- 1Molecular Biology and Biochemistry, University of California, Irvine, Irvine, CA, United States
- 2Biomedical Engineering, University of California, Irvine, Irvine, CA, United States
- 3Individualized Interdisciplinary Studies, Simon Fraser University, Burnaby, BC, Canada
- 4Cell and Molecular Biology, Tulane University, New Orleans, LA, United States
- 5Aracari Biosciences, Inc., Irvine, CA, United States
- 6Physiology, Tulane University, New Orleans, LA, United States
- 7Louisiana Cancer Research Center, New Orleans, LA, United States
Our group has developed and validated an advanced microfluidic platform to improve preclinical modeling of healthy and disease states, enabling extended culture and detailed analysis of tissue-engineered miniaturized organ constructs, or “organs-on-chips.” Within this system, diverse cell types self-organize into perfused microvascular networks under dynamic flow within tissue chambers, effectively mimicking the structure and function of native tissues. This setup facilitates physiological intravascular delivery of nutrients, immune cells, and therapeutic agents, and creates a realistic microenvironment to study cellular interactions and tissue responses. Known as the vascularized micro-organ (VMO), this adaptable platform can be customized to represent various organ systems or tumors, forming a vascularized micro-tumor (VMT) for cancer studies. The VMO/VMT system closely simulates in vivo nutrient exchange and drug delivery within a 3D microenvironment, establishing a high-fidelity model for drug screening and mechanistic studies in vascular biology, cancer, and organ-specific pathologies. Furthermore, the optical transparency of the device supports high-resolution, real-time imaging of fluorescently labeled cells and molecules within the tissue construct, providing key insights into drug responses, cell interactions, and dynamic processes such as epithelial-mesenchymal transition. To manage the extensive imaging data generated, we created standardized, high-throughput workflows for image analysis. This manuscript presents our image processing and analysis pipeline, utilizing a suite of tools in Fiji/ImageJ to streamline data extraction from the VMO/VMT model, substantially reducing manual processing time. Additionally, we demonstrate how these tools can be adapted for analyzing imaging data from traditional in vitro models and microphysiological systems developed by other researchers.
1 Introduction
Preclinical organ-on-a-chip models that closely replicate human physiology and pathology–and especially the blood vasculature–are indispensable to advance disease research, drug discovery, and personalized medicine (Hachey and Hughes, 2018; Low and Tagle, 2018; Low et al., 2021; Ewald et al., 2021; Ingber, 2022; Martier et al., 2024; Gaebler et al., 2024a; Gaebler et al., 2024b). To address the limitations of existing models that fail to recapitulate a vascularized tissue niche, we developed the vascularized micro-organ (VMO) platform, an advanced organ-on-a-chip system that supports long-term studies of tissue-engineered, miniaturized organ constructs with associated microvasculature. This dynamic microfluidic platform allows for the co-culture of multiple cell types in a controlled flow environment, enabling the self-assembly of perfused microvascular networks within 3D tissue chambers. The physiological relevance of the platform is further enhanced by its ability to deliver nutrients and therapeutic agents through functional vascular networks, creating a powerful tool for studying vascular biology, disease progression, and therapeutic responses in vitro (Phan D. T. et al., 2017; Urban et al., 2018; Bender et al., 2024; Hatch et al., 2024).
One application of the VMO platform is the vascularized micro-tumor (VMT) model, which integrates tumor cells and stromal components into a 3D extracellular matrix within the tissue chambers. Gravity-driven fluid flow facilitates the rapid formation of living, perfused microvascular networks that support tumor growth and drug delivery, closely mimicking the complexity of in vivo tumor biology (Sobrino et al., 2016; Phan D. T. T. et al., 2017; Hachey et al., 2021; 2022; 2023; 2024). The VMO/VMT system uniquely recreates the stromal-vascular interactions critical to understanding disease mechanisms and evaluating therapeutic strategies, overcoming many of the limitations of conventional drug-screening models.
Given the large and intricate spatial and temporal data generated from imaging experiments with the VMO/VMT platform and other organ-on-a-chip models, efficient and reproducible data analysis workflows are essential. To address this need, we developed Hughes Lab Tools, a suite of custom-designed image-processing algorithms implemented in ImageJ/Fiji, an open-source Java-based image processing program developed by the National Institutes of Health (Schindelin et al., 2012). ImageJ’s open architecture enables extensibility through Java plugins, recordable macros, and scripts written in various programming languages. Using these capabilities, Hughes Lab Tools incorporates user-friendly Jython scripts and ImageJ macros to automate and standardize image processing for VMO/VMT tissue constructs.
This set of tools enables high-throughput data extraction and automates critical tasks such as quantifying tumor growth, vascular remodeling, and flow dynamics. Hughes Lab Tools supports both fully automatic and semi-automatic workflows, allowing operators to verify intermediate results when necessary. Images can be processed from single directories or nested folder structures, and users can execute individual functions or run multiple tasks in series, vastly improving throughput over manual methods. Moreover, these tools are easily modifiable, offering flexibility to address a broad range of experimental questions beyond the VMO/VMT platform.
Here, we present the design and application of the Hughes Lab Tools suite on data generated from the VMO/VMT platform, demonstrating how it streamlines data extraction and analysis while maintaining accuracy and reproducibility. Furthermore, we highlight the broad applicability of Hughes Lab Tools for image-based analysis in other preclinical model systems, including organ-on-a-chip technologies and microphysiological platforms, making them a valuable resource for diverse areas of biomedical research.
2 Materials and equipment
1. Computation
• ImageJ/Fiji software
• AutoCAD (Autodesk Inc.)
• COMSOL Multiphysics software with the CFD Module
2. Equipment
• Biotek Lionheart automated fluorescent microscope; or Thermo Fisher EVOS 5000 fluorescent microscope
• Leica SP8 confocal microscope
3. Materials
• Cell culture medium
• EGM-2 (Endothelial Growth Medium-2)
• DMEM (Dulbecco’s Modified Eagle Medium)
• RPMI-1640 (Roswell Park Memorial Institute Medium-1640)
• Cell culture reagents
• HBSS (Hank’s Buffered Salt Solution)
• Dissociation enzyme (e.g., TrypLE)
• 0.1% gelatin in PBS
• 5 mg/mL fibrinogen
• 1 mg/mL laminin
• Thrombin
• Fabricated plates
• Cell types
• Endothelial cells
• Stromal cells
• Cancer cells (optional)
• Other cell types (optional)
• Cell sources
• Commercially purchased
• Primary derived
• iPSC-derived
3 Methods
3.1 Script development
The development of the tool suite was managed using Git version control (Community, 2025), with the complete version history and code accessible on GitHub (https://github.com/shachey13/HughesLabTools). Script development was conducted within the ImageJ and Fiji distributions (Schindelin et al., 2012), which provide a suite of tools to create macros and scripts (Schneider et al., 2012). These distributions include a Macro Recorder to assist in identifying command sequences for user interface operations. An integrated development environment (IDE) was employed for its advanced coding and debugging capabilities. Specifically, IntelliJ IDEA Community Edition (JetBrains) was utilized. This Java Virtual Machine (JVM)-based IDE (Lindholm et al., 2014) supports multiple programming languages, including Java and Python. Python served as the primary language for script development due to its compatibility with ImageJ and Fiji.
3.2 Cell culture
Human endothelial colony-forming cell-derived endothelial cells (ECFC-EC) were isolated from cord blood following an IRB-approved protocol. After selecting for the CD31+ population, ECFC-ECs were expanded in gelatin-coated flasks using EGM2 medium (Lonza) and used between passages 4 and 8. Alternatively, primary human ECFC-EC were purchased from StemBioSys. Normal human lung fibroblasts (NHLFs) were procured from Lonza and utilized between passages 6 and 10. Primary human adipose-derived perivascular support cells were a gift from Dmitry Traktuev (University of Florida), maintained in EGM2-MV medium (Lonza), and used between passages 4 and 8. The human non-small cell lung cancer line H1792 and colorectal cancer cell line HCT116 were obtained from ATCC and the K1 human thyroid carcinoma cell line was obtained from Sigma (92030501-1VL). ECFC-ECs and cancer cells were transduced with Generation II or Generation III lentiviruses packaged with expression vectors for mCherry (LeGO-C2, Addgene plasmid #27339), green fluorescent protein (pLV-eGFP, Addgene plasmid #36083; or LeGO-V2, plasmid #27340), or azurite (pLV-azurite, Addgene plasmid #36086). NHLFs and cancer cells were cultured in DMEM (Corning) or RPMI-1640 supplemented with 10% FBS (Gemini Bio). All cells were maintained at 37°C and 5% CO2.
3.3 Tumor spheroid generation
Tumor spheroid formation was performed using AggreWell™ plates (StemCell Technologies) to promote the aggregation of H1792 lung cancer cells or HCT116 colorectal cancer cells. Briefly, H1792 or HCT116 cells were seeded at a density of
3.4 3D spheroid culture and drug treatment
H1792 cancer spheroids were suspended in a 5 mg/mL fibrinogen solution at a density of
3.5 Microfluidic device fabrication
Device fabrication followed previously described methods (Sobrino et al., 2016; Phan D. T. T. et al., 2017; Hachey et al., 2023). Briefly, polydimethylsiloxane (PDMS) was prepared by mixing Sylgard 184 elastomer base with curing agent (10:1 ratio, Dow Corning), degassing the mixture, and casting it into a polyurethane master mold derived from a lithographically patterned silicon wafer. PDMS cast into the mold was cured at 70°C for 4 h, or 95°C for 2 h, after which inlets and outlets were punched and the platform was assembled in two steps. First, the PDMS layer was bonded to the base of a 96-well plate using chemical glue and oxygen plasma treatment. Second, a 150
3.6 Establishment of vascularized micro-organ (VMO) and vascularized micro-tumor (VMT) models
Establishment of the VMO and VMT models was performed according to published methods (Hachey et al., 2023). Briefly, to establish the VMO, endothelial colony-forming cell-derived endothelial cells (ECFC-EC)s and normal human lung fibroblasts (NHLF)s were resuspended in a
3.7 Drug treatment in the VMT
After four to five days of culturing, a perfused vascular network was established within each VMT, and the culture medium was replaced with drug-containing medium at the desired concentrations. Drug delivery to the tumor was achieved through the vascular bed via gravity-driven flow. Paclitaxel (a microtubule stabilizer) was purchased from SelleckChem. For H1792 VMTs, experimental groups were randomly assigned to one of three conditions: control (vehicle only), 200 nM paclitaxel, or 400 nM paclitaxel. Oregon green 488-conjugated paclitaxel was purchased from Invitrogen. The medium was replaced after 48 h. Fluorescent micrographs of VMTs were taken every 48 h for 6 days post-treatment, and tumor growth was quantified.
3.8 Fluorescence imaging and perfusion
Fluorescence imaging was conducted using a Biotek Lionheart fluorescent inverted microscope with automated acquisition and a standard 10× air objective, or with a Thermo Fisher EVOS 5000 inverted fluorescent microscope using a standard 4× or 10× air objective. Vessel perfusion and permeability were evaluated by adding 25 μg/mL FITC- or rhodamine-conjugated
3.9 Image segmentation using WEKA in Fiji
The Trainable Weka Segmentation plugin in Fiji/ImageJ was used for image segmentation. The plugin was installed via Fiji’s Update feature, and segmentation was performed by opening the image and launching Trainable Weka Segmentation. A Feature Set was selected, and the Brush Tool was used to manually annotate different regions. The Train Classifier function refined segmentation based on user-labeled samples. The trained model was saved and applied to new images via File–Load Classifier and–Apply Classifier. The final segmentation was generated using Create Probability Map, refined through Thresholding and Morphological Operations (Fill Holes, Watershed). Processed images were saved for analysis.
3.10 Finite element simulations
Finite element modeling of fluid flow within vascular networks was performed in COMSOL Multiphysics 5.2a (COMSOL AB, 2023). Vessel images processed in ImageJ were converted into. dxf files using the Hughes Lab Tools with custom code based on MATLAB DXFLib (Kwiatek, 2025) and refined in AutoCAD for integration into a 2D flow model. A stationary 2D Space Dimension model of Laminar Flow (spf) was used in COMSOL. Culture media flow was modeled as water with incompressible flow. The Bernoulli equation was used to convert fluid height to pressure, and pressure gradients were applied based on gravity-driven flow parameters.
To model interstitial flow through an empty device, a stationary 2D Space Dimension model of Free and Porous Media Flow (fp) was used with fibrin gel properties set to a porosity of 0.99 and a permeability of
3.11 Image analysis
Image processing and analysis were conducted using the Hughes Lab Tools script suite. This versatile suite facilitated the evaluation of area, circularity, and roundness for each tumor image, providing critical metrics for assessing tumor growth. All measurements were normalized to baseline levels. Tumor growth in the VMTs was assessed by analyzing total fluorescence intensity (mean gray value), circularity/roundness, and area within the color channel corresponding to tumor cells. This analysis accounted for both tumor area and depth, with thicker regions exhibiting higher brightness due to increased fluorescence intensity. Similarly, tumor spheroid growth was monitored by tracking changes in fluorescence intensity, spheroid roundness, and tumor area over time. The vessel parameters including area, length, diameter, junctions, and endpoints were quantified using the Hughes Lab Tools suite. All measures were normalized to their baseline values to enable accurate longitudinal comparisons.
Vessel permeability was assessed by fluorescence changes in extravascular regions as analyzed by selecting multiple regions of interest (ROI) per image. The permeability coefficients were calculated as described in Equation 1:
where
3.12 Vessel quantification
Vessel quantification was performed using a combination of built in ImageJ Commands and the AnalyzeSkeleton plugin. The input image was cleaned using a distance map before being skeletonized with the ImageJ skeletonization command. The number of junction points was determined with the AnalyzeSkeleton plugin. Junction points were filtered based on a distance threshold. To analyze each branch, the skeleton was broken at junction points and analyzed. The diameters were determined using a distance map. ImageJ’s particle analysis functions was used to determine the area and perimeter. Results are saved in CSV format, including a summary table and detailed skeleton values.
3.13 Statistical analysis
Statistical analyses were conducted using GraphPad Prism (Version 10.4.1). Data are represented as
3.14 Hughes Lab Tools user guide
3.14.1 Script installation
A detailed installation guide with accompanying screenshots is available in the Supplementary Material. The step-by-step summary is provided below.
a. The Hughes Lab Tools suite was validated in the Fiji ImageJ2 distribution (Schindelin et al., 2012) on macOS 10.14.4. Users are encouraged to use Fiji because of its inclusion of plugins that are not typically available in the base ImageJ distribution. To install Fiji on macOS, use the Homebrew package manager (Howell et al., 2025) with the command: brew cask install fiji
b. To facilitate the installation of Hughes Lab Tools, a shell script is provided. Follow these steps:
(1) Download the suite from GitHub and extract the compressed file.
(2) Navigate to the directory containing the scripts and execute the installer script with:
./hugheslabtools_install.sh
(3) Choose one of the following installation modes:
• “Copy Hughes Lab Tools to Fiji (End-user Mode)”: Copies source files into the Fiji.app directory.
• “SymLink Hughes Lab Tools to Fiji (Developer Mode)”: Creates symbolic links to the tools directory for easier development and testing.
• “Remove Hughes Lab Tools”: Removes installed files.
c. Once installed, a “Hughes Lab Tools” menu becomes accessible in Fiji’s menu bar. Users can create custom keyboard shortcuts using the “Add Shortcut … ” option in the “Plugins > Shortcuts” menu.
3.14.2 Workflow overview
a. Select Functions to Execute
• Start by using the checkboxes to select one or more image processing functions from the “Hughes Lab Tools” menu to build your customized image processing workflow.
• Click Next to proceed.
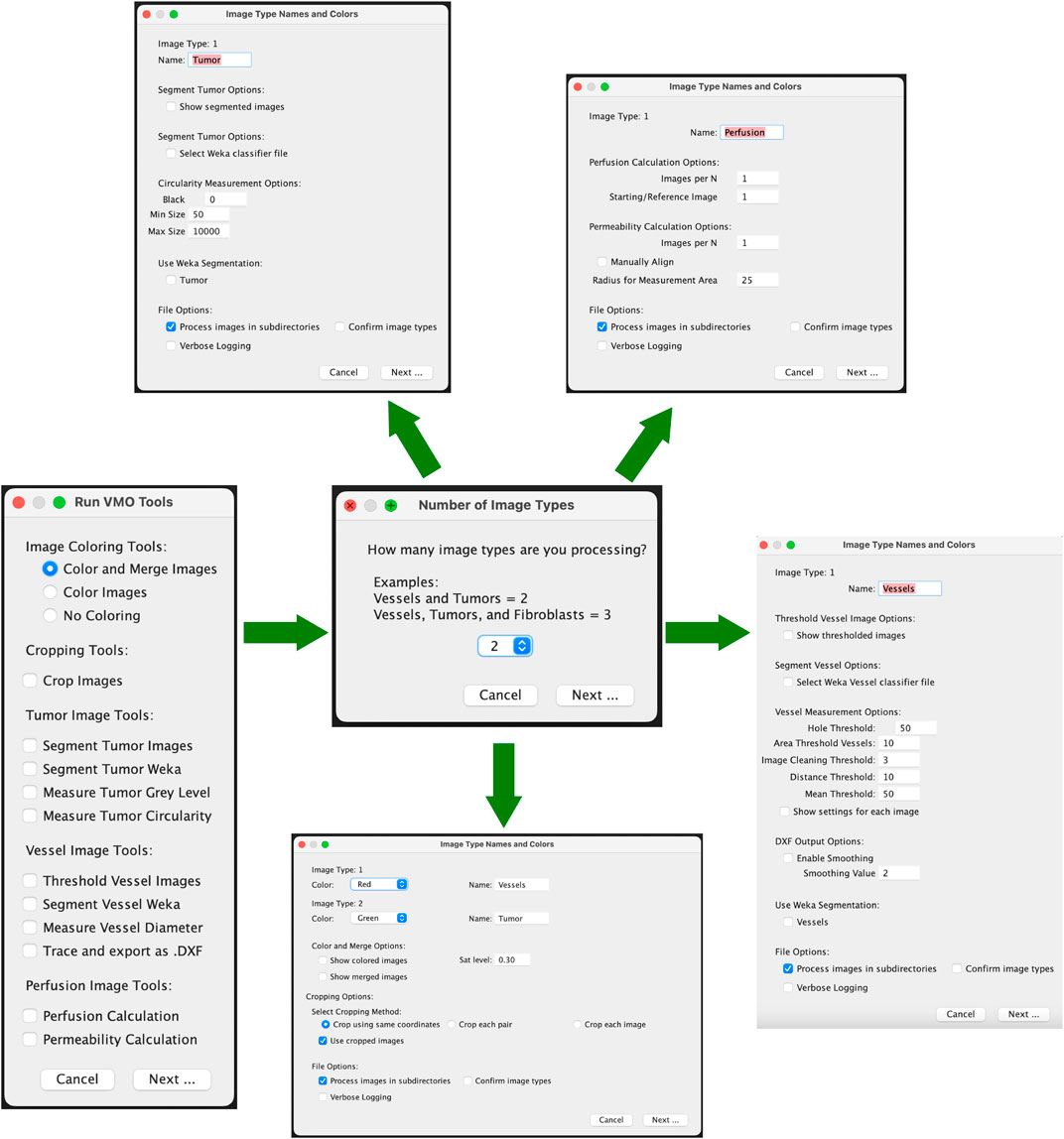
• Please see Figure 1 for an outline of the graphical user interface.
b. Specify Image Types in Sub-directories
• Indicate the number and classification of distinct image types present in the sub-directories (e.g., Vessels, Tumor, etc.). Currently, in order for the images to be analyzed, the text in the text box for the image type must exactly match “Vessels”, “Tumor”, or “Perfusion”. If there are additional image types in the folder to skip, the text box should be set to “Ignore”.
• Ensure that all sub-directories to be processed contain the same number of image types.
c. Image Classification Methods
• One classification method is currently available:
(1) Sequential Image Classification: Assumes images follow a sequential pattern.
d. Verify Image Classification (Optional)
• Check the “Confirm Image Types” option to manually verify tissue type classifications (e.g., Vessels, Tumor) during processing.
e. Select Function-Specific Options
• Adjust settings such as image coloring, output format, and processing verbosity via the options dialog.
f. Choose Image Directories
• Select the directory (and sub-directories) containing images to process. Images should be provided in TIF format.
g. Process Images
• Functions can run automatically or allow users to verify intermediate steps.
3.14.3 Tools and modules
a. Color and Merge Images: This module colors monochrome micrograph images (e.g., FITC- and mCherry-labeled cells) and optionally merges them into composite images. Steps:
(1) Specify colors for each image type in the options dialog.
(2) Colored images are saved in a “Colored” sub-directory as JPEG files.
(3) If merging is selected, composite images are saved in a “Merged” sub-directory as “composite_#” files.
b. Crop Images: This module allows efficient batch cropping of images. Steps:
(1) Select either the same coordinates (batch), each pair (images from same device) or each image.
(2) Images are loaded and a crop window must be manually drawn using the Rectangle Tool. This crop is applied to all related images, depending on selection. Images are saved as TIF files in the “crop” folder.
(3) An Optional checkbox to use cropped images for downstream analysis. Selecting this will change the main directory to the “crop” folder.
c. Segment Tumor Images: This module segments tumor portions from images using an iterative minimum cross-entropy thresholding algorithm Li and Lee (1993). Steps:
(1) Images are thresholded and converted to masks.
(2) Segmented images are saved in a “Tumor_Segmented” sub-directory as JPEG files.
(3) Measurement results (e.g., total area, mean gray value) are saved to a CSV file.
d. Segment Tumor Weka: This module segments tumor images using a user-trained classifier model with the Trainable Weka Segmentation tool (Arganda-Carreras et al., 2017). Users should generate and save a classifier file using Fiji’s Weka Segmentation tool prior to running the Hughes Lab Tools suite. Steps:
(1) User selects a classifier that is used to segment images and convert them to binary. They are saved as TIF files in the “Tumor_Segmented_Weka” folder.
(2) An optional dialog to use the segmented images in downstream steps is provided, though often not used for tumor images.
e. Measure Tumor Gray Level: This tool calculates mean, modal, minimum, and standard deviation of gray values for tumor images. Results are saved in a CSV file in the “measured_gray” folder that is generated by running this module.
f. Threshold Vessel Images: Thresholds vessel images using the same cross-entropy algorithm as described above for segmenting tumors. Steps:
(1) Images are thresholded and saved as TIF files in a “Vessel_Threshold” sub-directory with filenames appended with “_threshold.”
(2) If the Measure Vessel Diameter or Trace and export as .DXF output is selected at the same time, the thresholded images will automatically be used in all downstream analysis.
g. Segment Vessel Weka: This module segments vessel images from a user-trained classifier model with the Trainable Weka Segmentation tool. Users should generate and save a classifier file using Fiji’s Weka Segmentation tool prior to running the Hughes Lab Tools suite. Steps:
(1) User selects a classifier that is used to segment images and convert to binary. They are saved as TIF files in the “Vessel_Segmented” folder.
(2) If the Measure Vessel Diameter or Trace and export as .DXF output is selected at the same time, the segmented images will automatically be used in all downstream analysis.
(3) If Threshold Vessel Images and Segment Vessel Weka are both selected with either Measure Vessel Diameter or Trace and export as .DXF, a selection box for using either the Thresholded or Segmented images will be available. The selection will determine which images are used in the downstream analysis.
h. Measure Vessel Diameter: This cleans and filters vessel images before running the AnalyzeSkeleton plugin to skeletonized images prior to quantifying diameter, branch point, number of segments, area, and perimeter.
(1) Hole Filling Threshold: Default = 50; specifies the maximum size of holes to fill in the image.
(2) Vessel Area Threshold: Default = 10; defines the minimum vessel area to retain.
(3) Branch Mean Threshold: Default = 50; sets the minimum mean intensity for branches to be kept.
(4) Junction Distance Threshold: Default = 10; determines the maximum distance at which junction points are considered duplicates.
(5) Image Cleaning Threshold: Default = 3; sets the Euclidean Distance Map (EDM) threshold for edge pruning to clean the image.
i. Trace and export as .DXF: This tool converts the outlined vessels image into a .DXF file. It follows a similar methodology as Kwiatek (2025). Steps:
(1) Enable Smoothing: This runs the Shape Smoothing plugin to smooth the contours of binary images.
(2) Smoothing Value: This sets the Relative proportion FD percent that is used during Shape Smoothing.
j. Perfusion Coefficient/Permeability Calculation: This tool measures extravascular leak. Steps:
(1) Choose an ROI radius (default: 25 pixels) and specify the number of images in the time course (default: 3).
(2) Optionally align images manually.
(3) Place ROIs and confirm placement.
(4) Results are output to a CSV file, and labeled images are saved in a “Permeability” sub-directory.
k. Perfusion Quantification/Perfusion Calculation: This tool measures extravascular leak using a less accurate, but more automated process. Steps:
(1) Images per N. Set the number of images in the time series.
(2) Starting/Reference Image. Select the image to be used as the reference time point. The mask of the reference image will be subtracted from the other image masks, and the remaining regions will be quantified.
(3) Run Weka Segmentation. If selected, this will ask for a classifier model to segment the images before running the analysis.
(4) Results are output to a CSV file and saved in a “Perfusion” sub-directory.
4 Results
4.1 Vascularized micro-organs and tumors: physiologically relevant preclinical models
The VMO/VMT models integrate a living, perfused vascular network that transports oxygen and nutrients to a miniaturized tissue or organ construct. These models have been previously validated as robust in vitro systems for healthy tissue modeling, disease studies, and drug screening applications Sobrino et al. (2016); Phan D. T. T. et al. (2017); Hachey et al., 2021, 2022, 2023, 2024). Each high-throughput microfluidic platform incorporates multiple tissue units within a bottomless 96-well plate, enabling independent treatment of each VMO or VMT (Figures 2A,B). The device is fabricated from transparent, biocompatible polydimethylsiloxane (PDMS), providing an optically clear platform optimized for real-time microscopic imaging. With each tissue chamber measuring
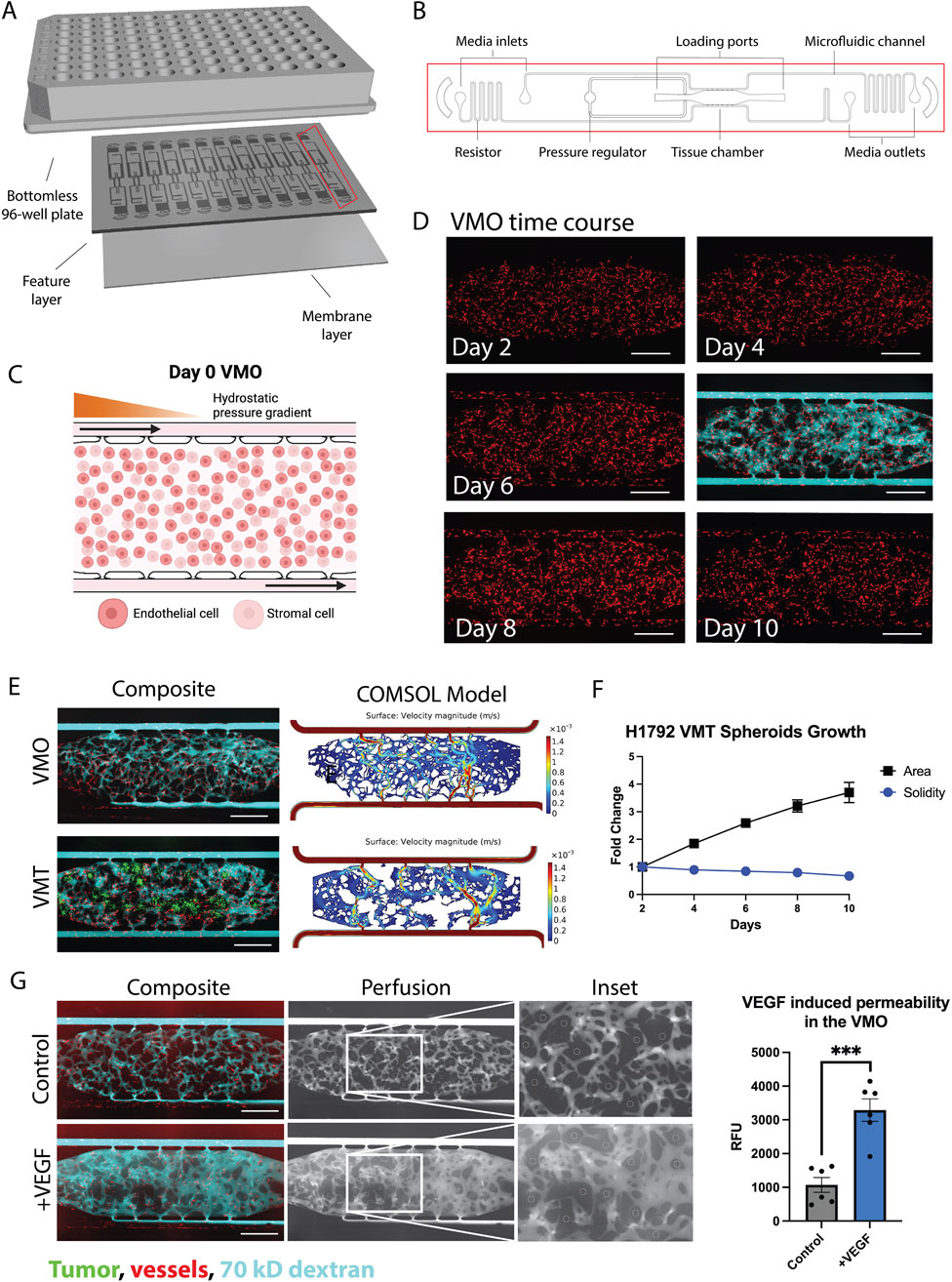
Figure 2. The VMO and VMT as a high-throughput platform for realistic tissue modeling and direct visualization of the vascular niche. (A) Schematic of a microfluidic platform consisting of a bottomless 96–well plate bonded to a feature layer and membrane layer. (B) Schematic of a single device unit with a single tissue chamber fed through microfluidic channels, 2 loading ports (L1-2), and uncoupled medium inlet and outlets (M1-2 and M3-4). A pressure regulator (PR) serves as a burst valve to release excess pressure from the tissue chamber during loading. (C) Schematic showing a zoom view of the chamber loaded on day 0 with endothelial cells and stromal cells, with hydrostatic pressure gradient predominantly from left to right driven across the microfluidic channels. (D) VMO time course of development from day 2 to day 10 with perfusion at day 6. Scale bar = 500 µm. (E) Composite micrographs of VMO and VMT with associated COMSOL models. Tumor shown in green, vessels in red, and 70 kD dextran in cyan. (F) Plot showing H1792 VMT spheroids growth with respect to area and solidity measures. (G) Left: Composite micrographs of perfused VMO (control and VEGF-treated), perfusion only greyscale and inset. Right: Quantification of permeability. Data represent mean
Physiological flow, driven by a hydrostatic pressure gradient across the tissue, enables endothelial cells, stromal cells, and—in the case of the VMT—cancer cells to self-organize within an extracellular matrix, forming a complex microecosystem within 5 days of culture (Figures 2C,D). The resulting vascularized tissue closely mimics an in vivo capillary bed, allowing for physiological drug delivery. To assess vessel patency and permeability changes, vascular networks are routinely perfused with 70 kD FITC- or rhodamine-dextran (Figure 2D). Multiphysics simulations using COMSOL on fully formed, anastomosed, and perfused vascular networks reveal heterogeneous surface velocities of medium flow, closely resembling the dynamic blood flow observed in capillary networks in vivo (Figure 2E). In the VMT model, tumor spheroids rely on the microvascular network for nutrient delivery, with their growth and survival closely tied to vascular perfusion. As the spheroids expand within the tissue chamber, they gradually disperse and migrate, leading to an increase in area and a corresponding decrease in solidity, a measure of sphericity, over time (Figure 2F).
Time-lapse imaging of dextran perfusion throughout the tissue chamber allows for the identification of disease-related vascular changes, such as increased permeability or “leaky” vessels in high-grade tumors. To evaluate the responsiveness of microvessels formed within the device, vascular endothelial growth factor (
4.2 Validation of Hughes Lab Tools for tumor measurement: assessing tumor response across models
We established VMTs using the H1792 non-small cell lung cancer (NSCLC) cell line and evaluated the robustness of the Hughes Lab Tools tumor measurement suite in detecting changes in tumor growth and morphology in response to paclitaxel, a microtubule-stabilizing drug commonly used in advanced NSCLC treatment. First, the IC50 in standard 2D monoculture was determined to be 17 nM (Figure 3A). H1792 NSCLC cell spheroids were monitored for 96 h using both manual methods and the Hughes Lab Tools suite. Measurements obtained from the software showed no significant differences compared to benchmark manual quantification, validating the precision of the tool for spheroid measurement (Figure 3B). Furthermore, the scripts were independently installed and tested by a naïve user using fluorescent micrographs of the HCT116 colorectal cancer cell line. Manual and script-based measurements of spheroids closely correlated, with no significant differences observed (Figure 3C). Dose response experiments in H1792 spheroid monocultures embedded in fibrin showed that paclitaxel treatment
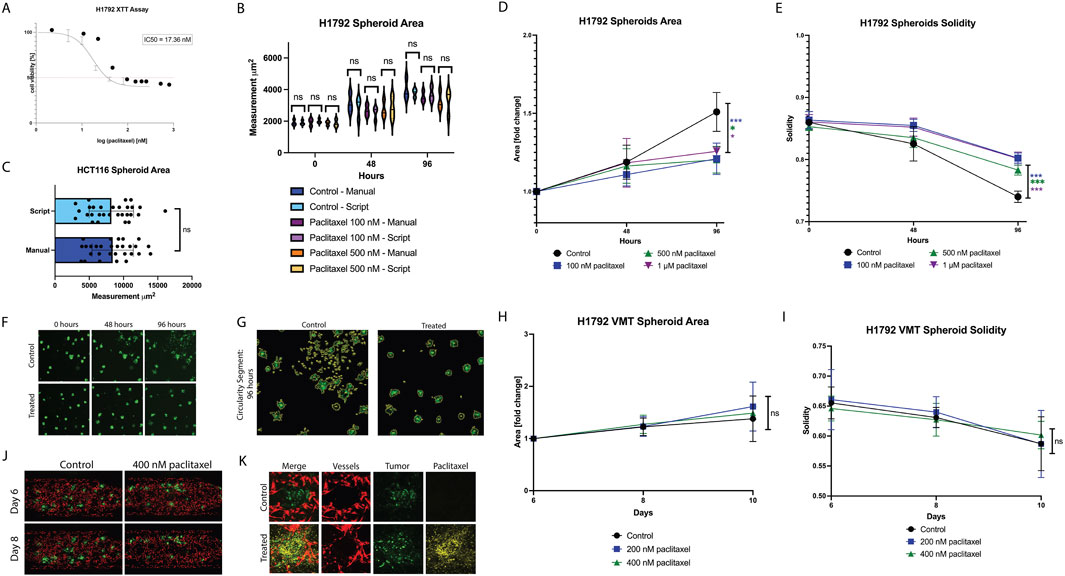
Figure 3. Differential paclitaxel response observed in non-small cell lung cancer spheroids vs Vascularized Micro-Tumors. (A) Plot showing 2D cytotoxicity results for H1792 treated with paclitaxel for 48 h. IC50 is 17 nM. (B) Plot showing external validation of the tumor scripts on HCT116 spheroids, comparing manual measurements to script-derived outputs. (C) Violin plot showing manual measurements of tumor area compared to script measurements for each spheroid condition over time. (D) Plot showing H1792 spheroid area in response to paclitaxel treatment at 48 h and 96 h. (E) Plot showing H1792 spheroid solidity in response to paclitaxel treatment at 48 h and 96 h. (F) Micrographs of spheroids (control and 1 µM paclitaxel treated) at time 0, 48, and 96 h. (G) Segmented tumors at 96 h for control and treated spheroids. (H) Plot showing H1792 VMT spheroid area in response to paclitaxel treatment for 48 h at day 6 (baseline), day 8, and day 10. (I) Plot showing H1792 VMT spheroid solidity in response to paclitaxel treatment for 48 h at day 6 (baseline), day 8, and day 10. (J) Fluorescent micrographs of VMT (control and 400 nM paclitaxel treated) on day 6 and day 8. Tumors shown in green, vessels in red. (K) Confocal micrographs of individual tumor spheroids in the VMT treated with 488 conjugated paclitaxel. Tumor shown in green, vessels in red, and paclitaxel in yellow. Data represent mean
In the VMT model, paclitaxel treatment did not significantly affect spheroid area (Figure 3H) or solidity (Figure 3I), as further illustrated by micrographs (Figure 3J). This lack of response was not due to insufficient drug exposure, as VMTs were fully perfused, and confocal microscopy confirmed FITC-conjugated paclitaxel accumulation in the tissue chamber and near tumors within 48 h post-treatment (Figure 3K). These findings suggest that the complex microenvironment within the VMT may influence drug sensitivity, shifting it toward peak plasma concentrations observed in patients, a phenomenon previously reported by our group (Sobrino et al., 2016; Hachey et al., 2021; 2023; 2024).
4.3 Validation of Hughes Lab Tools for vascular morphometry: comparison with existing software and external datasets
To validate the Hughes Lab Tools vascular morphometry suite, we compared its performance against REAVER (Robust and Efficient Analysis for Vessel Extraction and Reconstruction), a MATLAB-based computational tool for vessel analysis (Corliss et al., 2020). REAVER has been rigorously tested against other image analysis programs, demonstrating high accuracy and precision across various vessel architecture metrics. Manual measurements served as the gold standard for comparison. As shown in Figure 4A, Hughes Lab Tools achieves vessel segmentation and skeletonization comparable to REAVER when applied to the same raw image file. Error analysis of manual measurements versus outputs from both methods revealed no significant differences in diameter and segment measurements (Figure 4B). Notably, Hughes Lab Tools outperformed REAVER in branch point quantification (Figure 4B).
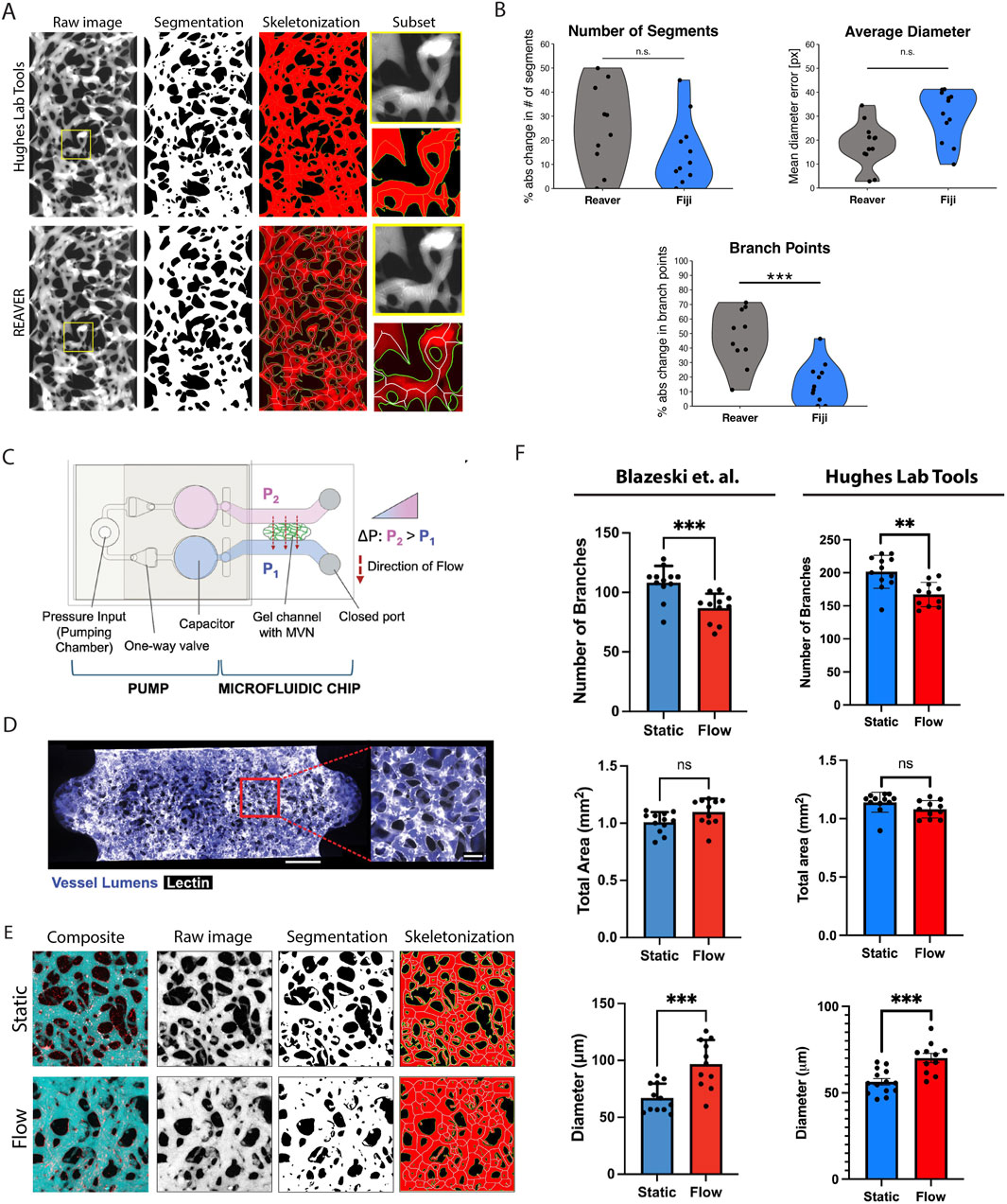
Figure 4. Hughes Lab Tools vascular morphometry suite results. (A) Hughes Lab Tools compared to REAVER for segmenting and skeletonizing raw images of the VMO. (B) Plots showing error comparisons between Hughes Lab Tools or REAVER and manual measurements for branch point, diameter, and segment number measures. (C) Microfluidic chip with pump system for generating microvascular networks (MVNs) (Blazeski et al., 2024). Reproduced with permission from Elsevier. (D) MVNs perfused with fluorescent dextran (purple) and stained with lectin (white) (Blazeski et al., 2024). Reproduced with permission from Elsevier. (E) Hughes Lab Tools processing of raw image files from Blazeski et al. (2024), showing composite micrographs of perfused MVNs in either static or flow conditions, segmentation and skeletonization. (F) Plots showing quantification of branch point numbers, vessel diameters, and area between static and flow MVNs, comparing between Blazeski et al. (2024) and Hughes Lab Tools. Reproduced with permission from Elsevier. ns = non significant, *
Further validation was performed using data from an independent research group studying a different organ-on-a-chip model. A recent study by Blazeski et al. utilized a pump-based microfluidic chip (Figure 4C) to generate microvascular networks (MVNs) perfused with fluorescent dextran (Figure 4D) and analyzed using a KLF2-based flow sensor to assess the effects of shear stress on endothelial cell function (Blazeski et al., 2024). Their findings demonstrated that flow conditions increased vessel diameter, reduced branching, and had no significant effect on total vessel area.
Blazeski et al. analyzed microvascular networks using ImageJ for image segmentation and fluorescent intensity measurements, quantifying the total vascular area from images of maximum intensity projection of dextran-perfused MVNs. Vessel morphology was assessed with AutoTube, a MatLab-based tool (Montoya-Zegarra et al., 2019), while the micro-Vasculature Evaluation System algorithm was applied to confocal z-stacks to perform vessel segmentation, skeletonization, and quantify branch number, length, and diameter (Rota et al., 2023). As shown in Figure 4E, Hughes Lab Tools effectively segments and skeletonizes micrographs from MVNs cultured under static conditions (no flow) and those exposed to flow for 48 h. Quantitative analysis using Hughes Lab Tools successfully replicates the key findings of the Blazeski et al. study: flow-exposed MVNs exhibit significantly fewer branches than static MVNs, flow conditions lead to a significant increase in average vessel diameter, and total vascular area remains unchanged between the two groups (Figure 4F).
4.4 Validation of Hughes Lab Tools: technology transfer to external lab
To confirm that Hughes Lab Tools can be transferred to external lab groups, naïve end-users were recruited at the Fang lab to use both the tumor and vascular morphometry suites to analyze vessel and tumor images from VMO and VMT experiments performed in their lab. The Fang lab was chosen because they use similar VMO/VMT devices, but they have not previously used the Hughes Lab Tools analytical suite for their image processing and morphometry workflow. Both VMO and VMT devices were established in microfluidic devices with diamond-shaped tissue chambers as previously described (Sobrino et al., 2016; Phan D. T. T. et al., 2017). VMO devices were established by co-seeding fluorescent endothelial cells alongside unlabeled perivascular support cells in the absence of co-seeded tumors, whereas VMT devices also included fluorescent reporter-expressing thyroid carcinoma cells (Figures 5A,B). Fluorescent images were captured at days 1, 3, and 5 for each device network. The fluorescent image set was then independently, rapidly, and reproducibly analyzed by two separate Fang lab users using the Hughes Lab Tools suite. Resulting vessel and tumor morphometry data reveal that vessels failed to robustly form in the VMT compared to VMO controls (Figure 5C), whereas tumor burden significantly increased over time in the VMT device (Figures 5D–G), suggesting that the presence of thyroid carcinoma cells disrupts microvessel network formation in this configuration of the VMT model. Additional optimization of cell seeding density and other factors may be necessary to overcome this challenge.
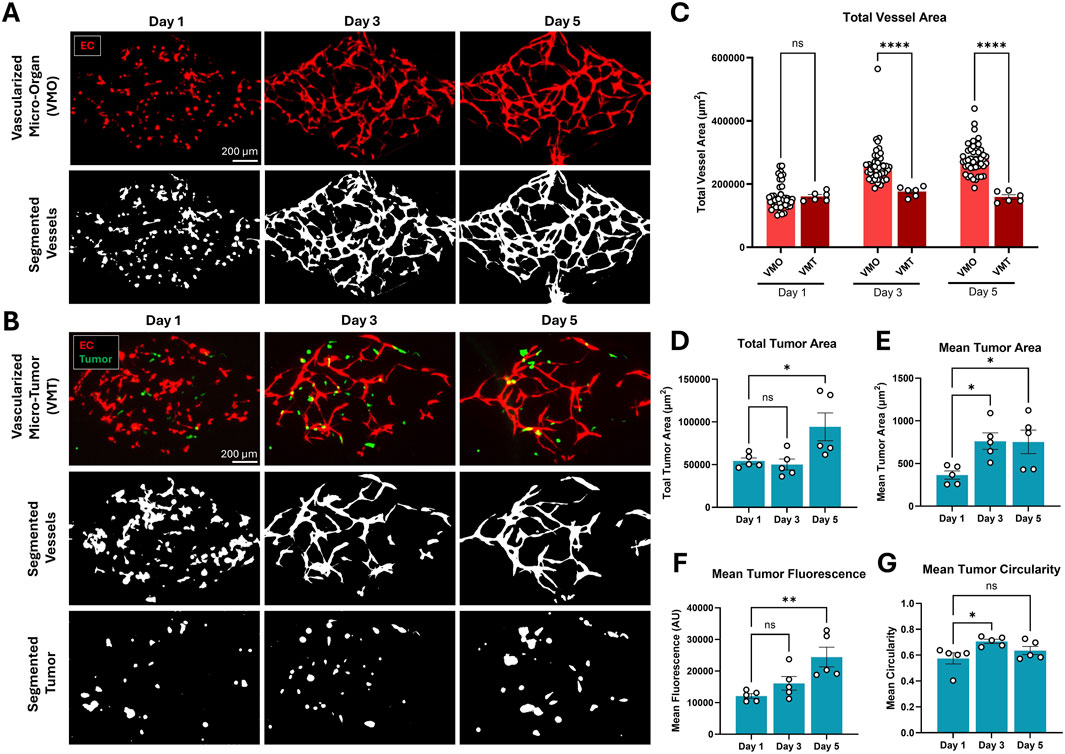
Figure 5. Thyroid carcinoma cells disrupt microvessel network formation in the VMT platform compared to VMO controls. (A) Microvessel networks were established from primary human endothelial and perivascular cells in the absence of tumor in control VMO devices, or (B) in the presence of co-seeded fluorescent reporter-expressing thyroid carcinoma cells. Resulting device images were segmented and analyzed using the Hughes Lab Tools suite. (C) Total vessel area increased over time in VMO, but not VMT, devices. (Statistics: 2-way ANOVA with post-hoc t-test, p < 0.0001, n = 45 VMO and n = 6 VMT devices). (D) Total and (E) mean tumor area also increased over time in the VMT, along with (F) mean tumor fluorescence. (G) Mean tumor circularity transiently increased at day 3. (Statistics: 1-way ANOVA with post-hoc t-test, * - p < 0.05, n = 5 VMT devices). All quantification is presented as mean
5 Discussion
Hughes Lab Tools is an ImageJ suite designed to streamline the processing and analysis of VMO/VMT micrographs, in vitro tumor spheroid models, and other microvascular systems. This tool provides a user-friendly, standardized, and high-throughput solution to extract imaging data, making it particularly valuable for therapeutic screening and large-scale analyses. Users can specify image locations or process entire subdirectories, enabling rapid analysis of large data sets in seconds. The suite also automates image coloring and merging, simplifying data visualization for experimental reference and publication. Benchmark tests highlight its efficiency: on a 2014 MacBook Pro, it processed 600 images (300 tumor/vessel pairs) in just 48 s, whereas manual analysis would take over 15 h, or up to 30 h with tumor segmentation, demonstrating its transformative impact on imaging workflows.
Beyond automation, Hughes Lab Tools supports longitudinal tumor and vascular analysis, allowing researchers to track changes in spheroid growth, vascular remodeling, and function in VMO/VMT and other preclinical or microphysiological models. A key feature is its vessel morphometry and thresholding function, which prepares images for COMSOL Multiphysics-based fluid flow modeling. The tool processes both total vessel and perfused vessel images, enabling tailored analyses for different experimental objectives, such as evaluating antiangiogenic treatments with total vessels or studying drug or immune cell delivery using perfused vessels. However, differences between thresholded vessel and perfused vessel images may arise from incomplete transduction of fluorescent proteins in endothelial cells or low-perfusion regions, emphasizing the need for careful experimental design and interpretation. Furthermore, while the current workflow supports independent and simultaneous assessment of vascular and tumor morphology, it does not yet provide integrated analysis across compartments. As such, spatial relationships between tumor and vasculature, such as proximity, co-localization, or invasion dynamics, are not directly quantified. Future extensions could incorporate spatial metrics to enable interdependent tumor-vascular morphometric analysis.
Validation studies confirm the robustness and broad applicability of Hughes Lab Tools, demonstrating its ability to accurately and reproducibly process external datasets and assess vessel and tumor structures in various imaging conditions and across end-users of varying familiarity with morphometric image analysis. Comparative benchmarking revealed that while overall trends were consistent between methodologies used in this study compared to Blazeski et al., absolute vascular branch counts differed due to sensitivity variations between tools. This discrepancy arises from methodological differences, as Hughes Lab Tools employs REAVER’s model-based approach, which extracts vessel centerlines and estimates radii via intensity profile analysis, making it highly effective for complex vascular networks, including bifurcations and irregular vessel shapes (Corliss et al., 2020). In contrast, AutoTube assumes a fixed tubular structure, which makes it better suited for well-defined cylindrical vessels but less precise in complex vascular environments (Corliss et al., 2020). Our prior work has shown that accurate modeling of small branch points and bifurcations in physiological vascular networks is crucial to understanding flow dynamics and vascular pruning, underscoring the biological importance of precise vessel segmentation (Hachey et al., 2021; Bender et al., 2024; Hatch et al., 2024).
This study focuses on the H1792 NSCLC cell line with supporting studies using K1 thyroid carcinoma cells and HCT116 colorectal cancer cells, revealing that these and other tumor types are known to vary widely in both vascular and growth characteristics, which can affect image-based analysis. For example, renal cell carcinoma and glioblastoma tend to be highly vascularized, while pancreatic and colorectal tumors are often hypovascular and stroma-rich (Carmeliet and Jain, 2000). These differences influence vascular metrics such as vessel density and perfusion. Tumor cell lines also differ in growth kinetics and morphology. Some, like MDA-MB-231 breast cancer cells, show invasive, diffuse growth, while others form compact nodular masses (Hachey et al., 2021; 2024). In contrast, K1 thyroid cells remained generally spheroidal (Figure 5G) suggesting non-invasiveness, despite these tumor cells significantly impairing vascular network formation in the device. These diverse cancer-specific traits affect segmentation and quantification, as infiltrative growth can produce less defined borders. Understanding these biological variations is important for the interpretation of image-derived tumor and vascular measurements.
Hughes Lab Tools brings advanced vascular analysis capabilities, previously restricted to licensed platforms such as MATLAB, into an accessible open-source environment based on Fiji/ImageJ. Beyond vascular quantification, the package includes extended functionality for tumor segmentation, image merging, color channel handling, and streamlined image file processing, all integrated within a single user-friendly interface. Moreover, the “tumor” channel can be designated for any labeled cell type, not limited to tumor cells, thereby expanding the suite’s applicability to a range of contexts, including co-cultures that incorporate liver cells, astrocytes, or immune cell populations, for example. The toolset’s modular design enables customization to accommodate a range of experimental workflows while maintaining reproducibility and compatibility with high-throughput imaging studies. Together, Hughes Lab Tools provides a robust and versatile platform for tumor-vascular image analysis that is accessible, efficient, and adaptable to diverse research applications.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by UC Irvine Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired as part of a previous study for which ethical approval was obtained. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
SH: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review and editing. CH: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – review and editing. DG: Data curation, Formal Analysis, Investigation, Visualization, Writing – review and editing. AF: Methodology, Software, Writing – review and editing. ME: Data curation, Methodology, Resources, Writing – review and editing. AC: Formal Analysis, Writing – review and editing. ZC: Formal analysis, Validation, Writing – review and editing. KT: Formal analysis, Validation, Writing – review and editing. MH: Formal analysis, Validation, Writing – review and editing. JF: Formal Analysis, Methodology, Software, Writing – review and editing. CH: Funding acquisition, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Institutes of Health, National Cancer Institute and National Center for Advancing Translational Sciences, Louisiana Board of Regents, Louisiana Cancer Research Center (LCRC), and Eye, Ear, Nose & Throat Foundation (EENT) of New Orleans through the following grants: UG3/UH3 TR002137, R61/R33 HL154307, 1R01CA244571, 1R01 HL149748, U54 CA217378 (CCWH) and TL1 TR001415 and W81XWH2110393 (SJH), a P30 pilot award to JSF through P30GM145498, Louisiana BOR RCS Award (091A-24 to JSF), EENT Foundation Generation Research Grant (EE221102 to JSF), and LCRC New Investigator Award (CR1305A6 to JSF).
Acknowledgments
We thank Guillermo Garcia-Cardena (Harvard University) for providing us with the raw image files from his groups’ study (Blazeski et al., 2024). We thank Dmitry Traktuev (University of Florida) for providing adipose-derived perivascular support cells for use in some of our VMO/VMT studies. Figure 2c was created using Biorender.
Conflict of interest
Authors MH and CH were employed by Aracari Biosciences, Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2025.1585003/full#supplementary-material
References
Ahn, S. I., Sei, Y. J., Park, H.-J., Kim, J., Ryu, Y., Choi, J. J., et al. (2020). Microengineered human blood–brain barrier platform for understanding nanoparticle transport mechanisms. Nat. Commun. 11, 175. doi:10.1038/s41467-019-13896-7
Arganda-Carreras, I., Kaynig, V., Rueden, C., Eliceiri, K. W., Schindelin, J., Cardona, A., et al. (2017). Trainable Weka Segmentation: a machine learning tool for microscopy pixel classification. Bioinformatics 33, 2424–2426. doi:10.1093/bioinformatics/btx180
Bender, R. H. F., O’Donnell, B. T., Shergill, B., Pham, B. Q., Tahmouresie, S., Sanchez, C. N., et al. (2024). A vascularized 3D model of the human pancreatic islet for ex vivo study of immune cell-islet interaction. Biofabrication 16, 025001. doi:10.1088/1758-5090/ad17d0
Blazeski, A., Floryan, M. A., Zhang, Y., Fajardo Ramírez, O. R., Meibalan, E., Ortiz-Urbina, J., et al. (2024). Engineering microvascular networks using a klf2 reporter to probe flow-dependent endothelial cell function. Biomaterials 311, 122686. doi:10.1016/j.biomaterials.2024.122686
Campisi, M., Shin, Y., Osaki, T., Hajal, C., Chiono, V., and Kamm, R. D. (2018). 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials 180, 117–129. doi:10.1016/j.biomaterials.2018.07.014
Carmeliet, P., and Jain, R. K. (2000). Angiogenesis in cancer and other diseases. Nature 407, 249–257. doi:10.1038/35025220
Comsol, A. B. (2023). COMSOL Multiphysics®, 6.3. Stockholm, Sweden. Available online at: https://www.comsol.com.
Corliss, B. A., Doty, R. W., Mathews, C., Yates, P. A., Zhang, T., and Peirce, S. M. (2020). REAVER: a program for improved analysis of high-resolution vascular network images. Microcirculation 27 (5), e126188. doi:10.1111/micc.12618
Ewald, M. L., Chen, Y.-H., Lee, A. P., and Hughes, C. C. (2021). The vascular niche in next generation microphysiological systems. Lab a Chip 21, 3244–3262. doi:10.1039/D1LC00530H
Gaebler, D., Hachey, S. J., and Hughes, C. C. W. (2024a). Improving tumor microenvironment assessment in chip systems through next-generation technology integration. Front. Bioeng. Biotechnol. 12, 1462293. doi:10.3389/fbioe.2024.1462293
Gaebler, D., Hachey, S. J., and Hughes, C. C. W. (2024b). Microphysiological systems as models for immunologically ‘cold’ tumors. Front. Cell Dev. Biol. 12, 1389012–1389021. doi:10.3389/fcell.2024.1389012
Hachey, S. J., Gaebler, D., and Hughes, C. C. W. (2023). Establishing a physiologic human vascularized micro-tumor model for cancer research. J. Vis. Exp., e65865doi. doi:10.3791/65865
Hachey, S. J., Hatch, C. J., Gaebler, D., Mocherla, A., Nee, K., Kessenbrock, K., et al. (2024). Targeting tumor–stromal interactions in triple-negative breast cancer using a human vascularized micro-tumor model. Breast Cancer Res. 26, 5. doi:10.1186/s13058-023-01760-y
Hachey, S. J., and Hughes, C. C. W. (2018). Applications of tumor chip technology. Lab a Chip 18, 2893–2912. doi:10.1039/C8LC00330K
Hachey, S. J., Movsesyan, S., Nguyen, Q. H., Burton-Sojo, G., Tankazyan, A., Wu, J., et al. (2021). An in vitro vascularized micro-tumor model of human colorectal cancer recapitulates in vivo responses to standard-of-care therapy. Lab a Chip 21, 1333–1351. doi:10.1039/D0LC01216E
Hachey, S. J., Sobrino, A., Lee, J. G., Jafari, M. D., Klempner, S. J., Puttock, E. J., et al. (2022). A human vascularized microtumor model of patient-derived colorectal cancer recapitulates clinical disease. Transl. Res. 255, 97–108. doi:10.1016/j.trsl.2022.11.011
Hajal, C., Offeddu, G. S., Shin, Y., Zhang, S., Morozova, O., Hickman, D., et al. (2022). Engineered human blood–brain barrier microfluidic model for vascular permeability analyses. Nat. Protoc. 17, 95–128. doi:10.1038/s41596-021-00635-w
Hatch, C. J., Piombo, S. D., Fang, J. S., Gach, J. S., Ewald, M. L., Van Trigt, W. K., et al. (2024). SARS-CoV-2 infection of endothelial cells, dependent on flow-induced ACE2 expression, drives hypercytokinemia in a vascularized microphysiological system. Front. Cardiovasc. Med. 11, 1360364. doi:10.3389/fcvm.2024.1360364
Helm, C.-L. E., Fleury, M. E., Zisch, A. H., Boschetti, F., and Swartz, M. A. (2005). Synergy between interstitial flow and VEGF directs capillary morphogenesis in vitro through a gradient amplification mechanism. Proc. Natl. Acad. Sci. 102, 15779–15784. doi:10.1073/pnas.0503681102
Hsu, Y.-H., Moya, M. L., Abiri, P., Hughes, C. C., George, S. C., and Lee, A. P. (2013). Full range physiological mass transport control in 3D tissue cultures. Lab. Chip 13, 81–89. doi:10.1039/C2LC40787F
Ingber, D. E. (2022). Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat. Rev. Genet. 23, 467–491. doi:10.1038/s41576-022-00466-9
Li, C. H., and Lee, C. (1993). Minimum cross entropy thresholding. Pattern Recognit. 26, 617–625. doi:10.1016/0031-3203(93)90115-D
Lindholm, T., Yellin, F., Bracha, G., and Buckley, A. (2014). The Java virtual machine specification. 8 Edition. Addison-Wesley Professional.
Low, L. A., Mummery, C., Berridge, B. R., Austin, C. P., and Tagle, D. A. (2021). Organs-on-chips: into the next decade. Nat. Rev. Drug Discov. 20, 345–361. doi:10.1038/s41573-020-0079-3
Low, L. A., and Tagle, D. A. (2018). You-on-a-chip’ for precision medicine. Expert Rev. Precis. Med. Drug Dev. 3, 137–146. doi:10.1080/23808993.2018.1456333
Martier, A., Chen, Z., Schaps, H., Mondrinos, M. J., and Fang, J. S. (2024). Capturing physiological hemodynamic flow and mechanosensitive cell signaling in vessel-on-a-chip platforms. Front. Physiol. 15, 1425618. doi:10.3389/fphys.2024.1425618
Montoya-Zegarra, J. A., Russo, E., Runge, P., Jadhav, M., Willrodt, A.-H., Stoma, S., et al. (2019). Autotube: a novel software for the automated morphometric analysis of vascular networks in tissues. Angiogenesis 22, 223–236. doi:10.1007/s10456-018-9652-3
Nahon, D. M., Vila Cuenca, M., van den Hil, F. E., Hu, M., de Korte, T., Frimat, J.-P., et al. (2024). Self-assembling 3D vessel-on-chip model with hiPSC-derived astrocytes. Stem Cell Rep. 19, 946–956. doi:10.1016/j.stemcr.2024.05.006
Phan, D. T., Bender, R. H. F., Andrejecsk, J. W., Sobrino, A., Hachey, S. J., George, S. C., et al. (2017a). Blood–brain barrier-on-a-chip: microphysiological systems that capture the complexity of the blood–central nervous system interface. Exp. Biol. Med. 242, 1669–1678. doi:10.1177/1535370217694100
Phan, D. T. T., Wang, X., Craver, B. M., Sobrino, A., Zhao, D., Chen, J. C., et al. (2017b). A vascularized and perfused organ-on-a-chip platform for large-scale drug screening applications. Lab. Chip 17, 511–520. doi:10.1039/C6LC01422D
Rota, A., Possenti, L., Offeddu, G. S., Senesi, M., Stucchi, A., Venturelli, I., et al. (2023). A three-dimensional method for morphological analysis and flow velocity estimation in microvasculature on-a-chip. Bioeng. Transl. Med. 8 (5), e105577. doi:10.1002/btm2.10557
Schindelin, J., Arganda-Carreras, I., Frise, E., Kaynig, V., Longair, M., Pietzsch, T., et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682. doi:10.1038/nmeth.2019
Schneider, C. A., Rasband, W. S., and Eliceiri, K. W. (2012). Nih image to imagej: 25 years of image analysis. Nat. Methods 9, 671–675. doi:10.1038/nmeth.2089
Sobrino, A., Phan, D. T. T., Datta, R., Wang, X., Hachey, S. J., Romero-López, M., et al. (2016). 3d microtumors in vitro supported by perfused vascular networks. Sci. Rep. 6, 31589. doi:10.1038/srep31589
Keywords: microphysiological system, tumor microenvironment, tumor on chip, microfluidic, bioengineering, image processing, therapeutic development, vasculature
Citation: Hachey SJ, Hatch CJ, Gaebler D, Forsythe AG, Ewald ML, Chopra AL, Chen Z, Thapa K, Hodanu M, Fang JS and Hughes CCW (2025) Methods for processing and analyzing images of vascularized micro-organ and tumor systems. Front. Bioeng. Biotechnol. 13:1585003. doi: 10.3389/fbioe.2025.1585003
Received: 28 February 2025; Accepted: 13 May 2025;
Published: 12 June 2025.
Edited by:
Jeong Ah Kim, Korea Basic Science Institute, Republic of KoreaReviewed by:
Lorna Ewart, Emulate Inc., United StatesJihoon Ko, Sungkyunkwan University, Republic of Korea
Copyright © 2025 Hachey, Hatch, Gaebler, Forsythe, Ewald, Chopra, Chen, Thapa, Hodanu, Fang and Hughes. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christopher C. W. Hughes, Y2NodWdoZXNAdWNpLmVkdQ==
 Stephanie J. Hachey
Stephanie J. Hachey Christopher J. Hatch
Christopher J. Hatch Daniela Gaebler
Daniela Gaebler Alexander G. Forsythe3
Alexander G. Forsythe3 Makena L. Ewald
Makena L. Ewald Alexander L. Chopra
Alexander L. Chopra Jennifer S. Fang
Jennifer S. Fang Christopher C. W. Hughes
Christopher C. W. Hughes