- Office of Occupational Safety and Health, Office of the Chief Scientist, Office of the Commissioner, United States Food and Drug Administration, Silver Spring, MD, United States
A laboratory quality management system (LQMS) enables the effective operation of laboratories of all types and sizes. With rapid advances in technology (e.g., artificial intelligence and machine learning, advanced manufacturing) comes the need for laboratories worldwide to conduct proper change management and process improvement to meet the continued demand amidst major changes. In order to do so while ensuring that results and data are accurate, timely, and reproducible, it is crucial for laboratories to sustain a foundational LQMS that accommodates laboratory processes, document and records management, and a path for continual improvement in the laboratory itself and within its contextual organization. A foundational LQMS provides a framework to address gaps in process or product performance and risks present throughout the laboratory’s workflow, any of which could lead to a critical error that compromises the organization’s credibility. There are many LQMS frameworks–benchmarks such as consensus standards or regulations (e.g., Good Laboratory Practices for Nonclinical Laboratory Studies) – that the laboratory can select from to govern its LQMS. While these frameworks vary in applicability, there are several common elements across these frameworks that can serve as the basic components of any LQMS. The aim of this study is to review and assess 12 widely-recognized, fundamental aspects of an LQMS to identify actionable examples and templates that can enable effective implementation of a robust LQMS. A robust LQMS is one that fosters long term success of the laboratory, and which ultimately ensures reliable results, efficient operations, and the protection of public health.
1 Introduction: what is a laboratory quality management system?
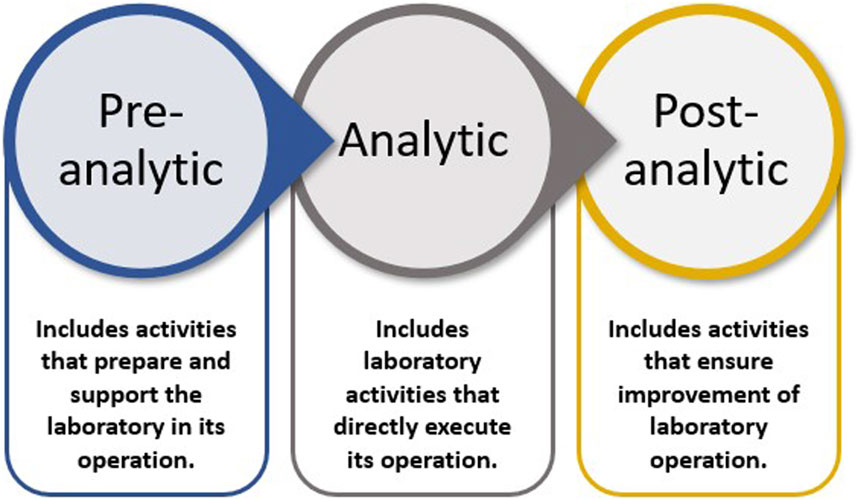
An LQMS is a formal system that documents the personnel, processes, and procedures by which laboratory management ensures the consistent quality of the processes conducted, outputs generated, and results reported. The entire set of operations that impacts a laboratory is considered the path of workflow. Workflow starts with the customer, ends with reporting, and results in interpretation, leading to appropriate actions and decisions. The path of workflow includes all steps before and after testing that affect laboratory outputs. There are three phases of the laboratory path of workflow: pre-analytic, analytic, and post-analytic (WHO, 2011), which Figure 1 graphically presents.
A Nature survey of 1,576 researchers found that 52% of responders agreed that there is a crisis of reproducibility; and over 70% have failed to reproduce another scientist’s data (Baker, 2016). To mitigate this crisis and protect public health through the generation of reliable and reproducible data, all aspects of laboratory operations, including organizational structure, processes, and procedures, must be addressed; an optimal solution for such is the implementation of a robust and efficient LQMS. To do so, consider establishing a quality policy and quality objectives; establishing processes, procedures, and systems to ensure that objectives and requirements are consistently fulfilled; and monitoring LQMS performance for continual improvement. To structure and guide effective LQMS implementation, activities can be grouped into components. Herein we describe one such framework, overviewed in the next section.
2 Overview of the 12 quality system essentials
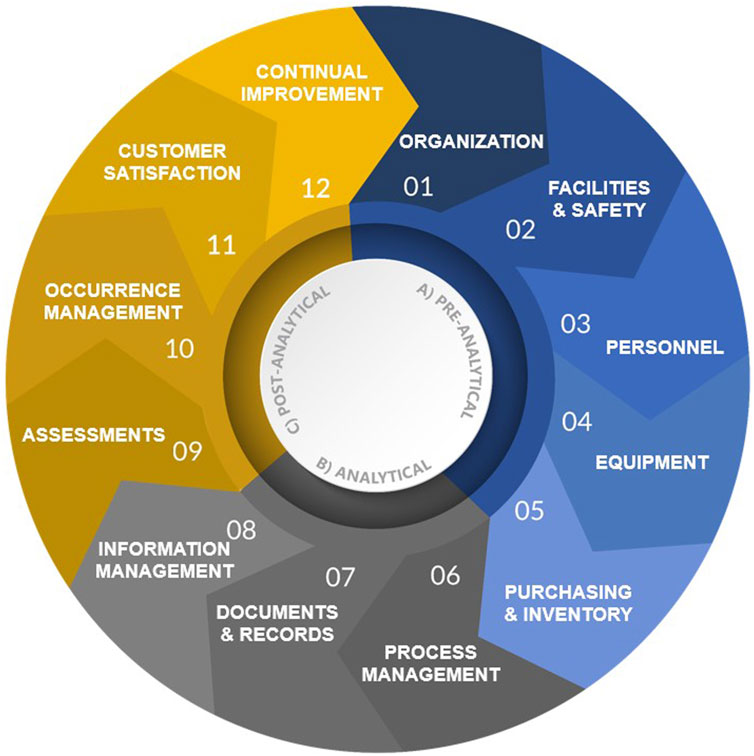
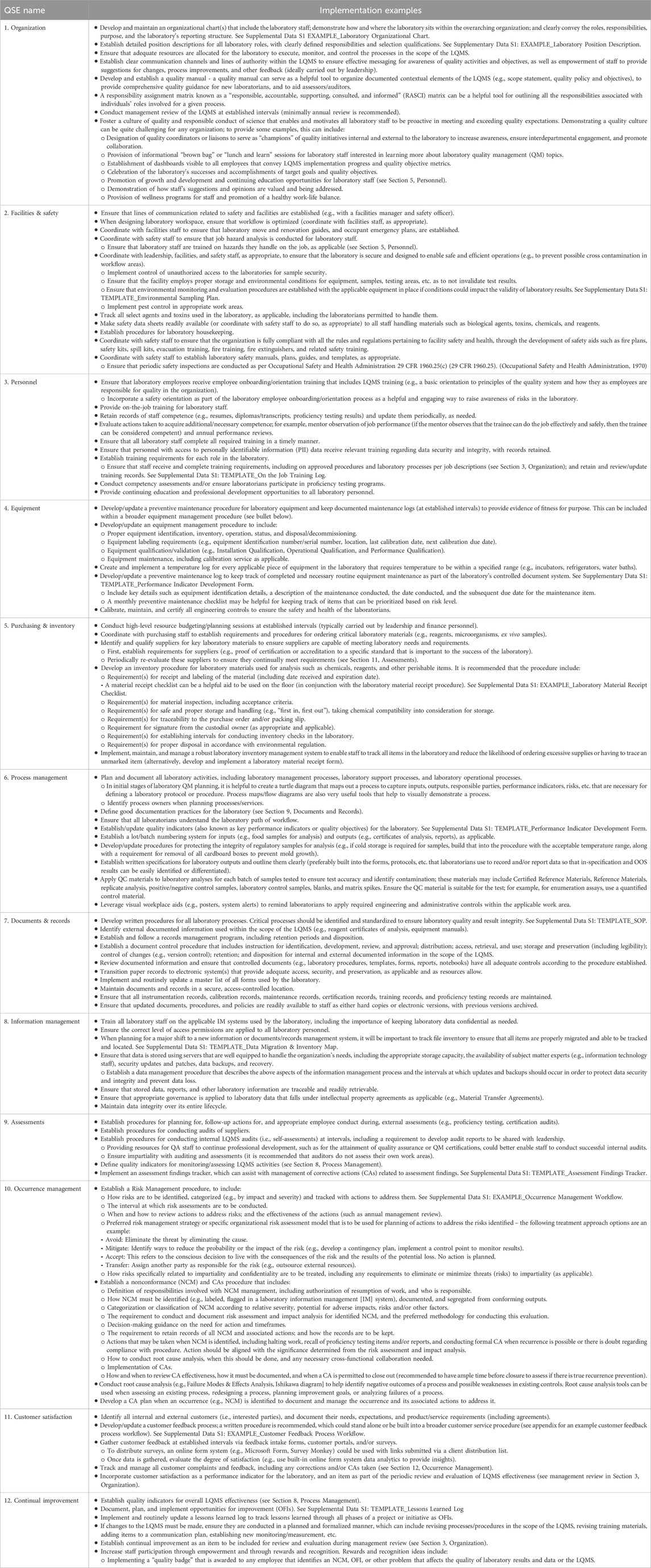
The purpose of this article is to detail the fundamental elements of an LQMS and provide examples in support of implementation and improvement of organization-specific LQMS processes, procedures, and manuals that meet a laboratory’s unique needs. Fundamentally, an LQMS can be framed around 12 quality system essentials (QSEs), which are graphically represented in Figure 2 and aligned with the appropriate laboratory path of workflow. The 12 QSEs, modified from the World Health Organization’s (WHO’s) Laboratory Quality Management System Handbook (WHO, 2011), include Organization, Facilities and Safety, Personnel, Equipment, Purchasing and Inventory, Process Management, Documents and Records, Information Management, Assessments, Occurrence Management, Customer Satisfaction, and Continual Improvement. While very beneficial for understanding LQMS implementation, one limitation of the WHO Handbook is that it does not include templates for on-the-job use in the laboratory. The templates and examples provided herein (please see Table 1 and Supplementary Material) provide added benefit to laboratory personnel at all levels by enabling consistent documentation and a starting point for effective LQMS implementation.
Implementing the 12 QSEs is beneficial for many laboratory types with the goal of assuring the continued generation of accurate, reliable, reproducible, and timely data. Pillai et al. reviewed five widely recognized quality management (QM) regulations and standards commonly applied in laboratories and found that at least 10 out of 12 QSEs (83%) are addressed in all five frameworks (Pillai et al., 2022). This finding highlights the significance of QSEs in laboratory QM. The subsequent sections provide descriptions and recommendations pertaining to each QSE, beginning with QSE 1: Organization.
3 QSE 1: organization
The first QSE, Organization, focuses on the laboratory staffing structure and how it sits within its organization. Leadership’s commitment plays a critical role in the successful implementation of an LQMS. If leadership does not clearly demonstrate interest in adherence to the LQMS, it will likely become difficult to instill a strong culture of quality in the laboratory. Designing a laboratory’s organizational structure in a manner that ensures quality objectives are met and aligned with organizational strategy can serve to fulfill its mission. For example, the role of a “Quality Liaison” can be established to uphold a culture of quality and promote best practices and policies. Implementation of the Organization QSE would also ensure that roles and responsibilities for monitoring and assessment are established to mitigate risks. With the appropriate resources and leadership’s dedication to the success of an LQMS, a laboratory is further equipped to protect public health.
4 QSE 2: facilities and safety
Facilities and Safety involves the laboratory environment and the processes, procedures, and plans that support its safe and secure operation. With this QSE, laboratories follow approved facility procedures, instructions, rules, national and state regulations, guidelines, and standards. A suitable environment for effective and conformant laboratory operations should be provided and maintained, including social, psychological, and physical factors that ensure staff safety and health. Consider the purpose and type of laboratory work when determining what is needed; for example, if environmental monitoring is conducted, ensure that proper facility systems are in place (e.g., air filtration, humidity monitoring, alarms for out-of-range measurements). Some may wonder, what does safety have to do with quality? While quality and safety practices have slightly different goals, they are complementary. Quality is defined as the “degree to which a set of inherent characteristics of an object fulfills requirements” (ISO, 2015a), and safety is an important laboratory requirement. It is recommended that safety and quality staff have separate, clearly delineated responsibilities, but also collaborate to achieve objectives. Leadership is responsible for establishing policies and resources to ensure safety and health of employees, the organization’s most critical asset. It is everyone’s duty to embrace safety and responsible behavior in their work. Regardless of the type of laboratory, a higher safety risk must inform enhanced safety protocol. Implementing this QSE is a proactive measure to sustain safety and quality in the laboratory.
5 QSE 3: personnel
The Personnel QSE focuses on defining job descriptions, qualifications, and training requirements for laboratory staff. This begins before they are hired–establishing competence requirements and position descriptions ensures that qualified staff conduct laboratory operations–and continues with enabling staff to grow professionally. Laboratory staff should receive appropriate training upon hiring and throughout employment, including safety training aligned with applicable regulatory requirements; hands-on training on laboratory procedures and equipment; and quality training needed for LQMS activities. Personnel must be fully trained and deemed competent to execute their responsibilities safely and accurately prior to beginning work. According to a 2019 review, a survey of 2,400 academic researchers found that while 70% received safety training, only 26% were trained within 30 days of onboarding; among other harrowing statistics presented, one study found that 25% of researchers had not been trained in the specific hazard with which they worked (Ménard and Trant, 2020). Hazard identification and risk mitigation strategies are foundational components of LQMS. To ensure training effectiveness, laboratory management should conduct competency assessments and/or proficiency testing at established intervals. This may reveal the need for additional or specific training, and incorporating safety requirements into assessments will help ensure that nothing is missed when it comes to the laboratory’s most important resource: its people.
6 QSE 4: equipment
Well-maintained equipment is crucial to safe laboratory operation and the quality of data generated. The Equipment QSE focuses on ensuring that policies and procedures are in place and available to appropriately receive, identify (label), qualify, inventory, calibrate, and maintain critical equipment throughout its lifecycle, including a decommissioning/retirement policy. Critical equipment refers to laboratory equipment or instrumentation used for scientific operations (e.g., sample analysis, processing, handling, and/or manipulation and data collection). It is important for leadership to prioritize equipment management and allocate resources to ensure it is done effectively. Routine preventive maintenance and calibration of critical equipment will help to ensure the validity of results. A preventive approach will not only minimize downtime from malfunction, but it can also reduce the likelihood of variability in test results, which can occur over time as equipment gradually degrades. Testing positive, negative, and calibration controls on analytical equipment, where applicable, ensures proper function. For equipment used to store valuable materials and conduct analysis—the loss of which can be detrimental—it is essential to implement redundant systems to manage equipment failure risks. Equipment must also be fit for purpose; fitness for purpose can be determined in many ways (e.g., calibration), but evidence should be documented (e.g., calibration records, temperature records). When traceability is required or essential, it becomes critical to identify (e.g., label), calibrate using measurement standards (e.g., National Institute of Standards and Technology), and safeguard equipment from anything that would invalidate it for use. If measurement equipment is found to be unfit (e.g., control results out-of-specification), the laboratory should determine if previous results that the equipment generated are valid or invalid, and take appropriate action as necessary (e.g., repeating analysis using newly calibrated equipment, recalling data). The above activities should be documented as evidence that they were performed–for example, an equipment receipt form may be completed when new equipment arrives, and a preventive maintenance log could be updated when routine service is performed (with certificates and receipts of service saved).
7 QSE 5: purchasing and inventory
Maintaining inventory and monitoring the quality of suppliers’ products and services helps ensure consistent quality of results. This QSE focuses on processes and procedures to appropriately receive, identify, use, store, and discard materials. Routine inventory should be conducted to ensure that chemicals, reagents, and other materials are within expiry and properly stored. Inventory management can prevent occurrence of testing interruptions (see Section 12, Occurrence Management). It can also assist with measurement of stock levels over time to optimize stock quantities of fast- and slow-moving materials. Another key aspect of this QSE is ensuring purchased material fitness-for-use. Establishing procedures and criteria for supplier qualification and continued capability, and coordinating with purchasing staff to implement guardrails (e.g., approved vendor lists), ensure that the laboratory receives suitable materials that support the validity and reproducibility of results. Additionally, handling requirements should be incorporated into procedures, including signing for receipt, confirming if the material is hazardous, inspecting for damage, and storing properly. For testing and research materials—the integrity of which is critical to scientific conduct—it is essential to manage risks associated with inventory such as incorrect storage, misidentification, and retention past expiry.
8 QSE 6: process management
Process management involves organizing and controlling laboratory processes and procedures to ensure accurate and reliable testing, which ultimately ensures the quality of data produced and the accurate interpretation of results. This QSE describes the planning, managing, and documenting of connected processes. Well-planned and managed processes help a laboratory to be more effective and efficient. This QSE involves establishing quality control (QC) activities. The purpose of QC is to monitor processes related to the analytic phase of testing and to allow for detecting errors in testing so that corrective action can be taken before flawed results are released from the laboratory. Variability in manufacturing processes is the result of many disruptions that occur during their implementation and by their nature cannot be 100% eliminated (Misztal and Ratajszczak, 2025). This natural variability further increases the need for QC. Whether the laboratory science is quantitative or qualitative, automated or manual, employing adequate QC procedures ensures the reliability of results generated. For a laboratory associated with product or method development, it is advisable to incorporate appropriate verification and validation procedures when implementing a new product or method or a significant change to an existing product or method.
9 QSE 7: documents and records
The Documents & Records QSE focuses on the development, control, and maintenance of written laboratory policies, processes, procedures, protocols, work instructions, forms, and records associated with laboratory operations. Records are documented information that serves as evidence of laboratory activities (e.g., laboratory notebooks, reports). Key aspects of analysis or research such as instrument used, maintenance/calibration data, and reagents used (including manufacturer, date of receipt, date of expiration, lot number, etc.), should be documented to support the reproducibility of results. Employees should be able to find documents and records when needed and easily understand the information conveyed. Document control procedures should ensure that applicable documents are identifiable; access-controlled; reviewed and approved before distribution and at periodic intervals; archived or destroyed as applicable; and that audit trails are in place to ensure accountability for edits or corrections (i.e., capturing who, when, and why, without obscuring the original entry). Procedures should also address correct format and media; storage and preservation; change control; and retention and disposition. It is recommended to maintain records electronically to the greatest extent possible. Records of evidence of conformity (i.e., records that prove requirements have been met), as well as non-conformity, should be protected from unauthorized alterations.
10 QSE 8: information management
The Information Management (IM) QSE focuses on management of data and its flow through the laboratory, from incoming to outgoing. IM systems may be paper-based, electronic, or a combination of both; whatever technology is employed, the IM QSE is essential and is closely related to the Documents and Records QSE (WHO, 2011). The main difference is that the IM QSE encompasses a broader system for management of information (e.g., security, data governance, how information moves through the path of workflow), while the Documents and Records QSE focuses on controlling written information about policies, processes, and procedures, and the completed information that serves as records (e.g., a batch record, a form that was filled out). The IM QSE involves reviewing and meeting information requirements (e.g., intellectual property, material transfer agreements) and disseminating information (e.g., test results) in approved system(s) to end users in a secure, timely, and accurate manner. Properly addressing this QSE ensures that data is accurate and confidential, and accessible to authorized users. Laboratories should establish procedures to ensure that information received, generated, disseminated, and published is effectively governed to safeguard its quality, integrity, reproducibility, security, and confidentiality based on sensitivity and/or privacy. Leadership (or designee) should assign access levels for laboratory personnel per job descriptions and work requirements, and for external users. Stored data, reports, and other information should be traceable and readily retrievable.
11 QSE 9: assessments
The Assessments QSE is a way to analyze an LQMS’s efficacy through internal and external assessments (e.g., audits), as well as through performance evaluation in an external quality assessment (EQA) program (Dhara et al., 2024) to verify conformance to regulatory, accreditation/certification, and customer requirements. The QSE involves evaluating laboratory performance compared to a standard, benchmark, or performance of other laboratories, as appropriate. Assessments and audits may be internal (e.g., self-assessments conducted within the organization by personnel trained for assessment, belonging to a department separate from the one being assessed) or external (e.g., conducted by a third-party entity). An internal assessment, for example, could be a process audit conducting by QA staff for the manufacturing department; and an external assessment could be an ISO/IEC 17025:2017 accreditation audit (International Organization for Standardization, 2017) for a calibration laboratory conducted by an ISO registrar. Standardized assessments conducted at established intervals help to evaluate the effectiveness of the LQMS. Properly addressing this QSE ensures comprehensive implementation of the LQMS, supports identification of opportunities for improvement, and improves the ability to achieve quality objectives. To implement this QSE, laboratories should establish procedures for internal assessments. Quality assurance (QA) staff (or appropriate designee) should also develop and implement procedures for supporting external assessments. Further, QA staff/designee(s) should define quality indicators for LQMS assessment.
12 QSE 10: occurrence management
In the complex world of laboratories, occurrences can and will naturally occur in any phase of the path of workflow. An occurrence is defined as any event that has a negative impact on an organization, including its personnel, the product of the organization, equipment, or the environment in which it operates (WHO, 2011). Unintended errors and other events in the laboratory can have serious consequences that affect the quality of its results and could negatively impact public health or trust. But when occurrences are properly managed through identification, reporting using appropriate channels, and application of corrective actions (CAs), the output of occurrence management is a continually improving organization. This QSE involves establishing procedures to investigate unintended consequences (e.g., non-conformances) from laboratory activities, identify root causes, and implement CAs to eliminate recurrence. Laboratories cannot prevent every error or incident, and events should be tracked to identify process gaps. But properly addressing this QSE can reduce risk of future occurrences, personnel injury, facilities damage, equipment or material loss, and events adverse to organizational decisions. Laboratory management should establish procedures for: identifying, reporting, documenting, and investigating occurrences; conducting risk assessments associated with occurrences; developing and implementing risk mitigation strategies; and implementing CAs properly with effectiveness checks.
13 QSE 11: customer satisfaction
The Customer Satisfaction QSE emphasizes laboratory customers, their expectations, and the importance of designing process(es) to meet those expectations. To uphold a laboratory’s organizational and public health missions, it is essential to consider the needs and expectations of its customers (i.e., interested parties). Customers can be both internal (e.g., leadership, another department that the laboratory interacts with such as manufacturing) and external (e.g., clients, regulators, proficiency testing providers). Anyone impacted by laboratory outputs could be considered a customer. Ultimately, the laboratory generates a product—data and results—for its customers. If the customer is not well served, the laboratory is not achieving its primary function. This QSE involves procedures to monitor customer needs through feedback mechanisms (e.g., surveys, emails, online chat), quality indicators, and assessments (see Section 11, Assessments). Leadership is responsible for ensuring that customer expectations are understood and met. Properly implementing this QSE can reduce customer dissatisfaction, ensure that the “voice” of the customer is heard, and improve internal processes through addressing feedback; it also demonstrates commitment to the customer. To do so, laboratory management should establish procedures to identify internal and external customers; document their needs, expectations, and requirements; manage satisfaction through feedback review and analysis; and address complaints in a timely manner.
14 QSE 12: continual improvement
The Continual Improvement QSE focuses on increasing LQMS effectiveness and efficiency to provide added benefit to the organization and its customers. Improvement strategies such as the Plan-Do-Check-Act approach, defined below, could be used to continually evaluate and improve laboratory processes:
Plan: establish the objectives of the system and its processes, and the resources needed to deliver results in accordance with requirements; identify and address risks and opportunities;
Do: implement what was planned;
Check: monitor and measure processes (as applicable) and the resulting outputs against objectives, requirements, and planned activities, and report the results;
Act: take actions to improve performance, as necessary (ISO, 2015b).
In support of organizational missions and the protection of public health, it is essential to take steps to implement and improve upon the LQMS to facilitate increased accuracy, reliability, reproducibility, and timeliness of data. Ensuring the quality of laboratory outputs is the main objective of an LQMS and ultimately relies on continual improvement. This QSE involves procedures to monitor and evaluate the effectiveness of the LQMS. Review of internal assessment reports, occurrences, customer satisfaction surveys, and data trends, are all ways to continually improve the laboratory. Leadership, QA staff, and laboratory management are responsible for monitoring LQMS compliance and evaluating the system overall for effectiveness. Implementing this QSE can help to identify and reduce risks in the laboratory, as well as increase productivity by fixing process inefficiencies; additionally, it enhances commitment to a culture of quality and responsible conduct of science.
15 Discussion
The foundational information described in this article and implementation examples provided in Table 1 support effective laboratory QM related to the 12 QSEs in the framework outlined. It is important that organizational leadership, laboratory management, QA staff, and laboratory staff have a foundational understanding of the measures available to ensure quality of laboratory operations, including the LQMS QSEs. Application of the QSEs can not only organize and improve the LQMS implementation process, but it can also ensure that all aspects of an LQMS are addressed to mitigate risks, allow for structured assessments, and prepare for third-party certification or accreditation. This study, including provision of examples and templates pertaining to each key area described, demonstrates that implementing a well-structured, robust framework that the 12 QSEs offer can support the continued success of the laboratory using consistent, standardized documentation and a clear understanding of what implementation could entail–this is critical for planning the LQMS. Implementation using the supplementary templates provided can also contribute to time and cost savings.
As laboratories conduct different types of work and have distinct needs and goals, further examination of the QSEs beyond this article may be beneficial, including identifying how to modify and apply their respective concepts to reflect a laboratory’s chosen LQMS framework (e.g., Good Laboratory Practices for Nonclinical Studies [GLP]; ISO standards) in order to improve applicability.
Table 1 below provides illustrative examples of how staff responsible for oversight of the LQMS, and leadership where appropriate, could apply each QSE within the organization to demonstrate conformance, should the organization decide to conform to the 12 QSE framework.
Author contributions
SP: Conceptualization, Formal Analysis, Methodology, Resources, Supervision, Visualization, Writing – original draft, Writing – review and editing. EF: Conceptualization, Formal Analysis, Methodology, Resources, Visualization, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The findings and conclusions in this paper are those of the author(s) and do not represent the official position of the United States Department of Health and Human Services or Food and Drug Administration.
Conflict of interest
The author(s) declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2025.1578654/full#supplementary-material
Abbreviations
CA, Corrective Action; CFR, Code of Federal Regulations; EQA, External Quality Assessment; GLP, Good Laboratory Practices; IEC, International Electrotechnical Commission; IM, Information Management; ISO, International Organization for Standardization; LQMS, Laboratory Quality Management System; NCM, Nonconformance; OFI, Opportunity for Improvement; PI, Principal Investigator; QA, Quality Assurance; QC, Quality Control; QM, Quality Management; QSE, Quality System Essential; WHO, World Health Organization.
References
Baker, M. (2016). 1,500 scientists lift the lid on reproducibility. Nature 533, 452–454. doi:10.1038/533452a
Dhara, P., Patel, M., Patel, G. H., and Meshram, D. B. (2024). A review on quality management system in laboratory testing. World J. Biol. Pharm. Health Sci. 19 (2), 173–188. doi:10.30574/wjbphs.2024.19.2.0460
International Organization for Standardization (2015a). ISO 9000 Quality management systems— fundamentals and vocabulary. Switzerland: ISO. Available online at: https://www.iso.org/standard/45481.html.
International Organization for Standardization (2015b). ISO 9001 Quality management systems—requirements. Switzerland: ISO. Available online at: https://www.iso.org/standard/62085.html.
International Organization for Standardization (2017). ISO/IEC 17025 General requirements for the competence of testing and calibration laboratories. Switzerland: ISO. Available online at: https://www.iso.org/standard/66912.html.
Ménard, A. D., and Trant, J. F. (2020). A review and critique of academic lab safety research. Nat. Chem. 12, 17–25. doi:10.1038/s41557-019-0375-x
Misztal, A., and Ratajszczak, K. (2025). Possibilities of using contemporary quality management methods and tools for the sustainable development of the organization. Sustainability 17 (2), 617. doi:10.3390/su17020617
Occupational Safety and Health Administration (1970). 29 CFR 1960 basic program elements for federal employee occupational safety and health programs and related matters. (Standard No. 1960.25, subpart D – inspection and abatement). Available online at: https://www.ecfr.gov/current/title-29/subtitle-B/chapter-XVII/part-1960/subpart-D (Accessed 29 October 2024).
Pillai, S., Calvert, J., and Fox, E. (2022). Practical considerations for laboratories: implementing a holistic quality management system. Front. Bioeng. Biotechnol. Sec. Biosaf. Biosecurity 10, 1040103. doi:10.3389/fbioe.2022.1040103
World Health Organization (2011). Laboratory quality management system handbook. Lyon, France: World Health Organization. Available online at: http://apps.who.int/iris/bitstream/10665/44665/1/9789241548274_eng.pdf.
Keywords: quality assurance, quality management, LQMS, laboratory, implementation, total quality, continual improvement, workflow
Citation: Pillai SP and Fox E (2025) Laboratory quality management system fundamentals. Front. Bioeng. Biotechnol. 13:1578654. doi: 10.3389/fbioe.2025.1578654
Received: 20 February 2025; Accepted: 02 May 2025;
Published: 21 May 2025.
Edited by:
Natalia Shubladze, National Center of Tuberculosis and Lung Diseases (NCTLD), GeorgiaReviewed by:
Anwar Abdullah Borai, King Saud bin Abdulaziz University for Health Sciences, Saudi ArabiaNagham Mahmood Aljamali, Ministry of Higher Education and Scientific Research, Iraq
Copyright © 2025 Pillai and Fox. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Segaran P. Pillai, c2VnYXJhbi5waWxsYWlAZmRhLmhocy5nb3Y=
†These authors have contributed equally to this work and share first authorship
 Segaran P. Pillai
Segaran P. Pillai Elizabeth Fox
Elizabeth Fox