- Wales Centre of Excellence for Anaerobic Digestion, Sustainable Environment Research Centre, University of South Wales, Pontypridd, United Kingdom
The persistence of fossil fuel-based plastics poses significant environmental challenges, prompting increased research into biodegradable polyhydroxyalkanoate (PHA) polymers derived from cost-effective and sustainable resources. Different microorganisms can produce PHA amongst carbon dioxide (CO2)-assimilating autotrophic organisms, particularly noteworthy in carbon capture and utilization (CCU). Autotrophic bacteria have evolved to utilize either light (photoautotrophy) or inorganic chemicals (chemolithoautotrophy) to capture CO2, which powers their primary and secondary metabolic activities. This review explores the diversity of PHA-producing autotrophs, the metabolic pathways implicated in autotrophic PHA accumulation, and recent progress in photoautotrophs and chemolithoautotrophs regarding PHA synthesis using CO2. Additionally, microbial electrosynthesis for converting CO2 to PHA is also discussed. Genetic engineering strategies are also emphasized for the autotrophic synthesis of PHA. This review also addresses the challenges and prospects for sustainable PHA production using CO2.
1 Introduction
Polyhydroxyalkanoates (PHAs) are a diverse group of microbial polyesters synthesized intracellularly by various microorganisms including bacteria, archaea, cyanobacteria as carbon and energy storage compounds (Rehm, 2010). These polyesters are of significant interest due to their biodegradability, thermoplasticity, and potential as sustainable alternatives to petrochemical-based plastics (Reddy et al., 2003; Możejko-Ciesielska and Kiewisz, 2016; Pandey et al., 2022). PHAs are accumulated as discrete granules within the cytoplasm under imbalanced growth conditions and are mobilized by cells under nutrient-limiting scenarios (Behera et al., 2022).
PHAs are structurally classified based on the number of carbon atoms in their monomer units. The two main categories are short chain-length PHAs (SCL-PHAs), which consist of three to five carbon atoms, and medium chain-length PHAs (MCL-PHAs), comprising six to fourteen carbon atoms (Anjum et al., 2016). SCL-PHAs include well known types such as poly-3-hydroxybutyrate (PHB), poly-3-hydroxyvalerate (PHV), and their copolymeric product poly-3-hydroxybutyrate-co-3-hydroxyvalerate (PHB-co-PHV). MCL-PHAs include polymers like poly-3-hydroxy octanoate (PHO), poly-3-hydroxy hexanoate (PHHx), poly-3-hydroxy decanoate (PHD), poly-3-hydroxy dodecanoate (PHDD), poly-3-hydroxy heptanoate (PHH). To date, over 150 PHA monomeric types have been discovered, underscoring PHAs as the most structurally diverse group of natural polyesters (Muneer et al., 2020).
The biosynthesis of PHA is primarily triggered under conditions of nutrient imbalance typically, an excess carbon sources combined with limitations in essential nutrients such as nitrogen, magnesium, phosphorous, sulphur, and oxygen (Sudesh et al., 2000; Reddy et al., 2003; Passanha et al., 2013; Sathiyanarayanan et al., 2013a). Additionally, environmental stressors such as temperature fluctuations, high osmotic pressure, and extreme pH conditions can induce PHA synthesis (Passanha et al., 2014; Obruca et al., 2020). These environmental triggers exploited in both natural ecosystems and controlled fermentation processes to maximise PHA production yields.
From an industrial perspective, PHAs represent a promising class of biodegradable polymers synthesised from renewable sources including agricultural residues, municipal wastes, and industrial by-products (Jiang et al., 2016). Moreover, PHAs are completely biodegradable and highly biocompatible, making them ideal for various applications (Pandey et al., 2022). In the biomedical sector, PHAs are employed in drug delivery systems, scaffolds for tissue engineering, and resorbable sutures due to their favourable degradation kinetics and non-toxic breakdown products (Gregory et al., 2022). In packaging, PHAs are being increasingly adopted as green alternatives to single-use plastics, offering compostable options for containers (Park et al., 2024). In agriculture, PHA-based films are used in the development of controlled-release fertilizers and biodegradable plant pots (Amelia et al., 2019). Furthermore, PHAs are used in the production of sustainable consumer goods, such as disposable cutlery, shopping bags, and cosmetic containers, contributing significantly to the global initiative against plastic pollution (Pandey et al., 2022).
The commercialisation of PHAs faces several key challenges. Majorly, high production costs, driven by expensive substrates (Sathiyanarayanan et al., 2013b; Li and Wilkins, 2020; Choi et al., 2023) and complex bacterial cultivation processes (i.e., heterotrophic) (Sathiyanarayanan et al., 2013c; Możejko-Ciesielska and Kiewisz, 2016), make PHA less competitive than petrochemical-based plastics. Also, optimising yields and productivity on an industrial scale remains difficult despite advances in metabolic engineering and process optimisation (Akaraonye et al., 2010; Behera et al., 2022). Therefore, the commercial viability of large-scale industrial PHA production depends on developing efficient fermentation processes that use low-cost carbon sources (Choi et al., 2023). Numerous attempts have been made to synthesise PHAs using cost-effective substrates, such as industrial and agricultural wastes, as carbon sources (Li and Wilkins, 2020; Choi et al., 2023; Kedia et al., 2014; Kumi et al., 2016; Tao et al., 2016). However, these production processes often have a significant carbon footprint (Baioli et al., 2019).
The climate and energy plans aim to reduce greenhouse gas (GHG) emissions in Europe by at least 40% below 1990 levels by 2030, with an ambition to further decrease emissions by 80%–95% by 2050 (European commission, 2021). Carbon dioxide (CO2) is the primary GHG released through anthropogenic activities. Currently, CO2 is an abundant resource on Earth and can be utilised to produce carbon-based chemicals (Francisco et al., 2019). Carbon Capture and Utilisation (CCU) technologies employ CO2 as a raw material for the synthesis of fuels, polymers, and building materials through chemical reduction processes (Muthuraj and Mekonnen, 2018; Grignard et al., 2019; Francisco et al., 2019; Liu C. et al., 2015). The biocatalytic reduction processes including gas fermentation and microbial electrosynthesis are also kind of CCU technologies that enables the conversion of C1 gaseous feedstocks (e.g., CO, CO2, CH4, syngas, or biogas) into valuable products by means of microorganisms (Teixeira et al., 2018). The production of bioplastics such as PHAs from C1 gas feedstocks represents a particularly compelling application of CCU. This technology has already achieved a semi-commercial scale, exemplified by microbial production of PHAs from CH4 (Newlight Technologies, 2024)
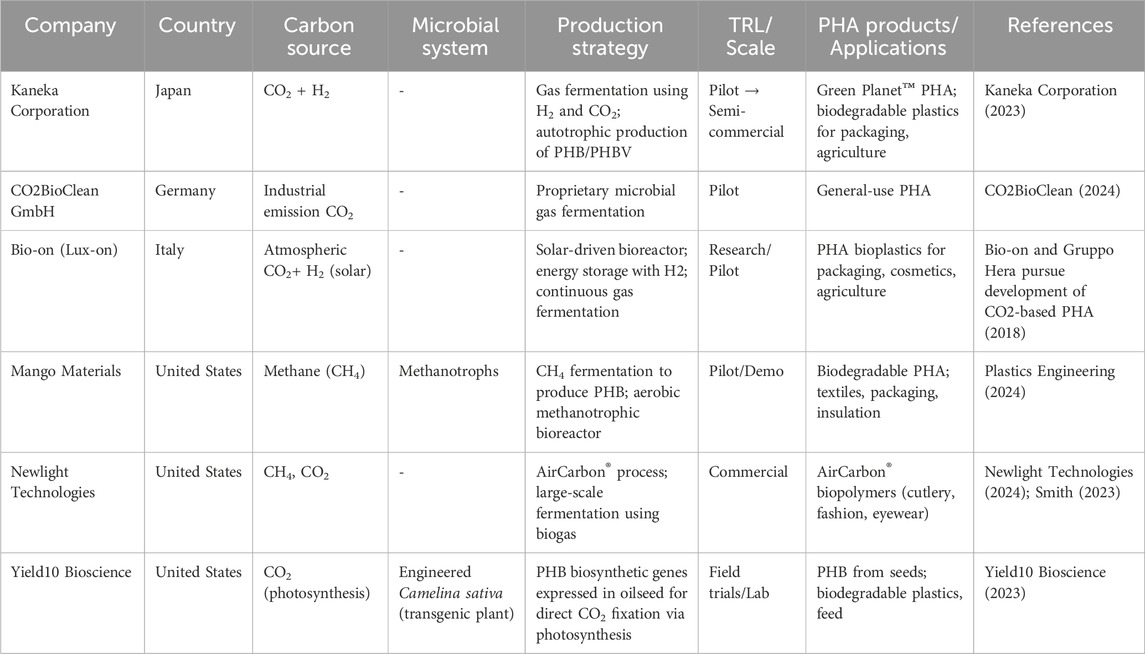
Using C1 gases as feedstocks is likely to result in the production of PHA with a low carbon footprint (Khosravi-Darani et al., 2013a; Azim et al., 2020). This approach offers the added benefits of consistent feed quality and reduced contamination risks compared to substrates derived from organic wastes (Ma et al., 2024). Additionally, certain bacteria, known as autotrophs, have the ability to reduce or fix CO2 into bio-based products, including PHA (Srisawat et al., 2022b). As the most oxidized C1 feedstock, CO2 requires a high energy input to be converted into more reduced chemical products like PHA. This energy can be supplied through light, as utilized by photosynthetic microorganisms (photoautotrophy) (Liebergesell et al., 1991; Carpine et al., 2020) or inorganic compounds such as hydrogen (H2) (chemolithoautotrophy) (Liebergesell et al., 1991) or via more efficient sources of reducing power, such as bio-electrocatalysis (microbial electrosynthesis) (Pepè Sciarria et al., 2018; Banu et al., 2019). Autotrophic metabolisms discussed in this review are illustrated in Figure 1.
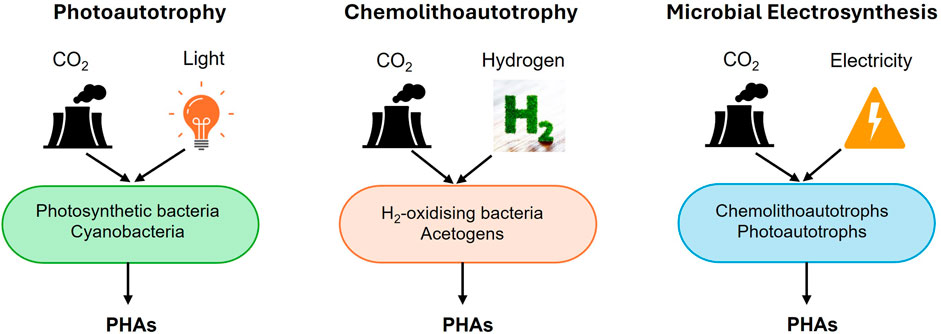
Figure 1. Production of PHAs from CO2 via different autotrophic metabolisms. Autotrophic microorganisms can fix and convert CO2 into cellular biomass and PHAs. This process requires an external energy source to drive CO2 reduction. Depending on the type of autotrophy, energy can be derived from light (photoautotrophy), inorganic electron donors like hydrogen (chemolithoautotrophy), or from an electrode at a poised potential (microbial electrosynthesis). In all cases, CO2 serves as the sole carbon source for both growth and PHA biosynthesis.
Autotrophic PHA synthesis from CO2 is known for its sustainable carbon utilization and energy efficiency compared to heterotrophic synthesis, wherein PHAs are produced from organic substrates. Despite extensive efforts to synthesize PHAs using both heterotrophic and autotrophic microorganisms, the application of autotrophic systems, particularly those relying on CO2 as a carbon source, remains underexplored. This is primarily due to the persistent challenges of achieving efficient production yields under autotrophic conditions. This review briefly discusses the biodiversity of PHA synthesizing autotrophs and autotropic PHA synthesis metabolisms. In addition, photoautotrophs, chemolithoautotrophs, and microbial electrosynthesis are highlighted for the autotrophic synthesis of PHA using CO2 as a substrate. Finally, genetic engineering strategies in developing CO2-fixing autotrophic microbial cell factories for PHA synthesis are also elucidated.
2 Biodiversity of PHA-producing heterotrophs and autotrophs
The earliest discovery of bacterial PHAs, specifically PHB, was documented in 1926 from the Priestia megaterium, previously classified as Bacillus megaterium (Lemoigne, 1926). Since then, various heterotrophic bacterial phyla, including Proteobacteria (α, β, γ, and δ), Firmicutes (Bacilli and Clostridia), Bacteroidetes, Actinobacteria, Deinococcus-Thermus, and Cyanobacteria, have been documented for PHA synthesis (Li and Wilkins, 2020; Behera et al., 2022; Saravanan et al., 2022). Currently, more than 92 bacterial genera are known for PHA synthesis. Most of them were isolated and screened from diverse environmental niches such as soil, freshwater, marine water, polar environments, and hydrothermal vents (Liu et al., 2024). Notable PHA-producing genera are Aeromonas, Alcaligenes, Azotobacter, Burkholderia, Cupriavidus, Chelatococcus, Comamonas, Corynebacterium, Enterobacter, Methylobacterium, Pseudomonas, Rhodobacter, Rhodopseudomonas, Sinorhizobium, and Thermus (Reddy et al., 2003; Li and Wilkins, 2020). SCL-PHAs are synthesized heterotrophically by numerous species, including Cupriavidus necator, Burkholderia cepacia, and Alcaligenes latus. Simultaneously, MCL-PHAs can be synthesized by fluorescent Pseudomonas species, including P. putida, P. oleovorans, and P. corrugate (Reddy et al., 2003; Behera et al., 2022). Some bacteria, including Aeromonas hydrophila (Możejko-Ciesielska et al., 2019) and Thiococcus pfennigii (Liebergesell et al., 2000), synthesize both SCL- and MCL-PHAs copolymers. In the Archaea domain, only haloarchaeal genera are known to produce PHAs, mainly Haloferax, Haloarcula, Halorubrum, Halobacterium, Haloterrigena, Halococcus, Haloquadratum, Natronobacterium, Natrialba, and Natronococcus (Koller and Rittmann, 2022). Among haloarchaea, Haloferax mediterranei is particularly noteworthy for its capability to synthesize substantial quantities of PHB-co-PHV copolymer (Poli et al., 2011; Koller and Rittmann, 2022). In recent decades, wild-type bacterial strains (i.e., Escherichia coli, P. putida, and C. necator) were also genetically/metabolically engineered for the commercial production of PHAs under heterotrophic cultivation (Li and Wilkins, 2020; Saravanan et al., 2022; Wang et al., 2023).
This review surveyed the vast diversity of PHA-synthesizing CO2-fixing autotrophic microorganisms (Figures 2, 3), and their phylogenetic tree generation methods have been emphasized in the supplementary material as supplementary data. Microbial biodiversity information is highly crucial for understanding PHA biosynthetic pathways and developing efficient PHA-producing autotrophic microbial cell factories. In the realm of CO2-fixing autotrophs, both photoautotrophic and chemolithoautotrophic group of microorganisms are prominent producers of PHAs (Srisawat et al., 2022b). Photoautotrophs can be classified into oxygenic (e.g., cyanobacteria) and anoxygenic bacteria (e.g., purple non-sulphur bacteria, PNSB). Within cyanobacteria, Anabaena sp. PCC 7120, Synechococcus elongatus PCC 7942, Synechococcus sp. PCC 7002 and Synechocystis sp. PCC 6803, and regarded as model organisms for photoautotrophic PHA production (Troschl et al., 2017a; Srisawat et al., 2022b; Ray et al., 2023). Anoxygenic PNSB, for example, freshwater Rhodobacter sphaeroides (Liebergesell et al., 1991; Schmid et al., 2021; Li et al., 2023), Rhodopseudomonas palustris (Ranaivoarisoa et al., 2019; Li et al., 2022), Rhodospirillum rubrum (Liebergesell et al., 1991; Revelles et al., 2016), Rhodobacter capsulatus (Liebergesell et al., 1991), Rhodomicrobium vannielii (Conners et al., 2023) and marine water Rhodovulum sulfidophilum (Higuchi-Takeuchi et al., 2016a; 2016b), have shown significant potential for PHA production, contributing to synthesizing biopolymers like PHB and other value-added chemicals. Chemolithoautotrophs, in contrast, utilize inorganic energy sources (i.e., H2, Fe2+, NH3, H2S) instead of light. Notable examples are C. necator (Tanaka et al., 1995; Volova et al., 2013) and Ideonella sp. O-1 (Tanaka et al., 2011) can grow on a gas mixture of CO2, H2, and O2, producing PHB very effectively. In addition to the above two groups, there are acetogens can fix CO2 to produce various bioproducts (Debabov, 2021; Flaiz and Sousa, 2024). Key acetogenic mixotrophs capable of fixing CO2 to produce biomolecules include Acetobacterium woodii, Butyribacterium methylotrophicum, Blautia producta, Clostridium aceticum, C. autoethanogenum, Clostridium ljungdahlii, and C. carboxidivorans (Salehizadeh et al., 2020). Acetogens are unable to synthesise PHAs naturally due to absence of PHA biosynthetic genes. However, genetically engineered C. coskatii has been shown to synthesize PHA via autotrophic CO2 reduction (Flüchter et al., 2019).
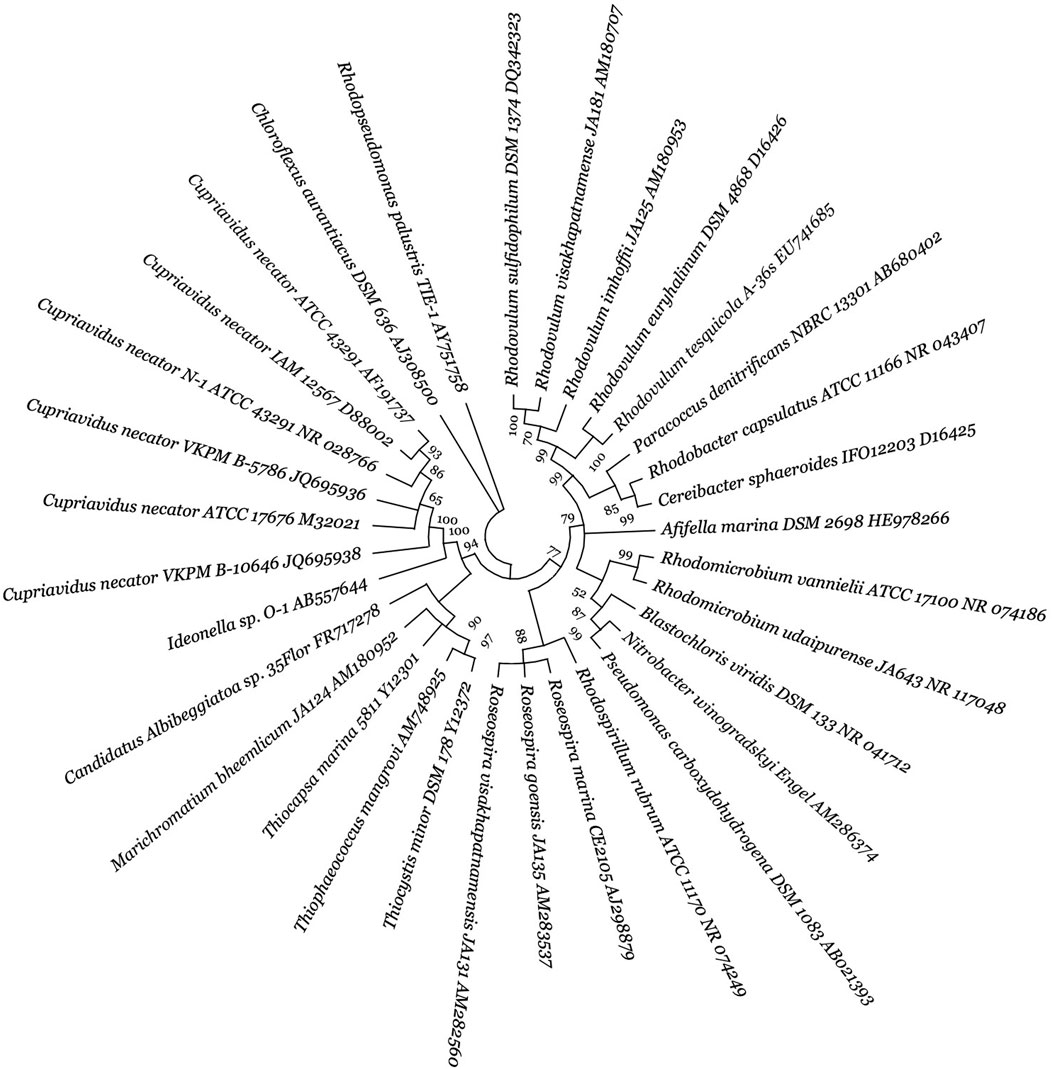
Figure 2. Diversity of autotrophic PHA-producing photosynthetic and chemolithotrophic bacteria. The figure presents a molecular phylogenetic analysis of 16S rRNA gene sequences using the neighbour-joining method for selected bacteria. For detailed methodology, refer to Supplementary data. The proportion of trees (≥70%) where the accompanying taxa clustered is indicated next to the branch nodes, based on 1,000 iterations. The strain number and GenBank accession numbers are properly designated for each microorganism.
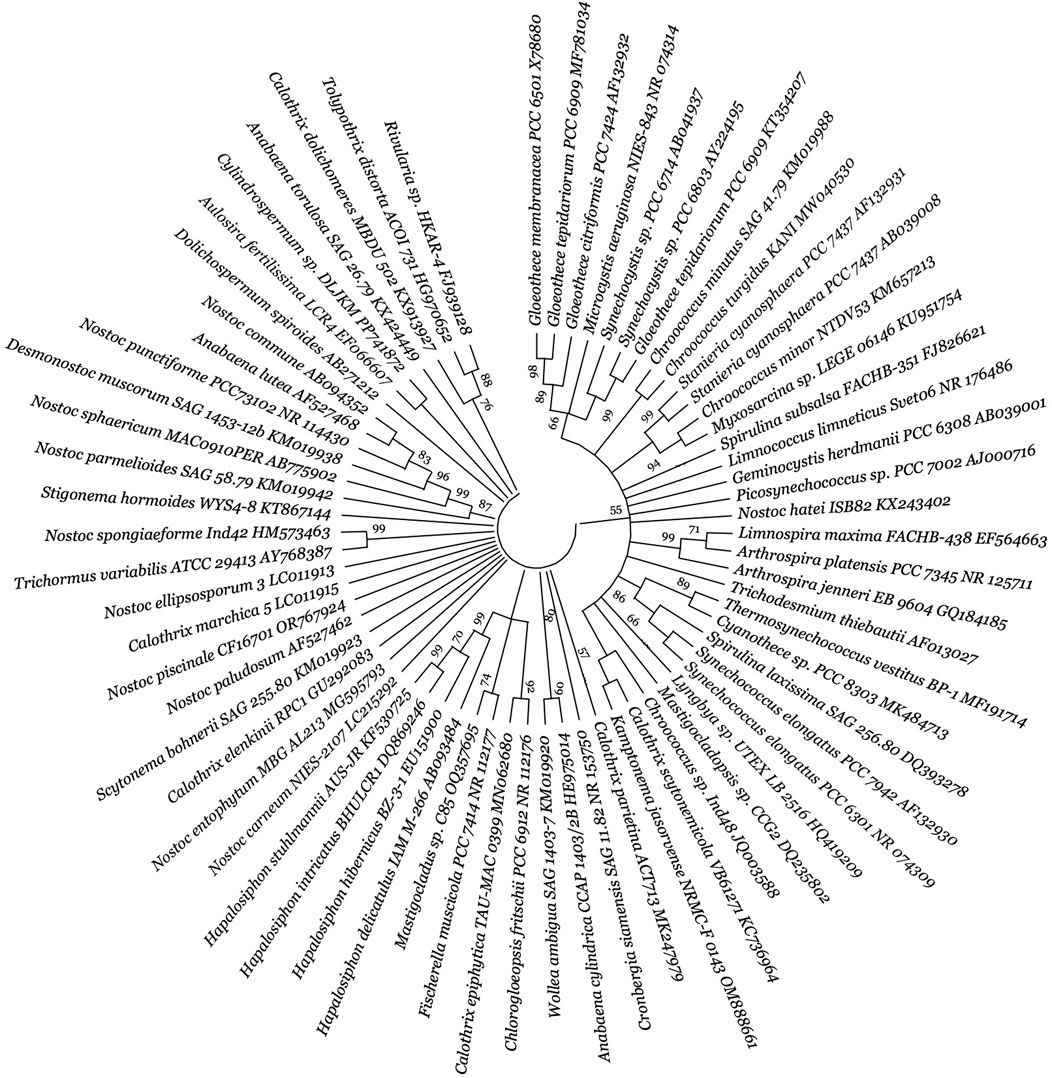
Figure 3. Diversity of autotrophic PHA-producing cyanobacteria. The figure presents a molecular phylogenetic analysis of 16S rRNA gene sequences using the neighbour-joining method for selected bacteria. For detailed methodology, refer to Supplementary data. The proportion of trees (≥70%) where the accompanying taxa clustered is indicated next to the branch nodes, based on 1,000 iterations. The strain number and GenBank accession numbers are properly designated for each microorganism.
3 Autotrophic PHA synthesis metabolisms
Extensive genomic and metabolic research has significantly enhanced our understanding of PHA biosynthesis and degradation. Understanding heterotrophic PHA synthesis is essential before exploring autotrophic pathways because it provides the foundational knowledge of the core biosynthetic enzymes, regulatory mechanisms, and metabolic fluxes (Chen and Jiang, 2018). This baseline is crucial, as autotrophic systems often rely on the heterologous expression of these genes (Kourmentza et al., 2017). Moreover, it allows for comparative evaluation of yields, substrate utilization, and process efficiency in engineered autotrophic platforms. The heterotrophic bacterial synthesis of PHA requires two primary stages: the generation of hydroxyacyl-CoA and its subsequent polymerization into PHA (Sudesh et al., 2000; Reddy et al., 2003). In the initial stage, three principal metabolic pathways facilitate PHA production: acetoacetyl-CoA generation, de novo lipogenesis, and β-oxidation (Haddadi et al., 2019). Acyl-CoA and acetyl-CoA are predominant intermediates across these metabolic pathways (Muneer et al., 2020) and regulate PHA production (Luengo et al., 2003). Depending on carbon sources, heterotrophic bacteria can synthesize PHA using different metabolic pathways. The acetoacetyl-CoA generation and de novo lipogenesis pathways execute the PHA synthesis when the medium is amended with a sugar substrate. In contrast, the fatty acid β-oxidation pathway significantly contributes to PHA production when fatty acids are the primary carbon source. All these pathways lead to the polymerization reaction catalysed by the enzyme PHA synthase.
Autotrophic PHA synthesis mainly relies on CO2 fixation in microorganisms. Until now, six distinct pathways have been recognized for microbial CO2 fixation such as reductive pentose phosphate cycle/Calvin-Benson-Bassham (CBB) pathway, Wood-Ljungdahl (WL) pathway, reductive tricarboxylic acid (R-TCA) pathway, 3-Hydroxypropionate pathway (3-HP/malyl-CoA), 3-Hydroxypropionate/4-Hydroxybutyrate (3HP-4HB) pathway, and dicarboxylate/4-Hydroxybutyrate (DC/4-HB) pathway (Figure 4). Mostly, cultivation conditions determine CO2 fixation pathways in microorganisms. Aerobic conditions enable 3HP-4HB, 3-HP/malyl-CoA, and CBB pathways, but anaerobic nature activates WL, DC/4HB, and R-TCA pathways. The other four pathways share similarities except for the WL and the DC/4HB pathways. The WL pathway is a linear carbon fixation mechanism that converts CO2 into acetyl-CoA and is predominantly found in acetogenic bacteria.
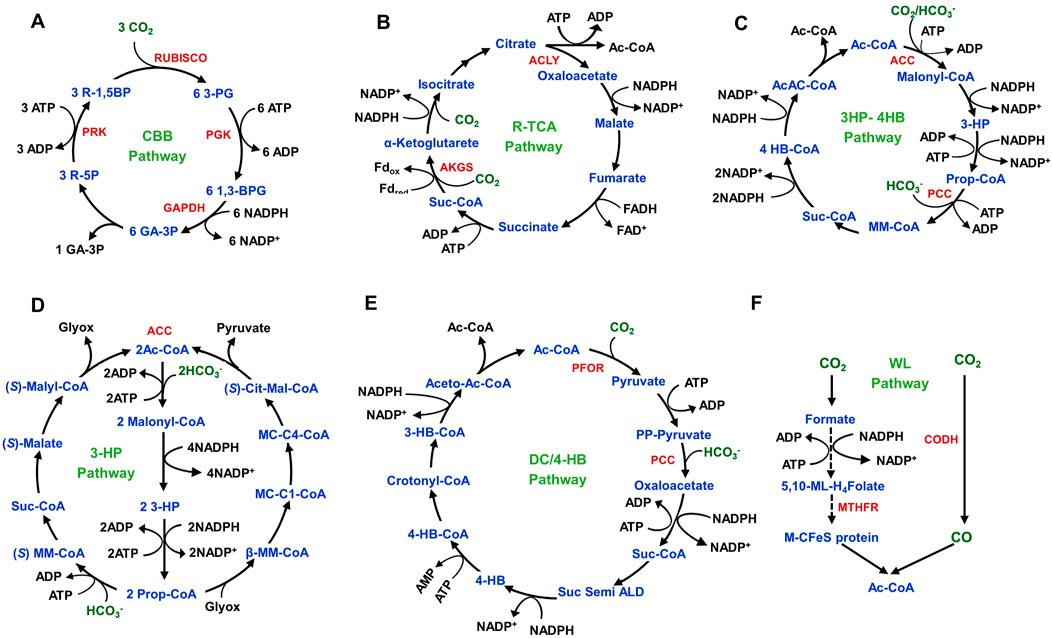
Figure 4. CO2-fixation metabolic pathways in autotrophic microorganisms. (A) Calvin‒Benson‒Bassham (CBB) pathway in cyanobacteria, algae, and proteobacteria. (B) Reductive TCA pathway in proteobacteria, green sulphur bacteria and Aquificae bacteria (C) 3-Hydroxypropionate/4-hydroxybutyrate (3HP-4HB) pathway in aerobic crenarchaeota. (D) 3-Hydroxypropionate (3-HP) pathway in green non-sulphur bacteria. (E) Dicarboxylate/4-hydroxybutyrate (DC/4-HB) pathway in anaerobic crenarchaeota. (F) Wood–Ljungdahl (WL) pathway in proteobacteria, spirochetes, planctomycetes and Euruarchaeota. The solid line represents a single reaction. The dashed line represents multiple reactions. The abbreviations of the metabolites (blue colour) are as follows: 3-PG, 3-Phosphoglycerate; 1,3-BPG: 1,3-Bisphophoglycerate; GA-3P: Glyceraldehyde-3-Phosphate; R-5P: Ribulose-5-Phosphate; R-1,5BP: Ribulose-1,5-Bisphosphate; Ac-CoA: Acetyl-CoA; 3-HP, 3-Hydroxypropionic acid; Prop-CoA, Propionyl-CoA; MM-CoA: Methylmalonyl-CoA; suc-CoA, Succinyl-CoA; 4 HB-CoA, 4-hydroxybutyryl-CoA; AcAc-CoA: Acetoacetyl-CoA; (S)-Cit-Mal-CoA: Citramalyl-CoA; MC-C4-CoA, Mesancolyl-C4-CoA; MC-C1-CoA, Mesancolyl-C1-CoA; β-MM-CoA: β-Methylmalyl-CoA; PP-Pyruvate, Phosphoenolpyruvate; Suc Semi ALD, Succinate Semialdehyde; 4-HB, 4-Hydroxybutyrate; 3-HB-CoA, 3-Hydroxybutyryl-CoA; Aceto-Ac-CoA, Acetoacetyl-CoA; 5,10-ML-H4Folate, 5,10-Methenyl-H4Folate; M-CFeS protein, Methyl-corrinoid iron-sulphur protein. The abbreviations of the metabolic enzymes (red colour) are as follows: RuBisCO, ribulose-1,5-bisphosphate carboxylase/oxygenase; PGK, phosphoglycerate kinase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; PRK, phosphoribulokinase; ACLY, ATP-citrate lyase; AKGS, α-ketoglutarate synthase; ACC, acetyl-CoA carboxylase; PCC, pyruvate carboxylase; PFOR, pyruvate ferredoxin oxidoreductase; CODH, carbon monoxide dehydrogenase; MTHFR, methylenetetrahydrofolate reductase.
Photoautotrophs, comprising both oxygenic and anoxygenic types, utilize light energy to produce ATP (Claassens et al., 2016). Oxygenic photoautotrophs, such as cyanobacteria, produce reducing power and create a proton gradient essential for ATP synthesis through the action of photosystem I and II complexes, which split water molecules and release oxygen. The light-dependent electron transport chain supplies the energy and reduces equivalents required for CO2 fixation via the CBB pathway, enabling the autotrophic synthesis of essential cellular components and various metabolites (Kanno et al., 2017). Anoxygenic photolithoautotrophs, such as PNSB, possess a single photosystem incapable of splitting water. Instead, they rely on organic and inorganic compounds such as H2 and sulphur compounds as electron donors to produce reducing power for light-driven CO2 fixation through the CBB pathway (Inui et al., 1998). PNSB also has a highly flexible metabolism, which allows them to thrive under aerobic and anaerobic conditions and exhibit both autotropic and heterotrophic growth. Their competence to adapt to extreme environments makes them model organisms for producing PHAs. The facultative chemolithoautotrophic bacterium C. necator can also fix CO2 via the CBB pathway while utilizing H2 as its exclusive energy source, even in the presence of O2 (Morlino et al., 2023). Therefore, the CBB pathway is predominantly present in most autotrophs, including cyanobacteria, algae, photoautotrophic, and chemolithoautotrophic bacteria. Thus, further exploring the CBB pathway is essential to understand autotrophic PHA synthesis.
In cyanobacteria, de nova lipogenesis (DNL) and nitrogen utilization are suggested mechanisms for producing PHAs (Troschl et al., 2017a; Carpine et al., 2020). Cyanobacteria can perform oxygenic photosynthesis through the CBB pathway, which generates ATP and NADPH to energise cellular activities. The cyanobacterial CBB pathway comprises three stages: the carboxylation of Ribulose-1,5-bisphosphate (RuBP), reduction of 3-phosphoglycerate (PGA), and regeneration of RuBP. In the carboxylation phase, three molecules of CO2 are fixed with six molecules of RuBP, forming six molecules of 3-phosphoglycerate (3-PGA). During the subsequent reduction stage, ATP and NADPH are utilized to transform 3-PGA into triose phosphate and dihydroxyacetone phosphate (DHAP). Finally, in the regeneration stage, five molecules of 3-PGA are used to regenerate three molecules of RuBP. Key enzymes involved in the CBB pathway are ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO), phosphoribulokinase (PrkA), and sedoheptulose bisphosphatase (SBPase) (Kumar M. et al., 2018). Cyanobacteria can assimilate CO2 and bicarbonate (HCO3−) through carbon dioxide-concentrating mechanisms (CCMs). This carbon assimilation involves five different transport systems. BicA, SbtA, and BCT1 enzymes facilitate the HCO3− transport, while NDH-I3 and NDH-I4 enable CO2 assimilation. The transport of CO2 takes place via CO2 transporters situated in the plasma membrane, while HCO3− transporters accelerate the translocation of intracellular HCO3− across the plasma membrane. Additionally, periplasmic carbonic anhydrase induces the transformation of HCO3− to CO2 (Durall and Lindblad, 2015). Depending on nutrient availability, the excess 3-PGA is channelled into synthesizing cellular materials. In nutrient-limited conditions, 3-PGA is diverted to synthesize PHAs. This process involves several key enzymes: β-keto thiolase (phaA), which catalyses the conversion of acetyl-CoA to acetoacetyl-CoA; acetoacetyl-CoA reductase (phaB), which condenses acetoacetyl-CoA to 3-hydroxy butyryl-CoA; and PHA synthase (phaC), which polymerizes 3-hydroxy butyryl-CoA into PHB (Gaspar et al., 2000). A simplified PHB synthesis metabolic route from the CO2 is illustrated in Figure 5.
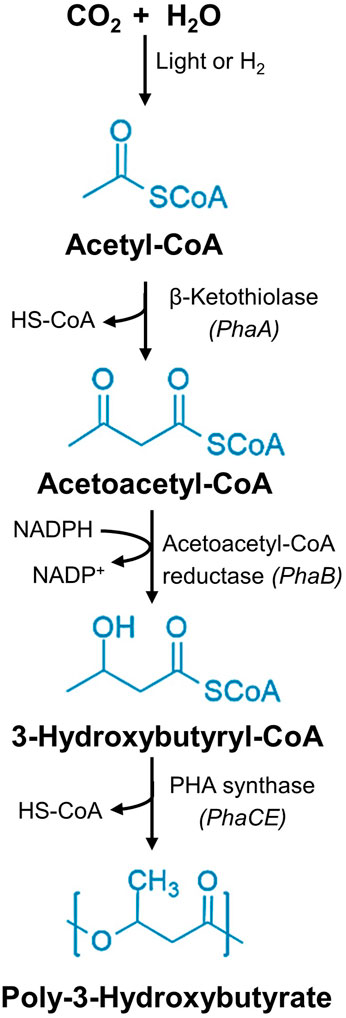
Figure 5. PHB synthesis route. Common metabolic pathway of the photoautotrophic (Cyanobacteria) and chemolithoautotrophic bacteria (C. necator) for the biosynthesis of PHB utilising CO2 as the primary carbon source.
Importantly, PHA synthase can integrate various hydroxy acid monomers into PHAs. This enzyme is categorized into four distinct classes: Class I (PhaC) found in C. necator, Class II (PhaC) present in P. oleovorans, Class III (comprising PhaC and PhaE subunits) found in Allochromatium vinosum and Thiocapsa pfennigii, and Class IV (consisting of PhaC and PhaR subunits) identified in B. megaterium (Luengo et al., 2003; Behera et al., 2022; Gregory et al., 2022). Cyanobacteria exclusively contain Class III PHA synthase (Carpine et al., 2020). Their genetic organization differs from other bacteria; in contrast to the single operon containing all four genes found in different bacterial species, cyanobacteria have two distinct operons. In the first operon, the phaA and phaB genes are co-expressed, while the phaE and phaC genes are in the second operon (Troschl et al., 2017a; Carpine et al., 2020).
In C. necator, the CBB pathway consists of 11 stages, where the RuBisCO enzyme primarily executes the CO2 fixation (Li et al., 2020). All enzymes necessary for CO2 fixation are encoded within the cbb operon, which appears in two copies in C. necator (Panich et al., 2021). Both copies of this operon are crucial for autotrophic growth. Additionally, the CBB pathway in C. necator demands significant energy input, requiring a net total of 7 mol of ATP to convert 3 mol of CO2 into 1 mol of pyruvate (Panich et al., 2021). Furthermore, RuBisCO in C. necator operates relatively slowly as a carboxylase and exhibits oxygenase activity, producing a toxic molecule called 2-phosphoglycolate (2-PG). This compound is not essentially required for further CBB mechanisms. Hence, it must be eliminated through a process known as 'phosphoglycolate salvage’ (Li et al., 2020; Panich et al., 2021). Most of the photoautotrophs execute the CCMs to balance the weak performance of the RuBisCO, whereas C. necator lacks typical CCM features. Instead, it captures CO2 using four metalloproteins (carbonic anhydrase), which help to accumulate adequate HCO3− in the cytoplasm, which activates the RuBisCO enzyme to perform the CO2 fixation. C. necator can also produce an alternate RuBisCO-like enzyme with a high affinity to CO2 (465 nmol/min/mg) and a median rate of ∼2.5 s-1 (Li et al., 2020; Panich et al., 2021). All these enzyme systems help the C. necator to fix CO2 effectively to synthesize PHA.
4 Photoautotrophic synthesis of PHA from CO2
Photoautotrophs are organisms that perform photosynthesis. In the natural environment, they capture the light energy from sunlight to transform CO2 and water into organic compounds, which are then utilized for cellular processes such as biosynthesis and respiration. Photoautotrophic microorganisms include anoxygenic photosynthetic bacteria, cyanobacteria, and microalgae, and all these microorganisms are known to accumulate PHA (Liebergesell et al., 1991; Troschl et al., 2017a; Costa et al., 2019). In this review, particular emphasis has been given to procaryotic microorganisms such as anoxygenic photosynthetic bacteria and cyanobacteria as the autotrophic microbial cell factories for PHA production. Hence, eucaryotic microalgae are excluded.
4.1 Anoxygenic photosynthetic bacteria
Anoxygenic photosynthetic bacteria are classified into four clusters depending on their pigments and electron donors: green sulphur, green non-sulphur, purple sulphur, and purple non-sulphur bacteria. These bacteria obtain electrons from organic compounds, sulphur, and H2. Most anoxygenic phototrophs can function as either photoautotrophs or photoheterotrophs in the presence of light. At the same time, some species can grow as chemoheterotrophs in the absence of light. This chemoheterotrophic nature has facilitated the exploration of these bacteria for various applications, including industrial wastewater purification and H2 production. A list of autotrophic PHA production studies on anoxygenic photosynthetic bacteria is listed in Table 1.
R. rubrum has been extensively studied for its competence in transforming syngas (a gas mixture of CO2, H2, CO, N2) into PHAs under photoautotrophic anaerobic conditions (Do et al., 2007; Revelles et al., 2016). R. rubrum can assimilate CO effectively as a sole carbon and energy source, wherein CO exposure induces a set of enzymes such as carbon monoxide dehydrogenase (CODH) and CO-tolerant hydrogenase, which further catalyse the oxidative conversion of CO to CO2 and H2, respectively. The resulting CO2 can be fixed by the CBB pathway for biomass production and subsequent PHA synthesis (Do et al., 2007). Moreover, when the syngas fermentation media was amended with acetate as an additional substrate, R. rubrum was shown to produce PHH up to 20% and 28% of its dry biomass under photoheterotrophic (light) and chemoheterotrophic (dark) settings, respectively (Revelles et al., 2016). In addition, R. rubrum had synthesized up to 16% of PHB from syngas derived from municipal solid waste when the process was amended with 10 mM acetate as a co-substrate (Revelles et al., 2017). In another study, diluted syngas and acetate combination also enhanced the PHB synthesis by up to 30% with a titre of 1.6 g/L under carbon and phosphorus limitation (Karmann et al., 2019).
Recently, a versatile nitrogen-fixing PNSB genus Rhodomicrobium was also identified as a PHA producer. Rhodomicrobium vannielii and Rhodomicrobium udaipurense have been shown to produce PHA under photoautotrophic (CO2) and photoheterotrophic (sodium butyrate) cultivation with either NH4Cl or N2 gas as nitrogen sources (Conners et al., 2024). During photoautotrophic cultivation with CO2, two different electron donors such as H2 (photohydrogenotrophy) and Fe2+ (photoferrotrophy), were used as an energy source. Photoferrotrophic growth resulted in a higher PHA synthesis in both species (4.64%–47.03% cdwprot) than photohydrogenotrophic growth (1.10%–6.19% cdwprot), where NH4Cl as a nitrogen source. N2-fixation promotes the PHA synthesis in photoheterotrophic growth but inhibits during the photoautotrophic condition in both species (Conners et al., 2024). A similar set of experiments was conducted on R. palustris TIE-1 (Ranaivoarisoa et al., 2019), where NH4Cl as a nitrogen source has produced higher PHB (7.23%, PHB carbon yield) in photo hydrogenotrophic growth than photoferrotrophic growth (5.77%, PHB carbon yield), whereas N2 fixing condition had shown a lower PHB yield (<3%, PHB carbon yield) in both photo hydrogenotrophic and photoferrotrophic growth under photoautotrophic condition (Ranaivoarisoa et al., 2019). In Rhodomicrobium and Rhodopseudomonas, N2-fixation does not effectively support the PHB synthesis under photoautotrophic CO2 reduction. Further, extensive studies may open new avenues for developing Rhodomicrobium and Rhodopseudomonas species as promising photoautotrophic platforms for PHA production.
Marine phototrophic bacteria are also considered excellent model organisms for the sustainable production of various products. They offer several benefits, including metabolic adaptability and tolerance to high salinity, which can help to develop low-cost, non-axenic fermentation processes (Higuchi-Takeuchi and Numata, 2019). Marine purple sulphur and PNSB have been explored for the synthesis of PHA under photoautotrophic conditions, where 1% sodium bicarbonate (NaHCO3, an inorganic source of CO2) is supplemented as a sole carbon source (Higuchi-Takeuchi et al., 2016a; 2016b). Among the species tested, very few PNSB (i.e., R. sulfidophilum, R. imhoffii, R. euryhalinum, and R. visakhapatnamense) were only able to synthesize PHA (up to <5%) under nitrogen-limited photoautotrophic conditions. Their biomass production was also lower than the photoheterotrophic condition (Higuchi-Takeuchi et al., 2016b). It was assumed that the low PHA production was due to the fluctuations in the cellular redox state and lower concentrations of NaHCO3 in the growth medium.
Photoautotrophic PHA synthesis in PNSB remains challenging since photoheterotrophic carbon assimilation pathways are less complex than photosynthetic carbon-fixation pathways. To overcome these hurdles, engineered nano-gel particles have been recently suggested to enhance the assimilation of NaHCO3 by R. sulfidophilum for the photoautotrophic synthesis of PHA (Srisawat et al., 2022a). Extensive screening of engineered anionic nano gel particles against the R. sulfidophilum biomass and PHA synthesis has increased up to 157-fold than control conditions without gel particles. Effective assimilation and subsequent incorporation of HCO3− in the autotrophic PHA synthesis confirmed by 13C tracing with gas chromatography-mass spectral analysis (Srisawat et al., 2022a). Therefore, engineered nanogel applications in different species of photosynthetic bacteria may expand our knowledge and efficiency of autotrophic PHA synthesis. Another interesting autotrophic PHA synthesis was identified in the green sulphur bacterium Chloroflexus aurantiacus while performing 13C isotope analysis (van der Meer et al., 2001). This bacterium had been shown to synthesize PHB, PHV, and PHB-co-PHV copolymers under photoautotrophic cultivation, where H2/CO2 (80:20) was fed continuously at 26 mg of carbon supplied/min (van der Meer et al., 2001). This bacterium is thought to fix CO2 using the 3-HP pathway (Strauss and Fuchs, 1993).
4.2 Cyanobacteria
Cyanobacteria (blue-green algae) are promising photoautotrophic hosts that produce various bioproducts, including organic acids, alcohols, fatty acids, bioplastics precursors, and biofuels (Bühler and Lindberg, 2023). As discussed earlier, cyanobacteria can flourish well with the help of CO2 fixation from the atmosphere by the CBB pathway. Some cyanobacterial species can tolerate even high concentrations of CO2 (i.e., Chlorella pyrenoidosa, C. vulgaris, Scenedesmus obliquus, Thermosynechococcus elongatus, and Rhodovulum viride). Their CO2 fixation ability mainly depends on the physical parameters, including pH, temperature, light intensity, cultivation mode, and type of bioreactors (Salehizadeh et al., 2020; Ray et al., 2023). During the nitrogen and phosphorous limitation in the growth environment, these photoautotrophs can synthesize a range of intracellular polymers, including glycogen, PHAs, cyanophycin, and polyphosphate (Bühler and Lindberg, 2023). Among these, glycogen and PHAs are carbon-rich energy storage biopolymers; more specifically, glycogen metabolism is conserved in all cyanobacteria. Glycogen biosynthesis is vital in maintaining cellular homeostasis and protecting against environmental stresses. At the same time, PHAs serve as long-term carbon reserves and contribute to managing environmental stress conditions (Bühler and Lindberg, 2023).
Cyanobacterial PHA occurrence was first documented in Chlorogloea fritschii under mixotrophic conditions (autotrophic and heterotrophic), where NaHCO3 and acetate were used together in the growth medium. (Carr, 1966). The predominant PHA producers are Anabaena, Aphanocapsa, Arthrospira, Calothrix, Chrococcus, Gleocapsa, Lyngbya, Mychrochaete, Nostoc, Phormidium, Synechocystis, Synechococcus, Spirulina, and Scytonema (Troschl et al., 2017a; Costa et al., 2018; Carpine et al., 2020; Bühler and Lindberg, 2023). All these genera accumulate only PHB while growing on NaHCO3 or CO2 (Costa et al., 2018). A detailed list of cyanobacteria that produce PHA under autotrophic growth is presented in Table 2. Photoheterotrophic PHB synthesis was also confirmed in Spirulina LEB18, wherein sodium acetate and glucose were mainly used as carbon sources. However, photoautotrophic media with NaHCO3 showed the highest PHB yield of about 44% compared to photoheterotrophic and mixotrophic conditions (Martins et al., 2014). Under nitrogen and phosphorous-limited conditions, wild-type Synechocystis sp. PCC 6714 has produced 16.4% of PHB (in dry cell weight) from CO2 (Kamravamanesh et al., 2017). Similarly, continuous aeration and CO2 addition have increased the PHB level to 21.5% in Nostoc muscorum under a phosphate-starved medium (Haase et al., 2012). Filamentous cyanobacterium Arthrospira subsalsa can produce up to 14.7% PHB from CO2 under high alkaline conditions (5% NaCl) (Shrivastav et al., 2010). In contrast, thermophilic cyanobacterium Synechococcus MA19 was reported to synthesize 55% of PHB while growing in a phosphate-limited autotrophic medium (Nishioka et al., 2001). However, most non-thermophilic cyanobacteria can synthesize only 2%–20% of PHB in the presence of CO2 (Troschl et al., 2017a; Costa et al., 2018). Transmission electron microscopic images clearly show that the autotropic synthesis of PHB from the wild-type Synechocystis sp. PCC 6803 using CO2 (0.03%–3%) as a sole carbon source (Damrow et al., 2016) (Figure 6A). The volumetric productivity of PHB (g/L) under photoautotrophic cultivation was not reported precisely in any of these studies except in Synechocystis sp. PCC 6803 with 16–27 mg/L (Monshupanee and Incharoensakdi, 2014) and Caltorix scytonemicola TISTR 8095 up to 356.5 mg/L of PHB (Kaewbai-ngam et al., 2016), respectively. So far, the C. scytonemicola TISTR 8095 strain has only shown a higher PHB yield under photoautotrophic cultivation. More interestingly, C. scytonemicola is thought to produce PHB by nitrogen fixation and CO2 reduction. However, this productivity is comparatively less than the commercial autotrophic PHA producer C. necator, which can produce up to 61 g/L of PHA using CO2 as a carbon source (Salehizadeh et al., 2020; Panich et al., 2021).
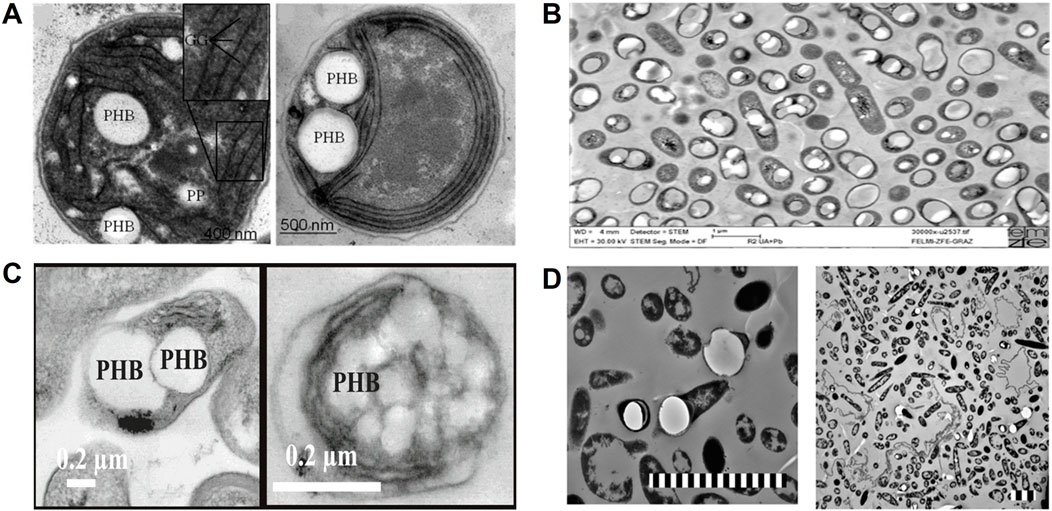
Figure 6. Transmission electron microscopy (TEM) images of autotrophic microorganisms involved in PHB production. (A) Photoautotrophic Synechocystis sp. PCC 6803 synthesizing PHB using 0.03%–3% v/v CO2; GG: glycogen granule, PP: polyphosphate (Damrow et al., 2016). (B) Chemolithoautotrophic C. necator DSM 545 displaying intracellular PHA granules (bright inclusions), imaged at ×30,000 magnification; scale bar: 1 μm (Koller, 2017). (C) PHB synthesis by R. palustris TIE-1 under photoautotrophic conditions using N2/CO2 (80%/20%) and +100 mV vs standard hydrogen electrode (SHE) via microbial electrosynthesis. TEM images (scale bar: 0.2 μm) show cells grown under photoferroautotrophic conditions with Fe(II) and photoelectroautotrophic conditions with a poised electrode, respectively (Ranaivoarisoa et al., 2019). (D) Genetically modified acetogen C. coskatii [p83_PHB_Scaceti] cultivated on syngas, showing PHB granules; scale bar: 3 μm Copyright permission was obtained from the publisher to reproduce this image (Flüchter et al., 2019).
Adding carbon sources like glucose, fructose, acetate, propionate, and valerate into the growth medium can achieve higher PHB content. Such photoheterotrophic condition immensely increased the PHA synthesis in Nostoc Muscorum Agardh (Bhati and Mallick, 2015) and Aulosira fertilissima (Samantaray and Mallick, 2012) up to 78% (PHB-co-PHV) and 85% (PHB) with volumetric productivity of 0.438 and 1.59 g/L, respectively. Introducing organic substrates causes a metabolic shift from autotrophic to heterotrophic growth. PHA copolymer synthesis under autotrophic conditions is not a feature of cyanobacteria. However, Anabaena spiroides TISTR 8075 was found to synthesize the PHB-co-PHV copolymer using CO2 as the sole substrate (Tarawat et al., 2020). The same strain has also produced PHB-co-PHV copolymer under mixotrophic cultivation, where acetate, propionate, and valerate were supplemented with CO2 (Tarawat et al., 2020). Like this, Oscillatoria okeni TISTR 8549 was found to synthesize 9%–14% PHB-co-PHV in their dry cell weight with 4.3–5.5 mol% of HV incorporation under nitrogen-limited photoautotrophic conditions (Taepucharoen et al., 2017). Photo-mixotrophic production of PHA from cyanobacteria is presented in the Supplementary Table S1. Nevertheless, photoheterotrophic growth did not improve the PHB-co-PHV accumulation, which suggests that PHA synthesis in cyanobacteria is strain-specific rather than the type of carbon source used (Taepucharoen et al., 2017).
The feast and famine strategies were also suggested for selecting autotrophic cyanobacterial mixed microbial culture (MMC) for PHB synthesis. The MMC was shown to produce PHB when the sequencing batch reactor (SBR) was entirely void of nitrogen (Arias et al., 2018a). Recent studies have intensified the autotrophic PHA synthesis in pilot-scale closed bioreactor (30L) using MMC mainly composed of cyanobacterial (abundance 60%–70%) species such as Aphanocapsa sp. and Chroococcidiopsis sp. The cyanobacterial MMC had undergone nitrogen and phosphorus limitation, resulting in 50 and 104 mg/L of PHB on photoautotrophic cultivation’s ninth and eighth day, respectively (Arias et al., 2018b). Most fermentation experiments were conducted in sterile laboratory environments, with very few reports on pilot-scale production of PHB under non-axenic settings. Austrian researchers have developed a 200-L photobioreactor (tubular) and cultured the Synechocystis sp. CCALA192 using CO2 under non-axenic conditions for over 75 days, with different growth cycles. After 16–20 days, Synechocystis sp. CCALA192 produced 1.0 g/L of biomass with 12.5% of PHB (Troschl et al., 2018). Similarly, another Austrian power company (Energie-Versorgung Niederosterreich AG) installed a small pilot-scale photobioreactor (tubular) to produce cyanobacterial biomass. It was subsequently processed for PHB extraction, and the residual biomass was allowed to produce biogas. Their initial results and theoretical calculations suggest that 1 ton of CO2 can be converted to 115 kg of PHB and 330 m3 of biogas, wherein 700 m2 of land may need to make 1 ton of PHB since the land area is one of the crucial factors for economic production (Zhang, 2015). Such numbers are promising for the sustainable production of PHA from cyanobacteria using CO2. Therefore, various large-scale cultivation strategies must be developed and assessed for autotrophic PHA production to achieve a high yield.
5 Chemolithoautotrophic PHA synthesis from CO2
Some procaryotic microorganisms obtain energy by oxidizing or reducing the inorganic compounds (electron donors) such as H2, H2S, Fe2+, CO, NO3, and NH3, thereby utilizing such energy to fix atmospheric CO2 via the CBB pathway. Those microorganisms are collectively called as chemolithotrophs, and most of the chemolithotrophs are obligate autotrophs. Facultative chemolithoautotrophs can adjust their biosynthetic pathways, enabling them to switch between autotrophic and heterotrophic lifestyles. One such example is hydrogen-oxidizing C. necator. Autotrophic PHA synthesis has been identified in hydrogen-oxidizing bacteria, acetogens, CO-oxidizing bacteria, sulphur-oxidizing, and nitrite-oxidizing bacteria. This section discusses recent advancements and progress in autotrophic PHA synthesis from CO2 by chemolithotrophic wild-type bacteria.
5.1 Hydrogen-oxidising bacteria
Hydrogen-oxidizing bacteria can only convert hydrogenous gas mixtures (H2, CO, CO2, and CH4) to bioproducts. Some facultative or obligate chemolithotrophic bacterial genera, including Cupriavidus, Comamonas, Ideonella, and Pseudomonas, are known for hydrogen oxidation and autotrophic PHA synthesis. All these bacteria are found to be resistant or tolerant to certain levels of CO; hence, they are also collectively known as CO-oxidizing bacteria. Cupriavidus is a well-studied genus for autotrophic PHA synthesis, where CO2 is a primary carbon source (Srisawat et al., 2022b; Ray et al., 2023). One of the best species is the C. necator H16, is a Gram-negative, non-pathogenic β-proteobacterium and facultative chemolithotroph, which oxidizes the H2 and assimilates the CO2 via the CBB pathway (Morlino et al., 2023), where O2 is an electron acceptor. This bacterium can naturally synthesize the PHAs up to >50% of its dry cell biomass on various carbon sources by autotrophic and heterotrophic routes (Ishizaki et al., 2001; Li and Wilkins, 2020; Behera et al., 2022; Morlino et al., 2023) (Figure 6B). Sometimes, this bacterium can accumulate up to 90% of PHA when the growth medium is amended with anaerobic digestate and 1% acetate (Passanha et al., 2013).
Over time, autotrophic cultivation of C. necator using H2 has gradually evolved since H2 is an insoluble and highly explosive gas substrate (Ishizaki et al., 2001). Two different cultivation systems have been developed to increase PHB production in C. necator, such as dead-end and recycled gas culture (Ishizaki et al., 2001). Dead-end cultivation is a process where the gas supply is not continuously replenished, which faces challenges with the gas-to-liquid mass transfer because it needs more aeration (Bongers, 1970). In contrast, the recycled gas closed circuit cultivation method offers several advantages, including continuous gas supply, operational safety, and reduced substrate gas loss (Schlegel et al., 1961; Kodama et al., 1975). Many researchers have explored the theoretical foundations, methodologies, stoichiometry, and realistic bioprocess systems for producing PHB from C. necator using CO2 as a substrate (Ishizaki and Tanaka, 1990; Tanaka and Ishizaki, 1994; Takeshita and Ishizaki, 1996; Sugimoto et al., 1999). Their studies also explored possible fermentation platforms for this bacterium from a manufacturing standpoint, focusing on challenges like the risk of detonation and inefficient gas utilization caused by exhaust gas flow from the bioreactor. While using an explosion-proof continued stir tank reactor (CSTR), researchers have successfully achieved a high cell density culture of C. necator with a yield of 91.3 g/L of dry biomass and 61.9 g/L of PHB (1.55 g/L/h) under autotrophic condition, wherein O2 was the limiting factor (Ishizaki et al., 1993; Tanaka et al., 1995). Later, a two-stage cultivation system was introduced along with carboxymethyl cellulose to enhance the mass transfer coefficient within an air-lift fermenter, which produced 56.4 g/L of PHB (0.613 g/L/h) from 69.3 g/L of biomass (Taga et al., 1997). Recently, high-pressure fermentation approaches have also been suggested to increase the gas-to-liquid mass transfer and avoid explosions during gas fermentation. Operating the reactors under elevated pressure from 1.5 to 3 bar, along with the O2 limitation, enables a lengthy exponential growth and further boosts the autotrophic PHB production from 10.8 g/L to 29.6 g/L (0.45 g/L/h), respectively (Vlaeminck et al., 2024). A comparison of PHA productivity among the C. necator autotrophic studies has been presented in Table 3. Bioengineering aspects of recycled gas systems have also been explored to develop an efficient bioprocess method for industrial cultivation of C. necator using inexpensive and instantly accessible gas substrates for the autotrophic production processes. Such studies have shown that high O2 levels may inhibit the specific growth rate of C. necator, whereas lower gas concentrations could stimulate PHB production (Darani et al., 2006).
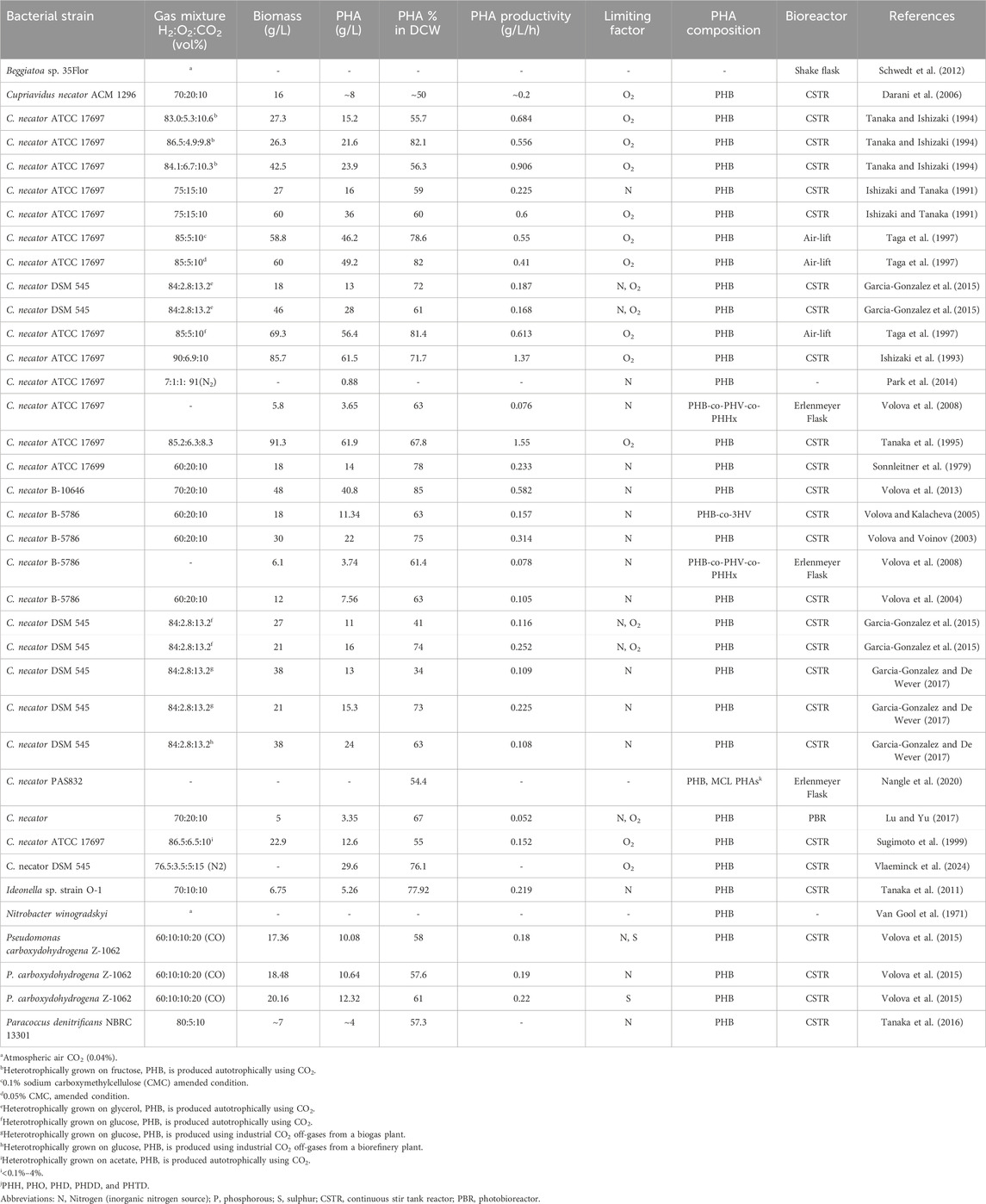
Table 3. Autotrophic PHA production from chemolithoautotrophic bacteria using CO2 and other gas mixtures.
PHB is a well-known SCL-PHA. However, its commercialization has some practical difficulties since this polymer is highly crystalline with high rigidity, brittleness, and low tensile power (Muneer et al., 2020). Such features can be enhanced by integrating different PHA monomers (Sudesh et al., 2000). For example, PHB-co-PHV copolymers have better flexibility and durability than PHB (Reddy et al., 2003; Philip et al., 2007). Moreover, MCL-PHAs and their copolymers are more elastomeric than SCL-PHAs (Anjum et al., 2016). Therefore, synthesizing different PHA copolymers from C. necator is inevitable, which can improve the polymer properties and applications. Recent studies have focused on mixotrophic PHA synthesis, where CO2 and other PHA precursors were supplied as carbon sources (Supplementary Table S2). For instance, the pulse feeding of valerate to the autotrophic medium effectively incorporated the valerate monomers and produced PHB-co-PHV from C. necator (Volova and Kalacheva, 2005; Park et al., 2014). In addition, MCL monomers were also incorporated while adding the MCL precursors (heptanoate, octanoate, and hexanoate) along with CO2 (Volova et al., 2008; 2013). Most autotrophic studies with CO2 have shown that either nitrogen or O2 limitation is a crucial factor for the PHB synthesis in C. necator (Panich et al., 2021; Morlino et al., 2023). Internal remobilization of PHB polymers is also observed when the bacterium faces a carbon-deficient condition, which is very common in all polymer-producing microorganisms in nature.
Valorisation of industrial exhaust gas (mainly CO, CO2, and H2) is an exciting subject for autotrophic PHA synthesis. However, CO-resistant strains can only tolerate such toxic gas composition since CO is lethal to most bacteria except CO-oxidizers. It has been found that the C. necator B5786 strain can exceptionally tolerate 5%–25% (v/v) of CO and produce 70%–75% of PHB-co-PHV copolymer under autotrophic conditions. The PHB-co-PHV polymer also had material properties like those produced from autotrophic fermentation using electrolytic H2 (Volova et al., 2002). Most of the wild-type C. necator lacks the CO dehydrogenase (CODH). Hence, C. necator cannot utilize the CO-containing syngas for PHA synthesis. Researchers have immobilized the CODH enzyme on the C. necator cell surface to overcome this, effectively utilizing CO-containing syngas and producing 14.2 g/L of PHB (Shin et al., 2021). Aerobic CO-oxidizing/H2-oxidising bacterium Pseudomonas carboxydohydrogena Z-1062 (formerly known as Seliberia carboxydohydrogena Z-1062) have been studied under autotrophic batch cultivation with a mixture of CO, H2, CO2, and O2 (Volova et al., 2015). This bacterium was shown to synthesize the PHA up to 52.6%–62.8% in dry cell biomass after 56h of process under the limitations of nitrogen and sulphur. PHA production has maximized as 0.13–0.22 g/L/h even though the medium was amended with 10%–30% CO v/v. The produced PHA comprises mainly PHB (99 mol%) with a small portion of PHV (0.24–0.48 mol%). However, 30% v/v of CO concentration affected the growth rate and cell concentration adversely (Volova et al., 2015). The CO-tolerating H2-oxidising bacterium Ideonella sp. O-1 has also been isolated from soil, which grows autotrophically by assimilating H2, O2, and CO2 as substrates (Tanaka et al., 2011). This bacterium has been shown to sustain up to 30% (v/v) of O2 and 70% (v/v) of CO and can produce 5.26 g/L of PHB from the 6.75 g/L of biomass under autotrophic conditions. Such a high tolerance of CO is highly comparable with well-known H2-oxidisers like C. necator and A. latus since they can tolerate up to 5% (v/v) CO (Tanaka et al., 2011). High tolerance of CO is a promising feature, and these strains can be used to produce PHB polymer from industrial exhaust gas, further boosting the circular economy.
5.2 Acetogens
Acetogens are metabolically diverse obligate anaerobes and comprise 23 bacterial genera with more than 100 species (Debabov, 2021). All acetogens can fix the C1 gases through the WL pathway (Claassens et al., 2016). During gas fermentation, acetogens can assimilate CO2 or CO as a substrate, whereas H2 or CO supplies reducing equivalents (Bae et al., 2022). Acetogens perform a series of reactions in the WL pathways to reduce the CO2 to acetyl-CoA and later synthesize the acetate as a terminal product. The WL pathway is the highest energy-efficient mechanism for CO2 reduction and relates to direct energy storage (Claassens et al., 2016; Bae et al., 2022). Their efficient autotrophic flux for synthesizing acetyl-CoA makes them promising candidates for producing value-added chemicals (i.e., organic acids and alcohol) through autotrophic processes since acetyl-CoA is a primary precursor for many biochemicals (Bae et al., 2022; Flaiz and Sousa, 2024). Among the 100 species, only a few are considered critical biocatalysts for producing biochemicals like butanol, 2,3-butanediol, and ethanol. The major acetate producers are C. aceticum (Sim and Kamaruddin, 2008), Acetobacterium woodie (Demler and Weuster-Botz, 2011), and Moorella thermoacetica (Daniell et al., 2012). A. woodie has been shown to produce 44 g/L of acetate, the highest titre achieved so far under H2/CO2 conditions (Demler and Weuster-Botz, 2011). In addition, C. ragsdalei, C. ljungdahlii, and C. autoethanogenum were used to make fuel-quality ethanol under autotrophic conditions (Bae et al., 2022). Another set of acetogens, including Butyribacterium methylotrophicum, Eubacterium limosum, and C. carboxidivorans, were explored to synthesize 2,3-butanediol (Michael et al., 2011) and butyrate (Bae et al., 2022) under gas fermentation, where C1 gases were used as carbon sources. Despite their promising potential, these organisms are not yet viable for industrial applications due to their slow growth rates and low efficiency in autotrophic production. Various cultivation strategies have been employed in gas fermentation to improve the capacity of acetogenic bacteria to transform C1 gases into valuable multi-carbon biochemicals. However, the product collection remains restricted to intrinsic chemicals, predominantly acetate and ethanol. The production of energy-dense compounds, including lipids, long-chain alcohols, and PHAs from C1 gases, poses significant challenges in acetogens. This difficulty arises from the energetic limitations of autotrophic growth and the lack of essential enzymes required for synthesizing these complex molecules (Bae et al., 2022). To address these challenges, research has been directed towards rechannelling the WL pathway by genetic and metabolic engineering methods, which will be discussed separately in this review. Recently, two-stage co-cultivation has emerged as a method for producing a broader array of biochemicals. This approach combines acetogenic gas fermentation with an acetate conversion process, thereby developing various products that can be synthesized from CO2 (Bae et al., 2022). Acetate-consuming microorganisms can flourish well on acetate and produce acetyl-CoA, a metabolic precursor for various biomolecules. Most acetate-converting bacteria are aerobes; growing them with acetogens in the same reactor is impossible. Therefore, two different fermentations must be conducted using two reactors with various parameters. Two-stage co-cultivation consists of an anaerobic reactor in which acetogens convert C1 gases into acetate during the first stage. The generated acetate is moved to an aerobic reactor in the subsequent stage for further transformation. Otherwise, the second stage can occur within the same reactor by modifying the operating parameters to facilitate aerobic growth (Bae et al., 2022). This approach produced acetate from S. ovata, where CO2 was used as a carbon source. It was later utilized by E. coli in the second stage, leading to a PHB productivity of 0.5 g/L (Liu Q. et al., 2015). Similarly, S. ovata have been used to convert CO2 into acetate (stage 1), which was later used as a substrate to produce PHB from Cupriavidus basilensis (stage 2). In the optimized media, S. ovata generated 10.4 mmol of acetate (L/day) under a CO2 environment. When the stage 1 fermented broth was used as a substrate, C. basilensis produced 12.54 mg of PHB (L/h), resulting in a net carbon profit of 11.06% from acetate (Cestellos-Blanco et al., 2021). Other metabolic intermediates from acetogens, including formic acid, have been utilized as a substrate for stage 2 bioprocess (Hwang et al., 2020). Initially, A. woodii was used to convert CO into formic acid, and then it was used as a substrate to produce PHB by Methylbacterium extorquens AM1 (Hwang et al., 2020). A similar two-stage bioprocess has also been demonstrated with A. woodii and C. necator H16, in which A. woodii produced 3 g/L of acetate using CO2 as a substrate, which was later used to produce 0.5 g/L PHB by C. necator H16 (Al Rowaihi et al., 2018a).
5.3 Other chemolithoautotrophs
The genus Beggiatoa contains large, thread-like filamentous bacteria in various sulphur-rich environments, including sediments, springs, and activated sludge. Beggiatoa obtains energy by oxidizing inorganic sulphur in the presence of oxygen. Recently, Beggiatoa sp. 35Flor isolated from marine environments has been shown to synthesize the PHA inclusion bodies while fixing the atmospheric CO2 during the movement between the oxygen-sulphide interface. Under anoxic conditions, PHA inclusion bodies were also remobilized during sulphur respiration (Schwedt et al., 2012). Nitrobacter winogradskyi, a well-studied nitrite-oxidizing bacterium, can also synthesize PHB, glycogen, and polyphosphate while growing autotrophically with CO2 as a carbon source. This bacterium can also depolymerize the PHB when the medium is depleted with nitrite (Van Gool et al., 1971). Denitrifying and sulphur-oxidizing bacterium Paracoccus denitrificans NBRC13301 have been studied for autotrophic growth under aerobic conditions when the culture was fed with a mixture of gases (H2/O2/CO2, 8:1:1). P. denitrificans exhibited growth at up to 15% oxygen levels, with an optimal growth concentration of 5%, and accumulated 57.3% w/w of PHB under nitrogen limitation (Tanaka et al., 2016). Iron-oxidizing acidophilic bacterium Acidithiobacillus ferroxidans can also fix the CO2 via the CBB pathway. This organism is mainly used in the biomining of metals. The ultrathin section of A. ferroxidans showed PHB-like inclusion bodies under transmission electron microscopy (TEM) (Matlakowska and Sklodowska, 2007). Although it lacks specific genes for PHA production, it stores glycogen as a carbon reserve material. With its complete genome sequence available, exploring genetic modifications to incorporate the PHA synthesis gene could be intriguing. This could potentially exploit this chemolithoautotrophic bacterium for PHA production.
6 Microbial electrosynthesis
Bio-electrochemical systems (BES) have previously been suggested for treating waste streams and recovering valuable products from waste materials. BES systems can be used for biological CO2 sequestration using electroactive microorganisms as self-sustaining and economic biocatalysts (Pepè Sciarria et al., 2018; Banu et al., 2019). One such BES method is microbial electrosynthesis (MES), where the CO2 is reduced into various organic products by electroactive bacteria (i.e., electrolithoautotrophs or chemolithoautotrophs) in the cathodic chamber. Hence, biocathode development is crucial for MES (Logan et al., 2019). Two types of electron transfer mechanisms have been identified in MES systems: direct and indirect electron transfer. Facultative electrolithoautotrophs can perform direct electron transfer where poised potential only acts as an electron source for CO2 reduction. In contrast, chemolithoautotrophs execute the indirect electron transfer, wherein metal ions (i.e., Fe2+/Fe3+, Mn2+/Mn3+, etc.), H2, formate or NH3 are used as diffusible electron carriers/mediators or additional electron donors to fix the CO2 via CBB pathway or WL pathway (Logan et al., 2019; Bian et al., 2020). In MES, both pathways can produce acetyl-CoA as a central precursor to produce various multi-carbon organic chemicals such as volatile fatty acids (VFAs) (e.g., formate, acetate, butyrate, valerate), ethanol, butanol, lactate, succinate, and 2,3-butanediol or gaseous substances (H2 and CH4) (Bian et al., 2020). These products can further act as precursors for biofuels, biopolymers (PHAs), polysaccharides, biomass/protein, and long-chain carboxylates with subsequent multi-step bioconversions (Bian et al., 2020). In MES, PHA can be produced in two different ways such as direct conversion of CO2 to PHA by photoautotrophic or chemolithoautotrophic bacteria and indirect conversion of CO2 to PHA (multi-step process), wherein CO2 is reduced into VFAs, followed its transformation to PHA by MMC (Bian et al., 2020; Stöckl et al., 2020). Direct conversion of CO2 to PHA through MES has been demonstrated in photoautotrophs, especially PNSB, and they have been shown to conduct a direct electron transfer, where the poised potential is only used as an electron source (photoelectroautotrophy) (Rengasamy et al., 2017). A comprehensive list of microorganisms producing PHAs by microbial electrosynthesis/electrolysis via CO2 reduction is presented Table 4. Photoelectroautotrophic PHB synthesis using N2/CO2 (80%/20%) has been demonstrated in R. palustris TIE-1, where the graphite-based cathode was continually supplied at an electric potential of +100 mV vs. standard hydrogen electrode (SHE). The MES system was shown to synthesize 4.48 mg/L and 5.49 mg/L of PHB while fed with NH4Cl and N2 gas as the nitrogen source, respectively (Ranaivoarisoa et al., 2019) (Figure 6C). When the graphite electrode was modified with an immobilized Prussian blue (i.e., Fe2+-based redox mediator), R. palustris TIE-1 showed a high electron transfer (mostly reversible redox reaction), which resulted in increased cathodic current density (5.6 ± 0.09 μA/cm2) and PHB synthesis (18.8 ± 0.5 g/L) compared to unmodified cathodic experiments (Rengasamy et al., 2017). Similarly, R. vannielii and R. udaipurense were shown to produce PHB from CO2 (2.02% and 0.78% cdwprot) when carbon felt was used as a working electrode with an electric supply of +100 mV vs. SHE under N2-fixation condition (Conners et al., 2024). In general, N2-fixing photoelectroautotrophy enhances the PHB synthesis in PSNB; however, PHB productivity is far less than in photoautotrophic or photoheterotrophic conditions (Rengasamy et al., 2017; Conners et al., 2024).
Direct conversion of CO2 to PHA is also recognized in C. necator H16 using industrial flue gas as a CO2 source derived from a coal-fired co-generation plant and H2 derived from water electrolysis (Langsdorf et al., 2024). Industrial flue gas does not affect the growth and PHB synthesis in C. necator H16; following electrochemical CO2 reduction has resulted in 333 ± 44 mg/L of PHB (43% ± 3% in dry cell weight) (Langsdorf et al., 2024). Furthermore, a MES-based one-pot carbon capture setup was recently developed to convert CO2 to formate (22 mM, at a pH of 7.5), and later the same formate was used to produce PHB by C. necator H16. PHB synthesis up to 25.2 mg/L (1.3 mol/h formate uptake) was recognized only when electrolysis functioned in the one-pot carbon capture system within 8h of the electrochemical process under a constant electric supply of −1.2V vs. reversible hydrogen electrode (RHE). Reactive oxygen species stress and nitrogen limitation might have triggered the PHB synthesis (as stress mitigation) instead of the co-generation of H2 during the electrolysis (Al Rowaihi et al., 2018b). Dinges et al. (2024) have demonstrated a two-step CO2 reduction process using a drop-in electrolysis process. Initially, CO2 was reduced to formate (441 ± 9 mmol/L) by C. necator H16, which further transformed into PHB by the same strain in a fed-batch reactor system, resulting in 63 ± 16 mg/L/OD of PHB (29.1% ± 7.1% in dry cell weight) (Dinges et al., 2024). Despite that, MMC were also used in MES systems for PHA synthesis. Pepè Sciarria et al. (2018) have used Clostridium-rich MMC to synthesize VFAs, especially acetate (43 mM carbon/L) and butyrate (103 mM carbon/L). The VFAs were extracted and concentrated, resulting 400 mM carbon/L (∼65% butyrate) was used as a feed to synthesize the PHA (74.4g/100g of volatile suspended solids) by MMC derived from activated sludge and the overall carbon conversion was estimated as 0.14 kg of PHA from 1 kg of CO2 (Pepè Sciarria et al., 2018). Though the number of studies on direct or indirect conversion of CO2 to PHA by MES is scarce, there are plenty of MES studies for only producing VFAs from CO2 using pure and mixed culture (Kumar P. et al., 2018). Researchers also have proposed that producing VFAs from CO2 is an indirect way of CO2 fixation, and it can be exploited further for PHA production. This approach offers a promising alternative to direct CO2 conversion, with potential advantages regarding CO2 fixation efficiency, H2 utilization, raw material costs, process performance, and safety (Garcia-Gonzalez and De Wever, 2018). Even though many technical factors influence MES productivity, including reactor configurations, cathode materials, microbial stability, and electron transfer mechanisms (Logan et al., 2019; Bian et al., 2020). In addition, MES also has its bottlenecks, mainly high energy consumption and poor CO2 assimilation efficiency. Despite all these drawbacks, MES is the best CCU strategy and sustainable route of CO2 reduction for producing diverse multi-carbon products (Pepè Sciarria et al., 2018; Banu et al., 2019).
7 Genetic engineering approaches for autotrophic PHA synthesis
Many possibilities exist to enhance the autotrophic PHA synthesis in photoautotrophs and chemolithoautotrophs. Tuning CO2 fixation pathways, introducing new enzymatic pathways, and overexpressing PHA synthesis enzymes through genetic engineering approaches could enhance CO2 bioconversion to PHAs in microbial systems. This chapter comprehensively reviews recent advancements and innovations in the genetic engineering of autotrophic microorganisms to enhance PHA production. A detailed list of genetic modifications and autotrophic PHA synthesis is summarized in Table 5.
7.1 Tuning of CO2 fixation pathways
The CBB pathway is a central metabolism for carbon fixation in photoautotrophs and some chemolithoautotrophs except acetogens. CCB pathway employs many key enzymes to reduce the CO2 in cellular biomaterials, including RuBisCO, PrkA, and SBPase. However, RuBisCO could not differentiate O2 from CO2, and it catalyzes the undesired oxygenation instead of carboxylation during the photorespiration/dark fermentation. To overcome this, cyanobacteria and PNSB execute the CCMs (see section 3), which decrease the overall CO2 fixation ability and energy transport of the CBB pathway. Therefore, genetic engineering of the RuBisCO enzyme may open avenues for increased CO2 fixation and following high titer of cellular products. Significant efforts have been undertaken to improve RuBisCO’s carboxylation efficiency. For example, creating a point mutation in the larger subunit of the RuBisCO (RbcLF140I) enzyme in Synechocystis sp. PCC 6803 has intensified carboxylation activity by 2.9-fold and enhanced the photorespiration rate by approximately 55% (Durão et al., 2015).
In addition, overexpression of RuBP regeneration enzymes also increased the CBB pathway carbon flux, further enhancing the autotrophic ethanol production from Synechocystis sp. PCC 6803 (Roussou et al., 2021). The engineering of CCMs has also improved the activity of RuBisCO, leading to significant changes in the HCO3− transport systems or the introduction of additional HCO3− transport. This enhancement has improved CO2 fixation and subsequent biomass production in Synechocystis sp. PCC 6803. As a result, there has been a high production of intra (∼50%) and extracellular polymers (3-fold), such as glycogen and exopolysaccharides, respectively (Kamennaya et al., 2015; Gupta et al., 2020). The reductive glycine mechanism was recently proposed to replace the CBB pathway for formate production, thereby enhancing C1 assimilation in C. necator H16. However, very little growth has been achieved compared to the native CBB pathway. Li et al. (2020) also focused on enhancing the CBB pathway and H2 utilization in C. necator to increase biomass and PHB synthesis. They achieved this by incorporating the RuBisCO enzyme from Synechococcus sp. PCC 7002 into C. necator system and adjusting the membrane-bound and soluble hydrogenase expression levels. As a result, the engineered strain showed up to 34% of PHB, in contrast to the wild-type strain (Li et al., 2020). Moreover, heterologous expression of CCMs-related enzymes has been accomplished in heterotrophs like E. coli and Corynebacterium glutamicum (Panich et al., 2021). Such alteration can be implemented in C. necator since this bacterium lacks the CCMs to overcome the RuBisCO inefficiency during the carboxylation phase of the CBB pathway.
7.2 Introducing new PHA enzymatic pathways by heterologous expression
PHA-producing model strain C. necator H16 does not have CODH by nature. Hence, wild-type strains cannot utilize the CO-containing syngas for PHA production. To overcome this hurdle, C. nectar H16 was genetically engineered by heterologous expression of CODH genes from the chemolithoautotrophic bacterium Oligotropha carboxidovorans OM5. The modification enabled C. necator H16 to use CO and CO2, leading to a 1.8-fold increase in biomass over the wild-type strain. PHB synthesis has significantly improved in C. necator H16 at 49.7% compared to the wild-type strain, which can synthesize only 40.8% (Heinrich et al., 2015). Most acetogens cannot naturally synthesize PHA; hence, PHA-synthesizing enzymes are required for heterologous production. The PHA synthetic pathway from C. necator H16 was expressed into Clostridium autoethanogenum. The genetically engineered C. autoethanogenum produced 5.58% of PHB by autotrophic gas fermentation while growing on a synthetic gas mixture (syngas) (de Souza Pinto Lemgruber et al., 2019). Flüchter et al. (2019) genetically engineered C. ljungdahlii and C. coskatii to utilize syngas for autotrophic PHB production. They introduce a novel PHA pathway containing thiolase (thlA), (R)-3-hydroxybutyrate dehydrogenase (bdhA), and CoA-transferase (ctfA/B) into C. ljungdahlii and C. coskatii. Consequently, C. coskatii produced 0.102 and 2.26 g/L of PHB under autotrophic and heterotrophic conditions, respectively. Meanwhile, C. ljungdahlii failed to produce PHB even after having new PHA synthetic genes (Flüchter et al., 2019). Similarly, C. coskatii was completely engineered with new PHA synthetic pathways, wherein crotonase (crt), 3-hydroxy butyryl-CoA dehydrogenase (hbd) and thiolase (thlA) genes were derived from C. scatologenes and PHA synthase (phaEC) and (R)-enoyl-CoA hydratase (phaJ) genes were derived from C. acetireducens, respectively. Subsequently, the engineered C. coskatii produced 1.12% PHB under autotrophic conditions using syngas as a substrate (Flüchter et al., 2019) (Figure 6D). The same PHB synthetic pathway was introduced into the acetogen A. woodie, which produced 1.9% of PHB during autotrophic cultivation (Höfele and Dürre, 2023). In addition, recombinant C. ljungdahlii was shown to synthesize 3-hydroxybutyrate (3-HB) as an unexpected product while incorporating the isopropanol synthetic pathway. Under autotrophic conditions, C. ljungdahlii produced 3 g/L of 3-HB along with ethanol (28.4 g/L) and isopropanol (13.4 g/L) (Jia et al., 2021). S. elongatus PCC 7942 is an outstanding cyanobacterial strain used for various biochemical production; however, it does not naturally possess a PHA synthesis pathway. However, it can be genetically engineered to synthesize PHA by establishing the mandatory gene cluster from another organism. The PHA synthetic gene cluster from C. necator H16 has been introduced into S. elongatus PCC 7942, resulting in 25% of PHB under autotrophic conditions (Takahashi et al., 1998). Similarly, Synechococcus sp. PCC 7002, also engineered with a phaABEC gene cluster obtained from C. necator H16, results in autotrophic PHB synthesis of up to 52% (Akiyama et al., 2011). Heterologous expression of the phaABEC gene cluster from Chlorogloeopsis fritschii PCC 9212 and deletion of the ccmR gene in Synechococcus sp. PCC 7002 enables the autotrophic synthesis of P (3HB-co-4HB) at 4.5% dry cell weight, with 4HB making up 12 mol% of the copolymer (Zhang et al., 2015). Roh et al. (2021) genetically expressed the whole C. necator PHA pathway into the S. elongatus UTEX 2973, resulting in PHB synthesis up to 420 mg/L (16.7% w/w) with a yield titer of 46.7 mg/L/d under photoautotrophic cultivation with industrial flue gas as a carbon substrate (Roh et al., 2021).
7.3 Overexpression of PHA synthase and other enzymes
Genetic modification, especially overexpression of PHA synthesis enzymes, has been employed in different microorganisms to enhance autotrophic PHA synthesis. Most of the PHA producers synthesize only PHB in autotrophic conditions. Genetically engineered strains can synthesize PHA copolymers. Recently, C. necator H16 was engineered with a different set of enzymes, such as monomer supplying gene (phaABRe) and 3-keto thiolase (bktB) from other C. necator strains and PHA synthase 1 (phaC1Ps) from Pseudomonas sp. 61-3. The genetically engineered C. necator H16 produced a PHA copolymer of about 0.14 ± 0.05 g/L with 57% of PHB along with 1.2 mol% 3-hydroxyvalerate and 3-hydroxy-4-methyl butyrate (Miyahara et al., 2020). Pseudomonas species are known for MCL-PHA synthesis by nature. Hence, MCL-PHA synthetic genes can be overexpressed in autotrophic hosts, including C. necator. Tanaka et al. (2021) engineered the C. necator with β-ketothiolase gene (bktB) encoding MCL-PHAs (C5-C14). They produced PHA copolymer up to 85.8% ± 13.2% from 8.52 ± 1.92 g/L of biomass under autotrophic conditions. The produced PHA copolymers comprise PHB and PHHx monomeric units with 96.7 ± 14 and 3.3 ± 1.4 mol%, respectively (Tanaka et al., 2021). Also, researchers have utilized metabolic engineering strategies to improve the productivity of PHA from CO2. For example, C. necator was engineered to produce PHA and grow effectively concurrently under autotrophic conditions. Tang et al. (2020) introduced a haemoglobin gene (vgb) from Vitreoscilla to enhance oxygen usage and knock out L-lactate dehydrogenase (ldh) genes in the CBB pathway to channel the carbon flow toward PHA synthesis in C. necator, which resulted in 0.55 g/L of biomass with 50.4% PHA under autotrophic condition (Tang et al., 2020).
Several genetic engineering approaches have been applied to photoautotrophs to increase PHA accumulation using CO2. A recombinant R. rubrum S1 strain, engineered with the pntAB gene from E. coli MG1655 and the phaB1 gene from C. necator H16, was able to accumulate a PHB-co-PHV copolymer at 5.1% concentration, with a PHV content of 28 mol%, during autotrophic fermentation using syngas (Heinrich et al., 2015). MCL-PHA homopolymers such as 3-hydroxy decanoate (3HD) and 3-hydroxy octanoate (3HO) have also been produced from R. rubrum while modifying the wild-type strain with MCL-PHA genes from Pseudomonas putida. Such modification resulted in the bioconversion of synthetic syngas to MCL-PHA up to 7% in dry cell weight (Daniel et al., 2016). Synechocystis is a natural PHA producer; however, its productivity is far lower than that of other autotrophic hosts. Efforts to overexpress C. necator PHA synthase genes (phaABEC) in Synechocystis sp. PCC 6803 resulted in a strain that produced 26% PHB under nitrogen-limiting conditions (Khetkorn et al., 2016). The same recombinant strain showed 35% PHB production under the mixotrophic conditions with 0.4% acetate as a co-substrate (Khetkorn et al., 2016). Overexpression of the phaEC genes from Microcystis aeruginosa NIES-843 in Synechocystis sp. PCC 6803 significantly boosted PHB accumulation to 7% of dry cell weight, with a productivity of 10.59 mg/L, a 12-fold increase over the wild-type strain (Hondo et al., 2015). Orthwein et al. (2021) developed a novel approach to enhance PHB synthesis using the Synechocystis sp. PCC 6803 mutant strain known as PPT1 (ΔpirC-REphaAB). Research revealed that the PirC protein influences glycolytic carbon flow in a PII-reliant behavior, thereby controlling the carbon flux in cyanobacteria (Orthwein et al., 2021). The overexpression of the phaCAB gene cluster from C. necator in Synechocystis sp. PCC 6803 (ΔpirC) led to an accumulation of PHB reaching up to 61%, representing a 6.1-fold increase compared to the wild-type strain (Koch et al., 2020). Acetyl-CoA is crucial for central carbon metabolism and PHA synthesis, so higher intracellular levels of acetyl-CoA may enhance PHA production in cyanobacteria. Carpine et al. (2017) demonstrated this concept by engineering a strain of Synechocystis sp. PCC 6803. They deleted the pta (phosphotransacetylase) and ach (acetyl-CoA hydrolase) genes while introducing the xfpk (phosphoketolase) gene from Bifidobacterium breve. The engineered strain generated 232 mg/L of PHB, 12% of its total weight, yielding 7.4 mg/L/day. The wild-type strain generated only 1.8% PHB, yielding 3.05 mg/L/day (Carpine et al., 2017).
Most cyanobacteria concurrently produce intracellular (i.e., glycogen and PHB) and extracellular polymers (exopolysaccharides). The knockout of one competitive pathway may increase the other polymer production. For example, while disrupting the glycogen production in Synechocystis sp. PCC 6803, by creating a glycogen defective mutant strain (ΔglgC), surprisingly increased the PHB synthesis up to 13%, whereas the wild-type strain showed only 8% of PHB under autotrophic cultivation (Damrow et al., 2016). Similarly, redirecting glycogen metabolism towards PHB synthesis was accomplished by overexpressing the RNA polymerase sigma factor (sigE) in Synechocystis sp. PCC 6803. This modification led to a 2.3-fold increase in autotrophic PHB synthesis, reaching approximately 14 mg/L (Osanai et al., 2013). Classical UV random mutagenesis can also be used as an alternative to genetic engineering approaches to generate more efficient PHA producers. Random mutagenesis strangely intensified the autotrophic growth of Synechocystis sp. PCC 6714 and increased PHB synthesis up to 37% with a yield of 134.2 mg/L/d/, thereby showing the potential of UV mutagenesis to improve cyanobacteria for efficient CO2 uptake and PHA synthesis. In addition, knockout of exopolysaccharide synthesis (ΔexoD) in Synechocystis sp. PCC 6714, resulting in higher PHB synthesis (∼16.5%) than the control strain (13%) under nitrogen/phosphorous limitation (Mittermair et al., 2021).
8 Effect of nutrients on autotrophic PHA synthesis
Autotrophic PHA production using CO2 is greatly influenced by the availability of macro and micronutrients essential for microbial metabolism and enzyme activity (Saravanan et al., 2022). Macro-nutrients such as nitrogen, phosphorus, and sulphur are essential for the synthesis of cellular components and energy production (Getino et al., 2024). Nitrogen is vital for synthesizing amino acids, nucleotides, and other cellular constituents. When nitrogen is limited, microorganisms often redirect their metabolic pathways toward accumulating storage compounds like PHAs as a survival strategy (Ma et al., 2024; Rueda et al., 2024). Similarly, phosphorus is a key component of nucleic acids and ATP, and its limitation can trigger PHA accumulation (Korkakaki et al., 2017). Sulfur, required for synthesizing certain amino acids and coenzymes, also influences PHA production, although its role is less pronounced than nitrogen and phosphorus (Tables 2, 3).
Micronutrients, including trace elements like magnesium (Mg), calcium (Ca), iron (Fe), and trace metals such as cobalt (Co), copper (Cu), and zinc (Zn), are critical for the function of various enzymes involved in PHA biosynthesis (Sakarika et al., 2023; Getino et al., 2024). For instance, magnesium is a cofactor for enzymes like PHA synthase, which catalyses hydroxyalkanoate monomers’ polymerization into PHAs (Ronďošová et al., 2022; Getino et al., 2024). Iron is essential for the activity of enzymes involved in the electron transport chain and oxidative phosphorylation processes that provide the energy required for PHA synthesis (Sakarika et al., 2023). The availability of these micronutrients can thus directly affect the efficiency of PHA production (Choi and Lee, 1999). Additionally, the balance between macro and micronutrients is crucial; an excess of one nutrient can lead to the depletion of another, thereby affecting overall microbial growth and PHA accumulation (Choi and Lee, 1999). For example, excess nitrogen can suppress PHA synthesis by promoting cell growth and division, while a balanced limitation can enhance PHA yield (Choi and Lee, 1999).
The type of microorganism used also plays a significant role, as different species have varying nutrient requirements and metabolic capabilities. Cyanobacteria and other photosynthetic bacteria, which can utilize CO2 directly, often require specific conditions of light and nutrient availability to optimize PHA production (Higuchi-Takeuchi et al., 2016b; Higuchi-Takeuchi and Numata, 2019; Rueda et al., 2024). Genetic and metabolic engineering approaches have enhanced these microorganisms’ nutrient utilization efficiency and PHA biosynthetic pathways (Madison and Huisman, 1999). Researchers can improve PHA yields even under nutrient-limited conditions by overexpressing key enzymes or knocking out competing pathways (Madison and Huisman, 1999). Environmental factors such as pH, temperature, and light intensity interact with nutrient availability to influence PHA production (González-Rojo et al., 2024). Optimal nutrient uptake and metabolic activity conditions must be maintained to achieve high PHA yields (González-Rojo et al., 2024). Hence, understanding and optimizing these nutrient interactions are essential for improving the efficiency and sustainability of PHA production processes.
9 Techno-economic analysis of PHA production from CO2
The utilization of CO2 as a feedstock for PHA production represents a promising pathway toward sustainable and carbon-negative biopolymer manufacturing. Hydrogenotrophic microorganism, such as C. necator and its engineered strains, have demonstrated effective CO2 fixation under autotrophic growth conditions. Under optimised settings, C. necator can achieve biomass yields of approximately 0.5–0.6 g dry cell weight (DCW) per Gram of CO2, with PHA accumulation reaching up to 80% of CDW, corresponding to 0.4–0.48 g PHA per g CO2 consumed (Troschl et al., 2017b; Khosravi-Darani et al., 2013b). Process configurations utilising CO2, H2, and O2 gas mixtures yield carbon conversion efficiencies (CCE) of 35%–40% on a molar basis, nearing the theoretical maximum of 60% defined by the CBB pathway (Tanaka et al., 2022; Claassens et al., 2016). Volumetric productivities reported for autotrophic gas fermentation systems range from 0.2 to 0.6 g/L/h (Karmann et al., 2017), although intensification beyond 1.0 g/L/h is necessary to achieve competitive economics.
Techno-economic analyses estimate that the cost of producing PHA via direct gas fermentation lies between $4.5–6.0/kg PHA when electrolytic H2 is priced at ∼$4/kg (Khosravi-Darani et al., 2013b; Haas et al., 2021). More recent projections suggest that if H2 costs are reduced to $2/kg, PHA production costs could decrease to $2.5–3.5/kg (Müller et al., 2023). Capital expenditure (CAPEX) requirements for a 10,000 ton/year plant are typically estimated at $80–120 million, largely driven by the need for specialised pressurised bioreactors and advanced gas management systems (Haas et al., 2021).
Innovations such as MES have emerged to further improve sustainability. In MES systems, electroautotrophic bacteria catalyse CO2 reduction at cathodes, producing VFAs like acetate and butyrate, which can subsequently be converted into PHAs (Sciarria et al., 2020). MES-derived VFA to PHA processes demonstrated overall carbon conversion efficiencies of up to 41% (kg C in PHB/kg C as CO2) (Alvarez Chavez et al., 2022). However, MES-based PHA production remains at an early technology readiness level with capital-intensive setups and relatively low current productivities (30–1330 mmol m-2 d-1) compared to conventional fermentations.
From an environmental standpoint, life cycle assessment (LCA) studies report net CO2 sequestration of approximately 2.2 kg CO2 per kg of PHA produced, when considering total system emissions (Narancic et al., 2020). The direct gross CO2 fixation, based on stoichiometric calculations, approximates 2.8–3.0 kg CO2 per kg of PHA synthesized (Volova et al., 2010). Commercially, Newlight Technologies (United States) has achieved notable advancements, reporting methane/CO2-derived AirCarbon® PHA production costs of approximately $2.5–$3.0/kg at pilot scale (Newlight Technologies, 2024). Market projections suggest that reaching PHB production costs below $3/kg and sustaining CO2 uptake efficiencies above 0.5 g PHA/g CO2 could open access to a global market exceeding 500,000 metric tons annually by 2030, driven by demand in biodegradable packaging, agricultural films, and biomedical applications (European Bioplastics, 2023).
10 Challenges and prospects
Harnessing microbial processes to fix CO2 offers a compelling eco-friendly approach to mitigating climate change while generating valuable products precisely from CO2. Bioconversion of CO2 is highly promising because it can lower costs associated with feedstock and operations and simultaneously address energy needs and CO2-related environmental risks. However, there are substantial obstacles that must be overcome for successful commercialization. These challenges range from biological constraints and metabolic inefficiencies to economic and engineering difficulties. The primary challenges in autotrophic PHA production using CO2 include.
- Bioprospecting of autotrophic PHA producers: A key challenge in producing PHAs is identifying robust strains that effectively utilize CO2 as a carbon source. Photosynthetic and H2-oxidising microorganisms are frequently employed due to their CO2 fixation potential. However, achieving commercially viable PHA yields requires selecting naturally proficient strains or applying advanced genetic/metabolic engineering techniques and adaptive evolution strategies to enhance PHA biosynthesis.
- Slow growth rate: Autotrophs typically exhibit slower growth rates than heterotrophs, which impacts biomass production and limits PHA yield over time. The extended doubling times observed in cyanobacteria and other autotrophic organisms lead to prolonged production cycles, which pose a significant challenge for large-scale industrial PHA production.
- Difficulties achieving optimal growth: Optimizing growth parameters is vital for enhancing PHA production in autotrophic systems. Critical factors like light intensity, CO2 concentration, temperature, and nutrient availability significantly influence the efficiency of PHA synthesis. Cyanobacteria require a steady supply of essential micronutrients like nitrogen and phosphorus to maintain growth and PHA synthesis. Managing the availability and recycling of these nutrients at an industrial scale presents logistical challenges, which can negatively impact productivity and increase operational costs. Therefore, fine-tuning these growth conditions is essential for maximizing yield while minimizing resource consumption.
- CO2 fixation issues: CO2 fixation via autotrophic pathways, mainly through the CBB pathway, is inherently slow, primarily due to the inefficiency of the RuBisCO. This enzyme catalyzes the initial step of the CBB pathway at a sluggish rate. It is prone to oxygenation reactions, which further hinder the conversion of CO2 into intermediates for PHA production. Overcoming these limitations necessitates targeted genetic and metabolic engineering approaches to improve RuBisCO’s efficiency. However, these strategies remain in the early stages of development.
- Metabolic constraints: In autotrophic organisms, most of the metabolic energy is directed towards carbon fixation, which can limit the resources available for PHA biosynthesis. Consequently, the synthesis of PHAs competes with critical cellular functions, including growth and maintenance. To enhance PHA production, regulating metabolic fluxes is essential to ensure adequate energy and precursor molecules are channelled toward PHA synthesis without compromising cell growth or viability. Achieving this balance is critical to improving the efficiency of PHA production in autotrophic systems.
- High energy inputs: Autotrophic PHA production often demands significant energy inputs, mainly light, CO2, and essential nutrients. Photoautotrophic systems rely on a constant supply of light energy. Still, the photosynthetic process is relatively inefficient in converting light into chemical energy, limiting these organisms’ overall productivity. Similarly, chemolithotrophs require a steady supply of H2 to drive CO2 reduction, as CO2 is the most oxidized form of carbon and necessitates an external energy source for conversion into more reduced compounds, such as PHAs. As a result, maintaining a continuous flow of these energy resources elevates operational costs, posing challenges for large-scale production.
- Low PHA yield: Autotrophic microorganisms generally achieve lower PHA yields than heterotrophic systems. For instance, heterotrophic bacteria can accumulate up to 90% of their DCW as PHA, while autotrophic systems typically demonstrate much lower accumulation levels. Nutrient-limiting conditions such as nitrogen or phosphorus stimulate heterotrophs to store PHAs as energy reserves. In contrast, inducing similar stress conditions in autotrophic systems while ensuring adequate CO2 fixation for growth presents a more intricate challenge. Consequently, achieving high PHA yields in autotrophic pathways requires careful management of both stress factors and growth conditions.
- CO2 availability limitations: Another major challenge is maintaining a reliable and sufficient supply of CO2. Large-scale industrial production demands substantial CO2, which can be obtained from industrial emissions or direct air capture sources. However, the associated costs and logistical complexities of CO2 capture and transportation can be significant barriers. In addition, maximizing the efficiency of CO2 utilization by microorganisms is crucial for ensuring the economic feasibility of the production process.
- Mass transfer challenges: Efficient CO2 mass transfer in bioreactors is a significant challenge. Unlike soluble carbon sources in heterotrophic PHA production, CO2 must be supplied continuously in gaseous form, and its low solubility in water limits availability to microorganisms. Addressing this in large-scale systems requires energy-intensive mixing or solubilization. Maintaining high CO2 concentrations while removing O2 and other by-products adds complexity to chemolithoautotrophic setups. Additionally, CO2 dissolution lowers pH, creating acidic conditions that can inhibit microbial growth and metabolism, thus reducing PHA productivity. Alternatively, pH buffering strategies will be required.
- Impact from other gases: Including gases like H2, CO, and CH4 alongside CO2 impacts PHA production. H2 supports ATP generation, while CO may inhibit metabolism. CH4 provides an alternative carbon source, boosting yields through methanotrophic activity. Hence, optimizing gas composition is crucial for substrate utilization, enhancing productivity, and avoiding metabolic inefficiencies.
Addressing these challenges may require the development of multidisciplinary engineering strategies. This involves integrating bioprocess engineering (gas fermentation), genetic engineering, metabolic engineering, and bioelectrochemistry. Some industries are actively developing biotechnological platforms to convert CO2 and CH4 into PHAs (Table 6). Although microbial production of PHA from CO2 is actively studied, it typically yields lower PHB accumulations than sugar-based methods or even through organic wastes as feedstocks. Several strategies must be developed to improve productivity, enhance CO2 utilization, and produce copolymers with excellent physical properties. Exploitation of industrial off-gases is a possible solution to address the CO2 availability issue. However, industrial waste gases and syngas usually contain CO, which is toxic to most organisms, making it necessary to engineer CO2-utilizing microbes for CO tolerance. Strain development through genetic engineering and metabolic pathway optimization has shown promise and needs further development, particularly for large-scale applications. Additionally, metabolic engineering should focus on producing a range of copolymers. Life cycle assessments are essential to evaluate the CO2 mitigation potential and the impact on the circular economy. Simulation modelling of bioprocesses could also play a role in optimizing CO2 mitigation and PHA production processes. Moreover, novel online monitoring systems and enhanced process control strategies are necessary for efficient scale-up. Techno-economic analyses should also ensure that CO2 conversion into value-added products is cost-effective and sustainable for long-term industrial application.
11 Conclusion
This review systematically examines recent advancements in utilizing autotrophic microorganisms for PHA production using CO2 as a carbon source. Despite the promising potential, these technologies are still in their infancy and require further expansion to achieve industrial-scale production. Identifying and addressing the critical challenges of the CO2-based autotrophic processes through integrated engineering approaches is crucial for advancing autotrophic PHA production platforms. Comprehensive research on improving CO2 fixation, PHA biosynthetic pathways, scale-up for process engineering, gas fermentation simulation models, and gas mass transfer could enhance the direct conversion of CO2 to PHA by autotrophic microorganisms. Concurrently, ongoing efforts to strengthen promising strains’ adaptability and genetic traits are necessary. These combined efforts will create an eco-friendly process for PHA production using CO2 by autotrophic microorganisms.
Author contributions
GS: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review and editing. SE: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the United Kingdom Research and Innovation (UKRI) and Engineering and Physical Sciences Research Council (EPSRC) for funding Marie Skłodowska-Curie Actions (MSCA) Postdoctoral Fellowships Guarantee 2021 for the CO2BIOPOL project. The authors would like to acknowledge funding provided by the European Regional Development Funding (ERDF) as administered by the Welsh Government SMART Expertise Programme through the SMART Circle project (Reference: 122016/COL/003); ERDF as administered by the Welsh European Funding Office (WEFO) through the Beacon Plus project (Reference: c80851); and ERDF as administered by the Welsh Government Sêr Cymru Programme BioPOL4Life project (Reference: 80761-USW-274, West).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2025.1545438/full#supplementary-material
References
Akaraonye, E., Keshavarz, T., and Roy, I. (2010). Production of polyhydroxyalkanoates: the future green materials of choice. J. Chem. Technol. Biotechnol. 85, 732–743. doi:10.1002/jctb.2392
Akiyama, H., Okuhata, H., Onizuka, T., Kanai, S., Hirano, M., Tanaka, S., et al. (2011). Antibiotics-free stable polyhydroxyalkanoate (PHA) production from carbon dioxide by recombinant cyanobacteria. Bioresour. Technol. 102, 11039–11042. doi:10.1016/j.biortech.2011.09.058
Al Rowaihi, I. S., Kick, B., Grötzinger, S. W., Burger, C., Karan, R., Weuster-Botz, D., et al. (2018a). A two-stage biological gas to liquid transfer process to convert carbon dioxide into bioplastic. Bioresour. Technol. Rep. 1, 61–68. doi:10.1016/j.biteb.2018.02.007
Al Rowaihi, I. S., Paillier, A., Rasul, S., Karan, R., Grötzinger, S. W., Takanabe, K., et al. (2018b). Poly(3-hydroxybutyrate) production in an integrated electromicrobial setup: Investigation under stress-inducing conditions. PLoS One 13, e0196079. doi:10.1371/journal.pone.0196079
Alvarez Chavez, B., Raghavan, V., and Tartakovsky, B. (2022). A comparative analysis of biopolymer production by microbial and bioelectrochemical technologies. RSC Adv. 12, 16105–16118. doi:10.1039/d1ra08796g
Amelia, T. S. M., Govindasamy, S., Tamothran, A. M., Vigneswari, S., and Bhubalan, K. (2019). “Applications of PHA in agriculture,” in Biotechnological applications of polyhydroxyalkanoates. Editor V. C. Kalia (Singapore: Springer Singapore), 347–361. doi:10.1007/978-981-13-3759-8_13
Anjum, A., Zuber, M., Zia, K. M., Noreen, A., Anjum, M. N., and Tabasum, S. (2016). Microbial production of polyhydroxyalkanoates (PHAs) and its copolymers: a review of recent advancements. Int. J. Biol. Macromol. 89, 161–174. doi:10.1016/j.ijbiomac.2016.04.069
Arias, D. M., Fradinho, J. C., Uggetti, E., García, J., Oehmen, A., and Reis, M. A. M. (2018a). Polymer accumulation in mixed cyanobacterial cultures selected under the feast and famine strategy. Algal Res. 33, 99–108. doi:10.1016/j.algal.2018.04.027
Arias, D. M., Uggetti, E., García-Galán, M. J., and García, J. (2018b). Production of polyhydroxybutyrates and carbohydrates in a mixed cyanobacterial culture: effect of nutrients limitation and photoperiods. N. Biotechnol. 42, 1–11. doi:10.1016/j.nbt.2018.01.001
Azim, A. A., Cordara, A., Battaglino, B., and Re, A. (2020). “Use of carbon dioxide in polymer synthesis,” in Conversion of carbon dioxide into hydrocarbons vol. 2 technology (Cham: Springer International Publishing), 1–43. doi:10.1007/978-3-030-28638-5_1
Bae, J., Song, Y., Lee, H., Shin, J., Jin, S., Kang, S., et al. (2022). Valorization of C1 gases to value-added chemicals using acetogenic biocatalysts. Chem. Eng. J. 428, 131325. doi:10.1016/j.cej.2021.131325
Baioli, F., Vogli, L., and Righi, S. (2019). “Carbon footprint and energy requirement of the biopolymers polyhydroxyalkanoates: a review,” in Conference: XIII convegno della Rete italiana LCAAt (Rome), 13–14.
Banu, J. R., Kumar, M. D., Gunasekaran, M., and Kumar, G. (2019). Biopolymer production in bio electrochemical system: literature survey. Bioresour. Technol. Rep. 7, 100283. doi:10.1016/j.biteb.2019.100283
Behera, S., Priyadarshanee, M., and Das, S. (2022). Polyhydroxyalkanoates, the bioplastics of microbial origin: properties, biochemical synthesis, and their applications. Chemosphere 294, 133723. doi:10.1016/j.chemosphere.2022.133723
Bhati, R., and Mallick, N. (2015). Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) copolymer production by the diazotrophic cyanobacterium Nostoc muscorum Agardh: process optimization and polymer characterization. Algal Res. 7, 78–85. doi:10.1016/j.algal.2014.12.003
Bhati, R., and Mallick, N. (2016). Carbon dioxide and poultry waste utilization for production of polyhydroxyalkanoate biopolymers by Nostoc muscorum Agardh: a sustainable approach. J. Appl. Phycol. 28, 161–168. doi:10.1007/s10811-015-0573-x
Bian, B., Bajracharya, S., Xu, J., Pant, D., and Saikaly, P. E. (2020). Microbial electrosynthesis from CO2: challenges, opportunities and perspectives in the context of circular bioeconomy. Bioresour. Technol. 302, 122863. doi:10.1016/j.biortech.2020.122863
Bio-on and Gruppo Hera pursue development of CO2-based PHA (2018). Bioplastics MAGAZINE. Available online at: https://www.bioplasticsmagazine.com/en/news/meldungen/20181213Bio-on-and-Gruppo-Hera-pursue-development-of-CO2-based-PHA.php.
Bongers, L. (1970). Energy generation and utilization in hydrogen bacteria. J. Bacteriol. 104, 145–151. doi:10.1128/jb.104.1.145-151.1970
Bühler, K., and Lindberg, P. (2023). in Cyanobacteria in biotechnology (Cham: Springer International Publishing). doi:10.1007/978-3-031-33274-6
Campbell, J. I., Stevens, S., and Balkwill, D. (1982). Accumulation of poly-beta-hydroxybutyrate in Spirulina platensis. J. Bacteriol. 149, 361–363. doi:10.1128/jb.149.1.361-363.1982
Carpine, R., Du, W., Olivieri, G., Pollio, A., Hellingwerf, K. J., Marzocchella, A., et al. (2017). Genetic engineering of Synechocystis sp. PCC6803 for poly-β-hydroxybutyrate overproduction. Algal Res. 25, 117–127. doi:10.1016/j.algal.2017.05.013
Carpine, R., Olivieri, G., Hellingwerf, K. J., Pollio, A., and Marzocchella, A. (2020). Industrial production of poly-β-hydroxybutyrate from CO2: can cyanobacteria meet this challenge? Processes 8, 323. doi:10.3390/pr8030323
Carr, N. G. (1966). The occurrence of poly-β-hydroxybutyrate in the blue-green alga, Chlorogloea fritschii. Biochimica Biophysica Acta (BBA) - Biophysics Incl. Photosynth. 120, 308–310. doi:10.1016/0926-6585(66)90353-0
Cestellos-Blanco, S., Friedline, S., Sander, K. B., Abel, A. J., Kim, J. M., Clark, D. S., et al. (2021). Production of PHB from CO2-derived acetate with minimal processing assessed for space biomanufacturing. Front. Microbiol. 12, 700010. doi:10.3389/fmicb.2021.700010
Chen, G.-Q., and Jiang, X.-R. (2018). Engineering microorganisms for improving polyhydroxyalkanoate biosynthesis. Curr. Opin. Biotechnol. 53, 20–25. doi:10.1016/j.copbio.2017.10.008
Chen, X., Cao, Y., Li, F., Tian, Y., and Song, H. (2018). Enzyme-assisted microbial electrosynthesis of poly(3-hydroxybutyrate) via CO2 bioreduction by engineered Ralstonia eutropha. ACS Catal. 8 (5), 4429–4437. doi:10.1021/acscatal.8b00226
Choi, J., and Lee, S. Y. (1999). Factors affecting the economics of polyhydroxyalkanoate production by bacterial fermentation. Appl. Microbiol. Biotechnol. 51, 13–21. doi:10.1007/s002530051357
Choi, S. Y., Lee, Y., Yu, H. E., Cho, I. J., Kang, M., and Lee, S. Y. (2023). Sustainable production and degradation of plastics using microbes. Nat. Microbiol. 8, 2253–2276. doi:10.1038/s41564-023-01529-1
Claassens, N. J., Sousa, D. Z., dos Santos, V. A. P. M., de Vos, W. M., and van der Oost, J. (2016). Harnessing the power of microbial autotrophy. Nat. Rev. Microbiol. 14, 692–706. doi:10.1038/nrmicro.2016.130
CO2BioClean (2024). Government of Hesse State Minister opens revolutionary CO2BioClean pilot plant. Available online at: https://co2bioclean.com/gvt-of-hesse-state-minister-opens-co2bioclean-pilot-plant/.
Conners, E. M., Rengasamy, K., and Bose, A. (2023). The phototrophic bacteria Rhodomicrobium spp. are novel chassis for bioplastic production. bioRxiv 2023 (17), 541187. doi:10.1101/2023.05.17.541187
Conners, E. M., Rengasamy, K., Ranaivoarisoa, T., and Bose, A. (2024). The phototrophic purple non-sulfur bacteria Rhodomicrobium spp. are novel chassis for bioplastic production. Microb. Biotechnol. 17, e14552. doi:10.1111/1751-7915.14552
Costa, J. A. V., Moreira, J. B., Lucas, B. F., Braga, V. da S., Cassuriaga, A. P. A., and Morais, M. G. de (2018). Recent advances and future perspectives of PHB production by cyanobacteria. Ind. Biotechnol. 14, 249–256. doi:10.1089/ind.2018.0017
Costa, S. S., Miranda, A. L., de Morais, M. G., Costa, J. A. V., and Druzian, J. I. (2019). Microalgae as source of polyhydroxyalkanoates (PHAs) — a review. Int. J. Biol. Macromol. 131, 536–547. doi:10.1016/j.ijbiomac.2019.03.099
Damrow, R., Maldener, I., and Zilliges, Y. (2016). The multiple functions of common microbial carbon polymers, glycogen and PHB, during stress responses in the non-diazotrophic cyanobacterium Synechocystis sp. PCC 6803. Front. Microbiol. 7, 966. doi:10.3389/fmicb.2016.00966
Daniel, H., Matthias, R., Philipp, F., T, K. S., Laura, M.-G., P, B. R., et al. (2016). Synthesis gas (Syngas)-Derived medium-chain-length polyhydroxyalkanoate synthesis in engineered Rhodospirillum rubrum. Appl. Environ. Microbiol. 82, 6132–6140. doi:10.1128/AEM.01744-16
Daniell, J., Köpke, M., and Simpson, S. (2012). Commercial biomass syngas fermentation. Energies 5, 5372–5417. doi:10.3390/en5125372
Darani, K. K., Vasheghani-Farahani, E., and Tanaka, K. (2006). Hydrogen oxidizing bacteria as poly(hydroxybutyrate) producers. Iran. J. Biotechnol. 4, 193–196. Available online at: https://www.ijbiotech.com/article_6996.html.
Debabov, V. G. (2021). Acetogens: biochemistry, bioenergetics, genetics, and biotechnological potential. Microbiol. (N Y) 90, 273–297. doi:10.1134/S0026261721030024
Demler, M., and Weuster-Botz, D. (2011). Reaction engineering analysis of hydrogenotrophic production of acetic acid by Acetobacterium woodii. Biotechnol. Bioeng. 108, 470–474. doi:10.1002/bit.22935
De Philippis, R., Sili, C., and Vincenzini, M. (1992). Glycogen and poly- -hydroxybutyrate synthesis in Spirulina maxima. J. Gen. Microbiol. 138, 1623–1628. doi:10.1099/00221287-138-8-1623
de Souza Pinto Lemgruber, R., Valgepea, K., Tappel, R., Behrendorff, J. B., Palfreyman, R. W., Plan, M., et al. (2019). Systems-level engineering and characterisation of Clostridium autoethanogenum through heterologous production of poly-3-hydroxybutyrate (PHB). Metab. Eng. 53, 14–23. doi:10.1016/j.ymben.2019.01.003
Dinges, I., Depentori, I., Gans, L., Holtmann, D., Waldvogel, S. R., and Stöckl, M. (2024). Coupling of CO2 electrolysis with parallel and semi-automated biopolymer synthesis – ex-cell and without downstream processing. ChemSusChem 17, e202301721. doi:10.1002/cssc.202301721
Do, Y. S., Smeenk, J., Broer, K. M., Kisting, C. J., Brown, R., Heindel, T. J., et al. (2007). Growth of Rhodospirillum rubrum on synthesis gas: conversion of CO to H2 and poly-β-hydroxyalkanoate. Biotechnol. Bioeng. 97, 279–286. doi:10.1002/bit.21226
Durall, C., and Lindblad, P. (2015). Mechanisms of carbon fixation and engineering for increased carbon fixation in cyanobacteria. Algal Res. 11, 263–270. doi:10.1016/j.algal.2015.07.002
Durão, P., Aigner, H., Nagy, P., Mueller-Cajar, O., Hartl, F. U., and Hayer-Hartl, M. (2015). Opposing effects of folding and assembly chaperones on evolvability of Rubisco. Nat. Chem. Biol. 11, 148–155. doi:10.1038/nchembio.1715
Dutt, V., and Srivastava, S. (2018). Novel quantitative insights into carbon sources for synthesis of poly hydroxybutyrate in Synechocystis PCC 6803. Photosynth Res. 136, 303–314. doi:10.1007/s11120-017-0464-x
Eberly, J. O., and Ely, R. L. (2012). Photosynthetic accumulation of carbon storage compounds under CO2 enrichment by the thermophilic cyanobacterium Thermosynechococcus elongatus. J. Ind. Microbiol. Biotechnol. 39, 843–850. doi:10.1007/s10295-012-1092-2
Esteve, I., Montesinos, E., Mitchell, J. G., and Guerrero, R. (1990). A quantitative ultrastructural study of Chromatium minus in the bacterial layer of Lake Cisó (Spain). Arch. Microbiol. 153, 316–323. doi:10.1007/BF00248999
European Bioplastics (2023). Bioplastics market development update 2023. Available online at: www.european-bioplastics.org (Accessed April, 2025).
European Commission (2021). 2030 climate and energy framework EC policies. Available online at: https://climate.ec.europa.eu/eu-action/climate-strategies-targets/2030-climate-targets_en.
Fink, P., Menzel, C., Kwon, J. H., and Forchhammer, K. (2025). A novel recombinant PHB production platform in filamentous cyanobacteria avoiding nitrogen starvation while preserving cell viability. Microb. Cell Fact. 24, 43. doi:10.1186/s12934-025-02650-y
Flaiz, M., and Sousa, D. Z. (2024). Accelerate acetogenic bioproduction: acetogens as sustainable producers of biocommodities. Curr. Opin. Syst. Biol. 37, 100500. doi:10.1016/j.coisb.2023.100500
Flüchter, S., Follonier, S., Schiel-Bengelsdorf, B., Bengelsdorf, F. R., Zinn, M., and Dürre, P. (2019). Anaerobic production of poly(3-hydroxybutyrate) and its precursor 3-hydroxybutyrate from synthesis gas by autotrophic Clostridia. Biomacromolecules 20, 3271–3282. doi:10.1021/acs.biomac.9b00342
Francisco, M., Rodríguez-Galán, M., Vega, F., Alonso-Fariñas, B., Vilches, L. F., and Navarrete, B. (2019). Carbon capture and utilization technologies: a literature review and recent advances. Energy Sources, Part A Recovery, Util. Environ. Eff. 41 (12), 1403–1433. doi:10.1080/15567036.2018.1548518
Garcia-Gonzalez, L., and De Wever, H. (2017). Valorisation of CO2-rich off-gases to biopolymers through biotechnological process. FEMS Microbiol. Lett. 364, fnx196. doi:10.1093/femsle/fnx196
Garcia-Gonzalez, L., and De Wever, H. (2018). Acetic acid as an indirect sink of CO2 for the synthesis of polyhydroxyalkanoates (PHA): comparison with PHA production processes directly using CO2 as feedstock. Appl. Sci. 8, 1416. doi:10.3390/app8091416
Garcia-Gonzalez, L., Mozumder, Md. S. I., Dubreuil, M., Volcke, E. I. P., and De Wever, H. (2015). Sustainable autotrophic production of polyhydroxybutyrate (PHB) from CO2 using a two-stage cultivation system. Catal. Today 257, 237–245. doi:10.1016/j.cattod.2014.05.025
Gaspar, T.-O., Koren, N., and Gregory, S. (2000). Identification and analysis of the polyhydroxyalkanoate-specific β-ketothiolase and acetoacetyl coenzyme A reductase genes in the cyanobacterium Synechocystis sp. strain PCC6803. Appl. Environ. Microbiol. 66, 4440–4448. doi:10.1128/AEM.66.10.4440-4448.2000
Getino, L., Martín, J. L., and Chamizo-Ampudia, A. (2024). A review of polyhydroxyalkanoates: characterization, production, and application from waste. Microorganisms 12, 2028. doi:10.3390/microorganisms12102028
González-Rojo, S., Paniagua-García, A. I., and Díez-Antolínez, R. (2024). Advances in microbial biotechnology for sustainable alternatives to petroleum-based plastics: a comprehensive review of polyhydroxyalkanoate production. Microorganisms 12, 1668. doi:10.3390/microorganisms12081668
Gracioso, L. H., Bellan, A., Karolski, B., Cardoso, L. O. B., Perpetuo, E. A., Nascimento, C. A. O. do, et al. (2021). Light excess stimulates Poly-beta-hydroxybutyrate yield in a mangrove-isolated strain of Synechocystis sp. Bioresour. Technol. 320, 124379. doi:10.1016/j.biortech.2020.124379
Gregory, D. A., Taylor, C. S., Fricker, A. T. R., Asare, E., Tetali, S. S. V., Haycock, J. W., et al. (2022). Polyhydroxyalkanoates and their advances for biomedical applications. Trends Mol. Med. 28, 331–342. doi:10.1016/j.molmed.2022.01.007
Grignard, B., Gennen, S., Jérôme, C., Kleij, A. W., and Detrembleur, C. (2019). Advances in the use of CO2 as a renewable feedstock for the synthesis of polymers. Chem. Soc. Rev. 48, 4466–4514. doi:10.1039/C9CS00047J
Gupta, J. K., Rai, P., Jain, K. K., and Srivastava, S. (2020). Overexpression of bicarbonate transporters in the marine cyanobacterium Synechococcus sp. PCC 7002 increases growth rate and glycogen accumulation. Biotechnol. Biofuels 13, 17. doi:10.1186/s13068-020-1656-8
Haas, R., Jin, B., Zepf, F., and Behrens, M. (2021). Economic feasibility of CO2-based bioprocesses: a case study. J. Clean. Prod. 300, 126994. doi:10.1016/j.jclepro.2021.126994
Haase, S. M., Huchzermeyer, B., and Rath, T. (2012). PHB accumulation in Nostoc muscorum under different carbon stress situations. J. Appl. Phycol. 24, 157–162. doi:10.1007/s10811-011-9663-6
Haddadi, M. H., Asadolahi, R., and Negahdari, B. (2019). The bioextraction of bioplastics with focus on polyhydroxybutyrate: a review. Int. J. Environ. Sci. Technol. 16, 3935–3948. doi:10.1007/s13762-019-02352-0
Hai, T., Hein, S., and Steinbüchel, A. (2001). Multiple evidence for widespread and general occurrence of type-III PHA synthases in cyanobacteria and molecular characterization of the PHA synthases from two thermophilic cyanobacteria: Chlorogloeopsis fritschii PCC 6912 and Synechococcus sp. strain MA19. Microbiology 147, 3047–3060. doi:10.1099/00221287-147-11-3047
Heinrich, D., Raberg, M., and Steinbüchel, A. (2015). Synthesis of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) from unrelated carbon sources in engineered Rhodospirillum rubrum. FEMS Microbiol. Lett. 362, fnv038. doi:10.1093/femsle/fnv038
Higuchi-Takeuchi, M., Morisaki, K., and Numata, K. (2016a). A screening method for the isolation of polyhydroxyalkanoate-producing purple non-sulfur photosynthetic bacteria from natural seawater. Front. Microbiol. 7, 1509. doi:10.3389/fmicb.2016.01509
Higuchi-Takeuchi, M., Morisaki, K., Toyooka, K., and Numata, K. (2016b). Synthesis of high-molecular-weight polyhydroxyalkanoates by marine photosynthetic purple bacteria. PLoS One 11, e0160981. doi:10.1371/journal.pone.0160981
Higuchi-Takeuchi, M., and Numata, K. (2019). Marine purple photosynthetic bacteria as sustainable microbial production hosts. Front. Bioeng. Biotechnol. 7, 258. doi:10.3389/fbioe.2019.00258
Höfele, F., and Dürre, P. (2023). Production of potential substitutes for conventional plastics using metabolically engineered Acetobacterium woodii. Fermentation 9, 799. doi:10.3390/fermentation9090799
Hondo, S., Takahashi, M., Osanai, T., Matsuda, M., Hasunuma, T., Tazuke, A., et al. (2015). Genetic engineering and metabolite profiling for overproduction of polyhydroxybutyrate in cyanobacteria. J. Biosci. Bioeng. 120, 510–517. doi:10.1016/j.jbiosc.2015.03.004
Hwang, H. W., Yoon, J., Min, K., Kim, M.-S., Kim, S.-J., Cho, D. H., et al. (2020). Two-stage bioconversion of carbon monoxide to biopolymers via formate as an intermediate. Chem. Eng. J. 389, 124394. doi:10.1016/j.cej.2020.124394
Inui, M., Roh, J. H., Momma, K., and Yukawa, H. (1998). “Studies on CO2 fixation in PNSB: analysis of CO2 metabolism in purple non-sulfur bacteria,” in Studies in surface science and catalysis. Editors T. Inui, M. Anpo, K. Izui, S. Yanagida, and T. Yamaguchi (Elsevier), 597–600. doi:10.1016/S0167-2991(98)80830-6
Ishizaki, A., and Tanaka, K. (1990). Batch culture of Alcaligenes eutrophus ATCC 17697T using recycled gas closed circuit culture system. J. Ferment Bioeng. 69, 170–174. doi:10.1016/0922-338X(90)90041-T
Ishizaki, A., and Tanaka, K. (1991). Production of poly-β-hydroxybutyric acid from carbon dioxide by Alcaligenes eutrophus ATCC 17697T. J. Ferment Bioeng. 71, 254–257. doi:10.1016/0922-338X(91)90277-N
Ishizaki, A., Tanaka, K., and Taga, N. (2001). Microbial production of poly- D- 3-hydroxybutyrate from CO2. Appl. Microbiol. Biotechnol. 57, 6–12. doi:10.1007/s002530100775
Ishizaki, A., Tanaka, K., Takeshita, T., Kanemaru, T., Shimoji, T., and Kawano, T. (1993). Equipment and operation for fermentative PHB production using gaseous substrate to guarantee safety from explosion. J. Chem. Eng. Jpn. 26, 225–227. doi:10.1252/jcej.26.225
Jia, D., He, M., Tian, Y., Shen, S., Zhu, X., Wang, Y., et al. (2021). Metabolic engineering of gas-fermenting Clostridium ljungdahlii for efficient Co-production of isopropanol, 3-hydroxybutyrate, and ethanol. ACS Synth. Biol. 10, 2628–2638. doi:10.1021/acssynbio.1c00235
Jiang, G., Hill, D. J., Kowalczuk, M., Johnston, B., Adamus, G., Irorere, V. U., et al. (2016). Carbon sources for polyhydroxyalkanoates and an integrated biorefinery. Int. J. Mol. Sci. 17, 1157. doi:10.3390/ijms17071157
Kadiyala, G. (2014). Isolation, purification and screening of biodegradable polymer PHB producing cyanobacteria from marine and fresh water resources. Iran. J. Energy Environ. 5, 94–100. doi:10.5829/idosi.ijee.2014.05.01.14
Kaewbai-ngam, A., Incharoensakdi, A., and Monshupanee, T. (2016). Increased accumulation of polyhydroxybutyrate in divergent cyanobacteria under nutrient-deprived photoautotrophy: an efficient conversion of solar energy and carbon dioxide to polyhydroxybutyrate by Calothrix scytonemicola TISTR 8095. Bioresour. Technol. 212, 342–347. doi:10.1016/j.biortech.2016.04.035
Kamennaya, N. A., Ahn, S., Park, H., Bartal, R., Sasaki, K. A., Holman, H.-Y., et al. (2015). Installing extra bicarbonate transporters in the cyanobacterium Synechocystis sp. PCC6803 enhances biomass production. Metab. Eng. 29, 76–85. doi:10.1016/j.ymben.2015.03.002
Kamravamanesh, D., Kovacs, T., Pflügl, S., Druzhinina, I., Kroll, P., Lackner, M., et al. (2018). Increased poly-β-hydroxybutyrate production from carbon dioxide in randomly mutated cells of cyanobacterial strain Synechocystis sp. PCC 6714: mutant generation and characterization. Bioresour. Technol. 266, 34–44. doi:10.1016/j.biortech.2018.06.057
Kamravamanesh, D., Pflügl, S., Nischkauer, W., Limbeck, A., Lackner, M., and Herwig, C. (2017). Photosynthetic poly-β-hydroxybutyrate accumulation in unicellular cyanobacterium Synechocystis sp. PCC 6714. Amb. Express 7, 143. doi:10.1186/s13568-017-0443-9
Kamravamanesh, D., Slouka, C., Limbeck, A., Lackner, M., and Herwig, C. (2019). Increased carbohydrate production from carbon dioxide in randomly mutated cells of cyanobacterial strain Synechocystis sp. PCC 6714: bioprocess understanding and evaluation of productivities. Bioresour. Technol. 273, 277–287. doi:10.1016/j.biortech.2018.11.025
Kaneka Corporation (2023). Development of polymer synthesis technology by microorganisms using CO2 as direct raw material. Available online at: https://www.kaneka.co.jp/en/topics/news/2023/ennr2303221.
Kanno, M., Carroll, A. L., and Atsumi, S. (2017). Global metabolic rewiring for improved CO2 fixation and chemical production in cyanobacteria. Nat. Commun. 8, 14724. doi:10.1038/ncomms14724
Karmann, S., Panke, S., and Zinn, M. (2017). Engineering Cupriavidus necator for CO2-based production of high-value chemicals. Microb. Cell Factories 16, 103. doi:10.1186/s12934-017-0727-6
Karmann, S., Panke, S., and Zinn, M. (2019). Fed-batch cultivations of Rhodospirillum rubrum under multiple nutrient-limited growth conditions on syngas as a novel option to produce poly(3-hydroxybutyrate) (PHB). Front. Bioeng. Biotechnol. 7, 59. doi:10.3389/fbioe.2019.00059
Kedia, G., Passanha, P., Dinsdale, R. M., Guwy, A. J., and Esteves, S. R. (2014). Evaluation of feeding regimes to enhance PHA production using acetic and butyric acids by a pure culture of Cupriavidus necator. Biotechnol. Bioprocess Eng. 19, 989–995. doi:10.1007/s12257-014-0144-z
Khetkorn, W., Incharoensakdi, A., Lindblad, P., and Jantaro, S. (2016). Enhancement of poly-3-hydroxybutyrate production in Synechocystis sp. PCC 6803 by overexpression of its native biosynthetic genes. Bioresour. Technol. 214, 761–768. doi:10.1016/j.biortech.2016.05.014
Khosravi-Darani, K., Mokhtari, Z.-B., Amai, T., and Tanaka, K. (2013a). Microbial production of poly(hydroxybutyrate) from C1 carbon sources. Appl. Microbiol. Biotechnol. 97, 1407–1424. doi:10.1007/s00253-012-4649-0
Khosravi-Darani, K., Mokhtari, Z. B., Amai, T., and Tanaka, K. (2013b). Production of polyhydroxyalkanoates from hydrogen and carbon dioxide by autotrophic bacteria. Int. J. Hydrogen Energy 38, 10244–10255. doi:10.1016/j.ijhydene.2013.06.040
Koch, M., Bruckmoser, J., Scholl, J., Hauf, W., Rieger, B., and Forchhammer, K. (2020). Maximizing PHB content in Synechocystis sp. PCC 6803: a new metabolic engineering strategy based on the regulator PirC. Microb. Cell Fact. 19, 231. doi:10.1186/s12934-020-01491-1
Kodama, T., Igarashi, Y., and Minoda, Y. (1975). Isolation and culture conditions of a bacterium grown on hydrogen and carbon dioxide. Agric. Biol. Chem. 39, 77–82. doi:10.1080/00021369.1975.10861578
Koller, M. (2017). Production of poly hydroxyalkanoate (PHA) biopolyesters by extremophiles? MOJ Poly Sci. 1, 69–85. doi:10.15406/mojps.2017.01.00011
Koller, M., and Rittmann, S. K.-M. R. (2022). Haloarchaea as emerging big players in future polyhydroxyalkanoate bioproduction: review of trends and perspectives. Curr. Res. Biotechnol. 4, 377–391. doi:10.1016/j.crbiot.2022.09.002
Korkakaki, E., van Loosdrecht, M. C. M., and Kleerebezem, R. (2017). Impact of phosphate limitation on PHA production in a feast-famine process. Water Res. 126, 472–480. doi:10.1016/j.watres.2017.09.031
Kourmentza, C., Plácido, J., Venetsaneas, N., Burniol-Figols, A., Varrone, C., Gavala, H. N., et al. (2017). Recent advances and challenges towards sustainable polyhydroxyalkanoate (PHA) production. Bioengineering 4, 55. doi:10.3390/bioengineering4020055
Ku, J. T., and Lan, E. I. (2018). A balanced ATP driving force module for enhancing photosynthetic biosynthesis of 3-hydroxybutyrate from CO2. Metab. Eng. 46, 35–42. doi:10.1016/j.ymben.2018.02.004
Kumar, M., Sundaram, S., Gnansounou, E., Larroche, C., and Thakur, I. S. (2018a). Carbon dioxide capture, storage and production of biofuel and biomaterials by bacteria: a review. Bioresour. Technol. 247, 1059–1068. doi:10.1016/j.biortech.2017.09.050
Kumar, P., Chandrasekhar, K., Kumari, A., Sathiyamoorthi, E., and Kim, B. (2018b). Electro-fermentation in aid of bioenergy and biopolymers. Energies 11, 343. doi:10.3390/en11020343
Kumi, P. J., Henley, A., Shana, A., Wilson, V., and Esteves, S. R. (2016). Volatile fatty acids platform from thermally hydrolysed secondary sewage sludge enhanced through recovered micronutrients from digested sludge. Water Res. 100, 267–276. doi:10.1016/j.watres.2016.05.030
Lama, L., Nicolaus, B., Calandrelli, V., Manca, M. C., Romano, I., and Gambacorta, A. (1996). Effect of growth conditions on endo- and exopolymer biosynthesis in Anabaena cylindrica 10C. Phytochemistry 42, 655–659. doi:10.1016/0031-9422(95)00985-X
Langsdorf, A., Schütz, J. P., Ulber, R., Stöckl, M., and Holtmann, D. (2024). Production of polyhydroxybutyrate from industrial flue gas by microbial electrosynthesis. J. CO2 Util. 83, 102800. doi:10.1016/j.jcou.2024.102800
Lemoigne, M. (1926). Produit de deshydratation et de polymerisation de l’acide β-oxybutyrique. Bull. Soc. Chim. Biol. 8, 770–782.
Li, M., Ning, P., Sun, Y., Luo, J., and Yang, J. (2022). Characteristics and application of Rhodopseudomonas palustris as a microbial cell factory. Front. Bioeng. Biotechnol. 10, 897003. doi:10.3389/fbioe.2022.897003
Li, M., and Wilkins, M. R. (2020). Recent advances in polyhydroxyalkanoate production: feedstocks, strains and process developments. Int. J. Biol. Macromol. 156, 691–703. doi:10.1016/j.ijbiomac.2020.04.082
Li, S., Kim, M., Kong, D. S., Min, K., Wu, G., Cui, M., et al. (2023). Electron uptake from solid electrodes promotes the more efficient conversion of CO2 to polyhydroxybutyrate by using Rhodobacter sphaeroides. Chem. Eng. J. 469, 143785. doi:10.1016/j.cej.2023.143785
Li, Z., Xin, X., Xiong, B., Zhao, D., Zhang, X., and Bi, C. (2020). Engineering the Calvin–Benson–Bassham cycle and hydrogen utilization pathway of Ralstonia eutropha for improved autotrophic growth and polyhydroxybutyrate production. Microb. Cell Fact. 19, 228. doi:10.1186/s12934-020-01494-y
Liebergesell, M., Hustede, E., Timm, A., Steinbüchel, A., Fuller, R. C., Lenz, R. W., et al. (1991). Formation of poly(3-hydroxyalkanoates) by phototrophic and chemolithotrophic bacteria. Arch. Microbiol. 155, 415–421. doi:10.1007/BF00244955
Liebergesell, M., Rahalkar, S., and Steinbüchel, A. (2000). Analysis of the Thiocapsa pfennigii polyhydroxyalkanoate synthase: subcloning, molecular characterization and generation of hybrid synthases with the corresponding Chromatium vinosum enzyme. Appl. Microbiol. Biotechnol. 54, 186–194. doi:10.1007/s002530000375
Liu, C., Gallagher, J. J., Sakimoto, K. K., Nichols, E. M., Chang, C. J., Chang, M. C. Y., et al. (2015a). Nanowire–bacteria hybrids for unassisted solar carbon dioxide fixation to value-added chemicals. Nano Lett. 15, 3634–3639. doi:10.1021/acs.nanolett.5b01254
Liu, J., Zhou, Z., Li, H., Yang, X., Wang, Z., Xiao, J., et al. (2024). Current status and challenges in the application of microbial PHA particles. Particuology 87, 286–302. doi:10.1016/j.partic.2023.08.011
Liu, Q., Wu, L., Jackstell, R., and Beller, M. (2015b). Using carbon dioxide as a building block in organic synthesis. Nat. Commun. 6, 5933. doi:10.1038/ncomms6933
Logan, B. E., Rossi, R., Ragab, A., and Saikaly, P. E. (2019). Electroactive microorganisms in bioelectrochemical systems. Nat. Rev. Microbiol. 17, 307–319. doi:10.1038/s41579-019-0173-x
Lu, Y., and Yu, J. (2017). Gas mass transfer with microbial CO2 fixation and poly(3-hydroxybutyrate) synthesis in a packed bed bioreactor. Biochem. Eng. J. 122, 13–21. doi:10.1016/j.bej.2017.02.013
Luengo, J. M., Garcı́a, B., Sandoval, A., Naharro, G., and Olivera, E. R. (2003). Bioplastics from microorganisms. Curr. Opin. Microbiol. 6, 251–260. doi:10.1016/s1369-5274(03)00040-7
Madison, L. L., and Huisman, G. W. (1999). Metabolic engineering of poly(3-hydroxyalkanoates): from DNA to plastic. Microbiol. Mol. Biol. Rev. 63, 21–53. doi:10.1128/mmbr.63.1.21-53.1999
Martins, R. G., Severo Gonçalves, I., de Morais, M. G., and Costa, J. A. V. (2014). Bioprocess engineering aspects of biopolymer production by the cyanobacterium Spirulina strain LEB 18. Int. J. Polym. Sci. 2014, 895237. doi:10.1155/2014/895237
Matlakowska, R., and Sklodowska, A. (2007). Adaptive responses of chemolithoautotrophic acidophilic Acidithiobacillus ferrooxidans to sewage sludge. J. Appl. Microbiol. 102, 1485–1498. doi:10.1111/j.1365-2672.2006.03208.x
Ma, R., Li, J., Tyagi, R. D., and Zhang, X. (2024). Carbon dioxide and methane as carbon source for the production of polyhydroxyalkanoates and concomitant carbon fixation. Bioresour Technol. 391 (Pt A), 129977. doi:10.1016/j.biortech.2023.129977
Meixner, K., Kovalcik, A., Sykacek, E., Gruber-Brunhumer, M., Zeilinger, W., Markl, K., et al. (2018). Cyanobacteria Biorefinery — production of poly(3-hydroxybutyrate) with Synechocystis salina and utilisation of residual biomass. J. Biotechnol. 265, 46–53. doi:10.1016/j.jbiotec.2017.10.020
Michael, K., Christophe, M., FungMin, L., H, T. J., S, A. M., J, C. J., et al. (2011). 2,3-Butanediol production by acetogenic bacteria, an alternative route to chemical synthesis, using industrial waste gas. Appl. Environ. Microbiol. 77, 5467–5475. doi:10.1128/AEM.00355-11
Mittermair, S., Richter, J., Doppler, P., Trenzinger, K., Nicoletti, C., Forsich, C., et al. (2021). Impact of exoD gene knockout on the polyhydroxybutyrate overaccumulating mutant Mt_a24. Int. J. Biobased Plastics 3, 1–18. doi:10.1080/24759651.2020.1863020
Miyahara, Y., Yamamoto, M., Thorbecke, R., Mizuno, S., and Tsuge, T. (2020). Autotrophic biosynthesis of polyhydroxyalkanoate by Ralstonia eutropha from non-combustible gas mixture with low hydrogen content. Biotechnol. Lett. 42, 1655–1662. doi:10.1007/s10529-020-02876-3
Miyake, M., Erata, M., and Asada, Y. (1996). A thermophilic cyanobacterium, Synechococcus sp. MA19, capable of accumulating poly-β-hydroxybutyrate. J. Ferment Bioeng. 82, 512–514. doi:10.1016/S0922-338X(97)86995-4
Mongili, B., and Fino, D. (2021). Carbon monoxide fermentation to bioplastic: the effect of substrate adaptation on Rhodospirillum rubrum. Biomass Convers. Biorefin 11, 705–714. doi:10.1007/s13399-020-00876-x
Monshupanee, T., and Incharoensakdi, A. (2014). Enhanced accumulation of glycogen, lipids and polyhydroxybutyrate under optimal nutrients and light intensities in the cyanobacterium Synechocystis sp. PCC 6803. J. Appl. Microbiol. 116, 830–838. doi:10.1111/jam.12409
Morlino, M. S., Serna García, R., Savio, F., Zampieri, G., Morosinotto, T., Treu, L., et al. (2023). Cupriavidus necator as a platform for polyhydroxyalkanoate production: an overview of strains, metabolism, and modeling approaches. Biotechnol. Adv. 69, 108264. doi:10.1016/j.biotechadv.2023.108264
Możejko-Ciesielska, J., and Kiewisz, R. (2016). Bacterial polyhydroxyalkanoates: still fabulous? Microbiol. Res. 192, 271–282. doi:10.1016/j.micres.2016.07.010
Możejko-Ciesielska, J., Marciniak, P., and Szacherska, K. (2019). Polyhydroxyalkanoates synthesized by Aeromonas species: trends and challenges. Polymers 11, 1328. doi:10.3390/polym11081328
Müller, J., Becker, J., and Wittmann, C. (2023). Economic and environmental evaluation of sustainable PHA production pathways. Sustain. Chem. Pharm. 30, 100846. doi:10.1016/j.scp.2023.100846
Muneer, F., Rasul, I., Azeem, F., Siddique, M. H., Zubair, M., and Nadeem, H. (2020). Microbial polyhydroxyalkanoates (PHAs): efficient replacement of synthetic polymers. J. Polym. Environ. 28, 2301–2323. doi:10.1007/s10924-020-01772-1
Muthuraj, R., and Mekonnen, T. (2018). Recent progress in carbon dioxide (CO2) as feedstock for sustainable materials development: Co-polymers and polymer blends. Polymer 145, 348–373. doi:10.1016/j.polymer.2018.04.078
Nangle, S. N., Ziesack, M., Buckley, S., Trivedi, D., Loh, D. M., Nocera, D. G., et al. (2020). Valorization of CO2 through lithoautotrophic production of sustainable chemicals in Cupriavidus necator. Metab. Eng. 62, 207–220. doi:10.1016/j.ymben.2020.09.002
Narancic, T., Verstichel, S., Reddy Chaganti, S., Morales-Gamez, L., Kenny, S. T., De Wilde, B., et al. (2020). Environmental impacts of bioplastic use: CO2 sequestration and end-of-life options. Mater. Today Sustain. 7 (8), 100031. doi:10.1016/j.mtsust.2020.100031
Nastro, R. A., Kuppam, C., Toscanesi, M., Trifuoggi, M., Pietrelli, A., Pasquale, V., et al. (2025). Bio-electrosynthesis of polyhydroxybutyrate and surfactants in microbial fuel cells: a preliminary study. Front. Microbiol. 16, 1372302. doi:10.3389/fmicb.2025.1372302
Newlight Technologies (2024). AirCarbon. Available online at: https://www.newlight.com/ (Accessed April, 2025).
Nishioka, M., Nakai, K., Miyake, M., Asada, Y., and Taya, M. (2001). Production of poly-β-hydroxybutyrate by thermophilic cyanobacterium, Synechococcus sp. MA19, under phosphate-limited conditions. Biotechnol. Lett. 23, 1095–1099. doi:10.1023/A:1010551614648
Obruca, S., Sedlacek, P., Slaninova, E., Fritz, vI., Daffert, C., Meixner, K., et al. (2020). Novel unexpected functions of PHA granules. Appl. Microbiol. Biotechnol. 104, 4795–4810. doi:10.1007/s00253-020-10568-1
Orthwein, T., Scholl, J., Spät, P., Lucius, S., Koch, M., Macek, B., et al. (2021). The novel PII-interactor PirC identifies phosphoglycerate mutase as key control point of carbon storage metabolism in cyanobacteria. Proc. Natl. Acad. Sci. 118, e2019988118. doi:10.1073/pnas.2019988118
Osanai, T., Numata, K., Oikawa, A., Kuwahara, A., Iijima, H., Doi, Y., et al. (2013). Increased bioplastic production with an RNA polymerase sigma factor SigE during nitrogen starvation in Synechocystis sp. DNA Res. 20, 525–535. doi:10.1093/dnares/dst028
Osanai, T., Oikawa, A., Numata, K., Kuwahara, A., Iijima, H., Doi, Y., et al. (2014). Pathway-level acceleration of glycogen catabolism by a response regulator in the cyanobacterium Synechocystis species PCC 6803. Plant Physiol. 164, 1831–1841. doi:10.1104/pp.113.232025
Panda, B., and Mallick, N. (2007). Enhanced poly-β-hydroxybutyrate accumulation in a unicellular cyanobacterium, Synechocystis sp. PCC 6803. Lett. Appl. Microbiol. 44, 194–198. doi:10.1111/j.1472-765X.2006.02048.x
Panda, B., Sharma, L., and Mallick, N. (2005). Poly-β-hydroxybutyrate accumulation in Nostoc muscorum and Spirulina platensis under phosphate limitation. J. Plant Physiol. 162, 1376–1379. doi:10.1016/j.jplph.2005.05.002
Pandey, A., Adama, N., Adjallé, K., and Blais, J.-F. (2022). Sustainable applications of polyhydroxyalkanoates in various fields: a critical review. Int. J. Biol. Macromol. 221, 1184–1201. doi:10.1016/j.ijbiomac.2022.09.098
Panich, J., Fong, B., and Singer, S. W. (2021). Metabolic engineering of Cupriavidus necator H16 for sustainable biofuels from CO2. Trends Biotechnol. 39, 412–424. doi:10.1016/j.tibtech.2021.01.001
Park, H., He, H., Yan, X., Liu, X., Scrutton, N. S., and Chen, G.-Q. (2024). PHA is not just a bioplastic. Biotechnol. Adv. 71, 108320. doi:10.1016/j.biotechadv.2024.108320
Park, I., Jho, E. H., and Nam, K. (2014). Optimization of carbon dioxide and valeric acid utilization for polyhydroxyalkanoates synthesis by Cupriavidus necator. J. Polym. Environ. 22, 244–251. doi:10.1007/s10924-013-0627-6
Passanha, P., Esteves, S. R., Kedia, G., Dinsdale, R. M., and Guwy, A. J. (2013). Increasing polyhydroxyalkanoate (PHA) yields from Cupriavidus necator by using filtered digestate liquors. Bioresour. Technol. 147, 345–352. doi:10.1016/j.biortech.2013.08.050
Passanha, P., Kedia, G., Dinsdale, R. M., Guwy, A. J., and Esteves, S. R. (2014). The use of NaCl addition for the improvement of polyhydroxyalkanoate production by Cupriavidus necator. Bioresour. Technol. 163, 287–294. doi:10.1016/j.biortech.2014.04.068
Pepè Sciarria, T., Batlle-Vilanova, P., Colombo, B., Scaglia, B., Balaguer, M. D., Colprim, J., et al. (2018). Bio-electrorecycling of carbon dioxide into bioplastics. Green Chem. 20, 4058–4066. doi:10.1039/c8gc01771a
Philip, S., Keshavarz, T., and Roy, I. (2007). Polyhydroxyalkanoates: biodegradable polymers with a range of applications. J Chem Technol Biotechnol. 82:233–247. doi:10.1002/jctb.1667
Pillot, G., Sunny, S., Comes, V., and Kerzenmacher, S. (2022). Optimization of growth and electrosynthesis of polyhydroxyalkanoates by the thermophilic bacterium Kyrpidia spormannii. Bioresour. Technol. Rep. 17, 100949. doi:10.1016/j.biteb.2022.100949
Plastics Engineering (2024). Mango Materials transforms waste methane into PHA. Plast. Eng. Mag. 9. Available online at: https://www.plasticsengineering.org/2024/08/mango-materials-transforms-waste-methane-into-pha-005952/.
Poli, A., Di Donato, P., Abbamondi, G. R., and Nicolaus, B. (2011). Synthesis, production, and biotechnological applications of exopolysaccharides and polyhydroxyalkanoates by archaea. Archaea 2011, 1–13. doi:10.1155/2011/693253
Ranaivoarisoa, T. O., Singh, R., Rengasamy, K., Guzman, M. S., and Bose, A. (2019). Towards sustainable bioplastic production using the photoautotrophic bacterium Rhodopseudomonas palustris TIE-1. J. Ind. Microbiol. Biotechnol. 46, 1401–1417. doi:10.1007/s10295-019-02165-7
Ray, S., Jin, J.-O., Choi, I., and Kim, M. (2023). Recent trends of biotechnological production of polyhydroxyalkanoates from C1 carbon sources. Front. Bioeng. Biotechnol. 10, 907500. doi:10.3389/fbioe.2022.907500
Reddy, C. S. K., Ghai, R., and Kalia, V. C. (2003). Polyhydroxyalkanoates: an overview. Bioresour. Technol. 87, 137–146. doi:10.1016/S0960-8524(02)00212-2
Rehm, B. H. A. (2010). Bacterial polymers: biosynthesis, modifications and applications. Nat. Rev. Microbiol. 8, 578–592. doi:10.1038/nrmicro2354
Rengasamy, K., Ranaivoarisoa, T. O., Singh, R., and Bose, A. (2017). Improving microbial electrosynthesis of polyhydroxybutyrate (PHB) from CO2 by Rhodopseudomonas palustris TIE-1 using an immobilized iron complex modified cathode. bioRxiv, 214577. doi:10.1101/214577
Revelles, O., Beneroso, D., Menéndez, J. A., Arenillas, A., García, J. L., and Prieto, M. A. (2017). Syngas obtained by microwave pyrolysis of household wastes as feedstock for polyhydroxyalkanoate production in Rhodospirillum rubrum. Microb. Biotechnol. 10, 1412–1417. doi:10.1111/1751-7915.12411
Revelles, O., Tarazona, N., García, J. L., and Prieto, M. A. (2016). Carbon roadmap from syngas to polyhydroxyalkanoates in Rhodospirillum rubrum. Environ. Microbiol. 18, 708–720. doi:10.1111/1462-2920.13087
Rippka, R., Neilson, A., Kunisawa, R., and Cohen-Bazire, G. (1971). Nitrogen fixation by unicellular blue-green algae. Arch. Mikrobiol. 76, 341–348. doi:10.1007/BF00408530
Roh, H., Lee, J. S., Choi, H.Il, Sung, Y. J., Choi, S. Y., Woo, H. M., et al. (2021). Improved CO2-derived polyhydroxybutyrate (PHB) production by engineering fast-growing cyanobacterium Synechococcus elongatus UTEX 2973 for potential utilization of flue gas. Bioresour. Technol. 327, 124789. doi:10.1016/j.biortech.2021.124789
Ronďošová, S., Legerská, B., Chmelová, D., Ondrejovič, M., and Miertuš, S. (2022). Optimization of growth conditions to enhance PHA production by Cupriavidus necator. Fermentation 8, 451. doi:10.3390/fermentation8090451
Roussou, S., Albergati, A., Liang, F., and Lindblad, P. (2021). Engineered cyanobacteria with additional overexpression of selected Calvin-Benson-Bassham enzymes show further increased ethanol production. Metab. Eng. Commun. 12, e00161. doi:10.1016/j.mec.2021.e00161
Rueda, E., García-Galán, M. J., Díez-Montero, R., Vila, J., Grifoll, M., and García, J. (2020). Polyhydroxybutyrate and glycogen production in photobioreactors inoculated with wastewater borne cyanobacteria monocultures. Bioresour. Technol. 295, 122233. doi:10.1016/j.biortech.2019.122233
Rueda, E., Gonzalez-Flo, E., Mondal, S., Forchhammer, K., Arias, D. M., Ludwig, K., et al. (2024). Challenges, progress, and future perspectives for cyanobacterial polyhydroxyalkanoate production. Rev. Environ. Sci. Biotechnol. 23, 321–350. doi:10.1007/s11157-024-09689-0
Sakarika, M., Kerckhof, F.-M., Van Peteghem, L., Pereira, A., Van Den Bossche, T., Bouwmeester, R., et al. (2023). The nutritional composition and cell size of microbial biomass for food applications are defined by the growth conditions. Microb. Cell Fact. 22, 254. doi:10.1186/s12934-023-02265-1
Salehizadeh, H., Yan, N., and Farnood, R. (2020). Recent advances in microbial CO2 fixation and conversion to value-added products. Chem. Eng. J. 390, 124584. doi:10.1016/j.cej.2020.124584
Samantaray, S., and Mallick, N. (2012). Production and characterization of poly-β-hydroxybutyrate (PHB) polymer from Aulosira fertilissima. J. Appl. Phycol. 24, 803–814. doi:10.1007/s10811-011-9699-7
Saravanan, K., Umesh, M., and Kathirvel, P. (2022). Microbial polyhydroxyalkanoates (PHAs): a review on biosynthesis, properties, fermentation strategies and its prospective applications for sustainable future. J. Polym. Environ. 30, 4903–4935. doi:10.1007/s10924-022-02562-7
Sathiyanarayanan, G., Kiran, G. S., Selvin, J., and Saibaba, G. (2013a). Optimization of polyhydroxybutyrate production by marine Bacillus megaterium MSBN04 under solid state culture. Int. J. Biol. Macromol. 60, 253–261. doi:10.1016/j.ijbiomac.2013.05.031
Sathiyanarayanan, G., Saibaba, G., Seghal Kiran, G., and Selvin, J. (2013b). A statistical approach for optimization of polyhydroxybutyrate production by marine Bacillus subtilis MSBN17. Int. J. Biol. Macromol. 59, 170–177. doi:10.1016/j.ijbiomac.2013.04.040
Sathiyanarayanan, G., Saibaba, G., Seghal Kiran, G., and Selvin, J. (2013c). Process optimization and production of polyhydroxybutyrate using palm jaggery as economical carbon source by marine sponge-associated Bacillus licheniformis MSBN12. Bioprocess Biosyst. Eng. 36, 1817–1827. doi:10.1007/s00449-013-0956-9
Schlegel, H. G., Gottschalk, G., and Von Bartha, R. (1961). Formation and utilization of poly-β-hydroxybutyric acid by knallgas bacteria (hydrogenomonas). Nature 191, 463–465. doi:10.1038/191463a0
Schmid, F., Novion Ducassou, J., Couté, Y., and Gescher, J. (2021). Developing Rhodobacter sphaeroides for cathodic biopolymer production. Bioresour. Technol. 336, 125340. doi:10.1016/j.biortech.2021.125340
Schwedt, A., Kreutzmann, A.-C., Polerecky, L., and Schulz-Vogt, H. N. (2012). Sulfur respiration in a marine chemolithoautotrophic Beggiatoa strain. Front. Microbiol. 2, 276. doi:10.3389/fmicb.2011.00276
Sciarria, T. P., Tenca, A., Goffredo, E., Colombo, A., Scaglia, B., Adani, F., et al. (2020). Combining microbial electrosynthesis and bioplastic production: a sustainable approach for biopolymer synthesis from CO2. J. Clean. Prod. 268, 122233. doi:10.1016/j.jclepro.2020.122233
Sharma, L., and Mallick, N. (2005). Accumulation of poly-β-hydroxybutyrate in Nostoc muscorum: regulation by pH, light–dark cycles, N and P status and carbon sources. Bioresour. Technol. 96, 1304–1310. doi:10.1016/j.biortech.2004.10.009
Shin, S. K., Ko, Y. J., Jeong, D. W., Lee, M.-E., You, S. K., Hyeon, J. E., et al. (2021). Enhanced production of polyhydroxybutyrate from syngas by using nanoscaled cellulose particles with a syngas-converting enzyme complex immobilized on Ralstonia eutropha. J. Clean. Prod. 285, 124903. doi:10.1016/j.jclepro.2020.124903
Shrivastav, A., Mishra, S. K., and Mishra, S. (2010). Polyhydroxyalkanoate (PHA) synthesis by Spirulina subsalsa from Gujarat coast of India. Int. J. Biol. Macromol. 46, 255–260. doi:10.1016/j.ijbiomac.2010.01.001
Siddiqui, P. J. A., Bergman, B., Björkman, P.-O., and Carpenter, E. J. (1992). Ultrastructural and chemical assessment of poly-β-hydroxybutyric acid in the marine cyanobacterium Trichodesmium thiebautii. FEMS Microbiol. Lett. 94, 143–148. doi:10.1016/0378-1097(92)90598-I
Sili, C. R. de P., Ena, A., Materassi, R., and Materassi, R. (1990). Occurrence of poly-beta-hydroxybutyrate in Spirulina species. J. Bacteriol. 172, 2791–2792. doi:10.1128/jb.172.5.2791-2792.1990
Sim, J. H., and Kamaruddin, A. H. (2008). Optimization of acetic acid production from synthesis gas by chemolithotrophic bacterium – Clostridium aceticum using statistical approach. Bioresour. Technol. 99, 2724–2735. doi:10.1016/j.biortech.2007.07.004
Smith, K. M. (2023). “AirCarbon: unlocking the potential of carbon emissions,” in Urth magazine. Available online at: https://magazine.urth.co/articles/air-carbon-interview.
Sonnleitner, B., Heinzle, E., Braunegg, G., and Lafferty, R. M. (1979). Formal kinetics of poly-β-hydroxybutyric acid (PHB) production in Alcaligenes eutrophus H 16 and Mycoplana rubra R 14 with respect to the dissolved oxygen tension in ammonium-limited batch cultures. Eur. J. Appl. Microbiol. Biotechnol. 7, 1–10. doi:10.1007/BF00522473
Srisawat, P., Higuchi-Takeuchi, M., Honda, R., Shirai, T., Kondo, A., Hoshino, Y., et al. (2022a). Engineered nanogel particles enhance the photoautotrophic biosynthesis of polyhydroxyalkanoate in marine photosynthetic bacteria. ACS Sustain Chem. Eng. 10, 4133–4142. doi:10.1021/acssuschemeng.1c07252
Srisawat, P., Higuchi-Takeuchi, M., and Numata, K. (2022b). Microbial autotrophic biorefineries: perspectives for biopolymer production. Polym. J. 54, 1139–1151. doi:10.1038/s41428-022-00675-3
Stal, L. J. (1992). Poly(hydroxyalkanoate) in cyanobacteria: an overview. FEMS Microbiol. Lett. 103, 169–180. doi:10.1016/0378-1097(92)90307-A
Stal, L. J., Heyer, H., and Jacobs, G. (1990). “Occurrence and role of poly-hydroxy-alkanoate in the cyanobacterium Oscillatoria Limosa,” in Novel biodegradable microbial polymers. Editor E. A. Dawes (Dordrecht: Springer Netherlands), 435–438. doi:10.1007/978-94-009-2129-0_38
Stöckl, M., Harms, S., Dinges, I., Dimitrova, S., and Holtmann, D. (2020). From CO2 to bioplastic – coupling the electrochemical CO2 reduction with a microbial product generation by drop-in electrolysis. ChemSusChem 13, 4086–4093. doi:10.1002/cssc.202001235
Strauss, G., and Fuchs, G. (1993). Enzymes of a novel autotrophic CO2 fixation pathway in the phototrophic bacterium Chloroflexus aurantiacus, the 3-hydroxypropionate cycle. Eur. J. Biochem. 215, 633–643. doi:10.1111/j.1432-1033.1993.tb18074.x
Sudesh, K., Abe, H., and Doi, Y. (2000). Synthesis, structure and properties of polyhydroxyalkanoates: biological polyesters. Prog. Polym. Sci. 25, 1503–1555. doi:10.1016/S0079-6700(00)00035-6
Sudesh, K., Taguchi, K., and Doi, Y. (2002). Effect of increased PHA synthase activity on polyhydroxyalkanoates biosynthesis in Synechocystis sp. PCC6803. Int. J. Biol. Macromol. 30, 97–104. doi:10.1016/S0141-8130(02)00010-7
Sugimoto, T., Tsuge, T., Tanaka, K., and Ishizaki, A. (1999). Control of acetic acid concentration by pH-stat continuous substrate feeding in heterotrophic culture phase of two-stage cultivation of Alcaligenes eutrophus for production of P(3HB) from CO2, H2, and O2 under non-explosive conditions. Biotechnol. Bioeng. 62, 625–631. doi:10.1002/(SICI)1097-0290(19990320)62:6<625::AID-BIT1>3.0.CO;2-D
Taepucharoen, K., Tarawat, S., Puangcharoen, M., Incharoensakdi, A., and Monshupanee, T. (2017). Production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) under photoautotrophy and heterotrophy by non-heterocystous N2-fixing cyanobacterium. Bioresour. Technol. 239, 523–527. doi:10.1016/j.biortech.2017.05.029
Taga, N., Tanaka, K., and Ishizaki, A. (1997). Effects of rheological change by addition of carboxymethylcellulose in culture media of an air-lift fermentor xon poly-3-hydroxybutyric acid productivity in autotrophic culture of hydrogen-oxidizing bacterium, Alcaligenes eutrophus. Biotechnol. Bioeng. 53, 529–533. doi:10.1002/(SICI)1097-0290(19970305)53:5<529::AID-BIT11>3.0.CO;2-B
Takahashi, H., Miyake, M., Tokiwa, Y., and Asada, Y. (1998). Improved accumulation of poly-3- hydroxybutyrate by a recombinant cyanobacterium. Biotechnol. Lett. 20, 183–186. doi:10.1023/A:1005392827791
Takeshita, T., and Ishizaki, A. (1996). Influence of hydrogen limitation on gaseous substrate utilization in autotrophic culture of Alcaligenes eutrophus ATCC 17697T. J. Ferment Bioeng. 81, 83–86. doi:10.1016/0922-338X(96)83127-8
Tanaka, K., Ishii, S., and Yoshida, Y. (2022). Polyhydroxyalkanoate synthesis using renewable electricity and CO2. Front. Bioeng. Biotechnol. 10, 835482. doi:10.3389/fbioe.2022.835482
Tanaka, K., and Ishizaki, A. (1994). Production of poly-d-3-hydroxybutyric acid from carbon dioxide by a two-stage culture method employing Alcaligenes eutrophus ATCC 17697T. J. Ferment Bioeng. 77, 425–427. doi:10.1016/0922-338X(94)90017-5
Tanaka, K., Ishizaki, A., Kanamaru, T., and Kawano, T. (1995). Production of poly(D-3-hydroxybutyrate) from CO2, H2, and O2 by high cell density autotrophic cultivation of Alcaligenes eutrophus. Biotechnol. Bioeng. 45, 268–275. doi:10.1002/bit.260450312
Tanaka, K., Miyawaki, K., Yamaguchi, A., Khosravi-Darani, K., and Matsusaki, H. (2011). Cell growth and P(3HB) accumulation from CO2 of a carbon monoxide-tolerant hydrogen-oxidizing bacterium, Ideonella sp. O-1. Appl. Microbiol. Biotechnol. 92, 1161–1169. doi:10.1007/s00253-011-3420-2
Tanaka, K., Mori, S., Hirata, M., and Matsusaki, H. (2016). Autotrophic growth of Paracoccus denitrificans in aerobic condition and the accumulation of biodegradable plastics from CO2. Environ. Ecol. Res. 4, 231–236. doi:10.13189/eer.2016.040407
Tanaka, K., Yoshida, K., Orita, I., and Fukui, T. (2021). Biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) from CO2 by a recombinant Cupriavidus necator. Bioengineering 8, 179. doi:10.3390/bioengineering8110179
Tang, R., Weng, C., Peng, X., and Han, Y. (2020). Metabolic engineering of Cupriavidus necator H16 for improved chemoautotrophic growth and PHB production under oxygen-limiting conditions. Metab. Eng. 61, 11–23. doi:10.1016/j.ymben.2020.04.009
Tao, B., Passanha, P., Kumi, P., Wilson, V., Jones, D., and Esteves, S. (2016). Recovery and concentration of thermally hydrolysed waste activated sludge derived volatile fatty acids and nutrients by microfiltration, electrodialysis and struvite precipitation for polyhydroxyalkanoates production. Chem. Eng. J. 295, 11–19. doi:10.1016/j.cej.2016.03.036
Tarawat, S., Incharoensakdi, A., and Monshupanee, T. (2020). Cyanobacterial production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) from carbon dioxide or a single organic substrate: improved polymer elongation with an extremely high 3-hydroxyvalerate mole proportion. J. Appl. Phycol. 32, 1095–1102. doi:10.1007/s10811-020-02040-4
Teixeira, L. V., Oliveira, L. M., Barbosa, M. A., Rodrigues, S., and Mendes, J. (2018). Gas fermentation of C1 feedstocks: commercialization status and future perspectives. Biofuels, Bioprod. Biorefining 12 (6), 1103–1115. doi:10.1002/bbb.1912
Troschl, C., Meixner, K., and Drosg, B. (2017a). Cyanobacterial PHA production—review of recent advances and a summary of three years’ working experience running a pilot plant. Bioengineering 4, 26. doi:10.3390/bioengineering4020026
Troschl, C., Meixner, K., and Drosg, B. (2017b). Microbial PHA production from waste streams: a review. Bioresour. Technol. 245, 1645–1657. doi:10.1016/j.biortech.2017.08.089
Troschl, C., Meixner, K., Fritz, I., Leitner, K., Romero, A. P., Kovalcik, A., et al. (2018). Pilot-scale production of poly-β-hydroxybutyrate with the cyanobacterium Synechocytis sp. CCALA192 in a non-sterile tubular photobioreactor. Algal Res. 34, 116–125. doi:10.1016/j.algal.2018.07.011
van der Meer, M. T. J., Schouten, S., van Dongen, B. E., Rijpstra, W. I. C., Fuchs, G., Damsté, J. S. S., et al. (2001). Biosynthetic controls on the 13C contents of organic components in the photoautotrophic bacterium Chloroflexus aurantiacus. J. Biol. Chem. 276, 10971–10976. doi:10.1074/jbc.M009701200
Vanessa, C., Silva, C., Ana, L., Costa, J. A., and Morais, M. (2015). Polyhydroxybutyrate production by Spirulina sp. LEB 18 grown under different nutrient concentrations. Afr. J. Microbiol. Res. 9, 1586–1594. doi:10.5897/ajmr2015.7530
Van Gool, A. P., Tobback, P. P., and Fischer, I. (1971). Autotrophic growth and synthesis of reserve polymers in Nitrobacter winogradskyi. Arch. Mikrobiol. 76, 252–264. doi:10.1007/BF00409120
Vlaeminck, E., Acuña Lopez, P., Uitterhaegen, E., Quataert, K., Delmulle, T., De Winter, K., et al. (2024). Pressure fermentation to boost CO2-based poly(3-hydroxybutyrate) production using Cupriavidus necator. Bioresour. Technol. 408, 131162. doi:10.1016/j.biortech.2024.131162
Volova, T., Kalacheva, G., and Altukhova, O. (2002). Autotrophic synthesis of polyhydroxyalkanoates by the bacteria Ralstonia eutropha in the presence of carbon monoxide. Appl. Microbiol. Biotechnol. 58, 675–678. doi:10.1007/s00253-002-0941-8
Volova, T., Zhila, N., and Shishatskaya, E. (2015). Synthesis of poly(3-hydroxybutyrate) by the autotrophic CO-oxidizing bacterium Seliberia carboxydohydrogena Z-1062. J. Ind. Microbiol. Biotechnol. 42, 1377–1387. doi:10.1007/s10295-015-1659-9
Volova, T. G., and Kalacheva, G. S. (2005). The synthesis of hydroxybutyrate and hydroxyvalerate copolymers by the bacterium Ralstonia eutropha. Microbiol. (N Y) 74, 54–59. doi:10.1007/s11021-005-0028-5
Volova, T. G., Kalacheva, G. S., Gorbunova, O. V., and Zhila, N. O. (2004). Dynamics of activity of the key enzymes of polyhydroxyalkanoate metabolism in Ralstonia eutropha B5786. Appl. Biochem. Microbiol. 40, 170–177. doi:10.1023/B:ABIM.0000018921.04863.d5
Volova, T. G., Kalacheva, G. S., and Steinbüchel, A. (2008). Biosynthesis of multi-component polyhydroxyalkanoates by the bacterium Wautersia eutropha. Macromol. Symp. 269, 1–7. doi:10.1002/masy.200850901
Volova, T. G., Kiselev, E. G., Shishatskaya, E. I., Zhila, N. O., Boyandin, A. N., Syrvacheva, D. A., et al. (2013). Cell growth and accumulation of polyhydroxyalkanoates from CO2 and H2 of a hydrogen-oxidizing bacterium, Cupriavidus eutrophus B-10646. Bioresour. Technol. 146, 215–222. doi:10.1016/j.biortech.2013.07.070
Volova, T. G., Shishatskaya, E. I., Sevastianov, V. I., and Efremov, S. N. (2010). Accumulation of polyhydroxyalkanoates by various microorganisms from CO2 and H2. Appl. Biochem. Microbiol. 46, 648–654. doi:10.1134/S0003683810070042
Volova, T. G., and Voinov, N. A. (2003). Kinetic parameters of a culture of the hydrogen-oxidizing bacterium Ralstonia eutropha grown under conditions favoring polyhydroxybutyrate biosynthesis. Appl. Biochem. Microbiol. 39, 166–170. doi:10.1023/A:1022585912873
Wang, B., Pugh, S., Nielsen, D. R., Zhang, W., and Meldrum, D. R. (2013). Engineering cyanobacteria for photosynthetic production of 3-hydroxybutyrate directly from CO2. Metab. Eng. 16, 68–77. doi:10.1016/j.ymben.2013.01.001
Wang, J., Liu, S., Huang, J., Cui, R., Xu, Y., and Song, Z. (2023). Genetic engineering strategies for sustainable polyhydroxyalkanoate (PHA) production from carbon-rich wastes. Environ. Technol. Innov. 30, 103069. doi:10.1016/j.eti.2023.103069
Wu, G. F., Shen, Z. Y., and Wu, Q. Y. (2002). Modification of carbon partitioning to enhance PHB production in Synechocystis sp. PCC6803. Enzyme Microb. Technol. 30, 710–715. doi:10.1016/S0141-0229(02)00044-3
Wu, G. F., Wu, Q. Y., and Shen, Z. Y. (2001). Accumulation of poly-β-hydroxybutyrate in cyanobacterium Synechocystis sp. PCC6803. Bioresour. Technol. 76, 85–90. doi:10.1016/S0960-8524(00)00099-7
Yield10 Bioscience (2023). PHA biopolymers. Available online at: https://www.yield10bio.com/commitment/pha-biopolymers.
Zhang, S., Liu, Y., and Bryant, D. A. (2015). Metabolic engineering of Synechococcus sp. PCC 7002 to produce poly-3-hydroxybutyrate and poly-3-hydroxybutyrate-co-4-hydroxybutyrate. Metab. Eng. 32, 174–183. doi:10.1016/j.ymben.2015.10.001
Zhang, X. (2015). Microalgae removal of CO2 from flue gas. Available online at: https://www.sustainable-carbon.org/report/microalgae-removal-of-co2-from-flue-gas-ccc-250/ (Accessed August 8, 2024).
Keywords: polyhydroxyalkanoates, carbon dioxide, CO2 fixation, autotrophs, cyanobacteria, photosynthetic bacteria, hydrogen-oxidizing bacteria, genetic engineering
Citation: Sathiyanarayanan G and Esteves S (2025) Autotrophic bacterial production of polyhydroxyalkanoates using carbon dioxide as a sustainable carbon source. Front. Bioeng. Biotechnol. 13:1545438. doi: 10.3389/fbioe.2025.1545438
Received: 15 December 2024; Accepted: 14 May 2025;
Published: 04 June 2025.
Edited by:
Jung Rae Kim, Pusan National University, Republic of KoreaReviewed by:
M. V. Rohit, Sardar Patel Renewable Energy Research Institute, IndiaShuwei Li, Xi’an Jiaotong University, China
Copyright © 2025 Sathiyanarayanan and Esteves. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ganesan Sathiyanarayanan, c2F0aGl5YW5hcmF5YW5hbi5nYW5lc2FuMUBzb3V0aHdhbGVzLmFjLnVr; Sandra Esteves, c2FuZHJhLmVzdGV2ZXNAc291dGh3YWxlcy5hYy51aw==
 Ganesan Sathiyanarayanan
Ganesan Sathiyanarayanan Sandra Esteves
Sandra Esteves