- 1Viral Therapeutics Center, Boehringer Ingelheim Pharma GmbH & Co. KG, Biberach an der Riss, Germany
- 2Global Innovation and Alliance Management, Boehringer Ingelheim Pharma GmbH & Co. KG, Biberach an der Riss, Germany
The oncolytic vesicular stomatitis (VSV)-GP virus is a promising therapeutic against cancer. To ensure clinical efficacy, doses with high titers are required, which poses a challenge for the manufacturing process. Perfusion cultivation processes with high cell densities have attracted great interest to improve the production titer. This work aimed to enhance the titer of the VSV-GP production process with suspension human embryonic kidney 293 (HEK293) cells by using perfusion with tangential flow filtration (TFF) and virus retention. For this purpose, six potential critical process parameters were evaluated using I-optimal design of experiments (DoE). The study showed that several input parameters and their interactions have significant impact on the infectious titer. Increasing the seeding cell density significantly improved the infectious titer, allowing infection at up to 46.6 × 106 cells mL-1 without decrease in the cell-specific virus yield. Keeping the perfusion pause after infection at minimum (1.1–1.3 h) and subsequently start the perfusion with a higher exchange rate (0.045–0.051 nL cell-1 d-1) was shown to be beneficial. The process was sensitive to shear stress and thus, the optimal crossflow rate was between 44 and 55 mL min-1, which corresponds to 950–1150 s-1 shear rate. By optimizing the perfusion process, the titer reached up to 5.1 × 1010 TCID50 mL-1, which is 17-fold higher than in batch cultivation. Overall, this work presents perfusion cultivation as an efficient technology to improve the VSV-GP titer with virus retention.
Introduction
Oncolytic virotherapy is an emerging therapeutic approach, which uses oncolytic viruses to selectively destroy malignant tissues (Shalhout et al., 2023). Vesicular stomatitis virus (VSV) is a promising candidate for oncolytic virotherapy. It has a broad cell tropism and a fast replication cycle, which ends in high titer (Hastie et al., 2013). VSV also has a capacity to carry transgenes such as tumor antigens or immunostimulatory molecules to enhance the therapeutic effect (Kimpel et al., 2018). Human infection with VSV is rare and only causes mild flu-like symptoms (Hastie et al., 2013). The tumor selectivity of the virus is based on the frequently aberrated type I interferon response in cancer cells that reduces the antiviral defense (Kimpel et al., 2018). The chimeric vesicular stomatitis virus, VSV-GP was created by substituting the envelope glycoprotein (G) of the VSV with the glycoprotein (GP) of the lymphocytic choriomeningitis virus (LCMV). Due to this modification, VSV-GP lacks VSV’s inherent neurotoxicity and is able to escape the humoral immune response (Muik et al., 2014). VSV-GP has shown potential to treat, e.g., glioblastoma (Muik et al., 2014), melanoma (Kimpel et al., 2018) and ovarian cancer (Dold et al., 2016).
VSV production processes have been described with adherent cell cultures on diverse growth matrices such as macroporous gelatin carriers (Lim et al., 1999), microcarriers (Kiesslich et al., 2020), Fibra-Cel disks (Rosen et al., 2022) and in Scale-X™ hydro fixed-bed bioreactor (Kiesslich et al., 2020). A GMP-conform production process in a 10-layer cell factory has also been reported (Ausubel et al., 2011). Due to the easier process scale-up and handling, adaptation of adherent cells to suspension growth has received a high interest. Accordingly, several recently published VSV production processes have utilized suspension cells (Paillet et al., 2009; Elahi et al., 2019; Gélinas et al., 2019; Kiesslich et al., 2021; Gautam et al., 2022; Göbel et al., 2022).
Perfusion is an advanced cultivation technique that enables continuous culture medium exchange while cells are retained in the bioreactor. Consequently, higher cell densities and higher titers can be achieved with perfusion compared to a classical batch cultivation process (Bock et al., 2011; Gálvez et al., 2012). Perfusion processes also have the potential to achieve higher volumetric productivities and operate with a smaller footprint compared to fed-batch processes due to the higher cell densities (Pollock et al., 2013; Tapia et al., 2016; Kiesslich et al., 2021). Hollow fiber modules with tangential flow filtration (TFF) or alternating tangential flow filtration (ATF) are widely used to retain cells in the bioreactor (Nikolay et al., 2018; Vázquez-Ramírez et al., 2018; Gränicher et al., 2019; Lavado-García et al., 2020). They are easy to scale up based on the surface area of the hollow fiber filter (Ozturk and Kompala, 2005). Previously, the TFF perfusion with a peristaltic pump was linked to higher shear stress and filter fouling compared to the ATF system (Schwarz et al., 2020). Nevertheless, exchanging the peristaltic pump to a low shear centrifugal pump presents new opportunities for the TFF technology (Wang et al., 2017). To achieve high virus titers, virus production conditions need careful optimization (Gálvez et al., 2012; Tapia et al., 2016; Elahi et al., 2019; Wu et al., 2021). Implementing an appropriate feeding or medium exchange strategy is crucial to promote high cell-specific virus yields at high cell densities (Lindsay and Betenbaugh, 1992; Maranga et al., 2003; Kamen and Henry, 2004).
Ambr® 250 High Throughput bioreactor systems (Sartorius Stedim Biotech) have gained popularity for accelerating process development using 12 or 24 disposable mini bioreactors (Tai et al., 2015; Joe et al., 2021). The reliability of the bioreactors as scale-down models has been demonstrated in several studies (Xu et al., 2017; Manahan et al., 2019; Zhang et al., 2019). One of the key features of the Ambr® 250 system is the individual automatic control of cultivation parameters, including dissolved oxygen (DO), pH, foam level, bolus addition and feeding. The automated sampling and sample analysis provide real-time data for controlling loops and substantially decrease manual work. These features promote the use of design of experiments (DoE) to analyze the impact of variables and their interactions on the process performance (Tai et al., 2015). Additionally, the high automatization minimizes human error and enables more consistent processes.
In this study, we aimed to improve the titer of the oncolytic VSV-GP production process with suspension HEK293 cells using TFF perfusion and Ambr®250 High Throughput bioreactors. Firstly, different medium exchange rates were investigated for cultivating suspension HEK293 cells in perfusion. Subsequently, a DoE approach was applied to identify process parameters with potential impact on the virus production, including the seeding cell density, time of infection (TOI), multiplicity of infection (MOI), perfusion pause after infection as well as the medium exchange rate and crossflow rate post infection. Finally, the perfusion process was optimized and compared to the batch production process in terms of titer and productivity.
Materials and methods
Cell and virus
HEK293-F cells (#11625019, Thermo Fisher Scientific, Waltham, MA, United States), hereafter referred to as HEK293 cells, were cultivated in a serum-free proprietary medium with 4 mM GlutaMAX™ (#35050061, Gibco, Thermo Fisher Scientific, Waltham, MA, United States) at 37°C, 5% CO2 and 120 rpm with 25 mm orbit shaker in non-baffled shaking flasks. Cells were passaged every 3 or 4 days with 2.5 × 105 or 4 × 105 cells mL-1 seeding density, respectively.
For infections, a recombinant VSV-GP virus vector stock (6.0 × 108 TCID50 mL-1) was used in which the glycoprotein (G) of the VSV was replaced by the glycoprotein (GP) of LCMV as described before (Muik et al., 2014). The virus seed stock had a titer of 6.0 × 108 TCID50 mL-1.
Batch cultivation
Batch cultivations were performed in 250 mL Ambr® 250 mammalian bioreactors (#001-5G25, Sartorius, Göttingen, Germany). Cells were seeded at 4.0 × 105 cells mL-1 in 200 mL working volume. The pH was set to 7.2 with a negative dead band of 0.2 and controlled with CO2 as well as 1 M Na2CO3. The cultivation temperature was 37°C. Cells were agitated at 492 rpm with two pitched blade impellers (d = 26 mm). The DO was maintained at 50% with a mixture of air and oxygen. Initially, only air was sparged up to 1.75 mL min-1. Then, oxygen was added up to 80 mL min-1 to keep the DO set point. Cultures were infected with an MOI of 0.0004 after 56 h of cultivation. The cultivation temperature was shifted from 37°C to 34°C directly before infection.
Perfusion cultivation without infection
Perfusion cultivations were conducted in 250 mL Ambr® 250 bioreactors with TFF and 30 kDa modified polyether sulfone (mPES) filter (#001-5G83, Sartorius, Göttingen, Germany). The TFF filter had 75 cm2 filter area with 20 cm effective length, 20 fiber count and 1 mm lumen bore. Cells were seeded at 4.0 × 105 cells mL-1 in 220 mL working volume. Cells were cultivated with the same pH, temperature, agitation and DO set points and control strategy as described by the batch cultivations. To eliminate present foam, 100 µL antifoam (FoamAway™, #A1036902, Thermo Fisher Scientific, Waltham, MA, United States) was added to the cultures.
Perfusion was started 16 h after seeding using the growth medium with increased, 10 g L-1 glucose content. The cell-specific perfusion rate (CSPR) was either 0.029, 0.051 or 0.073 nL cell-1 d-1. The minimum value of the medium exchange rate was set to 0.3 reactor volume per day (RV d-1). If the glucose concentration fell below 5 g L-1 during the perfusion, a glucose bolus was added up to twice a day from a 500 g L-1 stock solution to reach 5 g L-1. The crossflow rate was 40 mL min-1.
DoE for the virus production
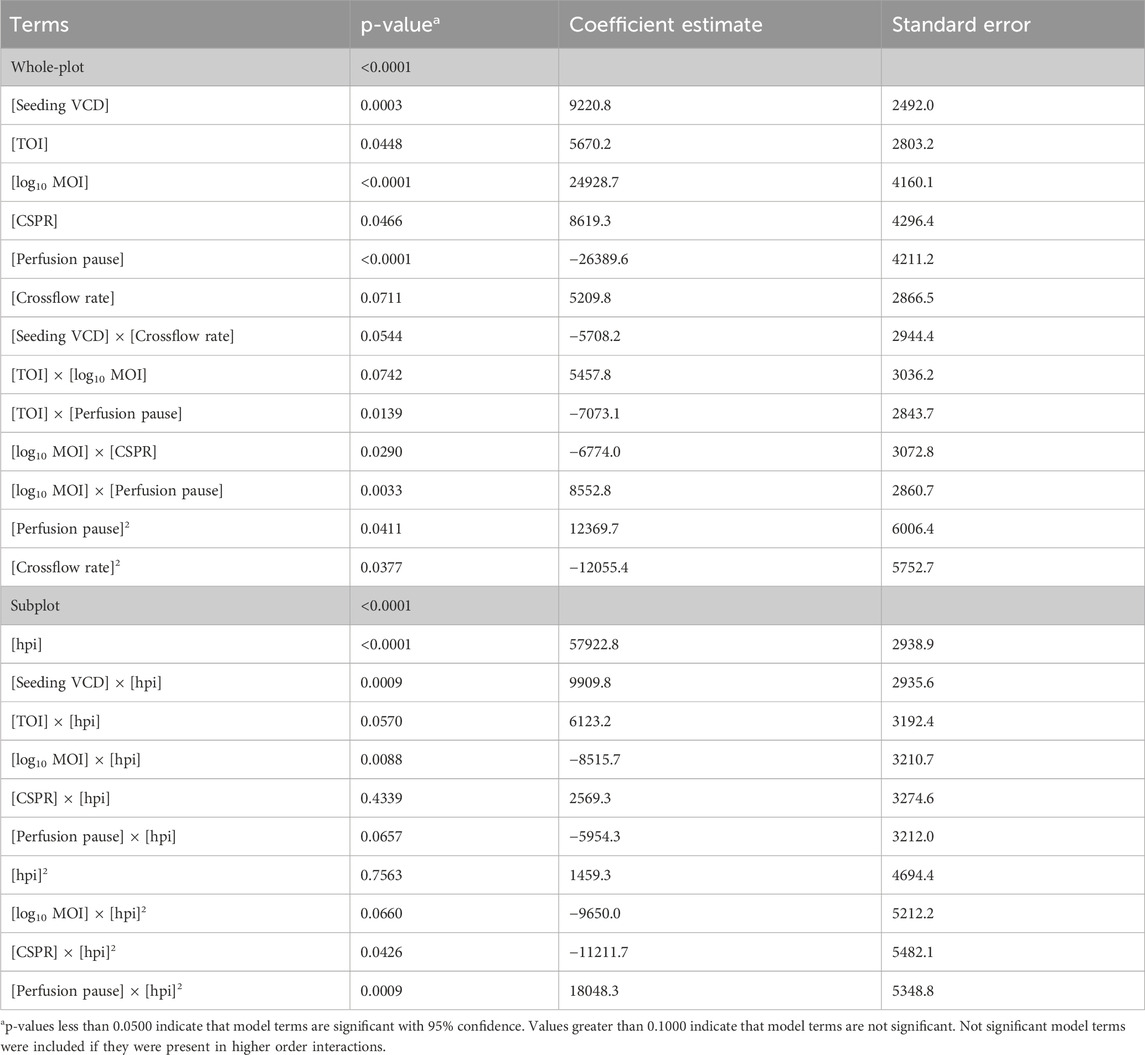
Six input parameters were varied at three levels to optimize the virus production in TFF perfusion vessels with 30 kDa mPES filter (#001-5G83, Sartorius, Göttingen, Germany). An I-optimal DoE was employed to develop a response surface model that considered interactions and quadratic effects of the parameters (Table 1). To test different cell densities for infection, the seeding viable cell density (VCD) (1 × 106, 2.5 × 106 or 4 × 106 cells mL-1) and the TOI (74, 98 or 122 h) were varied. Seed cultures were concentrated by centrifugation (180 g, 5 min, RT) prior to inoculation to ensure that a maximum split ratio of 20% (ratio of spent medium to fresh medium) was not exceeded. Perfusion was started 16 h after seeding with 0.051 nL cell-1 d-1 CSPR with a minimum medium exchange rate of 0.3 RV d-1. The same glucose strategy was adopted as in the perfusion cultivations without infection. The crossflow rate was set to 40 mL min-1. Before infection, the cultivation temperature was shifted from 37°C to 34°C. Then, the perfusion was halted by stopping the inflow and outflow of the medium as well as the crossflow within the system. Following this, cells were infected with an MOI of 0.04, 0.004 or 0.0004. Perfusion resumed 1, 8 or 16 h after infection with varying CSPRs (0.015, 0.033 and 0.051 nL cell-1 d-1) and crossflow rates (25, 40 and 70 mL min-1). Virus samples were collected at 22, 36, 40 and 46 h post infection (hpi).
30 experiments were planned in three blocks within the DoE. Additionally, three perfusion runs in block 3 and 12 runs in block 4 were performed to improve the quality and prediction of the statistical model. Up to 12 experiments were conducted within a single block. Input parameter settings of the perfusion cultivations are provided in the attachment (Supplementary Table S1).
Statistical analysis
The analysis and calculations for the DoE were performed with Design-Expert 13.0.5.0 software (Stat-Ease, RRID:SCR_022671).
A split plot design was used in the analysis to incorporate the dependency structure of the data, considering each DoE parameter as a hard-to-change factor and hpi as an easy-to-change factor. Therefore, additionally to the response surface model of the six DoE parameters, the linear and quadratic effect of hpi as well as their interactions with the DoE factors were considered.
Models were fitted using restricted maximum likelihood (REML) analysis and selected via stepwise backward selection with the Akaike information criterion corrected for small sample size (AICc) as implemented in Design-Expert. Model diagnostics were performed by visual inspection of residual normality and dispersion. Outlier detection was performed via difference in fits (DFFITs) and studentized residuals plots. Due to the presence of heteroscedasticity in the data, a square root transformation was applied for the analysis.
Analytical methods
Cell counting
Ambr® 250 bioreactor cultures were automatically counted with the integrated BioProfile® FLEX2 analyzer (Nova Biomedical, Waltham, MA, United States) to enable the automatic adjustment of the perfusion rate. Additionally, to account for cell aggregation during the cultivation process (Supplementary Figure S1), cells were also counted offline with the Nucleocounter® NC-202™ (Chemometec, Allerod, Denmark). When the aggregation level reached 20%, samples were treated with Lysis 1 buffer (#910–0010, Chemometec, Allerod, Denmark) to disaggregate cells before counting. A conversion factor of 1.36 was considered between the BioProfile® FLEX2 and Nucleocounter® NC-202™ for the automatic perfusion rate adjustment to account for method differences.
Metabolites
Glucose and lactate were automatically measured with the BioProfile® FLEX2 analyzer.
Virus titer
To harvest the virus from the cell-containing suspension, samples were incubated with sodium chloride as described previously (Gautam et al., 2022). Afterwards, samples were centrifuged (1000 g, 5 min, RT), and the supernatant was aliquoted and stored at −80°C until analysis. Infectious virus titer was determined with the automated, label-free tissue culture infectious dose 50 (TCID50) assay as described previously (Hochdorfer et al., 2022). The geometrical mean of the titer was calculated from single measurements on three different days.
Calculations
Cell-specific virus yield
Cell-specific virus yield (CSVY, [TCID50 cell-1]) was calculated according to Equation (1) to account for the cell growth after infection. Accordingly, CSVY was determined by dividing the maximum observed infectious titer (Virmax, [TCID50 mL-1]) by the maximum VCD (VCDmax, [cells mL-1]) observed at TOI or thereafter (Coronel et al., 2019; Gränicher et al., 2019).
Space-time yield
Space-time yield (STY, [TCID50 mL-1 d-1]) was determined based on the maximum infectious titer and process time (t, [d]) as shown in Equation 2:
Volumetric productivity
The volumetric productivity (PV, [TCID50 mL-1 d-1]) was calculated according to Equation 3 based on the maximum virus titer, the working volume of the bioreactor (VW, [mL]) as well as the consumed medium (VMedium, [mL]) and process time until Virmax was reached:
Results
Cell growth with perfusion cultivation
Perfusion cultivation supports cell growth to high cell densities. To test the impact of medium exchange on the suspension HEK293 cell growth, processes with different CSPRs (0.029, 0.051 and 0.073 nL cell-1 d-1) were tested and compared to the reference batch cultivation process (Figure 1).
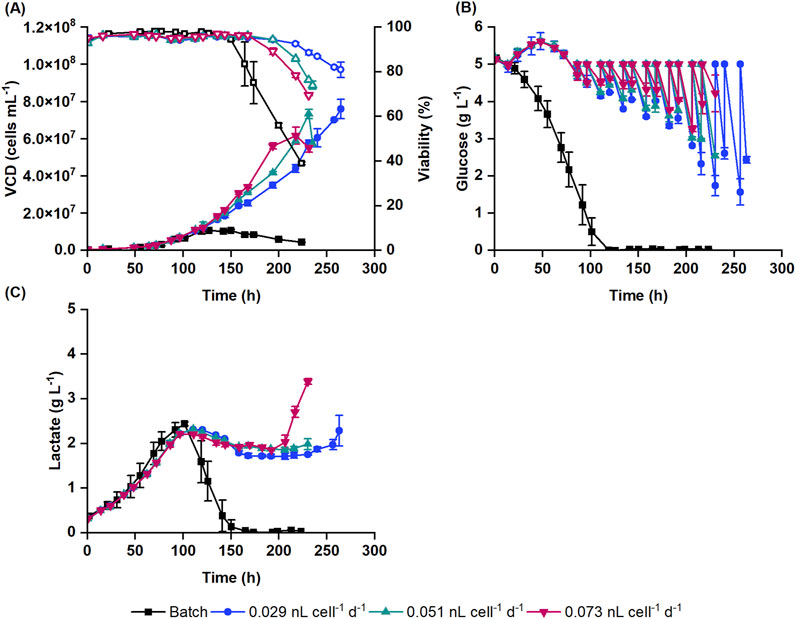
Figure 1. Suspension HEK293 cell cultivation in batch and perfusion cultivation mode with 0.029, 0.051 and 0.073 nL cell-1 d-1 cell-specific perfusion rates (CSPR). (A) Viable cell density (VCD) (filled symbols) and viability (empty symbols) obtained with Nucleocounter® NC-202™ are shown along with the (B) glucose and (C) lactate concentrations. Data represent the mean and standard deviation of biological duplicates.
Batch cultures reached a concentration of 10.5 ± 1.6 × 106 cells mL-1 at 119 h. In comparison, cells with the lowest perfusion rate (0.029 nL cell-1 d-1) reached a 6-fold higher VCD of 76.0 ± 5.2 × 106 cells mL-1. Similar VCD levels were reached at an earlier timepoint with 0.051 nL cell-1 d-1 perfusion rate (73.3 ± 2.4 × 106 cells mL-1). Cultures with 0.073 nL cell-1 d-1 medium exchange rate had a slightly lower final cell concentration (61.7 ± 4.6 × 106 cells mL-1) compared to other perfusion cultures (Figure 1A). This was accompanied by a faster increase in transmembrane pressure, which indicates filter clogging and decreased filtration efficiency. Overall, the growth rate of perfusion cultures slightly increased with the perfusion rate. The specific growth rates were 0.022, 0.025 and 0.027 h-1 at perfusion rates of 0.029, 0.051 and 0.073 nL cell-1 d-1, respectively. The viability of the perfusion cultures dropped below 90% under all conditions usually when the cell concentration reached between 56.1 × 106 and 59.0 × 106 cells mL-1 (Figure 1A). To cover the increasing oxygen demand of the cultures, the oxygen flow rate was continuously increased during the cultivation and reached 0.22–0.31 vessel volumes per minute (VVM) when the viability dropped below 90%. Consequently, this resulted in increased shear forces in the bioreactor due to bubble bursting, potentially causing higher cell death at higher cell densities. Additionally, bubbles trapped within the membrane may have reduced filtration efficiency, contributing to the decline in cell viability.
In batch cultures, glucose was completely depleted by 119 h, marking the end of the exponential cell growth phase (Figure 1B). Following this, cells began to consume lactate. As the lactate was depleted (from 150 h onwards), the cell density and viability started to decline (Figure 1C). In perfusion cultures, glucose was continuously supplied alongside the medium exchange, starting at 16 h post seeding. Initially, this led to a small increase in the glucose concentration. From 86 h onwards, as the glucose concentration dropped below 5 g L-1, a glucose bolus was applied to counterbalance the consumption of cells (Figure 1B). Cells produced lactate up to 2.2–2.3 g L-1. After 110 h, the lactate concentration gradually decreased to 1.7–1.9 g L-1 as the cells metabolized the lactate. Once the viability of the cells began to decline, the lactate concentration increased again in the bioreactor (Figure 1C).
For the subsequent DoE study, 0.051 nL cell-1 d-1 perfusion rate was selected, which promoted a higher specific growth rate than the 0.029 nL cell-1 d-1 perfusion rate, while also keeping the medium consumption within reasonable limits.
DoE and statistical model of the virus production
Process parameters with potential influence on the virus titer were evaluated in perfusion cultures with an I-optimal DoE. The seeding VCD (1 × 106, 2.5 × 106 and 4 × 106 cells mL-1) and TOI (74, 98 and 122 h) were varied to test different cell densities and timepoints for the infection. These levels were chosen to cover cell densities up to a maximum of 59.0 × 106 cells mL-1, where the culture’s viability begins to decline (Figure 1A). To ensure an efficient infection, the impact of MOI (0.0004, 0.004, 0.04) was also evaluated. The selection of MOI levels was based on the current MOI of 0.0004 in the reference batch process, which was set as a minimum threshold to maintain practical handling in large-scale production. A short perfusion pause of 1–15 h is frequently used after infection to facilitate virus entry into cells (Yuk et al., 2004; Gálvez et al., 2012; Genzel et al., 2014) and in certain instances, first virus replication cycles (Luitjens and Herk, 2011). Therefore, the study included perfusion pauses of 1, 8 and 16 h. Once the perfusion started again, the necessity of the medium exchange was tested with different CSPRs (0.015, 0.033 and 0.051 nL cell-1 d-1). The levels of CSPR were selected to balance between conditions known to support high viability and high cell density cultures (0.033 and 0.051 nL cell-1 d-1) (Figure 1A) and conditions (as low as 0.015 nL cell-1 d-1) to keep the medium consumption within an acceptable range. The shear-sensitivity of the virus and/or cells was tested by applying different crossflow rates (25, 40 and 70 mL min-1) that were either lower or higher than during the cell cultivation prior to infection and covered shear rates from 400 s-1 to 1500 s-1. The crossflow rate levels were chosen to fall within the recommended operational range of Ambr® 250 system specified by the manufacturer. In the reference batch process, the virus production kinetics reaches plateau within 46 hpi. Therefore, we expected to reach the maximum virus titer in perfusion by 46 hpi as well and collected virus samples at 22, 36, 40 and 46 hpi. 30 perfusion cultivations were planned for the DoE study and further 15 runs were added to improve the model quality and prediction (Supplementary Table S1).
The titers obtained in the DoE study varied in the range of two log10 steps between 7.1 × 108 and 5.6 × 1010 TCID50 mL-1 at 46 hpi (Figure 2). Raw data was fitted using REML, incorporating time as an easy-to-change factor to assess the time-dependency of the data and considering each bioreactor as a group. The residual analysis showed heteroscedasticity and therefore, the data was square root transformed. The validity of the fitted models was assessed by model diagnostics. A residual analysis was performed to verify assumptions of normally distributed and homoscedastic data as well as to detect outliers. A visual inspection was performed to assess whether the residuals of the datapoints approximate a straight line in the normal quantile plot and are randomly scattered around the zero line in the residuals vs predicted plot.
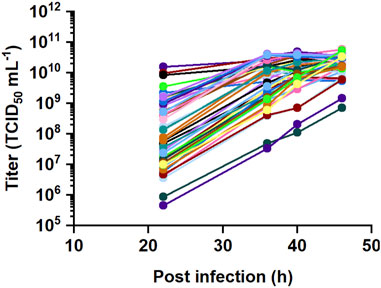
Figure 2. DoE study for recombinant VSV-GP production with 45 perfusion runs. Six input parameters were varied at three levels to maximize the virus titer, including seeding VCD, TOI, MOI as well as CSPR, perfusion pause and crossflow rate after infection.
The R2 of the model equaled 0.869, i.e., the model describes 86.9% of the variance within the data. Significance of the input parameters were analyzed considering main effects and interactions. The analysis of variance (ANOVA) of the whole-plot as well as the subplot (effect of DoE factors on kinetics) showed a significant model with p-values <0.0001 (Table 2). The coefficient of variation (CV) of the model, calculated as the root mean square error divided by the mean, was 32%, aligning well with the expected method variability. The datapoints followed the diagonal line in the actual vs predicted plot.
Increasing the seeding VCD or choosing a later timepoint for infection led to overall higher cell concentrations and significantly improved the titer (Figures 3A,B).
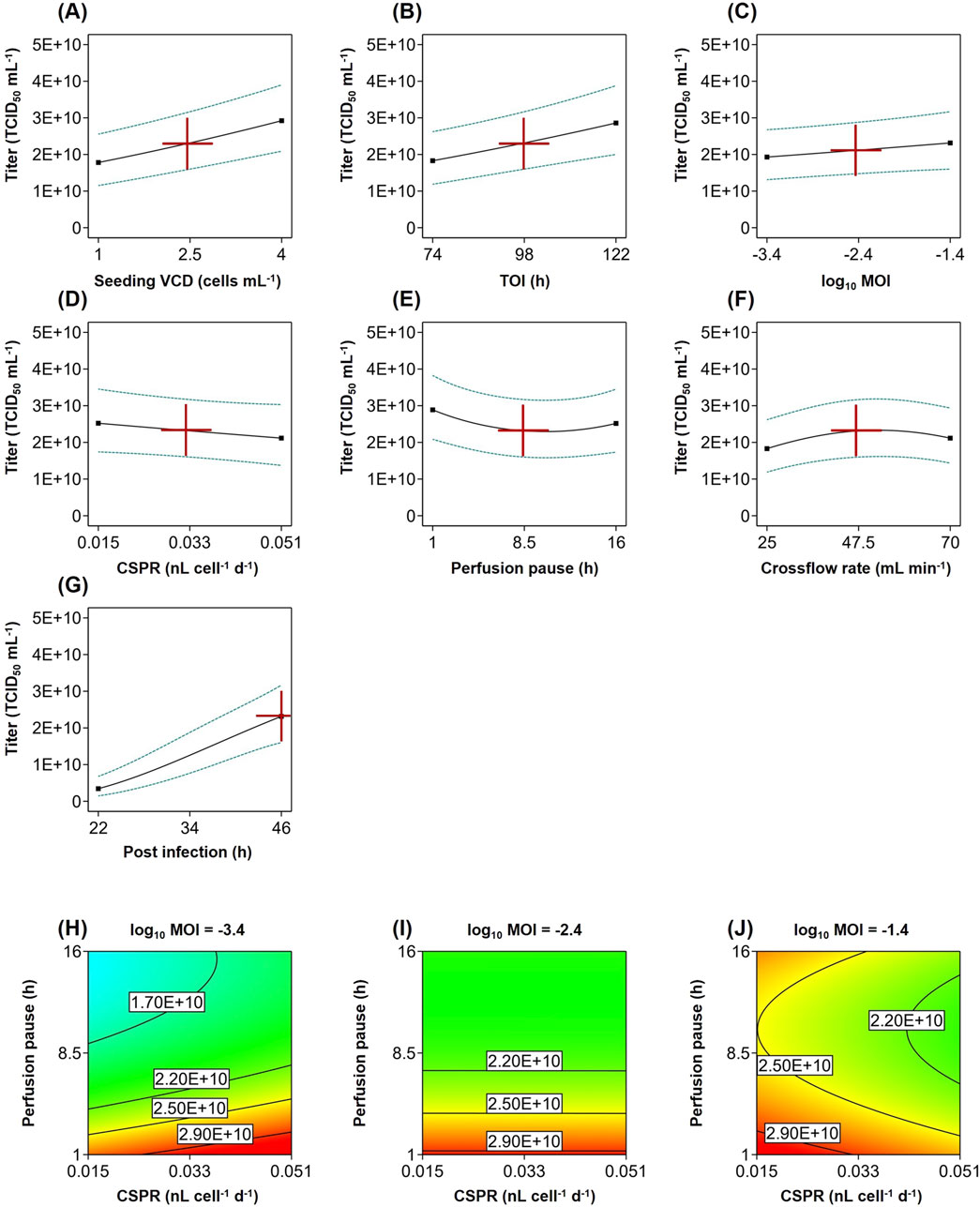
Figure 3. Statistical model of recombinant VSV-GP production in perfusion cultures. (A–G) Impact of input parameters on the infectious virus titer (solid line) with 95% confidence interval (dashed line) at center point condition at 46 hpi. (H–J) Interaction of MOI with the CSPR and perfusion pause after infection at three log10 MOI levels from −3.4 to −1.4 at center point condition at 46 hpi.
The MOI as well as its interaction with the CSPR and perfusion pause also had a significant effect on the titer (Table 2). While increasing the MOI generally had a positive impact (Figure 3C), the direction of the relationship with the titer depended on the parameter settings. For example, with 1 h perfusion pause and high 0.051 nL cell-1 d-1 perfusion rate, an MOI of 0.0004 (log10 MOI = −3.4) was more beneficial than a higher MOI of 0.04 (log10 MOI = −1.4) (Figures 3H,J). In processes with lower perfusion rate and longer perfusion pause, the titer increased with higher MOIs (Figures 3H–J).
Lowering the perfusion rate for the virus production generally decreased the titer (Figure 3D). Also, pausing the perfusion after the infection generally reduced the titer (Figure 3E). The interaction between the perfusion pause and the TOI suggests that pausing the perfusion had a greater impact on cultures, which were infected at later timepoints (Table 2).
The crossflow rate was only significant as a quadratic term, supporting higher titers in the middle of the tested range (Figure 3F).
Including the sampling time in the model enabled us to capture the virus production kinetics (Figure 3G) and its interaction with multiple input parameters. Applying a higher seeding cell density, higher MOI and higher cell-specific perfusion rate led to a faster increase in titer, whereas using a longer perfusion pause after infection hindered the virus production (Table 2).
Overall, all tested input parameters exhibited a substantial impact on the titer. The model analysis revealed multiple significant interactions between parameters as well as between parameters and time, underscoring the intricate relationships within the biological system. The fitted model showed a good quality, indicating that it can be used to optimize the input parameters to achieve high infectious titer.
Optimization of virus production with perfusion
The perfusion process was optimized to maximize the infectious virus titer at 46 hpi based on the fitted statistical model. Since the seeding cell density and the TOI both influence the medium consumption as well as the duration of the process, they also impact the PV. To assess this impact, four conditions were selected for optimization. Bioreactors were seeded at either 1 × 106 or 4 × 106 cells mL-1 and infected at either 74 or 122 h of cultivation. These optimized processes were compared to the batch production process in terms of titer, CSVY, STY and PV (Table 3; Figure 4).
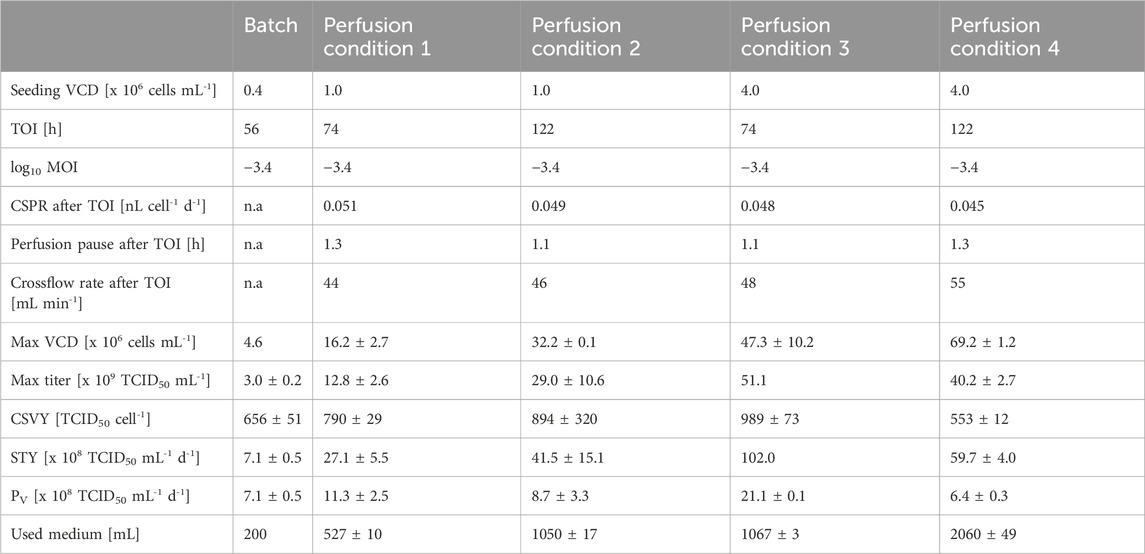
Table 3. Comparison of batch and perfusion processes for recombinant VSV-GP production. Perfusion cultures were seeded at 1 × 106 or 4 × 106 cells mL-1 and infected at either 74 or 122 h with an MOI of 0.0004. Values are reported as the mean and standard deviation of biological duplicates.
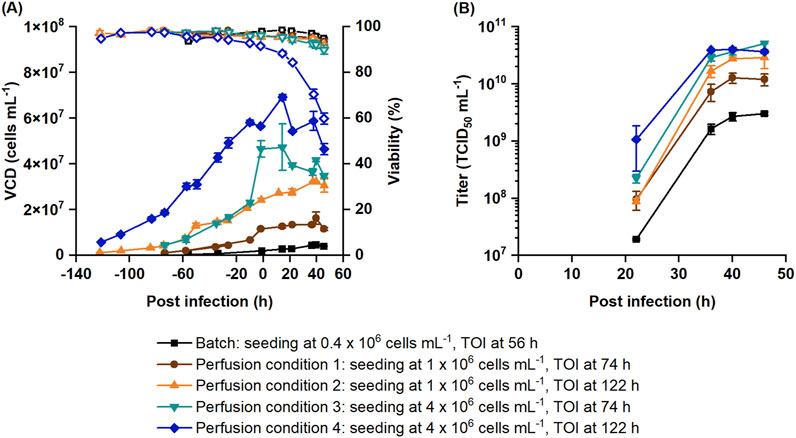
Figure 4. Optimized recombinant VSV-GP perfusion production processes in comparison to the batch production process. Cells were seeded at 1 × 106 or 4 × 106 cells mL-1 and infected at either 74 or 122 h. (A) VCD (filled symbols) and viability (empty symbols) obtained with Nucleocounter® NC-202™ are shown along with the (B) infectious titer. Data represent the mean and standard deviation of biological duplicates.
Regardless of the seeding VCD or TOI, the settings of the optimized parameters of the perfusion cultivations were similar (Table 3). Using the lower MOI of 0.0004, the perfusion should start 1.1–1.3 h after the infection with a comparable CSPR as before the infection (0.045–0.051 nL cell-1 d-1). The optimal range for crossflow rate was 44–55 mL min-1.
Reference batch cultures were infected at 2.0 ± 0.1 × 106 cells mL-1. Perfusion cultures reached VCDs between 11.6 ± 0.3 × 106 and 56.5 ± 0.1 × 106 cells mL-1 at the time of infection depending on the seeding VCD and time of infection. Batch and perfusion cultures with lower VCDs (condition 1 and 2) continued to grow after infection. Perfusion cultures with higher VCDs (condition 3 and 4) only showed minor cell growth post infection. The viability of perfusion cultures with the highest VCD (condition 4) rapidly decreased after infection to 59.8% ± 2.4% by 46 hpi. Presumably, this decrease in viability was triggered by the increased shear stress due to higher oxygen flow rates (0.29 ± 0.01 VVM) and bubble entrapment within the membrane (Figure 4A), which is consistent with earlier observations (Figure 1A).
While batch cultures had a titer of 3.0 ± 0.2 × 109 TCID50 mL-1, perfusion cultures produced between 1.3 × 1010 and 5.1 × 1010 TCID50 mL-1, which is 4.3–16.9-fold higher than in batch cultures (Table 3; Figure 4B). These results closely match the prediction of the statistical model (Supplementary Figure S2), i.e., the results were within the 95% prediction intervals of the model. As described in Table 3, the CSVY improved with perfusion, with a maximum observed for condition 3. However, perfusion cultures with the highest cell density (condition 4) had slightly lower CSVYs than the batch cultures (Table 3), which is possibly related to the lower cell viabilities after infection (Figure 4A).
Increasing the seeding cell density from 1 × 106 cells mL-1 to 4 × 106 cells mL-1 also increased the STY up to 3.8-fold and the PV by 1.9-fold. The highest STY of 1.0 × 1010 TCID50 mL-1 d-1 was achieved under perfusion condition 3, which is 14.4-fold higher than the STY with batch cultivation. Perfusion condition 3 also allowed to increase the PV by 3.0-fold compared to the batch cultivation. Perfusion conditions 1, 2 and 4 had a comparable PV to the batch cultures (Table 3).
In summary, perfusion cultivation allowed to infect cells up to 46.6 ± 3.6 × 106 cells mL-1 and increased both infectious titer and STY without a decrease in the CSVY compared to the reference batch cultivation. Also, increasing the seeding VCD and infecting cells at an earlier timepoint was beneficial to improve both STY and PV.
Discussion
This work aimed to increase the titer of the recombinant VSV-GP production process with suspension HEK293 cells by using TFF perfusion with virus retention. For this purpose, we initially investigated the growth of HEK293 cells with three different perfusion exchange rates. Subsequently, input parameters were screened and optimized for high infectious titer in Ambr® 250 bioreactors.
Perfusion cultivation enabled to reach high viability cell cultures (≥90%) up to maximum 59.0 × 106 cells mL-1, which is approximately 5-fold higher than in the classical batch cultivation process. Higher medium exchange rates slightly supported higher specific growth rates, reaching 0.022, 0.025 and 0.027 h-1 with perfusion rates of 0.029, 0.051 or 0.073 nL cell-1 d-1, respectively. These values are comparable to the previously reported specific growth rates of recombinant HEK293 cells obtained with ATF perfusion (up to approximately 0.6 d-1 or 0.025 h-1) (Schwarz et al., 2020) or perfusion with acoustic separator (0.03 h-1) (Henry et al., 2005). Once the cell cultures reached 56.1 × 106–59.0 × 106 cells mL-1, the viability gradually decreased while the cell density continued to increase. Schwarz et al. correlated the reduced viability of HEK293 cell cultures at high cell densities with the higher oxygen flow rate and stirring speed (Schwarz et al., 2020). While the stirring speed was constant in this study, the oxygen flow rate continuously increased to keep the DO at set point. Therefore, it is possible that high shear forces caused by bubble ruptures damaged the cells (Hu et al., 2011). In addition, it is well described that high shear stress should be avoided during virus production to minimize virus damage (Grein et al., 2019). Accordingly, cell cultures with viable cells densities above 59 × 106 cells mL-1 were not considered for oncolytic VSV-GP production in this study.
To optimize the perfusion cultivation process, a DoE was conducted, and the VSV-GP production was modeled as a function of the studied parameters and their influence on the kinetics. The model revealed a complex relationship between the parameters and the titer of the perfusion process. Each input parameter had a significant impact on the titer. Additionally, several significant interactions were observed, reflecting the complexity of the biological system, where the effect of one factor changes as another factor is varied. The model exhibited a high R2 value of 86.9%, indicating that the main sources of variance were identified. However, a portion of the variance remains unexplained, which might suggest even more complex kinetics in the perfusion process. Further investigation into these effects and the kinetics could provide additional insights.
The concentration of viable cells as available substrate for the virus production has a substantial impact on the attainable titer. Therefore, increasing the seeding cell density was beneficial to increase the infectious titer. Similarly, choosing a later timepoint for infection also improved the titer.
Besides the improved titer, increasing the seeding cell density from 1 × 106 cells mL-1 to 4 × 106 cells mL-1 also increased the STY up to 3.8-fold and the PV up to 1.9-fold. To meet the high cell demand for seeding bioreactors at higher cell densities, the volume of N-1 stage cultures had to be increased. Additionally, N-1 stage cultures had to be concentrated by centrifugation to ensure that a maximum split ratio of 20% is not exceeded in the N-stage bioreactor. The implementation of this strategy is challenging in large-scale production. Several authors have instead proposed N-1 perfusion to enable higher seeding cell densities for the N-stage fed-batch process. The goal has been to decrease the process time (Yang et al., 2014) or increase the titer (Stepper et al., 2020). Due to the high cell density of N-1 perfusion cultures, the number and size of the vessels in the seed train can also be reduced, which shortens the total cultivation time of the seed train (Stepper et al., 2020).
The optimal crossflow rate of the virus production process lay in the range between 44 and 55 mL min-1, which corresponds to a maximum shear rate of approximately 950–1150 s-1 in the perfusion loop as represented in the tubing and fittings. By increasing the crossflow rate, the shear rate also increased (1500 s-1 with 70 mL min-1 crossflow rate) and reduced the infectious virus titer. Zhang et al. recommended the threshold of 1266 s-1 shear rate to avoid shear-induced cell damage in hybridoma cell cultures during perfusion (Zhang et al., 1993). This threshold was exceeded in cultures with crossflow rate above 60 mL min-1. In another study, however, no considerable decrease in the cell growth has been observed at 2482 s-1 shear rate for HEK293 cells (Zhan et al., 2020). Hydrodynamic shear stress can also damage viruses (Loewe et al., 2019). Interestingly, crossflow rates below the optimal range (44–55 mL min-1) also led to a slight decrease in titer. This decrease may be attributed to non-specific virus adsorption to the mPES membrane. Additionally, applying a lower crossflow rate increases the residence time in the perfusion loop, which represents a non-controlled environment and might negatively impact the process (Chotteau, 2014). Further studies are necessary to understand the impact of residence time in more depth.
A short perfusion pause after infection is often used in virus production processes to minimize the virus washout in the external perfusion filter module. This practice is particularly important for processes with low MOIs to ensure an efficient virus infection (Yuk et al., 2004; Gálvez et al., 2012; Genzel et al., 2014). Although the filter cut-off in this study was smaller than the virus, pausing the perfusion after infection may prevent non-specific adsorption of the virus to the membrane, which could reduce the effective MOI. Our study indicates that a perfusion pause of 1.1–1.3 h is optimal for supporting VSV-GP entry into cells. Longer perfusion pauses were found to hinder the virus production. In an adenoviral vector production process, the decreased virus productivity was linked to the reduced metabolic activity of HEK293 cells (Henry et al., 2004). A following study found that HEK293 cells exhibited a lower ATP production rate and virus yield when subjected to low perfusion rates at high cell density (Henry et al., 2005). Therefore, it is possible that the metabolic activity of HEK293 cells also decreased during a longer perfusion pause, leading to a reduced virus titer. Furthermore, we also observed lower titers at lower medium exchange rates. Applying a higher MOI can counterbalance the impact of lower perfusion rate and decrease medium consumption. Alternatively, to spare on the expensive virus seed stock, a higher perfusion rate and a lower MOI can be employed.
Only a few perfusion cultivation processes have been reported for VSV production so far. Paillet et al. showed the feasibility to use suspension Vero cell cultures for VSV production in perfusion (Paillet et al., 2009). Recently, a perfusion process using tangential flow depth filtration (TFDF) was reported for producing VSV-based vectors with suspension BHK-21 and HEK293-SF cells with continuous virus harvest. In the HEK293-SF cell-based process, cells reached a maximum concentration of 11.3 × 106 cells mL-1 and exhibited 1.9 and 1.1-fold higher STY compared to the batch culture (Göbel et al., 2024). Our work additionally shows the feasibility of using TFF perfusion with virus retention to increase the viral output of the oncolytic VSV-GP production process. Furthermore, the optimized TFF process allowed to infect cultures at a wide range of cell density (up to 46.6 × 106 cells mL-1) without a decrease in CSVY. The optimized process exhibited up to 14.4-fold higher STY compared to the batch cultivation.
Conclusion
This work has improved the oncolytic VSV-GP production process with suspension HEK293 cells by using TFF perfusion. The optimized TFF process allowed to achieve up to 16.9-fold higher titer (5.1 × 1010 TCID50 mL-1), up to 14.4-fold higher STY and up to 3.0-fold increase in the PV compared to the batch cultivation. Due to the higher titers and higher levels of impurities (such as cell debris, host cell DNA, host cell protein) in high cell density culture harvests, modifications to the reference downstream process, based on batch cultivation, might become necessary. These modifications may involve adding a centrifugation step before depth filtration and optimizing the depth filtration and chromatography steps to handle higher loads and impurities. Overall, this work presents the potential of the TFF perfusion process for efficiently producing oncolytic VSV-GP.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
OH: Conceptualization, Formal Analysis, Investigation, Methodology, Visualization, Writing – original draft. AW: Formal Analysis, Methodology, Writing – review and editing. KH: Investigation, Writing – review and editing. SM: Investigation, Writing – review and editing. DB: Investigation, Writing – review and editing. IG: Writing – review and editing. AB: Conceptualization, Methodology, Writing – review and editing. JS-R: Supervision, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We would like to express our gratitude to Marco Kunzelmann for his valuable assistance in planning the experiments. Additionally, we would like to thank Pablo Urtlauf and Rebecca Habisch for their valuable support in conducting the experiments.
Conflict of interest
Authors OH, AW, KH, SM, DB, IG, AB, and JS were employed by Boehringer Ingelheim Pharma GmbH & Co. KG.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2025.1588293/full#supplementary-material
Abbreviations
AICc, Akaike information criterion corrected for small sample size; ANOVA, analysis of variance; ATF, alternating tangential flow filtration; BHK-21, baby hamster kidney 21 cells; CV, coefficient of variation; CSPR, cell-specific perfusion rate; DFFITs, difference in fits; DO, dissolved oxygen; DoE, design of experiments; G, glycoprotein of VSV; GP, glycoprotein of LCMV; HEK293, human embryonic kidney 293 cells; hpi, hours post infection; LCMV, lymphocytic choriomeningitis virus; MOI, multiplicity of infection; mPES, modified polyether sulfone; OLS, ordinary least square; PV, volumetric productivity; REML, restricted maximum likelihood analysis; RV, reactor volume; STY, space-time yield; TCID50, tissue culture infectious dose 50 assay; TFDF, tangential flow depth filtration; TFF, tangential flow filtration; TOI, time of infection; VCD, viable cell density; VSV, vesicular stomatitis virus; VSV-GP, vesicular stomatitis virus variant that was pseudotyped with LCMV-GP; VVM, vessel volumes per minute.
References
Ausubel, L. J., Meseck, M., Derecho, I., Lopez, P., Knoblauch, C., McMahon, R., et al. (2011). Current good manufacturing practice production of an oncolytic recombinant vesicular stomatitis viral vector for cancer treatment. Hum. Gene Ther. 22, 489–497. doi:10.1089/hum.2010.159
Bock, A., Schulze-Horsel, J., Schwarzer, J., Rapp, E., Genzel, Y., and Reichl, U. (2011). High-density microcarrier cell cultures for influenza virus production. Biotechnol. Prog. 27, 241–250. doi:10.1002/btpr.539
Chotteau, V. (2014). “Perfusion processes,” in Animal cell culture. Cell eng. Editor M. Al-Rubeau (Charm: Springer), 9, 407–443. doi:10.1007/978-3-319-10320-4_13
Coronel, J., Behrendt, I., Bürgin, T., Anderlei, T., Sandig, V., Reichl, U., et al. (2019). Influenza A virus production in a single-use orbital shaken bioreactor with ATF or TFF perfusion systems. Vaccine 37, 7011–7018. doi:10.1016/j.vaccine.2019.06.005
Dold, C., Urbiola, C. R., Wollmann, G., Egerer, L., Muik, A., Bellmann, L., et al. (2016). Application of interferon modulators to overcome partial resistance of human ovarian cancers to VSV-GP oncolytic viral therapy. Mol. Ther. - Oncolytics 3, 16021. doi:10.1038/mto.2016.21
Elahi, S. M., Shen, C. F., and Gilbert, R. (2019). Optimization of production of vesicular stomatitis virus (VSV) in suspension serum-free culture medium at high cell density. J. Biotechnol. 289, 144–149. doi:10.1016/j.jbiotec.2018.11.023
Gálvez, J., Lecina, M., Solà, C., Cairó, J. J., and Gòdia, F. (2012). Optimization of HEK-293S cell cultures for the production of adenoviral vectors in bioreactors using on-line OUR measurements. J. Biotechnol. 157, 214–222. doi:10.1016/j.jbiotec.2011.11.007
Gautam, S., Xin, D., Garcia, A. P., and Spiesschaert, B. (2022). Single-step rapid chromatographic purification and characterization of clinical stage oncolytic VSV-GP. Front. Bioeng. Biotechnol. 10, 992069. doi:10.3389/fbioe.2022.992069
Gélinas, J.-F., Azizi, H., Kiesslich, S., Lanthier, S., Perdersen, J., Chahal, P. S., et al. (2019). Production of rVSV-ZEBOV in serum-free suspension culture of HEK 293SF cells. Vaccine 37, 6624–6632. doi:10.1016/j.vaccine.2019.09.044
Genzel, Y., Vogel, T., Buck, J., Behrendt, I., Ramirez, D. V., Schiedner, G., et al. (2014). High cell density cultivations by alternating tangential flow (ATF) perfusion for influenza A virus production using suspension cells. Vaccine 32, 2770–2781. doi:10.1016/j.vaccine.2014.02.016
Göbel, S., Kortum, F., Chavez, K. J., Jordan, I., Sandig, V., Reichl, U., et al. (2022). Cell-line screening and process development for a fusogenic oncolytic virus in small-scale suspension cultures. Appl. Microbiol. Biotechnol. 106, 4945–4961. doi:10.1007/s00253-022-12027-5
Göbel, S., Pelz, L., Silva, C. A. T., Brühlmann, B., Hill, C., Altomonte, J., et al. (2024). Production of recombinant vesicular stomatitis virus-based vectors by tangential flow depth filtration. Appl. Microbiol. Biotechnol. 108, 240. doi:10.1007/s00253-024-13078-6
Gränicher, G., Coronel, J., Pralow, A., Marichal-Gallardo, P., Wolff, M., Rapp, E., et al. (2019). Efficient influenza A virus production in high cell density using the novel porcine suspension cell line PBG.PK2.1. Vaccine 37, 7019–7028. doi:10.1016/j.vaccine.2019.04.030
Grein, T. A., Loewe, D., Dieken, H., Weidner, T., Salzig, D., and Czermak, P. (2019). Aeration and shear stress are critical process parameters for the production of oncolytic measles virus. Front. Bioeng. Biotechnol. 7, 78. doi:10.3389/fbioe.2019.00078
Hastie, E., Cataldi, M., Marriott, I., and Grdzelishvili, V. Z. (2013). Understanding and altering cell tropism of vesicular stomatitis virus. Virus Res. 176, 16–32. doi:10.1016/j.virusres.2013.06.003
Henry, O., Dormond, E., Perrier, M., and Kamen, A. (2004). Insights into adenoviral vector production kinetics in acoustic filter-based perfusion cultures. Biotechnol. Bioeng. 86, 765–774. doi:10.1002/bit.20074
Henry, O., Perrier, M., and Kamen, A. (2005). Metabolic flux analysis of HEK-293 cells in perfusion cultures for the production of adenoviral vectors. Metab. Eng. 7, 467–476. doi:10.1016/j.ymben.2005.08.002
Hochdorfer, D., Businger, R., Hotter, D., Seifried, C., and Solzin, J. (2022). Automated, label-free TCID50 assay to determine the infectious titer of virus-based therapeutics. J. Virol. Methods 299, 114318. doi:10.1016/j.jviromet.2021.114318
Hu, W., Berdugo, C., and Chalmers, J. J. (2011). The potential of hydrodynamic damage to animal cells of industrial relevance: current understanding. Cytotechnology 63, 445–460. doi:10.1007/s10616-011-9368-3
Joe, C. C. D., Segireddy, R. R., Oliveira, C., Berg, A., Li, Y., Doultsinos, D., et al. (2021). Accelerating manufacturing to enable large-scale supply of a new adenovirus-vectored vaccine within 100 days. bioRxiv 2021. doi:10.1101/2021.12.22.473478
Kamen, A., and Henry, O. (2004). Development and optimization of an adenovirus production process. J. Gene Med. 6, S184–S192. doi:10.1002/jgm.503
Kiesslich, S., Kim, G. N., Shen, C. F., Kang, C. Y., and Kamen, A. A. (2021). Bioreactor production of rVSV-based vectors in Vero cell suspension cultures. Biotechnol. Bioeng. 118, 2649–2659. doi:10.1002/bit.27785
Kiesslich, S., Losa, J. P. V.-C., Gélinas, J.-F., and Kamen, A. A. (2020). Serum-free production of rVSV-ZEBOV in Vero cells: microcarrier bioreactor versus scale-XTM hydro fixed-bed. J. Biotechnol. 310, 32–39. doi:10.1016/j.jbiotec.2020.01.015
Kimpel, J., Urbiola, C., Koske, I., Tober, R., Banki, Z., Wollmann, G., et al. (2018). The oncolytic virus VSV-GP is effective against malignant melanoma. Viruses 10, 108. doi:10.3390/v10030108
Lavado-García, J., Cervera, L., and Gòdia, F. (2020). An alternative perfusion approach for the intensification of virus-like particle production in HEK293 cultures. Front. Bioeng. Biotechnol. 8, 617. doi:10.3389/fbioe.2020.00617
Lim, H. S., Chang, K. H., and Kim, J. H. (1999). Effect of oxygen partial pressure on production of animal virus (VSV). Cytotechnology 31, 265–270. doi:10.1023/a:1008060502532
Lindsay, D. A., and Betenbaugh, M. J. (1992). Quantification of cell culture factors affecting recombinant protein yields in baculovirus-infected insect cells. Biotechnol. Bioeng. 39, 614–618. doi:10.1002/bit.260390605
Loewe, D., Häussler, J., Grein, T. A., Dieken, H., Weidner, T., Salzig, D., et al. (2019). Forced degradation studies to identify critical process parameters for the purification of infectious measles virus. Viruses 11, 725. doi:10.3390/v11080725
Luitjens, A., and Herk, H. van (2011). Method for the production of AD26 adenoviral vectors. WO 2011/098592 A1.
Manahan, M., Nelson, M., Cacciatore, J. J., Weng, J., Xu, S., and Pollard, J. (2019). Scale-down model qualification of ambr® 250 high-throughput mini-bioreactor system for two commercial-scale mAb processes. Biotechnol. Prog. 35, e2870. doi:10.1002/btpr.2870
Maranga, L., Brazão, T. F., and Carrondo, M. J. T. (2003). Virus-like particle production at low multiplicities of infection with the baculovirus insect cell system. Biotechnol. Bioeng. 84, 245–253. doi:10.1002/bit.10773
Muik, A., Stubbert, L. J., Jahedi, R. Z., Geiβ, Y., Kimpel, J., Dold, C., et al. (2014). Re-engineering vesicular stomatitis virus to abrogate neurotoxicity, circumvent humoral immunity, and enhance oncolytic potency. Cancer Res. 74, 3567–3578. doi:10.1158/0008-5472.can-13-3306
Nikolay, A., Léon, A., Schwamborn, K., Genzel, Y., and Reichl, U. (2018). Process intensification of EB66® cell cultivations leads to high-yield yellow fever and Zika virus production. Appl. Microbiol. Biotechnol. 102, 8725–8737. doi:10.1007/s00253-018-9275-z
Ozturk, S., and Kompala, D. (2005). Optimization of high cell density perfusion bioreactors. Biotechnol. Bioprocess. 30, 387–416. doi:10.1201/9780849351068.ch11
Paillet, C., Forno, G., Kratje, R., and Etcheverrigaray, M. (2009). Suspension-Vero cell cultures as a platform for viral vaccine production. Vaccine 27, 6464–6467. doi:10.1016/j.vaccine.2009.06.020
Pollock, J., Ho, S. V., and Farid, S. S. (2013). Fed-batch and perfusion culture processes: economic, environmental, and operational feasibility under uncertainty. Biotechnol. Bioeng. 110, 206–219. doi:10.1002/bit.24608
Rosen, O., Jayson, A., Goldvaser, M., Dor, E., Monash, A., Levin, L., et al. (2022). Optimization of VSV-ΔG-spike production process with the Ambr15 system for a SARS-COV-2 vaccine. Biotechnol. Bioeng. 119, 1839–1848. doi:10.1002/bit.28088
Schwarz, H., Zhang, Y., Zhan, C., Malm, M., Field, R., Turner, R., et al. (2020). Small-scale bioreactor supports high density HEK293 cell perfusion culture for the production of recombinant Erythropoietin. J. Biotechnol. 309, 44–52. doi:10.1016/j.jbiotec.2019.12.017
Shalhout, S. Z., Miller, D. M., Emerick, K. S., and Kaufman, H. L. (2023). Therapy with oncolytic viruses: progress and challenges. Nat. Rev. Clin. Oncol. 20, 160–177. doi:10.1038/s41571-022-00719-w
Stepper, L., Filser, F. A., Fischer, S., Schaub, J., Gorr, I., and Voges, R. (2020). Pre-stage perfusion and ultra-high seeding cell density in CHO fed-batch culture: a case study for process intensification guided by systems biotechnology. Bioprocess Biosyst. Eng. 43, 1431–1443. doi:10.1007/s00449-020-02337-1
Tai, M., Ly, A., Leung, I., and Nayar, G. (2015). Efficient high-throughput biological process characterization: definitive screening design with the Ambr250 bioreactor system. Biotechnol. Prog. 31, 1388–1395. doi:10.1002/btpr.2142
Tapia, F., Vázquez-Ramírez, D., Genzel, Y., and Reichl, U. (2016). Bioreactors for high cell density and continuous multi-stage cultivations: options for process intensification in cell culture-based viral vaccine production. Appl. Microbiol. Biotechnol. 100, 2121–2132. doi:10.1007/s00253-015-7267-9
Vázquez-Ramírez, D., Genzel, Y., Jordan, I., Sandig, V., and Reichl, U. (2018). High-cell-density cultivations to increase MVA virus production. Vaccine 36, 3124–3133. doi:10.1016/j.vaccine.2017.10.112
Wang, S., Godfrey, S., Ravikrishnan, J., Lin, H., Vogel, J., and Coffman, J. (2017). Shear contributions to cell culture performance and product recovery in ATF and TFF perfusion systems. J. Biotechnol. 246, 52–60. doi:10.1016/j.jbiotec.2017.01.020
Wu, Y., Bissinger, T., Genzel, Y., Liu, X., Reichl, U., and Tan, W.-S. (2021). High cell density perfusion process for high yield of influenza A virus production using MDCK suspension cells. Appl. Microbiol. Biotechnol. 105, 1421–1434. doi:10.1007/s00253-020-11050-8
Xu, P., Clark, C., Ryder, T., Sparks, C., Zhou, J., Wang, M., et al. (2017). Characterization of TAP Ambr 250 disposable bioreactors, as a reliable scale-down model for biologics process development. Biotechnol. Prog. 33, 478–489. doi:10.1002/btpr.2417
Yang, W. C., Lu, J., Kwiatkowski, C., Yuan, H., Kshirsagar, R., Ryll, T., et al. (2014). Perfusion seed cultures improve biopharmaceutical fed-batch production capacity and product quality. Biotechnol. Prog. 30, 616–625. doi:10.1002/btpr.1884
Yuk, I. H. Y., Olsen, M. M., Geyer, S., and Forestell, S. P. (2004). Perfusion cultures of human tumor cells: a scalable production platform for oncolytic adenoviral vectors. Biotechnol. Bioeng. 86, 637–642. doi:10.1002/bit.20158
Zhan, C., Bidkhori, G., Schwarz, H., Malm, M., Mebrahtu, A., Field, R., et al. (2020). Low shear stress increases recombinant protein production and high shear stress increases apoptosis in human cells. iScience 23, 101653. doi:10.1016/j.isci.2020.101653
Zhang, S., Handa-Corrigan, A., and Spier, R. E. (1993). A comparison of oxygenation methods fro high-density perfusion culture of animal cells. Biotechnol. Bioeng. 41, 685–692. doi:10.1002/bit.260410702
Keywords: vesicular stomatitis virus, oncolytic virus, perfusion, tangential flow filtration, design of experiments
Citation: Hamusics O, Wittmann A, Hasler K, Müller S, Birk D, Gorr IH, Brix A and Soza-Ried J (2025) Perfusion process with tangential flow filtration for oncolytic VSV-GP production. Front. Bioeng. Biotechnol. 13:1588293. doi: 10.3389/fbioe.2025.1588293
Received: 05 March 2025; Accepted: 12 May 2025;
Published: 30 May 2025.
Edited by:
David W. Wood, The Ohio State University, United StatesReviewed by:
Ezhaveni Sathiyamoorthi, Yeungnam University, Republic of KoreaJuan Camilo Gonzalez Rivera, Bristol Myers Squibb, United States
Jianqi Nie, Jiangnan University, China
Copyright © 2025 Hamusics, Wittmann, Hasler, Müller, Birk, Gorr, Brix and Soza-Ried. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Orsolya Hamusics, b3Jzb2x5YS5oYW11c2ljc0Bib2VocmluZ2VyLWluZ2VsaGVpbS5jb20=
 Orsolya Hamusics
Orsolya Hamusics Anja Wittmann
Anja Wittmann Katrin Hasler1
Katrin Hasler1 Alexander Brix
Alexander Brix Jorge Soza-Ried
Jorge Soza-Ried