- 1Department of Rehabilitation Medicine, Zhengzhou Central Hospital Affiliated Zhengzhou University, Zhengzhou, China
- 2Department of Rehabilitation, Huashan Hospital, Fudan University, Shanghai, China
- 3Department of Rehabilitation, Hangzhou Linping Hospital of Traditional Chinese and Western Medicine, Hangzhou, China
- 4Department of Rehabilitation Medicine, Jing’an District Central Hospital of Shanghai, Shanghai, China
Objective: To investigate the effects of isokinetic strength training combined with manual lymphatic drainage (MLD) on leg circumference, walking ability and muscle strength in patients with secondary lymphedema following gynecologic cancer surgery.
Design: Randomized controlled trial.
Setting: Inpatient rehabilitation department.
Participants: Sixty-six patients with secondary lymphedema of the lower extremities following gynecologic cancer surgery were randomly allocated into an experimental group and a control group, each comprising 33 patients.
Interventions: The control group participated in a 4-week standardized MLD program. In addition to the MLD program, participants in the experimental group received additional isokinetic strength training for 20 min daily over the same 4-week period.
Outcomes: Lower limb volume derived from the circumference measurements, Holden Gait Scale and Lovett muscle strength grading.
Results: Prior to the intervention, no statistically significant differences were observed between the two groups across all outcomes (P > 0.05). Post-intervention, statistically significant improvements were noted in the experimental group compared to the control group with respect to reduced lower extremity volume, improved walking ability, and increased muscle strength (P < 0.05).
Conclusion: For patients with secondary lower limb lymphedema following gynecological tumor surgery, a combination of isokinetic strength training and MLD has been found to be more effective than MLD alone in reducing edema, improving walking ability, and enhancing muscle strength.
Introduction
A common complication of gynecological cancer treatment is secondary lower limb lymphedema, mainly affecting patients with cervical, endometrial, and ovarian cancers after surgery, radiotherapy, or chemotherapy (Bitler and Carpenter, 2017; Ida et al., 2019). Postoperative disruption of pelvic lymphatic drainage may result in the accumulation of lymphatic fluid in the lower extremities, ultimately leading to lymphedema (Caretto et al., 2022). According to a report by the Chinese Academy of Sciences, the incidence of secondary lower limb lymphedema as a complication following cervical cancer surgery ranges from 11.5% to 30.6% (Liu et al., 2023). Lower limb lymphedema can cause symptoms like heaviness, pain, muscle weakness, abnormal sensations and anxiety, significantly impacting the patient’s quality of life (Clinckaert et al., 2022) and causing substantial burdens and mental stress for both patients and their families (Ciudad et al., 2019; Dean et al., 2019).
For lower limb lymphedema, current treatments include conservative therapy and surgery (Reichart, 2017). Complex Decongestive Therapy (CDT) is a prominent non-surgical intervention for the management of lymphedema and is extensively utilized in clinical settings (Brandão et al., 2020). CDT consists of two phases: the intensive treatment phase and the maintenance phase. The intensive phase involves education, compression therapy, decongestive exercises, and manual lymphatic drainage (MLD). The maintenance phase focuses on sustaining and enhancing these results through compression garments, skin care, and home exercises (Liu et al., 2021). Although CDT has been extensively applied and studied for the management of upper limb lymphedema, its application for lower limb lymphedema remains underexplored, with insufficient evidence-based support. MLD, a critical component of CDT, entails the gentle facilitation of lymphatic fluid movement from congested regions to unaffected vessels through soft manual therapy (Thompson et al., 2021; Ali et al., 2022). The efficacy of MLD in the management of lymphedema has been demonstrated in observational studies and certain randomized controlled trials (RCTs). Nevertheless, there remains a paucity of high-quality RCTs with adequately powered sample sizes, especially for the management of lower limb lymphedema (Executive Committee of the International Society of Lymphology, 2020; Liang et al., 2020; Zhang et al., 2022).
Although limb lymphedema can be alleviated through MLD, a passive method to facilitate lymphatic drainage, active exercise is also crucial for increasing lymph fluid production and drainage, leading to more satisfactory long-term results (Wang and Dong, 2019). Research shows that isokinetic strength training, by activating muscles and enhancing contractions, increases pressure on lymphatic vessels. This promotes blood circulation and helps reduce edema by facilitating the absorption and drainage of interstitial fluid in patients (Zhu et al., 2023; Petrucci et al., 2024). Isokinetic strength training provides consistent muscle resistance throughout the range of motion, maintaining optimal muscle tension and ensuring thorough exercise (Wang et al., 2022). Strength training modulates sympathetic nerve activity, inducing self-contraction of lymphatic vessels. This mechanism is crucial for the long-term management of lymphedema (Omar et al., 2020). Recent systematic reviews have indicated that resistance strength training does not increase the risk of lymphedema or exacerbate its symptoms; instead, it improves physical function and quality of life (Baumann et al., 2018). These findings alleviate safety concerns regarding the application of isokinetic strength training for patients with lymphedema.
Given the limited reports on using isokinetic strength training with MLD for treating secondary lower limb lymphedema due to gynecological malignancies, we designed a randomized controlled study to evaluate the efficacy of this combined therapy. We hypothesized that combining isokinetic strength training with MLD would reduce lymphedema more effectively and improve lower limb mobility in these patients.
Materials and methods
Participants
Sixty-six patients with secondary lymphedema following gynecological malignant tumor surgery, admitted to the Rehabilitation Department of Zhengzhou Central Hospital from July 2022 to July 2023, were selected as research subjects. All participants provided written informed consent. This study was reviewed and approved by the Ethics Committee of Zhengzhou Central Hospital (approval number: 202,256). This study adhered to the principles of the Declaration of Helsinki and was conducted and reported in accordance with the recommendations provided by the CONSORT guideline (Schulz et al., 2010).
Inclusion/exclusion criteria
The inclusion criteria were the following: (1) Females diagnosed with unilateral lower limb lymphedema according to The diagnosis and treatment of peripheral lymphedema: 2020 Consensus Document of the International Society of Lymphology (Executive Committee of the International Society of Lymphology, 2020); (2) with unilateral lower limb lymphedema based on ≥5.7% circumference difference between affected and contralateral limbs (Campanholi et al., 2015; Cormier et al., 2010); (3) a history of lymph node dissection; (4) no deep vein thrombosis; (5) ability to cooperate with functional exercises, provide informed consent, and participate voluntarily.
The exclusion criteria were: (1) lower limb edema from cardiac, renal, or malnutrition causes; (2) lower limb venous thrombosis or superior vena cava obstruction; (3) acute cellulitis, erysipelas, or severe lower limb infection; (4) unstable hypertension; (5) untreated tuberculosis or malaria; (6) cognitive or behavioral issues preventing cooperation with treatment.
Randomization
Participants meeting the inclusion criteria were randomly allocated to either the experimental group (n = 33, MLD + isokinetic strength training) or the control group (n = 33, MLD alone). Baseline assessments were conducted post-randomization. A computer-generated random number list, managed by a researcher unaffiliated with participant recruitment or the study site, ensured allocation concealment. An independent investigator, not involved in randomization, intervention, or evaluation, recruited all participants. The allocation sequence remained concealed from all researchers and assessors.
Prior sample size estimation
The sample size was determined based on previous studies. We assumed a mean difference of 400 cm3 between groups in the changes of lower limb volumetric discrepancy between the affected and unaffected limbs, with a common standard deviation of 500 cm3. Anticipating a 10% dropout rate, we calculated a final target sample size of 33 participants, allocating 33 to each group to achieve 85% power at a 0.05 significance level.
Intervention
Participants underwent a 4-week inpatient intervention for lower extremity lymphedema. Both groups received the MLD program administered by therapists certified in international lymphedema therapy and comprehensive decongestive therapy (CDT). Additionally, the experimental group received isokinetic strength training in conjunction with MLD.
MLD
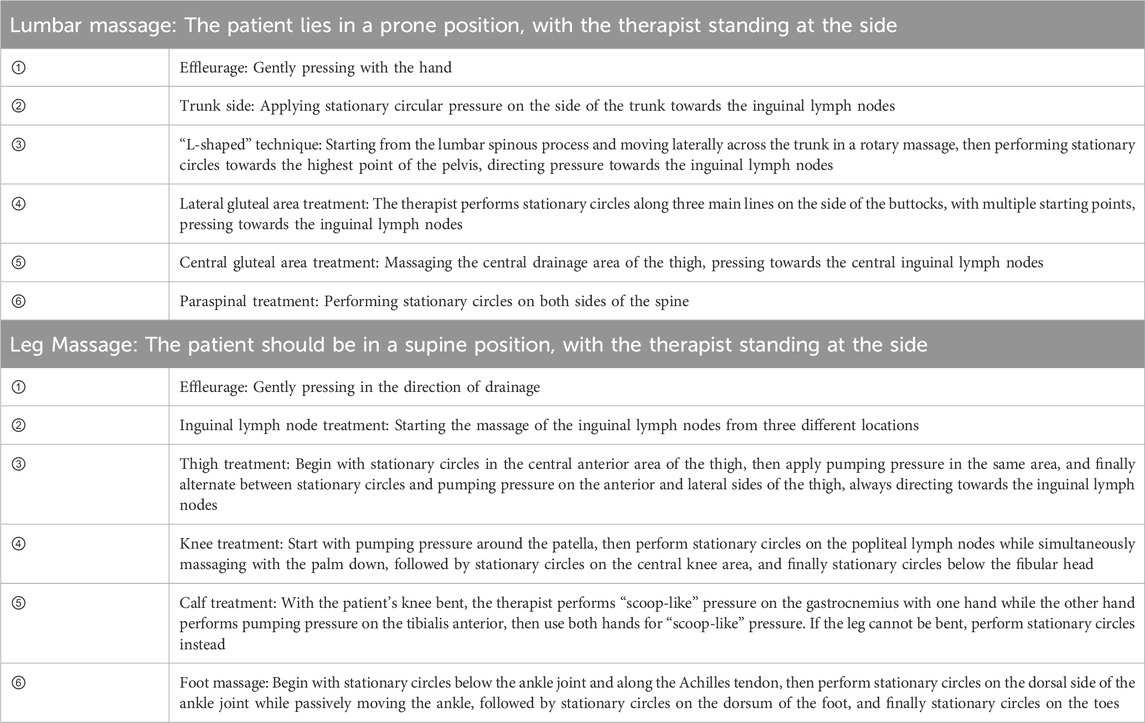
Conventional MLD technique were employed, utilizing four basic techniques: stationary circle, rotary technique, pump technique and scoop technique. The treatment began at the proximal end of the limb, then moved to the distal areas, and finally returned to the proximal end, following this sequence (Table 1) (Földi and Strossenreuther, 2005).
During the MLD procedure, the following points should be noted: The drainage force should be gentle and consistent, with traction applied between the skin without friction, and the pressure should be minimal, just enough to avoid skin redness; the operation speed should be slow and rhythmic, with each phase (working and resting) lasting at least 2 s; each treatment session should last 40 min, once a day, five times a week, for a total of 4 weeks (Kasseroller, 1998).
Isokinetic strength training
Isokinetic strength training was conducted using the LoopGO™ (China). Training sessions were performed on a bedside lower limb active-passive rehabilitation trainer, following an isokinetic prescription. Patients were placed in a supine position with hips and knees flexed, and their lower limbs were secured within the trainer’s groove. The movement speed and direction were adjusted to a moderate intensity based on individual patient tolerance and conditions. Each session lasted 20 min and was conducted twice daily, 5 days per week, over a 4-week period.
Outcomes
All outcomes were assessed by an independent trained physician blinded to the grouping information, before and after 4 weeks of treatment. The primary outcome was lower limb circumference. Secondary outcomes included gait function and lower limb muscle strength.
Lower limb volume derived from circumference measurements
A tape measure was used to assess the circumference of the affected lower limb in both groups before and after treatment. Measurements were conducted by the same therapist to ensure consistency and accuracy. Patients were positioned supine on a treatment bed in neutral alignment. Using the lateral malleolus as a bony landmark, measurements were taken from the ankle joint proximally at intervals of 10 cm, 20 cm, 30 cm, 40 cm, 50 cm, and 60 cm. Each measurement was performed three times, with the median value being recorded. Lower extremity volume was calculated using a formula reported by Casley-Smith: V = h (C12 + C1C2+ C22)/12π, where V is the volume of an extremity segment, C1 and C2 are circumferences at each end, and h is the distance between the ends (Figure 1). The volumetric discrepancy between the affected and unaffected limbs was calculated both before and after treatment.
Gait function assessment
The Holden Gait Scale, also referred to as the Functional Ambulation Categories (FAC), is a tool used to assess walking ability. This 6-level scale evaluates ambulation by determining the amount of human support required during walking, irrespective of assistive device use (Holden et al., 1984). Level 0 indicates non-functional ambulation (inability to walk). Levels I to III denote dependent ambulation, requiring assistance from another person: continuous manual contact (Level I), intermittent manual contact (Level II), or verbal supervision/guidance (Level III). Levels IV and V describe independent ambulation: walking on level surfaces only (Level IV) or on any surface (Level V).
Muscle Strength Test
The Lovett muscle strength grading system was utilized for manual muscle testing (MMT) (Cuthbert and Goodheart, 2007). Patients removed clothing that could affect assessment outcomes. The therapist stabilized the patient’s trunk or limb in an optimal position to facilitate specific actions and prevent unintended joint movements, focusing on the calf muscle group (ankle plantar flexors). Depending on individual conditions, gravity, muscle contraction, resistance, and range of motion tests were applied. Muscle strength was graded on a 0–5 scale before and after treatment, with higher scores indicating greater strength. To ensure data accuracy, all pre- and post-treatment assessments were conducted by the same therapist.
Statistical analysis
The statistical analysis was conducted by an independent statistician who was blinded to the interventions. The data were analyzed using SPSS (Version 26.0, Inc., Chicago, IL) statistical software. Continuous data were presented as the mean ± SD, while categorical variables were expressed as numbers (%). The homogeneity at baseline was verified through the implementation of the Student’s t-test, Wilcoxon rank-sum test and chi-square test. The normality of data distribution was assessed using the Shapiro-Wilk statistic. Independent samples t-tests were employed to evaluate differences between groups, while Wilcoxon rank-sum test was utilized in cases where values did not adhere to a normal distribution or for ordinal data. The significance level was set at α = 0.05. A per-protocol analysis was conducted, exclusively considering participants who successfully completed all assessments (Schoenfeld, 2005).
Results
Recruitment and retention
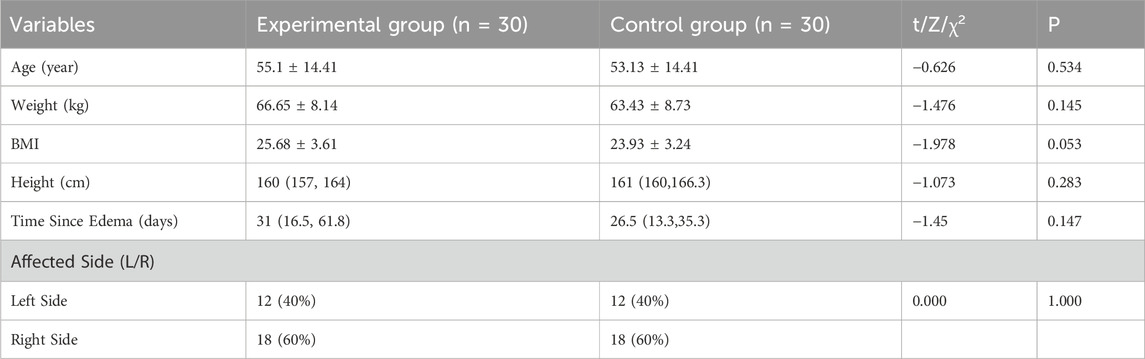
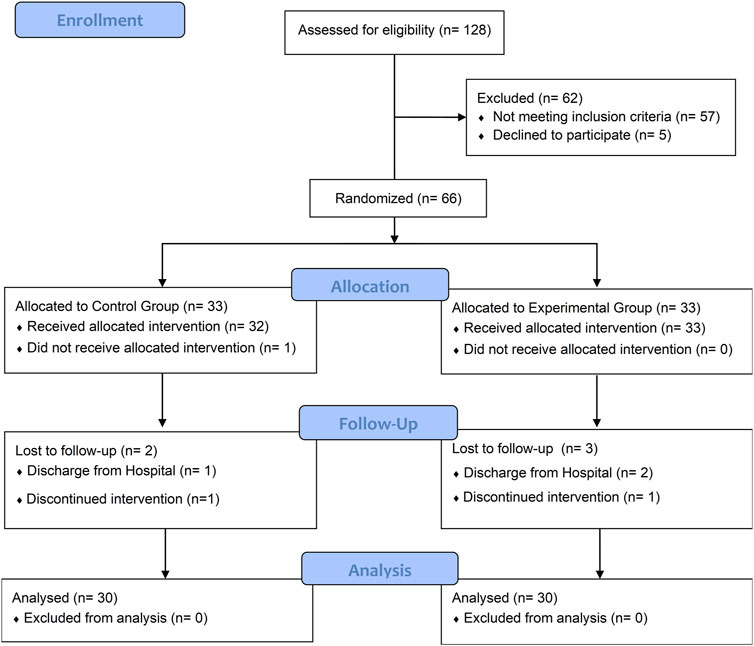
Among the initial pool of 188 patients who underwent eligibility screening, a total of 71 individuals met our study criteria, with 66 of them consenting to participate in this study and random equally allocated to the experiment and control group. At baseline assessment no significant differences (P > 0.05) were observed between the two groups in terms of baseline demographic characteristics and all outcomes (Table 2). The flow diagram (Figure 2) presents the detailed information regarding participant recruitment, allocation, and follow-up.
Lower limb volume
Before treatment, there was no statistically significant difference the volumetric discrepancy between the affected and unaffected limbs between the two groups (P > 0.05). After treatment, the volumetric discrepancy between the affected and unaffected limbs in both groups decreased compared to before treatment, and the experimental group showed a greater decrease than the control group, with a statistically significant difference (P < 0.05), as detailed in Table 3.
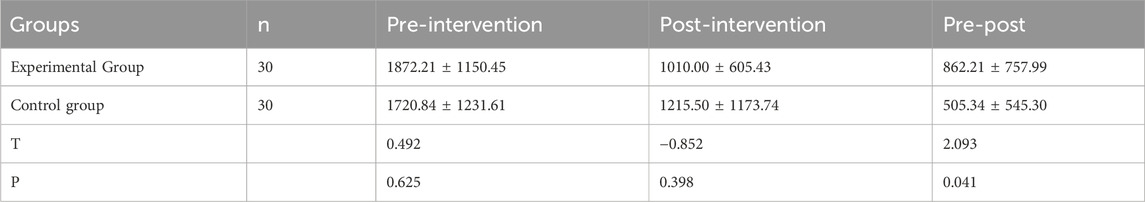
Table 3. Comparison of the pre- and post-intervention volumetric discrepancy between the affected and unaffected limbs between the two groups(
Gait function level score
Before treatment, no statistically significant difference was observed in gait function level scores between the two groups (P > 0.05). After treatment, gait function level scores increased significantly in both groups compared to pre-treatment levels (experimental group, P < 0.001; control group, P = 0.002). Moreover, the experimental group exhibited higher scores than the control group, with a statistically significant difference (P < 0.05), as shown in Table 4 and Figure 3a.
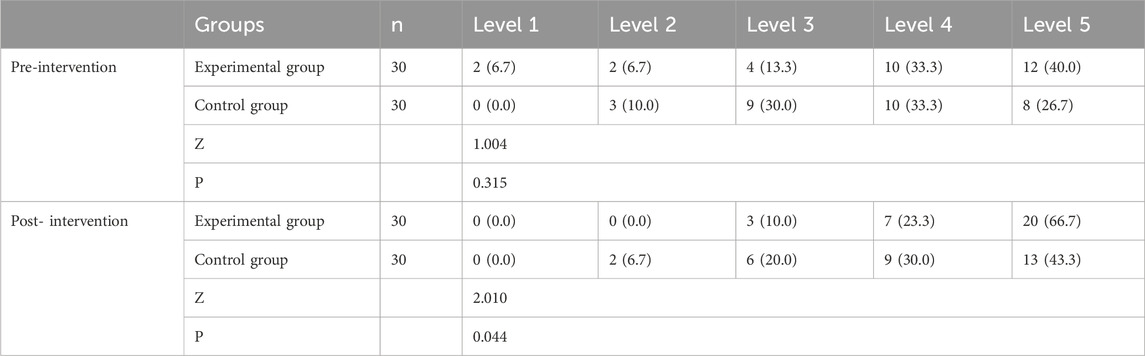
Table 4. Comparison of walking ability (FAC) between the two groups before and after 4 weeks of treatment (%).
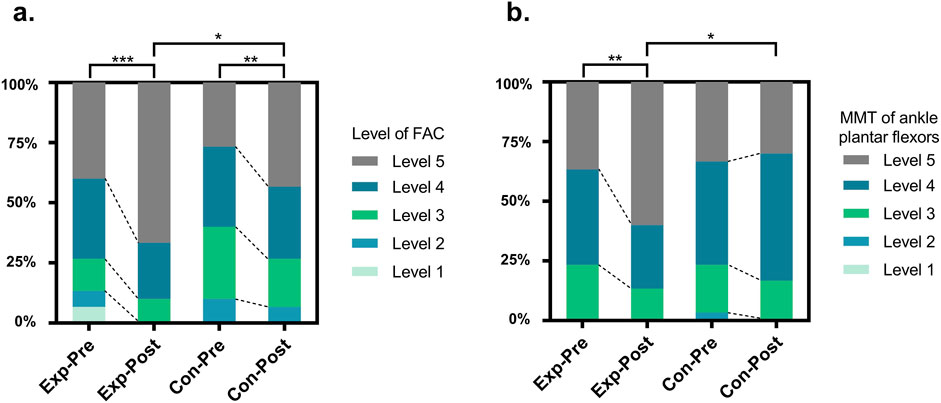
Figure 3. Changes of Gait Function Level Score (a) and Muscle Strength Test (b) between the groups before and after intervention. Exp, experimental group; Con, control group; FAC, Functional Ambulation Categories; MMT, Muscle Strength Test; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Muscle Strength Test
Before treatment, there was no statistically significant difference in the MMT of ankle plantar flexors on the affected side between the two groups (P > 0.05). After treatment, muscle strength increased in both groups compared to pre-treatment levels; however, a significant improvement was observed only in the experimental group (experimental group, P = 0.002; control group, P = 0.317). Furthermore, the experimental group exhibited higher scores than the control group, with a statistically significant difference (P < 0.05), as shown in Table 5 and Figure 3b.
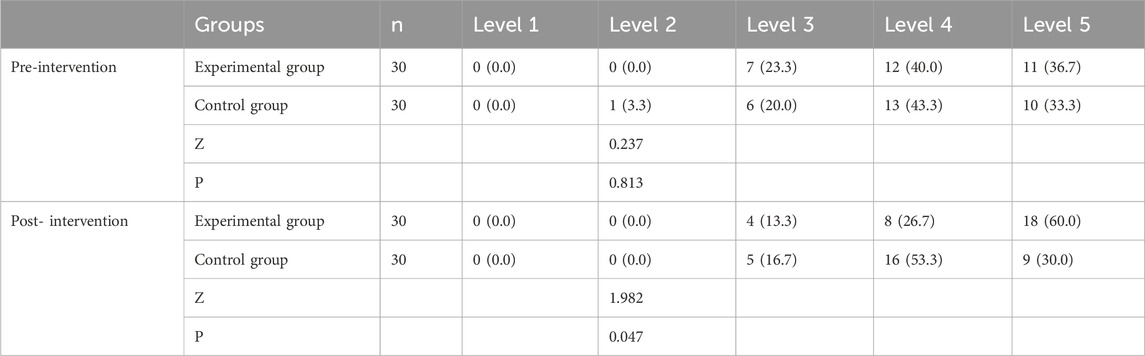
Table 5. Comparison of strength of calf muscle groups (MMT, ankle plantar flexors) between the two groups before and after 4 weeks of treatment (%).
Discussion
Secondary lymphedema is a chronic, irreversible condition with rising incidence. Up to 47% of patients undergoing surgery for gynecological cancers develop lower limb lymphedema (Wong et al., 2022). Lymphatic vessel damage or disruption of lymphatic circulation caused by surgical procedures is difficult to repair spontaneously (Aoishi et al., 2020). Lower limb lymphedema significantly restricts physical activity, such as daily walking, standing, and performing household chores (de Sire et al., 2022; Tedeschi, 2023). Without timely intervention, such damage is highly likely to progress to irreversible soft tissue fibrosis (Nakamura et al., 2017). To date, research on lymphedema has predominantly focused on upper limb lymphedema in breast cancer patients. Given the notable differences in tissue composition and function between the upper and lower limbs, it is crucial to develop effective treatment strategies specifically for lower limb lymphedema (Biglia et al., 2017). In this study, we demonstrated that a 4-week regimen integrating MLD with isokinetic strength training led to a reduction in lower extremity volume, an enhancement in walking ability, and an increase in muscle strength compared to MLD treatment alone. Our results highlight the importance of incorporating strength training into the management of lower limb lymphedema, as it not only improves walking ability but also facilitates lymphatic absorption and drainage.
MLD is a non-invasive, safe, effective, and convenient therapeutic approach that has been widely adopted for managing swelling of various etiologies (Lin et al., 2022). MLD addresses secondary lymphedema in the lower limbs by employing techniques analogous to those utilized for the upper limbs. Through gentle massage and manual maneuvers, MLD facilitates the circulation of lymphatic fluid, thus alleviating edema symptoms caused by lymphatic system dysfunction (Thompson et al., 2021). Unlike compression bandages or garments, which frequently induce localized heat retention and perspiration (particularly in lower limbs), limit joint mobility during daily activities, MLD overcomes these limitations through non-occlusive, anatomically adaptive techniques that synchronize with natural lymphatic rhythms, thereby sustaining therapeutic efficacy without compromising patient comfort or functional independence (Liang et al., 2020). However, MLD as a standalone treatment has inherent limitations. Its reliance on operator expertise can lead to variability in treatment quality, while prolonged therapy poses logistical and financial challenges for patients. Furthermore, individual responses to MLD vary significantly due to factors such as disease chronicity and the severity of tissue fibrosis (Thompson et al., 2021; Ali et al., 2022). Notably, MLD passively enhances lymphatic transport and drainage; however, if patients actively improve lymphatic return through exercise, it may yield more effective outcomes, particularly for lower limb lymphedema.
Isokinetic strength training, a method specifically designed to improve muscle strength and endurance, has recently been applied to edema treatment and has demonstrated promising results (Petrucci et al., 2024). This training method enhances lymphatic flow through sustained muscle contraction and relaxation in an isokinetic manner, thereby promoting the drainage of lymphatic fluid and alleviating edema symptoms. Systematic reviews have highlighted that, despite the limited number and quality of studies currently available, exercise is not only feasible and safe but also exhibits clinically significant efficacy in alleviating lymphedema-related symptoms in women post-gynecological cancer surgery (Hsu et al., 2024; Wittenkamp et al., 2025). Zhang et al. (2021) demonstrated that progressive resistance exercise is feasible and safe for post-cervical cancer surgery lymphedema, with no serious adverse reactions and no equipment or regional limitations. Research has also demonstrated that combining isokinetic strength training with MLD offers notable advantages in managing upper limb lymphedema following breast cancer surgery (Wang and Dong, 2019). Additionally, research has demonstrated that progressive resistance exercise training serves as an effective approach to prevent lower limb lymphedema following pelvic lymphadenectomy for cervical cancer (Zhang et al., 2024). Through isokinetic strength training, the controlled contraction and relaxation of the affected muscles, along with the compression of deep tissues, contribute to enhanced blood circulation and lymphatic return. This process may also facilitate the clearance of algogenic factors, thereby alleviating lymphedema and associated discomfort in patients (Wittenkamp et al., 2025). Furthermore, isokinetic strength training has been shown to enhance muscle strength and endurance, thereby improving exercise capacity and daily activity levels in patients (Xu and Liu, 2024).
However, strength training exhibits certain limitations when employed as a sole intervention in the treatment of lymphedema. This is due to the multifactorial nature of lymphedema, which involves complex pathological mechanisms. Repeated strength-training sessions may become impractical for patients with moderate to severe lower limb lymphedema and could potentially exacerbate symptoms. This exacerbation may result from increased local tissue pressure caused by sustained muscle contractions (Hayes et al., 2009). Therefore, integrating isokinetic strength training with other therapies based on individual condition is essential for comprehensive management and rehabilitation. Evidence suggests that MLD can significantly alleviate discomfort associated with muscle training, such as delayed onset muscle soreness and increased local tissue pressure resulting from prolonged muscle contractions, which may exacerbate edema symptoms (Földi and Strossenreuther, 2005). Meanwhile, rhythmic skeletal muscle contractions during active exercise generate a pump-like mechanism, exerting intermittent pressure on lymphatic vessels and veins to enhance lymph drainage and venous return, thereby improving fluid transport efficiency in the lymphatic system (Hsu et al., 2024; Wittenkamp et al., 2025).
Our study also explored the effects of combining isokinetic strength training with MLD to improve lower limb function in patients with secondary lymphedema. Results showed that the experimental group also had better outcomes in Holden gait scores and Lovett scores compared to the control group. Isokinetic training, by adjusting resistance to match patient strength, maximizes muscle loading while preventing atrophy and scar contracture associated with gynecologic cancer surgery (Hanson et al., 2016). This treatment further enhances the resilience of connective tissues and muscle elasticity, thereby promoting patient mobility, which is crucial for individuals with secondary lymphedema following gynecologic cancer surgery (Figueira et al., 2023).
Our study also has certain limitations that warrant acknowledgment. For instance, the relatively small sample size and single-center design may limit the generalizability of our findings. Additionally, long-term follow-up data are lacking. Furthermore, this study did not include the use of bandages, which constitute a critical component of CDT. Future research should aim to address these limitations by conducting larger, multicenter trials with extended follow-up periods and by exploring the effects of MLD in combination with isokinetic strength training and bandage application.
Conclusion
Following gynecological tumor surgery, patients who develop secondary lower limb lymphedema have shown greater benefits from a regimen that combines isokinetic strength training with MLD. This integrated approach has been more effective in reducing swelling, enhancing ambulatory function, and increasing muscle strength compared to MLD alone. Further research is essential to investigate its long-term efficacy and potential clinical applications.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Review Committee of Zhengzhou Central Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JM: Conceptualization, Writing – original draft. HW: Writing – review and editing. YL: Project administration, Writing – review and editing. XG: Data curation, Writing – review and editing. MX: Data curation, Writing – review and editing. XW: Supervision, Writing – review and editing. LM: Funding acquisition, Writing – review and editing. DX: Software, Writing – review and editing. LS: Formal Analysis, Writing – review and editing. DC: Data curation, Writing – review and editing. JW: Conceptualization, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This project is being supported by the Zhengzhou Science and Technology Bureau, Henan Province Science and Technology Development Program Project (222102310657), the Sailing Program supported by the Shanghai Committee of Science and Technology (21YF1404600); Department of Science and Technology of Henan Province and the Key Science and Technology Research Project of Henan Province (2018020778).
Acknowledgments
The sponsors were not involved in the study’s design, data collection, data analysis, interpretation of results, or manuscript writing. We thank all patients who volunteered to participate in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ali, K., Dibbs, R. P., Dougherty, C., Iacobas, I., and Maricevich, R. S. (2022). Treatment outcomes of manual lymphatic drainage in pediatric lymphedema. Ann. Vasc. Surg. 78, 263–271. doi:10.1016/j.avsg.2021.06.021j.avsg.2021.06.021
Aoishi, Y., Oura, S., Nishiguchi, H., Hirai, Y., Miyasaka, M., Kawaji, M., et al. (2020). Risk factors for breast cancer-related lymphedema: correlation with docetaxel administration. Breast Cancer 27 (5), 929–937. doi:10.1007/s12282-020-01088-x
Baumann, F. T., Reike, A., Hallek, M., Wiskemann, J., and Reimer, V. (2018). Does exercise have a preventive effect on secondary lymphedema in breast cancer patients following local treatment - a systematic review. Breast Care 13, 380–385. doi:10.1159/000487428
Biglia, N., Zanfagnin, V., Daniele, A., Robba, E., and Bounous, V. E. (2017). Lower body lymphedema in patients with gynecologic cancer. Anticancer Res. 37 (8), 4005–4015. doi:10.21873/anticanres.11785ticanres.11785
Bitler, M. P., and Carpenter, C. S. (2017). Effects of state cervical cancer insurance mandates on pap test rates. Health Serv. Res. 52 (1), 156–175. doi:10.1111/1475-6773.12477477
Brandão, M. L., Soares, HPDS, Andrade, M. D. A., Faria, A. L. S. d. C., and Pires, R. S. (2020). Efficacy of complex decongestive therapy for lymphedema of the lower limbs: a systematic review. J. Vasc. Bras. 19, e20190074. doi:10.1590/1677-5449.190074
Campanholi, L. L., Duprat, J. P., and Fregnani, J. H. T. G. (2015). Evaluation of inter-rater reliability of subjective and objective criteria for diagnosis of lymphedema in upper and lower limbs. J. Vasc. Bras. 14 (1), 16–21. doi:10.1590/1677-5449.20140037
Caretto, A. A., Stefanizzi, G., Garganese, G., Fragomeni, S. M., Federico, A., Tagliaferri, L., et al. (2022). Treatment of early-stage gynecological cancer-related lower limb lymphedema by lymphaticovenular anastomosis-the triple incision approach. Med. Kaunas. 58 (5), 631. doi:10.3390/medicina58050631
Ciudad, P., Sabbagh, M. D., Agko, M., Huang, T. C., Manrique, O. J., Román, L. C., et al. (2019). Surgical management of lower extremity lymphedema: a comprehensive review. Indian J. Plast. Surg. 52 (1), 081–092. doi:10.1055/s-0039-1688537
Clinckaert, A., Callens, K., Cooreman, A., Bijnens, A., Moris, L., Van Calster, C., et al. (2022). The prevalence of lower limb and genital lymphedema after prostate cancer treatment: a systematic review. Cancers(Basel) 14 (22), 5667. doi:10.3390/cancers14225667
Cormier, J. N., Askew, R. L., Mungovan, K. S., Xing, Y., Ross, M. I., and Armer, J. M. (2010). Lymphedema beyond breast cancer: a systematic review and meta-analysis of cancer-related secondary lymphedema. Cancer. 116 (22), 5138–5149. doi:10.1002/cncr.25458
Cuthbert, S. C., and Goodheart, Jr G. J. (2007). On the reliability and validity of manual muscle testing: a literature review. Chiropr. & osteopathy 15 (1), 4. doi:10.1186/1746-1340-15-41746-1340-15-4
Dean, T., Ransome, Y., and Moss, L. (2019). Between US breast cancer survivors with or without lymphedema. J. Cancer Surviv 13 (5), 804–814. doi:10.1007/s11764-019-00799-1
de Sire, A., Losco, L., Lippi, L., Spadoni, D., Kaciulyte, J., Sert, G., et al. (2022). Surgical treatment and rehabilitation strategies for upper and lower extremity lymphedema: a comprehensive review. Med. Kaunas. 58 (7), 954. doi:10.3390/medicina58070954
Executive Committee of the International Society of Lymphology (2020). The diagnosis and treatment of peripheral lymphedema: 2020 Consensus document of the international society of Lymphology. Lymphology 53 (1), 3–19. doi:10.2458/lymph.4649
Figueira, A. C. C., Pereira, A., Leitão, L., Ferreira, R., Oliveira, P. A., and Duarte, J. A. (2023). Effects of moderate exercise training on cancer-induced muscle wasting. MDPI 11 (19), 2652. doi:10.3390/healthcare11192652
Földi, M., and Strossenreuther, R. (2005). Foundations of manual lymph drainage. Elsevier Health Sciences.
Hanson, E. D., Wagoner, C. W., Anderson, T., and Battaglini, C. L. (2016). The independent effects of strength training in cancer survivors: a systematic review. Curr. Oncol. Rep. 18, 31–18. doi:10.1007/s11912-016-0511-3
Hayes, S., Reul-Hirche, H., and Turner, J. (2009). Exercise and secondary lymphedema: safety, potential benefits, and research issues. Med. Sci. sports Exerc. 41 (3), 483–489. doi:10.1249/MSS.0b013e31818b98fb
Holden, M. K., Gill, K. M., Magliozzi, M. R., Nathan, J., and Piehl-Baker, L. (1984). Clinical gait assessment in the neurologically impaired. Phys. Ther. 64, 35–40. doi:10.1093/ptj/64.1.35
Hsu, Y. Y., Nguyen, T. T. B., and Chou, Y. J. (2024). Effects of exercise on lower limb lymphedema in gynecologic cancer: a systematic review and meta-analysis. Eur. J. Oncol. Nurs. 70, 102550. doi:10.1016/j.ejon.2024.102550
Ida, K., Miyamoto, T., Higuchi, S., Takeuchi, H., Yamada, S., Ono, M., et al. (2019). Effectiveness of a genetic test panel designed for gynecological cancer: an exploratory study. Med. Oncol. 36 (7), 62. doi:10.1007/s12032-019-1286-9
Kasseroller, R. G. (1998). The Vodder school: the Vodder method. Cancer Interdiscip. Int. J. Am. Cancer Soc. 83 (S12B), 2840–2842. doi:10.1002/(SICI)1097-0142(19981215)83:12B+<2840::AID-CNCR37>3.0.CO;2-5
Liang, M., Chen, Q., Peng, K., Deng, L., He, L., Hou, Y., et al. (2020). Manual lymphatic drainage for lymphedema in patients after breast cancer surgery: a systematic review and meta-analysis of randomized controlled trials. Med. Baltim. 99 (49), e23192. doi:10.1097/MD.0000000000023192
Lin, Y., Yang, Y., Zhang, X., Li, W., Li, H., and Mu, D. (2022). Manual lymphatic drainage for breast cancer-related lymphedema: a systematic review and meta-analysis of randomized controlled trials. Clin. Breast Cancer 22 (5), e664–e673. doi:10.1016/j.clbc.2022.01.013
Liu, F., Liu, N. F., Wang, L., Chen, J., Han, L., Yu, Z., et al. (2021). Treatment of secondary lower limb lymphedema after gynecologic cancer with complex decongestive therapy. Lymphology 54 (3), 122–132. doi:10.2458/lymph.4786
Liu, G., Hu, J., Liu, Y., and Yuan, M. (2023). Factors influencing lower limb lymphedema after cervical cancer surgery:A case-control study. Lymphat. Res. Biol. 21 (2), 169–178. doi:10.1089/lrb.2022.0025
Nakamura, K., Masuyama, H., Ida, N., Haruma, T., Kusumoto, T., Seki, N., et al. (2017). Radical hysterectomy plus concurrent chemoradiation/radiation therapy is negatively associated with return to work in patients with cervical cancer. Int. J. Gynecol. Cancer 27 (1), 117–122. doi:10.1097/IGC.0000000000000840
Omar, M. T. A., Gwada, R. F. M., Omar, G. S. M., El-Sabagh, R. M., and Mersal, A. A. E. (2020). Low-intensity resistance training and compression garment in the management of breast cancer-related lymphedema: single-blinded randomized controlled trial. J. Cancer Educ. 35 (6), 1101–1110. doi:10.1007/s13187-019-01564-9
Petrucci, A., Guglielmino, D., Pecci, J., and Pareja-Galeano, H. (2024). The effects of isokinetic training in athletes after knee surgery: a systematic review. Phys. Sportsmed. 52 (4), 309–316. doi:10.1080/00913847.2023.2297666
Reichart, K. (2017). Lymphedema: improving screening and treatment among at-risk breast cancer survivors. Clin. J. Oncol. Nurs. 21 (1), 21–25. doi:10.1188/17.CJON.21-25
Schoenfeld, P. S. (2005). Evidence-based medicine in practice: applying intention-to-treat analysis and perprotocol analysis. Am. J. gastroenterology 100, 3–4. doi:10.1111/j.1572-0241.2005.41537.x
Schulz, K. F., Altman, D. G., and Moher, D. (2010). CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ Clin. Res. 340: c332. doi:10.1136/bmj.c332
Tedeschi, R. (2023). Biomechanical alterations in lower limb lymphedema: implications for walking ability and rehabilitation. Phlebology 38 (8), 496–502. doi:10.1177/02683555231188236
Thompson, B., Gaitatzis, K., Janse de Jonge, X., Blackwell, R., and Koelmeyer, L. A. (2021). Manual lymphatic drainage treatment for lymphedema: a systematic review of the literature. J. Cancer Surviv 15 (2), 244–258. doi:10.1007/s11764-020-00928-1
Wang, B., Zhang, X., Zhu, F., Zhu, W., Wang, X., Jia, F., et al. (2022). A randomized controlled trial comparing rehabilitation with isokinetic exercises and Thera-Band strength training in patients with functional ankle instability. PLoS One 17 (12), e0278284. doi:10.1371/journal.pone.0278284al.pone.0278284
Wang, Q. F., and Dong, L. M. (2019). Application of isokinetic muscle training combined with freehand lymphatic drainage in postoperative lymphedema of breast cancer. Nurs. Pract. Res. 16 (22), 82–84. doi:10.3969/j.issn.1672-9676.2019.22.034
Wittenkamp, M. C., Christensen, J., Vinther, A., and Juhl, C. B. (2025). The effect of exercise in patients with lower limb lymphedema: a systematic review. Acta Oncol. Stockh. Swed. 64, 484–498. doi:10.2340/1651-226X.2025.42560
Wong, M., Eaton, P. K., Zanichelli, C., Moore, C., Hegarty, C., and MacDonald, N. (2022). The prevalence of undiagnosed postoperative lower limb lymphedema among gynecological oncology patients. Eur. J. Surg. Oncol. 48 (5), 1167–1172. doi:10.1016/j.ejso.2021.12.464
Xu, H., and Liu, H. (2024). Effects of ankle isokinetic training on muscle strength and balance amongst older women with mild Parkinson's disease: a randomised trial. J. Back Musculoskelet. Rehabil. 37 (4), 1007–1014. doi:10.3233/BMR-230259
Zhang, H. Z., Zhong, Q. L., Zhang, H. T., Luo, Q. H., Tang, H. L., and Zhang, L. J. (2022). Effectiveness of six-step complex decongestive therapy for treating upper limb lymphedema after breast cancer surgery. World J. Clin. Cases 10 (25), 8827–8836. doi:10.12998/wjcc.v10.i25.8827.v10.i25.8827
Zhang, J., Ju, X., Feng, Z., Zhang, X., and Li, J. (2021). Progressive resistance exercise training to prevent lower-limb lymphedema after cervical cancer surgery: a feasibility study. Asia Pac J. Oncol. Nurs. 9 (1), 32–38. doi:10.1016/j.apjon.2021.12.002
Zhang, J., Zhou, C., Ma, Q., Zhang, Y., and Zhang, X. (2024). Preventing lower limb lymphedema after pelvic lymphadenectomy with progressive resistance exercise training: a randomized controlled trial. Asia-Pacific J. Oncol. Nurs. 11 (1), 100333. doi:10.1016/j.apjon.2023.100333
Zhu, T., Liu, K., Ni, B. Y., Li, L., Jin, H. P., and Wu, W. (2023). Effects of extracorporeal shock wave therapy combined with isokinetic strength training on spastic calf triceps in patients after a stroke: a double-blinded randomised controlled trial. Neurol. Res. 45 (11), 1019–1025. doi:10.1080/01616412.2023.2255413
Keywords: secondary lymphedema, gynecologic cancer, lower extremity, isokinetic strength training, manual lymphatic drainage
Citation: Ma J, Wang H, Li Y, Guo X, Xie M, Wang X, Mao L, Xing D, Shen L, Chen D and Wang J (2025) Effectiveness of isometric muscle training combined with manual lymphatic drainage on secondary lower extremity lymphedema following gynecologic cancer surgery. Front. Bioeng. Biotechnol. 13:1568003. doi: 10.3389/fbioe.2025.1568003
Received: 28 January 2025; Accepted: 23 April 2025;
Published: 14 May 2025.
Edited by:
Wujing Cao, Chinese Academy of Sciences (CAS), ChinaReviewed by:
Erich Brenner, Innsbruck Medical University, AustriaZhitao Gao, Xinxiang Medical University, China
Copyright © 2025 Ma, Wang, Li, Guo, Xie, Wang, Mao, Xing, Shen, Chen and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingxin Wang, a2Zrd2FuZ2p4YnNAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Jiahui Ma
Jiahui Ma Hewei Wang
Hewei Wang Yilan Li3
Yilan Li3 Xinxin Wang
Xinxin Wang Luxi Mao
Luxi Mao Li Shen
Li Shen Dan Chen
Dan Chen Jingxin Wang
Jingxin Wang