 ± 7.93°, p < 0.001). No significant differences emerged in combined anteversion, acetabular abduction, or femoral stem alignment parameters (all p > 0.05). DAA achieved shorter incisions (10.64 ± 0.94 vs. 15.21 ± 1.33 cm, p < 0.001) and hospital stays (7.59 ± 4.18 vs. 9.09 ± 3.65 days, p < 0.001) despite longer operative times (118.67 ± 26.95 vs. 53.27 ± 58.71 min, p < 0.001). Functional recovery favored DAA, with better VAS/Harris scores at 3 months and WOMAC scores at 1 month (all p < 0.05). No intergroup differences were observed in postoperative CK, CRP, Hb, or IL-6 levels (p > 0.05).
Conclusion: Both DAA and PLA approaches resulted in satisfactory postoperative outcomes; however, the DAA approach demonstrated enhanced early postoperative efficacy indicators, as well as improved femoral neck and acetabular anteversion compared to the PLA approach. This study advocates for the preferential adoption of the DAA technique for THA, while also emphasizing the importance of considering individual patient factors, as well as the surgeon’s preferences and expertise.
1 Introduction
Total Hip Arthroplasty (THA) is widely recognized as the most definitive and effective intervention for individuals diagnosed with Osteonecrosis of the Femoral Head (ONFH) (Filippo et al., 2021), osteoarthritis of the hip joint (OA) (Günther et al., 2021; Klaus Peter et al., 2021; Manon et al., 2021), Developmental Dysplasia of the Hip (DDH) (Zeng et al., 2017), and Fractures of the neck of the femur in the elderly (FNF) (Bhandari et al., 2019). The surgical techniques commonly employed in THA include the Posterolateral Approach (PLA), Direct Anterior Approach (DAA), among others (Lei et al., 2023). However, there exists considerable debate regarding the optimal surgical approach that yields the most favorable outcomes (Larry et al., 2018). The PLA is frequently favored due to its capacity to provide sufficient exposure for a diverse array of complex hip replacements, coupled with relatively straightforward surgical manipulation and a shorter learning curve, which has contributed to its status as the earliest and most prevalent approach in THA (Higgins et al., 2015). Nonetheless, this technique necessitates the disruption of the external rotator muscle group and the posterior joint capsule, often resulting in the requirement for early postoperative hip restrictions.
Conversely, the DAA employs muscle and neurovascular interspaces to access the hip joint through the muscular interval, thereby preserving the integrity of the posterior soft tissues. This approach is posited to offer several theoretical advantages, including reduced surgical trauma, diminished postoperative pain, and expedited recovery. Consequently, an increasing number of surgeons are adopting the DAA for THA (Dragan et al., 2018; Chechik et al., 2013), with data indicating that approximately half of the members of the American Association of Hip and Knee Surgeons (AAHKS) utilize this technique (Matthew et al., 2023). However, the DAA is not without its limitations, particularly concerning its relative lack of exposure and the challenges associated with elevating the proximal femur during the procedure, which may lead to suboptimal positioning of the femoral stem prosthesis.
The ongoing discourse regarding the DAA’s potential benefits in terms of postoperative neck anteversion, alignment of the femoral stem, early pain alleviation, and functional recovery remains significant (Fatih et al., 2019; Jia et al., 2019; Hirohito et al., 2015). Ultimately, the selection of surgical approach is predominantly influenced by the surgeon’s individual preferences (Meermans et al., 2017). Accurate alignment of the femoral stem, appropriate positioning of the acetabular cup, and correct anteversion angles of both the acetabular and femoral stems are essential for mitigating complications such as prosthesis dislocation, wear, and component loosening, thereby enhancing the overall quality of life for patients (Hirohito et al., 2015). Misalignment of the femoral stem can precipitate complications associated with hip impingement, including dislocation of the prosthesis, accelerated wear and failure of the liner, and loosening of the prosthesis. This risk is particularly pronounced in patients exhibiting hip varus, prominence of the iliac wing, and significant muscular strength, which may contribute to unsatisfactory alignment following femoral stem implantation. Improved alignment of the prosthesis not only significantly decreases the likelihood of related complications but also enhances the mobility of the patient’s joint (Hirohito et al., 2015).
The artificial intelligence preoperative planning system (AI-HIP) has exhibited remarkable reliability in forecasting component sizes, acetabular anteversion, and femoral anteversion for primary THA (Purbasari et al., 2023; Xuzhuang et al., 2021). Research conducted by Huo et al.reveals (Huo et al., 2021) that the AI-HIP system significantly outperforms Mimics software and radiographic assessments in accurately predicting acetabular and femoral stem prosthesis models. Furthermore, Ding et al. reported (Xuzhuang et al., 2021) that the accuracy rates for predicting acetabular cup prosthesis models using AI-HIP and X-ray template measurements were 87.7% and 58.9%, respectively, while the corresponding rates for femoral stem prosthesis models were 94.0% and 65.2%. These results indicate that AI-based systems, such as AI-HIP, demonstrate a high level of precision in preoperative planning for THA. In the present study, all THA procedures employed the AI-HIP system for the preoperative planning of prosthesis models, as well as for determining acetabular and femoral stem anteversion angles. It is posited that this approach contributes positively to reducing the incidence of intraoperative surgical manipulations and minimizing surgical trauma. In this study, we used the AI-HIP system for preoperative planning and compared AI-HIP preoperative planning with postoperative CT 3D reconstruction measurements to assess the impact of direct anterior approach (DAA) and posterolateral approach (PLA) on the positioning accuracy of prosthetic components. With this approach, combined with surgical efficiency, functional outcome, and biological metrics, we comprehensively assessed the differences between the two different approaches, DAA and PLA, in THA surgery.
2 Clinical data
2.1 Materials and methods
2.1.1 Criteria for inclusion and exclusion of cases
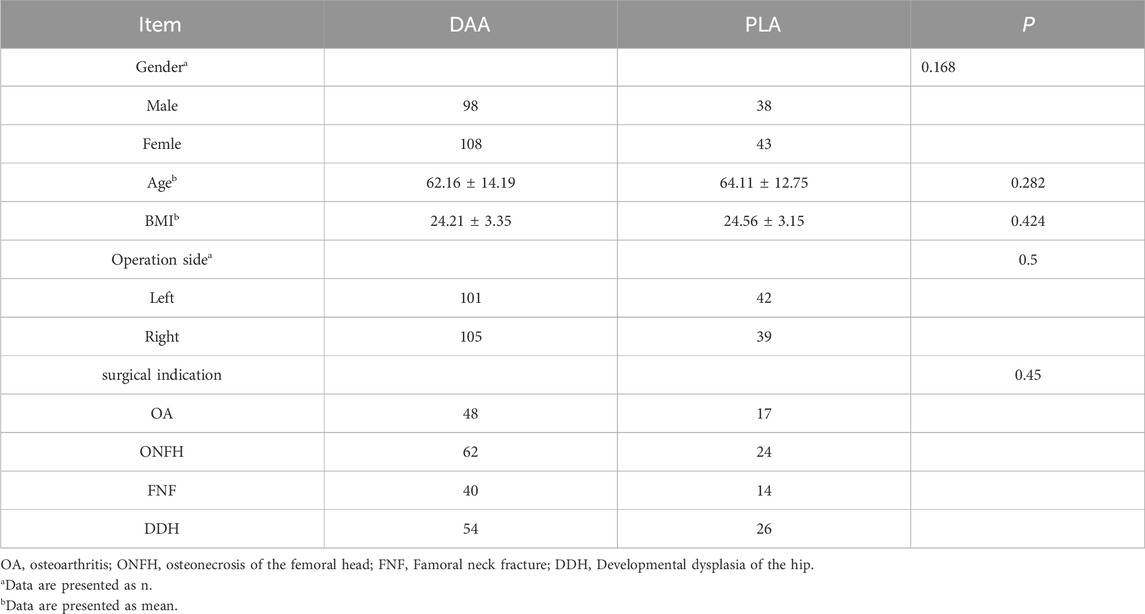
This research received approval from the Ethics Committee of Jiangsu Hospital of Traditional Chinese Medicine and was registered with the China Clinical Trial Center. The study adhered to the principles outlined in the Declaration of Helsinki and followed pertinent guidelines. Prior to participation, written informed consent was obtained from all subjects. Between May 2022 and July 2024, patients undergoing their primary THA at our institution were evaluated based on the following inclusion criteria: (1) Patients undergoing their first THA; (2) Individuals diagnosed with conditions such as developmental dysplasia of the hip, avascular necrosis of the femoral head (attributable to alcoholic, hormonal, traumatic, or idiopathic factors), femoral neck fractures, hip joint osteoarthritis, and other conditions with definitive surgical indications necessitating THA; (3) Preoperative and postoperative evaluations were performed, including a combined CT scan of the hip and knee. The preoperative scans were uploaded to the AI-HIP system for analysis and the postoperative scans were used to measure relevant angles and component alignment. In addition, reliable follow-up data was provided to ensure a comprehensive assessment. (4) Utilization of the AI-HIP system for preoperative planning; (5) Body Mass Index (BMI) of less than 30 kg/m2. A BMI greater than 30 kg/m2 is a surgical contraindication to the DAA approach for THA surgery and affects the visualization during the DAA approach; (6) Absence of significant deformities or defects in the pelvic bone structure. The exclusion criteria encompassed: (1) Inability to withstand surgical and anesthetic risks, presence of mental health disorders, or incapacity to engage in postoperative rehabilitation; (2) BMI of 30 kg/m2 or greater; (3) Bone metabolic disorders, rheumatoid arthritis, severe osteoporosis, or bone tumors; (4) Incomplete clinical data, with follow-up duration of less than 12 months; (5) Absence of preoperative planning utilizing the AI-HIP system. Following the screening process, 287 patients were identified and included in this study, with all surgical procedures conducted by a single, highly experienced lead surgeon. Comprehensive baseline information is provided in Table 1.
2.1.2 Choice of surgical method
In our study on total hip arthroplasty (THA), the selection of the surgical approach was guided by specific criteria to ensure optimal patient outcomes. All participants met the eligibility requirements for both the Direct Anterior Approach (DAA) and the posterolateral approach (PLA), allowing for an unbiased comparison between the two methods.
(1) Patient Eligibility: Each patient was thoroughly evaluated to confirm their suitability for both DAA and PLA, ensuring that they met all clinical indications necessary for either approach.
(2) Informed Decision-Making: Comprehensive preoperative education was provided to all patients, detailing the benefits, risks, and expected recovery profiles of each surgical method. This standardized education ensured that patients received consistent and accurate information.
(3) Autonomous Choice: Patients who demonstrated the capacity for informed decision-making were empowered to select their preferred surgical approach. This choice was made following detailed counseling and was formalized through the signing of an informed consent form.
(4) Study Enrollment: Of the 287 patients enrolled, 206 selected the DAA, while 81 opted for the PLA. This distribution highlights patient preferences when informed consent and autonomy are prioritized in the decision-making process.
2.2 Preoperative planning
Preoperative planning in this study was performed by the surgeon preoperatively by uploading the hip and knee joint scan to the AI-HIP preoperative planning system. The AI-HIP system is founded on the principles of deep learning and intelligent planning, facilitating the swift identification, rectification, and assessment of data through artificial intelligence, thus obviating the necessity for manual segmentation of computed tomography (CT) images. This system is capable of autonomously correcting pelvic images and is designed to be user-friendly (Maria et al., 2020). The procedural steps involved are as follows.
Preoperative scanning was performed using a low-dose CT program (specific parameters: electron tube voltage 100 kVp, electron tube current 20 mA, slice thickness 1 mm, voxel size 0.34 mm × 0.34 mm × 0.6 mm). The scanning area included only the hip and knee joints on the operated side, thus avoiding whole-body radiation in order to minimize the radiation exposure to the patient.
Import the scanned data in DICOM format into the AI-HIP software, and perform three-dimensional reconstruction of the hip joint utilizing the Transformer-unet algorithm (illustrated in Figure 1).
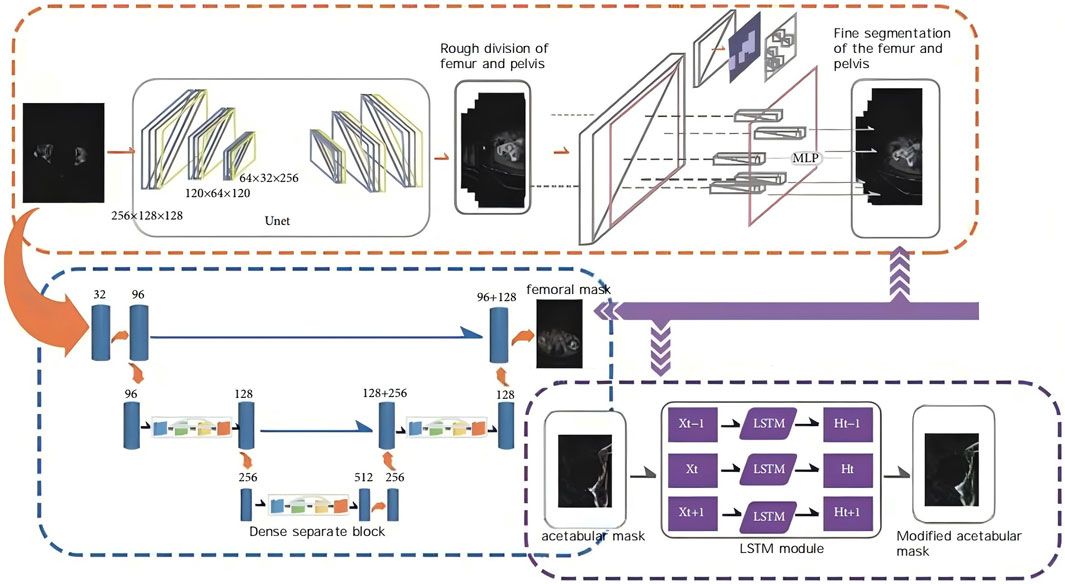
Figure 1. Schematic diagram of AIHIP self-developed unique algorithm (G-NET net-work) for skeletal segmentation.
Utilize the AI-HIP system to execute intelligent planning for both the femoral and acetabular components. For the acetabular side, position the acetabular cup prosthesis with an abduction angle of 40° and a cup anteversion of 20°. Based on the preoperative planning for the patient’s FNA, predict the suitable FNA implant for the femoral stem, and subsequently select an appropriate ball head based on the reconstructed model to finalize the simulated prosthesis placement.
Implement intelligent bone sawing simulation to ascertain the length of the femoral segment to be preserved and the distance from the tip of the greater trochanter to the shoulder of the femoral stem, ultimately producing the intelligent planning results that simulate the postoperative outcome.
The duration required for preoperative planning using the AI-HIP system is approximately 5 minutes, with the results of the preoperative planning illustrated in Figure 2.
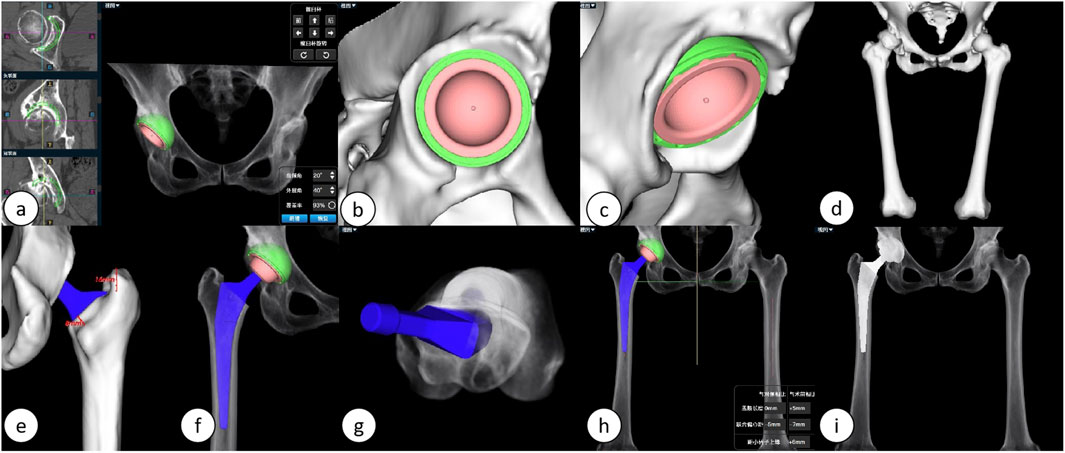
Figure 2. Illustrates the preoperative planning process using the AIHIP system: (A) The AI calculates the optimal placement position for the acetabular cup. (B, C) Detailed views of the acetabular side. (D) Three-dimensional reconstruction of the lower limb. (E, F) Detailed views of the femoral stem. (G) Predicted postoperative femoral stem anteversion angle. (H) Simulated postoperative overall three-dimensional view. (I) Simulated postoperative anteroposterior X-ray of the hip joint.
2.3 Surgical technique
The surgical procedure was performed by a single, highly skilled surgeon affiliated with the Department of Orthopedics at the Jiangsu Provincial Hospital of Traditional Chinese Medicine. The operation employed a total hip joint system, comprising the acetabular Pinnacle cup and the femoral Corail stem, both produced by the American corporation Johnson and Johnson (DePuy). After the administration of general anaesthesia, both the DAA approach and the PLA approach were performed in the lateral position. This position was chosen to optimise surgical access and visualisation for both approaches and to ensure consistency across procedures.
(1) DAA: The acetabulum should be exposed through the muscle gap (the gap between the broad fascia tensor/suture muscles and the gluteus medius), and direct observation of the bony landmarks on the anterior wall of the acetabulum (e.g., anterior inferior iliac spine, anterior edge of the transverse acetabular ligament) should be emphasized; however, due to the anterior capsule tension, the anterior angle of the acetabular filing should be finely controlled (15°–20° is recommended) in order to avoid insufficient anterior tilt. Because of the limited proximal femoral distraction, medullary preparation is prone to excessive anterior tilt of the prosthesis (requiring external rotation of the lower extremity and posterior extension of the hip), and internal rotation of the femoral stem needs to be avoided (Dargel et al., 2014; Lawrence and John, 2019). The DAA approach surgical procedure is shown in Figure 3.
(2) PLA: The posterior lateral approach enters through the gap between the gluteus maximus and the external rotator group, and needs to cut off part of the external rotator group, with the transverse acetabular ligament and sciatic tuberosity as the reference for posterior tilt; however, posterior displacement of the femur may obscure the acetabular view, and needs to be combined with the femoral lateral trial mold for temporary reset to assist in localization. The lateral femur is adequately exposed, but insufficient soft tissue release on the posterior side may lead to posterior tilt of the femoral stem, and balance stability should be partially preserved by the posterior joint capsule.
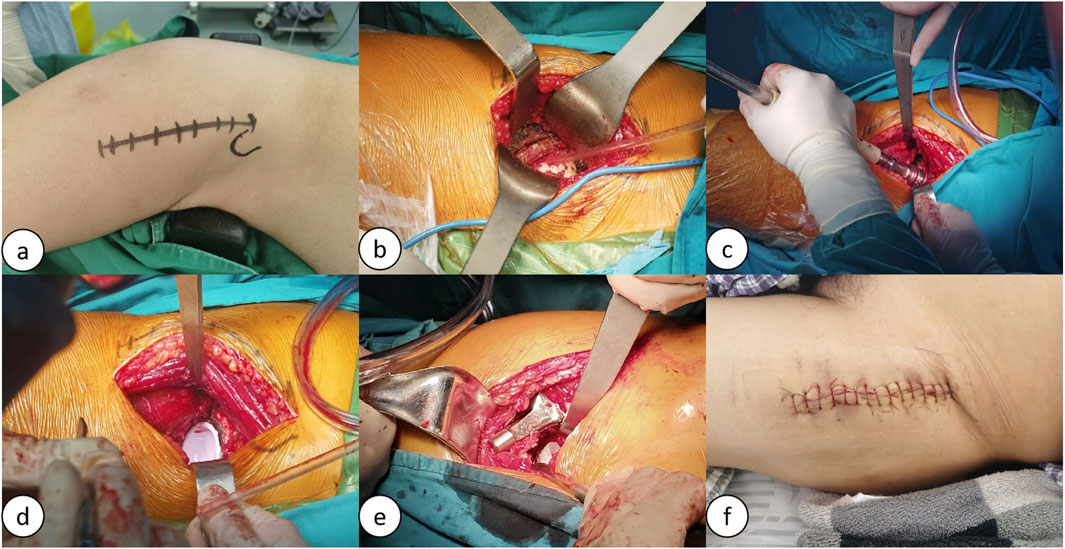
Figure 3. Schematic of the lateral decubitus position DAA procedure: (A) Preoperative markings for the DAA procedure; (B) Exposure of the joint cavity, osteotomy, and removal of the femoral head; (C) Reaming of the acetabulum; (D) Insertion of the acetabular prosthesis; (E) Canal broaching and insertion of the femoral stem prosthesis; (F) Suture incision.
2.4 Perioperative management
During the 24-h postoperative period, the patient’s vital signs are meticulously monitored, with a specific focus on the recovery of consciousness, sensory perception, and motor function on the surgical side. Simultaneously, patients receive a regimen that includes pantoprazole for gastric protection, ambroxol to aid in expectoration, citrus flavonoids to mitigate edema, flurbiprofen axetil for analgesia, ceftriaxone sodium for antibacterial prophylaxis, and enoxaparin sodium for anticoagulation purposes. For patients diagnosed with osteoporosis, denosumab injections are administered as clinically indicated. From the first to the third postoperative day, patients are instructed to engage in ankle pump exercises, leg raises, ambulation with crutches, and active flexion and extension exercises to facilitate recovery. The overarching objective is to enable patients to walk independently without the assistance of crutches and to achieve the capacity to bear weight effectively (Luc et al., 2020).
2.5 Efficacy indices
(1) Radiological Assessment Indices: All patients underwent de-noised computed tomography (CT) scans utilizing the Siemens SENSATION 256-slice spiral CT prior to discharge on the third postoperative day. The scanning protocol encompassed a range from the pelvis to the proximal femur, with a slice thickness of 0.64 mm. The acquired scan data were stored in DICOM format and subsequently recorded onto discs. Both preoperative and postoperative CT scans were imported into Mimics software (version 21.0, Materialise, Leuven, Belgium) in DICOM format, facilitating the three-dimensional reconstruction of the femoral stem and shaft. The reconstructed data were then transferred to the 3-matic Research software (version 18.0, Materialise, Leuven, Belgium) for further analysis.
(2) The following parameters were measured and documented: ① Femoral (prosthetic) neck anteversion (FNA): This parameter was defined as the angle between the surgical transepicondylar line—established by connecting the most prominent point of the lateral condyle and the deep groove of the medial condyle—and the axis of the femoral neck/stem neck. The axis of the femoral neck (prosthesis) was determined using the Design function of the 3-matic Research software. A plane was established based on the lateral and medial aspects of the distal femoral condyles and the lateral tuberosity, referred to as the posterior condylar plane. The angle between the axis of the femoral neck (prosthesis) and the posterior condylar plane was measured using the Measure tool in the 3-matic Research software, thereby providing a precise calculation of the anteversion angle, as depicted in Figures 4A–D. ② Acetabular (prosthetic) anteversion (AA): This was quantified as the angle between the lines connecting the superior and inferior margins of the acetabulum and the bilaterally lateral tears, Figure 4E shows preoperative acetabular anterior inclination and Figure 4F shows postoperative acetabular prosthesis anterior inclination Figure 4F. ③ Alignment: This parameter was defined as the angle between the distal axis of the femoral stem and the proximal axis of the femur. The axis of the femoral stem was selected for the prosthetic distal component, and the fitting procedure was executed using the Analytical command within the Design function of the 3-matic Research software. The proximal axis of the femur was characterized as a line connecting the midpoint of the horizontal canal of the lesser trochanter to the midpoint of the canal of the femoral trochanter, as shown in Figures 5A–E. ④ Femoral stem coronal alignment measurement: The femoral anatomical axis was assessed due to the anterior curvature of the femoral shaft and notable anatomical variations. The fitting procedure employed the Analytical command within the Design function of the 3-matic Research software to fit inertia axes. On the coronal plane, the angle between the axis of the femoral stem and the femoral axis provided the coronal alignment angle of the femoral stem, while the angle between the proximal axis of the femur and the femoral anatomical axis yielded the coronal inclination angle of the femur (Hirohito et al., 2015; Zurmuehle et al., 2017). A positive value indicated valgus alignment, whereas a negative value indicated varus insertion. An angle between −3° and 3° was classified as neutral insertion (Hirohito et al., 2015). The specific alignment is illustrated in Figure 5G. ⑤ The measurement of femoral stem sagittal alignment was conducted in a manner analogous to that of coronal alignment assessment. Specifically, the sagittal alignment of the femoral stem is characterized by the difference between the sagittal inclination angle of the femoral stem and that of the femur. A negative value of less than −3° is classified as a flexed insertion of the femoral prosthesis, while a positive value exceeding 3° is categorized as an extended insertion. Values falling between −3° and 3° are considered to represent neutral insertion (Hirohito et al., 2015; Zurmuehle et al., 2017). The details of the specific sagittal alignment are depicted in Figure 5F.

Figure 4. (A) Three-dimensional reconstruction model of the femoral shaft and femoral neck; (B–D): Different angled views of acetabular anteversion; (E) Preoperative anterior acetabular tilt angle; (F) Postoperative cup anteversion angle of the acetabular prosthesis.
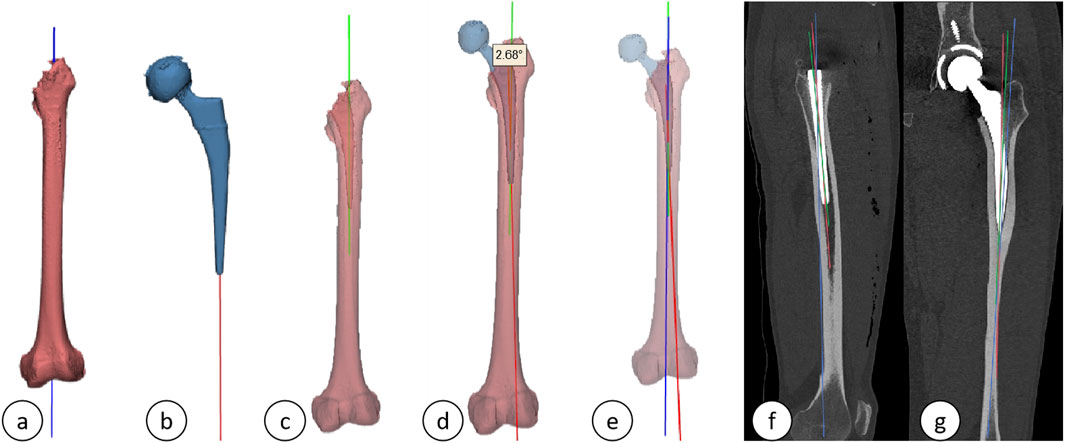
Figure 5. (A) Axis of the femur; (B) Axis of the femoral stem prosthesis; (C) Proximal femoral axis; (D) Alignment; (E) Three-dimensional schematic of the axes (green: proximal femoral axis; red: axis of the femoral stem prosthesis; blue: femoral axis); (F, G) Sagittal and coronal plane projections.
To ensure the reliability of the data, all measurements were conducted by two observers using the same methodology. These measurements were repeated at 1-month intervals, and inter-rater reliability was assessed using the intraclass correlation coefficient (ICC), with mean values calculated for analysis.
(1) Efficacy Indices: Postoperative functional recovery was evaluated utilizing the Hip Joint Harris Score and the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC Score). Elevated Harris Scores and diminished WOMAC Scores were indicative of enhanced postoperative recovery. The hip joint Harris Scores and WOMAC Scores for both cohorts were recorded prior to surgery and subsequently at 1 week, 4 weeks, 3 months, and 6 months postoperatively. The Visual Analog Scale (VAS) was utilized to assess pain levels, with VAS Scores documented at 24 h, 72 h, 1 week, 4 weeks, 3 months, and 6 months following the surgical procedure. Hemoglobin (Hb) levels were measured as an indicator of blood loss during the operation, with Hb levels recorded and subjected to statistical analysis at preoperative, first postoperative day, third postoperative day, and first postoperative month intervals. Additionally, levels of creatine kinase (CK), C-reactive protein (CRP), and interleukin-6 (IL-6) were quantified to evaluate the degree of tissue injury, with CK, CRP, and IL-6 levels recorded and statistically analyzed at preoperative and postoperative intervals of 2 h, 1 day, 3 days, and 1 week. Furthermore, the length of the surgical incision, duration of the surgery, and postoperative hospital stay were documented and statistically analyzed. Follow-up assessments were conducted post-discharge through a comprehensive disease management platform, as depicted in Figures 6, 7.
(2) Complications: The occurrence of postoperative adverse events, including sensory abnormalities, intraoperative fractures, delayed wound healing, postoperative infections, dislocations, and the formation of deep vein thrombosis (DVT), was carefully monitored. All clinical and follow-up data were recorded by two independent researchers who did not participate in the surgical procedures.
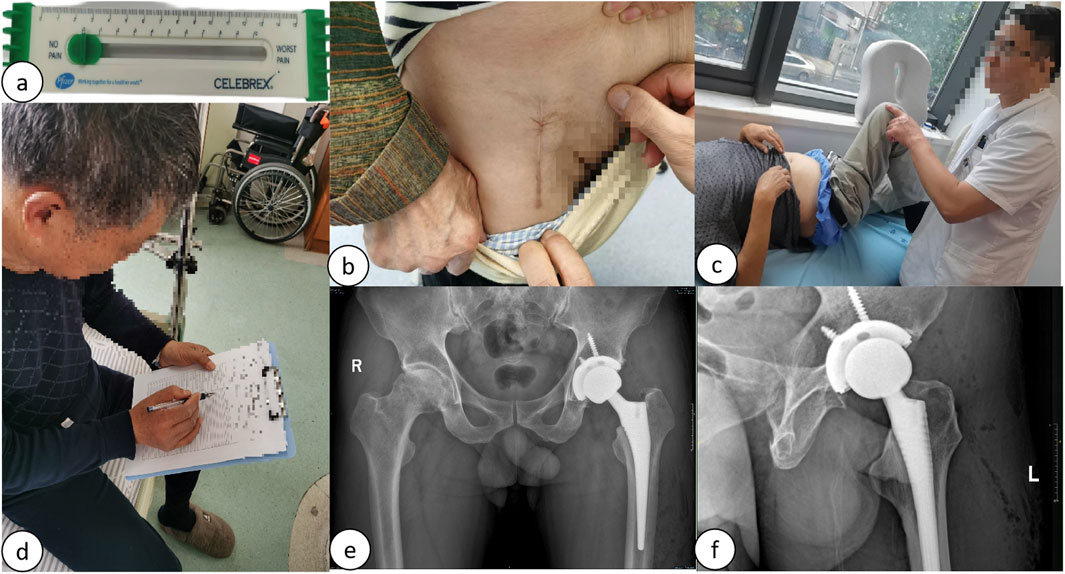
Figure 6. (A) VAS score measurement; (B) Follow-up of incision scars 6 months postoperatively; (C) Follow-up of functional range of motion 6 months postoperatively; (D) Follow-up of postoperative Harris Hip and WOMAC scores; (E) Follow-up of postoperative bilateral hip joint anteroposterior radiographs; (F) Follow-up of postoperative hip joint lateral radiographs.
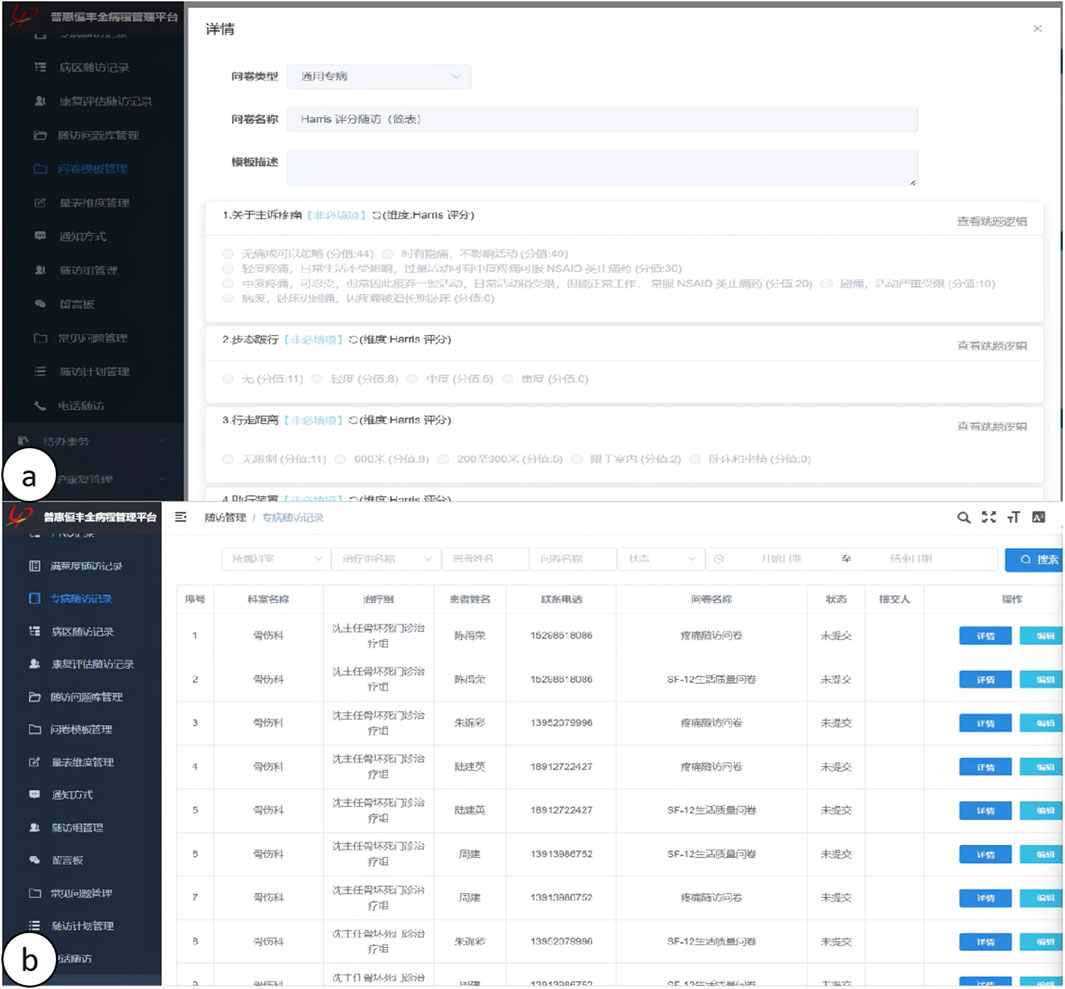
Figure 7. (A, B) Illustration of the online long-term follow-up on the HP CareCycle Management Platform.
3 Statistical methods
Statistical analyses were conducted using IBM SPSS Statistics software, version 27.0.1. Descriptive analysis was performed on continuous data, which are presented as mean ± standard deviation (x ± s). Independent sample t-tests were employed to compare differences between the two groups for Fine Needle Aspirations (FNA), Arthrocentesis (AA), changes from preoperative to postoperative FNA and AA values, the Harris score, the VAS score, hemoglobin (Hb) levels, incision length, surgical duration, hospital stay, time to ambulation postoperatively, and the level of significance (P-value = 0.05). Chi-square tests were used to compare the distribution of patient sex, surgical side, indications for surgery, incidence of DVT, rate of dislocation, and infection rates between the two groups.
4 Results
In this investigation, a total of 271 patients, encompassing 287 hips, were monitored, with follow-up durations extending a minimum of 6 months postoperatively. The differences in preoperative femoral anterior angulation (FNA) and acetabular anterior angulation (AA) were not significantly different (p > 0.05), the length of incision in patients in the DAA group was significantly shorter than that in the PLA group (DAA:10.64 ± 0.941 vs. PLA:15.21 ± 1.325, p < 0.001), and the duration of operation in patients in the DAA group was significantly longer than that in the PLA group (DAA. 118.67 ± 26.95 VS PLA:53.27 ± 58.71, p < 0.001), hospitalization time of patients in DAA group was significantly shorter than that of PLA group (DAA:7.59 ± 4.18 VS PLA:9.09 ± 3.65, p < 0.001), and early postoperative time to get down to the floor of the floor of the patients in DAA group was significantly earlier than that of the patients in PLA group (1.03 ± 0.16 vs. 1.18 ± 0.41, p = 0.05); postoperative FNA (DAA: 12.97 ± 6.93 vs. PLA:10.31 ± 9.46, p = 0.009) and postoperative AA (DAA: 20.93 ± 7.54 vs. PLA: 24.34 ± 7.93, p < 0.001), which were significantly different; and postoperative acetabular abduction angle (DAA: 44.52 ± 3.52 vs. PLA: 45.19 ± 4.02, p = 0.185) and postoperative CA (DAA: 32.75 ± 9.92 vs. PLA: 32.90 ± 12.72, p = 0.915), which were not significantly different; and the difference between preoperative AI-predicted FNA and postoperative FNA (DAA: 3.12 ± 5.88 vs. PLA: 5.59 ± 8.21, p = 0.005) and the difference between preoperative AI-predicted AA and postoperative AA (DAA: 0.93 ± 7.54 vs. PLA: 4.34 ± 7.93, p < 0.001) were significantly different, and the angle between the distal shaft of the femoral stem and the proximal shaft of the femur (DAA: 2.87 ± 1.86 vs. PLA: 2.62 ± 1.94, p = 0.001) was significantly different. 1.94, p = 0.435) and the angle between the coronal projection of the distal axis of the femoral stem and the proximal axis of the femur (DAA: 1.85 ± 0.76 vs. PLA: 1.74 ± 0.85, p = 0.522) and the angle between the sagittal projection of the distal axis of the femoral stem and the proximal axis of the femur (DAA: 2.20 ± 0.51 vs. PLA: 1.95 ± 0.64, p = 0.561) were not significantly different. Detailed findings are presented in Table 2.
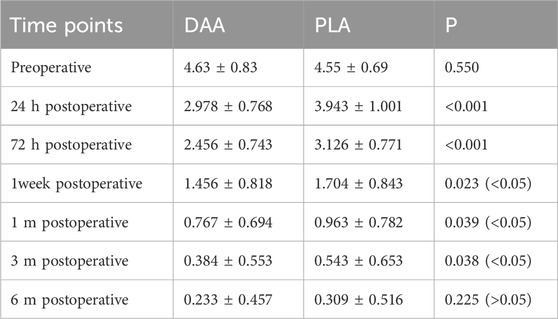
There were no significant differences in the levels of hemoglobin (Hb), creatine kinase (CK), C-reactive protein (CRP), and interleukin-6 (IL-6) between the two groups at 2 h, 1 day, 3 days, 1 week, and 4 weeks post-surgery (P > 0.05), as depicted in Tables 3, 4. Postoperative Visual Analog Scale (VAS) scores for both groups were significantly lower than their preoperative counterparts, with the VAS scores of the DAA group being lower than those of the PLA group at 24 h, 72 h, 1 week, 4 weeks, and 3 months postoperatively, demonstrating a statistically significant difference (P < 0.05), as illustrated in Table 5 and Figure 8. The Harris scores for the DAA group were significantly higher than those for the PLA group at both 4 weeks and 3 months postoperatively (P < 0.05), while the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores in the DAA group were significantly lower than those in the PLA group at 1 and 4 weeks postoperatively (P < 0.05), as indicated in Table 6. No statistically significant differences were observed in the comparison of VAS scores, WOMAC scores, and Harris scores between the two groups at the 6-month postoperative mark (P > 0.05).
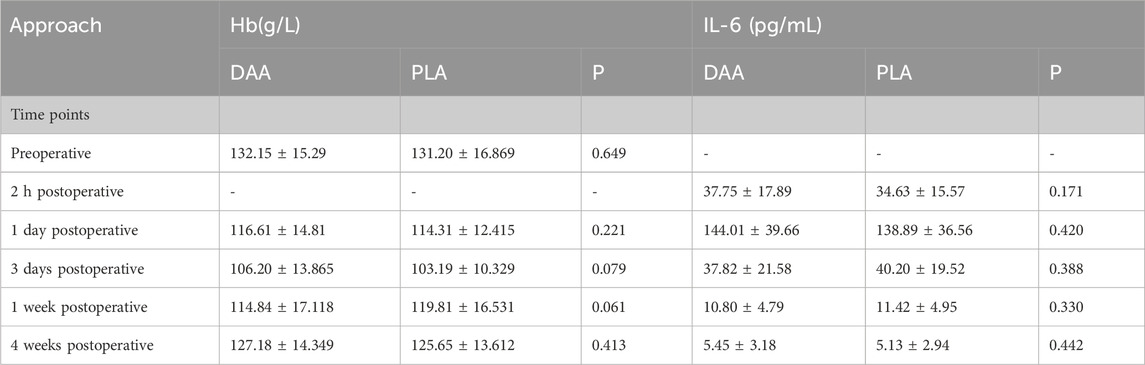
Table 4. Comparison of hemoglobin and Interleukin-6 levels at different time points between two approaches.
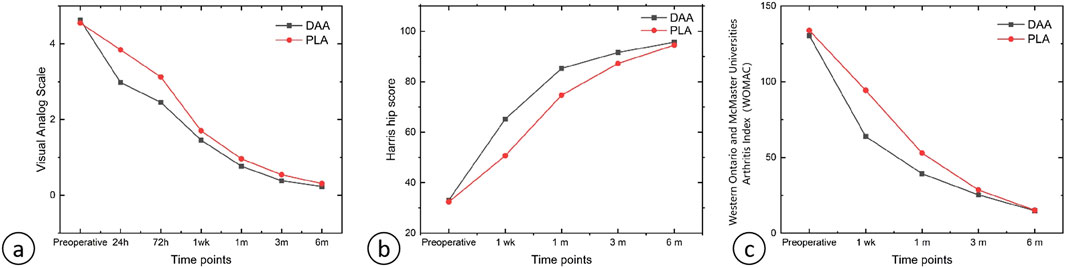
Figure 8. (A) Line graph of VAS scores; (B) Line graph of Harris Hip Scores; (C) Line graph of WOMAC scores.
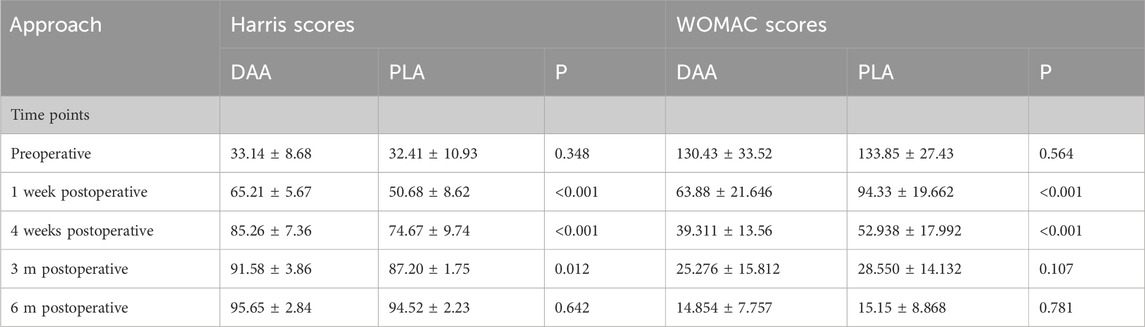
Table 6. Comparison of harris scores and WOMAC scores at different time points between two approaches.
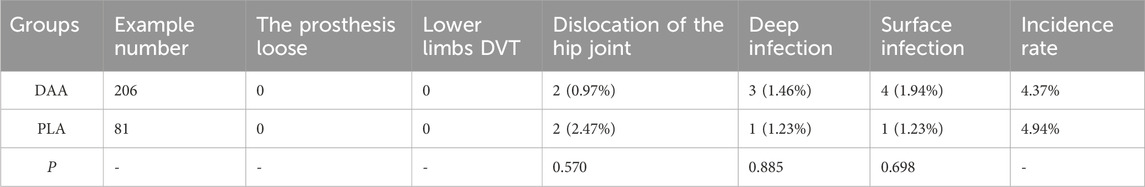
Within the DAA group, two patients experienced postoperative hip dislocation, and seven patients developed postoperative infections (three deep infections and four superficial infections). These cases were managed through one-stage and two-stage revision surgeries, guided by bacterial culture and macro gene detection, ultimately resulting in the resolution of the infections. The remaining patients in this cohort did not encounter any complications. In the PLA group, two patients experienced postoperative hip dislocation, and two patients developed postoperative infections (one deep infection and one superficial infection), both of which were successfully addressed through revision surgery. The analysis revealed no statistically significant difference in early complications between the two groups (P > 0.05), as presented in Table 7.
5 Discussion
This prospective study involving 287 patients indicates that individuals who underwent the DAA demonstrated significantly lower VAS and Western Ontario and WOMAC scores in the early postoperative period when compared to those who received the PLA. Moreover, the DAA cohort achieved notably higher Harris hip scores. At the 6-month follow-up, outcomes for both groups began to converge. Additionally, patients in the DAA group experienced shorter hospital stays and earlier ambulation following surgery, suggesting that the DAA is associated with reduced postoperative pain, decreased duration of hospitalization, and expedited recovery (Higgins et al., 2015; Jetse et al., 2017). Mao et al. found (Jin et al. 2023) that while the DAA resulted in longer operative times, it also led to reduced intraoperative bleeding and muscle damage. At 3 months post-surgery, patients who underwent DAA reported lower VAS scores and higher Harris scores compared to those who underwent PLA, with the differences being statistically significant in favor of the DAA group. Another research team (Sarhan et al., 2024) conducted a retrospective analysis of 731 DAA cases compared to 840 PLA cases in THA and found results consistent with those of the current study. The DAA approach was associated with shorter hospital stays, reduced pain following total hip replacement, accelerated recovery, and lower healthcare costs (Tze et al., 2017). Furthermore, the incision length for the DAA was significantly shorter than that for the PLA.
A study (McMullen et al., 2018) indicated that the smaller incision and soft tissue release associated with the DAA, along with the challenges in preparing the lateral femur, are likely to contribute to suboptimal prosthesis placement. In contrast, the presence of an intraoperative assistant during the PLA can facilitate the accurate positioning of the femoral stem. The placement of the femoral stem in the DAA is predominantly reliant on the operator’s judgment, which may render it more vulnerable to alignment errors. Another investigation (Batailler et al., 2018) revealed that, unlike cemented femoral prostheses where anteversion is managed by the surgeon, the positioning of compression-fit femoral prostheses is dictated by the morphology of the proximal medullary cavity. This characteristic restores the femur’s “natural anteversion,” leading to an increase in anteversion that exhibits considerable variability and limited adjustability. CA, defined as the sum of FNA and AA, is critical for the functional recovery of the hip joint following THA (Jolles et al., 2002). Dorr et al. Found (Lawrence et al., 2009) that patients with posterior hip dislocations exhibited a CA exceeding 50°, suggesting that maintaining CA within a range of 25°–50° during surgery may mitigate the risk of postoperative hip dislocation. In the present study, FNA and AA were assessed through three-dimensional reconstruction of postoperative hip structures, which demonstrated reduced measurement error compared to two-dimensional computed tomography (Sangeux et al., 2015). The findings indicated no statistically significant difference in preoperative FNA and AA between the DAA and PLA groups; however, a significant difference was observed in postoperative FNA and AA. When comparing postoperative FNA and AA with the preoperative values for AI planning, the DAA group exhibited postoperative FNA and AA that were more closely aligned with the preoperative planning angles, with no significant difference in CA between the two groups. A prior study concluded (Hirohito et al., 2015) that preoperative FNA was the primary determinant of postoperative FNA; however, this study found no significant difference in preoperative FNA and AA between the two approaches, suggesting that the primary factor influencing postoperative FNA and AA was the surgical access method. The authors posited that the enhanced accuracy of FNA and AA in the DAA group may be attributed to reduced interference with surrounding soft tissues (such as muscles and tendons), which minimizes distortion and displacement of anatomical markers, thereby improving the precision of angular measurements. Additionally, the DAA aligns with the natural anatomical planes of the hip joint, facilitating more accurate measurements by the attending surgeon. Furthermore, the proficiency of the surgeon is likely to play a significant role in these outcomes.
The analysis of our current statistical data revealed no significant differences in alignment between the DAA and the PLA groups. Additionally, there was no notable difference in the rates of inversion and eversion during implantation, with the majority of prostheses being positioned in a neutral alignment. Previous research indicates that minor misalignments in the coronal plane do not adversely affect long-term patient outcomes (Nicolas et al., 2019). Conversely, it has been established that sagittal flexion positioning of the prosthesis is associated with an increased risk of postoperative pain and dislocation (Michael et al., 2010). However, another study suggests (Junya et al., 2018) that sagittal flexion placement may decrease the incidence of intraoperative fractures and limit the range of extension and external rotation of the joint prosthesis. In the present study, the sagittal alignment of the lateral DAA was predominantly neutral, and there were no significant differences observed between the two surgical approaches regarding the angles of flexion and extension placement.
The DAA is often regarded as a minimally invasive surgical technique that circumvents the need to incise muscle tissue (Ahmed et al., 2023). However, anatomical investigations have demonstrated (van Oldenrijk et al., 2010; Meneghini et al., 2006) that THA performed via the DAA can still inflict damage on the anterolateral muscles of the hip. This assertion is further supported by a prospective study (Knut et al., 2015) that identified injury to the tensor fascia lata during the surgical intervention. Despite the classification of these techniques as minimally invasive, muscle damage can occur as a result of stretching and inadvertent dissection during the procedure. Additionally, when compared to the PLA, which involves the transection of a portion of the muscle, the excessive stretching of muscles during the DAA may lead to a more pronounced elevation in postoperative CK levels (Knut et al., 2015; Kyrill et al., 2017). While the muscle separation and incision associated with the PLA may result in limited damage to individual muscle fibers, the stretching involved in the DAA may cause more extensive muscle injury (Knut et al., 2015). In the present study, no significant differences were observed between the two surgical groups regarding CK, IL-6, CRP, and Hb levels, suggesting that both surgical approaches exhibit comparable levels of invasiveness.
Two systematic reviews (Ang et al., 2023; Wang et al., 2024) indicate that there is no statistically significant difference in the incidence of complications such as dislocation, periprosthetic fracture, or venous thromboembolism between the two surgical approaches, which aligns with the findings of the current study. However, existing literature suggests that the DAA for THA in obese patients (BMI >30) is associated with a heightened risk of wound complications and periprosthetic joint infections when compared to the PLA (Shah et al., 2022). Consequently, it is advisable to utilize the DAA for patients with lower BMI and simpler anatomical configurations, while reserving the PLA for those with higher BMI and more complex anatomical structures that necessitate extensive surgical exposure.
The AI-HIP preoperative planning system used in this study can improve the efficiency and accuracy of THA preoperative planning compared with human manual planning, and the AI-HIP system adopts the unique Transformer_unet algorithm, which can realize the automatic and accurate segmentation of hip CT images in a short period of time to improve the clinical efficiency, and it has a high utility and clinical application value (Zhu et al., 2025). Previous studies have shown (Wu et al., 2020) that the complete compliance rate of acetabular-side and femoral-side prostheses in the AI-HIP group was 90.0% (27/30) and 83.3% (25/30), respectively, and in the manual preoperative planning group, it was 56.7% (17/30) and 53.3% (16/30); the difference between the two groups was statistically significant (P < 0.05), and the time-consuming preoperative planning in the AI-HIP group (5.02 ± 1.22) min, which was significantly reduced compared with (8.29 ± 2.08) min in the manual preoperative planning group, and the difference was statistically significant (t = −7.431, P < 0.05). Compared with traditional 2D and 3D preoperative planning, preoperative planning using the AI-HIP system resulted in less variability in measurement angles, more accurate prosthesis models, and easier operation, thus improving surgical outcomes (Zhu et al., 2025; Chen et al., 2022). Currently, AI preoperative planning is becoming more common in other joint areas in addition to its use in hip revision, and the integration of artificial intelligence (AI) into decision support systems for the diagnosis and treatment of orthopaedic diseases is one of the main directions in the development of orthopaedic technology (Andriollo et al., 2024). In knee surgery, AI preoperative planning is also widely used, and studies have shown (Lan et al., 2024) that in total knee arthroplasty the accuracy of prosthesis size prediction in the AI group was significantly higher than that in the 2D group, with the complete compliance rates of femoral and tibial prostheses in the AI reconstruction group of 90% (27/30) and 86.7% (26/30), respectively. The corresponding rates in the 2D template group were 66.7% (20/30) and 60% (18/30), with high prediction accuracy.
This study does have several limitations. Firstly, it primarily focused on early postoperative imaging and efficacy indices, lacking a follow-up investigation into long-term imaging outcomes, clinical results, and associated complications, which necessitates extended follow-up to enhance the comparative analysis. Secondly, the angles of FNA, AA, and CA were measured using Mimics and other related software for three-dimensional reconstruction. This process required manual separation of joint structures to eliminate metal artifacts and impurities, which may not have been entirely effective, leading to potential measurement errors. Thirdly, the patients were discharged with a comprehensive management platform for care, rehabilitation, and follow-up, which necessitated the use of iPhones. For older, less educated, and economically disadvantaged patients, follow-up and rehabilitation guidance were conducted via telephone, potentially introducing bias. Fourthly, other functional outcomes, such as hip mobility and gait analysis, were not evaluated in this study. Fifthly, functional outcome indicators, including pain function post-THA, were not correlated with the pairwise line and the AA, FNA, and CA measurements, and a multifactorial logistic regression analysis to assess the influence of various factors on postoperative outcomes was not performed. Future research could enhance the analysis of these influencing factors. Additionally, the AIHIP system, like other artificial intelligence tools, is not immune to errors (e.g., AI hallucinations) in its output, necessitating that the surgeon make judgments based on the actual intraoperative context. Finally, while the AI-HIP system was employed for preoperative planning in this study, it did not facilitate the selection of the surgical access route based on the patient’s baseline characteristics. Future developments could include intelligent predictive models that recommend surgical access routes tailored to the patient’s BMI, age, and underlying health conditions.
6 Conclusion
Both the DAA and the PLA, when utilized in conjunction with AI-HIP preoperative planning, can yield improved surgical outcomes. Specifically, DAA-THA demonstrates functional outcomes and anatomical alignment that are more closely aligned with AI-HIP assisted preoperative planning compared to PLA. Furthermore, DAA exhibits advantages over PLA in terms of postoperative pain, duration of hospitalization, functional scores during early follow-up, and scar length. However, no significant differences were observed between the two approaches regarding invasiveness and postoperative complications. Both techniques resulted in favorable postoperative follow-up outcomes and high levels of surgical satisfaction. Based on the findings of this study, the authors advocate for the preference of DAA as the surgical approach for THA, while also emphasizing the importance of considering patient-specific factors, as well as the surgeon’s preferences and experience.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. Requests for data access can be directed to the corresponding author via email at ejE1NTAwNTI4ODc2QDE2My5jb20=
Ethics statement
This research received approval from the Ethics Committee of Jiangsu Hospital of Traditional Chinese Medicine. The study adhered to the principles outlined in the Declaration of Helsinki and followed pertinent guidelines. Prior to participation, written informed consent was obtained from all subjects. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SZ: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. JZ: Formal Analysis, Investigation, Software, Writing–original draft, Writing–review and editing. ZC: Resources, Supervision, Writing–original draft. XC: Validation, Visualization, Writing–review and editing. TX: Formal Analysis, Project administration, Resources, Supervision, Visualization, Writing–original draft, Writing–review and editing. CZ: Data curation, Funding acquisition, Resources, Visualization, Writing–review and editing. JS: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the National Natural Science Foundation of China (Grant No. 82274552), the Affiliated Hospital of Nanjing University of Chinese Medicine (Grant No. JSSZGCP2020-22), and the Science and Technology Development Program Project of Jiangsu Administration of Traditional Chinese Medicine (Grant No. ZD202315).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmed, S., Karthikeyan, P. I., Rajesh, B., and McBryde, C. (2023). Direct anterior approach to the hip joint. null, 61–82. doi:10.1007/978-3-031-35790-9_7
Andriollo, L., Picchi, A., Sangaletti, R., Perticarini, L., Rossi, S. M. P., Logroscino, G., et al. (2024). The role of artificial intelligence in anterior cruciate ligament injuries: current concepts and future perspectives. Healthc. (Basel) 12 (3), 300. doi:10.3390/healthcare12030300
Ang, J. J. M., Onggo, J. R., and Stokes, C. M. (2023). Comparing direct anterior approach versus posterior approach or lateral approach in total hip arthroplasty: a systematic review and meta-analysis. Eur J Orthop Surg Traumatol 33 (7), 2773–2792. doi:10.1007/s00590-023-03528-8
Bhandari, M., Einhorn, T. A., Guyatt, G., Schemitsch, E. H., Zura, R. D., Sprague, S., et al. (2019). Total Hip Arthroplasty or Hemiarthroplasty for Hip Fracture. N Engl J Med. 381 (23), 2199–2208. doi:10.1056/NEJMoa1906190
Batailler, C., Fary, C, Servien, E., and Lustig, S. (2018). Influence of femoral broach shape on stem alignment using anterior approach for total hip arthroplasty: A radiologic comparative study of 3 different stems. PLoS One 13 (10), e0204591. doi:10.1371/journal.pone.0204591
Chen, X., Liu, X., Wang, Y., Ma, R., Zhu, S., Li, S., et al. (2022). Development and validation of an artificial intelligence preoperative planning system for total hip arthroplasty. Front. Med. (Lausanne) 9, 841202. doi:10.3389/fmed.2022.841202
Chechik, O., Khashan, M., Lador, R., Salai, M., Amar, E., Li, S., et al. (2013). Surgical approach and prosthesis fixation in hip arthroplasty world wide. Arch Orthop Trauma Surg 133 (11), 1595–1600. doi:10.1007/s00402-2013-1828-0
Dragan, R., Vladimir, Z., Walaa, IKRAM E., and Azab, M. A. (2018). Patient’s perspective on direct anterior versus posterior approach total hip arthroplasty. Int. Orthop. 42, 2771–2775. doi:10.1007/s00264-018-4002-z
Dargel, J, Oppermann, J, Brüggemann, G. P., and Eysel, P. (2014). Dislocation following total hip replacement. Dtsch Arztebl Int. 111 (51–52), 884–90. doi:10.3238/arztebl.2014.0884
Fatih, K., Mohamed, S., and Javad, P. (2019). A meta-analysis comparing the direct anterior with other approaches in primary total hip arthroplasty. Surg. 17, 291–299. doi:10.1016/j.surge.2018.09.001
Filippo, M., Nicola, M., Alice, B., Eschweiler, J., Tingart, M., and Betsch, M. (2021). Failure and progression to total hip arthroplasty among the treatments for femoral head osteonecrosis: a Bayesian network meta-analysis. Br. Med. Bull. 138, 112–125. doi:10.1093/bmb/ldab006
Günther, K. P., Deckert, S., and Ltzner, C. (2021). Total Hip Replacement for Osteoarthritis-Evidence-Based and Patient-Oriented Indications. Dtsch Arztebl Int. 118 (43), 730–736. doi:10.3238/arztebl.m2021.0323
Huo, J., Huang, G., Han, D., Wang, X., Bu, Y., Chen, Y., et al. (2021). Value of 3D preoperative planning for primary total hip arthroplasty based on artificial intelligence technology. J Orthop Surg Res. 16 (1), 156. doi:10.1186/s13018-021-02294-9
Higgins, B. T., Barlow, D. R., Heagerty, N.E., and Lin, T. J. (2015). Anterior vs. posterior approach for total hip arthroplasty, a systematic review and meta-analysis. J Arthroplasty 30 (3), 419–434. doi:10.1016/j.arth.2014.10.020
Hirohito, A., Takashi, S., Masaki, T., Nishii, T., Nakamura, N., and Sugano, N. (2015). Difference in stem alignment between the direct anterior approach and the posterolateral approach in total hip arthroplasty. J. Arthroplasty 30, 1761–1766. doi:10.1016/j.arth.2015.04.026
Huo, J., Huang, G., Han, D., et al. (2024). Value of 3D preoperative planning for primary total hip arthroplasty based on artificial intelligence technology, 1749–799X.
Jetse, J., Rik, P., Harm, W. B., Eggen, P. J., Kleijn, L. L., Lacroix, H., et al. (2017). Limited benefits of the direct anterior approach in primary hip arthroplasty: a prospective single centre cohort study. J. Orthop. 14, 53–58. doi:10.1016/j.jor.2016.10.025
Jia, F., Guo, B., Xu, F., Hou, Y., Tang, X, Huang, L., et al. (2019). A comparison of clinical, radiographic and surgical outcomes of total hip arthroplasty between direct anterior and posterior approaches: a systematic review and meta-analysis. Hip Int 29 (6), 584–596. doi:10.1177/1120700018820652
Jin, M. W., Zhang, L., Chu, X. B., Lv, S. J., Zhang, J. J., Tong, P. J., et al. (2023). Comparison of clinical efficacy between direct anterior approach and posterolateral approach in primary total hip arthroplasty. Eur. Rev. Med. Pharmacol. Sci. 27 (12), 5604–5613. doi:10.26355/eurrev_202306_32799
Jolles, B. M., Philippe, Z., and Pierre-FrançOIS, L. (2002). Factors predisposing to dislocation after primary total hip arthroplasty. J. Arthroplasty 17, 282–288. doi:10.1054/arth.2002.30286
Junya, Y., Tamon, K., Yoshitomo, K., Takagi, T., Ohmori, T., Ueno, T., et al. (2018). The effect of flexion alignment in total hip arthroplasty with a cementless tapered-wedge femoral stem. Eur. J. Orthop. Surg. Traumatology 28, 1625–1632. doi:10.1007/s00590-018-2227-6
Klaus-Peter, G., Stefanie, D., Cornelia, L., et al. (2021). Total hip replacement for osteoarthritis. Dtsch. Arzteblatt Int.
Knut, ERIK M., Kjetil, K., Svein, S., Pripp, A. H., and Nordsletten, L. (2015). Comparison of markers for muscle damage, inflammation, and pain using minimally invasive direct anterior versus direct lateral approach in total hip arthroplasty: a prospective, randomized, controlled trial. J. Orthop. Research® 33, 1305–1310. doi:10.1002/jor.22911
Kyrill, R., Inge, H. F. R., Maurits, S. S., Knobben, B. A., and ten Have, B. L. (2017). Posterolateral vs direct anterior approach in total hip arthroplasty (polada trial): a randomized controlled trial to assess differences in serum markers. J. Arthroplasty 32, 3652–3658.e1. doi:10.1016/j.arth.2017.07.008
Lan, Q., Li, S., Zhang, J., Guo, H., Yan, L., and Tang, F. (2024). Reliable prediction of implant size and axial alignment in AI-based 3D preoperative planning for total knee arthroplasty. Sci. Rep. 14 (1), 16971. doi:10.1038/s41598-024-67276-3
Larry, E. M., Joseph, S. G., Samir, B., Kamath, A. F., Boettner, F., and Wright, J. (2018). Does surgical approach affect outcomes in total hip arthroplasty through 90 Days of follow-up? A systematic review with meta-analysis. J. Arthroplasty 33, 1296–1302. doi:10.1016/j.arth.2017.11.011
Lawrence, D. D., Aamer, M., Manish, D., and Wan, Z. (2009). Combined anteversion technique for total hip arthroplasty. Clin. Orthop. Relat. Res. 467, 119–127. doi:10.1007/s11999-008-0598-4
Lawrence, D. D., and John, J. C. (2019). Death of the lewinnek “safe zone”. J. Arthroplasty 34, 1–2. doi:10.1016/j.arth.2018.10.035
Lei, Y., Long, G., Shengjie, D., Saluja, K., Li, D., Reddy, K. S., et al. (2023). Evaluation of comparative efficacy and safety of surgical approaches for total hip arthroplasty. JAMA Netw. open 6, e2253942. doi:10.1001/jamanetworkopen.2022.53942
Luc, M. F., Zachary, A. R., Antonia, F. C., and Rajaee, S. S. (2020). Activity recommendations after total hip and total knee arthroplasty. J. Bone Jt. Surg. 103, 446–455. doi:10.2106/jbjs.20.00983
Manon, P., Anusha, J., Kee, B. P., and Meara, J. G. (2021). Osteoarthritis in 2020 and beyond. Lancet 397, 1059–1060. doi:10.1016/s0140-6736(21)00208-7
Maria, M., Anna, D. I. L., Johann, H., Hothi, H., and Hart, A. J. (2020). Three-dimensional pre-operative planning of primary hip arthroplasty: a systematic literature review. EFORT open Rev. 5, 845–855. doi:10.1302/2058-5241.5.200046
Matthew, P. A., Christopher, N. C., and Daniel, J. B. (2023). Current practice trends in primary hip and knee arthroplasties among members of the American association of hip and knee surgeons. J. Arthroplasty 38, 1921–1927.e3. doi:10.1016/j.arth.2023.08.005
McMullen, J., di Fagagna, F., Bernardo, B., Anderson, M., and Gililland, J. (2018). Complications and pitfalls of direct anterior approach total hip arthroplasty. Annals Of Joint. 3 (5), doi:10.21037/aoj.2018.04.05
Meneghini, R. M., Mark, W. P., Robert, T. T., and Hozack, W. J. (2006). Muscle damage during MIS total hip arthroplasty. Clin. Orthop. Relat. Res. 453, 293–298. doi:10.1097/01.blo.0000238859.46615.34
Meerman, G., Das, R., Volpin, A., and Haddad, F. S. (201). The direct anterior approach in total hip arthroplasty: a systematic review of the literature. Bone Joint J. 99-B (6), 732–740. doi:10.1302/0301-620x.99b6.38053
Michael, M., Dirk, C., Carsten, P., and Tohtz, S. (2010). The association between the sagittal femoral stem alignment and the resulting femoral head centre in total hip arthroplasty. Int. Orthop. 35, 981–987. doi:10.1007/s00264-010-1047-z
Nicolas, R., Christopher, G. S., Kevin, I. P., Hanssen, A. D., Berry, D. J., and Abdel, M. P. (2019). Mild coronal stem malalignment does not negatively impact survivorship or clinical results in uncemented primary total hip arthroplasties with dual-tapered implants. J. Arthroplasty 34, 1127–1131. doi:10.1016/j.arth.2019.01.055
Purbasari, I., Bayuseno, A., Isnanto, R., Winarni, T., Jamari, J., Tong, P. J., et al. (2023). Artificial Intelligence and Machine Learning in Prediction of Total Hip Arthroplasty Outcome: A Bibliographic Review. E3S Web of Conferences 448. doi:10.1051/e3sconf/202344802054
Sangeux, M., Pascoe, J., Graham, H. K., Ramanauskas, F., Cain, T., et al. (2015). Three-dimensional measurement of femoral neck anteversion and neck shaft angle. J Comput Assist Tomogr 39 (1). 83–85. doi:10.1097/rct.0000000000000161
Sarhan, O., Megalla, M., Imam, N., Ren, A. N., Redfern, R. E., Klein, G. R., et al. (2024). Improved patient reported outcomes with the direct anterior approach versus the posterior approach for total hip arthroplasty in the early post - operative period. Arch Orthop Trauma Surg. 144 (5), 2373–2380. doi:10.1007/s00402-024-05271-z
Shah, N. V., Huddleston, H. P., Wolff, D. T., Newman, J. M., Pivec, R., Naziri, Q., et al. (2022). Does surgical approach for total hip arthroplasty impact infection risk in the obese patient? A systematic review. Orthopedics 45 (2), e67–e72. doi:10.3928/01477447-20211227-03
Tze, E. C., Jason, A. W., Nicholas, F. T., Holden, C. T., Marks, P., Smith, C. L., et al. (2017). A prospective randomized clinical trial in total hip arthroplasty—comparing early results between the direct anterior approach and the posterior approach. J. Arthroplasty 32, 883–890. doi:10.1016/j.arth.2016.08.027
van Oldenrijk, J., Hoogland, P. V., Tuijthof, G. J., Corveleijn, R., Noordenbos, T. W., Schafroth, M. U., et al. (2010). Soft tissue damage after minimally invasive THA. Acta Orthop. 81 (6), 696–702. doi:10.3109/17453674.2010.537804
Wang, K., Zhang, T., Xia, R., Wang, J., Yu, J., and Wang, W. (2024). Efficacy of direct anterior approach versus posterolateral approach in total hip arthroplasty: a systematic review and meta-analysis. Arch Orthop. Trauma. Surg. 144 (9), 4443–4453. doi:10.1007/s00402-024-05547-4
Wu, D., Liu, X., Zhang, Y., Chen, J., Tang, P., and Chai, W. (2020). Research and application of artificial intelligence based three-dimensional preoperative planning system for total hip arthroplasty. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 34 (9), 1077–1084. Chinese. doi:10.7507/1002-1892.202005007
Xuzhuang, D., Bingshi, Z., Wenao, L., Huo, J., Liu, S., Wu, T., et al. (2021). Value of preoperative three-dimensional planning software (AI-HIP) in primary total hip arthroplasty: a retrospective study. J. Int. Med. Res. 49, 3000605211058874. doi:10.1177/03000605211058874
Zeng, W. N., Liu, J. L., Wang, F. Y., Zhang, X., Fan, H. Q., Chen, G. X., et al. (2017). Total hip arthroplasty for patients with Crowe type IV developmental dysplasia of the hip: Ten years results. Int J Surg 42, 17–21. doi:10.1016/j.ijsu.2017.04.029
Zhu, J., Zheng, S., Sun, J., Ma, B., Zhang, C., Zhang, C., et al. (2025). Efficacy of an artificial intelligence preoperative planning system for assisting in revision surgery after artificial total hip arthroplasty. BMC Surg. 25 (1), 58. doi:10.1186/s12893-024-02752-1
Keywords: total hip arthroplasty, combined anteversion angle, femoral anteversion angle, acetabular anteversion angle, direct anterior approach(DAA), posterolateral approach (PLA), three-dimensional reconstruction
Citation: Zheng S, Zhu J, Chen Z, Cao X, Xia T, Zhang C and Shen JR (2025) AI-assisted direct anterior approach versus posterolateral approach in total hip arthroplasty: a retrospective cohort study based on artifact-reduced CT 3D reconstruction. Front. Bioeng. Biotechnol. 13:1509200. doi: 10.3389/fbioe.2025.1509200
Received: 10 October 2024; Accepted: 14 March 2025;
Published: 14 April 2025.
Edited by:
Fabiano Bini, Sapienza University of Rome, ItalyReviewed by:
Abhimanyu Banerjee, Illumina, United StatesAhmed A. Khalifa, South Valley University, Egypt
Jax Luo, Harvard Medical School, United States
Copyright © 2025 Zheng, Zhu, Chen, Cao, Xia, Zhang and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ji Rong Shen, am9pbnQ2NjExOEBzaW5hLmNvbQ==; TianWei Xia, MTI2MzYzODYxMEBxcS5jb20=; Chao Zhang, MTM4MTM4NTgzMTRAMTYzLmNvbQ==
†These authors share first authorship
 ShanBin Zheng
ShanBin Zheng JiaQing Zhu†
JiaQing Zhu† TianWei Xia
TianWei Xia